Abstract
The development of sarcopenia in the elderly is associated with many potential factors and/or processes that impair the renovation and maintenance of skeletal muscle mass and strength as ageing progresses. Among them, a defect by skeletal muscle to respond to anabolic stimuli is to be considered. Common anabolic stimuli/signals in skeletal muscle are hormones (insulin, growth hormones, IGF-1, androgens, and β-agonists such epinephrine), substrates (amino acids such as protein precursors on top, but also glucose and fat, as source of energy), metabolites (such as β-agonists and HMB), various biochemical/intracellular mediators), physical exercise, neurogenic and immune-modulating factors, etc. Each of them may exhibit a reduced effect upon skeletal muscle in ageing. In this article, we overview the role of anabolic signals on muscle metabolism, as well as currently available evidence of resistance, at the skeletal muscle level, to anabolic factors, from both in vitro and in vivo studies. Some indications on how to augment the effects of anabolic signals on skeletal muscle are provided.
Keywords: exercise, intracellular signals, nutrition, protein foods, protein synthesis, sarcopenia, skeletal muscle
1. Introduction
The term “sarcopenia” (from the Greek words “sarx”, i.e., flesh, and “penia”, i.e., loss) was first proposed by Rosenberg in 1989 to describe the age-related loss of muscle mass [1]. Later, this term referred to the decrease in muscle mass and/or strength with ageing. Since 2016, the World Health Organization (WHO)’s International Statistical Classification of Diseases and Related Health Problems (ICD) has recognized sarcopenia as a disease (code ICD-10-CM) (M62.84) [2]. Sarcopenia, defined as an age-related loss in skeletal muscle mass and function, is one of the most pressing health problems in the elderly. Depending on the definition used for sarcopenia, the prevalence in 60- to 70-year olds is reported to be between 6% and 22%, while the prevalence is as high as 50% in people > 80 years old [2].
There are several definitions of sarcopenia. The three widely recognized ones are from the following International Working Groups or Societies [2,3,4]:
The European Working Group on Sarcopenia in Older People (EWGSOP): They defined sarcopenia in 2010 as “a syndrome characterized by progressive and generalized loss of skeletal muscle mass and strength, with a risk of adverse outcomes such as physical disability, poor quality of life, and death”.
The International Working Group on Sarcopenia (IWGS): The IWGS proposed a definition in 2011: “Sarcopenia is defined as a syndrome characterized by progressive and generalized loss of skeletal muscle mass and strength, and it is strictly correlated with physical disability, poor quality of life, and death”.
The Foundation for the National Institutes of Health (FNIH): In collaboration with various experts, they established a working definition in 2014: “Low muscle mass as measured by any method, and low muscle strength and/or low physical performance”.
Definition and Outcomes Consortium’s (SDOC) efforts, which involved a literature review, data analysis from multiple studies, and expert panel review to establish an evidence-based understanding of sarcopenia. They define sarcopenia as characterized by both weakness (defined by low grip strength) and slowness (defined by low usual gait speed) in older adults. These two components are considered important discriminators and predictors of various adverse health-related outcomes, including falls, self-reported mobility limitation, hip fractures, and mortality in community-dwelling older adults.
As the world’s older adult population increases, so does the number of individuals with sarcopenia. To ensure effective treatment, clinicians and operators must accurately measure sarcopenia before initiating treatment or interventions. It is essential to use an objective measurement tool for diagnosis and recommend utilizing any of the validated options we reported upstream.
Sarcopenia is an almost unavoidable process associated with ageing, albeit occurring at variable rates and different magnitudes. A variety of factors condition both its progression rate and the ultimate extent of the functional impairment [3,4]. Muscular loss has been estimated to occur at a rate of 1–3% yearly [5]. Besides sarcopenia, the overall impairment of neuromuscular function, defined as “dynapenia”, i.e., muscle weakness, is recognized as a practical, critical factor leading to the loss of physical independence in the elderly [4]. Loss of muscle fiber number is one of the main causes of sarcopenia, although fiber atrophy—particularly among type II fibers—is also involved [6,7]. Type II muscle fibers seem to play an important role in the ageing process of human skeletal muscles. According to this literature, loss of fibers, decrease in size, and fiber-type grouping represent major quantitative changes [8].
Risk factors commonly associated with the development of sarcopenia with ageing are a progressive decrease in physical activity; the coexistence of subtle or overt malnutrition and of age-related diseases; a higher body mass index with increased visceral fat area; smoking; sleep duration and poor quality; pharmacological therapies; and a chronic (sub)-inflammatory condition [9,10,11]. Each of these factors may impair muscle mass, function (i.e., strength, speed of contraction, and overall power), or both. These factors interact with each other, so that often it cannot be entirely clear which one comes first. A subjective reaction to perceived fatigue may also lead to an involuntary reduction in daily physical activity, thus amplifying muscle disuse in a negative loop. The mechanisms(s) for the increased fatigability occurring with ageing are incompletely understood [12].
Maintenance, wasting, and recovery of muscle mass depend on the relative rates of two opposite, ongoing processes, i.e., protein degradation (PD) and protein synthesis (PS), in other words, of protein turnover. These two processes occur simultaneously although at variable rates, and their regulation depends on different factors, operating in a complex network. Therefore, net protein anabolism (or “accretion”), i.e., the net increase in muscle mass, occurs anytime when synthesis exceeds degradation.
