Abstract
Introduction
The CAPTURE study estimated the global prevalence of established cardiovascular disease (CVD) and characterized the usage of glucose-lowering agents (GLAs) in adults with type 2 diabetes (T2D) across 13 countries. The purpose of this secondary analysis of data from the Japanese sites within CAPTURE (NCT03786406, NCT03811288) was to provide data about medication usage stratified by CVD status among Japanese participants with T2D.
Materials and methods
Data on GLA usage (including those with proven cardiovascular [CV] benefits) in Japanese participants with T2D managed in clinics or hospitals were collected and stratified by CVD subgroups.
Results
There were 800 Japanese participants in the CAPTURE study (n = 502 [no CVD group], n = 298 [CVD group], n = 268 [atherosclerotic CVD subgroup]). Oral antidiabetic agents and insulin were used by 88.5% and 23.4%, respectively, of participants overall. Among participants with established CVD, dipeptidyl peptidase-4 inhibitors (65.1%) were most frequently used, followed by biguanides (50.7%) and insulins (26.2%). The pattern was similar among participants with atherosclerotic CVD. A lower proportion of participants in the CVD group used glucagon-like peptide-1 receptor agonists (GLP-1 RAs) and sodium-glucose cotransporter-2 inhibitors (SGLT-2is) with proven CV benefits versus the no CVD group (GLP-1 RAs: 7.0% vs. 8.6%; SGLT-2is: 13.4% vs. 19.1%).
Conclusion
This analysis of the CAPTURE study provided a comprehensive overview of prescription patterns for the treatment of T2D in Japan. Use of GLAs with proven CV benefit was low, even in participants with established CVD, which was comparable to the findings from the global cohort.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13340-023-00638-w.
Keywords: Diabetes mellitus, type 2; Glucagon-like peptide-1; Sodium-glucose cotransporter-2 inhibitors
Introduction
The prevalence of diabetes (types 1 [T1D] and 2 [T2D]) in Japanese adults was 7.9% in 2019 according to the International Diabetes Federation [1]. A meta-analysis of 102 prospective studies reported that, independent of other conventional risk factors, patients with diabetes had a two-fold greater risk of numerous vascular diseases such as coronary heart disease (CHD) than those without diabetes [2]. Specifically, for example, according to the Japan Diabetes Complications Study, the crude incidence of myocardial infarction (MI) in patients with diabetes (3.84 per 1000 patient-years) was greater than in the general population (0.65–1.42 per 1000 patient-years) [3]. Diabetes is therefore considered among the top four healthcare priorities by the Japanese government [4]. However, a survey by the Japan National Health and Nutrition Surveys (2003–2012) in 51,128 Japanese adults reported that diabetes management was inadequate for preventing vascular complications [4].
The glucose-lowering agents (GLAs) commonly used in Japan include sulfonylureas (SUs), glinides, dipeptidyl peptidase-4 inhibitors (DPP-4is), biguanides, thiazolidinediones (TZDs), alpha-glucosidase inhibitors, sodium-glucose cotransporter-2 inhibitors (SGLT-2is), glucagon-like peptide-1 receptor agonists (GLP-1 RAs), and insulins. Several cardiovascular outcome trials (CVOTs) have shown cardiovascular (CV) risk reduction with some GLP-1 RAs or SGLT-2is versus placebo [5–10] , and this reduction may impact upon Asians more than Caucasians [11]. Unlike guidelines from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) [12, 13], the Japanese Clinical Practice Guideline for Diabetes 2019 does not specifically recommend agents with CV benefits [14], but a consensus statement from the Japanese Circulation Society (JCS) and the Japan Diabetes Society (JDS) includes such a recommendation [15].
The usage of GLAs in Japan appears to be different from other countries. While a few studies have investigated the use of GLAs within Japan [16], very few compare medication use simultaneously across several countries including Japan. Recently, the CAPTURE study, which was conducted across 13 countries simultaneously, estimated the global prevalence of established cardiovascular disease (CVD) in adults with T2D and compared GLAs and CVD medication use in participants with and without CVD [17]. The CAPTURE study also evaluated the usage of GLAs and CV medications in these participants with specific reference to GLAs demonstrating CV benefit [17].
The present analysis builds upon the CAPTURE Japan data previously published [18], which aimed to measure the prevalence and pattern of CVD among adults with T2D across 20 centers in Japan that took part in the CAPTURE study. The purpose of this secondary analysis was to provide data about medication usage stratified by CVD status among Japanese participants with T2D.
Materials and methods
Study design
The CAPTURE study was a cross-sectional, non-interventional study conducted at 214 centers in 13 countries between December 2018 and September 2019. Of these, 20 participating centers were from Japan. The full methods of the CAPTURE study (NCT03786406 and NCT03811288) have been previously published [17]. Adults (≥ 20 years) with diagnosis of T2D ≥ 180 days prior to providing informed consent were included, whereas adults with T1D or known congenital heart disease or malformation were excluded [17]. Available demographic, anthropometric, and clinical parameters were collected during a single visit [17, 18].
Diabetologists and general practitioners managing T2D in daily medical practice participated in the study. The clinical management of T2D and the treatments prescribed were entirely at the discretion of these physicians. In the current analysis, data specific to participants from Japan were analyzed.
Data recorded
Data on GLAs (current medications or those discontinued within the previous 3 months) used were the focus of this analysis; these medications included: biguanides, TZDs, SUs, glinides, DPP-4is, alpha-glucosidase inhibitors, SGLT-2is, GLP-1 RAs, and insulins. Participants were categorized based on CVD status: no CVD (patients without CVD), CVD (patients with CVD), and those with atherosclerotic cardiovascular disease (ASCVD; a subset of CVD). During this analysis, GLP-1 RAs and SGLT-2is were further categorized according to demonstrated CV benefit status, in line with the 2022 ADA guidelines [19, 20]. The GLAs with demonstrated CV benefit were three subcutaneous GLP-1 RAs (once-weekly dulaglutide, once-daily liraglutide, and once-weekly semaglutide) and three oral once-daily SGLT-2is (canagliflozin, dapagliflozin, and empagliflozin). The CVD medications used by participants were also analyzed.
