Highlights
-
•
Differences in flavor of milk from a wide variety of samples is demonstrated.
-
•
Higher fatty ethyl esters are reasoned for uniqueness of taste in breast milk.
-
•
Unique flavor in human milk appears due to products of lipid thermal oxidation.
-
•
Five key differential flavour compounds identified.
Keywords: Flavoromic, Thermal oxidation, Volatile compounds, Fatty acid ethyl ester, Relative odor activity value (ROAV)
Abstract
Breast milk plays a crucial role in the taste development of infants, which cannot be replicated by other mammalian milk or formulas. This study aimed to identify and characterize the flavor substances in 15 different types of milk and analyze the differences among them. The results showed that human milk contained high levels of esters, particularly fatty acid ethyl esters, which contribute to its unique flavor. The four substances that had the highest flavor contribution in all species were identified as 2,3-butanedione, trimethylamine, isophorone, and acetaldehyde. Furthermore, the analysis of differences revealed that thermal-oxidation of lipids could explain the variation between human milk and other species in terms of flavor compounds. The key differential flavor compounds identified in milk from all species were trimethylamine, propanal, 1-pentanol, pyridine 2-methyl, and 2-butanone. These findings can potentially aid in developing formulas that better meet the taste needs of infants.
1. Introduction
The mammalian taste process is complex and can be activated by various chemical entities. It is regulated by taste-receptor cells and the nervous system (Vincis & Fontanini, 2019) and plays a crucial role in evaluating the edibility of consumed substances throughout one's lifespan (Chandrashekar et al., 2006, De Cosmi et al., 2017). In infancy, breast milk presents an opportunity for infants to learn different tastes and establish lifelong taste preferences (Forestell, 2017). The preference for a particular food determines whether it will be consumed or not. The early stages of life are critical for preference learning, during which experiences with various flavors can shape future eating habits (Hausner, Philipsen, Skov, Petersen, & Bredie, 2009). Providing reasonable taste guidance during this period can promote the formation of healthy dietary habits. Moreover, appetizing flavors have positive nutritional significance, as evidenced by the interconnection between the gustatory and digestive systems demonstrated in Pavlov's experiments. This phenomenon, known as the cerebral phase reaction, may have significant nutritional implications (Bloomfield, Alexander, Muelbert, & Beker, 2017).
Milk and dairy products are popular due to their nutritional value. However, recent studies have highlighted the importance of exploring the milk of different species to diversify nutritional benefits. Milk flavor is a crucial aspect of milk quality, determined by a specific balance of volatile compounds (Jia et al., 2019). Breast milk has been proven to be the optimal milk for infant health due to its diverse flavor profiles at different stages (Krpan, Major, Satalic, & Hruskar, 2021). Unfortunately, not every infant can be breastfed due to various factors such as maternal health, diet, and genetics. In such cases, infant formula plays a vital nutritional role in the early growth of infants. The flavor of the formula is an essential factor influencing the rate of consumption by infants. Studies have found differences in the satiety levels and weight gain rates of infants consuming hydrolyzed protein formulas compared to cow's milk formulas (Mennella, Ventura, & Beauchamp, 2011). Although the physiological mechanism behind this effect is unknown, it has been proven that the chemical composition of formula has an impact on the intake and growth of babies. Unfortunately, the current experience of most formulas is monotonous, both in terms of nutrition and flavor (De Cosmi et al., 2017). Electronic tongue studies have shown that breast milk is richer in flavor compared to formula (Krpan et al., 2021). Therefore, the development of formulas that help infants establish taste preference during the initial stages of life is essential.
Infant formula is commonly based on cow's milk or soymilk with added supplements that mimic the nutrients found in breast milk (Martin, Ling, & Blackburn, 2016). Previous research has found that lipids, aldehydes, ketones, and alcohols are the primary components in infant formula that are similar to breast milk, but some lipid-derived compounds are unique to infant formula (Hausner et al., 2009). In addition to the dietary flavor provided by the mother, heat treatment can also accelerate lipid autoxidation, which may contribute to differences in flavor between breast milk and formula (He et al., 2022). Infants may have different reactions to different types of formula, such as protein hydrolysate formula, which is high in glutamate and provides a more savory taste than other formulas and is often preferred by infants (Mennella, 2014). However, not all protein hydrolysate formulas result in a similar taste for infants. Experimental studies by Giudice have demonstrated that the relative palatability of different hydrolyzed formulas varies, although the evaluation criteria are subjective (Miraglia Del Giudice et al., 2015). Overall, there is still much to be understood about the composition of flavor compounds in breast milk and formula milk.
Raw milk is the foundation for producing dairy products, and it significantly impacts the flavor profile of these products. Fresh cow’s milk was described as pleasing, slightly sweet, with little aroma and a pleasant mouth feel and aftertaste (Al-Attabi, D'Arcy, & Deeth, 2009), which aligns with the innate preference of infants for sweetness and aversion to bitterness (De Cosmi et al., 2017). Thus, cow's milk is generally accepted as an adequate base for formula due to its favorable flavor profile. While research on formula based on milk from other species is prevalent from a nutritional standpoint, little attention has been paid to flavor. Goat's milk, which is rich in short and medium-chain fatty acids, imparts waxy and “goaty” flavors that negatively impact its sensory attributes (Ranadheera et al., 2019) and is generally considered less acceptable than cow's milk (Ozawa, Takada, Nishitani, Fujita, & Blair, 2010). Buffalo milk cheese is considered more delicious than cow's milk cheese, owing to its higher fat content (a significant source of creamy flavor) (Murtaza, Rehman, Anjum, & Huma, 2013). Yak milk is a vital part of the Tibetan diet and is characterized by its unique flavor profile, which is largely influenced by major volatile flavor compounds such as aldehydes, lipids, acids, and alcohols with an odor activity value (OAV) >1 (Jia et al., 2019). Sheep's milk is known for its complex “sheepy flavor,” which is difficult to describe, and is primarily attributed to 4-methyloctanoic and 4-ethyloctanoic acid, along with p-cresol, m-cresol, and 3,4-dimethylphenol, which contribute most of its sensory attributes (Ochoa-Flores et al., 2021). However, there are mixed reviews on whether this flavor is considered pleasant or not (Ochoa-Flores et al., 2021, Teng et al., 2018). Although camel, donkey, and horse milk are often added to dairy products for their unique flavors and nutritional benefits, previous studies have not explored their raw milk's flavor profile. As our understanding of the flavor of various mammalian milk deepens, research is emerging on the flavor value of milk from different mammals, in addition to their nutritional value.
Flavoromics, a non-targeted approach to correlating the flavor composition of food with their flavor, has emerged as an alternative to traditional flavoromics (Pérez-Jiménez, Sherman, Pozo-Bayón, & Pinu, 2021). However, to date, no systematic approach has been employed to characterize and compare the overall flavor of milk from multiple mammals. In this study, we utilized a GC × GC TOF MS full-two-dimensional high-end chromatography-mass spectrometry platform to analyze differential flavor substances in the milk of 15 species, expanding the comparison range horizontally from the species level. Our aim is to provide new insights into the development of the dairy industry by comparing flavors at multiple levels with a systematic approach that has not been utilized before. This study could contribute to the development of new products or the improvement of existing ones.
2. Materials and methods
2.1. Test sample collection
Fifteen mammalian milk samples were collected for this experiment in 2021, with six replicates obtained for each species. The information related to the source and abbreviations of the milk samples used in this experiment can be seen in Table 1. In all species, milk samples should be collected after 2 weeks of birth, with a minimum of 50 mL of milk per dam. At the end of each milking, the milk sample is removed and placed in the same 10 mL plastic container and immediately placed in a refrigerator at −20 °C for a short period, not exceeding two weeks. The samples are then transported in un-defrosted dry ice and placed in a freezer at −80 °C for long-term storage, but not more than four months.
