Highlights
-
•
Pediococcus pentosaceus (pp) fermentation could effectively reduce the Tartary buckwheat allergenicity.
-
•
Tartary buckwheat sourdough fermented for 16 h (pp16) is recommended, with lower allergenicity.
-
•
There are 756 peptide fragments related to allergen protein. 213 fragments were upregulated after fermentation, and the expression decreased by 71.83%.
-
•
Six peptide fragments from Fagt 1 maybe one of the main reasons for the residual allergy of PP16.
Keywords: Tartary buckwheat, Allergenicity, HPLC/MS-MS, Fermentation
Abstract
Tartary buckwheat contains more valuable nutrients than common buckwheat, but it also contains allergenic proteins that induce allergic reactions through an IgE-mediated response. Our study demonstrated that fermentation by Pediococcus pentosaceus degrades allergenic proteins in Tartary buckwheat, as confirmed by HPLC-MS/MS analysis of polypeptides. Our results showed significant degradation of the protein after 16 h of Pediococcus pentosaceus fermentation (PP16), leading to a reduction in IgE-binding activity. Comparison with unfermented Tartary buckwheat (UTB) peptides yielded 2042 fragments, of which 756 fragments associated with allergenic proteins were upregulated. Among them, the expression of 213 fragments was reduced by 71.83%. By performing bioactivity prediction on potential allergenic peptide fragments, we identified six peptide fragments derived from Fagt 1, potentially contributing to the residual allergenicity in PP16. These suggest that Pediococcus pentosaceus fermentation can effectively destroy allergen epitopes and mitigate the allergenicity of Tartary buckwheat.
Introduction
Buckwheat, indigenous to southwestern China, has an extensive historical record of cultivation and widespread distribution throughout the region. Additionally, it has a well-established cultural tradition of cultivation and consumption in Japan, South Korea, Russia, and other nations (Zhu, 2016). The beneficial effects of buckwheat are well-documented and attributed to its rich content of flavonoids, phytosterols, dietary fiber, vitamins, minerals, and antioxidants (Sinkovič et al., 2022, Sytar et al., 2018). Buckwheat products decrease cholesterol levels, regulate blood lipids, and enhance lung capacity in humans (Sikder et al., 2014, Yang et al., 2014, Zhang et al., 2017). In addition, due to its gluten-free characteristics, buckwheat could even be offered to patients with celiac disease as an alternative to wheat (Chandrupatla, Kundu, & Aronson, 2005).
Most allergenic substances in food belong to the protein family, and the allergenic proteins of buckwheat are primarily distributed within the 10–70 kD range (Schatz, Sicherer, & Zeiger, 2018). However, the WHO/IUIS Allergen Nomenclature Sub-Committee has confirmed the presence of six allergens in buckwheat, including four in common buckwheat and two in Tartary buckwheat. The allergenic proteins in Tartary buckwheat are Fagt 2 and Fagt 6, with molecular weights of 16kD and 18kD, respectively (Geiselhart et al., 2018, Katayama et al., 2018, Zheng et al., 2018). Additionally, common buckwheat contains an allergenic epitope called Fage 1, derived from the 13 s globulin, which is considered the primary allergen of common buckwheat (Yoshioka, Ohmoto, Urisu, Mine, & Adachi, 2004). Fage 2 is known to have a cross-allergic reaction with latex, and other allergenic principles indicate a certain correlation between allergic reactions (Maruyama, Sato, Yanagida, Cabanos, Ito, Borres, et al., 2016).
Currently, the processing methods of hypersensitive food can be classified into two forms: thermal processing and nonthermal processing. Thermal processing often induces diverse modifications in food substrates, which can lead to a decrease in allergenicity through allergen epitopes masking or destruction, or an increase in allergenicity through the exposure of hidden epitopes or the generation of new epitopes (Shriver & Yang, 2011). Nonthermal processing demonstrates superior desensitization effects while minimizing nutrient loss and preserving food flavor. Fermentation is considered one of the most effective desensitization methods. It enzymatically degrades allergenic proteins into small molecular peptides and amino acids via proteolysis and acid-induced denaturation, thereby destroying or concealing allergen epitopes (Chizoba Ekezie, Cheng, & Sun, 2018). Rizzello, et al.,(2006) assessed the hydrolytic capacity of selected lactic acid bacteria, lactic acid bacteria mixtures, and Bifidobacterium for wheat protein sensitization during yeast bread production. The findings revealed that lactic acid bacteria fermentation was more conducive to the degradation of IgE epitopes in wheat protein. Additionally, microorganisms and their metabolites can retard bread quality and nutritional degradation (Zhou, Ouyang, Duan, Lv, & Zhou, 2022). The dominant bacteria isolated from spontaneously fermented wheat bran sourdough were Lactobacillus plantarum and Pediococcus pentosaceus, which possess exopolysaccharide-producing capabilities (Abedfar, Hosseininezhad, Sadeghi, Raeisi, & Feizy, 2018). Pediococcus pentosaceus exhibited favorable acidification, growth performance, and strong protein hydrolysis ability (up to 80% increase) in the whole wheat dough (Montemurro, Celano, De Angelis, Gobbetti, Rizzello, & Pontonio, 2020).
The primary methods employed for the detection of food allergens encompass enzyme-linked immunosorbent assay (ELISA) and polymerase chain reaction (PCR). However, the PCR method does not allow for quantitative analysis. It is worth noting that the ELISA method can yield false positives due to cross-reactivity concerns (Poms, Klein, & Anklam, 2004). On the other hand, liquid chromatography-tandem mass spectrometry (LC-MS-MS) serves as a non-immunological detection approach. This method involves the analysis of proteins and peptides in the samples to achieve the identification, characterization, and quantification of allergens. By bypassing immune reactions, the detection process effectively mitigates the risk of cross-allergic responses (Song, Sun, Xiao, Wang, Ding, Zhao, et al., 2019). Prandi et al., (2013) employed LC-MS-MS to analyze the sensitization of LTPs and X5 gliadin in wheat. Consequently, owing to its remarkable accuracy, specificity, and capacity for multi-target analysis, LC-MS-MS demonstrates promising prospects in the field of allergen detection.
