Abstract
Choline is an essential nutrient for human body, but dietary choline is metabolized into the hazard metabolite for the cardiovascular system. Because of the conflicting results between dietary choline intake and cardiovascular disease (CVD) risk in previous studies, we aimed to investigate this in US adults. Non-pregnant participants and those aged >20 years from National Health and Nutrition Examination Survey 2011–2016, with CVD assessment and reliable dietary recall status, were included. The dietary choline intake was assessed as a mean value of two total dietary choline intakes, including dietary choline intake and supplemental choline intake, in 24-h dietary recall interviews. The association between dietary choline intake and the presence of CVD was examined using logistic regression. We enrolled 14,323 participants. The participants without CVD had substantially higher dietary choline intakes (318.4 mg/d vs. 297.2 mg/d) compared to those with CVD (p < 0.05). After multivariable adjustments, the highest quartile of dietary choline intake was associated with a lower CVD risk, OR 0.693, 95%CI [0.520, 0.923], when compared to the lowest quartile. Consistent results were also found for stroke. Subgroup analyses also supported these, especially in participants aged ≥60 years and in those with BMI < 30 kg/m2. We found that a higher dietary choline intake was associated with a lower CVD risk, especially the risk of stroke. Further clinical trials are needed in order to confirm this finding and to provide dietary suggestions for the appropriate amount of choline intake.
Keywords: dietary choline intake, cardiovascular disease, NHANES
1. Introduction
Cardiovascular diseases (CVD), including chronic heart failure (CHF), coronary heart diseases (CHD), and stroke, are considered the primary cause of death worldwide [1]. Globally, 17.3 million people die from CVD each year, and that number is rising, placing a significant burden on the medical care system [2]. Therefore, it is of the utmost importance to reduce CVD incidence by controlling related risk factors as soon as possible. Choline is an essential ingredient that is required for many biological processes in the human body, including the formation of cell membranes, the preservation of liver and kidney function, and the production of neurotransmitters [3]. For humans, only a small amount of choline can be endogenously generated through the liver phosphatidylethanolamine N-methyltransferase pathway. It is vital to supplement it in the diet to prevent deficiency. Many foods, such as red meat, eggs, fish, green vegetables, and whole grains, are sources of choline. Nevertheless, the gut microbiome can convert dietary choline into trimethylamine, which is then metabolized to trimethylamine-n-oxide (TMAO) by the flavin monooxygenases enzymes in the liver [4]. A recent collection of evidence has suggested that TMAO could promote the development of adverse cardiovascular events [5]. A study found an increased risk of dietary consumption of choline causing all-cause and CVD mortality in the Nurses’ Health Study (1980–2012) and the Health Professionals Follow-Up Study (1986–2012) [6]. This result was later included in a meta-analysis involving 18,076 incident CVD events and 5343 CVD deaths from 184,010 total participants. However, the meta-analysis found there was no association between choline and incident CVD [7]. Considering the choline provided by food is regarded as a paramount necessity for humans, it is important to determine dietary advice related to choline. Herein, we investigated the association between dietary choline intake and CVD risk through additional data from the 2011–2016 cross-sectional National Health and Nutrition Examination Survey (NHANES).
2. Materials and Methods
2.1. Study Population
The NHANES was a survey conducted by the US Centers for Disease Control and Prevention (CDC) to monitor the health and nutrition statuses of the adults and children in the US. The survey was approved by the Institutional Review Board of the National Center for Health Statistics (NCHS), and all participants gave informed consent. NHANES used “stratified multistage probability sampling” to obtain a nationally representative sample. The details of survey administration and methods can be accessed on the NHANES website (http://www.cdc.gov/nchs/nhanes.htm, accessed on 18 March 2023).
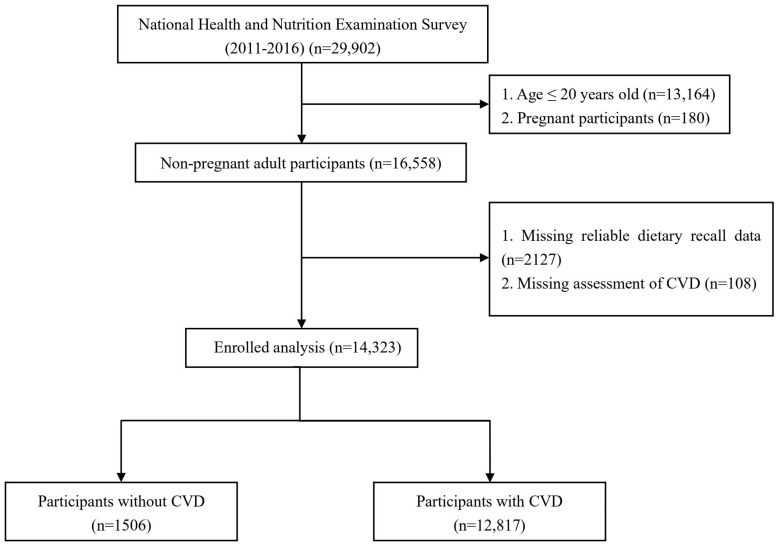
We combined 6 consecutive NHANES circles from 2011/2012 to 2015/2016 according to the NHANE analytical guidelines. The exclusion criteria were as follows: (1) participants aged ≤20 years, (2) participants who were pregnant, (3) participants missing an assessment of CVD, (4) participants without reliable dietary recall status. The selection process was shown in Figure 1. Finally, 14,323 participants were included in the subsequent analysis.
Figure 1.
Flowchart of participants included in the analysis.
2.2. Assessment of CVD
The CVD outcomes were diagnoses self-reported in a validated medical condition questionnaire during the personal interview, and participants were asked the following questions: “Has a doctor or other health professional ever told you that you have CHF/CHD/angina pectoris/heart attack/stroke?” Those who answered “yes” to the any of the questions were labeled as positive for CVD. In our study, we combined CHD, angina pectoris, and heart attack in the questionnaire regarding CHD, and the CVD outcomes included three types: CHF, CHD, and stroke.
2.3. Assessment of Dietary Choline Intake
Dietary choline intake was obtained from two 24-h dietary recall interviews, according to Food and Nutrient Database for Dietary Studies of United States Department of Agriculture. The two 24-h dietary recalls were collected by trained interviewers using the validated automated multiple pass Method. The first dietary recall interview was conducted in person at the Mobile Examination Center (MEC), and the second interview was conducted by telephone 3 to 10 days later. After the 24-h dietary recall, 24-h dietary supplement usage was collected, and the supplement amounts were obtained from NHANES’ dietary supplement database. In this study, the total choline intake included dietary intake and supplemental intake over 24 h, and we took the average of two days of total choline intake as the actual choline intake.
