Abstract
Over the past few years, evidence has accrued that demonstrates that terrestrial photochemical reactions could have provided numerous (proto)biomolecules with implications for the origin of life. This chemistry simply relies on UV light, inorganic sulfur species and hydrogen cyanide. Recently, we reported that, under the same conditions, reduced phosphorus species, such as those delivered by meteorites, can be oxidized to orthophosphate, generating thiophosphate in the process. Here we describe an investigation of the properties of thiophosphate as well as additional possible means for its formation on primitive Earth. We show that several reported prebiotic reactions, including the photoreduction of thioamides, carbonyl groups and cyanohydrins, can be markedly improved, and that tetroses and pentoses can be accessed from hydrogen cyanide through a Kiliani–Fischer-type process without progressing to higher sugars. We also demonstrate that thiophosphate allows photochemical reductive aminations, and that thiophosphate chemistry allows a plausible prebiotic synthesis of the C5 moieties used in extant terpene and terpenoid biosynthesis, namely dimethylallyl alcohol and isopentenyl alcohol.

Subject terms: Origin of life, Organic chemistry
The streamlined synthesis of multiple (proto)biomolecules from common starting materials is a key goal of prebiotic chemistry. Now, a one-pot synthesis of ribo-aminooxazoline (a precursor for prebiotic nucleotide synthesis) from HCN has been achieved. Additionally, the two moieties used in extant terpenoid biosynthesis have been accessed, with all carbon atoms also originating from HCN.
Main
When contemplating the chemistry that gave rise to life, one of the fundamental questions to be addressed is that concerning the set of molecules that comprised the basis from which life could emerge. As this question cannot be answered by inference from biology alone, chemical experiments are required to identify reaction pathways that could have led from simple, environmentally available feedstock molecules to (proto)biomolecules. For productive coupling of the various precursors, it is reasonable to assume that the prebiotic synthesis of the basis set of molecules occurred in reasonably close proximity on primitive Earth, rather than in disparate and distanced environments, and consequently a common type of chemistry would be expected to give rise to numerous (proto)biomolecules. Where the chemistry was confined to, at least initially, must have been defined by geology and geochemistry, so all the chemical steps must comport with a geochemical scenario and the boundaries it imposes. Once this preliminary identification has been made, refinement of the prebiotic pathway or geochemical scenario can be informed and refined by its counterpart. For example, cyanamide (NH2CN) is an important prebiotic reagent, and the thermal conversion of Ca2[Fe(CN)6] to CaNCN with ensuing hydrolysis has been suggested as a source of NH2CN (ref. 1). However, under CO2-rich atmospheres, CaCO3 would be expected to precipitate rather than Ca2[Fe(CN)6] (ref. 2). Thus, if Ca2[Fe(CN)6] is required, a reduced atmosphere must have been present, which is the expected outcome from the impact of a large, reduced meteorite3. Cycling between geochemistry and prebiotic chemistry in this way should aid the improvement and plausibility of reaction pathways and the discovery of new reactions and reagents, in effect, acting as a type of triangulation4.
Recent reports from this laboratory have described the prebiotic synthesis of purine and pyrimidine nucleosides, precursors to amino acids and acyl glycerol phosphates, the components of the Krebs cycle, and a means of harnessing and supplying chemical energy to potentially drive this inanimate collection of molecules towards life1,5–8. The photochemical reduction of nitrile groups and thioamides (which can be derived from nitriles) to aldehydes constitutes a key reaction in these syntheses, and is repeated multiple times (Supplementary Fig. 1)1. The resulting aldehydes can be employed for further prebiotic reactions, such as the Strecker synthesis of amino acids. Importantly, there is a systems chemistry aspect to this network, which would have allowed flexibility and access to alternative products from common starting materials, depending on the conditions at a particular time or in a particular location on primitive Earth (Supplementary Discussion 1).
The geochemical scenario we have proposed that supports this chemistry has been described in detail several times1,9–11, but broadly speaking is envisaged to be land-based, occurring in an impact or post-impact environment, with prebiotic chemistry taking place in small streams (or possibly pools) that occasionally mix. The reagents and reactions required for the whole prebiotic network (Supplementary Fig. 1) are derived from and conform to this scenario, and primarily involve ultraviolet (UV) light, cyanide, ferrous iron and inorganic sulfur species such as HS−. For the prebiotic scheme to work most efficiently, some separation of the chemistries is desirable, so an interconnected system of small streams or flowing water is invoked that could allow mixing of reactants at various stages, for example, at a confluence9–11. During the course of our studies, we found that phosphite (HPO32−) and hypophosphite (H2PO2−)—anoxic corrosion products of reduced Ni/Fe-P mineral species found in reduced meteorites12,13—could be oxidized to orthophosphate (PO43−) by UV light and HS− (ref. 14), thus providing one solution to the long-standing ‘phosphate problem’ and also complying with our geochemical and prebiotic model. We observed that thiophosphate (PSO33−) was formed as an inevitable intermediate during this oxidation chemistry, which was noteworthy as we had previously reported PSO33− to be an efficient reagent for the formation of thioamides from nitriles and for phosphorylation reactions15. As the means of production of PSO33− matched our geochemical model, a full evaluation of its potential in the context of our prebiotic reaction network (Supplementary Fig. 1)1 was warranted4. Additionally, further assessment of the prebiotic availability and stability of PSO33− was made, as well as its in situ production and use (Supplementary Discussion 2).
Results and discussion
Photochemical reduction of nitriles
Initially, we wondered whether PSO33− possessed similar photochemical properties to HS−, which could mean HS− and PSO33− were interchangeable in our prebiotic syntheses (Supplementary Fig. 1). This seemed an attractive possibility, as PSO33− is not volatile and can be formed at geologically relevant concentrations of H2S/HS− (ref. 14). As a representative example, we irradiated glycolonitrile 1 (20 mM) with low-pressure Hg lamps (principal emission at 254–256 nm) in the presence of PSO33− (20 mM) at pH 6.5. Reduction of 1 was efficient, the major products being glycolaldehyde 2 (∼19%) and glyceronitrile 4 (∼9%), with lesser amounts of acetaldehyde 3, EtOH and lactonitrile 5 also being formed (Fig. 1, horizontal reaction arrow; Supplementary Figs. 2–4 and Supplementary Table 1). Under identical conditions using NaSH as the reductant, 2 and 4 were produced in ∼6% and ∼5% yields, respectively, and only a trace amount of 3 was present (Supplementary Fig. 2 and Supplementary Table 1). Increasing the amount of PSO33− (1.5 equiv.) gave slightly improved yields after 1 h of irradiation (Supplementary Fig. 2 and Supplementary Table 1). Irradiating the reaction for longer did not really affect the yield of 2, as α-deoxygenation of 2 became competitive with the reduction of 1, giving increased yields of 3 and subsequent overreduction of 3 to EtOH (Supplementary Figs. 2 and 5 and Supplementary Table 1).
