Abstract
In adverse pregnancy a perturbed redox environment is associated with abnormal early-life cardiovascular development and function. Previous studies have noted alterations in the expression and/or activity of Nuclear Factor E2 Related Factor 2 (NRF2) and its antioxidant targets during human gestational diabetic (GDM) pregnancy, however to our knowledge the functional role of NRF2 in fetal ‘priming’ of cardiovascular dysfunction in obese and GDM pregnancy has not been investigated. Using a murine model of obesity-induced glucose dysregulated pregnancy, we demonstrate that NRF2 activation by maternal sulforaphane (SFN) supplementation normalizes NRF2-linked NQO1, GCL and CuZnSOD expression in maternal and fetal liver placental and fetal heart tissue by gestational day 17.5. Activation of NRF2 in utero in wild type but not NRF2 deficient mice improved markers of placental efficiency and partially restored fetal growth. SFN supplementation was associated with reduced markers of fetal cardiac oxidative stress, including Nox2 and 3-nitrotyrosine, as well as attenuation of cardiac mass and cardiomyocyte area in male offspring by postnatal day 52 and improved vascular function in male and female offspring by postnatal day 98. Our findings are the first to highlight the functional consequences of NRF2 modulation in utero on early-life cardiovascular function in offspring, demonstrating that activation of NRF2 affords cardiovascular protection in offspring of pregnancies affected by redox dysregulation.
Keywords: NRF2, Obesity, Diabetes, Cardiovascular, Pregnancy, Sulforaphane
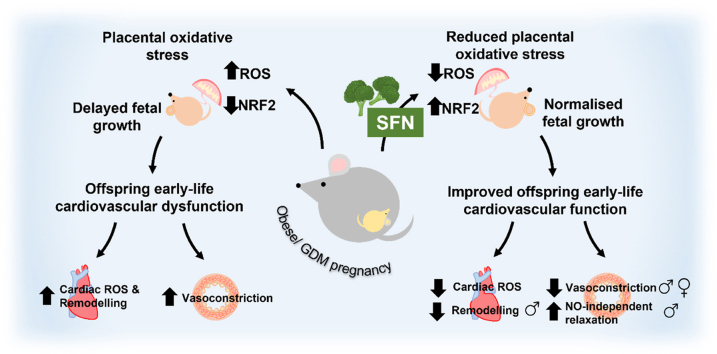
Graphical abstract
Highlights
-
•
Obesity induced glucose dysregulation reduces placental NRF2-linked gene expression.
-
•
Reduced placental NRF2 expression is associated with worsened pregnancy outcomes.
-
•
In utero sulforaphane restores fetal growth and placental efficiency in WT not NRF2KO pregnancies.
-
•
Developmental NRF2 modulation alters offspring cardiac redox status.
-
•
Sulforaphane attenuates early-life adverse cardiac remodelling and vascular dysfunction in offspring.
Abbreviations
- ARE
antioxidant response element
- AUC
area under curve
- CuZnSOD
copper zinc superoxide dismutase
- EC
endothelial cell
- ECL
enhanced chemiluminescence
- eNOS
endothelial nitric oxide synthase
- EpRE
electrophilic response element
- FBG
fasting blood glucose
- FOV
field of view
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GCLC (M)
glutamate-cysteine ligase catalytic (modifier) subunit
- GD
gestational day
- Grn
glucoraphanin
- GSH
reduced glutathione
- H&E
haematoxylin and eosin
- HUVEC
human umbilical vein endothelial cells
- i.pGTT
intra-peritoneal glucose tolerance test
- Keap1
Kelch-like ECH-associated protein 1
- l-NAME
L-NG-Nitro arginine methyl ester
- MC4R
melanocortin-4 receptor
- NA
noradrenaline
- Nox2
NADPH oxidase 2
- NRF2
nuclear factor erythroid 2–related factor 2
- NRFKO
NRF2 deficient/knockout
- NQO1
NAD(P)H quinone oxidoreductase 1
- 3-NT
3-nitrotryrosine
- PD
postnatal day
- PFA
paraformaldehyde
- ROS
reactive oxygen species
- SDS
sodium dodecyl sulfate
- SFN
sulforaphane
- SNP
sodium nitroprusside
- WT
wild type
1. Introduction
Several studies have reported that pregnancy complicated by obesity and GDM enhances in utero oxidative stress, with increased systemic [1] and placental reactive oxygen and nitrogen species (ROS/RNS) generation [2,3]. Maternal oxidative stress coincides with abnormal uterine artery perfusion and/or placental maladaptation [4,5] altering maternal:fetal transfer to influence fetal growth and development [6,7]. Pathological early life changes in cardiac development have been reported, including fetal ventricular hypertrophy, interventricular septal thickening, impaired myocardial function and increased heart rate in human [[8], [9], [10], [11]] and animal models of obesity and glucose dysregulation in pregnancy [[12], [13], [14]]. Moreover, vascular reactivity is compromised, with early-life vascular dysfunction and increases in systolic blood pressure leading to adult-onset hypertension and/or cardiovascular related death [[15], [16], [17], [18], [19], [20]], with perturbations in redox homeostasis influencing the phenotype of developing cardiac and vascular tissues [21,22].
We and others have reported that activation of the antioxidant transcriptional regulator NRF2 is altered in placenta and human fetal umbilical vein endothelial cells (HUVEC) derived from GDM pregnancies [22,23]. However, the functional consequences of NRF2 modulation during adverse pregnancy on early-life cardiovascular development and function remain to be elucidated. NRF2 is a member of the bZip transcription factor family, which under basal conditions is negatively regulated by Keap1, promoting its Cul-3 mediated ubiquitin-dependent degradation via the 26S proteasome [[24], [25], [26]]. Both oxidative stress and SFN promote NRF2 nuclear accumulation and binding to the antioxidant/electrophile response element (ARE/EpRE), an effect attenuated via specific Cys to Ser Keap1 point mutations [27,28]. SFN can also conjugate with glutathione (GSH) depleting the cellular thiol pool to trigger NRF2 activation. NRF2 induces a wide variety of antioxidant, detoxification and GSH-related genes including superoxide dismutase (SOD) [29], NAD(P)H:quinone oxidoreductase 1 (NQO1) [30] and glutamyl cysteine ligase (GCL) [31]. SFN is derived from glucoraphanin (Grn) found in Brassica vegetables, with NRF2 activation by SFN has well documented cardiometabolic protective actions [32] and preliminary reports suggest that Grn supplementation in human pre-eclamptic pregnancy is safe, with no influence of SFN on fetal heart rate [33].
The present study investigates the effects of dietary SFN on redox signaling and early-life cardiovascular function in wild type and global NRF2 deficient pregnant mice. Our findings establish that activation of NRF2 with dietary SFN during obese, glucose intolerant pregnancy suppresses oxidative damage, improves placental efficiency and fetal growth, attenuates cardiac oxidative damage, cardiac remodelling and vascular dysfunction in offspring. Our study suggests that activation of NRF2 targeted antioxidant gene expression during development in an adverse in utero environment is cardioprotective for offspring.
2. Materials and methods
2.1. Consumables and reagents
Mouse diets and the micronutrient mineral mix were purchased from LBS Biotech (Horley, UK). dl-Sulforaphane was obtained from Cayman Chemical (Ann Arbor, MI, USA). Sensitive mouse insulin ELISA was from Mercodia (Uppsala, Sweden). 6-Diamidino-2-phenylindole dihydro chloride (DAPI), Alexa Fluor™647 conjugated wheat germ agglutinin (WGA) and SuperFrost Menzel-Gläser microscope slides were from ThermoFisher Scientific (Waltham, MA, USA). The NAD(P)H quinone oxidoreductase 1 (NQO1) antibody was from Santa Cruz Biotechnology Inc. (Dallas, TX, USA). AlexaFluor® conjugated secondary antibodies, cytosolic copper zinc superoxide dismutase (CuZnSOD), and 3-nitrotryrosine (3-NT) antibodies were from Abcam (Cambridge, UK). Glutamate-cysteine ligase catalytic and modifier subunit (GCLC and GCLM) antibodies were a gift of Prof. Terrance Kavanagh (University of Washington, Seattle, WA, USA). Anti-mouse Cy3 secondary antibodies were from Jackson ImmunoResearch (Ely, UK). Enhanced chemiluminescence reagents (ECL) were from GE Healthcare Life Science (Amersham, UK). Anti-sarcomeric α-actinin antibodies and all other reagents were purchased from Merck (Darmstadt, Germany) or Millipore-Sigma (Burlington, MA, USA).
2.2. Experimental animals
All procedures were conducted in accordance with the UK Home Office Animals (Scientific Procedures) Act 1986 and ARRIVE guidelines and approved by the King's College London's AWERB. Although experimenter blinding was not possible due to obvious physical features/properties enabling genotype/intervention identification, where possible, data were validated by at least 2 independent experimenters. Dams were excluded based on failure to carry a viable litter or failure to develop diet-induced metabolic dysregulation, as sporadically noted in C57 mice [34,35]. All mice were maintained under humidity-controlled conditions (25 °C, 12 h light/dark cycle) with ad libitum access to food and water. Wild type C57BL/6J mice were purchased from Charles River and NRF2 deficient (NRF2KO) mice on a C57BL/6 background were bred in-house as previously described [36].
