Abstract
Aims
This study analyzed the gait patterns of diabetic peripheral neuropathy (DPN) patients and changes in the center of mass sway to prevent the formation and recurrence of foot ulcers.
Methods
Forty-two subjects were divided into the diabetes mellitus (DM), DPN, and diabetic foot ulcer (DFU) groups. We measured the range of motion (ROM) of the lower limb joints in the resting position and the center of mass sway in the standing position. Joint angles, ROM during walking, and distance factors were evaluated.
Results
In the DFU group, ROM limitation during walking was detected at the knee joint, and functional and ROM limitations were found at the ankle joint. The step length ratio and step width in the DFU group were significantly lower and higher than those in the DM group, respectively. The sway distances in the DFU group were greater than those in the DM and DPN groups.
Conclusions
Functional joint limitations and gait changes due to the decreased ability to maintain the center of gravity were observed in the DFU group. As DPN progressed, the patients’ gait became small, wide, and shuffled. Thus, supporting joint movement during walking may help reduce the incidence and recurrence of foot ulcers.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13340-023-00647-9.
Keywords: Type 2 diabetes mellitus, Foot ulcer, Diabetic neuropathy, Gait analysis, Center of mass sway, Balance
Introduction
The global prevalence of diabetic foot ulcers is 1.5–10%, and the incidence is 2.2–5.9% [1–3]. Because foot ulcers precede 80–85% of diabetes mellitus (DM)-related lower extremity amputations [4], preventing and managing them is closely related to the quality of life and prognosis of diabetic patients [5]. There are two main causes of diabetic foot ulcers: peripheral arterial disease and peripheral neuropathy. Patients with foot ulcers caused by peripheral neuropathy are reportedly more common than those with peripheral arterial disease [6]. Because neuropathic foot ulcers are mainly caused by sensory and motor neuropathy, which is difficult to treat, it is very important to suppress ulcer development and prevent recurrence after healing. Footwear and insoles with high decompression performance are reportedly effective in preventing the occurrence of foot ulcers, but the recurrence rate of foot ulcers remains as high as 40% [7].
In addition to plantar dysesthesia caused by sensory neuropathy, patients with DM reportedly suffer from muscle atrophy and limited joint range of motion (ROM) due to the glycosylation of proteins and lipids along with motor neuropathy [8]. Foot ulcers reportedly develop because of morphological changes in gait due to the combined effects of these factors [9–11]. Although there have been some studies in which detailed dynamic measurements and analyses of speed, pace, stride length, and gait patterns have been performed in patients with DM, the mechanism of foot ulcer development has not been elucidated [8]. Furthermore, the effect of body mass sway has been less considered in relation to gait.
This study aimed to examine how diabetic peripheral neuropathy (DPN) affects gait changes by analyzing joint movement at rest and during walking, and evaluating the center of mass sway in patients with DPN. These analyses can contribute to the elucidation of foot ulcer pathogenesis and the development of preventive strategies.
Subjects, materials and methods
Study participants
Subjects were recruited from among patients diagnosed with DM (type 1 and type 2) at Tokushima University Hospital between September 2017 and May 2019. All participants were classified into three groups: the DM (no DPN or foot ulcer history), DPN (with DPN and without foot ulcer history), and diabetic foot ulcer (DFU; with DPN and foot ulcer history) groups. The exclusion criteria were subjects who required any assistive apparatus to walk, suffered from peripheral arterial disease in which skin perfusion pressure was less than 40 mmHg at any one location, and could not provide consent for this study. Subjects with two of the following symptoms, loss of Achilles tendon reflex, decreased vibration perception, or decreased plantar perception, were considered to have DPN. This study was approved by the Clinical Research Ethics Review Committee of Tokushima University Hospital, and informed consent was obtained from all participants before the study. This study was conducted in compliance with the ethical principles of the Declaration of Helsinki.
ROM of the lower leg at resting position (ROM-Rest)
The ROM of the hip, knee, ankle, and metatarsophalangeal (MTP) joint of the first toe was measured in the supine position with the patient lying on a bed. The active and passive ROM for flexion and extension were measured for each joint, and the sum was defined as ROM-Rest (Supplemental Table).
