Abstract
Introduction
Life expectancy of patients with psoriasis is reduced by 4–5 years due to cardiovascular disease with an increased risk of myocardial infarction at an earlier age compared with the general population. This increased risk is independent of traditional cardiovascular risk factors and higher in moderate-to-severe forms of psoriasis. Inflammation may play a key role in the development of atherosclerosis in these patients.
Methods and analysis
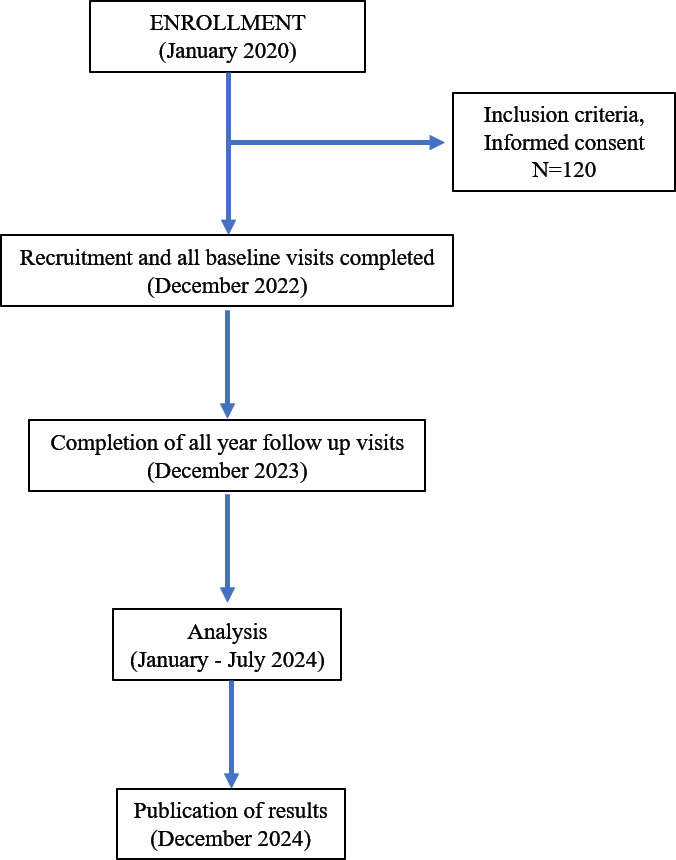
A prospective cohort study, Early Detection and Progression of Subclinical Atherosclerosis in Psoriasis (EDSAP), was initiated in January 2020 to investigate the presence and progression of subclinical atherosclerosis in patients with psoriasis. 120 patients aged 30–65 years and eligible for biological treatment have been recruited at Hospital Ramón y Cajal in Madrid, Spain. Patients undergo a baseline visit, and 1-year follow-up visit after starting biological therapy. Each visit includes: assessment of cardiovascular risk factors, screening for subclinical atherosclerosis by two-dimensional/three-dimensional ultrasound of carotid and femoral arteries, cardiac CT of coronary arteries and blood sampling. All baseline visits were completed by December 2022, and the remaining follow-up visits will be concluded by the end of 2023. The EDSAP study aims to identify new molecular and imaging markers associated with the presence of atherosclerosis and its progression in a chronic inflammatory state such as psoriasis. This has the potential to: (1) help improve primary cardiovascular prevention strategies in these patients; (2) understand the effect of biological drugs on the cardiovascular system; and (3) serve as a model for understanding atherosclerosis in other chronic inflammatory diseases.
Ethics and dissemination
The study protocol has been approved by the Institutional Review Board of the Hospital Ramón y Cajal in Madrid. We will present our findings at national and international congresses, and peer-reviewed journals.
Trial Registration number
Keywords: Psoriasis, Coronary heart disease, Computed tomography, Ultrasound
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Strict application of protocols, the collection of biobank samples and the prospective data collection which allows the evaluation of the impact of anti-inflammatory therapies on atheroma plaque characterisation and modulation.
The application of state-of-the-art scientific techniques to measure the anatomical and biological characteristics of subclinical atherosclerosis.
Being an observational study, it is more vulnerable to potential confounding factors compared with randomised controlled trials.
The open-label, non-randomised use of psoriasis treatments in a small sample size and with a short follow-up duration.
