Abstract
In this work we describe a straightforward approach for creating a nanocomposite comprising multiwalled carbon nanotubes (MWCNTs) and titanium dioxide (TiO2) using the hydrothermal technique, which is then characterized by scanning electron microscope (SEM), energy-dispersive X-ray spectrometer (EDS), X-ray diffraction analysis (XRD), Fourier transform infrared spectroscopy (FTIR), and thermal gravimetric analysis (TGA) to assess its properties. Nafion is employed as a reticular agent for the nanocomposite on the glassy carbon electrode (GCE), creating the MWCNT/TiO2/Nafion/GCE system. The electrochemical behavior of the system was evaluated using cyclic voltammetry, revealing its remarkable electrocatalytic activity for detecting hydrogen peroxide in water. The developed sensor showcased a broad linear response range of 14.00 to 120.00 μM, with a low detection limit of 4.00 μM. This electrochemical sensor provides a simple and highly sensitive method for detecting hydrogen peroxide in aqueous solutions and shows promising potential for various real-world applications, particularly in H2O2 monitoring.
Keywords: hydrogen peroxide, electrochemical sensor, nanocomposite, cyclic voltammetry, TiO2, MWCNTs
1. Introduction
Hydrogen peroxide (H2O2) is a simple but significant compound in various applications, such as pharmaceuticals, clinical, environmental, mining, textiles, and food manufacturing. H2O2 is a signaling molecule regulating essential biological processes, such as immune cell activation, vascular remodeling, and apoptosis. H2O2 is a secondary product of various enzymatic reactions [1,2,3,4]. Due to its importance as a regulating molecule, hydrogen peroxide is an excellent model molecule for applications in developing new electrochemical sensors.
Electrochemical methods are the most promising for detecting H2O2 because of their advantages, such as easy miniaturization, rapid response, simple instrumentation, and high specificity and sensitivity. The natural concentration of H2O2 varies from micromolar (µM) to tens of millimolar (mM) [5]. While various techniques exist for H2O2 determination, such as chromatography [6], chemiluminescence [7], colorimetry [8], and titrimetric analysis [8], these methods are often complex, expensive, and time consuming. In recent years, many electrochemical approaches have been developed to determine low concentrations of H2O2 in a high-throughput fashion in fast, simple, reliable, and inexpensive ways [8,9,10].
Electrochemical sensors are suitable for various matrices, require minimal sample preparation, and exhibit high sensitivity and a wide concentration range with low limits of detection [11,12]. Enzymatic and non-enzymatic sensors have been developed for detecting H2O2 [13,14]. Enzymatic approaches exhibit excellent selectivity and sensitivity but lack stability, require complex and expensive immobilization processes, and are highly dependent on experimental conditions. Therefore, developing non-enzymatic electrochemical H2O2 sensors is very interesting for various biomedical, industrial, and academic applications. Non-enzymatic electrochemical sensors have been developed by chemically modifying electrodes with nanomaterials, such as nanoparticles of noble metals, transition metals, metallic oxides/hydroxides, bimetallics/alloys, and carbon nanomaterials (CNT, graphene and its compounds) [5,15,16].
Among the promising metals for developing nanomaterials, titanium dioxide stands out. Titanium dioxide (TiO2) is a transition metal oxide belonging to the category of n-type semiconductors, with its bandgap energy ranging from 2.9–3.2 eV [17], depending on the crystal phase [18]. Due to its high electrochemical activity, excellent mechanical and chemical stability, and exceptional capacity for organic molecule adsorption, TiO2 is widely used in electrochemical sensors [19,20]. However, to further improve the sensing performance of TiO2, researchers are working on forming a hybrid structure with different materials with diverse functional properties. One such material extensively studied in electrochemical applications is multi-walled carbon nanotubes (MWCNTs), which possess remarkable physical and chemical properties, such as high electrical conductivity, large surface area, and high energy storage capacity [21]. Previous investigations have shown that modifying electrodes with MWCNTs substantially enhances the rates of electron and proton transfer, resulting in superior peak separation and enhanced electrode sensitivity compared to other types of modified electrodes [22,23]. Additionally, the anchoring of semiconductors’ metal oxide particles onto MWCNTs with homogenous distribution has exhibited improved electronic properties, making it a promising platform for selective sensing and catalytic processes.
Among various nanocomposites, the TiO2/MWCNTs nanostructures [24] and other notable composites, such as TiO2/MWCNTs/Pt [25,26,27], Au@TiO2/MWCNT [28], ZnS/Au10/f-MWCNT [29], and PB-TiO2/CNT [30], have been reported to exhibit exceptional electrocatalytic activity toward hydrogen peroxide (H2O2) and other analytes in biosensing applications. However, it should be mentioned that the growth of complex nanostructures and deposition of catalytic nanoparticles, as previously demonstrated in the literature, typically requires sophisticated techniques such as electrochemical anodization, electrochemical deposition, or photo-induced deposition. Although these techniques have enabled the creation of intricate nanostructures, they can be both time consuming and resource intensive [31].
In this context, the sol–gel technique combined with hydrothermal synthesis has emerged as a promising approach for the preparation of nanometer-sized particles and materials with high specific surface area [32]. TiO2/MWCNT nanocomposites can be prepared using this technique, which shows potential for developing chemical and electrochemical sensors or promoting novel applications in biosensing [33] and photodetection [33] thanks to its unique three-dimensional network texture, high surface area, and exceptional electrocatalytic activity.
This study presents a novel sensor for detecting H2O2 that employs a TiO2/MWCNT/Nafion nanocomposite as a surface modifier for a glassy carbon electrode (GCE) in conjunction with cyclic voltammetry (CV). The sensor exhibits high sensitivity and a low detection potential for H2O2. Notably, this is the first report on the use of TiO2/MWCNT/Nafion on GCE for electrochemical H2O2 detection in water using CV. Our findings indicate that the TiO2/MWCNT/Nafion nanocomposite holds promise for developing high-performance H2O2 sensors with a facile synthesis process, making it a viable candidate for creating sensors for various clinical and environmental applications.
2. Materials and Methods
2.1. Materials
All chemical reagents used in this study were not subjected to additional purification. Titanium (IV) isopropoxide (TiP, 97.00%) and hydrochloric acid (HCl, 36.00%) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Absolute ethanol (99.5%, CRQ Produtos Químicos, Diadema, Brazil), isopropanol (Sciavicco Comércio e Indústria Ltda, Belo Horizonte, Brazil), and sodium hydroxide micropellets (NaOH, Cromoline Química Fina, Diadema, Brazil). Multi-walled carbon nanotubes (MWCNTs) with an outer diameter of 8–25 nm and length ranging from 5.00 to 30.00 µm and a purity of ≥93% were provided by the Nanomaterials Laboratory of the Department of Physics, UFMG. Nafion-117 was purchased from Sigma-Aldrich (Oakville, ON, USA).
