Highlights
-
•
The matching feature between microorganism and food flavor was summarized.
-
•
The microbial mediated flavor regulation method was discussed.
-
•
The key pathways and regulation methods of microbial synthetic flavor compounds.
-
•
Communication and interaction between communities of different strains.
Keywords: Food, Microbial flora, Metabolism, Flavor, Regulation, Pathway
Abstract
The beneficial microorganisms in food are diverse and complex in structure. These beneficial microorganisms can produce different and unique flavors in the process of food fermentation. The unique flavor of these fermented foods is mainly produced by different raw and auxiliary materials, fermentation technology, and the accumulation of flavor substances by dominant microorganisms during fermentation. The succession and metabolic accumulation of microbial flora significantly impacts the distinctive flavor of fermented foods. The investigation of the role of microbial flora changes in the production of flavor substances during fermentation can reveal the potential connection between microbial flora succession and the formation of key flavor compounds. This paper reviewed the evolution of microbial flora structure as food fermented and the key volatile compounds that contribute to flavor in the food system and their potential relationship. Further, it was a certain guiding significance for food industrial production.
Introduction
Food contains abundant microbial resources. Various microorganisms grow and enrich the food system, gradually forming a stable unique flora in the food system. Under certain conditions, microorganisms produce expected flavor compounds in food through complex metabolic activities. Therefore, microorganisms are thought of as an essential factor determining the overall quality of food (Han et al., 2022, Kumar et al., 2022).
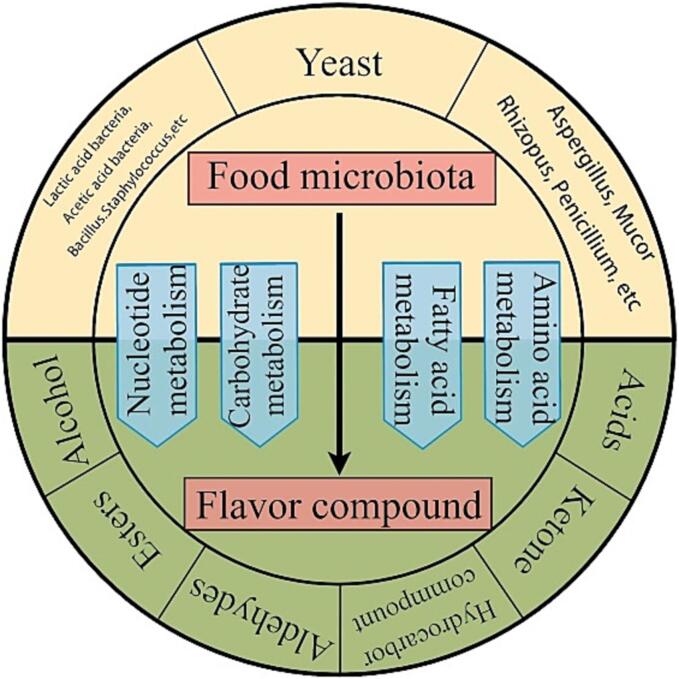
Traditional fermented foods exhibit a succession of communities of microorganisms that are complex and dynamic, including not only the bacteria that are conducive to fermentation but also some spoilage bacteria and pathogenic bacteria need to be inhibited (Lin et al., 2021). The key microbial genera that impact the generation of flavoring molecules differ based on the stage of food fermentation. The dominant bacteria in different fermented foods are also different. There may be symbiotic and alternate relationships among different microorganisms, which may affect metabolic pathways (Ai, Tong, Wen, Chen, Huang, & Zhao, 2022). The metabolic pathways of microorganisms in the fermentation process are also complex and changeable. Common metabolic routes include amino acid metabolism, fatty acid metabolism, carbohydrate metabolism, etc. All pathways interact with each other to form the unique flavor of food (Hu et al., 2022, Huang et al., 2022). Microflora accumulates small molecular flavor substances through metabolism, as shown in Fig. 1. Therefore, to analyze the changes in bacterial community composition and microbial diversity in food, reveal the metabolic pathway of microorganisms, study the characteristics of different microbial strains in different fermented foods and the characteristics of volatile compounds produced by different microbial strains through fermentation metabolism, and reveal the connection between microbial diversity and flavor chemicals were important. The investigation of the association between microbial diversity and flavor molecules, which are the identification of prospective flavor-forming microorganisms, and the control of food flavor are all positive relevance. Additionally, through the regulation of the microbial metabolic pathways, microbial metabolism can occur in the expected direction and produce the desired flavor substances, which has certain positive significance for improving food flavor and food nutritional value, reducing food safety risks. Further research on the interaction between microorganisms and characteristic flavor components can be applied to the screening and purification of dominant strains, to improve the flavor and security of fermented foods, and provide a guiding for the industrial standardized production of fermented food.
Fig. 1.
Summary of microbial metabolism and accumulation of flavor substances.
This review mainly started with traditional fermented food and reviewed the structure, metabolism, and succession of microbial flora in traditional fermented food and the regulation and control of key volatile flavor substances in fermented food. The relationship between microbial diversity and flavor formation were explained. The flavor metabolism mechanism of core microorganisms was also briefly covered. In conclusion, it aimed to provide a few guidelines for enhancing the quality of conventionally fermented foods and will outline potential future directions for fermented food research.
Types of microbial metabolism
The role of microbial metabolism in the food system is crucial. The microbial flora of various food systems can carry out various metabolic functions, to obtain different metabolites and produce a unique flavor. The metabolic pathways of microorganisms are shown in Table 1, mainly including the glycometabolic pathway, polysaccharide decomposition, proteolysis, lipid, fatty acid catabolism, etc.
Table 1.
Types of microbial metabolism.
| Metabolic Type | Specific approach | Metabolic microorganism |
|---|---|---|
| Glycometabolic pathway | Glycolysis pathway (EMP pathway) | Most microorganisms have an EMP pathway, including most anaerobic bacteria, facultative aerobic bacteria and obligate aerobic bacteria |
| Pentose phosphate pathway (HMP pathway) | Most aerobic and facultative anaerobic microorganisms have the HMP pathway | |
| 6-phosphogluconic acid pathway (ED pathway) | The ED pathway is widely distributed in Gram-negative Pseudomonas | |
| Phosphoketease pathway (PK pathway) | It is found in Leuconostoc and bifidobacterium | |
| Polysaccharide decomposition | Amylolysis (amylase) | Bacillus such as Bacillus subtilis, Bacillus licheniformis, etc. |
| Cellulose decomposition (cellulase) | Trichoderma such as Trichoderma viride, Trichoderma lignorum, etc. | |
| Pectinolysis (pectinase) | Aspergillus such as Aspergillus wentii, Aspergillus Niger, etc. | |
| Proteolysis | Protein → Peptide, amino acid (protease) | Bacillus, mycobacteria, etc. |
| Lipid and fatty acid catabolism | Fat → glycerol, fatty acids (lipase) → acetyl coenzyme A (β-oxidation) | Bacillus, pseudomonas, etc. Rhizopus oryzae, Aspergillus Niger, yeast, etc. |
The glycometabolic pathway in microbial metabolism is mainly divided into the Embden-Meyerhof-Parnas (EMP) pathway, Hexose Monophosphate (HMP) pathway, Entner-Doudoroff (ED) pathway, Pentose phosphate ketolyase (PK) pathway, etc. Glycolysis pathway is another name for the EMP pathway. After converting glucose into 1, 6-diphosphate fructose, it is cleaved into two three-carbon compounds under the action of fructose-bisphosphate aldolase. Finally, they converted into two molecules of pyruvate (You & Zhang, 2013). EMP pathway is the most fundamental and prevalent metabolic process. EMP pathway is regarded as the focal point of the synthesis and catabolism of various substances in microbial cells, along with TCA cycle (tricarboxylic acid cycle). The Pentose phosphate pathway is another name for the HMP pathway. When glucose is dehydrogenated by phosphorylation to produce 6-phosphoglucoce acid, it will carry on being degraded by 6-phosphoglucoce acid dehydrogenase into one molecule of pentose phosphate and one molecule of carbon dioxide which finally were also convert into pyruvate after a series of complex reactions (Horecker, 2002). The HMP pathway usually occurs simultaneously with the EMP pathway in microbial metabolism. Under the action of 2-keto-3-deoxy-6-phosphogluconate (KDPG) aldolase, 6-phosphogluconic acid in the ED pathway is broken down into pyruvate and 3-phosphoglyceraldehyde, which are then transformed into pyruvate via the EMP process (Gupta & Gupta, 2021). The ED pathway is an anaerobic metabolic pathway, usually co-existing with the HMP pathway. Pentose phosphate ketolyase pathway is another name for the PK pathway, exists only in a few microorganisms. Microorganisms convert monosaccharides into pyruvate through sugar metabolism, which can be further converted into some organic acids, alcohols and carbonyl compounds, and other flavors including ethanol, acetaldehyde, acetic acid, and diacetyl (Bartowsky & Pretorius, 2009).
Microbial polysaccharide metabolism is divided into amylolysis, cellulose decomposition, and pectinolytic. Polysaccharide macromolecules cannot be directly absorbed by microorganisms. They are often decomposed into small molecules for absorption and utilization. The decomposition of starch by microorganisms is catalyzed by amylase secreted by microorganisms, which can be produced by many bacteria, molds, and actinomyces. Many cellulase-producing microorganisms such as Trichoderma viride, Trichoderma reesei, and some actinomyces and bacteria can break down cellulose (Singhania, Adsul, Pandey, & Patel, 2017). Pectinase is widely found in plants, bacteria, molds, and yeasts, such as Aspergillus wentii, Aspergillus Niger, and other molds. Different pectinases play different roles in the decomposition of pectin by microorganisms. Microorganisms produce acids, alcohols, and other flavor substances through polysaccharide decomposition (Kashyap, Vohra, Chopra, & Tewari, 2001).
Proteins are macromolecular substances with a complex structure composed of amino acids. They can be used by microorganisms while being broken down into smaller molecules like peptides and amino acids. The microorganisms that produce protease mainly include bacteria, molds, actinomyces, etc. Different strains can produce different proteases. The same strain can also produce different types of proteases. Alkaline proteases are mainly produced by bacteria, of which Bacillus is the main producer of alkaline proteases, some other Bacillus can also produce neutral protease (Contesini, Melo, & Sato, 2018). Acid protease is mainly produced by Aspergillus Niger, Aspergillus oryzae, and other molds (Xu, Pan, Zhao, Zhao, Sun, & Liu, 2011). After protein decomposition into free amino acids and other small molecules, transamination, deamination, and decarboxylation are carried out to obtain the flavor substance such as aldehydes, acids, and alcohols.
Under the effect of lipase, microorganisms break down lipids into glycerol and fatty acids. Different microorganisms produce different lipases. Lipases are most abundant in bacteria, molds, and yeasts. The most common bacterial sources of lipase include Bacillus, Pseudomonas, and Staphylococcus (Gupta, Gupta, & Rathi, 2004). Lipase can be obtained from Rhizopus oryzae, Aspergillus Niger, etc. Candida and other yeasts are also able to produce lipase (Bhan and Singh, 2020, Gupta et al., 2015). Unsaturated free fatty acids after fat decomposition are prone to auto-oxidation, producing ketones, aldehydes, alkanes, etc. The aldehydes generate alcohols and acids through redox, and the two undergo esterification to produce esters. Fatty acid metabolism has a great contribution to flavor.
