Key Points
Question
Do pediatric patients diagnosed with unipolar depression who receive antidepressant treatment have an increased incidence of mania/hypomania compared with patients not treated with antidepressants?
Findings
In this cohort study including 43 677 children and adolescents diagnosed with unipolar depression, during a 12-week follow-up, patients treated with antidepressants did not have an increased incidence of mania/hypomania compared with patients who did not initiate antidepressant treatment. Hospitalizations, parental bipolar disorder, and use of antipsychotics and antiepileptics were the most important predictors of mania/hypomania by 12 weeks.
Meaning
This study found no evidence of treatment-emergent mania/hypomania in children and adolescents with unipolar depression.
Abstract
Importance
Antidepressants are increasingly prescribed to pediatric patients with unipolar depression, but little is known about the risk of treatment-emergent mania. Previous research suggests pediatric patients may be particularly vulnerable to this adverse outcome.
Objective
To estimate whether pediatric patients treated with antidepressants have an increased incidence of mania/hypomania compared with patients not treated with antidepressants and to identify patient characteristics associated with the risk of mania/hypomania.
Design, Setting, and Participants
In a cohort study applying the target trial emulation framework, nationwide inpatient and outpatient care in Sweden from July 1, 2006, to December 31, 2019, was evaluated. Follow-up was conducted for 12 and 52 weeks after treatment initiation, with administrative follow-up ending December 31, 2020. Data were analyzed between May 1, 2022, and June 28, 2023. Individuals aged 4 to 17 years with a diagnosis of depression, but without a prior diagnosis of mania/hypomania, bipolar disorder, or psychosis or treatment with mood stabilizer (lithium, valproate, or carbamazepine), prescriptions were included.
Exposures
The treatment group included patients who initiated any antidepressant medication within 90 days of diagnosis. The control group included patients who did not initiate antidepressants within 90 days.
Main Outcomes and Measures
Diagnosis of mania/hypomania or initiation of mood stabilizer therapy. Incidences were estimated with Kaplan-Meier estimator, and inverse probability of treatment weighting was used to adjust for group differences at baseline.
Results
The cohort included 43 677 patients (28 885 [66%] girls); 24 573 in the treatment group and 19 104 in the control group. The median age was 15 (IQR, 14-16) years. The outcome occurred in 96 individuals by 12 weeks and in 291 by 52 weeks. The cumulative incidence of mania was 0.26% (95% CI, 0.19%-0.33%) in the treatment group and 0.20% (95% CI, 0.13%-0.27%) in the control group at 12 weeks, with a risk difference of 0.06% (95% CI, −0.04% to 0.16%). At 52 weeks, the cumulative incidence was 0.79% (95% CI, 0.68%-0.91%) in the treatment group and 0.52% (95% CI, 0.40%-0.63%) in the control group (risk difference, 0.28%; 95% CI, 0.12%-0.44%). Hospitalizations, parental bipolar disorder, and use of antipsychotics and antiepileptics were the most important predictors of mania/hypomania by 12 weeks.
Conclusion
This cohort study found no evidence of treatment-emergent mania/hypomania by 12 weeks in children and adolescents. This corresponds to the time frame for antidepressants to exert their psychotropic effect. A small risk difference was found only with longer follow-up. Certain patient characteristics were associated with mania/hypomania, which warrants clinical attention.
This cohort study examines the risks for and incidence of mania episodes after 12 and 52 weeks in children and adolescents with unipolar depression who are prescribed antidepressants.
Introduction
Depression is a prevalent psychiatric disorder among children and adolescents,1,2,3,4 for which antidepressants, such as selective serotonin reuptake inhibitors (SSRIs), are the recommended first-line pharmacologic treatment.5 Some antidepressants, especially tricyclic antidepressants, may induce manic symptoms in a subset of patients.6,7,8 While considered a safer option, similar concerns have been raised for SSRIs and serotonin/norepinephrine reuptake inhibitors.7,9,10 Treatment-emergent switch, ie, the transition from depression into mania shortly after the initiation of antidepressant treatment, is estimated to occur in 3% to 10% of adult patients with bipolar disorder.11,12 However, it remains unclear whether antidepressants can induce mania in patients with unipolar depression. Randomized clinical trials (RCTs) showed no clinically meaningful risk difference between adults with unipolar depression receiving SSRIs vs placebo or tricyclic antidepressants vs placebo,12 whereas observational studies reported a clearly increased risk.7,9,10,13
A register-based study found a particularly increased risk of manic conversion in children medicated with SSRIs.14 This finding, along with reports of other adverse medication-related outcomes, such as aggression15,16,17,18 and suicidality,15,19,20 have raised concerns about the safety of antidepressants.21,22,23 This is particularly pressing given that antidepressants are increasingly prescribed to children and adolescents.24,25,26 Some studies suggest that the developing brain might be especially vulnerable to adverse drug reactions,23,27,28,29 although the mechanisms remain unknown.
It is critical to understand potential risks related to antidepressants in pediatric patients, but rare outcomes, such as incident mania, are difficult to investigate in RCTs. Randomized clinical trials often have insufficient sample sizes and strict inclusion criteria, which correspond poorly with the characteristics of the population actually receiving the medication in clinical practice.7,8,21,22 For instance, most pediatric antidepressant trials excluded individuals at risk of developing mania, such as those with a family history of bipolar disorder or who received inpatient treatment. Mania occurred in 0 to 4 patients and was not evaluated or was not reported in trials with a sample size from 96 to 376 individuals.21,22 Electronic health record data circumvent these limitations, but observational studies using such data may be confounded if not designed and analyzed appropriately.9,10,14,21 Furthermore, if the goal is to investigate whether antidepressant initiation is associated with the incidence of mania, it is important to specify a clinically meaningful follow-up time. This means that observational studies should specify the time frame after antidepressant initiation in which newly emergent mania could be linked with antidepressants and not to the natural course of bipolar disorder misdiagnosed as unipolar. The International Society for Bipolar Disorders Task Force suggests 8 weeks, which is analogous to the customary time frame in which antidepressant efficacy is determined.30,31,32 Most medication-induced adverse reactions also occur within the first months of treatment.33,34 Prior observational studies followed up patients over several years,13,14 making it unlikely that outcome differences can be attributed solely to medication initiation.
