Abstract
Ziziphus mauritiana Lam. (Rhamnaceae) (Chinee Apple, Indian Jujube, or Ber) is a significant woody weed in the drier tropics of northern Queensland, Western Australia, and the Northern Territory. Throughout these regions, its densely formed thickets influence the structure, function, and composition of rangeland ecosystems by outcompeting native pasture species. Despite this, the recent literature is heavily focused on the horticultural value of domesticated Ziziphus species in South Asia (China, India, and Pakistan), particularly its potential for poverty alleviation in arid or semi-arid areas. In fact, there has been comparatively little research undertaken on its invasiveness or associated ecological factors in pastoral contexts. Currently, the management of Z. mauritiana is limited to the application of synthetic herbicides or mechanical clearing operations. There is also considerable interest in the exploitation of host-specific, natural enemies (biological control agents, herbivorous insects, fungi, bacteria, or viruses) for limiting the vigour, competitiveness, or reproductive capacity of Z. mauritiana in northern Australia. The development of a “bioherbicide” in lieu of synthetic counterparts may foster a more resilient coexistence between agricultural systems and the natural environment owing to its reduced environmental persistence and increased target specificity. This review summarises the current literature on the weediness, ecological impacts, and current management of this problematic weed, thereby identifying (i) opportunities for further research and (ii) recommendations for improved management within its invasive range.
Keywords: Chinee apple, Indian jujube, Ziziphus mauritiana, woody weeds, invasive alien species
1. Introduction
Ziziphus mauritiana Lam. (Rhamnaceae) (Chinee apple, Indian jujube, or ber) is a deciduous thorny tree or shrub native to South Asia (China, India, and Pakistan) and eastern Africa [1,2,3,4,5], where it has a remarkable history of ancient cultivation (~3200 years). More recently, it has served as a crucial source of food security for poor and resource-scarce populations in these arid areas [6]. However, it has also established invasive populations in northern Australia [1,5], southern Africa (i.e., Zimbabwe and Zambia) [7,8], Fiji [7], and some Pacific and Indian Ocean islands [7,9]. In Australia, it was introduced to the early mining settlements of northern Queensland (e.g., Charters Towers, Ravenswood, Mingela, and Hughenden) in the late nineteenth century for its ornamental and horticultural value [1,2,3,5,7]. Its current distribution is densest in the northern parts of Queensland (Townsville–Charters Towers), Western Australia (Broome, Derby, and Kimberley), and the Northern Territory (Darwin, Daly River, and Roper River Catchment) [1,3,5]. Throughout these regions, its densely formed thickets influence the structure, function, and composition of rangeland ecosystems by outcompeting native pasture species [3,5,10]. This negatively affects the quality of services and pastoral operations (e.g., agronomic productivity, livestock carrying capacity, water accessibility, and mustering) obtainable from this diverse, natural resource [3,5,7,11,12]. Hence, it has been identified as a priority threat to the pastoral industry by both graziers and landholders [1,5,7]. The biodiversity of tropical woodlands is also threatened, whereby the herbaceous understorey of native vegetation is replaced by a dense layer of impenetrable thickets [3,13]. The transformation of these habitats is associated with the decline in or localised extirpation of endemic wildlife [14,15], such as the threatened black-throated finch (southern subspecies: Poephila cincta cincta) in north-eastern Queensland [15].
The management of Z. mauritiana is limited to the application of synthetic herbicides or mechanical clearing operations [3,5,7]. Manual removal of lower density (<50 plants/ha) or isolated infestations can be achieved by stick-raking, blade ploughing, or bulldozing of individual trees [5,16]. However, there are obvious limitations in terms of cost efficiency, labour supply, and its effectiveness on medium-scale (50 to 150 plants/ha) or large-scale populations (>150 plants/ha) [5,17,18]. The basal or cut-stump application of synthetic auxin herbicides (Group 4: triclopyr, fluroxypyr, or picloram) is the primary means for the effective suppression of Z. mauritiana in northern Australia [5,16]. Whist their efficacy is undisputed, there are concerns regarding the suitability of synthetic herbicides in ecologically sensitive or low-value habitats [19]. Their excessive or improper application is associated with adverse injury to neighbouring plant communities via herbicidal spray drift, runoff, or leaching. This recent appreciation for environmental stewardship has promoted significant developments in the field of woody weed management by reducing dosage levels or improving application methods [5,19]. There is also considerable interest in the exploration of host-specific, natural enemies for limiting the vigour, competitiveness, and reproductive capacity of Z. mauritiana in northern Australia [3,7].
The recent literature is heavily focused on the horticultural value of domesticated Ziziphus species (Z. mauritiana and Ziziphus jujuba Mill.) in South Asia [1,13] and its potential for poverty alleviation in arid or semi-arid climates [6,20,21]. There has been comparatively little research undertaken on its invasiveness in northern Australia [13]. In fact, a comprehensive review of Z. mauritiana has not been published since that by Grice (2002) [22], with the exception of a recent examination focused solely on prospective biological control agents [6]. The primary objective of this review is to summarise the current literature on the species’ weediness, ecological impacts, and management to identify knowledge gaps that could be the catalyst for further research on this problematic weed.
2. Historical and Global Significance of Ziziphus mauritiana Fruit Trees
The “ber” fruit (Z. mauritiana) has an ancient history of cultivation throughout the plains of northern India [23,24,25]. Perhaps the earliest reference to this fruit is in the Yajurveda (c. 1200–800 BCE), a primary religious Veda text [23,24,25]. The primary region of historical cultivation, the Deccan Plateau, also predated the Gangetic civilisation (c. 1500–500 BC) [25]. Much later in the late eighteenth century, the fruit was presented to King Raghoji Bhonsle II of the Kingdom of Nagpur by a local Muslim farmer, thereby cementing its popularity in present day India [26]. Ultimately, this historical trajectory underscores its cultural and horticultural significance in most of South Asia [26]. In India, it has been successfully cultivated in the arid and semi-arid zones, particularly Andhra Pradesh, Bihar, Gujarat, Haryana, Madhya Pradesh, Maharashtra, Punjab, Rajasthan and Uttar Pradesh [26,27,28,29,30,31]. It is considered the “king of arid-zone fruits” or the “poor man’s apple” because of its lowered cost of production, high-yielding nature, drought and salinity tolerance, nutritional value, and scope for value addition (e.g., beverages, jams, cake, bread, and porridge) [20,21,29]. In fact, the nutritive richness (i.e., ascorbic acid, vitamins, minerals, and polyphenols) of “ber” fruit has been well documented in the current literature [6,21,23,24,26,32] by Muhammad et al. (2022) [6], Prakash et al. (2020) [32], and Sharif et al. (2022) [21]. Several studies have also demonstrated the pharmacological potential of all parts of Z. mauritiana as an antioxidant, antimicrobial, antidiarrheal, antidepressant, immunomodulator, and hepatoprotective [25,32,33,34,35,36] (Table 1). These therapeutic properties are attributable to a diverse assortment of derivative metabolites, such as flavonoids, alkaloids, terpenoids, glycosides, and saponins [21,33,34,36].
Table 1.
The pharmacological potential of all parts (fruit, seed, leaves, bark, and roots) of Ziziphus mauritiana for the treatment of various ailments or diseases. The data were sourced from Morton 1987 [37], Meghwal et al. 2007 [23], Naaz et al. 2020 [33], and Butt et al. 2021 [36].

