Abstract
Significance:
This pilot randomized trial, the first to evaluate a specific base-in relieving prism treatment strategy for childhood intermittent exotropia, did not support proceeding to a full-scale clinical trial. Defining and measuring prism adaptation in children with intermittent exotropia is challenging and needs further study.
Purpose:
Determine whether to proceed to a full-scale trial of relieving base-in prism spectacles versus refractive correction alone for children with intermittent exotropia.
Methods:
Children 3- to <13-years-old with distance intermittent exotropia control score of ≥2 points on the Intermittent Exotropia Office Control Scale1 (0=phoria to 5=constant), ≥1 episode of spontaneous exotropia,16–35∆ by prism-and-alternate-cover test, who did not fully prism adapt on a 30-minute in-office prism-adaptation test were randomized to base-in relieving prism (40% of the larger of distance and near exodeviations) or non-prism spectacles for 8 weeks. A priori criteria to conduct a full-scale trial were defined for the adjusted treatment group difference in mean distance control: “proceed” (≥ 0.75 points favoring prism), “uncertain” (> 0 to < 0.75 points favoring prism), or “do not proceed” (≥ 0 points favoring non-prism).
Results:
57 children (mean age 6.6 ±2.2 years; mean baseline distance control 3.5 points) received prism (N=28) or non-prism (N=29) spectacles. At 8-weeks, mean control was 3.6 and 3.3 points in prism (N=25) and non-prism (N=25) groups, respectively; adjusted difference 0.3 points (95% confidence interval −0.5 to 1.1) favoring non-prism (meeting our a priori “do not proceed” criterion).
Conclusions:
Base-in prism spectacles, equal to 40% of the larger of the exodeviations at distance or near, worn for 8 weeks by 3- to 12-year-old children with intermittent exotropia did not yield better distance control than refractive correction alone, with the confidence interval indicating a favorable effect of 0.75 points or larger is unlikely. There was insufficient evidence to warrant a full-scale randomized trial.
Intermittent exotropia is a common form of strabismus characterized by periods of exotropia interspersed with periods of normal ocular alignment. Non-surgical management options beyond refractive correction are part-time patching,2, 3 over-minus lenses,4, 5 vision therapy/orthoptics,6, 7 and spectacles with base-in prism equal to (i.e., corrective) or less than (i.e., relieving or partial) the strabismic angle.8–10 Relieving prism treatment is prescribed for intermittent exotropia with the rationale of potentially restoring, improving, or maintaining normal binocular vision by optically reducing the fusional convergence demand, and thus making it easier to sustain fusion.8–10
If prism treatment results in better control of the intermittent exotropia and facilitates enhanced binocular fusion, thereby reducing the need for surgical or vision therapy/orthoptic treatment, there could be significant cost savings. In the absence of published reports evaluating the effectiveness of base-in relieving prism for intermittent exotropia, the Pediatric Eye Disease Investigator Group (PEDIG) designed a pilot randomized clinical trial to determine whether a full-scale randomized trial should be conducted.
The objective of this 8-week pilot randomized trial was to compare spectacles with relieving prism to spectacles without prism in controlling intermittent exotropia. Our goal was to determine whether to proceed to a full-scale trial by evaluating the short-term effect of relieving prism treatment on distance control of the intermittent exotropia and to assess feasibility aspects of recruitment, retention, treatment adherence, and masking. We also ascertained the proportion of participants reporting good to excellent adherence with wearing prism spectacles and the incidence of associated adverse effects.
METHODS
The study was conducted according to the tenets of the Declaration of Helsinki11 by PEDIG at 28 clinical sites. The protocol12 and Health Insurance Portability and Accountability Act-compliant informed consent forms were approved by the required institutional review boards. A parent or guardian of each participant gave written informed consent, and participants gave written assent when required. An independent Data and Safety Monitoring Committee (DSMC) provided oversight. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline13 and is listed on www.clinicaltrials.gov.14
Eligibility Criteria
Eligible children were 3 to <13 years old with intermittent exotropia meeting the following criteria: (1) intermittent or constant exotropia at distance with an Intermittent Exotropia Office Control Score1 of 2.0 points or worse on a scale ranging from 0 (phoria) to 5 (constant), with at least 1 score of 3, 4, or 5 (i.e., spontaneous tropia) (Appendix 1, available at [LWW insert link]), (2) exodeviation of 16 to 35 prism diopters (∆) at distance and 10 to 35∆ at near measured by the prism-and-alternate-cover test with the near exodeviation not exceeding the distance exodeviation by more than 10∆, and (3) no prior use of prism spectacles. Additional eligibility criteria are shown in Appendix 2, available at [LWW insert link].
Enrollment Testing
Before clinical testing, participants completed the Child Intermittent Exotropia Symptom Questionnaire15, 16 (Appendix 3, available at [LWW insert link]), and parents completed a 7-item survey (Appendix 4, available at [LWW insert link]) assessing potential issues with spectacles. Testing was performed sequentially by the same study-certified examiner through the participant’s habitual optical correction as follows: control #1 (Appendix 1), near stereoacuity, control #2, cover-uncover test, distance and near prism-and-alternate-cover tests (20/40 optotype at 6 meters and 20/30 optotype at 33 cm), control #3, and density of suppression (described in Table 1 footnote).17 Distance visual acuity was measured using the investigator’s usual optotype method.
Table 1.
Baseline Demographic and Clinical Characteristics by Treatment Group (N=57).
| Prism (N=28) |
Non-prism (N=29) |
|||
|---|---|---|---|---|
| N | % | N | % | |
| Sex: Female | 17 | 61% | 16 | 55% |
| Race/ethnicity | ||||
| Asian | 0 | 0% | 3 | 10% |
| Black/African American | 6 | 21% | 2 | 7% |
| Hispanic | 4 | 14% | 7 | 24% |
| White | 16 | 57% | 17 | 59% |
| More than one race | 2 | 7% | 0 | 0% |
| Age at randomization, years | ||||
| 3–<5 | 10 | 36% | 7 | 24% |
| 5–<7 | 7 | 25% | 10 | 34% |
| 7–<9 | 8 | 29% | 8 | 28% |
| ≥9 | 3 | 11% | 4 | 14% |
| Mean (SD) | 6.4 (2.4) | 6.8 (2.1) | ||
| Range | 3.2 to 12.3 | 3.7 to 12.6 | ||
| Refractive error, average of eyes (spherical equivalent, D) | ||||
| −6.00 to <−2.00 | 2 | 7% | 2 | 7% |
| −2.00 to <−1.00 | 2 | 7% | 0 | 0% |
| −1.00 to <−0.00 | 2 | 7% | 6 | 21% |
| 0.00 to <1.00 | 14 | 50% | 13 | 45% |
| 1.00 to <2.00 | 7 | 25% | 8 | 28% |
| Median (Range) | 0.6 (−3.80 to +2.0 D) | 0.5 (−4.00 to +1.80D) | ||
| Mean (SD) | 0.2 (1.3) | 0.2 (1.3) | ||
| Prior IXT Treatment | ||||
| Overminus | 2 | 7% | 3 | 10% |
| Patching | 7 | 25% | 5 | 17% |
| Patching and Atropine | 0 | 0% | 1 | 3% |
| Patching and Overminus | 1 | 4% | 0 | 0% |
| Vision Therapy and Overminus | 0 | 0% | 1 | 3% |
| None | 18 | 64% | 19 | 66% |
| Near Stereoacuity in arc seconds (log arcsec) a | ||||
| 40 (1.60) | 2 | 7% | 3 | 10% |
| 60 (1.78) | 6 | 21% | 8 | 28% |
| 100 (2.00) | 9 | 32% | 7 | 24% |
| 200 (2.30) | 3 | 11% | 4 | 14% |
| 400 (2.60) | 5 | 18% | 3 | 10% |
| 800 (2.90) | 2 | 7% | 2 | 7% |
| Nil (3.10) | 1 | 4% | 2 | 7% |
| Median (range) [log arcsec] | 2.0 (1.6 to 3.2) | 2.0 (1.6 to 3.2) | ||
| Mean (SD) (log arcsec) | 2.2 (0.4) | 2.2 (0.5) | ||
| Suppression b | ||||
| Negligible (0) | 3 | 12% | 3 | 12% |
| Mild (1) | 1 | 4% | 4 | 16% |
| Moderate (2) | 4 | 15% | 8 | 32% |
| Dense (3) | 18 | 69% | 10 | 40% |
SD = standard deviation; D = diopter(s); IXT = intermittent exotropia, log arcsec = logarithm of arc seconds
Randot Preschool Stereoacuity test (Stereo Optical Co., Inc., Chicago, IL). For analysis, stereoacuity was converted from seconds of arc scores to log arcsec values (in parentheses) as follows: 40 (1.60), 60 (1.78), 100 (2.00), 200 (2.30), 400 (2.60), 800 (2.90); participants with no detectable (nil) stereoacuity were assigned the value of 1600 (3.20).
