Abstract
Background
Preventing poor childhood asthma control is crucial for short-term and long-term respiratory health. This study evaluated associations between perinatal and early-life factors and early childhood asthma control.
Methods
This retrospective study used administrative health data from mothers and children born 2010–2012 with a diagnosis of asthma before age 5 years, in Alberta, Canada. The outcome was asthma control within 2 years after diagnosis. Associations between perinatal and early-life factors and risk of partly and uncontrolled asthma were evaluated by multinomial logistic regression.
Results
Of 7206 preschoolers with asthma, 52% had controlled, 37% partly controlled and 12% uncontrolled asthma 2 years after diagnosis. Compared with controlled asthma, prenatal antibiotics (adjusted risk ratio (aRR): 1.19; 95% CI 1.06 to 1.33) and smoking (aRR: 1.18; 95% CI 1.02 to 1.37), C-section delivery (aRR: 1.11; 95% CI 1.00 to 1.25), summer birth (aRR: 1.16; 95% CI 1.00 to 1.34) and early-life hospitalisation for respiratory illness (aRR: 2.24; 95% CI 1.81 to 2.76) increased the risk of partly controlled asthma. Gestational diabetes (aRR: 1.41; 95% CI 1.06 to 1.87), C-section delivery (aRR: 1.18; 95% CI 1.00 to 1.39), antibiotics (aRR: 1.32; 95% CI 1.08 to 1.61) and hospitalisation for early-life respiratory illness (aRR: 1.65; 95% CI 1.19 to 2.27) were associated with uncontrolled asthma.
Conclusion
Maternal perinatal and early-life factors including antibiotics in pregnancy and childhood, gestational diabetes, prenatal smoking, C-section and summertime birth, and hospitalisations for respiratory illness are associated with partly or uncontrolled childhood asthma. These results underline the significance of perinatal health and the lasting effects of early-life experiences on lung development and disease programming.
Keywords: Asthma, Asthma Epidemiology, Clinical Epidemiology, Paediatric asthma
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Asthma is the most common chronic condition in childhood in the developed world. The level of asthma control achieved shortly after the initial diagnosis at preschool age has been linked to future respiratory morbidity. While prenatal and early-life events have been linked to childhood asthma development, little is known regarding their impact on the level of asthma control during this critical time in a child’s development.
WHAT THIS STUDY ADDS
This study assessed the associations between a wide range of prenatal and early-life factors and the level of asthma control achieved at the preschool age in a population-level cohort.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study contributes to the growing knowledge on the significance of perinatal health and the lasting effects of early-life events on disease programming and lung development.
Introduction
Asthma is the most common chronic disease in childhood, affecting up to 13% of children in Canada.1 Preschool asthma accounts for the highest rate of emergency department (ED) visits and hospitalisations for asthma exacerbations among all age groups.2 By age 6, about 50%–60% of preschoolers with asthma become asymptomatic,3 yet many of these children already have impaired lung function that persists into adulthood,4 which is suggestive of early lung remodelling. Previous studies have found that children with unfavourable asthma control in the first 2 years after diagnosis are less likely to experience remission,5 and nearly twice as likely to have poor symptom control in adolescence.6 This dose–response relationship suggests a dynamic, potentially modifiable, pattern. In turn, poor asthma control and persistent wheezing during childhood and adolescence have been linked to further reduced lung function and respiratory problems in adulthood.7 8 Identifying the determinants of asthma control at the preschool age may thus have long-term benefits for respiratory health, either as predictors or modifiable factors.
The development of asthma is influenced by both genetic predisposition and environmental exposures, as well as the interaction between the two.9 The intrauterine environment during pregnancy and events in the first few years of life are crucial to healthy lung development over the life span. Unfavourable exposures both to the pregnant mother and young child have been linked to asthma development in childhood.10 11 While some evidence indicates that there are perinatal factors associated with more severe respiratory disease in children,12 13 most studies examining the link between the perinatal exposome, early life factors, and childhood lung health have focused on asthma development and prevalence rather than examining determinants of asthma control. Indeed, only recently has asthma control shortly after diagnosis been recognised as a potentially decisive aspect of disease evolution and a potentially modifiable factor that can be targeted for long-term societal impacts.
The objective of this study was to evaluate the associations between maternal, perinatal and early life factors and asthma control following diagnosis in preschoolers using retrospective population-based clinical and administrative health data.
Methods
Study design and setting
A population-based, retrospective cohort study was conducted using provincial maternal, perinatal and early-life data in Alberta, Canada; a province with a large population (4.4 million),14 approximately 50 000 births per year in 2010–2012,15 and a universal single-payer healthcare system. The study received ethical approval from the University of Alberta Research Ethics Board (Pro00105333) and followed the STrengthening the Reporting of OBservational studies in Epidemiology guidelines.16 Informed consent from individuals was not required due to the retrospective and unidentifiable nature of the data.
