Abstract
Background:
Previous studies have shown that environment and health can influence drug use trajectories and the effects of substance use disorder (SUD) treatments. We hypothesized that trajectories of drug use-related problems, based on changes in DSM-5 symptoms, would vary by type(s) of drugs used, health factors, and neighborhood characteristics.
Methods:
We assessed mental and physical health, stress, social instability, neighborhood characteristics (disorderliness and home value), and DSM-5 symptom counts at two study visits, 12 months apart, in a community sample (baseline N=735) in Baltimore, MD. Three prominent categories of drug-use trajectory were identified with K-means cluster analysis of symptom counts: Persistent (4 or more symptoms at both visits or at Visit 2), Improved (decrease from 4 or more symptoms at Visit 1 to 3 or fewer symptoms at Visit 2), and Low-Stable (3 or fewer symptoms at both visits). Baseline health and neighborhood measures were tested as predictors of trajectory in mediation and moderation models.
Results:
Among people with current opioid- and/or stimulant-use, odds of an Improved trajectory were (1) decreased with neighborhood disorder and social instability, or (2) increased with home value and social instability. Odds of a Low-Stable trajectory were decreased by social instability and stress but increased in those who were older or self-identified as white.
Conclusions:
Trajectories of drug use-related problems are influenced by sociodemographic variables, neighborhood factors, and health. Assessing DSM-5 symptom counts as an outcome measure may be valuable in monitoring or predicting long-term trajectories and treatment effectiveness.
Clinicaltrials.gov Identifier NCT01571752
Keywords: substance use disorder, DSM-5 symptoms, trajectory, neighborhood, mental health, stress
Introduction
Multiple factors influence the initiation and continuation of illicit drug use. Environment, including the neighborhoods where one lives and spends time, is widely recognized as one such factor. Neighborhood disadvantage, controlling for individual socioeconomic factors, has been associated with past-year drug use (Boardman et al., 2001)—a measure that presumably reflects, at least in part, the likelihood that lifetime drug use persists beyond initiation. The environments in which one lives and/or obtains and uses drugs can influence the tendency to continue use or to quit, reflecting both drug availability and social norms or acceptability of drug use (Chang, 2017; Sherman et al., 2004). Socioeconomic stressors, including housing (Dickson-Gomez et al., 2017), financial security (Moos et al., 1988), employment (Henkel, 2011), and family and social relationships (Moos, 2007), are closely related to the neighborhood environment, and can be risk factors for drug use as well.
Neighborhood environment may also impact stress and mood (Latkin & Curry, 2003; Scott et al., 2018) which in turn influence drug use (Boardman et al., 2001; Latkin et al., 2005). Relationships among these factors can vary across populations, at least at momentary time scales. For instance, momentary exposure to neighborhood disadvantage relative to one’s home neighborhood has been associated with increased momentary stress in adolescents with substance use (Mennis et al., 2016), but, among adults receiving methadone treatment for opioid use disorder, momentary exposure to neighborhood disorder was inversely related to self-reports of momentary stress and drug craving (Epstein et al., 2014). The role of mental health is also important to assess; among incarcerated men and women, mental health problem diagnosis mediated the relationship between fear of neighborhood violence and increased likelihood of having a substance use disorder (Rogers et al., 2012). Stress (Furnari et al., 2015; Panlilio et al., 2020; Panlilio et al., 2019; Panlilio, 2021; Preston & Epstein, 2011; Preston et al., 2017, 2018; Sinha, 2009), mental health (De Leon, 1989; Rounsaville et al., 1982), and physical health (Hasler et al., 2012; Klinkenberg et al., 2004; Rosenblum et al., 2003) are well-established as critical factors in drug-use trajectories, though further exploration and characterization of their interactions with neighborhood environment in broader populations is warranted.
Many studies have assessed drug-use trajectories in terms of treatment outcomes in those with substance use disorders (SUDs) (Grella & Lovinger, 2011; Hser et al., 2017; Ruglass et al., 2019), but treatment populations might only represent the “tip of the iceberg” of people who use drugs. Findings from treatment samples might not generalize to people who initiate or continue drug use but do not enter treatment or meet criteria for an SUD. In prior studies of use trajectories, trajectories have been defined as changes in use versus abstinence over time (Teesson et al., 2017) or as days used in the past month (Hser et al., 2008). In this study, we focused instead on the detrimental effects of drug use, by describing outcomes in terms of changes in Diagnostic and Statistical Manual of Mental Disorders (DSM) symptom counts (see (Stull et al., 2019)), which has been proposed as an alternative to sustained abstinence to assess treatment effectiveness (Epstein et al., 2009; Kiluk et al., 2019; Kiluk et al., 2018). Exploring drug-use trajectories across a range of substances and symptom severities can provide opportunities to develop new approaches to SUD prevention and management.
The purpose of this study was to examine the relationship between neighborhood environment and drug use status in terms of DSM symptomology over a one-year period, as well as the influence of perceived stress, mental and physical health, and social instability on this relationship, in different drug use groups. We hypothesized that: (1) mental health, physical health, stress, and social instability would be worse in people who use drugs than in people who do not use drugs; (2) objective indices of neighborhood poverty and disorder would predict deterioration in drug-use status over time; (3) these relationships would be partly mediated by mental health, physical health, and stress; (4) these relationships would be moderated by social instability (i.e., social instability would be a risk factor).
Methods
Participants
All participants included in the analysis enrolled in a 12-month observational study assessing health outcomes among neighborhood-matched people who use drugs and those who do not (ClinicalTrials.gov Identifier: NCT01571752). The Institutional Review Board (IRB) of the National Institute on Drug Abuse (NIDA) Intramural Research Program (IRP) approved the study, and participants gave written informed consent before enrollment. We recruited volunteers through IRB-approved newspaper and radio advertisements and personal referrals. Inclusion criteria were (1) being at least 18 years old and (2) living in Baltimore City, MD, USA, or one of its surrounding counties. Exclusion criteria were (1) inability to provide informed consent or valid self-report, and (2) medical illness severe enough to compromise study participation.
Procedures
Participants attended two study sessions or “visits,” at baseline (“Visit 1”) and 12–15 months later (“Visit 2”), at the NIDA IRP in Baltimore, MD, between 2012 and 2016. At each visit, participants were assessed for substance use, mental health, physical health, stress, and social instability. Each participant was assigned an objective neighborhood disorder score and mean neighborhood home value based on their home address. We planned to compare these measures at Visit 1 across drug-use groups (defined below) and to conduct parallel analyses on these groups to determine predictors of SUD symptomology trajectory over the 12-month period. We maintained bimonthly contact with participants by telephone between Visit 1 and Visit 2 to maximize follow-up rates. Participants who did not return within 15 months of Visit 1 were not eligible to provide follow-up data. Participants were compensated $100 at each of the two visits for answering questionnaires ($20 if Visit 2 was conducted by phone instead of in person), plus $10 for each bimonthly phone update. Several participants also enrolled in a concurrent treatment study in our clinic and received medication for opioid use disorder (MOUD) after Visit 1.
Measures
Drug use and DSM-5 SUD symptoms
We administered the Drug Use and Patterns of Substance Use interview, modified from the PhenX Toolkit (Hamilton et al., 2011) to assess current and lifetime drug use. We also administered the DSM Questionnaire, a semi structured interview assessing 11 DSM-5 criteria (American Psychiatric Association, 1994, 2013) for SUDs in the past 12 months and lifetime. Because the DSM-5 removed the criterion related to recurrent substance-related legal problems, we did not include this criterion in symptom counts, even for participants who completed the study prior to this change. Another change to the DSM-5 was the addition of the craving criterion, which we had included even prior to this change based on proposed DSM-5 criteria.
We categorized participants using items in the PhenX drug use interview. Participants who reported use of opioids and/or cocaine or other stimulants more than 11 times in their lifetime, and at least once in the past 30 days, were categorized in the current opioid/stimulant use group (COSU). We combined these drug classes into one group because they were the most commonly reported and most problematic drug use classes in our sample. A large proportion of COSU participants reported use of both opioids and stimulants at baseline (42%). COSU participants could also report use of other substances in addition to opioids and/or stimulants. Participants who reported marijuana use (and no opioid and/or stimulant use) more than 11 times in their lifetime and at least once in the past 30 days were categorized in the current marijuana use group (CMU). The CMU group included participants who used both marijuana and alcohol. Participants who did not report any substance use (except alcohol) more than 11 times in their lifetime, and who endorsed fewer than two DSM-5 symptoms for SUD (current and lifetime), were categorized in the non-drug use group (NDU). Participants who did not meet any of these criteria were categorized as “Unclassified.” The Unclassified group was composed of: (1) people who had used opioids, stimulants, or marijuana more than 11 times in their life, but not in the past 30 days, and also endorsed at least two DSM-5 symptoms for SUD (current or lifetime), including alcohol use, and (2) people who did not report any opioid, stimulant, or marijuana use more than 11 times in their lifetime, but endorsed at least two DSM-5 symptoms of SUD (current or lifetime), including alcohol use.