Nutrition and exercise are two major factors involved in the development of sarcopenia in the elderly. The aim of this narrative review is to provide an update of the current literature, addressing the role of (protein) nutrition and physical exercise, both as possible factors causing sarcopenia when defective, and as tools to prevent and/or to cure it when adequately provided. Due to the complex, sometimes contrasting body of existing data, we attempted to briefly and critically describe the experimental setting of each cited report, with the purpose of supporting each author’s conclusion. We provide also a review of the molecular mechanism(s) of muscle protein accretion, as well as the role of substrates and hormones on muscle protein turnover.
2. Part 1: Physiological Regulation of Muscle Metabolism and Growth
2.1. The Molecular Mechanisms behind Muscles Growth in Young Subjects: Exercise, Nutrients, and Hormones
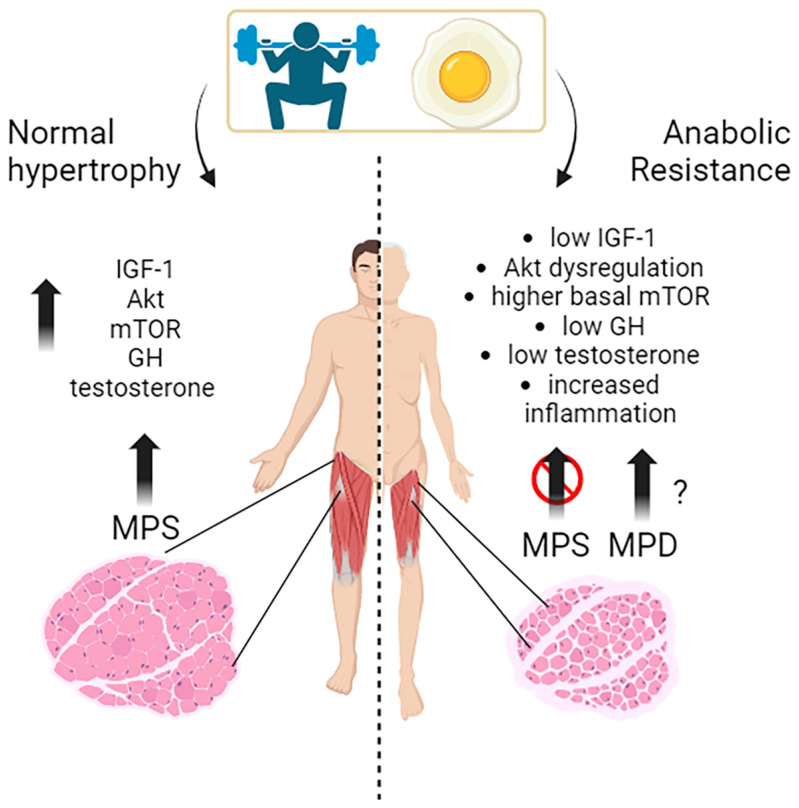
Muscle growth, or muscle hypertrophy, is a complex process regulated by several molecular pathways. The primary pathways that promote muscle growth in response to exercise, nutrients, and growth signals include the IGF-1/PI3K (phosphoinositide 3-kinase)/Akt/mTOR pathway and hormones like testosterone and GH (Figure 1).
Figure 1.
Here is a diagram showing the typical triggers for muscle growth in young people, which include exercise and high-quality protein intake. These stimuli activate various molecular pathways that lead to muscle hypertrophy. However, on the right-hand side of the diagram, you can see that the same stimuli do not have the same effect on skeletal muscle in cases of anabolic resistance.
The IGF-1/PI3K/Akt/mTOR pathway is a vital signaling cascade in muscle growth that involves various interconnected mechanisms. Its activation increases protein synthesis, reduces protein degradation, and improves cell growth. The molecular mechanisms and interactions within this pathway are still being studied in human muscle physiology. When activated, mTOR promotes muscle hypertrophy by stimulating protein synthesis and cell growth [13]. The mTOR kinase is the main regulator of muscle protein synthesis and responds to the availability of nutrients, particularly amino acids and growth factors. Indeed, resistance exercise and nutrient intake, especially leucine-rich amino acids, activate the mTOR pathway essential for exercise-induced muscle protein synthesis and are, thus, necessary for increasing muscle protein synthesis following resistance exercise in young men [14]. The key importance of mTOR is exemplified by an experiment, where young subjects were treated with a potent mTORC1 inhibitor (rapamycin) before performing a series of high-intensity muscle contractions to demonstrate this activation. Rapamycin treatment blocked the early, acute (after 1–2 h) contraction-induced increase in human muscle protein synthesis. Other studies have also shown that a high-protein diet enhances mTOR-mediated protein synthesis in young men following endurance exercise [15]. These studies indicate that mTOR activation is crucial in mediating the anabolic response to exercise and diet in humans (Figure 1).
Akt activation is crucial in promoting muscle protein synthesis in response to exercise and nutrient intake in young individuals [16]. In response to resistance training, significant hypertrophy (+10%) in the human quadriceps was demonstrated, with a parallel increase in phospho-Akt, phospho-GSK-3beta, and phospho-mTOR protein, and a decrease in the nuclear protein content of Foxo1, which is the master regulator of the atrophy program [16]. Akt is also influenced by nutrition, as excess leucine intake has been shown to enhance Akt signaling in young individuals, suggesting that Akt is sensitive to dietary amino acids and can modulate protein synthesis accordingly [17].
