Graphical abstract
Keywords: Rice flour, Proteins, Lipids, Heat-moisture treatment, Physicochemical properties, Starch digestibility
Highlights
-
•
HMT promoted the binding between starch, protein and lipid.
-
•
The gelatinization viscosity of HMT rice flour was sharply increased when the proteins were removed.
-
•
Proteins played a critical role in the physicochemical changes of HMT rice flour.
Abstract
This study investigated the effect of removing proteins, lipids and starch on the structure, physicochemical properties and digestion properties of rice flour (with 30% moisture) treated with heat moisture treatment (HMT). According to the results, HMT caused the adhesion and agglomeration of the rice flour, promoted the binding between starch, protein and lipid molecular chains and led to the formation of complexes (especially starch-lipid complexes), which hindered the removal of non-starch components. Compared to the untreated rice flour, the HMT treated lipid-removal rice flour had small changes in their crystallinity, gelatinization temperature and viscosity property. After removing protein, the crystallinity, peak viscosity, final viscosity, breakdown and starch digestibility were sharply increased. In particular, the peak viscosity increased from 811 cP to 1746 cP and the enthalpy change increased from 5.33 J/g to 10.18 J/g. These findings are helpful in understanding the contribution of removing endogenous proteins and lipids to the physicochemical changes of HMT treated rice flour during its heating process and thus can be helpful in controlling the quality of rice flour through HMT.
Introduction
Rice, which is primarily composed of starch, protein, and lipids, is one of the vital staple foods for most people in the world. Featured by wide distribution, large output and consumption, high nutrient content, good tastiness and digestibility, rice can be steamed or cooked directly or processed to instant rice, instant rice flour or quick-freeze rice products. In addition, it can be processed to powders for making featured rice products such as rice noodles and rice steamed sponge cakes.
Starch is the key component of rice, and the molecular organization of starch granules and the arrangement mode of glucan chains in lamellar piling determine the physiochemical properties of starch (Vamadevan & Bertoft, 2015), which further leads to important significance in processing adaptability of rice and quality of rice products(Chun et al., 2015, Khatun et al., 2019). In addition to the structure of starch, the presence of non-starch components in grains, such as endogenous proteins and lipids, and the interactions between the molecules of these substances are important internal determinants of the physiochemical properties of starch. It is generally accepted that endogenous lipids and proteins inhibit the expansion of starch granules and limit the access of digestive enzymes to the underlying starch molecules by encapsulating the starch granules (Ding, Cheng, Lin, Wang, Wang, & Yu, 2021; J. Ye, Hu, Luo, McClements., Julian., Liang, et al., 2018). However, the latter lead to a more significant effect on the digestibility of starch (Ding, Cheng, Lin, Wang, Wang, & Yu, 2021). Recent results have thus accumulated that the starch granule-associated proteins (SGAPs) is valuable for the manipulation of starch microstructure. If SGAPs was removed, although it may not lead to significant influences on morphological structure of starch, it may lead to reduction in particle stability, rough surface, and may generate ridge-and-valley structures and larger pores, with increased opportunity and time to contact with water, enzyme and chemical reagents, it showed the trend of being easier to be hydrolyzed by enzyme as well as changes in pasting behaviors such as rapid swelling, reduced pasting temperature and increased pasting viscosity (Mengting. Ma, Chen, Zhou, Li, Sui, & Corke, 2021; M. Ma, Xu, Li, Sui, & Corke, 2020).
To facilitate modern industrial production and quality control of rice products, food enterprises often produce conveniently cooked rice products of squeezing type, steaming or cooking type or baking type that are popular in eaters with the raw materials of rice flour grinded from rice. However, rice flour of natural form often leads to difficulty in production and quality control of rice products due to properties such as low solubility, poor resistance to shearing and high retrogradation. Heat moisture treatment (HMT) is an important physical modification technology which is widely used and can effectively improve nature and technical characteristics of starch due to advantages of being easy to control, safety and environmental friendliness. HMT can promote changes in amorphous regions and crystalline regions at the premise of keeping integrity of granules to different degrees, so as to effectively change the fine structure, crystallinity, pasting behaviors, thermal stability, digestive ability, and ultimate application characteristics (Fonseca et al., 2021, Schafranski et al., 2021). According to previous research findings, HMT promotes a mutual effect between denatured proteins and that between denatured proteins and starch, to further give rise to influence on pasting behaviors, gel characteristics and thermal properties of starch in rice flour (Puncha-arnon & Uttapap, 2013).
Most the existing research focused on the effect of non-starch components on the physiochemical properties of grain starch after removing endogenous lipids or proteins from raw powders of grains of different sources or further removing SGAPs from grain starch (Ding et al., 2021, Tan and Kong, 2020, Yang et al., 2021; J. Ye, et al., 2018). In addition, recent research discussed in the changes in physiochemical properties of the starch with removed SGAPs after toughening treatment (Sun, Xu, Song, Ma, Zhang, Chen, et al., 2021). However, there has been seldom discussion on the interactive effect between starch, endogenous proteins and endogenous lipids in grains during the high moisture HMT process. Therefore, the removal of endogenous lipids, the removal of endogenous proteins or the removal of both of them were carried out in the natural rice flour and that with 30% high moisture HMT treatment in this research, to measure the microstructure, the crystal structure, the pasting behaviors, the thermal properties and the digestive performance, so as to elaborate the effect of non-starch components on controlling the physiochemical properties of rice flour during HMT.
Materials and methods
Materials and chemical reagents
Rice was purchased from Hunan Huali Food Co., Ltd. (Hunan, China). The grains were milled to 100-mesh. α-amylase and amyloglucosidase were purchased from Sigma-Aldrich Co. LLC (Santa Clara, USA). A glucose oxidase/peroxidase (GOPOD) used to determine glucose content was obtained from Megazyme International Ireland (Bray Business Park, Bray, Co. Wicklow, Ireland). Alkaline protease (200 U /mg), Nile red and Nile blue were purchased from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China). All of the other reagents were of analytical grade.
Samples preparation
For protein removal, alkaline protease (800 U / mL) was dispersed in 1 L carbonate buffer (0.02 M, pH 9.0). After centrifugation, the supernatant was added to 100 g rice flour. The suspension was then hydrolyzed in a water bath at 45℃ for 2.5 h with continuously stirring and centrifuged at 4000 r/min for 10 min. After removing the upper brown pellet, the solid residue was hydrolyzed again with alkaline protease using the same procedure, and the resulting residues were washed with distilled water to neutral PH. Finally, in order to obtain rice flour without protein (RF-P), the pellet was dried in an oven at 40℃ overnight and collected through a 100-mesh.
Lipid removal, according to the Soxhlet extraction method, RF (20 g) was packed into filter paper bags, placed in an extraction thimble, and extracted using petroleum ether (500 mL) in a Soxhlet extractor at 50℃ for 10 h. Repeat the degreasing treatment as described above. Finally, in order to obtain the rice flour without lipids (RF-L), the solid residue was dried in an oven at 40℃ overnight and collected through a 100-mesh.