Table 1.
Samples Information Schedule.
| Samples | Sample Collection Site | Samples Abbreviations |
|---|---|---|
| Chinese human milk | Chinese mothers from Shenyang Maternal and Child Care Center | CHP |
| Holstein cow milk | Huishan Dairy Group | HST |
| Buffalo milk | Guangxi Buffalo Research Institute and Nanjing Agricultural University | GXB |
| Yak milk | Qinghai province and Lanzhou institute of husbandry and pharmaceutical science of cass | QHY |
| DeZhou Donkey milk | East Ajiao Donkey Farm in Fumeng County | DZD |
| Mongolian Horse milk | Inner Mongolia XilinGol League Science and Technology Association | MGH |
| Alxa camel milk | Alxa Alpaca Farm in Inner Mongolia | ALC |
| Saanen milk goat milk | Liaoyang dairy goat farm | SNG |
| Nubian milk goat milk | Liaoyang dairy goat farm | NBY |
| Toggenburg milk goat milk | Liaoyang dairy goat farm | TGB |
| Liaoning Cashmere goat milk | Liaoyang National Core Sheep Breeding Farm | LCG |
| Small Tail Han sheep milk | Inner Mongolia Horinger County sheep farm | OAS |
| Pig milk | Yangxiang Pig Group | YXP |
Ethical Statements: The Animal Welfare and Ethics Committee of Shenyang Agricultural University approved the participation of people and animals in this study. Approval No.: 20210601.
2.2. Reagents and instruments
Ethanol was purchased from Aladdin (Shanghai, China). n-Hexyl-d13 Alcohol was obtained from C/D/N Isotopes INC (Quebec, Canada). A solid phase micro-extraction fiber (SPME) coated with divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS, 50/30 μm × 1 cm) assembly was obtained from Supelco (Bellefonte, USA). H2O was obtained by Milli-Q ultra-pure water machine (Millipore, Bedford, MA). Agilent 8890A GC (Agilent Technologies, Palo Alto, CA, USA). LECO Pegasus BT 4D (LECO, St. Joseph, MI, USA).
2.3. Internal standard solution preparation
A stock solution with 1 mg/L of n-Hexyl-d13 Alcohol was prepared in 50 % ethanol and stored at 4℃ refrigerator.
2.4. Sample preparation
1 mL of sample is transferred into a 20 mL headspace vial. Each sample is added with 10 μL of the ISTD solution. The sample is incubated at 50℃ for 30 min. The SPME fiber is placed in the chamber at 270℃ for 10 min before the extracted sample. The SPME is transferred to the incubator at 50℃ for 30 min. The SPME fiber is desorbed at 250℃ for 5 min in the GC injector. The SPME fiber is put in the chamber at 270℃ for 10 min after the injection step.
2.5. Comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry (GC × GC-TOFMS)
2.5.1. GC × GC analysis
Analyses were carried out using a LECO Pegasus® 4D instrument (LECO, St. Joseph, MI, USA) consisting of an Agilent 8890A GC (Agilent Technologies, Palo Alto, CA, USA) system equipped with a split/splitless injector, and dual stage cryogenic modulator (LECO) coupled with TOFMS detector (LECO). A DB-Heavy Wax (30 m × 250 μm I.D., 0.5 μm) (Agilent, USA) was used as the first dimension column (1D) and Rxi-5Sil MS (2.0 m × 150 μm I.D., 0.15 μm) (Restek, USA) was used as a second-dimension column (2D). The carrier gas was high purity helium (>99.999%) at a constant flow rate of 1.0 mL/min. The temperature program of the oven was as followed: the oven temperature was held at 40 ℃ for 3 min at first, then raised to 150 ℃ at the rate of 4 ℃/min and held for 2 min, then raised to 200 ℃ at the rate of 6 ℃/min and held for 0 min, then raised to 230 ℃ at the rate of 10 ℃/min and held for 10 min. The secondary oven temperature was operated at 5 °C higher than the first oven. The temperature of the modulator is always 15 ℃ higher than that of the second column. The modulator was operated with a 5.0 s modulation period. The GC injector temperature was 250 ℃ (Cheng et al., 2021, Cordero et al., 2013).
2.5.2. Mass spectrum conditions
Analysis of flavor substance was performed on LECO Pegasus BT 4D. The transfer line and TOF MS ion source temperature were set at 250 ℃ and 250 ℃, respectively. The acquisition frequency was 200 spectra/s. The mass spectrometer was operated in the EI mode at 70 eV using a range of m/z 35–550 and the detector voltage of 1984 V (Cheng et al., 2021, Cordero et al., 2013).
2.6. Data analysis
The databases provided the sample related information, including the compound name, cas number etc. The final analysis result was obtained by integrating this interpretation information together. In order to enable data of different magnitudes to be compared, the total peak area normalization or internal standard normalization was performed on the raw data. Data analysis includes Hierarchical cluster analysis, Multivariate statistical analysis and Flavoromics analysis.
3. Result
3.1. Statistics on the number of flavor substances identified
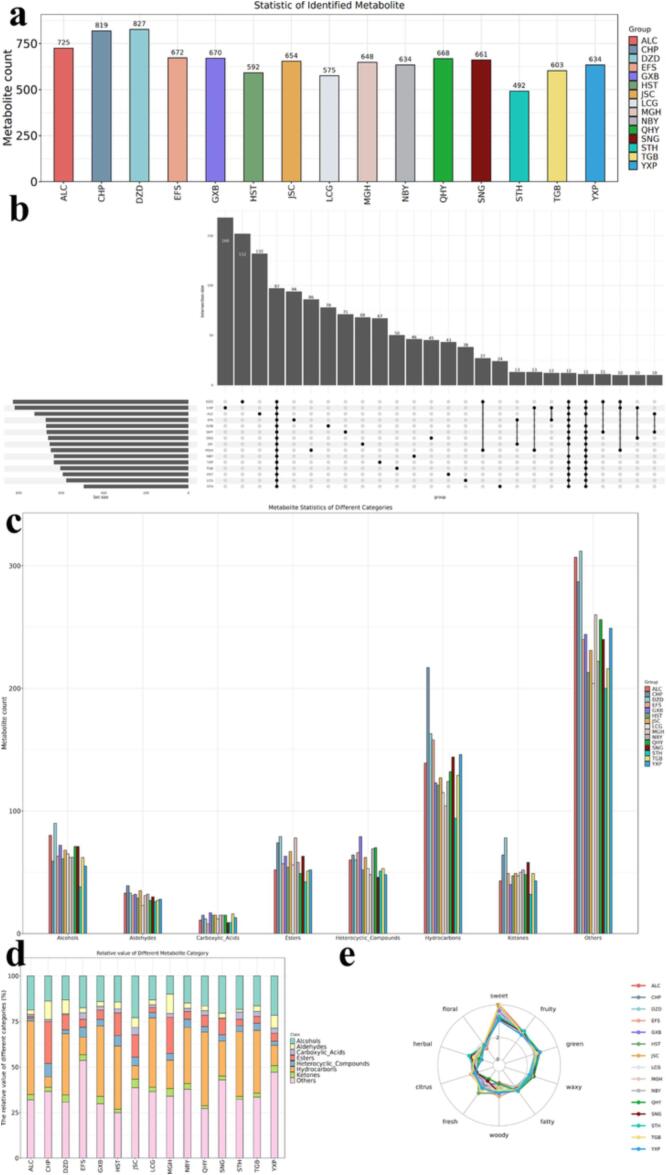
The original data of flavor compounds was analyzed using ChromaTOF software, and sensory annotations were retrieved from the NIST2020 database. After removing noise, the identification information for the flavor compounds was obtained. Fig. 1a displays the total number of compounds identified in the milk samples of each species. DZD had the highest number of identified flavor compounds, followed by CHP and ALC. The lowest number of flavor compounds was identified in STH.