Patient and public involvement
It was not feasible to involve patients or the public in the design, conduct or reporting of this study.
Study population, linkage and data sources
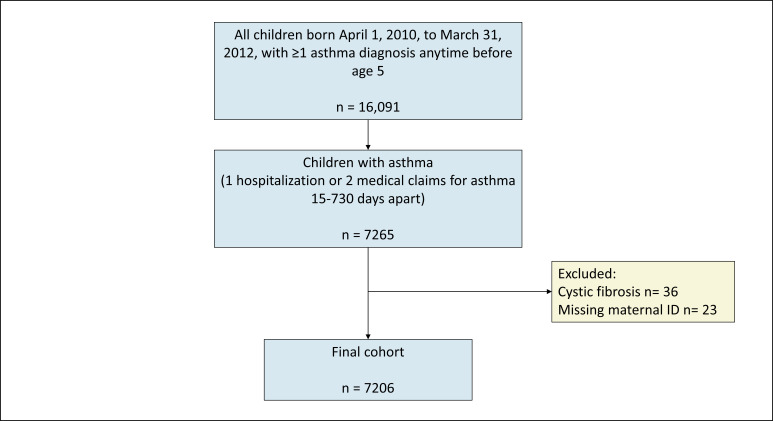
The study population were all children born in Alberta 2010–2012 diagnosed with asthma before age 5 years, based on validated criteria17 of one hospital admission or two outpatient medical claims with International Classification for Disease (ICD)-9 or ICD-10 diagnostic codes indicative of asthma (ICD-9 493 or ICD-10 J45). This definition has a sensitivity of 81% and a specificity of 90%18 and has been extensively applied in paediatric asthma research. The date of diagnosis was determined as the date of the first hospitalisation for asthma or the second date of an outpatient medical claim for asthma.19 The children were identified from the Alberta Perinatal Health Program, a provincial perinatal clinical registry that collects maternal and newborn data of all deliveries of 20 weeks or more of gestation occurring in a hospital or attended by a registered midwife at home in the province. Maternal health data were collected 2 years prior to the index pregnancy and follow-up data on the children was collected until 2019. Records with missing linkages or children diagnosed with cystic fibrosis were excluded. Figure 1 shows the flow diagram of the cohort creation.
Figure 1.
Flow chart of cohort creation.
Individual deidentified maternal, perinatal, health services use and medication data for the children and their birthmothers were linked through a unique personal health number assigned to Alberta residents and recorded at all healthcare visits. The linked data were retrieved from various sources, including the Discharge Abstract Database (for hospitalisations and inpatient visits); the National Ambulatory Care Reporting System (for ED and other ambulatory care visits); the Alberta Physician Claims Assessment System (for medical services); the Pharmaceutical Information Network (for dispensed medications), and 2016 Canadian census data were used to calculate the Pampalon Material and Social Deprivation Index.
Study variables
The primary study outcome was asthma control at preschool age categorised as controlled, partly controlled and uncontrolled asthma based on asthma control trajectories during the 2 years following the initial asthma diagnosis according to the validated Pharmacoepidemiologic Pediatric Asthma Control Index (PPACI).20 Briefly, the PPACI uses information on prescriptions filled for short-acting beta-agonists (SABA) and oral corticosteroids (OCS), ED visits and hospitalisations for asthma to define four categories of asthma control over a 6-month period: (1) well controlled: <4 SABA doses/week and no OCS, ED or hospital admissions; (2) partly controlled: ≥ 4 ≤ 7 SABA doses/week and no OCS, ED or hospital admissions; (3) poorly controlled: ≥7 SABA doses/week or ≥1 OCS or ≥ED visit but no hospital admission; and (4) very poorly controlled: ≥1 hospital admission for asthma. Asthma control trajectories were categorised over the four 6-month periods covering the 2 years immediately after the initial asthma diagnosis as (1) controlled throughout: the PPACI remained well controlled or partly controlled throughout; (2) improving control: PPACI poorly or very poorly controlled at 0–6 months and controlled or partly controlled at 18–24 months; (3) worsening control: PPACI controlled or partly controlled at 0–6 months and poorly controlled or very poorly controlled at 18–24 months; (4) out-of-control throughout: PPACI either poorly controlled or very poorly controlled throughout; or (5) fluctuating control: all other combinations of PPACI.5 6 Children with an asthma control trajectory characterised as controlled throughout the 2 years following the initial diagnosis were considered as ‘controlled’. Children with asthma control trajectories characterised as either improving control or fluctuating control were considered ‘partly controlled’. Finally, children with asthma control trajectories in the worsening control or out-of-control categories were considered having asthma that was ‘uncontrolled’.
