Graphical abstract
Keywords: Bacillus subtilis, Dark tea, Volatile organic compounds, Solid-state fermentation, Catechins composition, Multivariate statistical analysis
Highlights
-
•
B. subtilis was firstly used alone in the solid-state fermentation of dark tea.
-
•
Volatile and non-volatile metabolites of dark tea were comprehensively analyzed.
-
•
New flavor substances such as geranyl isovalerate are produced after fermentation.
-
•
The floral and fruit aroma of dark tea improved with fermentation.
-
•
B. subtilis fermentation transformed ester catechins into non-ester catechins.
Abstract
In this study, the solid-state fermentation (SSF) of dark tea was carried out using Bacillus subtilis LK-1, which was isolated from Fu brick tea (FBT). The effects of SSF with B. subtilis on volatile organic compounds (VOCs), non-volatile metabolites, and antioxidant activities of dark tea was investigated. A total of 45 VOCs were identified, primarily consisting of ketones (18), hydrocarbons (8), aldehydes (7), and alcohols (6). Following fermentation, the content of key odor active substances such as linalool, β-ionone, and 3,5-octadiene-2-one significantly increased, resulting in an enhanced floral and fruity aroma of dark tea. Furthermore, new flavor substances like geranyl isovalerate and decanal were produced during SSF, enriching the aroma profile of dark tea. Non-ester catechins demonstrated a drastic increase, while ester catechins remarkably decreased after SSF. Furthermore, SSF led to a slight decrease in the total polyphenols content and antioxidant activity of dark tea. There is a close relationship between VOCs and the main non-volatile metabolites during SSF. Overall, this study highlighted the great impact of SSF with B. subtilis on the metabolites of dark tea and provided valuable insights into the role of bacteria in shaping the metabolite profile of FBT.
1. Introduction
Fu brick tea (FBT), a traditional microbial post-fermented dark tea in China, has gained increasing attention due to its diverse bioactive substances and health-stimulating effects, such as anti-obesity, anti-oxidation, hypoglycemic properties, and regulation of intestinal flora (Zhu et al., 2023, Qi et al., 2023, Zhou et al., 2022). Additionally, FBT is renowned for its unique sensory characteristics. The manufacturing of FBT involves several key steps, including piling, steaming, pressing, microbial fermentation, and drying, using primary dark tea as the raw material. Notably, microbial fermentation has been identified as a crucial process for developing the distinctive quality attributes of FBT (Chen et al., 2022, Li et al., 2020, Li et al., 2021). Microorganisms secrete various extracellular enzymes that play a crucial role in transforming tea components and generating unique metabolites in FBT (Du, Yang, Yang, & Yang, 2022, Xiao et al., 2022c). Therefore, conducting a comprehensive investigation into the role of microorganisms in the formation of FBT metabolites is essential. This research can provide valuable insights into the control and enhancement of the quality of tea products.
In recent years, various technologies, such as polymerase chain reaction-denaturing gradient gel electrophoresis (PCR-DGGE), Illumina MiSeq sequencing of ITS gene amplicons, and high-throughput Illumina MiSeq sequencing combined with quantitative polymerase chain reaction (qPCR), have been employed to explore the microbial composition and dynamic changes during the fermentation process of FBT. These studies have revealed that Aspergillus consistently dominates the entire FBT production process, while Bacillus species are particularly abundant during the flowering stage, making them the dominant bacteria in FBT fermentation (Xu et al., 2011, Li et al., 2019, Li et al., 2021). Recently, researchers have begun fermenting dark tea with single strains to investigate the role of these dominant microorganisms in shaping the quality of dark tea. For instance, fermenting dark tea solely with the dominant fungus Eurotium cristatum resulted in a significant increase in volatile organic compounds (VOCs) with a stale and fungi flower fragrance, thereby enhancing the sensory quality of dark tea (Xiao et al., 2022b). At the same time, it also led to a substantial decrease in the content of total polyphenols, flavonoids, thearubins, theaflavins, and gallate catechins (Xiao et al., 2022a). Furthermore, fermenting dark tea exclusively with Aspergillus niger resulted in elevated levels of β-ionone, 9,12-octadecadienoic acid, linalool oxides, and geraniol (Cao et al., 2018).
Notably, the previous studies mentioned above primarily focused on the investigation of fungi, particularly research on E. cristatum (Xiao et al., 2021b, Xiao et al., 2022b). However, bacteria also play a significant role in the microbial fermentation of FBT (Li et al., 2021), and studies have identified Bacillus as the dominant microorganism in FBT using culture-dependent methods and high-throughput sequencing (Xiang et al., 2022, Li et al., 2019). Several previous studies have reported a close relationship between the change in chemical constituents and Bacillus during FBT manufacturing through correlation analysis. Bacillus has been found to promote the metabolism of tea polyphenols and the transformation of caffeine, which is associated with the production of 44 metabolites in FBT (Li et al., 2019, Xia et al., 2022). Furthermore, earlier research has demonstrated that Bacillus species contribute to the formation of aroma characteristics in tea (Pripdeevech et al., 2014, Zhu et al., 2022). Therefore, Bacillus species play a crucial role in the formation of metabolites during the manufacturing of FBT. B. subtilis, a member of the Bacillus genus, has received significant attention in recent decades because it is considered a probiotic with the ability to transform food constituents. B. subtilis secretes highly active extracellular enzymes, such as protease, amylase, and cellulase, which may play a key role in the transformation of tea metabolites (Li & Wang, 2021). Pripdeevech et al. (2014) found that fermentation of Green Oolong tea by B. subtilis significantly increased the content of most major VOCs, particularly 2-pentylfuran and limonene. Xu et al. (2019) explored the effects of mixed fermentation using B. subtilis and A. fumigatus on the quality of Qingzhuan brick tea and observed a significant decrease in the content of tea polyphenols, catechins, and theabrownin after solid-state fermentation (SSF). The aforementioned research demonstrates the significant impact of B. subtilis fermentation on the quality of tea. However, to date, no systematic study on the metabolic characteristics of dark tea during fermentation using B. subtilis as a pure culture has been conducted.
In this study, a strain of B. subtilis LK-1, which was isolated from the microbial fermentation process during FBT manufacturing, was utilized for SSF of primary dark tea, the raw material for FBT. The objective of this study was to investigate the dynamic changes in VOCs, non-volatile components, and antioxidant activity during the fermentation process. The main aim was to elucidate the role of B. subtilis in the formation of metabolites in FBT. By doing so, this study aimed to provide theoretical support for understanding the involvement of bacteria in the formation of distinctive aromatic compounds and non-volatile metabolites during the fermentation process of dark tea.
2. Materials and methods
2.1. Materials and chemicals
The gallic acid (GA), gallocatechin gallate (GCG), gallocatechin (GC), epigallocatechin (EGC), epigallocatechin gallate (EGCG), epicatechin gallate (ECG), epicatechin (EC), catechin (C), catechin gallate (CG), rutin, Folin–Ciocalteu’s reagent, diammonium 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) (ABTS), 2,2-diphenyl-1-picrylhydrazyl (DPPH), and ferrozine monosodium salt were supplied by Sigma–Aldrich Co. (St Louis, MO, USA). HPLC grade solvents, formic acid, and acetonitrile were provided by Fischer Scientific Co., Ltd. in Waltham, USA. All other chemicals and reagents used were of analytical grade. The B. subtilis LK-1 strain isolated from FBT in our laboratory was utilized as the inoculating starter for the fermentation of dark tea. The primary dark tea material was provided by Hunan Jiuyang Tea Co., Ltd. (Yiyang, China) and prepared according to the following procedure: fresh leaves → fixation → rolling → piling → drying. The related process parameters are detailed as follows: (1) fresh leaves: the raw material is one bud, three to five leaves, and the same tenderness of the leaves; (2) fixation: temperature is 220 ℃–260 °C, the fixation time is 4–5 min; (3) rolling: divided into three stages of “light-heavy-light”, the total time is approximately 15 min; (4) piling: piling lasting 12-18 h until the leaves color changes from dark green to yellow–brown; (5) drying: auto drying machine was used for drying the fermentation leaves at 110–150°C for two times until the moisture content of tea leaves is below 10%.
2.2. Strain identification
The morphological observation and Gram staining test of strains followed the method described by Dong & Cai (2001). For bacterial identification, physiological and biochemical tests were conducted on strain LK-1 according to the guidelines provided in Bergey's Manual of Determinative Bacteriology (Buchanan & Gibbons,1984). To perform molecular biological identification, the strain’s genome was extracted, and the 16S rDNA gene was amplified using PCR with universal primers 27F and 1492R. The amplified gene was then sequenced. The obtained sequence data were submitted to the NCBI GenBank database for BLAST analysis to determine its similarity to known sequences. Multiple sequence alignment was performed using ClusterX software, and the phylogenetic tree was constructed using MEGA-X software.
2.3. Preparation of starter inoculum
B. subtilis was inoculated into 50 mL of lysogeny broth (LB) medium and cultured at 37 °C for 24 h. Subsequently, 3 mL of the activated first generation was transferred into 150 mL of fresh LB broth medium and incubated for 12 h at 37 °C. The bacterial cells were harvested by centrifugation at 8000 rpm for 5 min at 4 °C and washed twice with sterilized physiological saline. The cells were then resuspended in sterilized physiological saline to achieve a concentration of 108 cells/mL.
2.4. Solid-state fermentation of dark tea
The preparation of fermented dark tea with B. subtilis involved the following steps: 20 g of primary dark tea were placed in flasks and sprayed with 12 mL of pure water. The flasks were then sterilized at 121 °C for 20 min. After the flasks cooled down to 25 °C, 4 mL of B. subtilis suspension was added to the dark tea leaves as a seeding culture. The flasks containing the dark tea and B. subtilis were placed in a microbiological incubator set to 37 °C. Sampling was conducted at various fermentation stages: initial stage (2 days, BDT2), middle stage (4 days, BDT4), and final fermentation stages (6 days, DT4; 8 days, BDT8). Dark tea sampled on day 0 (BDT0) was used as a control for comparison. The collected dark tea samples were freeze-dried and ground into powder form. The tea powders were screened through a 40-mesh filter and stored at −20 °C for further analysis.