Gait analysis
Nine reference points were set up on the floor at every 1 m in length and 0.5 m in width on a rectangular floor of 2 m in length and 1 m in width. Five points were set up vertically every 0.28 m in height from each reference point. Thus, 45 points were placed in the measuring area. The subjects, wearing sneakers, walked on a 2 m walking path with a 2 m approaching path at their own comfortable speed for gait analysis. The movements were recorded using a video camera (EX-100F CASIO, Japan) at 120 frames per second (fps) from the front and side directions. The captured video images were analyzed offline using Frame-DIAS 6 2D version (DKH, Q’sfix, Japan). The gait process was separated into seven phases and analyzed in the loading response, mid-stance, terminal stance, pre-swing, initial swing, mid-swing, and terminal swing phases [12].
Reflective markers were placed on the anterior superior iliac spine, greater trochanter, lateral epicondyle of the femur, lateral malleolus, lateral heel, lateral fifth toe MTP joint, and second toe phalanges. The angles of the hip, knee, ankle, and toe MTP joints were measured by analyzing the lateral-image gait movies. The hip joint angle was measured by connecting the three points of the anterior superior iliac spine, greater trochanter, and lateral epicondyle of the femur. Similarly, the knee, ankle, and toe angles of the MTP joints were measured.
The joint angles measured in the middle of each walking phase were compared among the three groups. The difference between each joint angle’s maximum and minimum points in a walking cycle was defined as the ROM during walking (ROM-Walk; Supplemental Figure, Supplemental Table).
The distance between the bilateral heel markers in a walking cycle was measured from the lateral video and was defined as the step length. The distance between the bilateral heel markers in a walking cycle was measured from the frontal video and was defined as the step width. Because there was a significant height difference between the groups, the measurements of distant factors were divided by height. The step length and width ratios for each height were calculated (Supplemental Table).
Center of mass sway on standing
The center of mass movement was recorded while the subjects stood on a measuring device (Wii Fit, Nintendo, Japan). Measurements were performed with eyes open and closed at two foot widths of 0 and 10 cm, respectively. The distance between the center of the device and the recorded center of mass of the subject was continuously measured for 30 s. The average sway distance was then calculated (Supplemental Table).
Statistical analysis
IBM SPSS Statistics 25.0 (2017, Stats Guild Inc. Japan) was used to perform the statistical tests. The Kruskal–Wallis test, with a significance level of 0.05, was performed on all mean values to reveal any differences among the three groups.
Results
Study participants
Forty-two subjects (men/women: 25/7, mean age ± standard deviation [SD]: 58.9 ± 14.8 years) participated in the study (Table 1). There were no significant inter-group differences in age, weight, and body mass index according to the Kruskal–Wallis test. The average height in the DFU group was significantly higher than that in the DM group as all participants in the former group were men. All participants in the DFU group had type 2 diabetes and two participants in the DPN group had type 1 diabetes.
Table 1.
Clinical characteristics of the study groups
| Group | DM | DPN | DFU | p-value |
|---|---|---|---|---|
| n (men/women) | 20 (8/12) | 15 (10/5) | 11 (7/0) | |
| Age (years) | 59.2 ± 13.9 | 57.8 ± 17.3 | 60.3 ± 13.8 | 0.943 |
| Body weight (kg) | 63.2 ± 16.4 | 65.1 ± 10.7 | 77.5 ± 18.2 | 0.058 |
| Height (cm) | 158.3 ± 8.9 | 163.1 ± 8.6 | 172.8 ± 9.2 | 0.007 |
| BMI (kg/m2) | 25.1 ± 5.0 | 24.4 ± 2.7 | 25.7 ± 4.2 | 0.690 |
Values are shown as mean ± SD. BMI body mass index
ROM-Rest
As the DPN progressed, a decrease in the ROM-Rest of each joint was observed among the three groups (Fig. 1). There was no significant difference in the ROM-Rest for both the hip and knee joints. In the ankle joint, however, the active and passive ROM-Rest in the DFU group were significantly smaller than those in the DM group (p < 0.01). In the MTP joint, both the active and passive ROM-Rest of the DFU group were significantly smaller than those of the DM (p < 0.01) and DPN (p < 0.05) groups.
Fig. 1.