Identifying individuals with an increased risk of developing mania may improve outcomes by providing information on which patients should be monitored closely during treatment. In addition to antidepressants, potential predictors of switch to mania include female sex, family history of mood disorders, psychotic features, emotional/behavioral dysregulation, hospitalization, and younger age.35 However, studies focusing on children and adolescents are scarce, and most prior work relied on small clinical samples.35
This study investigated the risk of incident mania/hypomania in pediatric patients with unipolar depression using nationwide register data from Sweden. We estimated the risk at 12 and 52 weeks of follow-up. We postulated that if antidepressants were to induce mania/hypomania, differences between the groups would emerge by 12 weeks. We used a time frame suggested by the International Society for Bipolar Disorders, adding 4 weeks to the customary 8 weeks to account for a potentially slower titration schedule in pediatric patients.36 It is less likely that the risk difference can be attributed to medication initiation if it emerges only in the longer follow-up period. Additionally, we investigated which patient characteristics predicted mania/hypomania in the 12-week follow-up.
Methods
Data Sources
In this cohort study, we used Swedish nationwide administrative registers linked with the unique personal identity number assigned at birth or upon immigration.37,38 Diagnoses were retrieved from the National Patient Register, which covers diagnoses from inpatient and outpatient care.39 Information concerning prescribed drugs was obtained from the Prescribed Drug Register, which contains data on all dispensed prescribed drugs since July 2005.40 Because the cohort was composed of children and adolescents, socioeconomic covariates were based on their parents’ information. Cohort members were linked to their parents via the Multi-Generation Register.41 We retrieved information on parental income and educational level from Statistics Sweden’s registers. The study was approved by the Swedish Ethical Review Authority. Informed consent requirement was waived by the Swedish Ethical Review Authority because the study was register based, and data were pseudonymized. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Study Cohort
We identified children and adolescents, aged 4 to 17 years, diagnosed with unipolar depression (International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ICD-10] codes F32 to F33, excluding F33.4) between July 1, 2006, and December 31, 2019. We required a washout period without use of any antidepressant medication (Anatomical Therapeutic Classification [ATC] code N06A) for at least 1 year preceding the depression diagnosis. Since the study focused on incident mania/hypomania, we excluded individuals with a prior diagnosis of mania (ICD-10 code F30), bipolar disorder (ICD-10 code F31), and psychosis (ICD-10 code F2) or prescriptions for lithium (ATC code N05AN), valproate (ATC code N03AG01), or carbamazepine (ATC code N03AF01) recorded any time before the depression diagnosis. We required that the prescribing indication was for mood-stabilizing purpose for the latter 2 medications. We selected the first diagnosis registered during the study period as the index diagnosis for eligible participants. Data were analyzed between May 1, 2022, and June 28, 2023.
Exposure
The treatment group consisted of patients who initiated any antidepressant medication within 90 days of diagnosis. The control group included patients who did not initiate antidepressant treatment within 90 days of diagnosis.
Outcome
The outcome was defined as any of the following: diagnosis of mania (ICD-10 code F30 [F30.0 hypomania]) or bipolar disorder (ICD-10 codes F31.0 [hypomania], F31.1, F31.2, and F31.6), or the initiation of lithium, valproate, or carbamazepine within 12 and 52 weeks of follow-up start.
Covariates
Several clinical and sociodemographic covariates were measured at baseline, including sex, age, year of diagnosis, source of diagnosis (inpatient vs outpatient), other diagnoses recorded any time before the follow-up start (anxiety disorders, attention deficit/hyperactivity disorder, drug use disorder, alcohol use disorder, personality disorders, conduct disorders, developmental disorders, autism spectrum disorder, suicide attempt/self-harm, and poisoning/overdose), other medication use within 4 months before the follow-up start (antipsychotics, hypnotics/sedatives, antiepileptics, stimulants, and opioids), number of mental health hospitalizations recorded any time before the follow-up start (as a proxy for the severity of mental health problems), parental income, parental educational level, and parental bipolar and depressive disorders. A detailed description of covariates is available in eTable 1 in Supplement 1. Information was missing for 1 or more covariates in a subset of individuals (n = 2197). If information was missing, the variable was coded as unknown and modeled as a separate response category.
Statistical Analysis
We applied the target trial emulation framework to guide the study design and analysis42 (eTable 2 in Supplement 1). Briefly, the first step for conducting a target trial emulation is articulating the causal question in the form of the protocol of a hypothetical randomized trial that would provide the answer. The protocol must specify certain key elements that define the causal estimands (eligibility criteria, treatment strategies, treatment assignment, start and end of follow-up, outcomes, and causal contrasts). The randomized trial described in the protocol becomes the target study, which is then emulated as closely as possible within the constraints of the observational data available. We provide the protocol in eTable 2 in Supplement 1. For the treatment group, follow-up started at the date of the first medication dispensation. The number of days from diagnosis to the first dispensation was assessed for participants who began treatment. For each person who did not initiate therapy, follow-up start was matched at random from this set and assigned to them (eFigure 1 in Supplement 1), which ensures the same distribution of time between diagnosis and start of follow-up in both groups, therefore accounting for immortal time bias.43 We estimated intention-to-treat (ITT) and per-protocol effect. In ITT, switching from antidepressant treatment to no treatment after the 90-day period and vice versa were not accounted for. In the per-protocol analysis, participants were censored when they switched from treatment to no treatment or vice versa. Details of this analysis are available in the eMethods in Supplement 1.
Follow-up continued until 12 weeks to investigate short-term outcomes. A time frame of 8 weeks is consistent with efficacy trials and suggested as the maximum time in which newly emergent mania may be considered antidepressant induced.30,31 We added 4 more weeks to account for a potentially slower titration schedule in pediatric patients36 as well as potential delay between medication dispensation and the time the patient began the medication. As a separate analysis, we extended follow-up to 52 weeks. Follow-up continued until emigration, death, outcome, censoring for psychosis hospitalization, administrative censoring at 12 and 52 weeks, administrative censoring on December 31, 2020, or censoring for treatment switch in the per-protocol analysis, whichever occurred first. We examined potential sex differences by estimating the models separately for boys and girls.
We used inverse probability of treatment weighting to adjust for differences between groups at baseline. The baseline covariates were used to estimate a propensity score, which is the probability of treatment conditional on covariates. We used stabilization and truncating to avoid extreme weights. For stabilized weights, the numerator of the weight is the probability of receiving the observed treatment. The denominator of the weight is the probability that given baseline confounders, an individual receives their observed treatment (ie, the numerator is the propensity score).44 The weights were then truncated at the 99th percentile. We used the resulting weights in the estimation of Kalbfleisch-Prentice cumulative incidence curves and Cox proportional hazards regression models. Confidence intervals were calculated using the infinitesimal jackknife variance estimator.