Data were collected on maternal factors including maternal age at delivery (categorised as<20 years, 20–35 years and >35 years), history of asthma (defined as at least one asthma diagnosis and one dispensed prescription for an asthma medication in the 2 years before or during the index pregnancy21), maternal atopy (defined as a medical encounter for either atopic dermatitis, urticaria and/or allergic rhinitis within the 2 years prior to or during the index pregnancy (online supplemental table S1), hypertensive disorders of pregnancy (including chronic hypertension, gestational hypertension and preeclampsia/eclampsia), gestational diabetes (yes/no), smoking during pregnancy (yes/no), antibiotic use in pregnancy (assessed based on prescriptions (online supplemental table S2)), and mode of delivery (vaginal vs Caesarean-section (C-section)).
bmjresp-2023-001928supp001.pdf (178.4KB, pdf)
The perinatal and early-life factors studied were low birth weight at term (<2500 g), gestational age at birth (preterm: <37 weeks of gestation), birth weight percentile relative to gestational age (small for gestational age (SGA): 10th percentile, appropriate for gestational age: 10th–90th percentile, and large for gestational age (LGA) 90th percentile),22 season of birth (December–February: winter; March–May: spring; June–August: summer; and September–November: fall), neonatal jaundice requiring phototherapy, bronchopulmonary dysplasia (including bronchopulmonary dysplasia (ICD-10 P27.1) and chronic lung disease originating in the perinatal period (P27.8, P27.9) among preterm babies),23 hospitalisation for respiratory illness (bronchiolitis, pneumonia or respiratory syncytial virus (online supplemental table S1)) in the first year of life, and antibiotic use from 30 days after birth until asthma diagnosis (online supplemental table S2).
The following child and maternal factors were considered as potential confounders in the study: the child’s sex, age at asthma diagnosis, season of diagnosis, use of controller medications before diagnosis, child’s atopy status (healthcare encounter for allergic rhinitis, urticaria, and/or atopic dermatitis anytime before asthma diagnosis (online supplemental table S1)), and maternal material deprivation (based on variables such as education, employment status and income levels) and social deprivation (based on variables such as household composition and marital status) at the time of delivery expressed in quintiles (Q1: least deprived, Q5: most deprived and grouped as Q1-3 and Q4-5).24
Statistical analysis
Baseline maternal, perinatal and early-life data were summarised using proportions and means for categorical and continuous variables, respectively. Multinomial logistic regression was applied to assess the relative risk of partly controlled and uncontrolled asthma, with controlled asthma as the reference group. Variables from the univariable analysis with a p value<0.25 were offered in the multivariable analysis along with child sex and maternal socioeconomic deprivation at the time of delivery as covariates, as determined by directed acyclic graphs (online supplemental figure 1).25 Unadjusted risk ratios and 95% CI were calculated for the dependent variable, and adjusted risk ratio (aRR) were reported after adjusting for covariates and other variables included in the model. The number and percentage of missing data were reported for each variable, and no imputation was performed. All statistical analyses were performed using Stata SE software V.17.0 (StataCorp LLC).
Results
Cohort creation and asthma control
The study cohort was derived from 16 091 children who had a recorded healthcare encounter for asthma within their first 5 years of life and their 16 068 birthmothers. After excluding children with cystic fibrosis and those with missing linked data, the final cohort consisted of 7206 children who had 1 or more hospitalisations for asthma, or 2 or more medical claims for asthma between 15 days and 2 years apart19 (figure 1). Of these, 51.7% had controlled asthma, 36.5% had partly controlled asthma and 11.8% had uncontrolled asthma in the 2 years after the initial diagnosis (table 1).
Table 1.
