Motor control has evolved in the nervous systems of animals and motor output is the final result of neural processing. Most previous studies have focused on the classical brain regions for motor coordination, including the cerebellum, motor cortex, and basal ganglia [1], while few studies have linked the claustrum to motor control. The claustrum is considered a global information integrator and coordinator, and many studies have focused on value, memory, and attention orientation [2]. However, clinical studies have shown a remarkable association between claustral pathologies and motor control disorders like Parkinson’s and Alzheimer’s disease [3]. Furthermore, electrical stimulation of the claustrum triggers movement [4], which heightens the clinical significance of the claustrum in motor control. Several recent studies on the claustrum have initially revealed some functions and operation modes of motor control [5–7], indicating that the motor control function of the claustrum will open up a new field for future research.
Shima et al. demonstrated in primate models that claustral neurons are involved in motor execution, hinting at the importance of premotor activity in motor function in the claustrum [8]. Subsequent exploration revealed connections with motor-related brain regions including the motor cortex (MC), prefrontal cortex (PFC), and parietal lobe, indicating its significant role in motor function [9, 10]. White et al. published two consecutive studies demonstrating that the claustrum primarily receives top-down input from the anterior cingulate cortex (ACC) and broadcasts to the downstream cortex. Then they identified neuronal types in the claustrum and discussed how microcircuits within the claustrum implement information processing [7, 11]. Recent research by Ollerenshaw et al. confirmed that G protein subunit beta4+ neurons in the anterior claustrum are strongly involved in behaviors related to sensory-motor transformation, rather than passively reflecting sensory stimuli [12]. In line with the conclusion above, Reus-García et al. found that the claustrum modulates the motor learning process of rabbit eyeblink conditioning and that inhibition of synaptic transmission in claustral neurons hinders the acquisition of the conditioned reflex [7]. Subsequently, Chevee et al. clearly pointed out that the activity of the neurons of the anterior claustrum in mice reflects the occurrence of licking movements [5]. Comparing the above results, we have summarized the mechanism and function of the claustrum in motor control.
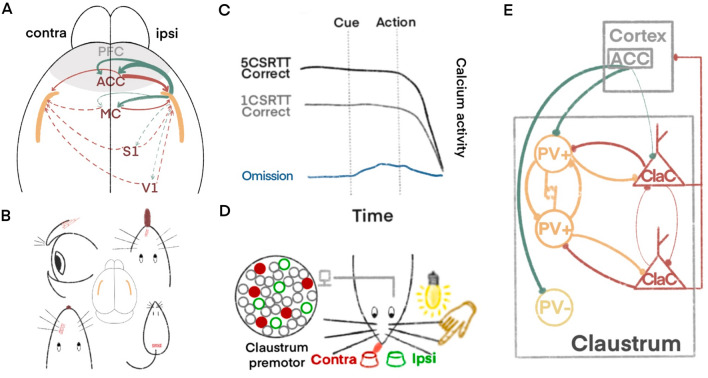
Regarding the input and output of motor control information in the claustrum, it has reciprocal projections with motor-related cortices such as the PFC, ACC, and MC, with stronger projections from the PFC and ACC [10, 13]. Similarly, White et al. reported that the ACC has significantly more projections to the claustrum than the somatosensory or motor cortex [6]. Notably, inputs to the claustrum from the ACC, prelimbic, primary motor cortex (M1), and secondary motor cortex tend to project to the contralateral side, while outputs have slightly stronger ipsilateral dominance [10, 14]. These connection patterns are consistent with motor control habits and may form the basis for bilateral motor coordination (Fig. 1A). Smith et al. made a conjecture about whether all types of movements are modulated by the claustrum. They found that both the M1 whisker retraction region and the caudal rhythmic whisking region in rats have projections to the contralateral claustrum, while the M1 forepaw region hardly has any projection [14]. Coincidentally, both the eyeblink reflexes [13] and licking movements [5] mentioned earlier belong to movements of sensory organs. This may suggest that the claustrum integrates information to rapidly regulate the movements of sensory organs (Fig. 1B).
Fig. 1.