The Office Suppression Test17 is a three-step procedure to grade density of suppression in patients with intermittent exotropia who are manifestly exotropic. While manifestly exotropic (spontaneous or induced), the participant viewed a 20/50 optotype at 6 meters, in normal room illumination, and was asked whether 1 or 2 letters were seen. If 2 letters were reported, suppression was classified as “negligible” (grade of 0). If 1 letter was reported (indicating suppression), a single-point light source was viewed at 6 meters with a red filter placed over the habitually fixing eye. If 2 lights were reported (one white and one red), suppression was classified as “mild” (grade 1). If 1 light was reported, the light source was viewed again in dim lighting with a red filter over the preferred eye. Suppression was classified as “moderate” (grade 2) if 2 lights were reported or dense (grade 3) if 1 light was reported. “Missing” refers to cases in which participants were unable to understand the test and/or gave unreliable responses.
Prism Adaptation Testing at Enrollment
After clinical testing and prior to randomization, an in-office prism adaptation test was administered so that children with “full” prism adaptation (i.e., deviation magnitude increases by the prism magnitude worn) would be excluded from the trial. The prism power to be prescribed if the participant were to be randomized to the prism group (40% of the larger deviation; see Treatment Regimens) was split equally (7Δ split as 3Δ and 4Δ) between the eyes and placed in a trial frame with the refractive correction, which the child wore for at least 30 minutes. Afterward, a near cover-uncover test was performed, and the distance and near prism-and-alternate-cover tests were measured through the trial-framed lenses and prisms. Children with full prism adaptation, any new esotropia at near, or a near esodeviation ≥6∆ were not eligible for randomization. Test procedure details are specified in the Intermittent Exotropia-6 Testing Procedures Manual (e-Supplement #1).
Treatment Regimens
Eligible participants were randomly assigned 1:1 through the study website to either prism or non-prism spectacles, using a permuted block design stratified by the mean of 3 measures of distance control (2 to <3, 3 to <4, 4 to 5 points). Prism group participants were prescribed spectacles with their refractive correction and ground-in base-in prism equal to 40% of the larger of the distance and near exodeviations (6Δ for a 16Δ exodeviation, 7Δ for 18Δ, 8Δ for 20Δ, 10Δ for 25Δ, 12 for 30Δ, and 14Δ for 35Δ) divided equally between the eyes. This prism magnitude (40% of the larger deviation) was the consensus opinion of the study planning committee after reviewing published guidelines.8,10,18 Non-prism group participants were prescribed spectacles with refractive correction only. Astigmatism, anisometropia, and myopia were fully corrected based on cycloplegic refraction within 7 months of enrollment. For spherical equivalent hyperopia or emmetropia, the sphere power resulting in plano spherical equivalent was prescribed for the less hyperopic eye, with a symmetrical reduction of hyperopic sphere power for the fellow eye (e.g., cycloplegic refraction of +3.00 −1.00 × 180 and +2.00 −2.00 × 175 resulted in a prescription of +2.00 −1.00 × 180 and +1.00 −2.00 × 175 for the right and left eyes, respectively). No other treatment for intermittent exotropia was allowed.
Masking
To facilitate masking, families were not informed of the child’s treatment assignment, and the dispensing optician was instructed not to disclose the prescription. Examiners masked to treatment assignment, were not apprised of the child’s refractive error or spectacle prescription, did not handle the study spectacles, and were queried after their examinations whether they had been unmasked to treatment assignment.
Follow-up Including Primary Outcome Examination
Follow-up (timed from randomization) consisted of a phone call at 3 weeks to verify receipt of study spectacles and the primary outcome visit at 8 weeks (±2 weeks). Because the coronavirus 2019 (COVID-19) pandemic resulted in restrictions on clinical research visits, the Data and Safety Monitoring Committee approved an amended protocol (April 2020) that allowed participants to continue treatment and extended the primary outcome visit window to 24 weeks.
At outcome, adherence with spectacle wear was determined to be excellent (>75% of waking hours), good (51% to 75%), fair (26% to 50%), or poor (≤25%) based on home calendar review and parental report. The Child Intermittent Exotropia Symptom Questionnaire15, 16 (Appendix 3) and the Parent Spectacle Symptom Survey (Appendix 4) were administered, and a masked examiner completed the same clinical testing performed at enrollment, with participants wearing their study spectacles. When study spectacles were unavailable, a trial frame and trial lenses with the imprinted prism and dioptric power designations concealed were used.
Statistical Methods
Prism Adaptation: Testing and Determination
For the prism adaptation test at enrollment, the in-prism exodeviation magnitude measurement used for analysis was defined as the prism-and-alternate-cover test measured through the trial-framed prism (the sum of prism in the trial frame and the measuring prism held in front of the trial frame needed to neutralize the deviation). For example, if 10∆ of base-in prism was placed in the trial frame and the measuring prism required to neutralize the deviation with the trial-framed 10∆ base-in prism remaining in place measured 25∆ base-in, then the total in-prism exodeviation magnitude was 35∆. Full prism adaptation was defined a priori as a total in-prism exodeviation magnitude that equaled or exceeded the sum of the pre-prism measurement of the prism-and-alternate-cover test and the magnitude of base-in prism in the trial frame, at both distance and near. The proportion of pre-randomized participants having full prism adaptation was calculated with an exact 95% confidence interval (CI).
Primary Analysis
The planned convenience sample size of 64 was expected to provide outcome data for at least 60 participants (30 per group). The primary analysis was the treatment group difference (prism minus non-prism; negative difference favors prism) (and 95% CI) in mean distance control at the 8-week outcome visit using an analysis of covariance (ANCOVA) adjusted for baseline distance control. Likelihood-based methods were used to account for missing 8-week outcomes. The following a priori criteria, based on the estimated treatment group mean difference, were established for advancing to a full-scale trial: “proceed” (≥ 0.75 points favoring prism), “uncertain” (> 0 to < 0.75 points favoring prism), or “do not proceed” ( 0 points favoring non-prism).
Three sensitivity analyses were conducted: 1) limited to participants who received their study spectacles at least 4 weeks before the outcome visit, 2) limited to participants evaluated in study spectacles (vs. trial frame) at outcome, and 3) including all participants and adjusting for baseline near control and baseline distance control.
Secondary Analyses
Two additional analyses were performed for the distance control outcome. Treatment-group differences and 95% CIs were calculated for the proportion of participants with a treatment response (i.e., ≥1 point improvement in mean distance control between baseline and outcome) and the proportion without a spontaneous tropia (i.e., did not score 3, 4, or 5 on any control assessment at distance or near).
For each continuous secondary outcome (treatment group comparisons of near control, near stereoacuity, and exodeviation magnitude at distance and near), we calculated the mean treatment group difference at 8 weeks (prism minus non-prism) using ANCOVA models parallel to the primary outcome. The distribution of each outcome and the change between baseline and outcome were tabulated for each treatment group. The total exodeviation (i.e., the sum of ground-in spectacle prism and measuring prism held in front of the spectacles to neutralize the prism-and-alternate-cover test) was used for analysis. As a post hoc outcome in the prism group only, full prism adaptation over 8 weeks was assessed by comparing: 1) the total in-prism exodeviation magnitude measurements at 8 weeks (prism magnitude worn added to the prism-and-alternate-cover test measurements using loose prism) at distance and near with 2) the baseline prism-and-alternate-cover test measurements (in spectacles without prism).
An additional post-hoc analysis of the Rasch-calibrated Child Intermittent Exotropia Symptom Questionnaire was conducted, calculating overall mean scores for each group at 8 weeks, based on published methods;16 scores ranging from 0 (no symptoms) to 100 (worst symptoms). The mean scores were compared using ANCOVA adjusting for baseline scores.
Analyses were conducted using SAS version 9.4. No adjustments were made for multiplicity because of the exploratory nature of the analyses.
RESULTS
Prism Adaptation Test at Enrollment
Between September 2019 and March 2020, 61 children with intermittent exotropia underwent in-office pre-randomization prism adaptation testing (Figure 1). Three participants (5%; 95% CI: 1%–14%) met the a priori definition of full prism adaptation, and 1 demonstrated a near esotropia through the prisms in the trial frame. These 4 participants were excluded from randomization.
Figure 1.