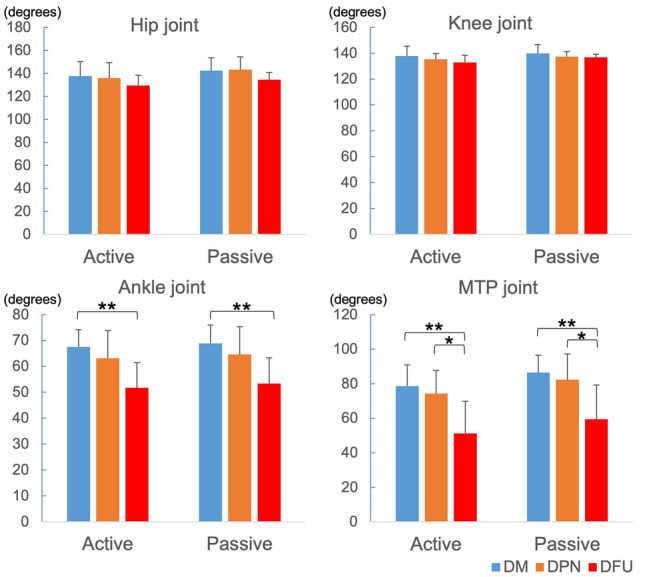
Active and passive range of motions at resting position (ROM-Rest) of 4 joints. DM diabetes mellitus, DPN diabetic peripheral neuropathy, and DFU diabetic foot ulcer. *p < 0.05, **p < 0.01
Gait analysis
Joint angle in each frame during walking
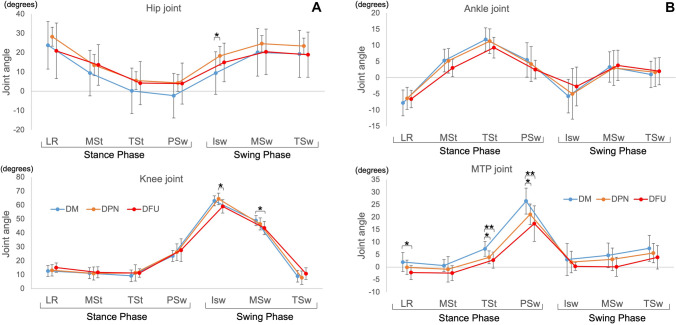
On comparing the joint angles during each walking phase among the three groups (Fig. 2), we found that the hip joint angles in the DM group were smaller than those in the DPN and DFU groups during almost all walking phases, with the hip joint angle in the DPN group being significantly larger than that in the DM group during the initial swing phase. Concerning the knee joint, the decrease in the joint angle in the DFU group was characteristic of the swing phase. Limitations of the ankle joint angle were observed in the DFU group during the stance and swing phases, although no significant difference was observed. Significant limitations of the MTP joint angle were observed in the DPN and DFU groups during the stance phase. The hip joint’s angle in the DPN group was greater than that in the DM group, and the joint angle of the knee joint was greater than that in the DFU group in the initial swing phase; however, the MTP joint’s angle was significantly lower than that in the DM group in the pre-swing phase.
Fig. 2.
A Joint angles recorded in a walking cycle of 3 groups. *p < 0.05 LR loading response phase, MSt mid-stance phase, TSt terminal stance phase, PSw pre-swing phase, ISw initial swing phase, MSw mid-swing phase, TSw terminal swing phase. DM diabetes mellitus, DPN diabetic peripheral neuropathy, and DFU diabetic foot ulcer. B Joint angles recorded in a walking cycle of 3 groups. LR loading response phase, MSt mid-stance phase, TSt terminal stance phase, PSw pre-swing phase, ISw initial swing phase, MSw mid-swing phase, TSw terminal swing phase. DM diabetes mellitus, DPN diabetic peripheral neuropathy, and DFU diabetic foot ulcer. *p < 0.05, **p < 0.01
ROM-Walk
There was no significant inter-group difference between the ROM-Walk angles of the hip joint (Fig. 3). The ROM-Walk angles in the DFU group were significantly smaller than those in the DM group in the knee, ankle, and MTP joints, while those in the MTP joint in the DPN group were significantly smaller than in the DM group.
Fig. 3.
