Abstract
By two PCR-based diagnostic methods, Plasmodium malariae infections have been rediscovered at two foci in the Sichuan province of China, a region where no cases of P. malariae have been officially reported for the last 2 decades. In addition, a variant form of P. malariae which has a deletion of 19 bp and seven substitutions of base pairs in the target sequence of the small-subunit (SSU) rRNA gene was detected with high frequency. Alignment analysis of Plasmodium sp. SSU rRNA gene sequences revealed that the 5′ region of the variant sequence is identical to that of P. vivax or P. knowlesi and its 3′ region is identical to that of P. malariae. The same sequence variations were also found in P. malariae isolates collected along the Thai-Myanmar border, suggesting a wide distribution of this variant form from southern China to Southeast Asia.
The four species of human malarial parasites differ greatly from each other in their clinical manifestations, responses to antimalarial drugs, and transmission potential and the nature of the immunity they elicit in human hosts. To design effective malaria control programs, an accurate and species-based epidemiological data set is a prerequisite both for avoiding suboptimal treatment of mixed infections and for implementing control measures (7, 18). In areas of hyperendemicity, mixed infections with two or more species are not uncommon (15). The less predominant species, Plasmodium malariae and P. ovale, tend to be overlooked because of low-level parasitemia and shortcomings of conventional microscopy in species identification. Thus, their distributions are often concealed by the other two predominant species, P. falciparum and P. vivax, in many areas of endemicity.
PCR methods based on detecting genus- or species-specific sequences of small-subunit (SSU) rRNA genes of Plasmodium spp. have been applied to the diagnosis and species-level identification of human malaria parasites collected in the field (13, 14, 16–20) and have revealed the underestimated prevalence of P. malariae and P. ovale in the past (4, 19, 22). By a nested-PCR-based method (12, 14), Kawamoto et al. (8) found that mutations in the target sequence of SSU rDNA account for the failure of a highly sensitive, PCR-based microtiter plate hybridization (MPH) method (2, 3) to detect malaria parasites morphologically resembling P. ovale. Zhou et al. (22) noticed that some P. malariae isolates collected in areas along the Thai-Myanmar border were MPH negative, with PCR-amplified products smaller than expected, suggesting the presence of a variant form of P. malariae. Here we report the rediscovery of P. malariae infection in the Sichuan province of China, a region that has been considered free of this species for the last 2 decades (21), and a variant which escaped earlier detection due to its genetic diversity in the sequence generally targeted for SSU rDNA-based diagnosis.
A total of 147 malarial samples were collected in transmission seasons from 1994 to 1996 in Junlian and Mingshan, counties located in the southeastern part and the middle part of Sichuan, respectively, and known as areas of hyperendemicity of tertian malaria (21). Thin and thick smears of finger or ear prick blood from febrile patients presenting in local public health stations or the county hospital were prepared and fixed with methanol. Microscopic examination for malaria diagnosis was performed by local microscopists on smears stained with Giemsa stain and acridine orange (AO) as described previously (9–11). Reexamination and species-level identification were performed in a double-blind manner, actually in different laboratories in China and Japan. A negative sample was defined as one for which no parasite was found in 300 fields at a magnification of ×1,000 under oil immersion for Giemsa-stained thick smears or by a 5-min observation at a magnification of ×400 for AO-stained thin smears. All of the stained samples were examined by two examiners experienced in these two methods and rechecked by two other experts at random. Venous blood samples were also collected in citrate-EDTA-anticoagulated tubes (1.5 ml) from volunteers (who gave informed consent) and were kept on ice in the field, kept at −20°C in the laboratory, and transported in dry-ice packages. All of the thin and thick smears were separately packaged and transported under dry conditions.