In sensitivity analyses, we assessed the robustness of the main results by using different cohort, exposure, outcome, and follow-up definitions. We (1) excluded individuals with missing information in any covariate; (2) excluded individuals who were dispensed antipsychotics or antiepileptics within 4 months before the start of follow-up; (3) extended the grace period to 120 days after diagnosis when defining the treatment group; (4) considered outcome to have occurred only if the individual had at least 2 diagnoses or 1 diagnosis and 1 medication dispensation; (5) extended the outcome to include olanzapine (ATC code N05AH03); included only prescriptions with an indication for a mood-stabilizing purpose (olanzapine was selected because, in addition to medications in the primary outcome, it is among the recommended treatment options for mania in pediatric patients in Sweden45); (6) extended the follow-up to 18 weeks; (7) censored individuals if they were hospitalized for any mental health reason (excluding the outcome); (8) conducted cloning-censoring-weighting analysis, in which follow-up starts at the index diagnosis46 to test whether our main approach was robust to immortal time bias (details in the eMethods in Supplement 1); and (9) used alternative ways to define treatment periods in per-protocol analyses (eMethods in Supplement 1).
We investigated which patient characteristics (covariates specified earlier) were associated with mania/hypomania in the 12-week follow-up. We used multivariable Cox proportional hazards regression to estimate a hazard ratio for each predictor. Some predictor variables did not have a sufficient number of observations to estimate a separate coefficient. We therefore recoded or combined a subset of them (details in the eMethods in Supplement 1). Predictive performance of the model was assessed with the concordance index. In the second step, we included the initiation of antidepressants to the model to test whether including it added incremental value in predicting mania/hypomania.
Results
The cohort included 43 677 patients (28 885 [66%] girls; 14 792 [34%] boys) of whom 24 573 (56%) initiated antidepressant treatment within 90 days of diagnosis; median age was 15 (IQR, 14-16) years. More than 95% of individuals who initiated treatment received SSRIs, with fluoxetine and sertraline being the most common types (eTable 3 in Supplement 1). Time from diagnosis to antidepressant initiation was short (mean, 10 days; median, 1 day [IQR, 0-11 days]), and 86% initiated antidepressants within 4 weeks. The estimated treatment periods lasted a mean (SD) of 398 (441) days (median, 250 [IQR, 113-513] days). A minority (11%) of the participants were dispensed only 1 antidepressant prescription. The outcome occurred in 96 individuals by 12 weeks and in 291 by 52 weeks.
Table 1 reports the nonweighted descriptive statistics of the cohort. The inverse probability of treatment-weighted descriptive statistics are available in eTable 4 in Supplement 1, showing that weighting produced excellent balance between groups in all covariates. A standardized mean difference smaller than 0.1 suggests a good balance between groups. The stabilized treatment weights had a mean of 0.995 (IQR, 0.751-1.113). A mean of 1 is considered to indicate no clear evidence of misspecification of the propensity model.47
Table 1. Nonweighted Cohort Characteristicsa.
| Characteristic | No. (%) | SMD (95% CI) | |
|---|---|---|---|
| Control (n = 19 104) | Treatment (n = 24 573) | ||
| Sex | |||
| Male | 6827 (35.7) | 7965 (32.4) | 0.070 (0.051 to 0.089) |
| Female | 12 277 (64.3) | 16 608 (67.6) | |
| Age, mean (SD), y | 14.66 (2.06) | 15.12 (1.72) | 0.240 (0.223 to 0.260) |
| Parental educational level | |||
| Primary school | 926 (4.8) | 855 (3.5) | 0.150 (0.130 to 0.168) |
| High school | 8081 (42.3) | 10 433 (42.5) | |
| University | 9197 (48.1) | 12 698 (51.7) | |
| Unknown | 900 (4.7) | 587 (2.4) | |
| Family income percentile | |||
| <20 | 1364 (7.1) | 1365 (5.6) | 0.153 (0.134 to 0.172) |
| 20-80 | 12 520 (65.5) | 16 152 (65.7) | |
| >80 | 4241 (22.2) | 6367 (25.9) | |
| Unknown | 979 (5.1) | 689 (2.8) | |
| Diagnosis year, mean (SD) | 2013.89 (3.71) | 2014.06 (3.70) | 0.047 (0.029 to 0.066) |
| Source of diagnosis | |||
| Outpatient | 17 422 (91.2) | 22 819 (92.9) | 0.071 (0.052 to 0.089) |
| Inpatient | 1432 (7.5) | 1566 (6.4) | |
| Unknown | 250 (1.3) | 188 (0.8) | |
| Parental bipolar disorder | |||
| No | 17 562 (91.9) | 23 021 (93.7) | 0.104 (0.085 to 0.123) |
| Yes | 804 (4.2) | 1035 (4.2) | |
| Unknown | 738 (3.9) | 517 (2.1) | |
| Parental depression | |||
| No | 15 742 (82.4) | 20 689 (84.2) | 0.104 (0.085 to 0.123) |
| Yes | 2623 (13.7) | 3368 (13.7) | |
| Unknown | 739 (3.9) | 516 (2.1) | |
| Prior hospitalizations | |||
| 0 | 16 655 (87.2) | 20 801 (84.6) | 0.078 (0.059 to 0.097) |
| 1 | 1862 (9.7) | 2887 (11.7) | |
| 2 or 3 | 475 (2.5) | 767 (3.1) | |
| ≥4 | 112 (0.6) | 118 (0.5) | |
| Diagnoses | |||
| Anxiety disorder | 4898 (25.6) | 8035 (32.7) | 0.156 (0.137 to 0.175) |
| Eating disorder | 1361 (7.1) | 1962 (8.0) | 0.033 (0.014 to 0.051) |
| Personality disorder | 240 (1.3) | 320 (1.3) | 0.004 (−0.015 to 0.023) |
| ADHD | 2908 (15.2) | 3317 (13.5) | 0.049 (0.030 to 0.068) |
| Developmental disorder | 1683 (8.8) | 2125 (8.6) | 0.006 (−0.013 to 0.025) |
| Autism spectrum disorder | 795 (4.2) | 1626 (6.6) | 0.109 (0.090 to 0.128) |
| Conduct disorder | 549 (2.9) | 574 (2.3) | 0.034 (0.015 to 0.053) |
| Alcohol use disorder | 431 (2.3) | 467 (1.9) | 0.025 (0.006 to 0.044) |
| Drug use disorder | 444 (2.3) | 410 (1.7) | 0.047 (0.028 to 0.066) |
| Poisoning by drugs | 1061 (5.6) | 1066 (4.3) | 0.056 (0.037 to 0.075) |
| Alcohol poisoning | 33 (0.2) | 32 (0.1) | 0.011 (0.037 to 0.075) |
| Suicidal behavior | 1323 (6.9) | 1418 (5.8) | 0.047 (0.028 to 0.066) |
| Medications | |||
| Antipsychotics | 294 (1.5) | 631 (2.6) | 0.073 (0.054 to 0.092) |
| Hypnotics/sedatives | 4365 (22.8) | 11 538 (47.0) | 0.523 (0.503 to 0.542) |
| Benzodiazepines | 40 (0.2) | 164 (0.7) | 0.069 (0.050 to 0.088) |
| Antiepileptics | 249 (1.3) | 198 (0.8) | 0.049 (0.030 to 0.068) |
| Opioids | 171 (0.9) | 241 (1.0) | 0.009 (−0.010 to 0.028) |
| Stimulants | 1719 (9.0) | 2017 (8.2) | 0.028 (0.009 to 0.047) |
Abbreviations: ADHD, attention deficit/hyperactivity disorder; SMD, absolute standardized mean difference.
