Abstract
Campylobacter is among the four main causes of gastroenteritis worldwide. Most reported Campylobacter infections are caused by C. jejuni and C. coli. However, other emerging Campylobacter pathogens have been recognized as important pathogens in humans and animals. A novel bacterial strain, PS10T, was isolated from the gastric mucous of pigs in 2022 in Beijing, China. The cell was Gram-negative, microaerobic, motile, and negative for catalase, oxidase, and urease. Phylogenetic and phylogenomic analyses based on the 16S rRNA gene and core genome indicated that this isolate belongs to the genus Campylobacter. There were low dDDH relatedness and ANI values shared within this strain and its closest species C. mucosalis below the cut-off values generally recognized for isolates of the same species. The draft genome size of PS10T is 2,240,910 bp in length with a percentage of DNA G+C contents of 37.72%. Comparing the phenotypic and phylogenetic features among this isolate and its related organisms, strain PS10T represents a novel species within the genus Campylobacter, for which the name Campylobacter gastrosuis sp. nov. (Type strain PS10T = GDMCC 1.3686T = JCM 35849T) is proposed.
Keywords: Campylobacter gastrosuis, novel species, genomic characteristics, phylogenetic analyses, antibiotic resistance
1. Introduction
The genus Campylobacter belongs to the family Campylobacteraceae and the order Campylobacterales, and they currently have over 50 species and subspecies, including many non-validly described species, which have been isolated from different times and sources according to the List of Prokaryotic names with Standing in Nomenclature (LPSN, https://lpsn.dsmz.de/genus/campylobacter) (accessed on 20 June 2023). Members of the Campylobacter genus are nutritionally fastidious and strictly grow under anaerobic or microaerobic conditions, and they are morphologically diverse, including spiral-, curved-, or rod-shaped. Campylobacter naturally colonizes humans, other mammals, birds, reptiles, and shellfish, particularly birds, and can be transmitted to humans through contaminated food or water [1,2,3,4].
Campylobacter is among the four main causes of gastroenteritis worldwide [5]. Most reported Campylobacter infections are caused by C. jejuni, which is a leading cause of bacterial gastroenteritis in humans, and symptoms typically include diarrhea, abdominal cramping, and fever, amongst others [6,7]. In addition, the antecedent infection of C. jejuni could trigger a Guillain–Barré Syndrome (GBS) outbreak [8]. And, to a lesser extent, C. coli accounts for 1–25% of all Campylobacter-related diarrheal diseases [9]. However, other emerging Campylobacter pathogens have been recognized as important pathogens in humans and animals [10]. C. upsaliensis has been isolated from patients with diarrhea, bacteremia, hemolytic uremic syndrome, and those who have undergone an abortion [5,11]. C. lari causes not only sporadic gastrointestinal infections in humans but also water outbreaks and bacteremia, and occasionally suppurative pleurisy, reactive arthritis, prosthesis, and urinary tract infections [5,12,13,14,15]. Therefore, the emerging Campylobacter pathogens and their pathogenicity to humans or animals need to be further discovered and studied.
Antimicrobial resistance in Campylobacter is a pressing concern, complicating the clinical treatment of infections caused by this bacterium. The prevalence of resistance to commonly used antibiotics of choice for the treatment of human campylobacteriosis, like fluoroquinolones, macrolides, and tetracyclines, is high, and multidrug-resistant strains are on the rise, meaning there is a risk of resistant strains spreading from animals to human, posing a significant threat to public health [16,17,18,19]. Notably, new antibiotic resistance mechanisms are continuously emerging in Campylobacter [20]. As such, continued monitoring and surveillance of Campylobacter antimicrobial resistance patterns is crucial to inform effective treatment strategies and to curb the rise and spread of resistance [20,21].
Poultry is the main source of human campylobacteriosis, and the pig is also considered an important source of C. coli-related human campylobacteriosis [22]. The study has found a possible link between human campylobacteriosis and pig-borne Campylobacter [23]. Moreover, Campylobacter spp. from pigs was found, possibly propagated in each slaughtering step, to have an average prevalence of 19.3% [24] and a high prevalence of resistance to multiple antibiotics, particularly macrolides, which is probably attributable to the overuse of antimicrobials in pig production [25]. As pigs are usually subclinically infected, the carcasses could have a high chance of being contaminated by Campylobacter spp. during the slaughter process, which can be transmitted to humans via contaminated pork [26].
This study described the phenotypic, taxonomic, antimicrobial susceptibility, and genomic characteristics of a novel Campylobacter-like isolate from the gastric mucous of pigs, and the phylogenetic and phylogenomic relationships between the isolated strain and other Campylobacter species were also clarified. Based on polyphasic taxonomic analyses, this novel isolate is proposed as a novel Campylobacter species, designated Campylobacter gastrosuis sp. nov. (PS10T).
2. Methods
2.1. Sampling, Isolation, and Culturing
In the exploration of Campylobacter spp. diversity in animals, the isolation process was executed utilizing the Campylobacter isolation kit (ZC-CAMPY-002, Qingdao Sinova Biotechnology Co., Ltd., Qingdao, China), which incorporates a membrane filter method. In a succinct overview, 0.4 mL of pig gastric mucous was added to 4 mL of enrichment buffer from the kit. The enriched suspension was then incubated at 37 °C for 24 h within a microaerophilic environment comprising 5% O2, 10% CO2, and 85% N2. Following this, approximately 300 μL of the cultured enrichment suspension was applied onto the filter’s surface affixed to the double medium plates. These plates contained Karmali and Columbia agar, each with 5% defibrinated sheep blood. Subsequently, the medium plates were placed within a microaerophilic atmosphere at 37 °C and incubated for 48 h [27].
At least 5 individual colonies exhibiting potential Campylobacter genus traits were carefully selected for Gram staining, subsequent passage cultivation, and purification. Those isolates that exhibited morphology consistent with Campylobacter were then subjected to initial characterization by PCR amplification and sequencing of the 16S rRNA gene [28]. Following this, the selected isolates were preserved at −80 °C in BHI medium containing 20% (v/v) glycerol, facilitating further analyses.
2.2. Morphological, Physiological, and Biochemical Characteristics
For the investigation of morphological and biochemical attributes, cells were cultivated and harvested during the late exponential growth phase. Gram staining was performed using a Gram staining kit (Baso) [29], and the samples were observed under a light microscope (Eclipse Ci-L, NIKON). Biochemical tests were conducted to elucidate the physiology and chemotaxonomy of this isolate. The catalase activity was assessed using a 3% (v/v) H2O2 solution to observe bubble production. Further biochemical characteristics specific to Campylobacter spp. were determined using the API Campy identification system (bio-Mérieux), adhering strictly to the manufacturer’s instructions. As a benchmark, C. jejuni ATCC 33560T was utilized as a control for comparison.
2.3. Antimicrobial Susceptibility Testing
The minimum inhibitory concentrations (MICs) for eleven antimicrobials (erythromycin, azithromycin, nalidixic acid, ciprofloxacin, gentamicin, streptomycin, chloramphenicol, florfenicol, tetracycline, telithromycin, and clindamycin) were determined for this Campylobacter isolate using the gradient strip diffusion method (E-test, bio-Mérieux) in accordance with the manufacturer’s instructions, as previously reported [30]. After 48 h of growth on Karmali blood agar plates, the isolate was suspended in sterile saline and adjusted to a McFarland turbidity standard of 1. The bacterial suspension was then evenly spread onto Karmali blood agar plates using a sterile cotton swab. Plates containing E-test strips were subsequently incubated under microaerobic conditions for 48 h. The MIC was determined as the lowest concentration that did not exhibit visible growth. The type strain C. jejuni ATCC 33560T was employed as the control.
2.4. Genome Extraction and Sequencing
Subsequent to cultivation, genomic DNA was extracted employing the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany), adhering to the manufacturer’s guidelines. The concentration and purity of the DNA samples were assessed using the NanoDrop spectrophotometer (Thermo Scientific, Waltham, MA, USA). Quality criteria encompassed a concentration of ≥20 ng/μL and a total amount exceeding 2 μg. Additionally, a purity requirement dictated that the OD260/OD280 value should fall within the range of 1.6 to 1.8.
