Highlights
-
•
Compared with goslings, adult geese had lighter, redder and chewier meat.
-
•
IMP, l-threonine, etc. increased with age and related to better flavor of adults.
-
•
Pyruvic acid, l-cysteine, etc. were elevated and important flavor traits of gosling.
-
•
The results provide biomarkers to determine goose meat quality at different ages.
Keywords: Yangzhou goose, Meat quality, Meat age, Nontargeted metabolomics
Abstract
The purpose of this study was to distinguish the effect of age on the meat quality and chemical composition of Yangzhou goose breast meat. Nontargeted metabolomics analysis (UHPLC-MS/MS) was used to distinguish the metabolic composition of goose meat at different ages, and Pearson’s correlations between differential metabolites and key meat parameters were assessed. Compared with goslings, adult geese had lighter, redder and chewier meat (p < 0.05). Metabolite analysis revealed significant differences in nucleosides, organic acids, amino acids and sugars. Levels of IMP, xanthosine, pretyrosine and l-threonine were significantly higher in older meat (p < 0.05) and positively correlated with meat freshness indicators. However, pyruvic acid, l-cysteine and glucose 6-phosphate were up-regulated in gosling meat (p < 0.05), which were important flavor compounds. These results facilitate the further investigation of changes in goose meat composition and provide biomarkers for determining goose meat quality at different ages.
Introduction
Meat products are important sources of protein in human diets and poultry meat provides eating attributes that fulfil expectations not normally achieved by other protein sources (Magdelaine et al., 2008). In recent years, the demand for poultry meat has risen rapidly worldwide and China is a major producer of goose meat (Razmaitė et al., 2022). Goose meat has high nutritional value, is a particularly good source of amino acids and has specific aroma and flavor traits unlike other poultry (Okruszek et al., 2013). Meat quality is a complex trait that is affected by many factors including diet, genotype, age, and sex (Biesek et al., 2020). Age of birds has a great influence on the functional properties of chicken and meat tenderness tends to decrease with poultry age (Schneider et al., 2012). Furthermore, with increasing age, there is an increase in lipid content of duck breast and breasts became darker and redder (Baéza et al., 2000). Xiao et al. (2019) suggested that chicken meat at 230 days contained more glucose, inosine 5′-phosphate (IMP), anserine and glutamine than younger meat. In duck, compared with 50-day-old meat, 170- and 500-day-old meat was superior in regards to meat color, tenderness, and an appropriate meat to fat ratio, resulting in better taste (Liu et al., 2013). Weng et al. (2021a) suggested that 120-day-old geese had a larger muscle fiber area, higher intramuscular fat and elevated protein content. However, detailed differences in meat quality between adult geese and goslings have not been specified.
Metabolomics is a widely employed omics techniques for studying food quality and food components. Gas chromatography mass spectrometry (GC–MS) and liquid chromatography MS (LC-MS) were used to assess goose meat quality (Fornal & Montowska, 2019) and ultra-high-performance LC tandem MS (UHPLC-MS/MS) was applied to examine metabolites changes in chilled chicken (Zhang et al., 2020). UHPLC offers high peak capacity, high resolution, sensitivity and high-speed analysis (de Villiers et al., 2006). Thus, combining UHPLC with tandem mass spectrometry (MS/MS) provides significant advantages including selectivity, sensitivity, and speed (Romero-González et al., 2008). UHPLC-MS/MS has been used to distinguish biomarkers of tropical fruits (Bataglion et al., 2015), fish muscle (Grande-Martinez et al., 2018) and egg yolk (Gao et al., 2021). However, metabonomic profiling of goose meat quality by UHPLC-MS/MS has not been reported.
Yangzhou goose, a major poultry species in Jiangsu Province, is renowned for high egg and good meat quality. The main purpose of the present study was to determine the effect of age on meat quality of Yangzhou goose and apply nontargeted metabolomics analysis (UHPLC-MS/MS) to determine metabolite profiles of geese at different ages. The results provide a theoretical basis for distinguishing differences in meat quality between young and adult Yangzhou geese, and provide biomarkers for distinguishing meat quality of geese at different ages.
Materials and methods
Ethics approval
This study was reviewed and approved by the Institutional Animal Care and Use Committee of the Department of Animal Science and Technology, Yangzhou University, China. All goose procedures were performed according to the Standards for the Administration of Experimental Practices (Jiangsu, China, 2008).
Goose rearing and sample preparation
Yangzhou geese were selected from the same commercial goose farm (Gaoyou, Yangzhou, China). Sixteen 70-day-old goslings (Youth) were randomly selected from a flock of 1000 goslings and 16 300-day-old healthy geese (Adulthood) were randomly selected from a flock of 300 adult geese, which were raised under a conventional method of stocking and supplementary feeding (Table S1). In addition to feed, geese were free to graze grass (Yu et al., 2020). Geese were maintained under natural daylight and temperature. Birds were stunned using a stun bath and exsanguinated by severing the jugular vein and carotid artery on one side of the neck. The left breast muscle of each goose was divided into two parts; one part was used to measure water-holding capacity, shear force, color, pH and for texture profile analysis (TPA) (Xiao et al., 2021); the other part was maintained in a sterile polythene bag and stored at 4 °C. Sterile breast muscle of geese was divided into two groups based on goose age (Y and A). There were eight replicates in each group with 50 g of breast muscle per sample.