Types and characteristics of microorganism producing flavor compounds
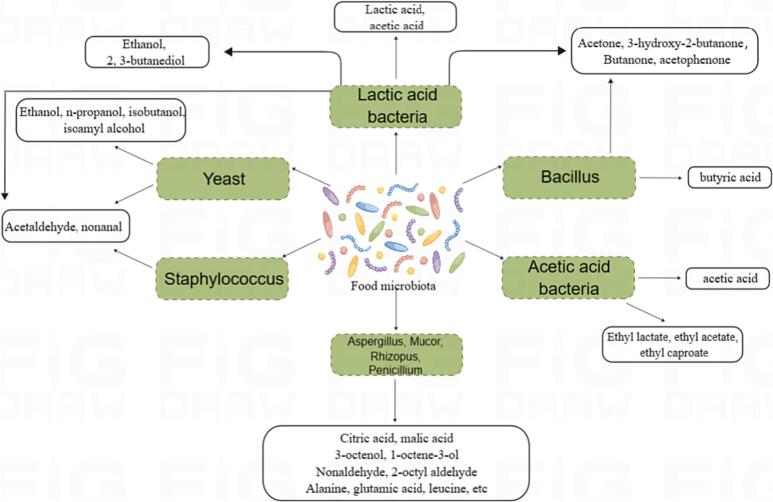
Fermented foods usually include dairy products, meat products, soy products, fermented wine, fermented vegetables, condiments, etc. Bacteria, mold, and yeast are the primary microbial species responsible for fermented foods. Different original microorganisms in the unprocessed materials cause differences in the microbial systems that are major infrastructure in the fermentation process. These differences contribute to the production of a variety of active substances that are beneficial to humans, which improve the taste of fermented foods and give them a variety of distinct flavors. Bacillus, lactic acid bacteria, and acetic acid bacteria are the most common forms of bacteria found in fermented foods (see Table 2). Mold is widely used in the fermentation industry and enzyme preparation industry. Aspergillus, Mucor, Rhizopus, etc. can be used in food industrial production. In food, medicine, and other industries, yeast is frequently employed. The main types of yeast used for food fermentation include Saccharomyces cerevisiae, Candida, Pichia pastoris, Wickerhamomyces, and Kazachstania Yeast, etc (Kaur & Dua, 2022).
Table 2.
Summary of flavor-producing microorganisms.
| Microorganism | Characteristic | Major strains | Flavor components | Reference |
|---|---|---|---|---|
| Lactic acid bacteria | Gram-positive, non-spore forming, non-reactive but gas tolerant, capable of producing lactic acid | Lactobacillus, Pediococcus, Streptococcus, Bifidobacterium, Leuconostoc, etc. | Lactic acid, acetic acid, ethanol, 2, 3-butanediol, acetone, nonaldehyde, benzaldehyde, etc. | Hu et al., 2022, Nsogning Dongmo et al., 2016, Pang et al., 2021. |
| Acetic acid bacteria | Gram-negative, spore - free, aerobic bacteria, capable of fermenting alcohol into acetic acid | Acetobacter, Gluconobacter, etc. | Ethyl lactate, ethyl acetate, ethyl caproate, etc. | Lynch et al., 2019, Raspor and Goranovič, 2008. |
| Bacillus | Gram-positive, spore-bearing bacteria | Bacillus subtilis, Bacillus flatus, Bacillus licheniformis, Bacillus globus, etc. | Butyric acid, butanone, acetophenone, pyrazines, etc | Kimura and Yokoyama, 2019, Zeng et al., 2023. |
| Staphylococcus | Gram-positive, spore-free, facultative anaerobes | Staphylococcus xylose, Staphylococcus succinis, etc. | Hexal, nononal, oleic acid, etc. | Stahnke et al., 2002, Wang et al., 2021. |
| Aspergillus | A variety of colors, generally relatively stable, aspergillus hypha is septal hypha, colorless or bright color; Conidial peduncle mostly without transverse septum, smooth, rough, or pitted. | Monascus, Aspergillus Niger, Aspergillus oryzae, Aspergillus wintii | Citric acid, malic acid, phenylacetaldehyde, 2-heptanone, 3-octanol, nonaldehyde, etc. | Li et al., 2023, Li et al., 2023. |
| Mucor | Mycelium developed, colony texture is loose, wadding, composed of many branching mycelium. Mycelium is generally white, without septum, and without pseudoroot. | Mucor mucedo, Mucor rouxii, Mucor racemose, Mucor actinomyces, etc. | 2-octenal, 1-octene-3-ol, 2-methylphenol, etc. | Chen et al., 2023, Morin-Sardin et al., 2017. |
| Rhizopus | Colonies are loose or dense and appear initially white, then grayish brown or dark brown. Mycelium creeping, colorless. Pseudoroot developed, branching finger-like or root-like, brown | Rhizopus oryzae, Rhizopus nigra, Rhizopus agaricus, etc. | Butyric acid, hexyl acetate, geranyl acetate, etc. | Londoño-Hernández et al., 2017, Wu et al., 2022. |
| Penicillium | Nutritive mycelium is colorless, monochromatic, or bright color, with transverse septa, air mycelium is silky felt-like, loose flocculent or partially save mycelium bundle. | Penicillium chrysogenum, Penicillium citrinum, Penicillium nalgiovense, etc. | Alanine, glutamic acid, leucine, and other free amino acids, 5-methyl-heptanone, 2, 6-dimethylpyrazine, etc. | Caron et al., 2021, Lee et al., 2022. |
| Yeast | The surface of the colony is smooth, moist, sticky, and easy to stir up. The colony is uniform in texture and color. Most of the colonies are milky white, a few are red, and some are black. | Saccharomyces cerevisiae, Baker's yeast, Kazakh yeast, Candida, etc. | Ethanol, isoamyl alcohol, hexal, phenylacetaldehyde, etc | Guneser et al., 2022, Wang et al., 2022. |
Characteristics of bacteria for producing flavor compounds in traditional fermented food
Lactic acid bacteria
Lactic acid bacteria are a class of Gram-positive, non-spore-forming, non-reactive but gas-tolerant microorganisms. They are usually characterized by their ability to produce lactic acid. Lactic acid bacteria, as an indispensable fermentation bacterium, are considered a kind of probiotic. They can produce anti-antibacterial activity and antioxidant activity. Additionally, lactic acid bacteria can promote the decomposition of proteins and lipids, to produce flavor precursor substances such as free amino acids or free fatty acids, give food unique flavor, and have a certain positive impact on the overall flavor of the finished product. Therefore, Lactic acid bacteria as a starter culture are frequently utilized in fermented dairy products, fermented meat products, fermented fruit juice, fermented vegetables, etc. The main microorganisms in the lactic acid bacteria starter culture are Lactobacillus, Pediococcus, Streptococcus, Bifidobacterium, Leuconostoc, etc. Lactic acid bacteria are classified as homozygotic or heterozygotic according to their fermentation ability. Homofermentative lactic acid bacteria can produce two molecules of lactic acid from a single glucose molecule, while heterofermentative lactic acid bacteria such as Streptococcus, Lactococcus, Weissella, and Candida albicans can produce lactic acid, ethanol, and carbon dioxide from one glucose molecule (Ayivi & Ibrahim, 2022). Streptococcus thermophilus and Lactobacillus bulgaricus subspecies are typically used in starter cultures for yogurt production since they are the two most common bacterial genera in dairy fermentation (Xu, Li, Gong, Liu, Wu, & Ma, 2015). Lactobacillus, Streptococcus, and Kluyveromyces are dominant bacteria in cheese fermentation production, among which Lactobacillus contributes more to the formation of flavor (Zheng et al., 2018). According to studies, the predominant lactic acid bacteria species found in fermented fruit and vegetable juices are Lactobacillus acidophilus, Lactobacillus casei, and Lactobacillus plantarum (Quan, Liu, Zuo, & Zhang 2022). The three Lactobacillus species metabolize and produce acid in the fruit and vegetable matrix as probiotics to enhance the fruit flavor. The microbial community composition of fermented vegetables was studied and the core flora was found to be Lactobacillus plantarum and Lactobacillus weisseri, which produce organic acids that impart a soft sour taste to fermented vegetables (Peng et al., 2018). The most prevalent lactic acid bacteria strains found in fermented meat products were Lactobacillus wine, Lactobacillus curvatus, and Lactobacillus plantarum (Parlindungan, Lugli, Ventura, van Sinderen, & Mahony, 2021). Among them, Lactobacillus plantarum can significantly improve the sweetness of salted duck leg meat by producing alanine (Cai et al., 2020).
Acetic acid bacteria
Acetic acid bacteria, as a kind of Gram-negative, non-spore, aerobic bacteria, can convert the alcohol into acetic acid. They are an important industrial bacterium, widely used in the brewing industry. They are also the main strain for brewing vinegar and fruit vinegar drinks. Acetic acid bacteria are mainly divided into Acetobacter and Gluconobacter, among which the Acetic acid bacteria used for brewing is mainly Acetobacter. The common Acetobacter species are Acetobacter aceti, Acetobacter schuezenbachii, Acetobacter pasteurianus, etc. Acetic acid bacteria have strong enzyme activity. They can not only convert ethanol and glucose into acetic acid and gluconic acid, but also oxidize other alcohols and sugars to produce corresponding acids, ketones, and other substances, such as organic acids like butyric acid, gluconic acid, pyruvic, succinic acid, lactic acid, etc., as well as oxidize glycerol to diketone, oxidize mannitol to fructose. Acetic acid bacteria and Lactic acid bacteria metabolize together to create a distinctive flavor. It was found that Acetobacter, Lactobacillus, and Aspergillus were dominant microorganisms in the acetic acid fermentation process of Zhenjiang vinegar. Bacteria contribute most to the flavor of vinegar. The core bacteria group of Zhenjiang vinegar fermentation was determined to include Acetobacter, Lactobacillus, Gluconoacetate, Bacillus, and Staphylococcus (Wang, Lu, Shi, & Xu, 2016). Additionally, research has shown that Acetic acid bacteria and Lactic acid bacteria predominate in the production of Shanxi aged vinegar. The main dominant bacteria genera are Acetobacter, Lactobacillus, Bacillus, and Weissenella, which are crucial for enhancing the umami and sweetness of vinegar (Fan, Xue, Bai, Xue, Lin, & Zhang, 2022). The microflora of apple cider vinegar, apricot vinegar, mulberry vinegar, plum vinegar, and other fruit vinegar was also studied. It was found that Acetobacter pasteurianus, Acetobacter ghanensis and Acetobacter fabarum, Lactobacillus paracasei, Lactobacillus plantarum, etc. were the dominant microorganisms. Acetobacillus and Lactobacillus worked together to enhance the aroma of fruit vinegar (Sengun, Kilic, Charoenyingcharoen, Yukphan, & Yamada, 2022).
Bacillus
Bacillus is a genus of Gram-positive, spore bacteria, widely existing in the natural environment. Bacillus subtilis and Bacillus licheniformis, and Bacillus amyloliquefaciens, etc. in the genus of Bacillus are widely used in the food industry. They can secrete many proteases and lipase to degrade proteins and fats in food and produce specific bioactive peptides. Bacillus is mainly used in the soybean production and the wine industry. Some studies have found that Bacillus subtilis, Bacillus amyloliquefaciens, Bacillus circulans, Bacillus licheniformis, and Bacillus sphaericus are predominant in fermented soybean food (Gopikrishna, Kumar, Perumal, & Elangovan, 2021). In fermented soy milk, pyrazine compounds produced by the metabolism of Bacillus subtilis give soy milk a special baking flavor (Gao et al., 2020). The microbial community diversity in fermented bean sauce was studied. Lactobacillus and Bacillus were the dominant groups of fermentation. Similarly, Bacillus subtilis, Bacillus cereus, and Bacillus amyloliquefaciens are the most prevalent microbes in bean curd (Li, Li, Liu, Feng, & Liu, 2014). In addition, Bacillus including Bacillus thermophilus, Bacillus subtilis, and Bacillus licheniformis have a strong ability to hydrolyze starch and protein. They are essential genera for the generation of flavor substances in sauce-flavor liquor. Bacillus subtilis and Bacillus licheniformis cooperate with Aspergillus Niger and Saccharomyces Cerevisiae to enrich flavor system of white wine. Tetramethylpyrazine produced by Bacillus gives the white wine a nutty flavor (Wang et al., 2023).