The resulting RF-P sample is prepared with removal of lipids as described above to obtain the RF without protein and lipids (RF-L-P), which is collected.
For the extraction of normal rice starch (NRS), the RF is dispersed in sodium hydroxide solution at a concentration of 0.2% (w/v), placed in a magnetic stirrer for stirring for 1 h, then left at room temperature and the sodium hydroxide solution is replaced every 4 h for 24 h. The filtrate was centrifuged twice at 4000 g for 15 min each and the top layer of yellow matter was scraped off, and the precipitate was washed with distilled water to neutral pH and then dried in an oven at 40℃ for 12 h.
Heat moisture treatment
The moisture content was adjusted by adding water to obtain rice samples with moisture contents of 30% (w/w). The samples were mixed thoroughly during the addition of water. Rice was sealed in polyethylene pouches and kept undisturbed for 24 h at 4℃, and then placed in an oven at 110 °C for 2 h. Occasionally containers were shaken during heating for uniform heat distribution within rice flour. Treated rice flour samples were cooled in a desiccator and dried at 40℃ for 6 to 8 h, ground and sieved with 100-mesh to obtain the heat moisture treated rice flour (RF-H).
According to the abovementioned methods, samples that remove lipids, proteins, and both are prepared by RF-H samples and named RF-H-L, RF-H-P and RF-H-L-P respectively.
Heat-moisture modified starch is prepared by NRS in accordance with the above methods and is called NRS-H.
Basic component analysis
The content of crude protein and crude lipid were determined according to the AACC 46–13.01 and 30–25.01 methods(AACC, 2000), respectively.
Scanning electron microscopy (SEM)
A scanning electron microscope (JEOL, Tokyo, Japan) was used to observe the granule micrographs of the rice flour samples. Test parameters: voltage of 20 kV, magnification of 2000 ×.
Confocal laser scanning microscopy (CLSM)
The CLSM of the samples was determined using the method of Ding et al.(Ding, Cheng, Lin, Wang, Wang, & Yu, 2021) with minor modifications. Nile Blue (25 μL, 0.1% w/v in water) was dispersed in 1 mL of sodium acetate buffer (1% w/v, pH 5.6) added over the samples. Nile Red (0.1% w/v in ethanol) was dispersed in the suspension of all samples as described above. The stained samples were then placed on microscope slides and observed with a confocal laser scanning microscope (Leica TCSSP8, Heidelberg, Germany). Excitation wavelengths of 488 nm (argon laser) and 552 nm (He-Ne laser) were used to observe lipids (red) and proteins (green), respectively.
X-ray diffraction (XRD)
The crystalline structure of rice flour samples was evaluated using a powder X-ray diffractometer (Rigaku Corporation, Tokyo, Japan). Measurement parameters: voltage of 40 kV, current of 40 mA, 2θ range from 5° to 40° (5°/min). The relative crystallinity (RC) was analyzed by peakfit and XRD software to find the crystalline area (AC) and the amorphous area (Aa) respectively, and calculated according to the following equation
| (1) |
Fourier transform infrared (FT-IR) spectroscopy
The infrared spectral properties of samples were determined by FTIR (IRTracer-100, Shimadzu, Japan). Weigh the starch sample and KBr and grind them according to the ratio of 1: 100. The scanning wavelength range is 4000 ∼ 400 cm−1 with a resolution of 4 cm−1 by 64 scans.
Rapid visco analyzer (RVA)
The pasting properties of the rice flour samples were determined based on the method of Xiao et al (Xiao, Liu, Wei, Shen, & Wang, 2017). Pasting properties of rice samples were determined using an RVA (Perten, Stockholm, Sweden). Measurement procedure: The mixture of sample (3 g, db) and distilled water (25 mL) in an aluminum RVA tank was held at 50 °C for 1 min, heated to 95 °C and held at 95 °C for 2.5 min, then cooled to 50 °C and held at 50 °C for 2 min. Pasting temperature (PT) and peak viscosity (PV), breakdown viscosity (BD), setback viscosity (SB) and final viscosity (FV) were measured.
Differential scanning calorimetry (DSC)
The thermal properties of the samples were measured in triplicate using a Q2000 DSC (TA Instruments, New Castle, DE, USA). Samples (3 mg) were equilibrated with deionised water (9 μL) in a sealed aluminium dish at 25℃ for 24 h. Scans were then performed from 30 °C to 110 °C (10 °C/min) and the onset temperature (To), peak temperature (Tp), conclusion temperature (Tc) and enthalpy change (ΔH) were determined from each analysis using thermal data analysis software.
In vitro starch digestibility
Starch nutrition fractions were analysed according to the method of Englyst, Goux, Meynier, Quigley, Englyst, Brack, et al. (2018) with minor modifications. Enzyme solution containing porcine pancreas a-amylase and amyloglucosidase was prepared immediately before use. Rice flour (100 mg) was dispersed in 15 mL of sodium acetate buffer (0.2 mol/L, pH 5.2) by vortexing. The mixture was equilibrated at 37 °C for 5 min, and 5 mL of the enzyme solution containing porcine pancreatic α-amylase (450 U/mg) and amyloglucosidase (51 U/mL) was added. Then, the mixture was immersed in a 37 °C water bath at 160 rpm for hydrolysis. At intervals of 0, 5, 10, 20, 40, 60, 90, 120 and 180 min, the mixture (500 μL) is treated with absolute ethanol (4 mL) to inactivate the enzyme and then centrifuged at 4000 × g for 10 min. Then, the glucose content (GT) was analyzed by using Gopod kit, and its absorbance was measured at 510 nm. The hydrolysis rate (%) was calculated as follows:
| (2) |
The contents of RDS, SDS and RS were determined according to the time course of digestion using the following equations:
| (3) |
| (4) |
| (5) |
where, G20 and G120 represent the amounts of glucose released after 20 min and 120 min, respectively. FG is the free glucose content of starch, and TS is total starch weight (Chen, Jia, Miao, Zeng, Guo, Zhang, et al., 2018).
Statistical analysis
All tests were performed in triplicate for each test sample and the results were represented as the mean value ± standard deviations. All data were analyzed by one-way analysis of variance (ANOVA) using SPSS 28.0. Differences were examined at a significant level of 95% (p < 0.05).
Results and discussion
Sample compositions
The composition of the samples is shown in Table 1. Prior to HMT, the protein removal rate in rice flour treated with alkaline protease was 85.7%, which was lower than that of alkaline extraction starch at 90.6%. This is because it is difficult to remove the internal proteins that are tightly bound to the starch substrates with alkaline proteins or alkaline liquids, at the premise of leading to no damage to the structure of starch granules (Ye, Zhang, Qiu, Corke, & Sui, 2019). The result also indicates that the abandoning of the yellow substance on top of sediments during extraction of starch can greatly reduce the contents of proteins and lipids (Singh & Okadome, 2000). After HMT treatment, the removal rate of proteins from RF-H-P decreased to 80.4%, which may be due to the interactive effect between proteins and that between proteins and starch or lipids enhanced by HMT, which promoted protein denaturation, leading to hindered removal of non-starch components (Puncha-arnon & Uttapap, 2013).