2.2. Synthesis of TiO2 Particles
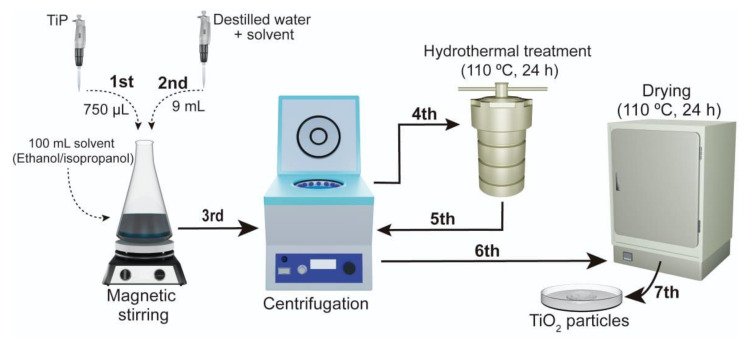
The TiO2 particles were prepared with modifications by the sol–gel method proposed by Ferreira-Neto, Elias et al. [20], represented in Figure 1. Initially, 750.00 μL of titanium isopropoxide (TiP) was slowly added to 100.00 mL of a mixture composed of ethanol-isopropanol [3:1 (v/v)] under magnetic stirring, which was maintained for three hours.
Figure 1.
Diagram of preparation of TiO2 nanoparticles by combined sol–gel and hydrothermal methods.
Subsequently, 9.00 mL of a mixture of deionized water and solvent (3.00 mL H2O:6.00 mL of the ethanol-isopropanol solvent) were added dropwise to form TiO2 particles through hydrolysis and condensation of titanium alkoxide species. After 2 h of magnetic stirring, the resulting colloidal suspension was centrifuged at 3500 rpm for 5 min and washed once with the solvent used in the reaction.
To crystallize the amorphous TiO2, the obtained material was resuspended in 32.00 mL of deionized water, then transferred to a 35.00 mL hermetic Teflon reactor and subjected to hydrothermal treatment at 110 °C for 24 h inside a flanged stainless steel hydrothermal reactor. After the hydrothermal treatment, the sample was centrifuged and washed twice with deionized water. Finally, the resulting precipitates were dried at 80 °C in an oven for 12 h.
2.3. Synthesis of TiO2/MWCNT Nanocomposite
The nanocomposite synthesis was adapted from the method Patel B.R. et al. proposed [34]. In this modified method, depicted in Figure 2, 100.00 mg of TiO2 particles obtained in the previous step were combined with 50 mg of MWCNTs and added to 10.00 mL of 10 M NaOH solution in a microtube with a screw cap. The resulting mixture was sonicated for 30 min at 60 °C to ensure proper dispersion of the particles. Next, the mixture was transferred to an autoclave container made of stainless steel, lined with Teflon material, and subjected to hydrothermal treatment at 130 °C for 24 h.
Figure 2.
Schematic diagram of the preparation of TiO2/MWCNT nanocomposite by hydrothermal method.
After cooling to room temperature, the nanocomposite was washed with 1 L of ultrapure water to remove impurities and unreacted species. The sample was then neutralized to a pH of 7.00 using a 0.50 M HCl solution. To ensure the complete removal of impurities, the nanocomposites were subjected to centrifugation at 5000 rpm with adding 0.50 L of ultrapure water. This washing step was repeated three times.
Finally, the nanocomposite was dried at 80 °C for 12 h to obtain a dry powder. The obtained material was named TiO2/MWCNT and was used for further studies.
2.4. Materials Characterization
X-ray diffraction analysis (XRD) has been carried out on the Shimadzu diffractometer (model 6100; Kyoto, Japan) using a Co Kα radiation source (λ = 1.788 Å, 40 kV/30 mA). Customary conditions included a 2θ scan from 10° to 70°, a 0.02° angular step, and a 1°/min scan speed. Data were converted to the Cu Kα wavelength using PowDLL software v 2.7 [35]. The crystallographic phase identification was carried out by comparing the obtained patterns with JCPDS standards.
The samples’ chemical structure and surface functional groups were analyzed using a Jasco FT/IR-4100 Fourier transform infrared spectrometer (FTIR) (Tokyo, Japan). The IR spectrum was recorded within the wavelength range of 4000 to 400 cm−1.
The structure and surface morphology of the synthesized samples were analyzed using a scanning electron microscope (SEM), model JEOL JSM-6380LV (Tokyo, Japan), equipped with a Thermo Scientific Noran System SIX energy-dispersive X-ray spectrometer (EDS) (Waltham, MA, USA) operated at 20 kV.
Thermogravimetric measurements were conducted using a simultaneous thermal analyzer, the TGA-DSC Netzsch STA 449F3 Jupiter (Selb, Germany), in synthetic air from 30 °C to 900 °C at a heating rate of 10 °C/min.
2.5. Sensor Fabrication and Evaluation
A 3.00 mm diameter electrode was polished using 0.05 μm alumina slurry and thoroughly rinsed with distilled water to prepare the glassy carbon electrode for surface modification. A 10.00 μL dispersion of either MWCNTs or TiO2/MWCNT nanocomposite in ultrapure water, each at a concentration of 1.00 mg/mL, was then added to the GCE surface. The active materials were immobilized with Nafion®–methanol (5.00% w/v) to create the MWCNT/Nafion/GCE and TiO2/MWCNT/Nafion/GCE sensors. One of the unique features of Nafion is its ability to facilitate proton transfer from its sulfonic groups to the perfluorinated hydrophobic backbone, which results in the formation of a highly conductive medium for protons [10,36] to provide greater reticulation of the nanocomposites on the surface of the GCE.
Electrochemical sensing was conducted at room temperature using a three-electrode electrochemical cell (30.00 mL) consisting of the MWCNT/Nafion/GCE or TiO2/MWCNT/Nafion/GCE as the working electrode, a Pt plate as the counter electrode, and Ag|AgCl as the reference electrode. The measurements were carried out using a portable bi-potentiostat/galvanostat μ-Autolab Type III (Metrohm Autolab, Utrecht, The Netherlands) controlled by Nova 2.1 software.
The electrochemical sensing was performed in different H2O2 concentrations in a 0.10 M Britton–Robinson (B-R) buffer at pH 7.00 under constant magnetic stirring. After each triplicate, the solutions were homogenized by stirring for 30 s, and any electrogenerated products were removed from the electrode surface. For the addition–recovery experiments, standard solutions of H2O2 were prepared using 0.01 M (mol·L−1) stock solutions in ultrapure water (R ≥ 18.2 MΩ cm).
3. Results
3.1. Characterization of MWCNT and TiO2/MWCNTs Nanocomposite
Figure 3 shows the XRD diffraction patterns of both pristine MWCNT and TiO2/MWCNT nanocomposite. Although some peaks present low intensity, smoothing has been applied to distinguish them better.
Figure 3.
XRD patterns of pristine MWCNT and TiO2/MWCNT nanocomposite.
The XRD patterns of pristine MWCNTs show diffraction peaks of graphite structures at 2θ = 25.8° and 43.5° (JCPDS card 41-1487) corresponding to reflections from crystallographic planes (002), the spacing between adjacent graphite layers, and ordering within the plane (100), respectively.
