Abstract
Monoamine oxidases (MAOs) are a family of flavin adenine dinucleotide-dependent enzymes that catalyze the oxidative deamination of a wide range of endogenous and exogenous amines. Multiple neurological conditions, including Parkinson’s disease (PD) and Alzheimer’s disease (AD), are closely correlated with altered biogenic amine concentrations in the brain caused by MAO. Toxic byproducts of this oxidative breakdown, including hydrogen peroxide, reactive oxygen species, and ammonia, can cause oxidative damage and mitochondrial dysfunction in brain cells. Certain MAO-B blockers have been recognized as effective treatment options for managing neurological conditions, including AD and PD. There is still a pressing need to find potent therapeutic molecules to fight these disorders. However, the focus of neurodegeneration studies has recently increased, and certain compounds are now in clinical trials. Chromones are promising structures for developing therapeutic compounds, especially in neuronal degeneration. This review focuses on the MAO-B inhibitory potential of several synthesized chromones and their structural activity relationships. Concerning the discovery of a novel class of effective chromone-based selective MAO-B-inhibiting agents, this review offers readers a better understanding of the most recent additions to the literature.
Keywords: chromones, neurodegenerative disorders, monoamine oxidase-B, structure-activity relationship, Parkinson’s disease, Alzheimer’s disease
1. Introduction
According to epidemiological data, Parkinson’s disease (PD) and Alzheimer’s disease (AD) are the most prevalent neurological illnesses. These conditions substantially negatively impact the suffering of individuals, relatives, caretakers, and the community. Unfortunately, only palliative treatments are currently available, which makes the design and creation of novel medications necessary [1,2,3]. AD is a neurological condition primarily affecting older people and is characterized by memory loss and dementia [4,5]. A range of illnesses, mostly related to neuronal cells in the human brain, are called neurodegenerative diseases. These disorders, often age-dependent, can be broadly characterized by the gradual degradation of the framework and functioning of the central or peripheral nervous systems [6,7,8,9]. Because neuronal cells are the basic units of the neurological system, they seldom reproduce or replenish themselves, and neuronal destruction or demise results in an inevitable loss of memory and cognitive impairments in people; however, under rare circumstances, it could lead to impairments in movement, speech, and breathing [10].
The World Health Organization (WHO) has projected that 50 million individuals will live with dementia worldwide by 2020, with approximately 10 million new cases occurring yearly. This number is expected to increase to 152 million by 2050. According to the WHO, AD may be a factor in 60–70% of dementia cases [6,11]. Despite numerous investigations into novel treatments in various phases of clinical trials, no effective therapies cure or reduce the progression of neurodegenerative illnesses [12,13,14]. Existing therapies may help alleviate most associated psychological and physical symptoms [12,15].
Monoamine oxidase (MAO) is a flavin adenine dinucleotide-dependent enzyme that is mainly found on the outer surface of mitochondria and is responsible for the oxidative breakdown of monoamines, including neurotransmitters such as dopamine, norepinephrine, as well as 5-hydroxytryptamine (serotonin) [16,17,18,19]. MAO-A and MAO-B are two subtypes that differ in tissue distribution, substrate particularity, susceptibility to particular inhibiting agents, and amino acid sequence [20,21]. Specifically, MAO-A deaminates noradrenaline, whereas MAO-B preferentially deaminates phenylethylamine, serotonin (5-hydroxytryptamine), and benzylamine [22]. MAO-B is primarily present in glial cells in the brain [23], whereas MAO-A is found in noradrenergic, serotonergic, and dopaminergic nerves and extra-neuronal compartment terminals [24]. Specific MAO-B blockers have been employed with levodopa to treat PD, whereas specific MAO-A-inhibiting agents have been utilized as antidepressants and anxiolytics [25,26,27,28]. MAO catalyzes the generation of hydrogen peroxide (H2O2) and reactive oxygen species (ROS), which may lead to oxidative stress and cell damage, ultimately leading to the progression of neurodegenerative disorders (ND); therefore, the concurrent inhibition of MAO may provide additional advantages for the treatment of ND [29,30,31]. MAO inhibitors are uncommon in clinical settings because only two medications, rasagiline and selegiline, have been approved for use as MAO-B blockers [32]. Both inhibitors have irreversible effects and are used to treat PD. Another MAO-B inhibitory compound, safinamide, has been clinically tested and functions as a reversible blocker [33]. Various scientific communities have focused on searching for novel MAO-B blockers with characteristics similar to those approved because of the limited number of -B blockers accessible for clinical use [34].
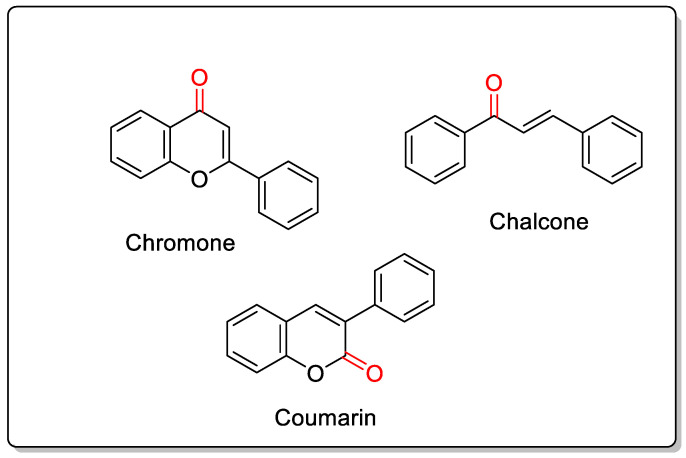
This review is solely concerned with substances belonging to the chromone class with MAO-inhibitory capabilities. The well-known MAO-B blockers chalcones and coumarins share structural similarities with chromones (Figure 1).
Figure 1.
Structurally similar oxygen-containing MAO-B inhibitors.
As shown in Figure 1, the structures of chalcones and coumarins are closely related to those of chromones. The MAO-B inhibitory properties of coumarins and chalcones have been reported in numerous studies [35,36,37,38,39]. Considering the similarities between these structures and chromones, further studies are needed to identify novel therapeutic candidates.
2. Chemistry of Chromones
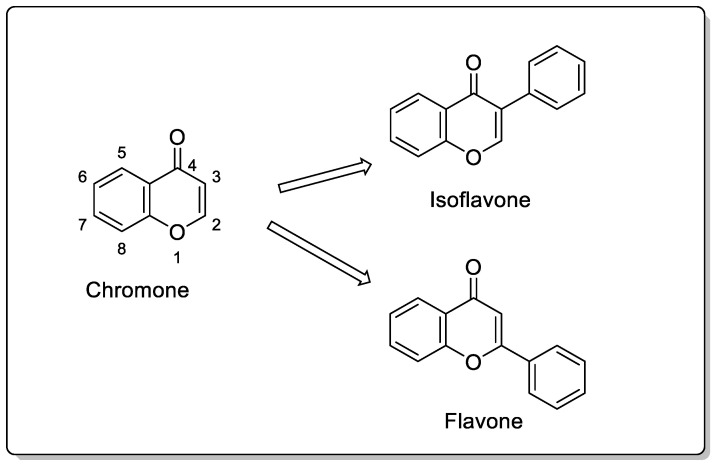
Chromones (4H-chromen-4-one,4H-1-benzopyran-4-one) are a significant class of oxygen-containing heterocyclic compounds with a benzoannelated-pyrone ring [40,41]. They belong to the flavonoid family, which includes isoflavones and flavones (Figure 2).
Figure 2.
Structure of a chromone, isoflavone, and flavone.
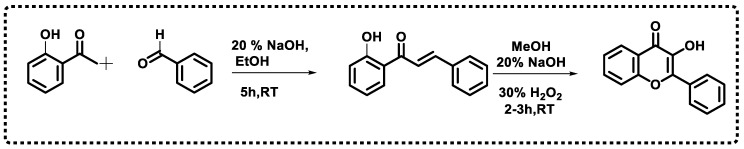
The development of substituted chromone analogs has an extensive background and is of great interest [42]. One of the most frequently used methods for producing chromones is the Claisen–Schmidt condensation of an aromatic aldehyde with an ortho-hydroxyarylketone, followed by cyclization [43,44] (Scheme 1). The small polar surface area (PSA) of chromones promotes blood–brain barrier crossing, which is primarily responsible for chromone-derived substances’ ability to exert their effects on the CNS. Numerous heterocyclic scaffolds have been considered for developing novel MAO-B inhibitors because isoform selectivity is a major concern. Isomers of coumarin, called chromones (4H-1-benzopyran-4-one), constitute the flavone backbone. The chromone ring system is regarded as a favored scaffold owing to its range of pharmacological and biological effects and the minimal risk of toxicity associated with chromone derivatives. Compounds derived from chromone scaffolds inhibit MAO. The AChE inhibitory potential of chromones was also investigated [45,46]. As dopamine D2 receptor agonists, chromones have been shown to mimic the actions of dopamine in the body, making them a potentially useful class of drugs for treating PD [47]. Chromones have been shown to have a significant capacity to adhere to adenosine receptors and have been studied clinically for managing PD and various CNS disorders [48,49,50,51,52].
Scheme 1.
The common route involved in the preparation of chromone scaffold.
Studies investigating the specificity of chromones have discovered that the methyl substitution on the chromone ring seemed more crucial for MAO-B inhibition than that of MAO-A. The current review, therefore, focuses on investigating the structure–activity relationship (SAR) and different methods of synthesis of various substituted chromones to determine how they affect MAO inhibitory activity.
3. SAR Studies of Chromone as MAO-B Inhibitors
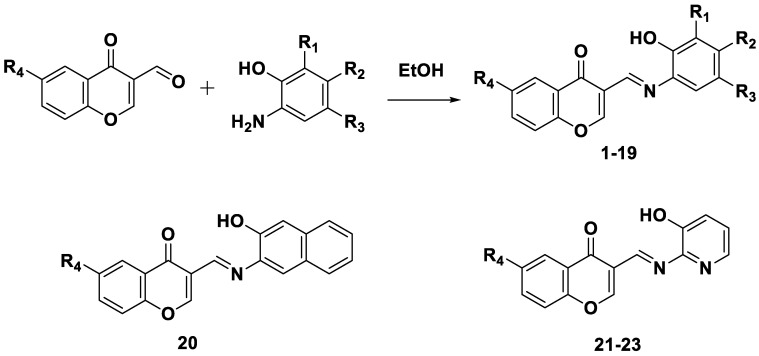
Li et al. (2017) reported the discovery of multipurpose chromone-containing ligands with the potential for MAO inhibition, reaction with β-amyloid, chelation with metals, antioxidant activity, and regulation of reactive oxygen species [29]. They employed phenolic hydroxyl groups and Schiff bases as metal chelators. Target multifunctional ligands (MLs) were modified with phenolic substituents because they are known to have antioxidant properties. Several unique chromone derivatives were obtained and positioned at the C3 position of the pyrone ring as a result of the functionalization of the chromone nucleus. The compounds were prepared by condensing an aldehyde with an aromatic amine in ethanol under reflux conditions, which is the traditional technique for imine synthesis shown in Scheme 2.
Scheme 2.
Synthesis of chromones based on Schiff’s base compounds (1–23).
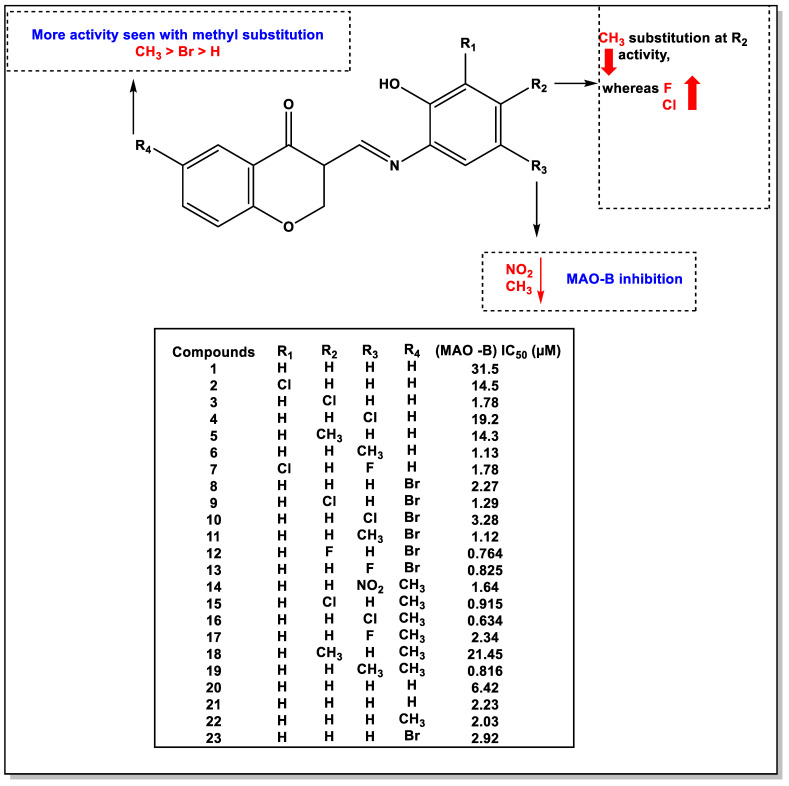
Figure 3 shows that adding different substituents to the phenyl ring or chromone moiety improved hMAO-B activity compared to compound 1 with no substitution (IC50 = 31.5 µM). It was found to be more effective in inhibiting MAOs when the bromine substitution was at the R4 position of the chromone, as in the cases of 8–13 and 12, with the F substitution at the R2 position of the phenyl ring. Introducing the methyl group in the R4 position of compounds 14–19 showed better activity than other analogs. Still, compound 18 showed the least inhibitory activity, with an IC50 value of 21.45 µM for hMAO-B, with the CH3 group at the R2 position. The highest inhibitory activity was demonstrated by compound 16, which had a methyl substitution at the R4 position, and in the R3 position of the phenyl ring, a chlorine group was introduced. The IC50 of 0.634 µM is roughly 12 times more active than the standard iproniazid (IC50 = 7.98 µM). A complete loss of inhibitory activity was observed when Cl group 15 was replaced with NO2 group 14 at the R2 position of the phenyl ring. Compounds 12, 13, 15, 16, and 19 demonstrated potent MAO inhibitory activities. The phenyl ring in the proposed compounds was changed to a different naphthalene ring 20 and pyridine rings 21–23, which were found to have less potent activity. According to this study, it can be concluded that the NO2 and CH3 groups at R3 decrease MAO activity, whereas halogens such as chlorine or fluorine can cause a marked increase in activity. More potency was found with a methyl substitution at R4.