The inverse probability of treatment-weighted cohort characteristics are available in eTable 4 in Supplement 1.
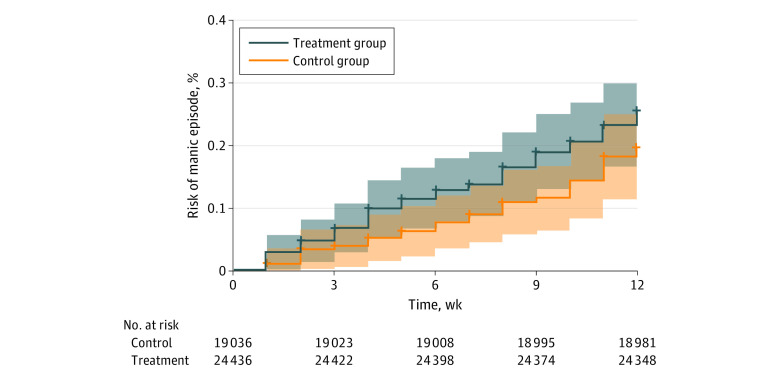
In the ITT analysis at 12 weeks, the estimated cumulative incidence of mania/hypomania was 0.26% (95% CI, 0.19%-0.33%) in the treatment group and 0.20% (95% CI, 0.13%-0.27%) in the control group, corresponding to a risk difference of 0.06% (95% CI, −0.04% to 0.16%) and a hazard ratio of 1.29 (95% CI, 0.83-2.03) (Table 2). The weighted risk curves are presented in the Figure. By 52 weeks, the cumulative incidence was 0.79% (95% CI, 0.68%-0.91%) in the treatment group and 0.52% (95% CI, 0.40%-0.63%) in the control group, with a risk difference of 0.28% (95% CI, 0.12%-0.44%) and a hazard ratio of 1.54 (95% CI, 1.18-2.00). The weighted risk curves are presented in eFigure 2 in Supplement 1. Nonweighted results show that weighting produced slightly attenuated associations (Table 2; eFigure 2 and eFigure 3 in Supplement 1). Boys in the treatment group appeared to have an increased risk of mania/hypomania relative to controls compared with the relative risk in girls (eTable 5 in Supplement 1). However, 95% CIs were highly overlapping. Per-protocol results were similar to the ITT estimates at 12 weeks, possibly showing attenuation at 52 weeks (Table 2).
Table 2. Main Analyses: Cumulative Incidence and Relative Risk of Mania/Hypomania at 12 and 52 Weeks.
| Outcome | No. of patients | No. of events | With IPT weighting (95% CI) | Without IPT weighting (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| Cumulative incidence, % | Risk difference, % | Hazard ratioa | Cumulative incidence, % | Risk difference, %e | Hazard ratioa | |||
| Intention to treat | ||||||||
| 12 wkb | ||||||||
| Control | 19 104 | 36 | 0.20 (0.13 to 0.27) | 0.06 (−0.04 to 0.16) | 1.29 (0.83 to 2.03) | 0.19 (0.13 to 0.25) | 0.06 (−0.03 to 0.14) | 1.30 (0.86 to 1.96) |
| Treatment | 24 573 | 60 | 0.26 (0.19 to 0.33) | 0.24 (0.18 to 0.31) | ||||
| 52 wkc | ||||||||
| Control | 19 104 | 95 | 0.52 (0.40 to 0.63) | 0.28 (0.12 to 0.44) | 1.54 (1.18 to 2.00) | 0.50 (0.39 to 0.60) | 0.30 (0.15 to 0.45) | 1.61 (1.25 to 2.05) |
| Treatment | 24 573 | 196 | 0.79 (0.68 to 0.91) | 0.80 (0.69 to 0.91) | ||||
| Per protocol | ||||||||
| 12 wk | ||||||||
| Control | 19 104 | 36 | 0.20 (0.08 to 0.31) | 0.02 (−0.13 to 0.17) | 1.11 (0.53 to 2.32) | 0.19 (0.12 to 0.26) | 0.02 (−0.06 to 0.11) | 1.12 (0.71 to 0.77) |
| Treatment | 24 573 | 49 | 0.22 (0.11 to 0.33) | 0.21 (0.16 to 0.27) | ||||
| 52 wk | ||||||||
| Control | 19 104 | 78 | 0.48 (0.28 to 0.68) | 0.15 (−0.11 to 0.41) | 1.31 (0.79 to 2.16) | 0.44 (0.35 to 0.54) | 0.21 (0.06 to 0.35) | 1.46 (1.11 to 1.93) |
| Treatment | 24 573 | 116 | 0.63 (0.44 to 0.82) | 0.65 (0.53 to 0.76) | ||||
Abbreviation: IPT, inverse probability of treatment.
Per-protocol analyses estimated risk ratios instead of hazard ratios because a discrete-time hazards model (a pooled logistic model) was used.
A total of 56% (n = 54) of the reported outcomes were based on diagnoses.
A total of 49% (n = 142) of the reported outcomes were based on diagnoses.
Figure. Estimated Cumulative Incidence of Mania/Hypomania During 12 Weeks of Follow-Up.