|

|

|

|
|
| FRUIT | SEED | LEAVES | BARK | ROOTS |
| Blood Disorders Constipation Dyspepsia Fevers Flatulence Hyperdipsia Indigestion Laxative Leprosy Ulcers Vomiting Wounds |
Abdominal Pains Asthma Cough Diarrhea Encephalopathy Insomnia Nausea Ophthalmopathy Rheumatism Sedative |
Asthma Blood Diseases Boils Conjunctivitis Diarrhoea Dysentery Fever Gum Bleeding Liver Issues Smallpox Stomatitis Syphilitic Ulcers Tuberculosis Typhoid Fever Wounds |
Boils Diarrhoea Dysentery Gingivitis Ulcers |
Analgesic Anti-Allergic Anti-Inflammatory Cephalalgia Dysentery Fever Gout Rheumatism Ulcers Wounds |
Ziziphus mauritiana is also cultivated on a smaller scale in other south Asian (Bangladesh, Bhutan, Nepal, Pakistan, and Sri Lanka), central Asian (Afghanistan, Iran, Iraq, and United Arab Emirates), and Arabian Gulf countries [24,25,26]. In these countries, the exacerbation of climate change has resulted in the expansion of arid land by altering precipitation patterns and intensifying drought conditions [6]. A recent study by Spinoni et al. (2021) predicted a significant climatic shift (~1.1% to 3.8%) in arid or semi-arid zones under four realistic “global warming levels” [38]. Therefore, the recent literature is heavily focused on domesticated Ziziphus varieties as a potential economic source in salt-affected soils or water-stressed conditions [6,29].
Although it has no commercial value in Australia [7], another Ziziphus species (Chinese jujube: Z. jujuba) has market potential in the southern states (New South Wales, South Australia, and Victoria) and southern parts of Western Australia [7,39,40,41]. Its commercial cultivation is mostly distributed throughout the Northern Rangelands, Perth Hills, and south-western region of Western Australia [39,40,41], where it is ecologically distinct from the invasive populations of Z. mauritiana in the northern states [7]. The production of “jujube” is a diversification opportunity for these farmers to build business resilience in the event of unpredictable salinity or seasonal drought [42]. Additionally, the proximity of these growing regions to South Asia creates a favourable scenario for the development of an export market to meet “off-season” demand in those countries [39,40,42]. For example, the largest producer of kiwifruit (Actinidia deliciosa) in Australia and New Zealand has recently invested in the production of commercial “jujube” varieties with the intention of launching an exportation industry [43].
3. Botany, Biology, and Ecology of Ziziphus mauritiana
Ziziphus mauritiana is a single- or multi-stemmed tree (height of <15 metres) with an intricately branched, spreading canopy (Figure 1A) [7,27]. The younger stems are covered in densely interwoven, woolly hairs and contain a single, curved thorn at each joint [22]. The upper side of its alternate, sub-elliptically shaped leaves (20 to 80 mm length) are dark-green, glossy, and glabrous (Figure 1B) [7,22], whilst the lighter-coloured underside is covered in white to rusty fine hairs (Figure 1D) [22]. Its small, inconspicuous flowers (5–8 mm width) are greenish-yellow with a hypanthium floral structure [7,27]. They are clustered in cyme inflorescences of twelve to fourteen flowers that are connected by short pedicles (<4 mm) [27]. The floral biology and development of Z. mauritiana has been described in further detail by Tel-Zur et al. 2009 [27].
Figure 1.
The invasive Ziziphus mauritiana in Northern Queensland, Australia: (A) branched, spreading canopy, (B) upper side of the leaf, (C) sub-globular, drupaceous fruit of varying degrees of ripeness, and (D) underside of the leaf.
The sub-globular, drupaceous fruit (20–50 mm diameter) have a leathery exocarp that varies in colouration between yellow-green and reddish-brown as an indication of ripeness (Figure 1C) [3,22,26]. They also have a lignified, irregularly furrowed endocarp surrounded by a white fleshy mesocarp that is palatable [3,26]. The intact endocarp encases one, sometimes two or three, rounded darker brown seeds (~6 mm) [1,3,7,13]. Despite this mechanism of physical dormancy, the seed viability and persistence are relatively short-lived (<2 years) in the soil [3,7]. In fact, Grice (1996) discerned that less than 10% of seeds at the soil surface or a shallow burial depth (~2 cm) were germinable after only twelve months [1]. However, the fecundity of Z. mauritiana has also been well documented in the literature [3,13]. In a single reproductive event, the production of more than 5000 seeds is typical of larger shrubs [1,3,7].
The consumption of seed by frugivorous birds is a potential avenue of localised dispersal [1,3]. A study by Grice (1996) observed the transportation of seed by pied currawongs (Strepera graculina), red-tailed black cockatoos (Calyptorhynchus banksii), and channel-billed cuckoos (Scythrops novaehollandiae) in the tropical woodlands of northern Australia [1]. They commonly feed on mature fruits in the canopy or harvest seed from naturally fallen fruits on the soil surface [1]. The partial consumption of fruit flesh has also been recorded in pale-headed rosellas (Platycerus adscius) and red-winged parrots (Aprosmictus erythropterus) [1,22]. However, the importance of avian seed transportation is largely unknown because of the lack of research on (1) their territorial behaviours or boundaries, (2) the time for seed material passage, and (3) the potential disgorgement of larger, woody endocarps [13].
Many studies have found that mammals likely have a significant role in the translocation of Z. mauritiana seeds. In Queensland, a large number of intact endocarps (<240 and µ = 17) [27] with viable seeds were collected from the faeces of domestic cattle (Bos indicus), feral pigs (Sus scrofa), and native agile wallabies (Notamacropus agilis) [1,13,22]. This is associated with the wider movement of seed material to nearby sites or over several kilometres [1,22]. Other mammals and birds are also involved in seed transportation, such as horses (Equus ferus), donkeys (Equus asinus), camels (Camelus dromedaries), goats (Capra aegagrus hircus), sheep (Ovis aries), emus (Dromaius novaehollandiae), and bustards (Ardeotis australis) [3]. Additionally, many seeds remain beneath or very close to the canopy of the parent plant [1,13]. This is evidenced by the frequent establishment of juvenile seedlings around reproductively mature plants at infestation sites throughout northern Australia [1].
Ziziphus mauritiana is a very hardy tree tolerant to extreme temperature variability (−5 °C to 49 °C) and dry conditions [7,23,27,29,30,31,44] and therefore is well suited to the seasonally variable rainfall patterns of northern Australia (average annual rainfall of 500 mm to 1500 mm) [45]. It is also successfully cultivated in the arid and semi-arid zones of north-western India (e.g., Gujarat, Haryana, and Rajasthan) [29,31], where the annual rainfall can be as low as 200 mm [30]. Clifford et al. (1998) investigated the physiological basis of drought tolerance in Z. mauritiana under glasshouse conditions [30]. This study found that a combination of solute accumulation and increased cell rigidity were the likely mechanisms for drought tolerance in this particular species [30]. However, a subsequent study by Arndt et al. (2000) indicated that it also accessed moisture deeper within the soil profile via the taproot system [46]. There are no specific soil requirements for Z. mauritiana throughout its native or naturalised distribution [7,22,44]. In fact, its successful establishment has been cited in deep coarse-textured sands, shallow-surfaced solodic soils, cracking clays, deep alluvials, and skeletal soils [22,23,44].
