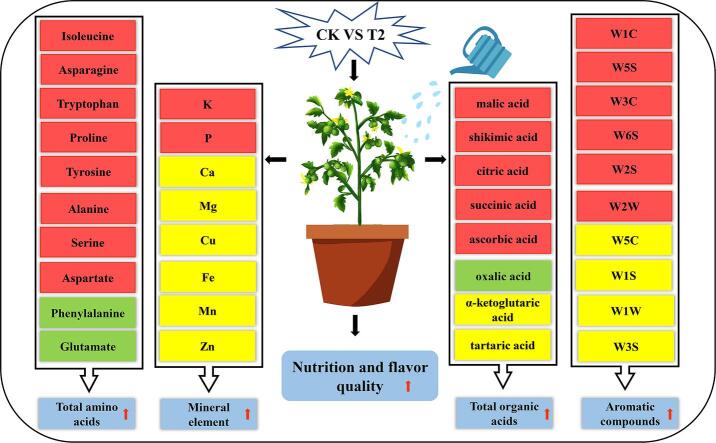
Graphical abstract
Keywords: Water deficient, Tomato fruit, Amino acids, Organic acids, Volatile compounds, Quality
Highlights
-
•
Water deficiency (WD) optimization improves consumer satisfaction and water waste.
-
•
WD improved the amino acid, organic acid, mineral elements, and volatile compounds.
-
•
Appropriate WD promotes primary/secondary metabolism, improving nutrition & flavor.
-
•
Our classification model provides a base for consumer preferences in tomato quality.
Abstract
Water deficit (WD) irrigation techniques to improve water use efficiency have been rapidly developed. However, the effect of WD irrigation on tomato quality has not been sufficiently studied. Here, we investigated the effects of varying water irrigation levels [T1–T4: 80%, 65%, 55%, and 45% of maximum field moisture capacity (FMC)] and full irrigation (CK: 90% of maximum FMC) on tomato fruits from the mature-green to red-ripening stages, to compare the nutritional and flavour qualities of the resulting tomatoes. The proline, aspartic, malic, citric, and ascorbic acid contents increased, phenylalanine and glutamic acid contents decreased, and the total amino and organic acid contents increased by 18.91% and 26.12%, respectively, in T2-treated fruits. Furthermore, the T2-treated fruits exhibited higher K and P contents alongside improved characteristic aromas. These findings provide novel insights for further improvements in tomato quality while also developing water-saving irrigation techniques.
Introduction
Tomatoes (Solanum lycopersicum L.) are well-known for their unique flavour and rich bioactive compounds, and are one of the most widely cultivated commercial crops worldwide (Lu & Zhu, 2022). Tomatoes contain a diverse range of compounds, including carotenoids (lycopene, β-carotene, and lutein), phenols (phenolic acids and flavonoids), vitamins (vitamins C, E, and A), amino acids, and minerals, which contribute substantially to their nutritional value. Additionally, these compounds endow tomatoes with anti-inflammatory, antioxidant, and anticancer activities, whilst also being highly effective for alleviation of coronary heart disease and lung cancer (Bruno et al., 2018, Muscatello et al., 2018). The eight essential amino acids that cannot be synthesized by humans include lysine, tryptophan, phenylalanine, methionine, threonine, isoleucine, leucine, and valine; therefore, they must be obtained from our diet, with tomatoes being a popular source of these amino acids. The flavour of tomatoes is a result of the combined interaction of many substances, with the ratio of soluble sugars to organic acids (mainly malic and citric acids) being the main determinant (Klee & Tieman, 2018). Additionally, the aroma—another critical component of the fruit flavour—is a complex mixture of volatile compounds derived from aromatic amino acids, fatty acids, and carotenoids (Wang, Baldwin, & Bai, 2016). Tomatoes are abundant in amino acids, organic acids, mineral elements, and volatile compounds, which are all important for nutrition and flavour. Furthermore, the nutritional value and flavour of tomatoes significantly correlates with consumer acceptance preferences. To satisfy the increasing demand, owing to the growing population, breeders compromise on the tomato quality by abandoning superior quality traits and varieties, in the pursuit of higher yields. Furthermore, factors such as climate change, excessive chemical fertilizer application, and irrigation have resulted in an additional deterioration in tomato quality (Ruiz-Nieves et al., 2021). It is also widely believed that the pursuit of high yields has led tomatoes to lose their ‘childhood taste’.
In recent years, increasing attention has been paid to improving tomato quality, with the increase in demand for the fruit. Consequently, several measures have been implemented to enrich the nutrition and flavour of tomatoes. Previous reports have stated that root or foliar sprays with suitable concentrations of exogenous substances, such as methyl jasmonate, paramylon, quercetin, chitosan, potassium, and selenium, can increase the content of soluble solids, sugars, carotenoids, and other nutrients in tomatoes (Liu et al., 2018). Several small-molecule gases are involved in maintaining nutrition value and improving tomato quality traits by regulating antioxidant metabolism (Zuccarelli et al., 2021). Furthermore, appropriate cultivation management practices significantly enhance tomato nutrition and flavour. For example, fertilization with arbuscular mycorrhizal fungi (AMF) has been shown to be effective for increasing mineral elements and volatile compounds in tomato fruit tissue (Pasković et al., 2021). Red and blue light emitting diode (LED) supplementation of tomato plants at different times during the morning and evening could also improve tomato fruit quality (Wang et al., 2022).
Irrigation (an important cultivation management practice) is considered a primary constraint to agricultural production because agricultural irrigation consumes 80 % of the world's total available water and is inefficiently managed by most farms (Farouk & El-Metwally, 2019). Water scarcity, especially in arid and semi-arid regions, has become a major issue that limits sustainable agricultural development. In response to increasing water scarcity, water conservation and efficient water use is becoming increasingly integrated with sustainable agricultural development. Furthermore, innovative water-saving irrigation methods, such as water deficit (WD) irrigation, have recently emerged. Plants have different water requirements during different development periods, and WD irrigation can focus on the entire plant growth cycle. However, WD irrigation has negative influences on seed germination, leaf photosynthetic physiology, seedling growth, and yields (Dietz, Zörb, & Geilfus, 2021). Nevertheless, responses to WD irrigation vary with plant species, genotype, growth period, and the intensity, timing, and duration of water stress (Mirás-Avalos & Intrigliolo, 2017). Incidentally, WD irrigation can benefit fruit quality by increasing the concentrations of primary and secondary metabolites. For example, WD irrigation improves apple, citrus, and mango flavour, fruit quality, and extends storage (Zhu, Yu, Brecht, Jiang, & Zheng, 2016). WD can also promote flowering, improve fruit colour, and increase the accumulation of soluble solids in ripe tomatoes (Shohat et al., 2021). Appropriate WD irrigation practices can balance the conflict between fruit yield and quality. For example, short-term water stress applied during the later growing period of nectarines effectively improved fruit quality (Lopez et al., 2016). Meanwhile, moderate water deficiency during cell division positively influenced the fresh quality and sugar content of tomatoes, possibly due to osmotic regulation (Ripoll, Urban, Brunel, & Bertin, 2016).
Previous research suggests the application of controlled water deficiency in the middle and late stages of plant growth may save water and balance the contradictions between fruit yield and quality (Lopez et al., 2016, Ripoll et al., 2016). To reduce the impacts of global water scarcity on tomato production, development of innovative water-saving and quality-enhancing irrigation management techniques is urgently required; consequently, improving our understanding of how WD affects nutritional and flavour compounds in tomatoes whilst also assessing the extent to which WD affects fruit quality is essential.
Herein, we assessed the variations in amino acids, organic acids, mineral elements, and volatile compounds in tomatoes (cv. 'Micro-Tom'), with a focus on the green to ripe red stages under full irrigation (CK) and four WD irrigation conditions. We also analysed the results using high-performance liquid chromatography (HPLC) and a portable electronic nose (E-nose). We successfully employed multivariate statistical analysis—a classification model based on principal component analysis and cluster analysis—to investigate the effects of different WD frequencies on tomato quality to scientifically classify the results to provide a base for improving the use of WD in tomato irrigation, and delve deeper into the experimental results (Devarajan et al., 2021, Jin et al., 2022). The classification model based on multivariate statistical analysis used here provides a base for consumer preferences in terms of the nutritional and flavour qualities of tomatoes. Our findings provide a theoretical basis for water-saving and high-quality cultivation of tomatoes grown in substrates.