Meat quality
Expressible moisture was determined using a meat quality pressure meter (Tenovo Meat-1, Beijing, China) and shear force was measured using a C-LM3B digital tenderness meter (Tenovo). The pH value was measured 45 min after slaughter using a pH-STAR pH meter (Matthaus, Berlin, Germany). Meat color was measured at three randomly selected positions using a CR-400 chroma meter (Konica Minolta, Osaka, Japan) and colorimetric parameters L*, a* and b* were recorded.
The TPA content of the goose breast muscle (30 × 30 × 25 mm) was measured by a TMS-PRO texture analyzer (FTC, Sterling, USA) equipped with a 2500 N load cell. A double compression cycle test was performed, TPA analysis was performed using the following feature detection parameters: test speed, 20 mm/min; sample deformation, 20%; height of the load cell, 20 mm; trigger force, 30 N. TPA parameters (hardness, elasticity, cohesiveness, and chewiness) were calculated from force-time curves generated from samples using FTC-PRO software (FTC, Sterling, USA). All measurements were performed in triplicate.
Sample preparation for metabolomics analysis
Metabolite extraction was carried out as described by Dunn et al. (2011). A 25 mg sample of breast muscle was extracted by adding 800 μL of precooled extraction reagent and internal standards mix 1 and 2 were added for quality control of sample preparation. After homogenizing for 5 min using a TissueLyser (JXFSTPRP, Shanghai, China), samples were sonicated for 10 min and incubated for 1 h at −20 °C. Samples were centrifuged for 15 min at 8000g and 4 °C and the supernatant was transferred for vacuum freeze-drying. Metabolites were resuspended in 200 μL of 10% methanol and sonicated for 10 min at 4 °C. After centrifuging for 15 min at 8000×g, supernatants were transferred to an autosampler vials for LC-MS analysis. A quality control (QC) sample was prepared by pooling the same volume of each sample to evaluate the reproducibility of the whole LC-MS analysis.
LC-MS analysis
Samples were analyzed on a Waters 2D UPLC instrument (Waters Corporation, MA, USA) coupled to a Q-Exactive MS instrument (Thermo Fisher Scientific, MA, USA) with a heated electrospray ionization source and controlled by Xcalibur 2.3 software (Thermo Fisher Scientific, MA, USA). Chromatographic separation was performed on a Waters ACQUITY UPLC BEH C18 column (1.7 μm, 2.1 mm × 100 mm; Waters) and the column temperature was maintained at 45 °C. MS settings for positive/negative ionization modes were as follows: spray voltage, 3.8/−3.2 kV; sheath gas flow rate, 40 arbitrary units (arb); aux gas flow rate, 10 arb; aux gas heater temperature, 350 °C; capillary temperature, 320 °C. The full scan range was 70–1050 m/z with a resolution of 70,000 and an MS/MS resolution of 17,500. The stepped normalized collision energy was set to 20, 40 and 60 eV.
Quality control, compound detection and annotation
Data quality was assessed according to the repeatability of QC sample detection, which was based on the base peak chromatogram (BPC) of all QC samples. Each sample was selected for BPC chromatogram display. The BPC chart should have good peak shape and large peak capacity. The reliability and stability of instrument performance were evaluated using principal component analysis (PCA) of all samples. Raw MS data collected by LC-MS/MS were imported into Compound Discoverer 3.1 (Thermo Fisher Scientific, MA, USA) for data processing. Identification of metabolites was performed using BGI self-built standard library, mzCloud and ChemSpider (HMDB, KEGG, LipidMaps) databases. Parameters for metabolite identification were precursor mass tolerance < 5 ppm, fragment mass tolerance < 10 ppm, retention time tolerance < 0.2 min.
Differential metabolite analysis
Multivariate statistical analyses principal coordinate analysis (PCA) and partial least squares discriminant analysis (PLS-DA), univariate analysis, fold-change and Kruskal-Wallis test were combined to screen for differential metabolites between groups. PCA and PLS-DA were used to establish a relationship model between metabolite expression and sample groups and thereby predict the sample group, then combined with fold-change and t-tests to determine differential metabolites. Differential metabolite screening criteria were as follows: variable importance in the projection (VIP) of the first two principal components of the PLS-DA model ≥ 1; fold-change ≥ 1.2 or ≤ 0.83; p < 0.05.
Statistical analysis
Data analyses were performed with SPSS 13.0 software (SPSS Inc., Chicago, USA). Results are expressed as mean ± standard error, the statistical significance of differences among the various groups was evaluated by one-way analysis of variance in the GLM procedure and p < 0.05 was considered statistically significant.
