Graphical abstract
Keywords: Maize germination, Seed part, Phenolic compound, Hypoglycemic activity
Highlights
-
•
Sprout treatment increases the content and type of phenolic compounds in maize.
-
•
There were obvious differences in phenolics in different parts of germinated maize.
-
•
Phenolic substances are involved in the synthesis and metabolism during sprouting.
-
•
The seed embryo, radicle and germ of sprouted maize have hypoglycemic abilities.
Abstract
In this study, qualitative and quantitative analyses of phenolic compounds in the maize germinating seed embryo, radicle, and germ were performed at 0, 48, and 96 h post-germination, followed by the evaluation of their hypoglycemic activity. The results revealed the accumulation of 80 phenolics in different parts of germinated maize, of which 47, 48, and 53 were present in the seed embryo, radicle, and germ. After germination 22, 26, and 34 polyphenols were found to differential accumulate in the seed embryo, radicle, and germ. At 96 h post-germination, the content of monomeric phenols in the germ was higher than that in the radicle and seed embryo. Moreover, the inhibitory activity of polyphenols in the germ towards α-glucosidase and α-amylase was higher than that in the radicle and seed embryo. These results indicate that germination can effectively improve the type and content of phenolic compounds in different parts of maize.
Introduction
Maize, also called corn, is one of the world's most significant cereals (Chen et al., 2022). In 2021, global maize production reached 1197 million tonnes, and it is used as a staple food in several regions, such as Africa, Asia and Latin America, for processing the products and providing feed in some parts of Asia (Erenstein, Jaleta, Sonder, Mottaleb & Prasanna, 2022). Maize kernels are rich in protein, fat, vitamins, phenolic compounds, and other active substances, which are suitable for people with high blood sugar and cholesterol (Carreño-Carrillo et al., 2021, Suriano et al., 2021). Moreover, maize is a high-quality source of dietary grains to produce extremely nutritious functional foods for humans (Jiao, Chen, Han, & Chang, 2022).
Phenolic compounds are critical active substances in maize, such as phenolic acids and flavonoids, which exert excellent hypoglycemic and hypolipidemic effects (Yong et al., 2022). It is considered as an active substance to control the postprandial blood glucose (PBG) levels and incidence of type II diabetes, unlike hypoglycemic drugs, such as acarbose, which induce several side effects, such as intestinal disturbances (Zeng et al, 2019). Maintaining normal PBG levels is imperative to control blood glucose vacillations and avoid and treat diabetes and its underlying complications (Umpierrez et al., 2018). However, the human body cannot synthesize phenolics independently, and therefore, developing safe and effective natural hypoglycemic supplements or dietary supplements from natural plant sources is of great significance.
Germination is a simple and valid approach to increasing the active substance content of grains (Ohanenye, Tsopmo, Ejike, & Udenigwe, 2020). After germination, the grain exploits its biosynthetic potential during this period. It synthesizes many hydrolytic enzymes and the biochemical activities that occur during germination to lead to the production of several bioactive ingredients, such as phenolics and γ-aminobutyric acid (GABA) (Liu et al., 2022). Many cereals are currently treated using germination to enhance their nutrient content, such as, barley (Tang et al., 2021), soybean (Ma et al.,2020), among others. Additionally, some studies have reported that germination not only causes changes in the ingredient and chemical structure of the bioactive components but also induces the synthesis of bioactive substances (Liu et al., 2022).
In recent years, there has been significant research interest in the germination treatment of grains. However, most of the previous studies have primarily focused on examining the overall nutrient composition of germinated grains, such as starch and protein. There is a lack of research specifically investigating the changes in nutrient composition, particularly phenolics, in different parts of the grain during germination. Therefore, the objective of this study was to assess, for the first time, the changes in phenolic metabolism in different parts of maize (seed embryo, radicle, and germ) throughout the germination process using a metabolomics approach. Two analytical modeling methods, PCA and PLS-DA, were employed to evaluate the phenolics isolated from different sites. Additionally, the study also examined the inhibitory effects of phenolic compounds extracted from different sites on α-glucosidase and α-amylase activities. We deem that the results of this research can offer a theoretical reference for the development of highly nutritious and functional maize foods in the near future.
Materials and methods
Materials
Horsetail yellow maize Jidan 66 (harvested at maturity in October 2020) was procured from Changchun, Jilin Province, China. Gallic acid, ferulic acid, caffeic acid, p-coumaric acid, GABA, o-coumaric acid, (+)-catechin, catechin, quercetin, and rutin (HPLC ≥ 97 %) were purchased from Sinopharm Chemical Reagent Co., Ltd, China. α-glucosidase (10 U/mg) and α-amylase (10 U/mg) were purchased from Sigma-Aldrich, USA. Both methanol and acetic acid were purchased from Sigma-Aldrich, USA. PBS, PNPG, DNS were purchased from Yuan-ye, China. Starch and Na2CO3 were purchased from Damao, China.
Germination of maize
A total of 50 g of maize kernels were soaked in 1 % sodium hypochlorite (Yuan-ye, Shanghai, China) for 40 min, rinsed several times with distilled water, and then placed in a germination tray (34 × 25 × 4.5 cm). Then, 1000 mL of distilled water was added into the germination tray which was placed at 25 °C for 48 h and 96 h in the dark conditions (pre-experiments revealed that 48 h and 96 h were the critical time points for assessing the changes in the phenolic content in different parts of germinated maize). Water was changed every 12 h during the germination culture. The seed embryo, radicle, and germ of germinated maize were harvested and stored at 4 °C separately and ungerminated treated maize was used as a control group. The fresh samples were flash-frozen in liquid nitrogen and stored at −80 °C for further testing.
Extraction of phenolic compounds
The phenolic compounds were extracted using the method described in the study conducted by Oroian et al. (2020), with minor modifications. The germinated maize was mixed with methanol (70 %) at a ratio of 1:30 to prepare the solution. After mixing, the solution was extracted using the ultrasonic machine (KQ3200DB, Kunshan, China) at 400 W for 45 min to obtain the soluble polyphenols. The solution was centrifuged at 7500 g and the supernatant was collected. Then, the supernatant was concentrated using a rotary evaporator (Zhengzhou Greatwall Scientific industrial and Trade Co. Ltd. Henan, China, R-1001VN, evaporating rate: 20 mL/min) at 45 °C. The concentrated solution was redissolved in 5 mL methanol and passed through a 0.22 μm filter membrane to conduct subsequent experiments.
Qualitative and quantitative determination of phenolic compounds
Qualitative analysis of phenolics was performed using liquid chromatography-mass spectrometry (LC-MS). Chromatographic column: Hypesil Gold column (C18), column temperature: 40 °C, flow rate: 0.2 mL/min. Positive mode mobile phase: 0.1 % formic acid (phase A) and methanol (phase B), negative mode mobile phase: 5 mM ammonium acetate (phase A) and methanol (phase B). Elution gradient: 98 % A and 2 % B (1.5 min), 0 % A and 100 % B (14 min), and 98 % A and 2 % B (17 min). The ESI source was set with the following parameters: spray voltage at 3.2 kV, sheath gas flow rate of 40 arb, auxiliary gas flow rate at 10 arb, and capillary temperature maintained at 320 °C.
