Highlights
-
•
EWP-based films were incorporated with EGCG to improve their antioxidant properties.
-
•
EGCG promoted the crosslinking of egg white protein-based films.
-
•
EWP-EGCG composite films can act as an environment friendly material in food packaging.
-
•
EWP-EGCG films could be applied in the preservation of chilled pork.
Keywords: Egg white protein film, Epigallocatechin gallate, Structural properties, Antioxidant activities, Biodegradability
Abstract
The aim of this study was to develop the composite films with antioxidant and biodegradable activity based on egg white protein (EWP) and epigallocatechin gallate (EGCG). Water susceptibility, light transmittance, microstructure and antioxidant properties of the composite films without and with EGCG were fully characterized. It was noted that the addition of EGCG might decrease the moisture content, water solubility and swelling capacity. SEM micrographs revealed that discontinuous blocks and rough surfaces were caused by increasing concentration of EGCG, whereas compact and homogeneous particles appeared when the concentration of EGCG reached to 80 μmol/L. Moreover, the biodegradability of the composite films was demonstrated by the soil degradation properties that they can be almost completely degraded within ten days. Experimental results on the application in chilled fresh pork showed that the EWP-based films could play an antioxidant role when incorporated with EGCG, indicating their great potential for food packaging.
Introduction
In recent years, people’s requirements for the environment have been continuously increasing, and the demands of ordinary consumers for food safety have also sharply increased. At present, a vast range of traditional polymer packaging materials, including polyethylene, polypropylene and polyamide, et al have been introduced in several scientific studies (Dai, et al., 2022). However, as plastic packaging materials, they are unsafe, non-environmental protection and difficult to decompose, whilst might be harmful to human health for long-time usage (Oliveira Filho et al., 2022), introducing to the plastic restriction for a period of time. In order to solve the problems induced by the plastic packaging materials, natural and sustainable materials could be created due to their biodegradability, biocompatibility and non-toxicity. Therefore, research of edible film packaging has been paid attention to and become a new research hotspot (Sun, Huang, Chen, Liu, & Leng, 2023).
As a typical kind of animal protein, egg white protein (EWP) possesses good film-forming ability. The components in EWP, i.e., ovalbumin, ovomucoid, ovomucin, ovotransferrin and lysozyme are natural antimicrobial substances (Deng, Xu, Hu, & Sheng, 2022). Besides, EWP can be used in food, pharmaceutical and cosmetic industries due to its excellent biodegradability. Liu, Huang, Geng, and Huang (2021) illustrated that the composite film prepared with EWP and k-carrageenan could be used as a new edible film in food packaging fields. Peng et al. (2017) proved that EWP-based films were much more transparent than the other protein films, e.g., soybean protein isolate film, whey protein film and pea protein isolate film (Rojas-Lema et al., 2023, Xiao et al., 2023). However, the mechanical properties and water susceptibilities of EWP films were reported to be poor, leading to a limited application in food packaging. In view of these two biggest shortcomings, some measures could be taken to improve their properties, including mechanical properties and functional properties. The physical–chemical characteristics of EWP-based films could be improved by the addition of glycerol as their plasticizer. Excessive moisture intake was illustrated to be easy to result in serious microbial contamination and a certain degree of oxidation in packaged food. In this sense, different kinds of bacteriostatic agents and antioxidants could be added to the films to improve their functional characteristics.
Polyphenols are recognized as good antioxidants and can be safely used in food materials since numerous researches have evaluated protein-based edible films which are enriched with polyphenols (Sun et al., 2022, Zhao et al., 2023). For example, the whey protein films activated with rosemary and sage extract were developed to keep their antioxidant activities and protect the soft cheese from spoilage (Kontogianni et al., 2022). Their prepared protein-based films were illustrated to own a layer to protect the cheese from oxygen of the environment. In yet another example, Wang et al. (2022) reported that the antioxidant properties of the chitosan/zein protein composite films were effectively improved with the incorporation of tea polyphenol. It was demonstrated that the zein protein-chitosan films had potential ability for maintaining the postharvest quality of mushroom.
In this article, epigallocatechin gallate (EGCG) was chosen as the antioxidant agent to cooperate with EWP in preparing films, aiming to characterize the antioxidant characteristics of EWP-EGCG films and verify their effects in fresh chilled meat preservation. In addition, the biodegradable properties of the EWP-EGCG films were determined in order to expand their application in the field of food in the future. Therefore, this article might provide useful information in preparing natural, degradable and antioxidant protein-based packaging materials in preservation of fresh chilled meat.
Materials and methods
Materials
Egg white powder was provided by Jiangsu Kangde Egg Industry Co., Ltd. (Nantong, Jiangsu, China). Glycerin, sodium hydroxide and hydrochloric acid were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Epigallocatechin gallate (EGCG), Trichloroacetic acid (TCA), boric acid, 1,1-diphenyl-2-trinitrophenylhydrazine (DPPH) and 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulphonate) (ABTS) were supplied by McLean Reagent Company. Fresh pork was obtained from New World Department Store (Yantai, China). Other chemicals used in this study were purchased from Sigma-Aldrich (St. Louis, MO, USA). All reagents used were analytically purified and the solvent used was Milli-Q water (Purified using Milli-Q apparatus, Millipore Corp Bedford, MA, USA).
Preparation of EWP-EGCG composite films
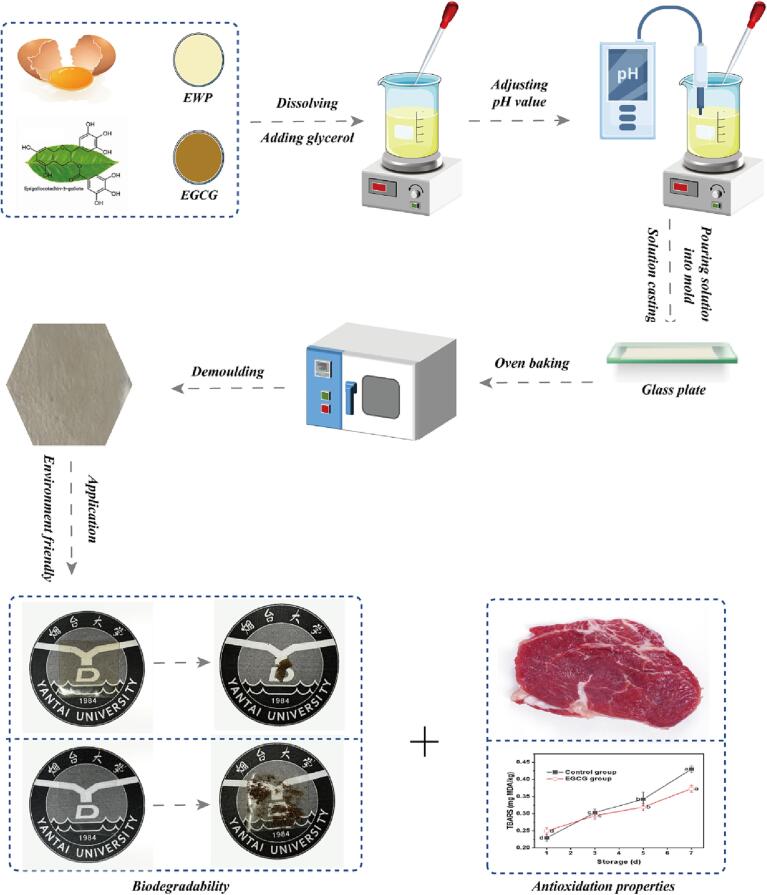
To obtain EWP solution, eight grams of egg white powder were added to 100 mL of deionized water at room temperature. The powder was allowed to dissolve for 3 h by magnetically stirring at a speed of 500 r/min. Then, the pH of the mixture was adjusted to 12.5. EGCG solutions were prepared by dissolving different mole numbers (0 μmol, 5 μmol, 10 μmol, 20 μmol, 40 μmol and 80 μmol) in 1 L 0.05 M Tris-HCl (pH 7.0) for 20 min with continuous stirring at room temperature. Thereafter, the EGCG solutions were gradually added to the EWP solution with continuous stirring to obtain mixed solution, followed by addition of the plasticizer (glycerin, 2.0%, w/v). The EWP-based films with 0% EGCG was prepared as a control. Film-forming solutions were heated in a water bath at 60℃ for 30 min with constant stirring and then ultrasonicated for 5 min to remove air bubbles. For preparation of the films, portions (30 mL) of the above solutions were cast onto glass plates (diameter: 12.5 cm) and dried for approximately 3 h at 60℃ in the drying-oven. The dried films were peeled off from the plates and equilibrated at 25℃±2℃ and 50%±5% relative humidity (RH) for at least 48 h before further analyses.