2.5. Analysis of volatile metabolites by HS-SPME-GC–MS
Headspace solid phase microextraction was employed to extract VOCs from the B. subtilis fermented dark tea (BDT) samples. The detailed method was carried out as follows: In a headspace vial with a capacity of 20 mL and equipped with a magnetic stirrer, 0.5 g of BDT sample, 0.5 g of sodium chloride, 10 μL of ethyl caprate (8.64 mg/L) as an internal standard solution, and 5 mL of boiled ultra-pure water were combined. The top of the glass bottle was sealed with a silicon diaphragm to create a sealed headspace environment. The headspace glass bottle was then heated to 80 °C, and the tea sample was stirred at a speed of 600 rpm using the magnetic stirrer. After a 10 min pre-balance period, a 50/30 μm DVB/CAR/PDMS fiber from American Supelco was inserted into the headspace vial to extract the VOCs. The extraction process lasted for 50 min. Subsequently, the fiber was desorbed for five minutes at the injection port of the GC–MS, operating in splitless injection mode at 250 °C.
For the separation of VOCs, a 30 m × 0.25 mm × 0.25 μm HP-5MS capillary column from Agilent (USA) was used. The gas chromatography-mass spectrometry (GC–MS) analysis of VOCs was performed using the Agilent 7000D system. The column temperature program was as follows: The initial temperature was set at 40 °C and maintained for 3 min. Subsequently, it increased to 80 °C at a rate of 2 °C/min, followed by an increase to 90 °C at the same rate. Subsequently, the temperature was further increased to 150 °C at a rate of 3 °C/min, then to 180 °C at 5 °C/min, and finally to 230 °C at 15 °C/min. The final temperature of 230 °C was held for 2 min. The mass spectrometry was performed using the electron impact ionization mode with the MS ion source temperature set to 230 °C. The electron energy used was 70 eV, and the mass spectrum scanning range was set from 35 to 400 m/z. To calculate the retention index (RI) for each peak, the retention time (RT) of n-alkanes (C6 ∼ C21) under the same GC–MS conditions was utilized. The identification of VOCs was accomplished by comparing the calculated RI values and the mass spectra of all detected metabolites with those present in the NIST 17 library. For quantification of VOCs, the peak area ratio of VOCs to the internal standard ethyl caprate was multiplied by the concentration of ethyl caprate.
2.6. Analysis of relative odor activity value
The relative odor activity value (ROAV) is a method used to identify key volatile compounds in samples, with a range from 0 to 100. The calculation of ROAV is performed using the following formula: ROAVi = 100 × (OAVi/OAVmax). The calculation formula for the odor activity value (OAV) is as follows: OAVi = Ci/OTi, where Ci represents the concentration of volatile compounds in the sample, and OTi represents the odor threshold in water (Zhu, Chen, Chen, Chen, & Deng, 2020). The odor threshold values can be obtained from previous reports (Xu et al., 2022, Xiao et al., 2022c). VOCs with an ROAV value greater than or equal to 1 are considered key volatile compounds. VOCs with an ROAV value ranging from 0.1 to<1 have a modifying effect on the overall flavor of the sample (Su, He, Zhou, Li, & Zhou, 2022).
2.7. Determination of major chemical composition changes in dark teas during SSF
A total of 1 g of ground BDT sample was transferred to a conical flask and mixed with 40 mL of distilled water. The mixture was then extracted in a 95 °C water bath for 30 min with intermittent shaking every 5 min. To obtain the supernatant, the extracted mixture was subjected to centrifugation using a centrifuge (3-18R, Hunan Hengnuo Instrument Equipment Co., Ltd., China) at 10,000 rpm for 15 min at 4 °C. The resulting supernatant was adjusted to a final volume of 50 mL and stored in a refrigerator at − 20 °C for further analysis. The concentration of tea polyphenols was measured using the Folin–Ciocalteu colorimetric method, as described by Xiao et al. (2021a). The data were expressed as gallic acid equivalents (GAE) in milligrams per gram (mg/g) of dried weight tea. The determination method for total flavonoids concentration followed the protocol outlined by Xiao et al. (2022a). The concentration was reported as rutin equivalents per gram of dried tea sample (mg RE/g d.w.).
The quantitative analysis of gallic acid (GA) and catechins was performed using the Agilent 1260 HPLC system from Agilent Technologies, USA. The tea liquids obtained previously were filtered using a 0.22 m PVDF membrane syringe filter. Then, a 10 μL sample was injected onto an Agilent C18 reverse-phase column (ZORBAX SB-C18, 4.6 × 250 mm) packed with 5 m particle size, which was maintained at a temperature of 30 °C. The HPLC system utilized Eluent A (acetonitrile) and Eluent B (0.1% formic acid in ultrapure water) as the mobile phases. The flow rate was set at 0.8 mL/min. The gradient elution program was presented as follows: from 0 to 40 min, the mobile phase composition changed from 90% to 65% B, and from 40 to 42 min, it returned to 90% B. To measure the analytes, a G7111A Quat Pump, AutoSampler, G7114A VWD detector, and detection at a wavelength of 280 nm were employed. By comparing the absorption spectra and retention times of the target catechins and GA with those of reference substances, the identification of these compounds was achieved. The results of the analysis were expressed as milligrams per 100 g of dried dark tea.
2.8. Evaluation of antioxidant activities
The antioxidant activities of the BDT samples were evaluated using several assays with different mechanisms. Following the method described in our previous study (Chen et al., 2020), the determination of reducing power (RP), DPPH radical scavenging activity (DPPH), and ABTS radical cation scavenging activity (ABTS) were conducted. The calibration curve was constructed using various concentrations of vitamin C. The results were expressed in milligrams of vitamin C equivalents per gram of dry weight dark tea flour (mg VCE/g d.w.). The chelating ability (CHA) of ferrous ions was assessed using the method previously published by Xiao et al. (2015). The calibration curve was constructed using ethylenediaminetetraacetic acid disodium salt (EDTA-2Na) solution at various concentrations. The results were expressed as milligrams of EDTA-2Na equivalents per gram of dry weight dark tea flour (mg EDTA-2Na equivalent/g d.w.).
2.9. Statistical analysis
The data were presented as mean ± standard deviation, and each measurement was performed at least three times. Statistical significance (p < 0.05) was determined using SPSS 26.0 software (Chicago, IL, USA) through single-factor ANOVA testing or independent sample t-tests. To analyze the correlations among the results in BDT, Pearson correlation analysis was conducted using SPSS 26.0 software. The correlations were visualized using Origin 2021. For data analysis, SIMCA 14.1 software was employed to create primary component analysis (PCA), hierarchical cluster analysis (HCA), and partial least-squares discriminant analysis (PLS-DA) models. These models were used to examine the distribution of VOCs and antioxidant activity in dark tea. The graphical display and creation of hot charts were performed using Origin 2021 (OriginLab Corporation, MA, USA) and TBtools version 1.108, respectively.
3. Results and discussion
3.1. Strain identification
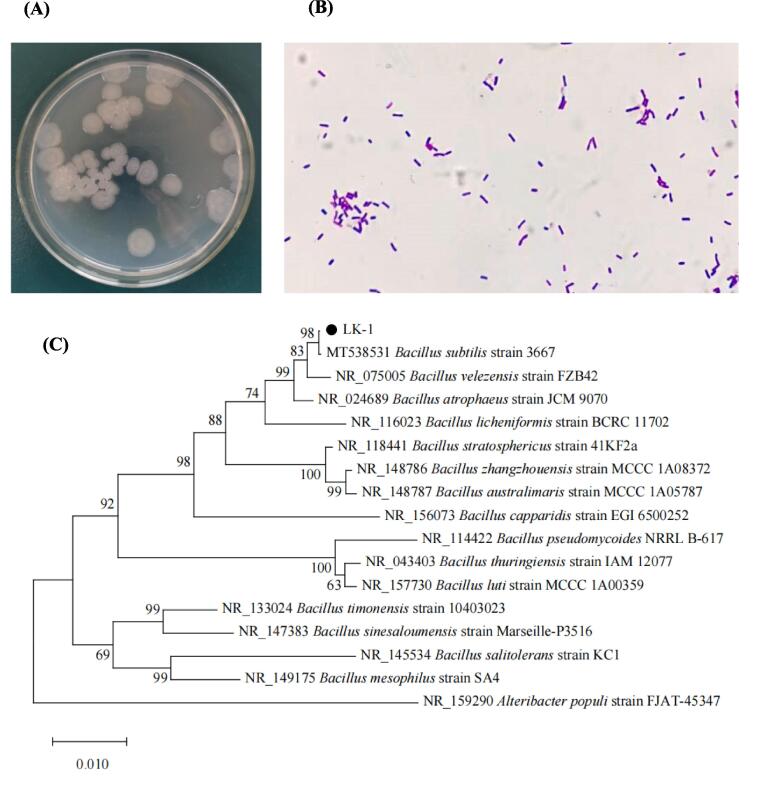
The colony morphology of LK-1 strain is depicted in Fig. 1A. The colonies appeared gray, approximately circular, opaque, slightly flattened on the surface, and exhibited saw-shaped edges. Clear concentric rings were observed, which are consistent with the morphological characteristics of B. subtilis reported in previous studies (Tasaki, Nakayama, & Shoji, 2017). The gram-staining result of the strain was positive (Fig. 1B). Microscopically, the strain exhibited a rod-shaped morphology with blunt and round ends. The cells did not show significant swelling. The results of physiological and biochemical identification for strain LK-1 are presented in Table S1. The strain did not liquefy gelatin but could utilize propionate. It tested positive for the catalase and methyl red tests, while the Voges–Proskauer (V-P) test yielded a negative result. Additionally, strain LK-1 demonstrated the ability to utilize various carbohydrates such as glucose, xylose, and mannitol, but it was unable to ferment fructose. These biochemical characteristics align with the identification criteria for B. subtilis. Further analysis involved sequencing the 16S rDNA gene of the LK-1 strain and comparing it with sequences in the NCBI GenBank database. The comparison revealed a high similarity of 99% between the 16S rDNA gene sequence of LK-1 and B. subtilis. A phylogenetic tree was constructed using 16 sequences with high similarity, as shown in Fig. 1C. LK-1 and B. subtilis strains clustered together, indicating a close genetic relationship. Therefore, LK-1 was identified as B. subtilis.