The timing of exercise and protein intake also affect Akt activation and subsequent muscle protein synthesis. While exercise alone did not increase Akt and mTOR phosphorylation, protein ingestion afterward did so in a dose-dependent manner. As a matter of fact, Akt activation is a complex process influenced by exercise, nutrition, and specific amino acids, and further research is being conducted to fully understand its role in muscle growth and adaptation [18].
The upstream controller in this axis is the insulin-like growth factor-1 (IGF-1), which is crucial in promoting growth and anabolic processes in skeletal muscles [13]. IGF-1 is a key growth factor that regulates both anabolic and catabolic pathways in skeletal muscle.
Studies indicate that IGF-1 induces hypertrophy of human myotubes in vitro, characterized by an increase in the mean number of nuclei per myotube, an increase in the fusion index, and an increase in myosin heavy chain (MyHC) content [19]. IGF-1 contributes to muscle protein turnover, protein synthesis, and the adaptation of skeletal muscles to resistance training [20]. Hormonal factors, such as ethinyl estradiol administration, can affect IGF-1 synthesis and degradation in skeletal muscles, potentially modulating muscle protein turnover and influencing responses to exercise and nutrient intake [21]. IGF1 is closely connected with growth hormone (GH), which stimulates muscle growth through several mechanisms and interactions with other signaling pathways. It stimulates protein synthesis by enhancing the uptake of amino acids into muscle tissue, providing the building blocks for muscle protein synthesis. This process is mediated, at least in part, by the GH/IGF-1 axis. GH stimulates the liver and other tissues to produce the insulin-like growth factor 1 (IGF-1), which promotes cell growth, protein synthesis, and the proliferation of satellite cells involved in muscle repair and growth [22].
GH promotes the uptake of essential nutrients, such as glucose and amino acids, into muscle cells for energy production and protein synthesis. It also affects metabolism by enhancing the breakdown of fats, providing additional energy sources for muscle growth [23]. However, pharmacological GH supplementation only increases muscle strength or size in individuals with clinical GH deficiency, and there is no evidence that exercise-induced changes in GH have the same effects in individuals with normal GH levels [24].
Testosterone is one of the most potent naturally secreted androgenic-anabolic hormones, and its biological effects include promoting muscle growth. In muscle, testosterone stimulates protein synthesis (anabolic effect) and inhibits protein degradation (anti-catabolic effect) [25,26,27,28,29]. Testosterone plays a crucial role in muscle growth in response to exercise and nutrition. Various studies have shed light on this topic. Vingren et al. (2010) explored the physiological aspects of testosterone in resistance exercise and training, highlighting its upstream regulatory elements [28]. They found that testosterone enhances muscle protein synthesis, stimulates satellite cell activation and proliferation, and modulates anabolic signaling pathways such as mTOR and IGF-1. These findings suggest that testosterone plays a key role in mediating the anabolic response to resistance exercise [28]. West and Phillips (2010) discussed the anabolic processes in human skeletal muscle, emphasizing the roles of growth hormone and testosterone (24). They concluded that testosterone acts directly on muscle tissue to promote muscle protein synthesis and hypertrophy.
The interactions between testosterone, growth hormone, and anabolic signaling pathways, such as mTOR and IGF-1, contribute to the overall anabolic response in muscle. West and Philips highlighted the importance of optimizing testosterone levels for maximizing muscle growth in response to exercise and nutrition [24]. In their work, the exercise paradigms are designed based on the assumption (not necessarily evidenced-based mechanisms) that GH and testosterone facilitate anabolic processes that lead to skeletal muscle protein accretion and hypertrophy. Furthermore, they pointed out that exercise-induced hormonal stimulation does not enhance intracellular markers of anabolic signaling or the acute post-exercise elevation of myofibrillar protein synthesis. Furthermore, they demonstrated that exercise-induced increases in GH and testosterone availability are unnecessary and do not enhance strength and hypertrophy adaptations. So, they concluded that local mechanisms, intrinsic to the skeletal muscle tissue performing the resistive contractions (i.e., weight lifting) are predominant in stimulating anabolism [24]. The regulation of satellite cells following myotrauma caused by resistance exercise is also related to testosterone, which plays a critical role in activating satellite cells, which are crucial for muscle repair and growth [29]. Finally, Bhasin et al. (2001) examined the dose–response relationships of testosterone in healthy young men, and they found that testosterone administration at varying doses increased dose-dependently muscle protein synthesis rates, resulting in greater muscle mass and strength gains [30]. These findings suggest that higher testosterone levels promote anabolic processes and contribute to muscle growth in response to exercise and nutrition
2.2. Effects of Substrates and Exercise on Skeletal Muscle Protein Synthesis in Young, Middle-Aged Subjects
Accretion of muscle mass depends on physiological, metabolic, and hormonal factors, as well as on physical activity. Conversely, it is hindered by inactivity, malnutrition, overt diseases, and/or subtle, chronic pathological conditions [31,32,33,34,35,36]. The main protein-anabolic factors, at both the whole-body and skeletal muscle level, are the proteins and/or the amino acids themselves (i.e., the protein building blocks), as well as physical activity/exercise. In addition, energy availability, anabolic hormones (insulin, human Growth Hormone (hGH), IGF-1, β-agonists, anabolic steroids, and see also above), adequate tissue perfusion, and, in general, a “healthy status” (i.e., the absence of both overt diseases and subtle pathological conditions, such as a chronic sub-inflammatory status) also condition skeletal muscle accretion. Furthermore, the effects of any of these factors could be divided into either “acute” (i.e., detectable under acute experimental conditions) or “chronic” (i.e., following repeated stimuli, with end-point results tested sometime after).