Health measures
Participants completed self-administered questionnaires addressing four main functional domains: stress, mental health, physical health, and social instability (See Table 1 for a list of questionnaires.). One of the secondary objectives of the present study was to provide neighborhood-matched control participants for a concurrent study on individuals receiving MOUD at NIDA-IRP; thus, we included questionnaires that were used in the treatment study. A composite score for each domain was calculated by expressing each participant’s score on each questionnaire as a percentile score for the entire study sample, and then averaging those percentile scores within each domain. For all domains, a higher score indicated a poorer outcome: more stress, worse mental health, worse physical health, and more social instability.
Table 1.
Stress, mental health, physical health, and social instability assessments
| Domain | Assessment | Reference |
|---|---|---|
| Stress | Life Events Questionnaire | (Norbeck, 1984) |
| Life Events Checklist | (Gray et al., 2004) | |
| Hassles and Uplifts Scale | DeLongis et al., 1988 | |
| Perceived Stress Scale | (Cohen et al., 1983) | |
| Coping Orientation to Problems Experienced Inventory | (Carver et al., 1989) | |
| Vulnerability to Stress subscale of the NEO Personality Inventory | (Costa & McCrae, 1985) | |
| Mental Health | 15-item Profile of Mood States | (Cranford et al., 2006) |
| Abbreviated Comprehensive Psychopathology Rating Scale | (Asberg et al., 1978) | |
| Mood Disorder Questionnaire | (Hirschfeld et al., 2000) | |
| Post-Traumatic Stress Disorder Checklist | (Weathers et al., 1991) | |
| Adult Attention Deficit Hyperactivity Disorder Self-Report Scale Symptom Checklist | (Adler et al., 2006) | |
| Physical Health | World Health Organization Quality of Life-BREF | (World Health Organization, 2004) |
| Pittsburgh Sleep Quality Index | (Buysse et al., 1989) | |
| National Health and Nutrition Examination (NHANES) Study Oral Health Questionnaire | (Centers for Disease Control and Prevention (CDC), 2009–2010) | |
| Brief Pain Inventory | (Keller et al., 2004) | |
| HIV Risk Taking Behavior Scale | (Ward, 1990) | |
| Nowinski and Lopiccolo Sexual History Form | (Creti et al., 1998) | |
| Social Instability | Social-Adjustment Scale-Self-Report | (Weissman & Bothwell, 1976) |
| NHANES Food-Security Questionnaire | (Centers for Disease Control and Prevention (CDC), 2009–2010) | |
| Housing-Security Questionnaire | (Jahiel, 1992) | |
| NHANES Health-Insurance Questionnaire | (Centers for Disease Control and Prevention (CDC), 2009–2010) | |
| NHANES Hospital-Utilization and Access-to-Care Questionnaire | (Centers for Disease Control and Prevention (CDC), 2009–2010) | |
| Perceived Neighborhood Scale | (Martinez et al., 2002) |
Environmental measures
We used each participant’s home address to assess two objective measures of neighborhood environment. Neighborhood disorder was measured with the Neighborhood Inventory for Environmental Typology (NIfETy), an onsite-observer-rated measure of physical and social disorder along 528 blockfaces in Baltimore City (Furr-Holden et al., 2010). The presence of each of 10 indicators of disorder (e.g., abandoned buildings, litter, drug paraphernalia) was summed to create a score of 0 to 10 for each blockface. Higher scores indicate greater neighborhood disorder. The methods used to calculate this value have been reported in detail in another study (Sarker et al., 2016). We also obtained the mean monetary (dollar) value of homes within a 500-meter radius of each participant’s home address from the Maryland State Department of Assessments and Taxation. We used these data in a previous study (Epstein et al., 2020). We log-transformed the home values to reduce skew. For any participant who had changed his or her neighborhood of residence during the year, a weighted average for each measure was calculated to reflect the amount of time lived in each neighborhood.
Statistical analyses
Group differences in domain composite measures and environmental measures at Visit 1 were analyzed with one-way ANOVAs. Substance-use trajectories were determined with longitudinal K-means cluster analysis (Genolini et al., 2013; Stull et al., 2019) on DSM symptom count in the past 12 months at Visit 1 and Visit 2. The symptoms for the primary substances used in each category were used in the analyses. For the COSU group, the symptom counts for opioids and stimulants were combined. For the Unclassified group, any substance use symptoms were combined and used in the analyses. For the CMU group, symptoms for marijuana use were used in the analyses. By definition, the CMU group did not report opioid or stimulant use or symptoms at Visit 1, but if they reported this at Visit 2, we included these symptoms as well. Based on this analysis, the Low-stable cluster consisted of participants who had low symptom counts (i.e., 3 or fewer symptoms) at both visits. The Improved cluster consisted of participants who improved from a moderate or high symptom count at Visit 1 (4–11 symptoms) to a low count at Visit 2 (3 or fewer symptoms). A small number of participants increased from a low count to a moderate or high count; this group was combined with those who had a moderate/high symptom count at both visits. This combined group was the Persistent cluster, consisting of participants who either maintained or developed a moderate or high level of symptoms by Visit 2 (4 or more). Participants who reported no current drug use at Visit 2 were labeled separately as “Abstinent.” The cluster analysis was conducted with the COSU, Unclassified, and CMU groups; most NDU participants did not change in drug use status at Visit 2. Due to lack of variability in the NDU group, the small N of the CMU group at Visit 2, and the heterogeneity in substance use history of the Unclassified group, additional analyses on substance use trajectory for these groups were not conducted.
Multinomial logistic regression analyses were conducted for the COSU group, with each environmental measure and functional-domain composite measure at Visit 1 tested separately as a predictor of trajectory. If trajectory category was predicted by stress, mental health, and/or physical health, and by environmental measures, then mediation analyses (MacKinnon et al., 2007) were conducted to determine whether the effect of environment was wholly or partly accounted for by the other significant predictors. If trajectory category was predicted by social instability, then moderation analyses were conducted with social instability as a predictor in a multinomial logistic regression model that included other significant predictors and an interaction term with each predictor, to test whether social stability protects against effects of environment and health measures on trajectory.
Trajectory categories were individually compared to a reference category, which was the category with the largest N. The reference category was the Persistent trajectory for the COSU group. Predictor variables were standardized to have a mean of zero and standard deviation of 1. Moderation and mediation analyses were conducted as multinomial (i.e., categorical) regressions with Bayesian regression models in RSTAN (R Core Team, 2018; Stan Development Team, 2022) with brms software (Bürkner, 2021) for mediation with a categorical outcome, and rstanarm software (Goodrich et al., 2022) for moderation with a binary outcome. Default priors were used: flat for predictors and a half-Student’s t distribution with 3 degrees of freedom for intercepts and sigmas in brms, and weakly informative with a Gaussian (mean = 0, standard deviation = 2.5) prior for intercepts, moderators and covariates in rstanarm. Other analyses were conducted using SPSS (IBM Corp, Released 2019).
Results
Baseline characteristics and differences across groups
There were 735 participants with sufficient data collected at baseline to determine drug-use category: 236 (32%) NDU, 102 (14%) CMU, 290 (39%) COSU, and 107 (15%) Unclassified. These groups differed in sex, race, employment, education, and age (Table 2). The NDU and CMU groups were younger than the COSU and Unclassified groups. The CMU group had more non-white participants than the other groups, and the NDU group had more female participants. The NDU group also had more participants with post-high school education. The COSU group had more unemployed participants compared to other groups. There were also significant differences across these groups in NIfETy score, home value, and composite scores for social instability, stress, physical health, and mental health (Table 2). Post hoc comparisons using Tukey’s HSD test showed that, compared to the NDU group, the COSU and CMU groups had lower home value scores, and the COSU group had higher NIfETy scores (i.e., more disordered neighborhood). The COSU, CMU and Unclassified groups had worse (higher) composite scores for social instability, stress, physical health, and mental health than the NDU group. The COSU group had worse social instability and mental health scores compared to the Unclassified group, and a worse physical health score compared to all other groups.
Table 2.