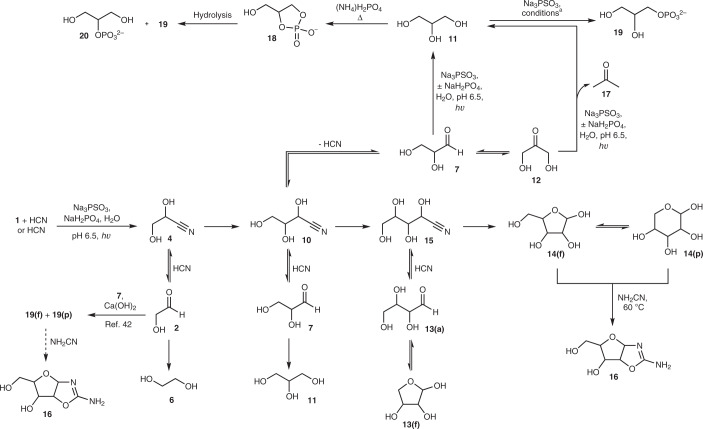
Fig. 1. Photochemical reduction of glycolonitrile 1 or 1 and HCN by PSO33− yields multiple products of prebiotic interest.

The major products of the reduction of 1 (horizontal reaction arrow) in the absence of phosphate are glycolaldehyde 2 and glyceronitrile 4, with lesser amounts of acetaldehyde 3, lactonitrile 5 and ethylene glycol 6. The reaction is more efficient in the presence of phosphate, with an increased yield of 6. In the presence of HCN (vertical reaction arrow), serine nitrile 8 and ethanolamine 9 are observed.
During the reaction the pH increased (pH ∼ 9.2 after 1 h of irradiation), so we repeated the reduction of 1 with the inclusion of phosphate (PO43−, 20 mM, 1 equiv.) as a pH buffer (pH 6.5), which is consistent with the geochemical scenario. Interestingly, reduction turned out to be much more efficient in the presence of phosphate, with glycolaldehyde 2 and glyceronitrile 4 being formed in ∼34% and ∼4% yields, respectively, after 1 h, in addition to increased amounts of ethylene glycol 6 (Supplementary Fig. 6 and Supplementary Table 1). Clearly, PSO33− is a far more effective reductant than HS− alone and is at least as efficient as the CuCN/HS− system we have previously reported1,16.
We then attempted the in situ generation and homologation of glycolaldehyde 2 to glyceraldehyde 7 starting from 1 (20 mM) and HCN (20 mM). After 1 h of irradiation, the crude 1H NMR spectrum indicated that 2, its cyanohydrin 4 and lactonitrile 5 had been formed in ∼4%, ∼24% and ∼3% yields, respectively (Fig. 1, vertical reaction arrow; Supplementary Fig. 7 and Supplementary Tables 2.1 and 2.2). Serine nitrile 8 (∼16%) was also present, suggesting that the nitrogen contained in HCN could be directly fixed into amino acids without the need for the addition of NH3. Additionally, a small amount of glycolaldehyde imine had been reduced to ethanolamine 9. Repeating the reaction in the presence of PO43− (60 mM) at pH 6.5, we detected 7 (∼5%) and its cyanohydrin 10 (∼13%), 2 (∼6%), 4 (∼9%), glycerol 11 (∼6%) and 6 (∼6%) after 1 h of irradiation (Fig. 2, Supplementary Fig. 8 and Supplementary Tables 2.1 and 2.2). Evaporation of hydrogen cyanide (HCN) from the crude reaction mixture gave 2 and 7 in ∼13% and ∼14% overall yields, respectively, and left 6 and 11 unchanged (Fig. 2 and Supplementary Fig. 9). This solution was then left for two weeks at room temperature, after which 7 had partially equilibrated to dihydroxyacetone 12 (giving a ratio of ∼1:1 of 7:12, although the end point for equilibration will favour 12 (ref. 17); Fig. 2 and Supplementary Fig. 9), thus allowing the prebiotic synthesis of valine and leucine as previously described1. Although HCN could have been potentially concentrated through the intermediacy of ferrocyanide1,9,10, and can undergo reductive homologation to 7 under the same conditions (Supplementary Fig. 10 and Supplementary Tables 2.1 and 2.2), glycolonitrile 1 may be an equally attractive starting material given its surprisingly high boiling point (102–104 °C at 6 mmHg (refs. 18,19)), which may have allowed its concentration in groundwater to some degree (Supplementary Fig. 11).
Fig. 2. Photochemical reductive homologation of HCN or glycolonitrile 1 + HCN by PSO33− yields various higher sugars, depending on slight differences in the starting conditions.
Unless stated, reactions were carried out at ambient temperature. For convenience, aldehydes are depicted as the carbonyl compounds, but would also exist as hydrates in water. a(±)CH2CHCN, formamide, 70 °C; formamide, CH2CHCN; Fe(CN)63−, formamide; hν, formamide (Supplementary Table 7).
As the Kiliani–Fischer-like homologation of 1 to glyceraldehyde 7 using thiophosphate had proven so efficient, we repeated the reaction to see if the reduction products of glyceraldehyde cyanohydrin 10 were formed. The tetroses 13 (threose 13-t and erythrose 13-e) were present in the crude reaction mixture (∼19%), in addition to 2 (∼8%) and 7 (∼8%; Fig. 2, Supplementary Figs. 12 and 13 and Supplementary Tables 3.1, 3.2 and 4). Little, if any, diastereoselectivity was observed, with 13-t:13-e of ∼0.9:1 (Supplementary Fig. 13). The total yield of identifiable products was ∼72%, comprising ∼4% C1, ∼27% C2, ∼22% C3 and ∼19 % C4 compounds (Supplementary Table 4).