Table 1.
Proximate compositions (g /100 g, dry weight), FTIR spectra analysis parameters and Digestibility parameters of HMT rice flour and native rice flour.
| Sample | Protein (%) | Fat (%) | FTIR R1022/995 cm-1 |
RDS (%) | SDS (%) | RS (%) |
|---|---|---|---|---|---|---|
| RF | 7.48 ± 0.03a | 0.99 ± 0.11a | 1.286 ± 0.007d | 18.70 ± 1.26f | 32.49 ± 1.26bc | 48.81 ± 0.30a |
| RF-L | 7.00 ± 0.12b | 0.20 ± 0.05de | 1.228 ± 0.019e | 20.92 ± 0.42e | 33.30 ± 1.99b | 45.78 ± 1.99b |
| RF-P | 1.07 ± 0.02 cd | 0.27 ± 0.06 cd | 1.470 ± 0.059b | 24.70 ± 0.95d | 28.28 ± 0.92de | 47.11 ± 0.95ab |
| RF-L-P | 1.04 ± 0.02 cd | 0.18 ± 0.01de | 1.476 ± 0.012b | 26.57 ± 0.96c | 27.99 ± 1.80de | 45.44 ± 1.80b |
| NRS | 0.70 ± 0.06d | 0.01 ± 0.00f | 1.579 ± 0.098a | 30.89 ± 0.91b | 26.64 ± 1.12e | 42.46 ± 1.12c |
| RF-H | 7.14 ± 0.33ab | 0.62 ± 0.04b | 1.126 ± 0.001f | 27.13 ± 1.74c | 36.81 ± 3.40a | 36.07 ± 3.40de |
| RF-H-L | 7.14 ± 0.09ab | 0.37 ± 0.10c | 1.090 ± 0.012f | 28.14 ± 1.65c | 36.77 ± 2.79a | 35.08 ± 2.79e |
| RF-H-P | 1.40 ± 0.12c | 0.17 ± 0.03de | 1.329 ± 0.014d | 31.45 ± 1.32b | 30.46 ± 0.00bcd | 38.09 ± 0.00d |
| RF-H-L-P | 1.33 ± 0.15c | 0.13 ± 0.05e | 1.332 ± 0.159d | 34.03 ± 1.24a | 29.53 ± 0.29cde | 36.44 ± 0.29de |
| NRS-H | 0.68 ± 0.12d | 0.01 ± 0.01f | 1.412 ± 0.001c | 34.43 ± 1.02a | 30.67 ± 2.09bcd | 34.91 ± 2.09e |
Means ± SD values followed by different lowercase letters in a column and different uppercase letters in the same column are significantly different (p < 0.05).
The lipid content of RF-H was reduced by 37.4% compared to RF, and the rate of lipid removal was reduced from 79.8% with HMT treatment to 40.3%. This may be caused by the compounds formed by mutual effect between free lipids and amylose or long branched chains of amylopectin promoted by HMT, which were removed or measured due to not dissolving in petroleum ether (Dhital, Brennan, & Gidley, 2019). In addition, the significant difference in lipid content between the RF-H-L with separate removal of lipids after HMT treatment and the RF-H-L-P with removal of both lipids and proteins further confirmed that the protein denaturation caused by HMT treatment significantly hindered the removal of lipids. The lower lipid content of RF-H-P and RF-H-L-P compared to that of RF-H-L indicated that HMT promoted the interaction between lipids and proteins, forming binary or ternary starch-protein-lipid compounds, and that this part of lipids was removed with the removal of proteins. The non-significant difference in protein content between RF-H-P and RF-H-L-P indicated that the changes in lipid state caused by HMT did not result in significant effects on proteins.
Granule morphology
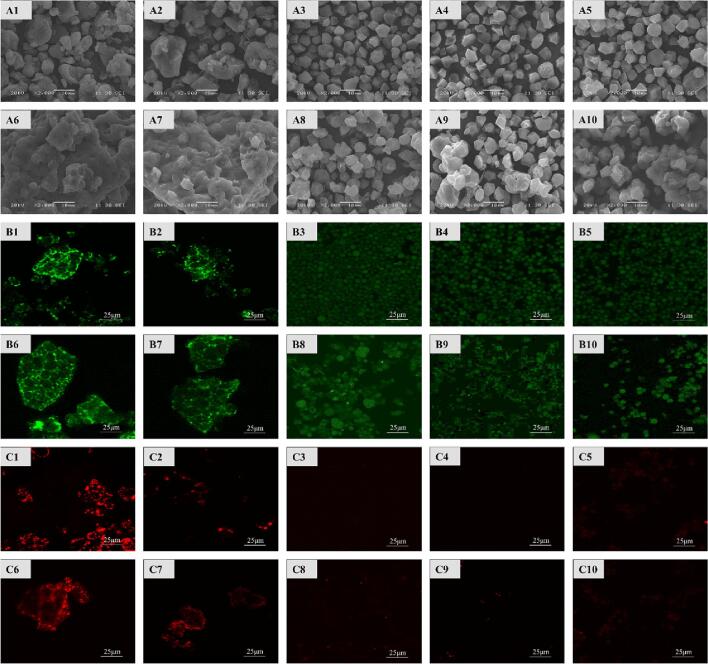
As shown in Fig. 1, RF showed typical compound amyloid granules consisting of piled granules of different sizes, irregular shapes and unsmooth surfaces with a crumbly structure. Removal of lipids (RF-L) significantly improved the aggregation of rice flour granules, and RF-P and RF-L-P with removed proteins showed polyhedral particle morphology close to NRS. The results indicated that most of the proteins that can be removed by protease or alkali liquid were substrates that starch granules were packed with, and the typical morphology of starch granules can be seen after removal, the same as the study results of Ye et al (Ye, et al., 2018). This showed that when proteins or lipids were removed, the shape of starch granules changed significantly. In addition, the removal of proteins had a significant influence on the distribution of starch granules than on that of lipids.
Fig. 1.
SEM images of starch granules at 2000 × magnification (A) and CLSM images of granules stained with Nile blue (B) and Nile red (C) (scale bars = 25 μm). 1, RF; 2, RF-L; 3, RF-P; 4, RF-L-P; 5, NRS; 6, RF-H; 7, RF-H-L; 8, RF-H-P; 9, RF-H-L-P; 10, NRS-H. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In all the samples with HMT modification, the surface of part of starch granules became rough, with pits and fissures, which may be caused by HMT, it was mechanical damages to starch granules. The agglomeration shape of RF-H and RF-H-L may be related to the fact that HMT treatment promoted a mutual effect between proteins and starch and proteins were further diffused on and adhere to the surface of starch granules due to their thermal denaturation. At the same time, the edge blurring and slight aggregation of starch granules in NRS-H may be related to slight pasting caused by slightly high content of moisture under the action of heat, as reported by Wang et al.(Wang, Liu, Chen, Li, Wang, & Xie, 2018).