Genomic DNA was prepared from methanol-fixed thin or thick smears or from blood as previously described (12, 22). Briefly, a blood layer scraped from the smear with a clean razor blade or 50 μl of blood was mixed with 100 μl of a 30% Chelex-100 (Bio-Rad Laboratories, Richmond, Calif.) suspension and 50 μl of HEPES-buffered saline (HBS) (140 mM NaCl, 10 mM KCl, 1 mM MgCl2, 10 mM HEPES; pH 7.2). Supernatant was collected after boiling the mixture in a water bath for 10 min and centrifugation at 23,000 × g for 10 min and was used as the template DNA for PCR amplification.
PCR and nested PCR were performed as previously described (14, 22). Briefly, the target fragment of Plasmodium SSU rDNA in a mixture containing 3 to 4 μl of template DNA, 0.5 U of Taq DNA polymerase (Takara Chemicals, Ohtsu, Japan), a 0.5 mM concentration of each universal primer (Fig. 1), a 125 mM concentration of each deoxynucleoside triphosphate, and reaction buffer (10 mM Tris-HCl [pH 8.3], 50 mM KCl, and 1.5 mM MgCl2) was amplified by using a profile of 3 min at 94°C for the first cycle; 1 min at 94°C, 2 min at 58°C, and 2 min at 72°C for 33 cycles; and 10 min at 72°C for the last cycle. Nested PCR was performed with primers specific for the four human malaria species (Fig. 1). Briefly, 1 μl of PCR product diluted (1:100) with distilled water was applied to the PCR mixture as described above except 0.4 mM concentrations of a universal forward primer and each of the four species-specific reverse primers were added (Fig. 1). The amplification was conducted by using a profile of 2 min at 92°C for the first cycle, 30 s at 92°C and 90 s at 60°C for 18 cycles, and 5 min at 60°C for the last cycle. DNAs extracted from cultured P. falciparum (3D7 strain) and from blood of patients infected with P. vivax, P. ovale, or P. malariae were used as positive controls. Distilled water and DNAs from uninfected local individuals were used as negative controls (Fig. 2). To avoid contamination, amplification reagents and PCR products were handled in separated laboratory rooms, and manipulations involving PCR products were done with aerosol-resistant filter tips (Molecular Bio-Products Inc., San Diego, Calif.). The size of PCR products was checked by gel electrophoresis on 2.5% NuSieve–1% SeaKem agarose (FMC BioProducts, Rockland, Maine) or 2.5% Gibco agarose (Gibco BRL, Grand Island, N.Y.).
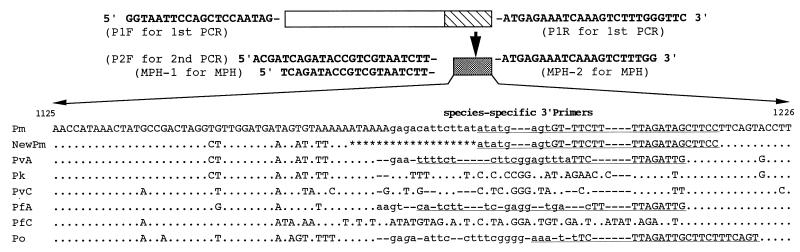
FIG. 1.
Partial sequences of the SSU rRNA genes of malaria species. Sequences are aligned with reference to that of P. malariae. Nucleotide identity is indicated by a dot; the lack of a corresponding nucleotide is indicated by a dash. Deleted nucleotides are shown as stars. Underlined regions are targeted by the species-specific 3′ primers used in nested PCR. Sequences in boldface type are those of the primers for the first PCR (P1F/P1R), nested PCR (P2F), and MPH (MPH-1/MPH-2). Lowercase letters are the probe-targeting regions for the MPH method. The italic letter (g) in the probe region of P. ovale should be an a in the originally designed specific probe based on the previously published partial sequence (2) (DDBJ accession number D17580). Abbreviations: PfA and PfC, A type and C type of P. falciparum, respectively; PvA and PvC, A type and C type of P. vivix, respectively; Pm, P. malariae; Pk, P. knowlesi; Po, P. ovale; NewPm, isolates with a 19-bp deletion and seven substitutions compared with P. malariae.