Participant characteristics by drug use category
| Non-drug use (NDU) n=236 |
Current Marijuana Use (CMU) n=102 |
Current Opioid/ Stimulant Use
(COSU) n=290 |
Unclassified n=107 |
Comparison | |
|---|---|---|---|---|---|
| Age | 34.0 (11.1) |
29.1 (8.3) |
42.7 (10.4) |
40.1 (11.0) |
Welch’s F(3, 295.98)=68.32, p<0.001 |
| Sex (% male) | 40.3 | 65.7 | 75.5 | 65.4 | χ2(3) = 70.404, p<0.001 |
| Race (% non-white) | 80.9 | 89.2 | 70.7 | 68.2 | χ2(3) = 21.045, p<0.001 |
| Education (% ≤ HS) | 39.4 | 66.7 | 72.8 | 48.6 | χ2 (3) = 66.287, p<0.0001 |
| Employment | χ2 2(6) = 28.942, p<0.0001 | ||||
| % currently employed | 81.8 | 81.4 | 63.8 | 80.4 | |
| % retired or disabled | 3.0 | 2.9 | 7.0 | 4.7 | |
| % unemployed | 15.3 | 15.7 | 29.3 | 15.0 | |
| Marital Status | χ2 (6) = 3.469, p=0.748 | ||||
| % unmarried | 84.7 | 86.3 | 83.4 | 79.4 | |
| % married | 8.9 | 5.9 | 9.7 | 10.3 | |
| % unknown | 6.4 | 7.8 | 6.9 | 10.3 | |
| NIfETy | 3.95 (2.15) |
4.55 (2.2) |
4.64 (2.08) |
4.22 (2.2) |
F(3, 532) =3.53, p<0.05 |
| Mean home value in 500 m radius (log transformed) | −.92 (.29) |
−1.04 (.34) |
−1.03 (.33) |
−.972 (.31) |
Welch’s F(3, 265.33) =5.837, p<0.001 |
| Mental health percentile | 36.32 (19.66) |
54.64 (20.43) |
59.06 (21.76) |
50.46 (19.35) |
F(3,551)=41.53, p<0.001 |
| Physical health percentile | 40.40 (14.14) |
48.32 (14.87) |
57.42 (15.17) |
46.57 (15.28) |
F(3,547)=44.42, p<0.001 |
| Stress percentile | 45.14 (11.89) |
51.76 (10.11) |
52.37 (11.58) |
51.45 (11.55) |
F(3,728)=19.41, p<0.001 |
| Social instability percentile | 42.99 (14.29) |
52.58 (13.06) |
54.77 (13.85) |
47.75 (14.77) |
F(3,596)=27.04, p<0.001 |
Table shows Mean (Standard Deviation) unless otherwise specified.
Higher percentile scores indicate worse conditions.
Changes in drug-use status at 12-month follow-up
Participants who completed Visit 2 (n=380) were older (39.2 vs. 36.0; t(733) = −3.80, p < .001) and had lower (better) mental health scores (48.2 vs. 52.0; t(733) = 1.97, p < .05) compared to the participants who only completed Visit 1. There were no differences by sex, race, employment, education, marital status, neighborhood measures, or other health measures.
There were 136 (58%) NDU participants who completed Visit 2. Drug-use group classification changed at Visit 2 for some NDU participants; 5 were classified as CMU, 1 was classified as COSU, and 18 were Unclassified, mainly due to reporting lifetime DSM-5 symptoms related to alcohol use. Three participants reported marijuana use with at least one DSM-5 symptom at Visit 2, but no other participants reported changes in past-12-month symptoms related to substance use (total number of symptoms were 0 or 1 for both visits).
There were 38 (37%) CMU participants who completed Visit 2. The cluster analysis resulted in three groups: 8 Persistent, 27 Low-stable, and 3 Abstinent. An average of 2.1 symptoms (SD=2.1, range 0–8) were reported at Visit 1 and 1.97 (SD=2.5, range 0–10) at Visit 2. Two participants reported opioid use at Visit 2, endorsing at least two DSM-5 symptoms. Further analyses were not conducted due to sample size. However, general trends in composite scores showed that the Persistent group tended to have worse mental and physical health, higher stress, and higher social instability, whereas they also had the lowest (i.e., least disordered) average NIfETy score (followed by Low-stable and then Abstinent).
In the COSU group, 140 (48%) participants completed Visit 2. The cluster analysis resulted in three groups: 37 Improved, 25 Low-stable, and 52 Persistent (reference group); additionally, 26 participants were abstinent at Visit 2. The average symptom count (based on total symptoms related to both opioid and stimulant use) at Visit 1 was 6.56 (SD=4.6, range 0–19) and 3.93 at Visit 2 (SD=4.36, range 0–19). Trajectory was related to NIfETy scores (χ2(3) =8.7, p<0.05), with a trend for higher (more disordered) NIfETy scores to be associated with membership in the Low-stable group (OR=1.4, p =.055) compared to the Persistent group. Higher average home value was more likely in the Improved group (OR=9.17, p<0.05). Lower stress was more likely in the Low-stable group (OR=.927, p<0.005). Lower social instability also was more likely in the Low-stable group (OR=.937, p<0.005) and the Improved group (OR=.96, p<0.05). Non-white participants were less likely to be in the Improved group (OR=.344, p<0.05) compared to the Persistent group (Table 3).
Table 3.
Multinomial logistic regression analyses by trajectory in the COSU group
| Low Stable | Improved | Abstinent | ||||
|---|---|---|---|---|---|---|
| OR | p | OR | p | OR | p | |
| NIfETy | 1.37 | .055 | .869 | .260 | 1.166 | .291 |
| Average home value | .701 | .614 | 9.17 | .007 | 2.313 | .272 |
| Physical health | .975 | .217 | .986 | .390 | .990 | .601 |
| Mental health | .979 | .116 | .996 | .737 | 1.008 | .534 |
| Stress | .927 | .003 | .976 | .251 | .993 | .783 |
| Social instability | .937 | .004 | .959 | .034 | .482 | .985 |
| Race | .837 | .774 | .344 | .033 | 1.151 | .830 |
| Sex | .612 | .387 | .496 | .158 | .450 | .140 |
| Age | 1.04 | .147 | 1.011 | .632 | 1.039 | .153 |
| Education | .402 | .087 | .559 | .234 | 1.476 | .544 |
| Employment | ||||||
| Currently employed vs. unemployed | 1.983 | .216 | 1.167 | .739 | 1.40 | .516 |
| Currently employed vs. retired or disabled | 3.50 | .400 | 8.750 | .060 | 2.625 | .513 |
Reference group = Persistent
Bolded values indicate significant effects (p<0.05).
In the Unclassified group, 66 (62%) participants completed Visit 2; 50 participants showed a Low-stable trajectory, and 15 participants showed a Persistent trajectory. One participant was classified as Improved. The average symptom count was 0.89 at Visit 1 (SD=1.7, range 0–9) and 1.73 at Visit 2 (SD=3.0, range 0–10). General trends in composite scores showed that the Persistent group tended to have worse mental and physical health, higher stress, higher social instability, and higher (i.e., more disordered) average NIfETy scores.
Of those who completed Visit 2, 32 (49.2%) of the Unclassified participants had a history of opioid and/or stimulant use, including 8 with current alcohol or marijuana DSM symptoms at Visit 1. Of the participants with a history of opioid and/or stimulant use, 11 (34.4%) had a Persistent trajectory and 21 (65.6%) had a Low-stable trajectory. Of the 33 Unclassified participants with no history of opioid and/or stimulant use, 4 (12.1%) had a Persistent trajectory and 29 (87.9%) had a Low-Stable trajectory. These participants included 7 with current alcohol DSM symptoms and 26 with past alcohol or marijuana DSM symptoms at Visit 1. Those with a history of opioid and/or stimulant use tended to have worse physical health and greater stress and social instability regardless of trajectory.
Mediation analyses
According to our analysis plan, we based the mediation analyses on the results of the initial multinomial regression analyses. If trajectory category was predicted by stress, mental health, and/or physical health, and by environmental measures, then mediation analyses were conducted to determine whether the effect of environment was wholly or partly accounted for by the other significant predictors.
For the COSU group, two mediation hypotheses were tested: (1) stress mediates the effect of NIfETy on trajectory, and (2) stress mediates the effect of home value on trajectory. Both models included the predictors that were significant in the multinomial logistic regression analyses described above for each group, and included age, race, sex, and education as covariates. Results were expressed as odds ratios (Table 4), with credible intervals indicating a reliable effect if they did not include a value of 1. In all models, the interval for the indirect effect did include 1, suggesting that mediation did not occur. However, the models indicate that there were direct effects, including a higher home value increasing the odds of having an Improved trajectory in COSU (Figure 1, right panel).