Quantitative analysis of phenolics was performed using high-performance liquid chromatography (HPLC). Chromatographic column: Agilent ZORBAX SB-C18 liquid chromatography column (150 mm × 4.6 mm, 5 μm), DAD detector, detection at 280 nm. The mobile phases were 100 % methanol (phase A) and 0.5 % glacial acetic acid (phase B), respectively. The elution gradients were 8 % A and 92 % B (8 min), 10 % A and 90 % B (17 min), 12 % A and 88 % B (20 min), 15 % A and 85 % B (25 min), 22 % A and 78 % B (33 min), and 50 % A and 50 % B (50 min).
Evaluation of hypoglycemic activity in vitro
α-glucosidase activity inhibition test
The α-glucosidase activity inhibition capacity of polyphenol compounds was measured using the method described by Zhao et al. (2019), with minor modifications. About 80 μL of PBS at a concentration of 0.1 mol/L pH 6.8, 40 μL of polyphenol extract, and 10 μL of α-glucosidase solution at 0.4 U/mL were added, and the reaction was performed at 37 °C for 15 min, following the addition of 100 μL of PNPG solution at a final concentration of 10 mmol/L. Thereafter, the reaction was continued at 37℃ for 15 min. The reaction was then terminated by adding 0.2 mol/L Na2CO3 solution, followed by the assessment of absorbance at 405 nm, a linear fit, to estimate the semi-inhibitory concentration (IC50).
α-amylase activity inhibition test
The α-amylase activity inhibition capacity of phenolic compounds was measured using the method depicted by Zhang et al. (2017), with minor modification. In 20 μL of polyphenol extract, 20 μL of α-amylase (enzyme activity 7 U/L) was added, and the reaction was conducted in water at 37 ℃ for 30 min. Thereafter, a 40 μL portion of 1 % ungelatinized soluble starch solution was introduced, and the reaction proceeded for an additional 3 min at 37 ℃. Following this, 80 μL of DNS display solvent was added, and the reaction mixture was subjected to boiling for 8 min before cooling down to 25℃. Subsequently, 800 μL of pure water was added to dilute the mixture, and the absorbance was measured at 450 nm. A control sample was concurrently assessed, and a linear fit was employed to estimate the IC50.
Statistical analysis
At least 3 independent tests were conducted for all experiments. Data from this experiment were transformed using the software metaX, which was then subjected to principal component analysis (PCA) and Partial Least Squares Discriminant Analysis (PLS-DA) permutation tests, and the R package Pheatmap, Spss-25, and Origin-2017 were used for data analysis and plotting the results.
Results and discussion
Qualitative analysis of phenolic compounds in different parts of germinated maize
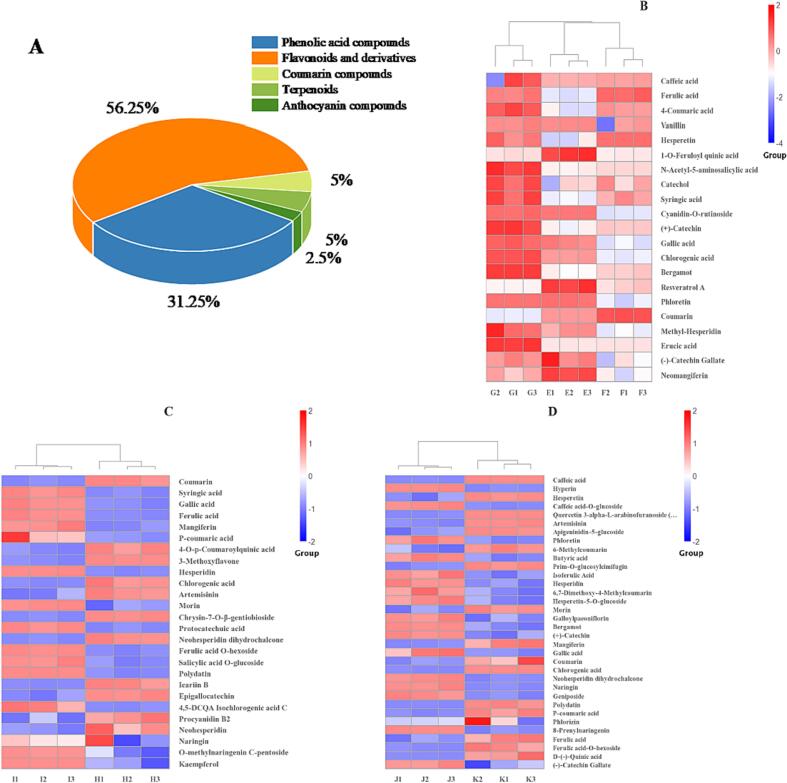
Polyphenol metabolites from three different parts of germinated maize were characterized by LC-MS. Table 1 shows the phenolic compounds present in different parts of germinated maize, which eluted well. The substances contained in the samples were determined by matching and analyzing the peak charge ratios and retention times appearing in the mass spectra. Using this test, 80 phenolic compounds were identified in germinated maize, including 40 flavonoids, and their derivatives (56.25%), 4 coumarins (5.00%), 4 terpenoids (5.00%) and 25 phenolic acids (31.25%), among others (2.50%).
Table 1.
Qualitative analysis of phenolic 611 compounds in different parts of germinated maize.