In addition to the diffraction peaks related to pristine MWCNTs, the MWCNT/TiO2 nanocomposite exhibits a diffraction peak at 2θ = 48.2° related to the (200) plane, indicating the formation of the anatase phase of TiO2 (JCPDS card no. 21-1272). The significant broadening of the peak indicates the nanometric characteristic of the TiO2 particles. Thus, the hydrothermal treatment employed was effective, as it promoted the crystallization of amorphous titania into the anatase phase.
An approximate measure of the crystallite size of the TiO2 phase in the sample was determined using the well-known Scherrer equation [37], which is based on the full width at half maximum (FWHM) Å obtained by a Gaussian function, diffraction angle θ and the wavelength λ associated with Cu Kα radiation. The average crystallite size of anatase (200) was found to be 5.50 nm based on the broadening of its diffraction peak, where the FWHM is 1.63 Å.
The FTIR spectra of pristine MWCNTs, bare TiO2 particles, and TiO2/MWCNT nanocomposite are shown in Figure 4.
Figure 4.
FTIR of MWCNT, TiO2 particles, and TiO2/MWCNTs nanocomposite.
The FTIR spectra of all samples showed a broad absorption band in the range of 3000 to 3500 , centered at 3219 , attributed to the O-H stretching vibration and the surface adsorbed water [38]. In the MWCNTs’ FTIR spectrum, distinct peaks were observed at 1539, 1728, and 3219 . The peak at 1539 confirmed the presence of graphitic carbon bonds (C=C stretching vibration), while the peak at 1728 indicated the presence of carbonyl (C=O) functional groups [39,40].
The FTIR spectrum of bare TiO2 particles shows a broad band of 1000 to 400 , which can be attributed to various stretching vibrations, including Ti–O and O–Ti–O bonds [41]. However, in the FTIR spectrum of the TiO2/MWCNT nanocomposite, in addition to the Ti–O and O–Ti–O bonds, the presence of Ti–O–C and Ti–O–C=O bonds is observed, indicating an interaction between TiO2 particles and the MWCNTs [42]. Furthermore, the anatase titania phase, as revealed by XRD analysis, contributes to the observed band [43,44]. Both the nanocomposite and bare TiO2 particles exhibit a distinct band ~1395 corresponding to TiO2 lattice vibrations [45,46]. In addition, the FTIR spectrum of the TiO2/MWCNT nanocomposite also displays a peak at 1633 , representing the deformative vibration of the Ti–OH stretching mode and the OH stretch of adsorbed water. Moreover, a band at 1100 has been attributed to alkoxy C-O stretching vibrations within the nanocomposite [47].
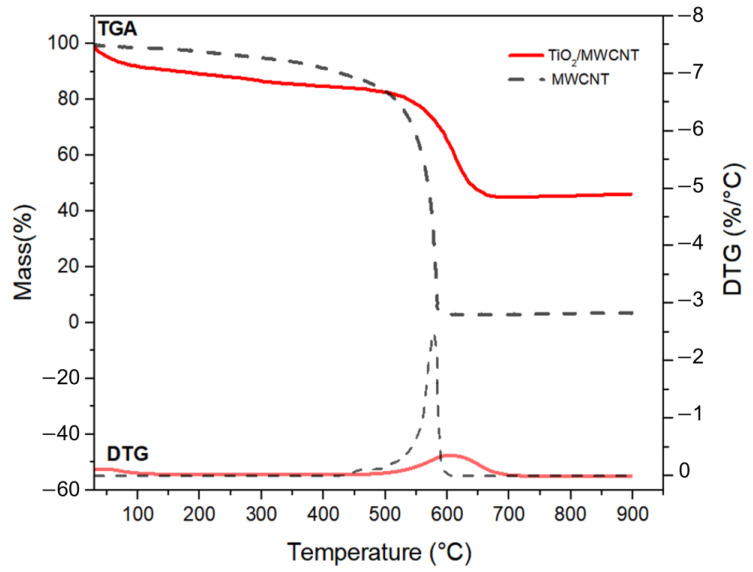
Thermogravimetric analysis was used to investigate the thermal stability of the studied materials. According to Figure 5, it can be observed that the MWCNT shows a mass loss caused by the oxidation of the nanotubes with a peak temperature at 575 °C, which agrees with values reported in the literature ranging from 550–650 °C [48]. The stability was achieved with a residue of 3.50%, resulting from the remaining catalysts from the synthesis of the nanotubes (MWCNT), as reported by the supplier of this material.
Figure 5.
TG and DTG curves for pristine MWCNT and TiO2/MWCNT nanocomposite.
The MWCNT/TiO2 nanocomposite exhibited a mass loss of 5.00% up to 100 °C, which can be attributed to adsorbed water on the material’s surface—this mass loss results from the desorption of the water molecules from the nanocomposite. Between the temperature range of 100 and 450 °C, a further mass loss of approximately 8.00% was observed. This additional mass loss is believed to be caused by the decomposition of oxygenated groups that were incorporated into the MWCNTs during the synthesis process of the nanocomposite [49]. The presence of these oxygenated groups provides evidence for the bonding mechanism between TiO2 and MWCNT, suggesting that the bonding occurs through the involvement of these functional groups. This bonding interaction between TiO2 and MWCNT is further supported by the presence of Ti–O–C and Ti–O–C=O bonds, as observed in FTIR analysis.
Furthermore, it was observed that the temperature range at which significant mass loss occurs because of the oxidation of nanotubes is between 500–690 °C. This can be easily seen in the DTG curve. The constant levels observed in the TGA curve after 690 °C indicate that the nanotubes underwent complete oxidation. The residue reflects the percentage of TiO2 present in the nanocomposite: approximately 41.50%.
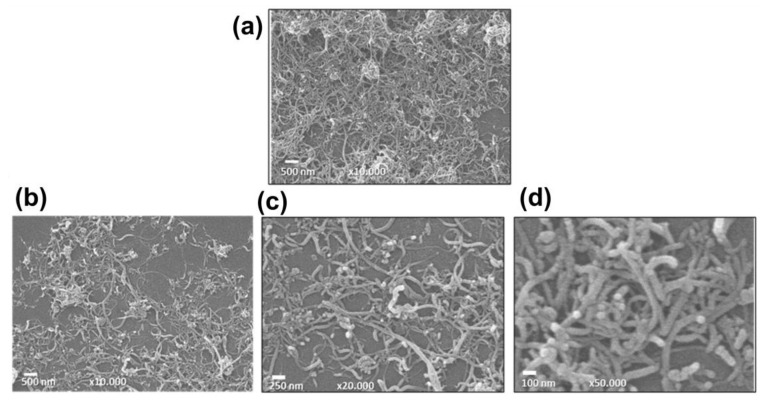
The morphology of pristine MWCNT and MWCNT/TiO2 nanocomposite samples were evaluated using SEM. Figure 6a shows that the pristine MWCNT has a smooth surface with entangled tube bundles. In Figure 6b–d, it can be observed that the MWCNT/TiO2 has morphology like the undecorated MWCNTs, i.e., no particles that could be attributed to TiO2 were marked.
Figure 6.