2.4. Covariates
Covariates in this study consisted of demographic characteristics (gender, age, race, education level, marital status, poverty/income ratio (PIR), physical activity category, body mass index (BMI), and smoking and drinking status), medical history (diabetes mellitus [DM], and hypertension), and laboratory results (white blood cells (WBCs), lymphocyte cells, monocyte cells, neutrophil cells, eosinophil cells, basophil cells, platelets (PLTs), red blood cells (RBC), hemoglobin (Hb), albumin (Alb), alanine transaminase (ALT), aspartate transaminase (AST), blood urea nitrogen (BUN), creatinine (Cr), uric acid (UA), estimated glomerular filtration rate (eGFR), glycohemoglobin (HbA1c), apolipoprotein B (Apo B), high-density lipoprotein cholesterol (HDL-C), triglyceride, and low-density lipoprotein cholesterol (LDL-C)).
The race categories included Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black, and other. Education levels were classified into below high school, high school, and above high school. Marital status was divided into three categories: married, separated, and never married. The PIR was calculated by dividing family (or individual) income by the poverty guidelines specific to the survey year, and a higher PIR indicated a better family income status. In this study, PIR was stratified as 0–1, 1–3, and >3 according to the original value. Weight and height were obtained from physical examinations, and BMI was calculated as weight in kilograms divided by height in meters squared. Participants who had smoked <100 cigarettes in their entire lives were defined as never smokers, those who had smoked ≥100 cigarettes but did not smoke at the time of survey were defined as former smokers, and those who had smoked ≥100 cigarettes and still smoked at the time of survey were defined as current smokers. Drinking status was categorized as non-drinker, low-to-moderate drinker (drinking <1 drink/day in women and <2 drinks/day in men), and heavy drinker (drinking ≥1 drink/day in women and ≥2 drinks/day in men) [8]. The terms of physical activity included work activity, walking or bicycling activity, and recreational activity. The weekly metabolic equivalent (MET) minutes of physical activity were recorded, and they were categorized as follows according to established standards: (1) below, i.e., <600 MET min/week or 150 min/week of moderate-intensity exercise; (2) meet, i.e., 600 to 1200 MET min/week or 150 to 300 min/week of moderate-intensity exercise; and (3) exceed, i.e., ≥1200 MET min/week or 300 min/week of moderate-intensity exercise [9]. DM was defined as a self-reported history of diabetes, HbA1c ≥ 6.5%, fasting plasma glucose ≥ 126 mg/dL, 2 h glucose in oral glucose tolerance test (OGTT) ≥200 mg/dL, taking antihyperglycemic agents, or taking insulin [10]. Hypertension was defined as a self-reported history of hypertension, blood pressure ≥ 140/90 mmHg, or taking antihypertensive agents [11]. The eGFR was calculated according to the four-variable Modification of Diet in Renal Disease (MDRD) [12]. The methodology for the laboratory tests is described in detail on the NHANES website (http://www.cdc.gov/nchs/nhanes/index.htm, accessed on 18 March 2023).
2.5. Statistical Analysis
The statistical analysis was performed in accordance with the analytic guidelines proposed by CDC. Considering the complex, multistage, probability sampling design of the NHANES, we integrated sample weights, stratification, and clustering into the statistical analysis. Continuous variables were presented in the form of weighted mean and standard error (SE), then compared using a survey-design-based t-test. Categorical variables were expressed by weighted percentages and compared using Rao-Scott χ2 statistics. The Proc Survey logistic regression analyses were applied to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) for the dietary choline intake and incidence of CVD. The dietary choline intake was categorized by quartiles, and the lowest quartile was used as the reference. In crude model 1, no covariates were included, and in model 2, age, gender, race, and BMI were included as covariates. In model 3, age, gender, race, BMI, education level, marital status, smoking status, drinking status, physical activity category, hypertension, DM, and PIR were included as covariates. Restricted cubic spline (RCS) regression was conducted with 4 default knots located at the 5th, 35th, 65th, and 95th percentiles of dietary choline intake to test the linearity/nonlinearity of the association between choline and CVD. We also explored the associations between dietary choline intake and three subtypes of CVD in model 3. Subgroup analyses, stratified by age, gender, and BMI, were performed among the different populations with the same adjustments as in model 3. All statistical analyses were conducted using R software version 3.6.0 (R Core Team, 2022, Vienna, Austria; version 4.1.6). p-value < 0.05 was considered as statistically significant.
3. Results
3.1. Baseline Characteristics of the Study Participants
A total of 14,323 participants from NHANES (2011–2016) were included in this study (Figure 1). The characteristics of the participants are presented in Table 1. The mean dietary choline intake was 316.5 ± 164.1 mg/d, and the incidence of CVD was 8.8% in the study participants. In Table 1, according to the quartiles of dietary choline intake, participants were equally classified into four groups. Participants in the Q4 group were more likely to be male and low-to-moderate or heavy drinkers, and they engaged in more physical activity. In addition, high dietary choline intake was associated with a slower heart rate; elevated levels of monocyte count, RBC, Hb, Alb, ALT, AST, BUN and UA; as well as lower levels of WBC and PLT. Table 2 summarizes the baseline characteristics of the participants with and without CVD.
Table 1.
Baseline characteristics of dietary choline intake among the general adult population in the National Health and Nutrition Examination Survey (NHANES), 2011–2016 (weighted).