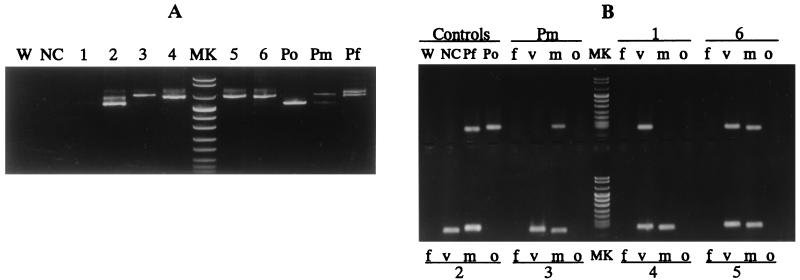
FIG. 2.
Detection of the four species of human malaria parasites by nested PCR. Results of the first PCR (A) and the second PCR (B) are shown. (A) A Sharp band(s) can be seen in positive controls and samples (lanes 2 to 6), while a faint band is seen in lane 1. No band is seen for any of the negative controls. (B) Positive controls show bands of the expected sizes, and no band is seen for the negative controls. Sample 1 is from a patient with a P. vivax infection. Samples 2 to 6 are from patients with mixed infections with P. vivax and P. malariae with a normal-size band (115 bp [sample 2]) or short band (96 bp [samples 3 to 6) of the latter species. Abbreviations: W, distilled water used as control; NC, negative control; Po, Pm, and Pf, positive controls for P. ovale, P. malariae, and P. falciparum, respectively; f, v, m, and o, products amplified by species-specific primers of P. falciparum, P. vivax, P. malariae, and P. ovale, respectively; MK, DNA size markers (pUCBM21 DNA digested by HpaII and DraI) (top to bottom, 1, 114, 900, 692, 501 and 489 [appears here as one band], 404, 320, 242, 190, 147, 124, and 110 bp).
A portion of the samples positive by PCR or nested PCR were further examined by the MPH method (2, 3). Briefly, Plasmodium-specific SSU rDNA in samples was amplified by using a pair of biotinylated universal primers (Fig. 1). The 138- to 150-bp products were then hybridized with species-specific probes immobilized in microtiter plate wells for detecting the four species of human malaria parasites (Fig. 1) and subjected to a colorimetric reaction by the alkaline phosphatase-conjugated streptavidin system. A405 measurements were made with a microplate reader (MPR-A4; Toshoh Co., Tokyo, Japan).
Nested-PCR products were subjected to sequence analysis to verify any variation in the target fragment, especially for those products that had positive results by the first PCR but indistinguishable results by MPH. As a direct strategy, DNA fragments of nested PCR products amplified from samples with single or mixed infections were electrophoretically separated on 2.5% agarose or 12% polyacrylamide gel and a single band was excised for purification with a Cleanmix Kit (Talent, Trieste, Italy). Otherwise, DNA fragments of the first PCR products were cloned into the pCR II plasmid from a TA Cloning Kit (Invitrogen Co., Carlsbad, Calif.). Screening for positive clones was performed by directly mixing the PCR mixture with a single transformed bacterial colony picked by a toothpick and amplifying the resulting mixture under the conditions of nested PCR. The target fragments from positive clones or uncloned samples were sequenced with a Dye Terminator Sequencing Kit (Applied Biosystems, Foster, Calif.) on an ABI-373A-90 sequencer (Applied Biosystems). Alignment of the obtained sequences and reported sequences was performed based on data from the GenBank/EMBL/DDBJ database (the accession numbers of P. malariae, a variant form of P. malariae, P. vivax (A/C forms), P. falciparum (A/C forms), P. ovale, and P. knowlesi are M54897, AB015654, U03079/U03080, M19172/M19173, L48986, and L07560, respectively).