Figure 3.
SAR study of chromone derivatives as MAO-B inhibitors.
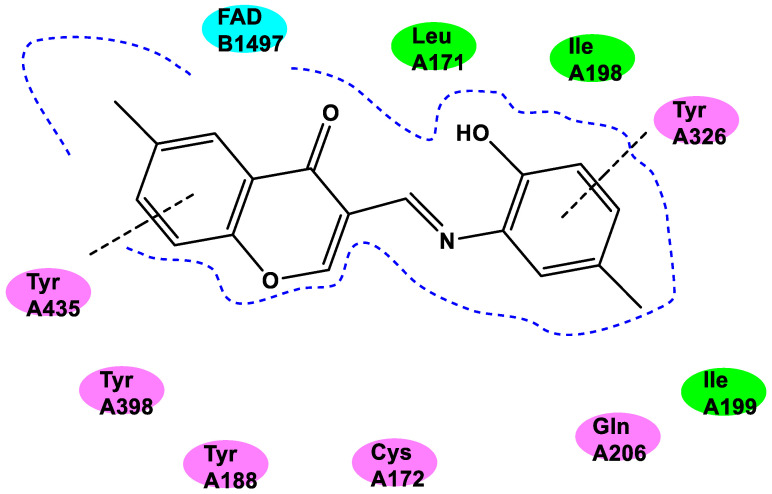
A docking study was performed using this protein (PDB ID:2V61). Figure 4 explains the two-dimensional interaction, which showed that the FAD cofactor was situated near the chromone moiety of 19 and that the phenyl group of 19 had a π-π stacking interaction with Tyr435 at a distance of 3.18 Å. Tyr326 was involved in a π-π stacking contact with the amide carbonyl of 19 separated by a distance of 3.49 Å. Additionally, the hydrophobic pocket in the entrance cavity created by Leu171, Ile198, and Ile199 was occupied by the 2-amino-4-methylphenol moiety in compound 19. These interactions may account for compound 19’s effective inhibitory activity against MAO-B.
Figure 4.
Docking study of compound 19. Green, hydrophobic amino acid residue; purple, hydrophilic amino acid residue; blue, FAD.
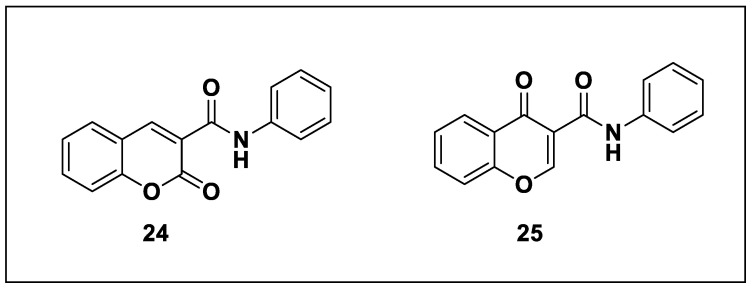
Fonseca et al. (2017) developed a set of novel coumarin and chromone derivatives and tested their biological activities against MAO-B [1]. With a focus on the lead optimization and under the guidance of the data obtained thus far, they carried out a detailed SAR examination of the two structural isomers. Compounds 34 and 54 were the most effective, selective, and reversible non-competitive MAO-B inhibitors. Searching for novel chemical compounds with pharmacological activities primarily involves using heterocyclic compounds. Benzopyrones are primarily coumarins and chromones. Chromones and coumarins are abundant and have useful therapeutic activities, such as antioxidant, anti-inflammatory, cardioprotective, and antibacterial effects. Coumarin-3-phenylcarboxamide 24 and chromone-3-phenylcarboxamide 25 (Figure 5) are desirable structures for the rational creation and identification of novel MAO-B inhibitors, according to Fonseca et al. and his research team.
Figure 5.
Coumarin-3-phenylcarboxamide (24) and chromone-3-phenylcarboxamide (25).
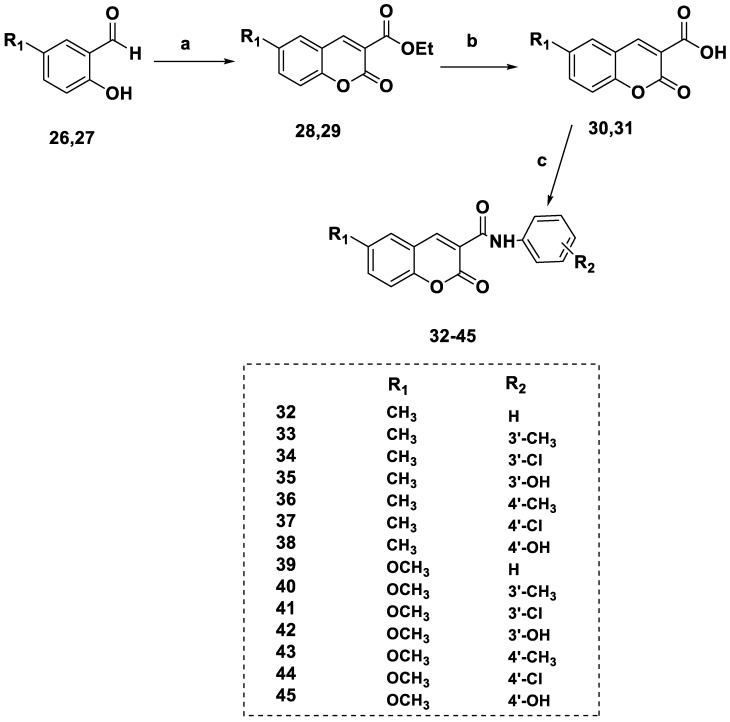
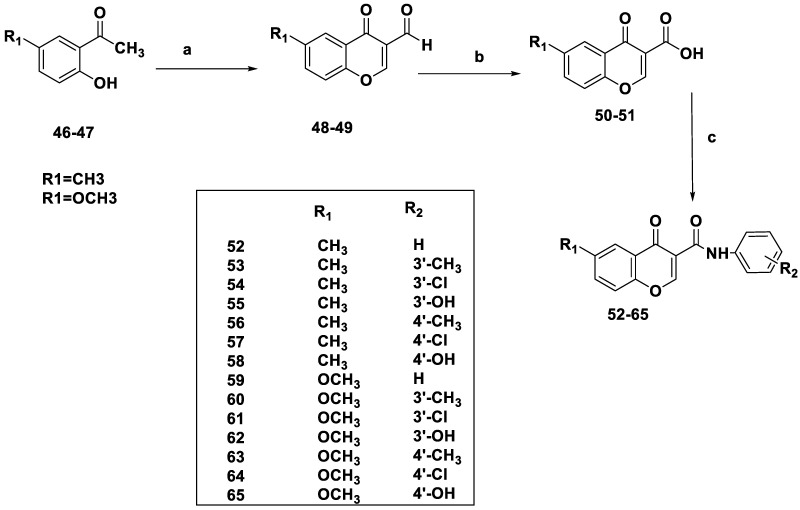
A library of novel coumarins 32–45 (Scheme 3) and chromones 52–65 (Scheme 4) was synthesized and evaluated for MAO-B inhibition. Scheme 3 shows the synthesis of coumarin derivatives by treating salicylaldehyde with diethylmalonate to obtain coumarin-3-carboxylates 28 and 29 and coumarin carboxylic acids 30 and 31. The coumarin-3-carboxamide derivatives 32–45 were created via the EDC-induced coupling of compounds 30 and 31 with phenylamines.
Scheme 3.
Synthesis of the coumarin derivatives 32–45. Reagents and conditions: (a) diethyl malonate, EtOH, piperidine, reflux, overnight; (b) NaOH (0.5% aq. ethanol), reflux, 4 h; (c) EDC, DMAP, DCM, substituted phenylamine, 0° to room temperature (rt), 4 h.
Scheme 4.
Synthesis of chromone-3-phenylcarboxamide 52–65. Reagents and conditions: (a) POCl3, DMF, −10 °C, 15 h; (b) H3NSO3, NaClO2, 0 °C, 12 h; (c) POCl3, DMF, phenylamine, rt, 1–5 h.
Scheme 4 explains the synthesis of chromone derivatives by treating acetophenone with phosphoryl chloride (POCl3) and N,N-Dimethyl formamide (DMF) at −10 °C for 15 h to obtain chromone-3-carbaldehydes (48 or 49) followed by oxidation of the formyl group with sodium chlorite to obtain chromone carboxylic acids (50–51). The synthesis of chromone-3-carboxamide derivatives (52–65) required the in situ formation of an acyl chloride intermediate, followed by the inclusion of a suitable phenylamine.
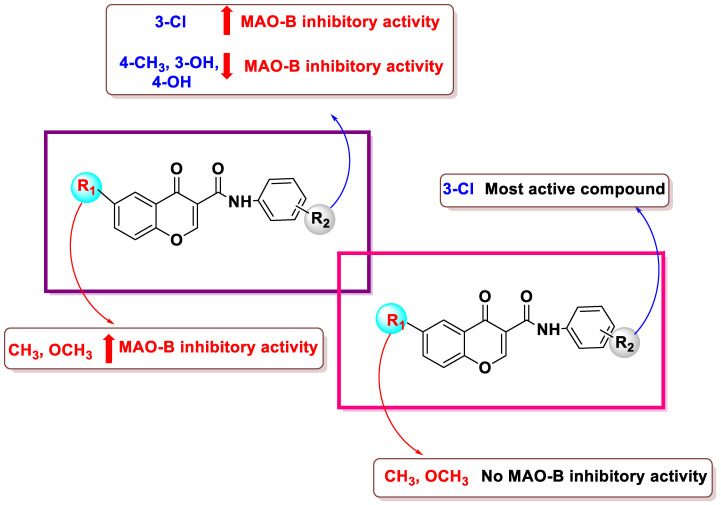
SAR analysis was conducted in response to earlier studies examining substituents’ effects at comparable positions on the isomeric scaffolds of coumarin- and chromone-based compounds as MAO inhibitors. Adding 6-CH3 or 6-OCH3 substituents to benzopyrone-3-phenylcarboxamide scaffolds led to strong MAO-B inhibition (Table 1). However, the 6-methylcoumarin derivatives (32–38) were more potent than their 6-methoxy analogs (39–45). The addition of a 6-methyl (52–58) or 6-methoxy (39–45) group to the chromone framework had no appreciable impact on the MAO-B inhibitory activity, except for compound 52 (which lacks substituents on the exocyclic ring). Derivatives with meta-alternatives on the exocyclic ring were shown to have increased potency in both series. Compounds 34, 54, and 61, all of which had m-chlorine substituents, were the major active compounds in both sets, with IC50 values of 5.07, 4.2, and 3.94 μM, respectively. The addition of substituents in the para-group resulted in decreased activity, except for compound 43 (IC50 = 19.43 μM), which had a p-CH3 group, as compared to compound 40 (IC50 = 47.24 μM), which had a m-methyl group. The activity was reduced by the influence of an OH group at either the meta- or para-position (Figure 6). The location of the benzopyrone carbonyl group on the pyrone ring did not appear to have a significant effect, although MAO-B inhibition was a function of this group. All compounds under investigation were selective for MAO-B (Table 1).
Table 1.
MAO inhibitory activities of benzopyrone derivatives 32–45 and 52–65.
| Compound | IC50 (μM) hMAO-B |
Compound | IC50 (μM) hMAO-B |
|---|---|---|---|
| 32 | 15.32 ± 1.02 | 52 | 21.35 ± 1.10 |
| 33 | 7.52 ± 1.05 | 53 | 17.10 ± 1.17 |
| 34 | 5.07 ± 1.25 | 54 | 4.20 ± 1.08 |
| 35 | 45.40 ± 1.30 | 55 | 78.22 ± 1.30 |
| 36 | 13.90 ± 1.30 | 56 | 151.6 ± 5.14 |
| 37 | 11.08 ± 1.20 | 57 | 45.42 ± 2.32 |
| 38 | 621.70 ± 1.8 | 58 | 512.6 ± 2.81 |
| 39 | 5.95 ± 1.28 | 59 | 41.8 ± 2.2 |
| 40 | 47.24 ± 1.12 | 60 | 21.80 ± 1.21 |
| 41 | 9.03 ± 1.07 | 61 | 3.94 ± 1.08 |
| 42 | 228.6 ± 1.26 | 62 | 113.5 ± 1.10 |
| 43 | 19.43 ± 1.19 | 63 | 210.8 ± 8.1 |
| 44 | 18.90 ± 1.01 | 64 | 10.31 ± 1.55 |
| 45 | * | 65 | 674.2 ± 1.72 |
| Deprenyl | 16.73 ± 1.48 | Safinamide | 23.07 ± 2.07 |
| Rasagiline | 49.66 ± 2.26 | Clorgyline | * |
(*) Inactive at 100 μM.
Figure 6.
SAR study of chromone and coumarin derivatives as MAO-B inhibitors.