| Variables | Total | Quartiles of Dietary Choline Intake | p-Value | |||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||
| Participants, n | 14,323 | 3566 | 3578 | 3583 | 3596 | |
| Male (%) | 7016 (48.8) | 1226 (32.2) | 1455 (40.1) | 1806 (49.9) | 2529 (70.8) | <0.001 |
| Age (mean ± SD) (years) | 48 ± 17 | 47 ± 17 | 49 ± 17 | 50 ± 16 | 48 ± 16 | <0.001 |
| Race (%) | <0.001 | |||||
| Mexican-American | 1936 (8.6) | 455 (8.6) | 445 (7.8) | 479 (8.2) | 557 (9.7) | |
| Other Hispanic | 1529 (6.0) | 419 (6.9) | 410 (6.3) | 358 (5.4) | 342 (5.4) | |
| Non-Hispanic White | 5598 (65.9) | 1300 (61.9) | 1427 (66.9) | 1456 (68.1) | 1415 (66.2) | |
| Non-Hispanic Black | 3208 (11.1) | 871 (13.0) | 807 (11.3) | 775 (10.2) | 755 (10.2) | |
| Other Race | 2052 (8.4) | 521 (9.7) | 489 (7.7) | 515 (8.1) | 527 (8.5) | |
| Education level (%) | <0.001 | |||||
| Below high school | 3175 (15.0) | 1006 (19.3) | 811 (15.3) | 686 (12.4) | 672 (13.4) | |
| High school | 3117 (20.9) | 825 (23.4) | 735 (20.6) | 752 (19.7) | 805 (20.1) | |
| Above high school | 8031 (64.1) | 1735 (57.2) | 2032 (64.1) | 2145 (67.9) | 2119 (66.5) | |
| Marital status (%) | <0.001 | |||||
| Married | 7327 (54.4) | 1608 (46.5) | 1797 (54.0) | 1991 (59.8) | 1931 (56.4) | |
| Separated | 3141 (19.0) | 938 (22.6) | 837 (19.8) | 738 (18.3) | 628 (15.7) | |
| Never married | 3855 (26.6) | 1020 (30.9) | 944 (26.2) | 854 (21.9) | 1037 (27.8) | |
| BMI (mean ± SD) (kg/m2) | 29.14 ± 6.86 | 29.22 ± 6.86 | 29.06 ± 7.19 | 29.14 ± 6.74 | 29.16 ± 6.67 | 0.9 |
| WBC (mean ± SD) (1000 cells/μL) | 7.29 ± 2.24 | 7.46 ± 2.34 | 7.26 ± 2.25 | 7.20 ± 2.22 | 7.26 ± 2.17 | 0.01 |
| Lymphocyte cells (mean ± SD) (1000 cells/μL) | 2.14 ± 0.99 | 2.21 ± 0.94 | 2.14 ± 0.95 | 2.10 ± 1.15 | 2.11 ± 0.90 | 0.001 |
| Monocyte cells (mean ± SD) (1000 cells/μL) | 0.57 ± 0.21 | 0.56 ± 0.21 | 0.56 ± 0.19 | 0.57 ± 0.21 | 0.59 ± 0.22 | 0.001 |
| Neutrophils cells (mean ± SD) (1000 cells/μL) | 4.33 ± 1.68 | 4.44 ± 1.78 | 4.31 ± 1.68 | 4.28 ± 1.58 | 4.31 ± 1.68 | 0.077 |
| Eosinophils cells (mean ± SD) (1000 cells/μL) | 0.20 ± 0.16 | 0.20 ± 0.16 | 0.20 ± 0.16 | 0.20 ± 0.15 | 0.21 ± 0.16 | 0.282 |
| Basophils cells (mean ± SD) (1000 cells/μL) | 0.05 ± 0.06 | 0.05 ± 0.06 | 0.05 ± 0.06 | 0.05 ± 0.05 | 0.05 ± 0.06 | 0.478 |
| PLT (mean ± SD) (1000 cells/μL) | 237.6 ± 60.2 | 245.9 ± 62.8 | 239.3 ± 59.5 | 236.1 ± 60.8 | 230.0 ± 57.0 | <0.001 |
| RBC (mean ± SD) (million cells/μL) | 4.67 ± 0.48 | 4.59 ± 0.48 | 4.64 ± 0.49 | 4.68 ± 0.47 | 4.76 ± 0.47 | <0.001 |
| Hb (mean ± SD) (g/dL) | 14.15 ± 1.46 | 13.81 ± 1.51 | 14.04 ± 1.42 | 14.20 ± 1.42 | 14.51 ± 1.42 | <0.001 |
| Alb (mean ± SD) (g/dL) | 4.33 ± 0.34 | 4.29 ± 0.35 | 4.33 ± 0.34 | 4.34 ± 0.34 | 4.37 ± 0.33 | <0.001 |
| ALT (mean ± SD) (U/L) | 25.5 ± 22.3 | 23.8 ± 15.9 | 24.9 ± 32.0 | 25.7 ± 18.0 | 27.5 ± 19.4 | <0.001 |
| AST (mean ± SD) (U/L) | 25.8 ± 16.1 | 25.2 ± 16.6 | 25.5 ± 17.7 | 25.7 ± 13.3 | 26.7 ± 16.7 | 0.013 |
| BUN (mean ± SD) (mg/dL) | 13.8 ± 5.5 | 12.8 ± 6.1 | 13.6 ± 5.2 | 14.0 ± 5.3 | 14.7 ± 5.3 | <0.001 |
| Cr (mean ± SD) (mg/dL) | 0.89 ± 0.37 | 0.88 ± 0.55 | 0.87 ± 0.29 | 0.88 ± 0.27 | 0.92 ± 0.31 | <0.001 |
| UA (mean ± SD) (mg/dL) | 5.44 ± 1.40 | 5.27 ± 1.41 | 5.35 ± 1.36 | 5.42 ± 1.42 | 5.68 ± 1.38 | <0.001 |
| HbA1c (mean ± SD) (%) | 5.65 ± 0.96 | 5.67 ± 1.03 | 5.64 ± 0.92 | 5.65 ± 0.93 | 5.66 ± 0.96 | 0.656 |
| Smoking status (%) | <0.001 | |||||
| Never smoker | 8086 (55.5) | 2075 (57.5) | 2157 (58.5) | 2012 (54.6) | 1842 (51.7) | |
| Former smoker | 3391 (25.1) | 687 (19.4) | 779 (23.1) | 949 (29.1) | 976 (28.1) | |
| Current smoker | 2846 (19.4) | 804 (23.1) | 642 (18.4) | 622 (16.3) | 778 (20.2) | |
| Drinking status (%) | <0.001 | |||||
| Non-drinker | 4563 (24.8) | 1470 (33.0) | 1275 (28.3) | 1087 (23.8) | 731 (15.3) | |
| Low-to-moderate drinker | 8479 (63.3) | 1871 (56.9) | 2036 (61.2) | 2173 (64.0) | 2399 (70.3) | |
| Heavy drinker | 1281 (11.8) | 225 (10.1) | 267 (10.5) | 323 (12.1) | 466 (14.4) | |
| eGFR (mean ± SD) (mL/min/1.73 m2) | 99.4 ± 29.7 | 101.1 ± 30.2 | 98.5 ± 27.8 | 99.6 ± 33.6 | 98.7 ± 26.5 | 0.132 |
| Physical activity category (%) | <0.001 | |||||
| Below | 5707 (35.9) | 1623 (40.7) | 1500 (37.9) | 1391 (35.7) | 1193 (29.9) | |
| Meet | 1536 (10.4) | 358 (9.5) | 410 (11.2) | 428 (12.5) | 340 (8.4) | |