Cohort characteristics
| Section | Variable | Category | N | % |
| Maternal perinatal | Livebirths | 7206 | 100 | |
| Maternal asthma | Yes | 617 | 8.6 | |
| No | 6589 | 91.4 | ||
| Missing | 0 | 0 | ||
| Maternal atopy | Yes | 1305 | 18.1 | |
| No | 5901 | 91.9 | ||
| Missing | 0 | 0 | ||
| Maternal age at delivery | <20 years | 264 | 3.7 | |
| 20–35 years | 5521 | 76.6 | ||
| >35 years | 1406 | 19.5 | ||
| Missing | 15 | 0.2 | ||
| Mode of delivery | Vaginal | 4916 | 68.2 | |
| C-section | 2290 | 31.8 | ||
| Missing | 0 | 0 | ||
| Hypertensive disorders of pregnancy | Yes | 446 | 6.2 | |
| No | 6760 | 93.8 | ||
| Missing | 0 | 0 | ||
| Gestational diabetes | Yes | 471 | 6.5 | |
| No | 6699 | 93.0 | ||
| Missing | 36 | 0.5 | ||
| Antibiotic use during pregnancy | Yes | 2259 | 31.4 | |
| No | 4947 | 68.7 | ||
| Missing | 0 | 0 | ||
| Smoking during pregnancy | Yes | 1223 | 17.0 | |
| No | 5953 | 82.6 | ||
| Missing | 30 | 0.4 | ||
| Socioeconomic deprivation—material | Q1-3 | 3696 | 51.3 | |
| Q4-5 | 3221 | 44.7 | ||
| Missing | 289 | 4.0 | ||
| Socioeconomic deprivation—social | Q1-3 | 3633 | 50.4 | |
| Q4-5 | 3284 | 45.6 | ||
| Missing | 289 | 4.0 | ||
| Child perinatal | Singletons | 6947 | 96.4 | |
| Multiple births | 259 | 3.6 | ||
| Sex | Male | 4639 | 64.4 | |
| Female | 2566 | 35.6 | ||
| Missing | 1 | 0.0 | ||
| Birth weight | Low (<2500 g) | 714 | 9.9 | |
| Adequate (>2500 g) | 6486 | 90.0 | ||
| Missing | 6 | 0.1 | ||
| Gestational age at birth | Term (≥37 weeks) | 6276 | 87.1 | |
| Pre-term (<37 weeks) | 929 | 12.9 | ||
| Missing | 1 | 0.0 | ||
| Birth weight percentiles | SGA | 768 | 10.7 | |
| AGA | 5767 | 80.0 | ||
| LGA | 670 | 9.3 | ||
| Missing | 1 | 0.0 | ||
| Season at birth | Spring (Mar–Apr) | 1775 | 24.6 | |
| Summer (Jun–Aug) | 1817 | 25.2 | ||
| Fall (Sep–Nov) | 1820 | 25.3 | ||
| Winter (Dec–Feb) | 1794 | 24.9 | ||
| Missing | 0 | 0 | ||
| Early life | Neonatal jaundice requiring phototherapy | Yes | 451 | 6.3 |
| No | 6755 | 93.7 | ||
| Missing | 0 | 0 | ||
| Bronchopulmonary dysplasia | Yes | 84 | 1.2 | |
| No | 7122 | 98.8 | ||
| Missing | 0 | 0 | ||
| Hospitalisation for lower respiratory illness | Yes | 481 | 6.7 | |
| No | 6725 | 93.3 | ||
| Missing | 0 | 0 | ||
| Antibiotics use prior to asthma diagnosis | Yes | 5723 | 79.4 | |
| Antibiotics during respiratory illness* | 4242 | 59.9 | ||
| No | 1483 | 20.6 | ||
| Missing | 0 | 0 | ||
| Child atopy | Yes | 3834 | 53.2 | |
| No | 3372 | 46.8 | ||
| Missing | 0 | 0 | ||
| Age at diagnosis, years | Mean (95% CI) | 2.6 (2.6, 2.6) | ||
| Missing | 0 | 0 | ||
| Season at diagnosis | Spring (Mar–Apr) | 1602 | 22.2 | |
| Summer (Jun–Aug) | 1956 | 27.1 | ||
| Fall (Sep–Nov) | 1812 | 25.2 | ||
| Winter (Dec–Feb) | 1836 | 25.5 | ||
| Missing | 0 | 0 | ||
| Asthma control | Categories | Controlled | 3728 | 51.7 |
| Partly controlled | 2629 | 36.5 | ||
| Uncontrolled | 849 | 11.8 | ||
| Missing | 0 | 0 |
*Antibiotics dispensed within 2 weeks of a healthcare encounters associated with respiratory morbidity.
Cohort characteristics
Table 1 shows the maternal, perinatal and early-life characteristics of the cohort. Of the 7206 child–mother dyads, 8.6% and 17.2% of the mothers reported a history of asthma and atopic disease, respectively. The majority of mothers (76.6%) were aged between 20 and 35 years at delivery. Hypertensive disorders of pregnancy occurred in 6.2% of pregnancies and gestational diabetes in 6.5%. Antibiotic use during pregnancy was reported for 31.4% of mothers, and 17.0% reported smoking at any point during pregnancy. 51.3% and 50.4% of mothers were in the lowest material and social deprivation quintiles, respectively, at the time of delivery.
The cohort consisted mainly of singleton pregnancies (96.4%) and resulting vaginal birth (68.2%). There was a higher proportion of children with asthma who were male (64.4) compared with female (35.6%). Nearly 10% (9.9%) of children had low birth weight (<2500 g) and 12.9% of children were born preterm. There was roughly equal representation of children born in each of the four seasons (25% each).
Approximately 6.3% of the children in the cohort developed neonatal jaundice requiring phototherapy, 0.7% were diagnosed with bronchopulmonary dysplasia, and 6.7% were hospitalised for respiratory illness within the first year of life. The majority (79.4%) of the children in the cohort had received at least one prescription for antibiotics before the asthma diagnosis, and 58.9% received antibiotics within 2 weeks of a healthcare encounter for a respiratory condition. Atopic disease other than asthma was prevalent in 53.3% of the children. The mean age at asthma diagnosis was 2.6 years (95% CI 2.6 to 2.6) with summer being the most common seasons of diagnosis (27.1%) and spring the least common (22.2%).