Data were weighted by the treatment propensity weights. Shaded areas represent 95% CIs; the gray shaded area indicates overlapping 95% CIs.
In the sensitivity analyses with different cohort, exposure, follow-up, and outcome definitions, the estimates remained similar to the main analyses (Table 3; nonweighted data given in eTable 6 in Supplement 1). Table 4 reports the association of baseline patient characteristics with the risk of mania/hypomania by 12 weeks. Hospitalization was associated with a 2.1-fold increased risk of mania/hypomania by 12 weeks, inpatient as the source of diagnosis with a 2.3-fold increased risk, parental bipolar disorder with a 4.1-fold increased risk, use of antipsychotics with a 4.4-fold increased risk, and use of antiepileptics with a 7.0-fold increased risk. The concordance index of the model was 0.77. Of all outcomes, 58% occurred in patients who had at least 1 of the top 5 predictors (8436 [20%] of the cohort). When antidepressant treatment was added to the model, it was not associated with the outcome, nor did the concordance index of the model change after its inclusion.
Table 3. Sensitivity Analyses: Cumulative Incidence and Relative Risk of Mania/Hypomania at 12 Weeksa .
| Outcome | Cumulative incidence, % (95% CI) | Risk difference (95% CI) | Hazard ratio | |
|---|---|---|---|---|
| Control | Treatment | |||
| Complete casesb | 0.20 (0.14 to 0.30) | 0.23 (0.17 to 0.30) | 0.03 (−0.07 to 0.13) | 1.15 (0.72 to 1.83) |
| Excluding certain medication usersc | 0.16 (0.10 to 0.22) | 0.20 (0.14 to 0.26) | 0.04 (−0.04 to 0.13) | 1.28 (0.78 to 2.09) |
| Extended grace periodd | 0.21 (0.15 to 0.28) | 0.26 (0.19 to 0.33) | 0.05 (−0.06 to 0.15) | 1.21 (0.78 to 1.89) |
| Restricted outcome definitione | 0.08 (0.04 to 0.11) | 0.06 (0.03 to 0.09) | −0.02 (−0.07 to 0.04) | 0.80 (0.36 to 1.74) |
| Extended outcome definitionf | 0.21 (0.14 to 0.28) | 0.27 (0.20 to 0.34) | 0.06 (−0.04 to 0.16) | 1.29 (0.83 to 2.00) |
| Extended follow-up lengthg | 0.25 (0.17 to 0.33) | 0.35 (0.27 to 0.43) | 0.10 (−0.01 to 0.21) | 1.39 (0.95 to 2.04) |
| Censoring for any hospitalizationh | 0.18 (0.11 to 0.24) | 0.24 (0.17 to 0.30) | 0.06 (−0.04 to 0.15) | 1.34 (0.83 to 2.15) |
| Cloning-censoring weighting, ITTi | 0.22 (0.16 to 0.30) | 0.22 (0.18 to 0.27) | 0.00 (−0.09 to 0.08) | 1.01 (0.68 to 1.48) |
| Cloning-censoring-weighting, PPi | 0.22 (0.16 to 0.31) | 0.21 (0.17 to 0.26) | −0.01 (−0.08 to 0.10) | 0.93 (0.62 to 1.39) |
| 60-d Gap treatment periods, PPj | 0.20 (0.08 to 0.31) | 0.22 (0.11 to 0.32) | 0.02 (−0.14 to 0.17) | 1.09 (0.51 to 2.32) |
| 1 Pill/d treatment periods, PPk | 0.20 (0.08 to 0.32) | 0.20 (0.09 to 0.32) | 0.00 (−0.15 to 0.16) | 1.01 (0.49 to 2.10) |
Abbreviations: ITT, intention to treat; PP, per protocol.
Estimates are from the ITT approach unless stated otherwise.
Individuals with missing information in any covariate were excluded (cohort n = 41 480).
Individuals who were dispensed any antipsychotic or antiepileptic medications within 4 months before initiation of follow-up were excluded (cohort n = 42 346).
The grace period was extended to 120 days after diagnosis in defining the treatment group.
Outcomes were restricted to those that occurred only in individuals who had at least 2 diagnoses or 1 diagnosis and 1 medication dispensation.
Outcome was extended to include dispensations for olanzapine (with indication for mood-stabilizing purpose).
Follow-up length was extended from 12 to 18 weeks.
Individuals were censored if they were hospitalized for any mental health reason (excluding the outcome).
Cloning-censoring weighting approach is described in detail in the eMethods in Supplement 1. Follow-up began from the time of the index diagnosis.
When defining antidepressant treatment periods, it was assumed that 2 dispensations falling within 60 days of each other belonged to the same treatment period. See the eMethods in Supplement 1 for details of this analysis.
Antidepressant treatment period lengths were estimated from the total quantity of pills dispensed and assuming that patients receive 1 pill per day. See the eMethods in Supplement 1 for details of this analysis.
Table 4. Patient Characteristics Associated With Mania/Hypomania in the 12-Week Follow-Up, Ranked According to Effect Size.
| Predictor | No. (%)a | HR (95% CI) |
|---|---|---|
| Use of antiepileptics | 410 (1.0) | 7.01 (3.31-14.84)b |
| Use of antipsychotics | 845 (2.0) | 4.42 (2.16-9.06)b |
| Parental bipolar disorder | 1810 (4.4) | 4.09 (2.24-7.44)b |
| Inpatient as source of diagnosis | 2790 (6.7) | 2.27 (1.13-4.58)b |
| Any prior hospitalization | 5709 (13.8) | 2.12 (1.08-4.17)b |
| Developmental disorder | 3618 (8.7) | 1.47 (0.79-2.73) |
| Use of stimulants | 3542 (8.5) | 1.39 (0.56-3.41) |
| Parental tertiary-level education | 21 564 (52.0) | 1.31 (0.84-2.02) |
| Personality or conduct disorder | 1524 (3.7) | 1.21 (0.50-2.89) |
| Alcohol or drug poisoning | 2018 (4.9) | 1.13 (0.35-3.61) |
| Age | NAc | 1.08 (0.96-1.22) |
| ADHD | 5915 (14.3) | 1.07 (0.47-2.40) |
| Anxiety disorder | 11 984 (28.9) | 1.03 (0.65-1.65) |
| Parental depression | 5873 (14.2) | 1.03 (0.59-1.80) |
| Parental income >80th percentile | 10 496 (25.3) | 1.02 (0.62-1.70) |
| Use of sedatives | 15 297 (36.9) | 1.01 (0.65-1.59) |
| Year of diagnosis | NAc | 0.93 (0.87-0.98)b |
| Female sex | 27 784 (67.0) | 0.87 (0.56-1.35) |
| Autism spectrum disorder | 2257 (5.4) | 0.45 (0.16-1.30) |
| Suicidal behavior | 2528 (6.1) | 0.34 (0.10-1.19) |
| Eating disorder | 3197 (7.7) | 0.32 (0.10-1.06) |
| Substance use disorder | 1512 (3.6) | 0.26 (0.06-1.10) |
Abbreviations: ADHD, attention deficit/hyperactivity disorder; HR, hazard ratio; NA, not applicable.