| Exceed | 7080 (53.7) | 1585 (49.7) | 1668 (51.0) | 1764 (51.7) | 2063 (61.7) | |
| Hypertension (%) | 5340 (33.6) | 1380 (33.7) | 1352 (34.5) | 1367 (34.5) | 1241 (31.8) | 0.355 |
| DM (%) | 9324 (62.8) | 2478 (68.8) | 2169 (56.4) | 2329 (63.5) | 2348 (63.1) | <0.001 |
| PIR (%) | <0.001 | |||||
| 0–1 | 3291 (16.0) | 1030 (22.2) | 832 (16.1) | 717 (12.9) | 712 (13.4) | |
| 1–3 | 5857 (36.2) | 1517 (39.7) | 1448 (36.6) | 1416 (34.4) | 1476 (34.4) | |
| >3 | 5175 (47.9) | 1019 (38.1) | 1298 (47.3) | 1450 (52.7) | 1408 (52.2) | |
| HR (mean ± SD) (bpm) | 73 ± 12 | 74 ± 12 | 73 ± 12 | 72 ± 12 | 72 ± 12 | <0.001 |
| SBP (mean ± SD) (mmHg) | 123 ± 17 | 123 ± 18 | 122 ± 17 | 122 ± 16 | 123 ± 16 | 0.428 |
| DBP (mean ± SD) (mmHg) | 71 ± 12 | 71 ± 12 | 70 ± 12 | 71 ± 11 | 71 ± 12 | 0.333 |
| Apo B (mean ± SD) (mg/dL) | 91.2 ± 24.7 | 91.7 ± 24.8 | 89.6 ± 24.2 | 91.7 ± 24.2 | 91.8 ± 25.4 | 0.01 |
| HDL-C (mean ± SD) (mg/dL) | 53.7 ± 16.8 | 53.9 ± 18.0 | 54.8 ± 17.1 | 54.1 ± 16.4 | 52.0 ± 15.8 | <0.001 |
| Triglyceride (mean ± SD) (mg/dL) | 187.5 ± 171.5 | 191.1 ± 172.9 | 179.3 ± 174.6 | 190.0 ± 166.1 | 189.9 ± 172.2 | 0.115 |
| LDL-C (mean ± SD) (mg/dL) | 102.6 ± 37.9 | 102.0 ± 38.0 | 101.8 ± 36.7 | 103.4 ± 38.3 | 103.2 ± 38.4 | 0.3 |
| Total cholesterol (mean ± SD) (mg/dL) | 193.7 ± 41.5 | 193.9 ± 41.6 | 192.3 ± 41.5 | 195.4 ± 41.4 | 193.1 ± 41.5 | 0.118 |
1. Values are weighted means ± SE for continuous variables or weighted percentages for categorical variables. 2. Choline: Q1: <197 mg/d, Q2: 197–282 mg/d, Q3: 282–392 mg/d, Q4: ≥392 mg/d. 3. PIR: poverty/income ratio; DM: diabetes mellitus; HR: heart rate; SBP: systolic blood pressure; DBP: diastolic blood pressure; BMI: body mass index; WBC: white blood cells; PLT: platelets; RBC: red blood cells; Hb: hemoglobin; Alb: albumin; ALT: alanine transaminase; AST: aspartate transaminase; BUN: blood urea nitrogen; Cr: creatinine; UA: uric acid; eGFR: estimated glomerular filtration rate; HbA1c:glycohemoglobin; Apo B: apolipoprotein B; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol. 4. The significance of differences between quartiles is indicated by the p-value. A p-value < 0.05 was considered as statistically significant.
Table 2.
Characteristics of participants with/without CVD (weighted).
| Non-CVD | CVD | p-Value | |
|---|---|---|---|
| n | 12,817 | 1506 | |
| Male (%) | 6190 (48.4) | 826 (53.2) | 0.026 |
| Age (mean ± SD) (years) | 47 ± 16 | 65 ± 14 | <0.001 |
| Race (%) | <0.001 | ||
| Mexican-American | 1806 (8.9) | 130 (5.1) | |
| Other Hispanic | 1384 (6.1) | 145 (4.0) | |
| Non-Hispanic White | 4853 (65.4) | 745 (71.1) | |
| Non-Hispanic Black | 2844 (11.0) | 364 (12.8) | |
| Other Race | 1930 (8.6) | 122 (7.0) | |
| Education level (%) | <0.001 | ||
| Below high school | 2721 (14.3) | 454 (21.6) | |
| High school | 2733 (20.4) | 384 (26.3) | |
| Above high school | 7363 (65.3) | 668 (52.2) | |
| Marital status (%) | <0.001 | ||
| Married | 6589 (54.5) | 738 (53.6) | |
| Separated | 2573 (17.7) | 568 (33.0) | |
| Never married | 3655 (27.8) | 200 (13.4) | |
| Choline intake (mean ± SD) (mg/d) | 318.4 ± 165.5 | 297.2 ± 148.1 | 0.001 |
| BMI (mean ± SD) (kg/m2) | 28.99 ± 6.78 | 30.77 ± 7.47 | <0.001 |
| WBC (mean ± SD) (1000 cells/μL) | 7.26 ± 2.16 | 7.55 ± 2.95 | 0.001 |
| Lymphocyte cells (mean ± SD) (1000 cells/μL) | 2.15 ± 0.79 | 2.05 ± 2.17 | 0.003 |
| Monocyte cells (mean ± SD) (1000 cells/μL) | 0.56 ± 0.20 | 0.63 ± 0.23 | <0.001 |
| Neutrophils cells (mean ± SD) (1000 cells/μL) | 4.31 ± 1.68 | 4.59 ± 1.68 | <0.001 |
| Eosinophils cells (mean ± SD) (1000 cells/μL) | 0.20 ± 0.15 | 0.24 ± 0.22 | <0.001 |
| Basophils cells (mean ± SD) (1000 cells/μL) | 0.05 ± 0.06 | 0.05 ± 0.06 | 0.073 |
| PLT (mean ± SD) (1000 cells/μL) | 239.2 ± 59.3 | 220.3 ± 67.1 | <0.001 |
| RBC (mean ± SD) (million cells/μL) | 4.68 ± 0.47 | 4.55 ± 0.52 | <0.001 |
| Hb (mean ± SD) (g/dL) | 14.18 ± 1.45 | 13.87 ± 1.59 | <0.001 |
| Alb (mean ± SD) (g/dL) | 4.35 ± 0.34 | 4.18 ± 0.35 | <0.001 |
| ALT (mean ± SD) (U/L) | 25.48 ± 18.00 | 26.06 ± 48.10 | 0.686 |
| AST (mean ± SD) (U/L) | 25.66 ± 14.53 | 27.06 ± 27.66 | 0.114 |
| BUN (mean ± SD) (mg/dL) | 13.47 ± 5.00 | 17.32 ± 8.53 | <0.001 |
| Cr (mean ± SD) (mg/dL) | 0.87 ± 0.34 | 1.07 ± 0.56 | <0.001 |
| UA (mean ± SD) (mg/dL) | 5.40 ± 1.38 | 5.81 ± 1.59 | <0.001 |