Staphylococcus
Staphylococcus is divided into coagulase-positive and coagulase-negative based on their capacity to produce coagulase. In general, coagulase-positive staphylococcus is pathogenic and can cause diseases like pneumonia, while coagulase-negative staphylococcus is generally considered benign and can be utilized as food starter cultures. Coagulase-negative staphylococci secrete proteases and lipases that produce flavor compound through hydrolysis of proteins and lipids (Heo, Lee, & Jeong, 2020). Based on this, they can inhibit lipid autoxidation (Hui, 2022), contributing to the improvement of sensory properties such as color, aroma, and taste of food. Therefore, they are commonly used as fermenters for soy or meat products. It has been discovered that Staphylococcus amber, when grown as starter culture during the fermentation of soybeans, can produce specific volatile compounds that enhance to food flavor (Jeong, Lee, Jeong, Kim, Shim, & Lee, 2019). The study on the microbial community in bean paste showed that the dominant bacteria in bean paste fermentation are Lactobacillus, Staphylococcus, Tetrastreptococcus, Acinetobacter, etc. (Li et al., 2017). In addition, coagulase-negative Staphylococcus can be used as a starting culture for meat products. It is crucial to the biochemical reactions involving flavor and color in fermented sausages (Lopez, Callegari, Patrone, & Rebecchi, 2020). In Harbin dry intestines, a mixture of Lactobacillus and Staphylococcus xylosus and produce aldehydes, ketones, alcohols through protein and fat metabolism (Hu, Chen, Wen, Wang, Qin, & Kong, 2019). Staphylococcus and micrococcus in Cantonese sausage jointly promoted the degradation and oxidation of protein and fat, enriched aldehydes, ketones and alcohols, and significantly improved the flavor of sausage (Zhang, Jiang, Xia, Li, & Cheng, 2015).
Characteristics of fungus for producing flavor compounds in traditional fermented food
Mould
Monascus, Aspergillus Niger, Aspergillus oryzae, and other strains of Aspergillus are the main strains utilized in the manufacturing of enzyme preparations and organic acids. They are also usually used in food fermentation industry. Sauces are commonly produced with Aspergillus oryzae. Aspergillus oryzae can produce protease, amylase, glutaminase, and other enzyme systems. Protease and amylase break down proteins and starches in raw materials into small molecules of amino acids and monosaccharides. Through intricate biochemical processes, they further produce volatile compounds like alcohols, aldehydes, phenols, and esters. During soy sauce fermentation, they promote the production of the specific flavorings, such ethanol, 2-phenyl ethanol, 2-octanone, 2-furaldehyde, acetic acid, and methyl benzoate, etc. (Xu, Liu, Hu, Zhou, Wang, & Li, 2016). Studies have shown that Aspergillus oryzae, Aspergillus Niger, and Aspergillus wentii can all produce citric acid. Among them, Aspergillus Niger have a strong ability to make citric acid and usually used as the primary bacteria for the manufacture of citric acid in industries (Hou & Bao, 2018). In fermented soybean products, mucor is used. Mucor mucedo can produce lipase to convert steroids into succinic acid. Mucor rouxii is a conventionally fermented bacterium that can produce protease to break down protein into amino acids and tiny peptides and provide flavor. The most important strains used in the fermentation of tofu are Mucor racemose, Mucor actinomyces, and Mucor Indus (Wei, Regenstein, Liu, & Zhou, 2020). A typical strain used in the brewing industry is Rhizopus. The genus Rhizopus contains strains that produce fermented grains and beans, including Rhizopus oryzae, Rhizopus nigra, and Rhizopus Agaricus. Rhizopus oryzae can be used in the production of l-lactic acid. Rhizopus Nigra is the primary strain for steroid transformation. In the production of bean curd, mixed fermentation of Mucor racemose and Rhizopus oryzae increased the yield of flavorful amino acids and enriched the flavor of bean curd (Li, 2017). It was found that Mucor and Rhizopus oryzae co-cultured and fermented during the fermentation of rice wine can improve the flavor by creating esters and higher alcohols with flowery and fruity flavors. (Xiang, Xu, Zhang, Rao, Zhu, & Zhang, 2019). Penicillium is rarely used in food fermentation. Penicillium chrysogenum is the main strain producing gluconic acid or glucose oxidase. Penicillium citrinum is the predominant strain producing antibiotics and umami. Penicillium nalgiovense created new aldehydes and alcohols in fermented duck meat, improving the flavor of the final product (Sun et al., 2022).
Yeast
Yeast is a single-celled organism, rich in protein, carbohydrates, lipids, vitamins, minerals, and a range of enzyme systems and active compounds. It has a high nutritional value, which can effectively promote digestion and absorption of food. Yeast has been used for a long time in the field of food and is widely used in the production of wine, bread, condiments, and food additives (Blandino et al., 2003, Tamang and Lama, 2022). Saccharomyces cerevisiae, used to make beer, drinks, and bread, contributes to a hypoglycemic effect during the brewing process while also generating carbon dioxide and alcohol. According to the beer fermentation method, yeast is separated into top-fermenting yeast and bottom-fermenting yeast. The yeast with a higher ferment degree and not easy to agglutinate precipitation is called top yeast. The yeast with a lower ferment degree and easy-to-agglutinate precipitation is called bottom yeast. Traditional Saccharomyces cerevisiae gives the beer a strong wheaten flavor (Chen et al., 2022). Baker's yeast is widely used in the food industry as an excellent starter and nutritive agent for bread, steamed buns, biscuits, and pastries. Baker's yeast belongs to the genus Cerevisiae. It can use the nutrients in the dough to ferment, turning the fermented carbohydrates into carbon dioxide, alcohol, alcohol, esters, and other volatile compounds (Arora, Ameur, Polo, Di Cagno, Rizzello, & Gobbetti, 2021). Thanks to this, the dough become puffiness and elasticity, as well as formats its unique color, fragrance, taste, and shape. Five commercial yeasts were examined for their impacts on bread and dough flavor. It was discovered that these yeasts can produce high levels of lactic, malic, and succinic acids as well as volatile flavor compounds like aldehydes, esters, and alcohols. Fermenting with them can give the bread a wine-like flavor and a strong fruity flavor. Yeast also plays an important role in the flavor of soy sauce. Candida sorbosivorans and Starmerella etchellsii can produce 4-hydroxy-2,5-dimethyl-3(2H)-furanone, 2,6-dimethylpyrazine, 3-hydroxy-2-methyl-4 h-pyran-4-one, etc. to improve the aroma of soy sauce (Wang, Zhao, Xie, Huang, & Feng, 2022). In addition to the effect of yeast on soy sauce fermentation process, its extract is also commonly used as soy sauce flavor compensation, especially Angel Yeast has developed a series of related products (Zhou et al., 2022). Besides, yeast can also be used to metabolize rice bran to synthesize flavor substances (Guneser, Yuceer, Hosoglu, Togay, & Elibol, 2022) and remove off-flavor of Allomyrina dichotoma through fermentation (Kim, Lee, Kim, Yang, Lee, & Jung, 2021).
Characteristics of microbial interaction for producing flavor compounds in traditional fermented food
The fermentation process involves complex microbial interactions between different types of microorganisms. Microorganisms, usually bacteria and fungi, coexist and interact based on the external fermentation environment to produce flavor compounds. It occurs spontaneously or through a controlled fermentation process. Many kinds of microorganisms coexist in the complex traditional fermented food system. Their interaction between the traditional fermented food environmental system together constitutes the characteristic flavor. The biochemical environment of food will affect the growth and reproduction of microorganisms. At the same time, the nutrients consumed and metabolites produced by the growth and reproduction of microorganisms can in turn change the food environment.
The interaction of microorganisms is mainly divided into synergistic effect and antagonistic effect. Microorganisms can promote the production of more different flavor substances and enhance the expression of flavor substances through synergistic effect or inhibit the production of some harmful substances and undesirable flavors through antagonistic effect. For example, Lactobacillus and yeast can enhance the aroma of liquor through synergistic effect on pyruvate metabolism (Song, Du, Zhang, & Xu, 2017). During fermentation, they promote the conversion of ethanol into acetic acid and ethyl acetate with the pyruvate metabolism and finally adjust the flavor of liquor. In fermented dairy products, lactic acid bacteria and yeast can also interact through pyruvate metabolism in promote growth and reproduction (Adesulu-Dahunsi, Dahunsi, & Olayanju, 2020). Through this synergistic effect, the content of lactic acid, acetic acid, diacetyl, acetoin, acetaldehyde, and another flavor compounds could be enhanced during dairy fermentation (McAuliffe, Kilcawley, & Stefanovic, 2019). As to fermented meat, molds and yeasts were also had synergistic effect on the flavor. During fermemtation, Wickerhamomyces, Kazachstania, Lactobacillus, Weissella, Brochothrix, etc. coexist and produce aroma compounds such as hexanal, ethyl lactate, 3-octen-2-one, 1-octanol, etc. to format the peculiar flavor of Sun zuo rou (Wang, Su, Mu, & Zhao, 2021).
In addition to improving traditional food flavor through synergistic effect, the off odor can also be eliminated through antagonistic effect. Lactic acid bacteria can inhibit Staphylococcus aureus through antagonistic effect and decrease the content of harmful substances and off odorants. It was found that Staphylococcus and Weissiella were the dominant bacteria in the fermented soya beans (Yang, Yang, Tu, & Wang, 2016). There were antagonistic effects between these two bacteria. Weissiella can produce organic acids to inhibit the growth of Staphylococcus and the generation of ethyl acetate and ethyl enanthate. Through this action, the aroma of fermented soya beans can be improved. In milk fermentation, products fermented with L. delbrueckii are too acidic and with too heavy acetaldehyde flavor. To solve this problem, Bifidobacterium is often added to adjust the flavor of the final product (Saarela, Mogensen, Fondén, Mättö, & Mattila-Sandholm, 2000). Besides, Lactobacillus lactis, Pediococcus acidilactici, Staphylococcus equorum, Staphylococcus xylosus, Candida deformans, Penicillium nalgiovense, etc. inhibit the growth of spoilage microorganism through their metabolites or competitive exclusion, and to improve aroma, taste, and palatability during fermenting ham (Zhou et al., 2022).
Flavor substances accumulated by microbial metabolism
Food flavor is an essential indicator to assess the sensory properties of food, and an important factor of consumer acceptability. Food flavor is heavily affected by the concentration and properties of volatile and non-volatile chemicals developed during processing. Microorganisms are crucial in the development of food flavor. Via intricate microbial metabolic processes, numerous distinct compounds are produced during the manufacture of food. The interaction of different flavor compounds results in the distinctive taste and aroma of food when the concentration of these compounds reaches a certain threshold. A significant part of the process of flavor building is played by microbial metabolism and accumulation of flavor compounds, particularly through microbial enzymes, protein, and lipid breakdown. Proteins are broken down by proteases into short peptides and free amino acids, which are later transformed into aromatic compounds. Lipids are hydrolyzed into free fatty acids by lipase. Short-chain fatty acids form cheesy flavors and long-chain fatty acids are precursors to flavors that can be further degraded into hydrocarbons, alcohols, aldehydes, ketones, and aromatic compounds. With the action of corresponding enzymes, polysaccharides are decomposed into small molecules through a glycometabolic pathway. The were further degraded into various flavor compounds. Fig. 2 illustrates the primary classifications of flavor compounds produced by microbial metabolism, including alcohols, aldehydes, esters, acids, ketones, hydrocarbons, aromatic compounds, sulfur, nitrogen compounds, etc.
Fig. 2.
Species of flavor compounds accumulated by microbial metabolism.
Alcohol compounds are volatile compounds that can be detected in most foods. In general, alcohol compounds have a low threshold value, which significantly aids in the formation of the unique flavors of food. Alcohols are primarily produced through the oxidation and breakdown of polyunsaturated fatty acids. Alcohol compounds in liquor are mainly advanced alcohols such as n-propanol, isobutanol, isoamyl alcohol, etc. Kazakh yeast is mainly closely related to the accumulation of alcohol, which converts sugar, acetic acid, and esters into ethanol. Alcohol can also enhance the sweetness and mellowness of liquor (Dong et al., 2022). The flavor of fish products is significantly influenced by alcohols as well. Linalool, the main volatile compound in Mandarin fish, is positively correlated with the metabolic accumulation of Hydrocephsalus. The most significant alcohol component in the majority of fermented fish products is 1-octen-3-ol. 1-Octen-3-ol is an essential flavoring ingredient detected in naturally fermented tilapia (Zhao et al., 2021), which has a mushroom odor and metallic odor. 1-Octen-3-ol is produced by Acinetobacter and Bacillus thuringiensis in fish through the autooxidation of unsaturated fatty acids like linoleic acid (Shen et al., 2021).