Motor control mechanisms of the claustrum. A The claustrum mainly receives contralateral projections from the PFC, ACC, and MC, with the strongest projections coming from the ACC. The output connections from the claustrum to the cortex tend to have ipsilateral dominance. B The claustrum regulates movements of sensory organs such as eyelids, the tongue, whiskers, and the tail. C The overall activation of the nucleus is higher when performing complex motor tasks than when performing simple motor tasks, and its overall neuronal activity runs through the task prompt until the end of the action. D Electrodes are implanted on the side of the claustrum and monitored, with two licking points located on the ipsilateral and contralateral sides of the implanted electrodes. Red indicates claustrocortical (ClaC) neurons with contralateral preference and green indicates neurons with ipsilateral preference. Red neurons are labeled as solid, indicating that ClaC neurons with contralateral preference are synchronously activated before the mouse licks the contralateral side. E The claustrum predominantly receives modulation from the ACC during motor control, which is tuned by synchronization of internal microcircuits followed by signal output to various parts of the cortex. Three types of claustrum neurons, aspiny PV+ interneurons, aspiny PV− interneurons, and ClaC are distinguished. The same color is used for homogenous neurons and synaptic markers. Extruded lines at the ends indicate chemical synapses, parallel lines close to each other indicate gap junction connections and thin lines indicate weak connections.
With regard to the activation pattern of claustrum-related circuits during motor execution, White et al. applied fiber photometry to monitor the Ca2+ activity of ACC-claustrum circuitry in a five-choice serial reaction time task (5CSRTT) to elucidate the activation pattern of this projection circuit during the motor process in mice. The ACC afferents had a higher degree of activation before the cue signal in correct response trails than incorrect trails and exhibited a sustained low degree of activation in neglect trails. This suggests that the claustrum is activated before the motor action occurrence, performing a guidance function in the correctness of movement choice (Fig. 1C). This was verified by a subsequent experiment in which the optoinhibiton of ACC terminals in the claustrum led to a decreased correct choice rate in mice [6]. This result is consistent with the rabbit eyeblink conditioning experiment of Reus-García et al. In the experiment, after giving an auditory cue, the air was blown onto the rabbit’s left cornea to train the rabbit to blink after a sound. By analyzing the recorded changes in claustral electrical signals, they identified two types of claustral neurons. Type A neurons began firing during the interval between the conditioned stimulus and unconditioned stimulus (US) and continued firing until 1 second after the end of the US. It is worth emphasizing that simply giving an auditory cue to untrained rabbits did not elicit this type of firing in type A neurons [7]. This demonstrates that this type of claustral neuron can prepare and respond to the upcoming US based on experience and initiate eyelid movement. Taken together, it can be inferred that the claustrum receives upstream cortical signals and prepares for upcoming movements.
Complex movements are encoded by the claustrum. In addition to being able to regulate movements of sensory organs, there is also evidence that the claustrum has the ability to influence complex movements. White et al. simplified the 5CSRTT task and found that the ACC afferents in the claustrum exhibited lower activity before cues by making correct choices on simple tasks compared with complex tasks, but higher activity than errors and neglect choices [6]. This suggests that the degree of this pre-motor pre-activation depends on the complexity of the task, but that an insufficient level of pre-activation detracts from the correct execution of the subsequent motor act. In addition, Liu et al. found that activating the claustrum promotes impulsive movements by inhibiting the PFC [15]. Subsequently, Chevee found that when performing tasks with chemogenetically inhibited claustral activity, mice have a reduced probability of impulsive errors while associative error rates remain unchanged [5]. It is worth emphasizing that Amengual et al. further explored this circuit in a macaque attention task experiment. They believe that the impulse control regulation of the claustrum-PFC pathway affects response tendencies when stimuli arrive, rather than attentional functions that discriminate the relevance of input information [16]. That is, premotor circuit activation involving the claustrum can inhibit the PFC and enhance subsequent impulsive movements rather than distract attention.
The orientation encoding of the claustrum: Chevee et al. found in a “cross-modal sensory selection task” in mice that, although the claustrum as a whole did not have a directional preference, some individual neurons showed a preference for the direction of licking. In other words, neurons that preferred a certain direction were activated only before moving in that direction, and this preference was also present in spontaneous and task-directed movement [5]. It is thus postulated that the claustrum encodes the direction of licking through a small group of neurons with orientation preferences (Fig. 1D). Coincidentally, in line with the preparatory regulation proposed by White et al. [6], Chevee found that neurons that preferred the correct orientation had higher basal activity before sensory stimulation in the corresponding blocks [5], suggesting that some individual claustral neurons are able to adjust the direction of movement to be performed in the same preparatory manner. How exactly do these neurons encode the direction of movement? It was also demonstrated that a small group of claustrum neurons is instantaneously synchronized within one second before sensory stimulation, and this proportion is independent of whether the mice need to ignore visual or tactile stimulation. This reveals that synchronization is an intrinsic characteristic of these neurons. Interestingly, compared with the ipsilateral recording side of the claustrum, the onset of neural activity in contralateral licking was faster than that in the ipsilateral licking; that is, the overall mean response time has a contralateral preference. This means that the neurons encoding contralateral licking are preferentially synchronized. This is similar to eyelid responses, where this learned task requires signal transmission through the hippocampus-PFC-claustrum circuit to achieve pre-activation of claustral neurons based on acquired experience [17]. Therefore, we hypothesize that the claustrum encodes the direction of motor execution through the synchronization of local neurons and that this dominance has a contralateral preference.