Fig. 1.
(A) Colony morphology of LK-1 strain inoculated on LB medium for 12 h, (B) Gram staining diagram of strain LK-1, (C) PCR Phylogenetic tree of the 16S rDNA from strain LK-1.
3.2. Analysis of VOCs during SSF with B. Subtilis
3.2.1. Qualitative and quantitative analysis of VOCs by GC–MS
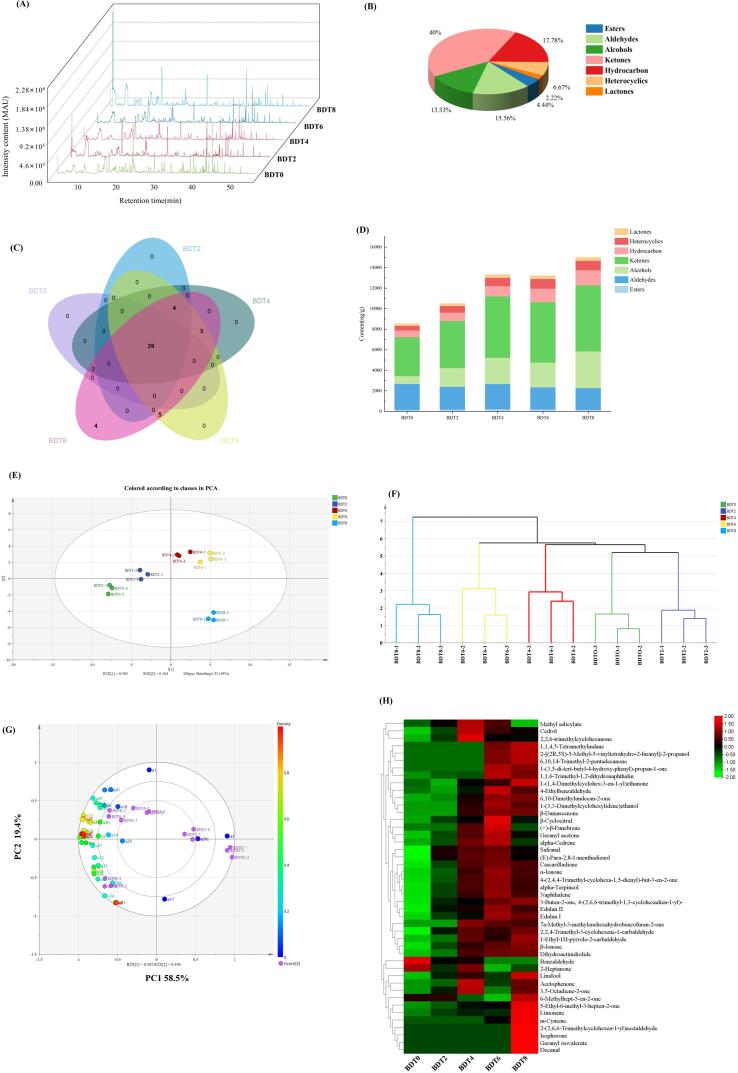
The typical total ion chromatogram of VOCs is depicted in Fig. 2A, while Table 1 provides qualitative and quantitative analysis of the identified VOCs. A total of 45 VOCs were identified using GC–MS, including aldehydes (7), esters (2), alcohols (6), ketones (18), hydrocarbons (8), heterocyclics (3), and lactones (1). The relative proportion of these categorized VOCs is illustrated in Fig. 2B, with ketones (40%), hydrocarbons (17.78%), aldehydes (15.56%), and alcohols (13.33%) constituting a significant portion. This result agrees with previous investigations of dark tea (Shi, Zhu, Zhang, Lin, & Lv, 2019). To analyze shared and distinctive traits among each sample, a Venn diagram (Fig. 2C) was employed. It reveals that there are 29 VOCs shared by all groups, and the number of VOCs increases with the progression of fermentation days. In Fig. 2D, the total content of each volatile category is depicted, indicating a significant increase in the levels of ketones and alcohols after fermentation (3790.40 ng/g → 6424.52 ng/g and 762.57 ng/g → 3579.28 ng/g, respectively). These levels remain elevated until the later stage of fermentation. This finding is consistent with previous studies that found the highest content of ketones in FBT (Lv, Wu, Li, Xu, Liu, & Meng, 2014). Overall, alcohol compounds are associated with fruity and floral aromas (Yun et al., 2021), while ketone compounds are linked to woody and floral scents (Zhang et al., 2021). This suggests that B. subtilis contributes to the enhancement of floral and woody aromas during the SSF of dark tea. Furthermore, it is evident from Fig. 2D that the total concentration of VOCs reaches its highest level on the eighth day of fermentation.
Fig. 2.
(A) GC–MS total ion chromatograms, (B) Venn diagram, (C) Classification of all VOCs, (D) Changes in the content of classified volatiles, (E) Principal component analysis, (F) Hierarchical cluster analysis, (G) Loading plot based on the signal intensity of VOCs of dark teas at different fermentation stages. (H) Heatmap analysis of all VOCs detected by GC–MS during the fermentation of dark tea by B. subtilis.
Table 1.
Concentration of volatile compounds among dark teas fermented by B. subtilis by GC–MS.
| No | Compounds | CAS | RIa/RIb | Identificationc | Odor description | Content (ng/g) | Thresholds (ng/g) | ROAV | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BDT0 | BDT2 | BDT4 | BDT6 | BDT8 | BDT0 | BDT2 | BDT4 | BDT6 | BDT8 | |||||||
| Esters | ||||||||||||||||
| a1 | Methyl salicylate | 119–36-8 | 1187/1192 | MS,RI | Almond, Caramel, Peppermint, Sharp | 144.77 ± 16.59c | 173.18 ± 30.43b | 218.98 ± 16.68a | 191.74 ± 13.74a | 118.26 ± 13.47c | 40 | 0.15 | 0.02 | 0.02 | 0.01 | 0.01 |
| a2 | Geranyl isovalerate | 109–20-6 | 1596/1606 | MS,RI | Apple, Fruit, Rose | n.d. | n.d. | n.d. | n.d. | 31.70 ± 4.5 | n.f. | – | – | – | – | – |
| Aldehydes | ||||||||||||||||
| a3 | Benzaldehyde | 100–52-7 | 957/962 | MS,RI | Bitter Almond, Burnt Sugar, Cherry, Malt, Roasted Pepper | 1899.22 ± 7.31a | 1228.25 ± 117.49b | 1302.68 ± 77.91b | 868.38 ± 31.37c | 859.01 ± 5.39c | 350 | 0.22 | 0.06 | 0.05 | 0.04 | 0.02 |
| a4 | 2,2,4-Trimethyl-3-cyclohexene-1-carbaldehyde | 127128–60-3 | 1137/1138 | MS,RI | – | n.d. | 100.57 ± 7.75c | 149.81 ± 11.87b | 131.47 ± 6.3b | 160.19 ± 14.22a | n.f. | – | – | – | – | – |
| a5 | 4-Ethylbenzaldehyde | 4748–78-1 | 1177/1180 | MS,RI | – | 127.26 ± 5.08c | 118.47 ± 7.4c | 144.36 ± 10.21b | 186.96 ± 11.44a | 165.04 ± 35.65a | n.f. | – | – | – | – | – |
| a6 | Safranal | 116–26-7 | 1193/1201 | MS,RI | Saffron | 268.24 ± 26.68b | 492.17 ± 53.86a | 535.25 ± 16.75a | 550.6 ± 47.83a | 477.88 ± 82.15a | 3 | 3.61 | 2.87 | 2.34 | 2.65 | 1.48 |
| a7 | Decanal | 112–31-2 | 1204/1206 | MS,RI | Floral, Fried, Orange Peel, Penetrating, Tallow | n.d. | n.d. | n.d. | n.d. | 41.45 ± 5.99 | 0.1 | – | – | – | – | 3.86 |
| a8 | β-Cyclocitral | 432–25-7 | 1217/1220 | MS,RI | Mint-like | 238.22 ± 59.76b | 284.86 ± 17.75b | 315.64 ± 31.73b | 418.02 ± 65.18a | 305.45 ± 31.23b | 3 | 3.20 | 1.66 | 1.38 | 2.01 | 0.95 |
| a9 | 2-(2,6,6-Trimethylcyclohexen-1-yl)acetaldehyde | 472–66-2 | 1264/1254 | MS,RI | – | n.d. | n.d. | n.d. | n.d. | 114.73 ± 6.96 | n.f. | – | – | – | – | – |
| Alcohols | ||||||||||||||||
| a10 | 2-[(2R,5S)-5-Methyl-5-vinyltetrahydro-2-furanyl]-2-propanol | 5989–33-3 | 1067/1074 | MS,RI | Floral | n.d. | n.d. | n.d. | 346.92 ± 34.09a | 423.92 ± 44.62a | n.f. | – | – | – | – | – |
| a11 | Linalool | 78–70-6 | 1096/1099 | MS,RI | Coriander, Floral, Lavender, Lemon, Rose | 477.12 ± 46.2d | 1259.62 ± 48.81c | 1677.78 ± 177.23b | 1133.73 ± 56.93c | 2363.71 ± 291.7a | 0.22 | 87.47 | 100.00 | 100.00 | 74.51 | 100.00 |
| a12 | (E)-Para-2,8–1-menthadienol | 7212–40-0 | 1108/1123 | MS,RI | – | n.d. | 72.08 ± 9.38a | 90.5 ± 19.07a | 97.54 ± 20.58a | 67.14 ± 6.69b | n.f. | – | – | – | – | – |
| a13 | alpha-Terpineol | 98–55-5 | 1184/1189 | MS,RI | Pleasant, floral | 170.03 ± 34.88d | 325.88 ± 56.29c | 468.88 ± 23.68a | 529.27 ± 13.78a | 451.48 ± 16.28b | 86 | 0.08 | 0.07 | 0.07 | 0.09 | 0.05 |
| a14 | 1-(3,3-Dimethylcyclohexylidene)ethanol | 26532–23-0 | 1227/1225 | MS,RI | – | n.d. | n.d. | 54.37 ± 1.39c | 88.32 ± 5.32a | 69.57 ± 5.23b | n.f. | – | – | – | – | – |
| a15 | Cedrol | 77–53-2 | 1601/1598 | MS,RI | Cedarwood, woody, dry, sweet, soft | 115.43 ± 7.74d | 170.15 ± 6.02c | 272.42 ± 12.66a | 221.17 ± 13.95b | 203.46 ± 39.97b | 1 | 4.66 | 2.97 | 3.57 | 3.20 | 1.89 |
| Ketones | ||||||||||||||||
| a16 | 2-Heptanone | 110–43-0 | 888/891 | MS,RI | Blue Cheese, Fruit, Green, Nut, Spice | 1030.41 ± 121.95a | 864.29 ± 104.16a | 997.62 ± 106.73a | 756.34 ± 8.62b | 845.06 ± 133.62a | 65 | 0.64 | 0.23 | 0.20 | 0.17 | 0.12 |