Film thickness
Film thickness was measured by a hand-held micrometer (211–101 k, SanLiang, Japan) with precision of 0.001 mm, as described by the previously published paper (Li, Liu, Ye, Wang, & Wang, 2015). Ten points of each film were randomly taken for measuring and thickness was calculated using the average value.
Water vapor permeability
Water vapor permeability (WVP) was conducted using gravimetric method (Wang, Liu, Ye, Wang, & Li, 2015) with some modifications. Each film was cut to 20 mm diameter, and fixed onto the suitable testing cup containing anhydrous calcium chloride with ∼ 0% RH, the initial weight was reported. Thereafter, the testing cups were transferred into a desiccator which was saturated with NaCl (75% RH) solution. The final weight was tested after 24 h at 25 ℃, changes in the weight of the testing cups were measured at intervals during the first 8 h and up to 24 h. WVP (g m−1 s−1 Pa−1) of the films was calculated as follows:
where Δw = the weight change of cup (g), L = thickness of the film (m), t = the time (s), A = the measuring area (m2), and Δp = the water vapor pressure difference across the two sides of the film (2376.39 Pa).
Water susceptibility
Equilibrated films were cut into 40 mm diameter (m0) and dried at 110℃ until constant weight (m1). The dried films were immersed in distilled water (40 mL) for 24 h at 25℃, then the films were weighted (m2) after wiping off the remaining water on the films with a filter paper. The remaining samples were dried again in an oven at 110℃ until they reached a final constant weight (m3). The moisture content (MC), water solubility (WS) and swelling capacity (SC) of each film were calculated as follows (Liu et al., 2023):
| (1) |
| (2) |
| (3) |
Optical properties
Color properties
The color properties, including Lightness (L*), redness-greenness (a*), yellowness-blueness (b*) and total color difference (ΔE) of the films were measured using a colorimeter (CS-800, 400-700n, Hangzhou, China) at 25℃. The total color difference (ΔE) was calculated as follows:
| (4) |
Light transmittance and opacity
Light transmittance of the films was recorded in the wavelength range of 600 nm by a UV–visible spectrometer (UV-2550, Shimadzu, Japan) according to a method described previously with minor modification (Moalla et al., 2021). The transparency (T%) and opacity of the films was defined with the following formula, respectively:
| (5) |
| (6) |
where A600 was the absorbance of the composite film, d was the thickness of the laminated film (mm).
Fourier transform infrared spectroscopy (FTIR)
FTIR spectroscopy was employed to analyze the chain interactions into films, the spectra were obtained in the region 4000 to 400 cm−1 at a resolution of 1 cm−1, using an IS50 spectrometer (Thermo Nicolet, Madison, Wisconsin, America).
Scanning electron microscopy (SEM)
The composite films were observed by a scanning electron microscope (SEM, JSM-IT 300 LV, Akishima, Tokyo, Japan) in order to study the microstructures. Prior to observation, samples were gold-plated with a vacuum sputtering coater. Thereafter, each sample was transferred to the cold stage of the SEM chamber and captured using an acceleration voltage of 20 kV.
Antioxidant activities
DPPH free radical-scavenging activity
The antioxidant activity of the EWP-EGCG composite films could be determined using DPPH free radical scavenging assay according to the method described by Kandi and Charles (2019) with some modifications. 1 g of each film was added into 100 mL deionized water, dissolving by stirring for 24 h at room temperature. The 0.06 mol/L DPPH was prepared by dissolving DPPH in the ethanol solution. Then, 2 mL sample solutions and 2 mL DPPH solutions were mixed and incubated for 30 min at room temperature in the dark. Control tubes were assessed in the same way without film samples. The absorbance values were measured at 517 nm with a visible spectrophotometer and calculated as follows:
| (7) |
where RDPPH was DPPH radical scavenging rate, A1 represented the absorbance of DPPH solution without addition of the film samples at 517 nm and A0 represented the absorbance of DPPH solution containing the film samples at 517 nm.
ABTS radical scavenging assay
For the ABTS assay, ABTS solution (7 mM) was mixed with potassium sulfate (2.4 mM) at a ratio of 2:1 (v/v) and stored in the dark at room temperature for 12 h. Then, the ABTS solution was diluted with distilled water to achieve an absorbance of 0.7 (±0.1) at 734 nm. Similar to the DPPH assay, approximately 2 mL film sample solution was placed in 5 mL of the ABTS solution in a dark location for 30 min, and then the film sample was removed to record the absorbance of the ABTS solution (Lee, Gwak, Chathuranga, Lee, Koo, & Park, 2023). The ABTS scavenging activity of all the samples was determined using the following formula:
| (8) |
where RABTS was ABTS radical scavenging rate, Ai represented the absorbance of ABTS solution without addition of the film samples at 734 nm and Af represented the absorbance of ABTS solution containing the film samples at 734 nm.
Degradability experiment
In order to determine their biodegradability, the antioxidant films were cut into squares pieces (50 mm × 50 mm) and buried in the soil to observe the degradation results. Meanwhile, the polyethylene membrane (50 mm × 50 mm) was taken as a control. All samples were dug out for photos each two days (0 d, 2 d, 4 d, 6 d, 8 d and 10 d).
Preservation experiment
The preservation experiment was performed as described in a previous study with some modification (Zhong, Hou, Li, Yang, Shu, & Wu, 2021). Fresh lean pork was cut into regular pieces, packed with the composite films and placed in the fresh-keeping cabinet (Baixian, BSC1200-2508, China) at 4℃, regarded as the experiment group (EGCG group). Simultaneously, the pork was wrapped with food-grade polyethylene (PE) plastic bag and was regarded as the control group. All samples were stored at 4℃ for seven days and pH values, total volatile basic nitrogen (TVB-N) and thiobarbituric acid reactive substances (TBARS) were analyzed in order to determine the antioxidant effects of the composite films during their storage, i.e., the first day, the third day, the fifth day and the seventh day.