Regulation of the motor process by microcircuits within the claustrum: It is worth noting that both activation and inhibition are encompassed in claustrum modulation. Reus-García et al. also identified a type of claustrum neurons whose discharge rate decreases during the conditioned reflex process, and their inhibition begins before the start of the eye-conditioned responses [7], implying that there is an internal inhibitory microcircuit in the claustrum regulating motor behavior. Previous studies have suggested that this regulatory mechanism is based on the microcircuits within the claustrum, in which neurons are mostly dominated by spiny excitatory projection neurons and aspiny inhibitory interneurons, while the latter can be subdivided into parvalbumin-positive (PV)+ and PV− interneurons. Since the connections between the claustral neurons projecting to the cortex are weak, and the PV+ interneurons have reciprocal connections to each other and contain gap junction connections, this pattern is speculated to be the basis for achieving synchronization tuning [18]. White et al. also noted that the optoactivation of PV+ interneurons eliminates the firing of spiny neurons along with aspiny PV− interneurons. Moreover, the large-scale and high-frequency spontaneous postsynaptic inhibitory currents mediated by γ-aminobutyric acid (GABA) type A receptors have been reported in spiny neurons, and this indicates that spiny claustral neurons are strongly inhibited [6], while the removal of inhibition would give rise to synchronized activation of spiny neurons. Thus, we argue that the strong innervation of spiny neurons by local PV+ interneurons is the microcircuit basis that synchronizes the direction of motor execution (Fig. 1E).
Based on these studied motor functions of the claustrum, what insights can we gain and how can we utilize these properties? First, claustral neurons encoding the direction of motor execution perform elevated basal activity before the movement occurs in a preparatory manner. Thus, the active state of the claustrum can predict whether the actual movement will occur next. Next, researchers can try to modulate the activity of microcircuits inside the claustrum and artificially increase or decrease the preparatory activity of a specific neuronal population to regulate the success rate of subsequent movements; or by regulate the claustrum-PFC circuit to influence the impulsiveness of movements. Then, as these populations of claustrum neurons with the same orientation preference are synchronized, they can be classified, in other words, we can predict the direction of motor execution by monitoring the activity of this group of neurons. In conclusion, with these characteristics of motor control in the claustrum, we can monitor the activity of the claustrum to predict the movement occurrence, action success rate, execution direction, and manipulate impulsive movement.
Conclusion
This article combines recent research on the claustrum and movement to summarize and emphasize the function and operating mechanism of the claustrum in motor control. In brief, the claustrum receives upstream cortical signals, processes them through its internal microcircuits, achieves synchronization tuning of the same type of neuronal populations, and then outputs back to various cortical regions to control movement. This process can directly control the occurrence and direction of movements of sensory organs, and even influence the success and impulsiveness of complex movements. Given that the preceding studies have been focused on the anterior part of the claustrum, future research will need to subdivide this region to confirm whether the middle and posterior parts play a similar role or function separately. In addition, there is an extensive bidirectional connection of the claustrum to the cortex [10, 11], as well as the complete opposite activation or inhibition effects of long activation and short activation of the claustrum on the downstream cortex [19]. These may provide a reference for studies on the downstream circuits through which the claustrum achieves motor control. In clinical motor-related disorders such as stroke and Parkinson’s disease, the specific functional impairment and compensatory mechanisms of the claustrum need to be further explored. This will provide important physiological references for clinical prognosis and rehabilitation.
Acknowledgements
This Insight was supported by grants from the Key-Area Research and Development Program of Guangdong Province (2019B030335001), the National Natural Science Foundation of China (32200815), the National Social Science Foundation of China (20&ZD296), and the China Postdoctoral Science Foundation (2022M721218).
Conflict of interests
The authors declare no competing interests.
