Abstract
Background
We evaluated the impact of infectious disease (ID) syndromes on US active duty (AD) servicemembers returning from overseas deployment (DEP), military training exercises (EXR), or short-term military travel (eg, temporary assignment of duty [TDY]).
Methods
We conducted a survey-based assessment of US AD servicemembers returning from DEP, EXR, or TDY between 2015 and 2019. Subjects completed a post-travel survey capturing symptoms of travelers’ diarrhea (TD), influenza-like illness (ILI), and febrile illness (FI). Risk factors associated with any ID syndrome (ie, either TD, ILI, or FI) that impacted daily activities were assessed using a logistic regression model with backward selection.
Results
One-third of servicemembers (654/1822) experienced an ID syndrome, and 26% (471/1822) reported a ≥50% reduction in activity level due to an ID syndrome (median duration, 3 days). TD was the most common ID syndrome experienced and accounted for 73% (346/471) of ID syndromes impacting daily activities. The greatest impact of ID syndromes was observed in servicemembers on DEP. Compared with servicemembers on EXR or TDY, those on DEP had a longer duration of travel and a delayed period of risk for ID syndromes. Multivariate analysis identified high-risk exposures (ie, environmental exposures, close contact with locals, consuming food from street vendors) and behaviors (ie, inability to sanitize hands before meals) that could be used to inform mitigation strategies.
Conclusions
ID syndromes result in a significant loss of productivity during military travel. Addressing modifiable risk factors and access to TD self-treatment in high-risk settings may help mitigate the impact of ID threats during military travel.
Keywords: geographic medicine, global health, military, prevalence, prevention, travel, travelers’ diarrhea
Infectious disease (ID) syndromes such as travelers’ diarrhea (TD), influenza-like illness (ILI), and undifferentiated febrile illness (FI) remain a persistent threat to military forces traveling to resource-limited countries. Estimating the risk and impact of ID syndromes on military operations is challenging due to the interplay of several factors including differences in the regional epidemiology, environmental factors, host risk behaviors, and access to mitigation strategies. The first year of US military operations in Iraq was significantly impacted by high TD attack rates (43.7 cases/100 person-months), particularly during the combat phase of operations (52.9 cases/100 person-months) [1–3]. The high attack rates and resulting decrease in work performance created transient critical shortages that impacted operational readiness. In contrast, a systematic review of publications on military and civilian travelers between 1990 and 2005 suggested a decline in TD incidence over time and a lower incidence in longer-term (>1 month) travelers (29 cases/100 person-months) [4, 5]. Varying estimates for respiratory infection attack rates and impact on military operations have also been reported. Among servicemembers deployed in support of Operation Iraqi Freedom and Operation Enduring Freedom, the cumulative incidence of respiratory infections and the proportion reporting decreased job performance due to the infection increased from 39% and 14%, respectively, in 2003–2004, to 69% and 34%, respectively, in 2005–2006 [3, 6]. In a study of US military personnel deployed to Southwest Asia and the Middle East between 2006 and 2007, respiratory infections were associated with more frequent health system utilization and decreased work performance despite a lower incidence than acute diarrhea [2].
Contemporary surveillance data evaluating the incidence and impact of ID syndromes on military travel, ranging from short-term assignments for a few weeks at single location (eg, temporary assignment of duty [TDY]) to military exercises (EXR) and deployments (DEP), are needed for informing mitigation strategies and Force Health Protection (FHP) decision-making. We conducted a subgroup analysis of US Department of Defense (DoD) active duty servicemembers enrolled in a prospective, multicenter observational study of DoD beneficiaries traveling outside the continental United States for ≤6.5 months: Deployment and Travel Related Infectious Disease Risk Assessment, Outcomes, and Prevention Strategies Among DoD Beneficiaries (TravMil). The survey-based study design allows for the capture of environmental exposures, behavioral risk factors, and symptom severity and treatment and provides a better estimate of the incidence rates, risk factors, and effectiveness of mitigation strategies compared with passive surveillance. The aim of this analysis was to evaluate the incidence of TD, ILI, and FI during military travel, their impact on daily activities, and the use of antibiotics for self-treatment. We also evaluated the risk factors associated with ID syndromes impacting daily activities.