Fig. 1.
A) statistic of identified compounds; b) UpSetPlot of identified compounds; c) Metabolite statistics of different categories; d) Stacked bar chart of the relative values of different compounds classes; e) Sensory flavor profile radar map.
The number of exclusive flavor compounds identified in a group and the number of common flavor compounds identified in multiple groups were displayed using UpSetPlot. Fig. 1b indicates that CHP contained the highest number of exclusive flavor compounds, with a total of 168, whereas STH had the lowest number of exclusive flavor compounds, with only 24 identified. Among the 15 species analyzed, 97 common flavor compounds were identified.
3.2. Grouping of flavor compound
Fig. 1c displays the number of flavor compounds corresponding to each class. This figure provides a summary statistic for the main classes, including Alcohols, Aldehydes, Carboxylic Acids, Esters, Heterocyclic Compounds, Hydrocarbons, and Ketones, which were present in all flavor compounds. Among the species analyzed, DZD contained the highest number of Alcohols, Esters, and Ketones. CHP had the most Aldehydes and Hydrocarbons, while GXB had the highest number of Carboxylic Acids and Heterocyclic Compounds, distinguishing it from other species.
As shown in Fig. 1d, the relative content of flavor compounds varied among different species. Alcohols were relatively more abundant in YXP, SNG, EFS and JSC, while aldehydes were relatively more abundant in CHP and MGH. Esters had a higher relative content in CHP and MGH than in other species. Hydrocarbons had a low relative content in CHP, EFS, YXP and JSC compared to other species.
3.3. Threshold analysis of volatile compounds
Odor activity value (OAV) is a measure of the minimum threshold at which humans can perceive a taste. However, when the samples contain a large number of flavor compounds, their relative percentage content can be used instead of their absolute content. The relative odor activity value (ROAV) can then be used to evaluate the contribution of each volatile component to the overall flavor. The formula for ROAV is as follows:
TA and PeakA were assumed to be the odor threshold and peak area of the compound with the minimum odor threshold in the sample, respectively. The ROAV for component A is set at the standard value of 100. TB and PeakB are the odor threshold and peak area of the compound to be measured, respectively. Where ROAV ≥ 1, the compound is considered to be the key flavor compound. Table 2 shows that TGB and HST had the highest number of key flavor compounds (19), while LCG had the lowest (2). The compounds with the highest ROAV values across all samples were 2,3-butanedione; trimethylamine; acetaldehyde; and isophorone. For most samples, 2,3-butanedione had the highest ROAV value, except for JSC, GXB, QHY, ALC and DZD (trimethylamine), TGB and HST (acetaldehyde), and YXP (isophorone).
Table 2.
Relative content statistics of high ROAV compounds.
| Name | CHP | YXP | SNG | TGB | NBY | LCG | EFS | STH | HST | JSC | GXB | QHY | ALC | MGH | DZD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2,3-Butanedione | 1.00E + 02 | 1.00E + 02 | 1.00E + 02 | 1.00E + 02 | 1.00E + 02 | 1.00E + 02 | 1.00E + 02 | 1.00E + 02 | 1.00E + 02 | 1.00E + 02 | 1.00E + 02 | 1.00E + 02 | 1.00E + 02 | 1.00E + 02 | 1.00E + 02 |
| Methylamine, N,N-dimethyl- | 6.62E + 01 | 5.04E + 01 | 1.42E + 01 | 4.45E + 01 | 5.60E + 00 | 8.25E-01 | 1.66E + 01 | 3.45E + 00 | 9.81E + 01 | 2.36E + 02 | 3.30E + 02 | 3.90E + 02 | 1.60E + 04 | 2.51E + 00 | 6.68E + 01 |
| 2-Heptanone | 9.69E + 00 | 1.16E + 01 | 5.74E + 00 | 2.86E + 01 | 8.24E + 00 | 2.29E-01 | 3.38E-01 | 6.84E + 00 | 6.12E + 01 | 1.43E + 01 | 1.00E + 01 | 1.08E + 01 | 6.67E + 00 | 2.57E + 00 | 1.37E + 00 |
| Acetic acid | 2.67E + 01 | 1.11E + 02 | 2.20E + 00 | 5.54E + 01 | 4.37E + 01 | 3.91E-03 | 2.74E + 01 | 3.50E + 01 | 8.39E + 01 | 4.34E + 01 | 3.96E + 01 | 3.37E + 01 | 5.94E + 01 | 1.11E + 00 | 9.38E-01 |
| Benzaldehyde | 2.12E + 01 | 1.68E + 02 | 2.04E + 01 | 7.02E + 01 | 1.30E + 01 | 8.14E-01 | 1.47E + 01 | 1.95E + 01 | 2.12E + 01 | 2.38E + 01 | 2.04E + 01 | 1.18E + 01 | 4.29E + 01 | 8.03E-01 | 8.73E-01 |