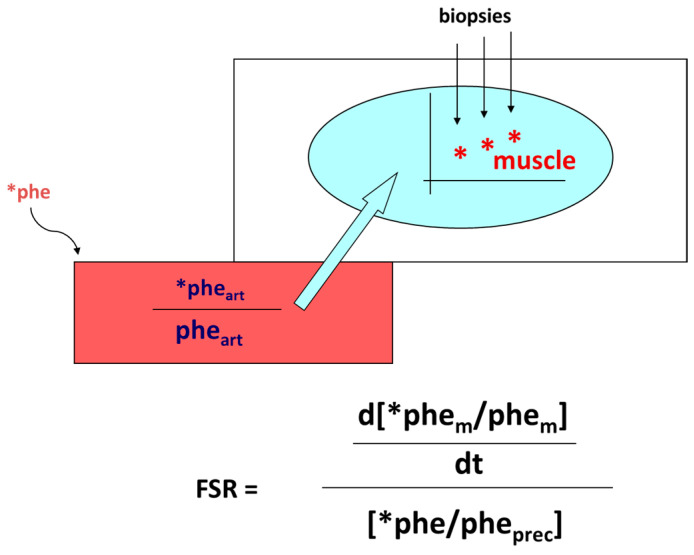
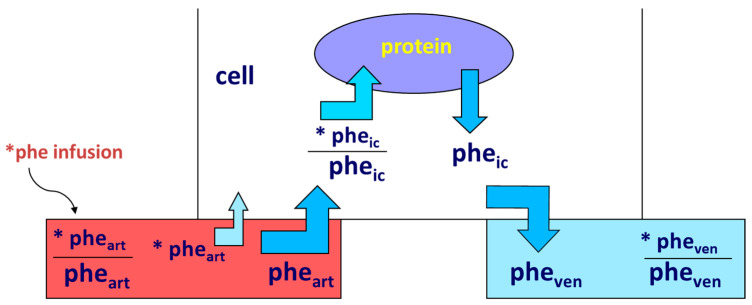
Protein anabolism is thus achieved through the stimulation of protein synthesis and/or the inhibition of protein degradation. In this narrative review, we will focus predominantly on the regulation of muscle protein synthesis (MPS). MPS can be determined in vivo either by measuring the incorporation of infused amino acid stable isotopes into muscle by biopsy (Figure 2) and/or through measurements of the A-V difference in labeled as well as unlabeled amino acid(s) across a sampled district predominantly constituted by skeletal muscle, typically a limb (either the leg or the forearm) [37] (Figure 3).
Figure 2.
The figure schematically illustrates the methodology commonly employed to determine skeletal muscle fractional protein synthesis rate (FSR) with the combined infusion of an amino acid tracer (in this example, phenylalanine, (phe)) and its timed incorporation into skeletal muscle (measured by biopsy). Absolute Muscle Protein Synthesis (MPS) rate is calculated as the product of FSR times muscle protein mass. The asterisks (*) indicate the labeled amino acid. Abbreviations used in the Figure: art: artery; m: muscle; prec.: the enrichment (or the specific activity) of the “precursor” (i.e., the labeled amino acid), used in the calculations.
Figure 3.
Measurement of skeletal muscle protein synthesis and degradation with arterial/venous combined with isotope infusion. The figure schematically depicts the measurements performed in the perfusing artery and in the deep vein draining blood from the sampled muscular-rich district (i.e., the leg or the forearm). In this example, the indicator essential amino acid is phenylalanine (Phe), which is utilized by muscle only for protein synthesis and is released from muscle only from protein degradation. Blood flow across the muscle district has to be measured too. Thus, from the measured phenylalanine utilization and release, it is possible to extrapolate protein degradation and synthesis. The asterisk (*) indicates the labeled amino acid. Abbreviations used in the Figure: art: artery; ven: vein; ic: intracellular. The equations used in the calculations are not reported here.
2.2.1. Effects of Proteins and Amino Acids
Definitely, protein ingestion stimulates skeletal muscle protein synthesis (MPS) [38]. The stimulation’s magnitude and duration depend on both the protein dose and its type/quality, which is closely associated with the concurrent post-ingestion rise in amino acid plasma concentrations [39,40]. Hyperaminoacidemia [41,42,43], specifically that of essential amino acids (EAAs) [44], among them, of the branched chain ones and of leucine in particular [31,45,46], largely condition tissue protein synthesis.
Leucine is particularly important as a key metabolic regulator of MPS through its activation of the mTOR pathway, and it acutely enhances skeletal MPS both in vitro [45,47,48,49,50] (also above) and in vivo [51]. In addition to the protein or the amino acids, other variables or factors, such as the coexistence of exercise, the pattern and/or the timing of nutrient administration, the age of subjects, the presence of comorbidities, etc., are important. Most reports have investigated the combined effect of protein and exercise. As an example, typing in a PubMed search the string, “Stimulation of muscle protein synthesis by protein ingestion” identified ≈ 600 papers. Conversely, typing, “Stimulation of muscle protein synthesis by protein ingestion and exercise” identified ≈ 250 papers, whereas the string, “Stimulation of muscle protein synthesis by protein ingestion at rest” identified 60 papers. Therefore, although there is a large overlap among these selections, such an example simply underlines the predominant interest in combining protein nutrition with exercise.