Materials and methods
Plant materials
Tomato seeds (cv. 'Micro-Tom', purchased from PanAmerican Seed) were surface sterilized with 4% (w/v) sodium hypochlorite for 15 min and then rinsed several times using sterile distilled water; subsequently, they were placed on moist filter paper for germination in 9 cm Petri dishes (Jin et al., 2022). The tomato seeds were then transferred into 50-hole cavity trays for cultivation, once 0.3 cm of radicle had emerged from the seeds, in an artificial climate chamber (RDN-400E-4; Ningbo Dongnan Instrument Co., Ltd., Zhejiang, China) maintained in the dark at 28 °C. Once the seedlings in the cavity trays developed three true leaves, they were transplanted into plastic pots (size: 7 × 7 × 8 cm) containing 2:1:1 (v/v/v) mixture of grass charcoal: vermiculite: pearl substrate. The cultivation environment was: 28 °C/20 °C for day/night cycle, 16/8h day/night cycle, 50% relative humidity, and 300 µmol m−2 s−1 photosynthetic photon flux density. Fruits from the same flowering stage were marked. WD was then applied once the tomato fruit entered the mature green stage, 20 days after flowering (DAF).
Experimental design
The experimental design involved five treatments with three replicates and 15 pots per replicate (5 × 3 × 15 = 225 samples). All pots were immersed in water until fully saturated (approximately 3 cm deep for 12 h) before initiating WD treatment. Excess water was drained, and the samples were weighed to obtain the maximum field moisture capacity (FMC) of the substrate (Liu et al., 2017). The experimental treatments were as follows: CK: Fully irrigated with a consistent substrate moisture content of 90% for maximum FMC; and T1–T4, maintained with a substrate moisture content of 80, 65, 55, 45% of the maximum FMC, respectively. The moisture content of the substrate was controlled using the sample weight (Tian, Lu, Gong, & Shah, 2014). All fruit quality indicators were measured upon experiment completion, once the red-ripening stage began (approximately 45 DAF); The maturity parameters of tomatoes during the red-ripening stage were measured using skin colour. Peel colour was measured using a colorimeter (CR-10 Plus, Konica Minolta, Inc., Tokyo, Japan), which provided colour surface coordinates L* (brightness), a* (range between green and red), and b* (range between blue and yellow). The a* and b* values were then processed to obtain the hue angle (h), which represents a composite indicator of colour variation, inversely proportional to a* and positively proportional to b*. The maturity parameters corresponding to the five water treated tomatoes were CK (L: 51.02, a*: 14.22, b*: 17.1, and h: 61.62), T1 (L: 48.68, a*: 16.22, b*: 16.08, and h: 56.86), T2 (L: 44.14, a*: 23.34, b*: 15.24, and h: 56.86), T3 (L: 42.86, a*: 30.98, b*: 14.86, and h: 45.92), and T4 (L: 42.48, a*: 34.62, b*: 13.96, and h: 44.4). Fig. 1S (supplementary materials) shows a flowchart of the experimental design. Uniformly sized tomato fruits (36 fruits per treatment) were collected, frozen in liquid nitrogen, and then stored at –70 °C, for determining the relevant indicators.
Determination of amino acids
The tomato samples were prepared and the liquid chromatograph-mass spectrometer (LC-MS) analysis of the free amino acid components followed the method described in Jin et al. (2021), with slight modifications. The 20 amino acid standards (purity: > 99%) were obtained from Merck and Sigma-Aldrich (St. Louis, MO, USA). Quantitative analysis was performed by external standard method and standard curves were generated with Agilent Mass Hunter (version 8.0) software. The standard curves, correlation coefficients, and recoveries of amino acids standards determined by LC-MS are shown in Table 1S (supplementary materials). Frozen tomato powder (0.1 g) was added to 1 mL of 0.5 M hydrochloric acid (analytical reagent) in the extraction water. Samples were then mixed at 6,600 × g for 20 min using a vortex mixer (MX-S, Scilogex, San Diego, CA, USA) before being extracted by ultrasonication (SB-800 DT, NingBo Scientz Biotechnology Co., China) at 25 °C for 20 min. Subsequently, the samples were centrifuged at 20,000×g for 20 min (using a 3–18 KS centrifuge, Sigma, Osterode am Harz, Germany). The supernatant was filtered through a 0.22 µm aqueous filter, with 5 µL of the supernatant being injected into HPLC-MS (LC-MS, Agilent 1290-6460, CA, United States) for quantitative analysis.
The HPLC instrument conditions were as follows: The column was an Agilent InfinityLab Poroshell 120 HILIC-Z (2.1 × 100 mm, 2.7 μm). A 200 mM ammonium formate (chromatographic purity) stock solution at pH 3 was prepared using water. The mobile phase A was a water: ammonium formate stock solution at 9:1. The mobile phase B was an acetonitrile (chromatographic purity): ammonium formate stock solution at 9:1 (the final concentration of mobile phases A and B was 20 mM). The flow rate was 0.5 mL·min−1. The column temperature was 25 °C. Meanwhile, the MS source conditions were as follows: Ionization mode, electron spray ionization (ESI) positive ion mode; A drying gas temperature of 330 °C; Atomizer at 35 psi; Gas flow rate of 13.0 L·min−1; Sheath gas temperature of 390 °C; Sheath gas flow rate of 12 L·min−1; A capillary voltage of 1500 V; Acquisition mode of multi-response monitoring model (MRM).
Determination of organic acids
Fresh sample (0.5 g) from three homogenised tomatoes was transferred to a 25-ml volumetric flask. This sample was then fixed with ultrapure water, shaken well, transferred to a 50-mL centrifuge tube, and centrifuged at 8500×g at 4 °C for 10 min (Wang et al., 2022). The supernatant (2 mL) was subsequently centrifuged and filtered through a 0.22-µm water system; samples were measured using an HPLC equipped with an ultraviolet (UV) detector (Agilent, 1260 Infinity II, Agilent Technologies, USA). All separations were performed using an X-Peonyx AQ-C18 column (250 × 4.6 mm, FeiniGen instrument, China) with a detection wavelength of 210 nm and a mobile phase of 0.2 mmol·L-1 sodium dihydrogen phosphate (analytical reagent) with isocratic elution at a flow rate of 1.2 mL·min−1 and a column temperature of 30 ℃; the injection volume was 5 µL. Table 2S (supplementary materials) presents the standard curves, correlation coefficients, and recoveries of organic acids standards determined by HPLC.
Determination of mineral elements
Fresh tomatoes (three replicates per treatment, three fruits per replicate) were heated at 105 °C and dried at 80 °C until a constant weight was obtained. Samples were then ground and sieved through 0.25-mm mesh and stored in self-sealing bags to determine mineral element content. Next, 0.5 g samples, for determination of total potassium (K) and phosphorus (P), were digested through wet digestion using H2SO4-H2O2. Additionally, 1 g of sample, used to measure the total calcium (Ca), magnesium (Mg), copper (Cu), iron (Fe), manganese (Mn), and zinc (Zn), was dried and washed in a muffle furnace. The total P was determined using the molybdenum-antimony colorimetric method. Meanwhile, total K, Ca, Mg, Cu, Fe, Mn, and Zn contents were measured using a ZEEnit 700P atomic absorption spectrometer (Analytik Jena AG, Germany). Table 3S (supplementary materials) shows the standard curves, correlation coefficients, and recoveries of the mineral element standards.