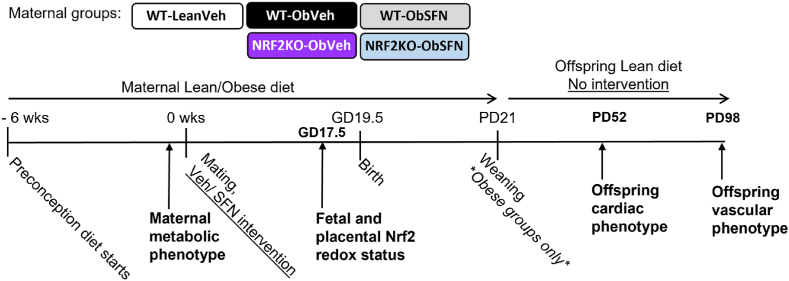
The animal model of diet-induced obesity and glucose dysregulation during pregnancy is illustrated in Fig. 1. As previously validated [17,37], proven breeders were fed ad libitum an obesogenic diet (Ob, 10% simple sugars, 20% animal lard, 28% polysaccharide, 23% protein, w/w%, 4.5 kcal/g, #824053, LBS Biotech) supplemented with sweetened condensed milk (∼55% simple sugars, 8% fat, 8% protein, w/w%, Nestle™) containing a micronutrient mineral mix (#AIN93G) for typically 6, maximally 8 weeks, until dams had gained ≥30% of their starting weight. A subset of wild type (WT) dams were maintained on a standard RM3 breeding diet analogous to RM1 control diet but with added micronutrient mineral mix incorporated (Lean, 7% simple sugars, 3% fat, 50% polysaccharide, 15% protein, w/w%, 3.5 kcal/g, LBS Biotech). Pre-pregnancy glucose dysregulation was confirmed by an intra-peritoneal glucose tolerance test (i.pGTT), where blood glucose was above 20 mM for ≥30min following a glucose bolus. Single housed dams were mated, with copulatory plug indicating gestational day 0.5 (GD 0.5). Animals on the Ob diet were randomized to receive either sulforaphane (SFN, 2.5 mg/kg) or vehicle (corn oil) administered orally in Nutella™ 6 days/week throughout gestation and lactation, with no intervention given on the remaining weekday. Litters were standardized to 5–6 pups by 48 h postpartum, and at postnatal day (PD) 21, male and female offspring were weaned onto RM1 control diet without any further intervention.
Fig. 1.
Study design
Proven breeder C57BL/6 wild type (WT) and NRF2 knockout (NRFKO) dams were fed a normal chow (RM1) diet (Lean) or were switched to an obesogenic (Ob) diet prior to pregnancy. Maternal metabolic dysregulation was confirmed when dams had gained ∼30% of their original body weight and developed glucose intolerance assessed ipGTT. Dams were randomly assigned to receive either dietary vehicle (corn oil, WT-LeanVeh/WT-ObVeh/NRF2KO-ObVeh) or SFN (2.5 mg/kg, WT-ObSFN/NRFKO-ObSFN) for 6 days/week throughout pregnancy and lactation. With the presence of a morning copulation plug signifying gestational day 0.5 (GD0.5), WT-LeanVeh dams fed RM3 and a subset of Ob dams were sacrificed at GD17.5 to assess fetal outcomes and placental development. Male and female offspring from the remaining Ob dams were weaned onto control RM1 diet without further veh/SFN intervention and sacrificed on postnatal day 52 (PD52, 7.5 weeks – males only) or PD98 (14 weeks) to assess cardiac morphology (PD52) and/or vascular reactivity (PD98).
2.3. i.pGTT and insulin measurement
Mice were fasted for 6 h, before tail vein glucometer sampling (Contour Next, Bayer) to assess fasting blood glucose (FBG) and glucose tolerance over 120min following a 2 g/kg d-glucose bolus (i.p.) [38]. Maternal plasma insulin was measured by ELISA.
2.4. Pregnancy outcomes
Dams were terminally anesthetized (4%v/v isoflurane) at gestational day 17.5 (GD17.5). Median wet weights of 2–3 viable fetuses and respective placentas were recorded, alongside the number of resorbed conceptuses, total litter size and/or pregnancy loss. The size of viable embryos was additionally calculated by multiplying the crown-to-rump length with the orofacial distance (CRL x OF mm2) [39]. For protein expression analyses, placentae or fetal tissues from the same litter were pooled, snap frozen and treated as an experimental sample.
2.5. Placenta and heart histology
Placental tissues (GD17.5) and hearts from male and female offspring (post-natal day PD52 male only or PD98 male and female) were weighed, rinsed in phosphate buffered saline (PBS, 4 °C) and preserved for either histological or tissue processing. Paraformaldehyde (PFA) fixed placental tissues were embedded along their long axis, with 5 μm midline sections stained with haematoxylin and eosin (H&E) and imaged. To evaluate the size of the maternal-fetal vascular interface zone, the labyrinth area was selected on placenta images using FIJI software [40]. The mean labyrinth and total placenta area of ≥3 individual placentae per litter was measured in mm2 and expressed as % area of the labyrinth zone to total placental area. Male offspring PD52 hearts were snap frozen in liquid N2 and stored at −80 °C until sectioning. Frozen hearts mounted in OCT were transversely cryosectioned (12 μm) starting from the heart apex. Mid-heart sections (×3) were subject to immunofluorescent staining with appropriate negative controls as described previously [41]. Immunofluorescence images were acquired with an inverted epifluorescence microscope (Nikon) and analysed with FIJI software as previously described [42]. Sarcomeric α-actinin staining was used to exclude longitudinally sectioned cardiomyocytes from analyses. Cardiomyocyte area along the WGA-stained cell border was measured using FIJI. Average cross-sectional area from ≥300 cardiomyocytes selected from 3 different fields of view (FOV) per heart is presented for each individual offspring.
2.6. Immunoblotting
Homogenized tissues (Tissue Lyser, Qiagen) were lysed in SDS buffer (2% w/v containing protease/phosphatase inhibitors), with proteins denatured (95 °C 5min). Equal amounts of protein were separated by gel electrophoresis, transferred onto PVDF membranes and blocked. Membranes were incubated overnight (4 °C) with primary antibodies raised against proteins of interest or housekeeper GAPDH. Protein expression was determined by ECL-based detection of horseradish peroxidase-conjugated secondary antibody luminescence visualized with the G-box gel documentation system (Syngene Bioimaging), with densitometric analysis performed using FIJI software [43].
2.7. Wire myography
Vascular reactivity of isolated male and female offspring mesenteric arteries (PD98, 300–400 μm) was assessed as previously described [44]. First and second order branches of the superior mesenteric artery were dissected in physiological salt solution (PSS [mM], NaCl 118, NaHCO3 24, NaH2PO4 0.53, MgSO4 1, KCl 4, d-glucose 5.5, CaCl2 1.8) and mounted with 40 μm wires on a 620 M Multi Myograph system (Danish Myo Technology, Denmark) continuously superfused with a 5% CO2/95% air gas mixture. Vasculature viability was determined by the presence of both an isometric contraction of >0.5 mN/mm in response to high K+ (80 mM) PSS solution and >60% vasorelaxation of noradrenaline (NA) pre-constricted vessels in response to acetylcholine (Ach, 10−5 M). Mesenteric resistance artery reactivity was subsequently determined by vasoconstriction responses to cumulative addition of NA (10−9 to 10−4 M) or vasorelaxation of sub-maximally pre-constricted vessels (80% of maximal NA constrictor tone) by Ach (10−9 to 10−4 M) or nitric oxide donor sodium nitroprusside (SNP, 10−9 to 10−4 M). To assess eNOS-independent vasorelaxation, a subset of vessels wwere incubated with the eNOS inhibitor l-NAME (20 min, 10−4 M) and Ach concentration-response relationships were repeated in NA pre-constricted vessels as described above.
2.8. Statistical analysis
Data points represent individual dams or offspring derived from different litters unless stated otherwise. Statistical comparisons between two independent groups were performed using an unpaired Student's t-test, while one- or two-way ANOVA with Tukey and Sidak post-hoc tests, respectively, were used to evaluate statistical differences between more than two conditions as applicable. P < 0.05 was considered statistically significant.
3. Results
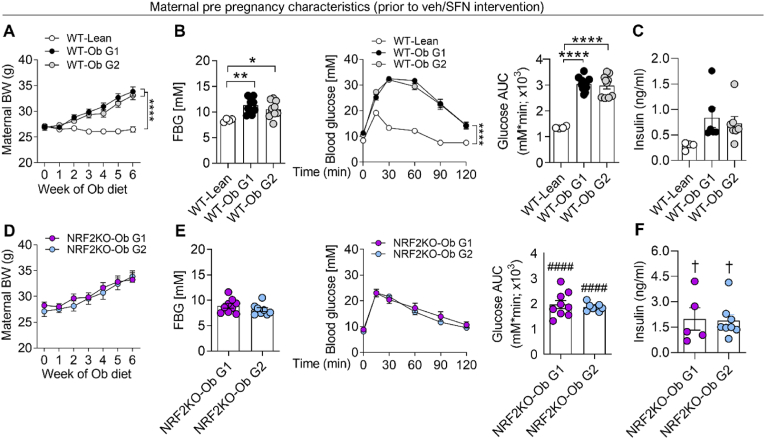
3.1. Obesity confers glucose dysregulation in WT and to a lesser extent NRF2 deficient dams
Replicating previous studies of diet-induced metabolic dysregulation in pregnancy [37], 6 weeks of Ob diet feeding prior to pregnancy resulted in WT dams becoming obese (Fig. 2A) and exhibiting marked glucose dysregulation. Compared with WT-Lean dams, WT-Ob dams had elevated fasting blood glucose (FBG), showed impaired glucose tolerance following i.pGTT (Fig. 2B) and trended to have raised fasting insulin (Fig. 2C). In agreement with previous studies [45], NRF2KO-Ob dams developed diet-induced obesity (Fig. 2D) and a significantly milder form of diet-induced glucose intolerance compared to WT-Ob groups (Fig. 2E), accompanied by a significant elevation in fasting insulin levels compared to WT-Lean dams, likely due to enhanced H2O2-mediated insulin release [46] (Fig. 2F). Critically, for both genotypes no baseline intergroup variability was observed between dams that went on to receive either veh (group 1) or SFN intervention (group 2) in subsequent studies.
Fig. 2.
