Abstract
We have investigated the role of two rapid PCR-based typing methods, IS6110-based PCR and spacer-oligonucleotide typing, within a national tuberculosis reference service. The validity of clusters with IS6110 restriction fragment length polymorphism fingerprints with less than 6 bands was also investigated in the context of referred isolates.
Molecular typing provides an important epidemiological tool with which to investigate the transmission of tuberculosis. By screening isolates from a defined community, the degree of ongoing transmission can be investigated and characterized, which can lead to the design of effective intervention strategies. On a more modest level, isolates for which there is a suspected epidemiological link or suspicion of laboratory contamination can be submitted for analysis by fingerprinting.
Restriction fragment length polymorphism (RFLP), which is based on the IS6110 insertion element (10), has become the standard method for fingerprinting of Mycobacterium tuberculosis. However, it has been shown that the apparent validity of RFLP clustering increases when the clusters of isolates have six or more bands (1, 11), and it has been proposed that all low-copy-number clusters from such studies should be confirmed by an alternative typing method. One problem with all RFLP-based approaches is that they can be complex, time-consuming procedures and require prior isolation by culture of the organism. For example, typing by IS6110 from the cultured organism can take 3 to 4 days. PCR-based methods such as spacer-oligonucleotide typing (spoligotyping) (6) or IS6110-based methods (4, 5, 7–9, 12) can be more rapid but can also be complex to perform and can involve steps which are difficult to optimize. More straightforward IS6110-based PCR methods have been described as good methods for rapid screening of isolates (3, 9).
As part of our reference service we receive isolates for typing analysis for which, in contrast with the use of IS6110 typing in large-scale epidemiological surveillance studies, there is already evidence for transmission or possible laboratory contamination events. Under these circumstances it is unclear whether clustering of low-IS6110-copy-number isolates is of significance or whether secondary typing is required. In this study we have investigated, by secondary typing, the validity of low-copy-number clusters which have been generated through IS6110 RFLP as part of the reference service of the laboratory. The role of alternative rapid PCR-based screening methods within a national reference service such as that produced by the Mycobacterium Reference Unit, London, United Kingdom, are also evaluated by selection of two PCR approaches for comparison with the RFLP.
A total of 73 isolates of M. tuberculosis from 21 different outbreak investigations were referred to the Mycobacterium Reference Unit over a period of 18 months for comparison by molecular typing. Each isolate was investigated by IS6110 RFLP and the two PCR-based methods. IS6110 RFLP was performed as described previously (10). For PCR-based typing, the DNA prepared for IS6110 RFLP was diluted 1/100, and 10 μl of this diluted DNA (about 10 ng) was used in each PCR. Spoligotyping was performed as described previously (6). An IS6110-based PCR approach was performed with a single primer, 5′-GAG TCT CCG GAC TCA CCG G-3′, targeted to the inverted repeat sequence of the IS6110 insertion. Reaction volumes were 40 μl and contained 40 pmol of primer. The PCR conditions were as follows: an initial denaturation at 93°C for 120 s; 1 cycle of 93°C for 20 s, 45°C for 360 s, and 72°C for 120 s; 30 cycles of 93°C for 20 s, 62°C for 30 s, and 72°C for 180 s; and a final extension at 72°C for 10 min. After PCR the products were analyzed by agarose gel electrophoresis.
In 16 out of the 21 investigations the clustering generated by all three methods was in agreement (Table 1). There were discrepancies in the clustering of 12 isolates within five investigations. The IS6110-based PCR results of all investigations which contained discrepant results are shown in Fig. 1.
TABLE 1.