DNA sequencing was conducted using the Illumina PE150 platform (Illumina Inc., San Diego, CA, USA) at the Novogene Corporation (Beijing, China), with coverage reaching a depth of 100×. A 350 bp paired-end library was established to facilitate genome sequencing, yielding 150 bp reads. To appraise and enhance the quality of raw sequence data, FastQC 0.11.9 [31] and fastp v0.23.4 [32] software tools were employed. Low-quality reads, defined by a sequence quality score ≤ Q30 across ≥3 consecutive bases, were subsequently excluded. The resultant high-quality reads were then assembled using SOAPdenovo2 [33].
2.5. Phylogenetic and Phylogenomic Analysis
To ascertain the strain’s phylogenetic placement, we initiated PCR amplification of the 16S rRNA gene. Following amplification, each PCR product underwent purification and subsequent subcloning into the pMD18-T vector. Subsequently, the vector was introduced into Escherichia coli DH5α, and the inserted 16S rRNA gene fragment was extracted from a single colony post-lysis and subjected to sequencing.
The resulting 16S rRNA gene sequences were then cross-referenced with other Campylobacter species using EzBioCloud’s identification service, enabling the taxonomic classification of this strain [34]. Employing the MAFFT 7.471 software [35], a multiple sequence alignment was performed on the 16S rRNA gene sequences of this strain and the type strains within the Campylobacter genus. Phylogenetic analysis ensued utilizing the MEGA X [36] software package, employing the neighbor-joining (NJ) [37], maximum-parsimony (MP) [38], and maximum-likelihood (ML) algorithms [39], all supplemented with a bootstrap analysis comprising 1000 replicates [40]. Furthermore, the type strain of Arcobacter butzleri ATCC 49616T was incorporated as an outgroup reference.
The assembled sequences underwent gene prediction and functional annotation using Prokka 1.14.6 software [41] and the tRNAscan-SE 2.0 tool [42]. The protein sequences of core genes sourced from this isolate and other Campylobacter species were extracted, leveraging CD-HIT 4.8.1 software [43] and .faa files from the Prokka results. The extraction was based on a 40% protein sequence similarity threshold, followed by alignment using MAFFT 7.471 software. Subsequently, the phylogenomic tree was constructed via FastTree 2.1.10 [44], and its visualization was facilitated using Dendroscope 3.8.3 software [45].
2.6. Genomic Analysis
The sequences of predicted proteins underwent assignment and annotation to the Clusters of Orthologous Groups (COGs) database through eggNOG-mapper v2 [46]. Genomic island and plasmid predictions for the isolate were performed using the online tools IslandViewer 4 [47] and PlasmidFinder 2.0 server [48], respectively. To identify prophage sequences, both the Phage Search Tool Enhanced Release (PHASTER) web server [49] and phiSpy 4.2.21 software [50] were employed. The Comprehensive Antibiotic Resistance Database (CARD) 3.2.7 and its Resistance Gene Identifier (RGI) 6.0.2 [51] were utilized for screening antimicrobial resistance genes and related mutations. Comparative analysis of antibiotic resistance gene clusters was carried out using Easfig v2.2.5 [52]. The VFanalyzer [53] was used to detect virulence genes in all genomes. Digital DNA-DNA hybridization (dDDH) relatedness calculations and comparisons were performed using the Genome-to-Genome Distance Calculator 3.0 [54]. Additionally, average nucleotide identity (ANI) values were determined using Pyani 0.2.10 software [55]. The visualization of COG classification, genomic islands, and virulence genes results was achieved using the ggplot2, genoPlotR, and pheatmap packages within R 4.2.2, respectively.
3. Results and Discussion
3.1. Isolation and Phenotypic Characterization
In 2022, a total of 20 Campylobacter isolates, like C. coli, C. fetus, C. hyointestinalis, and others, were isolated from 19 health gastric mucous samples collected from a pig slaughterhouse from Beijing, China (20/19, 105.26%). The strain PS10T is one of these isolates. This PS10T cell is Gram-negative, microaerobic, motile, and spiral-shaped (Figure S1). The colonies were circular, 2–3 mm in diameter, smooth, and grey after 2 days of growth on Karmali agar with 5% defibrinated sheep blood. Cells appear coccoid after 5–6 days of incubation or when exposed to air. No hemolysis was observed on the blood agar. The colony and morphological outcomes of this strain remained consistent with the fundamental attributes associated with Campylobacter [56].
The phenotypic and biochemical characteristics of PS10T exhibited distinct traits that set it apart from the standard profile of any other species within the Campylobacter genus. Notably, this strain tested negative for catalase, oxidase, and urease activities. Of particular significance is that the absence of oxidase activity is a trait shared with only a limited number of other members within the Campylobacter genus, such as C. gracilis and C. ornithocola [57], which makes it recognizable from closely related species C. mucosalis. The absence of H2S production further contributes to its distinctiveness from closely related species C. mucosalis. The type strain of this novel species also stands out due to its inability to hydrolyze hippurate, while it exhibits the capability to hydrolyze indoxyl acetate and reduce nitrate, setting it apart from C. suis. Finally, PS10T could be unequivocally differentiated from C. mucosalis due to its lack of oxidase activity and inability to generate H2S (Table 1) [57,58,59,60]. In the realm of biochemical phenotype, isolate PS10T showcases distinctive attributes within the Campylobacter genus, allowing for its precise differentiation from other Campylobacter species through its unique biochemical profiles.
Table 1.
Phenotypic characteristics of Campylobacter gastrosuis sp. nov. strain and the closely related Campylobacter species.
| Characteristic | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Catalase | − | − | − | − | + | + |
| Oxidase | − | + | − | − | − | + |
| Urease | − | − | − | − | + | + |
| Nitrate reduction | + | (−) | − | − | + | + |
| Indoxyl acetate hydrolysis | + | − | − | − | − | − |
| Hippurate hydrolysis | − | − | − | − | − | − |
| H2S | − | + | + | − | + | + |
1, C. gastrosuis sp. nov.; 2, C. mucosalis; 3, C.majalis; 4, C. suis; 5, C. pinnipediorum subsp. caledonicus; 6, C. pinnipediorum subsp. pinnipediorum. Data for other species were taken from previous publications [57]. +, 90–100% positive; (−), 11–25% positive; −, 0–10% positive.
3.2. Phylogenetic and Phylogenomic Analysis
The assessment against the EzTaxon-e database of nearly full-length 16S rRNA gene sequences (1510 bp) indicated that this isolate exhibited its closest affiliation with the representative specimen of the Campylobacter genus (Domain, Bacteria; Phylum, Pseudomonadota; Class, Epsilonproteobacteria; Order, Campylobacterales; Family, Campylobacteraceae). Strain PS10T was closest to C. mucosalis DSM 21682T (98.81% of 16S rRNA gene identity). The value of similarity was slightly higher than 98.70%,which was the generally accepted threshold for the species [61]. Thus, if no further identification methods were employed beyond the conventional 16S rRNA sequence comparison, this isolate could potentially be classified as C. mucosalis based on existing experience.