Definitions of CV variables used
Established CVD was defined as any of the following conditions listed as a diagnosis in a participant’s medical records: cerebrovascular disease, CHD, heart failure (HF), cardiac arrhythmia or conduction abnormalities, aortic diseases, peripheral arterial disease (PAD), or carotid artery disease [17]. ASCVD (a subgroup of CVD and an important cause of morbidity and mortality in patients with T2D [20]) was similarly defined as a diagnosis of cerebrovascular disease, CHD, PAD, or carotid artery disease [17].
Ethics
The CAPTURE study was conducted in accordance with the Declaration of Helsinki [21], International Society for Pharmacoepidemiology Good Pharmacoepidemiology Practices [22], and local regulations for clinical research in Japan. The study protocol was approved by the clinical research ethics committee and institutional review board at each site, and written informed consent was provided by all participants [17, 18].
Statistical analysis
Full details of the statistical methodology for CAPTURE have been published previously [17]. Demographic and clinical characteristics of the CAPTURE study sample in Japan were analyzed according to CVD status. Data were analyzed descriptively, with no statistical comparisons performed. Data on GLA usage were also stratified based on CVD subgroups, number of GLAs used, age, body mass index (BMI), estimated glomerular filtration rate (eGFR), and GLAs with proven CV benefits. Data on CVD status and GLAs used by participants in clinics (outpatients) and hospitals were measured. All statistical analyses were carried out using Statistical Analysis Software (SAS), version 9.4 (SAS Institute, NC, USA).
Results
Baseline characteristics of participants according to CVD status
The CAPTURE study enrolled 9823 adults with T2D, of whom 800 were from Japan [17, 18]. Of these, 502 (62.8%) did not have CVD (no CVD group), 298 (37.3%) had CVD (CVD group), and 268 (33.5%) had ASCVD (ASCVD subgroup) [17, 18]. Demographic and clinical characteristics are summarized in Table 1.
Table 1.
Demographic and clinical characteristics of the CAPTURE study sample in Japan stratified by CVD status
| Characteristica | Overall (n = 800) | By CVD status | ||
|---|---|---|---|---|
| No CVD (n = 502) | CVD (n = 298) | ASCVDb (n = 268) | ||
| Male, n (%) | 537 (67.1) | 334 (66.5) | 203 (68.1) | 185 (69.0) |
| Female, n (%) | 263 (32.9) | 168 (33.5) | 95 (31.9) | 83 (31.0) |
| Age, years | 65.6 (11.2) | 63.0 (11.0) | 70.0 (10.1) | 70.2 (9.9) |
| Diabetes duration, years | 13.5 (9.0) | 12.1 (7.9) | 16.0 (10.2) | 16.3 (10.3) |
| HbA1c, % | 7.2 (0.9) | 7.2 (1.0) | 7.2 (0.9) | 7.3 (0.9) |
| FPG, mg/dL | 141.8 (39.5) | 141.0 (38.2) | 143.7 (42.6) | 144.1 (41.8) |
| Body weight, kg | 67.8 (13.8) | 68.7 (14.0) | 66.3 (13.2) | 65.7 (12.4) |
| BMI, kg/m2 | 25.6 (4.2) | 25.8 (4.4) | 25.2 (4.0) | 25.0 (3.7) |
| Systolic blood pressure, mmHg | 132.4 (14.7) | 132.5 (14.6) | 132.4 (14.9) | 132.7 (15.0) |
| Diastolic blood pressure, mmHg | 75.8 (10.5) | 77.4 (10.1) | 73.2 (10.7) | 73.2 (10.7) |
| Total cholesterol, mg/dL | 186.9 (37.0) | 193.5 (35.8) | 173.9 (35.9) | 174.7 (36.7) |
| LDL cholesterol, mg/dL | 104.3 (31.1) | 109.8 (31.2) | 94.7 (28.6) | 93.9 (28.7) |
| HDL cholesterol, mg/dL | 56.6 (15.7) | 57.9 (15.9) | 54.3 (15.0) | 55.0 (15.2) |
| Triglycerides, mg/dL | 149.3 (89.4) | 150.0 (92.4) | 148.1 (83.9) | 147.2 (84.0) |
Data are mean (standard deviation) unless otherwise indicated
aNumber of observations differs for each characteristic. bASCVD is a subgroup of CVD
ASCVD atherosclerotic cardiovascular disease, BMI body mass index, CVD cardiovascular disease, FPG fasting plasma glucose, HbA1c glycated hemoglobin, HDL high-density lipoprotein, LDL low-density lipoprotein
GLA usage by CVD subgroups
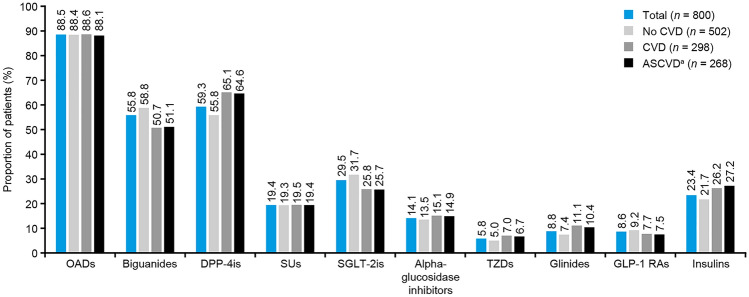
Oral antidiabetic drugs (OADs) were prescribed to a similar proportion of participants (88.1–88.6%) across the three CVD groups (Fig. 1). Insulin was prescribed to 21.7%, 26.2%, and 27.2% of the participants in the no CVD, CVD, and ASCVD subgroups, respectively (Fig. 1). Among participants with CVD, DPP-4is (65.1%) were most frequently used, followed by biguanides (50.7%), insulins (26.2%), and SGLT-2is (25.8%). Among those with ASCVD, the pattern was similar: DPP-4is (64.6%) were most frequently used, followed by biguanides (51.1%), insulins (27.2%), and SGLT-2is (25.7%). Most of the participants in the no CVD group (30.3%), CVD group (35.2%), and ASCVD subgroup (35.1%) received dual therapy (Fig. S1). Table S1 provides details on medication use in clinics and hospitals.