| Naphthalene | 2.67E + 01 | 7.88E + 01 | 2.09E + 01 | 5.19E + 01 | 1.91E + 01 | 4.22E-01 | 1.56E + 01 | 1.83E + 01 | 3.78E + 01 | 2.25E + 01 | 1.49E + 01 | 1.28E + 01 | 2.24E + 01 | 7.05E-01 | 6.88E-01 |
| Acetaldehyde | 3.69E + 01 | 1.67E + 02 | 5.74E + 01 | 2.06E + 02 | 6.93E + 01 | 1.70E + 00 | 2.19E + 01 | 1.26E + 00 | 2.40E + 02 | 5.97E + 01 | 8.00E + 01 | 9.02E + 01 | 1.50E + 02 | 2.82E + 00 | 5.51E-01 |
| Dimethyl sulfide | 1.14E + 00 | 1.67E + 00 | 2.94E + 00 | 1.67E + 00 | 6.45E-01 | 1.30E-02 | 4.94E + 00 | 5.74E-01 | 4.56E + 01 | 1.59E + 01 | 1.26E + 01 | 5.64E-01 | 2.21E + 01 | 4.14E-01 | 4.67E-01 |
| Ethanol | 5.23E + 00 | 1.52E + 01 | 5.03E + 00 | 2.19E + 01 | 7.57E + 00 | 1.75E-01 | 2.81E + 00 | 6.51E + 00 | 2.05E + 01 | 9.61E + 00 | 1.38E + 01 | 8.52E + 00 | 1.92E + 01 | 1.84E-01 | 1.94E-01 |
| Propanal, 2-methyl- | 5.98E-01 | 3.88E + 01 | 6.94E-01 | 2.98E + 00 | 9.30E-01 | 2.09E-02 | 8.59E-01 | 2.15E-01 | 1.52E + 00 | 2.05E + 00 | 1.06E + 00 | 9.49E-01 | 1.19E + 00 | 2.83E-02 | 1.63E-01 |
| a-Pinene | 3.12E + 01 | 3.33E + 00 | 1.66E + 01 | 3.55E + 01 | 1.16E + 00 | 4.30E-01 | 1.16E + 00 | 1.22E + 00 | 7.11E + 00 | 7.53E + 00 | 1.12E + 00 | 1.71E + 01 | 3.06E + 03 | 6.10E-02 | 1.38E-01 |
| Benzene, 1,2,4-trimethyl- | 8.72E-01 | 2.27E + 00 | 6.57E-01 | 1.70E + 00 | 8.44E-01 | 1.82E-02 | 2.84E-02 | 4.05E-01 | 1.53E + 00 | 4.04E-01 | 3.40E-01 | 5.74E-01 | 9.19E-01 | 2.02E-02 | 2.13E-02 |
| Acetic acid, butyl ester | 1.05E + 00 | 2.52E + 00 | 1.19E + 01 | 5.38E + 01 | 1.33E + 00 | 4.44E-01 | 1.27E + 01 | 7.42E + 00 | 7.85E + 00 | 1.66E + 01 | 5.17E-01 | 2.05E + 01 | 3.59E + 01 | 6.93E-02 | 1.79E-02 |
| Isophorone | 7.80E-01 | 3.82E + 02 | 6.55E-01 | 6.67E-01 | 7.50E-01 | 5.22E-03 | 2.33E-01 | 1.95E + 00 | 6.67E-01 | 4.60E-01 | 7.69E + 01 | 2.38E-01 | 8.62E-01 | 4.43E + 00 | 1.48E-02 |
| Dimethylamine | 1.80E-01 | 2.63E-01 | 9.32E-02 | 2.63E-01 | 9.19E-02 | 2.06E-03 | 9.19E-02 | 9.07E-02 | 1.63E + 00 | 1.82E-01 | 8.84E-02 | 8.91E-02 | 1.76E-01 | 4.81E-03 | 1.46E-02 |
| Propanal | 5.04E + 00 | 2.00E-01 | 5.22E-01 | 1.53E + 00 | 4.58E-01 | 1.57E-03 | 9.45E-01 | 7.82E-02 | 2.00E-01 | 1.18E + 00 | 7.90E-01 | 8.15E-01 | 2.84E + 00 | 4.09E-02 | 1.43E-02 |
| 2-Propenoic acid, butyl ester | 4.71E-01 | 2.28E + 00 | 2.77E + 00 | 1.51E + 01 | 1.59E + 00 | 6.48E-02 | 1.66E + 00 | 1.08E + 00 | 4.45E + 00 | 5.84E + 00 | 1.19E + 00 | 1.30E + 00 | 1.16E + 01 | 6.96E-02 | 1.41E-02 |
| Styrene | 2.06E + 00 | 4.63E + 00 | 4.08E + 00 | 8.45E + 00 | 3.30E + 00 | 3.18E-02 | 2.96E + 00 | 6.79E + 00 | 2.63E + 00 | 2.93E + 00 | 2.15E + 00 | 2.42E-02 | 7.15E + 00 | 1.44E-01 | 1.32E-02 |
| 1-Octene | 4.35E + 00 | 7.24E-01 | 7.09E-02 | 4.23E-01 | 6.99E-02 | 1.57E-03 | 5.05E-01 | 6.89E-02 | 2.96E-01 | 2.05E-01 | 6.72E-02 | 4.03E-01 | 6.97E-01 | 7.84E-03 | 1.30E-02 |
| Ethyl Acetate | 1.74E-01 | 8.49E-01 | 1.83E-01 | 6.03E-01 | 1.95E-01 | 5.50E-03 | 1.27E-01 | 2.69E-01 | 9.86E-01 | 4.01E-01 | 2.67E-01 | 2.91E-01 | 3.38E-01 | 9.06E-03 | 1.02E-02 |
| 1-Pentanol | 1.33E + 00 | 4.11E-01 | 1.81E-01 | 8.75E-01 | 4.09E-01 | 2.95E-03 | 1.97E-01 | 3.95E-01 | 1.28E + 00 | 4.89E-01 | 1.59E + 00 | 2.44E + 00 | 4.48E + 00 | 6.23E-02 | 8.58E-03 |
| Disulfide, dimethyl | 4.71E-01 | 9.20E-01 | 2.44E-01 | 2.54E + 00 | 2.56E-01 | 1.83E-02 | 2.41E-01 | 7.85E-01 | 6.90E-01 | 4.76E-01 | 2.32E-01 | 2.34E-01 | 4.62E-01 | 1.26E-02 | 8.03E-03 |
| Benzene, nitro- | 3.42E-01 | 5.00E-01 | 1.77E-01 | 5.00E-01 | 1.83E-01 | 3.91E-03 | 1.75E-01 | 1.72E-01 | 5.00E-01 | 3.45E-01 | 1.68E-01 | 1.69E-01 | 3.35E-01 | 9.14E-03 | 6.78E-03 |
| Pyridine | 2.10E-01 | 6.34E-01 | 5.26E-01 | 3.83E + 00 | 3.48E + 00 | 2.00E-02 | 1.66E-01 | 2.54E-01 | 2.08E + 00 | 1.12E + 00 | 1.23E + 00 | 1.53E + 00 | 1.21E + 00 | 7.60E-03 | 4.88E-03 |
| 2-Propenal | 6.86E-01 | 1.18E + 00 | 3.50E-01 | 8.79E-01 | 2.65E-01 | 4.35E-04 | 6.73E-01 | 1.91E-02 | 5.56E-02 | 6.94E-01 | 2.11E-02 | 2.37E-02 | 3.72E-02 | 1.02E-03 | 4.69E-03 |
| 1-Butanol | 4.29E-01 | 5.80E-01 | 1.28E-01 | 2.87E-01 | 1.30E-01 | 3.32E-03 | 2.98E-01 | 9.47E-02 | 3.19E-01 | 3.90E-01 | 4.10E-01 | 2.30E-01 | 5.13E-01 | 4.47E-03 | 4.66E-03 |
| Acetone | 4.31E-02 | 5.00E-04 | 1.12E-02 | 4.57E-01 | 1.13E-01 | 6.19E-03 | 2.03E-01 | 9.18E-02 | 3.60E-01 | 2.68E-01 | 2.62E-01 | 7.13E-02 | 3.23E-01 | 4.84E-03 | 3.63E-03 |