| Compound name | Molecular formula | RT(min) | Status |
|---|---|---|---|
| Coumarin | C9H6O2 | 8.827 | ▲★■ |
| Syringic acid | C9H10O5 | 1.343 | ▲★■ |
| Caffeic acid | C9H8O4 | 8.113 | ▲★■ |
| 3-Methoxyflavone | C16H12O3 | 10.259 | ▲ |
| Mangiferin | C19H18O11 | 8.372 | ▲★ |
| Chlorogenic acid | C16H18O9 | 7.152 | ▲★■ |
| Morin | C26H30O11 | 11.712 | ▲★ |
| (+)-Catechin | C15H14O6 | 8.354 | ▲★■ |
| 1-O-Feruloyl quinic acid | C17H20O9 | 9.021 | ▲★■ |
| Acetyl-trans-resveratrol | C20H18O6 | 12.365 | ▲★■ |
| Prim-O-glucosylcimifugin | C22H28O11 | 8.646 | ▲★■ |
| P-Coumaric acid ethyl ester | C11H12O3 | 12.391 | ▲★ |
| 7-O-Methylmangiferin | C20H20O11 | 8.989 | ▲ |
| Dihydromyricetin | C15H12O8 | 12.618 | ▲ |
| 6,7-Dimethoxy-4-Methylcoumarin | C12H12O4 | 11.113 | ▲★■ |
| Hesperetin | C16H14O6 | 10.958 | ▲★■ |
| 4-O-p-Coumaroylquinic acid | C16 H18O8 | 1.28 | ▲★■ |
| Artemisinin | C15H22O5 | 10.116 | ▲★ |
| Sinapinic acid | C11H12O5 | 9.133 | ▲★■ |
| D- (-)-Quinic acid | C7H12O6 | 1.279 | ▲★■ |
| Epigallocatechin | C15H14O7 | 1.289 | ▲ |
| Caffeic acid-O-glucoside | C16H20O9 | 6.956 | ▲★■ |
| 7,8-Dihydroxyflavone | C15H10O4 | 8.709 | ▲ |
| Coumalic acid | C6H4O4 | 1.333 | ▲★ |
| Chrysin-7-O-β-gentiobioside | C27H30O14 | 7.803 | ▲★■ |
| Carnosic acid | C20H28O4 | 14.641 | ▲★■ |
| (-)-Catechin Gallate | C22H18O10 | 1.512 | ▲★■ |
| Protocatechuic acid | C7H6O4 | 7.608 | ▲ |
| 3,4-Dihydroxybenzoic acid | C7H6O3 | 5.988 | ▲ |
| P-Aminocinnamic acid | C9H9NO2 | 8.282 | ▲ |
| Polydatin | C20H22O8 | 9.527 | ▲★■ |
| Geniposide | C17H24O11 | 7.584 | ▲★■ |
| Salicylic acid-O-glucoside | C13H16O8 | 6.346 | ▲★ |
| Kaempferol | C27H30O14 | 8.116 | ▲■ |
| Neohesperidin | C28H34O15 | 7.321 | ▲★■ |
| Gallic acid | C7H6O5 | 2.232 | ▲★■ |
| Neohesperidin dihydrochalcone | C28H36O15 | 9.133 | ▲★■ |
| Naringin | C27H3 O14 | 9.004 | ▲★■ |
| Procyanidin B2 | C30H26O12 | 6.936 | ▲ |
| Isoferulic Acid | C10H10O4 | 5.743 | ▲★ |
| Cucurbitacin IIA | C32H50O8 | 14.248 | ▲ |
| Saikosaponin B2 | C42H68O13 | 14.767 | ▲ |
| O-methylnaringenin-C-pentoside | C21H22O9 | 8.209 | ▲ |
| Icaritin | C21H22O7 | 4.32 | ▲★ |
| Ferulic acid | C10H10O4 | 7.879 | ▲★■ |
| N-acetyl-5-aminosalicylic acid | C9H9NO4 | 7.807 | ▲■ |
| P-coumaric acid | C9H8O3 | 7.629 | ▲★■ |
| O-coumaric acid | C9H8O3 | 9.037 | ■ |
| 3-Methoxycinnamic acid | C10 H10O3 | 14.824 | ■ |
| Cyanidin-O-rutinoside | C27H31O15 | 8.995 | ■ |
| Heptamethoxyflavone | C22H24O9 | 8.165 | ■ |
| Galloylpaeoniflorin | C30H32O15 | 8.663 | ■ |
| Resveratrol a | C21H22O7 | 7.516 | ■ |
| Methyl-Hesperidin | C29H38O16 | 9.047 | ■ |
| Phloretin | C15H14O5 | 9.043 | ■ |
| Quercetin3-alpha-l-arabinofuranoside (Avicularin) | C20H18O11 | 1.169 | ■★ |
| Hyperin | – | – | – |
| Apigeninidin-5-glucoside | C21H20NO12 | 7.805 | ■★ |
| Catechin | C21H21O9 | 8.309 | ■★ |
| Neomangiferin | C15H14O6 | 7.225 | ▲■★ |
| 4-Hydroxycoumarin | C25H28O16 | 1.214 | ■ |
| Hesperetin-5-O-glucoside | C9H6O3 | 8.169 | ■ |
| Caffeic acid -O-glucoside | C22H24O11 | 9.659 | ■★ |
| Catechol | C15H18O9 | 6.956 | ■ |
| 6-Methylcoumarin | C6H6O2 | 1.377 | ■★ |
| 4,5-DCQA Isochlorogenic acid C | C10H8O2 | 7.245 | ★ |
| Ligustroflavone | C25H24O12 | 8.33 | ★ |
| Phlorizin | C33H40O18 | 9.359 | ★ |
| 8-Prenylnaringenin | C21H24O10 | 9.992 | ★ |
| Ganoderic acid H | C20H20O5 | 6.87 | ★ |
| Apigenin-C-pentoside | C32H44O9 | 14.379 | ★ |
| Hesperidin | C20H18O9 | 9.088 | ★ |
| Ursolic acid | C28H34O15 | 9.345 | ★ |
| Camelliaside A | C30H48O3 | 7.41 | ★ |
| Luteoloside | C33H40O20 | 8.307 | ★ |
| Ellagic acid | C21H20O11 | 11.393 | ★ |
| Quercetin | C14H6O8 | 1.621 | ★ |
| Bergamot | C15H10O7 | 4.567 | ★ |
| Rutin | C12H8O4 | 8.967 | ■★ |
| C27H30O16 | 7.342 | ■ |
Note: “▲” represents radicle, “★” represents germ, “■” represents seed embryo.
A total of 47, 48, and 53 phenolic (Table 2) compounds were identified in the maize seed embryo, radicle, and germ, respectively. Twelve unique phenolic compounds were present in the seed embryo, such as 4-hydroxy-coumarin, neomangiferin, methyl-hesperidin, phlorizin, and among others. Twelve unique phenolic compounds were present in the radicle, such as epigallocatechin, protocatechuic acid, procyanidin B2, and others. Thirteen unique phenolics were present in the germ, such as ursolic acid, apigenin C-pentoside, hesperidin-5-O-glucoside, hesperidin, and among others. The results demonstrate that the germination treatment induced the production of new phenolic compounds in maize. Furthermore, the distribution of phenolic compounds exhibited a significant change following the germination treatment.
Table 2.
The quantitative analysis of phenolic compounds in different parts of germinated maize.