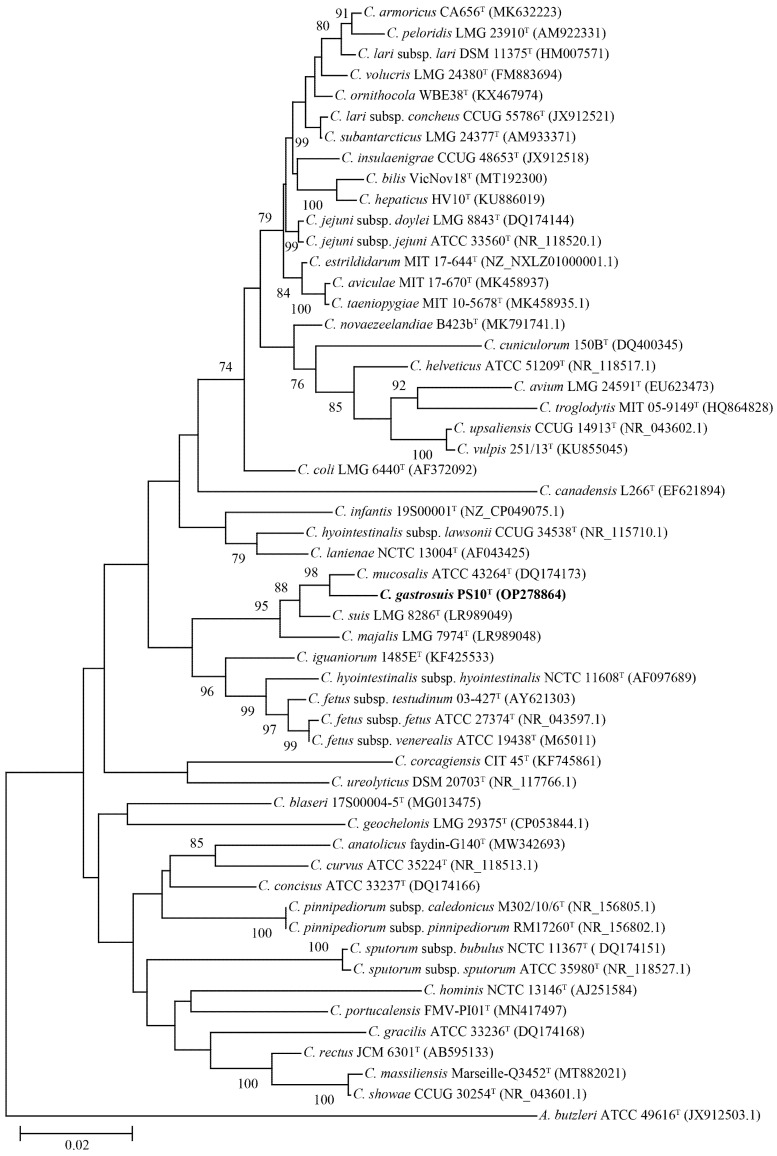
The phylogenetic tree constructed using the NJ algorithm (as shown in Figure 1) and based on the nearly complete 16S rRNA gene sequences demonstrated that PS10T is positioned within the Campylobacter genus. Notably, this strain, alongside C. mucosalis ATCC 43264T, C. majalis LMG 7974T, and C. suis LMG 8286T, formed a distinct cluster that exhibited a certain degree of independence. Among these strains, C. mucosalis ATCC 43264T emerged as the closest relative. Moreover, the phylogenetic trees generated using the ML and MP algorithms produced comparable topological outcomes, further corroborating these findings (Supplementary data Figures S2 and S3).
Figure 1.
Neighbor-joining phylogenetic tree based on nearly complete 16S rRNA gene showing the relationships between our isolates and the type strains of the genus Campylobacter. Bootstrap values (>70%) based on 1000 replicates are shown at branch nodes, with Arcobacter butzleri ATCC 49616T as an outgroup. Bar, 0.02 changes per nucleotide position. Novel strain is highlighted in bold.
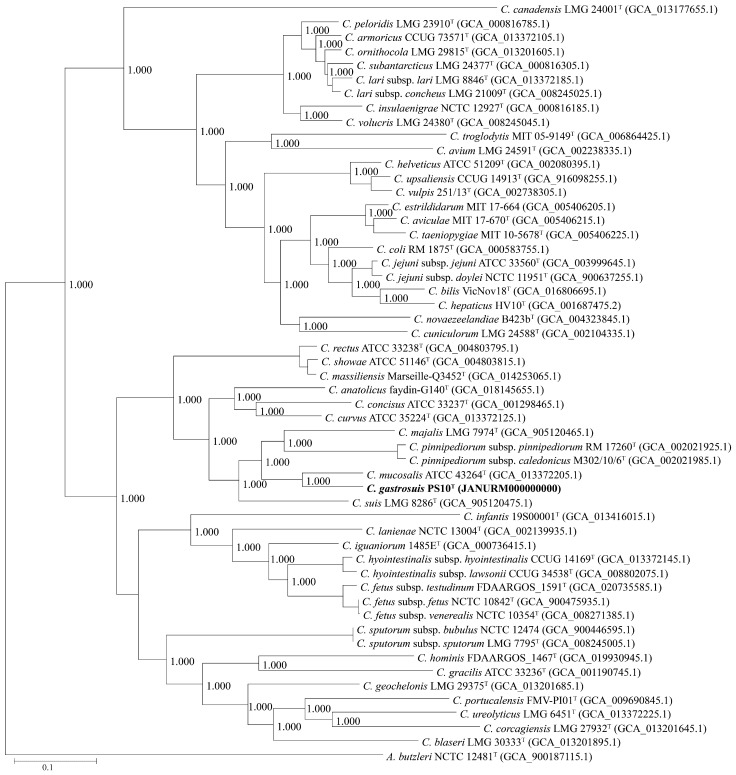
Utilizing a protein identity threshold of 40%, a set of 344 core genes, considered orthologous groups, were extracted and shared across this isolate and all accessible genomes of other species within the Campylobacter genus. This collection of core genes was then employed to construct a phylogenomic tree at the genome level (depicted in Figure 2). Analogous to the phylogenetic tree rooted in 16S rRNA gene sequences, PS10T was again positioned within an independent cluster alongside C. mucosalis ATCC 43264T, C. majalis LMG 7974T, and C. suis LMG 8286T. Notably, this cluster also encompassed strains C. pinnipediorum subsp. pinnipediorum RM 17260T and C. pinnipediorum subsp. caledonicus M302/10/6T. These findings collectively reinforce the classification of this isolate within the Campylobacter genus. Importantly, the consistent outcome underscores C. mucosalis as its closest species.
Figure 2.
Neighbor-joining phylogenomic tree based on 332 core genes of the genus Campylobacter. The outgroup is Arcobacter butzleri ATCC 49616T. Novel strain is highlighted in bold.
The amalgamation of outcomes from the comparison of 16S rRNA gene sequences, alongside the analyses performed at both phylogenetic and phylogenomic levels, unequivocally categorizes this isolate within the Campylobacter genus. However, due to its placement within a cluster housing several other Campylobacter species, notably C. mucosalis, the strain’s identification at the species level remains somewhat challenging. As a result, additional methods are warranted to discern this strain’s specific species designation with greater accuracy.
3.3. Genome Characteristics
The final genome assembly of strain PS10T contained 59 contigs with a draft genome size of 2,240,910 bp and a genomic GC content of 37.72%, which is slightly higher than the most closely related bacterium, C. mucosalis ATCC 43264T (GC content, 36.55%), and within the range of DNA base compositions previously reported for the members in the genus Campylobacter (GC content, 29–47%) [62]. The Prokka predicted 2325 coding genes in total with the draft genome, among which 1186 (51.01%) belong to predicted proteins and 1139 (48.99%) were assigned as hypothetical proteins. The genome contained 43 tRNAs, a tmRNA, a 16S rRNA, and a 23S rRNA. A total of three insertion sequence (IS) elements were found, namely IS1595, IS1380, and IS4. However, the presence of plasmids, phages, and phage-like elements was not predicted in the draft genome.
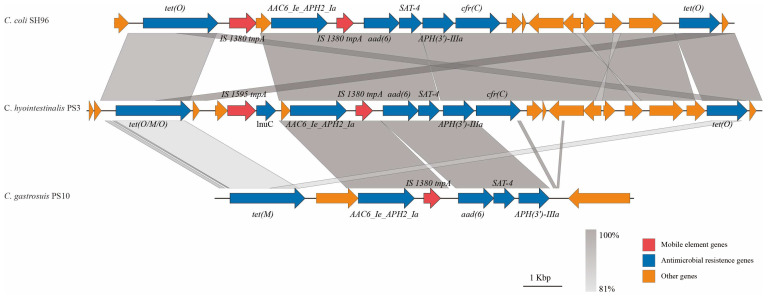
Moreover, a multi-drug resistance genomic island (MDRGI) was predicted, and it monopolizes a scaffold on the draft genome of this strain. The MDRGI contains a tet(M) gene, an AAC6_Ie_APH2_Ia gene, and an aad(6)-SAT-4-APH(3′)-IIIa gene cluster (Figure 3). These antibiotic resistance correlative genes could confer resistance to the antibiotics of tetracycline, nucleoside, and aminoglycoside. This MDRGI was in an independent genome contig, consisting of 10,736 bp bases, and the GC content was 33.7%, which was significantly lower than that of the draft genome of PS10T (37.72%), indicating that this fragment may be exogenously obtained from the other species [63]. Meanwhile, a predicted insertion sequence, IS1380, was distributed across this MDRGI. This type of MDRGI was shorter than that from PS3 (17,302 bp), another strain of C. hyointestinalis isolated in this project, and SH96 (15,885 bp), a strain of C. coli isolated from fecal samples of pigs [64]. The antibiotic resistance correlative genes in PS10T were also present in the other two strains, and both of them were AAC6_Ie_APH2_Ia gene and aad(6)-SAT-4-APH(3′)-IIIa gene cluster distributed at the ends of IS1380. In 2015, the isolation rate of aad(6)-SAT-4-APH(3′)-IIIa gene cluster in streptomycin-resistant strains has reached 10.86% [65]. However, the MDRGI in the SH96 strain was located on the plasmid, indicating that it may be horizontally transferred on chromosomes or plasmids between different strains as a whole and undergo evolutionary mutations and lead to receptor strains’ resistance to tetracycline, nucleoside, and aminoglycoside. The horizontal gene transfer (HGT) between host bacterial cells relies on the transfer of genetic material via mobile genetic elements (MGEs), such as phages, plasmids, transposons, and integrons [66,67]. These critical genes may facilitate the drug-resistant MGEs, particularly plasmids, which can be transmitted between bacterial populations [68]. Due to the lack of a strict restriction enzyme modification system, Campylobacter has a high mutation rate and easy access to exogenous genetic elements, which could lead to the acquisition or loss of antibiotic resistance correlative elements [69].