| HbA1c (mean ± SD) (%) | 5.60 ± 0.90 | 6.19 ± 1.31 | <0.001 |
| Smoking status (%) | <0.001 | ||
| Never smoker | 7477 (57.0) | 609 (39.4) | |
| Former smoker | 2845 (24.0) | 546 (36.7) | |
| Current smoker | 2495 (19.0) | 351 (23.9) | |
| Drinking status (%) | <0.001 | ||
| Non-drinker | 3972 (24.1) | 591 (32.6) | |
| Low-to-moderate drinker | 7682 (63.9) | 797 (57.6) | |
| Heavy drinker | 1163 (12.0) | 118 (9.8) | |
| eGFR (mean ± SD) (ml/min/1.73 m2) | 101.2 ± 29.1 | 80.9 ± 29.2 | <0.001 |
| Physical activity category (%) | <0.001 | ||
| Below | 4839 (34.2) | 868 (53.4) | |
| Meet | 1381 (10.4) | 155 (10.9) | |
| Exceed | 6597 (55.4) | 483 (35.7) | |
| Hypertension (%) | 4203 (29.9) | 1137 (72.2) | <0.001 |
| DM (%) | 8076 (60.9) | 1248 (82.7) | <0.001 |
| PIR (%) | <0.001 | ||
| 0–1 | 2872 (15.6) | 419 (20.2) | |
| 1–3 | 5143 (35.2) | 714 (46.6) | |
| >3 | 4802 (49.3) | 373 (33.2) | |
| HR (mean ± SD) (bpm) | 73 ± 12 | 70 ± 12 | <0.001 |
| SBP (mean ± SD) (mmHg) | 122 ± 17 | 129 ± 20 | <0.001 |
| DBP (mean ± SD) (mmHg) | 71 ± 11 | 67 ± 14 | <0.001 |
| Apo B (mean ± SD) (mg/dL) | 91.5 ± 24.7 | 88.4 ± 24.43 | 0.001 |
| HDL-C (mean ± SD) (mg/dL) | 54.1 ± 16.8 | 50.0 ± 16.1 | <0.001 |
| Triglyceride (mean ± SD) (mg/dL) | 188.2 ± 173.0 | 181.0 ± 154.0 | 0.212 |
| LDL-C (mean ± SD) (mg/dL) | 103.5 ± 37.9 | 93.3 ± 36.5 | <0.001 |
| Total cholesterol (mean ± SD) (mg/dL) | 195.1 ± 41.2 | 179.3 ± 42.1 | <0.001 |
Values are weighted means ± SE for continuous variables or weighted percentages for categorical variables. CVD: cardiovascular diseases; PIR: poverty/income ratio; DM: diabetes mellitus; HR: heart rate; SBP: systolic blood pressure; DBP: diastolic blood pressure; BMI: body mass index; WBC: white blood cells; PLT: platelets; RBC: red blood cells; Hb: hemoglobin; Alb: albumin; ALT: alanine transaminase; AST: aspartate transaminase; BUN: blood urea nitrogen; Cr: creatinine; UA: uric acid; eGFR: estimated glomerular filtration rate; HbA1c:glycohemoglobin; Apo B: apolipoprotein B; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol.
3.2. Association between Dietary Choline Intake and CVD
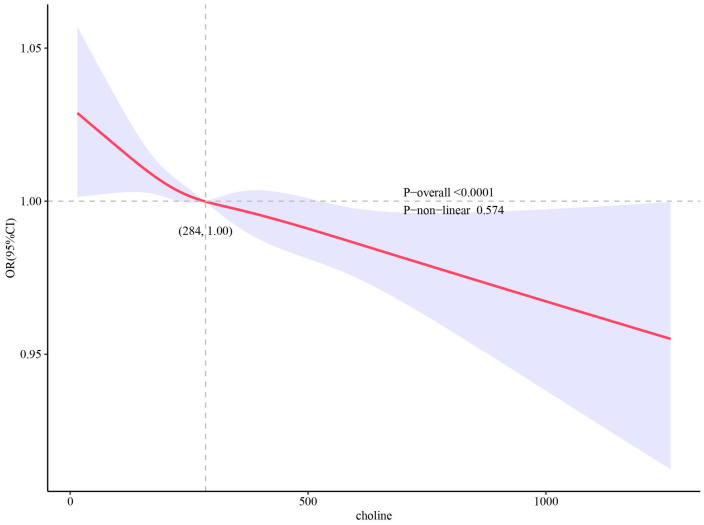
The association between the dietary choline intake and the presence of CVD are shown in Table 3. When compared with lowest quartile of dietary choline intake, the highest quartile was also associated with a lower incidence of CVD risk in all models: OR 0.728, 95%CI [0.580, 0.914] in unadjusted Model 1; OR 0.611, 95%CI [0.467, 0.799] in Model 2, adjusted for age, gender, race, and BMI; and OR 0.693, 95%CI [0.520, 0.923] in Model 3, adjusted for age, gender, race, BMI, education level, marital status, smoking status, drinking status, physical activity category, hypertension, DM, and PIR, respectively. The multivariable-adjusted RCS plot revealed that there was a linear association between dietary choline intake and the incidence of CVD (p-overall < 0.0001) (Figure 2). Table 4 shows the associations between dietary choline intake and subtypes of CVD. For different subtypes of CVD, a similar trend was found for stroke.
Table 3.
Logistic regression analysis of the association between choline and CVD (weighted).
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Choline | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value |
| Q1 | reference | reference | reference | |||
| Q2 | 0.904 (0.727, 1.125) | 0.359 | 0.758 (0.583, 0.985) | 0.039 | 0.863 (0.659, 1.131) | 0.271 |
| Q3 | 0.905 (0.739, 1.109) | 0.329 | 0.733 (0.572, 0.940) | 0.016 | 0.848 (0.654, 1.098) | 0.200 |
| Q4 | 0.728 (0.580, 0.914) | 0.007 | 0.611 (0.467, 0.799) | 0.001 | 0.693 (0.520, 0.923) | 0.014 |
Model 1: non-adjusted model; Model 2: adjusted age, gender, race and BMI; Model 3: adjusted age, gender, race, BMI, education level, marital status, smoking status, drinking status, physical activity category, hypertension, DM and PIR. Choline: Q1: < 197 mg/d, Q2: 197–282 mg/d, Q3: 282–392 mg/d, Q4: ≥ 392 mg/d.
Figure 2.
Restricted cubic spline (RCS) plot of the association between dietary choline intake levels and the presence of CVD. RCS regression was adjusted for age, gender, race, BMI, education level, marital status, smoking status, drinking status, physical activity category, hypertension, DM, and PIR (Model 3). The solid lines and shadow bands represent the OR and 95%CI.
Table 4.