In an attempt to make higher sugars, we increased the amount of PSO33− (100 mM) and HCN (45 mM) in the starting mixture and lowered the amount of glycolonitrile 1 (7.5 mM). After 2.75 h of irradiation, the total yield of identifiable products was ∼60%, comprising ∼1% C1, ∼8% C2, ∼11% C3, ∼11% C4 and ∼29% C5 compounds (Supplementary Figs. 14 and 15 and Supplementary Tables 3.1, 3.2 and 5). Although the presence of a small amount of 1 improved the efficiency of the synthesis of pentoses 14, its inclusion was not vital, with the Kiliani–Fischer-like process forming 14 in ∼19% yield starting from HCN alone (Supplementary Fig. 16 and Supplementary Tables 3.1 and 3.2). This constitutes an alternative one-pot, prebiotically plausible synthesis of the tetroses 13 and pentoses 14 from a C1 feedstock molecule to that reported by Butlerow in 1861 (ref. 20). If it is assumed that, at each step of HCN homologation, imine hydrolysis and cyanohydrin formation proceed quantitatively (which is clearly not the case given the presence of 6, 9 and 11), the photochemical reduction of nitriles proceeds at an average of ∼65% yield. Indeed, the similarity between a crude reaction mixture resulting from reduction of HCN, or 1 and HCN, and a roughly equimolar mixture of 2, 7, 13-t, 14, 6 and 11, is notable (Supplementary Fig. 17). Interestingly, the hexoses were not observed in 1H NMR spectra (Supplementary Fig. 18), even though the concentration of 14 was comparable to that of the C2, C3 and C4 sugars, and must be ascribed to the fact that the pentoses 14 exist almost exclusively in furanosyl 14(f) and pyranosyl 14(p) forms (in aqueous solution at circumneutral pH), with only traces (∼0.01–0.04%) of the carbonyl forms being present (Fig. 2 and Supplementary Fig. 19)21. Accordingly, a regulatory mechanism exists, and, by the time the pentoses 14 are being formed, the concentration of HCN is too low to force the equilibria in favour of pentose cyanohydrins and so further homologation of 14 is inhibited.
We then wondered if the transient protection of the carbonyls of 14, afforded by the cyclic hemiacetals 14(f) and 14(p), could have assisted the accumulation of 14 on early Earth. The crude reaction mixture resulting from the reduction of 1 and HCN by PSO33− was thus evaporated to the point of dryness and the solid dissolved in D2O. 1H NMR spectroscopy revealed that the C2, C3 and C4 sugars had decomposed (potentially via polymerization), but the pentoses 14 were still present (<30% decomposition of 14 taking place; Supplementary Fig. 20).
Competition experiments were then performed to examine the stability of 14 to photoreduction and photochemistry, and, in both the presence and absence of PSO33−, 14-x displayed excellent resistance to UV-promoted decomposition relative to 13-t and 2 (chosen as representative examples), with the partly ring-closed tetrose 13-t (∼90% 13(f))22 being approximately twice as stable as 2 (Supplementary Figs. 21 and 22 and Supplementary Table 6). Consequently, the photochemical reduction of 1 and HCN was repeated and the irradiation extended to 5 h. After this time, 2 and 7 were effectively absent from the crude reaction mixture and 13 had undergone partial decomposition, yet the concentration of 14 had increased relative to the 2.5 h time point (Supplementary Fig. 23). Although this presents a second potential mechanism by which the pentoses 14 could have been selected, it is not clear whether this enrichment is required, as the selective crystallization of ribo-aminooxazoline 16-r from a mixture of sugars has already been demonstrated (the aminooxazolines being formed by reaction of the sugars with cyanamide)23, and the elaboration of 16-r into purine and pyrimidine nucleosides has recently been reported5.
To connect to these previous studies, 1 and HCN were subjected to photoreduction using PSO33−, then excess HCN was evaporated from the solution and NH2CN added. After heating for 2 h at 60 °C, conversion of 14 to 16 was observed (Fig. 2 and Supplementary Fig. 24; CaCN2 was found to work equally efficiently as NH2CN (ref. 1), Supplementary Fig. 25). The procedure was then repeated using HCN alone as the carbon feedstock, and ribo-aminooxazoline 16-r was formed in ∼1% overall yield (Supplementary Fig. 26). Thus, telescoped (without work-up/purification/isolation of intermediates, but with addition of a reagent at a later point), prebiotically plausible syntheses of 16-r from feedstock molecules thought to have been available on early Earth have been achieved, in 12 steps from glycolonitrile 1 or 15 steps from HCN.
Photochemical reduction of carbonyl groups
Glycerol 11, a key component of all cell membranes, had been formed in several of the previous reactions, and probably resulted from the reduction of glyceraldehyde 7. We therefore assumed that 11 could be formed from the reduction of dihydroxyacetone 12 or glyceraldehyde 7, and consequently attempted the photoreduction of 12 (15 mM) by 2 equiv. of PSO33−. This yielded 11 in ∼51% yield after 0.5 h—almost doubling the yield previously reported, and produced approximately ten times faster, while requiring less than half of the amount of reductant (Supplementary Fig. 27)1. Reduction of glyceraldehyde 7 under the same conditions gave ∼63% of 11 after 45-min irradiation (Supplementary Fig. 28). The reductions of both 12 and 7 to 11 by PSO33− were accompanied by α-deoxygenation side reactions leading to acetone 17, and its 1,2-reduction product isopropanol, and smaller amounts of propan-1,2-diol, from 12, and propan-1,3-diol from 7 (Supplementary Figs. 27 and 28). As we had found previously (Supplementary Fig. 6), inclusion of PO43− increased the amount of 1,2-reduction, forming 11 in ∼64% yield from 12 after a 0.5-h reaction, or ∼71% yield starting from 7 after a 45-min reaction (Supplementary Fig. 29). Evaporation of water also removed the bulk of the side products and left glycerol 11 in a highly pure and concentrated form (Supplementary Fig. 30). The one-pot synthesis of 11 could be favoured by increasing the amount of reductant (PSO33−, 75 mM) and subjecting glycolonitrile 1 (10 mM) and HCN (10 mM) to irradiation for 2.5 h. Glycerol 11 was formed in ∼27% yield, alongside ethylene glycol 6 (∼32% yield) and minor amounts of the α-deoxygenated products mentioned above (Supplementary Fig. 31).
The ultimate fate of PSO33− is to be converted into PO43−, so the two components required to make glycerol phosphates 18, 19 and 20 under standard prebiotic phosphorylating conditions are formed in the same location. However, given that PSO33− itself is an effective phosphorylating agent15, we considered reactions of 11 with PSO33−. Heating 11 with PSO33− and acrylonitrile at 70 °C in formamide for 3 h (ref. 15), or in the absence of acrylonitrile for 10 h, gave 19 and 20, although we found that phosphorylation also occurred at room temperature in the presence of acrylonitrile (Fig. 2, Supplementary Figs. 32–35 and Supplementary Table 7). Low-temperature phosphorylations were also made possible by activation of PSO33− with ferricyanide or by photolysis in formamide (Supplementary Figs. 36 and 37 and Supplementary Table 7). Glycerol-1-phosphate 19 and glycerol-2-phosphate 20 were obtained in 14–30% yield and 4–9% yield, respectively, depending on the conditions used (Supplementary Figs. 32–37 and Supplementary Table 7). Furthermore, as low-temperature phosphorylations were possible, cyclic phosphates such as 18, generated under more conventional conditions1,24, could be avoided if desired.