Results
Meat quality
The meat quality of the breast muscle of geese at different ages is shown in Table 1. The cooking loss of 70-day-old goslings was 27.41%, which was very significantly higher than 300-day-old geese (p < 0.01), while the shear force was very significantly lower than that of adult geese (p < 0.01). The pH value of goslings was higher than that of adult geese (p < 0.05). Although the L* and b* breast muscle values were very significantly lower in adult geese than goslings (p < 0.01), the a* value showed no significant difference between the two groups (p > 0.05), suggesting adult geese had better meat color.
Table 1.
Breast meat quality of geese at different ages.
| Item | Adulthood | Youth |
|---|---|---|
| Cooking loss (%) | 20.34 ± 4.58 | 27.41 ± 3.25** |
| Shear force (N) | 86.04 ± 14.91 | 57.53 ± 5.66** |
| pH value | 6.31 ± 0.25 | 6.59 ± 0.14* |
| L* | 30.35 ± 1.15 | 50.21 ± 5.56** |
| a* | 14.62 ± 0.62 | 12.55 ± 3.06ns |
| b* | 4.47 ± 0.51 | 7.00 ± 1.84** |
Significant at P < 0.05.
Significant at P < 0.01.
Not significant at P > 0.05.
TPA parameters
The textural properties of the breast muscles of geese at different ages are shown in Table 2. Results from TPA analysis showed that the hardness and cohesiveness of meat from geese at different ages showed no significant difference (p > 0.05). The springiness and gumminess of adult geese was higher than that of goslings (p < 0.05). The chewiness of adult geese was 9.93 mJ, very significantly higher than for goslings (p < 0.01), confirming that adult geese were chewier.
Table 2.
TPA parameters for breast muscle of geese at different ages.
| Item | Adulthood | Youth |
|---|---|---|
| Hardness (N) | 52.28 ± 5.22 | 53.78 ± 2.72ns |
| Cohesiveness (%) | 0.55 ± 0.0.08 | 0.57 ± 0.02ns |
| Springiness (mm) | 0.28 ± 0.12 | 0.15 ± 0.02* |
| Gumminess (N) | 37.36 ± 4.23 | 33.42 ± 1.81* |
| Chewiness (mJ) | 9.93 ± 3.19 | 5.26 ± 0.82** |
Metabolic profiles of goslings and adult geese
The BPCs of all QC samples overlapped suggesting perfect repeatability and the signal was stable during detection and analysis (Fig. 1). A total of 4330 and 2213 peaks were detected using positive and negative ion modes (Tables S1 and S2). The PCA score plot showed a low dispersion of QC samples (Fig. 2), based on positive and negative ion modes. Furthermore, PLS-DA (R2Y = 0.92, Q2Y = 0.76) was used to confirm significant differences in metabolic profiles, R2-values were lower than the original point and the negative intercept of Q2 indicated the reliability of PLS-DA.
Fig. 1.
Multivariate statistical analysis of goose meat at different ages based on UPLC-MS profiles. (A) Principal component analysis (PCA) score plot based on positive ion mode results; (B) PCA score plot based on negative ion mode results; (C) Permutation testing of the PLS-DA model with 200 repetitions based on positive ion mode results; (D) Permutation testing of the PLS-DA model with 200 repetitions based on negative ion mode results.
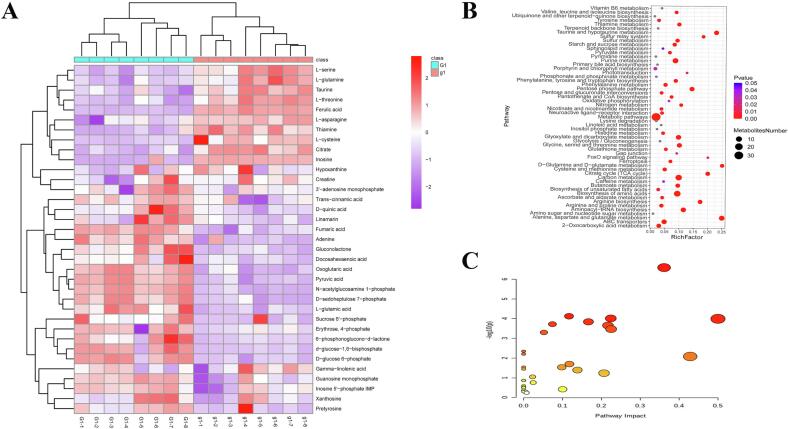
Fig. 2.
Changes in metabolites of goose meat at different ages. (A) Heatmap of differential metabolite; (B) Pathway prediction of differential metabolites based on KEGG analysis; (C) Pathway enrichment of differential metabolites.
Identification of differential metabolites
LC-MS/MS-based nontargeted metabolomics was used to detect changes in metabolic profiles of geese at different ages. It was obvious that the metabolic profiles were different between goose meat at different ages. Hierarchical clustering and heatmap analyses were performed to assesses metabolites in geese at different ages (Figs. S2 and S3), 733 and 615 metabolites from A and Y groups were identified in positive and negative ion modes, respectively. Additionally, 35 differential metabolites were identified, including fatty acids, organic acids, amino acids and nucleosides (Table 3). Furthermore, compared with adult geese, 17 metabolites were increased and 18 metabolites were decreased in young geese. Thiamine, l-glutamine, l-cysteine and l-asparagine were significantly increased in young geese, while γ-linolenic acid, creatine, IMP, hypoxanthine and cinnamic acid were higher in adult geese.