In non-exercising young subjects (i.e., “at rest”), the acute administration of either a protein-containing mixed meal or a pure protein load, stimulated skeletal muscle protein synthesis [52,53]. In dose–response studies, intake of 20 g of high-quality proteins, i.e., whey protein [54] or mixed egg protein [55], was sufficient to maximally stimulate postabsorptive rates of myofibrillar MPS in resting young men over 4 h [54].
In middle-aged men however, following the ingestion of graded amounts of beef (from 57 g, i.e., 12 g protein; 113 g, i.e., 24 g protein; or 170 g, i.e., 36 g protein), the stimulation of myofibrillar MPS was the greatest with 170 g of beef [56], thus apparently not achieving a plateau. Notably, in this study, exercise further and significantly enhanced the beef protein effect only at the highest administered dose (170 g beef). Similarly, following the administration to resting young volunteers, of a mixed meal containing both animal (beef) and vegetal proteins, at protein intakes of either 40 g or 70 g, the stimulation of skeletal muscle protein synthesis was similar at both doses, also independent of prior resistance exercise, thus leading to the conclusion that the ≈ 40 g mixed protein dose may attain the maximum effect on MPS [57]. The (marginal) inconsistency between the above-referenced studies could be due, besides possible experimental variations, either to the type of the administered protein (pure whey protein, i.e., a high quality, fast absorbable protein, vs. either beef, or mixed animal and vegetal proteins), or to the complex protein matrix of “natural” proteins that could retain specific, yet unappreciated effects.
The administration of free amino acids, either as bolus ingestion of 15 g EAA [58], or of a leucine-rich EAA and carbohydrate mixture [59], increased human muscle protein synthesis too. When dose–response curves between crystalline EAA and MPS were constructed, the literature data somehow contrasted. It was initially reported that 2.5 g crystalline EAA was sufficient to elicit an increase above basal of MPS in young subjects [60]. However, in subsequent studies using intact whey protein, the lower dose capable of eliciting a response in MPS in young muscle was set at >10 g (=5 g EAA), reaching saturation at 20–40 g EAA (Table 1).
Leucine, isoleucine, and valine, i.e., the branched-chain amino acids (BCAA), account for about one-third of all amino acid residues in muscle protein, and have been extensively studied regarding their direct regulatory role (due to leucine) on protein synthesis (see also above). Nevertheless, the demonstration of a clear-cut effect of BCAA alone, on skeletal muscle hypertrophy (i.e., a long-term effect) in humans is not sound. In a meta-analysis, leucine supplementation was reported to increase the muscle protein fractional synthesis rate, however, without changing either body lean mass or leg lean mass [61]. The effect of another amino acid, glutamine (non-essential), on skeletal muscle in humans, is yet uncertain and/or unwarranted [62,63,64]. Intravenous glutamine did not stimulate mixed muscle protein synthesis in healthy subjects [65].
The type of the administered protein is important too. Since the amount of the EAAs, and/or their relative proportions, are greater and/or more balanced in high- than in low-quality proteins, the amount and quality of the protein are relevant in the stimulation of protein synthesis. This issue is important in ageing, because the estimated recommended daily protein intake in aged people is ≈50% greater (1.2 g/kg BW) than that of young-adult subjects (0.8 g/kg BW) [66], and it could be better achieved by the intake of high-quality protein, such as whey protein, albumin, egg, or, to a lesser extent, of mixed milk protein, thus helping to maintain muscle mass and prevent sarcopenia.
Whey proteins (i.e., a soluble, fast-absorbable, high-quality milk protein) have been largely used as a test protein. In resting young volunteers, the stimulation of MPS after consumption of 10 g EAA hydrolysate from whey protein was ≈90% greater than that with casein, and ≈20% greater than that with soy [40]. Although the effect of whey protein might be short-lived [67] because of its fast absorption and the transient rise in plasma/blood amino acid concentrations, the stimulation of muscle PS following the ingestion of isonitrogenous quantities (20 g) of either casein or whey WP was sustained over 4 h in both cases, with that of WP, however, being 65% higher [68].
Ingestion of a single dose of 38 g mixed milk protein (i.e., including both fast- and slow-absorbable proteins) in young men, resulted in a time-dependent increase in postprandial muscle protein synthesis, detectable as soon as 60′ after and lasting at least up to 5 h. [69]. Also, the ingestion of 30–40 g of a vegetal protein, mycoprotein, stimulated skeletal muscle protein synthesis to an extent comparable to that of an isonitrogenous omnivorous diet [70].
Table 1.