Fig. 1.
Diabetes medication usage stratified by CVD groups among CAPTURE study participants in Japan. aASCVD is a subgroup of CVD. ASCVD atherosclerotic cardiovascular disease, CVD cardiovascular disease, DPP-4i dipeptidyl peptidase-4 inhibitor, GLP-1 RA glucagon-like peptide-1 receptor agonist, OAD oral antidiabetic drug, SGLT-2i sodium-glucose cotransporter-2 inhibitor, SU sulfonylurea, TZD thiazolidinedione
GLA usage stratified by age, BMI, and eGFR
Most of the participants aged 20–45 years received biguanides (76.9%), followed by SGLT-2is (59.0%) and DPP-4is (48.7%). The majority of participants aged ≥ 75 years received DPP-4is (71.0%), followed by biguanides (41.5%) and insulins (26.7%) (Fig. S2). Diabetes medication usage stratified by both age and CVD status is presented in Fig. S3. Insulin use varied more with age in those with CVD versus those without CVD (Fig. S3).
The majority of participants with normal BMI (18.5–24.9 kg/m2) and with BMI 25– ≤ 29.9 kg/m2 received DPP-4is (64.4% and 59.5%, respectively) (Fig. S4). Participants with BMI 30– ≤ 34.9 kg/m2 mostly received biguanides (59.4%), followed by DPP-4is (52.2%) and SGLT-2is (39.1%). Participants with BMI ≥ 35.0 kg/m2 mostly received biguanides (70.0%), followed by SGLT-2is (60.0%), DPP-4is (40.0%), and GLP-1 RAs (35.0%).
Most of the participants with eGFR > 89 mL/min/1.72 m2 and > 59– ≤ 89 mL/min/1.72 m2 received biguanides (70.3%) or DPP-4is (60.8%), respectively (Fig. S5). Insulin was the most used GLA among the participants with eGFR ≤ 29 mL/min/1.72 m2 (63.2%) (Fig. S5).
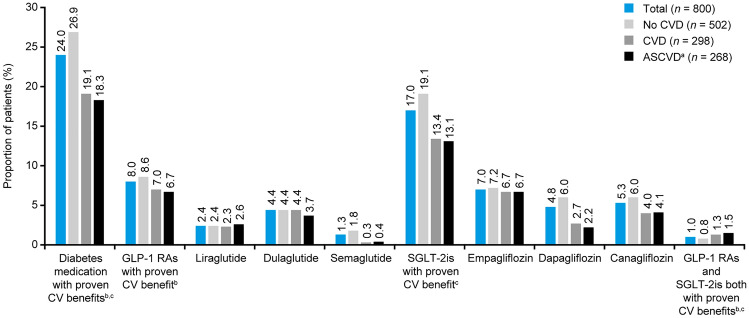
GLAs with proven CV benefit
Usage of SGLT-2is with proven CV benefit was lower in the CVD group versus the no CVD group (SGLT-2is: 13.4% vs. 19.1%), whereas that of GLP-1 RAs with proven CV benefit was similar (GLP-1 RAs: 7.0% vs. 8.6%) (Fig. 2). Overall, dulaglutide (4.4%) was the most frequently used GLP-1 RA, followed by liraglutide (2.4%) and semaglutide (1.3%). Empagliflozin (7.0%) was the most frequently used SGLT-2i, followed by canagliflozin (5.3%), and dapagliflozin (4.8%) (Fig. 2).
Fig. 2.
Usage of diabetes medication with proven CV benefit stratified by CVD groups among CAPTURE study participants in Japan. aASCVD is a subgroup of CVD. bSubcutaneous administration: once-weekly dulaglutide, once-daily liraglutide, once-weekly semaglutide. cOral administration (all once daily): empagliflozin, canagliflozin, dapagliflozin. ASCVD atherosclerotic cardiovascular disease, CVD cardiovascular disease, GLP-1 RA glucagon-like peptide-1 receptor agonist, SGLT-2i sodium-glucose cotransporter-2 inhibitor
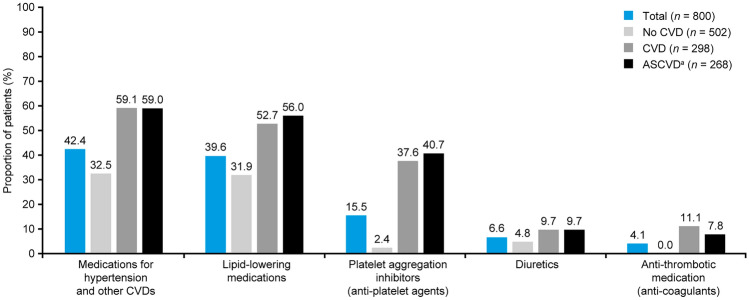
CVD medications commonly used
Overall, 42.4% of the participants were prescribed medications for hypertension and other CVDs. The proportions of participants receiving these medications were similar in the CVD and ASCVD subgroups (59.1% vs. 59.0%) (Fig. 3). After medications for hypertension and other CVDs, lipid-lowering agents were the next most frequently utilized in the no CVD (31.9%), CVD (52.7%), and ASCVD (56.0%) subgroups (Fig. 3); of these, statins were the most commonly used (n = 282 [35.3%]), irrespective of CVD subgroup. Details on commonly used CVD medications in clinics and hospitals are provided in Table S1.
Fig. 3.