Tartary buckwheat contains distinctive phytochemical constituents as a food source and exhibits potential for disease prevention. The rise in Tartary buckwheat allergy cases and associated food safety concerns have increasingly captivated consumer attention. However, complete avoidance of allergenic hazards proves challenging for allergic patients. Consequently, numerous scholars have explored various processing methods targeting common allergens, including heat treatment, enzymatic hydrolysis, and fermentation, aiming to reduce allergenicity. Research findings indicate that appropriate fermentation of Tartary buckwheat enhances its processability and augments its nutritional profile (Zhou, She, Zhu, & Zhou, 2022). Investigation into hypoallergenic buckwheat holds promise in facilitating access to this valuable pseudo-cereal for allergic individuals. Importantly, the development of hypoallergenic foods contributes to improving patients' quality of life and reducing the incidence of perilous allergic reactions. In summary, the application of Tartary buckwheat dough fermentation technology and the elucidation of its underlying mechanisms are of great significance to the advancement and utilization of Tartary buckwheat food and the broader domain of fermented grain products.
Materials and methods
Preparation of Tartary buckwheat sourdough
Under aseptic conditions, a ring of colonies was scraped from the seed plate of Pediococcus pentosaceus (JCM20453, Japan Collection of Microorganisms, PP) and inoculated into sterilized MRS liquid medium (Hopebio Technology Co. Ltd., Qingdao, China). The culture was incubated in a constant temperature shaker (SHZ-B, Bosun Industrial Co., Ltd., Shanghai, China) at 37 ℃ for 8 h. The bacterial suspension was then transferred to a sterile centrifuge tube, centrifuged at 5000g for 10 min, and washed twice with sterile saline solution followed by one wash with sterile water. After washing, the fermented seed liquid was prepared by dilution with water.
Referring to the processing technique described in “Laomian” (Gobbetti & Nzle, 2013), Tartary buckwheat sourdough was prepared by blending Tartary buckwheat flour and purified water at a mass ratio of 1:1.5. Subsequently, A 10% (v/w) inoculum of the prepared fermented seed liquid was added to the buckwheat dough and thoroughly mixed. At this point in time, Samples were taken to obtain unfermented Tartary buckwheat dough samples (UTB). Fermentation was carried out at 30 ℃ for 24 h. Samples were collected at 4-hour intervals. Upon completion of fermentation, the samples were freeze-dried using a freeze-dryer (Lab-1D-50, Biocool Instruments Co., Ltd., Beijing, China), pulverized, and prepared for further analysis. The Tartary buckwheat used was purchased from Yanmen Qinggao (Heifeng I), Shanxi, China. The purified water used was filtered drinking water.
pH and total titratable acidity (TTA) of fermented Tartary buckwheat sourdoughs
Determination of pH and Total Titratable Acidity (TTA) using the method of Liu et al., (2016). The pH value was measured using a pH meter (FE20, Mettler Toledo Instruments (Shanghai) Co., Ltd., China). The TTA value was measured through an automatic potentiometric titrator (809, Metrohm China Co., Ltd., Beijing, China), and the result was expressed as the volume (mL) of 0.1 M NaOH (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) standard solution required to reach a pH of 8.6 in the sample.
Microbiological analysis and counting
Referring to the method of Yan et al., (2019), MRS agar was used to count Pediococcus pentosaceus colonies in Tartary buckwheat dough. At 4-hour intervals during the fermentation process, 10 g of samples were suspended in 90 mL of 0.9% NaCl (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) saline solution and homogenized for 2 min in a vortex oscillator (DHP-100, U-ways smart technology Co., Ltd., Ningbo, China). The suspension was then subjected to a 10-fold serial dilution in sterile saline. The appropriate dilutions were plated onto MRS agar plates for colony counting. The plates were anaerobically incubated at 37 ℃ for 48 h. The agars were purchased from Hopebio Technology Co., Ltd., Qingdao, China.
Extraction of Tartary buckwheat protein
The extraction of Tartary buckwheat protein was conducted according to the method described by Park et al., (2000). Tartary buckwheat powder was used as the starting material. The protein extraction was performed using a column centrifugation protein extraction kit purchased from Invent BioTechnologies, Inc., Beijing, China.
SDS-PAGE and Western blot analysis
SDS-PAGE was performed according to the method of Carullo et al., (2020) with a few modifications. The Tartary buckwheat protein extracted by centrifugation was denatured with the sample buffer for 5 min at 100 ℃, followed by analysis on a separating gel of 12% acrylamide and a stacking gel of 5% acrylamide for 120 min at 120 V in a mini-protean gel apparatus (Bio-Rad, USA). The SDS-PAGE gel preparation kit was purchased from Beijing Solarbio Science & Technology Co., Ltd. Following electrophoresis, the gel was stained with Coomassie Brilliant Blue R-250, and recorded the electrophoresis results by the ChemStudio imaging system (Analytik Jena, Germany).
The supernatant was resuspended in buffer (20 mM Tris-HCl pH 8.0) and was separated by SDS-PAGE and blotted onto polyvinylidene fluoride membranes (0.45 μm). After blocking with 5% nonfat milk for 1 h at 25 ℃, the membrane was incubated with the serum of people allergic to buckwheat diluted 1:1000 times in TBST overnight at 25 ℃. The membrane was then washed three times with TBST for 5 min each. Goat Anti-Human IgE epsilon chain (Abcam) diluted in TBST (1:2000) were added to the membranes and incubated for 1 h at room temperature. After washing three times again, Donkey Anti-Goat lgG H&L (HRP) (Abcam) diluted in TBST (1:4000) was added to the membranes and incubated for 1 h at 25 ℃. The membranes were washed three times with TBST for 5 min each. The blotted membrane was reacted with the Super ECL detection reagent (Yeasen Biotechnology (Shanghai)) for 2 min.