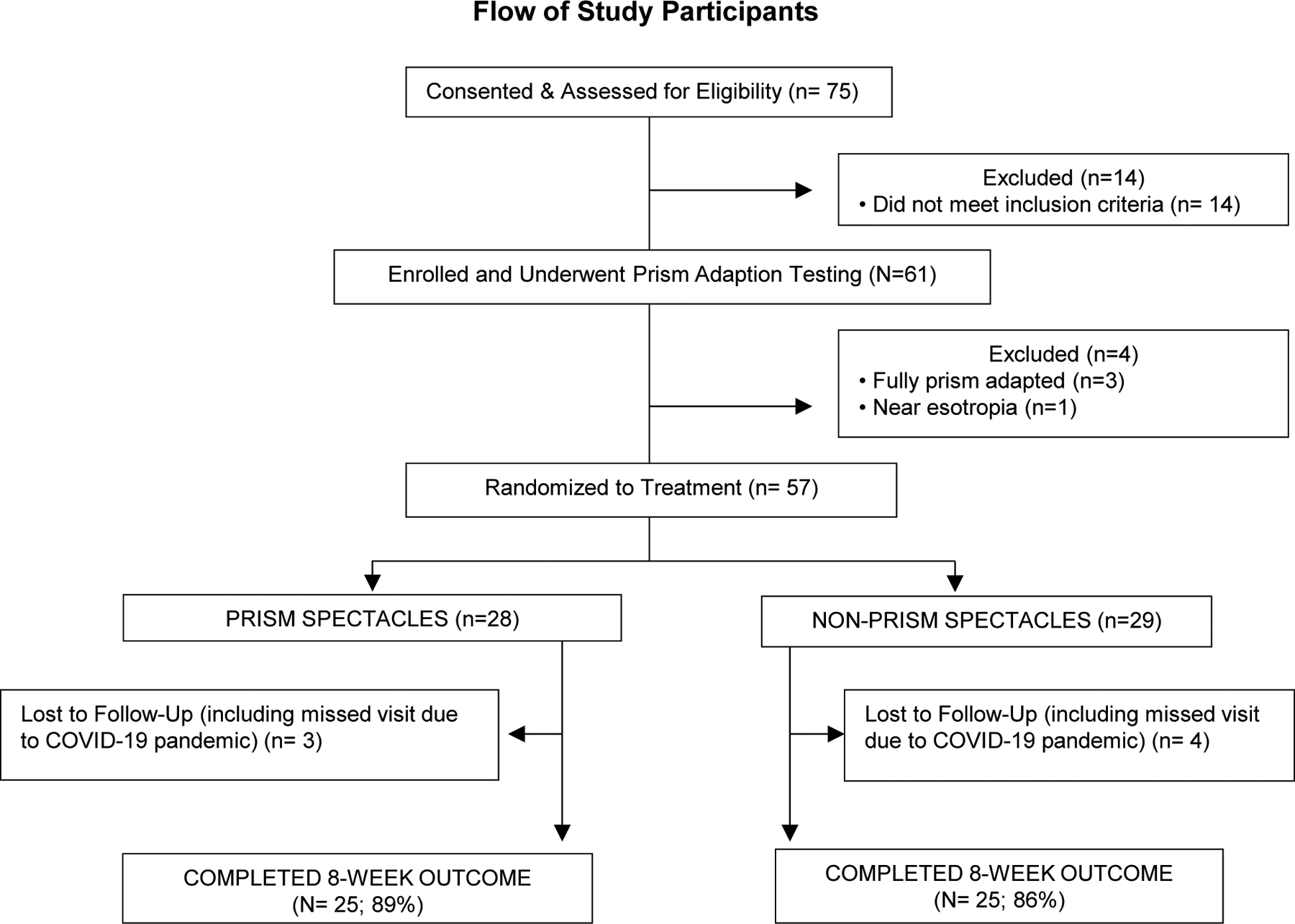
Flow of Study Participants.
Baseline Characteristics of Participants Randomized
Of the 57 participants randomized, 28 were assigned prism and 29 non-prism spectacles. Mean age was 6.6 (±2.2) years, and 33 (58%) were female. Baseline characteristics by treatment group are provided in Table 1. The mean control score at enrollment was 3.5 points at distance and 1.7 points at near in the non-prism group and 3.4 points at distance and 2.3 points at near in the prism group (Table 2).
Table 2.
Baseline Exotropia Control Score and Magnitude of Deviation by Treatment Group (N=57).
| Distance | Near | |||||||
|---|---|---|---|---|---|---|---|---|
| Prism (N=28) |
Non-prism (N=29) |
Prism (N=28) |
Non-prism (N=29) |
|||||
| N | % | N | % | N | % | N | % | |
| Exotropia controla (points) | ||||||||
| 0 to <1 | ---- | ---- | ---- | ---- | 2 | 7% | 6 | 21% |
| 1 to <2 | ---- | ---- | ---- | ---- | 9 | 32% | 9 | 31% |
| 2 to <3 | 8 | 29% | 8 | 28% | 6 | 21% | 9 | 31% |
| 3 to <4 | 10 | 36% | 10 | 34% | 6 | 21% | 4 | 14% |
| 4 to 5 | 10 | 36% | 11 | 38% | 5 | 18% | 1 | 3% |
| Mean (SD) | 3.4 (0.9) | 3.5 (0.9) | 2.3 (1.3) | 1.7 (1.1) | ||||
| Range | 2 to 5 | 2 to 5 | 0 to 4.3 | 0 to 4 | ||||
| Exodeviation by PACT (∆)b (in habitual correction) | ||||||||
| No exodeviation (orthophoria) | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- |
| 1–9 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- |
| 10–15 | ---- | ---- | ---- | ---- | 2 | 7% | 6 | 21% |
| 16–18 | 3 | 11% | 5 | 17% | 8 | 29% | 7 | 24% |
| 20–25 | 18 | 64% | 15 | 52% | 15 | 54% | 8 | 28% |
| 30–35 | 7 | 25% | 9 | 31% | 3 | 11% | 8 | 28% |
| 40–45 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- |
| Mean (SD) | 25.1 (4.7) | 24.9 (5.6) | 21.5 (5.1) | 21.1 (7.5) | ||||
| Range | 16 to 35 | 16 to 35 | 14 to 35 | 10 to 35 | ||||
∆ = prism diopter; PACT = prism-and-alternate-cover test; SD = standard deviation
---- indicates not applicable
Mean of 3 assessments performed throughout exam. Mean distance exotropia control of 2 points or greater (worse) was required for eligibility. Mean near exotropia control of less than 5 points (i.e., could not be constant on all 3 measures) was required for eligibility.
Eligibility criteria: Distance exodeviation at least 16∆ measured by PACT and no larger than 35∆. Near exodeviation at least 10∆ measured by PACT and no larger than 35∆. Near deviation does not exceed the distance deviation by more than 10∆ by PACT.
Prism Spectacles and Spectacle-wear Adherence
In the prism group, the magnitude of base-in prism prescribed (mean = 10.4Δ±1.9Δ) was 6Δ for 1 participant (4%,) 7Δ for 1 (4%), 8Δ for 2 (7%), 10Δ for 16 (57%), 12Δ for 5 (18%), and 14Δ for 3 (11%). Study spectacles were received at least 4 weeks before the outcome visit for 24 of 25 (96%) participants in each treatment group. Spectacle-wear adherence in the prism group was deemed excellent in 22 (88%) participants and good in 3 (12%). Adherence in the non-prism group was judged excellent in 22 (88%) participants, good in 2 (8%), and fair in 1 (4%).
Primary Outcome Visit Completion
The 8-week primary outcome visit was completed by 89% (25 of 28) of participants in both treatment groups. Forty percent (10 of 25) and 36% (9 of 25) of outcome visits were completed late (between 10 to 24 weeks) in the prism and non-prism groups, respectively.
Primary Outcome
At the primary outcome visit, mean intermittent exotropia distance control was 3.6 points for the 25 prism group participants versus 3.3 points for the 25 non-prism group participants (adjusted difference = 0.3 points [95% CI: −0.5 to 1.1]; Table 3). Mean change in distance control from baseline to outcome was 0.1 point and −0.2 point in the prism vs. non-prism groups. The three sensitivity analyses yielded similar results to the primary analysis (Table 4). Examiners reported being masked to treatment assignment for all outcome examinations.
Table 3.