| Phenolics | Different parts | 0 h | 48 h | 96 h |
|---|---|---|---|---|
| Gallic acid | 82.59 ± 0.91b | 85.79 ± 2.56b | 100.01 ± 3.65a | |
| Catechin | 66.26 ± 0.51b | 69.75 ± 0.73a | 70.51 ± 0.88a | |
| (+) - catechin | 58.82 ± 2.98c | 69.34 ± 2.73b | 83.39 ± 2.40a | |
| Syringic acid | 30.73 ± 0.40b | 34.05 ± 1.13a | 34.35 ± 2.15a | |
| P-coumaric acid | 269.21 ± 3.49c | 283.42 ± 2.47b | 302.19 ± 4.32a | |
| Ferulic acid | Seed embryo(μg/g) | 57.27 ± 3.04c | 62.83 ± 2.01b | 68.35 ± 5.34a |
| Caffeic acid | 169.92 ± 4.36c | 180.28 ± 3.19b | 191.55 ± 4.22a | |
| Rutin | 315.75 ± 6.56c | 326.35 ± 3.38b | 373.01 ± 11.05a | |
| O-coumaric acid | 36.47 ± 2.29c | 41.44 ± 1.96b | 54.65 ± 2.66a | |
| Quercetin | 34.55 ± 1.49c | 40.43 ± 1.89b | 46.27 ± 0.93a | |
| Gallic acid | 106.17 ± 1.02b | 111.15 ± 2.64a | ||
| Catechin | 74.69 ± 0.52b | 77.27 ± 1.23a | ||
| (+) - catechin | 105.11 ± 0.99b | 156.68 ± 8.47a | ||
| Syringic acid | 79.27 ± 3.62b | 124.38 ± 3.71a | ||
| P-coumaric acid | 328.24 ± 3.26b | 349.67 ± 4.20a | ||
| Ferulic acid | Radicle(μg/g) | 168.28 ± 4.29b | 480.61 ± 4.27a | |
| Caffeic acid | 130.24 ± 3.06a | 97.69 ± 1.21b | ||
| Rutin | – | – | ||
| O-coumaric acid | – | – | ||
| Quercetin | 50.02 ± 2.30b | 59.57 ± 1.47a | ||
| Gallic acid | 120.84 ± 0.66b | 126.29 ± 0.81a | ||
| Catechin | 81.40 ± 0.94b | 86.05 ± 0.76a | ||
| (+) - catechin | 187.35 ± 5.25b | 209.12 ± 1.45a | ||
| Syringic acid | 182.65 ± 1.54b | 239.55 ± 3.28a | ||
| P-coumaric acid | 344.79 ± 7.03b | 372.21 ± 2.96a | ||
| Ferulic acid | Germ(μg/g) | 318.77 ± 6.95b | 554.10 ± 4.59a | |
| Caffeic acid | 103.78 ± 2.35b | 142.59 ± 4.06a | ||
| Rutin | – | – | ||
| O-coumaric acid | – | – | ||
| Quercetin | 79.35 ± 0.88b | 82.26 ± 2.29a |
Note: All test data were performed three times and the results are expressed as mean ± standard deviation; different letters represent significant differences (p < 0.05).
Phenolic acids and flavonoids are abundant in germination-treated maize. This study aimed to identify various phenolic compounds present in maize kernels and their respective functions. Notably, ferulic acid, p-coumaric acid, and butyric acid exhibited promising anti-inflammatory and antioxidant properties. Resveratrol also demonstrated a positive impact on cardiovascular and neurological diseases (Kores et al., 2019). Hostnik et al. (2019) concluded that tannins, such as gallic acid and ellagic acid, possess strong scavenging abilities against chemical carcinogens and exhibit significant anticancer potential. Moreover, previous research has indicated that sprouting also induces changes in the composition and distribution of phenolics in quinoa, resulting in higher levels of trans-p-coumaric acid, trans-ferulic acid, kaempferol and quercetin derivatives in many sprouted quinoas (Lian et al., 2023).
PCA and PLS-DA analysis of metabolites in different parts of germinated maize
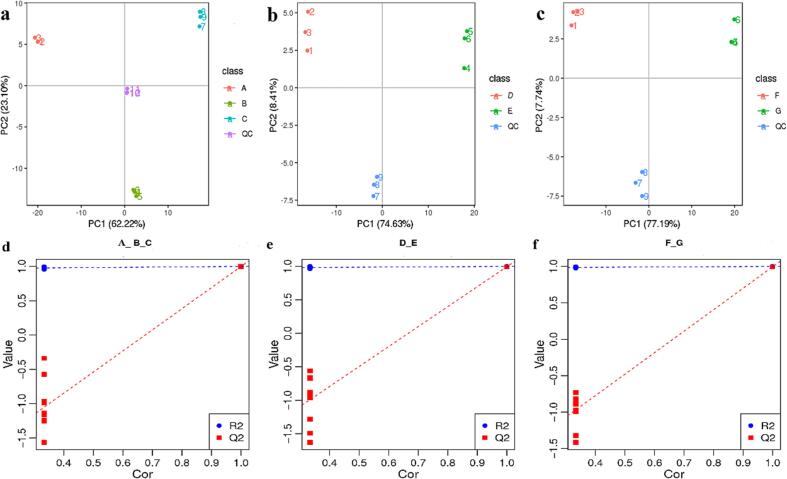
To assess the changes in the contents of phenolic compounds in the maize seed embryo, radicle, and germ during germination, metabolomics techniques were used to generate large-scale metabolic data, with QC samples clustered around the origin coordinates and treatment groups, showing data separation between the three biological replicates (Fig. 1). The PCA plots show that PC1 and PC2 scores were 62.22 % and 23.10 % in the seed embryo, 74.63 % and 8.41 % in radicle, 77.19 % and 7.74 % in germ, respectively. The positive effects were observed in seeds at 48 h and 96 h post-germination, and adverse effects were observed in ungerminated. Furthermore, the positive impacts in radicle and germ were observed at 96 h post germination; however, the negative effects were observed at 48 h germination. This outcome suggests that different germination times may lead to differences in the accumulation of metabolites at other sites (Fig. 1 a-c). The PCA scores could help assess the similarities and differences between the samples, ensuring that the data processing for each sample was repeatable and reliable. PCA also showed that the germination treatment significantly affected the chemicals in different parts of maize. However, it was impossible to clearly distinguish between other metabolites in individual parts. Therefore, the PLS-DA model was used to characterize different metabolites of the germinated maize seed embryo, radicle, and germ. In the replacement test model, the intercepts of the Q2 values on the y-axis for seed embryo, radicle, and germ were all less than zero, thereby indicating that the model was not over-fitted, had good predictive power, and was valid and usable (Fig. 1 d-f).
Fig. 1.
PCA and PLS-DA analysis of metabolites in different parts of germinated maize. (a-c) PCA (principal component analysis) plots of metabolites in the seed embryo, radicle, and germ; (d-f) PLS-DA substitution test plots of metabolites in the seed embryo, radicle, and germ; seed embryo: A, B, C (0 h, 48 h, and 96 h, respectively), radicle: D, and E (48 h, and 96 h, respectively), and germ: F, and G (48 h, and 96 h, respectively).
Heat map analysis of phenolic metabolites in different parts of germinated maize
Differentially accumulated metabolites were screened based on the PLS-DA model and after referring to the substances present before and after germination but exhibiting differences in content. Fig. 2 shows the metabolic thermogram of phenolics in different parts of germinated maize; the horizontal coordinates of the metabolic thermogram represent other germination times. Besides, each row of the vertical coordinates represents a polyphenol metabolite, with red for higher levels and blue for lower levels. After germination treatment, the phenolics characterized in different parts of maize were mainly flavonoids and phenolic acids. The relative content of differentially accumulated polyphenol metabolites was elevated in more than half of the other positions. A total of 22, 26, and 34 polyphenol metabolites were found to be differentially accumulated in the main seed embryo, radicle, and germ. At 96 h post-germination, 12 phenolic compounds were present at relatively higher levels than in the ungerminated seed embryos, like ferulic acid, caffeic acid, syringic acid, and (+)-catechin (Fig. 2 B). At 96 h post-germination, 15 phenolic compounds were present at relatively higher levels than at 48 h, like syringic acid, p-coumaric acid, gallic acid, and others (Fig. 2 C). The relative content of 20 phenolic compounds is higher at 96 h post germination in germs than 48 h post-germination, such as polydatin, ferulic acid, coumarins, and syringic acid (Fig. 2 D). The quantity and relative content of differentially accumulated polyphenolic metabolites in the seed embryo, radicle, and germ increased during maize germination. The upregulation was mainly noted in the contents of phenolic acids and flavonoids (Fig. 3).