Figure 3.
Genetic environments of MDRGIs in Campylobacter isolates. Arrows indicate the direction of transcription of the genes, and different genes are shown in different colors. IS, insertion sequence.
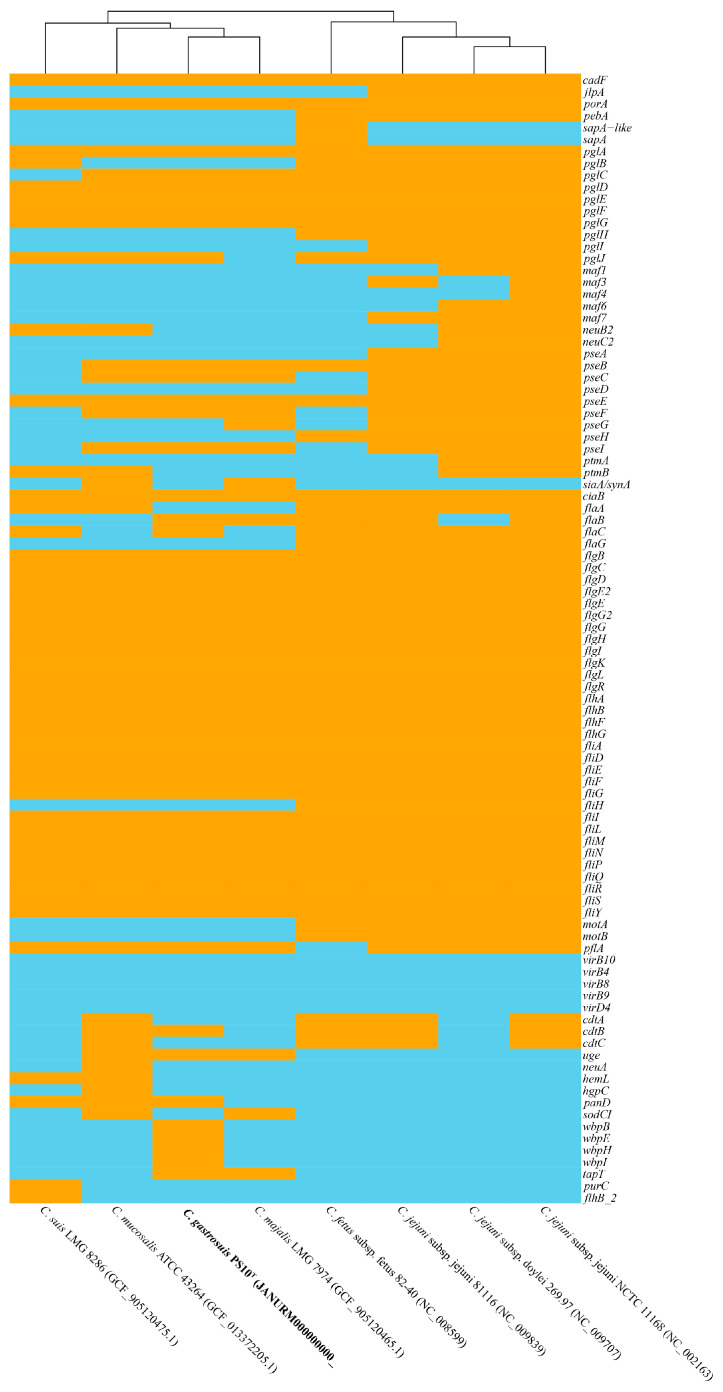
In genomes of PS10T, numerous Campylobacter virulence-associated genes were detected, which could encode genes related to adherence, colonization and immune evasion, glycosylation, invasion, motility and export apparatus (flagella and chemotaxis), secretion system, toxin, and other virulence factors. The virulence-associated gene profile of this strain is similar to that of C. mucosalis ATCC 43264T, C. majalis LMG 7974T, and C. suis LMG 8286T, mainly related to glycosylation, motility, and export apparatus. This type strain of PS10T has the incomplete cytolethal distending toxin (CDT, genes coding for the three subunits: cdtA, cdtB, and cdtC), while C. mucosalis ATCC 43264T has the complete CDT (Figure 4). And both PS10T with the other closely relative strain did not hold the type IV secretion system (T4SS) and iron uptake system (fur and cfrA genes). However, further experimental studies should confirm whether PS10T is a pathogen to the host. In silico screening results of the presence or absence of these virulence genes are presented in Figure 4.
Figure 4.
Heatmap of the distribution of virulence genes. Orange indicates the presence of virulence genes, and sky blue indicates the absence of virulence genes. Novel strain is highlighted in bold.
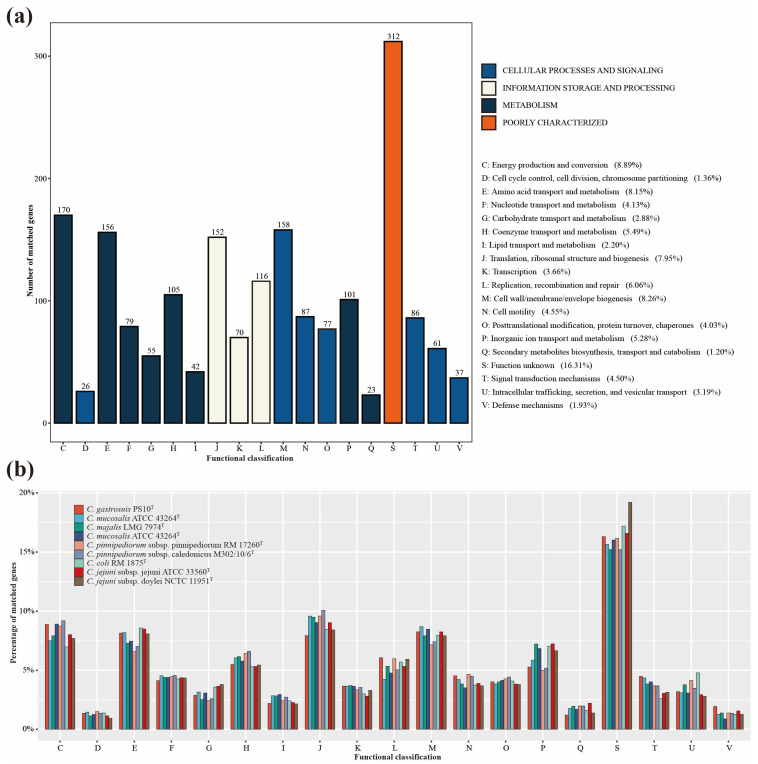
By employing the COG database, a total of 1601 proteins were successfully assigned functional classifications, while 312 remained unclassified (as depicted in Figure 5a). Specifically, the number of proteins attributed to “cellular processes and signaling”, “information storage and processing”, and “metabolism” was 532, 338, and 731, respectively. In comparison with closely related species on the phylogenomic tree and significant pathogenic species within the Campylobacter genus, PS10T exhibited a slight abundance of proteins within “Defense mechanisms” and “Signal transduction mechanisms”, both categorized under “cellular processes and signaling”. Conversely, it showcased a minor reduction in proteins for categories like “Lipid transport and metabolism”, “Translation, ribosomal structure, and biogenesis”, “Inorganic ion transport and metabolism”, and “Secondary metabolites biosynthesis, transport, and catabolism” (as depicted in Figure 5b). In alignment with the outcomes of the phylogenetic and phylogenomic analysis, the findings from the analysis of the COG database demonstrated a congruence in the annotation results between PS10T and its closely related species, exhibiting no notable differences.