Association of choline with the total and specific CVD.
| Subgroup | OR (95%CI) | p-Value |
|---|---|---|
| Overall | ||
| Q1 | Reference | |
| Q2 | 0.863 (0.659, 1.131) | 0.271 |
| Q3 | 0.848 (0.654, 1.098) | 0.200 |
| Q4 | 0.693 (0.520, 0.923) | 0.014 |
| CHF | ||
| Q1 | Reference | |
| Q2 | 0.807 (0.578, 1.127) | 0.197 |
| Q3 | 0.998 (0.668, 1.490) | 0.992 |
| Q4 | 0.743 (0.501, 1.103) | 0.133 |
| CHD | ||
| Q1 | Reference | |
| Q2 | 1.035 (0.733, 1.461) | 0.837 |
| Q3 | 0.874 (0.666, 1.146) | 0.314 |
| Q4 | 0.788 (0.539, 1.151) | 0.207 |
| Stroke | ||
| Q1 | Reference | |
| Q2 | 0.848 (0.609, 1.182) | 0.316 |
| Q3 | 0.858 (0.584, 1.262) | 0.421 |
| Q4 | 0.646 (0.457, 0.913) | 0.016 |
The model was adjusted for the parameters of age, gender, race, BMI, education level, marital status, smoking status, drinking status, physical activity category, hypertension, DM, and PIR. Choline: Q1: < 197 mg/d, Q2: 197–282 mg/d, Q3: 282–392 mg/d, Q4: ≥ 392 mg/d. CHF: congestive heart failure; CHD: coronary heart disease.
3.3. Subgroup Analysis of Dietary Choline Intake and CVD
A subgroup analysis, stratified by age, gender, and BMI, was carried out in this study (Table 5). For participants aged ≥60 years, high dietary choline intake in the Q4 group was negatively associated with the incidence of CVD: OR 0.669, 95%CI [0.479, 0.934], p = 0.020. Interestingly, for participants with BMIs < 30 kg/m2, the protective effect of high dietary choline against CVD was preserved, whereas for participants with BMIs ≥ 30 kg/m2, the protective effect was underpowered.
Table 5.
Subgroup analysis stratified by age, gender, and BMI.
| Subgroup | OR (95%CI) | p-Value |
|---|---|---|
| Overall | ||
| Q1 | Reference | |
| Q2 | 0.863 (0.659, 1.131) | 0.271 |
| Q3 | 0.848 (0.654, 1.098) | 0.200 |
| Q4 | 0.693 (0.520, 0.923) | 0.014 |
| Age < 60 years | ||
| Q1 | Reference | |
| Q2 | 0.914 (0.609, 1.370) | 0.650 |
| Q3 | 0.706 (0.475, 1.048) | 0.081 |
| Q4 | 0.819 (0.558, 1.202) | 0.294 |
| Age ≥ 60 years | ||
| Q1 | Reference | |
| Q2 | 0.871 (0.640, 1.185) | 0.364 |
| Q3 | 0.931 (0.685, 1.265) | 0.634 |
| Q4 | 0.669 (0.479, 0.934) | 0.020 |
| Female | ||
| Q1 | Reference | |
| Q2 | 0.768 (0.583, 1.011) | 0.059 |
| Q3 | 0.838 (0.605, 1.161) | 0.274 |
| Q4 | 0.788 (0.528, 1.176) | 0.232 |
| Male | ||
| Q1 | Reference | |
| Q2 | 1.079 (0.644, 1.808) | 0.764 |
| Q3 | 0.898 (0.617, 1.307) | 0.560 |
| Q4 | 0.722 (0.453, 1.152) | 0.163 |
| BMI < 30 kg/m2 | ||
| Q1 | Reference | |
| Q2 | 0.890 (0.642, 1.234) | 0.469 |
| Q3 | 0.794 (0.626, 1.007) | 0.056 |
| Q4 | 0.680 (0.487, 0.949) | 0.025 |
| BMI ≥ 30 kg/m2 | ||
| Q1 | Reference | |
| Q2 | 0.836 (0.571, 1.226) | 0.344 |
| Q3 | 0.935 (0.587, 1.488) | 0.767 |
| Q4 | 0.742 (0.489, 1.125) | 0.152 |
Logistic association between total choline intake and odds of adjusted age, gender, race, BMI, education level, marital status, smoking status, drinking status, physical activity category, hypertension, DM, and PIR. Choline: Q1: < 197 mg/d, Q2: 197–282 mg/d, Q3: 282–392 mg/d, Q4: ≥ 392 mg/d.
4. Discussion
The new findings of our study are as follows. (1) In contrast to previous studies, higher dietary choline intake was associated with a lower incidence of CVD, especially the incidence of stroke, in this large, nationally representative US population. (2) The protective role of higher dietary choline intake was accompanied by reduced inflammation and heart rate. (3) In the subgroup study, higher dietary choline intake in participants aged ≥60 years, and in participants with BMIs < 30 kg/m2, was found to be a protective factor for the presence of CVD. In summary, our results suggest that adequate choline intake acts against CVD and choline deficiency should be avoided.
There have been inconsistent findings regarding the relationship between dietary choline intake and CVD incidence, according to previous studies. Dalmeijer et al. [13] and Zheng et al. [6] found there was no association between dietary choline intake and incident CVD in post-menopausal Dutch women and in US women and men, respectively. The data from the Atherosclerosis Risk in Communities Study (ARIC) confirmed this [14]. A meta-analysis of four studies further supported that dietary choline was not associated with incident CVD (relative risk [RR] 1.00, 95% CI [0.98, 1.02]) [7]. However, data from 3924 Jackson Heart Study (JHS) showed a significant inverse association between dietary choline intake and incident stroke, β = −0.33 (p = 0.04) [15]. A cohort study involving 2606 subjects who were followed up for 10.6 years indicated that there was no association between total choline and the risk of CVD among adults, but a higher intake of free choline was associated with a lower risk of CVD (hazard rate [HR] 0.64, 95% CI [0.42, 0.98]) [16]. A PREDIMED-Plus trial found a longitudinal relationship between increased intake of dietary choline and improvements in cardiometabolic parameters [17]. Unlike the JHS study, which merely included African-American participants, and the PREDIMED-Plus trial, which evaluated cardiometabolic parameters, our study found for the first time that there was a protective effect of higher total choline intake on CVD incidence in 14,323 multi-ethnic participants.