Determination of volatile compounds
Tomato volatile components were assessed using a portable electronic nose (PEN3, Airsense Analytics GmbH, Schwerin, Germany), according to the method previously described by Wang et al. (2022) with minor modifications. The PEN3 E-nose was equipped with a sensor array consisting of 10 chemical sensing elements; Table 4S (supplementary materials) presents a description of the material types and performance of the sensors. The signal response of the sensor is expressed as (G/G0), which describes the ratio of the conductivity of the volatile substance to that of pure air (Cai et al., 2021). Subsequently, 0.5 g fresh samples from three homogenised tomato fruits and 1.5 g of anhydrous sodium sulphate (analytical reagent) were added to the headspace bottle. The tightly capped headspace vials were then placed in a magnetic mixer at 70 °C and heated for 15 min to equilibrate the internal headspace gas. An injection needle was then inserted into the headspace bottle to measure the volatile compounds. The E-nose detection conditions were as follows: Flush time, 60 s; Sensor zeroing time of 5 s; Pre-sampling time of 5 s; Injection flow rate of 400 mL·min−1, Measured at 180 s.
Statistical analysis
Data were analysed using one-way analysis of variance (ANOVA) in SPSS software (version 22.0; SPSS Institute Inc., Chicago, IL, USA); significant differences were compared using Duncan’s multiple range test (p < 0.05). Correlation analysis, principal component analysis (PCA), radar fingerprint chart, heat map generation, and hierarchical cluster analysis (HCA) were all performed using Origin 2021 (Origin Inc., San Francisco, CA, USA). Results are presented as the mean ± standard error (SE). In all analyses, a probability value below 0.05 was considered statistically significant (p < 0.05).
Results and discussion
Effect of varying water deficiency levels on tomato amino acid content
Variance analysis
Amino acids are important parameters reflecting the flavour and nutritional value of fruits and vegetables. Amino acids are the basic building blocks of proteins and are precursors to various metabolites and neurotransmitters that sustain the normal metabolism of life. In addition to their functions as nutrient and flavour compounds, amino acids are involved in plant responses to biotic and abiotic stresses through osmoregulation. Several amino acids are also rich in sweet (alanine, glycine, and serine), sour (aspartic acid and glutamic acid), and bitter (leucine, phenylalanine, tryptophan, and tyrosine) flavours, which are closely associated with human taste perceptions (Jin et al., 2021). In our study, 20 free amino acid compounds were quantified using LC-MS. The WD treatment groups (T1–T4) exhibited significantly higher levels of alanine and serine, which are associated with sweetness (Table 1), suggesting that WD treatment potentially enriched the taste of tomatoes. Numerous compounds have previously been identified such as amino acids (proline and aspartate), sugar alcohols (mannitol, polyols, sorbitol, and quercetin), and organic acids (mainly malic and citric acid) (González-Chavira, Herrera-Hernández, Guzmán-Maldonado, & Pons-Hernández, 2018). As presented in Table 1, proline levels in tomatoes were significantly higher by 22.02% (T1), 152.29% (T2), 156.88% (T3), and 174.31% (T4) under the WD treatments compared to CK. Furthermore, compared to those of CK, the aspartate contents were higher by 32.47% (T1), 87.36% (T2), 11.41% (T3), and 20.15% (T4) in WD treatments. Overall, it was shown that the levels of proline and aspartate—involved in osmoregulation—were significantly higher in the T2 treatment than in the CK treatment. Conversely, phenylalanine content was significantly lower by 8.08% (T1) and 11.78% (T2) in the WD treated tomatoes, when compared to CK tomatoes. In addition, glutamate content was significantly reduced by 28.82% in the T2 treated tomatoes when compared with CK tomatoes. Therefore, T2 treatment may have been advantageous in facilitating the conversion of glutamate, as glutamate is a precursor of many amino acids and amino acid-derived compounds (including proline, arginine, ornithine, thiamine, and lysine) and is converted to alpha-ketoglutarate in the tricarboxylic acid cycle (TCA) cycle (Ali et al., 2019). As a result of metabolite conversion, phenylalanine is depleted as an upstream metabolite of polyphenols. Here, this resulted in lower levels of phenylalanine, as observed in the T2 treatment compared to CK. Meanwhile in our previous study, we observed that the T2 treatment promoted the accumulation of polyphenol content, especially cinnamic acid (Jin et al., 2022). The total amino acid content was also significantly higher by 13.36% (T1), 18.91% (T2), 10.81% (T3), and 12.35% (T4) in WD treatments compared to CK, with the highest levels being observed in T2 treated tomatoes. These results imply that a suitable water deficiency (T2 treatment) could promote primary metabolism whilst significantly increasing the total amino acid content in tomatoes.
Table 1.
Effect of different levels of water deficit on the content of amino acid components (mg g−1 DW) in tomato fruit.
| Amino acid type | Treatments |
||||
|---|---|---|---|---|---|
| CK | T1 | T2 | T3 | T4 | |
| Threonine | 0.306 ± 0.007ab | 0.288 ± 0.005bc | 0.274 ± 0.008c | 0.315 ± 0.013ab | 0.319 ± 0.006a |
| Phenylalanine | 0.297 ± 0.003a | 0.273 ± 0.005b | 0.262 ± 0.005b | 0.303 ± 0.013a | 0.306 ± 0.003a |
| Leucine | 0.137 ± 0.003b | 0.144 ± 0.002b | 0.137 ± 0.002b | 0.189 ± 0.008a | 0.176 ± 0.004a |
| Isoleucine | 0.109 ± 0.001c | 0.113 ± 0.002c | 0.153 ± 0.001b | 0.147 ± 0.007b | 0.171 ± 0.003a |
| Asparagine | 0.107 ± 0.003c | 0.104 ± 0.005c | 0.153 ± 0.008b | 0.143 ± 0.012b | 0.181 ± 0.004a |
| Tryptophan | 0.214 ± 0.005c | 0.215 ± 0.006c | 0.247 ± 0.010b | 0.23 ± 0.004bc | 0.305 ± 0.010a |
| Methionine | 0.105 ± 0.001b | 0.123 ± 0.002a | 0.096 ± 0.006b | 0.129 ± 0.007a | 0.129 ± 0.008a |
| Proline | 0.109 ± 0.003d | 0.133 ± 0.004c | 0.275 ± 0.003b | 0.28 ± 0.004b | 0.299 ± 0.006a |
| Valine | 0.046 ± 0.001c | 0.060 ± 0.002b | 0.05 ± 0.001c | 0.067 ± 0.004a | 0.07 ± 0.002a |
| Tyrosine | 0.053 ± 0.003bc | 0.043 ± 0.003c | 0.061 ± 0.001b | 0.061 ± 0.001b | 0.074 ± 0.006a |
| Cysteine | 0.026 ± 0.007a | 0.033 ± 0.003a | 0.027 ± 0.002a | 0.032 ± 0.001a | 0.032 ± 0.003a |
| Alanine | 0.249 ± 0.004c | 0.343 ± 0.003ab | 0.328 ± 0.011b | 0.318 ± 0.009b | 0.367 ± 0.011a |
| Glycine | 0.028 ± 0.002ab | 0.035 ± 0.002ab | 0.023 ± 0.007c | 0.039 ± 0.004a | 0.038 ± 0.003a |
| Serine | 0.185 ± 0.006d | 0.240 ± 0.006b | 0.217 ± 0.005c | 0.267 ± 0.002a | 0.281 ± 0.005a |
| Glutamate | 33.309 ± 0.441b | 33.433 ± 0.449b | 23.71 ± 0.613c | 36.63 ± 0.54a | 34.494 ± 0.911b |
| Aspartate | 21.897 ± 0.314d | 29.006 ± 0.586b | 41.026 ± 1.36a | 24.395 ± 0.358c | 26.31 ± 0.748c |
| Histidine | 4.888 ± 0.533c | 5.762 ± 0.128bc | 7.159 ± 0.336a | 5.471 ± 0.128bc | 6.367 ± 0.226ab |
| Cystine | 0.013 ± 0.004a | 0.010 ± 0.001a | 0.012 ± 0.001a | 0.013 ± 0.003a | 0.015 ± 0.001a |
| Arginine | 0.529 ± 0.010a | 0.558 ± 0.010a | 0.517 ± 0.007a | 0.511 ± 0.049a | 0.406 ± 0.01b |
| Glutamine | 1.971 ± 0.036c | 2.289 ± 0.041a | 2.064 ± 0.067abc | 2.022 ± 0.088bc | 2.218 ± 0.101ab |
| Total amino acids | 64.579 ± 1.281c | 73.205 ± 1.185ab | 76.79 ± 2.382a | 71.562 ± 0.359b | 72.556 ± 1.56ab |
Note: The data are expressed as average values ± SE (n = 3). Different lowercase letters in the same row of the table indicate significant differences between treatments (p < 0.05).