CVD medication usage stratified by CVD groups among CAPTURE study participants in Japan. aASCVD is a subgroup of CVD. Of the lipid-lowering medications, statins were used by 282 (35.3%) in total, irrespective of CVD group. ASCVD atherosclerotic cardiovascular disease, CVD cardiovascular disease
Discussion
This secondary analysis of the CAPTURE study examined medication usage in the total population and in subgroups according to CVD status, among participants with T2D across 20 centers in Japan. Overall, 88.5% of the Japanese participants with T2D were prescribed OADs. DPP-4is were the most commonly used OADs among Japanese participants, whereas biguanides were the most used among global participants in the CAPTURE study [17]. Compared with the global participants, the CVD group within the Japanese population used DPP-4is more frequently (65.1% vs. 27.2%) and biguanides less frequently (50.7% vs. 70.6%) [17].
Japanese patients with T2D have lower insulin secretion compared with other ethnic populations and therefore insulin secretagogues like DPP-4is are a preferred treatment option [23, 24]. The findings from our analysis agree with earlier reports on high DPP-4i usage in Japan compared with other countries [25–28]. The preference to use DPP-4is in Japan might be due to their efficacy and safety [29, 30], infrequent dosing, fewer adverse events, and weight neutrality [31, 32]. Unlike in the USA (ADA) and Europe (EASD) [33], the Japanese guideline does not recommend any specific antidiabetic agent in the first-line treatment of patients with T2D [14, 34]. It is thus not surprising that differences in prescribing patterns in T2D between Japan and other countries were evident in this study.
In comparison with the global CAPTURE participants, a lower proportion of Japanese participants used insulin (37.7% vs. 23.4%), despite having a mean diabetes duration of ≈14 years [17]. Within the CVD group, insulin usage was lower in the Japanese participants (26.2%) compared with global participants (44.8%) [17]. Other studies have cited that the lower insulin usage in the Japanese participants may be attributed to fear of hypoglycemia and non-suitability to the Japanese lifestyle [35–37]. As per the Japanese Clinical Practice Guideline for Diabetes 2019, within the clinical setting, insulin therapy is only recommended in Japanese patients who do not reach their glycemic goal with diet, exercise therapy, and OADs [14].
Apart from the lower insulin usage, utilization of GLP-1 RAs was also lower among Japanese participants versus global participants [17], and was lower compared with other diabetes medications used in Japan. This is despite the fact that real-world evidence of GLP-1 RAs in improving glycated hemoglobin (HbA1c), body weight, and lipid profiles compared with baseline has been established using the Japan Diabetes Clinical Data Management Study Group (JDDM) database [38]. Furthermore, the GLP-1 RAs liraglutide, dulaglutide, and semaglutide, and the SGLT-2is empagliflozin and canagliflozin have shown positive outcomes in Japanese patients with T2D [39–43].
According to the JCS and JDS, GLP-1 RAs and SGLT-2is may be useful in the reduction of 3-point major adverse cardiovascular events (MACE) in patients with T2D [15]. Despite this, the present analysis revealed a risk-treatment paradox in use of GLP-1 RAs; the usage of such agents with beneficial CV outcomes was somewhat lower in the CVD and ASCVD groups compared with the no CVD group in the Japanese participants. Among these GLP-1 RAs, dulaglutide was used most frequently, followed by liraglutide and semaglutide. It is noteworthy that, in other countries, dulaglutide is approved at doses of 0.75 mg and 1.5 mg, with the latter providing CV benefits, while, in Japan, only the 0.75 mg dose is approved [8, 44, 45]. Furthermore, whereas liraglutide 1.8 mg, the dose approved for benefitting CV outcomes, was already in use in other countries [5, 46, 47], Japan did not grant approval until May 2019 [48], concurrent to the CAPTURE study. Subcutaneous semaglutide improved CV outcomes at doses of 0.5 mg and 1.0 mg in patients with T2D (post hoc analysis) [49], and was approved in Japan in 2018 [50]. Higher-dose GLP-1 RAs, which possess proven CV benefits, are relatively new in Japan, and therefore, presumably, the usage of such GLP-1 RAs for patients with CVD was limited at the time of the study and may have subsequently increased.
In contrast to the use of GLP-1 RAs, the use of SGLT-2is in Japanese participants was higher compared with the global population [17]. However, the use of SGLT-2is with beneficial CV outcomes was lower in the CVD and ASCVD groups compared with the no CVD group in the Japanese participants. The most commonly used SGLT-2i with CV benefit was empagliflozin, followed by canagliflozin and dapagliflozin. SGLT-2is such as ipragliflozin, luseogliflozin, and tofogliflozin, which are prescribed in Japan for patients with T2D [51–54], have not been investigated in CVOTs for patients with CVD or at high risk of CVD.
As reported in analyses of CVOTs with Asian participants, Asians with T2D might have greater CV benefits from SGLT-2is and GLP-1 RAs compared with Caucasians [11]. The low use both of GLP-1 RAs and of SGLT-2is in patients with CVD and ASCVD–the risk-treatment paradox–(which results in high morbidity and mortality in patients with T2D [20]) may suggest a lack of awareness of their proven CV benefits among physicians; however, physicians in Japan may gradually increase the use of these GLAs if the benefits are more widely known.
Our analysis adds to the few published studies that provide an overview of medications prescribed for T2D in Japan. Our results on the pattern of GLA usage in Japan are similar to those of the retrospective analyses of the Japan Diabetes compREhensive database project based on an Advanced electronic Medical record System (J-DREAMS) database, which analyzed the prevalence of comorbidities and complications in Japanese patients with T2D [55]. According to this study, despite the presence of comorbidities and complications, patients with T2D in Japan mainly received DPP-4is (37.2%) and biguanides (36.1%) in referral centers. In addition, GLP-1 RAs (7.0%) and SGLT-2is (12.6%) were not widely prescribed in Japan for the management of T2D [55]. It is possible that Japanese doctors might prescribe GLP-1 RAs and SGLT-2is anticipating not only secondary prevention of CVD but also as primary prevention for participants without CVD, due to the results from some CVOTs [5–10]. There is a strong need to explore the expanded use of these antidiabetic agents within Japan.