Determination of pH values
The chilled pork (10.0 g) after storage was cut into small pieces and homogenized with potassium chloride solution (0.1 mol/L) for 2 min, and then measured by pH meter (S220-Micro, Mettler Toledo, Germany).
Determination of TVB-N
TVB-N was characterized based on the Chinese standard GB 5009.228–2016. In simple terms, the chilled pork (about 10.0 g) was cut into small pieces and submerged in the ultrapure water (100 mL), shaking for 30 min and then filtered. 10 mL filtrate was mixed with 5 mL of 10 g/L magnesia suspension and diluted for 5 min, and the same volume of deionized water was used as the control group. The distillate was collected in a conical flask containing 10 mL boric acid (20 g/L), and then titrated with the hydrochloric acid (0.01 mol/L) to the end point (Zhang et al., 2023). The amount of TVB-N was calculated as following equation:
| (9) |
where V1 and V2 were the titration volumes (mL) for the tested samples and the control group, respectively, c was the concentration of hydrochloric acid (mol/L), m was the accurate weight of the chilled meat (g).
Determination of TBARS
The antioxidant effect of the prepared composite films on lipid could be expressed by the determination of TBARS, given by Chinese standard GB 5009.181-2016. Detail methods were also reported by Zeng, et al. (2023). Each 5.0 g sample was homogenized with 45 mL of 7.5% trichloroacetic acid solution for 30 min and filtered by Whatman No. 1 filter water. Afterwards, 5 mL of the filtrate was added with 5 mL thiobarbituric acid solution (0.02 mol/L) and pipetted into a 25 mL colorimetric tube with a stopper. It was heated at 90 ℃ for 30 min and cooled to room temperature. The absorbance was measured at 535 nm using a spectrophotometer and the TBARS values were expressed in terms of mg malondialdehyde/kg meat.
Statistical analysis
The flow chart of sample preparation and determination is shown in Fig. 1. All experiments were performed in triplicate and the significance level of the differences between means was evaluated at p < 0.05. SPSS software version 20 was used to analyze the obtained results by subjecting to one-way analysis of variance (ANOVA). The results were reported by drawing with software Origin 2019b.
Fig. 1.
Flow chart of sample preparation and determination.
Results and discussion
Film thickness, moisture content, water solubility and swelling capacity
Film thickness is a fundamental physical indicator; therefore, the thickness data for the EWP-EGCG composite films are listed in Table 1. The film thickness significantly decreased when the concentration of EGCG increased from 0 to 20 μmol/L. However, when the EGCG concentration was ˃20 μmol/L, no significant decreasing trend was observed in the film thickness. This phenomenon could be caused by the tight binding of the macromolecular substances in EWP to glycerol, water molecular, and EGCG with the increased addition of EGCG, which promoted the formation of a compact structure and compressed the three-dimensional structure of the composite films.
Table 1.
Thickness, moisture content (MC), water solubility (WS), and swelling capacity (SC) of the antibacterial composite films based on egg white protein incorporated with EGCG.
| EGCG (μmol/L) | Film thickness (mm) | MC (%) | WS (%) | SC (%) |
|---|---|---|---|---|
| 0 | 0.247 ± 0.01a | 12.11 ± 0.79a | 27.07 ± 3.38a | 3.99 ± 0.25a |
| 5 | 0.211 ± 0.01b | 9.25 ± 0.76b | 25.07 ± 1.78ab | 3.57 ± 0.18b |
| 10 | 0.179 ± 0.01c | 7.76 ± 1.21bc | 21.89 ± 1.19bc | 3.56 ± 0.13b |
| 20 | 0.172 ± 0.01cd | 7.49 ± 1.17bc | 18.62 ± 1.06 cd | 3.26 ± 0.11c |
| 40 | 0.165 ± 0.04 cd | 6.60 ± 0.17c | 17.27 ± 0.27d | 3.08 ± 0.07c |
| 80 | 0.161 ± 0.02d | 6.33 ± 0.05c | 17.10 ± 0.33d | 3.03 ± 0.03c |
Values are presented as mean ± standard deviation. Different letters in the same column indicate significant differences (p < 0.05).
Water susceptibility is an important parameter of protein-based films, as it determines the applicability of these films in food industry. Table 1 lists the water susceptibility, moisture content (MC), water solubility (WS), and swelling capacity (SC) of the EWP-EGCG films. The incorporation of EGCG (0–5 μmol/L) into the EWP-based films resulted in a decrease in the MC from 12.11 ± 0.79 to 9.25 ± 0.76. However, increasing the concentration of EGCG from 10 to 80 μmol/L did not significantly change the MC of the composite films (7.76 ± 1.21 to 6.33 ± 0.05). Additionally, the WS of the composite films showed a similar trend as that of the MC, which was consistent with the trends observed in kappa-carrageenan electrospun fiber mats enriched with Prunus domestica anthocyanins and EGCG Goudarzi, Moshtaghi, and Shahbazi (2023). These results were primarily attributed to the hydrophilic characteristics of glycerol and proteins, which were not significantly changed after EGCG addition. SC also affects the water susceptibility and functional properties of protein-based film. As presented in Table 1, incorporating EGCG into the EWP-based films resulted in the reduction of SC from 3.99%±0.25% to 3.03%±0.03%, which was probably due to the formation of a dense and compact network with the polyphenol. This result could be attributed to the hydrogen bonding interactions between EWP-glycerol matrix and EGCG (Zioga, Papantonopoulou, & Evageliou, 2023).
