Abstract
We describe a simple procedure for detecting fluconazole-resistant yeasts by a disk diffusion method. Forty clinical Candida sp. isolates were tested on RPMI-glucose agar with either 25- or 50-μg fluconazole disks. With 25-μg disks, zones of inhibition of ≥20 mm at 24 h accurately identified 29 of 29 isolates for which MICs were ≤8 μg/ml, and with 50-μg disks, zones of ≥27 mm identified 28 of 29 such isolates. All 11 isolates for which MICs were >8 μg/ml were identified by using either disk. Disk diffusion may be a useful screening method for clinical microbiology laboratories.
Advances in supportive therapy for immunocompromised patients have led to increased rates of fungal infections (9, 20). With the widespread use of fluconazole for treatment and prevention of oropharyngeal candidiasis, the most common fungal infection in patients infected with human immunodeficiency virus (18, 22), clinical resistance is becoming a serious problem (5, 11, 18, 22). Consequently, a rapid, reproducible method of fluconazole susceptibility testing would be useful in determining the epidemiology of and optimal treatment for infection with resistant isolates (4, 8, 13, 21). Candida albicans is usually acutely susceptible to fluconazole; fluconazole MICs for approximately 90% of C. albicans isolates are ≤1 μg/ml (16, 19). Some non-C. albicans yeasts have been noted to have decreased susceptibility or resistance to fluconazole (19, 22); these species include C. glabrata and C. krusei, which are being isolated more frequently (7, 24).
Screening clinical yeast isolates for decreased susceptibility to fluconazole has been difficult. Methodologies such as the standard National Committee for Clinical Laboratory Standards (NCCLS) broth macrodilution test (12) and alternative methods such as the E test or microdilution adaptations of the NCCLS method generally compare favorably for determining MICs for isolates (3, 10, 15, 23); however, these are not easily adapted to the screening of yeasts for fluconazole susceptibility. A disk diffusion method analogous to that used for testing antibacterial agents could be easily incorporated into a clinical laboratory and serve as an effective means for fluconazole susceptibility screening. Previously described disk diffusion methods utilize disks or employ media not available for routine use (2, 24). We have developed a disk diffusion method for susceptibility screening which uses disks that are simple to prepare and standard media, such that it could be easily implemented in routine clinical mycology laboratories.
(This study was presented in part at the 97th General Meeting of the American Society for Microbiology, Miami Beach, Fla., 4 to 8 May 1997 [25]).
Medium.
RPMI 1640 with l-glutamine and morpholinepropanesulfonic acid (MOPS) organic buffer (ABI, Niagara Falls, N.Y.) was prepared from a powdered medium at double the desired concentration with 500 ml of deionized H2O, and the solution was sterile filtered. Bacto Agar (Difco Laboratories, Detroit, Mich.) was also prepared at a 2× concentration by adding 20 g of agar to 500 ml of deionized H2O (14). The solution was mixed over heat to dissolve the agar, autoclaved, and then cooled to 48°C in a water bath. The agar and RPMI 1640 solutions were combined at 48°C and stirred, and approximately 20 ml was dispensed into sterile 100-mm-diameter petri plates. The medium was allowed to cool and harden at room temperature for 3 to 5 days, and the plates were then stored at 4°C and used within 1 to 2 weeks.
Organisms and reference methods.
Forty clinical isolates of Candida spp., representing forty consecutive clinical samples, which were submitted to the Fungus Testing Lab (The University of Texas Health Science Center, San Antonio, Tex.) for MIC determination by both the NCCLS broth macrodilution procedure (12) and a broth microdilution adaptation (1, 6) were selected for evaluation. Both 24- and 48-h MIC readings were evaluated, coordinating, respectively, with 24- and 48-h disk results. The breakdown of Candida species was as follows: 35 isolates were C. albicans, 2 were C. krusei, 1 was C. tropicalis, 1 was C. parapsilosis, and 1 was C. glabrata.
Fluconazole disks.
Twenty-five-microgram disks were prepared by pipetting 12.5-μl volumes of stock fluconazole (2 mg/ml; Pfizer-Roerig, New York, N.Y.) onto sterile blank disks (Difco Laboratories), while 50-μg disks were similarly prepared by using 25 μl of fluconazole solution. The disks were dried and then stored at 4°C until use within 1 to 2 weeks.
Inoculum.