METHODS
The TravMil study was approved by the Uniformed Services University Institutional Review Board (Bethesda, MD, USA). Consenting adults traveling outside the continental US were enrolled pretravel or within 8 weeks of return at 1 of 8 US military facilities (7 in the United States and 1 in Landstuhl, Germany). Travelers with itineraries limited to regions with a low risk of travel-related infections (ie, Western or Northern Europe, Canada, or New Zealand) were excluded. Demographic and itinerary data including the purpose of travel and operation name were collected and used to categorize military travel as DEP, TDY, or EXR. Geographic regions were based on the US DoD areas of responsibility (AOR) for command and control of US military forces (Figure 1): US Southern Command (SOUTHCOM), US Africa command (AFRICOM), US Central Command (CENTCOM), US Northern Command (NORTHCOM—limited to Mexico and the Caribbean in this analysis as travel to Canada was exclusionary), US European Command (EUCOM), and US Indo-Pacific Command (INDOPACOM). Subjects traveling to >1 AOR were assigned to a multiregional category. Subjects could enroll in the study for multiple trips, and each trip counted as a unique enrollment. Travel to a malaria-endemic region and prescriptions for malaria chemoprophylaxis were abstracted from the medical encounter. Pretravel enrollees were instructed to complete a travel diary, noting any diarrheal episodes or subjective fever during travel, and complete a post-travel survey within 8 weeks of return. Subjects enrolled post-travel completed a post-travel survey. The post-travel survey captured risk behaviors, compliance with malaria chemoprophylaxis, and episodes of TD, ILI, or FI. Syndromic definitions of TD (“Did you experience TD, defined as ≥3 loose stools in 24 hours or >2 loose stools in 24 hours with associated nausea/vomiting, abdominal pain, fever, or visible blood in stool during travel?”), ILI (“Did you experience symptoms of a flu-like illness [defined as a fever associated with either a sore throat or cough] during travel?”), and FI (“Did you develop a fever not associated with diarrhea or a flu-like illness during travel?”) were provided in the survey. Subjects reporting an ID syndrome were questioned about specific symptoms of TD (nausea, blood in stool, abdominal cramps, subjective fever), ILI (subjective fever, sore throat, cough, stuffy nose, headache, rash, muscle pain, joint pain, nausea/vomiting), FI (fatigue, jaundice, appetite loss, abdominal pain, headache, rash, muscle/joint pain, rapid breathing, nausea/vomiting), the impact of symptoms on daily activities (number of days that their activity level was reduced by ≥50% and number of days they felt completely unable to participate in daily activities), and treatment. The current analysis included active duty servicemembers enrolled between March 2015 and December 2019 who traveled for a military purpose and completed a post-travel survey.
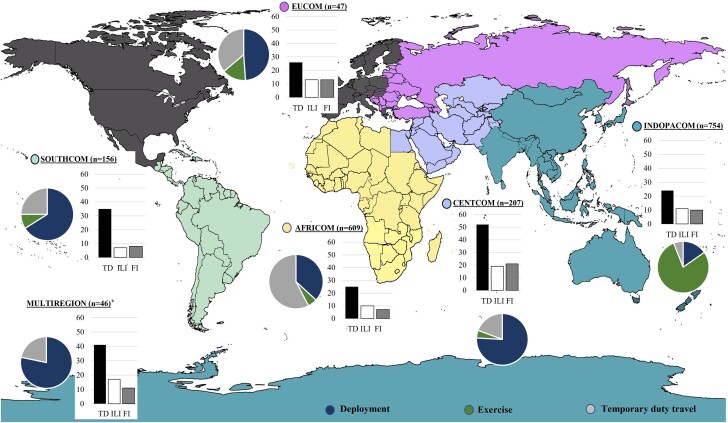
Figure 1.
Proportion of servicemembers on deployment (DEP), military exercises (EXR), and temporary duty assignments (TDY) and proportion reporting travelers’ diarrhea (TD), influenza-like illness (ILI), or febrile illness (FI) in each region. Travel limited to the continental United States, Canada, or Western Europe was excluded (shaded in gray)a. aGeographic regions were based on the United States Department of Defense areas of responsibility for command and control of US military forces: US Southern Command (SOUTHCOM), US Africa command (AFRICOM), US Central Command (CENTCOM), US European Command (EUCOM), and US Indo-Pacific Command (INDOPACOM). Three subjects went to regions assigned to US Northern Command (NORTHCOM) and did not report any infections and were excluded from the figure. bMultiregion travel included AFRICOM + CENTCOM: 16 subjects (35%); AFRICOM + EUCOM: 14 subjects (30%); CENTCOM + EUCOM + AFRICOM: 4 subjects (9%). The original file prior to adaptation for the study (File: White World Map Blank.png) is licensed under the Creative Commons Attribution-Share Alike 3.0 Unported license via Wikimedia Commons and freedom was granted to copy, distribute, and remix (adapt) the work. Original author of the unaltered file is $200inaire.
Analysis Plan
We evaluated the proportion of servicemembers who experienced TD, ILI, or FI by geographic region and type of travel (DEP, TDY, or EXR), incidence rate, use of antibiotics, and impact on daily activities. TD was further categorized as mild (ie, acute watery diarrhea with a normal activity level), moderate (ie, acute watery diarrhea with a ≥50% decrease in daily activities), and severe (ie, dysentery or febrile diarrhea). For pretravel enrollees who completed a post-travel survey and diary, report of an ID syndrome in either the diary or survey and the highest severity reported were included in the analysis. Incidence rates were calculated as the number of cases/100 person-months of travel. A chi-square or Fisher exact test was used for categorical values, and the Wilcoxon-Mann-Whitney test for continuous variables. For subjects who experienced multiple ID syndromes in a trip, the first episode of each syndrome was counted as a unique illness when calculating the cumulative incidence and incidence rates.
