Abstract
A 49-year-old male with Pneumocystis carinii pneumonia was seen at Bellevue Hospital in New York, N.Y. Sputum samples yielded cultures of Candida lusitaniae, Mycobacterium avium, and a filamentous fungus, Trichophyton fischeri. T. fischeri is a nonpathogenic fungus which resembles the dermatophyte Trichophyton rubrum. This is the first record of the species from U.S. sources. This case exemplifies the ecological differences between T. fischeri and T. rubrum and illustrates how correct identification of the former species can minimize diagnostic confusion. The two species are distinguished from each other by the type of growth on Casamino Acids-erythritol-albumin agar and by micromorphological differences.
Trichophyton fischeri was described by Kane (3) as a nonpathogenic species resembling the common dermatophyte Trichophyton rubrum. The original isolations were airborne contaminants. Later isolates obtained by one of us (J.K.) in the Ontario Ministry of Health diagnostic reference mycology laboratory were often from clinical specimens; however, in each case, investigation revealed that T. fischeri was medically insignificant and apparently represented only a chance contamination of the clinical material. A cluster of some recent cases of this kind, which were apparently related to an environmental source of T. fischeri contamination in a commercial diagnostic laboratory, are documented in Table 1.
TABLE 1.
T. fischeri cases (possible cluster) seen over a 2-month period at a private medical laboratory
| Source (patient sex and age) | Specimen receipt date (1996) | Microscopic finding (NaOH) | Conformity with key T. fischeri characteristicsa | Physician’s final diagnosis |
|---|---|---|---|---|
| Sole (M, 52) | 4 January | − | + | Psoriasis |
| Feet (M, 15) | 11 January | − | + | Atopic eczema |
| Hand (M, 38) | 12 January | − | + | Pyoderma |
| Toenail (M, 61) | 24 January | − | + | Onychodystrophy |
Key T. fischeri characteristics included red reverse pigment on SAB but not on CEA medium, heavy production of microconidia, broom-like hyphal branching on BHI, few or no thin macroconidia, urease negativity, and no alkalinity and no clearing on BCPMSG (see text for details).
Since T. fischeri is relatively uncommon and since its distinction from T. rubrum is facilitated by the use of Casamino Acids-erythritol-albumin agar (CEA), which has not been readily available, recognized isolations have almost entirely been from Canada, where CEA originated. This study reports the first isolation of T. fischeri from the United States. It was found as a contaminant in the sputum of a patient with pneumonia caused by Pneumocystis carinii. Its unequivocal status as a nonpathogen in this case helps reinforces the evidence for its status as a potential airborne contaminant in indoor situations. This in turn helps to clarify its significance when isolated from skin, a situation in which confusion with the pathogenic T. rubrum may be especially problematic.
Case report.
A 49-year-old male patient with AIDS suffering from pneumonia was seen at Bellevue Hospital. Sputum samples yielded cultures of Candida lusitaniae and a filamentous fungus which resembled T. rubrum. The latter was provisionally identified as T. fischeri Kane by one of us (S.A.R.) and was sent to J.K. for confirmation. The cause of the pneumonia was determined to be P. carinii. Three months following the initial examination, additional sputum samples yielded Mycobacterium avium. Fungal infection other than pneumocystosis was ruled out in the clinical diagnosis.
Laboratory analysis.
The New York isolate was subcultured on a variety of media (4, 5) in order to identify it. These methods and their applications are outlined below.
(i) CEA.
CEA (sold commercially as Candida Inhibitory Agar; Biomedia Unlimited, Toronto, Canada). CEA was introduced by Fischer and Kane (1) to selectively inhibit the growth of Candida albicans by depleting the medium of biotin for which C. albicans has an absolute, exogenous need. This allows dermatophytes to be isolated from clinical specimens when there is a dermatophyte-Candida mixture (5). Subsequent to its introduction in the clinical laboratory for this purpose, CEA was discovered to facilitate the distinction of T. rubrum from T. fischeri; the former produces a typical, red undersurface pigment on CEA, while the latter does not. It should be noted that some variant isolates of T. rubrum fail to produce red pigment entirely; these cannot be confused either with typical isolates of the species or with T. fischeri, which specifically fails to form red pigment on CEA but forms it on Sabouraud-peptone-glucose agar (SAB) and many other media.