The yeast isolates were stored at room temperature in sterile deionized H2O, subcultured onto Sabouraud dextrose agar (BBL, Cockeysville, Md.) to ensure purity and viability (2), and then subcultured again to select for isolated colonies. Three to five colonies were then suspended in 5.0 ml of sterile deionized H2O and mixed thoroughly on a vortex mixer. The suspension was adjusted to a 0.5 McFarland turbidity standard (106 CFU/ml) by using a spectrophotometer (17). Serial log10 dilutions of the adjusted suspension were prepared and quantitatively cultured to determine counts of CFU. A 1-ml inoculum from a suspension of 104 CFU/ml yielded optimal confluent growth for lawn formation.
Paired RPMI-glucose plates were individually inoculated with 1 ml of suspension formed from each yeast isolate, which was spread on the surface of the medium with a bent, sterile glass rod. A disk containing 25 μg of fluconazole was then applied to one inoculated plate, and a 50-μg fluconazole disk was applied to the other by using flamed forceps. Duplicate sets of plates were incubated at 30°C, and zone diameters were measured at 24 and 48 h. For each yeast isolate, the procedure was performed three separate times and the results were compared for reproducibility.
Zone determination and comparative MIC interpretation.
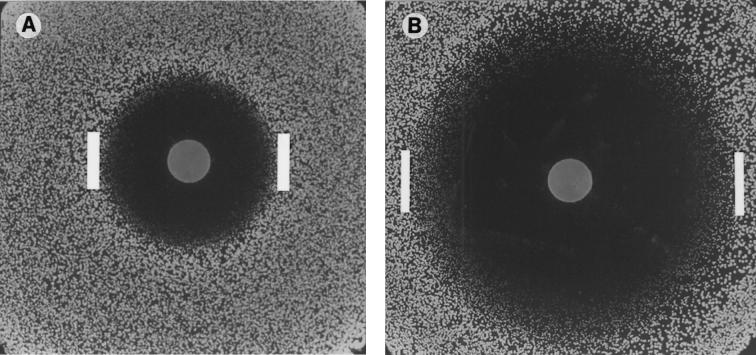
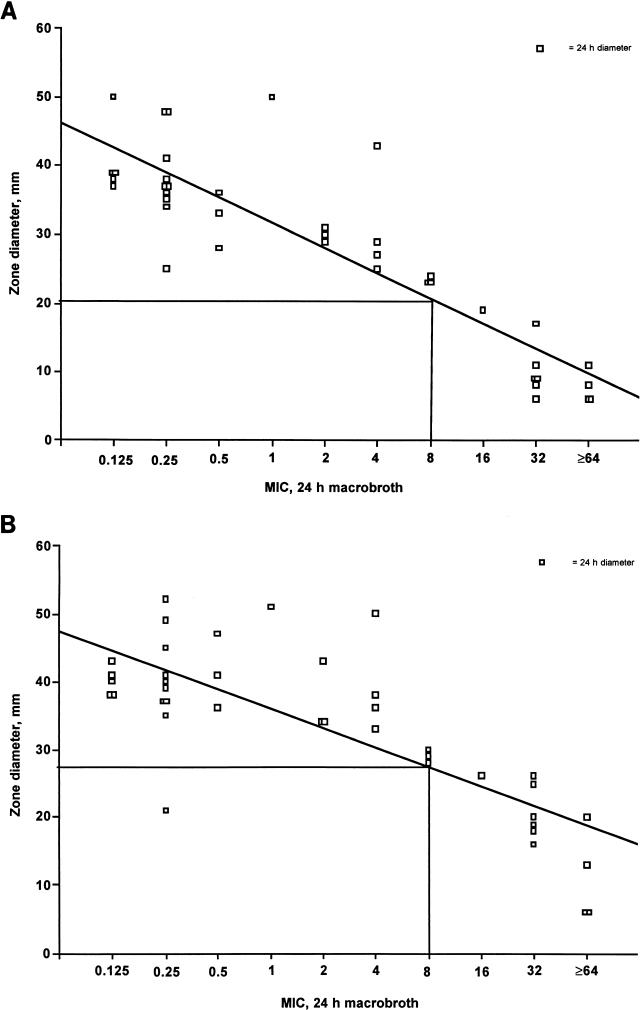
Inhibitory zone diameters were measured at the transitional point where growth abruptly decreased, as determined by a marked reduction in colony sizes (Fig. 1). The zone sizes measured at 24 and 48 h were then plotted against respective 24- and 48-h MICs determined by the reference methods. To interpret zone diameters, the NCCLS method was followed, in which MIC breakpoints of ≤8 μg/ml define susceptibility and ≥64 μg/ml define resistance, with MICs of between 16 and 32 μg/ml reflecting dose-dependent susceptibility (12). Zones corresponding to these MIC breakpoints were determined graphically through regression analysis for 25-μg (Fig. 2A) and 50-μg (panel B) disks at 24 and 48 h (data not shown).