| a17 | 6-Methylhept-5-en-2-one | 110–93-0 | 985/986 | MS,RI | Citrus, Mushroom, Pepper, Rubber, Strawberry | 768.36 ± 91.24a | 772.92 ± 143.61a | 656.57 ± 48.26b | 571.1 ± 54.88c | 894.34 ± 56.99a | 68 | 0.46 | 0.20 | 0.13 | 0.12 | 0.12 |
| a18 | 2,2,6-trimethylcyclohexanone | 2408–37-9 | 1028/1036 | MS,RI | Floral | 141.58 ± 17.37b | 176.56 ± 11.25b | 256.18 ± 44.29a | 192.85 ± 35.39b | 190.08 ± 19.99b | 100 | 0.06 | 0.03 | 0.03 | 0.03 | 0.02 |
| a19 | Acetophenone | 98–86-2 | 1060/1065 | MS,RI | Almonds, Flower, Meat, Must | 221.5 ± 15.85b | 239.22 ± 25.68b | 351.07 ± 5.24a | 254.82 ± 17.59b | 317.74 ± 24.62a | 65 | 0.14 | 0.06 | 0.07 | 0.06 | 0.05 |
| a20 | 3,5-Octadiene-2-one | 38284–27-4 | 1089/1091 | MS,RI | Fruit, Fat, Mushroom-like | 182.09 ± 20.93 | 227.43 ± 14.87 | 302.6 ± 39.72 | 192.2 ± 6.43 | 273.74 ± 31.25 | 0.15 | 48.96 | 26.48 | 26.45 | 18.53 | 16.99 |
| a21 | Isophorone | 78–59-1 | 1113/1124 | MS,RI | Sweet, Fruit | n.d. | n.d. | n.d. | n.d. | 78.39 ± 14.32 | 11 | – | – | – | – | 0.07 |
| a22 | 5-Ethyl-6-methyl-3-hepten-2-one | 57283–79-1 | 1144/1144 | MS,RI | – | 195.95 ± 22.94c | 217.09 ± 27.28b | 245.81 ± 23.93b | 250.5 ± 29.82b | 338.7 ± 23.76a | n.f. | – | – | – | – | – |
| a23 | 1-(1,4-Dimethylcyclohex-3-en-1-yl)ethanone | 43219–68-7 | 1150/1149 | MS,RI | Fruit | 164.82 ± 7.22c | 145.95 ± 6.04d | 192.99 ± 26.28b | 205.82 ± 21.64b | 243.39 ± 7.09a | n.f. | – | – | – | – | – |
| a24 | 7a-Methyl-3-methylenehexahydrobenzofuran-2-one | 67498–53-7 | 1300/1302 | MS,RI | – | n.d. | n.d. | 118.77 ± 8.2a | 103.12 ± 2.83b | 94.34 ± 7.34b | n.f. | – | – | – | – | – |
| a25 | β-Damascenone | 23726–93-4 | 1382/1386 | MS,RI | Floral, fruity, honey, cooked apples | n.d. | n.d. | 46.28 ± 6.08b | 63.21 ± 6.65a | 59.85 ± 5.67a | n.f. | – | – | 303.40 | 456.99 | 278.54 |
| a26 | 6,10-Dimethylundecan-2-one | 1604–34-8 | 1401/1408 | MS,RI | – | 38.59 ± 0.35c | 46.12 ± 4.21c | 77.92 ± 15.42b | 101.59 ± 17.29a | 96.22 ± 9.6a | n.f. | – | – | – | – | – |
| a27 | α-Ionone | 127–41-3 | 1424/1426 | MS,RI | Violet, Wood | 233.88 ± 39.86c | 350.02 ± 25.72b | 506.19 ± 50.06a | 583.73 ± 43.17a | 514.57 ± 35.33a | 3.7 | 2.55 | 1.65 | 1.79 | 2.28 | 1.29 |
| a28 | 4-(2,4,4-Trimethyl-cyclohexa-1,5-dienyl)-but-3-en-2-one | 1000187–51-9 | 1428/1423 | MS,RI | – | 209.56 ± 26.08c | 356.87 ± 13.3b | 541.73 ± 58.83a | 620.65 ± 56.3a | 532.48 ± 50.79a | n.f. | – | – | – | – | – |
| a29 | Geranyl acetone | 3796–70-1 | 1449/1453 | MS,RI | Fruit | 107.77 ± 4.24d | 202.3 ± 8.38c | 325.2 ± 42.97b | 398.16 ± 21.02a | 249.87 ± 45.4c | 60 | 0.07 | 0.06 | 0.07 | 0.10 | 0.04 |
| a30 | 3-Buten-2-one, 4-(2,6,6-trimethyl-1,3-cyclohexadien-1-yl)- | 1203–08-3 | 1479/1485 | MS,RI | – | n.d. | 73.87 ± 13.7b | 89.13 ± 2.73b | 127.68 ± 17.07a | 140.89 ± 10.74a | n.f. | – | – | – | – | – |
| a31 | β-Ionone | 14901–07-6 | 1482/1485 | MS,RI | Floral, Violet | 495.89 ± 36.78d | 909.94 ± 43.57c | 1264.14 ± 153.54b | 1383.16 ± 135.82a | 1486.21 ± 47.83a | 0.2 | 100.00 | 79.46 | 82.88 | 100.00 | 69.16 |
| a32 | 1-(3,5-di-tert-butyl-4-hydroxy-phenyl)-propan-1-one | 14035–34-8 | 1636/1640 | MS,RI | – | n.d. | n.d. | n.d. | 35.21 ± 3.5a | 30.16 ± 0.6a | n.f. | – | – | – | – | – |
| a33 | 6,10,14-Trimethyl-2-pentadecanone | 502–69-2 | 1844/1844 | MS,RI | Jasmine-like, oily, herbal | n.d. | n.d. | n.d. | 31.13 ± 1.79b | 38.49 ± 3.51a | n.f. | – | – | – | – | – |
| Hydrocarbon | ||||||||||||||||
| a34 | m-Cymene | 535–77-3 | 1019/1023 | MS,RI | Citrus | n.d. | n.d. | n.d. | 98.27 ± 48.09b | 364.93 ± 18.65a | n.f. | – | – | – | – | – |
| a35 | Limonene | 138–86-3 | 1025/1026 | MS,RI | Citrus, lemon, orange,green, etherel | 239.43 ± 19.53b | 250.82 ± 23.54b | 256.2 ± 21.14b | 253.99 ± 19.22b | 302.71 ± 22.78a | 200 | 0.05 | 0.02 | 0.02 | 0.02 | 0.01 |
| a36 | Naphthalene | 91–20-3 | 1172/1182 | MS,RI | Pungent | 153.13 ± 13.64b | 179.95 ± 24.68a | 201.22 ± 16.29a | 216.93 ± 26.88a | 202.58 ± 32.04a | 6 | 1.03 | 0.52 | 0.44 | 0.52 | 0.31 |
| a37 | 1,1,6-Trimethyl-1,2-dihydronaphthalin | 30364–38-6 | 1348/1354 | MS,RI | – | 95.78 ± 19.48b | 114.03 ± 11.07b | 119.15 ± 11.24b | 212.04 ± 36.73a | 190.51 ± 28.49a | n.f. | – | – | – | – | – |
| a38 | 1,1,4,5-Tetramethylindane | 16204–57-2 | 1352/1355 | MS,RI | – | n.d. | n.d. | n.d. | 50.34 ± 1.42b | 66.17 ± 1.44a | n.f. | – | – | – | – | – |
| a39 | Cascarilladiene | 59742–39-1 | 1379/1372 | MS,RI | – | n.d. | 65.7 ± 11.23b | 127.39 ± 1.95a | 141.9 ± 17.94a | 125.29 ± 17.96a | n.f. | – | – | – | – | – |
| a40 | alpha-Cedrene | 469–61-4 | 1406/1411 | MS,RI | Woody, cedar, sweet, fresh | 94.03 ± 4.37b | 122.15 ± 6.7b | 182.17 ± 34.17a | 202.98 ± 29.23a | 130.28 ± 25.72b | n.f. | – | – | – | – | – |
| a41 | (+)-β-Funebrene | 79120–98-2 | 1415/1414 | MS,RI | – | 73.05 ± 11.73b | 75.7 ± 5.64b | 117.14 ± 18.36a | 143.36 ± 26.74a | 83.26 ± 5.74b | n.f. | – | – | – | – | – |
| Heterocyclics | ||||||||||||||||
| a42 | 1-Ethyl-1H-pyrrole-2-carbaldehyde | 2167–14-8 | 1044/1046 | MS,RI | Savory | 216.24 ± 3.19c | 254.88 ± 17.87c | 365.48 ± 23.84a | 336.07 ± 19.76b | 392.65 ± 42.92a | n.f. | – | – | – | – | – |
| a43 | Edulan II | 41678–30-2 | 1258/1247 | MS,RI | – | 109.16 ± 6.17c | 135.07 ± 11.36b | 140.06 ± 8.99a | 155.00 ± 3.5a | 145.19 ± 11.19a | n.f. | – | – | – | – | – |
| a44 | Edulan I | 41678–29-9 | 1310/1314 | MS,RI | – | 124.64 ± 21.82c | 258.62 ± 23.49b | 302.35 ± 30.88b | 446.12 ± 36.06a | 400.19 ± 5.46a | n.f. | – | – | – | – | – |
| Lactones | ||||||||||||||||
| a45 | Dihydroactinidiolide | 17092–92-1 | 1529/1532 | MS,RI | Musk, coumarin | 189.87 ± 33.16b | 218.67 ± 20.45b | 297.11 ± 8.79a | 311.61 ± 10.86a | 315.02 ± 18.35a | 3.8 | 2.02 | 1.01 | 1.03 | 1.19 | 0.77 |
* Odor descriptions were from FEMA database. a: Retention index of compounds on HP-5MS; b: Retention index of compounds in references; c:“MS”, mass spetrum comparison using NIST17 library. “RI”, retention index in agreement with literature value; ‘n.f.’: threshold was not found in the literature; ‘n.d.’: data was not detected in the sample. The threshold of volatile compounds in water referred to the literatures Xu et al. (2022), Xiao et al. (2022c).