Obesogenic diet induces pre-gestational obesity and glucose intolerance in WT and to a lesser extent in NRF2 KO dams
WT (A-C) and NRF2 KO (D-F) dams were maintained on normal chow RM1 (Lean) or fed an obesogenic diet (Ob) prior to mating. For obese groups of each genotype, littermate dams were randomly assigned to one of two groups; Group 1 (G1) subsequently went on to receive vehicle, while Group 2 (G2) subsequently received dietary SFN (2.5 mg/kg) during pregnancy. Prior to pregnancy and veh/SFN intervention, dam body weight was recorded weekly during Ob or Lean RM3 diet exposure (A, D). During the final week of pre-pregnancy diet exposure, dams were fasted for 6 h to assess fasting blood glucose (FBG), glucose tolerance (ipGTT 2 g/kg, 0–120 min) with data displayed as time course and corresponding area under curve (AUC) (B, E) and fasting insulin (C, F). Data are summarised as mean ± S.E.M., n = 4–20, with data points representing individual dams. *P < 0.05, **P < 0.01 and ***P < 0.0001 in WT-Ob vs WT-Lean, ####P < 0.0001 Nrf2KO-Ob vs WT-Ob †P < 0.05 Nrf2KO-Ob vs WT-Lean.
3.2. SFN improves pregnancy outcomes and placental efficiency
WT-ObVeh dams had significantly reduced gestational weight gain and enhanced frequency of fetus resorption compared to WT-Lean and WT-ObSFN groups (Supplementary Figs. 1A–B), although the number of total conceptuses was not altered (data not shown). Fetal weight (Table 1) and fetal size (Supplementary Fig. 1C) were significantly reduced in WT-ObVeh vs WT-Lean dams, which was largely corrected in WT-ObSFN dams. In contrast, NRF2KO Ob dams were not protected by SFN, having smaller fetuses and enhanced incidence of resorption (Supplementary Fig. 1B). Despite no overall effects on placental weight between any group (Table 1), WT-ObVeh litters exhibited markers of placental insufficiency, including a reduced fetal:placental ratio and a reduced labyrinth zone exchange region in relation to total placental area (Table 1, H&E images shown in Supplementary Fig. 2A). Supplementation with SFN throughout the gestational period improved the fetal:placental ratio and the relative labyrinth area in WT but not NRF2KO Ob dams.
Table 1.
Fetal and placental weights and measurements demonstrating that SFN improves placental efficiency partly by restoring a normal fetal growth trajectory in WT but not NRF2KO dams.
| Fetal weight (g) | Placental weight (g) | Fetal:placental ratio | Labyrinth zone area (% of total placental area) | |
|---|---|---|---|---|
| WT-LeanVeh (n = 8) | 0.921 ± 0.019b | 0.093 ± 0.004 | 9.94 ± 0.28 | 58.81 ± 1.99 |
| WT-ObVeh (n = 9) | 0.708 ± 0.015a, b | 0.097 ± 0.004 | 7.42 ± 0.3a, b | 44.53 ± 0.63a, b |
| WT-ObSFN (n = 8) | 0.83 ± 0.025a | 0.086 ± 0.003 | 9.68 ± 0.33 | 51.15 ± 2.32 |
| NRF2KO-ObVeh (n = 4) | 0.725 ± 0.027 | 0.088 ± 0.011 | 8.35 ± 1.17 | 50.17 ± 1.65 |
| NRF2KO-ObSFN (n = 5) | 0.722 ± 0.028b | 0.096 ± 0.005 | 7.6 ± 0.81b | 47.03 ± 0.38 |
Values denote mean ± S.E.M., n = 4–9, for measurements labyrinth zone area measurements n = 3 across treatment groups. aP < 0.05, 0.01, 0.0001 vs WT-LeanVeh, bP < 0.05, 0.01, 0.001 vs WT-ObSFN.
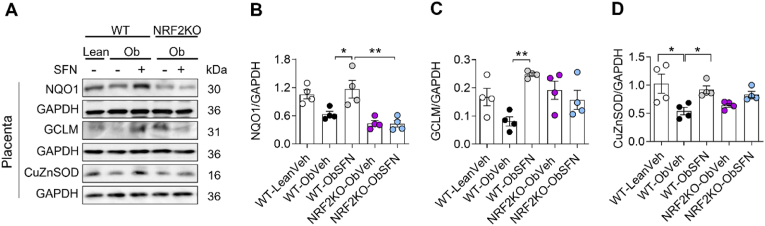
3.3. SFN induces maternal and fetal NRF2 target antioxidant genes
In the present study, SFN partially restored fetal growth and markers of placental efficiency. We next determined whether this was linked to NRF2 target gene upregulation in fetal and placental tissues. Obesity suppresses placental NRF2 targets NQO1, GCLM and CuZnSOD, associated with phase II, GSH-mediated and constitutive antioxidant defenses. Notably, SFN supplementation in WT Ob dams restored NRF2 target gene expression (Fig. 3A–D), which remained suppressed in NRF2KO Ob dams. Obesity-induced suppression of NRF2 targeted genes in maternal and fetal liver was also reversed by SFN in WT but not NRF2KO Ob pregnant mice (Supplementary Fig. 3). In placenta of WTOb mice, suppression of NRF2 gene expression was paralleled by enhanced 3-NT and to a lesser extent Nox2 expression. These markers of oxidative stress trended to increase in NRF2KO compared to WT placentas (Supplementary Figs. 1D–F).
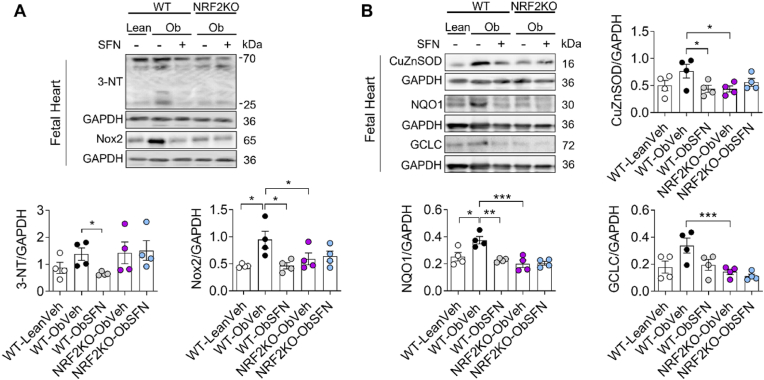
Fig. 3.
SFN restores expression of inducible and constitutive NRF2-linked antioxidant genes in placenta from WT but not NRF2 deficient dams
At mating, WT-lean dams were supplemented with veh and WT and NRFKO obese dams were supplemented with veh or SFN (2.5 mg/kg) for 6 days/week throughout pregnancy before termination at gestational day 17.5 (GD17.5). A-D, Representative immunoblots (A) and summary data showing normalised protein expression of NQO1 (B) and GCLM (C) and constitutive CuZnSOD (D) in placenta. Data denote mean ± S.E.M of n = 4 individual dam litters. *P < 0.05 and **P < 0.01.
3.4. SFN attenuates obesity and GDM primed in utero cardiovascular oxidative stress and abnormal cardiac development in WT offspring
We next characterized the impact of maternal obesity and SFN supplementation on offspring cardiac development. Male offspring cardiomyocyte cross-sectional area (Fig. 4) at PD52 and normalised heart size at PD98 (Table 2) were enlarged in offspring of WT-ObVeh compared to WT-ObSFN treated dams, with a similar non-significant trend observed in female offspring (Supplementary Table 1). Interestingly, male and female offspring of NRF2KO Ob dams did not exhibit adverse cardiac remodelling normally associated with Ob pregnancy, with SFN supplementation in NRF2KO dams also having no effect on heart morphology (Fig. 4, Table 2, Supplementary Table 1). We next determined whether SFN altered cardiac oxidative stress in offspring.
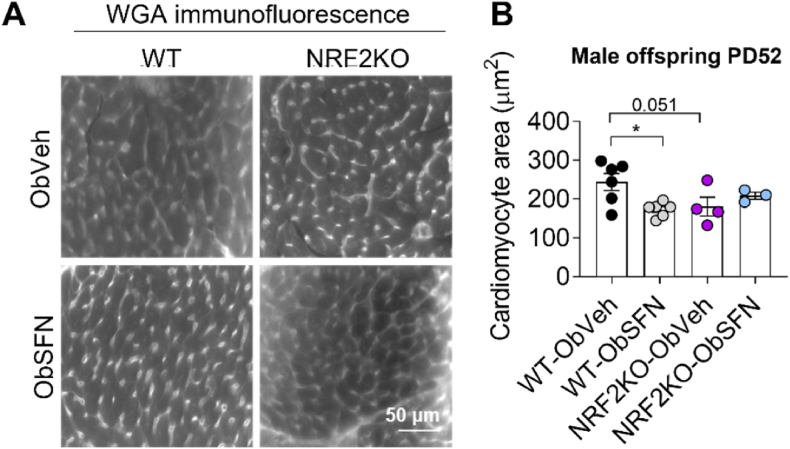
Fig. 4.
Maternal dietary SFN during obese pregnancy reduces cardiomyocyte area in hearts of male offspring
Hearts from the 7-week old male offspring of WT-Ob and NRF2KO-Ob dams supplemented with veh or SFN (2.5 mg/kg) throughout pregnancy and weaning were fixed and frozen sections (12 μm) stained with wheat germ agglutinin (WGA) for measurements of cardiomyocyte cross-sectional area. Representative WGA immunofluorescence grayscale images (A) and quantitative analysis of cardiomyocyte area (B) determined transverse sections. Data denote mean ± S.E.M from measurements of at least 500 cells and 3 separate FOV from n = 3–6 individual dam litters per group. *P < 0.05.
Table 2.