Protein (and) amino acid doses (in grams, g) that increase muscle protein synthesis (MPS) in young adults.
| Type of Food/Protein/AA | Subjects | Dose A: Either the Threshold or the Lowest Dose that Increased MPS | Dose B: Either the Highest Dose Tested or that Maximally Stimulated MPS | Exercise Status | Refs. |
|---|---|---|---|---|---|
| Whey protein | Y (≈21 y) males | >20 g | ≈20 to 40 g | +Ex | [71] |
| Whey protein | Y (20–22 y) males | 10 g | 40 g | −/+Ex | [54] |
| Whey protein | Y adults | 5–20 g | 20 g | −Ex | [72] |
| Combination of 5 Whey Protein and 1 Egg studies (original data recalculated to body weight.) |
Y (22 y) males | 8 g | ≈20 g | −/+Ex | [73] |
| Milk protein + CHO | Y (27 y) | 15 g | 45 g | +Ex | [74] |
| Milk protein concentrate | Y (22 y) males | / | 38 g | −Ex | [69] |
| Egg protein | Y (22 y) males | 5–10 g | 20 g | +Ex | [72] |
| Egg protein | Y males | 5–10 g | 20 g | +Ex | [55] |
| Whey protein Hydrolysate (dose achieving the greatest stimulation of MPS in [40].) (=AA) | Y (23 y) males | ≈10 g (as AA) | / | +Ex | [40] |
| Casein (micellar) (dose achieving an intermediate stimulation of MPS in [40].) | Y (23 y) males | ≈10 g | / | +Ex | [40] |
| Soy Hydrolysate (=AA) (dose achieving the lowest stimulation of MPS in [40].) | Y (23 y) males | ≈10 g (as AA) | / | +Ex | [40] |
| Beef (beef contains ≈ 22% of weight as protein.) | M males | >113 g | >170 (further enhanced by exercise) g | −/+Ex | [56] |
| Mixed animal (beef) and vegetal protein | Y (≈ 30 y) males | 40 g | 40–70 g | −/+Ex | [57] |
| Cristalline EAA | Y (34 y) | 15 g (as EAA) | −Ex | [58] | |
| EAA + Leucine + CHO | Y (≈26 y) | ≈20 g (as EAA) (Published data are reported as 0.35 g/FFM (Free Fat Mass). Here, they have been recalculated per mean subject assuming that FFM equals LBM (Lean Body Mass).) | −Ex | [59,75] | |
| Mycoprotein concentrate (corresponding to a total of 70 g whole mycoprotein.) | Y (21 y) | 38 g | +Ex | [76] |
Abbreviations used in the Table: AA: amino acid(s); CHO: carbohydrate; EAA: Essential Amino Acid(s); Ex: exercise; g: gram; M: Middle age; Y: young; y: years.
The pattern of protein administration may be important too. Diets are usually consumed as three main, bolus meals, plus occasional daily snacks. Alternatively, in some conditions and/or for experimental purposes, nutrition can be administered in a (sub)continuous pattern, i.e., at a near-constant rate over the day. In specific studies, the potential difference in the effects between these two patterns concerning the protein-anabolic effects has been investigated. Such a theoretical, experimental milieu, can be approached in vivo also by administering proteins with either a “fast” or “low” absorption pattern
In young subjects, the total protein-anabolic effect of meal ingestion, measured using leucine tracers at the whole-body level, was more pronounced with “slow” (i.e., casein) than with “fast” (i.e., whey) protein administration [67,77]. The mechanisms leading to these effects were different, too: the “fast” protein markedly stimulated amino acid oxidation and protein synthesis but did not change proteolysis, whereas the “slow” protein increased amino acid oxidation and protein synthesis to a lesser extent but strongly inhibited proteolysis. Therefore, since the effects of whey proteins may be more rapidly vanishing, it would be useful to combine the effects of fast and slow proteins, irrespective of whether they are vegetal or animal ones. In young adults, a supplement containing a vegetal, antioxidant-rich soy protein mixed with whey protein, could prolong the improvement of the AA net balance across the leg up to ≈ 2 h post-ingestion, compared with the 20 min attained with whey alone [78].
2.2.2. Effects of Exercise and Nutrition on Muscle Protein Synthesis and Accretion
Exercise is a potent stimulator of muscle protein synthesis, particularly in the recovery phase [79], and it positively interacts with protein/amino acid ingestion in the stimulation of skeletal muscle anabolism.
Most studies agree in reporting a powerful amplification by either protein or amino acid ingestion of the anabolic effects of concurrent exercise. An abundant supply of amino acids can also enhance amino acid transport and muscle protein synthesis following leg resistance exercise [80].
Muscle mass accretion following resistance exercise combined with food ingestion is observed even following an adequate habitual protein intake (≥0.8 g kg−1 day−1) [81]. A greater protein intake (1.8–3.0 g kg−1 day−1) further augments lean body mass (i.e., protein) accretion without increasing fat mass, when compared to an energy-rich, low-protein intake (≈5% of energy as protein) [81]. The high-quality whey protein, combined with resistance exercise in young adults, exerted a greater effect on MPS than equivalent doses of lower-quality proteins, such as soy protein or casein, an effect, however, still present up to 3–5 h post-exercise [55].
As reported above, a 20 g dose of whey protein was sufficient for the maximal stimulation of post-absorptive myofibrillar MPS in young men, whether or not exercising [54], and it was effective up to 3–5 h after exercise [40,82]. Conversely, others reported that the response of muscle protein synthesis following whole-body resistance exercise is greater following 40 g rather than 20 g of ingested whey protein [71]. After the ingestion of incremental doses of mixed milk protein (0, 15, 30, or 45 g) together with 45 g carbohydrate, the 30 g protein dose was sufficient to maximize the myosin synthesis rates during recovery from a single bout of endurance exercise in young men [74]. Therefore, a whey (milk) protein dose between 20 and 40 g seems sufficient for the maximal stimulation of MPS following exercise (Table 1). The greater potency of whey protein over other protein types is likely due to its nature of leucine-rich, high-quality protein, as well as to its rapid digestion and absorption, henceforth producing earlier and greater hyperaminoacidemia and hyperleucinemia [67,83].