Table 4.
Odds ratios for mediation effects of NIfETy and home value on stress in the COSU group.
| Effect | Median | 5% limit | 95% limit |
|---|---|---|---|
| NIfETy mediating effect of Stress (reference = Persistent) | |||
| Outcome = Abstinent | |||
| Indirect | 1.00 | 0.95 | 1.09 |
| Direct | 1.21 | 0.74 | 2.02 |
| Total | 1.22 | 0.75 | 2.04 |
| Outcome = Improved | |||
| Indirect | 1.03 | 0.96 | 1.16 |
| Direct | 0.64 | 0.39 | 1.00 |
| Total | 0.66 | 0.41 | 1.05 |
| Outcome = Low Stable | |||
| Indirect | 1.06 | 0.94 | 1.25 |
| Direct | 1.57 | 0.91 | 2.74 |
| Total | 1.63 | 0.93 | 2.87 |
| Home Value mediating effect of Stress | |||
| (reference = Persistent) | |||
| Outcome = Abstinent | |||
| Indirect | 1.00 | 0.96 | 1.06 |
| Direct | 1.35 | 0.88 | 2.09 |
| Total | 1.35 | 0.81 | 2.33 |
| Outcome = Improved | |||
| Indirect | 1.01 | 0.91 | 1.12 |
| Direct | 2.16 | 1.38 | 3.47 |
| Total | 2.17 | 1.29 | 3.94 |
| Outcome = Low Stable | |||
| Indirect | 1.00 | 0.96 | 1.03 |
| Direct | 0.89 | 0.59 | 1.34 |
| Total | 0.90 | 0.53 | 1.49 |
Figure 1.
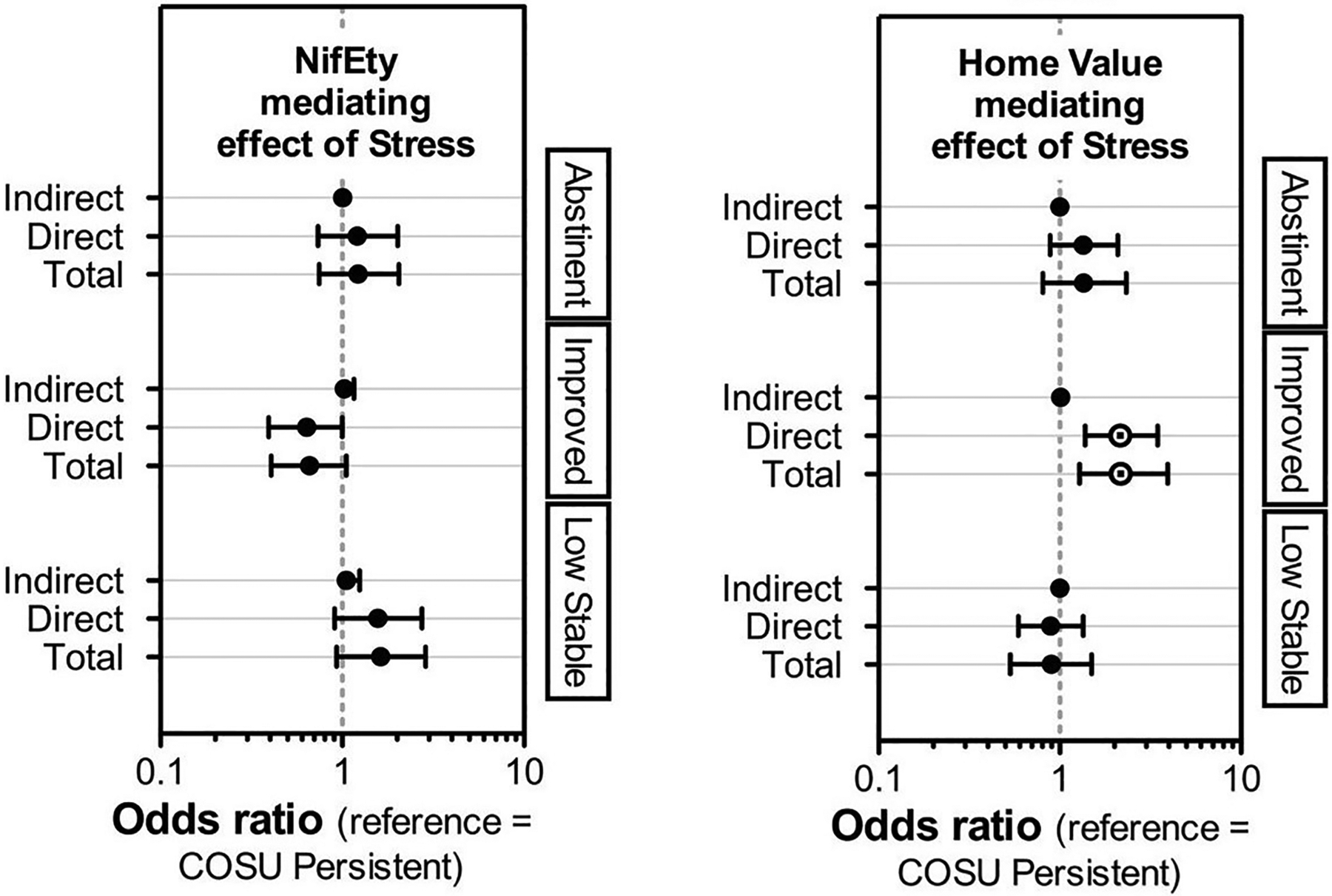
Odds ratios with Bayesian 90% credible intervals for mediation effects for the COSU group. Mediation models compared Abstinent, Improved, and Low-Stable trajectories to the Persistent trajectory (reference category). Total and direct effects were detected for home value and stress (right panel; Improved vs. Persistent).
Moderation analyses
According to our analysis plan, we based the moderation analyses on the results of the initial multinomial regression analyses. If trajectory category was predicted by social instability, then moderation analyses were conducted with social instability as a predictor in a multinomial logistic regression model that included other significant predictors and an interaction term with each predictor, to test whether social stability protects against effects of environment and health measures on trajectory.
In the COSU group, social instability was examined as a moderator in two models: Model 1 (NIfETy, stress, social instability, and their interactions predict trajectory); and Model 2 (home value, stress, and social instability predict trajectory). In Model 1, the interaction of NIfETy x Stress x Social instability affected odds of having an Improved trajectory (Figure 2). This effect involved high probability of having an Improved trajectory, compared to the Persistent trajectory, in participants with low neighborhood disorder and low social instability regardless of stress, or with low neighborhood disorder, moderate social instability, and low stress (see Supplementary Figure 1, Supplemental file). Older age and white race increased the probability of an Improved trajectory.
Figure 2.