SEM images of pristine MWCNT (a) and TiO2/MWCNT nanocomposite (b–d). Scale bars in (a,b) 500.00 nm, (c) 250.00 nm, and (d) 100.00 nm.
The results from the EDS analysis, as shown in Figure 7, support the presence of titanium in the nanocomposite and its successful integration into the carbon nanotube matrix. These findings are consistent with the XRD and FTIR analysis results.
Figure 7.
SEM image of the MWCNT/TiO2 nanocomposite (a) and corresponding EDS elemental mapping with separate maps shown for (b) C, (c) Ti, and (d) O.
The EDX analysis of the TiO2/MWCNT nanocomposite demonstrated its atomic percent (at.%) and weight percent (w.%) composition. The analysis results are as follows: carbon (C)—73.50% (at.%) and 86.72% (w.%), titanium (Ti)—9.22% (at.%) and 8.17% (w.%), and oxygen (O)—17.28% (at.%) and 5.11% (w.%). These findings validate its chemical composition.
3.2. Electrochemical Behavior of GCE, MWCNT/Nafion, and TiO2/MWCNT/Nafion-Modified GCEs
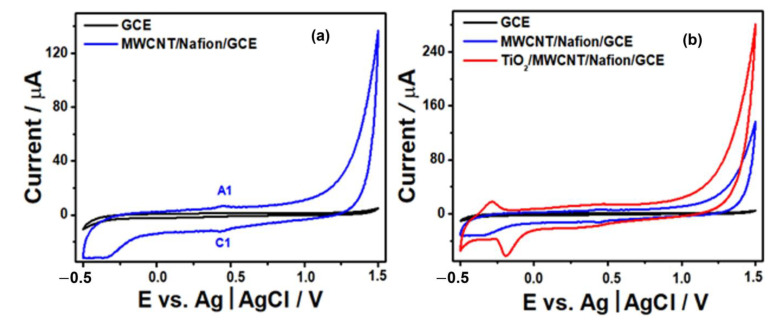
The electrocatalytic activity of modified working electrodes was examined by measuring the cyclic voltammograms (CVs) in 0.50 M H2SO4 within the potential range of −0.5 to 1.5 V vs. Ag|AgCl at a scan rate of 50 mVs−1. As depicted in Figure 8a, the unmodified GCE was determined to be electrochemically inactive under these conditions, which aligns with the earlier observations reported by Benck et al. [50]. The CV of the bare CGE displayed no redox pair, resulting in a narrow and almost flat line in the graph. This implies that no electrochemical reactions were taking place on the surface of the CGE electrode under the specified conditions.
Figure 8.
Cyclic voltammograms measured in 0.50 M H2SO4 within the potential range of 0.5 V to 1.5 V vs. Ag|AgCl at a scan rate of 50 mV using bare GCE, MWCNT/Nafion-modified GCE (a), and TiO2/MWCNT/Nafion-modified GCE (b).
0.5 V to 1.5 V vs. Ag|AgCl at a scan rate of 50 mV using bare GCE, MWCNT/Nafion-modified GCE (a), and TiO2/MWCNT/Nafion-modified GCE (b).
However, in Figure 8a, the modification of the CGE electrode with MWCNT altered the electrochemical profile of the surface, broadening the area of the voltammogram, as the carbon nanotubes provided a surface and electroactive increment. In addition, two peaks, A1 and C1, are observed, which correlate with the redox reactions of metallic iron resulting from the catalyst used to synthesize MWCNTs [51,52]. Notably, the potential scan toward more negative values for the GCE/MWCNT/TiO2 electrode gives rise to redox current peaks in the potential region between and 0.0 V, which may be associated with the oxidation/reduction in Ti ions [53,54,55,56].
Moreover, the presence of oxygenated functional groups, such as hydroxyls (OH) and carbonyls (C=O), in the TiO2/MWCNT nanocomposite plays a significant role in its electrochemical behavior. These functional groups contribute to increased adsorption capacity for species and provide additional active sites for the adsorption of molecules and ions. Consequently, electrochemical reactions, including the oxidation of H2O2, can take place more efficiently. This makes the modified GCE electrode an up-and-coming candidate for H2O2-sensing applications where accurate and sensitive detection of H2O2 is required [57,58,59].
3.3. Evaluation of TiO2/MWCNT/Nafion/GCE Electrode as an Electrochemical Sensor for H2O2 Determination
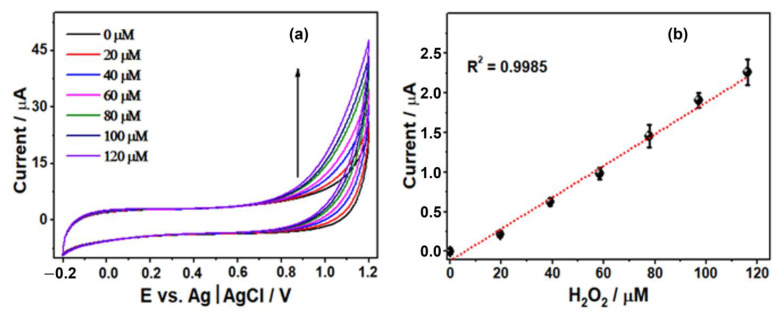
The electrocatalytic activity of the TiO2/MWCNT/Nafion/GCE sensor toward the oxidation of H2O2 was investigated by CV. Figure 9a shows the cyclic voltammograms of the electrode in a Britton–Robinson (B-R) buffer solution at pH 7, under a potential scan range of −0.2 V to 1.0 V, in the absence of and after successive additions of hydrogen peroxide (from 0 up to 120 μM) to the cell solution, and the corresponding calibration plot.
Figure 9.
(a) Cyclic voltammograms of the TiO2/MWCNT/Nafion/GCE to successive addition of H2O2 concentrations (0.00 up to 120.00 μM) into a continuously stirred B-R buffer solution, pH = 7.00. (b) The calibration curve of current vs. H2O2 concentration.
The cyclic voltammograms demonstrated the direct proportionality of the peak current to H2O2 concentration within 14.00–120.00 µM. The linear regression equation illustrated Ip (µA) = 1.0145x − 0.0346, (R2 = 0.9985) (Figure 9b). The detection limits (LD) and quantification limits (LQ) were calculated according to the recommendations of the IUPAC as three times the standard deviation of the blank signal—Britton–Robinson buffer—(σB) divided by the slope of the calibration curve (m): LD = 3 σB/m. The LQs were calculated similarly, with 10 replacing 3 in each equation, i.e., LQ = 10 σB/m. Therefore, the LD and LQ limits obtained were 4.00 µM and 14.00 µM, respectively.
The fabricated sensor’s accuracy was evaluated using the standard addition method, in distilled deionized water, at three different concentration levels of H2O2 (20.00, 40.00, and 60.00 µM) Britton–Robinson buffer solution at a pH of 7.00, using cyclic voltammetry.
The results are presented in Table 1, demonstrating a range of variation between 67.30% and 101.90%. Our findings indicate that this analytical method is only sufficiently accurate for concentrations greater than 20.00 µM.
Table 1.