Abbreviations: DW: dry weight; CK: the control with full irrigation of 90% the maximum field moisture capacity (FMC); T1: substrate maintained at 80% maximum FMC; T2: substrate maintained at 65% maximum FMC; T3: substrate maintained at 55% maximum FMC; T4: substrate maintained at 45% maximum FMC.
Correlation analysis
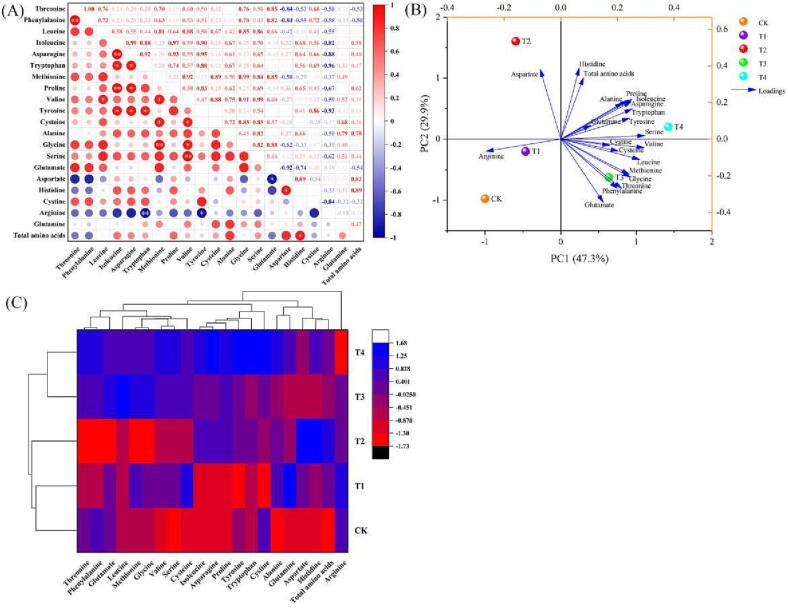
The shikimate pathway plays an important role in plant growth and development, pest defence, and plant response to various environmental stresses. Tryptophan, phenylalanine, and tyrosine are all produced in the shikimate pathway and are considered the three central molecules involved in plant metabolism (Galili & Höfgen, 2002). In plants, glutamate, glutamine, aspartate, and asparagine are all involved in the assimilation and transport of nitrogen (N) for use in growth, defence, and reproduction processes (Zemanová, Pavlík, Pavlíková, Hnilička, & Vondráčková, 2016). These studies have demonstrated the existence of intrinsic relationships between amino acid components. Similarly, our Pearson’s correlation analysis revealed multiple sets of significant or highly significant correlations between amino acid components (Fig. 1A). Of them, highly significant positive correlations were observed between isoleucine and asparagine (r = 0.99), and isoleucine and proline (r = 0.97). Additionally, phenylalanine and threonine exhibited highly significant, positive correlations (r = 1). Further, serine and valine also exhibited highly significant, positive correlations, whereas arginine and tryptophan showed significantly negatively correlation (r = –0.96). Asparagine was significantly positively correlated with tryptophan (r = 0.92), proline (r = 0.93), and tyrosine (r = 0.95), and histidine was significantly positively correlated with aspartate (r = 0.89). Aspartate, which plays a pivotal role in the aspartate-glutamate pathway in amino acid metabolism and can be transformed into glutamate and asparagine (Azevedo et al., 2006, González-Chavira et al., 2018), was significantly negatively correlated with glutamate (r = −0.92), possible because WD irrigation may have inhibited the aspartate-glutamate conversion.
Fig. 1.
Pearson’s correlation analysis (A), principal component analysis (B) and cluster analysis (C) of amino acids in tomatoes under different water deficiencies. The data are expressed as average values (n = 3). The * and ** represent significant correlations at p < 0.05 and p < 0.01 levels, respectively (two-tailed). Abbreviations: CK: The control with full irrigation of 90% the maximum field moisture capacity (FMC); T1: Substrate maintained at 80% maximum FMC; T2: Substrate maintained at 65% maximum FMC; T3: Substrate maintained at 55% maximum FMC; T4: Substrate maintained at 45% maximum FMC.
Principal component and cluster analyses
Previous studies have found that amino acid metabolism was closely linked to abiotic stress tolerance. For example, Zhang et al. (2017) found that amino acid metabolism and carbohydrate metabolic pathways played an essential role in the response to salt stress in tomatoes. Free amino acids are an important component of the osmoregulatory substances involved in tomato response to water deficit. The classification model for the amino acid contents of tomatoes under varying levels of WD, using a principal component analysis, is presented in Fig. 1B. This analysis involved the five water treatments and their 21 amino acid parameters. The first two principal components accounted for 77.2% of the variation, with PC1 and PC2 explaining 47.3% and 29.9% of the total variance, respectively. Serine, valine, and leucine were the main factors contributing to the first principal component. Meanwhile, aspartate, histidine, and total amino acids were the main factors in the second principal component and were considered proxy factors for the amino acids in the tomatoes. The CK, T1, T2, T3, and T4 treatment groups were all visibly separated in PC1, while T2, T1, and CK were visibly separated in PC2. These classification results were corroborated by the results of the cluster analysis. The classification model based on the similarities in the cluster analysis classified the five treatments into two broad categories: One contained the T3 and T4 treatments and the other contained the T1, T2, and CK treatments (Fig. 1C). A previous study on the influences of tomato quality under well-watered conditions and drought stress also identified significant differences among free amino acid components (Klunklin & Savage, 2017).
Effects of varying water deficiency levels on tomato organic acid content
Variance analysis
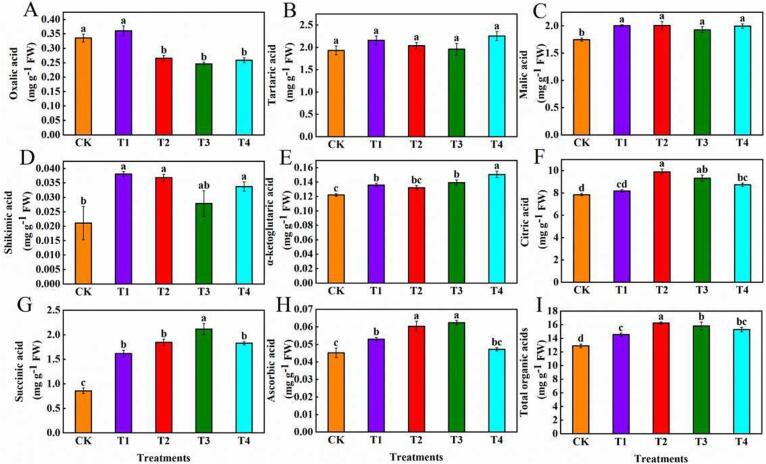
Several previous studies have demonstrated that moderate WD could improve the nutritional and flavour quality of fruits by promoting primary (amino acids and organic acids) and secondary (phenolics and volatile compounds) metabolites (González-Chavira et al., 2018). Organic acids (as the main intermediates of carbon metabolism in plant cells) contribute substantially to flavour, by altering acidity. Notably, the concentration of primary/secondary metabolites positively correlated with the degree of WD within a certain WD range (Bertin & Génard, 2018). In the present study, the total amino (Table 1) and organic acids (Fig. 2I) tended to initially increase before decreasing, as the degree of WD increased, with the peak in total amino acid and total organic acid contents being observed in the T2 treatment. In addition, the application of different degrees of WD increased the content of malic, shikimic, α-ketoglutaric, citric, succinic, and ascorbic acid (Fig. 2C–H) in tomatoes at the red ripening stage, with the T2 treatment providing the optimal results. Our results are in line with those of Bai et al. (2023) but contradictory to those of Medyouni et al. (2021) in terms of investigating the influence of water deficiency on tomato organic acids. These differing results may have been due to varying tomato genotypes and growth periods at which the water treatment was initiated. However, WD irrigation increased the organic acid content without causing an increase in the sour taste of tomatoes, as the balance of sugars and acids in combination determines fruit taste (Davies, Hobson, & McGlasson, 1981). Furthermore, Ripoll et al. (2016) previously identified that the organic acid content in the fresh matter of tomato was higher after WD treatment than when fully irrigated. However, Ripoll et al. (2016) observed the opposite trend for the organic acid content in the dry matter of tomatoes. Our results support the presence of a higher organic acid content in WD treated tomatoes, which may been a dilution effect. Organic acid compounds contain a large number of hydroxyl groups and facilitate hydrogen bonding with water molecules, thereby maintaining macromolecule functions in solution. Similarly, the levels of citric and malic acids, which are osmoregulatory substances, were significantly higher in the T2 treatment than in the CK treatment. Ascorbic acid is well known for its powerful antioxidant activity, reactive oxygen species (ROS) scavenging capacity, and the ability to enhance plant stress tolerance (Lu & Zhu, 2022). In our study, the ascorbic acid content was significantly higher in the T2 treatment than in the CK treatment, suggesting that T2-treated tomatoes may have a higher antioxidant capacity. The TCA—also known as the citric acid cycle—is the final metabolic pathway for the three major nutrients, sugars, lipids, and amino acids, while also being the central hub of their metabolism. Oxaloacetate (OAA) is a substrate in citric acid biosynthesis in the TCA cycle and is converted from aspartic acid by aspartate transaminase (Fang et al., 2019). Notably, the T2 treatment showed the highest accumulation of aspartic and citric acid. Therefore, the T2 treatment may be a suitable WD strategy for the promotion of the TCA cycle.