Aldehydes usually have a low threshold in foods and contribute to the overall flavor. Aldehydes are mostly formed by the oxidation and breakdown of lipids. Short-chain aldehydes can also be generated by the Strecker or microbial metabolism. Aldehydes often have pleasant flavors, such as malty, fruit, and cheese (Zhao et al., 2021). 1-Nonanal is the main flavor aldehyde detected in traditional dry sausages in northeast China. 1-Nonanal and hexanal produced by Lactococcus lactis promote lipid autooxidation and are generally considered the main flavor source of processed meat products, providing the aroma of fat and citrus (Hu, Zhang, Liu, Wang, Chen, & Kong, 2020). The main aldehydes in traditionally fermented fish identified are hexanal, nonanal, and capraldehyde. Hexanal and capraldehyde are mostly produced by Acinetobacter and Bacillus thuringiensis. These aldehydes impart herbaceous, grassy, and pungent scents that helped to shape the overall flavor (Shen et al., 2021). Vanillin, a flavor aldehyde compound, is extensively applied in the food industry as a spice. It can be produced through biosynthesis using various carbon sources by Bacillus, Aspergillus Niger, and Yeast (Ma et al., 2022).
Ester compounds are common volatile substances in food and important compounds to promote the food flavor. The production of ester compounds generally involves the non-enzymatic esterification of alcohols and organic acids as well as the enzymatic catalysis of microorganisms, which can impart a fruity flavor. The content of ester in wine is relatively high. Ethyl lactate and ethyl caproate are the main esters in fermented liquor. Lactic acid bacteria produce lactic acid and caproic acid. They esterify with ethanol to produce ethyl lactate and ethyl caproate under the action of acetobacter (Dong et al., 2022). The flavor of dairy products is significantly influenced by ester compounds. Ester components in fermented dairy products like ethyl acetate, ethyl hexanoate, and octyl acetate are directly linked to the metabolism of Lactobacillus, Acetobacter, and Leuconostoc (Walsh et al., 2016). The fermented sour meat was also found to contain a few ester compounds, including ethyl lactate, ethyl butyrate, ethyl caproate, ethyl oenanthate, ethyl caprylate, and ethyl caprate. These esters usually impart sour meat a fruit and cream aroma. The synthesis of these esters is mainly closely related to Lactobacillus, Staphylococcus, and Candida (Zhong et al., 2021).
Acid compounds, which are commonly found in fermented wine, can give food an acidic flavor. They are crucial to the quality and flavor of the wine. Propionic acid, butyric acid, valeric acid, caproic acid, lactic acid, malic acid, citric acid, succinic acid, lauric acid, pyruvate, and others are common organic acid in wine in general. The main acids involved in the fermented liquor are lactic acid, butyric acid, and caproic acid. Lactobacillus can produce lactic acid and acetic acid, while Acetobacter can oxidize ethanol to acetic acid. The content of these acid come from Lactobacillus and Acetobacter is related to the brewing process (Dong et al., 2022). Industrial production of some acidulants is also possible using microbial synthesis. The Aspergillus Niger is widely used in the industrial synthesis of citric acid. Through active glycolytic pathways, Aspergillus Niger has the potential to massive accumulation citric acid (Tong, Zheng, Tong, Shi, & Sun, 2019). Filamentous fungi like Aspergillus Niger, Rhizopus oryzae, Saccharomyces cerevisiae, etc. can generate malic acid. These microorganisms biosynthesize malic acid through the tricarboxylic acid cycle or glyoxylic acid cycle (Wu et al., 2022). Citric acid and malic acid are frequently utilized as acidulants in foods and beverages because of their pleasant flavor.
The primary approaches that generate ketones are the degradation of amino acids or the oxidation of unsaturated fatty acids. Ketones are a crucial flavor ingredient in dry-cured meat products. High concentrations of ketones often present creamy, fruity, and spicy flavors (Zhu, Zhao, Tian, Liu, Li, & Zhao, 2018). Studies have found that Pseudomonas and Mucor produce the 2,3-octanedione, the primary ketone in dried salted ducks, which has a distinctive fruity aroma (Li et al., 2022). Additionally, it was discovered by comparing Jinhua fat ham with lean ham that the fat ham had higher levels of 2-butanone and acetone ketones, which were mostly connected to Staphylococcus and Psychrobacter. Acetone also gave meat products a buttery flavor and a fruity scent (Zhang et al., 2023). Studies have found that ethyl ketone and 2,3-pentanedione in fermented carp have fruit flavor (Zang, Yu, Li, Xu, Regenstein, & Xia, 2022). Ketones in fish sauce also have fruit and cheese flavor, while 2, 5- octandione is related to fishlike odor (Han et al., 2023).
The basic types of hydrocarbons are alkanes, alkenes, terpenoids, etc. Due to their relatively high odor threshold, hydrocarbons are not usually considered as aroma contributions. However, alkanes can help enhance the flavor of food. The effect on food flavor is small. Hydrocarbons in food are usually naturally occurring. Terpenoids are produced during metabolism also called isoprene. Numerous terpenoids, including decene, myrcene, limonene, linalool, and geraniol, are volatile ingredients in food and have distinct orange, incense, and lemon flavors. Terpenoids can be produced by the biosynthesis of Escherichia coli and Yeast (Wang et al., 2018). The breakdown of aromatic amino acids results in the generation of aromatic molecules, while phenolic compounds are the dominant aromatic compounds and usually produce a particular aromatic odor. Microorganisms increase the accumulation of phenolic compounds by inducing the metabolic decomposition of certain aromatic amino acids such as tryptophan, phenylalanine, and tyrosine.
Sulfur-nitrogenous compounds exist in some foods, which are usually generated by the catabolism of sulfur-nitrogenous amino acids. These flavor compounds are relatively tiny and have a low threshold for flavor. However, they significantly affect the flavor of food. The principal sulfur flavoring ingredients in fermented dairy products include methyl mercaptan, dimethyl sulfide, and other sulfur-containing compounds. Sulfur-containing compounds, such as diallyl disulfide, mercaptan, and dimethyl disulfide, are found in trace amounts in fermented vegetables. It was found that sulfur compounds include hydrogen sulfide, 2-methyl-3-furan mercaptan, etc. were the main flavor compounds in Spanish ham (Carrapiso, Martin-Cabello, Torrado-Serrano, & Martin, 2015). In addition, nitrogen compounds such as trimethylamine and indole were found in fermented fish, presenting ammonia and fishy flavor. Under anaerobic conditions, some bacteria, including Salmonella, Shewanella, and Vibrio, can convert trimethylamine N-Oxide oxide to trimethylamine. Indoles are metabolized by Fusobacterium, which is helpful in the flavor development of fermented meat products (Shen et al., 2021).
Mechanisms of microbial metabolism to accumulation of flavor substances
The flavor is crucial for the food senses. The primary mechanisms of microbial metabolism to accumulate flavor substance are amino acid metabolism, fatty acid metabolism, carbohydrate metabolism, nucleotide metabolism, and other metabolic pathways. Protein and fat hydrolysis releases free amino acids and free fatty acids, which are the precursors of flavoring compounds. In the food system, protein degradation, fat oxidation, glycolysis, and other reactions occur through the action of microorganisms and enzymes to produce primary metabolites like amino acids, short-chain peptides, fatty acids, nucleotides. These primary metabolites can be utilized by microorganisms to develop and generate secondary metabolites, which can then be broken down further to produce volatile compounds.
Amino acid metabolism is crucial for flavor development. Alkaline protease is mostly secreted by Bacillus subtilis. The serine protease it produces is stable. Acid protease produced by Mucor, Rhizopus, Aspergillus oryzae, and other Molds has high activity (Han et al., 2020). These microbial proteases interact with endogenous enzymes of the food itself to degrade proteins into free amino acids and small molecular peptides, which are crucial precursors of flavor compounds. Through ammonia conversion, decarboxylation, and dehydrogenation processes, these amino acids are converted into certain aromatic compounds, such as aldehydes, ketones, alcohols, acids, indoles, phenols, etc. (Wei, Chitrakar, Regenstein, Sang, & Zhou, 2023). Amino acids are metabolized by microorganisms in two ways in general (Feng et al., 2021). The first is the destruction of amino acid side chain under the catalysis of amino acid lyase and decompose the amino acid into phenol, indole, and methyl mercaptan. This pathway is present in Yeast, Micrococcus, and Brevibacterium. It is mainly involved in the metabolism of aromatic amino acids like tyrosine, tryptophan, and methionine. The second is microbial metabolism through transamination reaction to form flavor, which is also the primary method of amino acid metabolism. Through amino transferase, α-ketoic acid is ammoniated, and then degraded in the metabolism process of amino acids to generate aldehydes, ketones, alcohols, carboxylic acids, and others. Isoleucine, leucine, and valine, three branched amino acids, can be metabolized to crease specific flavor compounds, such as 3-methyl-butyral and 2-methyl-butyral. 3-methyl-butyral has the fruity flavor while 2-methyl-butyral has the malty flavor. The amino acid metabolism pathway is an important flavor source of cheese, fermented wine, and fermented sausage. It was discovered that Wickerhamomyces and Clostridium were the predominant bacteria involved in the metabolism of amino acids in fermented pomfret. These bacteria can produce hexanal, 3-methyl-butyral, benzaldehyde, 2-butanone, and acetone by participating in the metabolism of glycine, glutamic acid, alanine, leucine, and other amino acids (Qiu et al., 2022).
Fatty acid metabolism is also crucial for food flavor. Oxidation of lipids is the principal way to produce volatile flavor compounds in the fermented meat products. Fatty acid metabolism is an important metabolic pathway. Lipids are solvents of many aromatic components, which can affect aroma through a series of biochemical reactions and maintain a relatively stable texture of food (Anast, Dzieciol, Schultz, Wagner, Mann, & Schmitz-Esser, 2019). The degradation of lipids mainly involves endogenous enzymes produced by the food system and lipases produced by microorganisms, which catalyze autooxidation and enzymatic oxidation. Bacillus, Pseudomonas, Candida, and other bacteria and yeast are the preferred microorganisms to produce lipase. When lipid is hydrolyzed by lipase, small molecules of free fatty acids are formed. Through β-oxidation, these molecules can further generate flavor compounds including fatty aldehydes, ketones, alcohols, and more. The lipid oxidation pathway is a complicated free radical chain reaction that produces hydroperoxides with the participation of oxygen molecules. Free fatty acids are produced because of the instability of hydroperoxides and go on to form hydrocarbons, alcohols, aldehydes, ketones, acids, and other chemicals via various pathways. It has been found that Staphylococcus equine or Staphylococcus xylose in sour meat with low salt can degrade fatty acids to produce linoleic acid, octadecenoic acid, palmitic acid, and other fatty acids, which are further oxidized and degraded to produce linear aldehydes such as nonanal and 2-heptanal. Linear aldehydes give fermented sour meat its unique cheesy, fruity, and sweet flavor (Wang et al., 2022).