The primary end point for our multivariate analysis was defined as subject reported reduction in activity by ≥50% due to an episode of TD, ILI, or FI. A bivariate and multivariate logistic regression model with the stepwise selection method (entry significance level of P < .10) was used to estimate the association between candidate factors and the primary end point (significance level for independent risk factors was P < .05). Time from departure to the end point was compared between the DEP, TDY, and EXR groups using a Kaplan-Meier analysis with a log-rank test. In subjects experiencing multiple ID syndromes per trip, the first episode of any ID syndrome with a ≥50% reduction in activities was categorized as the end point event for the Kaplan-Meier estimate and logistic regression model. We compared diary and survey data in pretravel subjects and conducted a sensitivity analysis to ensure the robustness of associations between risk factors and outcomes when using survey data alone or a combination of survey and diary data for the multivariate analysis (Supplementary Table 1). All analyses were done on SAS, version 9.4.
RESULTS
Two thousand thirty-two servicemembers were enrolled, and 1822 (90%) were included in the analysis. All subjects completed a post-travel survey, and 92% (997/1087) of the pretravel enrollees completed a travel diary (Supplementary Table 1). The majority of servicemembers traveling for EXR (n = 659) were recruited from 2 EXR in INDOPACOM (Koa Moana and Pacific Pathways). Servicemembers on DEP (n = 659) had a longer median (interquartile range [IQR]) duration of travel (175 [152–187] days) compared with EXR (77 [35–106] days) and TDY (18 [8–34] days; P < .01) (Table 1) and were comprised of Special Operations Forces, Marines, and Navy personnel. The TDY group (n = 504) consisted of Army, Air Force, and Special Operations personnel, with predominantly short-term travel to AFRICOM in support of logistics and operations (Figure 1; Supplementary Figure 1).
Table 1.
Demographics, Itinerary, and Risk Behaviors Reported During Military Travel
| Overall (n = 1822) | Deployment (n = 659) | Exercise (n = 659) | Temporary Duty Assignment (n = 504) | P Value | |
|---|---|---|---|---|---|
| Median age (range), y | 29 (18–60) | 30 (19–60) | 24 (18–56) | 33 (19–49) | <.001 |
| Male gender | 1571 (86) | 594 (90) | 593 (90) | 384 (76) | <.001 |
| Race | <.001 | ||||
| Caucasian | 1433 (79) | 538 (82) | 481 (73) | 414 (82) | |
| Black/other | 389 (21) | 121 (18) | 178 (27) | 90 (18) | |
| Branch | <.001 | ||||
| Air Force | 279 (15) | 4 (<1) | 15 (2) | 260 (52) | |
| Army | 763 (42) | 30 (6) | 575 (87) | 158 (31) | |
| Marine Corps | 310 (17) | 209 (32) | 54 (8) | 47 (9) | |
| Navy | 463 (25) | 415 (63) | 15 (2) | 33 (7) | |
| Trip duration, median (IQR), d | 77 (22–169) | 175 (152–187) | 77 (35–106) | 18 (8–34) | <.001 |
| Risk behaviors during travel | |||||
| Hotel without air conditioning, tent, or dormitory/barracks | 1488 (82) | 544 (83) | 617 (94) | 327 (65) | <.001 |
| Travel to malaria-endemic region and partial compliance or noncompliance with malaria chemoprophylaxisa | 497 (27) | 313 (48) | 76 (12) | 108 (21) | <.001 |
| Environmental exposures—wading in fresh water, rodent exposure, working in abandoned buildings or an animal slaughter areab | 693 (38) | 333 (51) | 181 (28) | 179 (36) | <.001 |
| Close contact with localsc | 1191 (65) | 372 (57) | 474 (72) | 345 (69) | <.001 |
| Unable to wash hands or use alcohol-based sanitizer before mealsd | 354 (19) | 120 (18) | 171 (26) | 63 (13) | <.001 |
| Consuming food from street vendors | 552 (30) | 265 (40) | 236 (36) | 51 (10) | <.001 |
Abbreviation: IQR, interquartile range.
Travel to malaria-endemic region and malaria chemoprophylaxis prescription abstracted from predeployment visit. Dosing frequency and adherence with malaria prophylaxis abstracted from post-travel survey. Partial or noncompliance defined as missing any doses of a weekly antimalarial or >2 doses of a daily antimalarial.
Abstracted from post-travel survey: “Did you have any exposure to rats including old, abandoned buildings or working in an animal slaughter area?” and “Did you have contact with fresh water from lakes, streams, or rivers (wading/bathing/swimming/etc.)? Do not include swimming pools.”
Abstracted from post-travel survey; definition provided: “being within approximately 6 feet (2 meters) or within the room or care area for a prolonged period of time (eg, healthcare personnel, household members) or having direct contact with secretions.”
Abstracted from post-travel survey: “unable to wash hands or use alcohol-based sanitizer regularly before eating meals.”