Percentage represents the proportion of individuals with the predictor.
Statistically significant coefficient.
Continuous variable for which the percentage cannot be calculated.
Discussion
This study investigated the risk of incident mania/hypomania in a cohort of pediatric patients with unipolar depression in Sweden. We found no evidence to suggest that antidepressants induce mania/hypomania in this patient group. The risk was similar in treatment and control groups by 12 weeks, which is the time window during which treatment-emergent mania is expected to occur. We found a small risk difference only in the longer follow-up analysis, suggesting that the risk increase may be attributable to factors other than the medication. We identified several patient characteristics that predicted mania/hypomania.
Our results contrast with an earlier register-based study reporting a substantially increased risk of mania in pediatric patients with unipolar depression treated with antidepressants.14 Differences in the results are likely explained by differences in study design. By using the target trial emulation framework, we aimed to minimize common biases in observational cohort studies.42 The most important differences, however, relate to the length of follow-up. The prior study followed up patients up to 5 years and excluded outcomes within the first 2 months. We used a shorter follow-up of 12 weeks consistent with the International Society for Bipolar Disorders Task Force consensus for defining treatment-emergent manic switch31,32 and did not exclude early outcomes. Conversion to mania long after treatment initiation may rather reflect selection bias, as patients with underlying bipolar disorder are also more likely to be prescribed antidepressants, and mania would have occurred eventually regardless of medication. We also observed a modest risk difference of 0.28% in the 52-week follow-up, which was further attenuated in the per-protocol analysis. The increased risk in this longer follow-up was likely produced by the combination of unadjusted time-varying confounding and the mentioned selection mechanism.
However, our findings are in line with RCTs.12,21,22 Similarly to RCTs, our cohort study found incident mania to be rare in the clinical practice setting. Furthermore, RCTs have found no clear evidence of antidepressants increasing the risk of mania compared with placebo.21,22 There have been concerns of clinical trials having inadequate data on adverse outcomes and for excluding individuals with the highest risk of developing mania.21,22 Our data offer complementary information to RCTs from a large cohort of patients treated in actual clinical practice. In addition, our findings converge with a recent study in adults with bipolar disorder, which found no increased risk of new-onset mania following antidepressant initiation in a community setting.48
Several patient characteristics predicted mania/hypomania by 12 weeks. In line with previous literature,8,13,35 psychosis (for which antipsychotics were an indicator), hospitalizations, and family history of bipolar disorder were associated with 2- to 4-fold increased risks. Additionally, we found the use of antiepileptics to be associated with mania/hypomania. The association may indicate that the patients had shown signs of mood instability shortly after the time of diagnosis.49 Antidepressant treatment was unrelated to the risk of mania/hypomania, suggesting that these other characteristics are more relevant when evaluating which patients may have an increased risk of switching from unipolar depression to mania. Our model using administrative information had a moderate predictive ability, suggesting it is possible to identify patients at high risk for mania/hypomania with a prognostic clinical prediction model. The model has potential to be improved in later work.
Strengths and Limitations
A major strength of this study is its large sample size and recent data covering pediatric psychiatric care in Sweden nationwide. We minimized common biases using the target trial emulation framework and adjusted for several important confounders, such as parental mood disorders. Our results were consistent across 11 robustness checks, including analyses to address potential immortal time bias.
The study also has limitations. Our findings apply primarily to SSRIs. Because we used diagnoses and dispensed medications to measure mania/hypomania, it is possible that some outcomes were misclassified. We did not have information concerning medication dosing and thus cannot rule out that increased dosing contributed to the increased risk in the 52-week follow-up. However, most dosage increases occur early in treatment during titration. Lack of dosing information may also have caused imprecision in the estimated medication exposure periods in per-protocol analyses. In addition, the time frame used for treatment-emergent mania is based on expert consensus. This time frame may be arbitrary since we do not yet know the biological mechanism underlying antidepressant-related adverse reactions. Nevertheless, the data were consistent with no increased risk up to 18 weeks.
Conclusions
In this cohort study, by 12 weeks of follow-up, we found no significant differences in the risk of mania/hypomania between pediatric patients with unipolar depression treated with antidepressants and those not treated with antidepressants. If antidepressants were to induce mania/hypomania, differences would be expected to emerge by then. A small risk difference was found only in the longer follow-up of 52 weeks, suggesting that the risk increase may be attributable to factors other than the medication.