Analysis of Tarary buckwheat polypeptides by HPLC-MS/MS
The sample was redissolved with 100 μL 0.1% TFA and then a Millipore10 kDa ultrafiltration tube (15 mL, Micoron, USA) was used to remove macromolecular proteins. The obtained polypeptide mixture was desalted by Empore solid-phase extraction column C18 (7 mm, 3 mL, Sigma, USA), and the polypeptide components were re-dissolved with 40 μL 0.1% TFA after freeze-vacuum drying for HPLC-MS/MS analysis.
The mixture of polypeptides was separated by HPLC liquid phase system Easy nLC (LC-20AD XR, Shimadzu, Japan) with a flow rate of 0.25 mL/min. The liquid gradient setting was as follows: 0 min-50 min, B liquid linear gradient from 4% to 50%; 50 min-54 min, B liquid linear gradient from 50% to 100%; 54 min-60 min, B liquid maintained at 100%. The mobile phase A used in the liquid phase was 0.1% formic acid aqueous solution and liquid B was 0.1% formic acid acetonitrile aqueous solution (84% acetonitrile). The liquid chromatographic column (75 um × 150 mm, RP-C18, Column Technology Inc) was balanced with 95% A solution before injection.
The sample was separated by high performance liquid chromatography and analyzed by mass spectrometry with a Q Exactive mass spectrometer (Thermo Fisher, USA). The analysis time for each component was 120 min. Positive ion detection mode was used to acquire spectra at the following parameters: precursor ion scan: 300–1800 m/z; the resolution of the primary mass spectrometry: 70,000(m/z = 200); AGC (Automatic gain control) target: 1e6; Maximum IT: 10 ms; Dynamic exclusion: 20.0 s. MS2 Activation Type: HCD; Isolation window: 1.6 m/z; The parameters of the secondary mass spectrometry: 17,500 (m/z = 200); Normalized collision energy: 27 eV; Underfill ratio: 0.1%.
Data analysis
Statistical analysis
All treatments and analyses were performed in triplicates, and the figures were drawn using Origin (Version 2021). Values were expressed as mean values and standard deviations. The results were subjected to a one-way ANOVA analysis and significant differences among values were calculated based on Duncan’s multiple range test by SPSS (Version 24.0, SPSS Inc, Chicago, IL, USA) (p < 0.05).
Kinetic modeling
The first-order fractional conversion model, one of the empirical kinetic models, was carried out by using (Eq. (1)) in Origin (Version 2021) to evaluate the pH, TTA, and microbiological counting during Tartary buckwheat dough fermentation (Shang, Ye, Li, Ren, Cai, Hu, et al., 2022).
| (1) |
where C is the parameter value at fermentation time(hours), C∞ is the value of the stable fraction, C0 is the initial value at the start of fermented 0 h, k is the reaction rate constant (hours−1), and t is the number of fermentation time (hours).
Correlation analysis
The original data of HPLC-MS/MS (Raw File) was processed by the MaxQuant 1.5.5.1 to search the Uniport database. The related parameters used in the database are as follows: Peptide mass tolerance: 20 ppm; MS/MS tolerance: 0.1 Da; Enzyme = None; Max Missed Cleavages: 2; Variable modification: Oxidation (M); database: UniProt_Polygonaceae_9309_20220228 and UniProt_Pediococcus pentosaceus_10501_20220228 combined with anti-database, results filtering parameters: FDR < 0.01.
Results and discussion
The pH value, TTA value, and microbiological counting of Tartary buckwheat sourdoughs
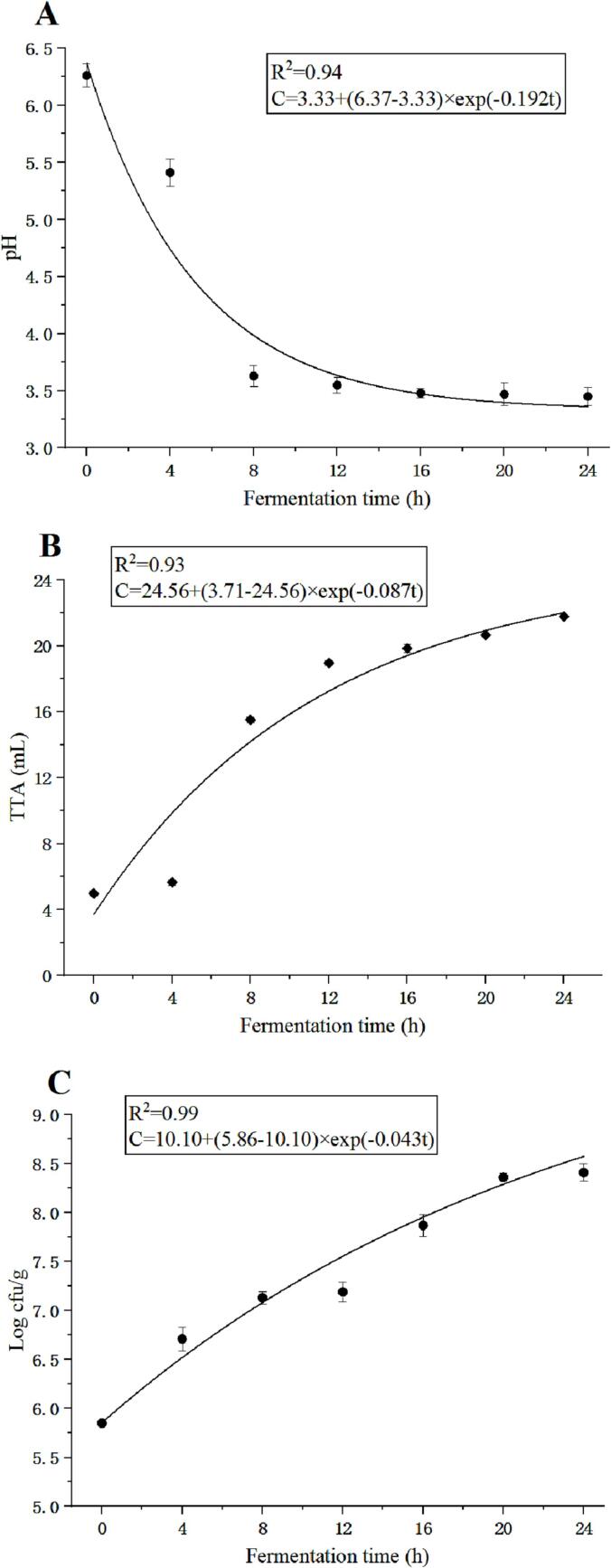
The changes in pH value represented the amount of strong acid produced by microbial metabolism during sourdough fermentation, while the TTA value represented the change in the total acidity of the sourdoughs (Zhou, She, Zhu, & Zhou, 2022). The results were shown in Fig. 1. The evolution of the pH value (R2 > 0.94), TTA (R2 > 0.93), and microbial profile (R2 > 0.99) during the fermentation of Tartary buckwheat sourdoughs could be accurately modeled using the first-order fractional conversion model. The rate of pH change (k = 0.192) was higher than that of the TTA value (k = 0.087). During the fermentation process, the pH value steadily decreased, with a rapid decrease in the initial 8 h followed by a slower decline. The initial pH value of Tartary buckwheat was 6.26, which significantly decreased to 3.45 after 24 h of fermentation. As shown in Fig. 1B, the trend of the TTA value was opposite to that of the pH value. Over time, the TTA value gradually increased from 4.98 mL to 21.77 mL. The changes in pH value and TTA value were associated with the dynamic succession of microorganisms and the production of organic acids during fermentation. In Fig. 1C, Pediococcus pentosaceus grew constantly during fermentation. After 4 h, the proliferation rate of microorganisms exceeded the rate of decrease after 12 h, further confirming the ease of Pediococcus pentosaceus growth in sourdoughs.