Male offspring heart weight at PD52 and PD98 following obese WT and NRF2KO pregnancy supplementation with SFN (2.5 mg/kg) versus vehicle.
| Male | PD52 Wet heart weight (g) | PD52 Heart weight/BW (mg/g) | PD98 Wet heart weight (g) | PD98 Heart weight/BW (mg/g) |
|---|---|---|---|---|
| WT-ObVeh | 0.166 ± 0.008 | 7.2 ± 0.4 | 0.182 ± 0.010 | 6.9 ± 0.4 |
| WT-ObSFN | 0.155 ± 0.01 | 6.3 ± 0.4 | 0.159 ± 0.009 | 5.7 ± 0.3* |
| NRF2KO-ObVeh | 0.154 ± 0.018 | 6.4 ± 0.7 | 0.156 ± 0.013 | 5.2 ± 0.3** |
| NRF2KO-ObSFN | 0.142 ± 0.007 | 5.8 ± 0.2 | 0.162 ± 0.008 | 5.3 ± 0.2 |
Values denote mean ± S.E.M., n = 5 (PD52) – 10 (PD98), *P < 0.05 and **P < 0.01 vs WT-ObVeh at PD98. Wet heart weight was normalised to total body weight (BW).
In fetal hearts from WT-ObSFN but not NRF2KO-ObSFN litters, SFN suppressed 3-NT and Nox2 expression, indices of cardiac oxidative stress (Fig. 5A) previously reported in models of diabetic or nutritional embryopathy [21,47]. In agreement with the development of a milder phenotype of obesity-induced metabolic dysregulation in NRF2KO Ob dams (Fig. 2), markers of oxidative stress were not enhanced basally in NRF2KO-ObVeh vs WT-ObVeh fetal heart tissue despite a trend for enhanced 3-NT levels. In addition to enhanced oxidative stress markers, fetal hearts from WT-ObVeh litters showed basal upregulation of NRF2-linked genes CuZnSOD, NQO1 and GCLC. These findings contrast with our results in placenta and liver, suggesting metabolic dysregulation in pregnancy is accompanied by differential temporal patterning of oxidative stress in the fetal heart compared with other tissues. (Fig. 5B). Conversely, fetal hearts of WT-ObSFN pregnancies maintained a lower basal expression of NRF2-linked genes at GD17.5 mirroring the redox profile of WT-Lean dams, and consistent with decreased expression of 3-NT and Nox2 compared with WT-ObVeh pregnancies (Fig. 5A–B).
Fig. 5.
SFN suppresses oxidative stress in fetal hearts of WT obese litters resulting in normalization of NRF2-linked antioxidant enzyme expression
At mating, WT-Lean dams were supplemented with veh and WT and NRF2KO obese dams were supplemented with veh or SFN (2.5 mg/kg) for 6 days/week throughout pregnancy before termination at gestational day 17.5 (GD17.5). A-B, Representative immunoblots and densitometric analysis of 3-NT and Nox2 (markers of oxidative stress, panel A), constitutive CuZnSOD and inducible NRF2 targets NQO1 and GCLC (B) expression relative to GAPDH. Data denote mean ± S.E.M of n = 4 individual dam litters per group. *P < 0.05, **P < 0.01 and ***P < 0.001.
3.5. SFN supplementation in pregnancy improves vascular function in offspring from obese mice
To further define the impact of NRF2 modulation on offspring cardiovascular health, we assessed the reactivity of isolated mesenteric arteries in male and female offspring at 3 months of age (Fig. 6), with maximal constrictor and vasodilatory responses plotted in Supplementary Fig. 4. Developmental SFN supplementation reduced the hypercontractile responses to noradrenaline (NA) in male and female WT-Ob offspring, with female but not male NRF2KO-ObSFN offspring showing enhanced contractility compared with NRF2KO-Ob offspring (Fig. 6A, E). While in WT-Ob male and female offspring overall relaxation responses to Ach or the nitric oxide donor SNP were unaltered (Fig. 6B–C, 6E-G), male WT-ObSFN arteries pre-treated with nitric oxide synthase inhibitor l-NAME demonstrated enhanced endothelium-dependent but NO-independent relaxation to Ach (Fig. 6D).
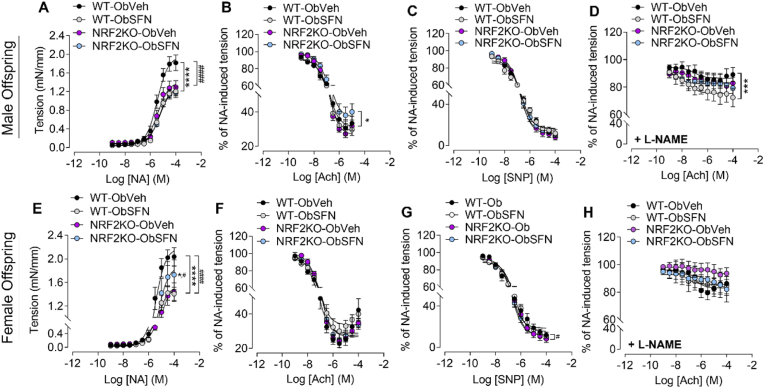
Fig. 6.
Dietary SFN attenuates resistance artery contractility in WT male and female 3-month-old offspring and improves nitric oxide independent vasorelaxation only in males
A-G, Wire myography used to assess vascular reactivity of small mesenteric arteries isolated at PD98 from male (A-D) and female (E-G) offspring of WT and NRF2KO obese dams given Veh or SFN (2.5 mg/kg) throughout pregnancy and weaning. Vasoconstriction in response to increasing concentrations of noradrenaline (NA, 10−9-10−4 M) as an isometric tension (A, E). Vasorelaxation of mesenteric arteries pre-constricted with NA in response to increasing concentrations of acetylcholine (Ach, 10−9-10−4 M, panels B, F), NO donor sodium nitroprusside (SNP, 10−9-10−4 M, panels C, G) or Ach in the presence of eNOS inhibitor l-NAME (10−5 M, 30 min, panels D, H). Results are expressed as % of NA pre-constriction tone. Data denote mean ± S.E.M. of measurements in offspring from n = 5–9 individual litters per group. *p < 0.05, ***P < 0.001 and ****P < 0.0001 vs maximal responses Veh vs SFN, #P < 0.05 ####P < 0.0001 WT vs NRF2KO corresponding condition.
Compared to NRF2KO-ObVeh, NRF2KO-ObSFN male offspring showed no change in NA-induced contractility, however female NRF2KO-ObSFN pregnancies exhibited am exaggerated contractile response (Fig. 6A, E). Similarly, dietary administration of SFN to NRF2KO obese mice led to impaired Ach induced NO-dependent relaxation in male offspring mesenteric arteries (Fig. 6B, Supplementary Fig. 4B) suggesting that NRF2 deficiency may worsen relaxation responses in male offspring.
4. Discussion
The present study provides the first evaluation of the impact of NRF2 signaling in diet-induced obese and GDM pregnancy on murine pregnancy outcomes, fetal growth and early-life cardiovascular development in offspring. We show that NRF2 positively influences obese pregnancy outcomes, including the ability to maintain viable fetuses, placental efficiency and to improve fetal growth. Notably, dietary SFN restores NRF2 target gene expression in a range of maternal and fetal tissues from obese mice, validating previous findings that SFN crosses the placenta to enter the fetal circulation and activate NRF2-linked gene transcription [48]. Somewhat surprisingly, our study highlighted that NRF2 signaling in the fetal mouse heart in late gestation is unique, being characterized by presumably a sustained upregulation rather than suppression of NRF2 genes in response to obesity, coinciding with enhanced markers of fetal cardiac ROS generation and nitrosylative stress. Our study is also the first to report that dietary SFN in pregnancy attenuates fetal cardiac oxidative stress, affording protection against early-life cardiac remodelling and improving vascular reactivity in offspring of obese dams.
In the context of NRF2 signaling in pregnancy, NRF2 deficient rodents are capable of reproducing and show a limited developmental phenotype indicating that under non-stressed conditions NRF2 is somewhat in essential for development [49]. Proteomic screening has however suggested a role for NRF2 in metabolic tissue adaptation to pregnancy, with β-cell NRF2 levels increasing by GD15 in mice [50]. A role for NRF2 in regulating the transgenerational phenotype of fetal epidermal keratinocytes has also been reported [48] and NRF2KO mice exhibit exacerbated neonatal lung injury [51] as well as a higher incidence of congenital intrahepatic shunt formation reducing hepatic perfusion [52]. Non-obese NRF2KO litters have also been shown to be more prone to oxidative insult leading to fetal resorption, reduced fetal growth and placental insufficiency, including reduced placental vascularisation and/or a reduction in the labyrinth maternal:fetal exchange region and total placental area [51,53].
Here, obesity reduced the ability of obese NRF2KO proven breeder dams to carry a successful timed pregnancy through to GD17.5 and more craniofacial abnormalities (particularly ocular) were noted in NRF2KO-ObSFN versus WT-ObSFN litters (data not shown). Moreover, SFN reduced fetal resorption in WT but not NRF2KO dams (Supplementary Fig. 1B), providing further evidence that NRF2 activation during development improves obese pregnancy outcomes. Consistent with our findings, in diabetic murine C57BL/KsJ‐Lep db/+pregnancies, the NRF2 inducer tBHQ reportedly improves maternal glucose tolerance, reduces markers of maternal oxidative stress and enhances postnatal offspring survival [54]. Comparing the phenotype of NRF2 deficient and diet-induced WT obese pregnancies, several studies report maternal obesity increases fetal resorption [55], with delayed fetal growth within the late first and second trimester and normalizing at birth [[56], [57], [58]]. In our study, both WT-ObVeh and NRF2KO-ObVeh fetuses were growth restricted. Moreover, SFN largely recovered fetal growth in WT mice only (see Table 1, Supplementary Figs. 1A and C), highlighting the protective role of NRF2 in fetal development in the context of obese pregnancy.