CLSM
Fig. 1 showed the positions and mutual effects of endogenous proteins and lipids in samples and those of starch granules based on CLSM observations. Proteins were marked with green by Nile blue and lipids were marked with red by Nile red. According to Fig. 1B1 and Fig. 1C1, proteins and lipids were mainly distributed on the surface of RF starch granules, which may provide physical barriers for starch granules by forming dense protein substrates or forming binary compounds or ternary compounds around starch granules(Ding, Cheng, Lin, Wang, Wang, & Yu, 2021; J. Ye, et al., 2018). When proteins (Fig. 1B3, Fig. 1B4 and Fig. 1B5) or lipids (Fig. 1C2, Fig. 1C4 and Fig. 1C5) were removed, the corresponding fluorescent marks would be faded, indicating that these endogenous components had been effectively removed. The disperse distribution of starch granules in RF-P, RF-L-P and NRS was consistent with the SEM result, further confirming that proteins play an important role in maintaining the structure and stability of starch granules(Ding, Cheng, Lin, Wang, Wang, & Yu, 2021). It is worth noting that the 3 samples still showed very weak fluorescence signals, indicating the residue of a small amounts of proteins that cannot be removed by alkaline protease or alkaline liquids due to their tight embedding in the surface, channels and substrate of the granules.
The fluorescence signals of RF-H and RF-H-L marked by Nile blue after HMT modification were strengthened, which may be related to aggregated and increased starch granules and adhesion of proteins to the surface of starch granules after thermal denaturation (Fig. 1B1 and Fig. 1B6, Fig. 1B2 and Fig. 1B7). The fluorescence intensity of RF-H-P and RF-H-L-P was significantly reduced than the first two, and was slightly more significant than RF-P and RF-L-P, but with poor dispersion uniformity of the starch granules. In addition, more significant fluorescence signals of RF-H-L than RF-L may be caused by greater difficulty in removing lipids due to intensified aggregation of rice flour after HMT modification, as well as the formation of some starch-lipid compounds. There were also small amounts of unremoved lipids in RF-H-P and RF-H-L-P for the same reason. Such starch-lipid compounds may form an infusible membrane on the surface of starch granules to promote the hydrophobic property of starch granules, leading to influences on water swelling and pasting of starch as well as infusion of amylose(Dhital, Brennan, & Gidley, 2019).
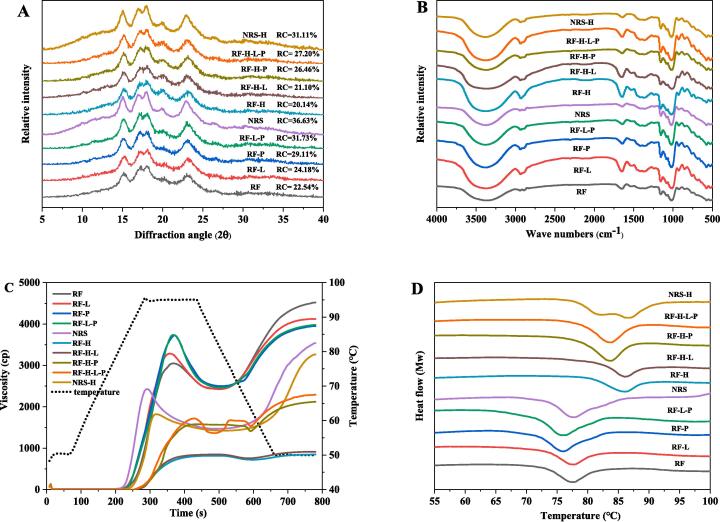
Crystalline structure
The X-ray diffraction diagram of rice flour was shown in Fig. 2A, and the samples had relatively strong diffraction peaks at the diffraction angle (2θ) of 15°, 17°, 18° and 23°, showing a typical A-type crystal structure. The small diffraction peaks appearing at about 20° (2θ) in the diffraction diagram of the unmodified samples were considered to be amorphous peaks formed by starch-lipid compounds, indicating the presence of starch-lipid compounds in natural starch, in agreement with the result of FT-IR. After removal of proteins and lipids, there was no deviation in the position of the feature diffraction peak of rice flour, indicating that the removal of non-starch components did not change the crystal type of rice flour. After removal of proteins and lipids, the RC in samples of the rice flour samples increased significantly (P < 0.05). This may be caused by a sharp decline in amylose in the amorphous region of starch and a rearrangement of the double helix structure of amylopectin after the removal of non-starch components(Israkarn, Na Nakornpanom, & Hongsprabhas, 2014). Yang et al. (Ding, Cheng, Lin, Wang, Wang, & Yu, 2021) found similar results, during the process of removal of non-starch components in adlay seed flour, the strength of all the peaks increased (except for 2 θ = 20°) (Yang, Jiao, Zhao, Liu, Fu, & Jin, 2021).
Fig. 2.
XRD (A), FTIR spectrum (B), RVA (C) and DSC (D) of HMT rice flour and native rice flour.
The samples after HMT still showed the A-type crystalline structure. However, the diffraction peaks in the starch-lipid compounds in the samples at 20° were more significant, indicating that HMT enhanced the combination of amylose and lipids. The decrease in relative crystallinity of rice flour after HMT was in agreement with the findings of earlier researches (Piecyk & Domian, 2021). This may be because that HMT increased the mobility of the double helix structure of amylopectin, and changed the orientation of the crystalline structure on starch granules and the relative crystallinity. Removal of proteins increased the intensity of the diffraction peak of 20°, indicating that the removal of proteins played positive roles in the formation of starch-lipid compounds. However, after the removal of lipids, the RC increased, indicating that HMT strengthened the binding of starch and lipid, resulted in the ordered molecular chain aggregation in amorphous amylose is more higher, made the double helices and A-type crystallite perfectly arranged, the bound lipids could not be removed during the removal process, and the crystallization characteristics of rice flour were also less affected(He, Zheng, Wang, Li, & Chen, 2020).
Short-range molecular structure determined by FTIR spectroscopy
All the samples showed almost similar Fourier infrared spectra (Fig. 2B). FT-IR of all the samples was measured within the spectrum section of 400–––4000 cm−1, in which the 960–––1060 cm−1 spectrum section was a specific band of starch caused by bending vibration of C-O and C-C, the narrow band at about 2850 cm−1 was mainly used for representing lipids, the bands at 1600–1700 cm−1 and about 1540 cm−1 were used for representing C O stretching vibration of protein peptide group and protein amide II band (Arslan, Akin, Karuk Elmas, Üner, Yilmaz, Janssen, et al., 2020). According to the figure, the peak of protein absorption of samples with removed proteins was weakened or disappeared at 1535 cm−1, indicating a decrease in protein content, which was mainly caused by bending vibration of stretching of ─NH and ─CN, the same as the research findings of Yang et al(Yang, Jiao, Zhao, Liu, Fu, & Jin, 2021). There was almost no change in the position of the absorption peak of the feature genes in samples before and after removal of lipids, indicating continuous existence of lipid compounds in starch. In addition, the position of the absorption peak of rice flour after heat-moisture modification moved to lower and wider bands, indicating a strengthened mutual effect between starch and other non-starch components in rice flour (Sun, Gong, Li, & Xiong, 2014).