Fig. 1.
Changes in pH (A), TTA (B), and the microbial profile (C) of Tartary buckwheat sourdoughs during fermentation for 24 h. The full lines represent the fitted values by the kinetic modeling and the symbols represent the experimental data. The estimated parameters of the kinetic model were shown in the box.
SDS-PAGE and Western blot analysis
The SDS-PAGE results are presented in Fig. 2A, revealing the molecular weight range of unfermented Tartary buckwheat proteins to be approximately 10–27 kD. Among them, the aggregation bands between 15 kD–20 kD and near 27 kD were much stronger. The protein bands near 10 kD were lighter in color and narrower in width. It has been proved that 10 kD, 16 kD, 19 kD, and 67 kD in Tartary buckwheat can bind to the IgE of patients with buckwheat allergy and cause allergic reactions (Fujino et al., 2001, Heffler et al., 2010, Matsumoto et al., 2015, Park et al., 2000). The distribution of similar protein bands could be observed from the results of SDS-PAGE. It was also found that after Pediococcus pentosaceus fermentation for 16 h, the number of protein bands decreased and the color became lighter, indicating that most Tartary buckwheat proteins were degraded during fermentation, but there was no significant change with the increase of fermentation time.
Fig. 2.
SDS-PAGE analysis of Pediococcus pentosaceus fermented Tartary buckwheat protein (A) and Western blot of the fermented samples (B).
In order to evaluate the effect of Pediococcus pentosaceus fermentation on the allergenicity of Tartary buckwheat proteins, the changes in the lgE-binding ability of human sera from patients with buckwheat allergy were determined by Western blotting. After fermentation, the IgE-binding activity to about 15–20 kD allergen decreased significantly, while the 10 kD and 27 kD allergen decreased partially (Fig. 2B). The reason might be that Pediococcus pentosaceus fermentation degraded allergen proteins into small molecular peptides and amino acids through the proteolysis and acid mutagenesis, resulting in the destruction or concealment of allergen epitopes and the decrease of some allergens in Tartary buckwheat (Chizoba Ekezie, Cheng, & Sun, 2018). It can be concluded that Pediococcus pentosaceus fermentation can effectively degrade allergen protein and destroy allergen epitopes so as to reduce the allergenicity of Tartary buckwheat.
Identification of Tarary buckwheat polypeptides by HPLC-MS/MS
A total of 2042 peptides from Tartary buckwheat were identified in PP16 and UTB samples. The differentially expressed peptides were selected based on a multiple expression factor of R > 1.5 and p < 0.05, resulting in 507 upregulated peptides found in both UTB and PP16 samples. This indicated an increase in polypeptide content in PP16. Moreover, through peptide sequence analysis, we discovered 756 peptides associated with allergenic proteins, with 213 of them being upregulated and linked to allergenic proteins. It is speculated that these 213 peptide fragments contribute to the residual allergenicity of PP16.
The peptides obtained from PP16 and UTB were compared with the Uniprot database. The results indicated that these peptides likely originated from 13 s globulin, 13 s globulin seed storage protein, routinize, α-amylase inhibitor, 16 kD buckwheat allergy protein, and so on. These peptide fragments ranged in length from 8 to 25 amino acids and had molecular weights ranging from approximately 790 to 3000 Da. The peptides corresponded to proteins identified as A9NJG2, E9NX73, Q56CY3, K4PY05, and Q8W3Y9 (Table 1). A total of 729 peptide fragments were associated with A9NJG2, followed by 15 peptide fragments related to E9NX73. Notably, no peptides were found for Q8W3Y9, which could be attributed to the complete hydrolysis of the protein during Pediococcus pentosaceus fermentation. Among the 213 upregulated peptides, 207 peptides were derived from A9NJG2, indicating that it may be the primary allergen in Tartary buckwheat.
Table 1.