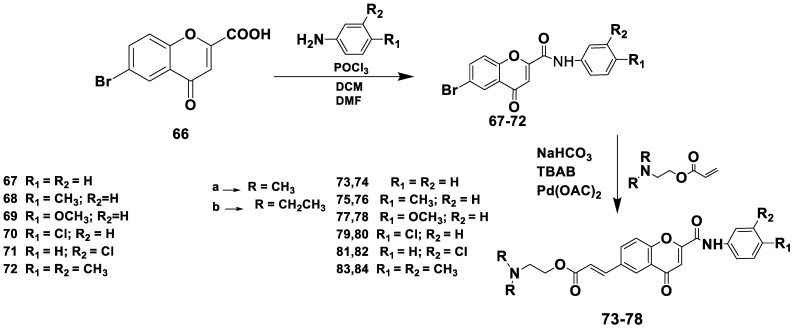
The Reis collection of chromone was developed, synthesized, and tested for MAO and choline esterase inhibition by Reis et al. [53]. Briefly, the chromone moiety was modified by adding a phenylcarboxamide group at position C2 or C3 and an acrylate moiety with a tertiary amine function at position C6. The potential byproducts of acrylate side-chain hydrolysis were also determined, and their biological activities were assessed in vitro. Additionally, testing was conducted on the most potent compounds, which were tested to determine their ability to permeate the blood–brain barrier, enzyme inhibition, kinetics and mechanisms, drug-like properties, and cytotoxicity profiles. Using models based on the crystal shapes of the targets, molecular modeling studies were performed to understand the interactions between the compounds and targets. The protein (PDB ID:2V5Z) was used as the receptor model for hMAO-B.
Scheme 5 shows the synthesis of chromone-2-phenylcarboxamide derivatives by treating 6-bromo-4-oxo-4H-chromene-2-carboxylic acid with phenylamine derivatives, followed by microwave-assisted Pd(II)-catalyzed Heck cross-coupling using 2-(dimethylamino)ethyl acrylates 73, 75, 77, 79, 81, and 83 or 2-(diethylamino)ethyl acrylates 74, 76, 78, 80, 82, and 84.
Scheme 5.
Synthesis of three substituted amide-based chromones.
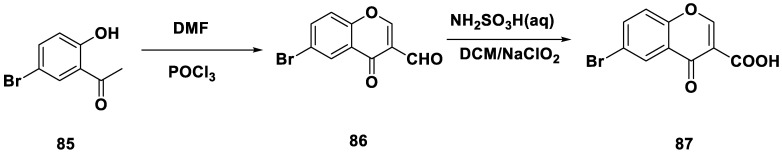
In Scheme 6, compound 86 (6-bromo-4-oxo-4H-chromene-3-carbaldehyde) was prepared from the starting material 5′-bromo-2′-hydroxyacetophenone 85 using POCl3-induced cyclization followed by subsequent oxidation in the presence of sodium chlorite and sulfamic acid to obtain bromo-4-oxo-4H-chromene-3-carboxylic acid (87). From 87, the derivatives 88–93 were prepared by the condensation of phenylamine derivatives, followed by a microwave-assisted Pd(II)-catalyzed Heck cross-coupling reaction, as described in Scheme 5, to obtain chromone 3-phenylcarboxamides 94–105.
Scheme 6.
Synthesis of 6-bromo-chromone-3-carboxylic acid.
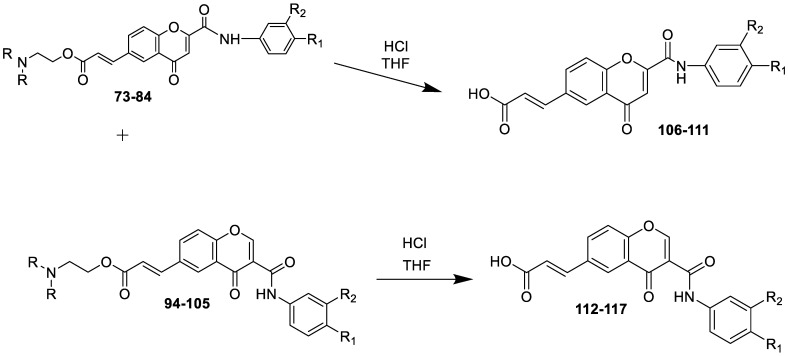
Scheme 7 shows the synthesis of chromones 106–111 and 112–117 prepared by the acid hydrolysis of chromone 2-phenylcarboxamides 73–84 and chromone 3-phenylcarboxamides 94–105.
Scheme 7.
Synthesis of chromone 3-phenylcarboxamides derivatives.
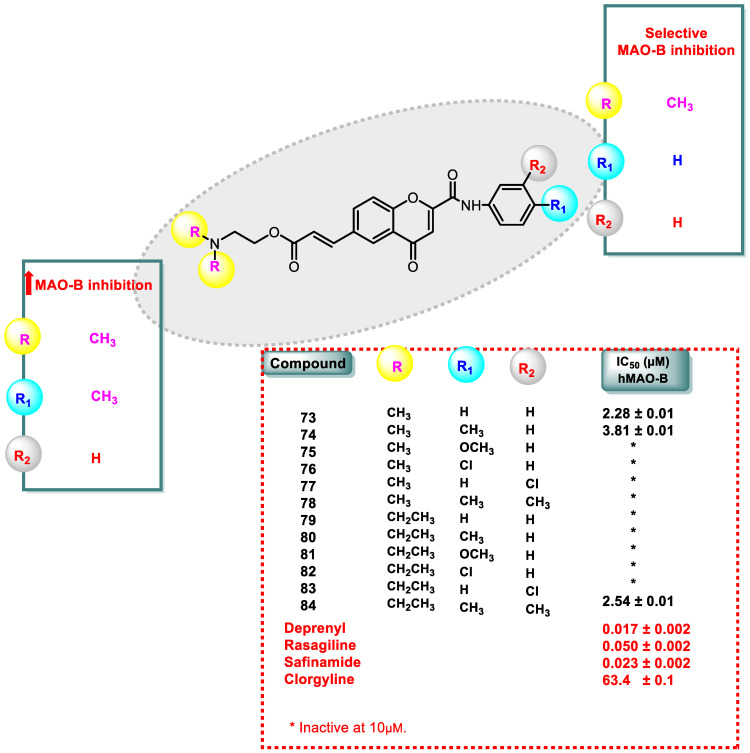
Compound 73, with two methyl (CH3) groups in the tertiary amine nucleus and no substituents on the exocyclic phenyl ring, specifically inhibited MAO-B with an IC50 value of 2.28 μM. Derivative 74 inhibited MAO-B, which has two -CH3 groups bound to the tertiary amine and a methyl group at the para position of the chromone exocyclic phenyl ring (Figure 7).
Figure 7.
SAR study of chromone 2-phenylcarboxamide derivatives (73–84) and its MAO-B inhibitory activity.
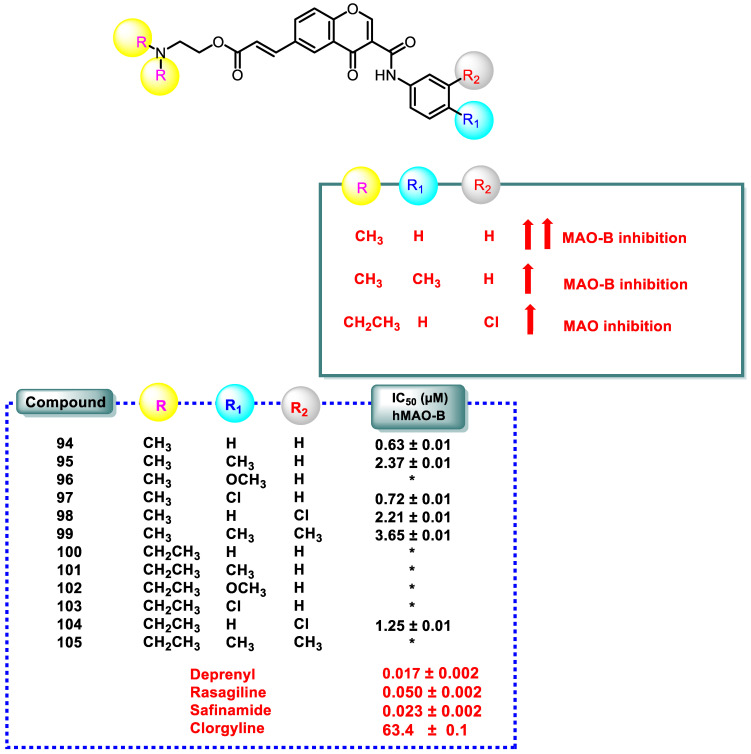
Except for compounds 96, 100–103, and 105, most of the chromone 3-carboxamide derivatives exhibited micromolar and sub-micromolar MAO-B-specific inhibition. In this sequence, the spatial volume of the substituent on the tertiary amine and/or in the exocyclic aromatic ring significantly influenced MAO-B inhibitory activity. Dimethyl-N-substituted compounds 100–105 were inert towards MAOs, except for compound 104. Notable MAO-B inhibitory activity was observed for compound 94, which had no derivatives on the exocyclic ring. The p-CH3 group on compound 95 allowed it to selectively inhibit MAO-B compared to MAO-A, with an SI value > 4.2 (Figure 8).
Figure 8.
SAR study of chromone-3-phenylcarboxamide derivatives (94–105) and its MAO-B inhibitory activity. *, not determined.
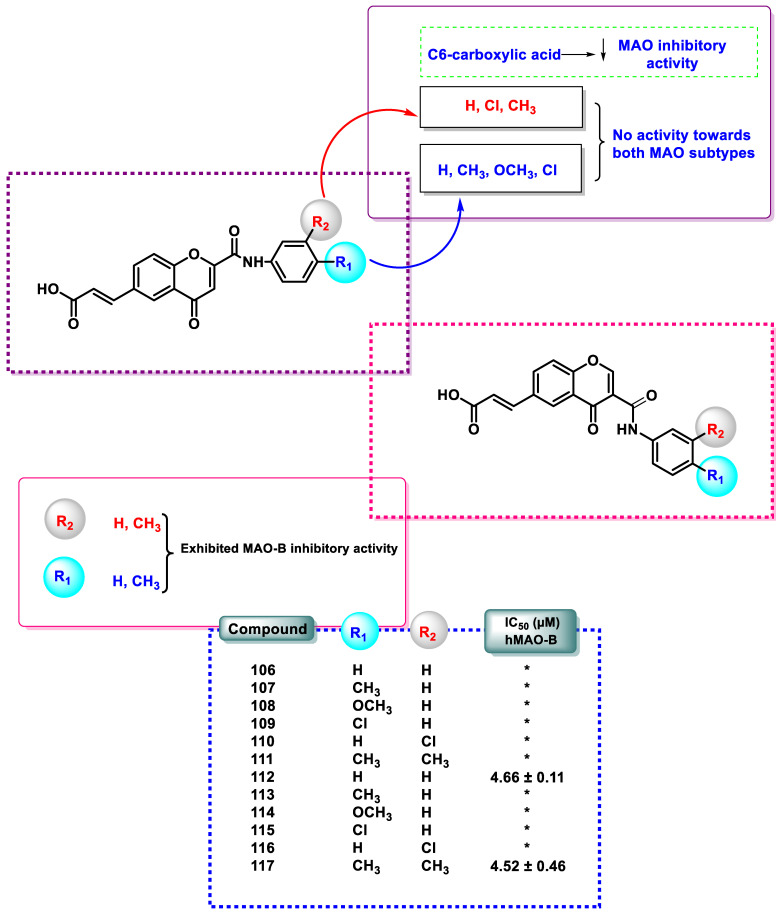
The potential compounds 106–117, synthesized by the hydrolysis of acrylate-substituted chromones, were examined for their MAO inhibitory potential (Figure 9). The results revealed that the presence of the C6-carboxylic acid group significantly reduced the inhibitory activity of MAO. In particular, the hydrolyzed compounds of chromone 2-phenylcarboxamide 106–111 showed no effect on either of the MAO isoforms. Compared to the related precursors, compounds 94 and 99, chromone 3-phenylcarboxamide 112 and 117 exhibited micromolar MAO-B inhibitory activity.
Figure 9.
SAR study of chromone-2-phenyl and 3-phenyl carboxamide carboxylic acid derivatives 106–117 as MAO-B inhibitors. *, not determined.
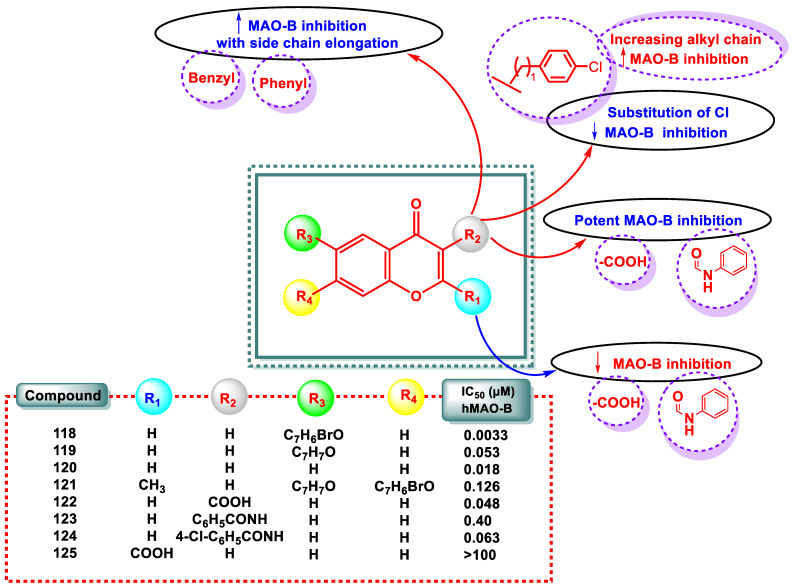
Mpitimpiti et al. developed a novel series of 15 chromone derivatives and tested their MAO inhibitory activity in light of earlier investigations on the possible inhibition of MAO by chromone compounds [54]. This study strongly emphasized the third position vs. the potential of MAO inhibition concerning the effect of flexible side chain replacement.