Associations between maternal, perinatal and early-life exposures and asthma control trajectories
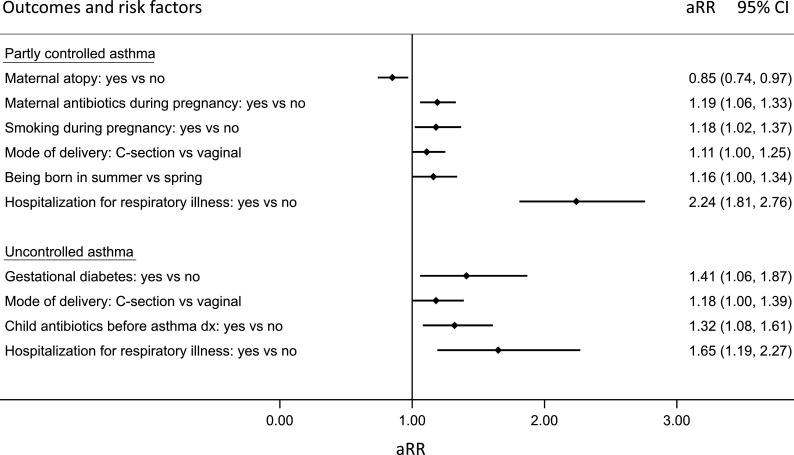
Figure 2 displays the relative risk of uncontrolled or partly controlled asthma compared with controlled asthma in relation to maternal, perinatal and child risk factors. A lower likelihood of having partly controlled asthma 2 years after diagnosis was seen among children with mothers with atopic disease (aRR: 0.85; 95% CI 0.0.74 to 0.97); however, maternal antibiotics use (aRR: 1.19; 95% CI 1.06 to 1.33) and maternal smoking (aRR: 1.18; 95% CI 1.02 to 1.37) during pregnancy were associated with an increased risk of partly controlled asthma in the child. Furthermore, being born via Caesarean section (aRR: 1.11; 95% CI 1.00 to 1.25), during the summer (aRR: 1.16; 95% CI 1.00 to 1.34), and having a history of early life hospitalisation for respiratory illness (aRR: 2.23; 95% CI 1.77 to 8.71) also increased the risk of partly controlled asthma.
Figure 2.
Relative risk of partly controlled (n=2629) and uncontrolled asthma (n=849) compared with controlled asthma (n=3728) assessed within the first 2 years of the initial diagnosis according to maternal and perinatal risk factors. The relative risk associated with each exposure and outcome is adjusted for all other exposures included in the figure, and covariates (child sex: male vs female, and maternal material socioeconomic status: low vs high). P value for model<0.0001, LR chi2: 118.89.
Having uncontrolled asthma 2 years following diagnosis was associated with gestational diabetes (aRR: 1.41; 95% CI 1.06 to 1.87), being born via caesarean section (aRR: 1.18; 95% CI 1.00 to 1.39), receiving antibiotics prior to asthma diagnosis (aRR: 132; 95% CI 1.08 to 1.61), and being hospitalised for respiratory illness in early life (aRR: 1.65; 95% CI 1.19 to 2.27). However, maternal asthma, maternal age at delivery, low birth weight and bronchopulmonary dysplasia were not associated with either partly controlled or uncontrolled asthma within the 2 years after diagnosis. Detailed results from both crude and adjusted multinominal logistic regression analyses can be found in online supplemental tables S3,S4.
Discussion
This study examined the association between perinatal and early-life factors and asthma control in newly diagnosed preschool children with asthma. Our findings suggest that perinatal factors such as antibiotic use, gestational diabetes, smoking in pregnancy, C-section birth and summer birth, as well as hospitalisations for respiratory illness in early life increase the risk of partly or uncontrolled asthma in preschoolers. These results underline the significance of maternal perinatal health and the lasting effects of early-life experiences on lung development and disease programming.
To our knowledge, this is the first study looking at a paediatric cohort with asthma where C-section delivery was linked to poor asthma control. The causes of childhood asthma are complex, but several links have been established between perinatal events and asthma development. C-section birth increases the susceptibility to early childhood asthma26 which is suggested to be due to an underdeveloped gut microbiota composition in the infant.27 28 Antibiotic use during pregnancy29 and early childhood30 are also known to disrupt the composition of the gut microbiota in children. We found that antibiotic use in early childhood appears to have a larger impact on asthma disease programming than fetal exposure: in our cohort of children with asthma, maternal antibiotic use during pregnancy was associated with a slight increase in the risk of partly controlled asthma (aRR: 1.11); however, we saw a higher likelihood of uncontrolled asthma in children who had antibiotics dispensed between 30 days of life and prior to their asthma diagnosis (aRR: 1.32). A large proportion of the antibiotics dispensations in children were associated with a healthcare encounter for respiratory disease but the associations between antibiotic use both during pregnancy and in childhood remained significant following adjustment for respiratory illness resulting in hospitalisation in early life. Furthermore, breastfeeding in early life can attenuate the risks of asthma and allergic disease associated with the suboptimal microbial colonisation in response to both antibiotics31 and C-section delivery,32 33 but data on breastfeeding status in the infants were not available in this study.