With regard to CVD medication usage, the CAPTURE study reported that statin use was lower in Japanese participants (35.3%) compared with global participants (51.0%) [17]. According to the JCS–JDS consensus report, statins are recommended for patients with T2D and CVD to achieve target low-density lipoprotein-cholesterol (LDL-C) of < 100 mg/dL, which is higher than the recommendation by the EASD guidelines (< 50 or < 75 mg/dL) [12, 15]. The statin use in this CAPTURE Japan analysis was consistent with the reported mean LDL-C levels of < 100 mg/dL in the CVD and ASCVD subgroups.
The Japanese clinical practice guideline recommends adopting a treatment strategy for patients with diabetes based on their characteristics and disease severity [14]. The majority of the Japanese participants in the CAPTURE study belonged to the age group 65–75 years. The mean age of the participants from Japan was comparable with that of the global participants, while their mean BMI was lower versus the global study participants [17]. It is important to note that the BMI of Asian populations is generally lower than that of Caucasians [56]. When diabetes medication usage was analyzed by BMI, an increased use of biguanides, SGLT-2is, and GLP-1 RAs was observed with increasing BMI. Furthermore, various studies have also reported greater weight loss due to SGLT-2is and GLP-1 RAs compared with placebo [9, 10, 57], which is likely to impact upon physicians’ decisions to prescribe these medications. When diabetes medication usage was analyzed by age, DPP-4is were the most prescribed medication in patients > 65 years. This may be related to DPP-4is being beneficial in controlling HbA1c levels in elderly patients with T2D with fewer side effects compared with other GLAs [58, 59]. Moreover, it was found that use of antidiabetic agents differed in participants according to their age, both in those with and those without CVD.
The present analysis has certain limitations. Japan has a unique healthcare system that includes clinic care and hospital healthcare systems [60]. The majority of the Japanese doctors participating in the CAPTURE study were diabetologists, which may have resulted in some bias. Hence, the data from Japanese centers cannot be directly compared with the global CAPTURE study due to differences in the healthcare settings. Since the prescribing pattern for use of GLAs in Japan seems to be governed by factors such as age, BMI, and comorbidities, without a specific guideline recommendation to use GLAs with proven CV benefit in patients with T2D, the findings may not be comparable with the global prescribing patterns. Although broad inclusion criteria were applied to ensure that the study sample was representative of the general adult T2D population, there were differences in age, antidiabetic agents used, and the prevalence of CVD between this cohort and other large Japanese cohort studies [26, 55, 61]. In addition, the sample size in this analysis was small (n = 800). As such, the generalization of our findings across the overall T2D population in Japan is a potential limitation of this analysis.
In conclusion, the present secondary analysis of the CAPTURE study provided a comprehensive overview of prescription patterns for the treatment of T2D in Japan. The analysis indicated that GLA usage in Japan differed from other countries. The overall use of GLAs with proven CV benefit was low, and comparable in participants with and without CVD in Japan; these findings are in line with those from the entire cohort of participants enrolled in the CAPTURE study [17]. Increased education, new evidence and updates to guidelines on the management of diabetes may enhance the use of GLAs with proven CV benefit.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This study was funded by Novo Nordisk A/S. The authors would like to thank the study participants, investigators, and coordinators. Medical writing support was provided by Dhara P. Patel and Beth Campbell, on behalf of Ashfield MedComms, and editorial assistance provided by Helen Marshall of Ashfield MedComms, an Inizio company, and funded by Novo Nordisk A/S.
Author contributions
YO, SS, and HS were investigators in the study and collected data. TS and KN contributed to statistical analyses required for the Japan data. All authors reviewed and edited drafts of the manuscript prior to submission. The authors confirm that they meet the International Committee of Medical Journal Editors uniform authorship requirements and that they have contributed to critical analysis and interpretation of the data, critically revised the article, and share in the final responsibility for manuscript content, as well as the decision to submit it for publication.
Funding
Novo Nordisk A/S.
Data availability
Upon reasonable request, the datasets used and/or analyzed during the current study are available from the lead author.
Declarations
Conflict of interest
YO has received honoraria/lecture fees from Novo Nordisk Pharma Ltd. and Sumitomo Pharma. SS has received honoraria/lecture fees from Eli Lilly Japan K.K., Novo Nordisk Pharma Ltd. and Sumitomo Pharma. KE and TS are employees of Novo Nordisk. KN holds shares in and is an employee of Novo Nordisk. HS has received research funding from Astellas Pharma Inc., Taisho Toyama Pharmaceutical, Takeda Pharmaceutical, Novo Nordisk Pharma, Sanofi, Mitsubishi Tanabe Pharma Co., Novartis Pharma K.K., Shionogi Pharma Co. Ltd., Boehringer Ingelheim, AstraZeneca K.K., and MSD; and honoraria from Shionogi Pharma Co., Mitsubishi Tanabe Pharma Co., Astellas Pharma Inc., Novartis Pharma K.K., Eli Lilly, Ono Pharmaceutical Co., MSD, and Sanofi.
Ethical approval
The protocol was approved by the IEC or other appropriate body and was provided by each investigator prior to undertaking any study-related activities. Specifically, the protocol was approved by: Institute for Adult Diseases, Asahi Life Foundation Institutional Review Board (approval date: 03 Dec 2018; approval number: 11000766); Medical Corporation Ichi YouKai Institutional Review Board Makato Honda Board (approval date: 13 Feb 2019; approval number: 14000077); Seino Naika Clinic Chairman, Ethical Review Board (approval date: 15 Dec 2018; approval number: 18000150); Shinagawa East One Medical Clinic Ethical Review Board Shinagawa East One Medical Clinic (approval date: 26 Nov 2018; approval number: 11000993); Nihonbashi Sakura Clinic Institutional Review Board (approval date: 05 Dec 2018; approval number: 11001007); Heiwadai Hospital Institutional Review Board (approval date: 21 Nov 2018; approval number: 11000861); Jinnouchi Hospital Ethical Review Board (approval date: 10 Dec 2018; approval number: 16000034); Kouhoukai Ethical Committee (approval date: 03 Dec 2018; approval number: 18000145); Hospital Joint Institutional Review Board (approval date: 21 Dec 2018; approval number: 14000050); and Institutional Review Board for Considering the Ethics of Special Non-Profit Entity Clinical Trials (approval date: 28 Jan 2019; approval number: 12000065).