Results for the recovery study in distilled-deionized water at three concentration levels for H2O2 in Britton–Robinson buffer solution at pH 7.00.
| Added (µM) | Found (µM) | Recovery (%) |
|---|---|---|
| 20.00 | 13.20 ± 0.11 | 67.30 |
| 40.00 | 36.02 ± 0.25 | 92.30 |
| 60.00 | 59.50 ± 0.12 | 101.90 |
A comparison between the TiO2/MWCNT/Nafion/GCE electrode and other GCE-modified electrodes reported in the literature is presented in Table 2. Our results align with those of other studies, indicating that this electrode coating is suitable for detecting H2O2. Furthermore, it is worth noting that the TiO2/MWCNT/Nafion/GCE electrode can be produced with fewer laborious steps than in some of the previous works.
Table 2.
Comparison of the proposed sensor with the recently reported H2O2 sensors.
| Modifier Material | Method | LD (µM) | References |
|---|---|---|---|
| MWCNT-POMAF | Amp | 0.33 | [60] |
| Co/MWCNT | DPV | 1.84 | [61] |
| AuNPs/PSi/Nafion | LSV | 14.84 | [62] |
| AuNPs/PSi/Nafion | SWV | 15.16 | [62] |
| Hb/MoS | CV | 6.70 | [63] |
| Ag/MWCNT | DPV | 3.30 | [61] |
| MoS2 | CV | 1.13 | [64] |
| Au@TiO2/MWCNT | DPV | 1.40 | [28] |
| PB–TiO2/fCN | CA | 0.088 | [30] |
| TiO2/MWCNT | Amp | 0.40 | [28] |
| Ni(OH)2/ERGO–MWNT | Amp | 4.00 | [65] |
| Ag@TiO2 | CV | 0.83 | [66] |
| MWCNT/TiO2 | CV | 4.00 | This work |
Abbreviations used: Amp (amperometry), DPV (differential pulse voltammetry), LSV (linear sweep voltammetry), SWV (square wave voltammetry), CV (cyclic voltammetry), CA (chronoamperometry), MWCNT-POMAF (multi-walled carbon nanotubes functionalized with polyoxometalate), Co/MWCNT (cobalt/multi-walled carbon nanotubes), AuNPs/PSi/Nafion (gold nanoparticles/porous silicon/Nafion), AuNPs/PSi/Nafion (gold nanoparticles/porous silicon/Nafion), Hb/MoS (hemoglobin/molybdenum sulfide), Ag/MWCNT (silver/multi-walled carbon nanotubes), MoS2 (molybdenum disulfide), Au@TiO2/MWCNT (gold@titanium dioxide/multi-walled carbon nanotubes), PB-TiO2/fCN (phosphate buffer-titanium dioxide/functionalized carbon nitride), TiO2/MWCNT (titanium dioxide/multi-walled carbon nanotubes), Ni(OH)2/ERGO-MWNT (nickel hydroxide/electrochemically reduced graphene oxide–multi-walled carbon nanotubes), Ag@TiO2 (silver@titanium dioxide), MWCNT/TiO2 (multi-walled carbon nanotubes/titanium dioxide).
Considering that hydrogen peroxide concentrations typically range from micromolar for in vivo conditions and residual levels in foodstuffs and drinking water to tens of millimolar for bleaching applications and molar for waste treatment applications [67], the TiO2/MWCNT/Nafion/GCE has the potential to determine H2O2 levels in water.
4. Conclusions
In summary, a novel H2O2 voltammetric sensor has been developed by modifying a glassy carbon electrode (GCE) with a TiO2/MWCNT/Nafion nanocomposite synthesized using a facile hydrothermal route. Incorporating semiconducting TiO2 nanoparticles and highly conducting MWCNTs synergistically enhanced the sensor’s performance toward H2O2 detection, surpassing the performance of both bare GCE and MWCNT/Nafion-modified GCE electrodes. The proposed modified electrode successfully detected and determined H2O2 concentrations in water ranging from 14.00–120.00 μM, with a detection limit of 4.00 μM.
These findings represent a significant advancement in the design and fabrication of highly efficient H2O2-based sensor devices and have important implications for various applications such as water treatment, food safety, and biomedical diagnostics. Using the TiO2/MWCNT/Nafion nanocomposite as a sensing material holds great promise for developing cost-effective, reliable, and sensitive H2O2 sensors with a wide range of detection capabilities.
Further research could explore the potential of this novel sensor platform for other analytes and environmental conditions, paving the way for the development of next-generation sensing technologies. However, it is essential to note that extensive testing and validation of the sensor’s performance under different conditions will be necessary to ensure its practical applicability before commercialization.
Acknowledgments
We are very grateful for the financial support from CAPES. We also would like to thank INCT Nanocarbono for supporting this work.
Author Contributions
R.H.d.O. and D.A.G. conceived and designed the experiments and performed the experiments; R.H.d.O. analyzed the data and wrote the paper; D.D.d.R. and D.A.G. contributed with materials, supervision, and review. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Mattos I.L.D., Shiraishi K.A., Braz A.D., Fernandes J.R. Peróxido de Hidrogênio: Importância e Determinação. J. Química Nova. 2003;26:373–380. doi: 10.1590/S0100-40422003000300015. [DOI] [Google Scholar]
- 2.Geiszt M., Leto T.L. The Nox Family of Nad (P) H Oxidases: Host Defense and Beyond. J. Biol. Chem. 2004;279:51715–51718. doi: 10.1074/jbc.R400024200. [DOI] [PubMed] [Google Scholar]
- 3.Giorgio, Marco, Mirella Trinei, Enrica Migliaccio, and Pier Giuseppe Pelicci Hydrogen Peroxide: A Metabolic by-Product or a Common Mediator of Ageing Signals? J. Nat. Rev. Mol. Cell Biol. 2007;8:722–728. doi: 10.1038/nrm2240. [DOI] [PubMed] [Google Scholar]
- 4.Martinez-Calatayu J. Flow Injection Analysis of Pharmaceuticals: Automation in the Laboratory. CRC Press; Boca Raton, FL, USA: 1996. [Google Scholar]
- 5.Dhara K., Mahapatra D.R. Recent Advances in Electrochemical Nonenzymatic Hydrogen Peroxide Sensors Based on Nanomaterials: A Review. J. Mater. Sci. 2019;54:12319–12357. doi: 10.1007/s10853-019-03750-y. [DOI] [Google Scholar]