Tartary buckwheat allergen protein identification results.
| Protein IDs | Protein names | Protein sequence length/aa | Protein molecular weight/Da | Allergen protein | Protein domain | Protein domain location | Source |
|---|---|---|---|---|---|---|---|
| A9NJG2 | 11S Globulin | 515 | 58,356 | Fagt 1 | Cupin | 52–272/333–482 | Tartary buckwheat |
| E9NX73 | 2S Albumen | 149 | 17,280 | Fagt 2 | AAI | 68–139 | Tartary buckwheat |
| Q56CY3 | 2S Albumen | 127 | 14,612 | Fage 2 | – | – | Buckwheat |
| K4PY05 | 13S Globulin | 537 | 61,033 | Fage 1 | Cupin | 48–300/359–508 | Buckwheat |
| Q8W3Y9 | Starch inhibitor | 133 | 15,642 | Fage 10 kD | AAI | 27–123 | Buckwheat |
These peptides are derived from the main allergen proteins in buckwheat or Tartary buckwheat (Table 1). A9NJG2 corresponds to the 11 s globulin of Tartary buckwheat, known as Fagt 1. K4PY05 corresponds to the 13 s globulin of buckwheat, known as Fage 1. Eight IgE binding epitopes have been identified in Fage1 (Yoshioka, Ohmoto, Urisu, Mine, & Adachi, 2004). Studies have indicated that most food allergen proteins belong to the Cupin protein superfamily, including 11 s globulin and 7 s globulin. These proteins not only contain allergen epitopes that can trigger allergic reactions but also have specific biological functions. The protein structure is relatively stable, and its high-temperature tolerance and digestibility are also influenced by the protein structure (Yang, Li, Li, & Wang, 2012). By comparing the protein sequences, it is observed that Fage 1 and Fagt 1 share a high degree of homology and have more similar fragments, suggesting the possibility of cross-allergic reactions. Yang, Li, Li, and Wang (2012) obtained the corresponding derivatives by recombining Fagt 1 and found that modifying the spatial structure of Fagt 1 could identify its allergenicity. This indicates that Fagt 1 contains not only linear epitopes but also conformational epitopes.
E9NX73 and Q56CY3 are allergen proteins with a molecular weight of about 16kD in Tartary buckwheat and buckwheat, known as Fagt 2 and Fage 2. Fage 2 has a molecular weight of about 14.7kD and exhibits pepsin resistance. Additionally, the protein is associated with immediate hypersensitivity to buckwheat and may induce anaphylactic shock in allergic patients (Koyano, Takagi, Teshima, & Sawada, 2006). Fagt 2 is a Tartary buckwheat allergen protein consisting of 127 amino acids, sharing 50% homology with an 8kD allergen protein reported in the literature (Lee, Hong, Park, Choi, Sohn, & Lee, 2007). Epitope prediction reveals the presence of six allergen epitopes in Fagt 2, with each allergen epitope's key amino acid considered to be relevant to its allergenicity (Bei, Zhang, Lei, Guo, & Peng, 2018). The identified allergen epitopes and predicted epitopes for all allergenic proteins are presented in Table 2.
Table 2.
Identified allergen epitope information.
| Allergen protein | Allergen epitope sequence | Location of allergen epitope | Sequence length |
|---|---|---|---|
| Fage 1 | QNVNRPSR | 359–366 | 8 |
| NNLPILEF | 383–390 | 8 | |
| WNLNAH | 410–415 | 6 | |
| EGRSVF | 432–437 | 6 | |
| KAGREG | 459–464 | 6 | |
| IAGKTSVLRA | 481–490 | 10 | |
| KEAFRL | 505–510 | 6 | |
| SRDEKERERF | 525–543 | 10 | |
| Fagt 2 | EEKCLRGCCVAM | 80–91 | 12 |
| FIILATATLLIAATQAKY | 4–21 | 18 | |
| PELVKCNRY | 47–55 | 9 | |
| CVCEW | 96–102 | 7 | |
| LPNKCGI | 129–135 | 7 | |
| EALSRI | 67–72 | 6 | |
Note: The amino acid with red in the table is the key amino acid in the allergen epitope, which has an important effect on the allergenicity of buckwheat.
Prediction of residual allergenicity of Tarary buckwheat polypeptides
The prediction of residual allergenicity of Tartary buckwheat polypeptides was conducted based on the results of peptide identification and comparison with known allergen epitopes. Six residual peptides were found to coincide with the known allergen epitopes. These peptide fragments were primarily detected in the PP16 samples (Table 3), and they exhibited high protein identification scores (score ≥ 20). Among the identified residual peptides, four contained the QNVNRPSR fragments from Fage 1. The other two peptides included the linear epitope IFRVREGDV found in the TBb protein of Fagt 1 and the amino acid sequence 345–357 from the E1 region of the TBa protein in Fagt 1. However, this fragment does not contain the key amino acids in the E1 region.
Table 3.
Tartary buckwheat peptide screening results.
| Polypeptide sequence | Protein name | Sequence length/aa | Score | Experiment | Ratio (PP/UTB) | P value (PP/UTB) | |
|---|---|---|---|---|---|---|---|
| UTB | PP16 | ||||||
| FRQNVNRPSRADVFNPRAGRIN | Fage 1 | 22 | 108.71 | 2 | 2 | 125.255 | 0.00292 |
| FRQNVNRPSRADVFNPRAGRINT | 23 | 99.407 | 0 | 2 | – | 0.0189 | |
| FRQNVNRPSRADVF | 14 | 69.716 | 0 | 2 | – | 0.00113 | |
| FRQNVNRPSRADVFNPRAG | 19 | 66.335 | 0 | 1 | – | 0.374 | |
| IFRVREGDVIPS | Fagt 1 | 12 | 44.299 | 0 | 1 | – | – |
| NPRAGRINTVNSN | 13 | 39.424 | 0 | 1 | – | 0.0397 | |
Note: “-”the value cannot be calculated.