Fig. 2.
Heat map analysis of phenolic metabolites in different parts of germinated maize. A depicts the percentage of polyphenol metabolites in different parts of germinated maize. B represents the differential accumulation of polyphenol metabolites in seed embryo: E, F, and G (0 h, 48 h, and 96 h, respectively); C represents the differential accumulation of polyphenol metabolites in radicle: H, and I (48 h, and 96 h, respectively); D represents differential polyphenol metabolites in germ: J, and K (48 h, and 96 h, respectively).
Fig. 3.
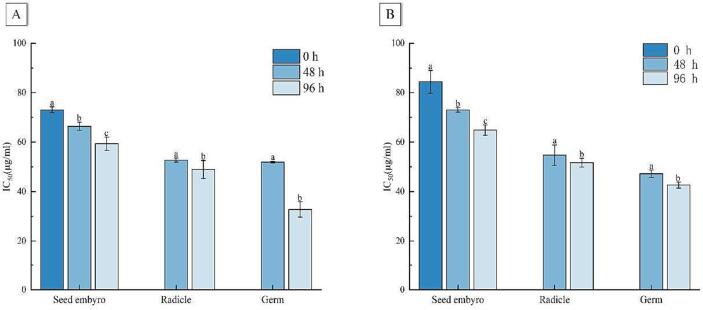
Hypoglycemic activities analysis of phenolic compounds from germinated maize in vitro. A: Inhibition activities of α-glucosidase of samples; B: inhibition activities of α-amylase of samples; lowercase letters represent significant differences (p < 0.05).
Some differences in the relative content of phenolics in the seed embryo, radicle, and germ during germination of maize have also been found in germinating grains also vary significantly in polyphenol species and content (Ikram et al., 2021) and even in different varieties of buckwheat seed embryos and germs, with significant differences in phenolics (Zhang et al., 2015). The phenol propane metabolic pathway is the main pathway for synthesizing phenolic compounds, and various enzymes and genes regulate the entire metabolic pathway (Hu, Sarengaowa, Guan, & Feng, 2022). It is noteworthy that the contracts within the sorts and sums of phenolic compounds in the grains, roots, and buds of maize post-germination are related to the enzymes and genes of the metabolic pathways of their respective phenolic compounds. The germination treatment could increase the rate of phenolic compound synthesis and metabolism by upregulating the enzymes and genes related to different branches in metabolic pathways. Differences in the synthesis and metabolic pathways of phenolic compounds are critical factors in the different types and contents of phenolic compounds (Ge, Jing et al., 2021).
Quantitative analysis of phenolic compounds
Quantitative analysis of five phenolic acids and five flavonoids during maize germination (these 10 substances are the phenolic compounds with high content in maize) helped detect remarkable differences (p < 0.05) in the content of monomeric phenols in different parts of maize. The content of gallic acid, catechin, (+)-catechin, p-coumaric acid, caffeic acid, o-coumaric acid, and quercetin in the seed embryo, radicle, and germ increased with an increase in germination time, and the order of monomeric phenolic content in different parts was: germ > radicle > seed embryo. At 96 h post germination germ, the content of p-coumaric acid, (+)-catechin, and ferulic acid increased more significantly (p < 0.05), reaching 372.21 ± 2.96 μg/g, 209.12 ± 1.45 μg/g, and 554.10 ± 4.59 μg/g, respectively, which were 7.9 %, 11.62 %, and 73.82 % higher than at 48 h germination in germ. Ge, Saleh et al. (2021) observed a notable rise in the ferulic acid content in germinated barley, which aligns with our own findings. Furthermore, they suggested that the phenolic content could potentially contribute to the various health advantages associated with germinated maize. O-coumaric acid and rutin were not present at detectable levels in the radicle and germ. In the seed embryo, the content of these two compounds gradually increased with an increase in germination time, which was 373.01 ± 11.05 μg/g and 54.65 ± 2.66 μg/g, respectively at 96 h post-germination. Caffeic acid content increased with an increase in germination time in the seed embryo and germ but decreased in the radicle. Previous studies have indicated that phenylalanine ammonia-lyase (PAL), chalcone isomerase (CHI), cinnamate-4-hydroxylase (C4H), and 4-coumaroyl-CoA-conjugating enzyme (4CL) play a role in the synthesis of phenolics and flavonoids. It has been suggested that the increase in phenolics observed during germination may be closely associated with the enhanced activities of these enzymes (Wu, Li, Li & Tan, 2022).
The phenolic content in various parts of maize demonstrated a positive correlation with the duration of germination, indicating an increase as germination time extended. Previously, significantly higher levels of chlorogenic acid, caffeic acid, syringic acid, and ferulic acid were detected in the germinated black barley than in ungerminated seeds (Guan, et al., 2019), which is consistent with the results of the present research. The increase in free phenolics during grain germination can be attributed to the synthesis and transformation of new substances. Glucose acts as a precursor substance for the synthesis of phenolics. It then enters into the metabolic pathways, such as glycolysis, mangiferin acid, and the phenyl propane pathway, which are involved in synthesizing and converting disparate phenolic compounds (Jan, Asaf, Numan, Lubna, & Kim, 2021).
During seed germination, the proliferation of new plant cells leads to the formation of fresh cell walls. Simultaneously, newly synthesized free phenolic compounds are released into these cell walls, resulting in the formation of bound phenolics. Therefore, bound phenolics are involved in the dynamic process, as their release and binding rates both contribute to the increased phenolic content in different parts of germinating maize (Tomé-Sánchez et al., 2021). Nevertheless, when tested, it was found that the caffeic acid content in maize radicles maintained a low level due to the phenolics in the radicle, which are transferred to the germ or seed embryo during seed growth, thereby resulting in a decrease in the content of some of the phenolics (Aguilar, Miano, Obregón, Soriano-Colchado & Barraza-Jáuregui, 2019).
In vitro analysis of the hypoglycemic activity of phenolic compounds
Postprandial hyperglycemia is a critical cause of type II diabetes and is mainly controlled by reducing the inhibitory activity of alpha-amylase and alpha-glucosidase (Dirir, Daou, Yousef & Yousef, 2022). The inhibition of α-glucosidase by phenolic compounds originally from different parts of germinated maize was significantly higher (p < 0.05) than that of α-amylase, which is consistent with the previous findings in millet and jute leaves (Pradeep & Sreerama, 2018). The order of glucose-lowering effect of polyphenols in different parts was: germ > radicle > seed embryo. At 96 h post-germination, the smallest IC50 values for α-glucosidase and α-amylase were 32.75 ± 3.07 μg/mL and 42.65 ± 1.24 μg/mL, respectively, thereby indicating that phenolics had the most significant inhibitory effect on α-glucosidase and α-amylase at this time point, with an increase of 58.52 % and 10.62 %, respectively compared to 48 h post germination in germ.