Introduction
Psoriasis is a complex chronic inflammatory and immune-mediated disease of the skin and joints associated with multiple comorbidities.1 2 The life expectancy of patients with psoriasis is reduced by 4–5 years due to cardiovascular (CV) disease, and there is an increased risk of myocardial infarction at an earlier age compared with individuals without the disease.3 4 This elevated CV risk could be due to systemic inflammation characteristic, especially in the moderate-to-severe forms of the disease.5 6 Therefore, classical screening methods such as the Framingham risk score, which is based on classical cardiovascular risk factors (CVRFs), do not reliably assess the risk of coronary heart disease in patients with psoriasis.4 7 8
Detection of atherosclerosis in its subclinical stage may help to identify strategies to halt the development of the disease. Many imaging studies in patients with psoriasis assessed subclinical atherosclerosis in individual vascular territories,4 9–13 but given the systemic nature of atherosclerosis, a multiterritorial analysis has the potential to provide a more comprehensive overview of the distribution and burden of atherosclerosis in these patients.14 The natural history of atherosclerosis involves a prolonged subclinical phase, where the disease is usually detected only at an advanced stage or after a CV event. Early detection of subclinical atherosclerosis and adoption of primary prevention measures, including adequate control of systemic inflammation, may minimise the risk of CV disease in patients with psoriasis. It has therefore been proposed that these patients should undergo comprehensive screening for subclinical atherosclerosis,4 a proposal that has arisen from the need to find a non-invasive, simple and widely available biomarker for its early detection.15
The most widely used and validated technique for screening subclinical atherosclerosis is vascular ultrasound (VUS), a reproducible, non-invasive technique with no side effects. In recent years, there has been a particular interest in the study of subclinical atherosclerosis in the femoral arteries. Studies in healthy adults have shown that femoral plaques are more prevalent than carotid plaques and are more associated with traditional CVRFs and coronary calcium,15 16 as well as being an independent predictor of future CV events.14 17 Interestingly, in the Progression of Early Subclinical Atherosclerosis (PESA) study (middle-aged participants from the general population), the presence of iliofemoral disease increases the risk of concurrent coronary calcium and is predictive of disease elsewhere.14 In fact, screening femoral arteries with VUS has been introduced in current clinical practice guidelines as a risk modifier in individuals at low or moderate risk individuals.18 In this regard, our research group evaluated the usefulness of femoral artery ultrasound for the detection of subclinical atherosclerosis in psoriasis. We observed that screening of femoral plaques improves the detection of subclinical atherosclerosis in these patients, whereas carotid artery scanning was not sufficiently accurate.15 19 Semiautomated three-dimensional vascular ultrasound (3DVUS) has been proposed as a better method for quantifying peripheral atherosclerotic burden. 3DVUS is a feasible, reproducible and novel imaging technique to quantify early carotid and femoral atherosclerotic burden in large populations. Furthermore, 3DVUS offers incremental value over the presence of plaque alone in its association with CV risk.16 20 In the last decade, the advent of coronary CT angiography (CCTA) has emerged as a promising non-invasive tool to assess coronary artery structure over time. It has been proposed that the ability of CCTA to identify and quantify the morphology of high-risk plaques, together with therapy monitoring, will eventually become the cornerstone of treatment personalisation.21
Several studies have shown the potential benefits of biological therapies on CV disease risk in patients with psoriasis. Biological therapy in severe psoriasis has been associated with a favourable modulation of coronary plaque indices by CCTA.19 22 These results support the need to expand our knowledge on the potential effects of biological therapies in atherosclerosis.
In addition to imaging techniques, there is a need to discover and validate new molecular markers that have practical value for clinical intervention as well as for identifying and elucidating CV disease processes at the individual level. Therefore, proteomic studies are needed to gain further insights in psoriasis-associated accelerated atherosclerosis in order to obtain a comprehensive overview of this high-risk population.
This article describes the rationale, aims and methods of the Early Detection and Progression of Subclinical Atherosclerosis in Psoriasis (EDSAP) study protocol, a longitudinal cohort study to decipher the molecular, imaging and clinical characteristics of this accelerated atherosclerosis phenotype associated with psoriasis and to explore the effect of anti-inflammatory therapies on it.
Study objectives
The objectives of the EDSAP study are: (1) to assess the prevalence, vascular distribution and burden of subclinical atherosclerosis in patients with psoriasis and its relationship with inflammatory biomarkers and CV risk algorithms using two-dimensional vascular ultrasound (2DVUS) of carotid and femoral arteries, 3DVUS of carotid and femoral arteries and CCTA; (2) to characterise the composition of atherosclerotic plaques by CCTA and 3DVUS of the carotid and femoral arteries; (3) to evaluate the effect of different treatments used in psoriasis on the progression and characterisation of subclinical atherosclerosis in different arterial territories assessed by non-invasive imaging techniques; and (4) to characterise the atherosclerosis process in patients with psoriasis using laboratory analysis and ‘-omics’ technologies, as well as to evaluate changes at the molecular level after treatment of the skin disease.
Study design and population
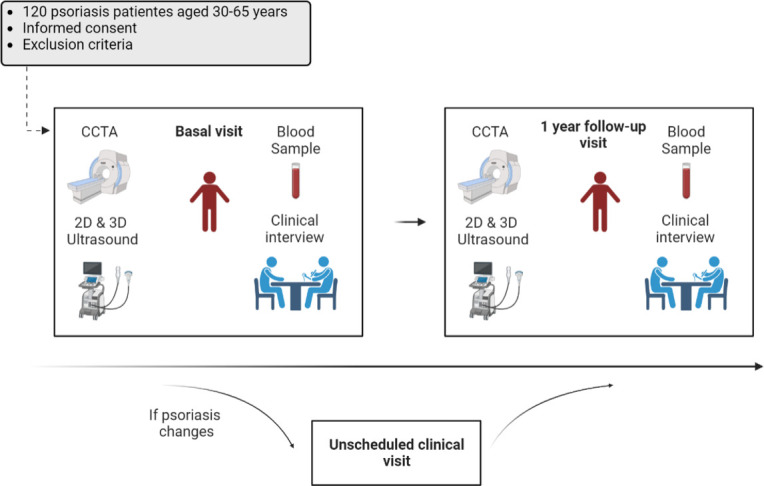
The EDSAP study is an observational, longitudinal, prospective cohort study that includes patients with psoriasis who will undergo a 1-year medical follow-up (figure 1). Recruitment is voluntary among patients attending dermatology consultations at the Hospital Ramón y Cajal, Madrid (Spain).