Water vapor permeability
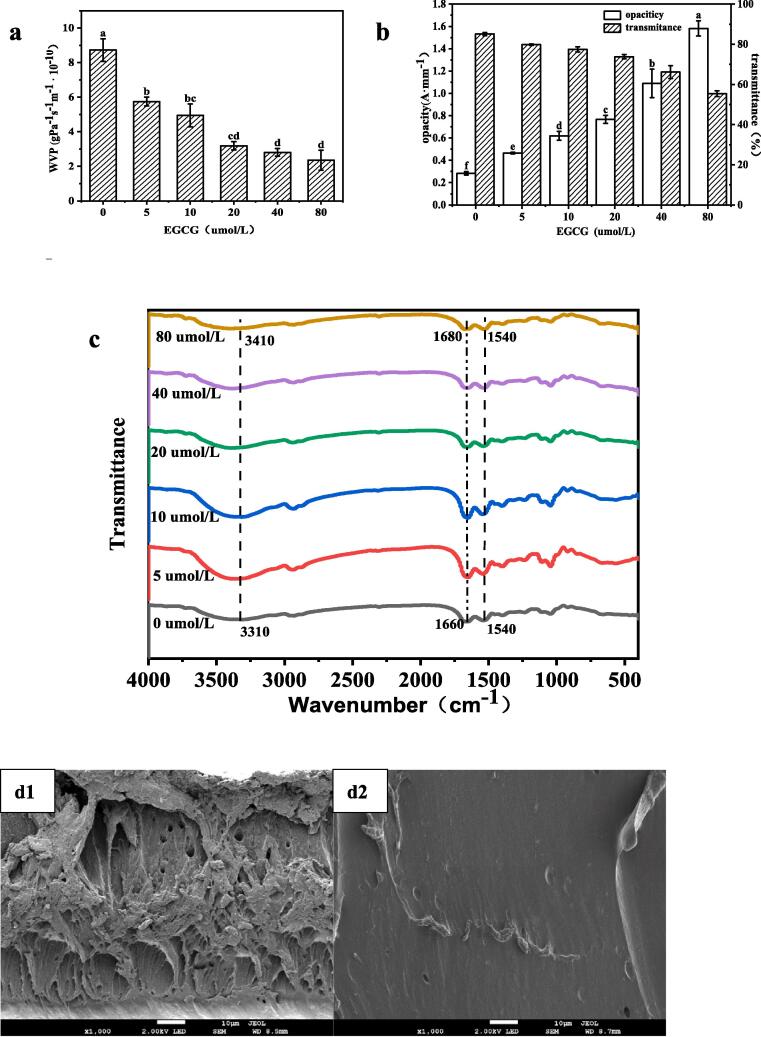
Water vapor permeability (WVP) of the prepared films with and without EGCG was used to determine their barrier properties towards water vapor, and the results were shown in Fig. 2a. A significantly lower WVP (p < 0.05) was observed when the antioxidant functional agent EGCG was added at concentration ranging from to 40 μmol/L. This result may be due to the increased migration rate of water molecular in contact with the composite film. As reported (Arciello et al., 2021), a relatively lower WVP value was essential for the packaging materials to maintain freshness and prolong the shelf life of different kinds of food. However, no significant differences in the WVP are observed in Fig. 2a when the EGCG increased from 40 to 80 μmol/L. This could be explained by the strong interactions between hydrogen bonds in the films, which leading to a denser network, thereby preventing water vapor diffusion (Li, Qin, Liu, Li, & Zhong, 2022).
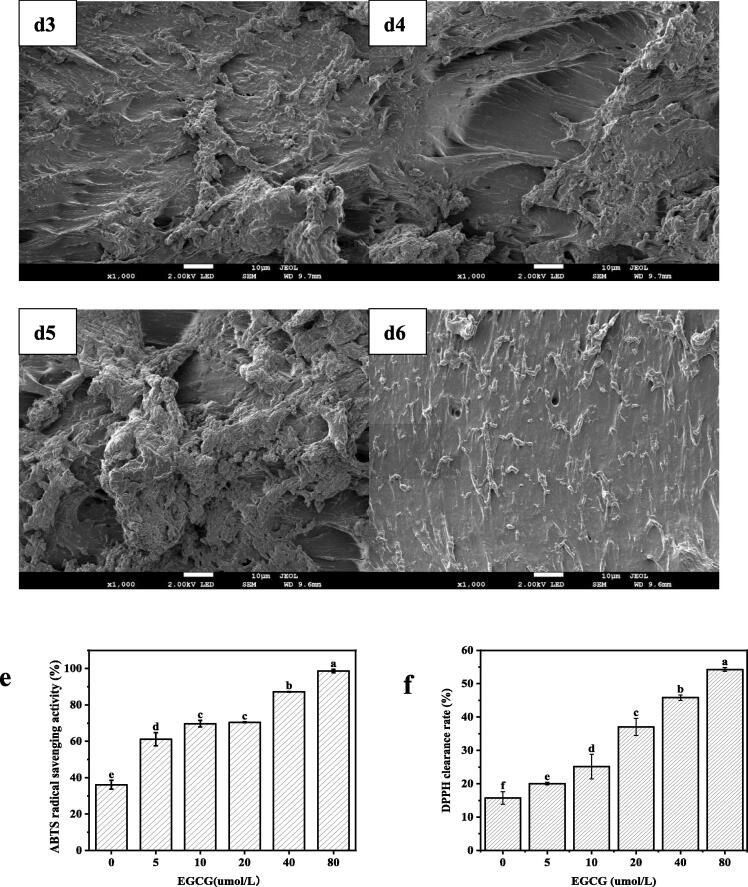
Fig. 2.
(a)WVP, (b) opacity and transmittance, (c)FTIR, (d1-d6) SEM micrographs of the cross-sectional images of composite films with different concentration of EGCG (0 to 80 μmol/L), (e) ABTS clearance rate and (f) DPPH clearance rate of antibacterial composite films based on egg white protein incorporated with EGCG.
Optical properties
The optical properties of packaging materials, including color parameter, opacity and transmittance are vital parameters for consumers when selecting foods. Color attributes (L*, a*, b* and ΔE) of the composite films were shown in Table 2. The L* value significantly decreased with increasing addition of EGCG in the composite films, which may be attributed to the yellow and dark colors of EGCG. Besides, negative values appeared on a* values which represented red to green from positive to negative values. The low absolute value of a* indicates that the films turned red with an increase in the EGCG concentration. Similarly, the significant increase in the b* value was attributed to the color of EGCG itself, which also indicated the facilitation of interactions between egg white protein and phenolic compounds. Therefore, a significant improvement in ΔE was observed in the composite films. These findings are consistent with the opacity and transmittance results shown in Fig. 2b. As reported (Dai, et al., 2022), the visible light can accelerate the oxidative deterioration of foods, thereby reducing their nutritional value and edibility. As presented in Fig. 2b, the opacities of the films increase from 0.283 ± 0.016 to 1.582 ± 0.068, whereas the transmittances at the wavelength of 600 nm of the films significantly decrease from 85.11 ± 0.79 to 55.43 ± 1.41, with the increase in the EGCG concentration from 0 to 80 μmol/L. This is primarily due to the visible light adsorption ability of the polyphenol (L. Wang, Lin, Guo, Long, Mu, & Pang, 2020). Another probable reason is that the EGCG-induced conformational changes facilitate the formation of compact structures in the composite films (Dai, et al., 2022). The transparency and appearance of the prepared films (Fig. 2b) would be acceptable to customers (Xiao, Liu, Kang, Cui, & Xu, 2021). Therefore, our results indicate that EWP-EGCG antioxidant films with a light-barrier function might prevent quality losses induced by the light in food packaging materials. Similar results were also observed by F. Wang, Yu, Yang, Yi, Fu, and Wang (2022) for the development of polysaccharide-based edible films.
Table 2.
Color attributes of antibacterial composite films based on egg white protein incorporated with EGCG.
| EGCG (μmol/L) | L* | a* | b* | Δ E |
|---|---|---|---|---|
| 0 | 33.22 ± 1.19a | −1.19 ± 0.11e | 3.67 ± 0.29e | 55.52 ± 1.04d |
| 5 | 32.57 ± 0.37a | −0.92 ± 0.03d | 4.42 ± 0.18de | 56.59 ± 0.26d |
| 10 | 30.53 ± 1.03b | −0.74 ± 0.06c | 5.29 ± 0.38 cd | 57.88 ± 0.34c |
| 20 | 29.86 ± 1.52bc | −0.64 ± 0.09bc | 5.93 ± 0.15c | 58.40 ± 0.26bc |
| 40 | 29.82 ± 1.16bc | −0.52 ± 0.07b | 6.15 ± 0.14b | 59.27 ± 0.19b |
| 80 | 28.27 ± 1.45c | −0.21 ± 0.09a | 6.48 ± 0.16a | 60.95 ± 1.21a |
Values are presented as mean ± standard deviation. Different letters in the same column indicate significant differences (p < 0.05).