Figure 5.
COG functional classification. (a) COG functional classification of genes belonging to PS10T. (b) COG functional classification of genes belonging to PS10T and closely related Campylobacter species.
The dDDH scores of this isolate with the other species in the genus Campylobacter were below 70%, the threshold for species demarcation [54]. Meanwhile, as the gold standard for the delineation of bacterial species, the ANI values between this isolate and all established species of Campylobacter were below 95%, the cutoff for species demarcation (Table 2 and Table S1) [70]. These results suggested that strain PS10T represented a novel species of the genus Campylobacter. Although 16S rRNA sequencing is widely used for strain identification, it could not accurately identify the correct species of PS10T, and the identification at the whole genome level is accurate. With the advancement of sequencing technology, microbial whole genome sequencing (WGS) technology has become the most effective means of bacterial species identification, and the gold standard of bacterial species identification has been updated from the DDH to the ANI [70]. In the current identifications of isolates, these genomic-level identification methods make up for the errors caused by the traditional identification of the 16S rRNA short sequence.
Table 2.
ANI (lower diagonal) and dDDH (upper diagonal) among novel Campylobacter strains and closely related Campylobacter species.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 1 | 1 | 27.90% | 19.10% | 19.40% | 20.20% | 20.00% | 20.00% | 20.90% | 19.00% |
| 2 | 78.58% | 1 | 71.37% | 18.00% | 19.50% | 19.70% | 66.17% | 66.26% | 66.35% |
| 3 | 72.14% | 20.80% | 1 | 20.40% | 18.40% | 17.90% | 66.60% | 66.53% | 66.71% |
| 4 | 71.17% | 72.82% | 71.41% | 1 | 70.36% | 70.29% | 65.96% | 65.82% | 65.90% |
| 5 | 71.16% | 71.02% | 71.68% | 17.90% | 1 | 56.90% | 67.60% | 67.60% | 67.61% |
| 6 | 70.92% | 71.04% | 71.51% | 19.30% | 94.27% | 1 | 67.78% | 67.74% | 67.92% |
| 7 | 66.21% | 22.80% | 20.60% | 21.80% | 22.00% | 21.40% | 1 | 27.60% | 27.70% |
| 8 | 66.06% | 22.30% | 23.50% | 21.80% | 21.80% | 22.40% | 84.02% | 1 | 67.40% |
| 9 | 66.34% | 22.30% | 22.90% | 20.40% | 20.80% | 23.60% | 84.08% | 95.98% | 1 |
1, C. gastrosuis sp. nov.; 2, C. mucosalis; 3, C. majalis; 4, C. suis; 5, C. pinnipediorum subsp. caledonicus; 6, C. pinnipediorum subsp. pinnipediorum; 7, C. coli; 8, C. jejuni subsp. doylei; 9, C. jejuni subsp. jejuni; The sequence used here is the same as in the phylogenomic analysis.
3.4. Antibiotic Resistance
Antibiotic resistance was demonstrated through the fact that strain PS10T has high MIC values to five types of antibiotics, namely macrolides (erythromycin and azithromycin), quinolones (nalidixic acid), tetracyclines (tetracycline), ketolides (telithromycin), and lincosamides (clindamycin) (Table 3). A part of our results was consistent with previous reports showing that Campylobacter species are highly resistant to macrolides, quinolones, and tetracyclines [18,20,21,27,71]. Macrolides and fluoroquinolones have been the mainstays of therapy and are used in the production of agricultural animals. However, resistance into these antibiotics, particularly fluoroquinolones such as ciprofloxacin, is common [17,72]. C. coli was the predominant strain of Campylobacter isolated from the feces of pigs, and resistance to tetracycline was also common [25,57,73]. As foodborne pathogens, Campylobacter require continued monitoring and surveillance in antibiotic usage and the prevalence of antimicrobial resistance patterns.
Table 3.
MICs of novel strain PS10T to antimicrobials.
| Antimicrobial Class | Antimicrobial Agent | MIC (μg mL−1) |
|---|---|---|
| Macrolides | Erythromycin | >256 |
| Azithromycin | >256 | |
| Quinolones | Nalidixic acid | >256 |
| Ciprofloxacin | 3 | |
| Aminoglycosides | Gentamicin | 3 |
| Streptomycin | 8 | |
| Chloramphenicol | Chloramphenicol | 12 |
| Florfenicol | 8 | |
| Tetracyclines | Tetracycline | 32 |
| Ketolides | Telithromycin | >256 |
| Lincosamides | Clindamycin | >256 |
In the PS10T genome, in addition to the antibiotic resistance relative genes on the MDRGI, another adeF gene that was detected to mediate tetracycline resistance exists in another contig. However, there are large differences between resistance phenotypes and antibiotic resistance relative genes, and only the resistance of tetracycline is consistent among them. Although there were tetracycline, nucleoside, and aminoglycoside resistance genes on the MDRGI, the isolate did not exhibit corresponding phenotypes, which may be caused by the functional dormancy of this isolate [64]. On the other hand, tetracycline resistance may be mediated by the adeF gene, which is not present in the MDRGI. However, antibiotic resistance genes related to macrolides, quinolones, ketolides, and lincosamides were also not found in the genome, suggesting that further research about drug resistance mechanisms is needed to understand the resistance mechanism of these drugs.
The expression and activity of antibiotic resistance genes may be related to the status of bacteria, and drug-resistant MGEs may play an important role in the development of bacterial resistance as a risk factor for drug resistance. Therefore, it is necessary to detect the drug-resistant phenotype, drug-resistant related genes, and drug-resistant related gene mutations of pathogenic bacteria to monitor the resistance of pathogens.
4. Conclusions
Using a polyphasic approach, including DNA sequencing and analysis (16S rRNA gene and whole genome sequencing), and a wide range of phenotypic tests, as suggested by On et al. [56], provided sufficient evidence to distinguish this isolate from its closely related type strains and to confirm that this isolate represents a novel species in the genus Campylobacter. With PS10T as the type strain, we suggest the name Campylobacter gastrosuis sp. nov. for this novel member of the genus Campylobacter. Meanwhile, we also tested the antibiotic sensitivity of this isolated strain and found that it was resistant to a variety of antibiotics commonly used in Campylobacter, and we found an MDRGI on the genome. Additional investigation into the pathogenesis, prevalence among pigs, as well as the potential for human infection through contact with pigs, is imperative. Such research could aid in better understanding and managing diseases attributed to this novel species. A description of Campylobacter gastrosuis sp. nov. is presented in Table 4.
Table 4.
Description of Campylobacter gastrosuis sp. nov.
| Genus name | Campylobacter |
| Species name | Campylobacter gastrosuis |
| Specific epithet | gastrosuis |
| Species status | sp. nov. |
| Species etymology | (gas.tro.su’is., Gr. n. gaster gastros, stomach; L. n. sus suis, a pig; L. gen. n. gastrosuis, from a pig’s stomach) |
| Description of the new taxon and diagnostic traits | The cell is Gram-negative, motile, and spiral-shaped after 48 h of growth on Karmali or Columbia agar with 5% defibrinated sheep blood in a microaerophilic atmosphere at 37 °C. The colonies are wet, flat, grey, circular, and smooth but may vary in size and morphology after a long incubation. No hemolysis on blood agar was observed. Cells are negative activities for catalase, oxidase, and urease, and could not produce H2S. No hydrolysis of hippurate. Nitrate can be reduced and indoxyl acetate can be hydrolyzed. The isolate was resistant to different types of antibiotics, namely erythro-mycin, azithromycin, nalidixic acid, tetracycline, telithromycin, and clindamycin, and carries multiple resistance relative genes and has an MDRGI. |
| Country of origin | China |
| Region of origin | Beijing |
| Source of isolation | the gastric mucous of pigs |
| Sampling date (dd/mm/yyyy) | 14 March 2022 |
| Latitude (xx°xx′xx″ N/S) | 116°18′40″ N |
| Longitude (xx°xx′xx″ E/W) | 40°11′27″ E |
| Altitude (meters above sea level) | About 30 m |
| 16S rRNA gene accession nr. | OP278864 |
| Genome accession number | JANURM000000000 |
| Genome status | Draft |
| Genome size | 2240 kbp |
| GC mol% | 37.72 |
| Designation of the Type Strain | PS10T |
| Strain Collection Numbers | GDMCC 1.3686T; JCM 35849T |
Acknowledgments
We thank our colleagues from the Chinese Center for Disease Control and Prevention.