Carbohydrate metabolism is also important in the development of food flavor. The tricarboxylic acid cycle, glycolysis, pentose phosphate pathway, pyruvate pathway, and citric acid cycle are the major metabolic pathways for carbohydrates. In general, lactic acid can be generated by microbial fermentation of carbohydrates, which causes the pH of food to drop, inhibits the reproduction of spoilage bacteria and pathogenic bacteria, and gives food a sour flavor. The microbial carbohydrate metabolism generates pyruvate, a crucial flavor intermediate, primarily by the action of phosphofructokinase, hexokinase, and pyruvate kinase. Pyruvate can be converted into flavor compounds through the tricarboxylic acid cycle, citric acid cycle, and other metabolic pathways. Carbohydrate metabolism of horse milk wine mainly includes glycometabolism, glycolysis and gluconeogenesis. Lactic acid bacteria produce lactic acid, free amino acids, organic acids, and extracellular polysaccharides through carbohydrate metabolism, which are crucial for the acidity, taste, aroma, and texture of horse milk wine (Xia, Yu, Liu, Feng, & Shuang, 2021). Most of the microorganisms in the fish sauce are involved in the metabolism of carbohydrates. Pseudomonas, Bacillus, Staphylococcus, and Tetragenococcus in fermented fish sauce participate in carbohydrate metabolism and produce a variety of volatile acids, including acetic acid, propionic acid, butyric acid, and 4-methyl valeric acid (Long, Wang, Wu, Qi, Zhang, & Wang, 2017).
Nucleotides and their derivatives are essential flavor substances that can impart food an umami flavor. The overall flavor of food is improved in a significant way by nucleotide metabolism. AMP, GAP, and IMP can express obvious umami at lower concentrations. AMP is helpful in the expression of sweet, umami, and complex flavors at low concentrations. GMP and IMP are flavor enhancers, usually expressing umami and thick flavor (Gao, Liu, Li, Liu, Zhou, & Yuan, 2023). 5′-Nucleotide is an important taste substance produced by the degradation of adenosine triphosphate (ATP) and free fatty acids. The synergistic interaction of 5′-nucleotide and amino acids considerably enhances the umami flavor of fish products (Zhao, Hu, & Chen, 2022). The expression of flavor nucleotides such as IMP, GMP, and AMP is directly correlated with metabolic activity of microorganisms. Microorganisms degrade nucleic acids by nuclease (phosphodiesterase) to form nucleotides, which can synergize with amino acids such as aspartic acid and glutamic acid to enhance umami flavor. Additionally, these nucleotides are also participated in the Maillard reaction, which helps to produce more flavorful chemicals. Lactobacillus, Staphylococcus, and other microbes are utilized in the fermentation of sour meat with low salt to break down IMP and produce hypoxanthine, which helps to produce the bitter flavor (Wang et al., 2022).
Regulation of microbial metabolism to accumulation of flavor substances
The complicated biochemical reactions that make up a microbial metabolic pathway are almost all enzymatic reactions that are catalyzed by enzymes. Controlling the activity of enzymes is a key component in regulating microbial metabolism. There are two strategies to control microbial metabolism. One is to regulate enzyme activity, and the other is to regulate enzyme synthesis. Enzyme activity regulation and enzyme synthesis regulation coexist in a normal metabolic pathway and collaborate to complete the regulation of microbial metabolism. Microbial enzyme activity regulation is to change the catalytic activity of enzyme molecules through intermediate metabolites or final products to control the metabolic rate. This regulation can prevent the excessive synthesis of some metabolites and insufficient synthesis of other metabolites in cells. The regulation of enzyme activity can be divided into activation and inhibition. Microorganisms can quickly adapt to the mutation of the metabolic environment by regulating enzyme activity. The regulation of enzyme synthesis is that regulates metabolic rate by regulating the amount of enzyme synthesis. The regulation of enzyme synthesis is also divided into the feedback repression of metabolic end products inhibiting enzyme synthesis and the induction of metabolic end products promoting enzyme synthesis. This regulation can save raw materials and energy for biosynthesis by preventing excessive enzyme synthesis. Taking microbial synthesis of malic acid as an example, filamentous fungi and some yeast can create a large amount of malic acid through the natural synthetic pathway that the reductive tricarboxylic acid pathway (Wei, Xu, Xu, Cao, Huang, & Liu, 2021). Usually, pyruvate carboxylase in Saccharomyces cerevisiae and filamentous fungi carboxylates pyruvate into oxaloacetic acid, which is further reduced to malic acid. The reductive TCA pathway was redesigned to enhance the malic acid yield in filamentous fungi and yeast, as well as by enhancing the overexpression of malate transporter. In addition, increasing precursors like oxaloacetic acid is also crucial to further increase the yield of malic acid. The introduction of oxaloacetic acid without heteromerization blocks the polymerization of oxalic acid and citric acid, which finally increases the production of malic acid. Therefore, the most popular and efficient strategy for increasing malic acid yield in yeast and filamentous fungi is synthesizing the reductive TCA pathway and malic acid transport.
Microbiotas have an extremely fine metabolic regulation system. It is necessary to break the original metabolic balance of microbiota to make microbiota metabolism in the desired direction and produce more expected metabolic substances. Therefore, the central idea of microbial metabolism regulation is to break the metabolic regulation mechanism of microorganisms themselves, to enable them to accumulate many certain metabolites and eliminate the accumulation of by-products. There are three potential strategies to eliminate by-products, namely deleting or reducing the competitive pathways in the biosynthesis or transportation, discovering key enzymes that are more efficient in metabolism, and optimizing the fermentation process parameters. Escherichia coli generally cannot accumulate malic acid. However, it is essential to stop the synthesis of significant amounts of byproducts like acetate and lactate during glucose fermentation, which are derived from pyruvate, a key intermediate in the synthesis of malic acid. As a result, inhibiting pyruvate secretion is an effective way to decrease byproduct biosynthesis and increase malic acid yield. To eliminate the genes encoding the essential enzymes of the competitive route, E. coli was genetically altered. Succinic acid was eliminated when fumarate reductase was inactivated. This results in pyruvate increasing and acetic acid decreasing. Pyruvate production was virtually abolished and malic acid production increased after the malase gene was deleted (Wei, Xu, Xu, Cao, Huang, & Liu, 2021). Malic acid, citric acid, and other organic acids can all be produced by Aspergillus Niger which is a significant strain in the synthesis of organic acids. The biosynthesis of citrate in Aspergillus Niger is promoted in the production of citric acid. The biosynthetic citric acid synthetase is polymerizing with the transporter, which can enhance the yield of citric acid after overexpression (Hossain, Hendrikx, & Punt, 2019). Contrarily, citric acid is the primary by-product when Aspergillus Niger is used to create malic acid. Decreasing the synthesis of citric acid is the key to enhancing malic acid. Studies have found that the destruction of citric acid transporters can eliminate the accumulation of citric acid (Xu, Zhou, Cao, & Liu, 2020).
Application
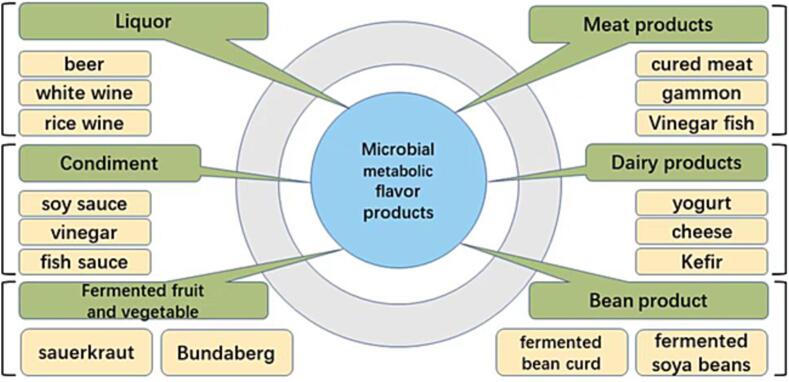
Microorganisms have been used in many foods to produce flavors and aromas. Extracting flavor ingredients produced by microorganisms can be used as food additives to improve the flavor of foods. Lactones, for example, have a wide range of flavor components, making dairy products present rich flavors including butter, coconut, cream, fruit, nut, and more. Decadelactone is produced from castor oil by microorganisms such as Aspergillus Niger, Purpureocillium, and Pichia pastoris, which has a strong creamy flavor and can be used as a dairy and fruit essence (Kumar Verma et al., 2022). Octonolactone is produced by Mortiform fermentation in coconut oil and has a coconut aroma, which can be used as essence for dessert production. Butanedione produced by the metabolism of Lactococcus lactis has a buttery aroma (Liu, Chen, Jensen, & Solem, 2021). The classification of products that use microbial metabolism to produce flavor is shown in Fig. 3. The potential connection between the formation of flavor chemicals and microbial metabolism can be determined. Main flavor-forming microorganisms can be isolated and screened out as excellent starter cultures for use in food fermentation. Functional starter cultures are developed according to different needs, which enhance the security and flavor of fermented food.
Fig. 3.
Classification diagram of microbial metabolism flavor products.
The application in traditional beverages
The production of wine is inseparable from microorganisms. The unique flavor of wine is produced by the fermentation of different microorganisms. Based on the identification and screening results of fermentation microorganisms, the wine flavor can be controlled by adding certain kinds of microorganisms. The lactic acid bacteria isolates (Celik, Con, Saygin, Sahin, & Temiz, 2021) screened from traditional yogurt were identified at the molecular level, and the flavor characteristics of 5 of them were better than commercial lactic acid bacteria, suggesting that they could be applied as starter cultures for fermented dairy products like yogurt. Mixed starter cultures were applied to rice wine. The results showed that the rice wine brewed by inoculating the starter cultures of yellow rot bacteria, black rot bacteria, small brown rot bacteria, and Saccharomyces cerevisiae at the same time had better flavor and fermentation performance, which was beneficial to produce high-quality rice wine (Liu et al., 2019). Traditional acetic acid fermentation uses mature vinegar cultures as starter cultures, and the quality and yield of the vinegar produced are not uniform.
The application in traditional sauce
The production and processing of soy sauce, vinegar, fish sauce etc. are inseparable from biological fermentation. In the process of sauce fermentation, the diversity of microbial flora and the complex metabolic reactions of microorganisms are accompanied by the formation of flavor substances. During soy sauce fermentation, T. halophilus and Z.rouxii were usually used to produce various aroma compounds, such as acetic acid, formic acid, benzaldehyde, methyl acetate, ethyl 2-hydroxypropanoate, etc. via lactic acid and alcoholic pathway (Devanthi & Gkatzionis, 2019). At present, it is a common method to improve the production of flavor substances in the fermentation process of fish sauce by digging the dominant bacteria and adding the dominant bacteria in the fermentation process. In low-salt fish sauce, Tetragenococcus muriaticus was found that it could improve the volatile flavor formation of the fish sauce (Li, Li, Li, Chen, Wu, & Qi, 2022).
The application in traditional dairy products
Fermented dairy products were usually fermented with cow, sheep, and yak milk by Debaryomyces, Lactobacillus, Issatchenkia, Streptococcus, Kluyveromyces, etc. Fermented dairy products are already common in daily life, such as yoghurt, cheese, fermented yak milk, etc (Hu, Zhang, Wen, Chen, & Kong, 2022). Some studies (Dan, Hu, Li, Dai, He, & Wang, 2022) used different Lactobacillus bulgaricus and Streptococcus thermophilus with good fermentation characteristics to mix fermented dairy products with different combination strains. By detecting volatile flavor substances from fermented dairy products, the mixed starter conducive to fermented dairy products flavor was screened, and the quality of fermented dairy products was controlled, which would promote to develop of innovative fermented dairy products.
The application in traditional bean products
Fermented soybean curd is one of famous traditional food in China with highly flavor. Bacillus, Lactobacillus, Stenotrophomonas, Sphingobacterium, Fusarium, Candida, etc. were the main microorganisms in fermenting soybean curd and producing flavor substance (He & Chung, 2020). During fermentation, Lactococcus and Acinetobacter could be used to produce esters and acids, while Enterobacteriaceae and Enterococcus could increase the content of amino acids and sugars that contribute to taste (Huang, Yu, Han, & Chen, 2018). Streptococcus were also found that it could do for the aroma compounds of fermented soybean curd such as maltol, linoleic acid ethyl ester, 2-methoxy-4-vinylphenol etc. (Liang et al., 2019).