3.2.2. Changes of VOCs in dark teas during SSF
The principal component analysis was employed for data visualization, as shown in Fig. 2E. Comparing the samples at different fermentation times, noticeable changes in the flavor components of dark tea can be observed. The most significant separation is observed between BDT0 and BDT6/BDT8, indicating that B. subtilis has a significant impact on the flavor components of dark tea during the later stages of fermentation. BDT0 and BDT2 samples are close to each other, suggesting that dark tea fermented for 0 and 2 days share similar flavor components. Hierarchical cluster analysis (HCA) also divides the dark tea samples into four groups (BDT8, BDT6, BDT4, and BDT2-BDT0) (Fig. 2F), consistent with the PCA results. Furthermore, a partial least-squares discriminant analysis (PLS-DA) model was constructed, and a loading plot (Fig. 2G) was generated to examine the contribution of various VOCs to the flavor of dark tea.
To observe the changes in VOCs during the SSF of BDT more clearly, a heatmap was constructed and the results are displayed in Fig. 2H. The heatmap represents the concentration levels of different VOCs, with red color indicating high levels and green color indicating low levels. Esters are commonly present in tea and other plants, and methyl salicylate, in particular, plays a crucial role in promoting the “mint” attribute of FBT (Li et al., 2020). In the SSF of dark tea by B. subtilis, the concentration of methyl salicylate initially increased, reached its maximum in the middle stage of fermentation, and gradually decreased in the later stage. This trend is consistent with previous findings in the fermentation of dark tea by E. cristatum (Xiao et al., 2022b). Aldehydes make up a relatively large proportion of the compounds. Benzaldehyde, which imparts floral aroma, is a key flavor compound contributing to the characteristic aroma of tea leaves (Hu et al., 2021). The content of 2,2,4-trimethyl-3-cyclohexene-1-carbaldehyde, a methyl derivative of benzaldehyde, increased, while the content of benzaldehyde significantly decreased. β-Cyclocitral and safranal are characteristic aroma components of dark tea, and their concentration increases after fermentation. They are primarily produced through the oxidation and degradation of β-carotene (Hu et al., 2021). Alcohol compounds play an important role in the formation of dark tea aroma (Ma et al., 2021). After fermentation, the content of linalool and alpha-terpineol increased, with linalool becoming the compound with the highest content. Enzymatic hydrolysis of their tea aroma precursor glycosides during SSF contributes to the production of linalool and alpha-terpineol (Zheng, Li, Xiang, & Liang, 2016). Linalool has a great contribution to the “fungus flower” aroma of FBT (Li et al., 2020, Ma et al., 2021). The rich alpha-terpineol in dark tea can release a pleasant smell similar to lilacs. Ketone compounds have the largest proportion and highest content. 6-Methylhept-5-en-2-one, α-ionone, β-ionone, geranyl acetone, and isophorone are the main ketone compounds in dark tea, and their content significantly increases after SSF. The increase in α-ionone and β-ionone content is associated with the degradation of carotenoids (Wang et al., 2021). β-Ionone can be further oxidized to 5,6-epoxy-β-ionone, which after two reduction steps is converted to a saturated triol that undergoes intramolecular cyclization followed by an oxidation reaction to produce dihydroactinidiolide (Ho, Zheng, & Li, 2015), the content of dihydroactinolide increased to a maximum (315.02 ± 18.35 ng/g) on the eighth day of SSF. Dihydroactinidiolide is detected in a variety of tea samples (Ma et al., 2021), and it imparts a musky, coumarinaceous aroma to tea, which is considered to be a key aroma in determining the quality of dark tea (Ho et al., 2015). Acetophenone, another important volatile substance, contributes to the aroma characteristics of FBT. Its increase in SSF process related to heat and humidity effect and microbial metabolism (Lv et al, 2014). Geranyl acetone and isophorone contribute to the sweetness and fruitiness of dark tea's characteristic aroma (Hu et al., 2021). M−cymene is produced in the later stages of SSF and imparts dark tea a citrus aroma. Overall, the SSF process with B. subtilis influences the levels of various VOCs, contributing to the formation of the distinctive aroma characteristics of dark tea.
3.2.3. Critical volatile compounds
It is worth noting that not all VOCs detected in dark tea samples fermented at different times contribute significantly to the flavor of dark tea. The ROAV analysis was conducted to assess the contribution of different volatiles to dark tea aroma during SSF. The ROAV values indicate the importance of key aroma active compounds, with higher scores indicating a greater contribution to the aroma qualities of dark tea (Zhu et al.,2020). Among the VOCs analyzed (Table 1), seven key VOCs with ROAV > 1 were identified in BDT8: α-ionone (ROAV = 1.29), safranal (ROAV = 1.48), cedrol (ROAV = 1.89), decanal (ROAV = 3.86), 3,5-octadiene-2-one (ROAV = 16.99), β-ionone (ROAV = 69.16), and linalool (ROAV = 100). Linalool has the highest ROAV and contributes the most to the flavor of dark tea. Its content increases after SSF, giving dark tea a sweet and fresh floral fragrance with hints of bergamot (Su et al., 2022). β-Ionone is the second most important odor active substance, contributing to the floral and violet aroma of dark tea. α-Ionone imparts a violet and woody aroma, consistent with previous research indicating the significant impact of ionone on tea aroma (Hu et al., 2021). 3,5-Octadiene-2-one provides a fruit aroma to dark tea and ranks third in terms of odor activity. Decanal, despite being present in low concentrations, has a high ROAV due to its low odor threshold and contributes citrus and floral aromas to dark tea. Cedrol, a sesquiterpene alcohol, imparts a sweet and soft cedarwood odor and is a representative compound of FBT (Ma et al., 2023). Safranal provides a saffron aroma to dark tea. The concentration of all these VOCs increases significantly after fermentation, enhancing the floral and fruit aroma of dark tea. VOCs with 0.1 ≤ ROAV < 1 include β-cyclocitral, 2-heptanone, 6-methylhept-5-en-2-one, and naphthalene, which have a moderating effect on the aroma characteristics of dark tea. β-Cyclocitral and 6-methylhept-5-en-2-one increase in content during fermentation, imparting mint and citrus aromas to dark tea. On the other hand, 2-heptanone significantly decreases in content after fermentation, reducing its contribution to the blue cheese aroma of dark tea.
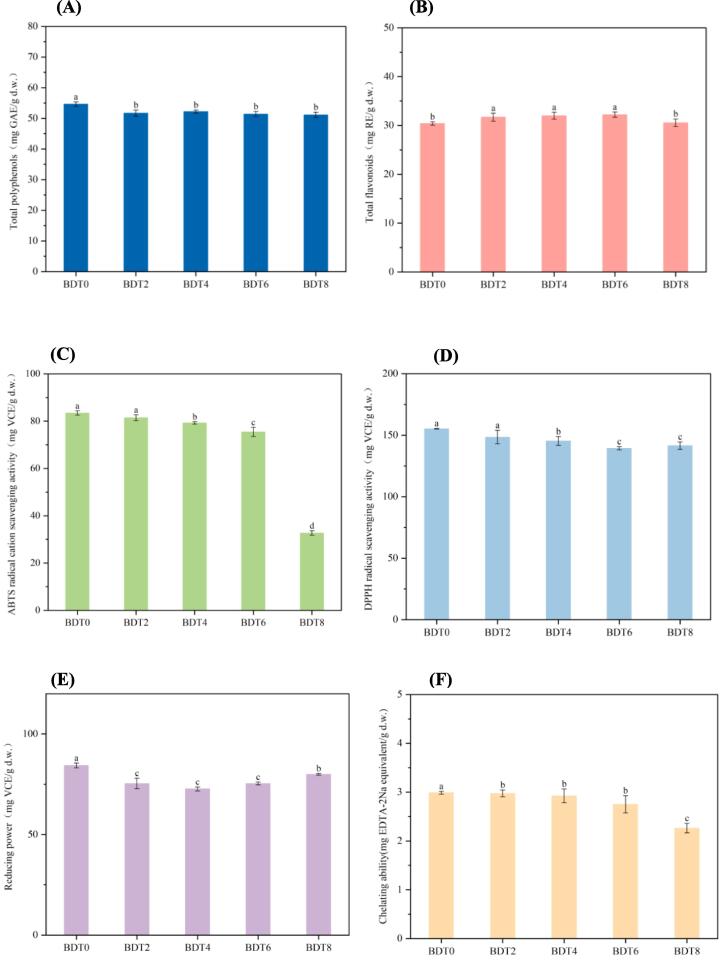
3.3. Alteration of main metabolites of dark tea during SSF
As shown in Fig. 3, the total polyphenols content decreased during SSF, and the total flavonoids content slightly increased in the middle of fermentation and decreased in the later stage. Catechins belong to flavonoid alcohols and is the main component of tea polyphenols. Catechins are divided into ester and non-ester types, which play crucial roles in the formation of tea flavor. The content changes in GA, GCG, EGCG, EGC, GC, ECG, CG, C, and EC in BDT samples during fermentation were identified and quantified by HPLC, which are listed in Table 2. The result revealed that the content of ester catechins remarkably decreased while the content of non-ester catechins greatly increased. Compared with raw dark tea (BDT0), GCG, CG, EGCG, and ECG decreased 15.3%, 10.0%, 12.4%, and 17.3% after 8 days of fermentation, respectively. However, GC, C, and EGC greatly increased 66.8%, 22.9% and 35.3%, respectively. Among them, EGCG, as one of the richest and most active gallic acid catechins, continued to decrease during fermentation, which is in line with the evolution of EGCG of dark tea fermented by E. cristatum (Xiao et al., 2022a). EGCG is the major contributor to the bitter and astringent taste of tea infusions (Cao et al., 2019). Xiao et al. (2021b) used E. cristatum (MF800948) to ferment autumn green tea to reduce the content of EGCG, thereby reducing the astringency of tea. Speculating that B. subtilis fermented dark tea can improve the bitterness and astringency is reasonable. EGCG has a low bioavailability in the small intestine. After oral administration, only 0.1%–0.32% of EGCG can be directly bioavailable (Liu, Bruins, Ni, & Vincken, 2018). After EGCG enters the small intestine, it needs to undergo gallic acid ester hydrolysis of intestinal microbiota, C-ring cleavage and further modification of reaction products to form simple phenolic acids that are quickly absorbed by the intestinal mucosa (Xiang et al., 2018). In this study, B. subtilis plays a similar role to intestinal microorganisms, decomposing non-absorbable high molecular weight tea catechins into easily absorbable phenolic metabolites, and improving the biological effects related to the consumption of tea catechins. Under aerobic conditions, EGCG is hydrolyzed into EGC and gallic acid, which is the main pathway of GA biosynthesis (Tanaka, Umeki, Nagai, Shii, Matsuo, & Kouno, 2012). Indeed, as the fermentation progressed, the content of EGCG decreases, and is further metabolized, GA also increases considerably. GA has anti-inflammatory, anti-hyperlipidemic, and anti-cirrhotic effects and used in the treatment of fatty liver and diabetes (Liu et al., 2020).