Summary of results of the three typing strategies with the study isolates
| Investigation no. | Isolate | No. of RFLP bands | RFLP cluster | Spoligotype cluster | IS6110-based PCR result | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1a | 9 | NCc | NC | NC | |||||||
| 1b | 11 | NC | 2a | NC | ||||||||
| 1c | 14 | NC | NC | NC | ||||||||
| 2 | 2a | 4 | NC | 2a | NC | |||||||
| 2b | 10 | NC | NC | NC | ||||||||
| 3 | 3a | 4 | 1 | 2a | Cd | |||||||
| 3b | 4 | 1 | 2a | C | ||||||||
| 3c | 4 | 1 | 2a | C | ||||||||
| 3d | 4 | 1 | 2a | C | ||||||||
| 3e | 4 | 1 | 2a | C | ||||||||
| 3f | 4 | 1 | 2a | C | ||||||||
| 3g | 4 | 1 | 2a | C | ||||||||
| 3h | 4 | 1 | NC | NC | ||||||||
| 4 | 4a | 2 | NC | NC | NC | |||||||
| 4b | 6 | NC | 1a | NC | ||||||||
| 4c | 11 | 2 | 5 | C | ||||||||
| 4d | 11 | 2 | 5 | C | ||||||||
| 4e | 11 | 2 | 5 | C | ||||||||
| 4f | 11 | 2 | 5 | C | ||||||||
| 4g | 11 | 2 | 5 | C | ||||||||
| 5 | 5a | 3 | 3a | 7a | C | |||||||
| 5b | 3 | 3a | 7a | C | ||||||||
| 5c | 3 | 3a | 7a | C | ||||||||
| 5d | 14 | NC | 15a | NC | ||||||||
| 5e | 12 | NC | NC | NC | ||||||||
| 5f | 15 | NC | NC | NC | ||||||||
| 6 | 6a | 3 | 4 | 1a | C | |||||||
| 6b | 3 | 4 | 1a | C | ||||||||
| 7 | 7a | 1 | NC | NC | NC | |||||||
| 7b | 11 | NC | 11a | NC | ||||||||
| 8 | 8a | 6 | NC | NC | NC | |||||||
| 8b | 13 | NC | NC | NC | ||||||||
| 9 | 9a | 2 | 5 | 10 | C | |||||||
| 9b | 2 | 5 | 10 | C | ||||||||
| 10 | 10a | 12 | 6 | 11a | C | |||||||
| 10b | 12 | 6 | 11a | C | ||||||||
| 10c | 10 | NC | 11a | NC | ||||||||
| 10d | 11 | NC | NC | C |
| Investigation no.
|
Isolate
|
No. of RFLP bands
|
RFLP cluster
|
Spoligotype cluster
|
IS6110-based PCR result
|
|---|---|---|---|---|---|
| 10e | 4 | 7a | 12a | C | |
| 10f | 12 | NC | NC | NC | |
| 11 | 11a | 4 | 7a | 12a | C |
| 11b | 4 | 7a | 12a | C | |
| 12 | 12a | 2 | 8 | 7a | C |
| 12b | 2 | 8 | 7a | C | |
| 13 | 13a | 7 | NC | NC | NC |
| 13b | 11 | NC | NC | NC | |
| 14 | 14a | 8 | NC | 3a | NC |
| 14b | 8 | 9 | 4 | C | |
| 14c | 8 | 9 | 4 | C | |
| 14d | 10 | 10 | 8 | C | |
| 14e | 10 | 10 | 8 | C | |
| 15 | 15a | 5b | NC | 13 | C |
| 15b | 4b | NC | 13 | C | |
| 16 | 16a | 9b | NC | 3a | NC |
| 16b | 6b | NC | 3a | NC | |
| 17 | 17a | 10 | 11 | 9 | C |
| 17b | 10 | 11 | 9 | C | |
| 17c | 10 | 11 | 9 | C | |
| 18 | 18a | 3 | 3a | 7a | C |
| 18b | 3 | 3a | 7a | C | |
| 18c | 11 | 12 | 16 | C | |
| 18d | 11 | 12 | 16 | C | |
| 19 | 19a | 15 | NC | NC | NC |
| 19b | 8 | NC | 2a | NC | |
| 20 | 20a | 12 | 13 | 2a | C |
| 20b | 12 | 13 | 2a | C | |
| 21 | 21a | 13 | 14 | 14 | C |
| 21b | 13 | 14 | 14 | C | |
| 21c | 13 | NC | NC | NC | |
| 21d | 9 | NC | NC | NC | |
| 21e | 12 | NC | NC | NC | |
| 21f | 15 | NC | 15a | NC | |
| 21g | 16 | NC | 15a | NC |
This pattern is identical to patterns found for isolates in other investigations.
This pattern has several bands in common with the other isolate from the same investigation.
NC, no clustering.
C, clustering.