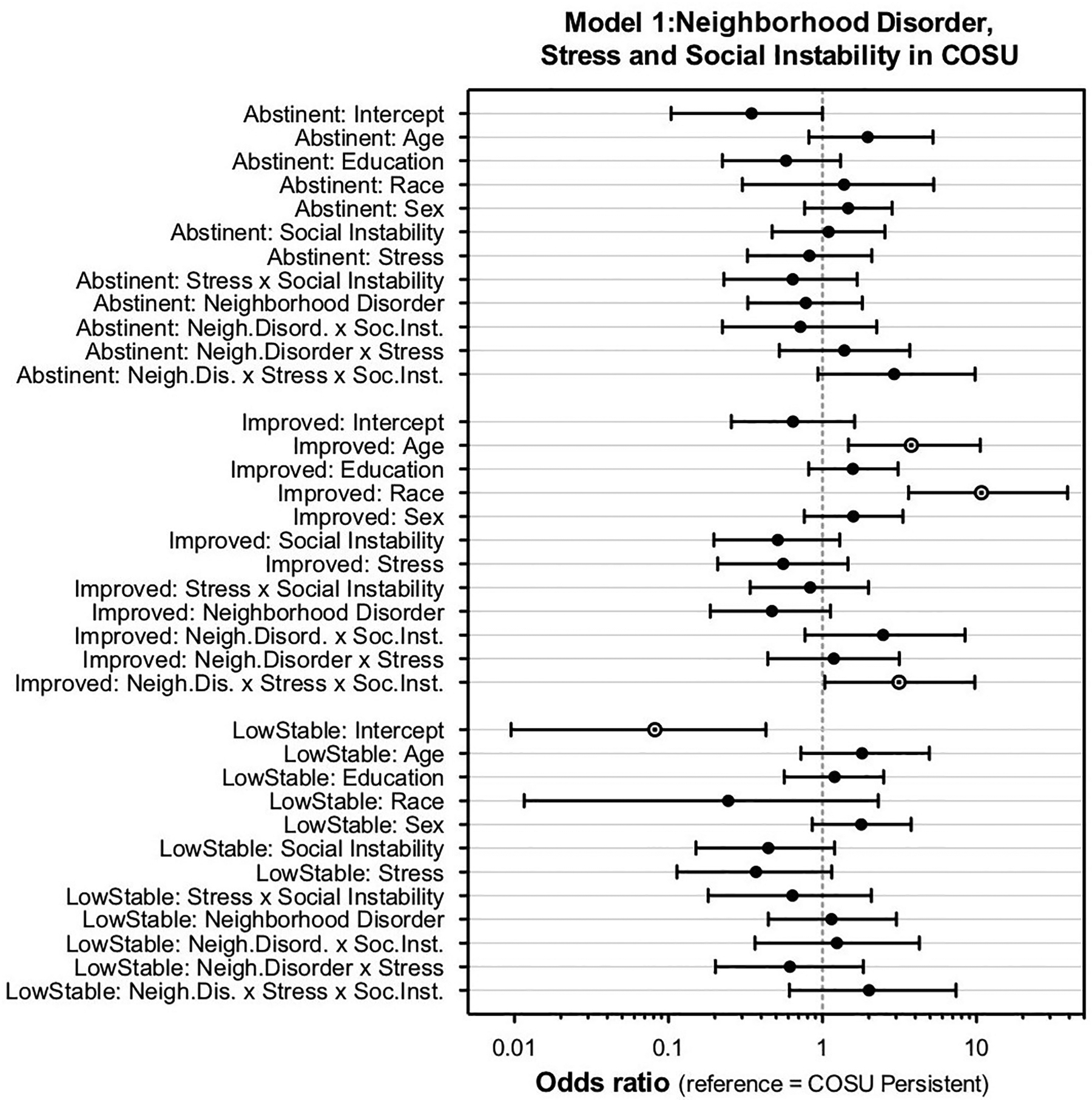
Odds ratios with Bayesian 90% credible intervals for moderation model 1: NIfETy (neighborhood disorder), stress, and social instability in COSU (reference category = Persistent). There was a NIfETy x stress x social instability interaction and effects of age and race that increased odds of the Improved trajectory.
In Model 2, the interaction of Home value x Stress x Social instability affected the odds of being in the Abstinent or Improved trajectories, compared to the Persistent trajectory (Figure 3). Low stress and moderate/high social instability increased the probability of an Improved trajectory (and to a lesser extent, the Abstinent trajectory) in those with a high home value (see Supplementary Figure 2, Supplemental file). White race increased the probability of the Improved trajectory. Odds of the Low-stable trajectory were decreased by social instability and stress but increased by age and white race.
Figure 3.
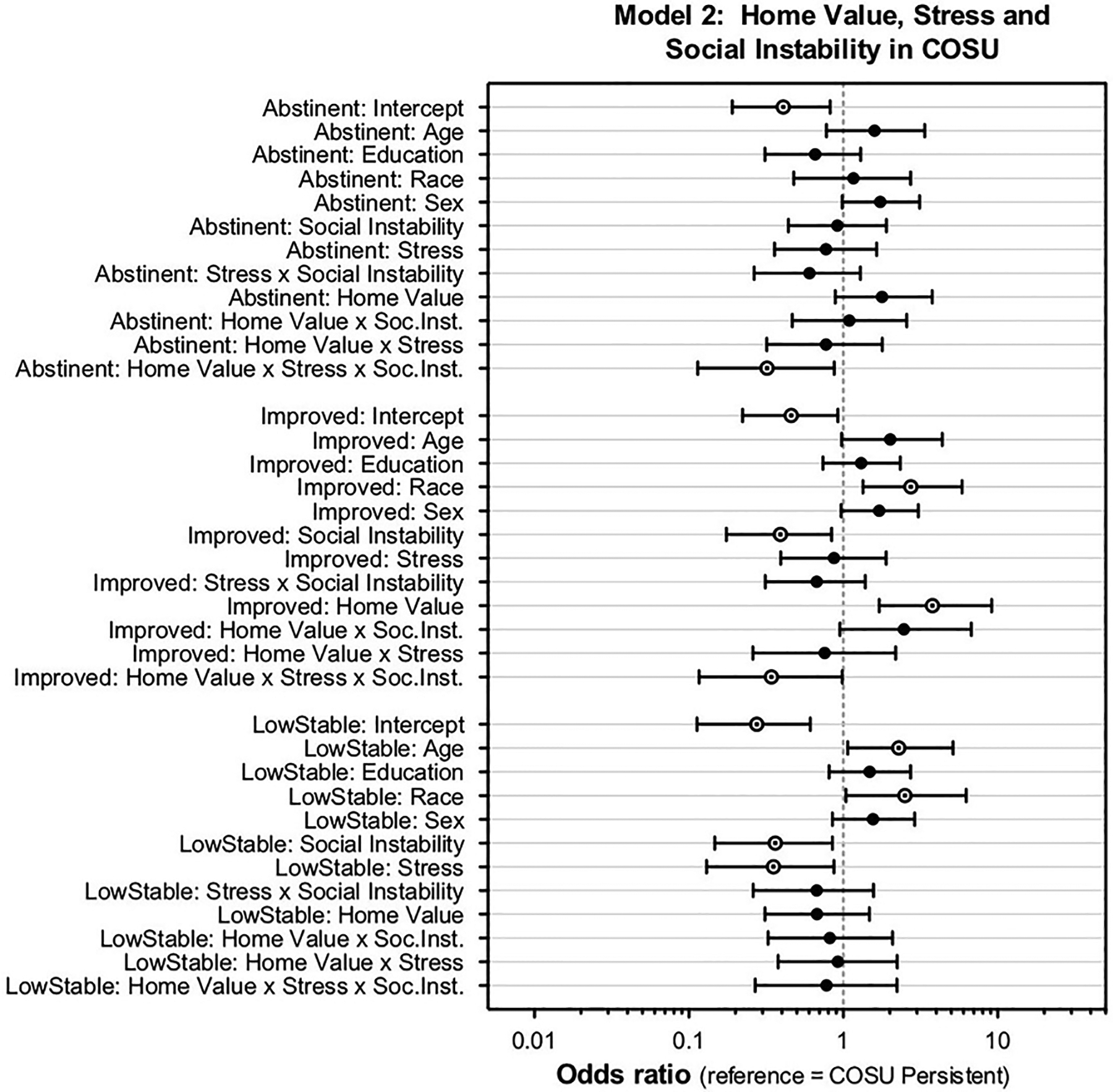
Odds ratios with Bayesian 90% credible intervals for moderation model 2: home value, stress, and social instability in COSU (reference category = Persistent). There was a home value x stress x social instability interaction which increased the odds of the Abstinent and Improved trajectory groups. Social instability, home value, and race effects also increased odds of the Improved trajectory. Social instability, stress, age, and race effects increased odds of the Low-stable trajectory.
Discussion
In this sample of people in the Baltimore, MD area, we found baseline differences across drug-use groups in measures of health and neighborhood. For the COSU group, changes in drug-use status over the 12-month study period were related to baseline measures of objective neighborhood disorder, home value, stress, and social instability. Social instability moderated the relationships among participants’ neighborhood, stress, and trajectory.
In our study protocol, we initially planned to define trajectory by absolute increases or decreases in DSM-5 symptom count from baseline to the 12-month follow-up, but this method did not account for baseline symptom count. Therefore, we developed a data-driven method to identify trajectory categories using k-means cluster analysis, and we tested the criterion validity of this approach using some of the predictor variables measured in the study. Using this method, the trajectories for the COSU group were previously described as “Symptomatic” (persistent), “Chippers” (low-stable), “Converted chippers” (improved), or “Quitters” (abstinent) (Stull et al., 2019). These trajectories were significantly related to a subset of factors that were measured for the study, including demographic variables, treatment history, psychological health, and neighborhood poverty and disorder (Stull et al., 2019). For the present report, we extended this method, (1) to determine trajectory across other drug use groups and (2) to include all domains of health and environment measured in the study as predictors of trajectory.