- 6.Mingrui S., Junli W., Baiyang C., Lei W. A Facile, Nonreactive Hydrogen Peroxide (H2O2) Detection Method Enabled by Ion Chromatography with Uv Detector. Anal. Chem. 2017;89:11537–11544. doi: 10.1021/acs.analchem.7b02831. [DOI] [PubMed] [Google Scholar]
- 7.Al Lawati H.A., Hassanzadeh J., Bagheri N. A Handheld 3d-Printed Microchip for Simple Integration of the H2O2-Producing Enzymatic Reactions with Subsequent Chemiluminescence Detection: Application for Sugars. J. Food Chem. 2022;383:132469. doi: 10.1016/j.foodchem.2022.132469. [DOI] [PubMed] [Google Scholar]
- 8.Lu J., Zhang H., Li S., Guo S., Shen L., Zhou T., Zhong H., Wu L., Meng Q., Zhang Y. Oxygen-Vacancy-Enhanced Peroxidase-Like Activity of Reduced Co3o4 Nanocomposites for the Colorimetric Detection of H2O2 and Glucose. J. Inorg. Chem. 2020;59:3152–3159. doi: 10.1021/acs.inorgchem.9b03512. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad T., Iqbal A., Halim S.A., Uddin J., Khan A., El Deeb S., Al-Harrasi A. Recent Advances in Electrochemical Sensing of Hydrogen Peroxide (H2O2) Released from Cancer Cells. Nanomaterials. 2022;12:1475. doi: 10.3390/nano12091475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tran H.V., Huynh C.D., Tran H.V., Piro B. Cyclic Voltammetry, Square Wave Voltammetry, Electrochemical Impedance Spectroscopy and Colorimetric Method for Hydrogen Peroxide Detection Based on Chitosan/Silver Nanocomposite. Arab. J. Chem. 2018;11:453–459. doi: 10.1016/j.arabjc.2016.08.007. [DOI] [Google Scholar]
- 11.Natinan B., Baeumner A.J. Combining Electrochemical Sensors with Miniaturized Sample Preparation for Rapid Detection in Clinical Samples. Sensors. 2015;15:547–564. doi: 10.3390/s150100547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aicheng C., Chatterjee S. Nanomaterials Based Electrochemical Sensors for Biomedical Applications. J. Chem. Soc. Rev. 2013;12:5425–5438. doi: 10.1039/c3cs35518g. [DOI] [PubMed] [Google Scholar]
- 13.Kamyabi M., Hajari N. Low Potential and Non-Enzymatic Hydrogen Peroxide Sensor Based on Copper Oxide Nanoparticle on Activated Pencil Graphite Electrode. J. Braz. Chem. Soc. 2017;28:808–818. doi: 10.21577/0103-5053.20160232. [DOI] [Google Scholar]
- 14.Nestor U., Frodouard H., Theoneste M. A Brief Review of How to Construct an Enzyme-Based H2O2 Sensor Involved in Nanomaterials. J. Adv. Nanoparticles. 2020;10:1–25. [Google Scholar]
- 15.Portorreal-Bottier A., Gutiérrez-Tarriño S., Calvente J.J., Andreu R., Roldán E., Oña-Burgos P., Olloqui-Sariego J.L. Enzyme-Like Activity of Cobalt-Mof Nanosheets for Hydrogen Peroxide Electrochemical Sensing. Sens. Actuators B Chem. 2022;368:132129. doi: 10.1016/j.snb.2022.132129. [DOI] [Google Scholar]
- 16.Pang P., Yang Z., Xiao S., Xie J., Zhang Y., Gao Y. Nonenzymatic Amperometric Determination of Hydrogen Peroxide by Graphene and Gold Nanorods Nanocomposite Modified Electrode. J. Electroanal. Chem. 2014;727:27–33. doi: 10.1016/j.jelechem.2014.05.028. [DOI] [Google Scholar]
- 17.Bai J., Zhou B. Titanium Dioxide Nanomaterials for Sensor Applications. Chem. Rev. 2014;114:10131–10176. doi: 10.1021/cr400625j. [DOI] [PubMed] [Google Scholar]
- 18.Linsebigler A.L., Lu G., Yates J.T., Jr. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. J. Chem. Rev. 1995;95:735–758. doi: 10.1021/cr00035a013. [DOI] [Google Scholar]
- 19.Hu Y., Tsai H.L., Huang C.L. Phase Transformation of Precipitated TiO2 Nanoparticles. Mater. Sci. Eng. A. 2003;344:209–214. doi: 10.1016/S0921-5093(02)00408-2. [DOI] [Google Scholar]
- 20.Ferreira-Neto E.P., Ullah S., Simões M.B., Perissinotto A.P., de Vicente F.S., Noeske P.-L.M., Ribeiro S.J.L., Rodrigues-Filho U.P. Solvent-controlled deposition of titania on silica spheres for the preparation of SiO2@TiO2 core@shell nanoparticles with enhanced photocatalytic activity. Colloids Surf. A Physicochem. Eng. Asp. 2019;570:293–305. doi: 10.1016/j.colsurfa.2019.03.036. [DOI] [Google Scholar]
- 21.Andrews R., Jacques D., Qian D., Rantell T. Multiwall Carbon Nanotubes: Synthesis and Application. J. Acc. Chem. Res. 2002;35:1008–1017. doi: 10.1021/ar010151m. [DOI] [PubMed] [Google Scholar]
- 22.You J.M., Jeong Y.N., Ahmed M.S., Kim S.K., Choi H.C., Jeon S. Reductive Determination of Hydrogen Peroxide with Mwcnts-Pd Nanoparticles on a Modified Glassy Carbon Electrode. Biosens. Bioelectron. 2011;26:2287–2291. doi: 10.1016/j.bios.2010.09.053. [DOI] [PubMed] [Google Scholar]
- 23.Tavakkoli H., Akhond M., Ghorbankhani G.A., Absalan G. Electrochemical Sensing of Hydrogen Peroxide Using a Glassy Carbon Electrode Modified with Multiwalled Carbon Nanotubes and Zein Nanoparticle Composites: Application to Hepg2 Cancer Cell Detection. Microchim. Acta. 2020;187:105. doi: 10.1007/s00604-019-4064-7. [DOI] [PubMed] [Google Scholar]
- 24.Qiu J., Zhang S., Zhao H. Recent Applications of TiO2 Nanomaterials in Chemical Sensing in Aqueous Media. Sens. Actuators B Chem. 2011;160:875–890. doi: 10.1016/j.snb.2011.08.077. [DOI] [Google Scholar]
- 25.Pang X., He D., Luo S., Cai Q. An Amperometric Glucose Biosensor Fabricated with Pt Nanoparticle-Decorated Carbon Nanotubes/TiO2 Nanotube Arrays Composite. Sens. Actuators B Chem. 2009;137:134–138. doi: 10.1016/j.snb.2008.09.051. [DOI] [Google Scholar]
- 26.Santangelo S., Messina G., Faggio G., Donato A., De Luca L., Donato N., Bonavita A., Neri G. Micro-Raman Analysis of Titanium Oxide/Carbon Nanotubes-Based Nanocomposites for Hydrogen Sensing Applications. J. Solid State Chem. 2010;183:2451–2455. doi: 10.1016/j.jssc.2010.08.018. [DOI] [Google Scholar]