Retarded fetal growth has been linked to abnormal uterine artery perfusion and defective placentation, impeding maternal:fetal exchange. Previous studies by Guedes-Martins and colleagues highlighted that increased uterine artery pulsatility in human pregnancy is associated with increased placental bed ROS generation, detected by DHE fluorescence [5]. These authors speculated that the source of ROS was Nox, given the high prevalence of Nox2, Nox 1, Nox 4 and Nox 5 in the vascularised placenta. Our own data also highlights a non-significant trend for increased Nox2 expression in the placentas of WT-ObVeh dams by late pregnancy. Nox2 expression was reduced by SFN in WT but not NRF2KO dams, with NRF2KO SFN treated dams having enhanced markers of placental oxidative stress (Supplementary Fig. 1F). Providing further evidence of redox imbalance, in the present study obesity suppressed NRF2-dependent NQO1, GCL and CuZnSOD gene expression in placental, maternal and fetal hepatic tissues, which was fully restored upon SFN supplementation (Fig. 3, Supplementary Fig. 3). In preliminary studies, loss of NRF2-mediated gene expression in obese placentas was accompanied by a significant suppression of NRF2 and NQO1 mRNA (data not shown), suggesting that this suppression is likely transcriptionally linked although the underlying mechanism(s) remain to be determined. Constitutively expressed CuZnSOD is known to protect against diabetes-associated fetal loss [59] and is reportedly NRF2 regulated [29], as confirmed in our study. Placental SOD activity is reduced by maternal obesity and GDM [60] and has been correlated with smaller neonatal birth weight [23].
Several studies also highlight a reduction in the labyrinth placental exchange region as being associated with reduced fetal growth [56,57]. In our study, recovery of placental NRF2 gene expression and improved fetal growth in WT dams was associated with improved markers of placental efficiency (Table 1). Placental efficiency in obese dams was diminished but not further impacted by NRF2 deficiency, and notably SFN was only capable of recovering placental efficiency in WT dams. Although not addressed in our study, late gestational SFN supplementation has been shown to improve placental angiogenesis in WT and to a lesser extent NRF2KO pregnancies [51,61]. In our obese dams, NRF2 deficiency was not associated with a reduction in overall placental size (data not shown), unlike previous reports in non-obese NRF2 deficient litters [53]. Furthermore, in the context of obesity, exosomes derived from maternal visceral adipose tissue have been shown to enhance placental oxidative stress, altering fetal growth and inducing fetal cardiac oxidative stress and hypertrophy [62], presumably in part through changes in placental function. Collectively, such studies provide a rationale for how maternal environmental factors including redox imbalance and obesity can alter fetal development.
The influence of oxidative stress on fetal development is exemplified by studies of early life cardiac development. In rodents oxidative stress has been identified as a key pathway affecting cardiomyocyte development during the fetal and postnatal period. Although Nox2 expression is essential for normal cardiac development, particularly valvoseptal development [63], excessive oxidative stress resulting from hyperglycaemia has been associated with congenital abnormalities [21], and offspring of diabetic dams are more prone to cardiac ischemia-reperfusion injury mediated by mTOR-Nox2 signaling [64]. Oxidative stress also appears to alter cardiomyocyte proliferation in an oxygen dependent manner. Upregulation of cardiac ROS after birth due to transition to a comparatively oxygen enriched environment and metabolic switching to oxidative phosphorylation induces cardiomyocyte cell cycle arrest [65]. In contrast, exposure to hypoxia and/or noradrenaline reportedly induce neonatal cardiomyocyte proliferation, which can be attenuated by CuZnSOD overexpression, highlighting a role for superoxide in cardiomyocyte cell cycle regulation [66].
Early life cardiac hypertrophy in offspring of rodent obese pregnancy is widely reported [[12], [13], [14]] as is vascular dysfunction in murine adolescents and adults [18,37], mimicking echocardiography observations in neonates [8,9] and measured increases in systolic pressure in children and adults of obese/GDM mothers [15,20]. Our study provides the first evidence that developmental SFN supplementation is sufficient to diminish offspring cardiomyocyte area (Fig. 4) and reduce cardiac tissue mass (Table 2), consistent with SFN mediated protection in adult models of diabetic cardiomyopathy [[67], [68], [69]]. In mice unlike humans, anatomical fetal cardiac development continues during the early postnatal period. We are therefore unable to exclude the possibility that SFN mediated protection may be partially postnatally driven, since SFN and SFN-NAC conjugates are found in murine milk [51]. In support of SFN protection beginning in utero, fetal cardiac Nox2, 3-NT and NRF2-mediated gene expression were similar between WT-Lean and WT-ObSFN pregnancies (Fig. 5A–B). In contrast, WT-ObVeh fetal hearts showed enhanced markers of NRF2-linked genes at GD17.5, likely due to enhanced NRF2-dependent transcriptional activity due to ongoing oxidative stress, as suggested by the strong upregulation of 3-NT and Nox2 in obese fetal hearts and similar to findings in adult offspring of diabetic dams [64]. In GDM pregnancies a reduction in pro-inflammatory cytokines combined with increased placental antioxidants in term placentae further support the premise that maternal tissues can adapt long-term to oxidative stress [70].
This study is also the first to show that developmental SFN exposure can attenuate early markers of vascular dysfunction in male and female offspring of WT-ObVeh dams, including increased contractility in response to NA (Fig. 6A, D). Enhanced sympathetic tone has previously been noted in offspring of WT-Ob dams and is linked to enhanced leptin-mediated melanocortin-4 receptor (MC4R) activation [17]. While we did not find evidence of gross structural changes in offspring vessel morphology resulting from maternal obesity and/or SFN exposure (vessel diameter and high K+ responses unaltered, data not shown), SFN may be able to reduce sympathetic activity through modulation of offspring leptin signalling in our model. Previous studies have shown that NRF2 activation reduces plasma leptin in offspring of diabetic C57BL/KsJ‐Lep db/+ murine dams [54], with SFN able to reduce plasma leptin and attenuate injury-induced vascular smooth muscle cell proliferation [71]. Supporting our observations, in spontaneously hypertensive stroke prone rats the SFN precursor Grn can reduce hypertension in offspring of dams given Grn throughout pregnancy [72]. In addition to attenuation of vascular contractility, our data also demonstrated developmental SFN exposure enhanced NO-independent relaxation to Ach in male but not female 3 months old offspring (Fig. 6D and H). Though this sex-specific difference resolves with increasing age (data not shown), it is presently unclear whether enhanced endothelial dependent hyperpolarisation factor(s) (EDH) and/or prostacyclin-mediated relaxation responses were responsible. Enhanced sensitivity to Ach in female compared to male mesenteric vasculature was previously shown to be endothelial dependent and linked to estradiol-mediated vasodilatation pathways involving both K+-activated Ca2+ channels and prostacyclin [73,74] leaving us to speculate whether in our offspring the EDH/prostacyclin-dependent component of Ach-mediated vasodilation may increase with age.
Lastly, somewhat surprisingly our findings also showed NRF2KO-ObVeh offspring exhibited a somewhat improved cardiovascular phenotype compared to WT-ObVeh litters, with reduced cardiac mass/ cardiomyocyte area (Fig. 4 Table 2) and reduced contractile responses to NA, although contractility was increased in NRF2KO-ObSFN vs NRF2KO-ObVeh female offspring (Fig. 6A, E) and Ach induced relaxation was impaired in NRF2KO-ObSFN vs male offspring NRF2KO-ObVeh (Fig. 6B). While these data may indeed suggest developmental global NRF2 loss per se is somewhat cardioprotective, potentially agreeing with reports that mild oxidative stress in pregnancy improves offspring metabolic outcomes [75], the fact that SFN treatment enhanced contractility to NA in female and impaired Ach-induced relaxation in male NRF2KO offspring suggests that SFNs cardiovascular protective actions are indeed NRF2 mediated. In adult models of diabetic cardiomyopathy and vascular dysfunction NRF2 deficiency is reported to exacerbate pathology whereas NRF2 activation attenuates this phenotype [[32], [69], [76]], agreeing in part with our findings. Moreover, in WT dams we have previously noted that sporadic resistance to diet-induced glucose dysregulation (as noted by others [[34], [35]]) results in a milder cardiovascular phenotype in offspring (data not shown). Since NRF2KO dams were markedly protected against obesity-induced glucose intolerance (Fig. 2E), we hypothesise that it is the fundamentally improved maternal metabolic phenotype of NRF2 KO dams which has ultimately prevented the transmission of cardiovascular dysfunction to offspring of NRF2KO-Ob dams.
Previous studies have demonstrated that NRF2 deficiency [77] as well as hyperglycaemia [78] results in the upregulation of cardiac proliferation marker Nox2. Here, NRF2KO litters exhibited oxidative stress markers, including Nox2, only in placentas and not fetal cardiac tissue. We cannot discount the possibility that tissue specific differences in NRF2-dependent Nox2 regulation may in part account for this discrepancy. However, based on the robust induction of Nox2 in fetal hearts derived from NRF2-competent hyperglycaemic WT-ObVeh pregnancies, we suggest that the failure NRF2KO dams to develop overt glucose dysregulation (Fig. 2E–F) is likely the reason for lack of Nox2 upregulation in NRF2KO fetal hearts. Improved glucose tolerance in NRF2KOs has been linked to enhanced hydrogen peroxide-dependent phosphorylation of insulin receptor kinase, increasing insulin sensitivity [45,79] as well as reduced SIRT-dependent gluconeogenesis [45]. Nevertheless, our data in NRF2KO pregnancies has revealed a distinct role for NRF2 in regulating fetal growth and placentation, linked presumably to its role in modulating anti-angiogenic genes [51].