Another condition which has also been linked to asthma development in the child is gestational diabetes.34 35 The results from the current study show a considerable increase in the risk of uncontrolled asthma in the offspring when the mother had gestational diabetes (aRR: 1.41), suggesting that hyperglycaemia in pregnancy may be also a determinant of asthma disease programming in the child. As clinical details on disease severity or disease management of women with gestational diabetes were not available, this study could not further explore separate subanalyses based on level of hyperglycaemia or treatment options. Mothers with gestational diabetes, especially those with more severe hyperglycaemia or elevated fasting glucose,36 are more likely to give birth to LGA infants, but we did not find a relationship between birth weight percentiles and asthma control in preschoolers. The impact of hyperglycaemic severity and/or treatment options on asthma control in early childhood warrants further investigation.
This study found that being born in summer was linked to a small increased risk of partly controlled asthma. This is possibly due to heighten susceptibility to seasonal virus infections as the natural nadir of maternal antibodies occurring 3–6 months after birth coincide with the start of viral season for children born in summer. Additionally, environmental factors such wildfires smoke that have become increasingly more common during the summer months.37 The association between birth season and asthma control has been studied before, with poor control being linked to winter births, an association that could be partly mediated by respiratory infections.38 However, we found that being born in winter was not associated with asthma control after adjusting for severe respiratory illness in the first year of life and other factors. Maternal smoking during pregnancy was only slightly associated with partly controlled asthma in this study, and no relationship was found with uncontrolled asthma. Further research is needed to examine the impact of indoor and outdoor environmental effects on respiratory health in early childhood, including postnatal smoking.
We acknowledge the following strengths and limitations. This large population-based cohort study derived from administrative health provided significant power. While asthma control could be conceptually considered an ordinal outcome, in the current study the proportional odds assumption was not met and multinominal regression was chosen as the more appropriate methods of analysis. We included all children with asthma according to a validated case-finding algorithm anytime before age 5.18 Of note, respiratory symptoms such as wheezing often occur in young children in the absence of a later asthma diagnosis and the case-finding algorithm has not been validated in children younger than 1 year of age. The use of administrative health data provided access to multiple perinatal and early-life exposures, avoiding sampling and recall biases and overcoming small sample size issues. However, some important variables were not available in the datasets, including maternal body composition during pregnancy,39 childhood weight,40 and breastfeeding,13 all of which have been linked to asthma development. Moreover, although this study focused on the perinatal period and early years, other factors such as maternal medical history, childcare attendance, pet ownership, physical activity, lifestyle choices and environmental exposures may have confounded the results.
Conclusion
Maternal perinatal and early-life factors including antibiotic use in pregnancy and childhood, gestational diabetes, smoking in pregnancy, being born via C-section and in summer, and hospitalisations for respiratory illness are linked to an elevated risk of poor asthma control in preschool-aged children. These findings suggest that poor asthma control in childhood may in part be connected to alterations in the infant development brought on by perinatal events and exposures and/or influenced by environmental interactions during the early years. The study contributes to the growing knowledge of the risks associated with perinatal and early-life factors that may be prevented or further explored to improve respiratory health in the long term.
Acknowledgments
The authors would like to acknowledge and thank Zoe Hsu at Alberta Health Services for data linkage. Data access and linkage were provided by Alberta Health Services. Inquiries about data or access can be sent to research.administration@ahs.ca.
Footnotes
Contributors: RJR, ALK, RC, AH, FMD and MBO contributed to the conception of the project. LEM, MBO, SC and JB contributed to data acquisition. LEM drafted the manuscript. LEM, JS-L, RJR and MBO contributed to the analysis and interpretation. LEM, JS-L, RJR, ALK, RC, SC, JB, AH, FMD and MBO contributed to the design of the work, revised the intellectual content, approved of the final version of the manuscript and agree to be accountable for all aspects of the final product. MBO is the guarantor of the study and accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: This study was funded by the Canadian Lung Association, the Canada Research Chairs Program (Ospina), and the Stollery Children’s Hospital Foundation and the Alberta Women’s Health Foundation through the Women and Children’s Health Research Institute (Moore, Ospina).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Inquiries about data or access can be sent to research.administration@ahs.ca.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Statistics Canada GR, Kohen D. Health Reports, Changes in the prevalence of childhood asthma,. 2008Available: https://www150.statcan.gc.ca/n1/daily-quotidien/080416/dq080416b-eng.htm [Accessed 7 Apr 2022].