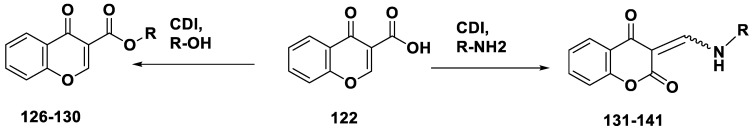
Scheme 8 illustrates the preparation of ester 126–130 and amino 131–141 derivatives of chromone by treating the chromone 3-carboxylic acid 124 with aromatic/aliphatic amines and alcohols in the presence of carbonyldiimidazole (CDI). Although the target esters were successfully synthesized, it is not surprising that the reaction of 122 with the amine compounds produced chromane-2,4-diones (131–141). The results of the MAO inhibition studies revealed that the ester derivatives were ineffective MAO inhibitors; however, several chromane-2,4-diones showed promising MAO-B inhibition potencies. The most effective MAO-B inhibitor was compound 133, with an IC50 value of 0.638 µM. Compound 131 is a reversible MAO-B inhibitor. However, compound 131 was a less potent MAO inhibitor than lazabemide, a reversible MAO-B-specific inhibitor (IC50 = 0.091 μM), assessed under comparable laboratory conditions. Similar to previously reported C6- and C7-substituted chromones, 135 had a much lower MAO-B-inhibiting potential. Chromones 118–120, at least one order of magnitude more effective MAO-B inhibitors than 131, serve as indicators.
Scheme 8.
Synthesis of ester- and amino-based chromones.
For example, a group of chromone compounds with C6 and C7 substitutions were shown to be efficient reversible MAO-B inhibitors. These studies have led to the development of effective MAO-B inhibitors, including compounds 118–121. Although some of these chromones also displayed IC50 values for MAO-A inhibition in the nanomolar range, these derivatives were specific inhibitors of the MAO-B isoform.
Intriguingly, chromone substitution at position C5 resulted in modest MAO-B inhibition, in contrast to the C6- and C7-substituted derivatives [55]. Chromone 3-carboxylic acid 122 is an effective and selective MAO-B inhibitor (IC50 = 0.048 μM), despite that the COOH group being present in location 2 of the 4-pyrone nucleus resulted in a decrease in activity compound 125 [56,57,58]. Similarly, a phenylcarboxamide substitution at position 3 of the 4-pyrone nucleus resulted in significant MAO-B inhibition, with derivatives 123 and 124 exhibiting IC50 values of 0.40 and 0.063 μM (Figure 10) [57,58,59].
Figure 10.
SAR study of chromone derivatives and the inhibition of MAO-B by chromone derivatives 118–125.
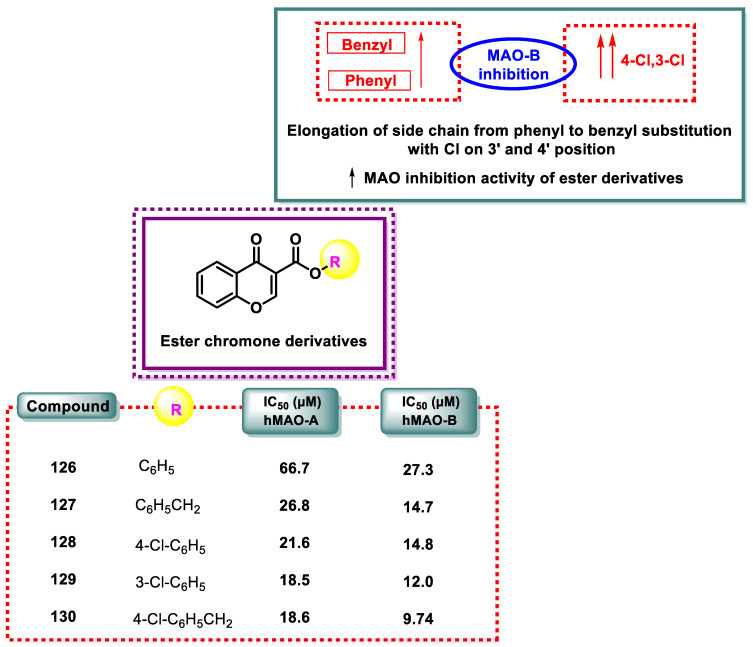
Figure 11 illustrates the results of the MAO inhibition experiments. Ester derivatives often exhibit IC50 values between 18.6 and 66.7 μM and 9.74 and 27.3 μM, which shows that the ester analogs are poor MAO-A and -B inhibitors, as shown in Figure 11. The benzyl derivative was more effective than the phenyl analog when comparing the potencies of compound 127 (benzyl-substituted) and compound 126 (phenyl-substituted). The MAO inhibitory efficacy increased as the chain lengthened from phenyl to benzyl. A similar phenomenon was observed when the activity of derivative 128 was compared with that of compound 126. The MAO inhibitory action was enhanced when comparing the 4-chlorophenyl substitution 128 to the phenyl side chain without substitution 126. A comparison of compound 130 with compound 127 revealed a similar pattern. Compound 130 was a derivative of the 4-chlorobenzyl replacement. The introduction of chlorine had little to no impact on the MAO inhibitory action, as shown by comparing 4-chlorophenyl and 3-chlorophenyl substitutions 128 and 129. Therefore, it can be deduced that extending the side chain from phenyl to benzyl and adding a Cl atom to position 3 or 4 of the side chain improved the MAO inhibitory activity of the ester analogs.
Figure 11.
SAR study of ester chromone derivatives 126 and the inhibition of MAO-A and MAO-B.
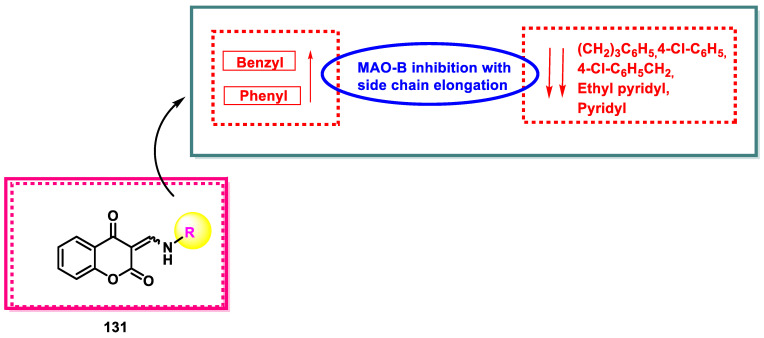
Table 2 illustrates that chromane-2,4-diones are specific MAO-B inhibitors, with IC50 values of 0.638–16.66 µM. Compared to ester derivatives, chromane-2,4-diones often have higher MAO-B inhibitory effects. Compound 133, with an IC50 value of 0.638 μM, is the most potent MAO-B inhibitor. Side chain elongation increased with MAO-B inhibition from compounds 132 to 133 (phenyl to benzyl); however, from compounds 134 to 135, as chain elongation increased, MAO-B inhibitory activity decreased. Compounds 136, 137, and 138 did not affect MAO-A or MAO-B, which could be attributed to adding a sterically large chlorine atom. When the MAO inhibitory activities of compounds 132–133 were compared with those of compounds 136–138, it was concluded that Cl substitution decreased the MAO-B inhibitory activity. When comparing the compounds with benzyl/phenyl substitutions to those with pyridyl substitutions, the former showed superior inhibition (Figure 12). Chain elongation from pyridyl 140 to ethyl pyridyl 141 led to a two-fold increase in MAO inhibition compared to compounds with pyridine-containing side chains. The results also demonstrate that chromane-2,4-diones are more effective MAO-B inhibitors than ester chromone analogs, with an IC50 value of 0.638 µM for 133 compared to 14.7 µM for 127. For instance, phenyl-substituted molecule 132 was approximately 28 times more potent than 126. Similarly, the MAO-B inhibitory potencies of chromane-2,4-diones 133 and 137 were much greater than those of their corresponding ester derivatives 127 and 128. The 3-aminomethylidene-2,4-chromandiones are inseparable mixtures of E- and Z-isomers, and the MAO inhibitory potencies indicated are those of the mixtures, which should be emphasized. According to a study by Cagide et al., the MAO inhibitory characteristics of four 3-(phenylamino)methylidene chromane-2,4-dione derivatives and chromane-2,4-diones are weak MAO-A inhibitors [59]. These compounds failed to suppress human MAO-A at the highest tested concentration of 10 μM. However, as reported by Cagide et al., the IC50 values for 132 (0.268 µM) and 137 (0.065 µM) to inhibit human MAO-B are considerably different from those found in the current study. Although the exact cause of this mismatch is unknown, it could be related to the different experimental methods employed to determine the MAO activity.
Table 2.
Inhibition of MAO by 3-aminomethylidene-2,4-chromandione derivatives.
| Code | R | IC50 (µM) | |
|---|---|---|---|
| MAO-A | MAO-B | ||
| 132 | C6H5 | 79.6 | 0.947 |
| 133 | C6H5CH2 | 77.9 | 0.638 |
| 134 | C6H5(CH2)2 | 101 | 0.897 |
| 135 | C6H5(CH2)3 | 312 | 1.43 |
| 136 | C6H5(CH2)4 | 155 | 142 |
| 137 | 4Cl-C6H5 | 72.1 | 3.08 |
| 138 | 4-Cl-C6H5cH2 | 288 | NI |
| 139 | 3-Cl-C6H5(CH2)2 | NI | NI |
| 140 | C5H4N | 73.1 | 38.7 |
| 141 | C5H4N(CH2)2 | 41.6 | 16.66 |
NI, no inhibition at a maximum tested concentration of 100 μM.
Figure 12.
SAR study of 3-aminomethylidene-2,4-chromandiones.
Over the past few decades, in both academic and professional contexts, molecular docking has been frequently used as a quick and affordable approach. There is still no easy and accurate way to quickly identify real ligands among a group of molecules or to precisely pinpoint the right ligand conformation inside the binding pocket of MAO, despite the fact that this discipline has had enough time to consolidate in many ways [60]. Based on the crystal structures of MAO-B in combination with reversible inhibitors, it is possible to predict that chromane-2,4-diones will bind to the active site of their moiety close to FAD, the most polar area. The chromane-2,4-dione moiety forms hydrogen bonds with the water molecules and amino acid residues. The 3-aminomethylidene side chain is intended to fill the entrance cavity, lined mostly with nonpolar residues. Interestingly, the binding positions of the trans- and cis-isomers are comparable. A π-π stacking interaction with Tyr-398 and a π-sulfur interaction with Cys-172 are both seen, despite normal hydrogen bonding (cis isomer). The side chain phenyl ring is stabilized by π-alkyl interactions with Ile-199 and Ile-316, while π alkyl interactions between the chromane-2,4-dione and Leu-171 and Ile198 and Cys-172 (trans-isomer) create the π alkyl interactions. By forming a carbon-hydrogen bond with the benzylic H of the side chain and the C2 carbonyl oxygen of chromane-2,4-dione, Ile-199 becomes even more significant. The phenolic oxygen of Tyr-326 additionally forms a carbon–hydrogen bond with benzylic H. Both isomers of 132 can bind to and interact with MAO-B; according to this analysis, the binding mechanisms and interactions are relatively comparable. Thus, both isomers are involved in MAO-B inhibition.
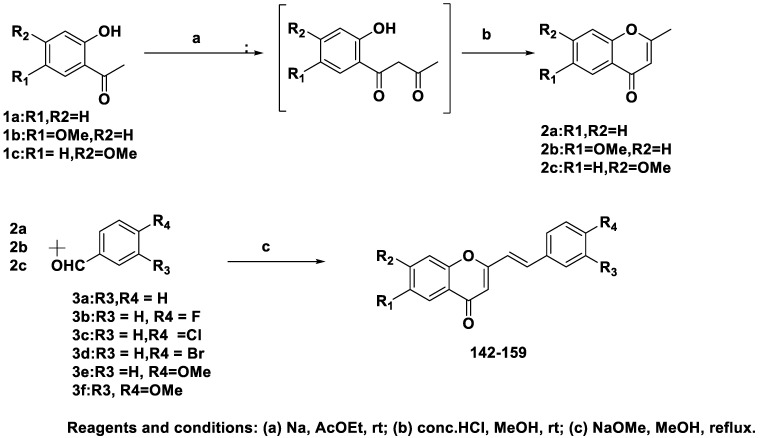
Takao et al. synthesized and assessed the MAO-A and MAO-B inhibitory properties of 18 2-styrylchromone derivatives [61]. In the prepared series, compound 150 had the greatest MAO-B inhibitory capacity and specificity with an –OCH3 substitution at R1 and a Cl substitution at R4. Compound 150 inhibited MAO-B competitively and reversibly. They used the pIC50 values of 2-styrylchromone derivatives to perform quantitative structure–activity relationship (QSAR) studies using the molecular operating environment (MOE) and dragon, which revealed significant relationships (p < 0.05). Through 3D-QSAR investigations using AutoGPA, based on a molecular field analysis method using MOE, 2-styrylchromone structures were investigated as valuable scaffolds. Based on these findings, the 2-styrylchromone moiety may be a good building block for novel MAO-B antagonists.
Scheme 9 explains the synthesis of the 2-styrylchromone derivatives involved in the reaction of acetophenone derivatives with ethyl acetate, followed by intramolecular cyclization. The generated IIa-IIc then interacted with benzaldehyde derivatives IIIa–f in the presence of a base.
Scheme 9.
Synthesis of 2-styrylchromone derivatives.