Figure 1.
EDSAP study flow. CCTA, coronary CT angiography; 2D, two-dimensional; 3D, three-dimensional; EDSAP, Early Detection and Progression of Subclinical Atherosclerosis in Psoriasis.
The study includes participants aged between 30 and 65 years, diagnosed with psoriasis clinically by an expert physician and deemed suitable for biological therapy by the investigator. Exclusion criteria are as follows: history of CV disease (myocardial infarction, angina pectoris, peripheral vascular disease, aortic aneurysm, angioplasty, cardiac surgery, atrial fibrillation or any other cardiological condition), current oncological treatment, history of transplantation with active immunosuppressive or immunomodulatory treatment, morbid obesity (body mass index ≥40 kg/m2), diabetes mellitus, chronic liver disease, chronic kidney disease (glomerular filtration rate <60 mL/min/1.73 m2), other chronic inflammatory disease, presence of any pathology that decreases life expectancy to less than 3 years or any disease or condition that could affect adherence to study procedures. In addition, participants will be excluded if they have had a chest CT scan in the previous year, are pregnant or breast feeding.
Data collection
Two study visits are scheduled for each participant: at baseline and 1-year follow-up. Both of them include a clinical interview, physical examination (height, weight, waist circumference, blood pressure and psoriasis severity through Psoriasis Area Severity Index (PASI)), fasting blood draw and assessment of atherosclerotic disease by non-invasive vascular imaging tests (2D/3DVUS and CCTA). Participants who experience primary or secondary failure of the initial treatment may undergo an unscheduled clinical visit as part of standard clinical practice, given the observational nature of this study. The attending physician will make the decision to change treatment based on the patient’s previous medical history and clinical presentation. This visit will include a clinical interview, a physical examination and a fasting blood draw. Imaging studies will not be repeated due to the radiation exposure associated with CCTA. Training sessions and certification of all personnel involved in data collection are repeated throughout the study. Inclusion started in January 2020 with baseline visits completed for all 120 patients by the end of 2022. The remaining 1-year follow-up visits are expected to be completed by the end of 2023 (figure 2).
Figure 2.
Participant timeline. This flow diagram illustrates the participant timeline including enrolment, baseline and 1-year follow-up visits, the analysis of data and publication of results.
Clinical interview: psoriasis medical history, CVRFS, diet and lifestyle habits
Regarding CVRFs, patients are assessed for diabetes mellitus, hypertension, hyperlipidaemia, obesity, smoking, metabolic syndrome and sedentary lifestyle.23 To assess the impact of the Mediterranean diet on the CV risk in patients with psoriasis, a questionnaire from the PREDIMED (Prevention with Mediterranean Diet) study is used. This is a validated tool that assesses the degree of adherence to the Mediterranean dietary pattern with 14 simple questions.24 In order to measure the impact of psoriasis on patients’ daily activities, the Dermatology Life Quality Index questionnaire is employed.25 It is a unidimensional scale consisting of a short, simple and easy-to-complete 10-question self-administered questionnaire. The questions are related to the perception of the impact of the skin disease on quality of life in the last week. All questionnaires are provided at all visits. Patients will also be asked about psoriasis duration, previous disease treatments and psoriasis comorbidities including psoriasis arthritis and intestinal bowel disease.
Vascular imaging studies
Carotid and femoral arteries are explored using 2DVUS and 3DVUS (figure 3). Staff performing and interpreting the images are blinded to the variables of the study and any other imaging procedures. A Philips iU22 ultrasound system (Philips Healthcare Andover, Massachusetts, USA), using a 2D L9-3 MHz high-resolution linear transducer is employed for 2DVUS image acquisition. The study protocol includes cross-sectional and longitudinal views of both carotid and femoral arteries to detect plaques and standardised longitudinal views for intima-media thickness measurements. 2DVUS images will be analysed using QLAB software (IntelliSpace Portal, Philips Healthcare). 2DVUS analysis includes assessment of carotid and femoral Intima-Media Thickness (IMT) (values >0.9 mm will be considered abnormal), the presence and extension of atherosclerotic plaques in the carotid and femoral territories (plaques are defined as a focal protrusion into the arterial lumen of thickness >0.5 mm or >50% of the surrounding IMT or a diffuse thickness >1.5 mm measured between the media-adventitia and intima-lumen interfaces), maximal plaque thickness (maximal distance between the plaque-lumen and the plaque-adventitia interfaces) and plaque stenosis severity (significant stenosis will be considered when luminal narrowing is >50%).26
Figure 3.