| Naphthalene, 2-methyl- | 3.28E-01 | 3.22E + 00 | 1.81E + 00 | 2.90E-01 | 1.01E-01 | 1.27E-02 | 1.01E-01 | 1.09E-01 | 2.90E-01 | 6.95E-01 | 1.05E + 00 | 1.05E-01 | 1.94E-01 | 5.30E-03 | 3.37E-03 |
| 2-Butanone | 7.95E-02 | 9.70E-01 | 7.76E-02 | 3.94E-01 | 1.01E-01 | 2.78E-03 | 9.99E-02 | 8.97E-02 | 1.44E + 00 | 7.00E-01 | 2.51E + 00 | 7.14E-02 | 4.02E-01 | 3.83E-03 | 3.22E-03 |
| 2-Hexanone, 5-methyl- | 6.51E-02 | 1.31E-01 | 3.43E-02 | 9.52E-02 | 3.95E-02 | 7.45E-04 | 3.33E-02 | 3.28E-02 | 9.52E-02 | 6.57E-02 | 3.20E-02 | 3.27E-01 | 6.38E-02 | 2.20E-03 | 2.32E-03 |
| 2-Hexanone | 7.81E-02 | 1.11E-02 | 4.22E-03 | 1.24E-01 | 6.01E-03 | 7.55E-04 | 2.18E-01 | 3.07E-03 | 3.54E-02 | 5.75E-03 | 3.52E-02 | 2.82E-03 | 7.02E-02 | 1.52E-04 | 1.90E-03 |
| Camphor | 8.11E-02 | 7.69E-02 | 2.73E-02 | 7.69E-02 | 2.82E-02 | 6.02E-04 | 2.69E-02 | 2.65E-02 | 7.69E-02 | 5.31E-02 | 2.58E-02 | 2.61E-02 | 3.13E-01 | 2.29E-03 | 1.67E-03 |
| Phthalic anhydride | 2.85E-02 | 2.94E-02 | 2.87E-02 | 1.29E-01 | 1.83E-02 | 1.26E-03 | 1.32E-03 | 1.35E-03 | 1.37E-01 | 1.75E-02 | 5.81E-02 | 6.04E-02 | 1.02E-01 | 1.78E-03 | 1.57E-03 |
| Pyridine, 2-methyl- | 1.20E-01 | 1.42E-01 | 6.19E-02 | 2.25E-01 | 2.43E + 00 | 1.82E-03 | 2.77E-02 | 5.21E-02 | 1.97E-01 | 7.87E-02 | 2.11E-01 | 1.05E-01 | 3.62E-01 | 1.41E-03 | 1.51E-03 |
| 1-Butanol, 3-methyl- | 8.09E-02 | 7.24E-01 | 4.19E-02 | 1.18E-01 | 4.13E-02 | 9.26E-04 | 4.72E-02 | 4.08E-02 | 1.91E-01 | 8.16E-02 | 3.98E-02 | 4.89E-02 | 7.92E-02 | 2.16E-03 | 1.38E-03 |
| Formic acid | 5.22E-02 | 3.85E-04 | 1.36E-04 | 2.24E-01 | 1.34E-04 | 9.70E-04 | 2.29E-03 | 9.11E-01 | 1.24E-01 | 8.84E-03 | 1.29E-04 | 7.55E-01 | 2.58E-04 | 1.97E-03 | 1.30E-03 |
| Limonene | 7.59E-02 | 1.11E-01 | 3.94E-02 | 1.88E + 01 | 3.88E-02 | 8.70E-04 | 3.88E-02 | 3.83E-02 | 1.11E-01 | 7.66E-02 | 3.98E-02 | 3.76E-02 | 7.44E-02 | 2.03E-03 | 1.29E-03 |
3.4. Flavor characteristics analysis
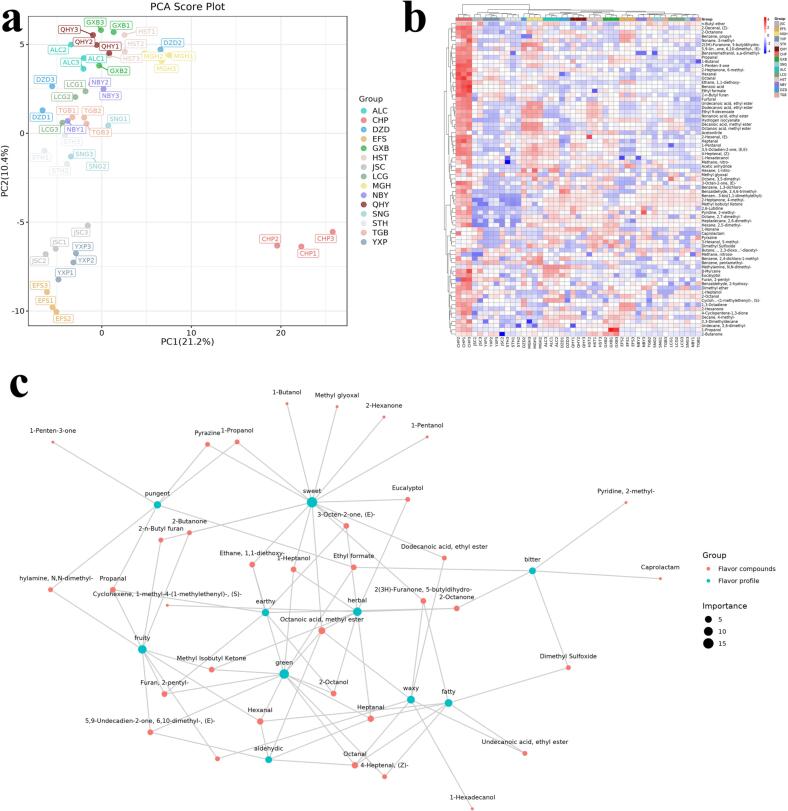
Milk flavor consists of two parts: identifiable taste and smell characteristics, and a complex of characteristics that cannot be separated. We used FlavorDB (It comprises of 25,595 flavor molecules representing an array of tastes and odors, https://cosylab.iiitd.edu.in/flavordb) to analyze and compare the sensory flavors of the compounds in different mammalian milks. We described the flavor using ten attributes: sweet, fruity, green, waxy, fatty, woody, fresh, citrus, herbal and floral. Fig. 1e shows the radar plots of the flavor profiles of each species. Most mammals have similar flavor profiles except for herbal and woody attributes. The most dominant flavor characteristics of mammalian milk are sweet, fruity and green.
3.5. Multivariate statistical analysis
Principal component analysis (PCA) is widely used as a multivariate statistical analysis tool for sample variance analysis as it simplifies the data and highlights the interrelationships between different samples. The first two principal components of the current study contributed 31.6%. Fig. 2a shows the results of PCA, where all species were grouped into three clusters and can be summarized as follows: CHP clustered alone; JSC, YXP, and EFS clustered in one cluster, and the other species clustered in one cluster.
Fig. 2.
A) pca score plot; b) hierarchical clustering heat map of differential compounds; c) the network of sensory flavor characteristics and flavor compounds.
3.6. Analysis of differential compounds
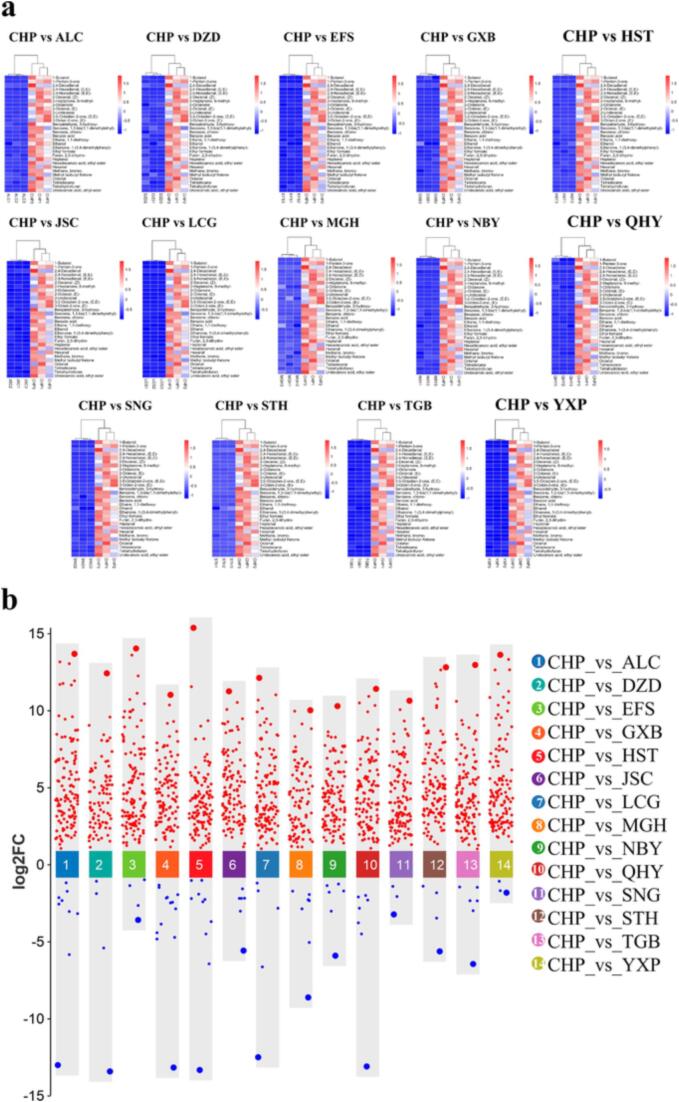
We used a t-test with p-value < 0.05 and VIP > 1 to select relevant differential organic compounds. We identified 75 differential compounds and plotted their relative content in a heatmap to show the variation between species. Fig. 2b shows that CHP had significantly higher abundance of these compounds than the other species, while JSC, YXP and STH had significantly lower abundance. This agrees with the PCA results, suggesting that these compounds are key to the flavor differences between species. We also identified 30 differential compounds that were unique to human milk and plotted them in another heatmap (Fig. 3a).
Fig. 3.
A) heat map of the hierarchical clustering of unique human flavor compounds with other species; b) dynamic multi-group scatter plots of different compounds.