Furthermore, only one out of the six peptide fragments showed up-regulation, indicating that this particular peptide sequence is resistant to degradation. As the Western blot results revealed residual allergenicity in PP16, the six peptides were subjected to activity prediction using PeptideRanker (https://distilldeep.ucd.ie/PeptideRanker/). Considering a prediction threshold of ≥0.5 as the criterion for determining biological activity, only the peptide sequence FRQNVNRPSRADVFNPRAGRIN had a prediction threshold of 0.510, suggesting potential biological activity. The remaining sequences had prediction thresholds below 0.5. Therefore, the residual allergenicity observed in PP16 may be associated with the peptide fragment FRQNVNRPSRADVFNPRAGRIN.
During the fermentation process, protein degradation can lead to the exposure of hidden allergen epitopes or the production of new allergen proteins through microbial metabolism. Therefore, the peptide fragments present in PP16 were screened, and their biological activities were predicted. Excluding the peptide fragments listed in Table 3, six peptides with biological activity were identified (Table 4). It has been observed in studies that hydrophobic amino acids tend to be located in the interior of proteins, while hydrophilic amino acids are typically found on the surface of the protein. Allergen proteins, particularly water-soluble globulins, often have their surface composed of hydrophilic amino acid residues, and allergen epitopes are frequently distributed in these regions (Rougé, Culerrier, Sabatier, Granier, & Barre, 2009). This indicated a close relationship between the hydrophilic sites of the protein and antibody binding. Therefore, in addition to predicting the biological activity of these peptide fragments, the content of hydrophobic and hydrophilic amino acids in these fragments was also analyzed. The results presented in Table 4 demonstrated that, except for SNAPYITF, all six peptides contain a hydrophilic amino acid content of over 50%. Further experiments are required to determine whether the residual allergenicity in PP16 is associated with these peptide fragments.
Table 4.
Tartary buckwheat peptide activity prediction results.
| Peptide sequence | Allergen protein | Activity prediction threshold | Hydrophobic amino acid content | Hydrophilic amino acid content |
|---|---|---|---|---|
| SNAPYITF | Fagt 1 | 0.736 | 37.50% | 37.50% |
| IWDHNTPEF | 0.658 | 33.33% | 55.56% | |
| PSYSNAPYITF | 0.608 | 18.18% | 54.55% | |
| ADVFNPRAGRIN | 0.579 | 25.00% | 58.30% | |
| PSYSNAPYI | 0.526 | 11.11% | 55.56% | |
| YVIQPGGLLL | 0.524 | 20.00% | 70.00% |
Furthermore, the experiments revealed that, following Pediococcus pentosaceus fermentation, allergen proteins other than Fagt 1 are degraded, and the expression of polypeptides is down-regulated or rendered biologically inactive. This finding also highlighted the primary reason for the significant reduction in allergenicity observed in PP16.
Conclusion
Based on the results obtained from SDS-PAGE and Western blot analysis, the molecular weight distribution of Tartary buckwheat protein after PP16 fermentation was mainly concentrated in the range of 10 kD, 15–20 kD, and 27 kD, and a significant decrease in IgE-binding activity was observed. HPLC-MS/MS analysis of Tartary buckwheat peptides resulted in the identification of a total of 2042 peptides, among which 213 peptides were up-regulated and associated with allergen proteins. Fagt1 was identified as the primary allergen in Tartary buckwheat through protein identification. Upon screening these peptide fragments, it was found that only FRQNVNRPSRADVFNPRAGRIN contained the corresponding allergen epitope, and its expression was up-regulated. This suggested a potential association between the residual allergenicity in PP16 and this specific peptide fragment. Furthermore, the predicted biological activity thresholds of other peptides in PP16, namely IWDHNTPEF, PSYSNAPYITF, ADVFNPRAGRIN, PSYSNAPYI, and YVIQPGGLLL, were all above 0.5, indicating high activity, and their hydrophilic amino acid content was also notable. This may contribute to the overall allergenicity of PP16 and warrants further investigation through additional experiments. Additionally, apart from Fagt1, all allergen-related peptides in PP16 were either completely degraded or exhibited no biological activity, which serves as one of the main factors contributing to the reduced allergenicity of PP16. This study confirmed the potential of fermentation in reducing sensitization to Tartary buckwheat and provided new avenues for its processing and utilization. However, it is important to note that fermentation can also lead to the formation of new allergen epitopes, potentially enhancing sensitization to Tartary buckwheat. Therefore, caution should be exercised to avoid excessive pursuit of low allergenicity and over-fermentation, which may result in the loss of the original nutritional value of Tartary buckwheat or the development of unfavorable flavors. Furthermore, this study focused solely on the variable of fermentation time, and further optimization of fermentation conditions is necessary to achieve the optimal desensitization state.
CRediT authorship contribution statement
Yiming Zhou: Conceptualization, Writing – review & editing. Siyuan Yu: Investigation, Writing – original draft. Xuanming She: Data curation, Software. Xiaoli Zhou: Conceptualization, Project administration, Writing – review & editing, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Capacity-Building Project of Local Universities of SSTC (22010504000), the Shanghai Natural Science Foundation (20ZR1455800), and China Agriculture Research System (CARS-07-E-2).
Data availability
No data was used for the research described in the article.