Flavonoids and phenolic acids exhibit comparable inhibitory mechanisms by affecting the enzymes. Phenolics, through their interaction with amino acid residues near the active site, modify the secondary structure of the enzyme. This modification hinders substrate entry, resulting in changes to the active site and subsequent reduction in enzyme activity (Zeng et al., 2019). It has previously been reported that the inhibition of α-glucosidase is associated with phenolic acids, such as caffeic acid and ferulic acid (Aleixandre, Gil, Sineiro & Rosell, 2022), and that the amount and content of phenolic acids in the germinated germ are higher than that in the radicle and seed embryo, and therefore, the polyphenols in the germ exert the most potent effect on α-glucosidase inhibition. However, the inhibition of α-amylase is closely related to the content and type of flavonoids, such as quercetin, catechin, and rutin, which strongly inhibit α-amylase (Martinez, Díaz, Rosa, Bustos & Alvarez, 2019).
To assess the connection between the content of phenolics in different parts of germinated maize and their hypoglycemic activity, correlation analyses were performed, which are shown in Supplementary Table 2. The correlation between the inhibition of both α-glucosidase and α-amylase and the contents of caffeic acid, (+)-catechin, rutin, and quercetin was highly significant in the seed embryo (p < 0.01). However, in the radicle, the contents of p-coumaric acid, catechin, and quercetin were significantly correlated with the inhibition of α-glucosidase inhibition (p < 0.05) but not with that of α-amylase. Ferulic acid and syringic acid were highly correlated with the inhibition of α-glucosidase (p < 0.01) and α-amylase (p < 0.05), while o-coumaric acid and rutin exhibited no detectable correlation in the radicle and germ. There was a correlation between the phenolics in different parts of germinated maize and the lowering of blood glucose levels, which suggests that germination prompts other parts of the maize to produce large amounts of phenolics that exhibit a potent hypoglycemic effect.
Possible metabolic mechanisms of phenolics during maize germination
The metabolic pathways of polyphenols are primarily produced through the shikimic acid and phenylpropane metabolic pathways, along with subsequent branching pathways. Glucose, as the main nutrient for the phenylpropane synthesis pathway, undergoes glycolysis, pentose phosphate, and shikimic acid pathways. It is metabolized to produce phenylalanine, which then enters the phenylpropane metabolic pathway to synthesize phenolic acids, flavonoids, and other substances (Yan et al., 2022).
PAL is the key enzyme of the phenylpropane pathway and plays a crucial role in the metabolism of this pathway. It directly converts l-phenylalanine's amino group into trans-cinnamic acid, and its activity is closely linked to changes in phenolic content (Kianfar & Salimi, 2020). The expression of the PAL gene in plants is both spatio-temporal, occurring in specific plant parts at specific times, such as radicles and leaves. Additionally, PAL is involved in the plant's response to pests, diseases, and external conditions like temperature, humidity, and water. Under stressful external conditions, the plant not only exhibits increased antioxidant activity but also produces substances such as polyphenols and flavonoids to resist the damage caused by these stresses (Zhou et al., 2018). C4H is a critical enzyme in the phenylpropane metabolism pathway. It acts after the PAL metabolism, decomposing cinnamic acid into p-coumaric acid. This process indirectly influences the levels of phenolic acids like ferulic acid and caffeic acid. According to the metabolic pathway of polyphenols, phenylalanine is converted into cinnamic acid by the PAL enzyme. Subsequently, a series of phenolic acids are produced through the action of the C4H enzyme. It is hypothesized that the increase in C4H enzyme activity during germination is due to two factors. Firstly, germination may prompt an increase in C4H enzyme activity. Secondly, germination affects the previous metabolizing enzyme, leading to increased activity of the PAL enzyme in different parts. This results in the breakdown of more precursors for the subsequent metabolism. Simultaneously, the activity of the C4H enzyme also increases, allowing for the breakdown of more cinnamic acid and the production of a series of phenolic acids, such as p-coumaric acid. Ma et al. (2019) conducted a study on the effects of GABA mediated changes in phenolic compounds in germinating hull-less barley under NaCl stress. They found that the content of monomeric phenols, such as p-coumaric acid, caffeic acid, and erucic acid, significantly increased. Additionally, they observed an up-regulation in the relative expression of PAL, C4H, and 4CL genes. The activity of this enzyme varied significantly among different sites and stages of growth, with the highest activity observed in the terminal buds (Li et al., 2020). However, the activity decreased sharply with increasing maturity. The 4CL enzyme is located at the turning point of the phenylpropane metabolic pathway-specific metabolites and plays a role in regulating the synthesis of phenolic acids and serving as a precursor for the flavonoid metabolic pathway. Enzymes are essentially proteins, and their steric effects depend on interactions such as hydrogen bonds, van der Waals forces, and ionic bonds. After undergoing germination treatment, seeds absorb water and swell, leading to the activation of endogenous proteases. This activation causes the molecular structure of the proteins to unfold, thereby altering the spatial structure of the enzyme. As a result, the enzyme activity of 4CL increases, leading to the decomposition of more p-coumarate and the generation of additional precursors for synthesizing flavanones. Consequently, this promotes the synthesis of flavonoids in various parts of the plant and enhances the overall amount of polyphenols. Our previous quantitative analysis of phenolics revealed an increase in the content of catechin, (+)-catechin, and quercetin, which can be attributed to the influence of the 4CL enzyme. The phenylpropane pathway is regulated by a variety of enzymes. Among these enzymes, CHI enzymes play a crucial role in influencing the metabolism of flavonoids. In a study conducted by Li et al. (2019), it was observed that certain genes, both structural and regulatory, exhibit differential expression during seed development and are associated with plant flavonoids. Another study by Ma et al. (2021) focused on investigating the changes in polyphenols during microwave-assisted germination of buckwheat. The results showed that after microwave-assisted germination, the relative expression of CHI and PAL genes increased significantly. Moreover, the content of rutin, chlorogenic acid, and oysterin reached its maximum level after 5 days of germination.
Conclusions
In this study, different parts of maize were found to be enriched in various phenolic compounds post-germination. The qualitative and quantitative analyses of phenolic metabolites in different parts of germinated maize using LC-MS and HPLC techniques revealed 80 phenolics, of which 47, 48, and 53 accumulated in the seed embryo, radicle, and germ, respectively, with 12, 12, and 13 unique phenolic metabolites. After germination, 22, 26, and 34 polyphenols exhibited differential accumulation in the seed embryo, radicle, and germ, respectively. At the same time, the relative contents of 12, 15, and 20 phenolic metabolites were found to be increased, and the polyphenols that were upregulated in different parts mainly included phenolic acids and flavonoids. The order of monomeric phenolic content at 96 h of germination was: germ > radicle > seed embryo, with a significant increase in the contents of p-coumaric acid, (+)-catechin, and ferulic acid (p < 0.05). Moreover, the polyphenols isolated from the germ exhibited potent hypoglycemic activity. Conclusively, by means of germination, we found that germination treatment increased the content and type of polyphenols in maize and that the in vitro hypoglycemic activity of maize increased with prolonged germination, so we consider germination to be an effective means of increasing the nutritional properties of maize.