For further analysis of the differential compounds, Dynamic multi-group scatter plot with FC > 1.5 or FC < 0.67 were used as screening conditions to display the differential compounds. Dynamic multi-group scatter plot (Fig. 3b) explains the up and down regulation information of various metabolites between humans and other species. The differential flavors of CHP compared to ALC, DZD, EFS, GXB, HST, JSC, LCG, MGH, NBY, QHY, SNG, STH, TGB and YXP were 127 (118up/9down), 89 (85up/4down), 127 (123up/4down), 134 (121up/13down), 125 (115up/10down), 122 (116up/6down), 129 (123up/6down), 101 (93up/8down), 118 (112up/6down), 123 (116up/7down), 114 (111up/3down), 124 (120up/4down), 126 (119up/7down), 132 (129up/3down). From Fig. 3b, we can see that most of the differential compounds were up regulated in human milk compared to other species. The expression of Cyclohexane‑d12 was much lower in CHP than in other species. The expression of 1-Nonanol, 2(3H)-Furanone, dihydro-4-methyl-, Undecanoic acid and ethyl ester were much higher in CHP than in other species.
3.7. Analysis of flavor characterization of differential compounds
We used Igraph and Flavordb to build a network graph of flavor compounds and their sensory characteristics. We selected the 10 most frequent flavors and the 39 compounds associated with them for the graph (Fig. 2c). Five differential compounds with ROAV ≥ 1 were key flavor compounds: Trimethylamine; Propanal; 1-Pentanol; Pyridine,2-methyl-; and 2-Butanone.
4. Discussion
The scarcity of literature on flavor compounds in raw milk highlights the importance of characterizing the nutritional and flavor profiles of different kinds of milk to optimize production strategies. To achieve this, we utilized GC × GC TOF MS, which offers higher throughput, precision, and sensitivity than conventional GC–MS, and identified more flavor compounds than previous studies. Our findings revealed that human milk and donkey milk had a higher number of flavor compounds, while Small-Tail Han sheep milk had the lowest. In particular, there were 168 and 24 exclusive flavor compounds in human milk and cow milk, respectively. It is widely accepted that the flavors produced in the diet are transferred to breast milk in a way that changes over time, resulting in a unique flavor (Spahn, Callahan, Spill, Wong, Benjamin-Neelon, Birch, & Casavale, 2019). The rich diet and nutritional structure of lactating women likely explain the high content of exclusive flavor compounds in breast milk. What is surprising is that higher content of esters and lower content of hydrocarbons in human milk. Most of the esters detected were fatty acid ethyl ester (FAEE). The majority of the detected esters were fatty acid ethyl ester (FAEE), which are commonly found in fruits, rice, and processed products. One possible explanation for this is that women obtain fatty acid esters from a diverse diet during pregnancy, which are then transported to breast milk through the bloodstream. On the other hand, other animals may not have as varied a diet as humans. Another explanation is that the fatty acid ester synthesis enzyme system of humans is not as well-developed as that of other mammals examined. The ability to utilize FAEE may vary from animal to animal and may be linked to their metabolism and gut microbiota. The formation of FAEE is catalyzed by acyl-coenzyme A: ethanol O-acyltransferase and FAEE synthase, which use acyl-CoA and free fatty acids as substrates, respectively (Diczfalusy, Björkhem, Einarsson, Hillebrant, & Alexson, 2001). Previous studies have reported that increased levels of FAEE can be found in serum shortly after alcohol consumption. FAEE synthesis in humans is believed to be more of an ethanol clearance mechanism. The higher levels of FAEE in human milk compared to other mammals may be attributed to the lack of carboxylesterase ES-4, which is found in the mouse liver. This lack of enzyme may explain the higher FAEE levels observed in human milk (Diczfalusy et al., 2001). It is worth noting that elevated levels of FAEE have a negative impact on infant growth and health, as well as adolescent behavior patterns (Singer et al., 2021). The literature on FAEE ingestion in breast milk is scarce, so the relationship between FAEE intake from breast milk and infant growth cannot be determined. Nevertheless, from a flavor perspective, FAEE can bring fruit flavors, and even slight changes in concentration can affect the final sensorial quality (Yin et al., 2019). Early exposure to rich flavors is beneficial to infants. Our study also revealed that Cyclohexane‑d12 is a major component of hydrocarbons. This compound has health and environmental hazards, and high concentrations can be fatal with a narcotic effect on specific target organs. This finding suggests that high concentrations of Cyclohexane‑d12 may result from pollution of the feeding environment and unhealthy sources of feed, indicating a newly discovered problem that the Chinese farming industry desperately needs to address.
ROAV was used to further explain the flavor characteristics of different mammalian milk. ROAV, an effective flavor evaluation criterion, can help identify the main contributors to flavor that can be detected by the taste buds. It is important to note that substances with ROAVs<1 can still have an impact on flavor, as they may have a synergistic effect. In analyzing the data, the most prominent flavor components found in mammalian milk were 2,3-butanedione, trimethylamine, isophorone, and acetaldehyde. Previous studies have shown that 2,3-butanedione is a crucial flavor compound that contributes to the “pleasing” and “buttery” sensory characteristics of dairy products (Liu et al., 2022). This observation is in line with our results, which indicate that it is a fundamental and enjoyable aspect of milk. Trimethylamine, on the other hand, has been described as fishy and pungent, and excessive amounts of it in dairy products may produce an unpleasant taste. It is believed that dietary choline, betaine, and l-carnitine are degraded by microbiota in the rumen and small intestine to form trimethylamine (Craciun & Balskus, 2012). Previous studies have demonstrated that trimethylamine oxides in Holstein cows are excreted primarily in the urine and that the limited presence of trimethylamine oxides in milk does not affect taste (Myers et al., 2021). Our research has shown that trimethylamine is not the predominant flavor characteristic in Holstein milk, but it is considered to be the most important component of flavor in the milk of buffaloes, yaks, Jersey cows, and camels. Interestingly, our research has also revealed that camel milk has a very high concentration of trimethylamine, which overwhelmingly dominates all other flavor compounds. This result may be due to the unique intestinal microbiota of camels and the fact that dietary choline is not well-protected when passing through the rumen and small intestine. These findings suggest that trimethylamine may be a significant deterrent to consumer acceptance of these animal milk and may be unfriendly to early infant flavor exposure. Furthermore, acetaldehyde, which is produced during the spontaneous oxidation of milk, is considered a health hazard and induces unpleasant “young” or “green” off-tastes (Kucharczyk, Żyła, & Tuszyński, 2020). High levels of acetaldehyde indicate reduced antioxidant levels in milk and accelerated propagation of oxidation in milk (Steffensen, Andersen, & Nielsen, 2002). Therefore, reducing the spontaneous oxidation of milk is crucial for improving milk quality and flavor. Lastly, we found isophorone to be the predominant flavor compound in pig milk, which was unexpected. This suggests that pig metabolism may be different from that of other mammals. Isophorone has a “camphor-like” and “sharp” odor and can be a health hazard when consumed in excess (Waddell, 2002). This may be an important reason why pig milk is different from other milk and reduces consumers' desire to buy pig dairy products. this result must be interpreted with caution because very little was found in the literature on the question of flavor compounds of pig milk.