The intake of another high-quality protein, i.e., egg protein, as low as 5–10 g (approximating that contained in a single ≈ 60 g egg: ≈ 6.8 g total protein), increased MPS above basal following resistance exercise, reaching a maximum with a dose of 20 g egg protein [72].
The structure/form of nutrient intake (free AA vs. intact protein) with respect to the timing of administration (either before, or 1 to 3 h after, exercise) on the stimulation of skeletal muscle protein accretion is relevant too. While net amino acid uptake by skeletal muscle (measured by means of femoral arteriovenous sampling) was greater when free essential amino acids plus carbohydrates were ingested before, rather than after, resistance exercise [84], the ingestion of intact whey protein was similar irrespective of the administration time [85].
2.2.3. Effect of Other Substrates
Protein synthesis is an energy-requiring process [86]; therefore, energy-providing substrates such as glucose and fat may affect protein turnover too. The activities of the cellular pathways controlling protein turnover are bio-energetically expensive and therefore depend on intracellular energy availability (i.e., macronutrient intake) [87].
Glucose
Glucose increased muscle protein synthesis in vitro [88]. Testing glucose-induced or derived substrates separately, tissue ATP decreased during incubation with lactate, and lactate + pyruvate supported protein synthesis better than pyruvate or glucose. The data on the effects in humans of either glucose or fat on protein metabolism, specifically on skeletal muscle, are scarce, complex, and not univocal [42]. Enteral glucose administration did not affect either duodenal mucosal protein FSR or the activities of mucosal proteases [89]. An oral glucose load, and the simultaneous glucose-induced stimulation of insulin secretion, did not alter the rate of whole-body protein synthesis or breakdown [90]. Similarly, glucose ingestion added to a protein dose that maximally stimulated MPS, despite greater insulin increments, did not show either additive or synergistic effects on either the stimulation of MPS or the inhibition of muscle protein breakdown [91]. The co-ingestion of carbohydrates with protein did not further augment the post-exercise stimulation of muscle protein synthesis [92]. In humans, an acute intravenous glucose infusion decreased whole-body protein degradation, an effect possibly mediated by insulin [93]. In contrast, hyperglycemia did not inhibit whole-body protein degradation in humans under insulin-controlled conditions [94]. To our knowledge, no data are available on the direct effects of glucose per se on skeletal muscle protein synthesis in humans. The co-ingestion of carbohydrates with protein did not further increase the post-exercise stimulation of muscle protein synthesis [92]. However, the diet-induced modulation of intramuscular carbohydrate (i.e., glycogen) availability affected skeletal muscle and skeletal muscle and whole-body protein synthesis, degradation, and net balance during prolonged exercise in humans [95]. Skeletal muscle CHO stores were depleted by previous exercise in the Low-CHO group, but were replenished with a High-CHO diet for 2 days. In the Low-CHO, the net leg protein balance was the decreased group compared with both the pre-exercise rest and the High-CHO condition, primarily due to increased protein degradation and decreased protein synthesis late in exercise. Whole-body leucine oxidation increased above rest in the Low-CHO group only, and was higher than in the H-CHO group. Whole-body net protein balance was reduced in the Low-CHO group, largely due to decreased general protein synthesis. Overall, these observations suggest an effect of a High-CHO diet to improve the net protein balance in skeletal muscle undergoing exercise.
Lipids and Ketones
The effects of either lipid or ketone infusion/administration in humans are complex. Lipid infusion in humans did not affect proteolysis [96]. In contrast, medium-chain fatty acid infusion apparently increased leucine oxidation and, therefore, net protein catabolism [97]. Thus, the effects on whole-body protein degradation may depend on the fatty acid length [98]. The increase in FFA decreased basal muscle protein synthesis, but not the anabolic effect of leucine [99]. When associated with dietary protein ingestion, neither acute nor short-term dietary fat overload impaired skeletal MPS in overweight/obese men in the post-prandial phase, thus excluding a role by dietary accumulation of intramuscular lipids on the anabolic response to meal ingestion [100]. The infusion of 3OHButyrate decreased both whole body and forearm protein turnover (measured by phenylalanine/tyrosine tracers), as well as phenylalanine catabolism, in post-absorptive conditions, whereas it did not modify the insulin-induced effects following an euglycemic clamp [96]. In another study, 3OHButyrate infusion did not change whole-body leucine turnover, decreased leucine oxidation, and increased the non-oxidative portion of leucine disposal, while muscle PS increased, demonstrating an anabolic effect of 3OHButyrate at this level [101].
The leucine metabolite β-hydroxyl, β-methylbutyrate (HMB) was recently identified as a potentially anabolic substrate [102]. Using combined stable isotope amino acid infusion, isotope incorporation into muscle myofibrils and arterial–venous femoral sampling in humans, oral administration of 2.42 g of pure HMB increased muscle protein synthesis by ≈ 70%. In contrast, it decreased muscle protein degradation by ≈60%, in an insulin-independent manner, whereas an oral 3.42 g leucine load increased muscle protein synthesis by ≈110% [103].