Table 3.
Differential metabolites of breast meat of geese at different ages.
| Metabolite | Molecular weight | Retention time (s) | VIP | ESI | Direction (Y/A) |
|---|---|---|---|---|---|
| Docosahexaenoic acid | 328.2392 | 9.881 | 1.3751** | pos | down |
| Thiamine | 264.1044 | 0.755 | 1.5399** | pos | up |
| γ-Linolenic acid | 278.2244 | 8.652 | 1.5** | pos | down |
| N-Acetyl-glucosamine 1-phosphate | 301.0562 | 0.785 | 1.2221** | neg | up |
| d-Glucose 1,6-bisphosphate | 339.9961 | 0.586 | 1.9099** | neg | down |
| Sucrose 6-phosphate | 422.083 | 0.898 | 1.4387** | pos | down |
| d-glucose 6-phosphate | 260.0294 | 0.774 | 1.4317** | neg | up |
| Pretyrosine | 227.0795 | 4.848 | 2.1477** | pos | down |
| Quinic acid | 192.0643 | 0.84 | 1.8146** | pos | down |
| l-serine | 105.0426 | 0.654 | 1.3871** | neg | up |
| l-cysteine | 121.0198 | 5.661 | 1.0934** | neg | up |
| Creatine | 131.0695 | 0.701 | 1.3603** | neg | down |
| l-threonine | 119.0583 | 0.672 | 1.2095** | neg | down |
| l-asparagine | 132.0532 | 0.695 | 1.7922** | pos | up |
| Inosine | 268.0804 | 1.497 | 2.2946** | neg | up |
| Xanthosine | 284.0756 | 2.842 | 2.0988** | neg | down |
| Inosine 5′-phosphate | 348.0471 | 0.717 | 1.9028** | neg | down |
| Guanosine monophosphate | 363.0579 | 0.706 | 1.6217** | neg | up |
| 3′-Adenosine monophosphate | 347.0631 | 2.356 | 1.5655** | pos | down |
| Hypoxanthine | 136.0386 | 1.067 | 1.2616** | neg | down |
| Adenine | 135.0546 | 1.639 | 1.0263** | neg | down |
| l-glutamine | 146.0692 | 0.658 | 1.4908** | neg | up |
| l-glutamic acid | 147.0532 | 0.783 | 1.3842 | neg | up |
| Trans-cinnamic acid | 148.0525 | 2.672 | 1.3926** | neg | down |
| Taurine | 125.0147 | 0.648 | 1.3938** | neg | up |
| Gluconolactone | 178.0479 | 0.801 | 1.5903** | pos | down |
| 6-Phosphonoglucono-d-lactone | 258.0141 | 0.596 | 1.5646** | neg | down |
| Erythrose 4-phosphate | 200.0086 | 0.609 | 1.5468** | neg | down |
| d-sedoheptulose 7-phosphate | 290.0401 | 0.773 | 1.1544** | neg | up |
| Fumaric acid | 116.011 | 0.604 | 1.9497** | neg | up |
| Citrate | 192.0271 | 0.603 | 1.511** | neg | up |
| Oxoglutaric acid | 146.0215 | 0.743 | 1.3612** | neg | up |
| Pyruvic acid | 88.0161 | 0.768 | 1.5394** | neg | up |
| Ferulic acid | 194.0579 | 4.717 | 1.5437** | neg | up |
| Linamarin | 247.1056 | 0.851 | 1.8836** | pos | down |
VIP, variable importance in the projection; ESI, electrospray ionization; pos, positive ion mode; neg, negative ion mode; Y/A, youth/adulthood.
Metabolic pathways of differential metabolites between goslings and adults
To explore reveal the pathways of differential metabolites in the breast muscle of geese at different ages, we performed enrichment analysis based on the 35 important metabolites (Fig. 2). Of the 16 KEGG pathways, purine metabolism was the most enriched, which included seven metabolites. Compared with adult geese, inosine and guanosine monophosphate were significantly increased in young geese, by 2.92-fold and 1.6-fold, respectively, while l-threonine, IMP, hypoxanthine and xanthosine were significantly decreased in young geese. l-threonine was significantly increased in adult geese and was enriched in seven pathways including glycine, serine and threonine metabolism, valine, leucine and isoleucine biosynthesis, porphyrin and chlorophyll metabolism and biosynthesis of amino acids, suggesting l-threonine plays an important role in meat flavor of goose meat at different ages.