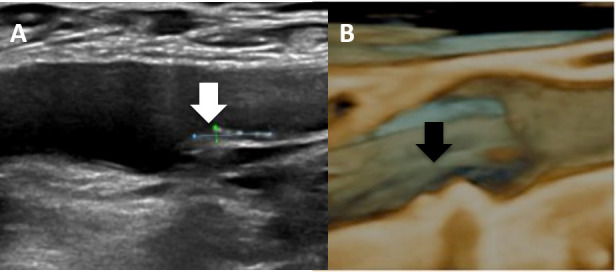
(A) Two-dimensional vascular ultrasound of atherosclerotic plaque (white arrow) in the right femoral artery and (B) and three-dimensional vascular ultrasound of another atherosclerotic plaque (black arrow).
A Philips iU22 ultrasound system equipped with a VL13-5 2D/3D volume linear array transducer (Philips Healthcare) is used for the 3DVUS protocol. The acquisition protocol for the carotid arteries consists of a 30° automatic sweep (explored vessel segment=6 cm long) centred at the carotid bulb to include the distal common carotid artery, the bulb, the bifurcation and the proximal internal and external carotid artery segments. For the femoral arteries, acquisition is centred at the bifurcation and includes the mid-distal common femoral artery, the bifurcation and the proximal superficial and deep femoral artery segments. Images will be analysed using the Vascular Plaque Quantification feature of QLAB software (IntelliSpace Portal, Philips). The 3D variables quantified include: plaque volume, which will be determined by measuring the volumes of each atherosclerotic plaque visualised in the standardised 3D acquisition of each carotid and femoral artery individually as well as their sum, number of plaques by vascular site and participant and 3D vessel wall volume or burden, as the plaque volume between the outer and inner wall boundaries.12
Coronary imaging studies
CCTA is performed with a 320-detector CT scanner (Aquilion ONE ViSION, Toshiba, Japan) at the Hospital Universitario HM Sanchinarro in Madrid, following the guidelines of the National Institutes of Health (NIH) Radiation Exposure Committee. Scans are performed with prospective or retrospective electrocardiogram (EKG) gating according to heart rate, tube potential of 100 or 120 kV, tube current of 100-850 mA adjusted to the patient’s body size, with a gantry rotation time of 275 ms. Images are being acquired with a slice thickness of 0.5 mm and a slice increment of 0.25 mm. Patient characteristics, such as date of visit and treatment, will not be considered when reading the scans. Coronary plaque quantification and characteristics will be analysed in each of the main coronary arteries (with a diameter >2 mm) using specific software (QAngio CT, Medis; The Netherlands).4 27 Automated longitudinal contouring of the inner lumen and outer wall will be performed, manually adjusting the results when there are clear deviations. Results of the automated contouring will also be reviewed on transverse reconstructed cross-sections of the artery on a section-by-section basis at 0.25 mm increments. Lumen attenuation will be adaptively corrected for each scan using gradient filters and intensity values within the artery.
Plaque volume (in cubic millimetres) will be divided by the corresponding segment length (in millimetres), to obtain a plaque index to take into account variable coronary artery lengths. Total plaque burden is defined as the sum of calcified plaque burden and non-calcified plaque burden, assessed in square millimetres. Non-calcified plaque volume and subcomponents will be obtained after adaptively correcting for lumen attenuation and represented as a function of the software-derived Hounsfield units as previously described.28 Also, high-risk plaque features, defined as positive remodelling (remodelling index >1.10), low-attenuation (<30 HU) or spotty calcification will be evaluated.
All CCTA images will be analysed in the Advanced Cardiac Imaging Unit at HM Sanchinarro, Madrid, and in The Laboratory of Inflammation and Cardiometabolic Diseases, National Heart, Lung and Blood Institute (Maryland, USA) for specific analysis. Figure 4 shows an example of coronary arteries with (green arrow) and without subclinical atherosclerosis by CCTA.
Figure 4.
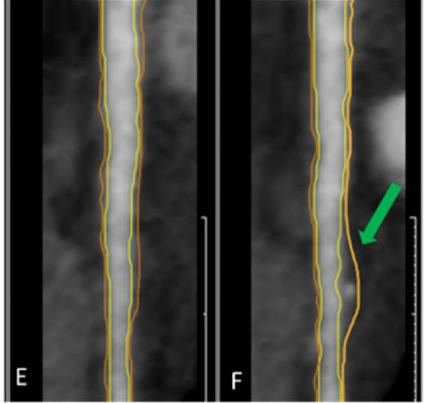
Left anterior descending coronary artery (E) without atheroma plaque (F) with atheroma plaque by computed CT angiography.
Physical examination, laboratory analysis and biobanking
Patients undergo basic clinical studies in which they are weighed, measured, have their blood pressure taken, their waist circumference measured and their PASI evaluated. Moreover, fasting blood samples are collected at baseline, 1-year follow-up and flare-up visits as part of the clinical process. We will collect data of the results of the analysis of serum glucose, uric acid, alkaline phosphatase, bilirubin, apolipoprotein A1, apolipoprotein B, triglycerides, GlycA, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, C reactive protein, C reactive protein ultra, lipoprotein (a), haptoglobin, insulin, haemoglobin, lymphocytes, neutrophils, monocytes and leucocytes. In addition, blood samples are collected in each scheduled visit to be processed and stored at −80°C for high-throughput ‘omics’ analysis and biobanking. Each aliquot is assigned a unique identifier by using a laboratory information management system (Bio-e-Bank), managed by the Ramón y Cajal Institute for Health Research (IRYCIS), to ensure adequate tracking of all procedures.