The application in traditional meat products
Fermented meat products are a traditional food deeply loved by consumers all over the world. The use of starter cultures is already common in fermented meat products. Typical strains used for meat fermentation include lactic acid bacterial species, micrococci, and staphylococci (Ashaolu, Khalifa, Mesak, Lorenzo, & Farag, 2023). As to Jinhua dry-cured ham one of the famous fermented meat products, staphylococci could contribute to its flavor by producing aldehyde compounds, such as hexanal, nonanal, benzaldehyde, etc. (Wang et al., 2021). In Suan zuo rou another traditional fermented meat products, Wickerhamomyces, Kazachstania, Lactobacillus, Weissella, Brochothrix, Debaryomyces, Staphylococcus, Pediococcus, Pichia, Candida, Leuconostoc, and Torulaspora were the main microorganisms doing for the flavor (Wang, Su, Mu, & Zhao, 2021). They are correlated with ethyl acetate, 2-ethylhexanol, isobutyric acid, ethanol, hexyl alcohol, and 2-methyl-1-propanol, etc.
The application in traditional vegetable products
Fermented vegetable products are famous in China such as Sichuan paocai, Jiangxi yancai, Dongbei suancai, etc. Lactobacillus, Pediococcus, Leuconostoc, Weissella, Tetragenococcus, Lactococcus, etc. were regarded as the dominant genera microorganisms responsible for the flavor of fermented vegetable products (He et al., 2020, Xiao et al., 2020). Among them, during fermenting Sichuan paocai, Lactobacillus could produce flavor substances such as lactic acid, formic acid, 3-mercaptohexyl butanoate, etc. while Leuconostoc is correlated with diamyl sulfite, 2,4-dimethylthiazole lavandulyl acetate, etc. Besides, Pediococcus and Lactococcus were associated with 2,4-di-tert-butylphenol, 1-hexanol, etc. when making Dongbei suancai.
Conclusion
The flavor of traditional fermented foods is directly related to microorganisms. The microbial floras contribute to the flavor of traditional fermented foods are mainly Lactic acid bacteria, Acetic acid bacteria, Bacillus, Staphylococcus, Aspergillus, Saccharomyces, etc. The main flavor substances include alcohols, aldehydes, esters, acids, ketones, hydrocarbons, aromatic compounds, sulfur-nitrogenous compounds, among which alcohols, esters, acids. The dominant microorganisms produce special flavor substances mainly through amino acid metabolism, fatty acid metabolism, carbohydrate metabolism, nucleotide metabolism, and other metabolic pathways. In addition, the regulation of microbial metabolic accumulation of flavor substances can be achieved by regulating metabolic key enzymes. It is necessary to break the metabolic regulation mechanism of microorganisms themselves, eliminate the accumulation of by-products, and realize the efficient control of certain flavor products for improving the quality of traditional fermented foods. At present, the application of microbial metabolism accumulation of flavor substances is mainly concentrated on the use microorganisms to produce the essence, screening of excellent starter, and culture of functional starter. Therefore, detailed research and summary of microbial flora structure and succession rule, flavor formation mechanism, and regulation can better develop flavored food and have extremely important significance in promoting the development of food industrialization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (32202084), the Basic Scientific Research Project of Colleges and Universities of Liaoning Provincial Department of Education (LJKMZ20220881), the Natural Science Foundation of Liaoning Province (2021-BS-228).
Data availability
No data was used for the research described in the article.
References
- Adesulu-Dahunsi A.T., Dahunsi S.O., Olayanju A. Synergistic microbial interactions between lactic acid bacteria and yeasts during production of nigerian indigenous fermented foods and beverages. Food Control. 2020;110 [Google Scholar]
- Ai C., Tong A., Wen J., Chen R., Huang Y., Zhao C. Variations in the substrate composition and microbial community structure in the anaerobic fermentation process using the green algae enteromorpha prolifera. Food Production, Processing and Nutrition. 2022;4(1):32. [Google Scholar]
- Anast J.M., Dzieciol M., Schultz D.L., Wagner M., Mann E., Schmitz-Esser S. Brevibacterium from austrian hard cheese harbor a putative histamine catabolism pathway and a plasmid for adaptation to the cheese environment. Scientific Reports. 2019;9:12. doi: 10.1038/s41598-019-42525-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora K., Ameur H., Polo A., Di Cagno R., Rizzello C.G., Gobbetti M. Thirty years of knowledge on sourdough fermentation: A systematic review. Trends in Food Science & Technology. 2021;108:71–83. [Google Scholar]
- Ashaolu T.J., Khalifa I., Mesak M.A., Lorenzo J.M., Farag M.A. A comprehensive review of the role of microorganisms on texture change, flavor and biogenic amines formation in fermented meat with their action mechanisms and safety. Critical Reviews in Food Science and Nutrition. 2023;63(19):3538–3555. doi: 10.1080/10408398.2021.1929059. [DOI] [PubMed] [Google Scholar]
- Ayivi R.D., Ibrahim S.A. Lactic acid bacteria: An essential probiotic and starter culture for the production of yoghurt. International Journal of Food Science & Technology. 2022;57(11):7008–7025. [Google Scholar]
- Bartowsky E.J., Pretorius I.S. In: Biology of microorganisms on grapes, in must and in wine. König H., Unden G., Fröhlich J., editors. Springer, Berlin Heidelberg; Berlin, Heidelberg: 2009. Microbial formation and modification of flavor and off-flavor compounds in wine; pp. 209–231. [Google Scholar]
- Bhan C., Singh J. Role of microbial lipases in transesterification process for biodiesel production. Environmental Sustainability. 2020;3(3):257–266. [Google Scholar]
- Blandino A., Al-Aseeri M.E., Pandiella S.S., Cantero D., Webb C. Cereal-based fermented foods and beverages. Food Research International. 2003;36(6):527–543. [Google Scholar]
- Cai Z., Ruan Y., He J., Dang Y., Cao J., Sun Y.…Tian H. Effects of microbial fermentation on the flavor of cured duck legs. Poult Sci. 2020;99(9):4642–4652. doi: 10.1016/j.psj.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron T., Piver M.L., Péron A.-C., Lieben P., Lavigne R., Brunel S.…Chassard C. Strong effect of Penicillium roqueforti populations on volatile and metabolic compounds responsible for aromas, flavor and texture in blue cheeses. International Journal of Food Microbiology. 2021;354 doi: 10.1016/j.ijfoodmicro.2021.109174. [DOI] [PubMed] [Google Scholar]
- Carrapiso A.I., Martin-Cabello L., Torrado-Serrano C., Martin L. Sensory characteristics and consumer preference of smoked dry-cured iberian salchichon. International Journal of Food Properties. 2015;18(9):1964–1972. [Google Scholar]
- Celik O.F., Con A.H., Saygin H., Sahin N., Temiz H. Isolation and identification of Lactobacilli from traditional yogurts as potential starter cultures. LWT-Food Science and Technology. 2021;148(8) [Google Scholar]
- Chen Y., Yang Y., Cai W., Zeng J., Liu N., Wan Y., Fu G. Research progress of anti-environmental factor stress mechanism and anti-stress tolerance way of Saccharomyces cerevisiae during the brewing process. Critical Reviews in Food Science and Nutrition. 2022:1–16. doi: 10.1080/10408398.2022.2101090. [DOI] [PubMed] [Google Scholar]
- Chen Z., Song J., Ren L., Wang H., Zhang Y., Suo H. Effect of the succession of the microbial community on physicochemical properties and flavor compounds of mucor wutungkiao-fermented sufu. Food Bioscience. 2023;51 [Google Scholar]
- Contesini F.J., Melo R.R.D., Sato H.H. An overview of bacillus proteases: From production to application. Critical Reviews in Biotechnology. 2018;38(3):321–334. doi: 10.1080/07388551.2017.1354354. [DOI] [PubMed] [Google Scholar]
- Zhang D., Jiang A., Xia L., Li J., Cheng W. Effect of adding staphylococcus and micrococcus on quality of cantonese sausage. Science and Technology of Food Industry. 2015;36(11):176–180. [Google Scholar]
- Dan T., Hu H.M., Li T., Dai A.N., He B.B., Wang Y.A. Screening of mixed-species starter cultures for increasing flavour during fermentation of milk. International Dairy Journal. 2022;135 [Google Scholar]
- Devanthi P.V.P., Gkatzionis K. Soy sauce fermentation: Microorganisms, aroma formation, and process modification. Food Research International. 2019;120:364–374. doi: 10.1016/j.foodres.2019.03.010. [DOI] [PubMed] [Google Scholar]
- Dong W.W., Zeng Y.T., Cui Y.X., Chen P., Cai K.Y., Guo T.T.…Zhao S.M. Unraveling the composition and succession of microbial community and its relationship to flavor substances during xin-flavor baijiu brewing. International Journal of Food Microbiology. 2022;372:11. doi: 10.1016/j.ijfoodmicro.2022.109679. [DOI] [PubMed] [Google Scholar]
- Feng L., Tang N.I., Liu R.J., Gong M.Y., Wang Z.T., Guo Y.W.…Chang M. The relationship between flavor formation, lipid metabolism, and microorganisms in fermented fish products. Food & Function. 2021;12(13):5685–5702. doi: 10.1039/d1fo00692d. [DOI] [PubMed] [Google Scholar]
- Gao R.C., Liu H.J., Li Y., Liu H.Y., Zhou Y., Yuan L. Correlation between dominant bacterial community and non-volatile organic compounds during the fermentation of shrimp sauces. Food Science and Human Wellness. 2023;12(1):233–241. [Google Scholar]
- Gao Y., Ni N., Xu B., Fan H., Zhang M., Lu C.…Li S. Exploration on the peculiar flavor of fermented soymilk by bacillus subtilis bsnk-5. Food and Fermentation Industries. 2020;46(15):258–264. [Google Scholar]
- Gopikrishna T., Kumar H.K.S., Perumal K., Elangovan E. Impact of bacillus in fermented soybean foods on human health. Annals of Microbiology. 2021;71(1):1–16. doi: 10.1186/s13213-021-01641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guneser O., Yuceer Y.K., Hosoglu M.I., Togay S.O., Elibol M. Production of flavor compounds from rice bran by yeasts metabolisms of Kluyveromyces marxianus and Debaryomyces hansenii. Brazilian Journal of Microbiology. 2022;53(3):1533–1547. doi: 10.1007/s42770-022-00766-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R., Gupta N. Springer; Singapore: 2021. Fundamentals of bacterial physiology and metabolism. [Google Scholar]
- Gupta R., Gupta N., Rathi P. Bacterial lipases: An overview of production, purification and biochemical properties. Applied Microbiology and Biotechnology. 2004;64(6):763–781. doi: 10.1007/s00253-004-1568-8. [DOI] [PubMed] [Google Scholar]
- Gupta R., Kumari A., Syal P., Singh Y. Molecular and functional diversity of yeast and fungal lipases: Their role in biotechnology and cellular physiology. Progress in Lipid Research. 2015;57:40–54. doi: 10.1016/j.plipres.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Han J., Kong T., Jiang J., Zhao X., Zhao X., Li P., Gu Q. Characteristic flavor metabolic network of fish sauce microbiota with different fermentation processes based on metagenomics. Frontiers in Nutrition. 2023;10 doi: 10.3389/fnut.2023.1121310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Kong T., Wang Q., Jiang J., Zhou Q., Li P.…Gu Q. Regulation of microbial metabolism on the formation of characteristic flavor and quality formation in the traditional fish sauce during fermentation: A review. Critical Reviews in Food Science and Nutrition. 2022:1–20. doi: 10.1080/10408398.2022.2047884. [DOI] [PubMed] [Google Scholar]