CRediT authorship contribution statement
Lipeng Liu: Investigation, Formal analysis, Writing – review & editing. Xiaomin Fang: Investigation, Formal analysis, Writing – original draft. Shida Ren: Investigation. Rui Jia: Writing – review & editing. Qiannan Liu: Investigation, Writing – review & editing. Huimin Liu: Investigation, Writing – review & editing. Lin Xiu: Methodology, Writing – review & editing. Sanabil Yaqoob: . Dan Cai: Conceptualization, Supervision, Formal analysis, Writing – review & editing. Jingsheng Liu: Conceptualization, Supervision, Formal analysis, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by Scientific and technological innovation team project for outstanding young and middle-aged of Jilin Province (20230508014RC).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2023.100848.
Contributor Information
Dan Cai, Email: dan1980623@163.com.
Jingsheng Liu, Email: liujingsheng@jlau.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
The data that has been used is confidential.
References
- Aguilar J., Miano A.C., Obregón J., Soriano-Colchado J., Barraza-Jáuregui G. Malting process as an alternative to obtain high nutritional quality quinoa flour. Journal of Cereal Science. 2019;90 doi: 10.1016/j.jcs.2019.102858. [DOI] [Google Scholar]
- Aleixandre A., Gil J.V., Sineiro J., Rosell C.M. Understanding phenolic acids inhibition of α-amylase and α-glucosidase and influence of reaction conditions. Food Chemistry. 2022;372 doi: 10.1016/j.foodchem.2021.131231. [DOI] [PubMed] [Google Scholar]
- Carreño-Carrillo C.V., Sánchez E.V., Verduzco C.-P., J e. Polyphenol-based nuclear magnetic resonance non-targeted metabolomics of temperature- and time-controlled blue and red maize sprouting. SN Applied Sciences. 2021;3:300. doi: 10.1007/s42452-021-04171-w. [DOI] [Google Scholar]
- Chen X.X., Jiao J.Y., Cao W.X., Yu B.G., Liu Y.M., Zou C.Q. A sustainable phosphorus management in agriculture: Assessing trade-offs between human health risks and nutritional yield regarding heavy metals in maize grain. Environmental Research. 2022;203 doi: 10.1016/j.envres.2021.111792. [DOI] [PubMed] [Google Scholar]
- Dirir A.M., Daou M., Yousef A.F., Yousef L.F. A review of alpha-glucosidase inhibitors from plants as potential candidates for treatment of type-2 diabetes. Phytochemistry Reviews. 2022;21:1049–1079. doi: 10.1007/s11101-021-09773-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erenstein O., Jaleta M., Sonder K., Mottaleb K., Prasanna B.M. Global maize production, consumption and trade: Trends and R&D implications. Food Security. 2022;14:1295–1319. doi: 10.1007/s12571-022-01288-7. [DOI] [Google Scholar]
- Ge X., Jing L., Zhao K., Su C., Zhang B., Zhang Q.…Li W. The phenolic compounds profile, quantitative analysis and antioxidant activity of four naked barley grains with different color. Food Chemistry. 2021;335 doi: 10.1016/j.foodchem.2020.127655. [DOI] [PubMed] [Google Scholar]
- Ge X.Z., Saleh A.S.M., Jing L.Z., Zhao K., Su C.Y., Zhang B.…Li W.H. Germination and drying induced changes in the composition and content of phenolic compounds in naked barley. Journal of Food Composition and Analysis. 2021;95 doi: 10.1016/j.jfca.2020.103594. [DOI] [Google Scholar]
- Guan Q., Ding X.W., Jiang R., Ouyang P.L., Gui J., Feng L.…Song L.H. Effects of hydrogen-rich water on the nutrient composition and antioxidative characteristics of sprouted black barley. Food Chemistry. 2019;299 doi: 10.1016/j.foodchem.2019.125095. [DOI] [PubMed] [Google Scholar]
- Hostnik G., Gladovic M., Bren U. Tannin Basic Building Blocks as Potential Scavengers of Chemical Carcinogens: A Computational Study. Journal of Natural Products. 2019;82:3279–3287. doi: 10.1021/acs.jnatprod.9b00435. [DOI] [PubMed] [Google Scholar]
- Hu, W., Sarengaowa, Guan, Y & Feng, K. (2022). Biosynthesis of Phenolic Compounds and Antioxidant Activity in Fresh-Cut Fruits and Vegetables. Frontiers in Microbiology. 13, 906069. Doi: 10.3389/fmicb.2022.906069. [DOI] [PMC free article] [PubMed]
- Ikram A., Saeed F., Afzaal M., Imran A., Niaz B., Tufail T.…Anjum F.M. Nutritional and end-use perspectives of sprouted grains: A comprehensive review. Food Science & Nutrition. 2021;9:4617–4628. doi: 10.1002/fsn3.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan R., Asaf S., Numan M., Luban, Kim K.M. Plant Secondary Metabolite Biosynthesis and Transcriptional Regulation in Response to Biotic and Abiotic Stress Conditions. Agronomy. 2021;11:968. doi: 10.3390/agronomy11050968. [DOI] [Google Scholar]
- Jiao Y., Chen H.D., Han H., Chang Y. Development and Utilization of Corn Processing by-Products: A Review. Foods. 2022;11:3709. doi: 10.3390/foods11223709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kianfar, E. & Salimi, M. (2020). A Review on the Production of Light Olenfins from Hydrocarbons Cracking and Methanol Consversion. Advances in Chemistry Research, 59, 1-82. Chapter: 1.