In the FT-IR spectrum diagram, the feature diffraction peaks at 995 cm−1 and 1022 cm−1 were related to the ordered structure and amorphous structure rearranged in the starch molecules of the microscopic structure of starch. Therefore, the ratio of peak intensity of 1022 cm−1 and 995 cm−1 (R1022 cm−1/995 cm−1) was regarded as an indicator of a short-range ordered structure of starch(Warren, Gidley, & Flanagan, 2016). Based on the R1022cm−1/995cm−1 (Table 1) calculated from the FT-IR spectrum diagram, HMT significantly reduced the R1022 cm−1/995cm−1 (p < 0.05) of rice flour. However, after removal of proteins, R1022 cm−1/995 cm−1 showed a significant rise trend. It showed that HMT led to a reduced degree of short-range order of starch granules, with an almost disordered structure, and the presence of proteins damaged the short-range order of rice starch. Besides, the R1022 cm−1/995cm−1 of sample RF or RF-H increased significantly after the removal of proteins, which may be caused by the destruction of the original crystal arrangement mode by different treatment methods during sample preparation, leading to the priority of degradation or faster degradation rate in the amorphous region, and an increase in the degree of order of starch(Tang, Wang, Cheng, Wu, & Ouyang, 2019).
Pasting properties
The RVA spectrogram of all the samples was shown in Fig. 2C, and the corresponding pasting parameters were concluded in Table 2. According to the table, after the removal of proteins or lipids in the original rice flour, the starch “barriers” were correspondingly removed, accelerating the leaching of amylose during the pasting process, leading to a significant increase in the peak viscosity of RF-L and RF-P (p < 0.05). The influences caused on pasting property of starch from proteins were significantly higher than that of lipids, indicating that proteins in rice were primary reasons for difference in pasting property between rice flour and rice starch, in agreement with the research findings of Zhu et al (Zhu, Liu, Sang, Gu, & Shi, 2010). BD showed a tendency for swelling starch granules to collapse under continuous shear at high temperature(Saleh, 2017). The BD of RF-L and RF-P had a sharp rise than RF, increasing from 593 cP to 882 cP and 1213 cP respectively.
Table 2.
Pasting parameters of HMT rice flour and native rice flour.
| Characteristics | Pasting temperature (℃) | Peak viscosity (cP) | Breakdown (cP) | Setback (cP) | Final viscosity (cP) |
|---|---|---|---|---|---|
| RF | 84.85 ± 0.01de | 3048.00 ± 2.83c | 593.00 ± 25.46e | 2065.50 ± 17.68b | 4520.50 ± 4.95a |
| RF-L | 83.30 ± 0.00ef | 3339.50 ± 75.66b | 882.50 ± 31.82d | 1734.00 ± 56.57c | 4191.00 ± 100.41ab |
| RF-P | 84.50 ± 0.64de | 3712.00 ± 25.46a | 1213.50 ± 21.92b | 1447.50 ± 4.95d | 3946.00 ± 8.49b |
| RF-L-P | 84.10 ± 0.00e | 3811.00 ± 104.65a | 1307.50 ± 45.96a | 1515.50 ± 0.71d | 4019.00 ± 57.98b |
| NRS | 81.68 ± 0.004f | 2424.00 ± 9.90d | 976.00 ± 19.80c | 2072.00 ± 7.07b | 3520.00 ± 22.63c |
| RF-H | 93.80 ± 1.20ab | 811.50 ± 20.51f | 100.00 ± 14.14 h | 164.00 ± 7.07f | 875.50 ± 41.72e |
| RF-H-L | 94.68 ± 0.004a | 821.50 ± 13.44f | 99.00 ± 11.31 h | 168.50 ± 7.78f | 891.00 ± 32.53e |
| RF-H-P | 92.60 ± 1.77c | 1746.50 ± 249.61e | 363.00 ± 60.81 g | 982.50 ± 159.10e | 2366.00 ± 347.90d |
| RF-H-L-P | 91.45 ± 1.27c | 1881.50 ± 246.78e | 434.00 ± 15.56f | 1112.50 ± 150.61e | 2560.00 ± 381.84d |
| NRS-H | 86.18 ± 0.60d | 1823.00 ± 1.41e | 403.50 ± 6.36 fg | 1869.50 ± 38.89c | 3289.00 ± 33.94c |
Means ± SD values followed by different lowercase letters in a column and different uppercase letters in the same column are significantly different (p < 0.05).
The rice flour after HMT had significantly reduced peak viscosity and increased pasting temperature. It was related to generation of starch-lipid compounds during HMT process, which increased the thermal stability of rice flour granules and reduced leaching quantity of amylose, leading to reduction in viscosity of samples. In addition, during the HMT process, the mutual effect among starch molecular links was intensified, promoting tighter starch molecular structure, and such rigid structure and the starch-lipid compounds generated during HMT reduced the swelling capacity of starch, in line with the research findings of rice starch(Puncha-arnon & Uttapap, 2013). The reduction in BD indicated that the modified samples were stable during the continuous heating and shearing period. However, the rigidity result formed by rearrangement among starch chain molecules restrained the content of leached amylose in starch granules (Liu, Guo, Li, Wang, Lv, Peng, et al., 2015). The effect of lipid removal on the pasting properties of rice flour before and after HMT was not significant. However, the effect of protein removal after HMT on its FV and BD was more significant than that of natural rice flour, and the peak viscosity of RF-H-P samples was significantly lower than that of RF-P, which may be caused by the exposure of hydrophobic groups folded in protein molecules, and the surface hydrophobicity was increased due to protein denaturation in rice (Mine, 1997). The mutual effect between denatured proteins and that between proteins and starch granules led to a combination of protein network structure and starch particle surface. Such a protein layer was coordinated with increased hydrophobicity, delaying the swelling of HMT starch granules in rice flour. Therefore, under the same conditions, the pasting degree of RF-P was far lower than that of RF-H-P.
Thermal properties
The thermal properties of all the samples were shown in Fig. 2D, and corresponding thermal characteristic parameters were shown in Table 3. RF had a relatively high pasting temperature, indicating that it has a high anti-swelling capacity. RF-L had reduced pasting temperature, indicating reduced thermal stability, which was associated with reduced generation of starch-lipid compounds under thermal interaction. The pasting temperature of RF-P was slightly reduced because the proteins interacted with each other and by themselves or with starch on the surface of the starch granules, restraining the accessibility of water and increasing pasting temperature. At the same time, after the removal of proteins and lipids (RF-L-P), the TO value changed to a lower temperature, indicating poor thermal stability, which may be due to the fact that the starch granules without physical barriers of non-starch components promoted leaching of amylose, as well as the swelling and dissolution of starch granules, leading to a greater tendency to pasting of starch granules (Zhan, Ye, Zhang, Kong, Bao, Corke, et al., 2020).
Table 3.