As planned in our study’s protocol, we ran mediation models to see if the effects of neighborhood disorder and home value on SUD symptom trajectory would be mediated by stress in the COSU group. Mediation effects were not detected, but our planned moderation models showed that at different levels of social instability, the probabilities of certain trajectories were differentially affected by stress, neighborhood disorder, and home value. For the COSU group, reduced symptoms (Improved trajectory) or becoming abstinent may be partly attributed to living in less disordered and impoverished neighborhoods. The probability of the Improved trajectory decreased with neighborhood disorder in those with low or moderate social instability. Although the probability of the Improved trajectory increased with home value across all levels of social instability, the relationship was, surprisingly, most pronounced for those with moderate or high social instability. The social instability composite score intentionally reflects a broad array of factors, including access to food, housing, and healthcare, as well as neighborhood perceptions, performance at work, and personal relationships. Experiencing certain social stressors could be a driving factor in reducing problematic substance use within the protective context of a less impoverished neighborhood, where access to treatment or other resources might be greater.
Although mental health disorders are frequently comorbid with SUDs (Grella et al., 2009; Rounsaville et al., 1982), the COSU group in our study did not show a direct relationship between mental health indices and SUD-symptom trajectory. However, it should be noted that our analyses were conducted according to the protocol for the study, which was designed to be conservative by only testing for interactions involving variables that had a direct effect; thus, it is possible that mental health affected trajectory in the COSU group through interaction with neighborhood variables. In addition, to avoid capitalizing on chance in these per protocol analyses, we only tested effects on composite score, not individual measures. Further exploration of each measure, or a principal components analysis, could highlight other specific aspects of mental health, and of other domains, that may have the most influence on drug-use trajectory.
Without accounting for environment, mental health tended to be worse in participants who had a Persistent trajectory in the Unclassified group. Many of the Unclassified participants who were in the Persistent trajectory had a large (3+) increase in symptoms at follow-up, whereas the COSU participants with Persistent trajectories generally had a high symptom count at both visits. For the Unclassified group, deterioration in drug-use status may be preceded by or simply related to mental health issues (Angst et al., 2010; Crum et al., 2018; Lopez-Quintero et al., 2011). In contrast, stress was associated with the Persistent trajectory for the COSU group, and thus may contribute more to the maintenance of a moderate to severe level of symptoms. This result is consistent with our EMA studies on participants in methadone or buprenorphine treatment, which showed that real-time self-reports of stress coincided with reports of heroin craving (Panlilio, 2021; Preston et al., 2017) and exposure to drug cues (Preston & Epstein, 2011), and that stressful events can increase in severity in the days preceding cocaine use (Furnari et al., 2015). End-of-day EMA reports of daily hassles are also associated with opioid and cocaine use (Preston et al., 2018), as well as treatment dropout (Panlilio et al., 2019).
Limitations of this study include the use of self-report measures and composite scores for each domain, the psychometric properties of which have not been established. Composite scores were created to avoid capitalizing on chance with multiple analyses. Another limitation is the low follow-up rate of the marijuana-use group, which was not sufficient to analyze changes in, or predictors of changes in, drug-use status. The overall follow-up rate of 51.7% was close to what we expected (50%) given previous experience with similar populations and results from other studies (Genberg et al., 2011; Murphy et al., 2010). Participants who completed Visit 2 were slightly older with better mental health scores compared to the participants who only completed Visit 1, but there were no differences by sex, race, employment, education, marital status, neighborhood measures, or other health measures, supporting the generalizability of the results. The current study was powered to detect medium effects for testing predictors of drug-use trajectory, and while the results support certain moderation effects, additional studies would be useful to clarify relationships detected here. In addition, sampling of DSM-5 symptoms over a more extended period and with more time points would provide additional information about longer-term trajectories (Hser et al., 2008; Hser et al., 2007). However, we were able to observe changes in drug-use status with a 12-month follow-up period, which may reflect the beginning of various types of longer-term trajectories. We used the DSM definition of current symptomology of “within the past 12 months” at both visits, but assessment of symptoms occurring within a more recent timeframe could help to clarify current symptom status and give a more accurate characterization at a 12–15 month follow-up visit.
Another limitation of this study is that our sample was composed primarily of residents of Baltimore City, which reduces generalizability. Also, only those who lived in the city (536 of 750 study participants) had a NIfETy score assigned to them, which limited the sample size for analyses related to this measure. Using property-tax data as an objective measure of neighborhood poverty circumvented this problem, because estimates could be determined for all participants, regardless of whether they lived in the city or a surrounding county. Additional objective measures of neighborhood environment will also be important to consider for future studies, to include participants from a greater variety of geographic contexts.
With our previously defined methods for categorizing drug-use trajectory (Stull et al., 2019), we used DSM-5 symptoms counts, as well as substance type and history of use, to assess risk and protective factors for changes in drug-use status in subgroups of people with drug use. Further use of DSM-5 symptom counts may be valuable in monitoring long-term trajectories, as other studies have suggested they may be a meaningful alternative to sustained abstinence as an outcome measure to assess treatment effectiveness (Epstein et al., 2009; Kiluk et al., 2019; Kiluk et al., 2018). Relationships among sociodemographic variables, environment, health, and drug use may not be apparent without comparing drug-use status at baseline to follow-up periods, which can be defined using DSM-5 criteria (Angst et al., 2010; Swendsen et al., 2009). Likewise, changes (or absence of changes) in symptom counts must be considered within the context of various social, environmental, and individual factors, to better adapt prevention and treatment efforts to specific populations.
Supplementary Material
Funding
This study was supported by the Intramural Research Program of NIH, NIDA. The views and opinions expressed in this manuscript are those of the authors only and do not necessarily represent the views, official policy or position of the U.S. Department of Health and Human Services or any of its affiliated institutions or agencies.
Footnotes
Declaration of Interest Statement
The authors report there are no competing interests to declare.
Data Availability Statement
The data presented here are not publicly available because other components of the study protocol are still ongoing: https://clinicaltrials.gov/ct2/show/NCT01571752.
References
- Adler LA, Spencer T, Faraone SV, Kessler RC, Howes MJ, Biederman J, & Secnik K (2006). Validity of pilot Adult ADHD Self-Report Scale (ASRS) to rate adult ADHD symptoms. Ann Clin Psychiatry, 18(3), 145–148. 10.1080/10401230600801077 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (1994). Diagnostic and Statistical Manual of Mental Disorders (4th ed.). [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). [Google Scholar]
- Angst J, Cui L, Swendsen J, Rothen S, Cravchik A, Kessler RC, & Merikangas KR (2010). Major depressive disorder with subthreshold bipolarity in the National Comorbidity Survey Replication. Am J Psychiatry, 167(10), 1194–1201. 10.1176/appi.ajp.2010.09071011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asberg M, Montgomery SA, Perris C, Schalling D, & Sedvall G (1978). A comprehensive psychopathological rating scale. Acta Psychiatr Scand Suppl(271), 5–27. 10.1111/j.1600-0447.1978.tb02357.x [DOI] [PubMed] [Google Scholar]