Reductive aminations and in situ azole synthesis
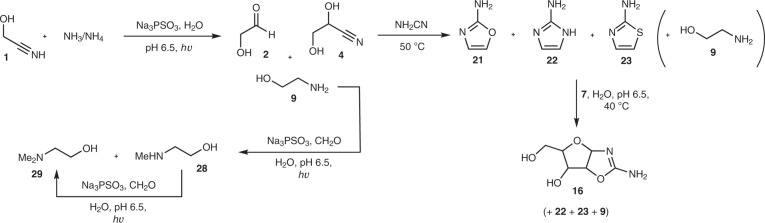
The production of ethanolamine 9 (Fig. 1 and Supplementary Fig. 7), alongside glycolaldehyde 2, interested us, as it has been found that 9 is an effective catalyst for the polymerization of nucleoside-2′,3′-cyclic phosphates25, and it has also been shown that 2 is a precursor for the prebiotic synthesis of ribo-nucleoside-2′,3′-cyclic phosphates via 2-aminooxazole 21 (refs. 26,27). Initially, we irradiated glycolaldehyde 2 (25 mM) in the presence of NH3/NH4 (150 mM) and PSO33− (100 mM) at pH 9.2 and observed ∼14% of ethanolamine 9 after 6 h of irradiation, in addition to EtOH (∼16%) and ethylene glycol 6 (∼11%; Supplementary Fig. 38 and Supplementary Table 8). Such high concentrations of ammonia on primitive Earth may have occurred after dissolution of Mg3N2 (refs. 1,9), for example, but would probably only have been short-lived given the volatility of NH3, and so we repeated the reaction at pH 7.0 in the presence of phosphate buffer when the majority of ammonia is protonated. Gratifyingly, 9 was still formed, albeit in reduced yield (∼6% after 6 h), in addition to EtOH (∼12%), but the most dramatic change was the yield of 6, ∼82% after 6 h of reaction (Supplementary Fig. 38 and Supplementary Table 8). Unsurprisingly, increasing the concentration of NH3/NH4 increased the yield of 9.
We then attempted the telescoped synthesis of ethanolamine 9 and 2-aminooxazole 21 starting from glycolonitrile 1 (25 mM) and NH4Cl (75 mM) at neutral pH. After irradiating the starting mixture for 1.5 h, 2 (and its cyanohydrin 4) and 9 were present in ∼32% and ∼1% yield, respectively (Fig. 3). Cyanamide (50 mM) was added to the crude reaction, and the solution was heated to 50 °C for 20 h, after which time 2-aminooxazole 21 could be seen in the 1H NMR spectrum in ∼8% yield (based on 1, Fig. 3; Supplementary Fig. 39 and Supplementary Table 9). In addition to 21, we determined that 2-aminoimidazole 22 and 2-aminothiazole 23 were also present and had been formed in ∼2% and ∼9% yield, respectively (ethanolamine 9 was unchanged after reaction with cyanamide, Fig. 3; Supplementary Fig. 39 and Supplementary Table 9). Omitting ammonia from the reaction yielded 21 and 23 in ∼7% and ∼8% yield (3 equiv. PSO33−) or ∼16% and ∼2% yield (1 equiv. PSO33−), respectively (Supplementary Fig. 40 and Supplementary Table 9). It would seem unlikely that mercaptoacetaldehyde would form under the reaction conditions, which may give some indication of the mechanism(s) that lead to azole formation from 2 and NH2CN (Supplementary Fig. 41).
Fig. 3. Prebiotic photochemical reductive aminations and telescoped synthesis of azoles 21, 22 and 23.
Irradiation of glycolonitrile 1 and NH4Cl in the presence of PSO33− generates glycolaldehyde 2 (and its cyanohydrin 4) and ethanolamine 9. Addition of cyanamide results in the generation of azoles 21, 22 and 23. Irradiation of 9 and formaldehyde in the presence of PSO33− affords N-methyl ethanolamine 28 and N,N-dimethyl ethanolamine 29.
Ethanolamine 9, 2-aminooxazole 21, 2-aminoimidazole 22 and 2-aminothiazole 23 could thus have been localized on early Earth. This may have implications for the prebiotic synthesis of (oligo)nucleotides, as dihydroxyacetone 12 (the thermodynamically preferred triose isomer) can be converted to glyceraldehyde 7 by 23 (ref. 17)—the triose isomer required for reaction with 21 to yield ribo-aminooxazoline 16-r (ref. 26). This in turn, can be converted to ribo-nucleoside-2′,3′-cyclic phosphates27, and 9 is a catalyst for the polymerization of ribo-nucleoside-2′,3′-cyclic phosphates, producing short oligonucleotides25. Finally, nucleotides activated by 22 have been suggested as labile surrogates for nucleotide triphosphates, allowing both the non-enzymatic copying of oligonucleotides28 and ribozyme-catalysed RNA ligation29. Although the full ramifications of this result warrant more detailed investigation, it is beyond the scope of the current paper. We do note, however, that the reaction of 7 (50 mM) with 2-aminooxazole 21 (50 mM) to give the pentose aminooxazolines 16 in the presence of 9 (6 mM), 22 (19 mM) and 23 (60 mM, constituting the ratio in which 9, 21, 22 and 23 were formed; Supplementary Fig. 39) was still possible, giving 16 in ∼30% yield (cf. ∼40% yield in the absence of 9, 21, 22 and 23; Fig. 3 and Supplementary Fig. 42). Thus, the synthesis of short oligonucleotides via ribo-nucleoside-2′,3′-cyclic phosphates is potentially still viable from this mixture.