Thermal properties of HMT rice flour and native rice flour.
| Samples | TO (℃) | TP (℃) | TC (℃) | TC-TO (℃) | ΔH (J/g) |
|---|---|---|---|---|---|
| 1st peak | |||||
| RF | 68.32 ± 0.00e | 77.07 ± 0.05e | 84.80 ± 0.16e | 16.48 ± 0.15de | 8.05 ± 0.21d |
| RF-L | 66.01 ± 0.38 g | 77.63 ± 0.17e | 83.98 ± 0.37ef | 17.97 ± 0.02bc | 8.36 ± 1.29 cd |
| RF-P | 66.74 ± 0.34 fg | 75.86 ± 0.09f | 84.41 ± 0.41ef | 17.67 ± 0.75 cd | 12.20 ± 0.05b |
| RF-L-P | 64.55 ± 0.23 h | 75.61 ± 0.06f | 83.55 ± 0.05f | 18.99 ± 0.18ab | 13.16 ± 0.87ab |
| NRS | 67.24 ± 13.96ef | 76.71 ± 6.22e | 87.42 ± 3.57d | 19.21 ± 3.59a | 13.96 ± 4.17a |
| RF-H | 77.77 ± 0.03b | 85.34 ± 0.04b | 91.35 ± 0.42b | 13.59 ± 0.45 g | 5.33 ± 0.75e |
| RF-H-L | 77.57 ± 0.02b | 86.21 ± 0.21ab | 92.58 ± 0.5a | 15.01 ± 0.52f | 4.87 ± 0.23e |
| RF-H-P | 73.95 ± 0.43 cd | 83.63 ± 0.08c | 89.35 ± 0.36c | 15.40 ± 0.78ef | 10.18 ± 0.11c |
| RF-H-L-P | 74.22 ± 0.16c | 83.44 ± 0.19 cd | 88.78 ± 0.16c | 14.56 ± 0.00 fg | 8.80 ± 0.82 cd |
| NRS-H | 72.96 ± 1.90d | 82.74 ± 3.99d | 84.07 ± 1.65ef | 11.11 ± 3.71 h | 1.90 ± 2.27f |
| 2nd peak | |||||
| NRS-H | 84.09 ± 1.68a | 86.92 ± 3.67a | 93.28 ± 1.25a | 9.19 ± 1.57i | 1.68 ± 2.52fe |
Means ± SD values followed by different lowercase letters in a column and different uppercase letters in the same column are significantly different (p < 0.05). RF: rice flour, RF-L: rice flour without lipids, RF-P: rice flour without protein, RF-L-P: rice flour without protein and lipids, NRS: normal rice starch, RF-H: rice flour after heat moisture treatment, RF-H-L: rice flour with lipids removed after heat moisture treatment, RF-H-P: rice flour with protein removed after heat moisture treatment, RF-H-L-P: rice flour with protein and lipids removed after heat moisture treatment, NRS-H: normal rice starch after heat moisture treatment.
The samples after HMT had significantly increased pasting temperature and reduced enthalpy value (ΔH), and the rise in pasting temperature were related to the increase in the integrity of starch granules caused by amylose–lipid compounds. This DSC trend was consistent with the research findings of Wang et al. (Wang, Liu, Chen, Li, Wang, & Xie, 2018), in addition, it was pointed out that ΔH was positively correlated with the amount of long-range and short-range ordered structures in starch molecules. Therefore, it can be speculated that the destruction in the double helix structure during HMT may be the reason for the reduced value of ΔH. Besides, HMT led the amylose and the amylopectin to achieve a mutual effect, promoting a reduction in the mobility of amylopectin and an increase in the thermal stability of starch granules(Sharma, Yadav, Singh, & Tomar, 2015). Removal of lipids after HMT hardly affected the pasting temperature compared to the significantly lower TO of RF-L in natural rice flour, and the removal of proteins after HMT resulted in a lower pasting temperature and more pronounced changes in the thermal properties brought about by RF-H-P compared to RF-P. It was indicating that the binding of proteins and lipids to starch changes during the moist heat treatment, with lipids binding more tightly to starch and proteins binding is weaker, which was because that hydrophilic groups and hydrophobic amino acid exposed by denatured proteins can promote forming of hydrogen bonds and hydrophobic interaction on the surface of starch granules, leading to increase in pasting temperature(Puncha-arnon & Uttapap, 2013). Under the same treatment conditions, RF-H showed a single-peak spectrogram, and starch after HMT showed a double-peak spectrogram with a significantly reduced pasting enthalpy value. This may be because that the very high moisture content in the samples led to partial pasting of rice flour, leading to greater nonuniformity of starch structure after HMT. Generally speaking, the initial pasting temperature of rice flour and rice flour after HMT was reduced by removing the non-starch components.
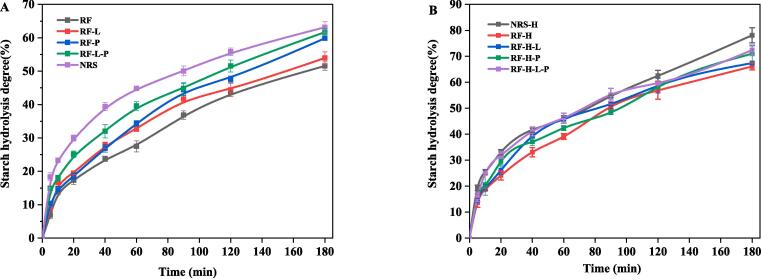
In vitro starch digestibility
Fig. 3 showed the in vitro digestibility curve of all the samples, with the corresponding digestibility parameters as shown in Table1. As shown in the Fig. 3A, the samples were digested rapidly within 20 min, and the increase in starch hydrolysis between 20 and 180 min was relatively slow. Within the first 60 min, the starch hydrolysis of RF-L was almost the same as that of RF-P; however, considering that the content of proteins in rice flour was about 7 times of that of lipids, it can be concluded that lipids may lead to greater influences on starch hydrolysis than proteins. This may be because the lipids on the surface of starch granules restrained the diffusion of moisture or prevented them from permeating into starch granules, leading to a limitation on the swelling of starch granules. At the same time, starch-lipid compounds can resist to erosion of amylase effectively, leading to a reduction in the sensitivity of starch digestive enzyme in the samples, in line with the research findings of Ye et al (Ye, et al., 2018). Removal of proteins significantly improved the in vitro digestibility of starch in rice, in addition, SEM and CLSM also showed that proteins can slightly accumulate around starch granules and formed physical barriers, which restrained the effect of the enzyme on starch granules. Removal of proteins and lipids improved the final hydrolysis, with RF-L-P achieving the highest level (61.7%), suggesting that non-starch components (proteins and lipids) may inhibit enzyme activity or interact with starch to create complex effects on the spatial arrangement, forming a protective layer leading to antagonism to a digestive enzyme (Khatun, Waters, & Liu, 2019).
Fig. 3.