In summary, our study is the first to show that in the context of redox perturbed obese/glucose dysregulated pregnancy, SFN restores NRF2 signaling in maternal and fetal tissues to improve pregnancy outcomes and placental function in WT but not NRF2KO mice. Moreover, maternal obesity and hyperglycaemia are associated with enhanced markers of fetal cardiovascular oxidative stress resulting in cardiovascular dysfunction. In WT pregnant mice, SFN exposure affords early-life cardiovascular protection in offspring, including attenuating cardiac redox imbalance, cardiac mass and cardiomyocyte area, reducing NA induced vasoconstriction in male and female offspring and further improving NO-independent Ach-mediated relaxation in male offspring. Our study therefore suggests that NRF2 modulation with compounds such as SFN may be an effective strategy to prevent the developmental programming of cardiovascular dysfunction in the next generation, through restoration of redox balance in obese/GDM pregnancy.
Funding sources
This work was supported by the British Heart Foundation [PG/17/38/33024 and FS/20/8/34984] and a Physiological Society Research Grant [S.J.C.]. Work in the Ehler lab was supported by UKRI-MRC (MR/R017050/1) and by the BHF.
Authors contributions
P-M.P., G.E.M., P.D.T. and S.J.C. designed the experimental protocols, wrote the MS and are guarantors of the study. All other authors read and provided comments on the manuscript. P-M.P., J.K.M. and S.J.C. performed the experiments. L.S. and J.B. assisted with GTT experiments and E.E. assisted with cardiomyocyte studies and analysis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2023.102883.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Suhail M., Patil S., Khan S., Siddiqui S. Antioxidant vitamins and lipoperoxidation in non-pregnant, pregnant, and gestational diabetic women: erythrocytes osmotic fragility profiles. J. Clin. Med. Res. 2010;2(6):266–273. doi: 10.4021/jocmr454w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramirez-Emiliano J., Fajardo-Araujo M.E., Zuniga-Trujillo I., Perez-Vazquez V., Sandoval-Salazar C., Ornelas-Vazquez J.K. Mitochondrial content, oxidative, and nitrosative stress in human full-term placentas with gestational diabetes mellitus. Reprod. Biol. Endocrinol. 2017;15(1):26. doi: 10.1186/s12958-017-0244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coughlan M.T., Vervaart P.P., Permezel M., Georgiou H.M., Rice G.E. Altered placental oxidative stress status in gestational diabetes mellitus. Placenta. 2004;25(1):78–84. doi: 10.1016/S0143-4004(03)00183-8. [DOI] [PubMed] [Google Scholar]
- 4.Bugatto F., Quintero-Prado R., Visiedo F.M., Vilar-Sanchez J.M., Figueroa-Quinones A., Lopez-Tinoco C., Torrejon R., Bartha J.L. The influence of lipid and proinflammatory status on maternal uterine blood flow in women with late onset gestational diabetes. Reprod. Sci. 2018;25(6):837–843. doi: 10.1177/1933719117698576. [DOI] [PubMed] [Google Scholar]
- 5.Guedes-Martins L., Silva E., Gaio A.R., Saraiva J., Soares A.I., Afonso J., Macedo F., Almeida H. Fetal-maternal interface impedance parallels local NADPH oxidase related superoxide production. Redox Biol. 2015;5:114–123. doi: 10.1016/j.redox.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanley J.L., Cheung C.C., Rueda-Clausen C.F., Sankaralingam S., Baker P.N., Davidge S.T. Effect of gestational diabetes on maternal artery function. Reprod. Sci. 2011;18(4):342–352. doi: 10.1177/1933719110393029. [DOI] [PubMed] [Google Scholar]
- 7.Sferruzzi-Perri A.N., Vaughan O.R., Haro M., Cooper W.N., Musial B., Charalambous M., Pestana D., Ayyar S., Ferguson-Smith A.C., Burton G.J., Constancia M., Fowden A.L. An obesogenic diet during mouse pregnancy modifies maternal nutrient partitioning and the fetal growth trajectory. FASEB J. 2013;27(10):3928–3937. doi: 10.1096/fj.13-234823. [DOI] [PubMed] [Google Scholar]
- 8.Ingul C.B., Loras L., Tegnander E., Eik-Nes S.H., Brantberg A. Maternal obesity affects fetal myocardial function as early as in the first trimester. Ultrasound Obstet. Gynecol. 2016;47(4):433–442. doi: 10.1002/uog.14841. [DOI] [PubMed] [Google Scholar]
- 9.Garg S., Sharma P., Sharma D., Behera V., Durairaj M., Dhall A. Use of fetal echocardiography for characterization of fetal cardiac structure in women with normal pregnancies and gestational diabetes mellitus. J. Ultrasound Med. 2014;33(8):1365–1369. doi: 10.7863/ultra.33.8.1365. [DOI] [PubMed] [Google Scholar]
- 10.Taylor P.D., Gu H., Saunders H., Fiori F., Dalrymple K.V., Sethupathi P., Yamanouchi L., Miller F., Jones B., Vieira M.C., Singh C., Briley A., Seed P.T., Pasupathy D., Santosh P.J., Groves A.M., Sinha M.D., Chowienczyk P.J., Poston L., Consortium U. Lifestyle intervention in obese pregnancy and cardiac remodelling in 3-year olds: children of the UPBEAT RCT. Int. J. Obes. 2022;46(12):2145–2155. doi: 10.1038/s41366-022-01210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Bernardo S.C., Lava S.A.G., Epure A.M., Younes S.E., Chiolero A., Sekarski N., G. MySweetHeart Research Consequences of gestational diabetes mellitus on neonatal cardiovascular health: MySweetHeart Cohort study. Pediatr. Res. 2022;94:231–238. doi: 10.1038/s41390-022-02390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaughan O.R., Rosario F.J., Chan J., Cox L.A., Ferchaud-Roucher V., Zemski-Berry K.A., Reusch J.E.B., Keller A.C., Powell T.L., Jansson T. Maternal obesity causes fetal cardiac hypertrophy and alters adult offspring myocardial metabolism in mice. J. Physiol. 2022;600(13):3169–3191. doi: 10.1113/JP282462. [DOI] [PubMed] [Google Scholar]
- 13.Blackmore H.L., Niu Y., Fernandez-Twinn D.S., Tarry-Adkins J.L., Giussani D.A., Ozanne S.E. Maternal diet-induced obesity programs cardiovascular dysfunction in adult male mouse offspring independent of current body weight. Endocrinology. 2014;155(10):3970–3980. doi: 10.1210/en.2014-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez-Twinn D.S., Blackmore H.L., Siggens L., Giussani D.A., Cross C.M., Foo R., Ozanne S.E. The programming of cardiac hypertrophy in the offspring by maternal obesity is associated with hyperinsulinemia, AKT, ERK, and mTOR activation. Endocrinology. 2012;153(12):5961–5971. doi: 10.1210/en.2012-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Derraik J.G., Ayyavoo A., Hofman P.L., Biggs J.B., Cutfield W.S. Increasing maternal prepregnancy body mass index is associated with reduced insulin sensitivity and increased blood pressure in their children. Clin. Endocrinol. 2015;83(3):352–356. doi: 10.1111/cen.12665. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds R.M., Allan K.M., Raja E.A., Bhattacharya S., McNeill G., Hannaford P.C., Sarwar N., Lee A.J., Bhattacharya S., Norman J.E. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow-up of 1 323 275 person years. BMJ. 2013;347:f4539. doi: 10.1136/bmj.f4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samuelsson A.S., Mullier A., Maicas N., Oosterhuis N.R., Eun Bae S., Novoselova T.V., Chan L.F., Pombo J.M., Taylor P.D., Joles J.A., Coen C.W., Balthasar N., Poston L. Central role for melanocortin-4 receptors in offspring hypertension arising from maternal obesity. Proc. Natl. Acad. Sci. U. S. A. 2016;113(43):12298–12303. doi: 10.1073/pnas.1607464113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torrens C., Ethirajan P., Bruce K.D., Cagampang F.R., Siow R.C., Hanson M.A., Byrne C.D., Mann G.E., Clough G.F. Interaction between maternal and offspring diet to impair vascular function and oxidative balance in high fat fed male mice. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0050671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tam W.H., Ma R.C., Yip G.W., Yang X., Li A.M., Ko G.T., Lao T.T., Chan J.C. The association between in utero hyperinsulinemia and adolescent arterial stiffness. Diabetes Res. Clin. Pract. 2012;95(1):169–175. doi: 10.1016/j.diabres.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 20.Yu Y., Arah O.A., Liew Z., Cnattingius S., Olsen J., Sorensen H.T., Qin G., Li J. Maternal diabetes during pregnancy and early onset of cardiovascular disease in offspring: population based cohort study with 40 years of follow-up. BMJ. 2019;367:l6398. doi: 10.1136/bmj.l6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Y., Reece E.A., Zhong J., Dong D., Shen W.B., Harman C.R., Yang P. Type 2 diabetes mellitus induces congenital heart defects in murine embryos by increasing oxidative stress, endoplasmic reticulum stress, and apoptosis. Am. J. Obstet. Gynecol. 2016;215(3):366 e1–e366 e10. doi: 10.1016/j.ajog.2016.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng X., Chapple S.J., Patel B., Puszyk W., Sugden D., Yin X., Mayr M., Siow R.C., Mann G.E. Gestational diabetes mellitus impairs Nrf2-mediated adaptive antioxidant defenses and redox signaling in fetal endothelial cells in utero. Diabetes. 2013;62(12):4088–4097. doi: 10.2337/db13-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang C., Yang Y., Chen R., Wei Y., Feng Y., Zheng W., Liao H., Zhang Z. Aberrant expression of oxidative stress related proteins affects the pregnancy outcome of gestational diabetes mellitus patients. Am. J. Transl. Res. 2019;11(1):269–279. [PMC free article] [PubMed] [Google Scholar]