- Boardman JD, Finch BK, Ellison CG, Williams DR, & Jackson JS (2001). Neighborhood disadvantage, stress, and drug use among adults. J Health Soc Behav, 42(2), 151–165. https://www.ncbi.nlm.nih.gov/pubmed/11467250 [PubMed] [Google Scholar]
- Bürkner PC (2021). brms: Bayesian regression models using ‘Stan’. https://CRAN.R-project.org/package=brms
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res, 28(2), 193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- Carver CS, Scheier MF, & Weintraub JK (1989). Assessing coping strategies: a theoretically based approach. J Pers Soc Psychol, 56(2), 267–283. 10.1037//0022-3514.56.2.267 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). (2009–2010). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Questionnaire. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Retrieved from https://wwwn.cdc.gov/Nchs/Nhanes/continuousnhanes/questionnaires.aspx?BeginYear=2009 [Google Scholar]
- Chang JS (2017). Health in the Tenderloin: A resident-guided study of substance use, treatment, and housing. Soc Sci Med, 176, 166–174. 10.1016/j.socscimed.2017.01.014 [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R (1983). A global measure of perceived stress. J Health Soc Behav, 24(4), 385–396. https://www.ncbi.nlm.nih.gov/pubmed/6668417 [PubMed] [Google Scholar]
- Costa J, P. T, & McCrae RR (1985). The NEO Personality Inventory Manual. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Cranford JA, Shrout PE, Iida M, Rafaeli E, Yip T, & Bolger N (2006). A procedure for evaluating sensitivity to within-person change: can mood measures in diary studies detect change reliably? Pers Soc Psychol Bull, 32(7), 917–929. 10.1177/0146167206287721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creti L, Fichten CS, Amsel R, Brender W, Schover LR, Kalogeropoulos D, & Libman E (1998). Global sexual functioning: A single summary score for Nowinski and LoPiccolo’s Sexual History Form (SHF). In Davis CM, Yarber WL, Bauseman R, Schreer G, & Davis SL (Eds.), Handbook of Sexuality-Related Measures (pp. 261–267). Thousand Oaks, CA: Sage Publications. [Google Scholar]
- Crum RM, Green KM, Stuart EA, La Flair LN, Kealhofer M, Young AS, Krawczyk N, Tormohlen KN, Storr CL, Alvanzo AAH, Mojtabai R, Pacek LR, Cullen BA, & Reboussin BA (2018). Transitions through stages of alcohol involvement: The potential role of mood disorders. Drug Alcohol Depend, 189, 116–124. 10.1016/j.drugalcdep.2018.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLongis A, Folkman S, Lazarus RS (1988). The impact of daily stress on health and mood: psychological and social resources as mediators. Journal of Personality and Social Psychology, 54(3), 486–495. 10.1037/0022-3514.54.3.486 [DOI] [PubMed] [Google Scholar]
- De Leon G (1989). Psychopathology and substance abuse: what is being learned from research in therapeutic communities. J Psychoactive Drugs, 21(2), 177–188. 10.1080/02791072.1989.10472158 [DOI] [PubMed] [Google Scholar]
- Dickson-Gomez J, McAuliffe T, & Quinn K (2017). The Effects of Housing Status, Stability and the Social Contexts of Housing on Drug and Sexual Risk Behaviors. AIDS Behav, 21(7), 2079–2092. 10.1007/s10461-017-1738-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Schmittner J, Umbricht A, Schroeder JR, Moolchan ET, & Preston KL (2009). Promoting abstinence from cocaine and heroin with a methadone dose increase and a novel contingency. Drug Alcohol Depend, 101(1–2), 92–100. 10.1016/j.drugalcdep.2008.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Tyburski M, Craig IM, Phillips KA, Jobes ML, Vahabzadeh M, Mezghanni M, Lin JL, Furr-Holden CDM, & Preston KL (2014). Real-time tracking of neighborhood surroundings and mood in urban drug misusers: Application of a new method to study behavior in its geographical context. Drug Alcohol Depend, 134, 22–29. 10.1016/j.drugalcdep.2013.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Tyburski M, Kowalczyk WJ, Burgess-Hull AJ, Phillips KA, Curtis BL, & Preston KL (2020). Prediction of stress and drug craving ninety minutes in the future with passively collected GPS data. NPJ Digit Med, 3, 26. 10.1038/s41746-020-0234-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furnari M, Epstein DH, Phillips KA, Jobes ML, Kowalczyk WJ, Vahabzadeh M, Lin JL, & Preston KL (2015). Some of the people, some of the time: Field evidence for associations and dissociations between stress and drug use. Psychopharmacology (Berl), 232(19), 3529–3537. 10.1007/s00213-015-3998-7 [DOI] [PubMed] [Google Scholar]
- Furr-Holden CD, Campbell KD, Milam AJ, Smart MJ, Ialongo NA, & Leaf PJ (2010). Metric properties of the Neighborhood Inventory for Environmental Typology (NIfETy): An environmental assessment tool for measuring indicators of violence, alcohol, tobacco, and other drug exposures. Eval Rev, 34(3), 159–184. 10.1177/0193841X10368493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genberg BL, Gange SJ, Go VF, Celentano DD, Kirk GD, & Mehta SH (2011). Trajectories of injection drug use over 20 years (1988–2008) in Baltimore, Maryland. Am J Epidemiol, 173(7), 829–836. 10.1093/aje/kwq441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genolini C, Pingault JB, Driss T, Cote S, Tremblay RE, Vitaro F, Arnaud C, & Falissard B (2013). KmL3D: a non-parametric algorithm for clustering joint trajectories. Comput Methods Programs Biomed, 109(1), 104–111. 10.1016/j.cmpb.2012.08.016 [DOI] [PubMed] [Google Scholar]
- Goodrich B, Gabry J, Ali I, & Brilleman S (2022). rstanarm: Bayesian applied regression modeling via Stan In R package version 2.21.3 https://mc-stan.org/rstanarm/
- Gray MJ, Litz BT, Hsu JL, & Lombardo TW (2004). Psychometric properties of the life events checklist. Assessment, 11(4), 330–341. 10.1177/1073191104269954 [DOI] [PubMed] [Google Scholar]
- Grella CE, Karno MP, Warda US, Niv N, & Moore AA (2009). Gender and comorbidity among individuals with opioid use disorders in the NESARC study. Addict Behav, 34(6–7), 498–504. 10.1016/j.addbeh.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grella CE, & Lovinger K (2011). 30-year trajectories of heroin and other drug use among men and women sampled from methadone treatment in California. Drug Alcohol Depend, 118(2–3), 251–258. 10.1016/j.drugalcdep.2011.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton CM, Strader LC, Pratt JG, Maiese D, Hendershot T, Kwok RK, Hammond JA, Huggins W, Jackman D, Pan H, Nettles DS, Beaty TH, Farrer LA, Kraft P, Marazita ML, Ordovas JM, Pato CN, Spitz MR, Wagener D, … Haines J (2011). The PhenX Toolkit: get the most from your measures. Am J Epidemiol, 174(3), 253–260. 10.1093/aje/kwr193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Smith LJ, Cousins JC, & Bootzin RR (2012). Circadian rhythms, sleep, and substance abuse. Sleep Med Rev, 16(1), 67–81. 10.1016/j.smrv.2011.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel D (2011). Unemployment and substance use: a review of the literature (1990–2010). Curr Drug Abuse Rev, 4(1), 4–27. 10.2174/1874473711104010004 [DOI] [PubMed] [Google Scholar]
- Hirschfeld RM, Williams JB, Spitzer RL, Calabrese JR, Flynn L, Keck PE Jr., Lewis L, McElroy SL, Post RM, Rapport DJ, Russell JM, Sachs GS, & Zajecka J (2000). Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry, 157(11), 1873–1875. 10.1176/appi.ajp.157.11.1873 [DOI] [PubMed] [Google Scholar]
- Hser YI, Huang D, Brecht ML, Li L, & Evans E (2008). Contrasting trajectories of heroin, cocaine, and methamphetamine use. J Addict Dis, 27(3), 13–21. 10.1080/10550880802122554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser YI, Huang D, Chou CP, & Anglin MD (2007). Trajectories of heroin addiction: growth mixture modeling results based on a 33-year follow-up study. Eval Rev, 31(6), 548–563. 10.1177/0193841X07307315 [DOI] [PubMed] [Google Scholar]
- Hser YI, Huang D, Saxon AJ, Woody G, Moskowitz AL, Matthews AG, & Ling W (2017). Distinctive trajectories of opioid use over an extended follow-up of patients in a multisite trial on buprenorphine + naloxone and methadone. J Addict Med, 11(1), 63–69. 10.1097/ADM.0000000000000274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp. (Released 2019). IBM SPSS Statistics for Windows, Version 26.0 In Armonk, NY: IBM Corp. [Google Scholar]
- Jahiel RI (1992). Homelessness: a prevention-oriented approach. Johns Hopkins University Press. [Google Scholar]
- Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, & Cleeland CS (2004). Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain, 20(5), 309–318. 10.1097/00002508-200409000-00005 [DOI] [PubMed] [Google Scholar]
- Kiluk BD, Fitzmaurice GM, Strain EC, & Weiss RD (2019). What defines a clinically meaningful outcome in the treatment of substance use disorders: Reductions in direct consequences of drug use or improvement in overall functioning? Addiction, 114(1), 9–15. 10.1111/add.14289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiluk BD, Frankforter TL, Cusumano M, Nich C, & Carroll KM (2018). Change in DSM-5 Alcohol Use Disorder Criteria Count and Severity Level as a Treatment Outcome Indicator: Results from a Randomized Trial. Alcohol Clin Exp Res, 42(8), 1556–1563. 10.1111/acer.13807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkenberg WD, Sacks S, & HIV/AIDS Treatment Adherence, Health Outcomes and Cost Study Group. (2004). Mental disorders and drug abuse in persons living with HIV/AIDS. AIDS Care, 16 Suppl 1, S22–42. 10.1080/09540120412331315303 [DOI] [PubMed] [Google Scholar]