Structural properties
FTIr
The FTIR spectra of the EWP films prepared with glycerol and different quantities of EGCG, along with those of control films without EGCG, are shown in Fig. 2c, with wavenumbers ranging from 4000 to 400 cm−1. The spectra exhibited major bands at the range of 3600–3000, 1700–1600, and 1600–1500 cm−1, which represented amide A and the amide Ⅰ region (mainly C O stretching vibrations), and amide Ⅱ region (primarily N—H bending and of C—N stretching vibrations) respectively, owing to the strong interaction among films. Similar characteristic peaks with slight differences in the position and intensity could be observed in the spectra. However, compared to that in the spectrum of the EWP-based film without EGCG addition, the intensity of the peak at wavenumber 3600–3000 cm−1 of composite films with EGCG increased from 3310 to 3410 cm−1 when the concentration of EGCG reached 80 μmol/L (Fig. 2c). The highest intensity of this broad adsorption band was observed in the spectra of the composite film containing 10 μmol/L EGCG. These results demonstrated that incorporating EGCG firstly enhanced the interactions within the protein network and subsequently increased the interaction with water molecules when the concentration was ˃10 μmol/L. Additionally, the amide Ⅰ region progressively shifted to a high-wavenumber region with increasing EGCG concentration because the secondary structure of egg white protein was affected by the incorporation of EGCG. This was coincidental with the results of soy protein isolate films incorporated with another kind of polyphenol (i.e., carvacrol) (Tao, Sedman, & Ismail, 2022). Simultaneously, the shift in the position of the absorption peak at 1660 cm−1 indicated that the amino group of egg white protein participated in the reaction with EGCG, and the formation of egg white protein-EGCG covalent complex was primarily due to the covalent reaction. Additionally, the disappearance of characteristic peaks of EGCG at 3410 cm−1 and 3310 cm−1 indicated that phenolic hydroxyl groups participated in the reaction between protein and polyphenols. Therefore, adding EGCG altered the secondary structure of EWP in the composite films.
Morphology (SEM)
The morphological characteristics of the composite films were determined using a field emission scanning electron microscopy (JSM-7900F, Japan), and the cross-section morphology of the EWP films with and without EGCG are shown in Fig. 2d. As plotted in Fig. 2d1, the uneven spatial network structure and thick holes could be clearly seen in the cross-sectional morphology of the pure egg white protein film incorporated with the plasticizer glycerol. Conversely, uniform and smooth surface are observed, as shown in Fig. 2d2, when the protein-based film is loaded with 5 μmol/L EGCG. This result was probably due to the homogenous distribution of the protein films owing to the presence of an appropriate amount of EGCG pellets. However, discontinuous blocks and rough surfaces are supported when the EGCG concentration is increased from 10 to 40 μmol/L, as shown in Fig. 2d3 and 2d4, owing to the increased tortuosity of the cross-section caused by the incompletely wrapped EGCG pellets throughout the protein-film matrix. Similar results were proposed in chitosan- epigallocatechin gallate films by Mittal et al. (2021). Nevertheless, following the addition of 80 μmol/L EGCG, the morphology of EWP-EGCG films comprised aligned chains in a film network (Fig. 2d6), with only compact and homogeneous particles covering the surfaces. This phenomenon is probably due to the strong interactions between the EGCG molecules and egg white protein when the concentration of EGCG reached 80 μmol/L, and the EWP-glycerol chains were easily disturbed by EWP-EGCG.
Antioxidant properties
Studies have demonstrated that the antioxidant ability is a significant feature of EGCG as well as its composite films (Q. Wang, Chen, Ma, Chen, Liu, & Liu, 2022). The antioxidant properties were determined by ABTS radical scavenging activity and DPPH radical scavenging activity, and the data are presented in Fig. 2e and Fi. 2f. Compared to the EWP film, the EWP-based composite films with EGCG supplementation demonstrated high antioxidant capacities because of their ability to capture both ABTS free radicals and DPPH free radicals. At higher EGCG concentrations, the composite films exhibited higher antioxidant abilities, which could be ascribed to the higher degree of EGCG encapsulation in the films and stronger interactions between EWP and EGCG (Li, et al., 2022). This result also implies an increase in the amount of EGCG involved in the preparation of the films. EGCG fully reacted with the proteins and was embedded within the produced films. However, compared with that toward ABTS+, the scavenging ability of the composite films toward DPPH showed a more significant increasing trend, as shown in Fig. 2f. This result is probably due to the strong electron-donating capacity of EGCG, allowing it to react with the DPPH free radicals and prevent its chain reaction, thereby resulting in a stable structure and improved antioxidant ability as evidenced by the DPPH free radical clearance rate (Chen, Zhao, Zhang, Shang, Gao, Li, et al., 2022). In addition, EGCG has been reported to be the most abundant derivative that is rich in antioxidants in tea (Muniandy, Shori, & Baba, 2016). Therefore, the data pertaining to the antioxidant abilities of the composite films are coincidental with the data obtained for the antioxidant properties of EGCG. Based on the abovementioned results, antioxidant composite films can be effectively applied in food packaging materials to prevent the quality degradation caused by the oxidation and deterioration.
Biodegradation evaluation
It is known that most commercial plastic packaging materials have extremely weak degradation capability and may require more than thousand years to decompose. Therefore, the evaluation of the biodegradation capabilities of the prepared composite films is essential for assessing the feasibility of future applications of these films. The biodegradable properties of the prepared films were investigated by using the soil-burial method, as described by Yuan, Tan, Lin, Zhang, Li, and Guo (2022). A commercial plastic wrap (i.e., polyethylene, PE) was used for comparison. The visual appearance of the tested films is depicted in Fig. 3a to evaluate their degradation because the accurate determination of changes in their weight is not practical, owing to the difficulty of removing the soil residues adhered to the films (Ferreira Nogueira, Matta Fakhouri, & de Oliveira, 2019). From Fig. 3a, no wrinkles are observed in the PE film following 10 d of burial in the soil, suggesting the difficulty in biodegradation of petroleum-based packaging materials. Notably, all the experimental films (egg white protein film incorporated with EGCG) gradually roughened after 4 d and started to lose their structural integrity after 6 d, indicating the beginning of degradation of EWP-EGCG films by the microorganisms present in the soil. The degradation rate of the developed films increased rapidly after 8 and 10 d, which may be because EWP-EGCG act as a carbon source for metabolic growth of soil microorganisms. Furthermore, the destruction of hydrogen bond interactions between EWP and glycerol could accelerate the degradation of the films. Moreover, adding EGCG may have affected the crystallization of the composite film, rendering it highly susceptible to microbial invasion and degradation upon contact with the soil (Chen, Zheng, Tan, Lin, Chen, & Zhu, 2022). Therefore, the prepared EWP-EGCG composite films could be considered as biodegradable packaging materials owing to their facile degradation behavior in soil.
Fig. 3.