Although the regions in the present study had been considered exclusively P. vivax-malarious areas (21), where species-level identification was routinely not conducted with Giemsa stain examination, P. malariae in the form of mixed infection with P. vivax was revealed in 43 patients by AO staining of thin smears. Unfortunately, most of the P. malariae parasites in the samples were atypical in morphology and hard to differentiate from P. vivax by the morphological characteristics of parasites and infected erythrocytes, though typical band forms, schizonts, and basket forms of P. malariae were found in a few samples. Therefore, diagnoses by species-specific PCR methods or sequence analysis were quite necessary to confirm the presence of this species.
A total of 147 malarial samples detected by Giemsa and AO staining were examined by nested PCR (Table 1, Fig. 2). Of the 147 samples, 127 were positive in the first PCR with a sharp band(s) at ∼0.7 kb (Fig. 2A) and 37 (29.1%) yielded a single band in the nested PCR with P. vivax- and P. malariae-specific primers (Fig. 2B). Among the 37 samples from patients with mixed infection, 29 (78.4%) showed a single band (96 bp) smaller than the expected size (115 bp), while 8 others showed a band with the expected normal size. There was no size variation among the samples positive for P. vivax (103 bp) and the controls for P. falciparum (101 bp) and P. ovale (114 bp) amplified with each of the species-specific primers (Fig. 2B). Negative controls were steadily negative in all of the experiments, and no cross-reaction was observed among positive controls and human DNA controls in the nested PCR.
TABLE 1.
Detection of Plasmodium spp. in malarial samples from Sichuan, Chinaa
| Method | No. of samples with result
|
No. of samples containing:
|
|||||
|---|---|---|---|---|---|---|---|
| + | − | P. falciparum | P. vivax | P. malariae | P. ovale | P. vivax + P. ma-lariae | |
| Giemsa stain | 147 | 0 | 0 | 147 | 0 | 0 | 0 |
| AO stain | 118 | 0 | 0 | 75 | 0 | 0 | 43 |
| Nested PCR | 127 | 20 | 0 | 90 | 0 | 0 | 37b |
A total of 147 samples were tested by each of the three methods shown. The numbers of samples with positive (+) and negative (−) results are given. Malaria-causing species were then determined.
Eight of these isolates showed normal-sized bands in the nested-PCR product.
To ascertain the presence of P. malariae and sequence variation in the target fragment, 16 samples with short or normal-size bands were subjected to sequence analysis by either the direct strategy or the cloning strategy. A 19-bp deletion and seven substitutions of base pairs in the target sequences of P. malariae were revealed in 11 samples (Fig. 1), corresponding to the short band (96 bp) which was also found in the other 18 samples by nested PCR (Table 1). Sequences of the normal-sized bands (115 bp) were identical to that previously reported (5). These results suggest that the variant form in the SSU rRNA gene of P. malariae is commonly present in areas local to us.
The MPH method was applied to samples from 12 patients with mixed infections with P. vivax and P. malariae as diagnosed by AO staining and nested PCR. Among them, 7 samples showing the shorter band resulted in no hybridization with the P. malariae-specific probe but had a positive reaction with the P. vivax-specific probe. The other samples with normal-size bands resulted in weakly positive reactions with the P. malariae-specific probe. These results confirmed that the variations were located just on the probe site in the target sequence (Fig. 1) and correlated well with the nested-PCR results and the sequencing data. In addition, we also analyzed samples collected from four P. malariae patients from the Thai-Myanmar border area (samples showed the short band in the nested-PCR product) (22). All of them revealed the same sequence variation as that found in Chinese samples.