Analysis of key meat parameters and differential metabolites
Differential metabolites are key factors affecting the quality of goose meat at different ages. A total of 35 differential metabolites with high VIP values and eight important meat parameters were selected and correlations were assessed using the Pearson’s method (Fig. 3). The results showed that l-glutamic acid, l-cysteine and thiamine had extremely strong positive correlations with L*, while pretyosine, xanthosine, gluconolactone and linamarin had extremely strong positive correlations with L*. Furthermore, pretyosine and xanthosine also had extremely strong positive correlations with chewiness, springiness, and shear force. However, l-cysteine had strong negative correlations with chewiness, springiness and shear force. Pyruvic acid and l-glutamic acid had strong positive correlations with cooking loss.
Fig. 3.
Correlations between key meat parameters and differential metabolites of goose meat at different ages.
Discussion
Meat color is one of the most important fresh meat characteristics at the point of purchase (Gracia & de Magistris, 2013). In China, people prefer redder poultry meat (Guo et al., 2018). In our study, meat from 300-day-old geese was redder meat than that from 70-day-old geese. Moreover, older geese not only had redder meat (a*), but also had lighter (L*) and yellower (b*) meat. Li et al. (2019) reported similar results for chicken meat. Age is an important factor affecting meat color and texture since myoglobin levels increase with age (Lyon et al., 2004). Furthermore, a longer growth season results in chewier goose meat, which is popular in China (Weng et al., 2021a), and older geese produced chewier meat in the present study. Consistent with the results of Saláková et al. (2009), gosling meat had a higher muscle pH, which was associated with darker meat. Furthermore, higher shear force and lower cooking loss were observed in breast meat from older geese, similar to the results of Weng et al. (2021b). Li (2006) concluded that gumminess is a proxy of hardness and cohesiveness in hens, but in present study, gumminess and springiness were significantly higher in adult goose meat, and hardness and cohesiveness were not significantly lower in goslings, which may be related to differences in breeds and sex.
In this study, the results of metabolite analysis of breast meat of geese at different ages revealed significant differences in organic acids, nucleosides, sugars and amino acids. Organic acids, including pyruvic acid and γ-linoleic acid, have a strong influence on meat quality. Pyruvic acid is a cellular metabolite at a key biochemical junction of glycolysis (Maleki & Eiteman, 2017). In our study, pyruvic acid was elevated in gosling. Welzenbach et al. (2016) indicated that a high rate of glycolysis results in a high L* value, which was similar to that of the gosling meat in our study. Furthermore, pyruvic acid had a strong positive correlation with cooking loss in this study, and numerous studies concluded that a high glycolytic potential in muscles results in a high drip loss (Sieczkowska et al., 2010), indicating that pyruvic acid is an important biomarker related to meat quality of geese at different ages. Linoleic acid is the most highly consumed polyunsaturated fatty acid (PUFA) found in the human diet, and it can serve as both a source of energy and a structural component (Whelan & Fritsche, 2013). Linoleic acid was higher in adult geese than goslings in the present work, and della Malva et al. (2016) reported similar results in lamb meat. Conjugated linoleic acid requirements in humans are mainly met by the consumption of animal-derived products, especially poultry products (Grashorn, 2007). Furthermore, it also showed positive correlations with chewiness and springiness, suggesting meat from adult geese can be consumed as a source of linoleic acid.
Nucleotides also affect meat flavor. IMP, xanthosine and hypoxanthine, involved in purine metabolism, were significantly elevated in adult geese. IMP plays a key role in the development of umami taste in chicken meat (Jung et al., 2013). It is hydrolyzed to hypoxanthine, which has a positive correlation with sweetness in cooked lamb (Bi et al., 2021). Furthermore, Huang et al. (2022) concluded that the IMP content of chicken muscle increased with increasing age, and meat quality was improved, consistent with better taste for adult geese in this study. Vani et al. (2006) found that phosphate hydrolysis from IMP to inosine was more rapid at lower pH, but adult geese had higher IMP levels and lower pH in the present study, and further research is needed to explore the transformation mechanism of IMP. Both xanthosine and inosine are indicators of meat freshness and play important roles in IMP metabolism (Fang et al., 2022). Xanthosine was up-regulated in adult geese and positively correlated with meat freshness indicators (chewiness, springiness, gumminess and shear force), while inosine was up-regulated in gosling and was only weakly correlated with freshness indicators, suggesting that xanthosine can be used as a freshness indicator of goose meat.
Xiao et al. (2019) concluded that amino acids are the most abundant metabolites affecting meat quality, and they are important flavor and flavor precursor substances in chicken meat. l-cysteine levels are higher in goslings and reaction of cysteine and sugars generates unique chicken flavor (Ames et al., 2001). Both cysteine and glucose 6-phosphate were elevated in goslings, and these two indicators had strong positive correlations with L*, indicating that they may affect the meat flavor and quality of goslings. In adult geese, both l-threonine and pretyrosine were elevated. l-threonine is sweet and can improve meat quality (Jiang et al., 2020), but it showed only a weak correlation with meat freshness indicators of goose meat. Pretyrosine is an obligatory intermediate of l-tyrosine biosynthesis, Leggio et al. (2012) found that l-tyrosine is closely related to meat flavor and can be used as an indicators of meat quality and freshness. Consistently, pretyrosine was strongly positively correlated with meat freshness in the present work.