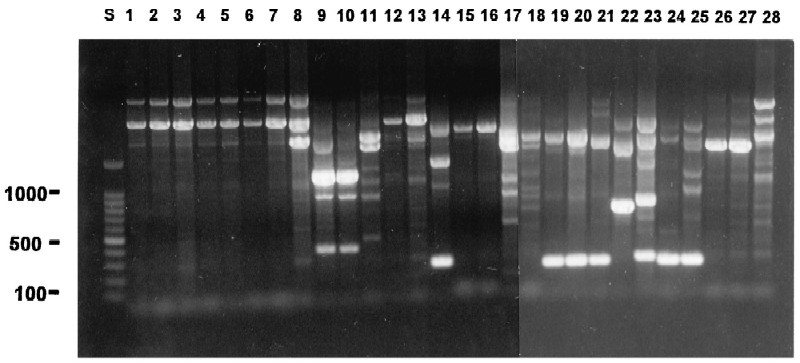
FIG. 1.
The IS6110-based PCR patterns of all investigations with discrepant results and some isolates with a low number of IS6110 copies are shown following agarose gel electrophoresis on a 1% (wt/vol) agarose gel. Discrepant isolates are shown in lanes as follows: lanes 1 to 8, investigation 3, isolates 3a to 3h; lanes 9 to 14, investigation 10, isolates 10a to 10f; lanes 15 and 16, investigation 15, isolates 15a and 15b; lanes 17 and 18, investigation 16, isolates 16a and 16b; and lanes 19 to 25, investigation 21, isolates 21a to 21g. Low-IS6110-copy-number isolates are shown in lanes as follows: lanes 26 and 27, investigation 9, isolates 9a to 9b (two copies of IS6110), and lane 28, investigation 7, isolate 7a (one copy of IS6110). S, DNA size standards (in base pairs) are indicated in the margin.
The RFLP and IS6110-based PCR results differed in only two investigations (investigations 3 and 10). Isolate 3h was clustered by RFLP with the rest of the isolates in investigation 3 (RFLP cluster 1) but by IS6110-based PCR had 3 bands (Fig. 1, lane 8), two of which were identical to those found in the other isolates of that investigation (Fig. 1, lanes 1 to 7). This isolate was also differentiated by spoligotyping though it had a high degree (97%) of similarity to the other isolates. The IS6110-based PCR clustered isolates 10d and 10e (Fig. 1, lanes 12 and 13), but the RFLP and spoligotyping did not.
In one investigation, investigation 15, the spoligotyping and IS6110-based PCR were in agreement but differed from the IS6110 RFLP, in which isolate 15a had one extra band compared to isolate 15b.
In three investigations (investigations 10, 16, and 21) the RFLP and IS6110-based PCR typing methods were in agreement but differed from the spoligotyping, which failed to differentiate some isolates.
Across investigations, spoligotyping appears to be less discriminatory than either RFLP or IS6110-based PCR and clusters isolates across different investigations which are not clustered by RFLP. For example spoligotype 2 occurred in investigations 1, 2, 3, 19, and 20, involving 12 isolates, none of which are clustered by the RFLP analysis.
In investigation 7, isolate 7a had only one IS6110 insert yet was still able to generate a PCR product by IS6110-based PCR (Fig. 1, lane 28). In a similar finding reported by Ross and Dwyer (9), PCR products generated by IS6110-specific primers on single-IS6110-copy-containing isolates were investigated by sequencing and found to have IS6110 at both, one, or neither end of the product. The recent investigation of the M. tuberculosis genome (2) has shown that there are many previously uncharacterized IS elements with some sequence homology to IS6110, which may explain these alternative priming sites. Alternately, nonspecific priming may be involved, and we have reduced the primer annealing temperature in the first cycle of our PCR to encourage such nonspecific interactions in order to generate product from isolates with one or few IS6110 elements.
In our study, out of 21 investigations 7 had clustered isolates which possessed five bands or less. All seven low-copy-number RFLP clusters in this study were confirmed by spoligotyping and IS6110-based PCR, which suggests that prior epidemiological suspicion of linkage is a major consideration when assessing the validity of low-copy-number clusters and that clustering of low-copy-number isolates by IS6110 RFLP is valid in the context of a reference service.
In its present format, using the standard spacer regions as probes, spoligotyping is not discriminatory enough to be used as a sole typing method but is of value when used in conjunction with other techniques. Similarly, IS6110-based PCR generates only one to three major bands after agarose gel electrophoresis, and isolates cannot be clustered by identity of a single band only. For these reasons, a double-typing strategy seems appropriate for population screening studies. Spoligotyping might be used as the primary screening method as it is more rapid than RFLP, but evidence from this study suggests that many false clusters, which would subsequently be resolved by RFLP, would be generated.