FIG. 1.
(A) A 25-μg fluconazole disk on a lawn of 104 CFU of C. albicans after 24 h of incubation. (B) A 50-μg fluconazole disk on a lawn of 104 CFU of C. albicans after 48 h of incubation. Inhibitory zone diameters were measured at the transitional point where growth abruptly decreased (interior edges of bars), as determined by a marked reduction in colony sizes.
FIG. 2.
(A) Regression analysis correlating zones of inhibition (24 h) obtained with 25-μg fluconazole disks with NCCLS 24-h broth macrodilution MICs. (B) Regression analysis correlating zones of inhibition (24 h) obtained with 50-μg fluconazole disks with NCCLS 24-h broth macrodilution MICs.
Regression analysis on curve plots for 25- and 50-μg disk zones versus macrodilution MICs defined interpretive breakpoints of ≥20 mm for susceptibility (MIC ≤ 8 μg/ml) with 25-μg disks and ≥27 mm with 50-μg disks at both 24 and 48 h. Zones which were smaller than these breakpoints were defined as indicating either dose-dependent susceptibility or resistance.
Three separate trials with 25- and 50-μg disks were performed on all 40 isolates for verification of reproducibility. Regression analysis indicated that zones could vary up to 4 mm and remain within ±1 reference tube dilution (NCCLS broth macrodilution method). Zones from all three trials met these criteria for 95% of isolates at 24 h, but at 48 h criteria were met for only 86% (data not shown).
Zone diameters measured at 24 h for 25- and 50-μg disks showed excellent correlation with predicted macrodilution 24-h MICs for susceptible yeasts. Diameters for 25-μg drug concentration disks accurately identified 29 of 29 (100%) of the isolates for which MICs were ≤8 μg/ml. Disks with 50 μg of fluconazole provided similar results, identifying 28 of 29 (97%) of the isolates for which MICs were ≤8 μg/ml. The zone diameters also correlated well with macrodilution 24-h MICs in predicting either dose-dependent susceptibility or resistance in yeast strains. Zones at 24 h with either 25- or 50-μg disks identified 11 of 11 (100%) isolates for which MICs were ≥16 μg/ml. Although yeast strains for which MICs were ≥16 μg/ml were accurately identified by the disk method, results could not adequately differentiate strains with dose-dependent susceptibility (MICs of 16 to 32 μg/ml) from fully resistant strains (MICs of ≥64 μg/ml). Comparison of disk diameters to microdilution MICs showed correlation similar to the correlation with broth macrodilution reference method MICs (data not shown).
Zone diameters after 48 h correlated less well with the reference macrodilution 48-h MICs at either drug concentration, with the 25- or 50-μg disks accurately predicting MICs of ≤8 μg/ml for 23 of 26 (88%) isolates. Sensitivity in identification of yeasts for which MICs were ≥16 μg/ml was much lower at 48 h: predictions were correct for only 11 of 14 (79%) isolates with 25-μg disks and 12 of 14 (86%) isolates with 50-μg disks. As with 24-h results, comparison of disk diameters to microdilution MICs showed similar correlation (data not shown).
As a screening tool, the disk diffusion procedure correlated well with the NCCLS reference broth macrodilution method and the microdilution method in identifying fluconazole-susceptible yeasts. In this study, both 25- and 50-μg fluconazole disks allowed correct determination of all fluconazole-susceptible isolates examined. Quick discernment of susceptibility would allow clinical laboratories to focus more effort on characterization of less-susceptible isolates. Yeasts which have dose-dependent susceptibility or frank resistance could not be readily differentiated from each other based on disk diffusion results. Such isolates could be reexamined by other, more discriminating tests such as the E test or a broth microdilution adaptation of the NCCLS method.