- 2.Canadian Institute for Health Information . CIHI Ottawa, ON; Asthma Hospitalizations Among Children and Youth in Canada: Trends and Inequalities,. 2018Available: from:https://www.cihi.ca/sites/default/files/document/asthma-hospitalization-children-2018-chartbook-en-web.pdf [Accessed 7 Apr 2022]. [Google Scholar]
- 3.Ducharme FM, Dell SD, Radhakrishnan D, et al. Diagnosis and management of asthma in Preschoolers: A Canadian Thoracic society and Canadian Paediatric society position paper. Paediatr Child Health 2015;20:353–71. 10.1093/pch/20.7.353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez FD, Wright AL, Taussig LM, et al. Asthma and wheezing in the first six years of life. N Engl J Med 1995;332:133–8. 10.1056/NEJM199501193320301 [DOI] [PubMed] [Google Scholar]
- 5.Longo C, Blais L, Brownell M, et al. Association between asthma control Trajectories in Preschoolers and disease remission. Eur Respir J 2021;57:2001897. 10.1183/13993003.01897-2020 [DOI] [PubMed] [Google Scholar]
- 6.Longo C, Blais L, Brownell M, et al. Association between asthma control Trajectories in Preschoolers and long-term asthma control. J Allergy Clin Immunol Pract 2022;10:1268–78. 10.1016/j.jaip.2021.12.033 [DOI] [PubMed] [Google Scholar]
- 7.Mogensen I, Hallberg J, Ekström S, et al. Uncontrolled asthma from childhood to young adulthood Associates with airflow obstruction. ERJ Open Res 2021;7:00179-2021. 10.1183/23120541.00179-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sears MR, Greene JM, Willan AR, et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med 2003;349:1414–22. 10.1056/NEJMoa022363 [DOI] [PubMed] [Google Scholar]
- 9.Subbarao P, Mandhane PJ, Sears MR. Asthma: epidemiology, etiology and risk factors. CMAJ 2009;181:E181–90. 10.1503/cmaj.080612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmad K, Kabir E, Ormsby GM, et al. Are wheezing, asthma and Eczema in children associated with mother’s health during pregnancy. Arch Public Health 2021;79. 10.1186/s13690-021-00718-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nasreen S, Wilk P, Mullowney T, et al. The effect of gestational diabetes mellitus on the risk of asthma in offspring. Ann Epidemiol 2021;57:7–13. 10.1016/j.annepidem.2021.02.003 [DOI] [PubMed] [Google Scholar]
- 12.Davidson R, Roberts SE, Wotton CJ, et al. Influence of maternal and perinatal factors on subsequent Hospitalisation for asthma in children: evidence from the Oxford record linkage study. BMC Pulm Med 2010;10:14. 10.1186/1471-2466-10-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Owora AH, Zhang Y. Childhood wheeze trajectory-specific risk factors: A systematic review and meta-analysis. Pediatr Allergy Immunol 2021;32:34–50. 10.1111/pai.13313 [DOI] [PubMed] [Google Scholar]
- 14.Government of Alberta . [Website]. Population statistics, 1 January. 2022Available: https://www.alberta.ca/population-statistics.aspx [Accessed 7 Apr 2022].
- 15.Statlista Research Department . Number of births in Alberta, Canada from 2000 to 2022. 2022. Available: https://www.statista.com/statistics/578589/number-of-births-in-alberta-canada/2022 [Accessed 6 Jun 2023].