4. Native and Naturalised Distribution of Ziziphus mauritiana
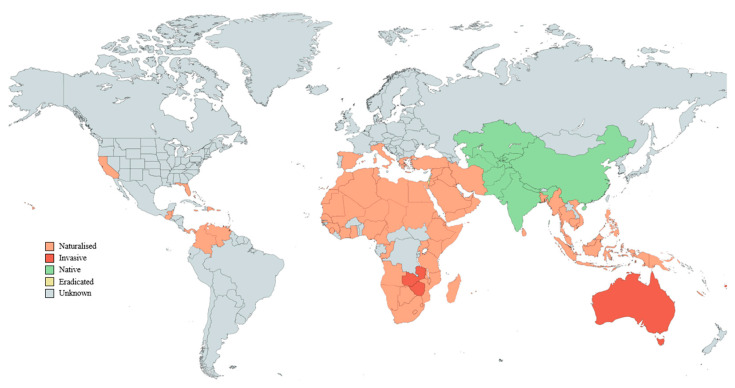
Ziziphus mauritiana is native to South Asia (China, India, and Pakistan) and eastern Africa [1,2,3,4,5] (Figure 2). However, it has established invasive populations in northern Australia [1,5,44], southern Africa (i.e., Zimbabwe and Zambia) [7,8], Fiji [7], and on some Pacific and Indian Ocean islands (e.g., Seychelles) [7,9,44] (Figure 2). Its naturalisation has also been recorded in the arid or semi-arid zones of South America [7,37,44], Central America [7,37], United States of America [7,37,44,47], Western Africa, the Middle East [22,37], the Caribbean [44], Cape Verde Islands, and La Reunion [7] (Figure 2).
Figure 2.
A presence-only global distribution map of Ziziphus mauritiana based on species’ records from the Centre for Agriculture and Bioscience International (CABI) [48], World Agroforestry Centre [49], Pacific Islands Ecosystems at Risk (PIER) [50], and Morton (1987) [37].
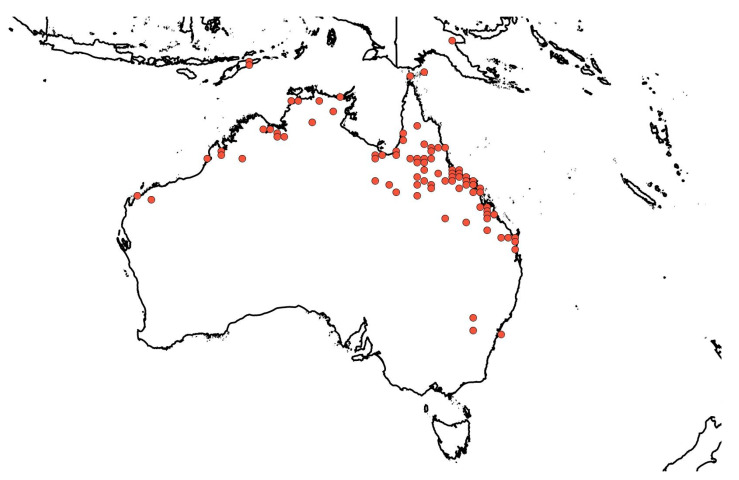
In Australia, the densest populations of Z. mauritiana are associated with former mining settlements (e.g., Charters Towers, Hughenden, Mingela, and Ravenswood) in northern Queensland [1,2,3,22], where it was initially introduced in the late nineteenth century (1863) for its horticultural value [1,2,3,5,7,22,44] (Figure 3). A study by Grice et al. (2000) examined the regional and landscape patterns of this invasive shrub in the Charters Towers region (area of 68,388 km2) of northern Queensland [2]. It was recorded in 32% of sites within a 20 km radius of Charters Towers [2]. A similar pattern of occurrence has been documented in the Northern Territory (e.g., Darwin, Daly River, and Rope River Catchment) and Western Australia (e.g., Broome, Derby, Kimberley, and Kununurra) [1,22], as shown in Figure 3.
Figure 3.
The distribution of Ziziphus mauritiana in northern Australia from the national and state herbarium occurrence records (~332 records from 1909 to 2019) of New South Wales, Queensland, South Australia, Victoria, Western Australia, and the Northern Territory [51]. Its presence in Timor-Leste has also been documented [51].
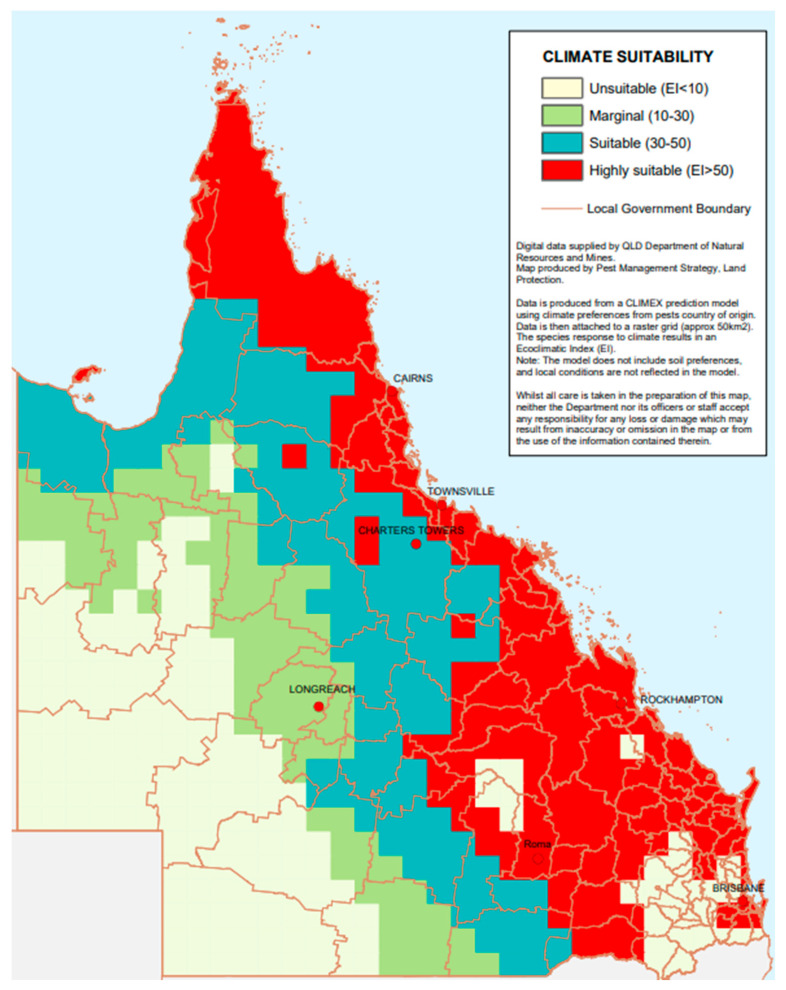
A CLIMEX model was developed by the Queensland Government for estimation of the potential distribution or relative climatic suitability of Z. mauritiana in Queensland (Figure 4) [52]. The entire eastern coast of Queensland (from Brisbane to Cape York Peninsula) was deemed highly suitable for invasion by this species, with a high probability of occurrence predicted in most of the dry and wet tropics (Figure 4). A similar distribution was also documented in parts of south-central Queensland, as well as west of Rockhampton (Figure 4). A lowered suitability was predicted further inland towards central-west and north-west Queensland (Figure 4). These species distribution models (SDMs) are valuable in the identification of prospective locations for the exploration or targeted establishment of biological control agents [53,54,55].
Figure 4.
A CLIMEX prediction model of the potential distribution or relative climatic suitability of Ziziphus mauritiana in Queensland, Australia. The data are attached to a raster grid (approx. 50 km2) [52].
5. Agricultural, Environmental, and Economic Impacts of Ziziphus mauritiana
In Australia, this species is a problematic weed in both grassland and rangeland environments [22]. Its densely formed thickets influence the structure, function, or composition of rangeland ecosystems by outcompeting native pasture species [3,5,7,10,44]. This negatively affects the quality of services and pastoral operations obtainable from this diverse, natural resource, such as the agronomic productivity, livestock carrying capacity, water accessibility, and mustering activities of the land [3,5,7,12]. Hence, it has been identified as a priority threat to the pastoral industry by graziers and other landholders [1,5,7]. There has been no quantitative assessment of the economic costs associated with Z. mauritiana in pastoral contexts [22]. The realisation of these costs will assist in (1) the prioritisation of research efforts, (2) resource allocation, (3) policy formation, (4) stakeholder collaboration, and (5) the development or rationalisation of management decisions [56,57].
From a conservation perspective, the biodiversity of tropical woodlands is also threatened by Z. mauritiana [3,13]. These habitats of scattered eucalypts are vulnerable to invasion by several invasive shrubs, particularly Chinee apple (Z. mauritiana), parkinsonia (Parkinsonia aculeata), prickly acacia (Vachellia nilotica), mesquite (Prosopis spp.), and rubber vine (Cryptostegia grandiflora) [58], whereby the herbaceous understorey is replaced by a dense layer of impenetrable thickets [15,58]. The transformed architecture of these woodlands is associated with the localised extirpation of or reduction in native wildlife [14,15]. For example, a recent study by Laguna et al. (2019) found that the proliferation of two invasive shrubs (Z. mauritiana and Lantana camara) in grassy woodlands coincided with a declined population in the endangered black-throated finch (southern subspecies: Poephila cincta cincta) [15].
In Queensland, Z. mauritiana is a Category 3 restricted species under the Biosecurity Act 2014 [59], meaning that the introduction, release, or commercial use of any plant matter is prohibited without an authorised permit [7,59]. Similarly, this invasive shrub is declared a Class A (i.e., must be eradicated) and Class C (i.e., cannot be introduced) weed in the Northern Territory as part of the Weeds Management Act 2001 [22,60]. The eventual eradication of Z. mauritiana is deemed feasible because of its limited or isolated establishment in Darwin, Daly River, and the Rope River Catchment. The only other state with a current declaration status is Western Australia [22,61], whereby some form of management is required (C3) under the Biosecurity and Agriculture Management Act 2007 [61].
Currently, Z. mauritiana is more problematic in agricultural production contexts, particularly in extensive livestock systems. This means that there are very few examples of its control in other situations (e.g., tropical woodlands or forestry environments). However, the management of many invasive woody weeds amongst native vegetation must be undertaken in compliance with vegetation management regulations, such as the Vegetation Management Act 1999 in Queensland [62], whereby the conservation and biodiversity of remnant vegetation is prioritised [63]. This can be achieved via the establishment of Area Management Plans (AMPs) by natural resource management organisations [62]. For example, the treatment of Z. mauritiana in environmentally sensitive habitats is approved and outlined under the “Dry Tropics Weed AMP” of northern Queensland [64].