Reading inoculated plates with 25-μg disks after 24 h resulted in the most sensitive method, accurately identifying 100% of susceptible Candida spp. with a diameter of ≥20 mm. Although 50-μg disks also yielded good results, no distinct advantage of using the higher-concentration disk rather than the 25-μg disk was found. Zone diameter results noted after 48 h were much less sensitive than the corresponding 24-h results for disks of both drug concentrations. Disk diffusion testing could probably be performed at 24 h with most Candida species; however, some species, such as C. krusei, do not always show resistance at 24 h and results should be read at 48 h (2).
The fluconazole disk diffusion procedure showed very good reproducibility at 24 h (95%), with some decline after 48 h (86%). The presence of microcolonies necessitated measurement of zone diameters at the transitional point where growth abruptly decreased from normal-sized colonies (Fig. 1). Thus, a certain amount of subjectivity was introduced into zone determination, as is the case for endpoint determination decisions made with the E-test (4) or NCCLS M27-A (12) methods.
The use of the fluconazole disk diffusion procedure appears to be a convenient and sensitive method for susceptibility screening or detection of fluconazole-resistant yeasts. Additional studies with a wider variety of yeast isolates should be conducted to determine the appropriateness of screening clinical isolates in this manner.
REFERENCES
- 1.Barchiesi F, Colombo A L, McGough D A, Rinaldi M G. Comparative study of broth macrodilution and microdilution techniques for in vitro antifungal susceptibility testing of yeasts by using the National Committee for Clinical Laboratory Standards’ proposed standard. J Clin Microbiol. 1994;32:2494–2500. doi: 10.1128/jcm.32.10.2494-2500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry A L, Brown S D. Fluconazole disk diffusion procedure for determining susceptibility of Candida species. J Clin Microbiol. 1996;34:2154–2157. doi: 10.1128/jcm.34.9.2154-2157.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen S C A, O’Donnell M L, Gordon S, Gilbert G L. Antifungal susceptibility testing using the E-test: comparison with the broth macrodilution technique. J Antimicrob Chemother. 1996;37:265–273. doi: 10.1093/jac/37.2.265. [DOI] [PubMed] [Google Scholar]
- 4.Colombo A L, Barchiesi F, McGough D A, Rinaldi M G. Comparison of E-test and National Committee for Clinical Laboratory Standards broth macrodilution method for azole antifungal susceptibility testing. J Clin Microbiol. 1995;33:535–540. doi: 10.1128/jcm.33.3.535-540.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dermoumi H. In vitro susceptibility of yeast isolates from the blood to fluconazole and amphotericin B. Chemotherapy. 1992;38:112–117. doi: 10.1159/000238950. [DOI] [PubMed] [Google Scholar]
- 6.Espinel-Ingroff A, Kish G W, Kerkering T M, Fromtling R A, Bartizal K, Galgiani J N, Villareal K, Pfaller M A, Gerarden T, Rinaldi M G, Fothergill A W. Collaborative comparison of broth macrodilution and microdilution antifungal susceptibility tests. J Clin Microbiol. 1992;30:3138–3145. doi: 10.1128/jcm.30.12.3138-3145.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan-Harvard P, Capano D, Smith S M, Mangia A, Eng R H. Development of resistance in Candida isolates from patients receiving prolonged antifungal therapy. Antimicrob Agents Chemother. 1991;35:2302–2305. doi: 10.1128/aac.35.11.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fromtling R A, Galgiani J N, Pfaller M, Espinel-Ingroff A, Bartizal K F, Bartlett M S, Body B A, Frey C, Hall G, Roberts G D, Nolte F B, Odds F C, Rinaldi M G, Sugar A M, Villareal K. Multicenter evaluation of a broth macrodilution antifungal susceptibility test for yeasts. Antimicrob Agents Chemother. 1993;37:39–45. doi: 10.1128/aac.37.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghannoum M A, Fu Y, Ibrahim A S, Mortara L A, Shafiq M C, Edwards J E, Jr, Criddle R S. In vitro determination of optimal antifungal combinations against Cryptococcus neoformans and Candida albicans. Antimicrob Agents Chemother. 1995;39:2459–2465. doi: 10.1128/aac.39.11.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghannoum M A, Rex J H, Galgiani J N. Susceptibility testing of fungi: current status of correlation of in vitro data with clinical outcome. J Clin Microbiol. 