Conclusion
In summary, this study demonstrated that springiness, gumminess, chewiness and shear force were significantly higher in 300-day-old geese, with lower L*, b* and higher a*, consistent with lighter, redder and chewier meat. The results suggest that age plays a vital role in the quality of goose meat. Furthermore, metabolites differed in geese between the ages of 70 and 300 days. Xanthosine was elevated in adult geese and positively correlated with meat freshness. Levels of pretyrosine and l-threonine were higher in adult geese and pretyrosine had a strong positive correlation with meat freshness. However, pyruvic acid was elevated in gosling meat and had a strong positive correlation with cooking loss. Cysteine and glucose 6-phosphate had strong positive correlations with L*, and they are important flavor compounds of gosling meat that can be used as biomarkers. The findings provide new insight into the molecular mechanisms underlying changes in metabolites in Yangzhou geese at different ages, and biomarkers for determining goose meat quality.
CRediT authorship contribution statement
Ying Wang: Conceptualization, Methodology, Validation, Writing – original draft. Wanqing Li: Methodology, Validation. Chi Zhang: Methodology, Data curation. Fushi Li: Methodology, Validation. Haiming Yang: Conceptualization, Writing – review & editing, Supervision, Funding acquisition. Zhiyue Wang: Conceptualization, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was funded by the Jiangsu Agricultural Industry Technology System [JATS(2023)496], Priority Academic Program Development of Jiangsu Higher Education Institutions [PAPD]. The funding body did not play any role in study design, data collection, analysis, or interpretation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2023.100775.
Contributor Information
Ying Wang, Email: dkwangying@yzu.edu.cn.
Haiming Yang, Email: yhmdlp@qq.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Fig. S1.
Quality control and metabolic profile analysis of samples. (A) Positive ion chromatograms of quality control samples; (B) negative ion chromatograms of quality control samples.
Supplementary Fig. S2.
Heatmap clustering of different groups. (A = positive ion mode, B = negative ion mode).
Supplementary Fig. S3.
Volcano map of differential metabolites of different groups (A = positive ion mode, B = negative ion mode).
Ingredients and nutrient composition of experimental diets.
Peaks detected using positive mode (partial; fold-change >30).
Peaks detected using negative mode (partial; fold-change >30).
Data availability
Data will be made available on request.
References
- Ames J.M., Guy R.C.E., Kipping G.J. Effect of pH and temperature on the formation of volatile compounds in cysteine/reducing sugar/starch mixtures during extrusion cooking. Journal of Agricultural and Food Chemistry. 2001;49:1885–1894. doi: 10.1021/jf0012547. [DOI] [PubMed] [Google Scholar]
- Baéza E., Salichon M.R., Marche G., Wacrenier N., Dominguez B., Culioli J. Effects of age and sex on the structural, chemical and technological characteristics of mule duck meat. British Poultry Science. 2000;41:300–307. doi: 10.1080/713654934. [DOI] [PubMed] [Google Scholar]
- Bataglion G.A., da Silva F.M., Eberlin M.N., Koolen H.H. Determination of the phenolic composition from Brazilian tropical fruits by UHPLC–MS/MS. Food Chemistry. 2015;180:280–287. doi: 10.1016/j.foodchem.2015.02.059. [DOI] [PubMed] [Google Scholar]
- Bi S., Chen L., Sun Z., Wen Y., Xue Q., Xue C.…Liu H. Investigating influence of aquaculture seawater with different salinities on non-volatile taste-active compounds in Pacific oyster (Crassostrea gigas) Journal of Food Measurement and Characterization. 2021;15:2078–2087. doi: 10.1007/s11694-020-00807-4. [DOI] [Google Scholar]
- Biesek J., Kuźniacka J., Banaszak M., Giuseppe M., Małgorzata G., Marek A. The effect of various protein sources in goose diets on meat quality, fatty acid composition, and cholesterol and collagen content in breast muscles. Poultry Science. 2020;99:6278–6286. doi: 10.1016/j.psj.2020.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Villiers A., Lestremau F., Szucs R., Gélébart S., David F., Sandra P. Evaluation of ultra-performance liquid chromatography. Part I. Possibilities and limitations. Journal of Chromatography A. 2006;1127:60–69. doi: 10.1016/j.chroma.2006.05.071. [DOI] [PubMed] [Google Scholar]