The inhibitory effects of 2-styrylchromone compounds 142–159 (Table 3) on MAO (A and B) were assessed. Modifications to R3 and R4 phenyl rings and R1 and R2 chromone rings showed several intriguing structure–activity relationships, as shown in Figure 13. We discovered that these compounds had inhibitory effects on MAO-A.
Table 3.
Inhibition of MAO by 2-styryl chromone derivatives.
| Compound | R1 | R2 | R3 | R4 | TC50 (µM) MAO-A | IC50 (µM) MAO-B |
|---|---|---|---|---|---|---|
| 142 | H | H | H | H | 0.95 | 0.24 |
| 143 | H | H | H | F | 0.59 | 0.17 |
| 144 | H | H | H | Cl | 0.29 | 0.079 |
| 145 | H | H | H | Br | 0.33 | 0.069 |
| 146 | H | H | H | OMe | 2.3 | 0.049 |
| 147 | H | H | OMe | OMe | 25 | 2.8 |
| 148 | OMe | H | H | H | 0.20 | 0.18 |
| 149 | OMe | H | H | F | 0.12 | 0.064 |
| 150 | OMe | H | H | Cl | 26 | 0.017 |
| 151 | OMe | H | H | Br | 0.53 | 0.024 |
| 152 | OMe | H | H | OMe | 0.21 | 0.19 |
| 153 | OMe | H | OMe | OMe | 21 | 0.68 |
| 154 | H | OMe | H | H | 26 | 0.22 |
| 155 | H | OMe | H | F | 35 | 0.12 |
| 156 | H | OMe | H | Cl | >100 | 0.27 |
| 157 | H | OMe | H | Br | >100 | 0.45 |
| 158 | H | OMe | H | OMe | 72 | 1.4 |
| 159 | H | OMe | OMe | OMe | >100 | 10 |
Figure 13.
SAR study of 2-styrylchromone derivatives.
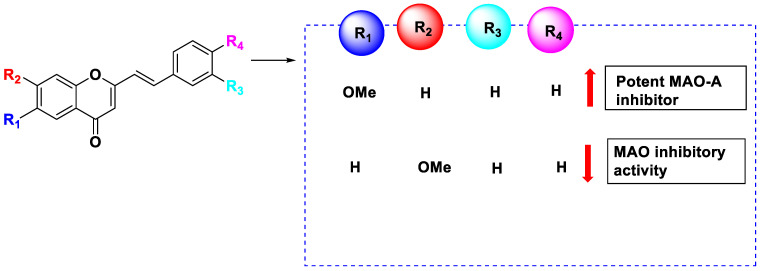
Compounds 142–146 and 148–151 displayed inhibitory activity, with compound 151 showing the most effective inhibition of MAO-A. Except for compound 144 vs. 150, adding a methoxy substituent at R1 appeared to increase the MAO-A-inhibiting properties of compounds 142 vs. 147, 143 vs. 149, 145 vs. 151, and 146 vs. 152. All MAO-A inhibitory activities, including those of compounds 142 vs. 154, 143 vs. 155, 144 vs. 153, 145 vs. 157, 146 vs. 158, and 147 vs. 159, were reduced when the –OCH3 group was at position R2. The ability of the derivatives to inhibit MAO-B was assessed, and it was found that all derivatives inhibited MAO-B more potently than MAO-A. The most effective inhibitor was compound 150, which showed inhibitory activity nearly 13 times greater than that of pargyline, used as a positive control. Compared to pargyline, compounds 144–146, 149, and 151 exhibited more potent inhibition, while compounds 142, 143, 148, and 152–157 displayed comparable inhibition. Additionally, 146 vs. 152, 143 vs. 149, 144 vs. 150, 145 vs. 151, and 147 vs. 153 appeared to have stronger MAO-B inhibitory effects when the methoxy group at position R1 was substituted. Furthermore, compounds 156, 157, 158, and 159 exhibited reduced MAO-B inhibitory effects when the methoxy group at position R2 was replaced.
Derivatives of 2-styrylchromone demonstrated strong and selective MAO-B blocking effects. The computational analysis of the statistical significance (p < 0.05) of each substituted group revealed that the methoxy substitution at position R2 substantially influenced both the selectivity and MAO-B blocking activity.
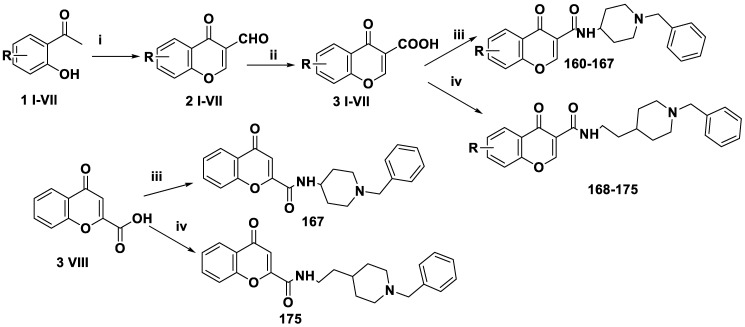
Wang et al. developed several donepezil-chromone hybrids 160–167 and 168–175 (Table 4) and tested their biological activity, including MAO inhibition and central nervous system penetration in vitro [62]. Molecular modeling studies were also conducted to evaluate the novel hybrid compounds’ interaction mechanism, structure–activity connections, and binding strategy.
Table 4.
Inhibitory activity of donepezil-chromone hybrids.
| hMAO-A | hMAO-B | ||
|---|---|---|---|
| Compound | R | IC50 (µM) | IC50 (µM) |
| 160 | H | 63.8 | 17.68 |
| 161 | 6-OCH3 | 7.5 | 46.27 |
| 162 | 6-OBn | 11.6 | 0.035 |
| 163 | 6 cH3 | 48.1 | 19.46 |
| 164 | 6-Br | 35.5 | 22.82 |
| 165 | 7-OCH3 | 37.4 | 28.64 |
| 166 | 7-Br | 31.6 | 19.73 |
| 167 | H | 29.7 | 33.29 |
| 168 | H | 68.6 | 35.29 |
| 169 | 6-OCH3 | 72.4 | 11.23 |
| 170 | 6-OBn | 67.2 | 0.272 |
| 171 | 6-CH3 | 30.6 | 52.45 |
| 172 | 6-Br | 48.2 | 26.13 |
| 173 | 7-OCH3 | 32.4 | 30.93 |
| 174 | 7-Br | 30.3 | 29.46 |
| 175 | H | 29.5 | 20.03 |
Scheme 10 shows the formation of donepezil-chromone hybrids 160–167 and 168–175; they were prepared by treating 2-hydroxyacetophenone with phosphorus oxychloride (DMF) by a modified Harnisch procedure that resulted in the formation of 3-formylchromones, followed by oxidation with sulfamic acid and sodium chlorite to obtain the appropriate 4-oxo-4H-chromene-3-carboxylic acids, which were then treated with acetyl chloride and a catalytic amount of DMF in CH2Cl2 to produce acyl chloride and react with 1-benzylpiperidin-4-amine or 2-(1-benzylpiperidin-4-yl)ethanamine in CH2Cl2, resulting in the target compounds.
Scheme 10.
Synthesis of donepezil-chromone hybrids 160–167 and 168–175. Reagent and condition, (i) POCl3, DMF, 0 °C, 2 h; (ii) NaClO2, NH2HSO3, CH2Cl2, 0 °C, 3 h; (iii) thionyl chloride, reflux; 1-benzylpiperidin-4-amine, K2CO3, CH2Cl2, rt, 8 h; (iv) thionyl chloride, reflux; 2-(1-benzylpiperidin-4-yl)ethanamine, K2CO3, CH2Cl2, rt, 8 h.
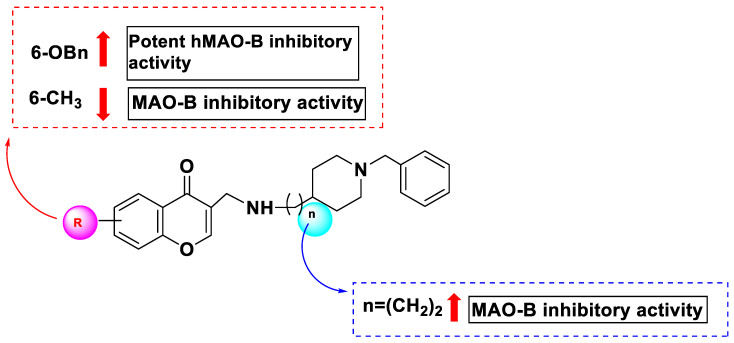
According to the SAR (Figure 14), hybrids containing a benzyloxy substitution at the sixth position of the chromone were more prone to have an inhibitory effect on hMAO-B. In particular, compound 162, which had a benzyloxy substitution at C6 of the chromone, demonstrated the greatest inhibition with an IC50 value of 0.035 μM, demonstrating an activity that was nearly three and 200 times more than that of the standards pargyline and iproniazid, respectively. All other analogs produced less activity when other groups were added instead of the benzyloxy substitution. Compound 171, which contained a methyl substitution at the same position, exhibited the weakest performance. Additionally, there was a strong correlation between the alkylene chain length and MAO-B inhibitory efficacy. Compound 170, an excellent MAO-B inhibitor, had an N-ethylcarboxamide connection between the chromone and benzylpiperidine. This compound had an IC50 value of 0.272 μM and was 7.8 times less effective than its counterpart, compound 162, with a carboxamide linker. These findings suggest that steric parameters affect the inhibitory effects of hMAO-B. Compound 170, which showed the most balanced ability to selectively block MAO-B based on the findings of MAO inhibitory activity, is thought to be an appealing multi-purpose blocker for future research.
Figure 14.
SAR of donepezil and chromone hybrids.
They assessed the ability of these hybrids to penetrate the BBB because brain crossing is a requirement for efficient anti-AD drugs. Di et al. developed an artificial membrane permeability test for the BBB (PAMPA-BBB) to achieve this. The three most efficient compounds (162, 169, and 170) were chosen as candidates to investigate the possible toxicological effects on rat pheochromocytoma (PC12) cells and the potential for therapy with these derivatives. Compound 170, which had high MAO-B selectivity, was the most intriguing variant of the generated compounds. Compound 170 crossed the BBB and demonstrated minimal cell toxicity when tested in vitro on rat pheochromocytoma (PC12) cells. Overall, the multifunctional ligand 170, which possesses balanced MAO-B inhibitory activities, may be considered a potential anti-AD target for future studies.
In the substrate cavity of the enzyme, the chromone moiety of compound 170 is located adjacent to the FAD cofactor. Its benzyloxy substitution interacts with Tyr435 and Tyr398 via π–π stacking interactions. Tyr326 interacted with the amide carbonyl of compound 172 via a hydrogen bond. In contrast, the benzylpiperidine moiety of compound 172 resides in the hydrophobic pocket formed by Pro102, Leu88, Ser200, Gly101, Glu84, Thr201, Ile199, and Ile316 in the entrance cavity. Additionally, Glu84 and the quaternary nitrogen present in piperidine form hydrogen bonds.
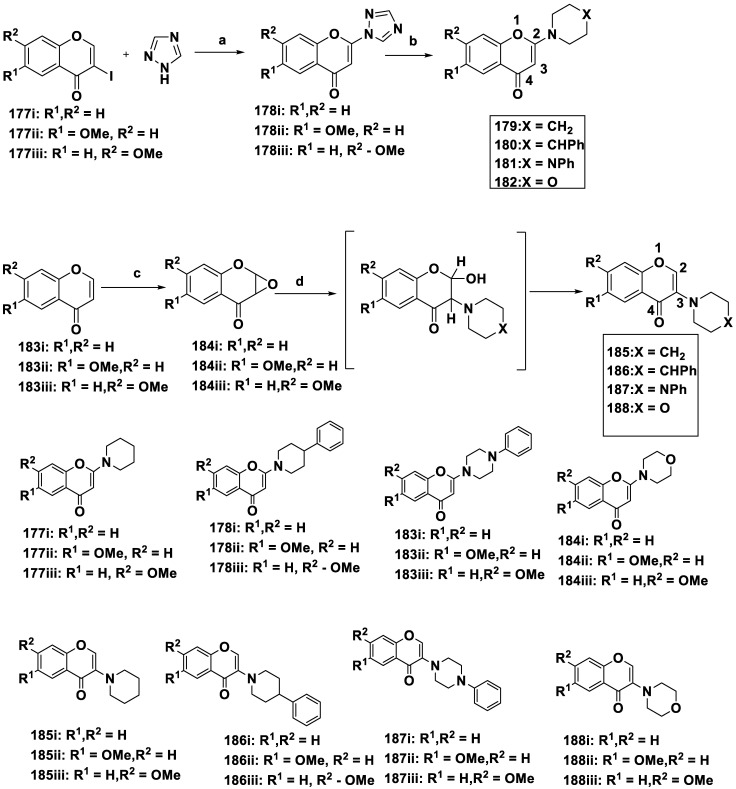
Takao et al. (2020) synthesized a collection of 2- and 3-(N-cyclicamino) chromone compounds and investigated their inhibitory activities against MAO [63]. Among the derivatives tested, including safinamide, which was employed as the control substance, compound 187iii, 7-methoxy-3-(4-phenyl-1-piperazinyl)-4H-1-benzopyran-4-one, demonstrated a major antagonistic action, demonstrated the largest specificity towards MAO-B, and functioned reversibly and competitively. These findings implied that the lead substances for MAO-B inhibitory studies could be 3-(N-cyclicamino) chromone derivatives.