Fig. 3.
Contents of (A) total polyphenols, (B) total flavonoids. Antioxidant activities of (C) ABTS radical cation scavenging activity, (D) DPPH radical scavenging activity, (E) reducing power, (F) chelating ability of dark tea fermented by B. subtilis at different times. Data were recorded as the mean value ± standard deviation of three replicates. Mean values marked by the different letters among the samples denoted significant difference (p < 0.05).
Table 2.
Content of gallic acid and catechin compostions in dark tea fermented by B. subtilis.
| Compounds | Contents (mg/100 g) | ||||
|---|---|---|---|---|---|
| BDT0 | BDT2 | BDT4 | BDT6 | BDT8 | |
| GA | 364.11 ± 5.44c | 362.08 ± 1.98c | 376.44 ± 8.21b | 400.76 ± 8.36a | 411.09 ± 1.82a |
| GC | 106.58 ± 1.75c | 182.02 ± 4.59a | 173.12 ± 3.00b | 170.79 ± 6.03b | 177.80 ± 3.46a |
| EGC | 69.55 ± 0.30b | 113.39 ± 3.58a | 103.99 ± 10.14a | 107.72 ± 20.61a | 94.10 ± 4.25a |
| C | 344.06 ± 6.85b | 381.69 ± 23.52a | 377.84 ± 19.40a | 419.54 ± 37.39a | 422.71 ± 27.21a |
| EC | 29.59 ± 0.83b | 35.38 ± 0.24a | 30.55 ± 0.47b | 30.01 ± 0.46b | 28.40 ± 0.25c |
| EGCG | 156.29 ± 1.64a | 151.06 ± 3.57b | 150.00 ± 0.44b | 136.54 ± 2.26c | 136.88 ± 2.53c |
| GCG | 342.00 ± 6.06a | 315.98 ± 5.53b | 302.18 ± 2.81b | 290.83 ± 1.69c | 289.70 ± 5.25c |
| ECG | 124.08 ± 3.36a | 122.62 ± 5.19a | 109.50 ± 4.69b | 104.40 ± 1.67b | 102.58 ± 2.08b |
| CG | 120.70 ± 2.31a | 109.51 ± 7.52b | 97.00 ± 1.14c | 114.27 ± 3.26a | 108.61 ± 4.37b |
| Non-ester catechins | 549.79 ± 8.49b | 712.48 ± 20.14a | 685.51 ± 17.92a | 728.06 ± 53.86a | 723.00 ± 27.87a |
| Ester catechins | 743.07 ± 6.38a | 699.17 ± 15.28b | 658.67 ± 1.36c | 646.04 ± 5.70c | 637.77 ± 4.09d |
GA, gallic acid; GC, gallocatechin; EGC, epigallocatechin; C, catechin; EC, epicatechin; EGCG, epigallocatechin gallate; GCG, gallocatechin gallate; ECG, epicatechin gallate; CG, catechin gallate. Each tea sample was determined with three replications. Letters indicate Duncan’s pairwise differences among different samples (p < 0.05).
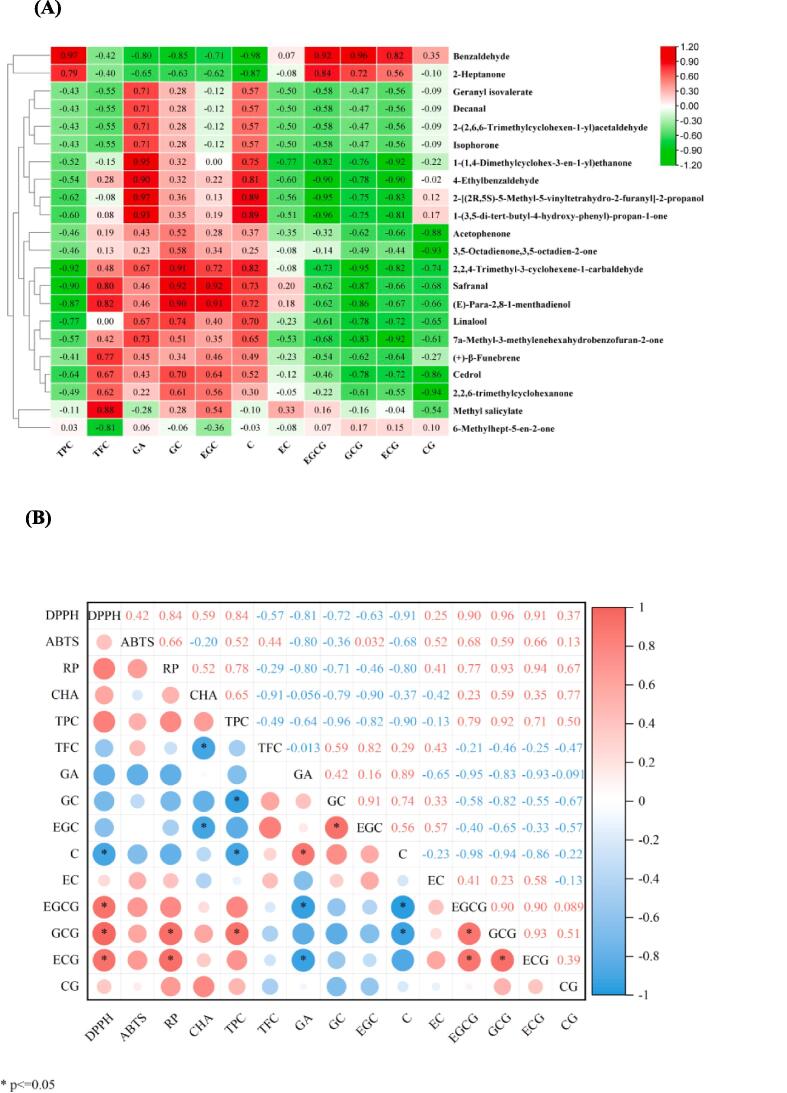
Phenolic compounds are known to be the main precursors of certain volatile compounds in tea and play a crucial role in flavor development (Ayseli & Ayseli, 2016). Using GC–MS analysis, changes in VOCs content were examined, and PLS-DA analysis was conducted to identify VOCs with VIP > 1. Pearson correlation analysis was performed between these VOCs and major non-volatile metabolites (Fig. 4A). The results revealed significant positive correlations between methyl salicylate and total flavonoids, benzaldehyde and total flavonoids, EGCG, and GCG, as well as safranal and EGC, GC. On the other hand, significant negative correlations were observed between 2,2,4-trimethyl-3-cyclohexene-1-carbaldehyde and total polyphenols, GCG, 4-ethylbenzaldehyde and EGCG, ECG. Moreover, safranal showed a negative correlation with total polyphenols, while CG displayed negative correlations with 2,2,6-trimethylcyclohexanone, acetophenone, and 3,5-octadiene-2-one. These findings suggest a close relationship between VOCs and the changes observed in these non-volatile metabolites.
Fig. 4.
(A) Correlation analysis between VOCs and the main non-volatile metabolites during SSF with B. subtilis. (B) The correlation between the antioxidant activity of dark teas fermented by B. subtilis and the content of catechins, total phenols and total flavonoids. Note: the detail names of the VOCs are shown in Table 1. GA, gallic acid; CG, catechin gallate; C, catechin; EC, epicatechin; EGC, epigallocatechin; GCG, gallocatechin gallate; ECG, epicatechin gallate; EGCG, epigallocatechin gallate; GC, gallocatechin; TCs, total catechins; TPC, total polyphenols; TFC, total flavonoids. DPPH, DPPH radical scavenging activity; ABTS, ABTS radical cation scavenging activity; RP, reducing power; CHA, chelating ability.