- della Malva A., Albenzio M., Annicchiarico G., Caroprese M., Muscio A., Santillo A., Marino R. Relationship between slaughtering age, nutritional and organoleptic properties of Altamurana lamb meat. Small Ruminant Research. 2016;135:39–45. doi: 10.1016/j.smallrumres.2015.12.020. [DOI] [Google Scholar]
- Dunn W.B., Broadhurst D., Begley P., Zelena E., Francis-McIntyre S., Anderson N., Brown M., Knowles J.D., Halsall A., Haselden J.N., Nicholls A.W., Wilson I.D., Kell D.B., Goodacre R., The Human Serum Metabolome (HUSERMET) Consortium Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nature Protocols. 2011;6:1060–1083. doi: 10.1038/nprot.2011.335. [DOI] [PubMed] [Google Scholar]
- Fang J., Feng L., Lu H., Zhu J. Metabolomics reveals spoilage characteristics and interaction of Pseudomonas lundensis and Brochothrix thermosphacta in refrigerated beef. Food Research International. 2022;156 doi: 10.1016/j.foodres.2022.111139. [DOI] [PubMed] [Google Scholar]
- Fornal E., Montowska M. Species-specific peptide-based liquid chromatography–mass spectrometry monitoring of three poultry species in processed meat products. Food Chemistry. 2019;283:489–498. doi: 10.1016/j.foodchem.2019.01.074. [DOI] [PubMed] [Google Scholar]
- Gao B., Hu X., Li R., Zhao Y., Tu Y., Zhao Y. Screening of characteristic umami substances in preserved egg yolk based on the electronic tongue and UHPLC-MS/MS. LWT. 2021;152 doi: 10.1016/j.lwt.2021.112396. [DOI] [Google Scholar]
- Gracia A., de Magistris T. Preferences for lamb meat: A choice experiment for Spanish consumers. Meat Science. 2013;95:396–402. doi: 10.1016/j.meatsci.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Grande-Martinez A., Moreno-Gonzalez D., Arrebola-Liebanas F.J., Garrido-Frenich A., Garcia-Campana A.M. Optimization of a modified QuEChERS method for the determination of tetracyclines in fish muscle by UHPLC–MS/MS. Journal of Pharmaceutical and Biomedical Analysis. 2018;155:27–32. doi: 10.1016/j.jpba.2018.03.029. [DOI] [PubMed] [Google Scholar]
- Grashorn M.A. Functionality of poultry meat. Journal of Applied Poultry Research. 2007;16:99–106. doi: 10.1093/japr/16.1.99. [DOI] [Google Scholar]
- Guo Y., Huang J., Sun X., Liu Q., Huang M., Zhou G.H. Effect of normal and modified atmosphere packaging on shelf life of roast chicken meat. Journal of Food Safety. 2018;38 doi: 10.1111/jfs.12493. [DOI] [Google Scholar]
- Huang Z., Zhang J., Gu Y., Cai Z., Feng X., Yang C., Xin G. Research progress on inosine monophosphate deposition mechanism in chicken muscle. Critical Reviews in Food Science and Nutrition. 2022;62:1062–1078. doi: 10.1080/10408398.2020.1833832. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Xie M., Tang J., Zhou Z., Zhang Y., Chen G., Hou S. Effects of genetic selection and threonine on meat quality in Pekin ducks. Poultry Science. 2020;99:2508–2518. doi: 10.1016/j.psj.2019.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S., Bae Y.S., Kim H.J., Jayasena D.D., Lee J.H., Park H.B.…Jo C. Carnosine, anserine, creatine, and inosine 5’-monophosphate contents in breast and thigh meats from 5 lines of Korean native chicken. Poultry Science. 2013;92:3275–3282. doi: 10.3382/ps.2013-03441. [DOI] [PubMed] [Google Scholar]
- Leggio A., Belsito E.L., De Marco R., Liguori A., Siciliano C., Spinella M. Simultaneous extraction and derivatization of amino acids and free fatty acids in meat products. Journal of Chromatography A. 2012;1241:96–102. doi: 10.1016/j.chroma.2012.04.029. [DOI] [PubMed] [Google Scholar]
- Li C.T. Myofibrillar protein extracts from spent hen meat to improve whole muscle processed meats. Meat Science. 2006;72:581–583. doi: 10.1016/j.meatsci.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Li, J., Yang, C., Peng, H., Yin, H., Wang, Y., Hu, Y., Yu, C., Jiang, X., Du, H., Li, Q., & Liu, Y. (2019). Effects of slaughter age on muscle characteristics and meat quality traits of Da-Heng meat type birds. Animals (Basel), 10, 69. https://doi: 10.3390/ani10010069. [DOI] [PMC free article] [PubMed]
- Liu C., Pan D., Ye Y., Cao J. 1H NMR and multivariate data analysis of the relationship between the age and quality of duck meat. Food Chemistry. 2013;141(2):1281–1286. doi: 10.1016/j.foodchem.2013.03.102. [DOI] [PubMed] [Google Scholar]
- Lyon B.G., Smith D.P., Lyon C.E., Savage E.M. Effects of diet and feed withdrawal on the sensory descriptive and instrumental profiles of broiler breast fillets. Poultry Science. 2004;83:275–281. doi: 10.1093/ps/83.2.275. [DOI] [PubMed] [Google Scholar]