After analyzing the flavor compound information, the current study found some noteworthy results that merit discussion. While sweetness is the predominant characteristic of most mammalian milk, variations in fresh, herbal, and woody flavors were observed in different animal species. It is likely that these differences are attributed to the dietary structures of each species. However, the study lacks sufficient evidence to determine whether these highly variable flavor attributes positively or negatively impact infant taste guidance. Multivariate statistical analysis and differential analysis revealed the specificity of human milk and identified several flavor compounds with significant interspecies differences. These compounds are likely sourced from spices, plant and animal metabolites, and thermal-oxidation products of lipids. Although the origin of these compounds is difficult to trace, they are found in low quantities across all species except humans. Of particular interest was the discovery that thermal-oxidation products of lipids are a significant source of flavor differences. Thermal-oxidation refers to a chemical reaction that occurs when a substance is exposed to heat and oxygen, leading to the degradation or alteration of its molecular structure. This process can cause changes in the flavor, aroma, and overall quality of the substance. The study identified several thermal-oxidation primary and secondary products in breast milk, with cholesterol and omega-6 fatty acids likely being the main forms of thermal-oxidation. Generally, thermal oxidation leads to the formation of hydrocarbons, aldehydes, ketones, alcohols, and organic acids (Cardenia et al., 2015, Fu et al., 2022). These substances are commonly found in cooked meat and are produced by the Maillard reaction, interactions with lipid oxidation products, and vitamin degradation (Arshad et al., 2018). Different cooking methods may lead to changes in flavor composition due to variations in time, temperature, and pH value. These differences may explain within-group differences in these substances in human milk (Fu et al., 2022). Maternal consumption of Thermal-oxidation products from cooked foods can contribute to the development of specific flavors that are subsequently transferred to breast milk, thereby inducing alterations in its taste profile. In China, cooked meat is rarely used as feed for mammals due to factors such as cost and disease prevention. However, the identified flavor compounds may be an important factor in the differences between human milk and mammalian milk.
Sensory flavor profiles and flavor substance association networks were developed to explain the associations between compounds and flavor profiles and to capture more comprehensive flavor profile information. The study's findings show that sweet, fruity, and green flavors had the most significant sensory contribution. The study identified five key differential flavor compounds based on the ROAV and differential expression results. Two possible differential production pathways for propionaldehyde were observed: one being a result of rapid decomposition of lipid-derived aldehydes in the presence of air and buffers (Zamora, Navarro, Aguilar, & Hidalgo, 2015), and the other being the fermentation of propylene glycol by ruminant rumen microorganisms leading to propionaldehyde production (Kristensen & Raun, 2007). The natural origin of 1-pentanol and 2-butanone results from intestinal microbial metabolism, but the specific metabolic pathway remains unstudied. It has been demonstrated that different feed conditions have an effect on the content of 1-Pentanol in milk (Villeneuve et al., 2013). Differences in gut microbiota and dietary structure may explain the variations in flavor substances found in the milk. Pyridine,2-methyl- may be produced as a consequence of reactive carbonyl oligomerization in the presence of ammonia (Villeneuve et al., 2013). The study suggests that aldehydes and ketones play a significant role in the flavor composition and that flavor components may interact with each other, ultimately resulting in overall flavor differences.
5. Conclusion
Compared to human milk, other mammalian milk contains lower levels of esters, primarily fatty acid ethyl esters, but has higher levels of cyclohexane‑d12. The most important flavor contributors to the milk of these species are 2,3-butanedione, trimethylamine, isophorone, and acetaldehyde. Thermal oxidation of lipids is associated with most of the flavor compounds that differed significantly between human milk and other species, likely due to the transfer of flavor compounds from cooked foods to human milk. The sensory flavor profile identified five key flavor compounds that play a crucial role in milk flavor: trimethylamine, propanal, 1-pentanol, pyridine,2-methyl-, and 2-butanone. These findings reveal the complex interactions between in-milk flavor substances of different species and provide insights for subsequent studies on in-milk flavor differences.
Chemical compounds studied in this article
Cyclohexane‑d12 (PubChem CID123129); 2,3-butanedione (PubChem CID650); Trimethylamine (PubChem CID1146); Isophorone (PubChem CID6544); Acetaldehyde (PubChem CID177); 1-Pentanol (PubChem CID6276); 2-Butanone (PubChem CID6569); Pyridine, 2-methyl- (PubChem CID7975).
Funding
Our research is supported by the following 3 projects: 1. The National Natural Science Foundation of China (No. 32272836); 2. Postdoctoral Science Foundation of China: Genetic trajectory and expression localization of key genes of cashmere fineness by multi-omics (2021 M693859); 3. Liaoning Province “the open competition mechanism to select the best candidates” Science and Technology Research Project: Selection and breeding of special advantageous livestock and poultry breeds in Liaoning and key technology of whole industry chain production (2021JH1/10400033).
CRediT authorship contribution statement
Yinggang Sun: Conceptualization, Methodology. Yanzhi Wu: Writing – review & editing, Writing – original draft. Ben Liu: Resources. Rui Chen: Software. Yanjun Qiao: Formal analysis. Qiu Zhang: Investigation. Qian Li: Data curation. Xiaowei Wang: Visualization. Zeying Wang: Supervision, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
References
- Al-Attabi Z., D'Arcy B.R., Deeth H.C. Volatile sulphur compounds in UHT milk. Critical Reviews in Food Science and Nutrition. 2009;49(1):28–47. doi: 10.1080/10408390701764187. [DOI] [PubMed] [Google Scholar]
- Arshad M.S., Sohaib M., Ahmad R.S., Nadeem M.T., Imran A., Arshad M.U.…Amjad Z. Ruminant meat flavor influenced by different factors with special reference to fatty acids. Lipids in Health and Disease. 2018;17(1):223. doi: 10.1186/s12944-018-0860-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield F.H., Alexander T., Muelbert M., Beker F. Smell and taste in the preterm infant. Early Human Development. 2017;114:31–34. doi: 10.1016/j.earlhumdev.2017.09.012. [DOI] [PubMed] [Google Scholar]
- Cardenia V., Olivero G., Rodriguez-Estrada M.T. Thermal oxidation of cholesterol: Preliminary evaluation of 2-methyl-6-heptanone and 3-methylbutanal as volatile oxidation markers. Steroids. 2015;99(Pt B):161–171. doi: 10.1016/j.steroids.2015.03.017. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J., Hoon M.A., Ryba N.J., Zuker C.S. The receptors and cells for mammalian taste. Nature. 2006;444(7117):288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- Cheng Z., Mannion D.T., O'Sullivan M.G., Miao S., Kerry J.P., Kilcawley K.N. Comparison of automated extraction techniques for volatile analysis of whole milk powder. Foods. 2021;10(9) doi: 10.3390/foods10092061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero C., Cagliero C., Liberto E., Nicolotti L., Rubiolo P., Sgorbini B., Bicchi C. High concentration capacity sample preparation techniques to improve the informative potential of two-dimensional comprehensive gas chromatography-mass spectrometry: Application to sensomics. Journal of Chromatography. A. 2013;1318:1–11. doi: 10.1016/j.chroma.2013.09.065. [DOI] [PubMed] [Google Scholar]