By the nested PCR, the target fragment of P. malariae SSU rDNA was detected at a relatively high frequency (29.1%; 37 of 127) from patients with mixed infections involving P. vivax in two areas of endemicity in Sichuan, China. Since only 22 cases of P. malariae in southern provinces (Guangdong, Yunnan, and Guangxi) were officially recorded from 1991 to 1996 (1–1e), the rediscovery of P. malariae in Sichuan—which is responsible for more than 1/10 of the total annual reported malaria cases of the whole country (1–1e) and where no cases of P. malariae have been reported in the last 2 decades—indicates that the prevalence of P. malariae has been underestimated or overlooked in China. Similar situations have also been reported in Africa (18) and Southeast Asia (15, 22). Thus, our data, which reveal that the Plasmodium species composition in patients is neglected, may draw attention to the prevalence of P. malariae in our region, at least in southwestern China, where routine microscopic examination has failed to detect this species, possibly because it is present in subpatent infections or at low levels in mixed infections with P. vivax.
The target fragment in this study is located in block 9, one of the four highly variable blocks which display considerable sequence variation among plasmodial SSU rRNA genes (16). The variations are not only species specific but also type specific, making them useful in distinguishing the so-called A gene and C gene of Plasmodium species (Fig. 1) (16). The 19-bp deletion and seven substitutions of base pairs in this target region are the first mutations reported in the P. malariae SSU rRNA gene, and the mutations at the most-variable site of this target region lead to an alteration of the domain’s predicted secondary structure, resulting in ∼27% heterogeneity (data not shown) compared to previously reported sequences (6). More interestingly, alignment analysis with corresponding sequences of four human malaria and monkey malaria isolates (P. knowlesi and P. cynomolgi) showed that the 5′ region of the variant sequence with seven base pair substitutions is identical to the corresponding region of P. vivax or P. knowlesi and to the 3′ region of that of P. malariae (Fig. 1). Whether this sequence is from a variant of the blood-stage-expressed A form of the P. malariae SSU rRNA gene, from the yet-unknown mosquito-stage-expressed C form of the P. malariae SSU rRNA gene, or, less possibly, from a recombinant SSU rRNA gene of two malaria species warrants further investigation. The fact that this variant form of P. malariae was also detected in the Thailand-Myanmar border area (22) suggests that it may be steadily distributed in the region from southern China to Southeast Asia.
In this study, the MPH method detected P. vivax and P. falciparum with good efficiency but failed to detect P. malariae in most of the samples. The mutations in the probe region might be the cause of the failure to anneal the species-specific probe. Generally, high specificity corresponds to high variability in regions of the target gene, and most PCR-based specific diagnostic or differentiation methods are based on the detection of genus-, species-, or type-specific sequences. Based on our experience, negative results obtained by such a highly specific method should not be simply interpreted as the absence of the target gene, since sequence variations other than those reported thus far may be present. In other words, it is not unlikely for a highly specific PCR-based method to fail to detect parasites in samples with sequence variations in the target region.
Acknowledgments
This study was supported by Grants-in-Aid for International Scientific Research: Field Survey (07041159 and 09041179) and Grants-in-Aid for Scientific Research on Priority Areas (08281207, 09270210, and 10166209) from the Ministry of Education, Science, Culture and Sports, Japan, and by a grant from the Toyota Foundation (96B3-011) to F.K.