References
- 1.Wang T, Chen Y, Cui H. From parametric representation to dynamical system: Shifting views of the motor cortex in motor control. Neurosci Bull. 2022;38:796–808. doi: 10.1007/s12264-022-00832-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith JB, Lee AK, Jackson J. The claustrum. Curr Biol. 2020;30:R1401–R1406. doi: 10.1016/j.cub.2020.09.069. [DOI] [PubMed] [Google Scholar]
- 3.Nikolenko VN, Rizaeva NA, Beeraka NM, Oganesyan MV, Kudryashova VA, Dubovets AA, et al. The mystery of claustral neural circuits and recent updates on its role in neurodegenerative pathology. Behav Brain Funct. 2021;17:8. doi: 10.1186/s12993-021-00181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bickel S, Parvizi J. Electrical stimulation of the human claustrum. Epilepsy Behav. 2019;97:296–303. doi: 10.1016/j.yebeh.2019.03.051. [DOI] [PubMed] [Google Scholar]
- 5.Chevée M, Finkel EA, Kim SJ, O'Connor DH, Brown SP. Neural activity in the mouse claustrum in a cross-modal sensory selection task. Neuron. 2022;110:486–501.e7. doi: 10.1016/j.neuron.2021.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White MG, Panicker M, Mu C, Carter AM, Roberts BM, Dharmasri PA, et al. Anterior cingulate cortex input to the claustrum is required for top-down action control. Cell Rep. 2018;22:84–95. doi: 10.1016/j.celrep.2017.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reus-García MM, Sánchez-Campusano R, Ledderose J, Dogbevia GK, Treviño M, Hasan MT, et al. The claustrum is involved in cognitive processes related to the classical conditioning of eyelid responses in behaving rabbits. Cereb Cortex. 2021;31:281–300. doi: 10.1093/cercor/bhaa225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shima K, Hoshi E, Tanji J. Neuronal activity in the claustrum of the monkey during performance of multiple movements. J Neurophysiol. 1996;76:2115–2119. doi: 10.1152/jn.1996.76.3.2115. [DOI] [PubMed] [Google Scholar]
- 9.Chia Z, Augustine GJ, Silberberg G. Synaptic connectivity between the cortex and claustrum is organized into functional modules. Curr Biol. 2020;30:2777–2790.e4. doi: 10.1016/j.cub.2020.05.031. [DOI] [PubMed] [Google Scholar]
- 10.Wang Q, Wang Y, Kuo HC, Xie P, Kuang X, Hirokawa KE, et al. Regional and cell-type-specific afferent and efferent projections of the mouse claustrum. Cell Rep. 2023;42:112118. doi: 10.1016/j.celrep.2023.112118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White MG, Mathur BN. Claustrum circuit components for top-down input processing and cortical broadcast. Brain Struct Funct. 2018;223:3945–3958. doi: 10.1007/s00429-018-1731-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ollerenshaw D, Davis J, McBride EG, Shelton AM, Koch C, Olsen SR. Anterior claustrum cells are responsive during behavior but not passive sensory stimulation. BioRxiv. 2021 doi: 10.1101/2021.03.23.436687. [DOI] [Google Scholar]
- 13.Gao L, Liu S, Gou L, Hu Y, Liu Y, Deng L, et al. Single-neuron projectome of mouse prefrontal cortex. Nat Neurosci. 2022;25:515–529. doi: 10.1038/s41593-022-01041-5. [DOI] [PubMed] [Google Scholar]
- 14.Smith JB, Alloway KD. Functional specificity of claustrum connections in the rat: Interhemispheric communication between specific parts of motor cortex. J Neurosci. 2010;30:16832–16844. doi: 10.1523/JNEUROSCI.4438-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Wu R, Johnson B, Vu J, Bass C, Li JX. The claustrum-prefrontal cortex pathway regulates impulsive-like behavior. J Neurosci. 2019;39:10071–10080. doi: 10.1523/JNEUROSCI.1005-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amengual JL, Di Bello F, Ben Hadj Hassen S, Ben Hamed S. Distractibility and impulsivity neural states are distinct from selective attention and modulate the implementation of spatial attention. Nat Commun 2022, 13: 4796. [DOI] [PMC free article] [PubMed]
- 17.Parras GG, Leal-Campanario R, López-Ramos JC, Gruart A, Delgado-García JM. Functional properties of eyelid conditioned responses and involved brain centers. Front Behav Neurosci. 2022;16:1057251. doi: 10.3389/fnbeh.2022.1057251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J, Matney CJ, Roth RH, Brown SP. Synaptic organization of the neuronal circuits of the claustrum. J Neurosci. 2016;36:773–784. doi: 10.1523/JNEUROSCI.3643-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McBride EG, Gandhi SR, Kuyat JR, Ollerenshaw DR, Arkhipov A, Koch C, et al. Influence of claustrum on cortex varies by area, layer, and cell type. Neuron. 2023;111:275–290.e5. doi: 10.1016/j.neuron.2022.10.026. [DOI] [PubMed] [Google Scholar]