- Craciun S., Balskus E.P. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(52):21307–21312. doi: 10.1073/pnas.1215689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cosmi V., Scaglioni S., Agostoni C. Early taste experiences and later food choices. Nutrients. 2017;9(2) doi: 10.3390/nu9020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diczfalusy M.A., Björkhem I., Einarsson C., Hillebrant C.G., Alexson S.E. Characterization of enzymes involved in formation of ethyl esters of long-chain fatty acids in humans. Journal of Lipid Research. 2001;42(7):1025–1032. [PubMed] [Google Scholar]
- Forestell C.A. Flavor perception and preference development in human infants. Annals of Nutrition & Metabolism. 2017;70(Suppl 3):17–25. doi: 10.1159/000478759. [DOI] [PubMed] [Google Scholar]
- Fu Y., Cao S., Yang L., Li Z. Flavor formation based on lipid in meat and meat products: A review. Journal of Food Biochemistry. 2022;46(12):e14439. doi: 10.1111/jfbc.14439. [DOI] [PubMed] [Google Scholar]
- Hausner H., Philipsen M., Skov T.H., Petersen M.A., Bredie W.L.P. Characterization of the volatile composition and variations between infant formulas and mother's milk. Chemosensory Perception. 2009;2(2):79–93. doi: 10.1007/s12078-009-9044-6. [DOI] [Google Scholar]
- He Y.Z., Chen L., Zheng L.F., Cheng F., Deng Z.Y., Luo T., Li J. A comparative study of volatile compounds in breast milk and infant formula from different brands, countries of origin, and stages. European Food Research and Technology. 2022;248(11):2679–2694. doi: 10.1007/s00217-022-04077-w. [DOI] [Google Scholar]
- Jia W., Zhang R., Shi L., Zhang F., Chang J., Chu X. Accurate determination of volatile-flavor components in bos grunniens milk by high-throughput dynamic headspace gas chromatographic-mass spectrometry. Journal of Chromatography. A. 2019;1603:67–82. doi: 10.1016/j.chroma.2019.06.058. [DOI] [PubMed] [Google Scholar]
- Kristensen N.B., Raun B.M. Ruminal and intermediary metabolism of propylene glycol in lactating Holstein cows. Journal of Dairy Science. 2007;90(10):4707–4717. doi: 10.3168/jds.2007-0295. [DOI] [PubMed] [Google Scholar]
- Krpan M., Major N., Satalic Z., Hruskar M. Human breast milk, infant formula, and follow-up milks comparison by electronic tongue. Acta Alimentaria. 2021;50(1):33–41. doi: 10.1556/066.2020.00122. [DOI] [Google Scholar]
- Kucharczyk K., Żyła K., Tuszyński T. Simultaneous optimization of acetaldehyde and DMS concentrations for better sensory quality of beer fermented on an industrial scale. Foods. 2020;9(8) doi: 10.3390/foods9081043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A., Liu Q., Bu Y., Hao H., Liu T., Gong P.…Yi H. Aroma classification and characterization of Lactobacillus delbrueckii subsp. bulgaricus fermented milk. Food Chem X. 2022;15 doi: 10.1016/j.fochx.2022.100385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C.R., Ling P.R., Blackburn G.L. Review of infant feeding: key features of breast milk and infant formula. Nutrients. 2016;8(5) doi: 10.3390/nu8050279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella, J. A. (2014). Ontogeny of taste preferences: basic biology and implications for health. American Journal of Clinical Nutrients, 99(3), 704s-711s. doi:10.3945/ajcn.113.067694. [DOI] [PMC free article] [PubMed]
- Mennella J.A., Ventura A.K., Beauchamp G.K. Differential growth patterns among healthy infants fed protein hydrolysate or cow-milk formulas. Pediatrics. 2011;127(1):110–118. doi: 10.1542/peds.2010-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miraglia Del Giudice M., D'Auria E., Peroni D., Palazzo S., Radaelli G., Comberiati P.…Riva E. Flavor, relative palatability and components of cow's milk hydrolysed formulas and amino acid-based formula. Italian Journal of Pediatrics. 2015;41:42. doi: 10.1186/s13052-015-0141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtaza M.A., Rehman S.U., Anjum F.M., Huma N. Descriptive sensory profile of cow and buffalo milk Cheddar cheese prepared using indigenous cultures. Journal of Dairy Science. 2013;96(3):1380–1386. doi: 10.3168/jds.2012-5992. [DOI] [PubMed] [Google Scholar]
- Myers W.A., Wang F., Chang C., Davis A.N., Rico J.E., Tate B.N.…McFadden J.W. Intravenous trimethylamine N-oxide infusion does not modify circulating markers of liver health, glucose tolerance, and milk production in early-lactation cows. Journal of Dairy Science. 2021;104(9):9948–9955. doi: 10.3168/jds.2021-20169. [DOI] [PubMed] [Google Scholar]
- Ochoa-Flores A.A., Hernández-Becerra J.A., Velázquez-Martínez J.R., Piña-Gutiérrez J.M., Hernández-Castellano L.E., Toro-Mujica P.…Vargas-Bello-Pérez E. Chemical and fatty acid composition of Manchego type and Panela cheeses manufactured from either hair sheep milk or cow milk. Journal of Dairy Science. 2021;104(7):7457–7465. doi: 10.3168/jds.2020-19301. [DOI] [PubMed] [Google Scholar]
- Ozawa T., Takada R., Nishitani J., Fujita M., Blair H.T. A comparative analysis of acceptance by Japanese females and price of goat milk from different sources. Animal Science Journal. 2010;81(2):271–275. doi: 10.1111/j.1740-0929.2009.00735.x. [DOI] [PubMed] [Google Scholar]
- Pérez-Jiménez M., Sherman E., Pozo-Bayón M.A., Pinu F.R. Application of untargeted volatile profiling and data driven approaches in wine flavoromics research. Food Research International. 2021;145 doi: 10.1016/j.foodres.2021.110392. [DOI] [PubMed] [Google Scholar]
- Ranadheera C.S., Evans C.A., Baines S.K., Balthazar C.F., Cruz A.G., Esmerino E.A.…Vasiljevic T. Probiotics in goat milk products: delivery capacity and ability to improve sensory attributes. Comprehensive Reviews in Food Science and Food Safety. 2019;18(4):867–882. doi: 10.1111/1541-4337.12447. [DOI] [PubMed] [Google Scholar]
- Singer L.T., Min M.O., Momotaz H., Powers G., Minnes S., Bearer C.F. Association of fatty acid ethyl esters in meconium with behavior during childhood. Drug and Alcohol Dependence. 2021;218 doi: 10.1016/j.drugalcdep.2020.108437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spahn, J. M., Callahan, E. H., Spill, M. K., Wong, Y. P., Benjamin-Neelon, S. E., Birch, L., . . . Casavale, K. O. (2019). Influence of maternal diet on flavor transfer to amniotic fluid and breast milk and children's responses: a systematic review. American Journal of Clinical Nutrients, 109(Suppl_7), 1003s-1026s. doi:10.1093/ajcn/nqy240. [DOI] [PubMed]
- Steffensen C.L., Andersen H.J., Nielsen J.H. Aldehyde-induced xanthine oxidase activity in raw milk. Journal of Agricultural and Food Chemistry. 2002;50(25):7392–7395. doi: 10.1021/jf020376r. [DOI] [PubMed] [Google Scholar]
- Teng F., Reis M.G., Ma Y., Day L. Effects of season and industrial processes on volatile 4-alkyl-branched chain fatty acids in sheep milk. Food Chemistry. 2018;260:327–335. doi: 10.1016/j.foodchem.2018.04.011. [DOI] [PubMed] [Google Scholar]
- Villeneuve M.P., Lebeuf Y., Gervais R., Tremblay G.F., Vuillemard J.C., Fortin J., Chouinard P.Y. Milk volatile organic compounds and fatty acid profile in cows fed timothy as hay, pasture, or silage. Journal of Dairy Science. 2013;96(11):7181–7194. doi: 10.3168/jds.2013-6785. [DOI] [PubMed] [Google Scholar]
- Vincis R., Fontanini A. Central taste anatomy and physiology. Handbook of Clinical Neurology. 2019;164:187–204. doi: 10.1016/b978-0-444-63855-7.00012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell W.J. Thresholds of carcinogenicity of flavors. Toxicological Sciences. 2002;68(2):275–279. doi: 10.1093/toxsci/68.2.275. [DOI] [PubMed] [Google Scholar]
- Yin H., Liu L.P., Yang M., Ding X.T., Jia S.R., Dong J.J., Zhong C. Enhancing medium-chain fatty acid ethyl ester production during beer fermentation through EEB1 and ETR1 overexpression in saccharomyces pastorianus. Journal of Agricultural and Food Chemistry. 2019;67(19):5607–5613. doi: 10.1021/acs.jafc.9b00128. [DOI] [PubMed] [Google Scholar]
- Zamora R., Navarro J.L., Aguilar I., Hidalgo F.J. Lipid-derived aldehyde degradation under thermal conditions. Food Chemistry. 2015;174:89–96. doi: 10.1016/j.foodchem.2014.11.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.