Exotropia Control Score at the 8-week Outcome Visit (N=50)a
| Distance | Near | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Prisme (N=25) |
Non-prismf (N=25) |
Prisme (N=25) |
Non-prismf (N=25) |
||||||
| N | % | N | % | N | % | N | % | ||
| Control Score (points) | |||||||||
| 0 to <1 | 1 | 4% | 1 | 4% | 5 | 20% | 7 | 28% | |
| 1 to <2 | 1 | 4% | 3 | 12% | 9 | 36% | 7 | 28% | |
| 2 to <3 | 6 | 24% | 5 | 20% | 7 | 28% | 6 | 24% | |
| 3 to <4 | 2 | 8% | 6 | 24% | 3 | 12% | 3 | 12% | |
| 4 to 5 | 15 | 60% | 10 | 40% | 1 | 4% | 2 | 8% | |
| Mean (SD) | 3.6 (1.3) | 3.3 (1.5) | 1.7 (1.1) | 1.7 (1.3) | |||||
| Range | 0.7 to 5.0 | 0.0 to 5.0 | 0.0 to 4.0 | 0.0 to 5.0 | |||||
| Adjusted difference in means (95% CI)b | 0.3 (−0.5, 1.1) | 0.01 (−0.7, 0.7) | |||||||
| Change from Baseline to Outcome (points) c | |||||||||
| Mean (SD) | 0.1 (1.4) | −0.2 (1.5) | −0.6 (1.3) | −0.1 (1.2) | |||||
| Range | −2.0 to 2.7 | −4.0 to 2.0 | −4.0 to 1.7 | −3.7 to 1.7 | |||||
| Improved ≥1 Point on Control Score d | 8 | 32% | 6 | 24% | 11 | 44% | 4 | 16% | |
| Difference (95% CI) | 8% (−17% to 33%) | 28% (4% to 52%) | |||||||
| Stayed within 1 point | 9 | 36% | 14 | 56% | 9 | 36% | 15 | 60% | |
| Worsened by ≥1 point | 8 | 32% | 5 | 20% | 5 | 20% | 6 | 24% | |
Mean of 3 distance control scores and mean of 3 near control scores were analyzed.
Difference in mean control (prism – non-prism) and 95% confidence intervals (CI) are from ANCOVA model adjusting for corresponding baseline control score.
Change is 8 weeks minus baseline; thus, a negative value indicates improvement. Maximum range of change possible is −5 (improvement) points to 3 points (worsening) for distance control and −5 (improvement) to 5 points (worsening) for near control.
Control score improvement of ≥1 point between baseline and 8 weeks is the a priori definition of treatment response.
4 of 25 (16%) of prism group participants were assessed in trial frame (2 had not brought their spectacles to the visit, 1 had spectacles that did not contain prism, and 1 had spectacles with refractive error that was slightly off but within tolerances); 21 of 25 (86%) were tested in prism spectacles (1 should have been tested in trial frame due to incorrect refractive correction in spectacles).
25 of 25 (100%) of non-prism group participants were assessed in study prescribed spectacles
Table 4.
Sensitivity Analyses for 8-week Distance Control Primary Outcome
| Sensitivity Analyses | N | 8-week Control
Score (Mean) |
Mean Change from Baseline | Adjusted
Difference (Prism-Non-prism) |
|||
|---|---|---|---|---|---|---|---|
| Prism | Non-prism | Prism | Non-prism | Prism | Non-prism | Mean (95% CI) | |
| Limited to participants who received study spectacles at least 4 weeks before the outcome visit | 24 (96%) | 24 (96%) | 3.5 | 3.3 | 0.1 | −0.2 | 0.3 (−0.5, 1.1) |
| Limited to participants who were tested in study spectacles (not in trial frame) | 21 (84%) | 25 (100%) | 3.6 | 3.3 | 0.0 | −0.2 | 0.3 (−0.5 1.1) |
| Adjusting for baseline near control in addition to baseline distance control | 25 (100%) | 25 (100%) | 3.6 | 3.3 | 0.1 | −0.2 | 0.3 (−0.5 1.1) |
Secondary Outcome for Distance Control
Treatment response (i.e., distance control improved ≥1 point from baseline) occurred in 8 (32%) and 6 (24%) of those wearing prism spectacles and those not, respectively (treatment group difference = 8%; 95% CI: −17% to 33%); Table 3.
Additional Secondary Outcomes
At the primary outcome visit, the mean near control scores were 1.7 points for both the prism and non-prism groups (treatment group difference = 0.01 [95% CI = (−0.7 to 0.7)] (Table 3). The proportion of participants whose near control improved ≥1 point from baseline was 44% (N=11) and 16% (N=4) of those wearing prism spectacles and those not, respectively (treatment group difference = 28%; 95% CI: 4% to 52%). The proportion of participants who did not have a spontaneous tropia during distance or near control testing was 21% (N=6) in the prism and 17% (N=5) in the non-prism group (difference = 4%; 95% CI: −19% to 27%).
Mean total exodeviation magnitude by prism-and-alternate-cover test at the outcome visit was 30.6∆ vs. 26.5∆ at distance (adjusted treatment group difference of 4.1∆ [95% CI: −0.8 to 9.0]) and 28.7∆ vs.19∆ at near (adjusted group difference of 9.7∆ [95% CI: 4.5 to 14.9]) in the prism and non-prism groups, respectively (Table 5). The mean increase in total exodeviation magnitude from baseline to outcome was 5.3∆ vs. 1.1∆ at distance and 7.0∆ vs. −2.8∆ at near, in the prism and non-prism groups, respectively (Table 5). Near stereoacuity and suppression data at outcome are found in Table 5.
Table 5.
Secondary Outcomes at 8 Weeks and Change from Baseline According to Treatment Group (N=50).a
| Prism Groupb, c (N=25) |
Non-prism Group (N=25) |
|||
|---|---|---|---|---|
| Distance Magnitude by PACT (∆) | ||||
| At 8 weeks | ||||
| Esodeviation | 0 | 0% | 0 | 0% |
| <10 Exodeviation | 1 | 4% | 0 | 0% |
| 10–15 Exodeviation | 0 | 0% | 0 | 0% |
| 16–18 Exodeviation | 0 | 0% | 5 | 20% |
| 19–25 Exodeviation | 3 | 12% | 10 | 40% |
| 26–35 Exodeviation | 14 | 56% | 9 | 36% |
| 36–45 Exodeviation | 6 | 24% | 1 | 4% |
| >45 Exodeviation | 1 | 4% | 0 | 0% |
| Mean (SD) | 30.6 (9.3) | 26.5 (8.1) | ||
| Range | 1 to 47 | 16 to 45 | ||
| Change between baseline and 8 weeksd | ||||
| Mean (SD) | 5.3 (9.0) | 1.1 (6.4) | ||
| Range | −24 to 27 | −10 to 12 | ||
| Near Magnitude by PACT (∆) d | ||||
| At 8 weeks | ||||
| Esodeviation | 0 | 0% | 0 | 0% |
| <10 Exodeviation | 1 | 4% | 6 | 24% |
| 10–15 Exodeviation | 0 | 0% | 3 | 12% |
| 16–18 Exodeviation | 1 | 4% | 5 | 20% |
| 19–25 Exodeviation | 6 | 24% | 4 | 16% |
| 26–35 Exodeviation | 15 | 60% | 6 | 24% |
| 36–45 Exodeviation | 1 | 4% | 1 | 4% |
| >45 Exodeviation | 1 | 4% | 0 | 0% |
| Mean (SD) | 28.7 (8.2) | 19.0 (9.8) | ||
| Range | 6 to 47 | 6 to 40 | ||
| Change between baseline and 8 weeks | ||||
| Mean (SD) | 7.0 (9.1) | −2.8 (9.1) | ||
| Range | −14 to 27 | −22 to 16 | ||
| Stereoacuity e | ||||
| At 8 weeks, arcseconds (log arcseconds) | ||||
| 40” (1.6) | 2 | 8% | 9 | 36% |
| 60” (1.78) | 5 | 20% | 4 | 16% |
| 100” (2.0) | 7 | 28% | 7 | 28% |
| 200” (2.3) | 5 | 20% | 2 | 8% |
| 400” (2.6) | 4 | 16% | 1 | 4% |
| 800” (2.9) | 2 | 8% | 2 | 8% |
| Mean (SD), log arcseconds | 2.2 (0.4) | 1.9 (0.4) | ||
| Range | 1.6 to 2.9 | 1.6 to 2.9 | ||
| Change between baseline and 8 weeks | ||||
| Mean (SD) | −0.1 (0.5) | −0.2 (0.3) | ||
| Range | −1.3 to 0.9 | −1.2 to 0.3 | ||
| Suppression f | ||||
| Negligible (0) | 1 | 4% | 5 | 23% |
| Mild (1) | 5 | 21% | 3 | 14% |
| Moderate (2)) | 6 | 25% | 4 | 18% |
| Dense (3) | 12 | 50% | 10 | 45% |
PACT = prism-and-alternate-cover test, SD = standard deviation, CI = confidence interval
All values are unadjusted for baseline measures.