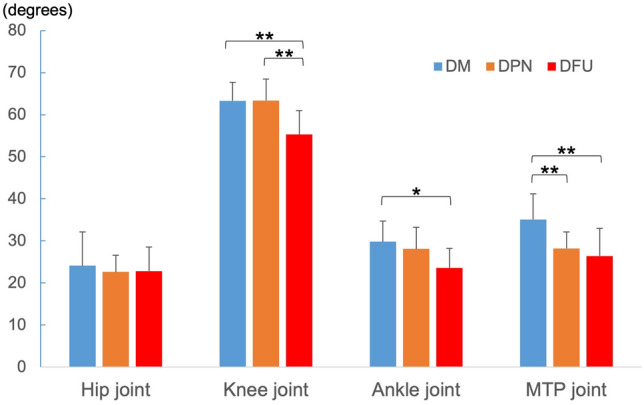
Results of ROM-walk in each joint. DM diabetes mellitus, DPN diabetic peripheral neuropathy, and DFU diabetic foot ulcer. *p < 0.05, **p < 0.01
Distance factors
The step length ratio in the DFU group was significantly smaller than that in the DM and DPN groups (Fig. 4). The step width ratio in the DFU group was significantly larger than that in the DM group.
Fig. 4.
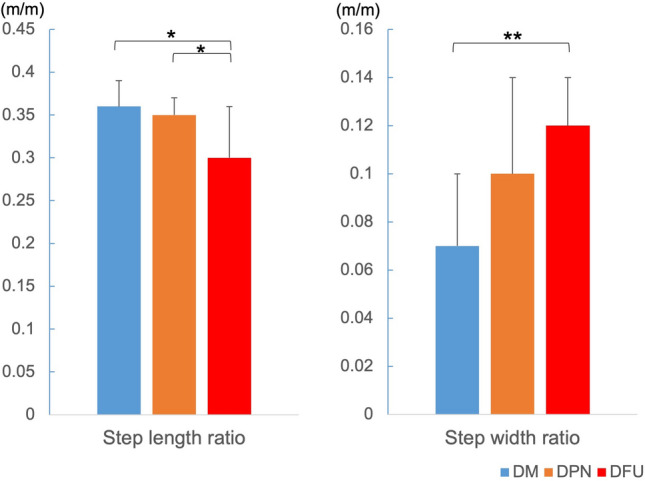
Distance factors in each group. DM diabetes mellitus, DPN diabetic peripheral neuropathy, and DFU diabetic foot ulcer. *p < 0.05, **p < 0.01
Center of mass sway
The average sway distances in the DFU group were larger than those in the DM and DPN groups (Fig. 5). Significant differences were observed between the measurements in the condition of foot width 10 cm with eyes closed, and in the condition of foot width 0 cm with eyes open and closed.
Fig. 5.
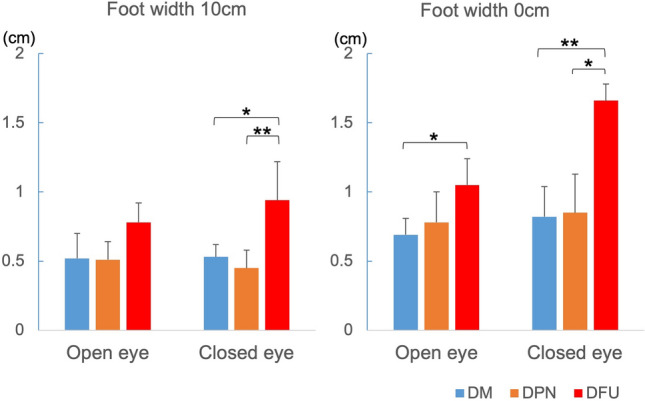
Average sway distances in each group. DM diabetes mellitus, DPN diabetic peripheral neuropathy, and DFU diabetic foot ulcer. *p < 0.05, **p < 0.01
Discussion
This study measured the ROMs of the four leg joints in two ways: ROM-Rest and ROM-Walk. Decrease in ROM-Rest and ROM-Walk showed joint function limitation at rest and joint ROM limitation while walking, respectively. The ROM-Rest and ROM-Walk angles in both the ankle and MTP joints tended to decrease as the severity of DPN progressed. The limitations of these ROMs were found to be more advanced at the MTP joint than at the ankle joint among the three groups. This finding is consistent with distal axonal degeneration in which DPN emerges from the periphery [13]. On the other hand, no significant between-group difference was detected in the ROM-Rest of the hip and knee joints. However, we found a significant difference in knee joint ROM-Walk in the DFU group.