Although nucleotide-5′-phosphoro-2-aminoimidazolides are good substrates for the abiotic copying of oligomeric nucleotides28, a source of chemical activation is still required. We recently showed that MeNH2 24 can be converted to methyl isonitrile (MeNC) 25 under prebiotic conditions using ferrocyanide salts (nitroprusside), and that the intermediate isonitrile complex 26 is stable and can be concentrated by evaporation (Supplementary Fig. 43)7. UV light causes 26 to release 25, which, in the presence of an aldehyde or under mildly acidic conditions, can activate 5′-nucleotide monophosphates (Supplementary Fig. 43)7,8,30. The activated nucleotides can be intercepted in a highly efficient manner by imidazoles, such as 22, which form nucleotide-5′-phosphoro-2-aminoimidazolides in up to 76% yield (Supplementary Fig. 43)7,8,30. Consequently, we attempted the reductive amination of formaldehyde 27 (25 mM) with NH4Cl (150 mM) using PSO33− (75 mM) and UV light at either pH 9.2 or pH 7.0 in the presence of phosphate. We were pleased to observe MeNH2 24 was formed in ∼11% yield after 2-h reaction at pH 9.2 and in ∼26% yield at pH 7.0 (Supplementary Fig. 44 and Supplementary Table 10). Halving the concentration of NH4Cl returned ∼10% of 24 at either pH 7.0 or 9.2 (Supplementary Table 10).
Given the success of the reductive methylation of ammonia using 27 and PSO33−, we next considered the methylation of ethanolamine 9. Irradiation of 9 (30 mM) and 27 (90 mM) in the presence of PSO33− (120 mM) gave N-methyl ethanolamine 28 in ∼46% yield and N,N-dimethyl ethanolamine 29 in ∼3% yield after 4 h of reaction (Supplementary Fig. 45). Addition of more reductant (40 mM) followed by two cycles of addition of 27 (20 mM) then irradiation resulted in further methylation of the amines, giving 28 in ∼56% yield and 29 in ∼25% yield with only ∼19% of 9 remaining (Supplementary Fig. 45), a process that could presumably be repeated further. Phosphorylation of 9, 28 and 29 (50 mM each) was achieved using PSO33− (50 mM) and Fe(CN6)3− (100 mM) in formamide, leading to O-phosphorylethanolamine 30, N-methyl ethanolamine phosphate 31 and N,N-dimethyl ethanolamine phosphate 32 in ∼16%, ∼12% and ∼26% yield, respectively (Supplementary Figs. 46 and 47). These products are reminiscent of the intermediates used by phosphatidylethanolamine N-methyltransferase in bacteria31, and so a facile transition to the biosynthesis of primitive versions of phosphatidylcholine can be imagined.
Prebiotic synthesis of terpene precursors
We then considered thioamides, functional groups that are integral to our protometabolic network (Supplementary Fig. 1)1. We had already shown that cyanohydrins can be cleanly converted to α-hydroxythioamides by incubation with PSO33− (ref. 15), and it now appeared that the products of thiolysis could be reduced by the same reagent. As a representative example, glycolonitrile 1 (20 mM) was reacted with PSO33− (80 mM) at 65 °C and pH 6.5 for 20 h, which gave α-hydroxythioacetamide 33 in ∼97% yield (Fig. 4 and Supplementary Fig. 48). Addition of a second portion of PSO33− (80 mM) followed by irradiation at 254 nm for 4 h gave the expected reduction products thioacetamide 34 (∼23%) and acetaldehyde 3 (∼17%), and unexpectedly glycolaldehyde 2 (∼14%; Fig. 4 and Supplementary Fig. 48). Previously, when an α-hydroxythioamide, such as 2,2-dimethyl-2-hydroxythioacetamide 35, was subjected to HS−/CuCN photoreduction, clean α-deoxygenation occurred to furnish the corresponding thioamide (that is, isobutyryl thioamide 36), and ensuing reduction then afforded the corresponding aldehyde (that is, isobutyraldehyde 37), and α-hydroxyaldehydes were not observed (Fig. 4)1. This unexpected mode of reactivity of thiophosphate presented an intriguing possibility. If the reduction of 35 yielded some 2-hydroxy-2-methylpropanal 38, homologation (via cyanide addition, thiolysis and reduction) could lead to 3-methyl-1,3-butanediol 39 (Fig. 4). We expected phosphorylation of 39 under heating conditions to temporarily form the cyclic phosphate 40, which should be primed for elimination of the phosphate dianion at the tertiary centre, thereby affording dimethylallyl phosphate 41 and isopentenyl phosphate 42. These structures are analogous to the biosynthetic precursors of terpenes (dimethylallyl pyrophosphate and isopentenyl pyrophosphate), which are generally used to make secondary metabolites. Archaea, however, are absolutely dependent on linear isoprenoids for cell-membrane formation, constituting the hydrophobic moiety of their phospholipids.
Fig. 4. The unexpected synthesis of glycolaldehyde 2 from photochemical reduction of α-hydroxythioacetamide 33 by thiophosphate, and a possible route to potential isoprenoid precursors 41 and 42.

The synthesis of glycolaldehyde 2 is shown at the top, and the possible route to potential isoprenoid precursors 41 and 42 is shown by dotted arrows. Unless stated, reactions were carried out at ambient temperature. For convenience, unidirectional reaction arrows are used and aldehydes are depicted as carbonyl compounds, although they would also exist as hydrates in water.
Acetone 17 (50 mM), a by-product from glycerol 11 synthesis (Fig. 2, Supplementary Fig. 27 and ref. 1), was incubated with HCN (100 mM) and PSO33− (250 mM) at 50 °C for 4 h, which afforded ∼29% of 35 (Fig. 5 and Supplementary Fig. 49). Gentle sparging with N2 left 35 as the sole product (Supplementary Fig. 49). The remaining solution was then irradiated for 4 h, after which time all of 35 had been consumed (Supplementary Fig. 49). Addition of HCN (30 mM) resulted in the formation of cyanohydrin 43 in ∼27% yield starting from 35, or ∼8% overall yield from starting from acetone 17 (four steps, Fig. 5 and Supplementary Fig. 49). Although some α-deoxygenation took place, none of the resulting thioamide 36 or its secondary reduction product (isobutyraldehyde 37) were observed in the 1H NMR spectrum; only the fully reduced product, isobutanol, was present (∼20% yield; Supplementary Fig. 49).
Fig. 5. Prebiotic route to potential isoprenoid precursors.

Reactions were carried out at ambient temperature unless stated otherwise. For convenience, unidirectional reaction arrows are used and aldehydes are depicted as carbonyl compounds, although they would also exist as hydrates in water.
We investigated the next sequence of reactions starting from a prepared sample of 43 (90 mM). Thiolysis of 43 by PSO33− (545 mM) proceeded more slowly than some cyanohydrins, but α-hydroxythioamide 44 was obtained in good yield (∼64%) after two days (Supplementary Fig. 50 and Supplementary Table 11). Although lower concentrations of PSO33− could be used for the reaction, thiolysis was slower. Using PSO33− (200 mM) under analogous conditions, ∼41% of 44 had formed after three days of reaction (Supplementary Table 11). Addition of further PSO33−, followed by UV irradiation, fortuitously gave the α-deoxygenated, fully reduced compound 39 as the major product (∼40% yield starting from 38, Fig. 5 and Supplementary Fig. 50; 3-methyl-1,2,3-butanetriol was present, but only in ∼16% yield, Supplementary Fig. 50). Although the steps in this synthesis are high-yielding, sequential addition of cyanide would probably be required to form 39 most efficiently, and this could potentially have been achieved by the confluence of a cyanide-rich stream with the reaction stream9–11.