In the prism group, the PACT values are the sum of the in-prism PACT measurement plus the ground-in prism magnitude in the spectacles.
Lensometry indicated the prism correction was accurate for 95% (21 of 22) of the prism-group participants who brought their spectacles to the outcome visit; 1 participant’s spectacles did not contain any prism.
Adjusted treatment group difference (prism – non-prism) in mean exodeviation magnitude at 8 weeks is 4.1∆ (95% CI: −0.8 to 9.0) at distance and 9.7∆ (95% CI: 4.5 to 14.9) at near. Change in PACT is 8 weeks PACT minus baseline PACT; thus, a negative change indicates improvement.
Adjusted treatment group difference (prism – non-prism) in mean near stereoacuity at 8 weeks is 0.2 (95% CI: −0.01 to 0.4). Change in stereoacuity is calculated as 8 weeks minus baseline; thus, a negative change indicates an improvement.
The Office Suppression Test17 is a three-step procedure to grade density of suppression in patients with intermittent exotropia who are manifestly exotropic. While manifestly exotropic (spontaneous or induced), the participant viewed a 20/50 optotype at 6 meters in normal room illumination, and was asked whether 1 or 2 letters were seen. If 2 letters were reported, suppression was classified as “negligible” (grade of 0). If 1 letter was reported (indicating suppression), a single-point light source was viewed at 6 meters with a red filter placed over the habitually fixing eye. If 2 lights were reported (one white and one red), suppression was classified as “mild” (grade 1). If 1 light was reported, the light source was viewed again in dim lighting with a red filter over the preferred eye. Suppression was classified as “moderate” (grade 2) if 2 light were reported or dense (grade 3) if 1 light was reported.
In a post hoc analysis, full prism adaptation (increase in magnitude by at least the amount of prism worn) between baseline and outcome was found in 6 of 25 (24%; 95% CI: 9 to 45%) participants in the prism group.
Participant and Parent Questionnaires
Participant-reported intermittent exotropia-related symptoms and parent-reported symptoms possibly related to spectacle wear by treatment group at baseline and outcome are found in Tables 6 and 7, respectively. According to the parental and child questionnaires, none of the intermittent exotropia- or treatment-related symptoms appeared to worsen in the prism group between baseline and the primary outcome visit. At outcome, the mean Child Intermittent Exotropia Symptom Questionnaire score was 23.7 in the prism group vs. 20.6 in the non-prism group (adjusted difference = −2.7 (95% CI = −12.6 to 7.3).
Table 6.
Child Assessment of Symptoms Associated with Intermittent Exotropia at Baseline and Outcome.
| Baseline | 8-Week Outcomea | |||||||
|---|---|---|---|---|---|---|---|---|
| “In the past 2
weeks…” (baseline) “Since enrolling in the study…” (outcome) |
Prism (N=28) |
Non-prism (N=28) |
Prism (N=25) |
Non-prism (N=25) |
||||
| N | % | N | % | N | % | N | % | |
| Do your eyes hurt? | ||||||||
| Never | 17 | 61% | 21 | 75% | 15 | 60% | 22 | 88% |
| Sometimes | 9 | 32% | 6 | 21% | 6 | 24% | 3 | 12% |
| Almost Always | 2 | 7% | 1 | 4% | 4 | 16% | 0 | 0% |
| Do your eyes feel funny? | ||||||||
| Never | 16 | 57% | 21 | 75% | 17 | 68% | 20 | 80% |
| Sometimes | 10 | 36% | 6 | 21% | 7 | 28% | 3 | 12% |
| Almost Always | 2 | 7% | 1 | 4% | 1 | 4% | 2 | 8% |
| Do you have double vision (do you see two of things when you know there is really only one)? one?? | ||||||||
| Never | 10 | 36% | 20 | 71% | 17 | 68% | 17 | 68% |
| Sometimes | 12 | 43% | 6 | 21% | 8 | 32% | 7 | 28% |
| Almost Always | 6 | 21% | 2 | 7% | 0 | 0% | 1 | 4% |
| Is it hard for you to stare at things? | ||||||||
| Never | 12 | 43% | 19 | 68% | 16 | 64% | 15 | 60% |
| Sometimes | 10 | 36% | 8 | 29% | 7 | 28% | 8 | 32% |
| Almost Always | 6 | 21% | 1 | 4% | 2 | 8% | 2 | 8% |
| Do you have problems with your eyes in the sun? | ||||||||
| Never | 9 | 32% | 13 | 46% | 11 | 44% | 14 | 56% |
| Sometimes | 11 | 39% | 9 | 32% | 8 | 32% | 5 | 20% |
| Almost Always | 8 | 29% | 6 | 21% | 6 | 24% | 6 | 24% |
| Do your eyes go in and out? | ||||||||
| Never | 16 | 57% | 19 | 68% | 20 | 80% | 19 | 76% |
| Sometimes | 10 | 36% | 7 | 25% | 2 | 8% | 3 | 12% |
| Almost Always | 2 | 7% | 2 | 7% | 3 | 12% | 3 | 12% |
| Is it hard to focus your eyes? | ||||||||
| Never | 12 | 43% | 15 | 54% | 16 | 64% | 12 | 48% |
| Sometimes | 10 | 36% | 12 | 43% | 5 | 20% | 11 | 44% |
| Almost Always | 6 | 21% | 1 | 4% | 4 | 16% | 2 | 8% |
The Rasch-calibrated overall mean score on the Child IXT Symptom Questionnaire16 at 8 weeks was 23.7 in the prism group vs. 20.6 in the non-prism group (adjusted difference = −2.7 (95% CI = −12.6 to 7.3).
Table 7.
Parental Survey: Assessment of Symptoms and Spectacle Wear Issues at Baseline and Outcome
| Baseline | 8-Week Outcome | |||||||
|---|---|---|---|---|---|---|---|---|
| “In the past 2
weeks…” (baseline) “Since enrolling in the study…” (outcome) |
Prism (N=28) |
Non-Prism (N=29) |
Prism (N=25) |
Non-Prism (N=25) |
||||
| N | % | N | % | N | % | N | % | |
| Symptoms | ||||||||
| Has your child had headaches? | ||||||||
| Never | 12 | 43% | 12 | 41% | 15 | 60% | 14 | 56% |
| Almost never | 8 | 29% | 10 | 34% | 8 | 32% | 6 | 24% |
| Sometimes | 8 | 29% | 6 | 21% | 2 | 8% | 5 | 20% |
| Often | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Almost always | 0 | 0% | 1 | 3% | 0 | 0% | 0 | 0% |
| Has your child had eyestrain (tired, sore, or uncomfortable eyes)? | ||||||||
| Never | 8 | 29% | 7 | 24% | 16 | 64% | 11 | 44% |
| Almost never | 5 | 18% | 7 | 24% | 5 | 20% | 4 | 16% |
| Sometimes | 12 | 43% | 11 | 38% | 4 | 16% | 9 | 36% |
| Often | 3 | 11% | 3 | 10% | 0 | 0% | 1 | 4% |
| Almost always | 0 | 0% | 1 | 3% | 0 | 0% | 0 | 0% |
| Has your child avoided reading or doing things up close? | ||||||||
| Never | 18 | 64% | 16 | 55% | 22 | 88% | 15 | 60% |
| Almost never | 8 | 29% | 5 | 17% | 2 | 8% | 6 | 24% |
| Sometimes | 2 | 7% | 5 | 17% | 0 | 0% | 3 | 12% |
| Often | 0 | 0% | 3 | 10% | 1 | 4% | 1 | 4% |
| Almost always | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Has your child reported blurry vision? | ||||||||
| Never | 13 | 46% | 20 | 69% | 21 | 84% | 21 | 84% |
| Almost never | 5 | 18% | 4 | 14% | 2 | 8% | 3 | 12% |
| Sometimes | 9 | 32% | 5 | 17% | 1 | 4% | 1 | 4% |
| Often | 1 | 4% | 0 | 0% | 1 | 4% | 0 | 0% |
| Almost always | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Spectacle Wear Problems | ||||||||
| Has your child looked over their spectacles? | ||||||||
| Never | 7 | 25% | 5 | 17% | 12 | 48% | 14 | 56% |
| Almost never | 4 | 14% | 2 | 7% | 7 | 28% | 3 | 12% |
| Sometimes | 0 | 0% | 3 | 10% | 5 | 20% | 5 | 20% |
| Often | 0 | 0% | 0 | 0% | 0 | 0% | 3 | 12% |
| Almost always | 0 | 0% | 0 | 0% | 1 | 4% | 0 | 0% |
| Never | 17 | 61% | 19 | 66% | 0 | 0% | 0 | 0% |
| Has your child taken their spectacles off when they should be wearing them? | ||||||||
| Never | 7 | 25% | 3 | 10% | 5 | 20% | 10 | 40% |
| Almost never | 1 | 4% | 3 | 10% | 11 | 44% | 5 | 20% |
| Sometimes | 3 | 11% | 3 | 10% | 6 | 24% | 8 | 32% |
| Often | 0 | 0% | 1 | 3% | 3 | 12% | 1 | 4% |
| Almost always | 0 | 0% | 0 | 0% | 0 | 0% | 1 | 4% |
| Not wearing spectacles | 17 | 61% | 19 | 66% | 0 | 0% | 0 | 0% |
| Has your child complained that the spectacles hurt their ears and/or nose? | ||||||||
| Never | 9 | 32% | 4 | 14% | 17 | 68% | 14 | 56% |
| Almost never | 2 | 7% | 2 | 7% | 3 | 12% | 5 | 20% |
| Sometimes | 0 | 0% | 3 | 10% | 5 | 20% | 5 | 20% |
| Often | 0 | 0% | 1 | 3% | 0 | 0% | 1 | 4% |
| Almost always | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Not wearing spectacles | 17 | 61% | 19 | 66% | 0 | 0% | 0 | 0% |
DISCUSSION
The results of this pilot randomized clinical trial of relieving base-in prism treatment using a magnitude equal to 40% of the larger exodeviation at distance or near for childhood intermittent exotropia did not meet our pre-determined criterion for proceeding to a full-scale randomized trial. The prism prescribing protocol used in our study did not yield better distance control than refractive correction alone, with our confidence interval indicating that a 0.75 point or larger favorable effect of prism on distance control is unlikely.