Abbreviations
ANI, average nucleotide identity; dDDH, digital DNA-DNA hybridization; ML, maximum-likelihood; MP, maximum parsimony; NJ, neighbor-joining; GBS, Guillain–Barré Syndrome; MDRGI, multi-drug resistance genomic island; COGs, Clusters of Orthologous Groups; WGS, whole genome sequencing; HGT, horizontal gene transfer; MGEs, mobile genetic elements.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms11092278/s1, Figure S1: Photomicrograph of Gram-stained exponentially growing Campylobacter gastrosuis sp. Nov. PS10T cells. A light microscope with 100× magnification was used; Figure S2: Maximum-likelihood phylogenetic tree based on nearly complete 16S rRNA gene showing the relationships between PS10T and the type strains of the genus Campylobacter. Bootstrap values (>70%) based on 1000 replicates are shown at branch nodes, with Arcobacter butzleri ATCC 49616T as an outgroup. Novel strain is highlighted in bold; Figure S3: Maximum-parsimony phylogenetic tree based on nearly complete 16S rRNA gene showing the relationships between PS10T and the type strains of the genus Campylobacter. Bootstrap values (>70%) based on 1000 replicates are shown at branch nodes, with Arcobacter butzleri ATCC 49616T as an outgroup. Novel strain is highlighted in bold. Table S1: ANI (lower diagonal) and dDDH (upper diagonal) among the novel Campylobacter strains and other Campylobacter species.
Author Contributions
H.W. and M.Z. contributed to the conception and design of the work; Y.G., L.H., and L.S. contributed to the acquisition of data; G.Z., X.C. and X.Z. participated in the analysis and interpretation of data; H.W. drafted the manuscript; Z.S., J.Z. and M.Z. revised the manuscript and provided important suggestions. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The GenBank/EMBL/DDBJ accession numbers of the National Center for Biotechnology Information (NCBI, https://www.ncbi.nlm.nih.gov/) (accessed on 20 June 2023) for the nearly full-length 16S rRNA gene and the draft genome sequences of this isolate PS10T are OP278864 and JANURM000000000, respectively.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Sponsored by the National Key Research and Development Program of China (2021YFC2301000), the Project for Novel Detection Techniques of Bacterial Pathogens (32073), the Enhancement of Comprehensive Monitoring, Prevention, and Control Capabilities for Traditional Infectious Diseases Such as Plague, Cholera, and Brucellosis (102393230020020000002), and the Prevention and Intervention of Bacterial and Fungal Infectious Diseases (102393220020020000031).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Wang H., Li Y., Gu Y., Zhou G., Chen X., Zhang X., Shao Z., Zhang J., Zhang M. Isolation and Genomic Characteristics of Cat-Borne Campylobacter felis sp. nov. and Sheep-Borne Campylobacter ovis sp. nov. Microorganisms. 2023;11:971. doi: 10.3390/microorganisms11040971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ju C., Ma Y., Zhang B., Zhou G., Wang H., Yu M., He J., Duan Y., Zhang M. Prevalence, genomic characterization and antimicrobial resistance of Campylobacter spp. isolates in pets in Shenzhen, China. Front. Microbiol. 2023;14:1152719. doi: 10.3389/fmicb.2023.1152719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soto-Beltra N.M., Lee B.G., Amezquita-Lopez B.A., Quinones B. Overview of methodologies for the culturing, recovery and detection of Campylobacter. Int. J. Environ. Health Res. 2023;33:307–323. doi: 10.1080/09603123.2022.2029366. [DOI] [PubMed] [Google Scholar]
- 4.Corcionivoschi N., Gundogdu O. Foodborne Pathogen Campylobacter. Microorganisms. 2021;9:1241. doi: 10.3390/microorganisms9061241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costa D., Iraola G. Pathogenomics of Emerging Campylobacter Species. Clin. Microbiol. Rev. 2019;32:e00072-18. doi: 10.1128/CMR.00072-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francois Watkins L.K., Laughlin M.E., Joseph L.A., Chen J.C., Nichols M., Basler C., Breazu R., Bennett C., Koski L., Montgomery M.P., et al. Ongoing Outbreak of Extensively Drug-Resistant Campylobacter jejuni Infections Associated With US Pet Store Puppies, 2016–2020. JAMA Netw. Open. 2021;4:e2125203. doi: 10.1001/jamanetworkopen.2021.25203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang M., Li Q., He L., Meng F., Gu Y., Zheng M., Gong Y., Wang P., Ruan F., Zhou L., et al. Association study between an outbreak of Guillain-Barre syndrome in Jilin, China, and preceding Campylobacter jejuni infection. Foodborne Pathog. Dis. 2010;7:913–919. doi: 10.1089/fpd.2009.0493. [DOI] [PubMed] [Google Scholar]
- 8.Shahrizaila N., Lehmann H.C., Kuwabara S. Guillain-Barre syndrome. Lancet. 2021;397:1214–1228. doi: 10.1016/S0140-6736(21)00517-1. [DOI] [PubMed] [Google Scholar]
- 9.Man S.M. The clinical importance of emerging Campylobacter species. Nat. Rev. Gastroenterol. Hepatol. 2011;8:669–685. doi: 10.1038/nrgastro.2011.191. [DOI] [PubMed] [Google Scholar]
- 10.Kaakoush N.O., Castano-Rodriguez N., Mitchell H.M., Man S.M. Global Epidemiology of Campylobacter Infection. Clin. Microbiol. Rev. 2015;28:687–720. doi: 10.1128/CMR.00006-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohkoshi Y., Sato T., Murabayashi H., Sakai K., Takakuwa Y., Fukushima Y., Nakajima C., Suzuki Y., Yokota S.I. Campylobacter upsaliensis isolated from a giant hepatic cyst. J. Infect. Chemother. 2020;26:752–755. doi: 10.1016/j.jiac.2020.02.015. [DOI] [PubMed] [Google Scholar]
- 12.Mori E., Hashimoto T., Yahiro T., Miura M., Ishihara T., Miyazaki M., Komiya K., Takahashi N., Nishizono A., Hiramatsu K. Campylobacter lari Vertebral Osteomyelitis. Jpn. J. Infect. Dis. 2022;75:322–324. doi: 10.7883/yoken.JJID.2021.532. [DOI] [PubMed] [Google Scholar]
- 13.Gourmelon M., Boukerb A.M., Nabi N., Banerji S., Joensen K.G., Serghine J., Cormier A., Megraud F., Lehours P., Alter T., et al. Genomic Diversity of Campylobacter lari Group Isolates from Europe and Australia in a One Health Context. Appl. Environ. Microbiol. 2022;88:e0136822. doi: 10.1128/aem.01368-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boukerb A.M., Penny C., Serghine J., Walczak C., Cauchie H.M., Miller W.G., Losch S., Ragimbeau C., Mossong J., Megraud F., et al. Campylobacter armoricus sp. nov., a novel member of the Campylobacter lari group isolated from surface water and stools from humans with enteric infection. Int. J. Syst. Evol. Microbiol. 2019;69:3969–3979. doi: 10.1099/ijsem.0.003836. [DOI] [PubMed] [Google Scholar]
- 15.Igwaran A., Okoh A.I. Human campylobacteriosis: A public health concern of global importance. Heliyon. 2019;5:e02814. doi: 10.1016/j.heliyon.2019.e02814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sahin O., Yaeger M., Wu Z., Zhang Q. Campylobacter-Associated Diseases in Animals. Annu. Rev. Anim. Biosci. 2017;5:21–42. doi: 10.1146/annurev-animal-022516-022826. [DOI] [PubMed] [Google Scholar]