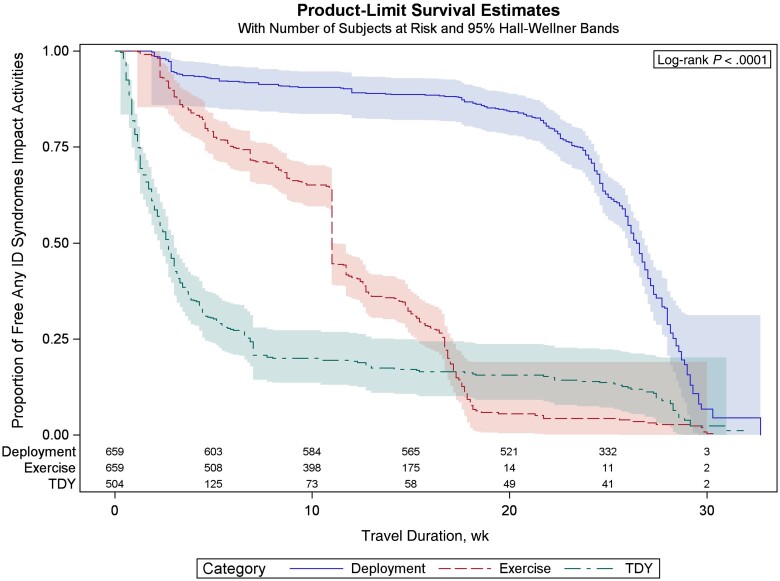
Thirty-six percent (654/1822) of servicemembers experienced an episode of TD, ILI, or FI during military travel (incidence rate, 11.7/100 person-months; 95% CI, 11.65–11.69) (Supplementary Table 2). Multiple ID syndromes per trip were reported by 10% of servicemembers. ID syndromes significantly impacted daily activities: 26% (n = 471/1822) reported either a ≥50% decrease in daily activities (n = 261) or a complete inability to perform daily activities (n = 210) (Table 2). The cumulative incidence and incidence rate of ID syndromes varied significantly by region and type of military travel. DEP to CENTCOM had the highest cumulative incidence of ID syndromes (64%) (Figure 1), although the incidence rate was low (10.6/100 person-months; 95% CI, 10.5–10.8). In contrast, the incidence rate was highest among TDY travelers to SOUTHCOM (32.9 cases/100 person-months) (Supplementary Table 2), although the cumulative incidence (23%) was lower than the estimate for the overall cohort. This disparity between the cumulative incidence and incidence rate was due to a longer duration of travel in the DEP group and significantly lower rates of infection in the initial 15–20 weeks of travel compared with the EXR and TDY groups (Figure 2). The TDY group had the shortest duration of travel and the highest incidence rate in the first 5 weeks of travel.
Table 2.
Infectious Disease Syndromes Reported During Military Travel
| ID Syndromes Reported During Travela | Overall (n = 1822) | Deployment (n = 659) | Exercise (n = 659) | TDY (n = 504) | P Value |
|---|---|---|---|---|---|
| TD: No. of cases (%) (95% CI) | 522 (29) (26.6–30.8) | 288 (44) (39.9–47.6) | 135 (21) (17.5–23.8) | 99 (20) (16.3–23.4) | <.001 |
| Cases/100 person-mo (95% CI) | 9.3 (9.29–9.33) | 8.5 (8.50–8.54) | 8.5 (8.4–8.5) | 15.7 (15.6–15.8) | <.001 |
| No. of participants reporting ≥50% reduction in daily activities due to TD (%) | 346 (19) | 207 (31) | 77 (12) | 62 (12) | <.001 |
| Median duration of decrease in daily activities (IQR), d | 3 (1–9) | 4 (1–10) | 3 (<1–10) | 2 (<1–4) | .019 |
| No. of participants who took antibiotics for TD (%)b | 17 (3) | 2 (1) | 4 (<1) | 11 (11) | <.001 |
| No. of participants seeking care for TD (%)b | 103 (20) | 66 (23) | 21 (16) | 16 (16) | <.001 |
| ILI: No. of cases (%) (95% CI) | 207 (11) (9.9–12.9) | 116 (18) (14.8–20.7) | 68 (10) (8.1–12.9) | 23 (5) 2.9–6.8 | <.001 |
| Cases/100 person-mo (95% CI) | 3.7 (3.68–3.71) | 3.4 (3.42–3.45) | 4.3 (4.24–4.29) | 3.7 (3.6–3.7) | <.001 |
| No. of participants reporting ≥50% reduction in daily activities due to ILI (%) | 175 (10) | 105 (16) | 53 (8) | 17 (3) | <.001 |
| Median duration of decrease in daily activities (IQR), d | 4 (2–8) | 5 (2–8) | 4 (2–9) | 3 (2–8) | .934 |
| No. of participants who took antibiotics for ILIb (%) | 60 (30) | 8 (7) | 4 (6) | 7(30) | .005 |
| No. of participants seeking care for ILI (%)b | 55 (27) | 33 (28) | 14 (21) | 8 (35) | .0008 |
| FI: No. of cases (%) (95% CI) | 182 (10) (8.7–11.5) | 104 (16) (13.1–18.8) | 52 (8) (6.0–10.2) | 26 (5) (3.4–7.5) | <.001 |
| Cases/100 person-mo (95% CI) | 3.3 (3.2–3.3) | 3.1 (3.06–3.09) | 3.3 (3.24–3.28) | 4.1 (4.1–4.2) | <.001 |
| No. of participants reporting ≥50% reduction in daily activities due to FI | 72 (4) | 38 (6) | 15 (2) | 19 (4) | .005 |
| Median duration of decrease in daily activities (IQR),b d | 3 (1–8) | 2 (1–6) | 2 (2–7) | 6 (4–18) | .088 |
| No. of participants who took antibiotics for FIb | 10 (5) | 4 (4) | 2 (4) | 4 (15) | .081 |
| No. of participants seeking care for FI | 35 (19) | 20 (19) | 8 (15) | 7 (27) | .033 |
Abbreviations: FI, febrile illness; ID, infectious diseases; ILI, influenza-like illness; IQR, interquartile range; TD, travelers’ diarrhea; TDY, temporary assignment of duty.