Although base-in prism treatment is long-recognized as a management option for intermittent exotropia, we could find only one small (n=12) retrospective report of children who received base-in prism as their sole treatment modality.19 However, full corrective rather than relieving prisms were prescribed in that study,19 and there were numerous other differences in methodology precluding comparison with our study. In contrast to the present study evaluating relieving base-in prism treatment to facilitate normal sensorimotor fusion, most prior reports on base-in prism and intermittent exotropia have focused on whether a pre-operative prism adaptation test is useful in uncovering the maximum angle of exodeviation on which to base surgical dosage in the hope of improved surgical outcomes.20–25 Generally, these studies defined prism adaptation as an increase in exodeviation of 5∆ (or 10∆) or more after incrementally increasing the initial corrective prism magnitude over 30 minutes to 3 hours until no additional prism was required to neutralize the deviation. The frequency of prism adaptation using this method ranges from 8% to 100%,20–26 with this phenomenon often interpreted to be similar to the uncovering of larger latent angles of exodeviation that are sometimes found with a monocular occlusion test.21 It is unknown how the mechanisms underlying prism adaptation in intermittent exotropia might differ from those reported in patients with constant esotropia and anomalous retinal correspondence.27–29 Further study of prism adaptation in patients with intermittent exotropia and other forms of strabismus is needed.
In evaluating for change in magnitude between baseline and 8 weeks in the present study, we found the prism group’s mean exodeviation increased relative to the non-prism group at distance (+5.3∆ vs.+1.1∆) and at near (+7∆ vs.−2.8∆) with 24% of prism-group participants having shown full prism adaptation over this time, indicating that their distance and near angles at outcome exceeded their respective baseline angles by the amount of prism worn or greater. We do not know if the increase in angle was temporary or sustained because we did not remeasure the exodeviation after discontinuing prism wear. Prism adaptation (full or partial) during the study might be one reason why the prism treatment did not improve distance control outcomes or the mean near control outcome, although a significantly greater proportion (28%) of participants in the prism group showed ≥1 point improvement in near control from baseline.
In the present pilot study, we employed a standardized prism magnitude of 40% of the larger of the distance or near exodeviation for all participants. Other clinical prism prescribing strategies, such as prescribing a larger magnitude or fully corrective prism,19, 30 or prescribing the minimum prism power necessary to improve control of the exodeviation or to demonstrate better fusion on tests of suppression or stereoacuity,8, 10, 18, 31 may yield different results.
Our study has several important limitations. The study definition of full prism adaptation did not account for the wide test-retest repeatability for prism-and-alternate-cover test measurements in children with intermittent exotropia,32 potential partial prism adaptation, and for the true biological variability of intermittent exotropia magnitudes;33 consequently, some participants might have had full or partial prism adaptation at enrollment. Prism-and-alternate-cover testing after prolonged monocular occlusion and through +3.00 D lenses at near were not completed at baseline, precluding determination of the maximum angle of exodeviation and classification of exotropia.21 In addition, because this pilot study had a small convenience sample size, the analyses should be interpreted with caution. The sample size also precludes evaluating whether a relationship exists between prism adaptation and the depth of suppression. We did not determine whether anomalous retinal correspondence was present when the intermittent exotropia was manifest.
For future studies or use of other clinical prescribing strategies for base-in prism treatment for childhood intermittent exotropia, we suggest that an in-office prism adaptation test be performed, using a broader definition of full prism adaptation that includes test-retest repeatability for the prism-and-alternate-cover test.32 Clinicians and researchers should be aware that not only do a proportion of children with intermittent exotropia show immediate prism adaptation, but others adapt to prism over time, and thus, one should not only evaluate for short-term prism adaptation but also monitor for long-term prism adaptation.
In terms of the feasibility objective of conducting a randomized trial of base-in prism spectacles for children with intermittent exotropia, we found that recruitment was straightforward, outcome visit completion was reasonable despite the COVID-19 pandemic, and examiners remained masked to treatment assignment. Any concerns by investigators that children would have difficulty wearing prism spectacles were unfounded because parent-reported adherence with prism spectacle wear of the magnitudes prescribed (6∆−14∆) was excellent. Possible treatment-associated symptoms and issues with spectacles appeared similar between treatment groups. These aspects bode well for future studies that might evaluate different prism prescribing strategies for potential therapeutic effects, and that might better identify and exclude children with prism adaptation.
CONCLUSIONS
Our a priori criterion for progressing to conduct a full-scale trial was not met. Base-in prism spectacles equal to 40% of the larger exodeviation (distance or near) worn for at least 8 weeks did not result in better distance control than non-prism spectacles in 3- to 12-year-old children with intermittent exotropia. Our findings suggest that a 0.75-point or larger favorable effect on distance control is unlikely with our prism prescribing protocol. It is unknown whether results might differ with other prism-prescribing regimens or with different eligibility criteria. The phenomenon of prism adaptation in children with intermittent exotropia is worthy of further study.
Supplementary Material
ACKNOWLEDGMENTS
Supported by National Eye Institute of National Institutes of Health, Department of Health and Human Services EY011751 and EY024333. Dr. Summers notes funding from NIH P30EY010572 grant. Dr. Summers notes an unrestricted grant to the department from Research to Prevent Blindness, New York, New York. The funding organization had no role in the design or conduct of this research.
This manuscript was presented in part at the American Academy of Ophthalmology Annual Meeting 2020, American Academy of Ophthalmology Annual Meeting 2021, and American Academy of Optometry Annual Meeting 2020.
Robert Henderson had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Appendix 1. Intermittent Exotropia Office Control Score1
Control of exodeviation will be measured at distance and near using the Office Control Score.1
Distance (6 meters) – fixing on an accommodative target such as a video or reading optotype letters
Near (1/3 meter – fixing on Lang near-viewing stick or similar accommodative target)
The scale below applies to both distance and near separately.
Intermittent Exotropia Control Scale
5 = Constant Exotropia
4 = Exotropia > 50% of the 30-second period before dissociation
3 = Exotropia < 50% of the 30-second period before dissociation
2 = No exotropia unless dissociated, recovers in >5 seconds
1 = No exotropia unless dissociated, recovers in 1–5 seconds
0 = No exotropia unless dissociated, recovers in <1 second (phoria)
Not applicable = No exodeviation present
Directions:
Step1: Assessment before any dissociation: Levels 5 to 3 are assessed during 30 seconds of observation; first at distance fixation and then at near fixation for another 30-second period. Both distance and near are assessed before any dissociation (i.e., before step 2, when assessing control scores of 0, 1, and 2). If the participant is spontaneously tropic (score 3, 4, or 5) at a specified test distance, then step 2 (assessment after standard dissociation) is skipped at that specific test distance.