Recognizing that forcing conditions would be needed to eliminate H2O from 3-methyl-1,3-butanediol 39, we dissolved 39 (100 mM) in formamide with ammonium phosphate (300 mM), and heated the reaction at 150 °C. After 22 h, isopentenyl alcohol 45 (∼48% yield), isopentenyl phosphate 42 (∼6% yield) and dimethylallyl alcohol 46 (∼3% yield) could be observed by 1H NMR spectroscopy (Fig. 5 and Supplementary Figs. 51 and 52). For laboratory convenience we heated the reactions to 150 °C, but the reaction gave comparable results when run for longer periods of time at lower temperatures (Supplementary Fig. 53 and Supplementary Table 12), and on the geologic timescale, even lower temperatures may have sufficed (although not explored, metals ions and acid catalysis may also lead to elimination of water from 39). It was not possible to determine whether the elimination step occurs via cyclic phosphate 40, although we did note the presence of 40 in the reaction mixture. As thiophosphate can phosphorylate alcohols under much milder conditions, we mixed 46 (50 mM) with PSO33− (100 mM) in formamide and activated PSO33− (either with ferricyanide or UV light), which gave dimethylallyl phosphate 41 in ∼10% yield (Supplementary Figs. 54 and 55).
Conclusions
Evaluation of the first prebiotic synthesis of activated pyrimidine ribonucleotides26, in terms of a geological/geochemical scenario that could satisfy the requirements of that synthetic route, led us to consider meteoritic impacts1. While potentially providing a localized, abundant source of phosphorus species12,13,32, HCN would also be generated by those same impacts33,34. This suggests that cyanometallates would be present in the same location. We then showed that these compounds are effective catalysts for the photochemical reduction of HCN and nitriles using inorganic sulfur species as the stoichiometric reductant1,9,16,35,36. Returning full circle, we reassessed the processing of meteoritie-derived phosphorus species under photochemical conditions in the presence of HS−/H2S and found that hypophosphite and phosphite could be oxidized to phosphate, during which a new reagent was formed, PSO33− (ref. 14). In a further iteration of this process, we examined PSO33− in the context of our cyanosulfidic network, which led to marked improvements over existing prebiotic pathways and allowed entirely different ones. Most notably, we have demonstrated a one-pot, prebiotic synthesis of C2–C5 sugars from a C1 feedstock and prebiotic access to isopentenyl alcohol 45 and dimethylallyl alcohol 46, or phosphates thereof—the biological precursors of terpenes.
Although the mixture of C2–C5 sugars can be enriched in pentoses 14, several reported prebiotic routes to ribonucleotides requiring (d)-ribose37–39 will be confounded by the lack of stereoselectivity in the current synthesis of 14. Ribo-aminooxazoline 16-r offers a potential solution to this problem given its diastereoselective, and even enantioselective, crystallization, which can occur under appropriate conditions23,40,41. Although 16-r can be easily accessed from the C2–C5 sugar mixture (Fig. 2 and Supplementary Figs. 24–26), it could also be derived after aldol reaction of glycolaldehyde 2 and glyceraldehyde 7 (Fig. 2)42, which can be formed in almost equimolar amounts from HCN or HCN and 1 using thiophosphate as the reductant (Supplementary Figs. 8–10). A third avenue for the synthesis of 16-r also exists; this proceeds through 2-aminooxazole 21 by reaction with 7 (Fig. 3 and Supplementary Fig. 42)26, possibly requiring 2-aminothiazole 23 (Supplementary Figs. 39 and 40) to separate 2 and 7 (ref. 17). Studies can now be undertaken to determine which of these routes is the most promising for a telescoped synthesis of (deoxy)ribonucleosides from feedstock molecules.
Methods
General experimental
All reactions were run at least twice, and the NMR spectra shown in the Supplementary Information are representative examples. Reagents and solvents were bought from Sigma-Aldrich, Alfa Aesar and Santa Cruz Biotechnology and were used without further purification, apart from thiophosphate, which had variable amounts of impurities and H2O present (Supplementary Information, page 1). Reagents were weighed using a Sartorius AX124 M-Pact analytical balance and small volumes were measured using a Gilson Pipetman system. Photochemical reactions were carried out using a Rayonet RPR-200 photochemical reactor chamber, with cooling fans switched on (the internal temperature of the unit when operational was ~40 °C) and fitted with low-pressure RPR-2537A mercury lamps purchased from Rayonet (principal emission at 254 nm). Hellman QS Spectrosil 10.0-mm quartz cuvettes with four UV-transparent windows were used for photochemical reactions. A Mettler Toledo SevenMulti pH/mV module fitted with a Thermo Scientific Orion 8103BN pH probe was used to measure pH, and deoxygenation of solvents and HCl/NaOH solutions was achieved by sparging with argon for 20–30 min before use. Although deoxygenation of HCl and NaOH solutions, used to adjust the pH of the reactions, may have altered the concentrations of these solutions, it was not deemed to be important, as adjustment of the pH of the reaction was our only consideration. Although using solvents that had not been deoxygenated was not anticipated to have a substantial effect on the outcome of the reactions, we wished to ensure that the reaction of O2 with any sulfur species was kept to a minimum, while also comporting with the expected anoxic environment of early Earth. The removal of dissolved O2 from aqueous media by sparging with an inert gas has been shown to be effective, although trace amounts of dissolved O2 may remain43. Rigorous exclusion of O2 from the solutions after deoxygenation was not possible, particularly when checking/adjusting the pH where the solution would typically be exposed to the atmosphere for ∼45 s. For comparison, a thiolysis and reduction reaction was run in ‘oxygenated’ solvents that were not deoxygenated before use, using thiophosphate (Supplementary Figs. 75 and 76). 1H, 31P and 13C NMR spectra were acquired using a Bruker Ultrashield 400 Plus instrument (at 400.1, 162.0 and 100.6 MHz, respectively); alternatively, 1H and 31P NMR spectra were recorded using a Bruker Ascend 400 instrument (at 400.2 and 162.0 MHz, respectively) using solvent suppression to collect 1H NMR data if reactions were run in a D2O/H2O mixture. If the spectra were unsatisfactory, a small amount of D2O was added to the NMR sample and the spectrum was reacquired. Yields were determined by the relative integration of signals in the 1H or 31P NMR spectra or by the addition of a standard of known volume and concentration, and relative integration to this signal. For quantitative integration of phosphorus NMR signals, we used a Bruker Avance-II 500 spectrometer with broadband cryogenic probe at a 31P frequency of 202.4 MHz. Quantitative integration of phosphorus NMR signals was achieved by determining the relaxation time (T1) for the nucleus, which was slowest to relax (thiophosphate, 8.4 s). 31P quantitative NMR (qNMR) spectra were acquired with a 30° pulse flip angle, >7 × T1 relaxation delay (giving >99% relaxation of 31P nuclei), 160-ppm spectral width, 128,000 acquisition data points and the spectrum offset close to the midpoint frequency of peaks being integrated. Spectra were processed and quantified using TopSpin version 3.2 software. Coupling constants (J) are given in hertz and the notations d, t and q represent the multiplicities doublet, triplet and quartet. Chemical shifts (δ) are given in ppm. Mass spectra were recorded with an Agilent Technologies 6130 Quadrupole LCMS using positive and negative electron spray ionization.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41557-023-01251-9.