The IS6110-based PCR method was shown to be more discriminatory than spoligotyping in these outbreak investigations, but as some nonspecific priming is involved, the pattern of bands may depend on the exact PCR conditions used. This is not a problem for comparing isolates side by side in the same PCR run, as would be the case with referred isolates received at a reference center, but may preclude the use of IS6110-based PCR in epidemiological surveys of large communities. One advantage of the IS6110-based PCR is that we routinely perform it directly on boiled suspensions of organisms taken from Löwenstein-Jensen slopes. In the context of the day-to-day functions of a reference facility, for dealing with referred samples, such a double-typing approach using a rapid and simple PCR-based primary typing method on the referred cultures prior to subculture offers a quick screen for unclustered, unlinked isolates, while any apparently clustered isolates can be confirmed by secondary typing by classical RFLP. The reference facility benefits from the more rapid result turnaround and a savings in labor due to the decreased number of isolates which need RFLP typing.
REFERENCES
- 1.Burman W J, Reves R R, Hawkes A P, Rietmeijer C A, Yang Z, el-Hajj H, Bates J H, Cave M D. DNA fingerprinting with two probes decreases clustering of Mycobacterium tuberculosis. Am J Respir Crit Care Med. 1997;155:1140–1146. doi: 10.1164/ajrccm.155.3.9117000. [DOI] [PubMed] [Google Scholar]
- 2.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry III C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail M A, Rajandream M-A, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston J E, Taylor K, Whitehead S, Barrell B G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 3.Friedman C R, Stoeckle M Y, Johnson W D, Jr, Riley L W. Double-repetitive-element PCR method for subtyping Mycobacterium tuberculosis clinical isolates. J Clin Microbiol. 1995;33:1383–1384. doi: 10.1128/jcm.33.5.1383-1384.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gutierrez M, Samper S, Gavigan J A, Garcia-Marin J F, Martin C. Differentiation by molecular typing of Mycobacterium bovis strains causing tuberculosis in cattle and goats. J Clin Microbiol. 1995;33:2953–2956. doi: 10.1128/jcm.33.11.2953-2956.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haas W H, Butler W R, Woodley C L, Crawford J T. Mixed-linker polymerase chain reaction: a new method for rapid fingerprinting of isolates of Mycobacterium tuberculosis complex. J Clin Microbiol. 1993;31:1293–1298. doi: 10.1128/jcm.31.5.1293-1298.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, Bunschoten A, Molhuizen H, Shaw R, Goyal M, van Embden J. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997;35:907–914. doi: 10.1128/jcm.35.4.907-914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel S, Wall S, Saunders N A. Heminested inverse PCR for IS6110 fingerprinting of Mycobacterium tuberculosis strains. J Clin Microbiol. 1996;34:1686–1690. doi: 10.1128/jcm.34.7.1686-1690.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plikaytis B B, Crawford J T, Woodley C L, Butler W R, Eisenach K D, Cave M D, Shinnick T M. Rapid amplification-based fingerprinting of Mycobacterium tuberculosis. J Gen Microbiol. 1993;139:1537–1542. doi: 10.1099/00221287-139-7-1537. [DOI] [PubMed] [Google Scholar]
- 9.Ross B C, Dwyer B. Rapid simple method for typing isolates of Mycobacterium tuberculosis by using the polymerase chain reaction. J Clin Microbiol. 1993;31:329–334. doi: 10.1128/jcm.31.2.329-334.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Embden J D A, Cave M D, Crawford J T, Dale J W, Eisenach K D, Gicquel B, Hermans P, Martin C, McAdam R, Shinnick T M, Small P M. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993;31:406–409. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Z, Chaves F, Barnes P F, Burman W J, Koehler J, Eisenach K D, Bates J H, Cave M D. Evaluation of method for secondary DNA typing of Mycobacterium tuberculosis with pTBN12 in epidemiologic study of tuberculosis. J Clin Microbiol. 1996;34:3044–3048. doi: 10.1128/jcm.34.12.3044-3048.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuen K Y, Chan C M, Chan K S, Yam W C, Ho P L, Chau P Y. IS6110 based amplityping assay and RFLP fingerprinting of clinical isolates of Mycobacterium tuberculosis. J Clin Pathol. 1995;48:924–928. doi: 10.1136/jcp.48.10.924. [DOI] [PMC free article] [PubMed] [Google Scholar]