- 27.Santangelo S., Faggio G., Messina G., Fazio E., Neri F., Neri G. On the Hydrogen Sensing Mechanism of Pt/TiO2/Cnts Based Devices. Sens. Actuators B Chem. 2013;178:473–484. doi: 10.1016/j.snb.2013.01.005. [DOI] [Google Scholar]
- 28.Jiang L.C., Zhang W.D. Electrodeposition of TiO2 Nanoparticles on Multiwalled Carbon Nanotube Arrays for Hydrogen Peroxide Sensing. J. Electroanal. 2009;21:988–993. doi: 10.1002/elan.200804502. [DOI] [Google Scholar]
- 29.Naik S.S., Lee S.J., Theerthagiri J., Yu Y., Choi M.Y. Rapid and Highly Selective Electrochemical Sensor Based on Zns/Au-Decorated F-Multi-Walled Carbon Nanotube Nanocomposites Produced Via Pulsed Laser Technique for Detection of Toxic Nitro Compounds. J. Hazard. Mater. 2021;418:126269. doi: 10.1016/j.jhazmat.2021.126269. [DOI] [PubMed] [Google Scholar]
- 30.Guerrero L.A., Fernández L., González G., Montero-Jiménez M., Uribe R., Díaz Barrios A., Espinoza-Montero P.J. Peroxide Electrochemical Sensor and Biosensor Based on Nanocomposite of TiO2 Nanoparticle/Multi-Walled Carbon Nanotube Modified Glassy Carbon Electrode. Nanomaterials. 2020;10:64. doi: 10.3390/nano10010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frontera P., Malara A., Stelitano S., Leonardi S.G., Bonavita A., Fazio E., Antonucci P., Neri G., Neri F., Santangelo S. Characterisation and H2O2 Sensing Properties of TiO2-Cnts/Pt Electro-Catalysts. Mater. Chem. Phys. 2016;170:129–137. doi: 10.1016/j.matchemphys.2015.12.030. [DOI] [Google Scholar]
- 32.Seck E.I., Doña-Rodríguez J.M., Melián E.P., Fernández-Rodríguez C., González-Díaz O.M., Portillo-Carrizo D., Pérez-Peña J. Comparative Study of Nanocrystalline Titanium Dioxide Obtained through Sol–Gel and Sol–Gel–Hydrothermal Synthesis. J. Colloid Interface Sci. 2013;400:31–40. doi: 10.1016/j.jcis.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 33.Kaur J., Vergara A., Rossi M., Gravagnuolo A.M., Valadan M., Corrado F., Conte M., Gesuele F., Giardina P., Altucci C. Electrostatically Driven Scalable Synthesis of Mos2–Graphene Hybrid Films Assisted by Hydrophobins. RSC Adv. 2017;7:50166–50175. doi: 10.1039/C7RA09878B. [DOI] [Google Scholar]
- 34.Patel B.R., Imran S., Ye W., Weng H., Noroozifar M., Kerman K. Simultaneous Voltammetric Detection of Six Biomolecules Using a Nanocomposite of Titanium Dioxide Nanorods with Multi-Walled Carbon Nanotubes. Electrochim. Acta. 2020;362:137094. doi: 10.1016/j.electacta.2020.137094. [DOI] [Google Scholar]
- 35.Kourkoumelis N. PowDLL, a Reusable.Net Component for Interconverting Powder Diffraction Data: Recent Developments. Powder Diffr. J. 2013;28:137–148. [Google Scholar]
- 36.Li S., Noroozifar M., Kerman K. Nanocomposite of Ferricyanide-Doped Chitosan with Multi-Walled Carbon Nanotubes for Simultaneous Senary Detection of Redox-Active Biomolecules. J. Electroanal. Chem. 2019;849:113376. doi: 10.1016/j.jelechem.2019.113376. [DOI] [Google Scholar]
- 37.Klung H.P., Alexander L.E. X-Ray Diffraction Procedures. Volume 1. John Wiley Sons; New York, NY, USA: 1962. p. 974. [Google Scholar]
- 38.Zhang Y., Zhang W., Yang K., Yang Y., Jia J., Liang Y., Guo L. Carbon Nano-Onions (Cnos)/Tio 2 Composite Preparation and Its Photocatalytic Performance under Visible Light Irradiation. J. Environ. Eng. 2020;146:04020009. doi: 10.1061/(ASCE)EE.1943-7870.0001662. [DOI] [Google Scholar]
- 39.Delekar S.D., Dhodamani A.G., More K.V., Dongale T.D., Kamat R.K., Acquah S.F., Dalal N.S., Panda D.K. Structural and Optical Properties of Nanocrystalline TiO2 with Multiwalled Carbon Nanotubes and Its Photovoltaic Studies Using Ru(Ii) Sensitizers. ACS Omega. 2018;3:2743–2756. doi: 10.1021/acsomega.7b01316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bahgat M., Farghali A.A., El Rouby W.M.A., Khedr M.H. Synthesis and Modification of Multi-Walled Carbon Nano-Tubes (Mwcnts) for Water Treatment Applications. J. Anal. Appl. Pyrolysis. 2011;92:307–313. doi: 10.1016/j.jaap.2011.07.002. [DOI] [Google Scholar]
- 41.Ashoka N.B., Swamy B.K., Jayadevappa H. Nanorod TiO2 Sensor for Dopamine: A Voltammetric Study. New J. Chem. 2017;41:11817–11827. doi: 10.1039/C7NJ02188G. [DOI] [Google Scholar]
- 42.Wang L., Guo J., Dang J., Huang X., Chen S., Guan W. Comparison of the Photocatalytic Performance of TiO2/Ac and TiO2/Cnt Nanocomposites for Methyl Orange Photodegradation. Water Sci. Technol. 2018;78:1082–1093. doi: 10.2166/wst.2018.374. [DOI] [PubMed] [Google Scholar]
- 43.Lu X., Lv X., Sun Z., Zheng Y. Nanocomposites of Poly(L-Lactide) and Surface-Grafted TiO2 Nanoparticles: Synthesis and Characterization. Eur. Polym. J. 2008;44:2476–2481. doi: 10.1016/j.eurpolymj.2008.06.002. [DOI] [Google Scholar]
- 44.Hashimoto M., Takadama H., Mizuno M., Kokubo T. Enhancement of Mechanical Strength of TiO2/High-Density Polyethylene Composites for Bone Repair with Silane-Coupling Treatment. J. Mater. Res. Bull. 2006;3:515–524. doi: 10.1016/j.materresbull.2005.09.014. [DOI] [Google Scholar]
- 45.Muduli S., Lee W., Dhas V., Mujawar S., Dubey M., Vijayamohanan K., Han S.-H., Ogale S. Enhanced Conversion Efficiency in Dye-Sensitized Solar Cells Based on Hydrothermally Synthesized TiO2−Mwcnt Nanocomposites. ACS Appl. Mater. Interfaces. 2009;1:2030–2035. doi: 10.1021/am900396m. [DOI] [PubMed] [Google Scholar]
- 46.Mali S.S., Betty C.A., Bhosale P.N., Patil P.S. Synthesis, Characterization of Hydrothermally Grown Mwcnt–TiO2 Photoelectrodes and Their Visible Light Absorption Properties. ECS J. Solid State Sci. Technol. 2012;1:M15. doi: 10.1149/2.004202jss. [DOI] [Google Scholar]