REFERENCES
- 1.Advisory Committee on Malaria, Ministry of Public Health. Malaria situation in the People’s Republic of China in 1991. Chin J Parasitol Parasitic Dis. 1992;10:161–165. [PubMed] [Google Scholar]
- 1a.Advisory Committee on Malaria, Ministry of Public Health. Malaria situation in the People’s Republic of China in 1992. Chin J Parasitol Parasitic Dis. 1993;11:161–164. [PubMed] [Google Scholar]
- 1b.Advisory Committee on Malaria, Ministry of Public Health. Malaria situation in the People’s Republic of China in 1993. Chin J Parasitol Parasitic Dis. 1994;12:161–164. [PubMed] [Google Scholar]
- 1c.Advisory Committee on Malaria, Ministry of Public Health. Malaria situation in the People’s Republic of China in 1994. Chin J Parasitol Parasitic Dis. 1995;13:161–164. [PubMed] [Google Scholar]
- 1d.Advisory Committee on Malaria, Ministry of Public Health. Malaria situation in the People’s Republic of China in 1995. Chin J Parasitol Parasitic Dis. 1996;14:169–172. [Google Scholar]
- 1e.Advisory Committee on Malaria, Ministry of Public Health. Malaria situation in the People’s Republic of China in 1996. Chin J Parasitol Parasitic Dis. 1997;15:129–132. [Google Scholar]
- 2.Arai M, Kunisada K, Kawai S, Kimura M, Wataya Y. DNA diagnosis of ovale malaria and malariae malaria using microplate hybridization. Nucleosides Nucleotides. 1994;13:1363–1374. [Google Scholar]
- 3.Arai M, Kunisada K, Kim H S, Miyake H, Mizukoshi C, Kakutani T, Yamane A, Nakagami S, Kawai S, Nakano H, Kawamoto F, Wataya Y. A colorimetric DNA diagnostic method for falciparum and vivax malaria: a field trial in the Solomon Islands. Nucleosides Nucleotides. 1996;15:719–731. [Google Scholar]
- 4.Bottius E, Guanzirolli A, Trape J F, Rogier C, Konate L, Druilhe P. Malaria: even more chronic in nature than previously thought; evidence for subpatent parasitemia detectable by the polymerase chain reaction. Trans R Soc Trop Med Hyg. 1996;90:15–19. doi: 10.1016/s0035-9203(96)90463-0. [DOI] [PubMed] [Google Scholar]
- 5.Goman M, Mons B, Scaife J G. The complete sequence of a Plasmodium malariae SSUrRNA gene and its comparison to other plasmodial SSUrRNA genes. Mol Biochem Parasitol. 1991;45:281–288. doi: 10.1016/0166-6851(91)90096-o. [DOI] [PubMed] [Google Scholar]
- 6.Gunderson J H, Sogin M L, Wollett G, Hollingdale M, de la Cruz V F, Waters A P, McCutchan T F. Structurally distinct, stage-specific ribosomes occur in Plasmodium. Science. 1987;238:933–937. doi: 10.1126/science.3672135. [DOI] [PubMed] [Google Scholar]
- 7.Hess F J, Kilian D A, Nothdurft H D, Loscher T. Problems in the therapy of mixed malarial infections: a case of infection with Plasmodium falciparum and P. malariae treated with mefloquine and halofantrine. Trans R Soc Trop Med Hyg. 1993;87:688. doi: 10.1016/0035-9203(93)90295-2. [DOI] [PubMed] [Google Scholar]
- 8.Kawamoto F, Miyake H, Kaneko O, Kimura M, Dung N T, Dung N T, Liu Q, Zhou M, Dao L D, Kawai S, Isomura S, Wataya Y. Sequence variation in the 18S rRNA gene, a target for PCR-based malaria diagnosis, in Plasmodium ovale from southern Vietnam. J Clin Microbiol. 1996;34:2287–2289. doi: 10.1128/jcm.34.9.2287-2289.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawamoto F. Rapid diagnosis of malaria by fluorescence microscopy using light microscope and interference filter. Lancet. 1991;337:200–202. doi: 10.1016/0140-6736(91)92159-y. [DOI] [PubMed] [Google Scholar]