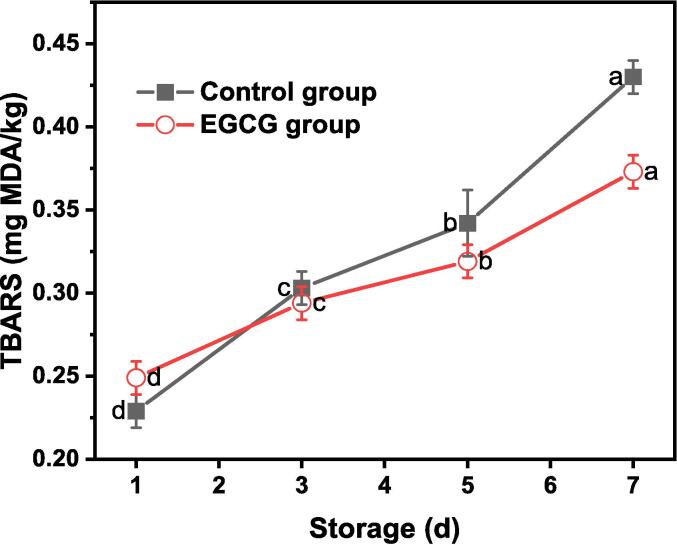
(a) Degradation of antibacterial films based on egg white protein incorporated with EGCG. (b) Changes in the pH values and (c) Changes in the thiobarbituric acid reactive substance (TBARS) value of the chilled meat during storage (mean ± standard error).
Food packaging application
pH values
With an increase in the storage time, the pH of the chilled pork wrapped in films significantly increased (Fig. 3b). However, the increasing pH of the sample wrapped in the as-prepared protein films was lower than that of the control group between 0 and 5 d. In addition, a pH ˃ 6.4 corresponds to meat spoilage according to Chinese standards (Zeng, et al., 2023). As shown in Fig. 3b, the pH value of the control group at the fifth day is 6.41 ± 0.27, whereas that of the EGCG group (i.e., EWP-based composite film) is 6.05 ± 0.02. This result was attributed to the effective production of protein films upon the incorporation of EGCG into pork.
TVB-N and TBARS
Total volatile basic nitrogen (TVB-N) and thiobarbituric acid reactive substances (TBARS) are commonly used parameters to evaluate meat oxidation during storage to determine the antioxidant effects of the composite films (Zeng, et al., 2023). The effect of different storage time on the TVB-N of the ordinary fresh-keeping film i.e., polyethylene (PE) plastic bag and EWP-based composite film cooperated with EGCG is summarized in Table 3. The initial TVB-N content of pork before storage was regarded as the same for all the cases. Following 1 d of storage, the TVB-N content of the ordinary fresh-keeping and composite film were 4.67 ± 1.46 and 9.10 ± 1.21 mg/100 g, respectively. This result indicates that the ordinary PE plastic bag could protect the fresh of the chilled pork from spoilage on the first day. However, the TVB-N value of the pork wrapped with PE film significantly increased to 9.33 ± 2.02, 12.37 ± 1.76 and 17.03 ± 1.46 mg/100 g after storage for 3, 5, and 7 d, respectively, Following storage for 7 d, whereas that of the fresh chilled pork wrapped with the EWP-based composite film increased to 9.33 ± 2.02, 9.80 ± 2.52 and 14.00 ± 3.50 mg/100 g after storage for 3, 5, and 7 d, respectively, with no significant differences (p > 0.05). Following storage for 7 d, the low TVB-N values were observed in the experiment group (i.e., chilled pork coated with EWP-based films). The increase in the TVB-N value was distinct, and the differences between the PE- and EWP-based composite films were significant. This phenomenon was attributed to the preservation and maintenance of meat freshness came from the antioxidant capacity of composite film. As reported by (Bekhit, Holman, Giteru, & Hopkins, 2021), the quality of pork is acceptable when the TVB-N accumulation level was <15 mg/100 g. Therefore, the as-prepared composite films presented better fresh-keeping ability than the other tested reference films in terms of meat preservation for an extended time and would be acceptable to consumers when used for wrapping pork for 7 d of chilled preservation. In other words, the composite films exhibited a better ability to inhibit microbial reproduction in pork than the PE plastic bags.
Table 3.
Changes in the total volatile basic nitrogen (TVB-N) value of the chilled meat during storage (mean ± standard error).
| Storage time (d) | Control group TVB-N (mg/100 g) |
EGCG group TVB-N (mg/100 g) |
|---|---|---|
| 1 | 4.67 ± 1.46b | 9.10 ± 1.21a |
| 3 | 9.33 ± 2.02a | 9.33 ± 2.02a |
| 5 | 12.37 ± 1.76a | 9.80 ± 2.52a |
| 7 | 17.03 ± 1.46c | 14.00 ± 3.50a |
Changes in the TBARS values of the chilled pork wrapped with ordinary fresh-keeping film (control group) and the composite film (EGCG group) are shown in Fig. 3c. Similar to the TVB-N content, the initial TBARS value of pork was considered the same for all cases before storage. Following storage for 1 d, the TBARS values of the control group and EGCG group were 0.229 ± 0.01 and 0.249 ± 0.01 mg MDA/100 kg meat, respectively. This result indicates the rapid-activated protective capacity of PE films. However, the effect of the PE film did not persist during the subsequent days of storage. As depicted in Fig. 3c, the TBARS values of the control group significantly increase to 0.303 ± 0.01, 0.342 ± 0.02, and 0.43 ± 0.01 mg MDA/100 kg meat after 3, 5, and 7 d, respectively. Compared to the TBARS values of the control group, the values of the EGCG group were lower at 0.294 ± 0.01, 0.319 ± 0.01, and 0.373 ± 0.01 mg MDA/100 kg meat after 3, 5, and 7 d, respectively. Therefore, the PE films of the control group were only effective in the antioxidation of the pork in the early stage of storage. In contrast, the slow release of EGCG in the composite film play an important role in antioxidant in the later period of preservation (within 7 d). Compared with that of the ordinary PE plastic bags, the antioxidant activity of EGCG in EWP-based films was more stable and continuous over a longer storage time. Similar results were also obtained by R. Zhao, Guan, Zhou, Lao, and Cai (2022), who reported that the TBARS value of fish fillets wrapped with the collagen/chitosan nanoparticles/anthocyanidin films was higher at the second storage day than the unwrapped film, which exhibited a relatively lower value during the last few days of storage. Therefore, the TBARS results are in good agreement with the pH and TVB-N values.
Conclusions
Physicochemical and antioxidant properties of egg white protein (EWP)-based films incorporated with epigallocatechin gallate (EGCG) were examined. Fourier transform infrared spectroscopy (FTIR) and scanning electron microscopy (SEM) results confirmed the highly efficient formation of the composite films. Moreover, the appropriate water susceptibility was conductive to the high biodegradability. The results also validated the effective antioxidant capacities of EWP-EGCG composite films in terms of ABTS and DPPH free radical scavenging abilities, which were significantly enhanced with increasing concentration of EGCG in the packaging films. Compared with commercially available polyethylene (PE) plastic films, the EWP-EGCG composite films significantly reduced TVB-N and TBARS values of the chilled pork during 7 d of storage. In summary, the fundamental insights presented herein would aid the preparation of new types of degradable, EWP-based packaging material with excellent potential for application in meat preservation.