3.4. Changes of antioxidant activity of dark tea during SSF
The antioxidant activity of dark tea samples fermented by B. subtilis was assessed using four different antioxidant capacity assays with distinct mechanisms (Fig. 3). The results indicated that the ABTS radical cation scavenging activity and chelating ability remained relatively stable during the early and middle stages of fermentation, retaining approximately 90.38% and 92.07% of the original antioxidant activity by the sixth day of fermentation. However, a significant decrease in ABTS radical cation scavenging activity was observed by the eighth day of fermentation. The DPPH radical scavenging activity and reducing power exhibited a slight decline throughout the fermentation process, but maintained approximately 91.14% and 94.78% of the original activity until the eighth day of fermentation. In summary, the optimal duration for maintaining the maximum antioxidant activity and enhancing the flavor of dark tea through B. subtilis fermentation was found to be 6 days.
Then, PCA was to examine the relationship between antioxidant activity, bioactive ingredients, and antioxidant capabilities. The PCA score plot (Fig. S1A) demonstrated clear distinctions among tea samples with different fermentation periods. The BDT4 and BDT6 sample located in the upper right quadrant of Fig. S1A was positively correlated with PC1 and PC2, suggesting a higher level of polyphenol metabolites and relatively strong antioxidant activity. To identify key contributors, a loading plot (Fig. S1B) was generated. The loading patterns of ABTS, DPPH, and CHA on PC1 indicated their association with antioxidant activity. Moreover, EGCG, ECG, GCG, TPC, CG, and EC showed higher loadings on PC1, indicating that polyphenols are effective antioxidants. The presence of polyphenols in dark tea samples of different fermentation durations is known to contribute to the variations in antioxidant capacity (Dai et al., 2015).
Correlation analysis was conducted to verify the relationship between antioxidant activity and polyphenols in tea products (Fig. 4B). Notably, there were significant positive correlations between EGCG, GCG, ECG, and DPPH. Similarly, GCG, ECG, and RP also exhibited significant positive correlations. The O-dihydroxy or trihydroxy structure, which enables chelation of metal ions and inhibition of free radical production, is one of the chemical structures responsible for the potent antioxidant activity of catechins. Among them, EGCG, with its four dihydroxy groups, is recognized as the most effective tea antioxidant (Dufresne & Farnworth, 2001). Despite the substantial decrease in EGCG and other ester-type catechins, dark tea retained its high antioxidant activity. This could be attributed to the degradation of EGCG into EGC, which possesses a high degree of hydroxylation on the B ring, resulting in great antioxidant capacity. Additionally, the degradation products of EGCG may exhibit synergistic effects with other components (e.g., ascorbic acid, vitamin E), further enhancing the antioxidant capacity.
4. Conclusions
The study revealed that the SSF of dark tea by B. subtilis LK-1 had great effects on the change of VOCs and catechins. The fermentation process led to a notable increase in the total content and abundance of VOCs, with ketones emerging as the major compounds. Several newly generated VOCs were detected in the BDT, including geranyl isovalerate and isophorone with fruity aroma, decanal with floral aroma, and m-cymene with citrus aroma, which enriched the overall aroma profile of dark tea. Key odor-active compounds such as linalool, β-ionone, and safranal contributed to the floral aroma of dark tea, while 3,5-octadiene 2-one and decanal imparted a fruit aroma, and cedrol and α-ionone contributed to the woody aroma. The contents of these compounds significantly increased during fermentation, particularly in the later stage, indicating that B. subtilis fermentation enhanced the intensity of floral, fruity, and woody aromas in dark tea. The ester-type catechins in dark tea exhibited a significant decrease, while the non-ester-type catechins showed a notable increase after SSF. This shift in catechin composition may contribute to the reduction in bitterness and astringency of the tea. The antioxidant activity of dark tea was positively correlated with the levels of EGCG, GCG, and ECG, and remained high on the 6th day of fermentation. This suggests that a 6-day fermentation period using B. subtilis is optimal for maintaining high antioxidant activity and enhancing the flavor of dark tea. The findings demonstrate that B. subtilis plays a crucial role in the metabolism and transformation of both volatile and non-volatile components during the production process of dark tea. These results provide a valuable foundation for future investigations on the influence of bacteria in the quality formation of fermented dark tea.
CRediT authorship contribution statement
Leike Xiao: Methodology, Data curation, Validation, Writing – original draft, Investigation. Chenghongwang Yang: Writing – original draft, Investigation. Xilu Zhang: Investigation. Yuanliang Wang: Resources. Zongjun Li: Resources. Yulian Chen: Formal analysis, Investigation, Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. Zhonghua Liu: Supervision. Mingzhi Zhu: Project administration, Resources, Writing – review & editing. Yu Xiao: Project administration, Formal analysis, Methodology, Supervision, Funding acquisition, Conceptualization, Data curation, Validation, Writing – original draft, Writing – review & editing, Investigation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors appreciate the financial support from the Key Technologies Research and Development Program of Hunan Province (2023NK2025), the National Key R&D Program (2022YFE0111200), the Science Research Project of Education Department of Hunan Province (No. 22C0108) and Natural Science Foundation of China (No. 32002095).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2023.100811.
Contributor Information
Yulian Chen, Email: chenylhn@163.com.
Mingzhi Zhu, Email: mzzhucn@hotmail.com.
Yu Xiao, Email: yuxiao_89@163.com, xiaoyu@hunau.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Ayseli M.T., Ayseli Y.İ. Flavors of the future: Health benefits of flavor precursors and volatile compounds in plant foods. Trends in Food Science & Technology. 2016;48:69–77. doi: 10.1016/j.tifs.2015.11.005. [DOI] [Google Scholar]
- Buchanan R.E., Gibbons N.E. Chinese ed. Science Press; Beijing: 1984. Bergey’s manual of determinative bacteriology (8th. [Google Scholar]
- Cao L., Guo X., Liu G., Song Y., Ho C.T., Hou R.…Wan X. A comparative analysis for the volatile compounds of various Chinese dark teas using combinatory metabolomics and fungal solid-state fermentation. Journal of Food and Drug Analysis. 2018;26(1):112–123. doi: 10.1016/j.jfda.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q.Q., Zou C., Zhang Y.H., Du Q.Z., Yin J.F., Shi J.…Xu Y.Q. Improving the taste of autumn green tea with tannase. Food Chemistry. 2019;277:432–437. doi: 10.1016/j.foodchem.2018.10.146. [DOI] [PubMed] [Google Scholar]
- Chen Y., Chen J., Chen R., Xiao L., Wu X., Hu L.…Xiao Y. Comparison of the fungal community, chemical composition, antioxidant activity, and taste characteristics of Fu brick tea in different regions of China. Frontiers in Nutrition. 2022;9:900138. doi: 10.3389/fnut.2022.900138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Wang Y., Chen J., Tang H., Wang C., Li Z., Xiao Y. Bioprocessing of soybeans (Glycine max L.) by solid-state fermentation with Eurotium cristatum YL-1 improves total phenolic content, isoflavone aglycones, and antioxidant activity. RSC. Advances. 2020;10(29):16928–16941. doi: 10.1039/c9ra10344a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W., Qi D., Yang T., Lv H., Guo L., Zhang Y.…Lin Z. Nontargeted analysis using ultraperformance liquid chromatography–quadrupole time-of-flight mass spectrometry uncovers the effects of harvest season on the metabolites and taste quality of tea (Camellia sinensis L.) Journal of Agricultural and Food Chemistry. 2015;63(44):9869–9878. doi: 10.1021/acs.jafc.5b03967. [DOI] [PubMed] [Google Scholar]
- Dong X.Z., Cai M.Y. Science press; 2001. Manual of identification of common bacterial systems. [Google Scholar]
- Du Y., Yang W., Yang C., Yang X. A comprehensive review on microbiome, aromas and flavors, chemical composition, nutrition and future prospects of Fuzhuan brick tea. Trends in Food Science & Technology. 2022;119:452–466. doi: 10.1016/j.tifs.2021.12.024. [DOI] [Google Scholar]
- Dufresne C.J., Farnworth E.R. A review of latest research findings on the health promotion properties of tea. The Journal of Nutritional Biochemistry. 2001;12(7):404–421. doi: 10.1016/S0955-2863(01)00155-3. [DOI] [PubMed] [Google Scholar]
- Ho C.T., Zheng X., Li S. Tea aroma formation. Food Science and Human Wellness. 2015;4(1):9–27. doi: 10.1016/j.fshw.2015.04.001. [DOI] [Google Scholar]
- Hu S., He C., Li Y., Yu Z., Chen Y., Wang Y., Ni D. The formation of aroma quality of dark tea during pile-fermentation based on multi-omics. LWT - Food Science and Technology. 2021;147 doi: 10.1016/j.lwt.2021.111491. [DOI] [Google Scholar]
- Li J., Xu R., Zong L., Brake J., Cheng L., Wu J., Wu X. Dynamic evolution and correlation between metabolites and microorganisms during manufacturing process and storage of Fu Brick Tea. Metabolites. 2021;11(10):703. doi: 10.3390/metabo11100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Wang T. Effect of solid-state fermentation with Bacillus subtilis lwo on the proteolysis and the antioxidative properties of chickpeas. International Journal of Food Microbiology. 2021;338 doi: 10.1016/j.ijfoodmicro.2020.108988. [DOI] [PubMed] [Google Scholar]