The formation of 2- and 3-(N-cyclic amino) chromone analogs is shown in Scheme 11. When 3-iodochromone derivatives 177i–iii were treated along with 1,2,4-triazole in the presence of K2CO3, DMF at 80 °C, it resulted in the generation of 2-(1,2,4-triazolyl)-chromone variants 178i–iii, which then underwent a substitution reaction by interacting with the corresponding cyclic amine (DMF at 80 °C), forming the compounds of interest. Next, 3-(N-cyclicamino) chromone variants 185–188 were generated by the epoxidation of chromone derivatives 183i–iii using an aqueous H2O2 solution under basic conditions to obtain 2,3-epoxychromone derivatives 184i–iii, which reacted with the corresponding cyclic amine to form the target compounds (Table 5).
Scheme 11.
Synthesis of 2- or 3-(N-cyclic amino) chromone derivatives: (a) K2CO3, DMF, and at 80 °C; (b) cyclic amine, DMF, and at 80 °C; (c) H2O2, PhCH2N+(CH3)3OH-, Et2O, and at 0 °C; and (d) cyclic amine, CH3CN, rt.
Table 5.
Inhibition of MAO by 2- or 3-(N-cyclic amino)chromone derivatives.
| Compound | R1 | R2 | IC50 (µM) | |
|---|---|---|---|---|
| MAO-A | MAO-B | |||
| 2-(N-Cyclic amino)chromone | ||||
| 177i | H | H | 38 | 59 |
| 177ii | OMe | H | 16 | 27 |
| 177iii | H | OMe | 57 | 36 |
| 178i | H | H | 31 | 22 |
| 178ii | OMe | H | 4.1 | 5.6 |
| 178iii | H | OMe | 8.8 | 7.4 |
| 183i | H | H | 38 | 22 |
| 183ii | OMe | H | 2.6 | 2.8 |
| 183iii | H | OMe | 59 | 12 |
| 184i | H | H | 66 | 58 |
| 184ii | OMe | H | 36 | 40 |
| 184iii | H | OMe | 34 | 31 |
| 3-(N-Cyclic amino)chromone | ||||
| 185i | H | H | 23 | 2.0 |
| 185ii | OMe | H | 23 | 0.99 |
| 185iii | H | OMe | 18 | 14 |
| 186i | H | H | >100 | 1.5 |
| 186ii | OMe | H | >100 | >100 |
| 186iii | H | OMe | >100 | 0.25 |
| 187i | H | H | >100 | 0.72 |
| 187ii | OMe | H | >100 | >100 |
| 187iii | H | OMe | >100 | 0.015 |
| 188i | H | H | 57 | 23 |
| 188ii | OMe | H | 34 | 8.0 |
| 18Siii | H | OMe | 25 | 7.3 |
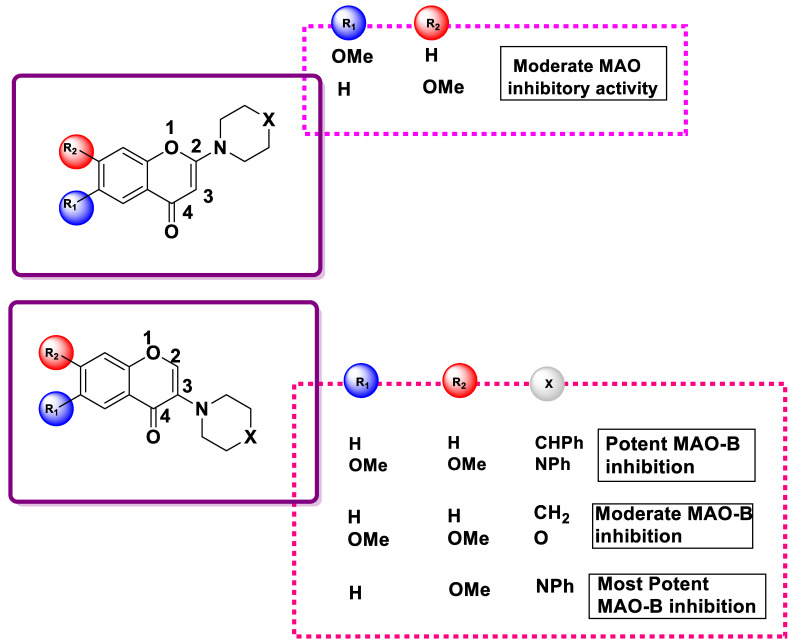
Compounds 178ii, 179iii, and 183ii displayed equivalent IC50 concentrations for MAO-B and mildly inhibited MAO-A and MAO-B. Due to the smaller site action of MAO-A compared to MAO-B, compounds 186 and 187 did not show any inhibiting effects against MAO-A at a concentration of 100 μM. The derivatives 186i, 186iii, 187i, and 187iii effectively and selectively inhibited MAO-B. Compounds 185i, 188ii, and 188iii moderately inhibited MAO-B. The most effective inhibition and MAO-B specificity were observed for compound 187iii. Safinamide used as a positive control, and it was nearly three times more effective than compound 188iii when its efficacy and selectivity were examined. Except for compounds 186ii and 187ii, the 3-(N-cyclic amino) chromone derivatives specifically inhibited MAO-B, whereas the 2-(N-cyclic amino) chromone derivatives did not. The current findings clearly distinguish the two sets of compounds, which is in line with earlier studies on chromone carboxylic acids and related amides, as well as the latest studies on the MAO-inhibitory activities of flavones (chrysin) and isoflavones (genistein). These results revealed that substitutions on the chromone ring at position 3 enhance the MAO-B inhibitory activity. According to the findings for compounds 186 and 187, a significant SAR (Figure 15), the methoxy group present at position 6 (R1) or 7 (R2) of the chromone ring was the difference between compounds 186ii or 187ii and 186iii or 187iii, respectively, in the selective blocking of MAO-B. This indicates that incorporating a methoxy group at position 6, as in compounds 186ii or 187ii, caused MAO-B to be removed from the active site.
Figure 15.
SAR of 2- and 3-(N-cyclic amino chromone derivatives).
In contrast, a methoxy substitution at C7, as in compounds 186iii or 187iii, led to close binding at the active site. Therefore, compounds 185i and 186i, which lack a methoxy group, were expected to exhibit moderate MAO-B inhibition. These results are consistent with their previous analysis of the impact of -OCH3 replacement on the chromone ring, which showed that diagonal substitutions on the chromone ring increased the MAO-B inhibitory action.
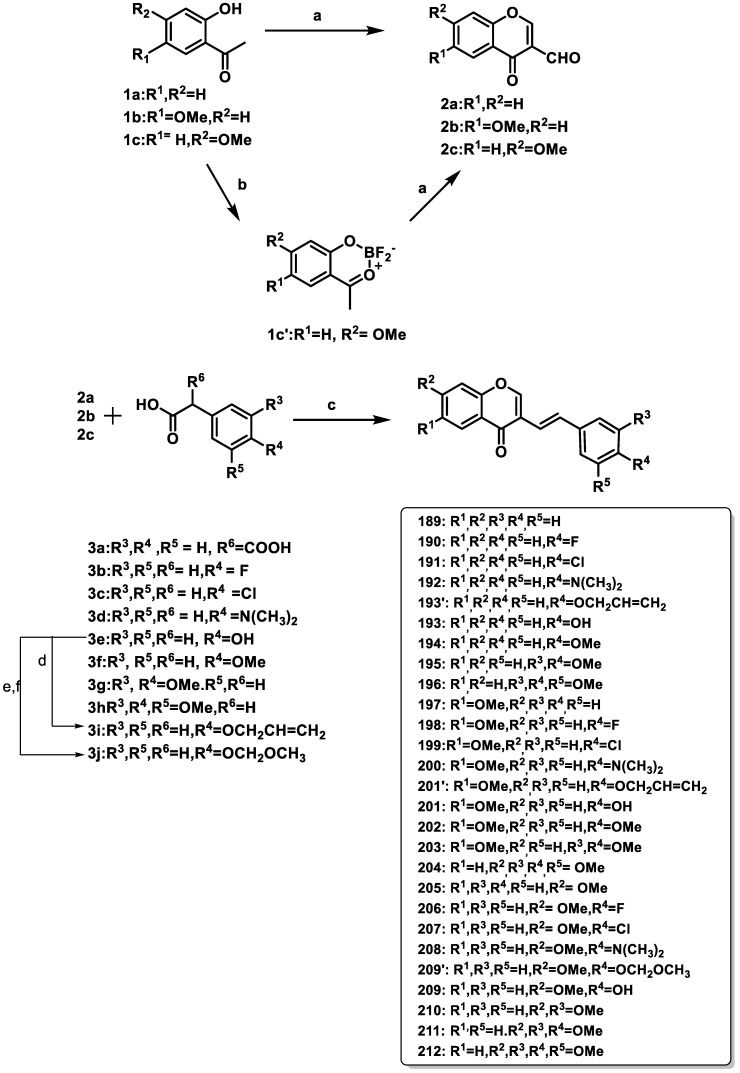
Takao et al. (2021) examined MAO-A and MAO-B inhibitory activities of several 3-styrylchromone derivatives [64]. Most derivatives inhibited MAO-B, except for compound 209, which had an OH group at position 4 and a methoxy substitution at position 2 in its chemical structure. The chlorine atoms were located at positions 4 and 2 on the phenyl and chromone rings 207. It efficiently inhibited MAO-B, with an IC50 level of 2.2 μM. Derivative 189 showed the highest MAO-B selectivity, with a selectivity index > 3700. Compounds 189 and 207 were found to be mixed-type, reversible MAO-B blockers, suggesting that tight-binding blocking of MAO-B may be part of their mechanism of action.
As shown in Scheme 12, to create 3-styrylchromone derivatives 190–213, various phenylacetic acid/phenylmalonic acid variants were combined with 3-formylchromone derivatives IIa–c. The condensation of 3-formylchromone compounds and protected 4-hydroxyphenylacetic acids IIIi and IIIj, followed by the removal of the protective group, produced 3-styrylchromones with hydroxy groups 193, 201, and 209. Acceptable yields were obtained in all cases. The Vilsmeier–Hack reagent converted 2-hydroxyacetophenone derivatives 1a–c to 3-formylchromone derivatives IIa–c.
Scheme 12.
Synthesis of 3-styrylchromone derivatives. Reagents and conditions: (a) DMF/POCl3; (b) (CH3CO)2O/BF3·O(C2H5)2; (c) tert-BuOK, dry pyridine, reflux; (d) allylbromide, K2CO3, EtOH, and then KOH; (e) conc.H2SO4, MeOH, reflux; (f) chloromethyl methyl ether, K2CO3, acetone, reflux, and then KOH, MeOH-H2O.
Chromone (4H-1-benzopyran-4-one) derivatives, which are extensively present in natural substances, including 2-styrylchromones, isoflavones, and flavones, are an important category of oxygenated heterocyclic compounds employed in the discovery of drugs. Researchers have developed several novel chromone compounds, such as 2-azolylchromone, 2-styrylchromone, and 3-styrylchromene, and tested their ability to inhibit MAO based on the observations of MAO inhibition by synthetic chromone derivatives. They also provided data on the production and MAO-blocking properties of 2- and 3-cyclicaminochromone analogs. Compared to 2-cyclicaminochromone derivatives, 3-cyclicaminochromone derivatives inhibited MAO-B more effectively. These outcomes led them to explore 3-styrylchromone derivatives comprising recently produced compounds for their MAO-B antagonistic action. This paper describes the synthesis of many 3-styrylchromone variants (Scheme 12) and their inhibitory actions on human MAO-A and MAO-B.
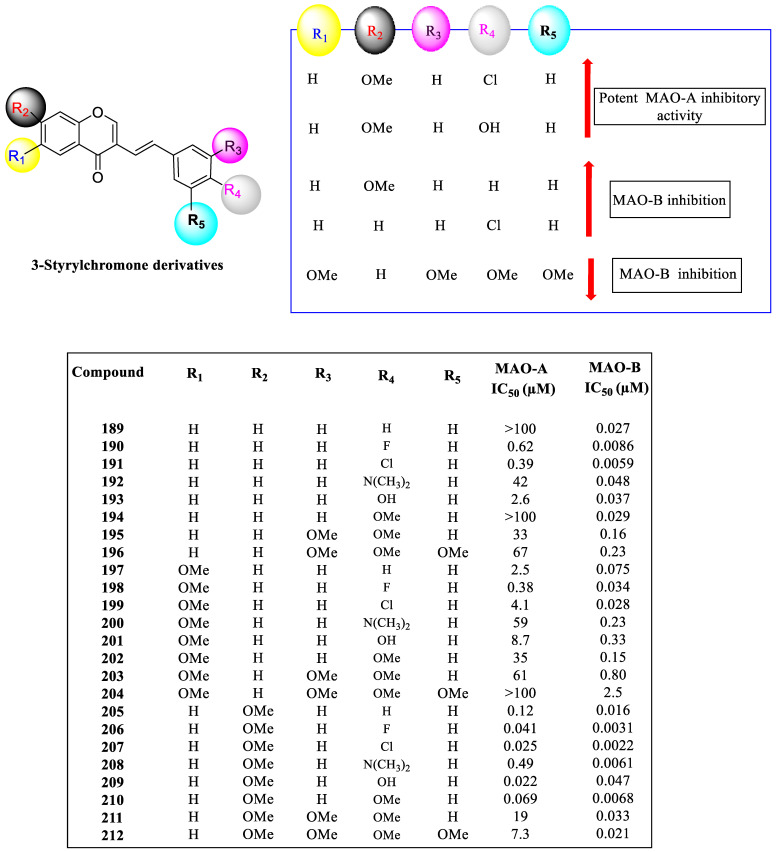
The inhibitory actions of MAO-A and MAO-B were tested on all the synthetic 3-styrylchromone derivatives 189–212, as shown in Figure 16. Structure–activity connections were discovered by studying the impact of the substituent on the phenyl ring at positions R3, R4, and R5, the effects on the chromone ring at positions R1 and R2, and their effects on MAO inhibitory activity.
Figure 16.