The proteomic analysis will be performed by qualified personnel from the vascular physiopathology department of the Hospital Nacional de Parapléjicos in Toledo. Building on the previous experience of our group, atherosclerosis in patients with psoriasis will be studied at the molecular level, trying to generate proteomic profiles that will allow us to obtain new data that shed light on this high-risk phenotype, incorporating the most recent advances in post-genomic sciences to identify panels of novel biomarkers that can serve as potential tools in the diagnosis and prognosis of the early phase of atherosclerosis, allowing to improve the primary, or even primordial, preventive strategies for the management of CV risk in these patients. To this end, the overall proteomic discovery strategy will consist of a discovery phase, a verification phase and a validation phase: (1) The discovery phase will be performed using Tandem Mass Tag (TMT) 10-plex reagents, followed by liquid chromatography-mass spectrometry (LC-MS/MS) using a reverse-phase C-18 nanocolumn. Identification of selected peptides will be performed using the likelihood ratio method29 and the false discovery rate (FDR) calculated using inverted databases and the refined method.30 Only peptides identified with FDR≤1% will be used to quantify the relative abundance of each protein from reporter ion intensities. For statistical analysis of quantitative data, the weighted spectrum, peptide and protein (WSPP) statistical model will be used.31 Finally, a functional analysis of the whole set of quantified proteins will be performed by analysing the coordinated protein responses in quantitative proteomics experiments—the systems biology triangle,32 which correlates the performance of groups of proteins within a biological process with their quantitative behaviour. (2) The verification phase will involve a targeted proteomics strategy to study the proteins and the mechanisms previously described by our group, which possibly could be involved in psoriasis, focusing on their relationship with atherosclerosis and CV risk stratification. (3) The Validation phase will consist of targeted proteomics and immunoassays. The results obtained in the discovery phase will be validated in an independent cohort of patients and will be analysed for a comprehensive assessment of lipoprotein biomarkers and inflammatory biomarkers. These last two phases will use the selected reaction monitoring (SRM) methodology—SRM design.
Clinical events
In addition to these examinations, patients will have their routine hospital visits, during which additional information will be collected on any clinical or health-related events affecting the participants during the study period. In any case, the continuity of the patient in the study will be assessed, as the patient’s health status will always be prioritised.
Statistical analysis plan
EDSAP is a longitudinal cohort study, in which measurements are made of different variables related to psoriasis and CV imaging at baseline and after 1 year of biological treatment. This type of design allows simultaneous testing of different hypotheses, which will involve a specific statistical analysis plan and the selection of the most appropriate data treatment and models to study associations and account for potential confounders. On the one hand, cross-sectional studies will be carried out at each visit between the independent variables and the different outcome variables. Analyses will be performed using linear regression models for continuous variables and logistic regression models for categorical variables. On the other hand, longitudinal studies of the progression of subclinical atherosclerosis based on different independent variables will be performed using the most appropriate models for longitudinal data.
The non-calcified coronary burden after 1 year of treatment was considered as the reference variable for the sample size calculation, given its clinical relevance and the existence of previous literature providing reference values.4 19 Accepting an α=0.05 and a β=0.2 in a bilateral contrast for repeated (paired) means, a total of 97 subjects are needed to detect a minimum difference of 0.03 mm, a more conservative value than that obtained in previous studies.4 19 We assumed a loss to follow-up rate of 10%. To date, we have recruited a total of 120 patients, far exceeding the minimum sample size needed to detect differences in the primary endpoint. Study results will be reported in accordance with Strengthening the Reporting of Observational Studies in Epidemiology guidelines.33
Patient and public involvement
None.
Discussion
The EDSAP study aims to provide information on the presence and progression of subclinical atherosclerosis in patients with psoriasis in order to help establish earlier and more personalised management of care for these patients, whose life expectancy is reduced due to the increased risk of CV disease at younger ages compared with the general population.