Dietary factors play an important role in reducing cardiovascular disease risk, and some food sources rich in antioxidants and with anti-inflammatory, hypolipidemic, and hypoglycemic properties are thought to protect against the development of CVD. There is a complex association between dietary choline intake and CVD, which might be related to food source, TMAO concentration, and other factors. The PREDIMED-Plus trial suggested that food sources of choline might show a shift towards a healthier diet, including more fiber, omega-3 fatty acids, and polyphenols [17]. These foods might act synergistically with choline to improve the blood lipid profile and cardiometabolic parameters. Studies have indicated that TMAO concentration is primarily determined by the dietary consumption of precursors, gut microbial flora, liver flavin monooxygenase activity, and kidney function [18]. Hence, after taking into consideration the differences in TMAO generation, we determined that the association between higher dietary choline intake and CVD risk might be negative. In addition, according to the National Academies of Medicine, the recommended adequate intake (AI) of choline is 550 mg/day in adult men and 425 mg/day in adult women [19]. In our study, the mean dietary choline intake for participants was 316.5 mg/d, which was below the corresponding dietary choline recommendation. Choline is involved in the formation of specific phosphatidylcholine species, such as endogenous peroxisome proliferator-activated receptor α ligand (PPAR-α). PPAR-α participates in fatty acid oxidation, gluconeogenesis, lipid transport, and ketogenesis [15]. With inadequate dietary choline for phosphatidylcholine synthesis, the production of very low-density lipoproteins would decrease in the liver, whereas triglycerides would accumulate in the liver, heart, and arterial tissues, increasing the risk of CVD [20]. Hence, a sufficient dietary choline intake might be protective against the risk of CVD.
Several studies have demonstrated that dietary choline is independently associated with a reduction in inflammation mediators such as CRP, interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) [21,22]. These inflammation mediators play crucial roles in CVD. Studies have shown that dietary choline intake could attenuate inflammatory responses by increasing S-adenosylmethionine (SAM) and reducing S-adenosylhomocysteine (SAH) [22,23]. The elevated SAM could inhibit inducible nitric oxide synthase and nuclear factor-B, and increase the production of glutathione [22]. The SAH could be converted to homocysteine, and the decreased homocysteine could contribute to the lower CVD risk [23]. In our analysis, we observed that dietary choline intake was inversely associated with WBC counts. Thus, dietary choline intake might protect against CVD by appeasing inflammation.
In our study, there was no obvious association of dietary choline intake with systolic or diastolic blood pressure, triglyceride, total cholesterol, or LDL-C. It was worth noting that a high dietary choline intake was significantly associated with a slowed heart rate in this study. The mechanism by which choline reduces the heart rate may be involved with enhanced endogenous production of a phosphatidylcholine molecule that is enriched in DHA. DHA is an ω-3 (n-3) long-chain polyunsaturated fatty acid that has been shown to reduce heart rate and improve vascular reactivity [24]. Moreover, as a substrate for acetylcholine, choline may relax vascular smooth muscles and reduce heart rate [25]. In general, HDL-C can neutralize the proinflammatory and pro-oxidant effects of monocytes to prevent atherosclerosis. The mechanism involves the inhibition of the migration of macrophages, oxidation of LDL, and efflux of cholesterol [26]. However, we found that higher dietary choline intake was associated with a lower HDL-C. This may arise from dietary choline intake collected at baseline and can be affected by multiple confounding factors, such as dietary covariates, total energy intake, and other lifestyle factors.
In this study, it was interesting to note that the protective effect of high dietary choline intake against CVD was significant in participants with BMIs < 30 kg/m2. Obese individuals were at a greater risk of numerous diseases, including DM, hypertension, and CVD. Considering lifestyle and dietary factors in the obese, the obese tended to engage in less exercise and to consume foods abundant in saturated fats and cholesterol. When dietary choline is mainly obtained through excessively high-fat foods, these unhealthy dietary habits might promote CVD. However, Donya’s study indicated that overweight/obese adolescents with higher dietary intakes of choline were less likely to be metabolically unhealthy [27]. Furthermore, we found that a higher dietary choline intake in participants aged ≥ 60 years was associated with lower incidence of CVD. Further studies are needed in order to confirm these subgroup findings.
There are some strengths of this study. Firstly, our study sample included a large and nationally representative sample of US adults. Secondly, the total choline intake was calculated by the average of total choline intake over two days, including dietary and supplemental intake per 24 h. Thirdly, considering multiple confounding factors, we performed the subgroup analyses stratified by age, gender, and BMI. Lastly, we found that reducing dietary choline intake to prevent CVD was not recommended. Our result provides new evidence for public dietary health.
There are several limitations of this study. Firstly, although we have adjusted many potential confounding factors, other unknown factors could not be completely ruled out, such as dietary covariates, total energy intake, and genetic factors. Secondly, dietary choline intake information was collected during two 24-h dietary recall interviews, but individual diet intake may change over time. Third, because the NHANES study was surveyed in the US population using the standardized health questionnaire, unavoidable recall and report bias may also have influenced the process of data acquisition. These are the limitations of the NHANES study.
5. Conclusions
We retrospectively analyzed 14,323 adults in the US from NHANES and found that higher dietary choline intake was associated with a lower CVD risk. Our current results may provide a new perspective on the correlation between dietary choline intake and CVD, but further studies are still needed in order to confirm our findings and titrate the appropriate amount of choline intake. In addition, pharmaceutical treatment is closely related to clinical outcomes of CVD, and future investigations could also consider to include pharmacological treatments.
Author Contributions
Conceptualization, R.Z. and J.Z.; methodology, Y.S. and Y.T.; software, Y.S.; validation, Y.S. and Y.T.; formal analysis, R.Z.; investigation, M.Y.; resources, C.Y.; data curation, R.Z.; writing—original draft preparation, R.Z.; writing—review and editing, J.Z.; visualization, L.Z.; supervision, S.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The National Centre for Health Statistics’ research ethics review board approved the NHANES study.
Informed Consent Statement
All the NHANES participants provided their written informed consent.
Data Availability Statement
The data used in this study are publicly available online (https://wwwn.cdc.gov/nchs/nhanes/, accessed on 18 March 2023).