- Kores K., Lešnik S., Bren U., Janežič D., Konc J. Discovery of Novel Potential Human Targets of Resveratrol by Inverse Molecular Docking. Journal of Chemical Information and Modeling. 2019;59:2467–2478. doi: 10.1021/acs.jcim.8b00981. [DOI] [PubMed] [Google Scholar]
- Li G., Liu X., Zhang Y., Muhammad A., Han W., Li D.…Cai Y. Cloning and functional characterization of two cinnamate 4-hydroxylase genes from Pyrus bretschneideri. Plant Physiology and Biochemistry. 2020;156:135–145. doi: 10.1016/j.plaphy.2020.07.035. [DOI] [PubMed] [Google Scholar]
- Li H., Lv Q., Ma C., Qu J.T., Cai F., Deng J.…Chen Q.F. Metabolite profiling and transcriptome analyses provide insights into the flavonoid biosynthesis in the developing seed of Tartary buckwheat (Fagopyrum tataricum) Journal of Agriculture and Food Chemistry. 2019;67:11262–11276. doi: 10.1021/acs.jafc.9b03135. [DOI] [PubMed] [Google Scholar]
- Lian Y., Zhang W., Liu F., Wang L., Yang X., Ma S.…Liu X. Recent advances in physiochemical changes, nutritional value, bioactivities, and food applications of germinated quinoa: A comprehensive review. Food Chemistry. 2023;426 doi: 10.1016/j.foodchem.2023.136390. [DOI] [PubMed] [Google Scholar]
- Liu S., Wang W., Lu H., Shu Q., Zhang Y., Chen Q. New perspectives on physiological, biochemical and bioactive components during germination of edible seeds: A review. Trend in Food Science & Technology. 2022;123:187–197. doi: 10.1016/j.tifs.2022.02.029. [DOI] [Google Scholar]
- Ma Y., Wang P., Wang M., Sun M.M., Gu Z.X., Yang R.Q. GABA mediates phenolic compounds accumulation and the antioxidant system enhancement in germinated hulless barley under NaCl stress. Food Chemistry. 2019;270:593–601. doi: 10.1016/j.foodchem.2018.07.092. [DOI] [PubMed] [Google Scholar]
- Ma H., Xu X.M., Wang S.M., Wang J.Z., Peng W.P. Effects of microwave irradiation on the expression of key flavonoid biosynthetic enzyme genes and the accumulation of flavonoid products in Fagopyrum tataricum sprouts. Journal of Cereal Science. 2021;101 doi: 10.1016/j.jcs.2021.103275. [DOI] [Google Scholar]
- Ma M., Zhang H.X., Xie Y.J., Yang M., Tang J.F., Wang P.…Gu Z.X. Response of nutritional and functional composition, anti-nutritional factors and antioxidant activity in germinated soybean under UV-B radiation. LWT-Food Science and Technology. 2020;118:1–13. doi: 10.1016/j.lwt.2019.108709. [DOI] [Google Scholar]
- Martinez G., Díaz S., Rosa L., Bustos J., Alvarez P. Inhibition of α-amylase by flavonoids: Structure activity relationship (SAR) Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2019;206:437–447. doi: 10.1016/j.saa.2018.08.057. [DOI] [PubMed] [Google Scholar]
- Ikenna C. Ohanenye, Apollinaire Tsopmo, Chukwunonso E.C.C. Ejike & Chibuike C. Udenigwe. (2020). Germination as a bioprocess for enhancing the quality and nutritional prospect of legume proteins. Trends in Food Science & Technology, 101, 213-222. Doi: 10.1016/j.tifs.2020.05.003.
- Oroian M., Dranca F., Ursachi F. Comparative evaluation of maceration, microwave and ultrasonic-assisted extraction of phenolic compounds from propolis. Journal of Food Science and Technology. 2020;57:70–78. doi: 10.1007/s13197-019-04031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradeep P.M., Sreerama Y.N. Phenolic antioxidants of foxtail and little millet cultivars and their inhibitory effects on α-amylase and α-glucosidase activities. Food Chemistry. 2018;247:46–55. doi: 10.1016/j.foodchem.2017.11.103. [DOI] [PubMed] [Google Scholar]
- Suriano S., Balconi C., Valoti P., Redaelli R. Comparison of total polyphenols, profile anthocyanins, color analysis, carotenoids and tocols in pigmented maize. LWT-Food Science and Technology. 2021;144 doi: 10.1016/j.lwt.2021.111257. [DOI] [Google Scholar]
- Tang Y., Xiao L., Wu X., Li W., Wu T., Zhang P. Impact of germination pretreatment on the polyphenol profile, antioxidant activities, and physicochemical properties of three color cultivars of highland barley. Journal of Cereal Science. 2021;97:1–11. doi: 10.1016/j.jcs.2020.103152. [DOI] [Google Scholar]
- Tomé-Sánchez I., Martín-Diana A.B., Peñas E., Frias J., Rico D., Jiménez-Pulido I., Martínez-Villaluenga C. Bioprocessed Wheat Ingredients: Characterization, Bioaccessibility of Phenolic Compounds, and Bioactivity During in vitro Digestion. Frontiers in Plant Science. 2021;12 doi: 10.3389/fpls.2021.790898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umpierrez G.E., Bailey T.S., Carcia D., Shaefer C., Shubrook J.H., Skolnik N. Improving postprandial hyperglycemia in patients with type 2 diabetes already on basal insulin therapy: Review of current strategies. Journal of Diabetes. 2018;10:94–111. doi: 10.1111/1753-0407.12576. [DOI] [PubMed] [Google Scholar]
- Wu N.N., Li R., Li Z.J., Tan B. Effect of germination in the form of paddy rice and brown rice on their phytic acid, GABA, γ-oryzanol, phenolics, flavonoids and antioxidant capacity. Food Research International. 2022;159 doi: 10.1016/j.foodres.2022.111603. [DOI] [PubMed] [Google Scholar]
- Yan Z., Wang H., Kou X., Wu C., Fan G., Li T., Zhou D. Metabolomics analysis reveals that MeJA treatment induces postharvest blueberry resistance to Botrytis cinerea. Postharvest Biology and Technology. 2022;194 doi: 10.1016/j.postharvbio.2022.112075. [DOI] [Google Scholar]
- Yong, Z., BK, A., Zhao, W., Shi, L., Wu, H., Barrow, C., Dunshea, F. & Suleria, H. A.R. (2022). Bioaccessibility and bioavailability changes of phenolic compounds in pumpkins (Cucurbita moschata): A review. Food Bioscience. 47, 101753. Doi: 10.1016/j.fbio.2022.101753.
- Zeng, L., Zhang, G., Lin, S., & Gong, D. (2019). Inhibitory mechanism of apigenin onα-glucosidase and synergy analysis of flavonoids. Journal of Agricultural and Food Chemistry,67, 6939–6949.http://doi.org/10.1021/acs.jafc.6b02314. [DOI] [PubMed]
- Zhang B., Xing Y., Wen C., Yu X., Sun W., Xiu Z., Dong Y. Pentacyclic triterpenes as a-glucosidase and a-amylase inhibitors: Structure-activity relationships and the synergism with acarbose. Bioorganic and Medicinal Chemistry Letters. 2017;27:5065–5070. doi: 10.1016/j.bmcl.2017.09.027. [DOI] [PubMed] [Google Scholar]
- Zhang G., Xu Z.C., Gao Y.Y., Huang X.X., Zou Y.P., Yang T.K. Effects of germination on the nutritional properties, phenolic profiles, and antioxidant activities of buckwheat. Journal of Food Science. 2015;80:H1111–H1119. doi: 10.1111/1750-3841.12830. [DOI] [PubMed] [Google Scholar]
- Zhao X.H., Tao J.H., Zhang T., Jiang S.R., Wei W., Han H.P.…Yue H.L. Resveratroloside alleviates postprandial hyperglycemia in diabetic mice by competitively inhibiting a-glucosidase. Journal of Agricultural and Food Chemistry. 2019;67:2886–2893. doi: 10.1021/acs.jafc.9b00455. [DOI] [PubMed] [Google Scholar]
- Zhou P.L., Li Q.Y., Liu G.L., Xu N., Yang Y.J., Zeng W.L.…Wang S.S. Integrated analysis of transcriptomic and metabolomic data reveals critical metabolic pathways involved in polyphenol biosynthesis in Nicotiana tabacum under chilling stress. Plant Function and Evolutionary Biology. 2018;46:30–43. doi: 10.1071/FP18099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that has been used is confidential.