- Magdelaine P., Spiess M.P., Valceschini E. Poultry meat consumption trends in Europe. World's Poultry Science Journal. 2008;64:53–64. doi: 10.1017/S0043933907001717. [DOI] [Google Scholar]
- Maleki N., Eiteman M.A. Recent progress in the microbial production of pyruvic acid. Fermentation. 2017;3:8. doi: 10.3390/fermentation3010008. [DOI] [Google Scholar]
- Okruszek A., Wołoszyn J., Haraf G., Orkusz A., Wereńska M. Chemical composition and amino acid profiles of goose muscles from native Polish breeds. Poultry Science. 2013;92:1127–1133. doi: 10.3382/ps.2012-02486. [DOI] [PubMed] [Google Scholar]
- Razmaitė V., Šiukščius A., Šveistienė R., Jatkauskienė V. Present conservation status and carcass and meat characteristics of Lithuanian Vištinės goose breed. Animals. 2022;12:159. doi: 10.3390/ani12020159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-González, R., Garrido Frenich, A., & Martínez Vidal, J. L. (2008). Multiresidue method for fast determination of pesticides in fruit juices by ultra-performance liquid chromatography coupled to tandem mass spectrometry. Talanta, 76, 211–25. https://doi:10.1016/j.talanta.2008.02.041. [DOI] [PubMed]
- Saláková A., Straková E., Válková V., Buchtová H., Steinhauserová I. Quality indicators of chicken broiler raw and cooked meat depending on their sex. Acta Veterinaria Brno. 2009;78:497–504. doi: 10.2754/avb200978030497. [DOI] [Google Scholar]
- Schneider B.L., Renema R.A., Betti M., Carney V.L., Zuidhof M.J. Effect of holding temperature, shackling, sex, and age on broiler breast meat quality. Poultry Science. 2012;91:468–477. doi: 10.3382/ps.2010-00952. [DOI] [PubMed] [Google Scholar]
- Sieczkowska, H., Koćwin-Podsiadła, M., Zybert, A., Krzęcio, E., Antosik, K., Kamiński, S., & Wójcik, E. (2010). The association between polymorphism of PKM2 gene and glycolytic potential and pork meat quality. Meat Science, 84, 180–185. https://doi: 10.1016/j.meatsci.2009.08.045. [DOI] [PubMed]
- Vani N.D., Modi V.K., Kavitha S., Sachindra N.M., Mahendrakar N.S. Degradation of inosine-5′-monophosphate (IMP) in aqueous and in layering chicken muscle fibre systems: Effect of pH and temperature. LWT-Food Science and Technology. 2006;39:627–632. doi: 10.1016/j.lwt.2005.05.003. [DOI] [Google Scholar]
- Weng K., Huo W., Gu T., Bao Q., Cao Z.F., Zhang Y.…Chen G.H. Quantitative phosphoproteomic analysis unveil the effect of marketable ages on meat quality in geese. Food Chemistry. 2021;361 doi: 10.1016/j.foodchem.2021.130093. [DOI] [PubMed] [Google Scholar]
- Weng K., Huo W., Gu T., Bao Q., Hou L.E., Zhang Y.…Chen G. Effects of marketable ages on meat quality through fiber characteristics in the goose. Poultry Science. 2021;100:728–737. doi: 10.1016/j.psj.2020.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welzenbach J., Neuhoff C., Looft C., Schellander K., Tholen E., Große-Brinkhaus C. Different statistical approaches to investigate porcine muscle metabolome profiles to highlight new biomarkers for pork quality assessment. PLoS One. 2016;11 doi: 10.1371/journal.pone.0149758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan, J., & Fritsche, K. (2013). Linoleic acid. Advances in Nutrition, 4, 311–312. https://doi: 10.3945/an.113.003772. [DOI] [PMC free article] [PubMed]
- Xiao Z., Ge C., Zhou G., Zhang W., Liao G. 1H NMR-based metabolic characterization of Chinese Wuding chicken meat. Food Chemistry. 2019;274:574–582. doi: 10.1016/j.foodchem.2018.09.008. [DOI] [PubMed] [Google Scholar]
- Xiao X., Liang J.R., Yang H.M., Wan X.L., Wang Z.Y. Vitamin A deficiency or critical excess has negative effects on the growth performance, slaughter performance, and meat quality of goslings. Animal Feed Science and Technology. 2021;280 doi: 10.1016/j.anifeedsci.2021.115064. [DOI] [Google Scholar]
- Yu J., Yang H.M., Wan X.L., Chen Y.J., Yang Z., Liu W.F.…Wang Z.Y. Effects of cottonseed meal on slaughter performance, meat quality, and meat chemical composition in Jiangnan White goslings. Poultry Science. 2020;99:207–213. doi: 10.3382/ps/pez451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Zhang S., Chen L., Ding H., Wu P., Zhang G.…Wang J. UHPLC–MS/MS-based nontargeted metabolomics analysis reveals biomarkers related to the freshness of chilled chicken. Foods. 2020;9:1326. doi: 10.3390/foods9091326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ingredients and nutrient composition of experimental diets.
Peaks detected using positive mode (partial; fold-change >30).
Peaks detected using negative mode (partial; fold-change >30).
Data Availability Statement
Data will be made available on request.