These results revealed that DFU patients do not show functional limitations of the knee joint in the resting position but do show limitations in the ROM of the knee joint in their walking behavior. Interestingly, no significant limitations in ankle joint ROM were found in any walking phase, although a significant difference was observed in ROM-Rest and ROM-Walk between the DM and DFU groups. Previous studies on clinical populations have yielded conflicting results, particularly for ankle joint motion [14–16]. This study, however, found both joint function and ROM limitation in the ankle joint of patients with DFU.
The joint angle data in each phase (Fig. 2) revealed the gait characteristics of each group. The joint angles at the ankle and MTP joints in the DM group tended to be greater than those in the DPN and DFU groups; however, hip joint flexion in the swing phase of the DM group was less than that of the DPN group, especially during the initial swing phase. However, the effect of diabetic neuropathy on hip motion remains unclear. Two studies found a decrease in hip flexion range in DPN patients compared to that in non-diabetic participants [5, 15]. However, Gomes et al. found an increase in hip flexion in patients with DPN, which they believed was due to a compensatory effect for the loss of motion at the distal joints [17]. Our results support the complementary motion of the hip joint observed in the DPN group.
Our analysis of distance factors revealed decreased step length and increased step width in the DFU group. The significant difference in the distance factors and joint ROM limitation observed between the DM and DFU groups in this study suggests that these changes manifest after the appearance of foot ulcers. However, similar changes have been reported even in DM patients without neuropathy compared to the participants without DM [15]. Few studies have simultaneously measured these distance factors among patient groups with or without DM and with or without neuropathy. Further research is needed to determine how advanced diabetes progresses to step length and width abnormalities.
Our four sway distance measurements showed significantly increased instability in the DFU group. DPM-associated damage to vestibular, autonomic, and somatic nerves reportedly affects gait stability [18, 19], and vestibular neuropathy often precedes the loss of sensation in the feet [20]. The group II afferent fibers, which are sensory nerves from muscle spindles, play an important role in feedback control under static and dynamic conditions, including the stance phase of walking. It is assumed that the conduction velocity of group II fibers is reduced in patients with DM peripheral neuropathy [21, 22]. The increase in the sway distance found in this study is thought to be related to peripheral neuropathy and associated with an increased step width ratio.
There is currently no validated, dedicated system that can accurately quantify center of gravity, a critical component of standing balance, that is inexpensive, portable, and widely available. Clark et al. reported that the minimum detectable change values for the standing balance test performed with Wii fit exceeded the minimum detectable change values for the Laboratory-level force platform in three of the four tests [23]. These results suggest that the Wii Fit is a valid tool for assessing standing balance, and since the Wii Fit is portable, widely available, and a fraction of the cost of a force platform, it may provide a suitable standing balance assessment tool.
We believe dynamic balance control during gait is impaired with the progression of DM peripheral neuropathy and that compensating for the instability by increasing the step width prevents falls during gait [24]. The primary measurements in previous articles were gait speed and step length, but the importance of step width has often been underestimated [25]. Furthermore, mass sway has been studied in relation to fall risk, but its association with gait style has been less frequently reported. We believe that body mass sway affects gait, especially step width. After combining the results of the distance factors, joint ROM limitations, and joint function limitations, it can be assumed that as DPN progresses, the gait becomes “shuffle walking” with a small step length, wide step width, and little movement below the knee joint. This study suggests that in the DFU group, there was a mixture of functional limitations of the joints and gait changes due to decreased ability to maintain the center of gravity. Although shuffle walking and an enlarged step width lead to a stable gait to avoid falling, the increase in pressure and shear force in the plantar region may induce callosities formation and lead to foot ulceration.
One limitation of this study was that no measurement of plantar pressure, which is affected by gait changes, was performed. Future studies should examine the relationship between gait change and plantar pressure. Another limitation was the significant height difference observed between the DFU and DM groups. Only the distance factors, which were thought to be most affected by height, were corrected, but the remaining data were considered to be unaffected by height differences.
In the present study, joint restrictions, distance factor, and mass sway abnormalities were not significantly different between the DPN and DM groups but were significantly more pronounced in the DFU group. It is thought to be important to detect DPN in the early stage of DM and delay or stop the transition to DFU by controlling blood glucose and using walking aids, such as insoles or orthopedic shoes.