-
Step 2: Assessment with standardized dissociation: If no exotropia is observed during step 1 (i.e., the 30-second period of observation at the specified test distance), levels 2 to 0 are then assessed as the worst of 3 rapidly successive trials of dissociation:
- An occluder is placed over the right eye for 10 seconds and then removed, measuring the length of time it takes for fusion to become re-established.
- The left eye is then occluded for 10 seconds (second assessment under dissociation), and the time to re-establish fusion is similarly measured.
- The third assessment under dissociation is performed, covering the eye (for 10 seconds) that required the longest time to re-fuse.
The worse level of control observed following the three 10-second periods of occlusion should be recorded. Since the level under dissociation is recorded as the worst of the three assessments, if a score of 2 (>5 seconds recovery) is noted on the first or second dissociation, then subsequent dissociation(s) are not needed.
If the participant has a micro-esotropia by the cover test, but an exodeviation by the prism-andalternate-cover test, the scale applies to the exodeviation.
Appendix 2. Eligibility Criteria.
| Eligibility Criteria for Enrollment |
|---|
| 1. Age 3 to < 13 years |
| 2. Intermittent exotropia meeting all of the following criteria: |
| a. Intermittent exotropia or constant exotropia at distance |
| i. Mean distance control score of >2.0 points (mean of 3 assessments over the exam) and with at least one measure of 3, 4, or 5, (spontaneous tropia) during control testing |
| b. Intermittent exotropia or exophoria at near |
| i. Child cannot have a score of 5 points on all 3 near assessments of control |
| c. Distance exodeviation at least 16A measured by PACT and no larger than 35A |
| d. Near exodeviation at least 10A measured by PACT and no larger than 35A |
| e. Near deviation does not exceed the distance deviation by more than 10A by PACT (i.e., convergence insufficiency-type excluded) |
| 3. Distance visual acuity (by any optotype method) in both eyes according to age normal values34, 35 as follows: |
| a. 20/50 or better for 3- to <4-year-olds |
| b. 20/40 or better for 4- to <5-year-olds |
| c. 20/32 or better for 5- to <7-year-olds |
| d. 20/25 or better for >7-year-olds |
| 4. Interocular difference of distance visual acuity < 2 lines (0.2 logMAR) |
| 5. Refractive error between −6.00D SE and +2.50D SE (inclusive) (based on a cycloplegic refraction performed within 7 months but prior to the day of enrollment) |
| 6. Refractive correction (pre-study spectacles) worn for at least 1 week if refractive error (based on cycloplegic refraction performed within 7 months) meets any of the following: |
| a. SE anisometropia >1.00D |
| b. Astigmatism >1.00D in either eye |
| c. SE myopia >−0.50D in either eye |
| 7. If spectacles are worn, they must meet the following pre-enrollment criteria: |
| a. SE anisometropia corrected within <1.00D of full SE anisometropic difference |
| b. Astigmatism corrected within <1.00D of full magnitude; axis within 10 degrees if <1.00D of astigmatism and within 5 degrees if >1.00D of astigmatism |
| 8. Gestational age > 32 weeks |
| 9. Birth weight >1500 grams |
| 10. Parent understands the protocol and is willing to accept randomization to prism spectacles or non-prism spectacles |
| 11. Parent has home phone (or access to phone) and is willing to be contacted by Jaeb Center staff and Investigator’s site staff |
| 12. Relocation outside of area of an active PEDIG site within next 8 weeks is not anticipated |
| Exclusion Criteria |
| 1. Dissociated vertical deviation (DVD) |
| 2. Vertical deviation >3A in primary gaze at distance or near |
| 3. Pattern (such as an “A” or “V” pattern) with a downgaze measurement of >10A difference from straight ahead by PACT, measured by investigator’s routine method |
| 4. Treatment for IXT or amblyopia (other than refractive correction) within the past 4 weeks, including vision therapy, orthoptics, patching, atropine, or other penalization |
| 5. Substantial overminus spectacles (spectacles overminused by more than 1.00D SE than the most recent cycloplegic refraction and ALSO results in minus SE power in the spectacles; i.e. underplussing is allowed) within the past 4 weeks |
| 6. Prior strabismus, intraocular, or refractive surgery (including BOTOX injection) |
| 7. Prior use of prism spectacles |
| 8. Current contact lens wear |
| 9. Abnormality of the cornea, lens, or central retina |
| 10. Down syndrome or cerebral palsy |
| 11. Developmental delay which would, in the opinion of the investigator, interfere with treatment or evaluation. Mild speech delays, reading disability, and learning disabilities are not excluded. |
| 12. Any condition that would, in the investigator’s opinion, result in poor spectacle compliance |
| 13. Disease known to affect accommodation, vergence, or ocular motility such as multiple sclerosis, Graves orbitopathy, myasthenia gravis, diabetes mellitus, or Parkinson disease |
| 14. Current use of any ocular or systemic medication known to affect accommodation or vergence, such as anti-anxiety agents (e.g., Librium or Valium), anti-arrhythmic agents (e.g., Cifenline, Cibenzoline), anticholinergics (e.g., motion sickness patch (scopolamine)), bladder spasmolytic drugs (e.g., propiverine), hydroxychloroquine, chloroquine, phenothiazines (e.g., Compazine, Mellaril, Thorazine), tricyclic antidepressants (e.g., Elavil, Nortriptyline, Tofranil) |
| Additional Criteria for Randomization (Based on 30-minute In-office Prism Adaptation Testing) |
| After 30 minutes of wearing 40% relieving prism in a trial frame, participants are eligible to be randomly assigned if all of the following are met: |
| • The participant has not fully adapted to prism at distance AND near |
| ◦ The participant’s PACT measurement at distance while wearing “trial” relieving prism is smaller than the originally measured deviation at distance OR near when not wearing prism (i.e., at least some reduction in deviation with prism) |
| • The participant has no NEW near esotropia by cover test while wearing the “trial” relieving prism (e.g., a micro-esotropia previously noted on near cover test during initial testing and also noted at this time is allowed) |
| • The participant has no esodeviation >6A on PACT at near while wearing “trial” relieving prism. |
PACT = prism-and-alternate-cover test, ∆ = prism diopter, DVD = dissociated vertical deviation, SE = spherical equivalent, D = diopter, IXT = intermittent exotropia, logMAR = logarithm of the minimum angle of resolution
Appendix 3. Child Intermittent Exotropia Symptom Questionnaire15,16
Instructions for Clinical Staff
Children should be positioned such that they are unable to view their parents during testing and parents should be advised not to influence their child’s responses.
For each question, clinic staff will read the question and record the child’s response on paper. Children can use the accompanying matching card or can respond verbally
Each question should be answered. If the child does not appear to understand a question, clinic staff should repeat the question verbatim. Clinic staff should not try to explain the question or elaborate on the question, but should encourage children to choose the answer that best reflects how they feel
Before proceeding, clinic staff should read the following instructions aloud to the child and should present the sample question to determine whether the child understands the test. If the child does not understand the sample question, indicate that the survey and questionnaire will not be completed because the child does not understand the sample question.
Instructions for Interviewer
I am going to ask you some questions about some things that you might notice about your eyes. I would like to know how much you notice any of these things. There are no right or wrong answers. Choose the answer that is closest to how you feel.
If you are not sure how to answer, please choose the answer you think is best.
I am going to read each question and then you can point to the picture to show me how much you notice it.
If you never notice it, point to the smiling face

If you notice it sometimes, point to the middle face

If you notice it all the time, point to the sad face

Let’s try a practice question:
Never
|
Sometimes
|
All the
time
|
Never
|
Sometimes
|
All the time
|
Never
|
Sometimes
|
All the time
|
Never
|
Sometimes
|
All the time
|
Never
|
Sometimes
|
All the time
|
Never
|
Sometimes
|
All the time
|
Never
|
Sometimes
|
All the time
|
Never
|
Sometimes
|
All the time
|
Appendix 4. Parent Spectacle Symptom Survey
Instructions for Clinical Staff
The parent should complete the SPECTACLE SYMPTOM SURVEY by themselves on paper. If they are unable to complete this on their own, they may ask for assistance. Each question should be answered.
The symptom survey is a short survey about your observations of your child over the last two weeks. If you are unable to complete this on your own, please ask for someone to assist you.