1996;34:489–495. doi: 10.1128/jcm.34.3.489-495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Millon L, Manteaux A, Reboux G, Drobacheff C, Monod M, Barale T, Michel-Briand Y. Fluconazole-resistant recurrent oral candidiasis in human immunodeficiency virus-positive patients: persistence of Candida albicans strains with the same genotype. J Clin Microbiol. 1994;32:1115–1118. doi: 10.1128/jcm.32.4.1115-1118.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard. NCCLS document M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 13.Patterson T F, Kirkpatrick W R, Revankar S G, McAtee R K, Fothergill A W, McCarthy D I, Rinaldi M G. Comparative evaluation of macrodilution and chromogenic agar screening for determining fluconazole susceptibility of Candida albicans. J Clin Microbiol. 1996;34:3237–3239. doi: 10.1128/jcm.34.12.3237-3239.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patterson T F, Revankar S G, Kirkpatrick W R, Dib O P, Fothergill A W, Redding S W, Sutton D A, Rinaldi M G. Simple method for detecting fluconazole-resistant yeasts with chromogenic agar. J Clin Microbiol. 1996;34:1794–1797. doi: 10.1128/jcm.34.7.1794-1797.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfaller M A, Barry A L. Evaluation of a novel colorimetric broth microdilution method for antifungal susceptibility testing of yeast isolates. J Clin Microbiol. 1994;32:1992–1996. doi: 10.1128/jcm.32.8.1992-1996.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfaller M A, Barry A L. In vitro susceptibilities of clinical yeast isolates to three antifungal agents determined by the microdilution method. Mycopathologia. 1995;130:3–9. doi: 10.1007/BF01104343. [DOI] [PubMed] [Google Scholar]
- 17.Pfaller M A, Burmeister L, Bartlett M A, Rinaldi M G. Multicenter evaluation of four methods of yeast inoculum preparation. J Clin Microbiol. 1988;26:1437–1441. doi: 10.1128/jcm.26.8.1437-1441.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Revankar S G, Kirkpatrick W R, McAtee R K, Dib O P, Fothergill A W, Redding S W, Rinaldi M G, Patterson T F. Detection and significance of fluconazole resistance in oropharyngeal candidiasis in human immunodeficiency virus-infected patients. J Infect Dis. 1996;174:821–827. doi: 10.1093/infdis/174.4.821. [DOI] [PubMed] [Google Scholar]
- 19.Rex J H, Pfaller M A, Barry A L, Nelson P W, Webb C D. Antifungal susceptibility testing of isolates from a randomized, multicenter trial of fluconazole versus amphotericin B as treatment of nonneutropenic patients with candidemia. Antimicrob Agents Chemother. 1995;39:40–44. doi: 10.1128/aac.39.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rex J H, Pfaller M A, Galgiani J N, Bartlett M S, Espinel-Ingroff A, Ghannoum M A, Lancaster M, Odds F C, Rinaldi M G, Walsh T J, Barry A L. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infections. Clin Infect Dis. 1997;24:235–247. doi: 10.1093/clinids/24.2.235. [DOI] [PubMed] [Google Scholar]
- 21.Rex J H, Pfaller M A, Rinaldi M G, Polak A, Galgiani J N. Antifungal susceptibility testing. Clin Microbiol Rev. 1993;6:367–381. doi: 10.1128/cmr.6.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rex J H, Rinaldi M G, Pfaller M A. Resistance of Candida species to fluconazole. Antimicrob Agents Chemother. 1995;39:1–8. doi: 10.1128/aac.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.To W, Fothergill A W, Rinaldi M G. Comparative evaluation of macrodilution and Alamar colorimetric microdilution broth methods for antifungal susceptibility testing of yeast isolates. J Clin Microbiol. 1995;33:2660–2664. doi: 10.1128/jcm.33.10.2660-2664.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Troillet N, Durussel C, Bille J, Glauser M P, Chave J P. Correlation between in vitro susceptibility of Candida albicans and fluconazole-resistant oropharyngeal candidiasis in HIV-infected patients. Eur J Clin Microbiol Infect Dis. 1993;12:911–915. doi: 10.1007/BF01992164. [DOI] [PubMed] [Google Scholar]
- 25.Turner T M, Kirkpatrick W R, Fothergill A W, McCarthy D I, Redding S W, Dib O P, Rinaldi M G, Patterson T F. Abstracts of the 97th General Meeting of the American Society for Microbiology 1997. Washington, D.C: American Society for Microbiology; 1997. Fluconazole disk diffusion susceptibility testing of Candida species, abstr. F-86; p. 274. [DOI] [PMC free article] [PubMed] [Google Scholar]