Not mutually exclusive—for subjects who experienced multiple ID syndromes in a trip, the first episode of each syndrome was counted as a unique illness; number with >1 syndrome reported: TD + ILI: 20; TD + FI: 25; ILI + FI: 60; all 3 syndromes reported: 76.
Denominator for proportion (%) is the number of subjects with TD, ILI, and FI, respectively.
Figure 2.
Kaplan-Meier curve of infectious disease (ID) syndromes in the deployment (DEP), exercise (EXR), and temporary duty assignment (TDY) groups.
TD was the most common ID syndrome reported, both in terms of cumulative incidence and incidence rate (TD: 29%; ILI: 11%; and FI: 10%; TD incidence rate, 9.3 cases/100 person-months; 95% CI, 9.29–9.33) and accounted for 73% (346/471) of ID syndromes impacting daily activities. The DEP group reported a longer median duration of decreased activity due to TD, compared with EXR and TDY (ie, 4 days, 3 days, 2 days, respectively; P = .02) (Table 2). The incidence rate of TD in the TDY group (15.7 cases/100 person-months) was almost twice as high as in the DEP and EXR groups (8.5 cases/100 person-months for each), and the highest TD incidence rates (>23 cases/100 person-months) were observed in TDY travelers going to AFRICOM, INDOPACOM, and SOUTHCOM and in the EXR subgroup going to AFRICOM (Supplementary Table 2). Differences between the cumulative incidence and incidence rates were also observed for ILI and FI. The cumulative incidence of ILI in the DEP group was significantly higher than in the EXR and TDY groups (Table 2), while the incidence rates were higher in the EXR and TDY groups (Supplementary Table 2). Although the overall incidence proportion and incidence rates of ILI and FI were lower than TD, a substantial proportion of infected servicemembers reported an impact on daily activities (ie, 84% of ILI cases, 175/207; median duration of impact, 4 days; and 39% of FI cases, 72/182; median duration of impact, 3 days). ILI cases were also more likely to take antibiotics (ILI: 30%, 60/207; TD: 3%, 17/522; FI: 5%, 10/182). Among servicemembers with an ID syndrome (n = 654), the proportion seeking care was higher among those who reported an impact on daily activities (145/471 = 25.8%) vs those reporting normal function (10/183 = 5.4%). Servicemembers who experienced >1 ID syndrome were also more likely to seek medical care (69/181 = 38.1% vs servicemembers who experienced 1 ID syndrome 86/473 = 18.2%).
Risk factors associated with ID syndromes impacting daily activities on multivariate analysis included DEP (vs TDY; OR, 1.7; 95% CI, 1.2–2.4), travel duration >6 weeks (OR, 2.2; 95% CI, 1.6–3.1), environmental exposures (ie, wading in fresh water, rodent exposure, working in abandoned buildings or an animal slaughter area [OR, 1.5; 95% CI, 1.1–1.9]), close contact with locals (OR, 1.7; 95% CI, 1.3–2.1), consuming food from street vendors (OR, 1.8; 95% CI, 1.4–2.4), and inability to sanitize hands before meals (OR, 1.6; 95% CI, 1.2–2.1; P < .05 for all comparisons). Travel to CENTCOM (OR, 2.5; 95% CI, 1.5–4.1), INDOPACOM (OR, 1.8; 95% CI, 1.1–3.1), and AFRICOM (OR, 1.6; 95% CI, 1.0–2.6) was also associated with a higher risk compared with SOUTHCOM (Table 3). Three subjects went to NORTHCOM (1 DEP, 2 TDY) and did not experience any illnesses and were excluded from the multivariate analysis.
Table 3.