Impact of proteins and lipids on the in vitro hydrolysis profiles of HMT rice flour (A) and native rice flour (B), RF: rice flour, RF-L: rice flour without lipids, RF-P: rice flour without protein, RF-L-P: rice flour without protein and lipids, NRS: normal rice starch, RF-H: rice flour after heat moisture treatment, RF-H-L: rice flour with lipids removed after heat moisture treatment, RF-H-P: rice flour with protein removed after heat moisture treatment, RF-H-L-P: rice flour with protein and lipids removed after heat moisture treatment, NRS-H: normal rice starch after heat moisture treatment.
The rice flour after HMT had significantly improved hydrolysis rate, which was because that slight pasting of part of the rice flour during HMT may lead to the destruction of the double helix crystal structure in starch, and gradual fracture of hydrogen bonds in non-crystal regions reduced the crystallinity of starch in rice and increased the sensitivity of α-amylase(Khatun, Waters, & Liu, 2019). In addition, the starch-lipid compounds generated during HMT can delay the crash of starch granules during pasting, to reduce the sensitivity to digestive enzyme, which was not related to the significance of the hydrolysis rate of RF-H-L than that of RF-H. The in vitro digestibility of samples protein removal was increased, which was related to the removal of the barrier formed by proteins. After HMT, the proteins are more encapsulated in the starch granules and the starch-protein interaction limits the hydrolysis of starch by α-amylase, while the structural stability of starch may lead to a reduction in RS. Other studies have shown that the removal of endogenous proteins may result in the change of RS to RDS (Ye, Zhang, Qiu, Corke, & Sui, 2019). It is worth noting that the starch hydrolysis rate spectrogram of the rice flour after HMT (Fig. 3B) showed narrower hydrolysis curves between samples compared to that of the original rice flour, which was due to the fact that the slight pasting of the rice flour during HMT had a more significant effect than other factors that led to influences on starch digestibility, such as the size of the rice flour granules and the additive effect of non-starch components.
Conclusions
According to the experiments, HMT showed significant changes in the microscopic structure of rice flour granules after HMT, with aggregated and adhered granules and significantly improved thermal stability and resistance to thermal decomposition. In addition, it was not easy to achieve pasting of the samples after HMT, with more stable pastes during continuous heating and shearing. The removal of the corresponding non-starch components (proteins and lipids) after HMT had a more significant effect on the physicochemical properties of the rice flour compared to the natural rice flour. The proteins, i.e., the firm barrier for starch pasting and digestibility, disappeared and starch granules appeared in an aggregating form, leading to an increase in swelling, degree of crystallinity, pasting enthalpy, peak viscosity, breakdown value and starch hydrolysis as well as a reduction in pasting temperature. In general, the mutual effect between non-starch components and starch molecules or between molecules had a major impact on the properties of rice flour after HMT.
CRediT authorship contribution statement
Guiyuan Xiang: Conceptualization, Data curation, Writing – original draft, Validation, Visualization. Jiangtao Li: Methodology, Validation, Investigation, Resources, Supervision, Project administration. Qinlu Lin: Writing – review & editing. Yili Zhang: Writing – review & editing, Software, Methodology. Yuqin Ding: Writing – review & editing, Software, Methodology. Xiaofeng Guo: Formal analysis, Investigation. Qianru Pan: Formal analysis, Investigation. Qiongxiang Liu: Formal analysis, Investigation. Xiangjin Fu: Methodology, Investigation. Ying Yang: Methodology, Investigation. Wenfang Han: Writing – review & editing, Project administration, Supervision, Funding acquisition. Yong Fang: Methodology, Investigation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research has been financially supported by the National Key R&D Program of China (Grant No. 2022YFD2101303), National Natural Science Foundation of China (Grant No. 31801497), the Scientific Research Fund of Hunan Provincial Education Department (Grant No. 21B0230) and Changsha Municipal Science and Technology Major Project (Grant No. kh2003013).
Contributor Information
Jiangtao Li, Email: ljthyd@163.com.
Wenfang Han, Email: hwfay@vip.163.com.
Data availability
Data will be made available on request.
References
- AACC. (2000). Approved methods of the American association of cereal chemists. In). St. Paul, MN, USA: AACC International.
- Arslan F.N., Akin G., Karuk Elmas Ş.N., Üner B., Yilmaz I., Janssen H.-G., Kenar A. FT-IR spectroscopy with chemometrics for rapid detection of wheat flour adulteration with barley flour. Journal of Consumer Protection and Food Safety. 2020;15(3):245–261. doi: 10.1007/s00003-019-01267-9. [DOI] [Google Scholar]
- Chen B., Jia X., Miao S., Zeng S., Guo Z., Zhang Y., Zheng B. Slowly digestible properties of lotus seed starch-glycerine monostearin complexes formed by high pressure homogenization. Food Chemistry. 2018;252:115–125. doi: 10.1016/j.foodchem.2018.01.054. [DOI] [PubMed] [Google Scholar]
- Chun A., Lee H.J., Hamaker B.R., Janaswamy S. Effects of ripening temperature on starch structure and gelatinization, pasting, and cooking properties in rice (Oryza sativa) Journal of Agricultural and Food Chemistry. 2015;63(12):3085–3093. doi: 10.1021/jf504870p. [DOI] [PubMed] [Google Scholar]
- Dhital S., Brennan C., Gidley M.J. Location and interactions of starches in planta: Effects on food and nutritional functionality. Trends in Food Science & Technology. 2019;93:158–166. doi: 10.1016/j.tifs.2019.09.011. [DOI] [Google Scholar]
- Ding, Y., Cheng, J., Lin, Q., Wang, Q., Wang, J., & Yu, G. (2021). Effects of endogenous proteins and lipids on structural, thermal, rheological, and pasting properties and digestibility of adlay seed (Coix lacryma-jobi L.) starch. Food Hydrocolloids, 111, 106254.10.1016/j.foodhyd.2020.106254.