Fig. 2.
Effect of different levels of water deficiency on the tomato content of: Oxalic acid (A), tartaric acid (B), malic acid (C), shikimic acid (D), α-ketoglutaric acid (E), citric acid (F), succinic acid (G), ascorbic acid (H), and total amino acids (I). The data are presented as the mean ± SE of three biological replicates. Different lowercase letters indicate significant differences in Duncan’s multiple range tests (p < 0.05). Abbreviations: CK: The control with full irrigation of 90% the maximum field moisture capacity (FMC); T1: Substrate maintained at 80% maximum FMC; T2: Substrate maintained at 65% maximum FMC; T3: Substrate maintained at 55% maximum FMC; T4: Substrate maintained at 45% maximum FMC.
Correlation analysis
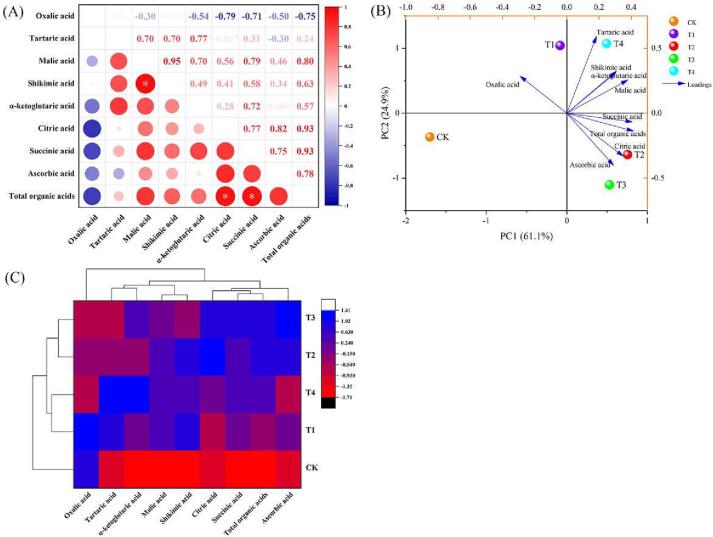
Oxalic, tartaric, malic, shikimic, α-ketoglutaric, citric, succinic, and ascorbic acids are all closely linked in the TCA cycle (Vitor & Sodek, 2019). The correlations between organic acids in tomato under WD conditions are presented in Fig. 3A. A positive correlation was observed among eight of the organic acid parameters. The only exception was oxalic acid, which was negatively correlated with the eight organic acids. In particular, shikimic and malic acid had a significant positive correlation (r = 0.95). Significant positive correlations were observed between the total organic acids and citric acid (r = 0.93) as well as the total organic acids with succinic acid (r = 0.93). Pearson's correlation analysis of amino acids and organic acids indicated an intrinsic relationship between these parameters, which resulted in overlapping information. These findings further support the use of PCA on the intrinsic relationships between indicators (Jin et al., 2021).
Fig. 3.
Pearson’s correlation analysis (A), principal component analysis (B) and cluster analysis (C) of organic acids under different levels of water deficiency. The data are expressed as average values (n = 3). The * and ** represent significant correlations at the p < 0.05 and p < 0.01 levels, respectively (two-tailed). Abbreviations: CK: The control with full irrigation of 90% the maximum field moisture capacity (FMC); T1: Substrate maintained at 80% maximum FMC; T2: Substrate maintained at 65% maximum FMC; T3: Substrate maintained at 55% maximum FMC; T4: Substrate maintained at 45% maximum FMC.
Principal component and cluster analyses
PCA is a valid approach for identifying primary metabolites in high-throughput profiles. Here, PCA was used to screen the organic acids in the major primary metabolites to determine variations in organic acid metabolites among the five water treatment groups. Fig. 3B shows the classification model for the organic acids in tomatoes under different WD treatments based on an intuitive visual representation of the PCA results. The first two principal components could explain 86.0% of the total variation, with PC1 and PC2 accounting for 61.1% and 24.9%, respectively. In addition, the total organic acids and succinic acid were the main contributors to the first principal component. Meanwhile, tartaric acid and shikimic acid were the main contributors to the second principal component. Therefore, these four components were used as proxies for the organic acid metabolites in tomatoes grown under the different WD treatments. The CK was distinctly separated from T1, T2, T3, and T4, based on the PC1 and PC2 results. Additionally, the T1 and T4 treatments and the T2 and T3 treatments were distinctly separated by PC2. T2 and T3 were grouped together, showing similar tomato organic acid contents. Similarly, the five treatments were classified into two categories: CK and T1–T4 using the classification model based on a cluster analysis (Fig. 3C). Within the T1–T4 category, the T2 and T3 treatments were most similar. Furthermore, PCA- and HCA-based classification models for amino acids and organic acids indicated that WD treatments could promote tomato nutritional and flavour quality, especially the T2 treatment. Controlled WD irrigation strategies could trigger abiotic stressor signalling pathways in primary metabolite synthesis to improve fruit quality by promoting the accumulation of bioactive compounds.
Effects of varying water deficiency levels on tomato mineral element content
Variance analysis
Both biotic and abiotic stresses can affect plant growth and development by impacting the nutrient status of the soil and how plant roots absorb mineral elements. Available mineral elements also determine the likelihood that a plant can survive extreme conditions, by protecting metabolism and directly participating in or inducing the synthesis of critical signalling molecules (Ahanger and Agarwal, 2017, Bista et al., 2018). The minerals required for the human diet are widely available in nature in inorganic forms. The effects of different levels of WD on the macro- and trace elements of tomatoes are provided in Table 5S (supplementary materials). In terms of the macro-elements, the K and P contents tended to be initially higher before decreasing with increasing WD. Conversely, the Ca and Mg contents tended to gradually increase. The K and P contents were significantly higher under the T2 treatment than under the CK treatment, with increases of 38.99% and 22.48%, respectively. For the trace elements, Fe content was the highest in the T2 treatment, being 2.52% higher than that in the CK. This is consistent with the findings of Lipan et al. (2019), who found that the mineral elements of almonds were lower under full irrigation and high water stress, whereas fruit mineral elements were higher under a suitable water-deficit irrigation regime. The relationship between water availability and mineral absorption by plants showed a correlation, as the uptake of soluble minerals depends on water flow in the soil-to-root pathway. Excess water can cause mineral leaching, while appropriate WD irrigation can reduce mineral leaching (Alikhani-Koupaei, Fatahi, Zamani, & Salimi, 2018), possibly explaining why appropriate WD irrigation enhanced mineral elements in tomatoes.