Risk Factors Associated With Infectious Disease Syndromes Impacting Daily Activities
| ID Syndrome Impacting Daily Activities (n = 471) | Univariate OR (95% CI) | Multivariate OR (95% CI) | P Value | |
|---|---|---|---|---|
| Age, y | 471 | 1.01 (0.99–1.02) | ||
| Male gender | 411 | 1.1 (0.8–1.5) | ||
| Race | ||||
| Caucasian (ref) | 386 | |||
| Black/other | 85 | 0.8 (0.6–0.99) | ||
| Type of travel | ||||
| Temporary duty assignment (ref) | 84 | |||
| Deployment | 272 | 3.5 (2.7–4.7) | 1.7 (1.2–2.4) | .006 |
| Exercise | 115 | 1.1 (0.8–1.4) | 0.5 (0.3–0.8) | .004 |
| Travel duration >6 wk | 387 | 3.2 (2.5–4.2) | 2.2 (1.6–3.1) | <.001 |
| Geographic regiona | ||||
| US Southern Command (SOUTHCOM) (ref) | 45 | |||
| US Indo-Pacific Command (INDOPACOM) | 157 | 0.7 (0.4–0.96) | 1.8 (1.1–3.1) | .021 |
| US Africa command (AFRICOM) | 141 | 0.7 (0.5–1.1) | 1.6 (1.0–2.6) | .032 |
| US Central Command (CENTCOM) | 95 | 2.1 (1.3–3.3) | 2.5 (1.5–4.1) | <.001 |
| US European Command (EUCOM) | 15 | 1.2 (0.6–2.3) | 1.7 (0.8–3.9) | .169 |
| Multiple geographic regions | 18 | 1.6 (0.8–3.1) | 1.8 (0.9–3.7) | .125 |
| Hotel without air conditioning, tent, or dormitory/barracks | 401 | 1.4 (1.0–1.9) | ||
| Environmental exposures—wading in fresh water, rodent exposure, working in abandoned buildings or an animal slaughter areab | 238 | 2.0 (1.6–2.5) | 1.5 (1.1–1.9) | .004 |
| Partial compliance or noncompliance with malaria chemoprophylaxisc | 153 | 1.4 (1.1–1.8) | ||
| Close contact with localsd | 330 | 1.3 (1.1–1.7) | 1.7 (1.3–2.1) | <.001 |
| Inability to wash or sanitize hands before meals | 127 | 1.8 (1.4–2.3) | 1.6 (1.2–2.1) | .001 |
| Consuming food from street vendors | 212 | 2.4 (1.9–3.0) | 1.8 (1.4–2.4) | <.001 |
Abbreviations: ID, infectious diseases; OR, odds ratio.
Geographic regions were based on the United States Department of Defense areas of responsibility for command and control of US military forces.
Abstracted from post-travel survey: “Did you have any exposure to rats including old, abandoned buildings or working in an animal slaughter area?” and “Did you have contact with fresh water from lakes, streams, or rivers (wading/bathing/swimming/etc.)? Do not include swimming pools.”
Travel to malaria-endemic region and malaria chemoprophylaxis prescription abstracted from predeployment visit. Dosing frequency and adherence with malaria prophylaxis abstracted from post-travel survey. Partial or noncompliance defined as missing any doses of a weekly antimalarial or >2 doses of a daily antimalarial.
Abstracted from post-travel survey; definition provided: “being within approximately 6 feet (2 meters) or within the room or care area for a prolonged period of time (eg, healthcare personnel, household members) or having direct contact with secretions.”
DISCUSSION
Despite improvements in public health and preventive measures, ID syndromes continue to impact US peacetime military operations through lost duty days, decreased work performance, and increased health care costs. We utilized an active surveillance strategy in a large cohort of military servicemembers by administering post-travel surveys that were completed within 8 weeks of return from travel (median [IQR], 20 [9–28] days). This approach allowed us to provide an assessment of the incidence and impact of ID syndromes on military travel while reducing the impact of recall bias. Approximately one-quarter of military travelers in our cohort reported a decrease of ≥50% in daily activities due to an ID syndrome for a median duration of 3 days. The greatest impact of ID syndromes was observed in the DEP servicemembers, and DEP was an independent risk factor for decreased daily activities due to an ID syndrome. Maintaining operational readiness is most critical during DEP, and our results suggest that this group also faces the greatest risk of disruptions and operational degradation from ID syndromes. We further explored the timing of ID syndromes using incidence rates and a Kaplan-Meier curve. Compared with servicemembers on EXR or TDY, the period of greatest risk for ID syndromes was delayed in the DEP group (Figure 2). The lower incidence rate in the initial 10–16 weeks of DEP is likely because military units reside in an overseas US military base during the initial phase of DEP, which has military dining facilities, developed infrastructure, and restrictions on off-base activities, and this is followed by deployment to remote locations with a greater risk of environmental exposures, contact with the local population, and difficulty in maintaining hygienic practices such as handwashing or cleaning of shared spaces (eg, restrooms, conference rooms) to mitigate the spread of infectious diseases. The inability to wash or sanitize hands before meals (OR, 1.6; 95% CI, 1.2–2.1) was identified as an independent risk factor for ID syndromes impacting activity in our multivariate model. Strategies that promote handwashing are effective in reducing diarrheal and respiratory infections among children in developing countries and could be applied in field settings [7]. In addition, the perception of risk in servicemembers may have declined over time, resulting in suboptimal compliance with preventive measures, while host susceptibility to infections remained unchanged due to the lack of exposure during the initial weeks of DEP. Our findings highlight the importance of assessing the incidence rates in addition to the cumulative incidence when evaluating individuals with differing itineraries and trip durations.