Supplementary information
Supplementary Figs. 1–76, Tables 1–12, Discussions 1 and 2 and experimental procedures.
Acknowledgements
We would like to thank S. J. Mojzsis for helpful discussions and T. Rutherford for invaluable assistance with NMR spectroscopy. This work was supported by the Medical Research Council, as part of United Kingdom Research and Innovation (also known as UK Research and Innovation; grant no. MC_UP_A024_1009 to J.D.S.), and a grant from the Simons Foundation (grant no. 290362 to J.D.S.). For the purpose of open access, the MRC Laboratory of Molecular Biology has applied a CC BY public copyright licence to any author accepted manuscript version arising.
Author contributions
D.J.R. and J.D.S. conceived and designed the experiments. D.J.R. performed the experiments. D.J.R. and J.D.S. analysed the results. D.J.R. and J.D.S. wrote the paper.
Peer review
Peer review information
Nature Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Data availability
All data associated with this study are available in the published Article and its Supplementary Information.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dougal J. Ritson, Email: dritson@mrc-lmb.cam.ac.uk
John D. Sutherland, Email: johns@mrc-lmb.cam.ac.uk
Supplementary information
The online version contains supplementary material available at 10.1038/s41557-023-01251-9.
References
- 1.Patel BH, Percivalle C, Ritson DJ, Duffy CD, Sutherland JD. Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism. Nat. Chem. 2015;7:301–307. doi: 10.1038/nchem.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toner JD, Catling DC. Alkaline lake settings for concentrated prebiotic cyanide and the origin of life. Geochim. Cosmochim. Acta. 2019;260:124–132. doi: 10.1016/j.gca.2019.06.031. [DOI] [Google Scholar]
- 3.Genda H, Iizuka T, Sasaki T, Ueno Y, Ikoma M. Ejection of iron-bearing giant-impact fragments and the dynamical and geochemical influence of the fragments re-accretion. Earth Planet. Sci. Lett. 2017;470:87–95. doi: 10.1016/j.epsl.2017.04.035. [DOI] [Google Scholar]
- 4.Powner MW, Sutherland JD. Prebiotic chemistry: a new modus operandi. Phil. Trans. R. Soc. B. 2011;366:2870–2877. doi: 10.1098/rstb.2011.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu J, et al. Selective prebiotic formation of RNA pyrimidine and DNA purine nucleosides. Nature. 2020;582:60–66. doi: 10.1038/s41586-020-2330-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ritson DJ. A cyanosulfidic origin of the Krebs cycle. Sci. Adv. 2021;7:eabh3981. doi: 10.1126/sciadv.abh3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mariani A, Russell DA, Javelle T, Sutherland JD. A light-releasable potentially prebiotic nucleotide activating agent. J. Am. Chem. Soc. 2018;140:8657–8661. doi: 10.1021/jacs.8b05189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Z, et al. Harnessing chemical energy for the activation and joining of prebiotic building blocks. Nat. Chem. 2020;12:1023–1028. doi: 10.1038/s41557-020-00564-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutherland JD. The origin of life—out of the blue. Angew. Chem. Int. Ed. 2016;55:104–121. doi: 10.1002/anie.201506585. [DOI] [PubMed] [Google Scholar]
- 10.Ritson DJ, Battilocchio C, Ley SV, Sutherland JD. Mimicking the surface and prebiotic chemistry of early Earth using flow chemistry. Nat. Commun. 2018;9:1821. doi: 10.1038/s41467-018-04147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sasselov DD, Grotzinger JP, Sutherland JD. The origin of life as a planetary phenomenon. Sci. Adv. 2020;6:eaax3419. doi: 10.1126/sciadv.aax3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasek MA, Lauretta DS. Aqueous corrosion of phosphide minerals from iron meteorites: a highly reactive source of prebiotic phosphorus on the surface of the early. Earth. Astrobiol. 2005;5:515–535. doi: 10.1089/ast.2005.5.515. [DOI] [PubMed] [Google Scholar]
- 13.Bryant DE, Kee TP. Direct evidence for the availability of reactive, water soluble phosphorus on the early Earth. H-Phosphinic acid from the Nantan meteorite. Chem. Commun. 2006;22:2344–2346. doi: 10.1039/b602651f. [DOI] [PubMed] [Google Scholar]
- 14.Ritson DJ, Mojzsis SJ, Sutherland JD. Supply of phosphate to early Earth by photogeochemistry after meteoritic weathering. Nat. Geosci. 2020;13:344–348. doi: 10.1038/s41561-020-0556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ritson DJ, Xu J, Sutherland JD. Thiophosphate—a versatile prebiotic reagent? Synlett. 2017;28:64–67. doi: 10.1055/s-0036-1589414. [DOI] [Google Scholar]
- 16.Ritson DJ, Sutherland JD. Synthesis of aldehydic ribonucleotide and amino acid precursors by photoredox chemistry. Angew. Chem. Int. Ed. 2013;52:5845–5847. doi: 10.1002/anie.201300321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Islam S, Bučar D-K, Powner MW. Prebiotic selection and assembly of proteinogenic amino acids and natural nucleotides from complex mixtures. Nat. Chem. 2017;9:584–589. doi: 10.1038/nchem.2703. [DOI] [Google Scholar]
- 18.Polstorff K, Meyer H. Über die Einwirkung von Cyankalium auf Formaldehyde. Ber. Dtsch. Chem. Ges. 1912;45:1905–1912. doi: 10.1002/cber.19120450263. [DOI] [Google Scholar]
- 19.Gaudry R. Glycolonitrile. Org. Synth. 1947;27:41. doi: 10.15227/orgsyn.027.0041. [DOI] [Google Scholar]