Correlation analysis
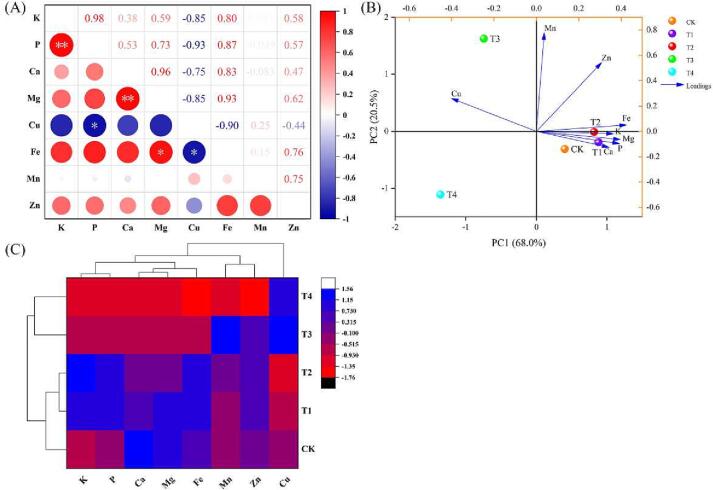
Pearson's correlation analysis was conducted to determine the strength of the relationship between the water treated mineral elements (Fig. 4A). Pillay & Fungo's (2016) investigation revealed a highly significant positive correlation between Fe and Mg, Mn, Na, and Zn. Fe and Mg also exhibited a significant positive correlation (r = 0.93). Furthermore, the P and K (r = 0.98) and Mg and Ca (r = 0.96) elements showed highly significant positive correlations. Anyasi et al. (2018) similarly reported a highly significant positive correlation of P with K and Mn.
Fig. 4.
Pearson’s correlation analysis (A), principal component analysis (B) and cluster analysis (C) of the mineral elements in tomato at different levels of water deficiency. The data are expressed as average values (n = 3). The * and ** represent significant correlations at the p < 0.05 and p < 0.01 levels, respectively (two-tailed). Abbreviations: CK: The control with full irrigation of 90% the maximum field moisture capacity (FMC); T1: Substrate maintained at 80% maximum FMC; T2: Substrate maintained at 65% maximum FMC; T3: Substrate maintained at 55% maximum FMC; T4: Substrate maintained at 45% maximum FMC.
Principal component and cluster analyses
The variable separations were examined, with PCA highlighting variations in mineral element contents among the five treatments. The components of PC1 and PC2 explained 88.5% of the total variation (PC1: 68.0%; PC2: 20.5%), indicating that the model correctly predicted the data. Clear separation between the mineral elements was observed among the five water treatments, while the CK, T1, T2, T3, and T4 treatment groups were clearly separated in PC1 (Fig. 4B). The loading plots also showed that Fe and Mg strongly influenced the first principal component. Similarly, Mn and Zn strongly influenced the second principal component. As demonstrated in Fig. 4C, the classification model based on cluster analysis classified the five water treatments into two categories using their mineral element contents: One group was CK, T1, and T2, and the other group contained T3 and T4 treatments. The PCA- and HCA- based model classified the five water treatments into two categories based on the mineral elements. The first category included CK, T1, and T2, and the second one contained the T3 and T4 treatment. The classification results indicated that excessive WD treatments significantly reduced mineral element accumulation in fruit, which may have resulted from excessive water stress leading to direct effects on root uptake kinetics and reduced mass flow and diffusion of nutrients from the soil (Bista et al., 2018).
Effect of varying water deficiency levels on tomato volatile compounds content
Variance analysis
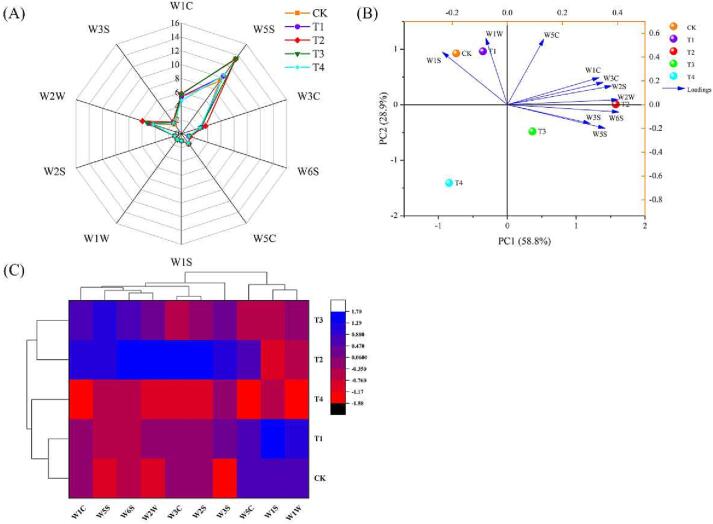
Fruits and vegetables can produce different aromas containing different volatile organic compounds. In contrast to traditional methods that rely on lengthy laboratory analysis, E-nose allows the rapid quality assessment of agricultural and food products by differentiating complex odours using a range of sensors (Peris & Escuder-Gilabert, 2016). Furthermore, E-nose is an instrument designed to mimic human olfactory perception, allowing the identification and classification of volatile mixtures while eliminating olfactory fatigue, which is common in humans (Wei et al., 2022). E-noses are widely used in industries such as the pharmaceutical, forestry, feed, dairy, and food industries, which determine odour-related qualities (Tan & Xu, 2020). Therefore, an E-nose equipped with 10 constant sensors investigated the effect of WD on the overall flavour characteristics of tomatoes. As demonstrated in Fig. 5A, W5S, W1C, W2W, W3C, and W3S were all sensitive to nitrogen oxides, aromatic benzene compounds, aromatic organic sulphide substances, aromatic ammonia compounds, and long-chain alkanes, respectively. All flavour characteristics had high responses in all tomato samples. The highest responses for W5S were observed in all tomatoes with WD treatments. The sensor W5S significantly contributed to tomato flavour. Notably, the aromas of W5S, W3C, W2W, W6S, and W2S sensors were significantly higher under the T2 treatment than under the CK treatment (Table 6S, supplementary materials). These results, consistent with those of Wang et al. (2022), indicate that WD altered the aroma composition of tomatoes.
Fig. 5.
Radar fingerprint chart (A), principal component analysis (B) and cluster analysis (C) of the aroma characteristics in tomato grown under different levels of water deficiency. Data are expressed as mean values (n = 3). The aromatic compounds include: W1C: Aromatic benzene compounds; W5S: High sensitivity, sensitive to nitrogen oxides; W3C: Sensitive aromatic components, ammonia; W6S: Mainly selective to hydride; W5C: Aromatic components of short-chain alkanes; W1S: Sensitive to methyl groups; W1W: Sensitive to sulphides; W2S: Sensitive to alcohols, aldehydes and ketones; W2W: Aromatic components, sensitive to organic sulphides; W3S: Sensitive to long-chain alkanes. Abbreviations: CK: The control with full irrigation of 90% the maximum field moisture capacity (FMC); T1: Substrate maintained at 80% maximum FMC; T2: Substrate maintained at 65% maximum FMC; T3: Substrate maintained at 55% maximum FMC; T4: Substrate maintained at 45% maximum FMC.
Principal component and cluster analyses
A strong relationship was observed between aroma and fruit or vegetable quality, which can be attributed to distinctive odours based on individual consumer preferences (Baietto & Wilson, 2015). PCA was performed using the E-nose data results to assess the impact of various WD treatments on the tomato sample groupings. A two-dimensional plot of the scores and loadings for the tomato samples with different WD levels is presented in Fig. 5B. PC1 and PC2 accounted for 58.8% and 28.9% of the total variance, respectively. Meanwhile, the cumulative contribution of the first two PCs accounted for 87.70% of the variation, indicating that they sufficiently differed between the tomato samples from different water treatments. The sample groups were clearly divided into two categories in PC1. One group included CK, T1, and T4 treatments, and the second category included the T2 and T3 treatments. The classification model based on the cluster analysis grouped the five treatments into two categories (Fig. 5C). The W2W, W6S, and W2S were all significantly positively correlated with PC1; the tomato samples in the T2 treatment highly contributed to this grouping. The classification results based on PCA and HCA revealed a clear separation between the WD (T2 and T3) treatments and CK, suggesting that moderate WD treatments altered the aroma characteristics of tomatoes, resulting in a more intense aroma that was most pronounced in the T2 treatment, which may also optimize consumer acceptance.