- Li Q., Li Y., Luo Y., Xiao L., Wang K., Huang J., Liu Z. Characterization of the key aroma compounds and microorganisms during the manufacturing process of Fu brick tea. LWT-Food Science and Technology. 2020;127 doi: 10.1016/j.lwt.2020.109355. [DOI] [Google Scholar]
- Li Q., Li Y., Luo Y., Zhang Y., Chen Y., Lin H.…Liu Z. Shifts in diversity and function of the bacterial community during the manufacture of Fu brick tea. Food Microbiology. 2019;80:70–76. doi: 10.1016/j.fm.2019.01.001. [DOI] [PubMed] [Google Scholar]
- Liu M., Xie H., Ma Y., Li H., Li C., Chen L.…Zhao M. High performance liquid chromatography and metabolomics analysis of tannase metabolism of gallic acid and gallates in tea leaves. Journal of Agricultural and Food Chemistry. 2020;68(17):4946–4954. doi: 10.1021/acs.jafc.0c00513. [DOI] [PubMed] [Google Scholar]
- Liu Z., Bruins M.E., Ni L., Vincken J.P. Green and black tea phenolics: Bioavailability, transformation by colonic microbiota, and modulation of colonic microbiota. Journal of Agricultural and Food Chemistry. 2018;66(32):8469–8477. doi: 10.1021/acs.jafc.8b02233. [DOI] [PubMed] [Google Scholar]
- Lv S., Wu Y., Li C., Xu Y., Liu L., Meng Q. Comparative analysis of Pu-erh and Fuzhuan teas by fully automatic headspace solid-phase microextraction coupled with gas chromatography–mass spectrometry and chemometric methods. Journal of Agricultural and Food Chemistry. 2014;62(8):1810–1818. doi: 10.1021/jf405237u. [DOI] [PubMed] [Google Scholar]
- Ma W., Zhu Y., Shi J., Wang J., Wang M., Shao C., Yan H., Lin Z., Lv H. Insight into the volatile profiles of four types of dark teas obtained from the same dark raw tea material. Food Chemistry. 2021;346 doi: 10.1016/j.foodchem.2020.128906. [DOI] [PubMed] [Google Scholar]
- Ma W., Zhu Y., Ma S., Shi J., Yan H., Lin Z., Lv H. Aroma characterisation of Liu-pao tea based on volatile fingerprint and aroma wheel using SBSE-GC-MS. Food Chemistry. 2023;414 doi: 10.1016/j.foodchem.2023.135739. [DOI] [PubMed] [Google Scholar]
- Pripdeevech P., Moonggoot S., Popluechai S., Chukeatirote E. Analysis of volatile constituents of fermented tea with Bacillus subtilis by SPME-GC-MS. Chiang Mai Journal of Science. 2014;41(2):395–402. http://epg.science.cmu.ac.th/ejournal/ [Google Scholar]
- Qi B., Zhang Y., Ren D., Qin X., Wang N., Yang X. Fu Brick Tea Alleviates Constipation via Regulating the Aquaporins-Mediated Water Transport System in Association with Gut Microbiota. Journal of Agricultural and Food Chemistry. 2023;71(8):3862–3875. doi: 10.1021/acs.jafc.2c07709. [DOI] [PubMed] [Google Scholar]
- Shi J., Zhu Y., Zhang Y., Lin Z., Lv H.P. Volatile composition of Fu-brick tea and Pu-erh tea analyzed by comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry. LWT-Food Science and Technology. 2019;103:27–33. doi: 10.1016/j.lwt.2018.12.075. [DOI] [Google Scholar]
- Su D., He J.J., Zhou Y.Z., Li Y.L., Zhou H.J. Aroma effects of key volatile compounds in Keemun black tea at different grades: HS-SPME-GC-MS, sensory evaluation, and chemometrics. Food Chemistry. 2022;373 doi: 10.1016/j.foodchem.2021.131587. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Umeki H., Nagai S., Shii T., Matsuo Y., Kouno I. Transformation of tea catechins and flavonoid glycosides by treatment with Japanese post-fermented tea acetone powder. Food Chemistry. 2012;134(1):276–281. doi: 10.1016/j.foodchem.2012.02.136. [DOI] [Google Scholar]
- Tasaki S., Nakayama M., Shoji W. Morphologies of Bacillus subtilis communities responding to environmental variation. Development, Growth & Differentiation. 2017;59(5):369–378. doi: 10.1111/dgd.12383. [DOI] [PubMed] [Google Scholar]
- Wang Z., Ma B., Ma C., Zheng C., Zhou B., Guo G., Xia T. Region identification of Xinyang Maojian tea using UHPLC-Q-TOF/MS-based metabolomics coupled with multivariate statistical analyses. Journal of Food Science. 2021;86(5):1681–1691. doi: 10.1111/1750-3841.15676. [DOI] [PubMed] [Google Scholar]
- Xia F., Hu S., Zheng X., Wang M.W., Zhang C.C., Wu Z.N., Sun Y.J. New insights into metabolomics profile generation in fermented tea: The relevance of bacteria and metabolites in Fuzhuan brick tea. Journal of the Science of Food and Agriculture. 2022;102(1):350–359. doi: 10.1002/jsfa.11365. [DOI] [PubMed] [Google Scholar]
- Xiang L.M., Liu Y.Q., Lai X.F., Li Q.H., Sun L.L., Chen W.P.…Sun S.L. Biochemical component analysis and antioxidant activities of different kinds of aged tea. Modern Food Science and Technology. 2018;34(4):56–62. doi: 10.13982/j.mfst.1673-9078.2018.04.010. [DOI] [Google Scholar]
- Xiang M., Chu J., Cai W., Ma H., Zhu W., Zhang X.…Liu X. Microbial succession and interactions during the manufacture of Fu Brick Tea. Frontiers in Microbiology. 2022;13 doi: 10.3389/fmicb.2022.892437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., He C., Chen Y., Ho C.T., Wu X., Huang Y.…Liu Z. UPLC–QQQ–MS/MS-based widely targeted metabolomic analysis reveals the effect of solid-state fermentation with Eurotium cristatum on the dynamic changes in the metabolite profile of dark tea. Food Chemistry. 2022;378 doi: 10.1016/j.foodchem.2021.131999. [DOI] [PubMed] [Google Scholar]
- Xiao Y., Huang Y., Chen Y., Zhu M., He C., Li Z.…Liu Z. Characteristic fingerprints and change of volatile organic compounds of dark teas during solid-state fermentation with Eurotium cristatum by using HS-GC-IMS, HS-SPME-GC-MS, E-nose and sensory evaluation. LWT-Food Science and Technology. 2022;169 doi: 10.1016/j.lwt.2022.113925. [DOI] [Google Scholar]
- Xiao Y., Huang Y., Chen Y., Xiao L., Zhang X., Yang C.…Wang Y. Discrimination and characterization of the volatile profiles of five Fu brick teas from different manufacturing regions by using HS-SPME/GC-MS and HS-GC-IMS. Current Research in Food Science. 2022;5:1788–1807. doi: 10.1016/j.crfs.2022.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., Wu X., Yao X., Chen Y., Ho C.T., He C.…Wang Y. Metabolite profiling, antioxidant and α-glucosidase inhibitory activities of buckwheat processed by solid-state fermentation with Eurotium cristatum YL-1. Food Research International. 2021;143 doi: 10.1016/j.foodres.2021.110262. [DOI] [PubMed] [Google Scholar]
- Xiao Y., Li M., Liu Y., Xu S., Zhong K., Wu Y., Gao H. The effect of Eurotium cristatum (MF800948) fermentation on the quality of autumn green tea. Food Chemistry. 2021;358 doi: 10.1016/j.foodchem.2021.129848. [DOI] [PubMed] [Google Scholar]
- Xiao Y., Rui X., Xing G., Wu H., Li W., Chen X.…Dong M. Solid state fermentation with Cordyceps militaris SN-18 enhanced antioxidant capacity and DNA damage protective effect of oats (Avena sativa L.) Journal of Functional Foods. 2015;16:58–73. doi: 10.1016/j.jff.2015.04.032. [DOI] [Google Scholar]
- Xu Q., Sun M., Ning J., Fang S., Ye Z., Chen J., Fu R. The core role of Bacillus subtilis and Aspergillus fumigatus in pile-fermentation processing of Qingzhuan Brick Tea. Indian Journal of Microbiology. 2019;59:288–294. doi: 10.1007/s12088-019-00802-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu A., Wang Y., Wen J., Liu P., Liu Z., Li Z. Fungal community associated with fermentation and storage of Fuzhuan brick-tea. International Journal of Food Microbiology. 2011;146(1):14–22. doi: 10.1016/j.ijfoodmicro.2011.01.024. [DOI] [PubMed] [Google Scholar]
- Xu J., Zhang Y., Yan F., Tang Y., Yu B., Chen B.…Chen H. Monitoring Changes in the Volatile Compounds of Tea Made from Summer Tea Leaves by GC-IMS and HS-SPME-GC-MS. Foods. 2022;12(1):146. doi: 10.3390/foods12010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun J., Cui C., Zhang S., Zhu J., Peng C., Cai H.…Hou R. Use of headspace GC/MS combined with chemometric analysis to identify the geographic origins of black tea. Food Chemistry. 2021;360 doi: 10.1016/j.foodchem.2021.130033. [DOI] [PubMed] [Google Scholar]
- Zhang W., Cao J., Li Z., Li Q., Lai X., Sun L.…Lai Z. HS-SPME and GC/MS volatile component analysis of Yinghong No. 9 dark tea during the pile fermentation process. Food Chemistry. 2021;357 doi: 10.1016/j.foodchem.2021.129654. [DOI] [PubMed] [Google Scholar]
- Zheng X.Q., Li Q.S., Xiang L.P., Liang Y.R. Recent Advances in Volatiles of Teas. Molecules. 2016;21(3):338. doi: 10.3390/molecules21030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W., Fang X., Wang W., Xu W., Chen W., Wu S.…Wang S. Aroma effects of critical volatile compounds during thermophilic bacteria pile-fermentation in dark tea using gas chromatography mass spectrometry and odor activity value. Food Science and Technology. 2022;42 doi: 10.1590/fst.87022. [DOI] [Google Scholar]
- Zhu Y., Chen J., Chen X., Chen D., Deng S. Use of relative odor activity value (ROAV) to link aroma profiles to volatile compounds: Application to fresh and dried eel (Muraenesox cinereus) International Journal of Food Properties. 2020;23(1):2257–2270. doi: 10.1080/10942912.2020.1856133. [DOI] [Google Scholar]
- Zhou X., Ge B., Zhang X., Wang K., Zhou C., Fu D. Metabolomics analysis reveals the effects of compound fuzhuan brick tea (CFBT) on regulating dyslipidemia and metabolic disorders in mice induced by high-fat diet. Nutrients. 2022;14(6):1128. doi: 10.3390/nu14061128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M., Ouyang J., Zhou F., Zhao C., Zhu W., Liu C.…Liu Z. Polysaccharides from Fu brick tea ameliorate obesity by modulating gut microbiota and gut microbiota-related short chain fatty acid and amino acid metabolism. The Journal of Nutritional Biochemistry. 2023;118 doi: 10.1016/j.jnutbio.2023.109356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.