(ii) BHI agar.
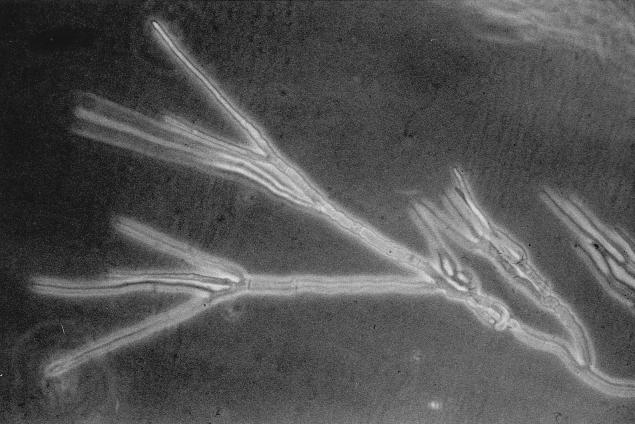
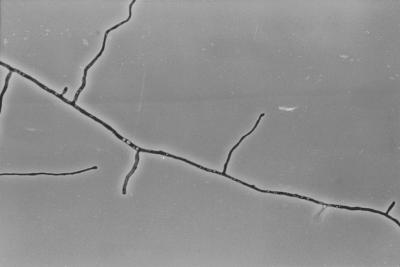
Microscopic examination of T. fischeri growing on brain-heart infusion agar (BHI) reveals a pattern of clustered branching points resembling verticillate, dichotomous, or broom-like branching in the mycelium of the colony margin (Fig. 1). There are many atypically short branch internodes intermixed with internodes of normal length (Fig. 1). T. rubrum produces strictly monopodial branching with elongated internodes and regularly spaced, clearly subdominant lateral branches (Fig. 2) (5).
FIG. 1.
Broom-like, verticillate, or dichotomous branching in the marginal mycelium of T. fischeri on BHI for 7 days. Magnification, ×1,000.
FIG. 2.
Strictly monopodial branching in the marginal mycelium of T. rubrum on BHI for 7 days. Magnification, ×392 (magnification less than that in Fig. 1 because of longer internode lengths in T. rubrum).
Other tests employed in the study of the New York isolate included those for urease production in Christensen’s urea broth (5), vitamin requirements, growth on bromcresol purple-milk solids-glucose agar (BCPMSG) (5), and hair perforation.
The isolate proved to be T. fischeri in every morphologic and physiologic characteristic. In brief, it was a deeply velvety colony, heavily sporulating with teardrop-shaped microconidia and a few thin macroconidia. It produced a red colony reverse on SAB but not on CEA. It was urease negative and negative for both alkalinity and clearing of suspended solids on BCPMSG at 7 days and at 25°C. It was negative for hair perforation. A comparison of the differences between T. fischeri and T. rubrum in general is given in Table 2.
TABLE 2.