The “dual nature” of most weeds is either rarely acknowledged or poorly understood in the current ecological literature [14,65]. However, some recent studies have demonstrated that the interaction between native biodiversity and exotic invasion is not always inevitably negative [14,65,66], a phenomenon referred to as “the invasion paradox” [62,63]. For example, Ward-Fear et al. (2017) observed that Z. mauritiana offered critical refuge to native rodents (pale field rats: Rattus tunneyi) from feral horses (E. ferus) in the remote floodplains of north-western Australia [14]. The spiny thorns excluded feral horses from the shaded area beneath the canopy, thereby providing the only sites for rat burrows within the landscape that were not subject to trampling and soil compaction [14]. Although this ecosystem is under simultaneous threat, this invasive shrub essentially buffers the impacts posed by the feral horses and subsequently enhances the survival of the threatened rodents [14]. Therefore, the eradication of Z. mauritiana in this degraded ecosystem may in fact cause population bottlenecks, local extinction, or trophic cascades [14,65].
6. Management of Invasive Ziziphus mauritiana in Australia
6.1. Manual and Mechanical Control Methods
The manual removal of lower density (<50 plants/ha) or isolated infestations can be achieved through stick-raking, blade ploughing, or bulldozing of individual trees [5,16]. The most suitable period for these activities is prior to fruit development or lowered root reserves [16]. However, there are obvious limitations in terms of cost efficiency, labour supply, and effectiveness for larger-scale populations [5,17,18]. For example, a blade plough (depth of 150–200 mm below ground level) is only effective on plants with a substantial root system, yet not too oversized for the capacity of the machinery [67]. The vigorous resprouting of roots or lignotubers is also highly likely following some types of mechanical disturbance (e.g., bulldozing), whereby the plant is severed at the base [16,22]. Therefore, the treatment of subsequent regrowth or exposed stems with synthetic herbicides is often needed [16,22].
In Australia, the estimated annual cost of woody weeds to the grazing industry is in the vicinity of AUD 12.3 billion [68]. Although a “trade-off” exists, the management of Z. mauritiana is largely very costly relative to the annual returns of rangeland environments (Table 2) [69,70]. A study by Zull et al. (2008) constructed an analytical framework for the optimal frequency of management by synthesising the complex relationships between population dynamics, direct weed costs, and the cost–benefit of different control methods [70]. This model suggested that mechanical means of management were largely uneconomic for Z. mauritiana, particularly in upland zones [70].
Table 2.
The variable, fixed, and total cost of four different control methods (i.e., no control, prescribed burning, synthetic chemicals, and mechanical) for Ziziphus mauritiana. The variable costs are density-dependent, whereby additional materials and labour are required for very dense infestations [69].
| Fixed Costs (ha−1) | Variable Costs (ha−1) | Total Cost (ha−1) | |
|---|---|---|---|
| No Control | AUD$0 | AUD$0 | AUD$0 |
| Burning | AUD$15 | AUD$0 | AUD$15 |
| Chemical | AUD$37.50 | AUD$112.50 | AUD$150 |
| Mechanical * | AUD$50 | AUD$50 | AUD$100 |
* Blade-Ploughing.
The encroachment of many invasive woody weeds can be controlled with “prescribed fire” either directly or as part of an integrated approach [71,72]. Some successful examples of this include rubber vine (C. grandiflora), bellyache bush (Jatropha gossypiifolia), mesquite (Prosopis spp.), parkinsonia (P. aculeata), lantana (L. camara), and prickly acacia (V. nilotica) [72]. A study by Grice (1997) investigated a controlled fire event on the survival and vegetative growth of Z. mauritiana in northern Queensland [58]. Although considerable mortality (>90%) has been previously observed in surface-located seeds [73], the survival rate of established plants was similar in burnt versus unburnt plots [58]. In fact, most plants resprouted vigorously within three months of fire treatment, even those of smaller height classes (<100 cm) [58]. Anecdotal reports suggest that they recover with increased vigour, similarly to other fire-tolerant woody weeds (e.g., calotrope: Calotropis procera and Gorse: Ulex europaeus). However, there is an opportunity for further research in the application of chemical defoliants following a single or repeated fire event [12,58,74].
6.2. Chemical Control Methods
Although costly, the basal or cut-stump application of synthetic auxin herbicides (Group 4: triclopyr, fluroxypyr, or picloram carried in diesel) is the most effective means for the aggressive eradication of higher density populations (>150 plants/ha) [5,16,60,61]. There are several chemical products registered for the management of Z. mauritiana in northern Australia [16,22,75,76], as shown in Table 3. In particular, the basal bark application of triclopyr (600 g/L), fluroxypyr (333 g/L), or a combination herbicide (triclopyr 240 g/L + picloram 120 g/L) is suitable for seedlings and juvenile plants in their active growth stage (Table 4) [16,22]. Alternatively, the same herbicide mixtures are also recommended for the treatment of cut-stumps at any time of year (Table 3) [16,22]. However, these methods may be constrained by the spiny lower branches or multi-stemmed character of some shrubs [22]. Therefore, a high-volume spray mixture in water (350 mL herbicide mixture to 100 L water) can be applied to actively growing juvenile or established plants (Table 3) [16,22].
Table 3.
The synthetic herbicides (trade names, active ingredient(s), and application rate) registered for the management of Ziziphus mauritiana in Queensland, Western Australia, and the Northern Territory [15,72,73].
| TRADE NAME | ACTIVE INGREDIENT(S) | APPLICATION RATE | ||
|---|---|---|---|---|
| BASAL BARK & CUT-STUMP APPLICATION |
Access® | Triclopyr (240 g/L) + Picloram (120 g/L) |
1 L/60 L Diesel | GROUP 4 (Disruptors of Plant Cell Growth) |
| Invader® 600 | Triclopyr (600 g/L) | 1 L/60 L Diesel | ||
| Garlon® 600 | ||||
| Redeem® 600 | ||||
| Acclaim® | Fluroxypyr (200 g/L) | 3 L/100 L Diesel | ||
| Flagship® 200 | ||||
| Starane® Advanced | Fluroxypyr (333 g/L) | 1.8 L/100 L Diesel | ||
| Comet® 400 | Fluroxypyr (400 g/L) | 1.5 L/100 L Diesel | ||
| Decoy® 400 | ||||
| HIGH VOLUME SPRAY |
Fightback® | Triclopyr (300 g/L) + Picloram (100 g/L) |
350 mL/100 L Water | |
| Conqueror® | ||||
| Grazon® Extra | Triclopyr (300 g/L) + Picloram (100 g/L) + Aminopyralid (8 g/L) |
350 mL/ 100 L Water | ||
| SOIL APPLICATION | TordonTM Granules | Picloram (20 g/kg) | 35 to 45 g/m2 |
Table 4.
A summary of the primary Ziziphus mauritiana reproductive events (i.e., flowering, fruiting, and germination) and their correlation with differing synthetic and manual management options in Northern Australia [16].
| JAN | FEB | MAR | APR | MAY | JUNE | JULY | AUG | SEP | OCT | NOV | DEC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FLOWERING |