Conclusions
In this study, different levels of WD irrigation treatments were found to increase amino acids, organic acids, mineral elements, and volatile compounds in tomatoes to varying degrees. Among them, the T2 treatment significantly increased proline, alanine, serine, aspartate, and asparagine, while significantly decreasing phenylalanine and glutamic acid contents. Meanwhile, the T2 treatment significantly increased the main organic acid contents (including malic, citric, and ascorbic acid) in tomatoes. The mineral elements and volatile compounds in the T2 treatment exhibited increased macro-elements (K and P) and characteristic aromas. These results indicate that T2 treatment is a suitable and promising WD irrigation strategy for improving the nutritional quality and enhancing flavour in tomatoes. This study provides direction for WD irrigation technology to regulate tomato quality for increased consumer satisfaction. Future research should focus on resolving the mechanisms by which moderate WD improves the nutritional and flavour quality of tomatoes at the molecular level.
Funding
This work was supported by the Major Science and Technology Special Projects in Gansu Province (22ZD6NA009); Gansu Top Leading Talent Plan (GSBJLJ-2021-14); Modern Silk Road Cold and Arid Agriculture Science and Technology Support Project (GSLK-2021-6); Fuxi Young Talents Fund of Gansu Agricultural University (GAUfx-04Y03); the Special Project of Central Government Guiding Local Science and Technology Development (ZCYD-2021-07), and the Soft Science Program of Gansu Provincial Science and Technology Department (20CX4ZA044).
CRediT authorship contribution statement
Ning Jin: Methodology, Writing – original draft, Writing – review & editing, Visualization. Dan Zhang: Methodology, Visualization. Li Jin: Methodology. Shuya Wang: Visualization, Supervision. Xiting Yang: Investigation. Yongzhong Lei: Methodology. Xin Meng: Validation, Formal analysis. Zhiqi Xu: Validation, Formal analysis. Jianhong Sun: Investigation. Jian Lyu: Conceptualization, Resources, Writing – review & editing, Supervision, Funding acquisition. Jihua Yu: Conceptualization, Resources, Writing – review & editing, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2023.100756.
Contributor Information
Jian Lyu, Email: lvjiangs@126.com.
Jihua Yu, Email: yujihuagg@163.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Ahanger M.A., Agarwal R. Salinity stress induced alterations in antioxidant metabolism and nitrogen assimilation in wheat (Triticum aestivum L) as influenced by potassium supplementation. Plant Physiology and Biochemistry. 2017;115:449–460. doi: 10.1016/j.plaphy.2017.04.017. [DOI] [PubMed] [Google Scholar]
- Ali Q., Haider M.Z., Shahid S., Aslam N., Shehzad F., Naseem J.…Hussain S.M. Plant tolerance to environmental stress. CRC Press; 2019. Role of amino acids in improving abiotic stress tolerance to plants; pp. 175–204. [Google Scholar]
- Alikhani-Koupaei M., Fatahi R., Zamani Z., Salimi S. Effects of deficit irrigation on some physiological traits, production and fruit quality of ‘Mazafati’date palm and the fruit wilting and dropping disorder. Agricultural Water Management. 2018;209:219–227. doi: 10.1016/j.agwat.2018.07.024. [DOI] [Google Scholar]
- Anyasi T.A., Jideani A.I., Mchau G.R. Phenolics and essential mineral profile of organic acid pretreated unripe banana flour. Food Research International. 2018;104:100–109. doi: 10.1016/j.foodres.2017.09.063. [DOI] [PubMed] [Google Scholar]
- Azevedo R., Lancien M., Lea P. The aspartic acid metabolic pathway, an exciting and essential pathway in plants. Amino Acids. 2006;30(2):143–162. doi: 10.1007/s00726-005-0245-2. [DOI] [PubMed] [Google Scholar]
- Bai C., Zuo J., Watkins C.B., Wang Q., Liang H., Zheng Y.…Ji Y. Sugar accumulation and fruit quality of tomatoes under water deficit irrigation. Postharvest Biology and Technology. 2023;195 doi: 10.1016/j.postharvbio.2022.112112. [DOI] [Google Scholar]
- Baietto M., Wilson A.D. Electronic-nose applications for fruit identification, ripeness and quality grading. Sensors. 2015;15(1):899–931. doi: 10.3390/s150100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertin N., Génard M. Tomato quality as influenced by preharvest factors. Scientia Horticulturae. 2018;233:264–276. doi: 10.1016/j.scienta.2018.01.056. [DOI] [Google Scholar]
- Bista D.R., Heckathorn S.A., Jayawardena D.M., Mishra S., Boldt J.K. Effects of drought on nutrient uptake and the levels of nutrient-uptake proteins in roots of drought-sensitive and-tolerant grasses. Plants. 2018;7(2):28. doi: 10.3390/plants7020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno A., Durante M., Marrese P.P., Migoni D., Laus M.N., Pace E.…Lenucci M.S. Shades of red: Comparative study on supercritical CO2 extraction of lycopene-rich oleoresins from gac, tomato and watermelon fruits and effect of the α-cyclodextrin clathrated extracts on cultured lung adenocarcinoma cells’ viability. Journal of Food Composition and Analysis. 2018;65:23–32. doi: 10.1016/j.jfca.2017.08.007. [DOI] [Google Scholar]
- Cai J.-S., Zhu Y.-Y., Ma R.-H., Thakur K., Zhang J.-G., Wei Z.-J. Effects of roasting level on physicochemical, sensory, and volatile profiles of soybeans using electronic nose and HS-SPME-GC–MS. Food Chemistry. 2021;340 doi: 10.1016/j.foodchem.2020.127880. [DOI] [PubMed] [Google Scholar]
- Davies J.N., Hobson G.E., McGlasson W. The constituents of tomato fruit—the influence of environment, nutrition, and genotype. Critical Reviews in Food Science & Nutrition. 1981;15(3):205–280. doi: 10.1080/10408398109527317. [DOI] [PubMed] [Google Scholar]
- Devarajan R., Jayaraman J.K., Somasundaram S.M., Ragupathy S., Raman P., Sathiamoorthy K., Subbaraya U. Genetic diversity in fresh fruit pulp mineral profile of 100 Indian Musa accessions. Food Chemistry. 2021;361 doi: 10.1016/j.foodchem.2021.130080. [DOI] [PubMed] [Google Scholar]
- Dietz K.J., Zörb C., Geilfus C.M. Drought and crop yield. Plant Biology. 2021;23(6):881–893. doi: 10.1111/plb.13304. [DOI] [PubMed] [Google Scholar]
- Fang Y., Deng X., Lu X., Zheng J., Jiang H., Rao Y.…Xue D. Differential phosphoproteome study of the response to cadmium stress in rice. Ecotoxicology and Environmental Safety. 2019;180:780–788. doi: 10.1016/j.ecoenv.2019.05.068. [DOI] [PubMed] [Google Scholar]
- Farouk S., El-Metwally I. Synergistic responses of drip-irrigated wheat crop to chitosan and/or silicon under different irrigation regimes. Agricultural Water Management. 2019;226 doi: 10.1016/j.agwat.2019.105807. [DOI] [Google Scholar]
- Galili G., Höfgen R. Metabolic engineering of amino acids and storage proteins in plants. Metabolic Engineering. 2002;4(1):3–11. doi: 10.1006/mben.2001.0203. [DOI] [PubMed] [Google Scholar]
- González-Chavira M.M., Herrera-Hernández M.G., Guzmán-Maldonado H., Pons-Hernández J.L. Controlled water deficit as abiotic stress factor for enhancing the phytochemical content and adding-value of crops. Scientia Horticulturae. 2018;234:354–360. doi: 10.1016/j.scienta.2018.02.049. [DOI] [Google Scholar]