eMethods. Detailed Methods
eReferences
eTable 1. Description of Covariates
eTable 2. Target Trial Emulation Protocol
eFigure 1. Prescription Time-Distribution Matching to Assign Start of Follow-Up
eTable 3. Frequencies of Different Antidepressant Medications in the Treatment Group
eTable 4. Weighted Descriptive Statistics of the Cohort
eFigure 2. The Estimated Cumulative Incidence of Mania/Hypomania Over 52 Weeks of Follow-Up
eFigure 3. The Estimated Cumulative Incidence of Mania/Hypomania Over 12 Weeks of Follow-Up
eTable 5. Sex-Stratified Analyses: Cumulative Incidence and the Relative Risk of Mania/Hypomania in the Treatment and Control Groups at 12 and 52 Weeks of Follow-Up
eTable 6. Sensitivity Analyses: Non-Weighted Cumulative Incidence and the Relative Risk of Mania/Hypomania in the Treatment and Control Groups at 12 Weeks of Follow-Up
Data Sharing Statement
References
- 1.Polanczyk GV, Salum GA, Sugaya LS, Caye A, Rohde LA. Annual research review. J Child Psychol Psychiatry. 2015;56(3):345-365. doi: 10.1111/jcpp.12381 [DOI] [PubMed] [Google Scholar]
- 2.Ghandour RM, Sherman LJ, Vladutiu CJ, et al. Prevalence and treatment of depression, anxiety, and conduct problems in US children. J Pediatr. 2019;206:256-267.e3. doi: 10.1016/j.jpeds.2018.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in US adolescents. J Am Acad Child Adolesc Psychiatry. 2010;49(10):980-989. doi: 10.1016/j.jaac.2010.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mojtabai R, Olfson M, Han B. National trends in the prevalence and treatment of depression in adolescents and young adults. Pediatrics. 2016;138(6):e20161878. doi: 10.1542/peds.2016-1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett K, Courtney D, Duda S, Henderson J, Szatmari P. An appraisal of the trustworthiness of practice guidelines for depression and anxiety in children and youth. Depress Anxiety. 2018;35(6):530-540. doi: 10.1002/da.22752 [DOI] [PubMed] [Google Scholar]
- 6.Wehr TA, Goodwin FK. Rapid cycling in manic-depressives induced by tricyclic antidepressants. Arch Gen Psychiatry. 1979;36(5):555-559. doi: 10.1001/archpsyc.1979.01780050065007 [DOI] [PubMed] [Google Scholar]
- 7.Allain N, Leven C, Falissard B, et al. Manic switches induced by antidepressants. Acta Psychiatr Scand. 2017;135(2):106-116. doi: 10.1111/acps.12672 [DOI] [PubMed] [Google Scholar]
- 8.Gill N, Bayes A, Parker G. A review of antidepressant-associated hypomania in those diagnosed with unipolar depression-risk factors, conceptual models, and management. Curr Psychiatry Rep. 2020;22(4):20. doi: 10.1007/s11920-020-01143-6 [DOI] [PubMed] [Google Scholar]
- 9.Patel R, Reiss P, Shetty H, et al. Do antidepressants increase the risk of mania and bipolar disorder in people with depression? BMJ Open. 2015;5(12):e008341. doi: 10.1136/bmjopen-2015-008341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacob L, Bohlken J, Kostev K. Incidence of and factors associated with manic episodes and bipolar disorder in the decade following depression onset in Germany. J Affect Disord. 2020;266:534-539. doi: 10.1016/j.jad.2020.02.004 [DOI] [PubMed] [Google Scholar]
- 11.Viktorin A, Lichtenstein P, Thase ME, et al. The risk of switch to mania in patients with bipolar disorder during treatment with an antidepressant alone and in combination with a mood stabilizer. Am J Psychiatry. 2014;171(10):1067-1073. doi: 10.1176/appi.ajp.2014.13111501 [DOI] [PubMed] [Google Scholar]
- 12.Peet M. Induction of mania with selective serotonin re-uptake inhibitors and tricyclic antidepressants. Br J Psychiatry. 1994;164(4):549-550. doi: 10.1192/bjp.164.4.549 [DOI] [PubMed] [Google Scholar]
- 13.Baldessarini RJ, Faedda GL, Offidani E, et al. Antidepressant-associated mood-switching and transition from unipolar major depression to bipolar disorder. J Affect Disord. 2013;148(1):129-135. doi: 10.1016/j.jad.2012.10.033 [DOI] [PubMed] [Google Scholar]
- 14.Martin A, Young C, Leckman JF, Mukonoweshuro C, Rosenheck R, Leslie D. Age effects on antidepressant-induced manic conversion. Arch Pediatr Adolesc Med. 2004;158(8):773-780. doi: 10.1001/archpedi.158.8.773 [DOI] [PubMed] [Google Scholar]
- 15.Sharma T, Guski LS, Freund N, Gøtzsche PC. Suicidality and aggression during antidepressant treatment. BMJ. 2016;352:i65. doi: 10.1136/bmj.i65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lagerberg T, Fazel S, Molero Y, et al. Associations between selective serotonin reuptake inhibitors and violent crime in adolescents, young, and older adults. Eur Neuropsychopharmacol. 2020;36:1-9. doi: 10.1016/j.euroneuro.2020.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hemminki E, Merikukka M, Gissler M, et al. Antidepressant use and violent crimes among young people. J Epidemiol Community Health. 2017;71(1):12-18. doi: 10.1136/jech-2016-207265 [DOI] [PubMed] [Google Scholar]
- 18.Healy D, Herxheimer A, Menkes DB. Antidepressants and violence. PLoS Med. 2006;3(9):e372. doi: 10.1371/journal.pmed.0030372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lagerberg T, Fazel S, Sjölander A, Hellner C, Lichtenstein P, Chang Z. Selective serotonin reuptake inhibitors and suicidal behaviour. Neuropsychopharmacology. 2022;47(4):817-823. doi: 10.1038/s41386-021-01179-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hetrick SE, McKenzie JE, Cox GR, et al. Newer generation antidepressants for depressive disorders in children and adolescents. BJPsych Adv. 2017;23(2):74. doi: 10.1192/apt.23.2.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joseph MF, Youngstrom EA, Soares JC. Antidepressant-coincident mania in children and adolescents treated with selective serotonin reuptake inhibitors. Future Neurol. 2009;4(1):87-102. doi: 10.2217/14796708.4.1.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheung AH, Emslie GJ, Mayes TL. Review of the efficacy and safety of antidepressants in youth depression. J Child Psychol Psychiatry. 2005;46(7):735-754. doi: 10.1111/j.1469-7610.2005.01467.x [DOI] [PubMed] [Google Scholar]
- 23.Murphy SE, Capitão LP, Giles SLC, Cowen PJ, Stringaris A, Harmer CJ. The knowns and unknowns of SSRI treatment in young people with depression and anxiety. Lancet Psychiatry. 2021;8(9):824-835. doi: 10.1016/S2215-0366(21)00154-1 [DOI] [PubMed] [Google Scholar]
- 24.Bachmann CJ, Aagaard L, Burcu M, et al. Trends and patterns of antidepressant use in children and adolescents from five Western countries, 2005-2012. Eur Neuropsychopharmacol. 2016;26(3):411-419. doi: 10.1016/j.euroneuro.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 25.Olfson M, Druss BG, Marcus SC. Trends in mental health care among children and adolescents. N Engl J Med. 2015;372(21):2029-2038. doi: 10.1056/NEJMsa1413512 [DOI] [PubMed] [Google Scholar]