References
- Abedfar A., Hosseininezhad M., Sadeghi A., Raeisi M., Feizy J. Investigation on “spontaneous fermentation” and the productivity of microbial exopolysaccharides by Lactobacillus plantarum and Pediococcus pentosaceus isolated from wheat bran sourdough. LWT. 2018;96:686–693. doi: 10.1016/j.lwt.2018.05.071. [DOI] [Google Scholar]
- Bei Z., Zhang H., Lei W., Guo Y., Peng C. Characterization of 16-kDa major allergen with α-amylase inhibitor domain in tartary buckwheat seeds. Molecular Immunology. 2018;94:121–130. doi: 10.1016/j.molimm.2017.12.024. [DOI] [PubMed] [Google Scholar]
- Carullo D., Donsì F., Ferrari G. Influence of high-pressure homogenization on structural properties and enzymatic hydrolysis of milk proteins. LWT. 2020;130 doi: 10.1016/j.lwt.2020.109657. [DOI] [Google Scholar]
- Chandrupatla C.V., Kundu R.V., Aronson I.K. Buckwheat allergy and atopic dermatitis. Journal of the American Academy of Dermatology. 2005;53(2):356–357. doi: 10.1016/j.jaad.2005.02.044. [DOI] [PubMed] [Google Scholar]
- Chizoba Ekezie F.-G., Cheng J.-H., Sun D.-W. Effects of nonthermal food processing technologies on food allergens: A review of recent research advances. Trends in Food Science & Technology. 2018;74:12–25. doi: 10.1016/j.tifs.2018.01.007. [DOI] [Google Scholar]
- Fujino K., Funatsuki H., Inada M., Shimono Y., Kikuta Y. Expression, cloning, and immunological analysis of buckwheat (Fagopyrum esculentum Moench) seed storage proteins. Journal of Agricultural & Food Chemistry. 2001;49(4):1825–1829. doi: 10.1021/jf0011485. [DOI] [PubMed] [Google Scholar]
- Geiselhart S., Nagl C., Dubiela P., Pedersen A.C., Bublin M., Radauer C.…Mortz C.G. Concomitant sensitization to legumin, Fag e 2 and Fag e 5 predicts buckwheat allergy. Clinical and Experimental Allergy. 2018;48(2):217–224. doi: 10.1111/cea.13068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbetti, M., & GNzle, M. (2013). Handbook on Sourdough Biotechnology || Sourdough and Cereal Beverages. 10.1007/978-1-4614-5425-0(Chapter 11), 265-278. https://doi.org/10.1007/978-1-4614-5425-0_11.
- Heffler E., Nebiolo F., Asero R., Guida G., Badiu I., Pizzimenti S.…Canaletti F. Clinical manifestations, co-sensitizations, and immunoblotting profiles of buckwheat-allergic patients. Allergy. 2010 doi: 10.1111/j.1398-9995.2010.02469.x. [DOI] [PubMed] [Google Scholar]
- Katayama S., Yamaguchi D., Suzuki Y., Al Athamneh A.M., Mitani T., Satoh R.…Nakamura S. Oral immunotherapy with a phosphorylated hypoallergenic allergen ameliorates allergic responses more effectively than intact allergen in a murine model of buckwheat allergy. Molecular Nutrition & Food Research. 2018;62(21) doi: 10.1002/mnfr.201800303. [DOI] [PubMed] [Google Scholar]
- Koyano S., Takagi K., Teshima R., Sawada J.I. Molecular cloning of cDNA, recombinant protein expression and characterization of a buckwheat 16-kDa major allergen. International Archives of Allergy & Immunology. 2006;140(1):73–81. doi: 10.1159/000092038. [DOI] [PubMed] [Google Scholar]
- Lee E.K., Hong C.S., Park J.W., Choi S.Y., Sohn J.H., Lee Y.W. Application of the 16-kDa buckwheat 2 S storage albumin protein for diagnosis of clinical reactivity. Annals of Allergy, Asthma & Immunology. 2007;99(3):254–260. doi: 10.1016/S1081-1206(10)60661-8. [DOI] [PubMed] [Google Scholar]
- Liu T., Li Y., Chen J., Sadiq F.A., Zhang G., Li Y., He G. Prevalence and diversity of lactic acid bacteria in Chinese traditional sourdough revealed by culture dependent and pyrosequencing approaches. LWT – Food Science and Technology. 2016;68:91–97. doi: 10.1016/j.lwt.2015.12.025. [DOI] [Google Scholar]
- Maruyama N., Sato S., Yanagida N., Cabanos C., Ito K., Borres M.P.…Ebisawa M. Clinical utility of recombinant allergen components in diagnosing buckwheat allergy. The Journal of Allergy and Clinical Immunology In Practice. 2016;4(2):322–323.e323. doi: 10.1016/j.jaip.2015.11.028. [DOI] [PubMed] [Google Scholar]
- Matsumoto R., Fujino K., Nagata Y., Hashiguchi S., Sugimura K. Molecular characterization of a 10-kDa buckwheat molecule reactive to allergic patients' IgE. Allergy. 2015;59(5):533–538. doi: 10.1046/j.1398-9995.2003.00412.x. [DOI] [PubMed] [Google Scholar]
- Montemurro M., Celano G., De Angelis M., Gobbetti M., Rizzello C.G., Pontonio E. Selection of non-Lactobacillus strains to be used as starters for sourdough fermentation. Food Microbiology. 2020;90 doi: 10.1016/j.fm.2020.103491. [DOI] [PubMed] [Google Scholar]
- Park J.W., Kang D.B., Kim C.W., Koh S.H., Lee K.Y. Identification and characterization of the major allergens of buckwheat. Allergy. 2000;55(11):1035–1041. doi: 10.1016/j.fochms.2022.100127. [DOI] [PubMed] [Google Scholar]
- Poms R.E., Klein C.L., Anklam E. Methods for allergen analysis in food: A review. Food Additives & Contaminants. 2004;21(1):1–31. doi: 10.1080/02652030310001620423. [DOI] [PubMed] [Google Scholar]
- Prandi B., Faccini A., Tedeschi T., Galaverna G., Sforza S. LC/MS analysis of proteolytic peptides in wheat extracts for determining the content of the allergen amylase/trypsin inhibitor CM3: Influence of growing area and variety. Food Chemistry. 2013;140(1):141–146. doi: 10.1016/j.foodchem.2013.02.039. [DOI] [PubMed] [Google Scholar]