TD was the most common ID syndrome and had the greatest operational impact. Subgroups with the highest incidence rate of ID syndromes (eg, TDY travelers to SOUTHCOM, INDOPACOM, and AFRICOM) were largely due to TD cases. In addition, TD incidence rates in these subgroups (Supplementary Table 2) were similar to rates reported in systematic reviews from 1990–2005 and 2005–2015 (29 and 30.3 cases/100 person-months respectively), indicating that certain subgroups remain at high risk of TD despite advances in mitigation strategies through the decades [4, 8]. TD also had the greatest operational impact, contributing ∼2282 lost duty days and millions in estimated, nonadjusted costs, based on a 2002 Congressional Budget Office report that estimated the cost of deployment in Iraq [9]. These data support the prioritization of vaccine development against the most common pathogens such as diarrheagenic Escherichia coli (particularly enterotoxigenic E. coli and enteroaggregative E. coli), Campylobacter, and norovirus as a means to maintain operational readiness during military travel [10]. Prescription of antibiotics before deployment is not required for individuals deploying as part of a unit, per DoD policy [11, 12]. Symptomatic servicemembers are advised to contact their unit’s medical corpsmen during military travel for evaluation and treatment. This approach may have contributed to the low rates of self-treatment observed in moderate and severe TD cases. We identified several high-risk settings (eg, inability to sanitize hands before meals, close contact with locals, eating street vendor food, environmental exposures, type of military travel, geographic location) and periods of increased risk during which servicemembers may benefit from the individual provision of standby therapy to mitigate the impact of TD while minimizing the inappropriate use of antibiotics.
It is reassuring to note that FI episodes were likely self-limited viral infections, as evidenced by the limited impact on daily activities and low proportion of medically attended events, rather than serious systemic infections such as typhoid or malaria. Although the incidence rate of ILI was comparable to that of FI, ILI cases were more likely to seek care and take antibiotics (Table 2). Approximately 10% of servicemembers experienced multiple ID syndromes during a trip, often during the same week of deployment, which can make it challenging to separate self-limited infections from serious infections such as dengue, malaria, and leptospirosis that can manifest with mild symptoms at the onset of infection.
There are limitations to our study design, not least of which is the effect of recall bias, particularly in surveys with greater delay to completion. We did not capture all prevalent ID syndromes (eg, skin and soft tissue infections, sexually transmitted infections), and the study was conducted before the 2020 outbreak of the severe acute respiratory syndrome coronavirus 2 pandemic. Additional risk factors including the impact of seasonality, close living quarters, and physical and mental stressors on disease incidence rates would be important to assess in future studies. There was significant heterogeneity in the travel duration, destinations, and exposures during military travel, and as this analysis focused on Marines, Special Forces, and other specialized military units going to high-risk regions prioritized by the DoD GEIS, the generalizability of our findings may be limited to a subset of military travelers [13]. We were unable to divide geographic regions into further subdivisions because servicemembers usually traveled to multiple countries within a geographic region, but the duration of travel within each country was not available. In addition, units involved in Special Operations did not provide country-specific itinerary data due to operational security. Nonetheless, our survey-based study design provided granular data on the risk of ID threats during military travel and their impact on operations. Only 25% of servicemembers reporting an ID syndrome and 30% of servicemembers with limitations in physical activity sought care, suggesting that passive surveillance limited to medically attended illness would likely underestimate the impact of ID syndromes [14].
In conclusion, ID syndromes have a significant impact on military travel, but there is substantial variability in the incidence rates based on the type and location of military travel and during high-risk periods during travel. Addressing modifiable risk factors and improving access to TD self-treatment in high-risk settings may help mitigate the impact of ID threats during military travel.
Supplementary Material
Acknowledgments
The authors would like to thank the study participants and the team of clinical research coordinators for their support of and dedication to the project.
Author contributions. Concept and design: M.A.B., T.L., D.R.T. Acquisition, analysis, or interpretation of data: M.A.B., H.C.K., D.A.L., R.O. Drafting of the manuscript: M.A.B., T.L., H.C.K. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: H.C.K.
Financial support. This study IDCRP-037 was conducted by the Infectious Disease Clinical Research Program (IDCRP), a Department of Defense (DoD) program executed by the Uniformed Services University of the Health Sciences (USUHS) through a cooperative agreement with The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. (HJF). This project has been funded in whole, or in part, with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), under Inter-Agency Agreement Y1-AI-5072 and from the U.S. Department of Defense via Global Emerging Infections Surveillance (Uniformed Services University of the Health Sciences Grant Agreement: HU00011820095).
Research disclaimer. The contents of this publication are the sole responsibility of the author(s) and do not necessarily reflect the views, opinions, or policies of Uniformed Services University of the Health Sciences (USUHS), the Department of Defense (DoD), the Departments of the Army, Navy, or Air Force, or Brooke Army Medical Center, the US Army Medical Department, the US Army Office of the Surgeon General, Naval Medical Center San Diego, Tripler Army Medical Center, Landstuhl Regional Medical Center, Madigan Army Medical Center, or Naval Medical Center Portsmouth. The views expressed in this manuscript are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the US Government. Mention of trade names, commercial products, or organizations does not imply endorsement by the US Government.