Traditional prediction systems based on classical CVRFs, underestimate the actual CV risk of patients with psoriasis and other chronic inflammatory states.8 34 There is a lack of markers that allow us to adequately predict those patients who will develop CV disease. In this scenario, numerous publications have emerged recently highlighting the value of detecting subclinical atherosclerosis through various imaging tests as a tool to overcome this gap. The use of ultrasound techniques has shown to be an accurate biomarker of atherosclerosis presence.4 Screening for subclinical atherosclerosis in patients with psoriasis with imaging techniques in more than one vascular territory, such as the carotid and especially the femoral arteries, has proven to be a reliable biomarker for CV risk assessment.15 In addition, 3DVUS is another well-established imaging technique for quantifying early carotid and femoral atherosclerotic burden, as well as being an accessible, novel and reliable technique.16 Recently, this technique has been further developed to obtain plaque characterisation and quantification, enabling risk stratification based on atherosclerotic plaque burden.16 Regarding CCTA, some studies have shown how biologics can modulate coronary plaque indices in psoriasis favourably, supporting further studies to qualify these results.19 21 22 These results have been recently validated in a systematic review and meta-analysis evaluating the impact of licensed biological treatments on blood and imaging biomarkers of CV risk in adult patients with psoriasis. The results demonstrated how some biological drugs were associated with a reduction in aortic vascular inflammation.35 In this study, one of the interesting aspects to explore, and which could provide more data on the complex relationship between cutaneous and vascular inflammation in these patients, is whether this modulation of coronary plaques by antipsoriatic therapies is parallel to or independent of the improvement in PASI, which would provide a more global view of the systemic inflammation affecting the patient with psoriasis. The EDSAP study is the first study that aims to have a comprehensive CV overview of the patient with psoriasis, using novel imaging techniques to study peripheral and coronary subclinical atherosclerosis to accurately assess individual CV risk and minimise this risk through prevention and early treatment. This global understanding of the disease, and the ability to see how antipsoriatic therapies modulate the patient’s internal inflammation, may represent a breakthrough in the treatment of the patient with psoriasis, as well as in the understanding of psoriasis as a human model of atherosclerosis in inflammatory states.
As a complement to multiterritorial imaging studies, EDSAP incorporates the use of new molecular techniques such as proteomics that could be the starting point to identify those individuals potentially predisposed to develop atherosclerosis, allowing us to find potential predictive and therapeutic targets. At present, few studies have evaluated the usefulness of proteomic strategies to identify a biomarker of atherosclerosis in patients with psoriasis.36 37 This approach aims to identify early markers of subclinical atherosclerosis that can, alone or in combination with imaging techniques, identify individuals at increased risk. The biological resource generated will also be available for future use in longitudinal association studies of any parameter of interest (eg, clinical events, response to treatment, development of risk factors, progression of atherosclerosis), which will advance the understanding of how CV disease initiates and progresses.
The main methodological advantages of this study are the strict application of protocols, the collection of biobank samples and the prospective data collection which allows the evaluation of the impact of anti-inflammatory therapies on atheroma plaque presence and characterisation. In addition, state-of-the-art scientific techniques are being applied to measure the anatomical and biological characteristics of subclinical atherosclerosis. However, there are limitations to be kept in mind: this is an observational study and more vulnerable to potential confounding factors compared with randomised controlled trials, and the open, non-randomised use of psoriasis treatments in a small sample and with a short follow-up duration. However, this could be the largest consecutive sample of patients with psoriasis followed over time using CCTA, 3D and 2DVUS. In addition, we will follow a consecutive sample to minimise any selection bias in longitudinal follow-up. Finally, we will use arterial plaque imaging technology to understand modulation in CV disease risk secondary to biological treatment.
In conclusion, the EDSAP study is designed to study psoriasis as a model of atherosclerosis in inflammatory states, as well as to provide clinical, imaging and molecular biomarkers, using innovative imaging techniques as well as omics technologies for the prevention and treatment of these high-risk patients for early CV events.
Ethics and dissemination of results
The study protocol has been approved by the Ethics Committee of Hospital Ramón y Cajal in Madrid. All participants will provide written informed consent that explicitly includes consent for biobanking of surplus biological materials, which will be provided for future research projects. We will present our findings at national and international congresses, and peer-reviewed journals.
Supplementary Material
Acknowledgments
We thank all the participants in the study and gratefully acknowledge the collaboration and assistance of the staff at Hospital Universitario Ramón y Cajal, Hospital HM Sanchinarro, National Institute of Health, Hospital 12 de Octubre and Atria Clinic. We would also like to thank the dedication and impeccable management of the biological samples to all the members of the Biobank of the Hospital Ramón y Cajal. Finally, we are very grateful to all the participants in the EDSAP study, without them it would not have been possible.
Footnotes
Twitter: @EmilioBeric
CA-JdA and EB-R contributed equally.
Correction notice: This article has been corrected since it was published. Joint authorship statement has been added.
Contributors: CA-JdA: Investigation, Conceptualisation, Methodology, Original draft preparation, Visualisation. EB-R: Conceptualisation, Methodology, Visualisation, Writing—Reviewing and Editing. MAB-M: Writing—Reviewing and Editing. PJ: Writing—Reviewing and Editing. JS: Writing—Reviewing and Editing. MGB: Software, Validation. LF-F: Writing—Reviewing and Editing. NNM: Writing—Reviewing and Editing. JMG: Writing—Reviewing and Editing. ÁG-C: Conceptualisation, Methodology, Writing—Reviewing and Editing, Funding acquisition, Supervision.
Funding: The EDSAP study is funded by competitive independent grants from the Instituto de Salud Carlos III and the National Psoriasis Foundation and also by non-competitive investigator-initiated studies (LEO Pharma, Almirall and Amgen).