1. Has your child had headaches?
| Never | Almost never | Sometimes | Often | Almost always |
2. Has your child had eyestrain (tired, sore, or uncomfortable eyes)?
| Never | Almost never | Sometimes | Often | Almost always |
3. Has your child avoided reading or doing things up close?
| Never | Almost never | Sometimes | Often | Almost always |
4. Has your child reported blurry vision?
| Never | Almost never | Sometimes | Often | Almost always |
5. Has your child looked over their spectacles?
| Never | Almost never | Sometimes | Often | Almost always | Not wearing spectacles |
6. Has your child taken their spectacles off when they should be wearing them?
| Never | Almost never | Sometimes | Often | Almost always | Not wearing spectacles |
7. Has your child complained that the spectacles hurt their ears and/or nose?
| Never | Almost never | Sometimes | Often | Almost always | Not wearing spectacles |
Footnotes
APPENDICES
Appendix 1, available at [LWW insert link]: Intermittent Exotropia Office Control Score4
Appendix 2, available at [LWW insert link]: Study Eligibility Criteria
Appendix 3, available at [LWW insert link]: Child Intermittent Exotropia Symptom Questionnaire15,16 is a 7-item questionnaire that assesses intermittent-exotropia-related symptoms. The version for children 5 to <8 years is shown. The similar version for children 8 to 13 years has text-only responses and does not include the face pictures.
Appendix 4, available at [LWW insert link] Parent Spectacle Symptom Survey is a previously unpublished 7-item survey that asks parents about their child’s intermittent exotropia-related symptoms and spectacle-wear issues.
REFERENCES
- 1.Mohney BG, Holmes JM. An Office-Based Scale for Assessing Control in Intermittent Exotropia. Strabismus 2006;14:147–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pediatric Eye Disease Investigator Group. A Randomized Trial Comparing Part-Time Patching with Observation for Children 3 to 10 Years of Age with Intermittent Exotropia. Ophthalmology 2014;121:2299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pediatric Eye Disease Investigator Group. A Randomized Trial Comparing Part-Time Patching with Observation for Intermittent Exotropia in Children 12 to 35 Months of Age. Ophthalmology 2015;122:1718–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pediatric Eye Disease Investigator Group. A Randomized Trial Evaluating Short-Term Effectiveness of Overminus Lenses in Children 3 to 6 Years of Age with Intermittent Exotropia. Ophthalmology 2016;123:2127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen AM, Erzurum SA, Chandler DL, et al. Overminus Lens Therapy for Children 3 to 10 Years of Age with Intermittent Exotropia: A Randomized Clinical Trial. JAMA Ophthalmol 2021;139:464–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asadi R, Falavarjani K, Sadighi N. Orthoptic Treatment in the Management of Intermittent Exotropia. Iran J Ophthalmol 2009;21:35–40. [Google Scholar]
- 7.Ma MM, Kang Y, Scheiman M, Chen X. Office-Based Vergence and Accommodative Therapy for the Treatment of Intermittent Exotropia: A Pilot Study. Optom Vis Sci 2019;96:925–33. [DOI] [PubMed] [Google Scholar]
- 8.Caloroso E, Cotter SA. Prescribing prisms for strabismus. In: Cotter SA, ed. Clinical Uses of Prism: A Spectrum of Applications. St. Louis: Mosby; 1995:193–234. [Google Scholar]
- 9.Coffey B, Wick B, Cotter S, et al. Treatment Options in Intermittent Exotropia: A Critical Appraisal. Optom Vis Sci 1992;69:386–404. [DOI] [PubMed] [Google Scholar]
- 10.Cotter SA, Franz KA. Therapeutic uses of prism for binocular vision disorders. In: Tasman W, Jaeger EA, eds. Duane’s Ophthalmology. Philadelphia: Lippincott Williams & Wilkins; 2009. [Google Scholar]
- 11.World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013;310:2191–4. [DOI] [PubMed] [Google Scholar]
- 12.JAEB Center for Health Research (JCHR). Pediatric Eye Disease Investigator Group: Public Web Site. https://public.jaeb.org/pedig/stdy/562. Accessed: December 14 2021.
- 13.Eldridge SM, Chan CL, Campbell MJ, et al. Consort 2010 Statement: Extension to Randomised Pilot and Feasibility Trials. BMJ 2016;355:i5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Institutes of Health (NIH) U. S. National Library of Medicine. Intermittent Exotropia Study 6: A Pilot Randomized Clinical Trial of Base-in Prism Spectacles for Intermittent Exotropia. Clinicaltrials.Gov Identifier: Nct03998670.;2021. Available at: https://clinicaltrials.gov/ct2/show/NCT03998670. Accessed: December 14 2021.
- 15.Hatt SR, Leske DA, Liebermann L, Holmes JM. Symptoms in Children with Intermittent Exotropia and Their Impact on Health-Related Quality of Life. Strabismus 2016;24:139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holmes JM, Leske DA, Hercinovic A, et al. Rasch-Calibrated Intermittent Exotropia Symptom Questionnaire for Children. Optom Vis Sci 2022;99:513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatt SR, Leske DA, Holmes JM, et al. Testing Depth of Suppression in Childhood Intermittent Exotropia. J AAPOS 2022;26:36–9.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roper-Hall G Optical Management in Strabismus: Simple, Advanced, and Unconventional Techniques. Am Orthopt J 2005;55:144–57. [DOI] [PubMed] [Google Scholar]
- 19.Pratt-Johnson JA, Tillson G. Prismotherapy in Intermittent Exotropia. A Preliminary Report. Can J Ophthalmol 1979;14:243–5. [PubMed] [Google Scholar]
- 20.Ohtsuki H, Hasebe S, Okano M, Furuse T. Comparison of Surgical Results of Responders and Non-Responders to the Prism Adaptation Test in Intermittent Exotropia. Acta Ophthalmol Scand 1997:528–31. [DOI] [PubMed] [Google Scholar]
- 21.Kushner BJ, Morton GV. Distance/near Differences in Intermittent Exotropia. Arch Ophthalmol 1998;116:478–86. [DOI] [PubMed] [Google Scholar]
- 22.Dadeya S, Kamlesh, Naniwal S. Usefulness of the Preoperative Prism Adaptation Test in Patients with Intermittent Exotropia. J Pediatr Ophthalmol Strabismus 2003;40:85–9. [DOI] [PubMed] [Google Scholar]
- 23.Takada R, Matsumoto F, Wakayama A, et al. Efficacies of Preoperative Prism Adaptation Test and Monocular Occlusion for Detecting the Maximum Angle of Deviation in Intermittent Exotropia. BMC Ophthalmol 2021;21:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiyak Yilmaz A, Kose S, Guven yilmaz S, Uretmen O. The Impact of Prism Adaptation Test on Surgical Outcomes in Patients with Primary Exotropia. Clin Exp Optom 2015;98:224–7. [DOI] [PubMed] [Google Scholar]
- 25.Ohtsuki H, Hasebe S, Kono R, et al. Prism Adaptation Response Is Useful for Predicting Surgical Outcome in Selected Types of Intermittent Exotropia. Am J Ophthalmol 2001;131:117–22. [DOI] [PubMed] [Google Scholar]
- 26.Yun YI, Kim SJ, Jung JH. Clinical Characteristics of Patients with Intermittent Exotropia According to the Response to Short-Term Prism Adaptation Test. Korean J Ophthalmol 2020;34:375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schildwächter-von Langenthal A, Kommerell G, Klein U, Simonsz HJ. Preoperative Prism Adaptation Test in Normosensoric Strabismus. Graefes Arch Clin Exp Ophthalmol 1989;227:206–8. [DOI] [PubMed] [Google Scholar]
- 28.Campos EC, Catellani T. Further Evidence for the Fusional Nature of the Compensation (or ‘Eating up’) of Prisms in Concomitant Strabismus. Int Ophthalmol 1978;1:57–62. [DOI] [PubMed] [Google Scholar]
- 29.Bagolini B, Capobianco NM. The effect of prisms in the treatment of squint (anomalous movements). In: Moore S, ed. Orthoptics, Past, Present, Future: Transactions of the Third International Orthoptic Congress. New York: Stratton Intercontinental Medical Book Corporation; 1976:493–9. [Google Scholar]
- 30.Ravault AP, Bongrand G, Bonamour G. The Utilization of Prisms in the Treatment of Divergent Strabismus. In. Orthoptics. Proceedings of the 2nd International Orthoptics Congress. Amsterdam: Excerpta Medica Foundation; 1972:77. [Google Scholar]
- 31.Veronneau-Troutman S. Prismotherapy. In. Prisms in the Medical and Surgical Management of Strabismus. St. Louis: Mosby; 1994:107–43. [Google Scholar]
- 32.Hatt SR, Leske DA, Liebermann L, et al. Variability of Angle of Deviation Measurements in Children with Intermittent Exotropia. J AAPOS 2012;16:120–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Economides JR, Adams DL, Horton JC. Variability of Ocular Deviation in Strabismus. JAMA Ophthalmol 2016;134:63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