- Jin N., Jin L., Luo S., Tang Z., Liu Z., Wei S.…Zhong Y. Comprehensive evaluation of amino acids and polyphenols in 69 varieties of green cabbage (Brassica oleracea L. var. capitata L.) based on multivariate statistical analysis. Molecules. 2021;26(17):5355. doi: 10.3390/molecules26175355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin N., Jin L., Wang S., Meng X., Ma X., He X.…Yu J. A comprehensive evaluation of effects on water-level deficits on tomato polyphenol composition, nutritional quality and antioxidant capacity. Antioxidants. 2022;11(8):1585. doi: 10.3390/antiox11081585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee H.J., Tieman D.M. The genetics of fruit flavour preferences. Nature Reviews Genetics. 2018;19(6):347–356. doi: 10.1038/s41576-018-0002-5. [DOI] [PubMed] [Google Scholar]
- Klunklin W., Savage G. Effect on quality characteristics of tomatoes grown under well-watered and drought stress conditions. Foods. 2017;6(8):56. doi: 10.3390/foods6080056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipan L., Martín-Palomo M.J., Sánchez-Rodríguez L., Cano-Lamadrid M., Sendra E., Hernández F.…Carbonell-Barrachina Á.A. Almond fruit quality can be improved by means of deficit irrigation strategies. Agricultural Water Management. 2019;217:236–242. doi: 10.1016/j.agwat.2019.02.041. [DOI] [Google Scholar]
- Liu H., Meng F., Miao H., Chen S., Yin T., Hu S.…Zhu C. Effects of postharvest methyl jasmonate treatment on main health-promoting components and volatile organic compounds in cherry tomato fruits. Food Chemistry. 2018;263:194–200. doi: 10.1016/j.foodchem.2018.04.124. [DOI] [PubMed] [Google Scholar]
- Liu M., Yu H., Zhao G., Huang Q., Lu Y., Ouyang B. Profiling of drought-responsive microRNA and mRNA in tomato using high-throughput sequencing. BMC Genomics. 2017;18(1):1–18. doi: 10.1186/s12864-017-3869-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez G., Echeverria G., Bellvert J., Mata M., Behboudian M.H., Girona J., Marsal J. Water stress for a short period before harvest in nectarine: Yield, fruit composition, sensory quality, and consumer acceptance of fruit. Scientia Horticulturae. 2016;211:1–7. doi: 10.1016/j.scienta.2016.07.035. [DOI] [Google Scholar]
- Lu Y., Zhu H. The regulation of nutrient and flavour metabolism in tomato fruit. Vegetable Research. 2022;2(1):1–14. doi: 10.48130/VR-2022-0005. [DOI] [Google Scholar]
- Medyouni I., Zouaoui R., Rubio E., Serino S., Ahmed H.B., Bertin N. Effects of water deficit on leaves and fruit quality during the development period in tomato plant. Food Science & Nutrition. 2021;9(4):1949–1960. doi: 10.1002/fsn3.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirás-Avalos J.M., Intrigliolo D.S. Grape composition under abiotic constrains: Water stress and salinity. Frontiers Plant Science. 2017;8:851. doi: 10.3389/fpls.2017.00851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatello M.R.A., Zoccali R.A., Bruno A. Polyphenols: Mechanisms of action in human health and disease. Elsevier; 2018. Citrus fruit polyphenols and flavonoids: Applications to psychiatric disorders; pp. 119–131. [DOI] [Google Scholar]
- Pasković I., Soldo B., Ban S.G., Radić T., Lukić M., Urlić B.…Rouphael Y. Fruit quality and volatile compound composition of processing tomato as affected by fertilisation practices and arbuscular mycorrhizal fungi application. Food Chemistry. 2021;359 doi: 10.1016/j.foodchem.2021.129961. [DOI] [PubMed] [Google Scholar]
- Peris M., Escuder-Gilabert L. Electronic noses and tongues to assess food authenticity and adulteration. Trends in Food Science & Technology. 2016;58:40–54. doi: 10.1016/j.tifs.2016.10.014. [DOI] [Google Scholar]
- Pillay M., Fungo R. Diversity of iron and zinc content in bananas from East and Central Africa. HortScience. 2016;51(4):320–324. doi: 10.21273/HORTSCI.51.4.320. [DOI] [Google Scholar]
- Ripoll J., Urban L., Brunel B., Bertin N. Water deficit effects on tomato quality depend on fruit developmental stage and genotype. Journal of Plant Physiology. 2016;190:26–35. doi: 10.1016/j.jplph.2015.10.006. [DOI] [PubMed] [Google Scholar]
- Ruiz-Nieves J.M., Ayala-Garay O.J., Serra V., Dumont D., Vercambre G., Génard M., Gautier H. The effects of diurnal temperature rise on tomato fruit quality. Can the management of the greenhouse climate mitigate such effects? Scientia Horticulturae. 2021;278 doi: 10.1016/j.scienta.2020.109836. [DOI] [Google Scholar]
- Shohat H., Cheriker H., Kilambi H.V., Illouz Eliaz N., Blum S., Amsellem Z.…Weiss D. Inhibition of gibberellin accumulation by water deficiency promotes fast and long-term ‘drought avoidance’responses in tomato. New Phytologist. 2021;232(5):1985–1998. doi: 10.1111/nph.17709. [DOI] [PubMed] [Google Scholar]
- Tan J., Xu J. Applications of electronic nose (e-nose) and electronic tongue (e-tongue) in food quality-related properties determination: A review. Artificial Intelligence in Agriculture. 2020;4:104–115. doi: 10.1016/j.aiia.2020.06.003. [DOI] [Google Scholar]
- Tian S.-L., Lu B.-Y., Gong Z.-H., Shah S.N.M. Effects of drought stress on capsanthin during fruit development and ripening in pepper (Capsicum annuum L.) Agricultural Water Management. 2014;137:46–51. doi: 10.1016/j.agwat.2014.02.007. [DOI] [Google Scholar]
- Vitor S.C., Sodek L. Products of anaerobic metabolism in waterlogged roots of soybean are exported in the xylem. Plant Science. 2019;284:82–90. doi: 10.1016/j.plantsci.2019.03.023. [DOI] [PubMed] [Google Scholar]
- Wang L., Baldwin E.A., Bai J. Recent advance in aromatic volatile research in tomato fruit: The metabolisms and regulations. Food and Bioprocess Technology. 2016;9(2):203–216. doi: 10.1007/s11947-015-1638-1. [DOI] [Google Scholar]
- Wang S., Jin N., Jin L., Xiao X., Hu L., Liu Z.…Lyu J. Response of tomato fruit quality depends on period of LED supplementary light. Frontiers in Nutrition. 2022;9 doi: 10.3389/fnut.2022.833723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L., Wei S., Hu D., Feng L., Liu Y., Liu H., Liao W. Comprehensive flavor analysis of volatile components during the vase period of cut lily (Lilium spp. ‘Manissa’) flowers by HS-SPME/GC–MS combined with E-nose technology. Frontiers Plant Science. 2022;13 doi: 10.3389/fpls.2022.822956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemanová V., Pavlík M., Pavlíková D., Hnilička F., Vondráčková S. Responses to Cd stress in two Noccaea species (Noccaea praecox and Noccaea caerulescens) originating from two contaminated sites in Mežica, Slovenia and Redlschlag, Austria. Archives of Environmental Contamination and Toxicology. 2016;70(3):464–474. doi: 10.1007/s00244-015-0198-8. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Mao C., Shi Z., Kou X. The amino acid metabolic and carbohydrate metabolic pathway play important roles during salt-stress response in tomato. Frontiers Plant Science. 2017;8:1231. doi: 10.3389/fpls.2017.01231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Yu J., Brecht J.K., Jiang T., Zheng X. Pre-harvest application of oxalic acid increases quality and resistance to Penicillium expansum in kiwifruit during postharvest storage. Food Chemistry. 2016;190:537–543. doi: 10.1016/j.foodchem.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Zuccarelli R., Rodríguez-Ruiz M., Lopes-Oliveira P.J., Pascoal G.B., Andrade S.C., Furlan C.M.…Rossi M. Multifaceted roles of nitric oxide in tomato fruit ripening: NO-induced metabolic rewiring and consequences for fruit quality traits. Journal of Experimental Botany. 2021;72(3):941–958. doi: 10.1093/jxb/eraa526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.