- 24.Itoh K., Wakabayashi N., Katoh Y., Ishii T., Igarashi K., Engel J.D., Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen T., Sheratt P.J., Huang H.-C., Yang C.S., Pickett C.B. Increased protein stability as a mechanism that enhances Nrf2-mediated transcriptional activation of the anitoxidant response element. Degradation of Nrf2 by the 26S proteasome. J. Biol. Chem. 2003;278(7):4536–4541. doi: 10.1074/jbc.M207293200. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi A., Kang M.I., Okawa H., Ohtsuji M., Zenke Y., Chiba T., Igarashi K., Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate for proteasomal degradation of Nrf2. Mol. Cell Biol. 2004;24(16):7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dinkova-Kostova A.T., Fahey J.W., Kostov R.V., Kensler T.W. KEAP1 and done? Targeting the NRF2 pathway with Sulforaphane. Trends Food Sci. Technol. 2017;69:257–269. doi: 10.1016/j.tifs.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang D.D., Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol. Cell Biol. 2003;23(22):8137–8151. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang W.D., Liu Y., Hu K., Jiang J., Li S.H., Feng L., Zhou X.Q. Copper exposure induces oxidative injury, disturbs the antioxidant system and changes the Nrf2/ARE (CuZnSOD) signaling in the fish brain: protective effects of myo-inositol. Aquat. Toxicol. 2014;155:301–313. doi: 10.1016/j.aquatox.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Bloom D.A., Jaiswal A.K. Phosphorylation of Nrf2 at Ser 40 by protein kinase C in response to antioxidants leads to the release of Nrf2 from INrf2, but is not required for Nrf2 stabilization/accumulation in the nucleus and transcriptional activation of antioxidant response element-mediated NAD(P)H:quinone oxidoreductase-1 gene expression. J. Biol. Chem. 2003;278(45):44675–44682. doi: 10.1074/jbc.M307633200. [DOI] [PubMed] [Google Scholar]
- 31.Yang H., Magilnick N., Lee C., Kalmaz D., Ou X., Chan J.Y., Lu S.C. Nrf 1 and Nrf2 regulate rat glutamate-cysteine ligase catalytic subunit transcription indirectly via NF-kappaB and AP-1. Mol. Cell Biol. 2005;25(14):5933–5946. doi: 10.1128/MCB.25.14.5933-5946.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel B., Mann G.E., Chapple S.J. Concerted redox modulation by sulforaphane alleviates diabetes and cardiometabolic syndrome. Free Radic. Biol. Med. 2018;122:150–160. doi: 10.1016/j.freeradbiomed.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Langston-Cox A.G., Anderson D., Creek D.J., Palmer K.R., Marshall S.A., Wallace E.M. Sulforaphane bioavailability and effects on blood pressure in women with pregnancy hypertension. Reprod. Sci. 2021;28(5):1489–1497. doi: 10.1007/s43032-020-00439-5. [DOI] [PubMed] [Google Scholar]
- 34.Boulange C.L., Claus S.P., Chou C.J., Collino S., Montoliu I., Kochhar S., Holmes E., Rezzi S., Nicholson J.K., Dumas M.E., Martin F.P. Early metabolic adaptation in C57BL/6 mice resistant to high fat diet induced weight gain involves an activation of mitochondrial oxidative pathways. J. Proteome Res. 2013;12(4):1956–1968. doi: 10.1021/pr400051s. [DOI] [PubMed] [Google Scholar]
- 35.Burcelin R., Crivelli V., Dacosta A., Roy-Tirelli A., Thorens B. Heterogeneous metabolic adaptation of C57BL/6J mice to high-fat diet. Am. J. Physiol. Endocrinol. Metab. 2002;282(4):E834–E842. doi: 10.1152/ajpendo.00332.2001. [DOI] [PubMed] [Google Scholar]
- 36.Itoh K., Chiba T., Takahashi S., Ishii T., Igarashi K., Katoh Y., Oyake T., Hayashi N., Satoh K., Hatayama I., Yamamoto M., Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997;236(2):313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 37.Samuelsson A.M., Matthews P.A., Argenton M., Christie M.R., McConnell J.M., Jansen E.H., Piersma A.H., Ozanne S.E., Twinn D.F., Remacle C., Rowlerson A., Poston L., Taylor P.D. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension. 2008;51(2):383–392. doi: 10.1161/HYPERTENSIONAHA.107.101477. [DOI] [PubMed] [Google Scholar]
- 38.Bowe J.E., Franklin Z.J., Hauge-Evans A.C., King A.J., Persaud S.J., Jones P.M. Metabolic phenotyping guidelines: assessing glucose homeostasis in rodent models. J. Endocrinol. 2014;222(3):G13–G25. doi: 10.1530/JOE-14-0182. [DOI] [PubMed] [Google Scholar]
- 39.Miner J.J., Cao B., Govero J., Smith A.M., Fernandez E., Cabrera O.H., Garber C., Noll M., Klein R.S., Noguchi K.K., Mysorekar I.U., Diamond M.S. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell. 2016;165(5):1081–1091. doi: 10.1016/j.cell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., Tinevez J.Y., White D.J., Hartenstein V., Eliceiri K., Tomancak P., Cardona A. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stathopoulou K., Schnittger J., Raabe J., Fleischer F., Mangels N., Piasecki A., Findlay J., Hartmann K., Krasemann S., Schlossarek S., Uebeler J., Wixler V., Blake D.J., Baillie G.S., Carrier L., Ehler E., Cuello F. CMYA5 is a novel interaction partner of FHL2 in cardiac myocytes. FEBS J. 2022;289(15):4622–4645. doi: 10.1111/febs.16402. [DOI] [PubMed] [Google Scholar]
- 42.Psefteli P.M., Kitscha P., Vizcay G., Fleck R., Chapple S.J., Mann G.E., Fowler M., Siow R.C. Glycocalyx sialic acids regulate Nrf2-mediated signaling by fluid shear stress in human endothelial cells. Redox Biol. 2021;38 doi: 10.1016/j.redox.2020.101816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chapple S.J., Keeley T.P., Mastronicola D., Arno M., Vizcay-Barrena G., Fleck R., Siow R.C.M., Mann G.E. Bach 1 differentially regulates distinct Nrf2-dependent genes in human venous and coronary artery endothelial cells adapted to physiological oxygen levels. Free Radic. Biol. Med. 2016;92:152–162. doi: 10.1016/j.freeradbiomed.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 44.Khan I.Y., Taylor P.D., Dekou V., Seed P.T., Lakasing L., Graham D., Dominiczak A.F., Hanson M.A., Poston L. Gender-linked hypertension in offspring of lard-fed pregnant rats. Hypertension. 2003;41(1):168–175. doi: 10.1161/01.hyp.0000047511.97879.fc. [DOI] [PubMed] [Google Scholar]
- 45.Meakin P.J., Chowdhry S., Sharma R.S., Ashford F.B., Walsh S.V., McCrimmon R.J., Dinkova-Kostova A.T., Dillon J.F., Hayes J.D., Ashford M.L. Susceptibility of Nrf2-null mice to steatohepatitis and cirrhosis upon consumption of a high-fat diet is associated with oxidative stress, perturbation of the unfolded protein response, and disturbance in the expression of metabolic enzymes but not with insulin resistance. Mol. Cell Biol. 2014;34(17):3305–3320. doi: 10.1128/MCB.00677-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pi J., Bai Y., Zhang Q., Wong V., Floering L.M., Daniel K., Reece J.M., Deeney J.T., Andersen M.E., Corkey B.E., Collins S. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes. 2007;56(7):1783–1791. doi: 10.2337/db06-1601. [DOI] [PubMed] [Google Scholar]
- 47.Tarry-Adkins J.L., Martin-Gronert M.S., Fernandez-Twinn D.S., Hargreaves I., Alfaradhi M.Z., Land J.M., Aiken C.E., Ozanne S.E. Poor maternal nutrition followed by accelerated postnatal growth leads to alterations in DNA damage and repair, oxidative and nitrosative stress, and oxidative defense capacity in rat heart. FASEB J. 2013;27(1):379–390. doi: 10.1096/fj.12-218685. [DOI] [PubMed] [Google Scholar]
- 48.Kerns M.L., DePianto D., Dinkova-Kostova A.T., Talalay P., Coulombe P.A. Reprogramming of keratin biosynthesis by sulforaphane restores skin integrity in epidermolysis bullosa simplex. Proc. Natl. Acad. Sci. U. S. A. 2007;104(36):14460–14465. doi: 10.1073/pnas.0706486104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chapple S.J., Puszyk W.M., Mann G.E. Keap1-Nrf2 regulated redox signaling in utero: priming of disease susceptibility in offspring. Free Radic. Biol. Med. 2015;88(Pt B):212–220. doi: 10.1016/j.freeradbiomed.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 50.Horn S., Kirkegaard J.S., Hoelper S., Seymour P.A., Rescan C., Nielsen J.H., Madsen O.D., Jensen J.N., Kruger M., Gronborg M., Ahnfelt-Ronne J., Resource Research. A dual proteomic approach identifies regulated islet proteins during beta-cell mass expansion in vivo. Mol. Endocrinol. 2016;30(1):133–143. doi: 10.1210/me.2015-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cho H.Y., Miller-DeGraff L., Blankenship-Paris T., Wang X., Bell D.A., Lih F., Deterding L., Panduri V., Morgan D.L., Yamamoto M., Reddy A.J., Talalay P., Kleeberger S.R. Sulforaphane enriched transcriptome of lung mitochondrial energy metabolism and provided pulmonary injury protection via Nrf2 in mice. Toxicol. Appl. Pharmacol. 2019;364:29–44. doi: 10.1016/j.taap.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skoko J.J., Wakabayashi N., Noda K., Kimura S., Tobita K., Shigemura N., Tsujita T., Yamamoto M., Kensler T.W. Loss of Nrf2 in mice evokes a congenital intrahepatic shunt that alters hepatic oxygen and protein expression gradients and toxicity. Toxicol. Sci. 2014;141(1):112–119. doi: 10.1093/toxsci/kfu109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kweider N., Huppertz B., Rath W., Lambertz J., Caspers R., ElMoursi M., Pecks U., Kadyrov M., Fragoulis A., Pufe T., Wruck C.J. The effects of Nrf2 deletion on placental morphology and exchange capacity in the mouse. J. Matern. Fetal Neonatal Med. 2017;30(17):2068–2073. doi: 10.1080/14767058.2016.1236251. [DOI] [PubMed] [Google Scholar]