- Rizzello C., De Angelis M., Coda R., Gobbetti M. Use of selected sourdough lactic acid bacteria to hydrolyze wheat and rye proteins responsible for cereal allergy. European Food Research and Technology. 2006;223:405–411. doi: 10.1007/s00217-005-0220-x. [DOI] [Google Scholar]
- Rougé P., Culerrier R., Sabatier V., Granier C., Barre A. Mapping and conformational analysis of IgE-binding epitopic regions on the molecular surface of the major Ara h 3 legumin allergen of peanut (Arachis hypogaea) Molecular Immunology. 2009;46(6):1067–1075. doi: 10.1016/j.molimm.2008.09.030. https://doi.org/molimm.2008.09.030. [DOI] [PubMed] [Google Scholar]
- Schatz M., Sicherer S.H., Zeiger R.S. The journal of allergy and clinical immunology: In practice 2017 year in review. The Journal of Allergy and Clinical Immunology In Practice. 2018;6(2):328–352. doi: 10.1016/j.jaip.2017.12.016. [DOI] [PubMed] [Google Scholar]
- Shang Z., Ye Z., Li M., Ren H., Cai S., Hu X., Yi J. Dynamics of microbial communities, flavor, and physicochemical properties of pickled chayote during an industrial-scale natural fermentation: Correlation between microorganisms and metabolites. Food Chemistry. 2022;377 doi: 10.1016/j.foodchem.2021.132004. [DOI] [PubMed] [Google Scholar]
- Shriver S.K., Yang W. Thermal and nonthermal methods for food allergen control. Food Engineering Reviews. 2011;3:26–43. doi: 10.1016/j.tifs.2018.01.007. [DOI] [Google Scholar]
- Sikder K., Kesh S.B., Das N., Manna K., Dey S. The high antioxidative power of quercetin (aglycone flavonoid) and its glycone (rutin) avert high cholesterol diet induced hepatotoxicity and inflammation in Swiss albino mice. Food & Function. 2014;5(6):1294–1303. doi: 10.1039/c3fo60526d. https://doi.org/1039/c3fo60526d. [DOI] [PubMed] [Google Scholar]
- Sinkovič L., Deželak M., Kopinč R., Meglič V. Macro/microelements, nutrients and bioactive components in common and Tartary buckwheat (Fagopyrum spp.) grain and stone-milling fractions. LWT. 2022;161 doi: 10.1016/j.lwt.2022.113422. [DOI] [Google Scholar]
- Song Y., Sun H., Xiao J., Wang F., Ding Y., Zhao J.…Wen A. Development of a liquid chromatography-tandem mass spectrometric (LC-MS/MS) method for simultaneous determination of epigallocatechin-3-gallate, silibinin, and curcumin in plasma and different tissues after oral dosing of Protandim in rats and its application in pharmacokinetic and tissue distribution studies. Journal of Pharmaceutical and Biomedical Analysis. 2019;170:54–62. doi: 10.1016/j.jpba.2019.03.024. [DOI] [PubMed] [Google Scholar]
- Sytar O., Biel W., Smetanska I., Brestic M. In: Buckwheat germplasm in the world. Zhou M., Kreft I., Suvorova G., Tang Y., Woo S.H., editors. Academic Press; 2018. Chapter nineteen - Bioactive compounds and their biofunctional properties of different buckwheat germplasms for food processing; pp. 191–204. [DOI] [Google Scholar]
- Yan B., Sadiq F.A., Cai Y., Fan D., Chen W., Zhang H., Zhao J. Microbial diversity in traditional type I sourdough and jiaozi and its influence on volatiles in Chinese steamed bread. LWT. 2019;101:764–773. doi: 10.1016/j.lwt.2018.12.004. [DOI] [Google Scholar]
- Yang N., Li Y.M., Zhang K., Jiao R., Ma K.Y., Zhang R.…Chen Z.-Y. Hypocholesterolemic activity of buckwheat flour is mediated by increasing sterol excretion and down-regulation of intestinal NPC1L1 and ACAT2. Journal of Functional Foods. 2014;6:311–318. doi: 10.1016/j.jff.2013.10.020. [DOI] [Google Scholar]
- Yang Z., Li Y., Li C., Wang Z. Synthesis of hypoallergenic derivatives of the major allergen Fag t 1 from tartary buckwheat via sequence restructuring. Food & Chemical Toxicology. 2012;50(8):2675–2680. doi: 10.1016/j.fct.2012.03.039. [DOI] [PubMed] [Google Scholar]
- Yoshioka H., Ohmoto T., Urisu A., Mine Y., Adachi T. Expression and epitope analysis of the major allergenic protein Fag e 1 from buckwheat. Journal of Plant Physiology. 2004;161(7):761–767. doi: 10.1016/j.jplph.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Zhang X.-Y., Chen J., Li X.-L., Yi K., Ye Y., Liu G.…Wang Z.-G. Dynamic changes in antioxidant activity and biochemical composition of tartary buckwheat leaves during Aspergillus niger fermentation. Journal of Functional Foods. 2017;32:375–381. doi: 10.1016/j.jff.2017.03.022. [DOI] [Google Scholar]
- Zheng B., Zhang H.N., Wang L., Guo Y.F., Chen P. Characterization of 16-kDa major allergen with alpha-amylase inhibitor domain in Tartary buckwheat seeds. Molecular Immunology. 2018;94:121–130. doi: 10.1016/j.molimm.2017.12.024. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Ouyang B., Duan M., Lv X., Zhou X. Biological characteristics of the gluten-free sourdough system fermented by Lactobacillus plantarum ST-III and its effect on dough quality and nutritional value during freezing. Food Chemistry: X. 2022;14 doi: 10.1016/j.fochx.2022.100350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., She X., Zhu S., Zhou X. The study of microbial diversity and volatile compounds in Tartary buckwheat sourdoughs. Food Chemistry: X. 2022;14 doi: 10.1016/j.fochx.2022.100353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F. Chemical composition and health effects of Tartary buckwheat. Food Chemistry. 2016;203:231–245. doi: 10.1016/j.foodchem.2016.02.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.