- He W., Chung H.Y. Exploring core functional microbiota related with flavor compounds involved in the fermentation of a natural fermented plain sufu (chinese fermented soybean curd) Food Microbiology. 2020;90 doi: 10.1016/j.fm.2019.103408. [DOI] [PubMed] [Google Scholar]
- He Z., Chen H., Wang X., Lin X., Ji C., Li S., Liang H. Effects of different temperatures on bacterial diversity and volatile flavor compounds during the fermentation of suancai, a traditional fermented vegetable food from northeastern china. LWT-Food Science and Technology. 2020;118 [Google Scholar]
- Heo S., Lee J.H., Jeong D. Food-derived coagulase-negative Staphylococcus as starter cultures for fermented foods. Food Science and Biotechnology. 2020;29:1023–1035. doi: 10.1007/s10068-020-00789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horecker B.L. The pentose phosphate pathway. Journal of Biological Chemistry. 2002;277(50):47965–47971. doi: 10.1074/jbc.X200007200. [DOI] [PubMed] [Google Scholar]
- Hossain A.H., Hendrikx A., Punt P.J. Identification of novel citramalate biosynthesis pathways in Aspergillus niger. Fungal Biology and Biotechnology. 2019;6(1):1–13. doi: 10.1186/s40694-019-0084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou W.L., Bao J. Simultaneous saccharification and aerobic fermentation of high titer cellulosic citric acid by filamentous fungus Aspergillus niger. Bioresource Technology. 2018;253:72–78. doi: 10.1016/j.biortech.2018.01.011. [DOI] [PubMed] [Google Scholar]
- Hu Y., Chen Q., Wen R., Wang Y., Qin L., Kong B. Quality characteristics and flavor profile of harbin dry sausages inoculated with lactic acid bacteria and Staphylococcus xylosus. LWT-Food Science and Technology. 2019;114 [Google Scholar]
- Hu Y., Zhang L., Liu Q., Wang Y., Chen Q., Kong B. The potential correlation between bacterial diversity and the characteristic volatile flavour of traditional dry sausages from northeast china. Food Microbiol. 2020;91 doi: 10.1016/j.fm.2020.103505. [DOI] [PubMed] [Google Scholar]
- Hu Y., Zhang L., Wen R., Chen Q., Kong B. Role of lactic acid bacteria in flavor development in traditional chinese fermented foods: A review. Critical Reviews in Food Science and Nutrition. 2022;62(10):2741–2755. doi: 10.1080/10408398.2020.1858269. [DOI] [PubMed] [Google Scholar]
- Huang Q., Dong K., Wang Q., Huang X., Wang G., An F.…Luo P. Changes in volatile flavor of yak meat during oxidation based on multi-omics. Food Chemistry. 2022;371 doi: 10.1016/j.foodchem.2021.131103. [DOI] [PubMed] [Google Scholar]
- Huang X., Yu S., Han B., Chen J. Bacterial community succession and metabolite changes during sufu fermentation. LWT-Food Science and Technology. 2018;97:537–545. [Google Scholar]
- Hui X. Hefei University of Technology; 2022. Study on effect and application of microorganism on flavor of dry cured ham. [Google Scholar]
- Jeong D.W., Lee H., Jeong K., Kim C.T., Shim S.T., Lee J.H. Effects of starter candidates and nacl on the production of volatile compounds during soybean fermentation. Journal of Microbiology and Biotechnology. 2019;29(2):191–199. doi: 10.4014/jmb.1811.11012. [DOI] [PubMed] [Google Scholar]
- Kashyap D.R., Vohra P.K., Chopra S., Tewari R. Applications of pectinases in the commercial sector: A review. Bioresource Technology. 2001;77(3):215–227. doi: 10.1016/s0960-8524(00)00118-8. [DOI] [PubMed] [Google Scholar]
- Kaur P., Dua K. In: Advances in dairy microbial products. Singh J., Vyas A., editors. Woodhead Publishing; 2022. Chapter 3 - recent trends in fungal dairy fermented foods; pp. 41–57. [Google Scholar]
- Kim J., Lee H.E., Kim Y., Yang J., Lee S.-J., Jung Y.H. Development of a post-processing method to reduce the unique off-flavor of allomyrina dichotoma: Yeast fermentation. LWT-Food Science and Technology. 2021;150 [Google Scholar]
- Kimura K., Yokoyama S. Trends in the application of bacillus in fermented foods. Current Opinion in Biotechnology. 2019;56:36–42. doi: 10.1016/j.copbio.2018.09.001. [DOI] [PubMed] [Google Scholar]
- Kumar V., Naik B., Kumar A., Khanduri N., Rustagi S., Kumar S. Probiotics media: Significance, challenges, and future perspective - a mini review. Food Production, Processing and Nutrition. 2022;4(1):17. [Google Scholar]
- Kumar Verma D., Thyab Gddoa Al-Sahlany T.G., Kareem Niamah A., Thakur M., Shah N., Singh S., Baranwal D., Patel A.R., Lara Utama G., Noe Aguilar C. Recent trends in microbial flavour compounds: A review on chemistry, synthesis mechanism and their application in food. Saudi Journal of Biological Sciences. 2022;29(3):1565–1576. doi: 10.1016/j.sjbs.2021.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.E., Lee H.J., Kim C.H., Ryu S., Kim Y., Jo C. Effect of Penicillium candidum and Penicillium nalgiovense and their combination on the physicochemical and sensory quality of dry-aged beef. Food Microbiology. 2022;107 doi: 10.1016/j.fm.2022.104083. [DOI] [PubMed] [Google Scholar]
- Li C., Li W., Li L., Chen S., Wu Y., Qi B. Microbial community changes induced by a newly isolated salt-tolerant tetragenococcus muriaticus improve the volatile flavor formation in low-salt fish sauce. Food Research International. 2022;156 doi: 10.1016/j.foodres.2022.111153. [DOI] [PubMed] [Google Scholar]
- Li J., Liu B., Feng X., Zhang M., Ding T., Zhao Y., Wang C. Comparative proteome and volatile metabolome analysis of Aspergillus oryzae 3.042 and Aspergillus sojae 3.495 during koji fermentation. Food Research International. 2023;165 doi: 10.1016/j.foodres.2023.112527. [DOI] [PubMed] [Google Scholar]
- Li J., Sun C., Shen Z., Tian Y., Mo F., Wang B.…Wang C. Untargeted metabolomic profiling of Aspergillus sojae 3.495 and Aspergillus oryzae 3.042 fermented soy sauce koji and effect on moromi fermentation flavor. LWT-Food Science and Technology. 2023;184 [Google Scholar]
- Li X., Li J., Liu X., Feng Y., Liu C. Analysis of bacterial community diversity in fermented soybean using pyrosequencing. Journal of Food Science and Biotechnology. 2014;33(2):137–142. [Google Scholar]
- Li X.M., Deng J.Y., Wu Y., Nie W., Wang Z.M., Zhou H., Xu B.C. Insight into the correlation between microbial diversity and flavor profiles of traditional dry-cured duck from the metabolomic perspective. Food Research International. 2022;156 doi: 10.1016/j.foodres.2022.111349. [DOI] [PubMed] [Google Scholar]
- Li Z.H., Rui J.P., Li X.Z., Li J.B., Dong L., Huang Q.L.…Chen F.S. Bacterial community succession and metabolite changes during doubanjiang-meju fermentation, a chinese traditional fermented broad bean (vicia faba l.) paste. Food Chemistry. 2017;218:534–542. doi: 10.1016/j.foodchem.2016.09.104. [DOI] [PubMed] [Google Scholar]
- Liang J., Li D., Shi R., Wang J., Guo S., Ma Y., Xiong K. Effects of microbial community succession on volatile profiles and biogenic amine during sufu fermentation. LWT-Food Science and Technology. 2019;114 [Google Scholar]
- Lin X., Tang Y., Hu Y., Lu Y., Sun Q., Lv Y.…Chi Y. Sodium reduction in traditional fermented foods: Challenges, strategies, and perspectives. Journal of Agricultural and Food Chemistry. 2021;69(29):8065–8080. doi: 10.1021/acs.jafc.1c01687. [DOI] [PubMed] [Google Scholar]
- Liu J.M., Chen L., Jensen P.R., Solem C. Food grade microbial synthesis of the butter aroma compound butanedione using engineered and non-engineered Lactococcus lactis. Metabolic Engineering. 2021;67:443–452. doi: 10.1016/j.ymben.2021.08.006. [DOI] [PubMed] [Google Scholar]
- Liu S., Yang L., Zhou Y., He S.D., Li J.L., Sun H.J.…Xu S.Y. Effect of mixed moulds starters on volatile flavor compounds in rice wine. LWT-Food Science and Technology. 2019;112 [Google Scholar]
- Londoño-Hernández L., Ramírez-Toro C., Ruiz H.A., Ascacio-Valdés J.A., Aguilar-Gonzalez M.A., Rodríguez-Herrera R., Aguilar C.N. Rhizopus oryzae – ancient microbial resource with importance in modern food industry. International Journal of Food Microbiology. 2017;257:110–127. doi: 10.1016/j.ijfoodmicro.2017.06.012. [DOI] [PubMed] [Google Scholar]
- Long S., Wang H., Wu S., Qi G., Zhang H., Wang J. Biological functions and mechanisms of docosahexaenoic acid and its application in poultry production. Chinese Journal of Animal Nutrition. 2017;29(4):1101–1109. [Google Scholar]
- Lopez C.M., Callegari M.L., Patrone V., Rebecchi A. Assessment of antibiotic resistance in Staphylococci involved in fermented meat product processing. Current Opinion in Food Science. 2020;31:17–23. [Google Scholar]
- Lynch K.M., Zannini E., Wilkinson S., Daenen L., Arendt E.K. Physiology of acetic acid bacteria and their role in vinegar and fermented beverages. Comprehensive Reviews in Food Science and Food Safety. 2019;18(3):587–625. doi: 10.1111/1541-4337.12440. [DOI] [PubMed] [Google Scholar]
- Ma Q., Liu L., Zhao S., Huang Z., Li C., Jiang S.…Gu P. Biosynthesis of vanillin by different microorganisms: A review. World Journal of Microbiology & Biotechnology. 2022;38(3):40. doi: 10.1007/s11274-022-03228-1. 1–9. [DOI] [PubMed] [Google Scholar]
- McAuliffe O., Kilcawley K., Stefanovic E. Symposium review: Genomic investigations of flavor formation by dairy microbiota. Journal of Dairy Science. 2019;102(1):909–922. doi: 10.3168/jds.2018-15385. [DOI] [PubMed] [Google Scholar]
- Morin-Sardin S., Nodet P., Coton E., Jany J.-L. Mucor: A janus-faced fungal genus with human health impact and industrial applications. Fungal Biology Reviews. 2017;31(1):12–32. [Google Scholar]
- Nsogning Dongmo S., Procopio S., Sacher B., Becker T. Flavor of lactic acid fermented malt based beverages: Current status and perspectives. Trends in Food Science & Technology. 2016;54:37–51. [Google Scholar]
- Pang X.-N., Chen C., Huang X.-N., Yan Y.-Z., Chen J.-Y., Han B.-Z. Influence of indigenous lactic acid bacteria on the volatile flavor profile of light-flavor baijiu. LWT-Food Science and Technology. 2021;147 [Google Scholar]
- Parlindungan E., Lugli G.A., Ventura M., van Sinderen D., Mahony J. Lactic acid bacteria diversity and characterization of probiotic candidates in fermented meats. Foods. 2021;10(7):23. doi: 10.3390/foods10071519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Q.N., Jiang S.M., Chen J.L., Ma C.C., Huo D.X., Shao Y.Y., Zhang J.C. Unique microbial diversity and metabolic pathway features of fermented vegetables from Hainan, China. Frontiers in Microbiology. 2018;9:12. doi: 10.3389/fmicb.2018.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y., Wu Y., Li L., Chen S., Zhao Y., Li C.…Wang Y. Elucidating the mechanism underlying volatile and non-volatile compound development related to microbial amino acid metabolism during golden pomfret (Trachinotus ovatus) fermentation. Food Research International. 2022;162 doi: 10.1016/j.foodres.2022.112095. [DOI] [PubMed] [Google Scholar]
- Quan Q., Liu W., Zuo M., Zhang J. Advances in the flavor of fruit and vegetable juices fermented by lactic acid bacteria. Food and Fermentation Industries. 2022;48(1):315–323. [Google Scholar]