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Kassebaum N.J., Arora M., Barber R.M., Bhutta Z.A., Brown J., Carter A., Casey D.C., Charlston F.J., Coates M.M., Coggeshall M., et al. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1603–1658. doi: 10.1016/S0140-6736(16)31460-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mozaffarian D., Benjamin E.J., Go A.S., Arnett D.K., Blaha M.J., Cushman M., de Ferranti S., Després J.-P., Turner M.B., Fullerton H.J., et al. Heart disease and stroke statistics—2015 update: A report from the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.Bekdash R.A. Neuroprotective Effects of Choline and Other Methyl Donors. Nutrients. 2019;11:2995. doi: 10.3390/nu11122995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Díez-Ricote L., Ruiz-Valderrey P., Micó V., Blanco R., Tomé-Carneiro J., Dávalos A., Ordovás J.M., Daimiel L. TMAO Upregulates Members of the miR-17/92 Cluster and Impacts Targets Associated with Atherosclerosis. Int. J. Mol. Sci. 2022;23:12107. doi: 10.3390/ijms232012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Witkowski M., Weeks T.L., Hazen S.L. Gut Microbiota and Cardiovascular Disease. Circ. Res. 2020;127:553–570. doi: 10.1161/CIRCRESAHA.120.316242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng Y., Li Y., Rimm E.B., Hu F.B., Albert C.M., Rexrode K.M., Manson J.E., Li Q. Dietary phosphatidylcholine and risk of all-cause and cardiovascular-specific mortality among US women and men. Am. J. Clin. Nutr. 2016;104:173–180. doi: 10.3945/ajcn.116.131771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer K.A., Shea J.W. Dietary Choline and Betaine and Risk of CVD: A Systematic Review and Meta-Analysis of Prospective Studies. Nutrients. 2017;9:711. doi: 10.3390/nu9070711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C., Ye Y., Zhang Y., Pan X.F., Pan A. Weight change across adulthood in relation to all cause and cause specific mortality: Prospective cohort study. BMJ. 2019;367:15584. doi: 10.1136/bmj.l5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bao W., Liu B., Rong S., Dai S.Y., Trasande L., Lehmler H.J. Association Between Bisphenol A Exposure and Risk of All-Cause and Cause-Specific Mortality in US Adults. JAMA Netw. Open. 2020;3:e2011620. doi: 10.1001/jamanetworkopen.2020.11620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Diabetes Association Professional Practice Committee Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45((Suppl. S1)):S17–S38. doi: 10.2337/dc22-S002. [DOI] [PubMed] [Google Scholar]
- 11.Williams B., Mancia G., Spiering W., Agabiti Rosei E., Azizi M., Burnier M. ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 12.Levey A.S., Coresh J., Greene T., Stevens L.A., Zhang Y.L., Hendriksen S., Kusek J.W., Van Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann. Intern. Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 13.Dalmeijer G.W., Olthof M.R., Verhoef P., Bots M.L., van der Schouw Y.T. Prospective study on dietary intakes of folate, betaine, and choline and cardiovascular disease risk in women. Eur. J. Clin. Nutr. 2008;62:386–394. doi: 10.1038/sj.ejcn.1602725. [DOI] [PubMed] [Google Scholar]
- 14.Bidulescu A., Chambless L.E., Siega-Riz A.M., Zeisel S.H., Heiss G. Usual choline and betaine dietary intake and incident coronary heart disease: The Atherosclerosis Risk in Communities (ARIC) study. BMC Cardiovasc. Disord. 2007;7:20. doi: 10.1186/1471-2261-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Millard H.R., Musani S.K., Dibaba D.T., Talegawkar S.A., Taylor H.A., Tucker K.L., Bidulescu A. Dietary choline and betaine; associations with subclinical markers of cardiovascular disease risk and incidence of CVD, coronary heart disease and stroke: The Jackson Heart Study. Eur. J. Nutr. 2018;57:51–60. doi: 10.1007/s00394-016-1296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Golzarand M., Mirmiran P., Azizi F. Association between dietary choline and betaine intake and 10.6-year cardiovascular disease in adults. Nutr. J. 2022;21:1. doi: 10.1186/s12937-021-00755-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Díez-Ricote L., San-Cristobal R., Concejo M.J., Martínez-González M.Á., Corella D., Salas-Salvadó J., Goday A., Martínez J.A., Alonso-Gómez Á.M., Wärnberg J., et al. One-year longitudinal association between changes in dietary choline or betaine intake and cardiometabolic variables in the PREvención con DIeta MEDiterránea-Plus (PREDIMED-Plus) trial. Am. J. Clin. Nutr. 2022;116:1565–1579. doi: 10.1093/ajcn/nqac255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartiala J., Bennett B.J., Tang W.H., Wang Z., Stewart A.F., Roberts R., Allayee H., McPherson R., Lusis A.J., Hazen S.L., et al. Comparative genome-wide association studies in mice and humans for trimethylamine N-oxide, a proatherogenic metabolite of choline and L-carnitine. Arterioscler. Thromb. Vasc. Biol. 2014;34:1307–1313. doi: 10.1161/ATVBAHA.114.303252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bidulescu A., Chambless L.E., Siega-Riz A.M., Zeisel S.H., Heiss G. Repeatability and measurement error in the assessment of choline and betaine dietary intake: The Atherosclerosis Risk in Communities (ARIC) study. Nutr. J. 2009;8:14. doi: 10.1186/1475-2891-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiedeman A.M., Barr S.I., Green T.J., Xu Z., Innis S.M., Kitts D.D. Dietary Choline Intake: Current State of Knowledge Across the Life Cycle. Nutrients. 2018;10:1513. doi: 10.3390/nu10101513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazidi M., Katsiki N., Mikhailidis D.P., Banach M. Dietary choline is positively related to overall and cause-specific mortality: Results from individuals of the National Health and Nutrition Examination Survey and pooling prospective data. Br. J. Nutr. 2019;122:1262–1270. doi: 10.1017/S0007114519001065. [DOI] [PubMed] [Google Scholar]
- 22.Detopoulou P., Panagiotakos D.B., Antonopoulou S., Pitsavos C., Stefanadis C. Dietary choline and betaine intakes in relation to concentrations of inflammatory markers in healthy adults: The ATTICA study. Am. J. Clin. Nutr. 2008;87:424–430. doi: 10.1093/ajcn/87.2.424. [DOI] [PubMed] [Google Scholar]
- 23.Purohit V., Abdelmalek M.F., Barve S., Benevenga N.J., Halsted C.H., Kaplowitz N., Kharbanda K.K., Liu Q.-Y., Lu S.C., McClain C.J., et al. Role of S-adenosylmethionine, folate, and betaine in the treatment of alcoholic liver disease: Summary of a symposium. Am. J. Clin. Nutr. 2007;86:14–24. doi: 10.1093/ajcn/86.1.14. [DOI] [PubMed] [Google Scholar]
- 24.Jones P.J.H., Senanayake V.K., Pu S., Jenkins D.J.A., Connelly P.W., Lamarche B., Couture P., Charest A., Baril-Gravel L., West S.G., et al. DHA-enriched high-oleic acid canola oil improves lipid profile and lowers predicted cardiovascular disease risk in the canola oil multicenter randomized controlled trial. Am. J. Clin. Nutr. 2014;100:88–97. doi: 10.3945/ajcn.113.081133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furchgott R.F., Zawadzki J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 26.Ganjali S., Gotto A.M., Ruscica M., Jr., Atkin S.L., Butler A.E., Banach M., Sahebkar A. Monocyte-to-HDL-cholesterol ratio as a prognostic marker in cardiovascular diseases. J. Cell. Physiol. 2018;233:9237–9746. doi: 10.1002/jcp.27028. [DOI] [PubMed] [Google Scholar]
- 27.Poursalehi D., Lotfi K., Mirzaei S., Asadi A., Akhlaghi M., Saneei P. Association between methyl donor nutrients and metabolic health status in overweight and obese adolescents. Sci. Rep. 2022;12:17045. doi: 10.1038/s41598-022-21602-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this study are publicly available online (https://wwwn.cdc.gov/nchs/nhanes/, accessed on 18 March 2023).