- 54.Song H., Xu Y., Yang X., Rong X., Wang Y., Wei N. Tertiary butylhydroquinone alleviates gestational diabetes mellitus in C57BL/KsJ-Lep db/+ mice by suppression of oxidative stress. J. Cell. Biochem. 2019;120(9):15310–15319. doi: 10.1002/jcb.28798. [DOI] [PubMed] [Google Scholar]
- 55.Baltayeva J., Konwar C., Castellana B., Mara D.L., Christians J.K., Beristain A.G. Obesogenic diet exposure alters uterine natural killer cell biology and impairs vasculature remodeling in micedagger. Biol. Reprod. 2020;102(1):63–75. doi: 10.1093/biolre/ioz163. [DOI] [PubMed] [Google Scholar]
- 56.Lager S., Samulesson A.M., Taylor P.D., Poston L., Powell T.L., Jansson T. Diet-induced obesity in mice reduces placental efficiency and inhibits placental mTOR signaling. Phys. Rep. 2014;2(2) doi: 10.1002/phy2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Barros Mucci D., Kusinski L.C., Wilsmore P., Loche E., Pantaleao L.C., Ashmore T.J., Blackmore H.L., Fernandez-Twinn D.S., Carmo M., Ozanne S.E. Impact of maternal obesity on placental transcriptome and morphology associated with fetal growth restriction in mice. Int. J. Obes. 2020;44(5):1087–1096. doi: 10.1038/s41366-020-0561-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Musial B., Vaughan O.R., Fernandez-Twinn D.S., Voshol P., Ozanne S.E., Fowden A.L., Sferruzzi-Perri A.N. A Western-style obesogenic diet alters maternal metabolic physiology with consequences for fetal nutrient acquisition in mice. J. Physiol. 2017;595(14):4875–4892. doi: 10.1113/JP273684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hagay Z.J., Weiss Y., Zusman I., Peled-Kamar M., Reece E.A., Eriksson U.J., Groner Y. Prevention of diabetes-associated embryopathy by overexpression of the free radical scavenger copper zinc superoxide dismutase in transgenic mouse embryos. Am. J. Obstet. Gynecol. 1995;173(4):1036–1041. doi: 10.1016/0002-9378(95)91323-8. [DOI] [PubMed] [Google Scholar]
- 60.Duan Y., Sun F., Que S., Li Y., Yang S., Liu G. Prepregnancy maternal diabetes combined with obesity impairs placental mitochondrial function involving Nrf2/ARE pathway and detrimentally alters metabolism of offspring. Obes. Res. Clin. Pract. 2018;12(Suppl 2):90–100. doi: 10.1016/j.orcp.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 61.Cox A.G., Gurusinghe S., Abd Rahman R., Leaw B., Chan S.T., Mockler J.C., Murthi P., Marshall S.A., Lim R., Wallace E.M. Sulforaphane improves endothelial function and reduces placental oxidative stress in vitro. Pregnancy Hypertens. 2019;16:1–10. doi: 10.1016/j.preghy.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 62.Liu Y., Wang Y., Wang C., Shi R., Zhou X., Li Z., Sun W., Zhao L., Yuan L. Maternal obesity increases the risk of fetal cardiac dysfunction via visceral adipose tissue derived exosomes. Placenta. 2021;105:85–93. doi: 10.1016/j.placenta.2021.01.020. [DOI] [PubMed] [Google Scholar]
- 63.Moazzen H., Wu Y., Engineer A., Lu X., Aulakh S., Feng Q. NOX2 is critical to endocardial to mesenchymal transition and heart development. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/1679045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang L., Wang X., Wu Y., Lu X., Chidiac P., Wang G., Feng Q. Maternal diabetes up-regulates NOX2 and enhances myocardial ischaemia/reperfusion injury in adult offspring. J. Cell Mol. Med. 2018;22(4):2200–2209. doi: 10.1111/jcmm.13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Puente B.N., Kimura W., Muralidhar S.A., Moon J., Amatruda J.F., Phelps K.L., Grinsfelder D., Rothermel B.A., Chen R., Garcia J.A., Santos C.X., Thet S., Mori E., Kinter M.T., Rindler P.M., Zacchigna S., Mukherjee S., Chen D.J., Mahmoud A.I., Giacca M., Rabinovitch P.S., Aroumougame A., Shah A.M., Szweda L.I., Sadek H.A. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell. 2014;157(3):565–579. doi: 10.1016/j.cell.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karliner J.S., Honbo N., Epstein C.J., Xian M., Lau Y.F., Gray M.O. Neonatal mouse cardiac myocytes exhibit cardioprotection induced by hypoxic and pharmacologic preconditioning and by transgenic overexpression of human Cu/Zn superoxide dismutase. J. Mol. Cell. Cardiol. 2000;32(10):1779–1786. doi: 10.1006/jmcc.2000.1212. [DOI] [PubMed] [Google Scholar]
- 67.Bai Y., Cui W., Xin Y., Miao X., Barati M.T., Zhang C., Chen Q., Tan Y., Cui T., Zheng Y., Cai L. Prevention by sulforaphane of diabetic cardiomyopathy is associated with up-regulation of Nrf2 expression and transcription activation. J. Mol. Cell. Cardiol. 2013;57:82–95. doi: 10.1016/j.yjmcc.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 68.Sun Y., Zhou S., Guo H., Zhang J., Ma T., Zheng Y., Zhang Z., Cai L. Protective effects of sulforaphane on type 2 diabetes-induced cardiomyopathy via AMPK-mediated activation of lipid metabolic pathways and NRF2 function. Metabolism. 2020;102 doi: 10.1016/j.metabol.2019.154002. [DOI] [PubMed] [Google Scholar]
- 69.Gu J., Cheng Y., Wu H., Kong L., Wang S., Xu Z., Zhang Z., Tan Y., Keller B.B., Zhou H., Wang Y., Xu Z., Cai L. Metallothionein is downstream of Nrf2 and partially mediates sulforaphane prevention of diabetic cardiomyopathy. Diabetes. 2017;66(2):529–542. doi: 10.2337/db15-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lappas M., Mitton A., Permezel M. Response to oxidative stress, the expression of inflammatory cytokines and antioxidant enzymes are impaired in placenta, but not adipose tissue, of women with gestational diabetes. J. Endocrinol. 2010;204(1):75–84. doi: 10.1677/JOE-09-0321. [DOI] [PubMed] [Google Scholar]
- 71.Shawky N.M., Pichavaram P., Shehatou G.S., Suddek G.M., Gameil N.M., Jun J.Y., Segar L. Sulforaphane improves dysregulated metabolic profile and inhibits leptin-induced VSMC proliferation: implications toward suppression of neointima formation after arterial injury in western diet-fed obese mice. J. Nutr. Biochem. 2016;32:73–84. doi: 10.1016/j.jnutbio.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Noyan-Ashraf M.H., Wu L., Wang R., Juurlink B.H. Dietary approaches to positively influence fetal determinants of adult health. FASEB J. 2006;20(2):371–373. doi: 10.1096/fj.05-4889fje. [DOI] [PubMed] [Google Scholar]
- 73.White R.M., Rivera C.O., Davison C.A. Nitric oxide-dependent and -independent mechanisms account for gender differences in vasodilation to acetylcholine. J. Pharmacol. Exp. Therapeut. 2000;292(1):375–380. [PubMed] [Google Scholar]
- 74.Sobrino A., Oviedo P.J., Novella S., Laguna-Fernandez A., Bueno C., Garcia-Perez M.A., Tarin J.J., Cano A., Hermenegildo C. Estradiol selectively stimulates endothelial prostacyclin production through estrogen receptor-alpha. J. Mol. Endocrinol. 2010;44(4):237–246. doi: 10.1677/JME-09-0112. [DOI] [PubMed] [Google Scholar]
- 75.Dimova L.G., Battista S., Plosch T., Kampen R.A., Liu F., Verkaik-Schakel R.N., Pratico D., Verkade H.J., Tietge U.J.F. Gestational oxidative stress protects against adult obesity and insulin resistance. Redox Biol. 2020;28 doi: 10.1016/j.redox.2019.101329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.He X., Ma Q. Disruption of Nrf2 synergizes with high glucose to cause heightened myocardial oxidative stress and severe cardiomyopathy in diabetic mice. J Diabetes Metab Suppl. 2012;7 doi: 10.4172/2155-6156.S7-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kovac S., Angelova P.R., Holmstrom K.M., Zhang Y., Dinkova-Kostova A.T., Abramov A.Y. Nrf2 regulates ROS production by mitochondria and NADPH oxidase. Biochim. Biophys. Acta. 2015;1850(4):794–801. doi: 10.1016/j.bbagen.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Balteau M., Tajeddine N., de Meester C., Ginion A., Des Rosiers C., Brady N.R., Sommereyns C., Horman S., Vanoverschelde J.L., Gailly P., Hue L., Bertrand L., Beauloye C. NADPH oxidase activation by hyperglycaemia in cardiomyocytes is independent of glucose metabolism but requires SGLT1. Cardiovasc. Res. 2011;92(2):237–246. doi: 10.1093/cvr/cvr230. [DOI] [PubMed] [Google Scholar]
- 79.Schmid E., Hotz-Wagenblatt A., Hacj V., Droge W. Phosphorylation of the insulin receptor kinase by phosphocreatine in combination with hydrogen peroxide: the structural basis of redox priming. FASEB J. 1999;13(12):1491–1500. doi: 10.1096/fasebj.13.12.1491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.