- 17.Poudel S., Li T., Chen S., Zhang X., Cheng W.H., Sukumaran A.T., Kiess A.S., Zhang L. Prevalence, Antimicrobial Resistance, and Molecular Characterization of Campylobacter Isolated from Broilers and Broiler Meat Raised without Antibiotics. Microbiol. Spectr. 2022;10:e0025122. doi: 10.1128/spectrum.00251-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gharbi M., Bejaoui A., Ben Hamda C., Ghedira K., Ghram A., Maaroufi A. Distribution of virulence and antibiotic resistance genes in Campylobacter jejuni and Campylobacter coli isolated from broiler chickens in Tunisia. J. Microbiol. Immunol. Infect. 2022;55:1273–1282. doi: 10.1016/j.jmii.2021.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Redondo N., Carroll A., McNamara E. Molecular characterization of Campylobacter causing human clinical infection using whole-genome sequencing: Virulence, antimicrobial resistance and phylogeny in Ireland. PLoS ONE. 2019;14:e0219088. doi: 10.1371/journal.pone.0219088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen Z., Wang Y., Zhang Q., Shen J. Antimicrobial Resistance in Campylobacter spp. Microbiol. Spectr. 2018;6 doi: 10.1128/microbiolspec.ARBA-0013-2017. [DOI] [PubMed] [Google Scholar]
- 21.Dai L., Sahin O., Grover M., Zhang Q. New and alternative strategies for the prevention, control, and treatment of antibiotic-resistant Campylobacter. Transl. Res. 2020;223:76–88. doi: 10.1016/j.trsl.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.European Food Safety Authority. European Centre for Disease Prevention and Control The European Union one health 2019 zoonoses report. Efsa J. 2021;19:e06406. doi: 10.2903/j.efsa.2021.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meistere I., Kibilds J., Eglite L., Alksne L., Avsejenko J., Cibrovska A., Makarova S., Streikisa M., Grantina-Ievina L., Berzins A. Campylobacter species prevalence, characterisation of antimicrobial resistance and analysis of whole-genome sequence of isolates from livestock and humans, Latvia, 2008 to 2016. Euro. Surveill. 2019;24:1800357. doi: 10.2807/1560-7917.ES.2019.24.31.1800357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang J., Zang X., Lei T., Ren F., Jiao X. Prevalence of Campylobacter spp. in Pig Slaughtering Line in Eastern China: Analysis of Contamination Sources. Foodborne Pathog. Dis. 2020;17:712–719. doi: 10.1089/fpd.2020.2800. [DOI] [PubMed] [Google Scholar]
- 25.Tang M., Zhou Q., Zhang X., Zhou S., Zhang J., Tang X., Lu J., Gao Y. Antibiotic Resistance Profiles and Molecular Mechanisms of Campylobacter From Chicken and Pig in China. Front. Microbiol. 2020;11:592496. doi: 10.3389/fmicb.2020.592496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abley M.J., Wittum T.E., Moeller S.J., Zerby H.N., Funk J.A. Quantification of campylobacter in swine before, during, and after the slaughter process. J. Food Prot. 2012;75:139–143. doi: 10.4315/0362-028X.JFP-11-334. [DOI] [PubMed] [Google Scholar]
- 27.Li Y., Gu Y., Lv J., Liang H., Zhang J., Zhang S., He M., Wang Y., Ma H., French N., et al. Laboratory Study on the Gastroenteritis Outbreak Caused by a Multidrug-Resistant Campylobacter coli in China. Foodborne Pathog. Dis. 2020;17:187–193. doi: 10.1089/fpd.2019.2681. [DOI] [PubMed] [Google Scholar]
- 28.Frank J.A., Reich C.I., Sharma S., Weisbaum J.S., Wilson B.A., Olsen G.J. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl. Environ. Microbiol. 2008;74:2461–2470. doi: 10.1128/AEM.02272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tripathi N., Sapra A. StatPearls. StatPearls Publishing LLC.; Orlando, FL, USA: 2023. Gram Staining. Disclosure: Amit Sapra Declares no Relevant Financial Relationships with Ineligible Companies. [PubMed] [Google Scholar]
- 30.Zhou G., Liang H., Gu Y., Ju C., He L., Guo P., Shao Z., Zhang J., Zhang M. Comparative genomics of Helicobacter pullorum from different countries. Gut Pathog. 2020;12:56. doi: 10.1186/s13099-020-00394-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andrews S. FastQC: A Quality Control Tool for High Throughput Sequence Data. [(accessed on 21 May 2022)]. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/
- 32.Chen S. Ultrafast one-pass FASTQ data preprocessing, quality control, and deduplication using fastp. iMeta. 2023;2:e107. doi: 10.1002/imt2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo R., Liu B., Xie Y., Li Z., Huang W., Yuan J., He G., Chen Y., Pan Q., Liu Y., et al. SOAPdenovo2: An empirically improved memory-efficient short-read de novo assembler. Gigascience. 2012;1:18. doi: 10.1186/2047-217X-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoon S.H., Ha S.M., Kwon S., Lim J., Kim Y., Seo H., Chun J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017;67:1613–1617. doi: 10.1099/ijsem.0.001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rozewicki J., Li S., Amada K.M., Standley D.M., Katoh K. MAFFT-DASH: Integrated protein sequence and structural alignment. Nucleic Acids Res. 2019;47:W5–W10. doi: 10.1093/nar/gkz342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stecher G., Tamura K., Kumar S. Molecular Evolutionary Genetics Analysis (MEGA) for macOS. Mol. Biol. Evol. 2020;37:1237–1239. doi: 10.1093/molbev/msz312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saitou N., Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 38.Fitch W.M. Toward Defining the Course of Evolution: Minimum Change for a Specific Tree Topology. Syst. Zool. 1971;20:406–416. doi: 10.2307/2412116. [DOI] [Google Scholar]
- 39.Felsenstein J. Evolutionary trees from DNA sequences: A maximum likelihood approach. J. Mol. Evol. 1981;17:368–376. doi: 10.1007/BF01734359. [DOI] [PubMed] [Google Scholar]
- 40.Felsenstein J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution. 1985;39:783–791. doi: 10.2307/2408678. [DOI] [PubMed] [Google Scholar]
- 41.Seemann T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 42.Chan P.P., Lowe T.M. tRNAscan-SE: Searching for tRNA Genes in Genomic Sequences. Methods Mol. Biol. 2019;1962:1–14. doi: 10.1007/978-1-4939-9173-0_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fu L., Niu B., Zhu Z., Wu S., Li W. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics. 2012;28:3150–3152. doi: 10.1093/bioinformatics/bts565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Price M.N., Dehal P.S., Arkin A.P. FastTree: Computing large minimum evolution trees with profiles instead of a distance matrix. Mol. Biol. Evol. 2009;26:1641–1650. doi: 10.1093/molbev/msp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huson D.H., Scornavacca C. Dendroscope 3: An interactive tool for rooted phylogenetic trees and networks. Syst. Biol. 2012;61:1061–1067. doi: 10.1093/sysbio/sys062. [DOI] [PubMed] [Google Scholar]
- 46.Cantalapiedra C.P., Hernandez-Plaza A., Letunic I., Bork P., Huerta-Cepas J. eggNOG-mapper v2: Functional Annotation, Orthology Assignments, and Domain Prediction at the Metagenomic Scale. Mol. Biol. Evol. 2021;38:5825–5829. doi: 10.1093/molbev/msab293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bertelli C., Laird M.R., Williams K.P., Simon Fraser University Research Computing G., Lau B.Y., Hoad G., Winsor G.L., Brinkman F.S.L. IslandViewer 4: Expanded prediction of genomic islands for larger-scale datasets. Nucleic Acids Res. 2017;45:W30–W35. doi: 10.1093/nar/gkx343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carattoli A., Hasman H. PlasmidFinder and In Silico pMLST: Identification and Typing of Plasmid Replicons in Whole-Genome Sequencing (WGS) Methods Mol. Biol. 2020;2075:285–294. doi: 10.1007/978-1-4939-9877-7_20. [DOI] [PubMed] [Google Scholar]