Human research
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and/or with the Helsinki Declaration of 1964 and later versions. Informed consent was provided by each participant prior to undertaking any study-related activities (during the first and only study visit).
Informed consent
Informed consent was provided by each participant prior to undertaking any study-related activities (during the first and only study visit).
Approval date of registry and registration no. of the study/trial
NCT03786406, 26 December 2018 and NCT03811288, 22 January 2019.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be submitted.
Prior presentation
Some of the results from this study were previously presented at the 64th Annual Meeting of the Japanese Diabetes Society, virtual meeting, 2021.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.International Diabetes Federation (IDF) (2019) IDF Diabetes Atlas, 9th edn. Brussels, Belgium: 2019. Available at: https://www.diabetesatlas.org. 2019 (Accessed 2 Sep 2021).
- 2.Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujihara K, Sone H. Cardiovascular disease in Japanese patients with type 2 diabetes mellitus. Ann Vasc Dis. 2018;11:2–14. doi: 10.3400/avd.ra.17-00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ikeda N, Nishi N, Noda H, Noda M. Trends in prevalence and management of diabetes and related vascular risks in Japanese adults: Japan National Health and Nutrition Surveys 2003–2012. Diabetes Res Clin Pract. 2017;127:115–122. doi: 10.1016/j.diabres.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, Jeppesen OK, Lingvay I, Mosenzon O, Pedersen SD, Tack CJ, Thomsen M, Vilsbøll T, Warren ML, Bain SC. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841–851. doi: 10.1056/NEJMoa1901118. [DOI] [PubMed] [Google Scholar]
- 7.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 8.Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riesmeyer JS, Riddle MC, Rydén L, Xavier D, Atisso CM, Dyal L, Hall S, Rao-Melacini P, Wong G, Avezum A, Basile J, Chung N, Conget I, Cushman WC, Franek E, Hancu N, Hanefeld M, Holt S, Jansky P, Keltai M, Lanas F, Leiter LA, Lopez-Jaramillo P, Cardona Munoz EG, Pirags V, Pogosova N, Raubenheimer PJ, Shaw JE, Sheu WH, Temelkova-Kurktschiev T. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 9.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 10.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 11.Lee MMY, Ghouri N, McGuire DK, Rutter MK, Sattar N. Meta-analyses of results from randomized outcome trials comparing cardiovascular effects of SGLT2is and GLP-1RAs in asian versus white patients with and without type 2 diabetes. Diabetes Care. 2021;44:1236–1241. doi: 10.2337/dc20-3007. [DOI] [PubMed] [Google Scholar]
- 12.Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, Huikuri HV, Johansson I, Jüni P, Lettino M, Marx N, Mellbin LG, Östgren CJ, Rocca B, Roffi M, Sattar N, Seferović PM, Sousa-Uva M, Valensi P, Wheeler DC. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2022. Diabetes Care. 2021;45:S125–S143. doi: 10.2337/dc22-S009. [DOI] [PubMed] [Google Scholar]
- 14.Araki E, Goto A, Kondo T, Noda M, Noto H, Origasa H, Osawa H, Taguchi A, Tanizawa Y, Tobe K, Yoshioka N. Japanese clinical practice guideline for diabetes 2019. Diabetol Int. 2020;11:165–223. doi: 10.1007/s13340-020-00439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Araki E, Tanaka A, Inagaki N, Ito H, Ueki K, Murohara T, Imai K, Sata M, Sugiyama T, Ishii H, Yamane S, Kadowaki T, Komuro I, Node K. Diagnosis, prevention, and treatment of cardiovascular diseases in people with type 2 diabetes and prediabetes - a consensus statement jointly from the Japanese circulation society and the japan diabetes society. Circ J. 2020;85:82–125. doi: 10.1253/circj.CJ-20-0865. [DOI] [PubMed] [Google Scholar]
- 16.Nishimura R, Kato H, Kisanuki K, Oh A, Hiroi S, Onishi Y, Guelfucci F, Shimasaki Y. Treatment patterns, persistence and adherence rates in patients with type 2 diabetes mellitus in Japan: a claims-based cohort study. BMJ Open. 2019;9:e025806. doi: 10.1136/bmjopen-2018-025806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosenzon O, Alguwaihes A, Leon J, Bayram F, Darmon P, Davis T, Dieuzeide G, Eriksen KT, Hong T, Kaltoft MS, engyel C, Rhee NA, Russo GT, Shirabe S, Urbancova K, Vencio S, CAPTURE: a multinational, cross-sectional study of cardiovascular disease prevalence in adults with type 2 diabetes across 13 countries. Cardiovasc Diabetol. 2021;20:154. doi: 10.1186/s12933-021-01344-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seino H, Onishi Y, Eguchi K, Nishijima K, Sato T, Shirabe S. Cardiovascular disease prevalence in adults with type 2 diabetes in Japan: results from the Japanese centers in the CAPTURE study. Diabetol Int. 2023 doi: 10.1007/s13340-022-00612-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Diabetes Association 10. Cardiovascular disease and risk management: standards of medical care in diabetes—2022. Diabetes Care. 2021;45:S144–S174. doi: 10.2337/dc22-S010. [DOI] [PubMed] [Google Scholar]
- 20.American Diabetes Association 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2020. Diabetes Care. 2020;43:S111–s134. doi: 10.2337/dc20-S010. [DOI] [PubMed] [Google Scholar]
- 21.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 22.Epstein M. Guidelines for good pharmacoepidemiology practices (GPP) Pharmacoepidemiol Drug Saf. 2005;14:589–595. doi: 10.1002/pds.1082. [DOI] [PubMed] [Google Scholar]
- 23.Fukushima M, Usami M, Ikeda M, Nakai Y, Taniguchi A, Matsuura T, Suzuki H, Kurose T, Yamada Y, Seino Y. Insulin secretion and insulin sensitivity at different stages of glucose tolerance: a cross-sectional study of Japanese type 2 diabetes. Metabolism. 2004;53:831–835. doi: 10.1016/j.metabol.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Yabe DKH, Iwasaki M, Seino Y. Why are incretin-based therapies more efficient in East Asians? Perspectives from the pathophysiology of type 2 diabetes and East aAian dietary habits. EMJ Diabet. 2015;3:57–65. doi: 10.33590/emjdiabet/10312637. [DOI] [Google Scholar]