- 26.Lagerberg T, Molero Y, D’Onofrio BM, et al. Antidepressant prescription patterns and CNS polypharmacy with antidepressants among children, adolescents, and young adults. Eur Child Adolesc Psychiatry. 2019;28(8):1137-1145. doi: 10.1007/s00787-018-01269-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andersen SL, Navalta CP. Altering the course of neurodevelopment. Int J Dev Neurosci. 2004;22(5-6):423-440. doi: 10.1016/j.ijdevneu.2004.06.002 [DOI] [PubMed] [Google Scholar]
- 28.Andersen SL, Navalta CP. Annual research review: new frontiers in developmental neuropharmacology J Child Psychol Psychiatry. 2011;52(4):476-503. doi: 10.1111/j.1469-7610.2011.02376.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Safer DJ, Zito JM. Treatment-emergent adverse events from selective serotonin reuptake inhibitors by age group: children versus adolescents. J Child Adolesc Psychopharmacol. 2006;16(1-2):159-169. doi: 10.1089/cap.2006.16.159 [DOI] [PubMed] [Google Scholar]
- 30.Goldberg JF, Truman CJ. Antidepressant-induced mania. Bipolar Disord. 2003;5(6):407-420. doi: 10.1046/j.1399-5618.2003.00067.x [DOI] [PubMed] [Google Scholar]
- 31.Tohen M, Frank E, Bowden CL, et al. The International Society for Bipolar Disorders (ISBD) Task Force report on the nomenclature of course and outcome in bipolar disorders. Bipolar Disord. 2009;11(5):453-473. doi: 10.1111/j.1399-5618.2009.00726.x [DOI] [PubMed] [Google Scholar]
- 32.Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society for Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170(11):1249-1262. doi: 10.1176/appi.ajp.2013.13020185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reinblatt SP, Dosreis S, Walkup JT, Riddle MA. Activation adverse events induced by the selective serotonin reuptake inhibitor fluvoxamine in children and adolescents. J Child Adolesc Psychopharmacol. 2009;19(2):119-126. doi: 10.1089/cap.2008.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilens TE, Biederman J, Kwon A, et al. A systematic chart review of the nature of psychiatric adverse events in children and adolescents treated with selective serotonin reuptake inhibitors. J Child Adolesc Psychopharmacol. 2003;13(2):143-152. doi: 10.1089/104454603322163862 [DOI] [PubMed] [Google Scholar]
- 35.Uchida M, Serra G, Zayas L, et al. Can manic switches be predicted in pediatric major depression? J Affect Disord. 2015;172:300-306. doi: 10.1016/j.jad.2014.09.046 [DOI] [PubMed] [Google Scholar]
- 36.Dwyer JB, Bloch MH. Antidepressants for pediatric patients. Curr Psychiatr. 2019;18(9):26-42F. [PMC free article] [PubMed] [Google Scholar]
- 37.Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number. Eur J Epidemiol. 2009;24(11):659-667. doi: 10.1007/s10654-009-9350-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ludvigsson JF, Almqvist C, Bonamy AKE, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol. 2016;31(2):125-136. doi: 10.1007/s10654-016-0117-y [DOI] [PubMed] [Google Scholar]
- 39.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish National Inpatient Register. BMC Public Health. 2011;11(1):450. doi: 10.1186/1471-2458-11-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wettermark B, Hammar N, Fored CM, et al. The new Swedish Prescribed Drug Register—opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16(7):726-735. doi: 10.1002/pds.1294 [DOI] [PubMed] [Google Scholar]
- 41.Ekbom A. The Swedish Multi-Generation Register. In: Dillner J, ed. Methods in Biobanking: Methods in Molecular Biology. Vol 675. Humana Press; 2011. doi: 10.1007/978-1-59745-423-0_10 [DOI] [PubMed] [Google Scholar]
- 42.Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183(8):758-764. doi: 10.1093/aje/kwv254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou Z, Rahme E, Abrahamowicz M, Pilote L. Survival bias associated with time-to-treatment initiation in drug effectiveness evaluation. Am J Epidemiol. 2005;162(10):1016-1023. doi: 10.1093/aje/kwi307 [DOI] [PubMed] [Google Scholar]
- 44.Matthews AA, Szummer K, Dahabreh IJ, et al. Comparing effect estimates in randomized trials and observational studies from the same population. J Am Heart Assoc. 2021;10(11):e020357. doi: 10.1161/JAHA.120.020357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Region Stockholm Barn- och Ungdomspsykiatri. Farmakainstruktion: kunskapsstöd för barn- och ungdomspsykiatrisk läkemedelsbehandling inom BUP Stockholm. January 17, 2023. Accessed May 15, 2023. https://www.bup.se/494342/contentassets/c5b865fe86d44fbd957c9d71eaaeadbe/farmakainstruktion-bup-stockholm.pdf
- 46.Maringe C, Benitez Majano S, Exarchakou A, et al. Reflection on modern methods: trial emulation in the presence of immortal-time bias. Int J Epidemiol. 2020;49(5):1719-1729. doi: 10.1093/ije/dyaa057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hernán MA, Robins JM. Estimating causal effects from epidemiological data. J Epidemiol Community Health. 2006;60(7):578-586. doi: 10.1136/jech.2004.029496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jefsen OH, Rohde C, Østergaard SD. Revisiting the association between treatment with antidepressants and mania. Bipolar Disord. 2023;00:1-9. doi: 10.1111/bdi.13353 [DOI] [PubMed] [Google Scholar]
- 49.Wolf RM, Hall M, Williams DJ, et al. Pharmacologic restraint use for children experiencing mental health crises in pediatric hospitals. J Hosp Med. 2023;18(2):120-129. doi: 10.1002/jhm.13009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Detailed Methods
eReferences
eTable 1. Description of Covariates
eTable 2. Target Trial Emulation Protocol
eFigure 1. Prescription Time-Distribution Matching to Assign Start of Follow-Up
eTable 3. Frequencies of Different Antidepressant Medications in the Treatment Group
eTable 4. Weighted Descriptive Statistics of the Cohort
eFigure 2. The Estimated Cumulative Incidence of Mania/Hypomania Over 52 Weeks of Follow-Up
eFigure 3. The Estimated Cumulative Incidence of Mania/Hypomania Over 12 Weeks of Follow-Up
eTable 5. Sex-Stratified Analyses: Cumulative Incidence and the Relative Risk of Mania/Hypomania in the Treatment and Control Groups at 12 and 52 Weeks of Follow-Up
eTable 6. Sensitivity Analyses: Non-Weighted Cumulative Incidence and the Relative Risk of Mania/Hypomania in the Treatment and Control Groups at 12 Weeks of Follow-Up
Data Sharing Statement