- 47.Stobinski L., Lesiak B., Kövér L., Tóth J., Biniak S., Trykowski G., Judek J. Multiwall Carbon Nanotubes Purification and Oxidation by Nitric Acid Studied by the Ftir and Electron Spectroscopy Methods. J. Alloys Compd. 2010;501:77–84. doi: 10.1016/j.jallcom.2010.04.032. [DOI] [Google Scholar]
- 48.Serp P., Corrias M., Kalck P. Carbon Nanotubes and Nanofibers in Catalysis. J. Appl. Catal. A Gen. 2003;253:337–358. doi: 10.1016/S0926-860X(03)00549-0. [DOI] [Google Scholar]
- 49.Datsyuk V., Kalyva M., Papagelis K., Parthenios J., Tasis D., Siokou A., Kallitsis I., Galiotis C. Chemical Oxidation of Multiwalled Carbon Nanotubes. Carbon. 2008;46:833–840. doi: 10.1016/j.carbon.2008.02.012. [DOI] [Google Scholar]
- 50.Benck J.D., Pinaud B.A., Gorlin Y., Jaramillo T.F. Substrate Selection for Fundamental Studies of Electrocatalysts and Photoelectrodes: Inert Potential Windows in Acidic, Neutral, and Basic Electrolyte. PLoS ONE. 2014;9:e107942. doi: 10.1371/journal.pone.0107942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fayemi O.E., Adekunle A.S., Ebenso E.E. Biosensors & Bioelectronics Metal Oxide Nanoparticles/Multi-Walled Carbon Nanotube Nanocomposite Modified Electrode for the Detection of Dopamine: Comparative Electrochemical Study. J. Biosens. Bioelectron. 2015;6:10–4172. [Google Scholar]
- 52.Poornajar M., Nguyen N.T., Ahn H.J., Büchler M., Liu N., Kment S., Zboril R., Yoo J.E., Schmuki P. Fe2O3 Blocking Layer Produced by Cyclic Voltammetry Leads to Improved Photoelectrochemical Performance of Hematite Nanorods. J. Surf. 2019;2:131–144. doi: 10.3390/surfaces2010011. [DOI] [Google Scholar]
- 53.Marken F., Bhambra A.S., Kim D.H., Mortimer R.J., Stott S.J. Electrochemical Reactivity of TiO2 Nanoparticles Adsorbed onto Boron-Doped Diamond Surfaces. Electrochem. Commun. 2004;6:1153–1158. doi: 10.1016/j.elecom.2004.09.006. [DOI] [Google Scholar]
- 54.Yu H., Ma J., Zhang Y., Zhang X., Shi W. Cyclic Voltammetry Studies of TiO2 Nanotube Arrays Electrode: Conductivity and Reactivity in the Presence of H+ and Aqueous Redox Systems. J. Electrochim. Acta. 2011;56:6498–6502. doi: 10.1016/j.electacta.2011.05.004. [DOI] [Google Scholar]
- 55.Hassaninejad-Darzi S.K., Shajie F. A Sensitive Voltammetric Determination of Anti-Parkinson Drug Pramipexole Using Titanium Dioxide Nanoparticles Modified Carbon Paste Electrode. J. Braz. Chem. Soc. 2017;28:529–539. doi: 10.5935/0103-5053.20160192. [DOI] [Google Scholar]
- 56.Silva-Galindo G., Zapata-Torres M. Synthesis and Characterization of TiO2 Thick Films for Glucose Sensing. J. Biosens. 2022;12:973. doi: 10.3390/bios12110973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Palisoc S.T., Natividad M.T., De Jesus N., Carlos J. Highly Sensitive Agnp/Mwcnt/Nafion Modified Gce-Based Sensor for the Determination of Heavy Metals in Organic and Non-Organic Vegetables. Sci. Rep. 2018;8:17445. doi: 10.1038/s41598-018-35781-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Da Silva E.P., Araujo M.D., Kunita M.H., Matos R., Medeiros R.A. Electrochemical Sensor Based on Multi-Walled Carbon Nanotubes and N-Doped TiO2 Nanoparticles for Voltametric Simultaneous Determination of Benserazide and Levodopa. J. Mol. 2022;27:8614. doi: 10.3390/molecules27238614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tarahomi S., Rounaghi G.H., Zavar M.H.A., Daneshvar L. Electrochemical Sensor Based on TiO2 Nanoparticles/Nafion Biocompatible Film Modified Glassy Carbon Electrode for Carbamazepine Determination in Pharmaceutical and Urine Samples. J. Electrochem. Soc. 2018;165:B946. doi: 10.1149/2.1061816jes. [DOI] [Google Scholar]
- 60.Mujica M.L., Sotomayor-Santander I., Hermosilla-Ibáñez P., Oyarzun-Ampuero F., Rodríguez M.C., Rivas G.A., Venegas-Yazigi D., Bollo S. Mwcnt-Organoimido Polyoxomolybdate Hybrid Material: Analytical Applications for Amperometric Sensing of Hydrogen Peroxide. Electroanalysis. 2021;33:2105–2114. doi: 10.1002/elan.202100149. [DOI] [Google Scholar]
- 61.Ohannesian L., Streeter A. Handbook of Pharmaceutical Analysis. CRC Press; Boca Raton, FL, USA: 2001. [Google Scholar]
- 62.Rashed M.A., Harraz F.A., Faisal M., El-Toni A.M., Alsaiari M., Al-Assiri M.S. Gold Nanoparticles Plated Porous Silicon Nanopowder for Nonenzymatic Voltammetric Detection of Hydrogen Peroxide. Anal. Biochem. 2021;615:114065. doi: 10.1016/j.ab.2020.114065. [DOI] [PubMed] [Google Scholar]
- 63.Liu H., Su X., Duan C., Dong X., Zhu Z. A Novel Hydrogen Peroxide Biosensor Based on Immobilized Hemoglobin in 3d Flower-Like Mos2 Microspheres Structure. Mater. Lett. 2014;122:182–185. doi: 10.1016/j.matlet.2014.02.047. [DOI] [Google Scholar]
- 64.Haritha V., Vijayan A., Kumar S.S., Rakhi R. Voltammetric Determination of Hydrogen Peroxide Using Mos2 Modified Glassy Carbon Electrodes. Mater. Lett. 2021;301:130258. doi: 10.1016/j.matlet.2021.130258. [DOI] [Google Scholar]
- 65.Gao W., Tjiu W.W., Wei J., Liu T. Highly Sensitive Nonenzymatic Glucose and H2O2 Sensor Based on Ni(OH)2/Electroreduced Graphene Oxide−Multiwalled Carbon Nanotube Film Modified Glass Carbon Electrode. Talanta. 2014;120:484–490. doi: 10.1016/j.talanta.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 66.Khan M.M., Ansari S.A., Lee J., Cho M.H. Novel Ag@TiO2 Nanocomposite Synthesized by Electrochemically Active Biofilm for Nonenzymatic Hydrogen Peroxide Sensor. Mater. Sci. Eng. C. 2013;33:4692–4999. doi: 10.1016/j.msec.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 67.Evans S.A., Elliott J.M., Andrews L.M., Bartlett P.N., Doyle P.J., Denuault G. Doyle, and Guy Denuault. Detection of Hydrogen Peroxide at Mesoporous Platinum Microelectrodes. Anal. Chem. 2002;74:1322–1326. doi: 10.1021/ac011052p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.