Patient consent. The patient's written consent was obtained as part of the study protocol. The design of the work has been approved by local ethical committees and conforms to standards currently applied in the country of origin (United States) and includes the name of the authorizing body, which is stated in the paper under the “Methods” section.
Ethics statement. The investigators have adhered to the policies for protection of human subjects as prescribed in 45 CFR 46.
Copyright statement. Some authors are military servicemembers and employees of the US Government. This work was prepared as part of their official duties. Title 17 U.S.C. 105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. 101 defines a United States Government work as a work prepared by a military servicemember or employee of the United States Government as part of that person's official duties.
Prior presentation. This work was presented in part at the ID Week Virtual Conference, September 29–October 3, 2021.
Contributor Information
Michael A Boatwright, Walter Reed National Military Medical Center, Bethesda, Maryland, USA.
Huai-Ching Kuo, Infectious Diseases Clinical Research Program, Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Bethesda, Maryland, USA.
David A Lindholm, Brooke Army Medical Center, Joint Base San Antonio-Fort Sam, Houston, Texas, USA; Department of Medicine, Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA.
Tara Griffith, Infectious Diseases Clinical Research Program, Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Bethesda, Maryland, USA.
Rhonda E Colombo, Infectious Diseases Clinical Research Program, Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Bethesda, Maryland, USA; Madigan Army Medical Center, Joint Base Lewis, McChord, Washington, USA.
David R Tribble, Infectious Diseases Clinical Research Program, Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA.
Robert O’Connell, Infectious Diseases Clinical Research Program, Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA.
Tahaniyat Lalani, Infectious Diseases Clinical Research Program, Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Bethesda, Maryland, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Riddle MS, Savarino SJ, Sanders JW. Gastrointestinal infections in deployed forces in the Middle East theater: an historical 60 year perspective. Am J Trop Med Hyg 2015; 93:912–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Riddle MS, Tribble DR, Putnam SD, et al. Past trends and current status of self-reported incidence and impact of disease and nonbattle injury in military operations in Southwest Asia and the Middle East. Am J Public Health 2008; 98:2199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sanders JW, Putnam SD, Frankart C, et al. Impact of illness and non-combat injury during Operations Iraqi Freedom and Enduring Freedom (Afghanistan). Am J Trop Med Hyg 2005; 73:713–9. [PubMed] [Google Scholar]
- 4. Riddle MS, Sanders JW, Putnam SD, Tribble DR. Incidence, etiology, and impact of diarrhea among long-term travelers (US military and similar populations): a systematic review. Am J Trop Med Hyg 2006; 74:891–900. [PubMed] [Google Scholar]
- 5. Black RE. Epidemiology of travelers’ diarrhea and relative importance of various pathogens. Rev Infect Dis 1990; 12(Suppl 1):S73–9. [DOI] [PubMed] [Google Scholar]
- 6. Soltis BW, Sanders JW, Putnam SD, Tribble DR, Riddle MS. Self reported incidence and morbidity of acute respiratory illness among deployed U.S. military in Iraq and Afghanistan. PLoS One 2009; 4:e6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luby SP, Agboatwalla M, Feikin DR, et al. Effect of handwashing on child health: a randomised controlled trial. Lancet 2005; 366:225–33. [DOI] [PubMed] [Google Scholar]
- 8. Olson S, Hall A, Riddle MS, Porter CK. Travelers’ diarrhea: update on the incidence, etiology and risk in military and similar populations—1990–2005 versus 2005–2015, does a decade make a difference? Trop Dis Travel Med Vaccines 2019; 5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Congressional Budget Office. Estimated costs of a potential conflict with Iraq. 2002. Available at: https://www.cbo.gov/sites/default/files/107th-congress-2001-2002/reports/09-30-iraq.pdf. Accessed May 9, 2023.
- 10. Riddle MS, Tribble DR, Cachafiero SP, Putnam SD, Hooper TI. Development of a travelers’ diarrhea vaccine for the military: how much is an ounce of prevention really worth? Vaccine 2008; 26:2490–502. [DOI] [PubMed] [Google Scholar]
- 11. Riddle MS, Connor BA, Burgess T, Ericsson CD. Guidelines for the prevention and treatment of travelers’ diarrhea: a graded expert panel report. J Travel Med 2017; 24(Suppl 1):S57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stagliano DR, Kuo H-C, Fraser JA, et al. Military and civilian sector practice patterns for short-term travelers’ diarrhea self-treatment in adults. Am J Trop Med Hyg 2022; 106:1156–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Department of Defense . Global Emerging Infection Surveillance Section, GEIS; Alignment With GCC Regional Priorities FY21, in Department of Defense: Defense Health Agency Memo. Department of Defense; 2021.
- 14. Porter CK, Olson S, Hall A, Riddle MS. Travelers’ diarrhea: an update on the incidence, etiology, and risk in military deployments and similar travel populations. Mil Med 2017; 182(Suppl 2):4–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.