IC50 values of 3-styryl chromone derivatives for the inhibition of MAO-A and MAO-B.
The 3-styrylchromone derivatives 190, 191, 198, 205, and 208 had IC50 values for MAO-A below 1 μM. In contrast, compounds 206, 207, 209, and 210 had IC50 values below 0.1 μM, showing that adding a substituent at position R2 as well as position R4 was successful in MAO-A inhibition. Derivatives 207 and 209 strongly inhibited MAO-A, with IC50 values of 25 and 22, respectively, compared to the positive control clorgyline, which had an IC50 value of 4.9 μM. Except for compound 209, all tested 3-styrylchromone derivatives had much lower IC50 values for MAO-B than for MAO-A. Compounds 206 and 207 showed significantly low IC50 values for MAO-B, with 3.1 and 2.2 μM, respectively, when contrasted with safinamide, which was a positive reference. This demonstrates the potential benefit of inhibiting MAO by substituting positions R2 and R4.
4. The Role of 3-Styryl Chromones’ Substituents in Inhibiting MAO-B
MAO-B was significantly inhibited by methoxy substitutions at positions R1, R2, or R3. A comparison was made between the pIC50 values of compounds with hydrogen and those of analogs with methoxy substitutions at positions R1, R2, or R3. The results showed that the OCH3 groups at positions R1 and R3 reduced MAO-B inhibition, whereas the methoxy groups at position R2 boosted it. The substituent at position R4 tends to increase the MAO-B inhibitory activity, which is also true for position R4 chloride. Compounds 196, 204, and 212 with substituents at position R5 appeared to reduce inhibitory activity. According to Takao et al., the phenyl rings of 2-styrylchromone and 3-styrylchromene were strengthened by adding chlorine, which improved their capacity to inhibit MAO-B.
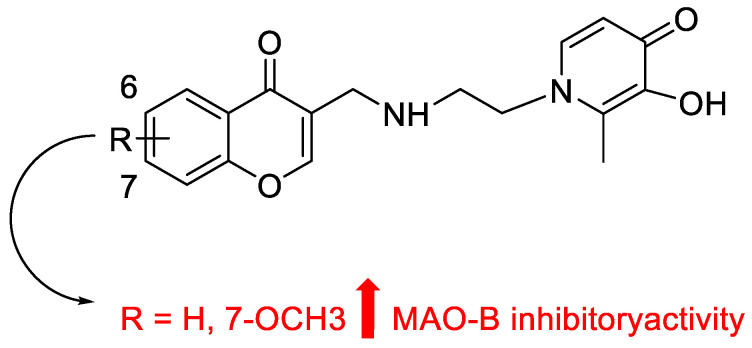
Zhang et al. developed, synthesized, and assessed several chromone-hydroxypyridinone hybrids (Table 6) as potential multimodal anti-AD ligands [65]. Compound 216 exhibited selective hMAO-B inhibitory activity, with an IC50 value of 67.02 nM. Derivative 216 considerably improved scopolamine-induced cognitive impairment in AD mice by crossing the BBB.
Table 6.
Inhibitory activity of chromone derivatives against MAO-B.
| Compound | R | IC50 (μM) hMAO-B |
|---|---|---|
| 213 | H | 0.0672 |
| 214 | 7-methyl | 0.0827 |
| 215 | 6-methyl | 0.0863 |
| 216 | 7-methoxy | 0.0670 |
| 217 | 6-methoxy | 0.0886 |
| Pargyline | 0.1113 |
The most promising chromone derivatives, with IC50 concentrations ranging from 0.067 to 0.088 μM, were modified by alkyl groups at C-6 or C-7 of the chromone nucleus (Figure 17). Compared to the reference medication (pargyline), compound 216 showed the strongest inhibitory action.
Figure 17.
SAR study of chromone derivatives.
5. Conclusions
PD therapy was the indication for when MAO-B inhibitors were first made available, and they are still a frequently prescribed cornerstone of therapy. Although not conclusive, the pre- and post-clinical evidence in support of a neuroprotective and disease-modifying effect for MAO-B inhibitors is unmatched by any other class of antiparkinsonian drugs to date. An additional reason to begin using MAO-B inhibitors early in the course of the disease and to keep using them over the long term is the potential that they may reduce the progression of PD. New medicines having MAO-B inhibitory as well as non-dopaminergic activity are still being developed and tested due to the potential of neuroprotection. Considering the limited number of MAO-B blockers currently approved for clinical application, many research projects have focused on developing new MAO-B blockers with greater efficacy. This review focused on the effects of various chromone ring substituents on MAO-B inhibition. The inhibitory effect of MAO-B on chromones has been explored in a small number of studies. Chromone is a MAO-B antagonist. The following is a summary of the SARs for the inhibition of MAO-B by chromone classes:
The methyl group at the R4 position of chromone was shown to be a beneficial substitution.
The R4 position of the chromone with a bromine group demonstrated a large increase in MAO inhibition and fluorine substitution at R2 of the phenyl ring.
NO2 and CH3 groups in R3 decreased MAO activity, whereas the electronegative halogens chlorine and fluorine caused a marked increase in activity.
Electronegative groups, such as Cl and F substitutions at the para position of styryl chromones, demonstrated higher MAO-B inhibition, as seen in the cases of compounds 207 and 206.
The amino chromone derivatives exhibited more powerful MAO-B inhibition than the ester derivatives 126 and 131. As demonstrated for compounds 131 and 132, phenyl-to-benzyl chain elongation increased MAO-B inhibition, but further chain elongation diminished activity, as seen for compound 135. The inhibition of MAO-B was reduced by compounds containing pyridyl side chains.
Derivatives with meta substituents on the exocyclic ring showed increased potency.
In the future perspective of view, researchers could modify and extend the alkyl chains at the R1 and R5 positions of the chromone ring to develop potent MAO-B inhibitors. The introduction of heterocyclic-based amide on the C-3 position of chromone was not explored so far for the development of MAO-B inhibition. This information will be beneficial for discovering and creating a novel category of powerful and specific MAO-B blockers based on chromones.
Author Contributions
Conceptualization, B.M. and H.K.; writing—original draft preparation, R.S.I., F.B., J.J., A.M., S.K. and S.T.S.; review and editing, G.G. and P.G.; supervision, H.K. and B.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used, analyzed, and reviewed were collected from the corresponding authors and online research databases.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Fonseca A., Reis J., Silva T., Matos M.J., Bagetta D., Ortuso F., Alcaro S., Uriarte E., Borges F. Coumarin versus Chromone Monoamine Oxidase B Inhibitors: Quo Vadis? J. Med. Chem. 2017;60:7206–7212. doi: 10.1021/acs.jmedchem.7b00918. [DOI] [PubMed] [Google Scholar]
- 2.Castellani R.J., Rolston R.K., Smith M.A. Alzheimer Disease. Disease-a-Month. 2010;56:484–546. doi: 10.1016/j.disamonth.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Lau L.M., Breteler M.M. Epidemiology of Parkinson’s Disease. Lancet Neurol. 2006;5:525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 4.Townsend M. When Will Alzheimer’s Disease Be Cured? A Pharmaceutical Perspective. J. Alzheimer’s Dis. 2011;24:43–52. doi: 10.3233/JAD-2011-110020. [DOI] [PubMed] [Google Scholar]
- 5.Mohsin N.U.A., Irfan M., Hassan S.U., Saleem U. Current Strategies in Development of New Chromone Derivatives with Diversified Pharmacological Activities: A Review. Pharm. Chem. J. 2020;54:241–257. doi: 10.1007/s11094-020-02187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madhav H., Jameel E., Rehan M., Hoda N. Recent Advancements in Chromone as a Privileged Scaffold towards the Development of Small Molecules for Neurodegenerative Therapeutics. RSC Med. Chem. 2022;13:258–279. doi: 10.1039/D1MD00394A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hou Y., Dan X., Babbar M., Wei Y., Hasselbalch S.G., Croteau D.L., Bohr V.A. Ageing as a Risk Factor for Neurodegenerative Disease. Nat. Rev. Neurol. 2019;15:565–581. doi: 10.1038/s41582-019-0244-7. [DOI] [PubMed] [Google Scholar]
- 8.Fernandopulle M.S., Lippincott-Schwartz J., Ward M.E. RNA Transport and Local Translation in Neurodevelopmental and Neurodegenerative Disease. Nat. Neurosci. 2021;24:622–632. doi: 10.1038/s41593-020-00785-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu H., Hardy J., Duff K.E. Selective Vulnerability in Neurodegenerative Diseases. Nat. Neurosci. 2018;21:1350–1358. doi: 10.1038/s41593-018-0221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Souza G.X., Rose S.E., Knupp A., Nicholson D.A., Keene C.D., Young J.E. The Application of in vitro-derived Human Neurons in Neurodegenerative Disease Modeling. J. Neurosci. Res. 2021;99:124–140. doi: 10.1002/jnr.24615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schikowski T., Altuğ H. The Role of Air Pollution in Cognitive Impairment and Decline. Neurochem. Int. 2020;136:104708. doi: 10.1016/j.neuint.2020.104708. [DOI] [PubMed] [Google Scholar]
- 12.Durães F., Pinto M., Sousa E. Old Drugs as New Treatments for Neurodegenerative Diseases. Pharmaceuticals. 2018;11:44. doi: 10.3390/ph11020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jenner P. Functional Models of Parkinson’s Disease: A Valuable Tool in the Development of Novel Therapies. Ann. Neurol. 2009;64:S16–S29. doi: 10.1002/ana.21489. [DOI] [PubMed] [Google Scholar]
- 14.Rowinska-Zyrek M., Salerno M., Kozlowski H. Neurodegenerative Diseases—Understanding Their Molecular Bases and Progress in the Development of Potential Treatments. Coord. Chem. Rev. 2015;284:298–312. doi: 10.1016/j.ccr.2014.03.026. [DOI] [Google Scholar]
- 15.Adan G., Mitchell J.W., Ziso B., Larner A.J. Diagnosis and Management of Seizures in Neurodegenerative Diseases. Curr. Treat. Options Neurol. 2021;23:1. doi: 10.1007/s11940-020-00656-y. [DOI] [Google Scholar]
- 16.Ebadi M., Brown-Borg H., Ren J., Sharma S., Shavali S., El ReFaey H., Carlson E. Therapeutic Efficacy of Selegiline in Neurodegenerative Disorders and Neurological Diseases. Curr. Drug Targets. 2006;7:1513–1529. doi: 10.2174/1389450110607011513. [DOI] [PubMed] [Google Scholar]
- 17.Dhiman P., Malik N., Sobarzo-Sánchez E., Uriarte E., Khatkar A. Quercetin and Related Chromenone Derivatives as Monoamine Oxidase Inhibitors: Targeting Neurological and Mental Disorders. Molecules. 2019;24:418. doi: 10.3390/molecules24030418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalgutkar A.S., Dalvie D.K., Castagnoli N., Taylor T.J. Interactions of Nitrogen-Containing Xenobiotics with Monoamine Oxidase (MAO) Isozymes A and B: SAR Studies on MAO Substrates and Inhibitors. Chem. Res. Toxicol. 2001;14:1139–1162. doi: 10.1021/tx010073b. [DOI] [PubMed] [Google Scholar]
- 19.Shih J.C., Chen K., Ridd M.J. Monoamine Oxidase: From Genes to Behavior. Annu. Rev. Neurosci. 1999;22:197–217. doi: 10.1146/annurev.neuro.22.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prah A., Purg M., Stare J., Vianello R., Mavri J. How Monoamine Oxidase A Decomposes Serotonin: An Empirical Valence Bond Simulation of the Reactive Step. J. Phys. Chem. B. 2020;124:8259–8265. doi: 10.1021/acs.jpcb.0c06502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bortolato M., Chen K., Shih J.C. Monoamine Oxidase Inactivation: From Pathophysiology to Therapeutics. Adv. Drug Deliv. Rev. 2008;60:1527–1533. doi: 10.1016/j.addr.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carradori S., Silvestri R. New Frontiers in Selective Human MAO-B Inhibitors. J. Med. Chem. 2015;58:6717–6732. doi: 10.1021/jm501690r. [DOI] [PubMed] [Google Scholar]
- 23.Benny F., Kumar S., Jayan J., Abdelgawad M.A., Ghoneim M.M., Kumar A., Manoharan A., Susan R., Sudevan S.T., Mathew B. Review of Β-carboline and Its Derivatives as Selective MAO-A Inhibitors. Arch. Pharm. 2023;356:e2300091. doi: 10.1002/ardp.202300091. [DOI] [PubMed] [Google Scholar]