- 49.Arndt D., Grant J.R., Marcu A., Sajed T., Pon A., Liang Y., Wishart D.S. PHASTER: A better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016;44:W16–W21. doi: 10.1093/nar/gkw387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akhter S., Aziz R.K., Edwards R.A. PhiSpy: A novel algorithm for finding prophages in bacterial genomes that combines similarity- and composition-based strategies. Nucleic Acids Res. 2012;40:e126. doi: 10.1093/nar/gks406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alcock B.P., Raphenya A.R., Lau T.T.Y., Tsang K.K., Bouchard M., Edalatmand A., Huynh W., Nguyen A.V., Cheng A.A., Liu S., et al. CARD 2020: Antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2020;48:D517–D525. doi: 10.1093/nar/gkz935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sullivan M.J., Petty N.K., Beatson S.A. Easyfig: A genome comparison visualizer. Bioinformatics. 2011;27:1009–1010. doi: 10.1093/bioinformatics/btr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu B., Zheng D., Jin Q., Chen L., Yang J. VFDB 2019: A comparative pathogenomic platform with an interactive web interface. Nucleic Acids Res. 2019;47:D687–D692. doi: 10.1093/nar/gky1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meier-Kolthoff J.P., Carbasse J.S., Peinado-Olarte R.L., Goker M. TYGS and LPSN: A database tandem for fast and reliable genome-based classification and nomenclature of prokaryotes. Nucleic Acids Res. 2022;50:D801–D807. doi: 10.1093/nar/gkab902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pritchard L., Glover R.H., Humphris S., Elphinstone J.G., Toth I.K. Genomics and taxonomy in diagnostics for food security: Soft-rotting enterobacterial plant pathogens. Analytical. Methods. 2016;8:12–24. doi: 10.1039/C5AY02550H. [DOI] [Google Scholar]
- 56.On S.L.W., Miller W.G., Houf K., Fox J.G., Vandamme P. Minimal standards for describing new species belonging to the families Campylobacteraceae and Helicobacteraceae: Campylobacter, Arcobacter, Helicobacter and Wolinella spp. Int. J. Syst. Evol. Microbiol. 2017;67:5296–5311. doi: 10.1099/ijsem.0.002255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lynch C., Peeters C., Walsh N., McCarthy C., Coffey A., Lucey B., Vandamme P. Campylobacter majalis sp. nov. and Campylobacter suis sp. nov., novel Campylobacter species isolated from porcine gastrointestinal mucosa. Int. J. Syst. Evol. Microbiol. 2022;72:5510. doi: 10.1099/ijsem.0.005510. [DOI] [PubMed] [Google Scholar]
- 58.Phung C., Scott P.C., Dekiwadia C., Moore R.J., Van T.T.H. Campylobacter bilis sp. nov., isolated from chickens with spotty liver disease. Int. J. Syst. Evol. Microbiol. 2022;72:5314. doi: 10.1099/ijsem.0.005314. [DOI] [PubMed] [Google Scholar]
- 59.Silva M.F., Pereira G., Carneiro C., Hemphill A., Mateus L., Lopes-da-Costa L., Silva E. Campylobacter portucalensis sp. nov., a new species of Campylobacter isolated from the preputial mucosa of bulls. PLoS ONE. 2020;15:e0227500. doi: 10.1371/journal.pone.0227500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parisi A., Chiara M., Caffara M., Mion D., Miller W.G., Caruso M., Manzari C., Florio D., Capozzi L., D’Erchia A.M., et al. Campylobacter vulpis sp. nov. isolated from wild red foxes. Syst. Appl. Microbiol. 2021;44:126204. doi: 10.1016/j.syapm.2021.126204. [DOI] [PubMed] [Google Scholar]
- 61.Rossi-Tamisier M., Benamar S., Raoult D., Fournier P.E. Cautionary tale of using 16S rRNA gene sequence similarity values in identification of human-associated bacterial species. Int. J. Syst. Evol. Microbiol. 2015;65:1929–1934. doi: 10.1099/ijs.0.000161. [DOI] [PubMed] [Google Scholar]
- 62.Debruyne L., Broman T., Bergstrom S., Olsen B., On S.L.W., Vandamme P. Campylobacter subantarcticus sp. nov., isolated from birds in the sub-Antarctic region. Int. J. Syst. Evol. Microbiol. 2010;60:815–819. doi: 10.1099/ijs.0.011056-0. [DOI] [PubMed] [Google Scholar]
- 63.Pendleton S., Hanning I., Biswas D., Ricke S.C. Evaluation of whole-genome sequencing as a genotyping tool for Campylobacter jejuni in comparison with pulsed-field gel electrophoresis and flaA typing. Poult. Sci. 2013;92:573–580. doi: 10.3382/ps.2012-02695. [DOI] [PubMed] [Google Scholar]
- 64.Tang Y., Lai Y., Yang X., Cao X., Hu Y., Wang X., Wang H. Genetic environments and related transposable elements of novel cfr(C) variants in Campylobacter coli isolates of swine origin. Vet. Microbiol. 2020;247:108792. doi: 10.1016/j.vetmic.2020.108792. [DOI] [PubMed] [Google Scholar]
- 65.Zhang A., Gu Y., Liang H., Deng Y., He L., Zhang J., Zhang M. Distribution of aminoglycoside resistance gene cluster aadE-sat4-aphA-3 in 607 Campylobacter isolates from different sources in China. Dis. Surveill. 2015;30:479–484. doi: 10.3784/j.issn.1003-9961.2015.06.012. [DOI] [Google Scholar]
- 66.Xu H., Chen Z., Huang R., Cui Y., Li Q., Zhao Y., Wang X., Mao D., Luo Y., Ren H. Antibiotic Resistance Gene-Carrying Plasmid Spreads into the Plant Endophytic Bacteria using Soil Bacteria as Carriers. Environ. Sci. Technol. 2021;55:10462–10470. doi: 10.1021/acs.est.1c01615. [DOI] [PubMed] [Google Scholar]
- 67.Meng M., Li Y., Yao H. Plasmid-Mediated Transfer of Antibiotic Resistance Genes in Soil. Antibiotics. 2022;11:525. doi: 10.3390/antibiotics11040525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.He T., Wang R., Liu D., Walsh T.R., Zhang R., Lv Y., Ke Y., Ji Q., Wei R., Liu Z., et al. Emergence of plasmid-mediated high-level tigecycline resistance genes in animals and humans. Nat. Microbiol. 2019;4:1450–1456. doi: 10.1038/s41564-019-0445-2. [DOI] [PubMed] [Google Scholar]
- 69.Guirado P., Miro E., Iglesias-Torrens Y., Navarro F., Campoy S., Alioto T.S., Gomez-Garrido J., Madrid C., Balsalobre C. A New Variant of the aadE-sat4-aphA-3 Gene Cluster Found in a Conjugative Plasmid from a MDR Campylobacter jejuni Isolate. Antibiotics. 2022;11:466. doi: 10.3390/antibiotics11040466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Richter M., Rossello-Mora R. Shifting the genomic gold standard for the prokaryotic species definition. Proc. Natl. Acad. Sci. USA. 2009;106:19126–19131. doi: 10.1073/pnas.0906412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang M., Gu Y., He L., Ran L., Xia S., Han X., Li H., Zhou H., Cui Z., Zhang J. Molecular typing and antimicrobial susceptibility profiles of Campylobacter jejuni isolates from north China. J. Med. Microbiol. 2010;59:1171–1177. doi: 10.1099/jmm.0.022418-0. [DOI] [PubMed] [Google Scholar]
- 72.Contreras-Omana R., Escorcia-Saucedo A.E., Velarde-Ruiz Velasco J.A. Prevalence and impact of antimicrobial resistance in gastrointestinal infections: A review. Rev. Gastroenterol. Mex. 2021;86:265–275. doi: 10.1016/j.rgmxen.2021.06.004. [DOI] [PubMed] [Google Scholar]
- 73.Sithole V., Amoako D.G., Abia A.L.K., Perrett K., Bester L.A., Essack S.Y. Occurrence, Antimicrobial Resistance, and Molecular Characterization of Campylobacter spp. in Intensive Pig Production in South Africa. Pathogens. 2021;10:439. doi: 10.3390/pathogens10040439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The GenBank/EMBL/DDBJ accession numbers of the National Center for Biotechnology Information (NCBI, https://www.ncbi.nlm.nih.gov/) (accessed on 20 June 2023) for the nearly full-length 16S rRNA gene and the draft genome sequences of this isolate PS10T are OP278864 and JANURM000000000, respectively.