|

|

|

|

|

|
||||||
| FRUITING |

|

|

|

|

|

|

|

|
||||
| GERMINATION |

|

|
||||||||||
| FOLIAR SPRAY |

|

|

|

|

|
|
|
|||||
| BASAL BARK |

|

|

|

|

|

|

|

|

|

|

|

|
| CUT-STUMP |

|

|

|

|

|

|

|

|

|

|

|

|
| MANUAL |
|
|
|
|
|
|
|
|

|

|

|

|
 Growth & Reproductive Events;
Growth & Reproductive Events;  Most Suitable Control Option;
Most Suitable Control Option;  Least Suitable Control Option.
Least Suitable Control Option.
The efficacy of synthetic herbicides is often influenced by the growth or reproductive cycles of the target species at the time of application [16,77]. For example, the sensitivity of some weeds is increased in the active reproductive stages (e.g., flowering or fruiting) [16] (Table 4). In this instance, the production of viable fruit is disrupted, thereby limiting the further spread and establishment of the weed (Table 4) [16]. Similarly, the treatment of seedlings or juvenile plants before the first seed set (e.g., the first two to five years) can inhibit critical metabolic and developmental processes within the plant (Table 4) [16]. The ideal time of varying herbicide application methods for Z. mauritiana is summarised in Table 4.
A proprietary stem implantation system has been developed by BioHerbicides Australia (BHA Pty Ltd.) for the encapsulated delivery of an endophytic fungal bioherbicide (Lasiodiplodia pseudotheobromae, Macrophomina phaseolina, and Neoscytalidium novaehollandiae) in parkinsonia (P. aculeata) [78,79,80]. This novel technology has since been expanded to the application of other endophytic organisms, as well as synthetic herbicide compounds available in dry formulations [5,79,81,82]. Several synthetic herbicide formulations have been trialed for the management of various invasive woody weeds: calotrope (C. procera) [81], camphor laurel (Cinnamomum camphora) [81], Chinese elm (Celtis sinensis) [18], leucaena (Leucaena leucocephala) [81], prickly acacia (V. nilotica) [81], and mimosa bush (Vachellia farnesiana) [82]. Recently, preliminary research was undertaken by O’Brien et al. (2022) on the compatibility of this technology with Z. mauritiana in rangeland environments [5]. The initial results are very promising, whereby three of the encapsulated synthetic herbicides (Di-Bak AM®: aminopyralid + metsulfuron-methyl, Di-Bak® M: metsulfuron-methyl, and Di-Bak P®: picloram) achieved a similar response to the “drill-and-fill” application of Tordon® RegrowthMaster (triclopyr, picloram, and aminopyralid) [5]. However, unlike its industry counterparts, a minimum recommended lethal dose is delivered directly into the vascular system, where the respective active ingredient is fully captured internally [5,79,81]. This targeted, readily calibrated herbicide application is associated with (1) a lowered active ingredient concentration (~20% to 30%), (2) a reduced likelihood of environmental exposure to plant protection compounds, and (3) improved safety for the human operator [79]. This technology could be a possible replacement for conventional methods of foliar or stem spraying, stem injection, or canopy application in environmentally sensitive habitats (e.g., tropical woodlands, native forestry, and riparian zones) [79].
6.3. Biological Control Methods
There is considerable interest in the exploitation of host-specific, natural enemies for limiting the vigour, competitiveness, and reproductive capacity of Z. mauritiana in northern Australia [3,7]. The development of a “bioherbicide” in lieu of their synthetic counterparts may foster a more resilient coexistence between agricultural systems and the natural environment owing to their reduced environmental persistence and increased target specificity [71,83]. It is noted that they are not a total replacement or “panacea” to conventional weed management [83]. Rather, they could be used concurrently with other control methods (e.g., synthetic herbicides or mechanical options) as part of a wider integrated approach with multiple modes of action [83].
Currently, Z. mauritiana has not been the focus of any biological control programs [3,7,22], although there is a plethora of published literature available on the pests and diseases of Ziziphus species [6]. In fact, Dhileepan (2017) explored the feasibility of biological control by cataloguing the phytophagous insects, mites, and pathogens of two wild Ziziphus species (Z. mauritiana and Z. jujuba) within their native range [7]. These opportunistic field surveys and literature searches identified a suite of prospective agents with differing feeding guilds, including the leaves, shoots, stem, fruit, and seed [7]. Of these, there were seven phytophagous arthropods (i.e., four leaf-feeding, two shoot-feeding, and one seed-feeding arthropods) with potential host specificity for Z. mauritiana (Table 5) [7]. In particular, a seed-feeding weevil (Aubeus himalayanus) and two shoot-galling mites (Aceria cernuus and Larvacarus transitans) were considered the most suitable biological control candidates for northern Australia [7].
Table 5.
The prospective agents with restricted host specificity identified by Dhileepan (2017) for the biological control of Ziziphus mauritiana in northern Australia [7].
| Prospective Agent(s) | PHYTOPHAGUS INSECTS | |
| Seed-Feeding Weevil | Aubeus himalayanus | |
| Leaf-Feeding Crambid Moth | Synclera univocalis | |
| Leaf-Feeding Gracillariid Moth | Phyllonorycter iochrysis | |
| Leaf-Galling Midge | Phyllodiplosis jujubae | |
| Stem-Galling Midge | Silvestrina jujubae | |
| PHYTOPHAGUS MITES | ||
| Shoot-Galling Mite | Aceria cernuus | |
| Shoot-Galling Mite | Larvacarus transitans | |
| FUNGAL PATHOGENS | ||
| Leaf Rust | Phakopsora zizyphi-vulgaris | |
| Powdery Mildew | Pseudoidium ziziphi | |
Among all the known fungal pathogens of Ziziphus species, leaf rust (Phakopsora zizyphi-vulgaris Diet.) and powdery mildew (Pseudoidium ziziphi) have the most restricted host specificity [7]. Autoecious rust (P. zizyphi-vulgaris) is a significant disease of commercial jujube (Z. jujuba) in China [84], associated with the formation of irregular reddish-brown pustules on the entire leaf surface and eventual defoliation [7,84]. In India, it has also been recorded in wild varieties of Z. mauritiana, wild jujube (Ziziphus nummularia), and jackal jujube (Ziziphus oenoplia) [7]. The latter is a native tree of environmental significance in northern Australia, and therefore P. zizyphi-vulgaris is an unsuitable candidate for biological control [7]. There are only two recorded hosts (Z. mauritiana and Z. nummularia) of powdery mildew (P. ziziphi) in India, Pakistan, and Bangladesh [7,84]. The younger leaves are often covered in a white powdery mass followed by shrinking and eventual defoliation [84,85]. The fruits are also significantly affected (yield loss of ~50% to 60%), whereby they become corky, cracked, or underdeveloped [84,85]. Of the two diseases, powdery mildew should be the focus of future surveys based on its host specificity and severity [7].
There is no current information on the susceptibility of various plant parts and growth stages (i.e., seedlings, juvenile, or adult plants) of Z. mauritiana to herbivory or diseases [7]. Furthermore, the literature available on its natural enemies is focused mostly on cultivated trees, and further research should be undertaken on the surveying of wild Z. mauritiana [7]. There are also two native Ziziphus species (Ziziphus quadrilocularis and Z. oenoplia) of environmental significance in northern Australia [7]. Therefore, the host specificity of any prospective agent(s) should be reviewed extensively to avoid deleterious impacts on valuable native flora [7].