CRediT authorship contribution statement
Yue Wu: Data curation, Formal analysis, Writing – original draft. Yuemeng Wang: Data curation, Supervision, Methodology, Writing – review & editing. Jianhao Lv: Investigation, Conceptualization, Data curation. Han Jiao: Software, Data curation, Validation. Jiahan Liu: Software, Data curation, Validation. Wenhui Feng: Data curation, Validation. Chengfeng Sun: Visualization. Xin Li: Supervision, Writing – review & editing, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially by the Natural Science Foundation of Shandong Province (Grant No. ZR2021QC065).
Data availability
Data will be made available on request.
References
- Arciello A., Panzella L., Dell’Olmo E., Abdalrazeq M., Moccia F., Gaglione R.…Giosafatto C.V.L. Development and characterization of antimicrobial and antioxidant whey protein-based films functionalized with Pecan (Carya illinoinensis) nut shell extract. Food Packaging and Shelf Life. 2021;29 doi: 10.1016/j.fpsl.2021.100710. [DOI] [Google Scholar]
- Bekhit A.-E.-D.-A., Holman B.W.B., Giteru S.G., Hopkins D.L. Total volatile basic nitrogen (TVB-N) and its role in meat spoilage: A review. Trends in Food Science & Technology. 2021;109:280–302. doi: 10.1016/j.tifs.2021.01.006. [DOI] [Google Scholar]
- Chen J., Zheng M., Tan K.B., Lin J., Chen M., Zhu Y. Polyvinyl alcohol/xanthan gum composite film with excellent food packaging, storage and biodegradation capability as potential environmentally-friendly alternative to commercial plastic bag. International Journal of Biological Macromolecules. 2022;212:402–411. doi: 10.1016/j.ijbiomac.2022.05.119. [DOI] [PubMed] [Google Scholar]
- Chen X., Zhao Z., Zhang C., Shang C., Gao L., Li C.…Liu L. Effect of epigallocatechin gallate on the fermentative and physicochemical properties of fermented milk. Journal of Dairy Science. 2022;105(9):7322–7333. doi: 10.3168/jds.2021-21709. [DOI] [PubMed] [Google Scholar]
- Dai W., Zhou L., Gu S., Wang W., Xu Z., Zhou X., Ding Y. Preparation and characterization of chitosan films incorporating epigallocatechin gallate: Microstructure, physicochemical, and bioactive properties. International Journal of Biological Macromolecules. 2022;211:729–740. doi: 10.1016/j.ijbiomac.2022.04.226. [DOI] [PubMed] [Google Scholar]
- Deng W., Xu Q., Hu X., Sheng L. Structure and properties of egg white protein films modified by high-intensity ultrasound: An effective strategy. Food Research International. 2022;157 doi: 10.1016/j.foodres.2022.111264. [DOI] [PubMed] [Google Scholar]
- Ferreira Nogueira G., Matta Fakhouri F., de Oliveira R.A. Incorporation of spray dried and freeze dried blackberry particles in edible films: Morphology, stability to pH, sterilization and biodegradation. Food Packaging and Shelf Life. 2019;20 doi: 10.1016/j.fpsl.2019.100313. [DOI] [Google Scholar]
- Goudarzi J., Moshtaghi H., Shahbazi Y. Kappa-carrageenan-poly(vinyl alcohol) electrospun fiber mats encapsulated with Prunus domestica anthocyanins and epigallocatechin gallate to monitor the freshness and enhance the shelf-life quality of minced beef meat. Food Packaging and Shelf Life. 2023;35 doi: 10.1016/j.fpsl.2022.101017. [DOI] [Google Scholar]
- Kandi S., Charles A.L. Statistical comparative study between the conventional DPPH spectrophotometric and dropping DPPH analytical method without spectrophotometer: Evaluation for the advancement of antioxidant activity analysis. Food Chemistry. 2019;287:338–345. doi: 10.1016/j.foodchem.2019.02.110. [DOI] [PubMed] [Google Scholar]
- Kontogianni V.G., Kasapidou E., Mitlianga P., Mataragas M., Pappa E., Kondyli E., Bosnea L. Production, characteristics and application of whey protein films activated with rosemary and sage extract in preserving soft cheese. LWT-Food Science and Technology. 2022;155 doi: 10.1016/j.lwt.2021.112996. [DOI] [Google Scholar]
- Lee S.J., Gwak M.A., Chathuranga K., Lee J.S., Koo J., Park W.H. Multifunctional chitosan/tannic acid composite films with improved anti-UV, antioxidant, and antimicrobial properties for active food packaging. Food Hydrocolloids. 2023;136 doi: 10.1016/j.foodhyd.2022.108249. [DOI] [Google Scholar]
- Li G., Lee Y.Y., Lu X., Chen J., Liu N., Qiu C., Wang Y. Simultaneous loading of (-)-epigallocatechin gallate and ferulic acid in chitosan-based nanoparticles as effective antioxidant and potential skin-whitening agents. International Journal of Biological Macromolecules. 2022;219:333–345. doi: 10.1016/j.ijbiomac.2022.07.242. [DOI] [PubMed] [Google Scholar]
- Li J., Qin X., Liu X., Li J., Zhong J. Enhanced mechanical, barrier and antioxidant properties of rice protein/sodium alginate-based films by incorporating cellulose nanocrystals and rosemary extract. Food Packaging and Shelf Life. 2022;34 doi: 10.1016/j.fpsl.2022.101000. [DOI] [Google Scholar]
- Li X., Liu A., Ye R., Wang Y., Wang W. Fabrication of gelatin–laponite composite films: Effect of the concentration of laponite on physical properties and the freshness of meat during storage. Food Hydrocolloids. 2015;44:390–398. doi: 10.1016/j.foodhyd.2014.10.014. [DOI] [Google Scholar]
- Liu J., Wang Y., Lv J., Wu Y., Guo Y., Sun C., Li X. Biodegradable composite films based on egg white protein and tea polyphenol: Physicochemical, structural and antibacterial properties. Food Packaging and Shelf Life. 2023;38 doi: 10.1016/j.fpsl.2023.101098. [DOI] [Google Scholar]
- Liu L., Huang X., Geng F., Huang Q. Optimization of preparation process of egg white protein/κ-carrageenan composite film. Journal of Food Processing and Preservation. 2021;46(9) doi: 10.1111/jfpp.16167. [DOI] [Google Scholar]
- Mittal, A., Singh, A., Benjakul, S., Prodpran, T., Nilsuwan, K., Huda, N., & Caba, K. d. l. (2021). Composite films based on chitosan and epigallocatechin gallate grafted chitosan: Characterization, antioxidant and antimicrobial activities. Food Hydrocolloids, 111, 106384. doi: 10.1016/j.foodhyd.2020.106384.