- 16.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Bull World Health Organ 2007;85:867–72. 10.2471/BLT.07.045120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.To T, Dell S, Dick PT, et al. Case verification of children with asthma in Ontario. Pediatr Allergy Immunol 2006;17:69–76. 10.1111/j.1399-3038.2005.00346.x [DOI] [PubMed] [Google Scholar]
- 18.Omand JA, Maguire JL, O’Connor DL, et al. Agreement between a health claims algorithm and parent-reported asthma in young children. Pediatr Pulmonol 2019;54:1547–56. 10.1002/ppul.24432 [DOI] [PubMed] [Google Scholar]
- 19.Gershon AS, Wang C, Guan J, et al. Identifying patients with physician-diagnosed asthma in health administrative databases. Can Respir J 2009;16:183–8. 10.1155/2009/963098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Després F, Ducharme FM, Forget A, et al. Development and validation of a Pharmacoepidemiologic pediatric asthma control index (PPACI) using administrative data. Canadian Journal of Respiratory, Critical Care, and Sleep Medicine 2021;5:261–9. 10.1080/24745332.2020.1727789 [DOI] [Google Scholar]
- 21.Blais L, Kettani FZ, Forget A. Associations of maternal asthma severity and control with pregnancy complications. J Asthma 2014;51:391–8. 10.3109/02770903.2013.879880 [DOI] [PubMed] [Google Scholar]
- 22.Kramer MS, Platt RW, Wen SW, et al. A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics 2001;108:E35. 10.1542/peds.108.2.e35 [DOI] [PubMed] [Google Scholar]
- 23.Landry JS, Croitoru D, Menzies D. Validation of ICD-9 diagnostic codes for Bronchopulmonary dysplasia in Quebec’s provincial health care databases. Chronic Dis Inj Can 2012;33:47–52. [PubMed] [Google Scholar]
- 24.Pampalon R, Hamel D, Gamache P, et al. A deprivation index for health planning in Canada. Chronic Dis Can 2009;29:178–91. [PubMed] [Google Scholar]
- 25.Textor J, van der Zander B, Gilthorpe MS, et al. Robust causal inference using directed Acyclic graphs: the R package 'Dagitty Int J Epidemiol 2016;45:1887–94. 10.1093/ije/dyw341 [DOI] [PubMed] [Google Scholar]
- 26.Darabi B, Rahmati S, HafeziAhmadi MR, et al. The association between Caesarean section and childhood asthma: an updated systematic review and meta-analysis. Allergy Asthma Clin Immunol 2019;15. 10.1186/s13223-019-0367-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stokholm J, Thorsen J, Blaser MJ, et al. Delivery mode and gut microbial changes correlate with an increased risk of childhood asthma. Sci Transl Med 2020;12:eaax9929. 10.1126/scitranslmed.aax9929 [DOI] [PubMed] [Google Scholar]
- 28.Chen YY, Zhao X, Moeder W, et al. Impact of maternal Intrapartum antibiotics, and Caesarean section with and without labour on Bifidobacterium and other infant gut Microbiota. Microorganisms 2021;9:1847. 10.3390/microorganisms9091847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou P, Zhou Y, Liu B, et al. Perinatal antibiotic exposure affects the transmission between maternal and neonatal Microbiota and is associated with early-onset sepsis. mSphere 2020;5. 10.1128/mSphere.00984-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Obiakor CV, Parks J, Takaro TK, et al. Early life antimicrobial exposure: impact on Clostridioides difficile Colonization in infants. Antibiotics (Basel) 2022;11:981. 10.3390/antibiotics11070981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dai DLY, Petersen C, Hoskinson C, et al. Breastfeeding enrichment of B. In: longum subsp. infantis mitigates the effect of antibiotics on the microbiota and childhood asthma risk. Med. New York, NY, 2022. [DOI] [PubMed] [Google Scholar]
- 32.Chu S, Zhang Y, Jiang Y, et al. Cesarean section without medical indication and risks of childhood allergic disorder, attenuated by Breastfeeding. Sci Rep 2017;7:9762. 10.1038/s41598-017-10206-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen YY, Tun HM, Field CJ, et al. Impact of cesarean delivery and Breastfeeding on Secretory immunoglobulin A in the infant gut is mediated by gut Microbiota and metabolites. Metabolites 2023;13:148. 10.3390/metabo13020148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adgent MA, Gebretsadik T, Reedus J, et al. Gestational diabetes and childhood asthma in a racially diverse US pregnancy cohort. Pediatr Allergy Immunol 2021;32:1190–6. 10.1111/pai.13523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicolosi BF, Vernini JM, Costa RA, et al. Maternal factors associated with hyperglycemia in pregnancy and perinatal outcomes: a Brazilian reference center cohort study. Diabetol Metab Syndr 2020;12. 10.1186/s13098-020-00556-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryan EA, Savu A, Yeung RO, et al. Elevated fasting vs post-load glucose levels and pregnancy outcomes in gestational diabetes: a population-based study. Diabet Med 2020;37:114–22. 10.1111/dme.14173 [DOI] [PubMed] [Google Scholar]
- 37.Henry S, Ospina MB, Dennett L, et al. Assessing the risk of respiratory-related Healthcare visits associated with Wildfire smoke exposure in children 0-18 years old: A systematic review. Int J Environ Res Public Health 2021;18:8799. 10.3390/ijerph18168799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Almqvist C, Ekberg S, Rhedin S, et al. Season of birth, childhood asthma and allergy in a nationwide cohort-mediation through lower respiratory infections. Clin Exp Allergy 2020;50:222–30. 10.1111/cea.13542 [DOI] [PubMed] [Google Scholar]
- 39.Forno E, Young OM, Kumar R, et al. Maternal obesity in pregnancy, gestational weight gain, and risk of childhood asthma. Pediatrics 2014;134:e535–46. 10.1542/peds.2014-0439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen YC, Dong GH, Lin KC, et al. Gender difference of childhood overweight and obesity in predicting the risk of incident asthma: a systematic review and meta-analysis. Obes Rev 2013;14:222–31. 10.1111/j.1467-789X.2012.01055.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2023-001928supp001.pdf (178.4KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Inquiries about data or access can be sent to research.administration@ahs.ca.