7. Conclusions
The recent literature is heavily focused on domesticated Ziziphus species (Z. mauritiana and Z. jujuba) as a potential economic source in salt-affected or water-stressed environments [6,20,21,86]. Despite its overt status as a problematic weed, there has been comparatively little research undertaken on its invasiveness or related ecological factors in other landscapes [13]. The management of Z. mauritiana is currently restricted to the application of synthetic herbicides or mechanical clearing operations [3,5,7]. However, they have limited effectiveness individually, and a more integrated weed management (IWM) approach is recommended [83,87]. There is also considerable interest in the expansion of control options through the exploration of host-specific, natural enemies for limiting the vigour, competitiveness, and reproductive capacity of many woody weeds, including Z. mauritiana [3,7]. To move forward with these biological control efforts, it is essential for future research to delve into the introduction history of Z. mauritiana in Australia [7]. The genetic diversity and relatedness of differing populations or biotypes (native, naturalised, and invasive ranges) should also be determined [7]. Furthermore, a species distribution model (SDMs: CLIMEX or MaxEnt) of the other northern states in Australia (i.e., Western Australia and the Northern Territory) is recommended to assess the relative climatic suitability of specific locations for the exploration or targeted establishment of biological control agents [7,53,54,55]. Although not yet published, the pathogenicity of several fungal endophytes recovered from healthy and diseased populations of Z. mauritiana in northern Queensland is under current assessment as prospective “bioherbicide” candidates.
Author Contributions
Conceptualization, C.J.O., S.C. and V.J.G.; investigation, C.J.O.; data curation, C.J.O., S.C.; writing—original draft preparation, C.J.O.; writing—review and editing, S.C., A.Y., W.V. and V.J.G.; supervision, S.C., A.Y., W.V. and V.J.G.; funding acquisition, V.J.G. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Grice A.C. Seed Production, Dispersal and Germination in Cryptostegia grandiflora and Ziziphus mauritiana, Two Invasive Shrubs in Tropical Woodlands of Northern Australia. Aust. J. Ecol. 1996;21:324–331. doi: 10.1111/j.1442-9993.1996.tb00615.x. [DOI] [Google Scholar]
- 2.Grice A.C., Radford I.J., Abbott B.N. Regional and Landscape-Scale Patterns of Shrub Invasion in Tropical Savannas. Biol. Invasions. 2000;2:187–205. doi: 10.1023/A:1010021515356. [DOI] [Google Scholar]
- 3.Bebawi F.F., Campbell S.D., Mayer R.J. Seed Bank Persistence and Germination of Chinee Apple (Ziziphus mauritiana Lam.) Rangel. J. 2016;38:17–25. doi: 10.1071/RJ15104. [DOI] [Google Scholar]
- 4.Liang T., Sun W., Ren H., Ahmad I., Vu N., Huang M., Huang J. Genetic Diversity of Ziziphus mauritiana Germplasm Based on SSR Markers and Ploidy Level Estimation. Planta. 2019;249:1875–1887. doi: 10.1007/s00425-019-03133-2. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien C.O., Campbell S., Vogler W., Galea V.J. Evaluation of Di-Bak® Herbicide Capsule System for Control of Chinee Apple (Ziziphus mauritiana) in North Queensland; Proceedings of the 22nd Australasian Weeds Conference; Adelaide, SA, Australia. 25–29 September 2022; Adelaide, SA, Australia: Council of Australasian Weed Societies Inc. (CAWS); 2022. [Google Scholar]
- 6.Muhammad N., Luo Z., Yang M., Liu Z., Liu M. The Nutritional, Medicinal, and Drought-Resistance Properties of Ziziphus Mill. Make It an Important Candidate for Alleviating Food Insecurity in Arid Regions—A Case of Pakistan. Horticulturae. 2022;8:867. doi: 10.3390/horticulturae8100867. [DOI] [Google Scholar]
- 7.Dhileepan K. Biological Control of Ziziphus mauritiana (Rhamnaceae): Feasibility, Prospective Agents, and Research Gaps. Ann. Appl. Biol. 2017;170:287–300. doi: 10.1111/aab.12338. [DOI] [Google Scholar]
- 8.Nyoka B.I. Biosecurity in Forestry: A Case Study on the Status of Invasive Forest Species in South Africa. Forestry Department of Food and Agriculture Organisation (FAO); Rome, Italy: 2003. [Google Scholar]
- 9.Weber E. Invasive Plant Species of the World: A Reference Guide to Environmental Weeds. 2nd ed. CABI International; Wallingford, UK: 2003. p. 548. [Google Scholar]
- 10.Grice A.C. Weeds and the Monitoring of Biodiversity in Australian Rangelands. Austral Ecol. 2004;29:51–58. doi: 10.1111/j.1442-9993.2004.01364.x. [DOI] [Google Scholar]
- 11.Ani O., Onu O., Okoro G., Uguru M. Chapter 2: Overview of Biological Methods of Weed Control. In: Radhakrishnan R., editor. Biological Approaches for Controlling Weeds. InTechOpen; London, England: 2018. pp. 5–16. [Google Scholar]
- 12.Vitelli J.S. Options for Effective Weed Management. Trop. Grassl. 2000;34:280–294. [Google Scholar]
- 13.Grice A.C. Ecology in the Management of Indian Jujube (Ziziphus mauritiana) Weed Sci. 1998;46:467–474. doi: 10.1017/S0043174500090913. [DOI] [Google Scholar]
- 14.Ward-Fear G., Brown G.P., Pearson D.J., Shine R. An Invasive Tree Facilitates the Persistence of Native Rodents on an Over-Grazed Floodplain in Tropical Australia. Austral Ecol. 2017;42:385–393. doi: 10.1111/aec.12454. [DOI] [Google Scholar]
- 15.Laguna J.M., Reside A.E., Kutt A., Grice A.C., Buosi P., Vanderduys E.P., Taylor M., Schwarzkopf L. Conserving the Endangered Black-Throated Finch Southern Subspecies: What Do We Need to Know? Emu—Austral Ornithol. 2019;119:331–345. doi: 10.1080/01584197.2019.1605830. [DOI] [Google Scholar]
- 16.Northern Territory Department of Environment, Parks and Water Security Chinee Apple Management Guide. [(accessed on 9 August 2020)]; Available online: https://nt.gov.au/__data/assets/pdf_file/0019/231418/chinee-apple-management-guide.pdf.
- 17.Abbas T., Zahir Z.A., Naveed M., Kremer R.J. Limitations of Existing Weed Control Practices Necessitate Development of Alternative Techniques Based on Biological Approaches. Adv. Agron. 2018;147:239–280. doi: 10.1016/bs.agron.2017.10.005. [DOI] [Google Scholar]
- 18.Moss S.R. Non-Chemical Methods for Weed Control: Benefits and Limitations; Proceedings of the 17th Australasian Weeds Conference; Christchurch, New Zealand. 26–30 September 2010; Karekare, New Zealand: New Zealand Plant Protection Society; 2010. [Google Scholar]
- 19.O’Brien C.J., Mellor V., Galea V.J. Controlling Woody Weed Chinese Elm (Celtis sinensis Pers.) with Stem-Implanted Herbicide Capsules. Plants. 2022;11:444. doi: 10.3390/plants11030444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marzuza I.M., Musemwa L., Mapurazi S., Matsika P., Munyati V.T., Ndhleve S. Future Prospects of Ziziphus mauritiana in Alleviating Household Food Insecurity and Illness in Arid and Semi-Arid Areas: A Review. World Dev. Perspect. 2017;5:1–6. doi: 10.1016/j.wdp.2017.01.001. [DOI] [Google Scholar]
- 21.Sharif M., Naz A., Farooq U., Razzaq K., Sher M.A., Khan M.A., Waheed U., Hayder Q., Naz N., Ahmad S., et al. A Comprehensive Investigation of Novel Ber (Ziziphus mauritiana) Products From South Punjab, Pakistan. J. Bioresour. Manag. 2022;9:15–31. [Google Scholar]
- 22.Grice A.C. The Biology of Australian Weeds—39. Ziziphus mauritiana Lam. Plant Prot. Q. 2002;17:2–11. [Google Scholar]