Comparison between T. fischeri and T. rubrum
| Organism | Result(s) with the following tests:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| SAB | CEA | BHI colony margin | Urease production | BCPMSG | Vitamin requirements | Hair perforation | Pathogenicity | |
| T. fischeri | Red undersurface pigment; many microconidia; few macroconidia | No red undersurface pigment | Broom-like-to-dichotomous branching | Negative | No alkalinity; no clearing | None | Negative | No |
| T. rubrum | Red undersurface pigment; few microconidia (in most cases); rare macroconidia | Red undersurface pigment | Monopodial branching | Negative | No alkalinity; no clearing | None | Negative | Yes |
The close morphological similarity between T. fischeri and T. rubrum may lead to the unnecessary treatment of patients for dermatophyte infection when the identification of the fungus is in error. The presence of the saprophyte T. fischeri should be considered when an unusually heavily sporulating T. rubrum-like colony grows from a KOH-negative skin scraping or when a similar colony is isolated from a source unlikely to yield T. rubrum, as in the present case. Investigators studying environmental materials such as room dust and public aquatic facilities for the presence of dermatophytes should be aware of the possibility of isolating this nonpathogenic organism. The isolate in the present case was almost certainly derived from airborne particulate material. Although the geographical distribution of T. fischeri is only beginning to be mapped, both the original finding of the organism as a contaminant of a medium preparation room (3) and the cluster of isolations noted in Table 1 suggest that growth indoors is likely. This species is, therefore, likely to be widely distributed; the present case aids in confirming this probability.
The small number of records to date of T. fischeri isolations, in comparison with the extreme abundance of T. rubrum, presents a practical problem. Clearly it is not practical to screen all T. rubrum-like isolates from KOH-negative skin and nail specimens to rule out this organism. Nor does a single isolation of T. fischeri from the United States justify such an addition to laboratory costs. It is possible that T. fischeri is indeed very rare or that, as with Onychocola canadensis, a regularly seen, cosmopolitan (2), onychomycotic pathogen that went unnoticed for decades until described by Sigler and Congly in 1990 (6), its apparent rarity may be an artifact of inadequate detection procedures. In laboratories already using CEA medium for other applications, the most practical approach is to screen only isolates deriving from KOH-negative specimens and matching the description of T. fischeri. Its heavy microconidial production alone separates it from the great majority of T. rubrum isolates, and its relative paucity of macroconidia separates it from granular-type T. rubrum isolates as well as the heavily macroconidial Trichophyton raubitschekii. In practice, the number of tests needed per annum for prospective T. fischeri isolates is very small. For example, the approximately 1,580 T. rubrum-like isolates obtained in one of our laboratories (that of J.K.) entailed an estimated 104 screening tests for T. fischeri (estimates are given because a proportion of the records are inaccessible due to changes resulting from laboratory mergers). That is, even with a high index of suspicion, only approximately 6.6% of T. rubrum-like isolates were tested. A total of seven T. fischeri isolates were confirmed.
Laboratories not using CEA may be justified in monitoring the situation until the abundance of T. fischeri in their geographic area is clarified by further CEA or other (e.g., molecular) studies. However, they run a risk that a small number of patients may be treated with antifungal drugs unnecessarily.
REFERENCES
- 1.Fischer J B, Kane J. The detection of contamination in Trichophyton rubrum and Trichophyton mentagrophytes. Mycopathol Mycol Appl. 1971;43:169–180. doi: 10.1007/BF02051718. [DOI] [PubMed] [Google Scholar]
- 2.Gupta, A. K., C. B. Horgan-Bell, and R. C. Summerbell. Onychomycosis associated with Onychocola canadensis: 10 case reports and a review of the literature. J. Am. Acad. Dermatol., in press. [DOI] [PubMed]
- 3.Kane J. Trichophyton fischeri sp. nov.: a saprophyte resembling Trichophyton rubrum. Sabouraudia. 1977;15:231–241. [PubMed] [Google Scholar]
- 4.Kane J, Smitka C M. A practical approach to the isolation and identification of members of the Trichophyton rubrum group. Pan Am Health Organ Sci Publ. 1980;396:121–134. [Google Scholar]
- 5.Kane J, Summerbell R C, Sigler L, Krajden S, Land G. Laboratory handbook of dermatophytes. Belmont, Calif: Star Publishing; 1997. [Google Scholar]
- 6.Sigler L, Congly H. Toenail infection caused by Onychocola canadensis gen. et sp. nov. J Med Vet Mycol. 1990;28:405–417. [PubMed] [Google Scholar]