- 25.Katakami N, Mita T, Takahara M, Yajima T, Wada F, Kawashima M, Shimomura I, Watada H. Baseline characteristics of patients with type 2 diabetes initiating second-line treatment in Japan: findings from the J-DISCOVER Study. Diabetes Ther. 2020;11:1563–1578. doi: 10.1007/s13300-020-00846-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morita Y, Murayama H, Odawara M, Bauer M. Treatment patterns of drug-naive patients with type 2 diabetes mellitus: a retrospective cohort study using a Japanese hospital database. Diabetol Metab Syndr. 2019;11:90. doi: 10.1186/s13098-019-0486-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kubota K, Kamijima Y, Kao Yang YH, Kimura S, Chia-Cheng Lai E, Man KKC, Ryan P, Schuemie M, Stang P, Su CC, Wong ICK, Zhang Y, Setoguchi S. Penetration of new antidiabetic medications in East Asian countries and the United States: a cross-national comparative study. PLoS ONE. 2018;13:e0208796. doi: 10.1371/journal.pone.0208796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murayama H, Imai K, Odawara M. factors influencing the prescribing preferences of physicians for drug-naive patients with type 2 diabetes mellitus in the real-world setting in Japan: insight from a web survey. Diabetes Ther. 2018;9:1185–1199. doi: 10.1007/s13300-018-0431-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mishriky BM, Cummings DM, Tanenberg RJ. The efficacy and safety of DPP4 inhibitors compared to sulfonylureas as add-on therapy to metformin in patients with Type 2 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2015;109:378–388. doi: 10.1016/j.diabres.2015.05.025. [DOI] [PubMed] [Google Scholar]
- 30.Kim YG, Hahn S, Oh TJ, Kwak SH, Park KS, Cho YM. Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: a systematic review and meta-analysis. Diabetologia. 2013;56:696–708. doi: 10.1007/s00125-012-2827-3. [DOI] [PubMed] [Google Scholar]
- 31.Kohro T, Yamazaki T, Sato H, Harada K, Ohe K, Komuro I, Nagai R. Trends in antidiabetic prescription patterns in Japan from 2005 to 2011. Int Heart J. 2013;54:93–97. doi: 10.1536/ihj.54.93. [DOI] [PubMed] [Google Scholar]
- 32.Oh A, Kisanuki K, Nishigaki N, Shimasaki Y, Sakaguchi K, Morimoto T. Comparison of persistence and adherence between DPP-4 inhibitor administration frequencies in patients with type 2 diabetes mellitus in Japan: a claims-based cohort study. Curr Med Res Opin. 2020;36:387–395. doi: 10.1080/03007995.2019.1699519. [DOI] [PubMed] [Google Scholar]
- 33.Buse JB, Wexler DJ, Tsapas A, Rossing P, Mingrone G, Mathieu C, D'Alessio DA, Davies MJ. 2019 update to: management of hyperglycemia in type 2 diabetes, 2018. a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2020;43:487–493. doi: 10.2337/dci19-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, Maruthur NM, Rosas SE, Del Prato S, Mathieu C, Mingrone G, Rossing P, Tankova T, Tsapas A, Buse JB. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American DIABETES association (ADA) and the European association for the Study of diabetes (EASD) Diabetes Care. 2022;45:2753–2786. doi: 10.2337/dci22-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harashima SI, Nishimura A, Inagaki N. Attitudes of patients and physicians to insulin therapy in Japan: an analysis of the global attitude of patients and physicians in insulin therapy study. Expert Opin Pharmacother. 2017;18:5–11. doi: 10.1080/14656566.2016.1260547. [DOI] [PubMed] [Google Scholar]
- 36.Patorno E, Pawar A, Bessette LG, Kim DH, Dave C, Glynn RJ, Munshi MN, Schneeweiss S, Wexler DJ, Kim SC. Comparative effectiveness and safety of sodium-glucose cotransporter 2 inhibitors versus glucagon-like peptide 1 receptor agonists in older adults. Diabetes Care. 2021;44:826–835. doi: 10.2337/dc20-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peyrot M, Barnett AH, Meneghini LF, Schumm-Draeger PM. Insulin adherence behaviours and barriers in the multinational global attitudes of patients and physicians in insulin therapy study. Diabetic Med. 2012;29:682–689. doi: 10.1111/j.1464-5491.2012.03605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishigaki Y, Strizek A, Aranishi T, Arai N, Imaoka T, Cai Z, Maegawa H. Glucagon-like peptide-1 receptor agonist utilization in type 2 diabetes in Japan: a retrospective database analysis (JDDM 57) Diabetes Ther. 2021;12:345–361. doi: 10.1007/s13300-020-00977-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seino Y, Rasmussen MF, Nishida T, Kaku K. Efficacy and safety of the once-daily human GLP-1 analogue, liraglutide, vs glibenclamide monotherapy in Japanese patients with type 2 diabetes. Curr Med Res Opin. 2010;26:1013–1022. doi: 10.1185/03007991003672551. [DOI] [PubMed] [Google Scholar]
- 40.Kaku K, Yamada Y, Watada H, Abiko A, Nishida T, Zacho J, Kiyosue A. Safety and efficacy of once-weekly semaglutide vs additional oral antidiabetic drugs in Japanese people with inadequately controlled type 2 diabetes: a randomized trial. Diabetes Obes Metab. 2018;20:1202–1212. doi: 10.1111/dom.13218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaku K, Chin R, Naito Y, Iliev H, Ikeda R, Ochiai K, Yasui A. Safety and effectiveness of empagliflozin in Japanese patients with type 2 diabetes: interim analysis from a post-marketing surveillance study. Expert Opin Drug Saf. 2020;19:211–221. doi: 10.1080/14740338.2020.1694659. [DOI] [PubMed] [Google Scholar]
- 42.Koike Y, Shirabe SI, Maeda H, Yoshimoto A, Arai K, Kumakura A, Hirao K, Terauchi Y. Effect of canagliflozin on the overall clinical state including insulin resistance in Japanese patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2019;149:140–146. doi: 10.1016/j.diabres.2019.01.029. [DOI] [PubMed] [Google Scholar]
- 43.Odawara M, Miyagawa J, Iwamoto N, Takita Y, Imaoka T, Takamura T. Once-weekly glucagon-like peptide-1 receptor agonist dulaglutide significantly decreases glycated haemoglobin compared with once-daily liraglutide in Japanese patients with type 2 diabetes: 52 weeks of treatment in a randomized phase III study. Diabetes Obes Metab. 2016;18:249–257. doi: 10.1111/dom.12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pharmaceuticals and Medical Devices Agency (2015) New Drugs Approved in FY 2015. Available at: https://www.pmda.go.jp/files/000229077.pdf (Accessed Sep 13, 2021).