Competing interests: NNM is a full-time US government employee and has served as a consultant for several pharmaceutical companies, receiving grants and/or research funding; and as a principal investigator for the NIH receiving grants and/or research funding. JMG served as a consultant for several pharmaceutical companies, receiving honoraria and research grants (to the Trustees of the University of Pennsylvania). JMG is a co-patent holder of resiquimod for treatment of cutaneous T-cell lymphoma. JMG receives honoraria from multiple organisms, for being a Deputy Editor for the Journal of Investigative Dermatology, Chief Medical Editor for Healio Psoriatic Disease. He is also a member of the Board of Directors for the International Psoriasis Council, receiving no honoraria. AG-C has served as a consultant for several pharmaceutical companies receiving grants/other payments. MAB-M has served as a consultant for several pharmaceutical companies receiving honoraria. LF-F, JS, EB-R, MGB, PJ and CA-JdA have no interests to disclose.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet 2007;370:263–71. 10.1016/S0140-6736(07)61128-3 [DOI] [PubMed] [Google Scholar]
- 2.Neimann AL, Shin DB, Wang X, et al. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol 2006;55:829–35. 10.1016/j.jaad.2006.08.040 [DOI] [PubMed] [Google Scholar]
- 3.JAMA Network . Risk of myocardial infarction in patients With psoriasis | acute coronary syndromes, Available: https://jamanetwork.com/journals/jama/fullarticle/203598 [Accessed 30 Nov 2022].
- 4.Lerman JB, Joshi AA, Chaturvedi A, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation 2017;136:263–76. 10.1161/CIRCULATIONAHA.116.026859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garshick MS, Barrett TJ, Wechter T, et al. Inflammasome signaling and impaired vascular health in psoriasis. Arterioscler Thromb Vasc Biol 2019;39:787–98. 10.1161/ATVBAHA.118.312246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.PubMed . Potential immunological links between psoriasis and cardiovascular disease. Available: https://pubmed.ncbi.nlm.nih.gov/29910818/ [Accessed 6 Dec 2022]. [DOI] [PMC free article] [PubMed]
- 7.Mehta NN, Krishnamoorthy P, Yu Y, et al. The impact of psoriasis on 10-year framingham risk. J Am Acad Dermatol 2012;67:796–8. 10.1016/j.jaad.2012.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berna-Rico E, Abbad-Jaime de Aragon C, Garcia-Aparicio A, et al. Cardiovascular screening practices and Statin prescription habits in patients with psoriasis among dermatologists, rheumatologists and primary care physicians. Acta Derm Venereol 2023;103:5087. 10.2340/actadv.v103.5087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez-Cantero A, Gonzalez-Cantero J, Sanchez-Moya AI, et al. Subclinical atherosclerosis in psoriasis. usefulness of femoral artery ultrasound for the diagnosis, and analysis of its relationship with insulin resistance. PLoS One 2019;14:e0211808. 10.1371/journal.pone.0211808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armstrong AW, Harskamp CT, Ledo L, et al. Coronary artery disease in patients with psoriasis referred for coronary angiography. Am J Cardiol 2012;109:976–80. 10.1016/j.amjcard.2011.11.025 [DOI] [PubMed] [Google Scholar]
- 11.Mansouri B, Kivelevitch D, Natarajan B, et al. Comparison of coronary artery calcium scores between patients with psoriasis and type 2 diabetes. JAMA Dermatol 2016;152:1244–53. 10.1001/jamadermatol.2016.2907 [DOI] [PubMed] [Google Scholar]
- 12.Hjuler KF, Böttcher M, Vestergaard C, et al. Increased prevalence of coronary artery disease in severe psoriasis and severe atopic dermatitis. Am J Med 2015;128:1325–34. 10.1016/j.amjmed.2015.05.041 [DOI] [PubMed] [Google Scholar]
- 13.Tinggaard AB, Hjuler KF, Andersen IT, et al. Prevalence and severity of coronary artery disease linked to prognosis in psoriasis and Psoriatic arthritis patients: a multi-centre cohort study. J Intern Med 2021;290:693–703. 10.1111/joim.13311 [DOI] [PubMed] [Google Scholar]
- 14.Fernández-Friera L, Peñalvo JL, Fernández-Ortiz A, et al. Prevalence, vascular distribution, and multiterritorial extent of subclinical atherosclerosis in a middle-aged cohort: the PESA (Progression of Early Subclinical Atherosclerosis) study. Circulation 2015;131:2104–13. 10.1161/CIRCULATIONAHA.114.014310 [DOI] [PubMed] [Google Scholar]
- 15.González-Cantero A, Gonzalez-Cantero J, Sanchez-Moya AI, et al. Femoral artery ultrasound for improving the detection of atherosclerosis in psoriasis. J Am Acad Dermatol 2019;80:784–6. 10.1016/j.jaad.2018.07.007 [DOI] [PubMed] [Google Scholar]
- 16.López-Melgar B, Fernández-Friera L, Oliva B, et al. Subclinical atherosclerosis burden by 3D ultrasound in mid-life: the PESA study. J Am Coll Cardiol 2017;70:301–13. 10.1016/j.jacc.2017.05.033 [DOI] [PubMed] [Google Scholar]