- 23.Meghwal P.R., Khan M.A., Tewari J.C. Growing Ber (Ziziphus mauritiana Lam.) for Sustainable Income and Employment in Arid and Semi-Arid Regions. ICAR—Central Arid Zone Research Institute (CAZRI); Jodhpur, India: 2007. [Google Scholar]
- 24.Janick J., Paull R.E. The Encyclopaedia of Fruit and Nuts. CABI; Oxfordshire, UK: 2008. p. 617. [Google Scholar]
- 25.Mishra T., Paice A.G., Bhatia A. Chapter 87: Use of Seeds of Malay Apple (Ziziphus mauritiana) and Related Species in Health and Disease. In: Preedy V.R., Watson R.R., Patel V.B., editors. Nuts and Seeds in Health and Disease Prevention. Academic Press; Cambridge, MA, USA: 2011. pp. 733–739. [Google Scholar]
- 26.Pareek S., Yahia E.M. Postharvest Biology and Technology of Ber Fruit. In: Janick J., editor. Horticultural Reviews. John Wiley & Sons; Hoboken, NJ, USA: 2013. pp. 201–231. [Google Scholar]
- 27.Tel-Zur N., Schneider B. Floral Biology of Ziziphus mauritiana (Rhamnaceae) Sex. Plant Reprod. 2009;22:73–85. doi: 10.1007/s00497-009-0093-4. [DOI] [PubMed] [Google Scholar]
- 28.Pareek S., Mukherjee S., Paliwal R. Floral Biology of Ber—A Review. Agric. Revolut. 2007;28:277–282. [Google Scholar]
- 29.Verma S.S., Verma R.P., Verma S.K., Yadav A.L., Verma A.K. Responses of Ber (Ziziphus mauritiana Lamk.) Varieties to Different Level of Salinity. Int. J. Curr. Microbiol. App. Sci. 2018;7:580–591. doi: 10.20546/ijcmas.2018.707.071. [DOI] [Google Scholar]
- 30.Clifford S.C., Arndt S.K., Corlett J.E., Joshi S., Sankhla N., Popp M., Jones H.G. The Role of Solute Accumulation, Osmotic Adjustment and Changes in Cell Wall Elasticity in Drought Tolerance in Ziziphus mauritiana (Lamk.) J. Exp. Bot. 1998;49:967–977. doi: 10.1093/jxb/49.323.967. [DOI] [Google Scholar]
- 31.Bhatt M.J., Patel A.D., Bhatti P.M., Pandey A.N. Effect of Soil Salinity on Growth, Water Status and Nutrient Accumulation in Seedlings of Ziziphus mauritiana (Rhamnaceae) J. Fruit Ornam. Plant Res. 2008;16:383–401. [Google Scholar]
- 32.Prakash O., Usmani S., Singh R., Singh N., Gupta A., Ved A. A Panoramic View on Phytochemical, Nutritional and Therapeutic Attributes of Ziziphus mauritiana Lam.: A Comprehensive Review. Phytother. Res. 2020;35:63–77. doi: 10.1002/ptr.6769. [DOI] [PubMed] [Google Scholar]
- 33.Naaz F., Agari N., Singh A. Medicinal Properties of Ziziphus mauritiana: A Review Article. Int. J. Pharm. Pharm. Res. 2020;19:219–230. [Google Scholar]
- 34.Dahiru D., Sini J.M., John-Africa L. Antidiarrheal Activity of Ziziphus mauritiana Root Extract in Rodents. Afr. J. Biotechnol. 2006;5:941–945. [Google Scholar]
- 35.Maaiden E.E., Kharrassi Y.E., Qarah N.A.S., Essamadi A.K., Moustaid K., Nasser B. Genus Ziziphus: A Comprehensive Review on Ethnopharmacological, Phyto-chemical and Pharmacological Properties. J. Ethnopharmacol. 2020;259:112950. doi: 10.1016/j.jep.2020.112950. [DOI] [PubMed] [Google Scholar]
- 36.Butt S.Z., Hussain S., Munawar K.S. Phytochemistry of Ziziphus mauritiana; Its Nutritional and Pharmaceutical Potential. Sci Inq. Rev. 2021;5:1–15. doi: 10.32350/sir/52. [DOI] [Google Scholar]
- 37.Morton J. Indian Jujube. In: Morton J.F., editor. Fruits of Warm Climates. Echo Point Books & Media; Brattleboro, VT, USA: 1987. pp. 272–275. [Google Scholar]
- 38.Spinoni J., Barbosa P., Cherlet M., Forzieri G., McCormick N., Naumann G., Vogt J.V., Dosio A. How Will the Progressive Global Increase of Arid Areas Affect Population and Land-Use in the 21st Century? Glob Planet Change. 2021;205:103597. doi: 10.1016/j.gloplacha.2021.103597. [DOI] [Google Scholar]
- 39.Crawford R., Shan F., McCarthy A. Chinese Jujube: A Developing Industry in Australia. Acta Hortic. 2013;993:29–36. doi: 10.17660/ActaHortic.2013.993.3. [DOI] [Google Scholar]
- 40.Johnstone R. Development of the Chinese Jujube Industry in Australia. Australian Government Rural Industries Research and Development Corporation; Barton, ACT, Australia: 2014. [Google Scholar]
- 41.Johnstone R.M., Shan F. Chinese Jujube Industry Takes Root in Western Australia. Acta Hortic. 2016;1116:31–34. doi: 10.17660/ActaHortic.2016.1116.5. [DOI] [Google Scholar]
- 42.Johnstone R. Overcoming Barriers to Development of the Australian Jujube Industry. AgriFutures Australia; New South Wales, Australia: 2017. [Google Scholar]
- 43.ABC Landline Jujube Expansion: Australia’s Biggest Kiwi Grower Invests in Jujubes. [(accessed on 12 July 2023)]. Available online: https://www.abc.net.au/news/rural/programs/landline/2023-05-07/jujube-expansion:-australias-biggest-kiwi-fruit/102314704.
- 44.Pasieznik N. Ziziphus Mauritiana (Jujube) CABI Compendium; Wallingford, UK: 2007. [Google Scholar]
- 45.Dight I. Burdekin Water Quality Improvement Plan. Burdekin Solutions Ltd.; Townsville, Australia: 2009. [Google Scholar]
- 46.Arndt S.K., Wanek W., Clifford S.C., Popp M. Contrasting Adaptations to Drought Stress in Field-Grown Ziziphus mauritiana and Prunus persica Trees: Water Relations, Osmotic Adjustment and Carbon Isotope Composition. Aust. J. Plant Physiol. 2000;27:985–996. doi: 10.1071/PP00022. [DOI] [Google Scholar]
- 47.Atlas of Florida Plants: Institute for Systematic Botany Ziziphus mauritiana. [(accessed on 3 May 2023)]. Available online: https://florida.plantatlas.usf.edu/Plant.aspx?id=255.
- 48.CABI . Forestry Compendium. CABI; Wallingford, UK: 2005. [Google Scholar]
- 49.Orwa C., Mutua A., Kindt R., Jamnadass R., Simons A. Agroforestree Database: A Tree Reference and Selection Guide (Version 4.0) World Agroforestry Centre; Kenya, Africa: 2009. [Google Scholar]
- 50.Pacific Islands Ecosystems at Risk (PIER) [(accessed on 5 May 2023)]. Available online: https://www.hear.org.au/pier/index.html.
- 51.Atlas of Living Australia (ALA) [(accessed on 6 May 2023)]. Available online: https://biocache.ala.org.au/occurrences/search?q=lsid%3Ahttps%3A%2F%2Fid.biodiversity.org.au%2Fnode%2Fapni%2F2894258#tab_mapView.
- 52.Pest Management Strategy. [(accessed on 8 May 2023)]; Available online: https://www.daf.qld.gov.au/__data/assets/pdf_file/0007/50389/IPA-Chinee-Apple-PM.pdf.
- 53.Kriticos D.J., Morin L., Leriche A., Anderson R.C., Caley P. Combining a Climatic Niche Model of an Invasive Fungus with its Host Species Distributions to Identify Risks to Natural Assets: Puccinia psidii Sensu Lato in Australia. PLoS ONE. 2013;8:e64479. doi: 10.1371/journal.pone.0064479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Byeon D.H., Jung S., Lee W.H. Review of CLIMEX and MaxEnt for Studying Species Distribution in South Korea. J. Asia-Pac. Biodivers. 2018;11:325–333. doi: 10.1016/j.japb.2018.06.002. [DOI] [Google Scholar]
- 55.Kour S., Khurma U., Brodie G., Singh S. Modelling the Potential Global Distribution of Suitable Habitat for the Biological Control Agent Heterorhabditis indica. Ecol. Evol. 2022;12:e8997. doi: 10.1002/ece3.8997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones R., Alemseged Y., Medd R., Vere D. The Distribution, Density and Economic Impact of Weeds in the Australian Annual Winter Cropping System. CRC for Weed Management Systems; Glen Osmond, Australia: 2000. [Google Scholar]
- 57.Walker P.T. Crop Losses: The Need to Quantify the Effect of Pests, Disease and Weeds on Agricultural Production. Agric Ecosyst Env. 1983;9:119–158. doi: 10.1016/0167-8809(83)90038-5. [DOI] [Google Scholar]
- 58.Grice A.C. Post-Fire Regrowth and Survival of the Invasive Tropical Shrubs Cryptostegia grandiflora and Ziziphus mauritiana. Aust. J. Ecol. 1997;22:49–55. doi: 10.1111/j.1442-9993.1997.tb00640.x. [DOI] [Google Scholar]