The pathogenesis of diabetic foot ulcers is quite different between peripheral neuropathy and peripheral arterial disease. The mechanisms of muscle atrophy and limitation of joint range of motion may also be different between peripheral neuropathy and peripheral arterial disease. It is clinically useful to investigate any different effect of the support of joint movement during walking to reduce the incidence of foot ulcers between the two main causes. In the present study, we examined gait change only in patients with peripheral neuropathy, but in the future, we would like to examine the effect of peripheral arterial disease on gait change. Moreover, the present cross-sectional study does not prove that joint support prevents the development and recurrence of foot ulcers. In order to draw more reliable conclusions, additional studies are needed, such as preparing groups with and without joint support to examine the development of foot ulcers.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
This work was supported by Research Clusters of Tokushima University (Grant Number: 1702007).
Declarations
Conflict of interest
All authors declare that they have no conflict of interest in the subject matter of that manuscript.
Ethical approval
This study was approved by the Clinical Research Ethics Review Committee of Tokushima University Hospital (Approval Number: 3002-2, Approval Date: December 21, 2020), and informed consent was obtained from all participants before the study. This study was conducted in compliance with the ethical principles of the Declaration of Helsinki.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abbott CA, Vileikyte L, Williamson S, Carrington AL, Boulton AJ. Multicenter study of the incidence of and predictive risk factors for diabetic neuropathic foot ulceration. Diabetes Care. 1998;21:1071–1075. doi: 10.2337/diacare.21.7.1071. [DOI] [PubMed] [Google Scholar]
- 2.Ramsey SD, Newton K, Blough D, McCulloch DK, Sandhu N, Reiber GE, Wagner EH. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care. 1999;22:382–387. doi: 10.2337/diacare.22.3.382. [DOI] [PubMed] [Google Scholar]
- 3.Abbott CA, Carrington AL, Ashe H, Bath S, Every LC, Griffiths J, Hann AW, Hussein A, Jackson N, Johnson KE, Ryder CH, Torkington R, Van Ross ER, Whalley AM, Widdows P, Williamson S, Boulton AJ. The North-West diabetes foot care study: incidence of, and risk factors for, new diabetic foot ulceration in a community-based patient cohort. Diabet Med. 2002;19:377–384. doi: 10.1046/j.1464-5491.2002.00698.x. [DOI] [PubMed] [Google Scholar]
- 4.Frykberg RG, Zgonis T, Armstrong DG, Driver VR, Giurini JM, Kravitz SR, Landsman AS, Lavery LA, Moore JC, Schuberth JM, Wukich DK, Andersen C, Vanore JV. Diabetic foot disorders. A clinical practice guideline revision (2006 revision) J Foot Ankle Surg. 2006;45:S1–S66. doi: 10.1016/S1067-2516(07)60001-5. [DOI] [PubMed] [Google Scholar]
- 5.Raspovic A. Gait characteristics of people with diabetes-related peripheral neuropathy, with and without a history of ulceration. Gait Posture. 2013;38:723–728. doi: 10.1016/j.gaitpost.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Moulik PK, Mtonga R, Gill GV. Amputation and mortality in new-onset diabetic foot ulcers stratified by etiology. Diabetes Care. 2003;26:491–494. doi: 10.2337/diacare.26.2.491. [DOI] [PubMed] [Google Scholar]
- 7.Bus SA, Waaijman R, Arts M, de Haart M, Busch-Westbroek T, van Baal J, Nollet F. Effect of custom-made footwear on foot ulcer recurrence in diabetes: a multicenter randomized controlled trial. Diabetes Care. 2013;36:4109–4116. doi: 10.2337/dc13-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wrobel JS, Najafi B. Diabetic foot biomechanics and gait dysfunction. J Diabetes Sci Technol. 2010;4:833–845. doi: 10.1177/193229681000400411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersen H. Motor dysfunction in diabetes. Diabetes Metab Res Rev. 2012;28:89–92. doi: 10.1002/dmrr.2257. [DOI] [PubMed] [Google Scholar]