- Raspor P., Goranovič D. Biotechnological applications of acetic acid bacteria. Critical Reviews in Biotechnology. 2008;28(2):101–124. doi: 10.1080/07388550802046749. [DOI] [PubMed] [Google Scholar]
- Saarela M., Mogensen G., Fondén R., Mättö J., Mattila-Sandholm T. Probiotic bacteria: Safety, functional and technological properties. Journal of Biotechnology. 2000;84(3):197–215. doi: 10.1016/s0168-1656(00)00375-8. [DOI] [PubMed] [Google Scholar]
- Fan S., Xue H., Bai B., Xue T., Lin J., Zhang J. Dynamic analysis of microbial community and selection of different genera during fermentation of Shanxi vinegar. Science and Technology of Food Industry. 2022;43(24):171–179. [Google Scholar]
- Sengun I.Y., Kilic G., Charoenyingcharoen P., Yukphan P., Yamada Y. Investigation of the microbiota associated with traditionally produced fruit vinegars with focus on acetic acid bacteria and lactic acid bacteria. Food Bioscience. 2022;47 [Google Scholar]
- Shen Y.Y., Wu Y.Y., Wang Y.Q., Li L.H., Li C.S., Zhao Y.Q., Yang S.L. Contribution of autochthonous microbiota succession to flavor formation during chinese fermented mandarin fish (Siniperca chuatsi) Food Chemistry. 2021;348 doi: 10.1016/j.foodchem.2021.129107. [DOI] [PubMed] [Google Scholar]
- Han S., Zhang J., Jing Y., Wang P., Wang G., Liu F.…Zhao Y. progress in research about the protease extracted from microorganisms. Science and Technology of Food Industry. 2020;41(13):321–327. [Google Scholar]
- Li S. Hefei University of Technology; 2017. Study on mixed fermentation of sufu with mucor racemosus and rhizopus oryzae. [Google Scholar]
- Singhania R.R., Adsul M., Pandey A., Patel A.K. In: Current developments in biotechnology and bioengineering. Pandey A., Negi S., Soccol C.R., editors. Elsevier; 2017. 4 - cellulases; pp. 73–101. [Google Scholar]
- Song Z., Du H., Zhang Y., Xu Y. Unraveling core functional microbiota in traditional solid-state fermentation by high-throughput amplicons and metatranscriptomics sequencing. Frontiers in Microbiology. 2017;8:1294. doi: 10.3389/fmicb.2017.01294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahnke L.H., Holck A., Jensen A., Nilsen A., Zanardi E. Maturity acceleration of italian dried sausage by staphylococcus carnosus—relationship between maturity and flavor compounds. Journal of Food Science. 2002;67(5):1914–1921. [Google Scholar]
- Sun P., Wang M., Wang S., Sun J., Miao C., Huang H.…Zhang J. Effect of fermentation with Enicilium roqueforti and Penicilium nalgiovense on physicochemical properties and microstructure of duck meat products. Journal of Light Industry. 2022;37(4):18–25. [Google Scholar]
- Tamang J.P., Lama S. Probiotic properties of yeasts in traditional fermented foods and beverages. Journal of Applied Microbiology. 2022;132(5):3533–3542. doi: 10.1111/jam.15467. [DOI] [PubMed] [Google Scholar]
- Tong Z., Zheng X., Tong Y., Shi Y.C., Sun J. Systems metabolic engineering for citric acid production by Aspergillus niger in the post-genomic era. Microbial Cell Factories. 2019;18(1):28. doi: 10.1186/s12934-019-1064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh A.M., Crispie F., Kilcawley K., O'Sullivan O., O'Sullivan M.G., Claesson M.J., Cotter P.D. Microbial succession and flavor production in the fermented dairy beverage kefir. mSystems. 2016;1(5):e00052–e00116. doi: 10.1128/mSystems.00052-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Liwei M., Park J.B., Jeong S.H., Wei G., Wang Y., Kim S.W. Microbial platform for terpenoid production: Escherichia coli and Yeast. Frontiers in Microbiology. 2018;9:2460. doi: 10.3389/fmicb.2018.02460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Su W., Mu Y., Zhao C. Correlation between microbial diversity and volatile flavor compounds of suan zuo rou, a fermented meat product from guizhou, china. Frontiers in microbiology. 2021;12 doi: 10.3389/fmicb.2021.736525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Yan C., Ma C., Huang S., Chang X., Li Z.…Li X. Effects of two kinds of bacillus on flavour formation of baijiu solid-state fermentation with pure mixed bacteria. International Journal of Food Science & Technology. 2023;58(3):1250–1262. [Google Scholar]
- Wang J., Zhao M., Xie N., Huang M., Feng Y. Community structure of yeast in fermented soy sauce and screening of functional yeast with potential to enhance the soy sauce flavor. International Journal of Food Microbiology. 2022;370 doi: 10.1016/j.ijfoodmicro.2022.109652. [DOI] [PubMed] [Google Scholar]
- Wang Q., Li X., Xue B., Wu Y., Song H., Luo Z.…Huang Q. Low-salt fermentation improves flavor and quality of sour meat: Microbiology and metabolomics. LWT-Food Science and Technology. 2022;171 [Google Scholar]
- Wang Y., Li F., Chen J., Sun Z., Wang F., Wang C., Fu L. High-throughput sequencing-based characterization of the predominant microbial community associated with characteristic flavor formation in Jinhua Ham. Food Microbiology. 2021;94 doi: 10.1016/j.fm.2020.103643. [DOI] [PubMed] [Google Scholar]
- Wang Z.M., Lu Z.M., Shi J.S., Xu Z.H. Exploring flavour-producing core microbiota in multispecies solid-state fermentation of traditional Chinese vinegar. Scientific Reports. 2016;6(1):26818. doi: 10.1038/srep26818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei G., Chitrakar B., Regenstein J.M., Sang Y., Zhou P. Microbiology, flavor formation, and bioactivity of fermented soybean curd (furu): A review. Food Research International. 2023;163 doi: 10.1016/j.foodres.2022.112183. [DOI] [PubMed] [Google Scholar]
- Wei G.M., Regenstein J.M., Liu X.M., Zhou P. Comparative aroma and taste profiles of oil furu (soybean curd) fermented with different mucor strains. Journal of Food Science. 2020;85(6):1642–1650. doi: 10.1111/1750-3841.15100. [DOI] [PubMed] [Google Scholar]
- Wei Z., Xu Y., Xu Q., Cao W., Huang H., Liu H. Microbial biosynthesis of L-malic acid and related metabolic engineering strategies: Advances and prospects. Frontiers in Bioengineering and Biotechnology. 2021;9 doi: 10.3389/fbioe.2021.765685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N., Zhang J., Chen Y., Xu Q., Song P., Li Y.…Liu H. Recent advances in microbial production of L-malic acid. Applied Microbiology and Biotechnology. 2022;106(24):7973–7992. doi: 10.1007/s00253-022-12260-y. [DOI] [PubMed] [Google Scholar]
- Xia Y., Yu J., Liu H., Feng C., Shuang Q. Novel insight into physicochemical and flavor formation in koumiss based on microbial metabolic network. Food Research International. 2021;149 doi: 10.1016/j.foodres.2021.110659. [DOI] [PubMed] [Google Scholar]
- Xiang W., Xu Q., Zhang N., Rao Y., Zhu L., Zhang Q. Mucor indicus and Rhizopus oryzae co-culture to improve the flavor of chinese turbid rice wine. Journal of Agriculture and Food Sciences. 2019;99(12):5577–5585. doi: 10.1002/jsfa.9831. [DOI] [PubMed] [Google Scholar]
- Xiao M., Huang T., Huang C., Hardie J., Peng Z., Xie M., Xiong T. The microbial communities and flavour compounds of jiangxi yancai, sichuan paocai and dongbei suancai: Three major types of traditional chinese fermented vegetables. LWT-Food Science and Technology. 2020;121 [Google Scholar]
- Xu D., Pan L., Zhao H., Zhao M., Sun J., Liu D. Breeding and identification of novel koji molds with high activity of acid protease by genome recombination between Aspergillus oryzae and Aspergillus niger. Journal of Industrial Microbiology and Biotechnology. 2011;38(9):1255–1265. doi: 10.1007/s10295-010-0904-5. [DOI] [PubMed] [Google Scholar]
- Xu N., Liu Y., Hu Y., Zhou M., Wang C., Li D. Autolysis of Aspergillus oryzae mycelium and effect on volatile flavor compounds of soy sauce. Journal of Food Science. 2016;81(8):1883–1890. doi: 10.1111/1750-3841.13396. [DOI] [PubMed] [Google Scholar]
- Xu Y., Zhou Y., Cao W., Liu H. Improved production of malic acid in Aspergillus niger by abolishing citric acid accumulation and enhancing glycolytic flux. ACS Synthetic Biology. 2020;9(6):1418–1425. doi: 10.1021/acssynbio.0c00096. [DOI] [PubMed] [Google Scholar]
- Xu Z., Li S., Gong G., Liu Z., Wu Z., Ma C. Influence of different acidifying strains of Lactobacillus delbrueckii subsp. bulgaricus on the quality of yoghurt. Food Science and Technology Research. 2015;21(2):263–269. [Google Scholar]
- You C., Zhang Y.H.P. In: Future trends in biotechnology. Zhong J.-J., editor. Springer, Berlin Heidelberg; Berlin, Heidelberg: 2013. Cell-free biosystems for biomanufacturing; pp. 89–119. [Google Scholar]
- Zang J.H., Yu D.W., Li T.R., Xu Y.S., Regenstein J.M., Xia W.S. Identification of characteristic flavor and microorganisms related to flavor formation in fermented common carp (Cyprinus carpio l.) Food Research International. 2022;155:12. doi: 10.1016/j.foodres.2022.111128. [DOI] [PubMed] [Google Scholar]
- Zeng X., Mo Z., Zheng J., Wei C., Dai Y., Yan Y., Qiu S. Effects of biofilm and co-culture with bacillus velezensis on the synthesis of esters in the strong flavor baijiu. International Journal of Food Microbiology. 2023;394 doi: 10.1016/j.ijfoodmicro.2023.110166. [DOI] [PubMed] [Google Scholar]
- Zhang J., Zhao K., Li H., Li S., Xu W., Chen L.…Tang H. Physicochemical property, volatile flavor quality, and microbial community composition of jinhua fatty ham and lean ham: A comparative study. Frontiers in Microbiology. 2023;14:1124770. doi: 10.3389/fmicb.2023.1124770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D.D., Hu J., Chen W.X. Analysis of the relationship between microorganisms and flavour development in dry-cured grass carp by high-throughput sequencing, volatile flavour analysis and metabolomics. Food Chemistry. 2022;368 doi: 10.1016/j.foodchem.2021.130889. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Wang Y., Li C., Li L., Yang X., Wu Y.…Zhao Y. Novel insight into physicochemical and flavor formation in naturally fermented tilapia sausage based on microbial metabolic network. Food Research International. 2021;141 doi: 10.1016/j.foodres.2021.110122. [DOI] [PubMed] [Google Scholar]
- Zheng X., Liu F., Shi X., Wang B., Li K., Li B., Zhuge B. Dynamic correlations between microbiota succession and flavor development involved in the ripening of kazak artisanal cheese. Food Research International. 2018;105:733–742. doi: 10.1016/j.foodres.2017.12.007. [DOI] [PubMed] [Google Scholar]
- Zhong A., Chen W., Duan Y., Li K., Tang X., Tian X.…Wang C. The potential correlation between microbial communities and flavors in traditional fermented sour meat. LWT-Food Science and Technology. 2021;149 [Google Scholar]
- Zhou X., Guo T., Lu Y., Hadiatullah H., Li P., Ding K., Zhao G. Effects of amino acid composition of yeast extract on the microbiota and aroma quality of fermented soy sauce. Food Chemistry. 2022;393 doi: 10.1016/j.foodchem.2022.133289. [DOI] [PubMed] [Google Scholar]
- Zhu C.Z., Zhao J.L., Tian W., Liu Y.X., Li M.Y., Zhao G.M. Contribution of histidine and lysine to the generation of volatile compounds in Jinhua Ham exposed to ripening conditions via Maillard reaction. Journal of Food Science. 2018;83(1):46–52. doi: 10.1111/1750-3841.13996. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.