- Moalla S., Ammar I., Fauconnier M.L., Danthine S., Blecker C., Besbes S., Attia H. Development and characterization of chitosan films carrying Artemisia campestris antioxidants for potential use as active food packaging materials. International Journal of Biological Macromolecules. 2021;183:254–266. doi: 10.1016/j.ijbiomac.2021.04.113. [DOI] [PubMed] [Google Scholar]
- Muniandy P., Shori A.B., Baba A.S. Influence of green, white and black tea addition on the antioxidant activity of probiotic yogurt during refrigerated storage. Food Packaging and Shelf Life. 2016;8:1–8. doi: 10.1016/j.fpsl.2016.02.002. [DOI] [Google Scholar]
- Oliveira Filho J.G., Bertolo M.R.V., Rodrigues M.A.V., Silva G.D.C., Mendonca G.M.N., Bogusz Junior S.…Egea M.B. Recent advances in the development of smart, active, and bioactive biodegradable biopolymer-based films containing betalains. Food Chemistry. 2022;390 doi: 10.1016/j.foodchem.2022.133149. [DOI] [PubMed] [Google Scholar]
- Peng N., Gu L., Li J., Chang C., Li X., Su Y., Yang Y. Films Based on Egg White Protein and Succinylated Casein Cross-Linked with Transglutaminase. Food and Bioprocess Technology. 2017;10(8):1422–1430. doi: 10.1007/s11947-017-1901-8. [DOI] [Google Scholar]
- Rojas-Lema S., Nilsson K., Langton M., Trifol J., Gomez-Caturla J., Balart R.…Moriana R. The effect of pine cone lignin on mechanical, thermal and barrier properties of faba bean protein films for packaging applications. Journal of Food Engineering. 2023;339 doi: 10.1016/j.jfoodeng.2022.111282. [DOI] [Google Scholar]
- Sun H., Huang Y., Chen Y., Liu X., Leng X. Effects of curcumin, phycocyanin, or modified lycopene colorants on the physicochemical and sensory properties of whey protein-cellulose nanocrystal packaging films. Food Chemistry. 2023;412 doi: 10.1016/j.foodchem.2023.135541. [DOI] [PubMed] [Google Scholar]
- Sun J., Liu T., Zhang F., Huang Y., Zhang Y., Xu B. Tea polyphenols on emulsifying and antioxidant properties of egg white protein at acidic and neutral pH conditions. LWT-Food Science and Technology. 2022;153 doi: 10.1016/j.lwt.2021.112537. [DOI] [Google Scholar]
- Tao R., Sedman J., Ismail A. Characterization and in vitro antimicrobial study of soy protein isolate films incorporating carvacrol. Food Hydrocolloids. 2022;122 doi: 10.1016/j.foodhyd.2021.107091. [DOI] [Google Scholar]
- Wang F., Yu G., Yang Q., Yi X., Fu L., Wang Y. Antibacterial Gelidium amansii polysaccharide-based edible films containing cyclic adenosine monophosphate for bioactive packaging. International Journal of Biological Macromolecules. 2022;212:324–336. doi: 10.1016/j.ijbiomac.2022.05.090. [DOI] [PubMed] [Google Scholar]
- Wang L., Lin L., Guo Y., Long J., Mu R.-J., Pang J. Enhanced functional properties of nanocomposite film incorporated with EGCG-loaded dialdehyde glucomannan/gelatin matrix for food packaging. Food Hydrocolloids. 2020;108 doi: 10.1016/j.foodhyd.2020.105863. [DOI] [Google Scholar]
- Wang Q., Chen W., Ma C., Chen S., Liu X., Liu F. Enzymatic synthesis of sodium caseinate-EGCG-carboxymethyl chitosan ternary film: Structure, physical properties, antioxidant and antibacterial properties. International Journal of Biological Macromolecules. 2022;222(Pt A):509–520. doi: 10.1016/j.ijbiomac.2022.09.138. [DOI] [PubMed] [Google Scholar]
- Wang X., Huang X., Zhang F., Hou F., Yi F., Sun X.…Liu Z. Characterization of chitosan/zein composite film combined with tea polyphenol and its application on postharvest quality improvement of mushroom (Lyophyllum decastes Sing.). Food Packaging and Shelf. Life. 2022;33 doi: 10.1016/j.fpsl.2022.100869. [DOI] [Google Scholar]
- Wang Y., Liu A., Ye R., Wang W., Li X. Transglutaminase-induced crosslinking of gelatin-calcium carbonate composite films. Food Chemistry. 2015;166:414–422. doi: 10.1016/j.foodchem.2014.06.062. [DOI] [PubMed] [Google Scholar]
- Xiao Y., Liu Y., Kang S., Cui M., Xu H. Development of pH-responsive antioxidant soy protein isolate films incorporated with cellulose nanocrystals and curcumin nanocapsules to monitor shrimp freshness. Food Hydrocolloids. 2021;120 doi: 10.1016/j.foodhyd.2021.106893. [DOI] [Google Scholar]
- Xiao Y., Xu H., Zhou Q., Li W., Gao J., Liao X.…Liu Y. Influence mechanism of wheat bran cellulose and cellulose nanocrystals on the storage stability of soy protein isolate films: Conformation modification and molecular interaction perspective. Food Hydrocolloids. 2023;139 doi: 10.1016/j.foodhyd.2023.108475. [DOI] [Google Scholar]
- Yuan Y., Tan W., Lin C., Zhang J., Li Q., Guo Z. Development of antioxidant chitosan-based films incorporated with chitooligosaccharide-caffeic acid conjugates. Food Hydrocolloids. 2022;138 doi: 10.1016/j.foodhyd.2022.108431. [DOI] [Google Scholar]
- Zeng Z., Yang Y.J., Tu Q., Jian Y.Y., Xie D.M., Bai T.…Liu A.P. Preparation and characterization of carboxymethyl chitosan/pullulan composite film incorporated with eugenol and its application in the preservation of chilled meat. Meat Science. 2023;198 doi: 10.1016/j.meatsci.2022.109085. [DOI] [PubMed] [Google Scholar]
- Zhang D., Zhu L., Jiang Q., Ge X., Fang Y., Peng J., Liu Y. Real-time and rapid prediction of TVB-N of livestock and poultry meat at three depths for freshness evaluation using a portable fluorescent film sensor. Food Chemistry. 2023;400 doi: 10.1016/j.foodchem.2022.134041. [DOI] [PubMed] [Google Scholar]
- Zhao R., Guan W., Zhou X., Lao M., Cai L. The physiochemical and preservation properties of anthocyanidin/chitosan nanocomposite-based edible films containing cinnamon-perilla essential oil pickering nanoemulsions. LWT-Food Science and Technology. 2022;153 doi: 10.1016/j.lwt.2021.112506. [DOI] [Google Scholar]
- Zhao X., Li C., Xue F. Effects of whey protein-polyphenol conjugates incorporation on physicochemical and intelligent pH-sensing properties of carboxymethyl cellulose based films. Future Foods. 2023;7 doi: 10.1016/j.fufo.2022.100211. [DOI] [Google Scholar]
- Zhong C., Hou P.-F., Li Y.-X., Yang W.-Y., Shu M., Wu G.-P. Characterization, antioxidant and antibacterial activities of gelatin film incorporated with protocatechuic acid and its application on beef preservation. LWT-Food Science and Technology. 2021;151 doi: 10.1016/j.lwt.2021.112154. [DOI] [Google Scholar]
- Zioga M., Papantonopoulou G., Evageliou V. High internal phase emulsions and edible films with high methoxyl pectin and pea protein isolate or sodium caseinate. Food Hydrocolloids. 2023;140 doi: 10.1016/j.foodhyd.2023.108605. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.