- 10.Giacomozzi C, D'Ambrogi E, Uccioli L, Macellari V. Does the thickening of Achilles tendon and plantar fascia contribute to the alteration of diabetic foot loading? Clin Biomech (Bristol, Avon) 2005;20:532–539. doi: 10.1016/j.clinbiomech.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Mueller MJ, Diamond JE, Delitto A, Sinacore DR. Insensitivity, limited joint mobility, and plantar ulcers in patients with diabetes mellitus. Phys Ther. 1989;69:453–459. doi: 10.1093/ptj/69.6.453. [DOI] [PubMed] [Google Scholar]
- 12.Perry J, Burnfield JM. Phases of Gait. In: Perry J, Burnfield JM, editors. Gait Analysis: Normal and Pathological Function. 2. New Jersey: SLACK Inc; 2010. pp. 9–18. [Google Scholar]
- 13.Cashman CR, Höke A. Mechanisms of distal axonal degeneration in peripheral neuropathies. Neurosci Lett. 2015;596:33–50. doi: 10.1016/j.neulet.2015.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rao S, Saltzman C, Yack HJ. Ankle ROM and stiffness measured at rest and during gait in individuals with and without diabetic sensory neuropathy. Gait Posture. 2006;24:295–301. doi: 10.1016/j.gaitpost.2005.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yavuzer G, Yetkin I, Toruner FB, Koca N, Bolukbasi N. Gait deviations of patients with diabetes mellitus: looking beyond peripheral neuropathy. Eura Medicophys. 2006;42:127–133. [PubMed] [Google Scholar]
- 16.Sacco IC, Amadio AC. A study of biomechanical parameters in gait analysis and sensitive cronaxie of diabetic neuropathic patients. Clin Biomech (Bristol, Avon) 2000;15:196–202. doi: 10.1016/s0268-0033(99)00060-1. [DOI] [PubMed] [Google Scholar]
- 17.Gomes AA, Onodera AN, Otuzi ME, Pripas D, Mezzarane RA, Sacco IC. Electromyography and kinematic changes of gait cycle at different cadences in diabetic neuropathic individuals. Muscle Nerve. 2011;44:258–268. doi: 10.1002/mus.22051. [DOI] [PubMed] [Google Scholar]
- 18.Resnick HE, Vinik AI, Schwartz AV, Leveille SG, Brancati FL, Balfour J, Guralnik JM. Independent effects of peripheral nerve dysfunction on lower-extremity physical function in old age: the women’s health and aging study. Diabetes Care. 2000;23:1642–1647. doi: 10.2337/diacare.23.11.1642. [DOI] [PubMed] [Google Scholar]
- 19.Petrofsky J, Lee S, Bweir S. Gait characteristics in people with type 2 diabetes mellitus. Eur J Appl Physiol. 2005;93:640–647. doi: 10.1007/s00421-004-1246-7. [DOI] [PubMed] [Google Scholar]
- 20.Di Nardo W, Ghirlanda G, Cercone S, Pitocco D, Soponara C, Cosenza A, Paludetti G, Di Leo MA, Galli I. The use of dynamic posturography to detect neurosensorial disorder in IDDM without clinical neuropathy. J Diabetes Complications. 1999;13:79–85. doi: 10.1016/s1056-8727(99)00032-x. [DOI] [PubMed] [Google Scholar]
- 21.Nardone A, Grasso M, Schieppati M. Balance control in peripheral neuropathy: are patients equally unstable under static and dynamic conditions? Gait Posture. 2006;23:364–373. doi: 10.1016/j.gaitpost.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Nardone A, Corna S, Turcato AM, Schieppati M. Afferent control of walking: are there distinct deficits associated to loss of fibres of different diameter? Clin Neurophysiol. 2014;125:327–335. doi: 10.1016/j.clinph.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Clark RA, Mentiplay BF, Pua YH, Bower KJ. Reliability and validity of the Wii balance board for assessment of standing balance: a systematic review. Gait Posture. 2018;61:40–54. doi: 10.1016/j.gaitpost.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 24.Allet L, Armand S, Golay A, Monnin D, de Bie RA, de Bruin ED. Gait characteristics of diabetic patients: a systematic review. Diabetes Metab Res Rev. 2008;24:173–191. doi: 10.1002/dmrr.809. [DOI] [PubMed] [Google Scholar]
- 25.Lamola G, Venturi M, Martelli D, Iacopi E, Fanciullacci C, Coppelli A, Rossi B, Piaggesi A, Chisari C. Quantitative assessment of early biomechanical modifications in diabetic foot patients: the role of foot kinematics and step width. J Neuroeng Rehabil. 2015;12:98. doi: 10.1186/s12984-015-0093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.