- 20.Butlerow A. Bildung einer zuckerartigen Substanz durch Synthese. Liebigs Ann. Chem. 1861;120:295–298. doi: 10.1002/jlac.18611200308. [DOI] [Google Scholar]
- 21.Drew KN, Zajicek J, Bondo G, Bose B, Serianni AS. 13C-Labeled aldopentoses: detection and quantitation of cyclic and acyclic forms by heteronuclear 1D and 2D NMR spectroscopy. Carbohydr. Res. 1998;307:199–209. doi: 10.1016/S0008-6215(98)00040-8. [DOI] [Google Scholar]
- 22.Serianni AS, Pierce J, Huang S-G, Barker R. Anomerization of furanose sugars: kinetics of ring-opening reactions by 1H and 13C saturation-transfer NMR spectroscopy. J. Am. Chem. Soc. 1982;104:4037–4044. doi: 10.1021/ja00379a001. [DOI] [Google Scholar]
- 23.Springsteen G, Joyce GF. Selective derivatization and sequestration of ribose from a prebiotic mix. J. Am. Chem. Soc. 2004;126:9578–9583. doi: 10.1021/ja0483692. [DOI] [PubMed] [Google Scholar]
- 24.Lohrmann R, Orgel LE. Urea-inorganic phosphate mixtures as prebiotic phosporylating agents. Science. 1971;171:490–494. doi: 10.1126/science.171.3970.490. [DOI] [PubMed] [Google Scholar]
- 25.Verlander MS, Lohrmann R, Orgel LE. Catalysts for the self-polymerisation of adenosine cyclic 2′,3′-phosphate. J. Mol. Evol. 1973;2:303–316. doi: 10.1007/BF01654098. [DOI] [PubMed] [Google Scholar]
- 26.Powner MW, Gerland B, Sutherland JD. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature. 2009;459:239–242. doi: 10.1038/nature08013. [DOI] [PubMed] [Google Scholar]
- 27.Xu J, et al. A prebiotically plausible synthesis of pyrimidine β-ribonucleosides and their phosphate derivatives involving photoanomerization. Nat. Chem. 2017;9:303–309. doi: 10.1038/nchem.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li L, et al. Enhanced nonenzymatic RNA copying with 2-aminoimidazole activated nucleotides. J. Am. Chem. Soc. 2017;139:1810–1813. doi: 10.1021/jacs.6b13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walton T, DasGupta S, Duzdevich D, Oh SS, Szostak JW. In vitro selection of ribozyme ligases that use prebioticially plausible 2-aminoimidazole-activated substrates. Proc. Natl Acad. Sci. USA. 2020;117:5741–5748. doi: 10.1073/pnas.1914367117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang SJ, Duzdevich D, Szostak JW. Potentially prebiotic activation chemistry compatible with nonenzymatic RNA copying. J. Am. Chem. Soc. 2020;142:14810–14813. doi: 10.1021/jacs.0c05300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geiger O, López-Lara IM, Sohlenkamp C. Phosphatidylcholine biosynthesis and function in bacteria. Biochim. Biophys. Acta. 2013;1831:503–513. doi: 10.1016/j.bbalip.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Pasek M, Lauretta D. Extraterrestrial flux of potentially prebiotic C, N and P to the Early Earth. Orig. Life Evol. Biosph. 2008;38:5–21. doi: 10.1007/s11084-007-9110-5. [DOI] [PubMed] [Google Scholar]
- 33.Fegley B, Prinn RG, Hartman H, Watkins H. Chemical effects of large impacts on the Earth’s primitive atmosphere. Nature. 1986;319:305–308. doi: 10.1038/319305a0. [DOI] [PubMed] [Google Scholar]
- 34.Parkos D, Pikus A, Alexeenko A, Melosh HJ. HCN production via impact ejecta during reentry during the late heavy bombardment. J. Geophys. Res. Planets. 2018;123:892–909. doi: 10.1002/2017JE005393. [DOI] [Google Scholar]
- 35.Ritson D, Sutherland JD. Prebiotic synthesis of simple sugars by photoredox systems chemistry. Nat. Chem. 2012;4:895–899. doi: 10.1038/nchem.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu J, et al. Photochemical reductive homologation of hydrogen cyanide using sulfite and ferrocyanide. Chem. Commun. 2018;54:5566–5569. doi: 10.1039/C8CC01499J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fuller WD, Orgel LE, Sanchez RA. Studies in prebiotic synthesis: VI. Solid-state synthesis of purine nucleosides. J. Mol. Evol. 1972;1:249–257. doi: 10.1007/BF01660244. [DOI] [PubMed] [Google Scholar]
- 38.Kim H, Benner SA. Prebiotic stereoselective synthesis of purine and noncanonical pyrimidine nucleotides from nucleobases and phosphorylated carbohydrates. Proc. Natl Acad. Sci. USA. 2017;114:11315–11320. doi: 10.1073/pnas.1710778114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Becker S, et al. Unified prebiotically plausible synthesis of pyrimidine and purine RNA ribonucleotides. Science. 2019;366:76–82. doi: 10.1126/science.aax2747. [DOI] [PubMed] [Google Scholar]
- 40.Anastasi C, Crowe MA, Powner MW, Sutherland JD. Direct assembly of nucleoside precursors from two- and three-carbon units. Angew. Chem. Int. Ed. 2006;45:6176–6179. doi: 10.1002/anie.200601267. [DOI] [PubMed] [Google Scholar]
- 41.Hein JE, Tse E, Blackmond DG. A route to enantiopure RNA precursors from nearly racemic starting materials. Nat. Chem. 2011;3:704–706. doi: 10.1038/nchem.1108. [DOI] [PubMed] [Google Scholar]
- 42.Harsch G, Bauer H, Voelter W. Kinetik, Katalyse und Mechanismus der Sekundärreaktion in der Schlußphase der Formose-Reaktion. Liebigs Ann. Chem. 1984;1984:623–635. doi: 10.1002/jlac.198419840402. [DOI] [Google Scholar]
- 43.Degenhardt S, et al. Comparison of the effectiveness of various deaeration techniques. Dissolution Technol. 2004;11:6–11. doi: 10.14227/DT110104P6. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figs. 1–76, Tables 1–12, Discussions 1 and 2 and experimental procedures.
Data Availability Statement
All data associated with this study are available in the published Article and its Supplementary Information.