- Latkin CA, & Curry AD (2003). Stressful neighborhoods and depression: A prospective study of the impact of neighborhood disorder. J Health Soc Behav, 44(1), 34–44. https://www.ncbi.nlm.nih.gov/pubmed/12751309 [PubMed] [Google Scholar]
- Latkin CA, Hua W, & Davey MA (2005). Exploring the role of needle selling in a drug-using community in Baltimore, Maryland. J Acquir Immune Defic Syndr, 38(1), 57–60. 10.1097/00126334-200501010-00011 [DOI] [PubMed] [Google Scholar]
- Lopez-Quintero C, Perez de los Cobos J, Hasin DS, Okuda M, Wang S, Grant BF, & Blanco C (2011). Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend, 115(1–2), 120–130. 10.1016/j.drugalcdep.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Fairchild AJ, & Fritz MS (2007). Mediation analysis. Annu Rev Psychol, 58, 593–614. 10.1146/annurev.psych.58.110405.085542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez ML, Black M, & Starr RH (2002). Factorial structure of the perceived neighborhood scale (PNS): A test of longitudinal invariance. J Community Psychol, 30(1), 23–43. 10.1002/jcop.1048 [DOI] [Google Scholar]
- Mennis J, Mason M, Light J, Rusby J, Westling E, Way T, Zahakaris N, & Flay B (2016). Does substance use moderate the association of neighborhood disadvantage with perceived stress and safety in the activity spaces of urban youth? Drug Alcohol Depend, 165, 288–292. 10.1016/j.drugalcdep.2016.06.019 [DOI] [PubMed] [Google Scholar]
- Moos RH (2007). Theory-based processes that promote the remission of substance use disorders. Clin Psychol Rev, 27(5), 537–551. 10.1016/j.cpr.2006.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos RH, Fenn CB, Billings AG, & Moos BS (1988). Assessing life stressors and social resources: applications to alcoholic patients. J Subst Abuse, 1(2), 135–152. 10.1016/s0899-3289(88)80017-8 [DOI] [PubMed] [Google Scholar]
- Murphy DA, Hser Y-I, Huang D, Brecht M-L, & Herbeck DM (2010). Self-report of longitudinal substance use: a comparison of the UCLA Natural History Interview and the Addiction Severity Index. J Drug Issues, 40(2), 495–515. 10.1177/002204261004000210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbeck JS (1984). Modification of life event questionnaires for use with female respondents. Res Nurs Health, 7(1), 61–71. 10.1002/nur.4770070110 [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Stull SW, Bertz JW, Burgess-Hull AJ, Kowalczyk WJ, Phillips KA, Epstein DH, & Preston KL (2020). Beyond abstinence and relapse: cluster analysis of drug-use patterns during treatment as an outcome measure for clinical trials. Psychopharmacology (Berl), 237(11), 3369–3381. 10.1007/s00213-020-05618-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Stull SW, Kowalczyk WJ, Phillips KA, Schroeder JR, Bertz JW, Vahabzadeh M, Lin JL, Mezghanni M, Nunes EV, Epstein DH, & Preston KL (2019). Stress, craving and mood as predictors of early dropout from opioid agonist therapy. Drug Alcohol Depend, 202, 200–208. 10.1016/j.drugalcdep.2019.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Stull SW, Bertz JW, Burgess-Hull AJ, Lanza ST, Curtis BL, Phillips KA, Epstein DH, Preston KL (2021). Beyond abstinence and relapse II: momentary relationships between stress, craving, and lapse within clusters of patients with similar patterns of drug use. Psychopharmacology (Berl), 238 (6), 1513–1529. 10.1007/s00213-021-05782-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, & Epstein DH (2011). Stress in the daily lives of cocaine and heroin users: relationship to mood, craving, relapse triggers, and cocaine use. Psychopharmacology (Berl), 218(1), 29–37. 10.1007/s00213-011-2183-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Kowalczyk WJ, Phillips KA, Jobes ML, Vahabzadeh M, Lin JL, Mezghanni M, & Epstein DH (2017). Context and craving during stressful events in the daily lives of drug-dependent patients. Psychopharmacology (Berl), 234(17), 2631–2642. 10.1007/s00213-017-4663-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Kowalczyk WJ, Phillips KA, Jobes ML, Vahabzadeh M, Lin JL, Mezghanni M, & Epstein DH (2018). Exacerbated craving in the presence of stress and drug cues in drug-dependent patients. Neuropsychopharmacology, 43(4), 859–867. 10.1038/npp.2017.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2018). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rogers JD, Ramaswamy M, Cheng CI, Richter K, & Kelly PJ (2012). Perceptions of neighborhood social environment and drug dependence among incarcerated women and men: a cross-sectional analysis. Subst Abuse Treat Prev Policy, 7, 39. 10.1186/1747-597X-7-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum A, Joseph H, Fong C, Kipnis S, Cleland C, & Portenoy RK (2003). Prevalence and characteristics of chronic pain among chemically dependent patients in methadone maintenance and residential treatment facilities. JAMA, 289(18), 2370–2378. 10.1001/jama.289.18.2370 [DOI] [PubMed] [Google Scholar]
- Rounsaville BJ, Weissman MM, Kleber H, & Wilber C (1982). Heterogeneity of psychiatric diagnosis in treated opiate addicts. Arch Gen Psychiatry, 39(2), 161–168. 10.1001/archpsyc.1982.04290020027006 [DOI] [PubMed] [Google Scholar]
- Ruglass LM, Scodes J, Pavlicova M, Campbell ANC, Fitzpatrick S, Barbosa-Leiker C, Burlew K, Greenfield SF, Rotrosen J, & Nunes EV Jr. (2019). Trajectory classes of opioid use among individuals in a randomized controlled trial comparing extended-release naltrexone and buprenorphine-naloxone. Drug Alcohol Depend, 205, 107649. 10.1016/j.drugalcdep.2019.107649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker H, Tyburski M, Rahman MM, Hovsepian K, Sharmin M, Epstein DH, Preston KL, Furr-Holden CD, Milam A, Nahum-Shani I, al’Absi M, & Kumar S (2016). Finding significant stress episodes in a discontinuous time series of rapidly varying mobile sensor data. Proc SIGCHI Conf Hum Factor Comput Syst, 2016, 4489–4501. 10.1145/2858036.2858218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SB, Munoz E, Mogle JA, Gamaldo AA, Smyth JM, Almeida DM, & Sliwinski MJ (2018). Perceived neighborhood characteristics predict severity and emotional response to daily stressors. Soc Sci Med, 200, 262–270. 10.1016/j.socscimed.2017.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SG, Hua W, & Latkin CA (2004). Individual and environmental factors related to quitting heroin injection. Subst Use Misuse, 39(8), 1199–1214. 10.1081/ja-120038683 [DOI] [PubMed] [Google Scholar]
- Sinha R (2009). Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addict Biol, 14(1), 84–98. 10.1111/j.1369-1600.2008.00134.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stan Development Team. (2022). RStan: the R interface to Stan. In R package https://mc-stan.org/
- Stull SW, Panlilio LV, Moran LM, Schroeder JR, Bertz JW, Epstein DH, Preston KL, & Phillips KA (2019). The chippers, the quitters, and the highly symptomatic: A 12-month longitudinal study of DSM-5 opioid- and cocaine-use problems in a community sample. Addict Behav, 96, 183–191. 10.1016/j.addbeh.2019.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swendsen J, Conway KP, Degenhardt L, Dierker L, Glantz M, Jin R, Merikangas KR, Sampson N, & Kessler RC (2009). Socio-demographic risk factors for alcohol and drug dependence: the 10-year follow-up of the national comorbidity survey. Addiction, 104(8), 1346–1355. 10.1111/j.1360-0443.2009.02622.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teesson M, Marel C, Darke S, Ross J, Slade T, Burns L, Lynskey M, Memedovic S, White J, & Mills KL (2017). Trajectories of heroin use: 10–11-year findings from the Australian Treatment Outcome Study. Addiction, 112(6), 1056–1068. 10.1111/add.13747 [DOI] [PubMed] [Google Scholar]
- Ward J, Darke S, Hall W (1990). The HIV Risk-Taking Behaviour Scale (HRBS) Manual. Sydney: National Drug and Alcohol Research Centre, University of New South Wales. https://ndarc.med.unsw.edu.au/sites/default/files/ndarc/resources/TR.010.PDF [Google Scholar]
- Weathers FW, Huska JA, & Keane TM (1991). PCL-C for DSM-IV. Boston, MA: National Center for PTSD—Behavioral Science Division. [Google Scholar]
- Weissman MM, & Bothwell S (1976). Assessment of social adjustment by patient self-report. Arch Gen Psychiatry, 33(9), 1111–1115. 10.1001/archpsyc.1976.01770090101010. [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2004). World Health Organization Quality of Life (WHO-QOL)-BREF.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented here are not publicly available because other components of the study protocol are still ongoing: https://clinicaltrials.gov/ct2/show/NCT01571752.