- 10.Kawamoto F. Rapid detection of Plasmodium by a new ‘thick smear method’ using transmission fluorescence microscopy: direct staining with acridine orange. J Protozool Res. 1991;1:27–34. [Google Scholar]
- 11.Kawamoto F, Billingsley P F. Rapid diagnosis of malaria by fluorescence microscopy. Parasitol Today. 1992;8:69–71. doi: 10.1016/0169-4758(92)90093-h. [DOI] [PubMed] [Google Scholar]
- 12.Kimura M, Kaneko O, Inoue A, Ishii A, Tanabe K. Amplification by polymerase chain reaction of Plasmodium falciparum DNA from Giemsa-stained thin blood smears. Mol Biochem Parasitol. 1995;70:193–197. doi: 10.1016/0166-6851(95)00006-m. [DOI] [PubMed] [Google Scholar]
- 13.Kimura M, Kaneko O, Liu Q, Zhou M, Kawamoto F, Wataya Y, Otani S, Yamaguchi Y, Tanabe K. Identification of the four species of human malaria parasites by nested PCR that targets variant sequences in the small subunit rRNA gene. Parasitol Int. 1997;46:91–95. [Google Scholar]
- 14.Lal A A, Changkasiri S, Hollingdale M R, McCuthan T F. Ribosomal RNA-based diagnosis of Plasmodium falciparum malaria. Mol Biochem Parasitol. 1989;36:67–72. doi: 10.1016/0166-6851(89)90201-6. [DOI] [PubMed] [Google Scholar]
- 15.Paxton L A, Slutsker L, Schultz L J, Luby S P, Meriwether R, Matson P, Sulzer A J. Imported malaria in montagnard refugees settling in North Carolina: implications for prevention and control. Am J Trop Med Hyg. 1996;54:54–57. doi: 10.4269/ajtmh.1996.54.54. [DOI] [PubMed] [Google Scholar]
- 16.Qari S H, Goldman I F, Pieniazek N J, Collins W E, Lal A A. Blood and sporozoite stage specific small subunit ribosomal RNA-encoding genes of the human malaria parasite Plasmodium vivax. Gene. 1994;150:43–49. doi: 10.1016/0378-1119(94)90855-9. [DOI] [PubMed] [Google Scholar]
- 17.Sethabutr O, Brown A E, Panyim S, Kain K C, Webster H K, Echeverria P. Detection of Plasmodium falciparum by polymerase chain reaction in a field study. J Infect Dis. 1992;166:145–148. doi: 10.1093/infdis/166.1.145. [DOI] [PubMed] [Google Scholar]
- 18.Snounou G, Pinheiro L, Goncalves A, Fonseca L, Dias F, Brown K N, Rosario V E. The importance of sensitive detection of malaria parasites in the human and insect hosts in epidemiological studies, as shown by the analysis of field samples from Guinea Bissau. Trans R Soc Trop Med Hyg. 1993;87:649–653. doi: 10.1016/0035-9203(93)90274-t. [DOI] [PubMed] [Google Scholar]
- 19.Snounou G, Viriyakosol S, Jarra W, Thaithong S, Brown K N. Identification of the four human malaria parasite species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Mol Biochem Parasitol. 1993;58:283–292. doi: 10.1016/0166-6851(93)90050-8. [DOI] [PubMed] [Google Scholar]
- 20.Snounou G, Viriyakosol S, Zhu X P, Jarra W, Pinheiro L, Rosario V E, Thaithong S, Brown K N. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61:315–320. doi: 10.1016/0166-6851(93)90077-b. [DOI] [PubMed] [Google Scholar]
- 21.Wang X Z, Zhu S H, Liu Q, Hu A Q, Zan Z X, Yu Q G, Yin Q L. Field evaluation of the QBC technique for rapid diagnosis of vivax malaria. Bull W H O. 1996;74:599–603. [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou M, Liu Q, Kimura M, Wongsrichanalai C, Suwonkerd W, Panart K, Prajakwong S, Pensiri A, Kimura M, Matsuoka H, Ferreira M U, Isomura S, Kawamoto F. High prevalence of Plasmodium malariae and presence of P. ovale along the Thai-Myanmar border, as revealed by fluorescence microscopy and nested PCR. Trop Med Int Health. 1998;3:304–312. doi: 10.1046/j.1365-3156.1998.00223.x. [DOI] [PubMed] [Google Scholar]