- 17.Kaur S, Kingo K, Zilmer M. Psoriasis and cardiovascular risk—do promising new biomarkers have clinical impact? Mediators Inflamm 2017;2017:7279818. 10.1155/2017/7279818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.European Heart Journal Oxford Academic . ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk, . 2019Available: https://academic.oup.com/eurheartj/article/41/1/111/5556353 [DOI] [PubMed]
- 19.Elnabawi YA, Dey AK, Goyal A, et al. Coronary artery plaque characteristics and treatment with biologic therapy in severe psoriasis: results from a prospective observational study. Cardiovasc Res 2019;115:721–8. 10.1093/cvr/cvz009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.López-Melgar B, Mass V, Nogales P, et al. New 3-dimensional volumetric ultrasound method for accurate quantification of Atherosclerotic plaque volume. JACC Cardiovasc Imaging 2022;15:1124–35. 10.1016/j.jcmg.2022.01.005 [DOI] [PubMed] [Google Scholar]
- 21.Elnabawi YA, Dey AK, Mehta NN. Emerging applications of coronary CT angiography in coronary heart disease: getting better with time. Eur Heart J 2018;39:3682–4. 10.1093/eurheartj/ehy645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hjuler KF, Bøttcher M, Vestergaard C, et al. Association between changes in coronary artery disease progression and treatment with biologic agents for severe psoriasis. JAMA Dermatol 2016;152:1114–21. 10.1001/jamadermatol.2016.1984 [DOI] [PubMed] [Google Scholar]
- 23.Hu SCS, Lan CCE. Psoriasis and cardiovascular comorbidities: focusing on severe vascular events, cardiovascular risk factors and implications for treatment. Int J Mol Sci 2017;18:2211. 10.3390/ijms18102211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martínez-González MA, Ros E, Estruch R. Primary prevention of cardiovascular disease with a mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med 2018;379:1388–9. 10.1056/NEJMc1809971 [DOI] [PubMed] [Google Scholar]
- 25.Finlay AY, Khan GK. Dermatology life quality index (DLQI)--A simple practical measure for routine clinical use. Clin Exp Dermatol 1994;19:210–6. 10.1111/j.1365-2230.1994.tb01167.x [DOI] [PubMed] [Google Scholar]
- 26.Touboul P-J, Hennerici MG, Meairs S, et al. Mannheim intima-media thickness consensus. Cerebrovasc Dis 2004;18:346–9. 10.1159/000081812 [DOI] [PubMed] [Google Scholar]
- 27.Kwan AC, May HT, Cater G, et al. Coronary artery plaque volume and obesity in patients with diabetes: the factor-64 study. Radiology 2014;272:690–9. 10.1148/radiol.14140611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sorokin AV, Patel N, Abdelrahman KM, et al. Complex association of apolipoprotein E-containing HDL with coronary artery disease burden in cardiovascular disease. JCI Insight 2022;7:e159577. 10.1172/jci.insight.159577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martínez-Bartolomé S, Navarro P, Martín-Maroto F, et al. Properties of average score distributions of SEQUEST: the probability ratio method. Mol Cell Proteomics 2008;7:1135–45. 10.1074/mcp.M700239-MCP200 [DOI] [PubMed] [Google Scholar]
- 30.Navarro P, Vázquez J. A refined method to calculate false discovery rates for peptide identification using decoy databases. J Proteome Res 2009;8:1792–6. 10.1021/pr800362h [DOI] [PubMed] [Google Scholar]
- 31.García-Marqués F, Trevisan-Herraz M, Martínez-Martínez S, et al. A novel systems-biology algorithm for the analysis of coordinated protein responses using quantitative proteomics. Mol Cell Proteomics 2016;15:1740–60. 10.1074/mcp.M115.055905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isern J, Martín-Antonio B, Ghazanfari R, et al. Self-renewing human bone marrow mesenspheres promote hematopoietic stem cell expansion. Cell Rep 2013;3:1714–24. 10.1016/j.celrep.2013.03.041 [DOI] [PubMed] [Google Scholar]
- 33.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495–9. 10.1016/j.ijsu.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez-Cantero A, Reddy AS, Dey AK, et al. Underperformance of clinical risk scores in identifying imaging-based high cardiovascular risk in psoriasis: results from two observational cohorts. Eur J Prev Cardiol 2022;29:591–8. 10.1093/eurjpc/zwaa033 [DOI] [PubMed] [Google Scholar]
- 35.González-Cantero A, Ortega-Quijano D, Álvarez-Díaz N, et al. Impact of biological agents on imaging and biomarkers of cardiovascular disease in patients with psoriasis: a systematic review and meta-analysis of randomized placebo-controlled trials. J Invest Dermatol 2021;141:2402–11. 10.1016/j.jid.2021.03.024 [DOI] [PubMed] [Google Scholar]
- 36.Kaiser H, Wang X, Kvist-Hansen A, et al. Biomarkers of subclinical atherosclerosis in patients with psoriasis. Sci Rep 2021;11:21438. 10.1038/s41598-021-00999-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qi F, Tan Y, Yao A, et al. Psoriasis to psoriatic arthritis: the application of proteomics technologies. Front Med 2021;8:681172. 10.3389/fmed.2021.681172 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.