- 24.Tong J., Rathitharan G., Meyer J.H., Furukawa Y., Ang L.-C., Boileau I., Guttman M., Hornykiewicz O., Kish S.J. Brain Monoamine Oxidase B and A in Human Parkinsonian Dopamine Deficiency Disorders. Brain. 2017;140:2460–2474. doi: 10.1093/brain/awx172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathew B., Mathew G.E., Petzer J.P., Petzer A. Structural Exploration of Synthetic Chromones as Selective MAO-B Inhibitors: A Mini Review. Comb. Chem. High Throughput Screen. 2017;20:522–532. doi: 10.2174/1386207320666170227155517. [DOI] [PubMed] [Google Scholar]
- 26.Saura J., Luque J.M., Cesura A.M., Da Prada M., Chan-Palay V., Huber G., Löffler J., Richards J.G. Increased Monoamine Oxidase b Activity in Plaque-Associated Astrocytes of Alzheimer Brains Revealed by Quantitative Enzyme Radioautography. Neuroscience. 1994;62:15–30. doi: 10.1016/0306-4522(94)90311-5. [DOI] [PubMed] [Google Scholar]
- 27.Cesura A.M., Pletscher A. Progress in Drug Research/Fortschritte der Arzneimittelforschung/Progrès Des Recherches Pharmaceutiques. Birkhäuser; Basel, Switzerland: 1992. The New Generation of Monoamine Oxidase Inhibitors; pp. 171–297. [DOI] [PubMed] [Google Scholar]
- 28.Amrein R., Martin J.R., Cameron A.M. Moclobemide in Patients with Dementia and Depression. Adv. Neurol. 1999;80:509–519. [PubMed] [Google Scholar]
- 29.Li F., Wu J.-J., Wang J., Yang X.-L., Cai P., Liu Q.-H., Kong L.-Y., Wang X.-B. Synthesis and Pharmacological Evaluation of Novel Chromone Derivatives as Balanced Multifunctional Agents against Alzheimer’s Disease. Bioorganic Med. Chem. 2017;25:3815–3826. doi: 10.1016/j.bmc.2017.05.027. [DOI] [PubMed] [Google Scholar]
- 30.Youdim M.B.H., Lavie L. Selective MAO-A and B Inhibitors, Radical Scavengers and Nitric Oxide Synthase Inhibitors in Parkinson’s Desease. Life Sci. 1994;55:2077–2082. doi: 10.1016/0024-3205(94)00388-2. [DOI] [PubMed] [Google Scholar]
- 31.Sayre L.M., Perry G., Smith M.A. Oxidative Stress and Neurotoxicity. Chem. Res. Toxicol. 2008;21:172–188. doi: 10.1021/tx700210j. [DOI] [PubMed] [Google Scholar]
- 32.Ramsay R.R. Inhibitor Design for Monoamine Oxidases. Curr. Pharm. Des. 2013;19:2529–2539. doi: 10.2174/1381612811319140004. [DOI] [PubMed] [Google Scholar]
- 33.Knudsen Gerber D.S. Selegiline and Rasagiline: Twins or Distant Cousins? Guidelines. Consult. Pharm. 2011;26:48–51. doi: 10.4140/TCP.n.2011.48. [DOI] [PubMed] [Google Scholar]
- 34.Fariello R.G. Safinamide. Neurotherapeutics. 2007;4:110–116. doi: 10.1016/j.nurt.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Binda C., Wang J., Pisani L., Caccia C., Carotti A., Salvati P., Edmondson D.E., Mattevi A. Structures of Human Monoamine Oxidase B Complexes with Selective Noncovalent Inhibitors: Safinamide and Coumarin Analogs. J. Med. Chem. 2007;50:5848–5852. doi: 10.1021/jm070677y. [DOI] [PubMed] [Google Scholar]
- 36.Chimenti F., Secci D., Bolasco A., Chimenti P., Bizzarri B., Granese A., Carradori S., Yáñez M., Orallo F., Ortuso F., et al. Synthesis, Molecular Modeling, and Selective Inhibitory Activity against Human Monoamine Oxidases of 3-Carboxamido-7-Substituted Coumarins. J. Med. Chem. 2009;52:1935–1942. doi: 10.1021/jm801496u. [DOI] [PubMed] [Google Scholar]
- 37.Delogu G., Picciau C., Ferino G., Quezada E., Podda G., Uriarte E., Viña D. Synthesis, Human Monoamine Oxidase Inhibitory Activity and Molecular Docking Studies of 3-Heteroarylcoumarin Derivatives. Eur. J. Med. Chem. 2011;46:1147–1152. doi: 10.1016/j.ejmech.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 38.Secci D., Carradori S., Bolasco A., Chimenti P., Yáñez M., Ortuso F., Alcaro S. Synthesis and Selective Human Monoamine Oxidase Inhibition of 3-Carbonyl, 3-Acyl, and 3-Carboxyhydrazido Coumarin Derivatives. Eur. J. Med. Chem. 2011;46:4846–4852. doi: 10.1016/j.ejmech.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 39.Chimenti F., Fioravanti R., Bolasco A., Chimenti P., Secci D., Rossi F., Yáñez M., Orallo F., Ortuso F., Alcaro S. Chalcones: A Valid Scaffold for Monoamine Oxidases Inhibitors. J. Med. Chem. 2009;52:2818–2824. doi: 10.1021/jm801590u. [DOI] [PubMed] [Google Scholar]
- 40.Silva C.F.M., Pinto D.C.G.A., Silva A.M.S. Chromones: A Promising Ring System for New Anti-Inflammatory Drugs. ChemMedChem. 2016;11:2252–2260. doi: 10.1002/cmdc.201600359. [DOI] [PubMed] [Google Scholar]
- 41.Silva C.F.M., Batista V.F., Pinto D.C.G.A., Silva A.M.S. Challenges with Chromone as a Privileged Scaffold in Drug Discovery. Expert. Opin. Drug Discov. 2018;13:795–798. doi: 10.1080/17460441.2018.1494720. [DOI] [PubMed] [Google Scholar]
- 42.Horton D.A., Bourne G.T., Smythe M.L. The Combinatorial Synthesis of Bicyclic Privileged Structures or Privileged Substructures. Chem. Rev. 2003;103:893–930. doi: 10.1021/cr020033s. [DOI] [PubMed] [Google Scholar]
- 43.Roy T., Boateng S.T., Banang-Mbeumi S., Singh P.K., Basnet P., Chamcheu R.-C.N., Ladu F., Chauvin I., Spiegelman V.S., Hill R.A., et al. Synthesis, Inverse Docking-Assisted Identification and in Vitro Biological Characterization of Flavonol-Based Analogs of Fisetin as c-Kit, CDK2 and MTOR Inhibitors against Melanoma and Non-Melanoma Skin Cancers. Bioorganic Chem. 2021;107:104595. doi: 10.1016/j.bioorg.2020.104595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ganguly A.K., Kaur S., Mahata P.K., Biswas D., Pramanik B.N., Chan T.M. Synthesis and Properties of 3-Acyl-γ-Pyrones, a Novel Class of Flavones and Chromones. Tetrahedron Lett. 2005;46:4119–4121. doi: 10.1016/j.tetlet.2005.04.010. [DOI] [Google Scholar]
- 45.Brühlmann C., Ooms F., Carrupt P.-A., Testa B., Catto M., Leonetti F., Altomare C., Carotti A. Coumarins Derivatives as Dual Inhibitors of Acetylcholinesterase and Monoamine Oxidase. J. Med. Chem. 2001;44:3195–3198. doi: 10.1021/jm010894d. [DOI] [PubMed] [Google Scholar]
- 46.Fernández-Bachiller M.I., Pérez C., Monjas L., Rademann J., Rodríguez-Franco M.I. New Tacrine–4-Oxo-4 H-Chromene Hybrids as Multifunctional Agents for the Treatment of Alzheimer’s Disease, with Cholinergic, Antioxidant, and β-Amyloid-Reducing Properties. J. Med. Chem. 2012;55:1303–1317. doi: 10.1021/jm201460y. [DOI] [PubMed] [Google Scholar]
- 47.Erickson R.H., Natalie K.J., Bock W., Lu Z., Farzin F., Sherrill R.G., Meloni D.J., Patch R.J., Rzesotarski W.J. (Aminoalkoxy)Chromones. Selective Sigma Receptor Ligands. J. Med. Chem. 1992;35:1526–1535. doi: 10.1021/jm00087a005. [DOI] [PubMed] [Google Scholar]
- 48.Gaspar A., Reis J., Matos M.J., Uriarte E., Borges F. In Search for New Chemical Entities as Adenosine Receptor Ligands: Development of Agents Based on Benzo-γ-Pyrone Skeleton. Eur. J. Med. Chem. 2012;54:914–918. doi: 10.1016/j.ejmech.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 49.Gaspar A., Reis J., Kachler S., Paoletta S., Uriarte E., Klotz K.-N., Moro S., Borges F. Discovery of Novel A3 Adenosine Receptor Ligands Based on Chromone Scaffold. Biochem. Pharmacol. 2012;84:21–29. doi: 10.1016/j.bcp.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 50.Cagide F., Gaspar A., Reis J., Chavarria D., Vilar S., Hripcsak G., Uriarte E., Kachler S., Klotz K.N., Borges F. Navigating in Chromone Chemical Space: Discovery of Novel and Distinct A 3 Adenosine Receptor Ligands. RSC Adv. 2015;5:78572–78585. doi: 10.1039/C5RA14988F. [DOI] [Google Scholar]
- 51.Andrews S.P., Mason J.S., Hurrell E., Congreve M. Structure-Based Drug Design of Chromone Antagonists of the Adenosine A 2A Receptor. Med. Chem. Commun. 2014;5:571–575. doi: 10.1039/C3MD00338H. [DOI] [Google Scholar]
- 52.Cagide F., Reis J., Gaspar A., Chavarria D., Kachler S., Klotz K.N., Gomes L.R., Low J.N., Vilar S., Hripcsak G., et al. Discovery of the First A 1 Adenosine Receptor Ligand Based on the Chromone Scaffold. RSC Adv. 2016;6:46972–46976. doi: 10.1039/C6RA02347A. [DOI] [Google Scholar]
- 53.Reis J., Cagide F., Valencia M.E., Teixeira J., Bagetta D., Pérez C., Uriarte E., Oliveira P.J., Ortuso F., Alcaro S., et al. Multi-Target-Directed Ligands for Alzheimer’s Disease: Discovery of Chromone-Based Monoamine Oxidase/Cholinesterase Inhibitors. Eur. J. Med. Chem. 2018;158:781–800. doi: 10.1016/j.ejmech.2018.07.056. [DOI] [PubMed] [Google Scholar]
- 54.Mpitimpiti A.N., Petzer J.P., Petzer A., Jordaan J.H.L., Lourens A.C.U. Synthesis and Evaluation of Chromone Derivatives as Inhibitors of Monoamine Oxidase. Mol. Divers. 2019;23:897–913. doi: 10.1007/s11030-019-09917-8. [DOI] [PubMed] [Google Scholar]
- 55.Legoabe L.J., Petzer A., Petzer J.P. Selected Chromone Derivatives as Inhibitors of Monoamine Oxidase. Bioorganic Med. Chem. Lett. 2012;22:5480–5484. doi: 10.1016/j.bmcl.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 56.Alcaro S., Gaspar A., Ortuso F., Milhazes N., Orallo F., Uriarte E., Yáñez M., Borges F. Chromone-2- and -3-Carboxylic Acids Inhibit Differently Monoamine Oxidases A and B. Bioorganic Med. Chem. Lett. 2010;20:2709–2712. doi: 10.1016/j.bmcl.2010.03.081. [DOI] [PubMed] [Google Scholar]
- 57.Gaspar A., Silva T., Yáñez M., Vina D., Orallo F., Ortuso F., Uriarte E., Alcaro S., Borges F. Chromone, a Privileged Scaffold for the Development of Monoamine Oxidase Inhibitors. J. Med. Chem. 2011;54:5165–5173. doi: 10.1021/jm2004267. [DOI] [PubMed] [Google Scholar]
- 58.Gaspar A., Reis J., Fonseca A., Milhazes N., Viña D., Uriarte E., Borges F. Chromone 3-Phenylcarboxamides as Potent and Selective MAO-B Inhibitors. Bioorganic Med. Chem. Lett. 2011;21:707–709. doi: 10.1016/j.bmcl.2010.11.128. [DOI] [PubMed] [Google Scholar]
- 59.Cagide F., Silva T., Reis J., Gaspar A., Borges F., Gomes L.R., Low J.N. Discovery of Two New Classes of Potent Monoamine Oxidase-B Inhibitors by Tricky Chemistry. Chem. Commun. 2015;51:2832–2835. doi: 10.1039/C4CC08798D. [DOI] [PubMed] [Google Scholar]
- 60.Torres P.H., Sodero A.C., Jofily P., Silva F.P., Jr. Key topics in molecular docking for drug design. Int. J. Mol. Sci. 2019;20:4574. doi: 10.3390/ijms20184574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takao K., Endo S., Nagai J., Kamauchi H., Takemura Y., Uesawa Y., Sugita Y. 2-Styrylchromone Derivatives as Potent and Selective Monoamine Oxidase B Inhibitors. Bioorganic Chem. 2019;92:103285. doi: 10.1016/j.bioorg.2019.103285. [DOI] [PubMed] [Google Scholar]
- 62.Wang X.-B., Yin F.-C., Huang M., Jiang N., Lan J.-S., Kong L.-Y. Chromone and Donepezil Hybrids as New Multipotent Cholinesterase and Monoamine Oxidase Inhibitors for the Potential Treatment of Alzheimer’s Disease. RSC Med. Chem. 2020;11:225–233. doi: 10.1039/C9MD00441F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takao K., Sakatsume T., Kamauchi H., Sugita Y. Syntheses and Evaluation of 2- or 3-(N-cyclicamino) Chromone Derivatives as Monoamine Oxidase Inhibitors. Chem. Pharm. Bull. 2020;68:1082–1089. doi: 10.1248/cpb.c20-00579. [DOI] [PubMed] [Google Scholar]
- 64.Takao K., Takemura Y., Nagai J., Kamauchi H., Hoshi K., Mabashi R., Uesawa Y., Sugita Y. Synthesis and Biological Evaluation of 3-Styrylchromone Derivatives as Selective Monoamine Oxidase B Inhibitors. Bioorganic Med. Chem. 2021;42:116255. doi: 10.1016/j.bmc.2021.116255. [DOI] [PubMed] [Google Scholar]
- 65.Zhang C., Zhang Y., Lv Y., Guo J., Gao B., Lu Y., Zang A., Zhu X., Zhou T., Xie Y. Chromone-Based Monoamine Oxidase B Inhibitor with Potential Iron-Chelating Activity for the Treatment of Alzheimer’s Disease. J. Enzyme Inhib. Med. Chem. 2023;38:100–117. doi: 10.1080/14756366.2022.2134358. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used, analyzed, and reviewed were collected from the corresponding authors and online research databases.