- 45.Frias JP, Wynne AG, Matyjaszek-Matuszek B, Bartaskova D, Cox DA, Woodward B, Li YG, Tham LS, Milicevic Z. Efficacy and safety of an expanded dulaglutide dose range: a phase 2, placebo-controlled trial in patients with type 2 diabetes using metformin. Diabetes Obes Metab. 2019;21:2048–2057. doi: 10.1111/dom.13764. [DOI] [PubMed] [Google Scholar]
- 46.Ito D, Iuchi T, Kurihara S, Inoue I, Katayama S, Inukai K. Efficacy and clinical characteristics of liraglutide in Japanese patients with type 2 diabetes. J Clin Med Res. 2015;7:694–699. doi: 10.14740/jocmr2237w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaku K. Liraglutide for the treatment of diabetes mellitus in Japan. Diabetes Manage. 2011;1:451–463. doi: 10.2217/dmt.11.34. [DOI] [Google Scholar]
- 48.Pharmaceuticals and Medical Devices Agency (2019) New Drugs Approved in FY 2019. Available at: https://www.pmda.go.jp/files/000235289.pdf. Accessed on June 10, 2021.
- 49.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 50.Pharmaceuticals and Medical Devices Agency (2017) List of approved products FY 2017. Available at: https://www.pmda.go.jp/files/000232769.pdf. (Accessed on Aug 3, 2022).
- 51.Poole RM, Dungo RT. Ipragliflozin: first global approval. Drugs. 2014;74:611–617. doi: 10.1007/s40265-014-0204-x. [DOI] [PubMed] [Google Scholar]
- 52.Poole RM, Prossler JE. Tofogliflozin: first global approval. Drugs. 2014;74:939–944. doi: 10.1007/s40265-014-0229-1. [DOI] [PubMed] [Google Scholar]
- 53.Markham A, Elkinson S. Luseogliflozin: first global approval. Drugs. 2014;74:945–950. doi: 10.1007/s40265-014-0230-8. [DOI] [PubMed] [Google Scholar]
- 54.Pharmaceuticals and Medical Devices Agency (2013) List of approved products FY 2013. Available at: https://www.pmda.go.jp/files/000232771.pdf. (Accessed on Sep 2, 2021).
- 55.Ohsugi M, Eiki JI, Iglay K, Tetsuka J, Tokita S, Ueki K. Comorbidities and complications in Japanese patients with type 2 diabetes mellitus: retrospective analyses of J-DREAMS, an advanced electronic medical records database. Diabetes Res Clin Pract. 2021;178:108845. doi: 10.1016/j.diabres.2021.108845. [DOI] [PubMed] [Google Scholar]
- 56.Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr. 2000;72:694–701. doi: 10.1093/ajcn/72.3.694. [DOI] [PubMed] [Google Scholar]
- 57.de Boer SA, Lefrandt JD, Petersen JF, Boersma HH, Mulder DJ, Hoogenberg K. The effects of GLP-1 analogues in obese, insulin-using type 2 diabetes in relation to eating behaviour. Int J Clin Pharm. 2016;38:144–151. doi: 10.1007/s11096-015-0219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu DN, Qiu L, Ning SY, Guo LX. Evaluation of efficacy and safety of DPP-4 inhibitors for Chinese elderly patients with type 2 diabetes mellitus. Diabetol Metab Syndr. 2020;12:35. doi: 10.1186/s13098-020-00543-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Viljoen AMC, Gadsby R, Viljoen S, Langerman H, Sinclair AJ. The tolerability and safety of DPP-4 inhibitors for the treatment of older people with type 2 diabetes mellitus: an observational study. British J Diabetes Vascular Dis. 2013;13:187–191. doi: 10.1177/1474651413500698. [DOI] [Google Scholar]
- 60.Hata J, Ninomiya T, Hirakawa Y, Nagata M, Mukai N, Gotoh S, Fukuhara M, Ikeda F, Shikata K, Yoshida D, Yonemoto K, Kamouchi M, Kitazono T, Kiyohara Y. Secular trends in cardiovascular disease and its risk factors in Japanese: half-century data from the Hisayama Study (1961–2009) Circulation. 2013;128:1198–1205. doi: 10.1161/CIRCULATIONAHA.113.002424. [DOI] [PubMed] [Google Scholar]
- 61.Tajima A, Tobe K, Eiki JI, Origasa H, Watada H, Shimomura I, Tokita S, Kadowaki T. Treatment patterns and satisfaction in patients with type 2 diabetes newly initiating oral monotherapy with antidiabetic drugs in Japan: results from the prospective real-world observational study on patient outcomes in diabetes (RESPOND) BMJ Open Diabetes Res Care. 2022 doi: 10.1136/bmjdrc-2022-003032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon reasonable request, the datasets used and/or analyzed during the current study are available from the lead author.