- 59.Queensland Department of Agriculture and Fisheries Restricted Plants of Queensland. [(accessed on 16 June 2023)]; Available online: https://www.daf.qld.gov.au/__data/assets/pdf_file/0004/383818/restricted-invasive-plants.pdf.
- 60.Northern Territory Department of Environment, Parks and Water Security Weed Management Plan for Chinee Apple 2012–2022. [(accessed on 16 June 2023)]; Available online: https://nt.gov.au/__data/assets/pdf_file/0020/231419/chinee-apple-weed-management-plan.PDF.
- 61.Western Australia Department of Primary Industries and Regional Development Ziziphus mauritiana Lam. [(accessed on 16 June 2023)]; Available online: https://www.agric.wa.gov.au/organisms/119159.
- 62.Queensland Government Area Management Plans (AMPs) [(accessed on 14 August 2023)]; Available online: https://www.qld.gov.au/environment/land/management/vegetation/clearing-approvals/area-management-plans.
- 63.Queensland Government Legislation: Vegetation Management Act 1999. [(accessed on 14 August 2023)]; Available online: https://www.stateoftheenvironment.des.qld.gov.au/biodiversity/management-responses/legislation/vegetation-management-act-1999#:~:text=The%20Vegetation%20Management%20Act%201999,prevents%20loss%20of%20biodiversity.
- 64.Queensland Department of Natural Resources and Mines . Area Management Plan for the Control of Pest Plants in the Dry Tropics Region. Queensland Government; Brisbane, Australia: 2015. [Google Scholar]
- 65.Martin-Forés I., Guerin G.R., Lowe A.J. Weed Abundance is Positively Correlated With Native Plant Diversity in Grasslands of Southern Australia. PLoS ONE. 2017;12:e0178681. doi: 10.1371/journal.pone.0178681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fridley J.D., Stachowicz J.J., Naeem S., Sax D.F., Seabloom E.W., Smith M.D., Stohlgren T.J., Tilman D., Von Holle B. The Invasion Paradox: Reconciling Pattern and Process in Species Invasions. Ecology. 2007;88:3–17. doi: 10.1890/0012-9658(2007)88[3:TIPRPA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 67.Zull A., Cacho O., Lawes R. A Matrix Model for the Management of Perennial Weeds in the North Queensland Rangelands System: Application to Ziziphus mauritiana (Lam); Proceedings of the 15th Australian Weeds Conference; Adelaide, SA, Australia. 24–28 September 2006; Torrens Park, SA, Australia: Weed Management Society of South Australia; 2006. pp. 671–674. [Google Scholar]
- 68.Zull A.F., Cacho O.J., Lawes R.A. Optimising the Control of Rangeland Woody Weeds; Proceedings of the 17th Australasian Weeds Conference; Christchurch, New Zealand. 26–30 September 2010; Karekare, New Zealand: New Zealand Plant Protection Society; 2010. pp. 414–417. [Google Scholar]
- 69.Zull A.F., Cacho O.J., Lawes R.A. Optimising Woody-Weed Control; Proceedings of the 53rd Australian Agricultural and Resource Economics Society (AARES) Annual Conference; Cairns, Queensland, Australia. 11–13 February 2009; Vermont, VIC, Australian: Australian Agricultural and Resource Economics Society; 2009. [Google Scholar]
- 70.Zull A.F., Lawes R.A., Cacho O.J. Optimal Frequency for Woody Weed Management for North Queensland Grazing Properties: An Economic Perspective; Proceedings of the 16th Australian Weeds Conference; Cairns, Queensland, Australia. 18–22 May 2008; Clifford Gardens, QLD, Australian: Queensland Weed Society; 2008. [Google Scholar]
- 71.DiTomaso J.M., Brooks M.L., Allen E.B., Minnich R., Rice P.M., Kyser G.B. Control of Invasive Weeds with Prescribed Burning. Weed Technol. 2006;20:535–548. doi: 10.1614/WT-05-086R1.1. [DOI] [Google Scholar]
- 72.Queensland Department of Agriculture, Fisheries and Forestry Fire: Management of Native and Invasive Woody Weeds in the Northern and Southern Gulf. [(accessed on 20 June 2023)]. Available online: https://futurebeef.com.au/wp-content/uploads/fire_factsheet_web1.pdf.
- 73.Grice A.C., Brown J.R. Fire and Population Ecology of Invasive Shrubs in the Tropical Woodlands. In: Floyd R.B., Sheppard A.W., De Barro P.J., editors. Frontiers of Population Biology, Canberra. CSIRO Publishing; Melbourne, Australia: 1996. pp. 589–597. [Google Scholar]
- 74.Nobel J.C., Grice A.C., MacLeod N.D., Mulle W.J. Integration of Prescribed Fire and Sub-Lethal Chemical Defoliation for Controlling Shrub Populations in Australian Semi-Arid Woodlands; Proceedings of the First International Weed Control Conference; Monash University, Melbourne, Australia. 17–21 February 1992; pp. 362–364. [Google Scholar]
- 75.Queensland Government Chinee Apple (Ziziphus mauritiana) [(accessed on 23 June 2023)]; Available online: https://www.daf.qld.gov.au/__data/assets/pdf_file/0008/52766/chinee-apple.pdf.
- 76.Western Australia Department of Primary Industries and Regional Development Chinee Apple Control. [(accessed on 23 June 2023)]; Available online: https://www.agric.wa.gov.au/herbicides/chinee-apple-control.
- 77.Chauhan B.S., Abugho S.B. Effect of Growth Stage on the Efficacy of Postemergence Herbicides on Four Weed Species of Direct-Seeded Rice. Sci. World J. 2012;2012:123071. doi: 10.1100/2012/123071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Galea V.J. Use of Stem Implanted Bioherbicide Capsules to Manage an Infestation of Parkinsonia aculeata in Northern Australia. Plants. 2021;10:1909. doi: 10.3390/plants10091909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Galea V.J. Stem-Implanted Capsules—A Targeted, Efficient and Sustainable Method for Delivery of Synthetic and Biological Agents to Plantation Crops; Proceedings of the 2nd International Plantation Conference (IPC2021); Online. 9–10 March 2021; Shah Alam, Malaysia: Faculty of Plantation and Agrotechnology, Universiti Teknologi MARA; 2021. [Google Scholar]
- 80.Morin L. Progress in Biological Control of Weeds with Plant Pathogens. Annu. Rev. Phytopathol. 2020;58:201–223. doi: 10.1146/annurev-phyto-010820-012823. [DOI] [PubMed] [Google Scholar]
- 81.Goulter K.C., Galea V.J., Riikonen P. Encapsulated Dry Herbicides: A Novel Approach for Control of Trees; Proceedings of the 21st Australasian Weeds Conference; Sydney, New South Wales, Australia. 9–13 September 2018; Muswellbrook, NSW, Australian: The Weed Society of New South Wales Inc.; 2018. [Google Scholar]
- 82.Limbongan A.A., Campbell S.D., Galea V.J. Novel Encapsulated Herbicide Delivery Mechanism: Its Efficacy in Mimosa Bush (Vachellia farnesiana) Plants. 2021;10:2505. doi: 10.3390/plants10112505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hasan M., Ahmad-Hamdani M.S., Rosli A.M., Hamdan H. Bioherbicides: An Eco-Friendly Tool for Sustainable Weed Management. Plants. 2021;10:1212. doi: 10.3390/plants10061212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mirzaee M.R. An Overview of Jujube (Ziziphus jujuba) Diseases. Arch. Phytopathol. 2014;47:82–89. doi: 10.1080/03235408.2013.804238. [DOI] [Google Scholar]
- 85.Jamadar M.M., Balikai R.A., Sataraddi A.R. Status of Diseases on Ber (Ziziphus mauritiana Lamarck) in India and their Management Options. Acta Hortic. 2009;840:383–390. doi: 10.17660/ActaHortic.2009.840.53. [DOI] [Google Scholar]
- 86.Mungate P.N.P., Masocha M., Dube T. Modelling the Distribution of the Invasive Ziziphus mauritiana Along Road Corridors. Afr. J. Ecol. 2018;57:122–129. doi: 10.1111/aje.12572. [DOI] [Google Scholar]
- 87.Harding D.P., Raizada M.N. Controlling Weeds with Fungi, Bacteria and Viruses: A Review. Front. Plant. Sci. 2015;6:659. doi: 10.3389/fpls.2015.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.