- Englyst K., Goux A., Meynier A., Quigley M., Englyst H., Brack O., Vinoy S. Inter-laboratory validation of the starch digestibility method for determination of rapidly digestible and slowly digestible starch. Food Chemistry. 2018;245:1183–1189. doi: 10.1016/j.foodchem.2017.11.037. [DOI] [PubMed] [Google Scholar]
- Fonseca L.M., Halal S., Dias A.R.G., Zavareze E.D.R. Physical modification of starch by heat-moisture treatment and annealing and their applications: A review. Carbohydrate Polymers. 2021;274 doi: 10.1016/j.carbpol.2021.118665. [DOI] [PubMed] [Google Scholar]
- He H., Zheng B., Wang H., Li X., Chen L. Insights into the multi-scale structure and in vitro digestibility changes of rice starch-oleic acid/linoleic acid complex induced by heat-moisture treatment. Food Research International. 2020;137 doi: 10.1016/j.foodres.2020.109612. [DOI] [PubMed] [Google Scholar]
- Israkarn K., Na Nakornpanom N., Hongsprabhas P. Physicochemical properties of starches and proteins in alkali-treated mungbean and cassava starch granules. Carbohydrate Polymers. 2014;105:34–40. doi: 10.1016/j.carbpol.2014.01.054. [DOI] [PubMed] [Google Scholar]
- Khatun A., Waters D.L.E., Liu L. A Review of Rice Starch Digestibility: Effect of Composition and Heat-Moisture Processing. Starch - Stärke. 2019;71(9–10):1900090. doi: 10.1002/star.201900090. [DOI] [Google Scholar]
- Liu H., Guo X., Li W., Wang X., Lv M., Peng Q., Wang M. Changes in physicochemical properties and in vitro digestibility of common buckwheat starch by heat-moisture treatment and annealing. Carbohydrate Polymers. 2015;132:237–244. doi: 10.1016/j.carbpol.2015.06.071. [DOI] [PubMed] [Google Scholar]
- Ma M., Chen X., Zhou R., Li H., Sui Z., Corke H. Surface microstructure of rice starch is altered by removal of granule-associated proteins. Food Hydrocolloids. 2021;121 doi: 10.1016/j.foodhyd.2021.107038. [DOI] [Google Scholar]
- Ma M., Xu Z., Li P., Sui Z., Corke H. Removal of starch granule-associated proteins affects amyloglucosidase hydrolysis of rice starch granules. Carbohydrate Polymers. 2020;247 doi: 10.1016/j.carbpol.2020.116674. [DOI] [PubMed] [Google Scholar]
- Mine Y. Effect of Dry Heat and Mild Alkaline Treatment on Functional Properties of Egg White Proteins. Food Chemistry. 1997;45:2924–2928. doi: 10.1021/jf970158b. [DOI] [Google Scholar]
- Piecyk M., Domian K. Effects of heat-moisture treatment conditions on the physicochemical properties and digestibility of field bean starch (Vicia faba var. minor) International Journal of Biological Macromolecules. 2021;182:425–433. doi: 10.1016/j.ijbiomac.2021.04.015. [DOI] [PubMed] [Google Scholar]
- Puncha-arnon, S., & Uttapap, D. (2013). Rice starch vs. rice flour: differences in their properties when modified by heat-moisture treatment. Carbohydrate Polymers, 91(1), 85-91.10.1016/j.carbpol.2012.08.006. [DOI] [PubMed]
- Saleh M.I. Protein-starch matrix microstructure during rice flour pastes formation. Journal of Cereal Science. 2017;74:183–186. doi: 10.1016/j.jcs.2017.02.005. [DOI] [Google Scholar]
- Schafranski K., Ito V.C., Lacerda L.G. Impacts and potential applications: A review of the modification of starches by heat-moisture treatment (HMT) Food Hydrocolloids. 2021;117 doi: 10.1016/j.foodhyd.2021.106690. [DOI] [Google Scholar]
- Sharma M., Yadav D.N., Singh A.K., Tomar S.K. Effect of Heat-Moisture Treatment on Resistant Starch Content as well as Heat and Shear Stability of Pearl Millet Starch. Agricultural Research. 2015;4(4):411–419. doi: 10.1007/s40003-015-0177-3. [DOI] [Google Scholar]
- Singh V., Okadome H. Thermal and Physicochemical Properties of Rice Grain, Flour and Starch. Food Chemistry. 2000;48:2639–2647. doi: 10.1021/jf990374f. [DOI] [PubMed] [Google Scholar]
- Sun L., Xu Z., Song L., Ma M., Zhang C., Chen X.…Corke H. Removal of starch granule associated proteins alters the physicochemical properties of annealed rice starches. International Journal of Biological Macromolecules. 2021;185:412–418. doi: 10.1016/j.ijbiomac.2021.06.082. [DOI] [PubMed] [Google Scholar]
- Sun Q., Gong M., Li Y., Xiong L. Effect of dry heat treatment on the physicochemical properties and structure of proso millet flour and starch. Carbohydrate Polymers. 2014;110:128–134. doi: 10.1016/j.carbpol.2014.03.090. [DOI] [PubMed] [Google Scholar]
- Tan, L., & Kong, L. (2020). Starch-guest inclusion complexes: Formation, structure, and enzymatic digestion. Critical Reviews in Food Science and Nutrition, 60(5), 780-790.10.1080/10408398.2018.1550739. [DOI] [PubMed]
- Tang, M., Wang, L., Cheng, X., Wu, Y., & Ouyang, J. (2019). Non-starch constituents influence the in vitro digestibility of naked oat (Avena nuda L.) starch. Food Chemistry, 297, 124953.10.1016/j.foodchem.2019.124953. [DOI] [PubMed]
- Vamadevan V., Bertoft E. Structure-function relationships of starch components. Starch-Stärke. 2015;67(1–2):55–68. doi: 10.1002/star.201400188. [DOI] [Google Scholar]
- Wang H., Liu Y., Chen L., Li X., Wang J., Xie F. Insights into the multi-scale structure and digestibility of heat-moisture treated rice starch. Food Chemistry. 2018;242:323–329. doi: 10.1016/j.foodchem.2017.09.014. [DOI] [PubMed] [Google Scholar]
- Warren F.J., Gidley M.J., Flanagan B.M. Infrared spectroscopy as a tool to characterise starch ordered structure-a joint FTIR-ATR, NMR, XRD and DSC study. Carbohydrate Polymers. 2016;139:35–42. doi: 10.1016/j.carbpol.2015.11.066. [DOI] [PubMed] [Google Scholar]
- Xiao Y., Liu H., Wei T., Shen J., Wang M. Differences in physicochemical properties and in vitro digestibility between tartary buckwheat flour and starch modified by heat-moisture treatment. LWT - Food Science and Technology. 2017;86:285–292. doi: 10.1016/j.lwt.2017.08.001. [DOI] [Google Scholar]
- Yang Y., Jiao A., Zhao S., Liu Q., Fu X., Jin Z. Effect of removal of endogenous non-starch components on the structural, physicochemical properties, and in vitro digestibility of highland barley starch. Food Hydrocolloids. 2021;117 doi: 10.1016/j.foodhyd.2021.106698. [DOI] [Google Scholar]
- Ye J., Hu X., Luo S., McClements, Julian D., Liang L., Liu C. Effect of endogenous proteins and lipids on starch digestibility in rice flour. Food Research International. 2018;106:404–409. doi: 10.1016/j.foodres.2018.01.008. [DOI] [PubMed] [Google Scholar]
- Ye X., Zhang Y., Qiu C., Corke H., Sui Z. Extraction and characterization of starch granule-associated proteins from rice that affect in vitro starch digestibility. Food Chemistry. 2019;276:754–760. doi: 10.1016/j.foodchem.2018.10.042. [DOI] [PubMed] [Google Scholar]
- Zhan Q., Ye X., Zhang Y., Kong X., Bao J., Corke H., Sui Z. Starch granule-associated proteins affect the physicochemical properties of rice starch. Food Hydrocolloids. 2020;101 doi: 10.1016/j.foodhyd.2019.105504. [DOI] [Google Scholar]
- Zhu L., Liu Q., Sang Y., Gu M., Shi Y. Underlying reasons for waxy rice flours having different pasting properties. Food Chemistry. 2010;120(1):94–100. doi: 10.1016/j.foodchem.2009.09.076. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.