Other Nutritional Interventions
β-alanine supplementation may increase physical performance in middle-aged individuals [104] and improve physical performance during exercise [105]. Creatine may increase muscle mass in combination with resistance exercise, although the mechanism(s) of action remain elusive. Short-term creatine monohydrate (CrM) supplementation may exert anti-catabolic actions on selected proteins in men, but it did not enhance either whole-body or mixed-muscle protein synthesis [106]. Acute metabolic studies testing various substances may provide useful information for estimating the efficacy of potentially anabolic agents [107].
2.3. Hormones and Related Drug Interventions
2.3.1. Insulin
Although insulin has an undisputed anabolic effect on tissue protein, its experimental demonstration in vivo has challenged the investigators over time, mainly because its administration induces a decrease in amino acid plasma concentration, thus possibly obscuring its direct effect in muscle. A major advancement was the maintenance of the “amino acid “clamp” at baseline during insulin infusion or injection, also achieved when insulin was directly infused into the artery perfusing a muscle-rich organ (such as the leg or the forearm), thus avoiding a system perturbation of the aminoacidemia and allowing, at the same time, the insulin effect in the perfused limb to be studied selectively, using any of these techniques. Muscle protein synthesis and degradation were determined by combining amino acid isotope infusion with arterial–venous limb catheterization, often complemented by muscle biopsy in some studies. A systemic insulin infusion, with no prevention of hypoaminoacidemia, suppressed whole-body protein degradation [43,108], and improved limb (either the forearm or the leg) net protein balance mainly by decreasing protein degradation [43]. However, when insulin-induced hypoaminoacidemia was prevented, insulin was shown to stimulate muscle protein synthesis [109,110]. In addition, insulin enhanced the amino-acid-induced stimulation of protein synthesis [43]. In summary, the protein anabolic effect of insulin in skeletal muscle requires sufficient plasma amino acid levels; a condition that is physiologically attained following meal ingestion.
2.3.2. Glucocorticoids
Cortisol, often referred to as a stress hormone, has both catabolic and anabolic effects, depending on the context. Cortisol-induced secondary sarcopenia (i.e., specifically induced either by either an exogenous administration or an endocrine disease) is a frequent finding in the clinical setting. Chronic glucocorticoid exposure induces loss of lean body mass by decreasing protein synthesis and increasing degradation [111,112,113]. The protein-catabolic effect of prednisone is antagonized by growth hormone [112]. In humans, the administration of 8 mg dexamethasone daily for 4 days antagonized the anti-proteolytic effect of insulin in the forearm [114].
2.3.3. Human Growth Hormone (hGH) and IGF-1
In humans, hGH administration increased whole-body [112] and muscle protein synthesis [115]. The insulin-like growth factor-binding proteins (IGFBPs) bind to IGFs, modulating their activity and availability. They help in regulating IGFs’ actions in various tissues, including muscle. When rhIGF-I was infused at a rate achieving plasma IGF-I concentrations close to those observed following rhGH treatment, and yet avoiding the IGF-1-induced hypoglycemia, proteolysis and protein synthesis were not affected, even in the presence of prednisone treatment [116]. However, when rhGH and rhIGF-I were administered simultaneously, nitrogen balance was remarkably improved [116]. Thus, the exact anabolic effect of combined rhGH and rhIGF administration remains to be fully elucidated. Data in vitro may help to address this point.
IGF-1 exhibits several splicing variants, IGF-1Ea, which is a circulating factor synthesized in the liver, and IGF-1Eb and IGF-1Ec, recognized as Mechano-Growth Factors (MGFs), which manifest in skeletal muscles of rodents and humans, respectively [117]. The act of stretching or overloading skeletal muscles triggers a rise in IGF-1 mRNA, particularly emphasizing the specific IGF-1Ec variant (MGF) [117]. The extent to which IGF-1 alternative splicing variants might induce greater hypertrophy compared to IGF-1 per se remains partially unresolved [118]. Notably, the effects of the synthetic E-domain peptide mimetic have been elucidated: the MGF-24aa-E peptide (YQPPSTNKNTKSQRRKGSTFEEHK) activates satellite cells, prompting their replication [117]. These cells are subsequently hindered from progressing until they successfully merge with muscle fibers and assume a myogenic program [119].
2.3.4. Catecholamines
Epinephrine has both an α- and β-receptor affinity. Epinephrine infusion in humans depressed plasma amino acid concentrations, particularly the essential ones, however without changing leucine or phenylalanine flux [120], nor did it impair the disposal of exogenous amino acids in humans [121]. However, in another study, the increase in plasma epinephrine concentrations inhibited proteolysis and leucine oxidation in humans via beta-adrenergic mechanism, compatible with an anabolic effect [122]. In the human forearm, physiological epinephrine exerted an anticatabolic action on muscle protein, however, with unknown mechanism(s) [123].
The β-agonist clenbuterol may exert anabolic effects in skeletal muscle [124]. However, its use in humans is hampered by unacceptable large cardiovascular side effects [125].
2.3.5. Estrogens
Although estrogens are female sex hormones, they are also present in smaller amounts in males. Estrogen plays a role in maintaining bone health, promoting protein synthesis and muscle growth, and influencing body composition, in both males and females. While the exact mechanisms by which estrogens affect muscle growth are still being studied, research suggests that they can, directly and indirectly, affect muscle tissue. One way estrogens may influence muscle growth is by promoting protein synthesis, interacting with receptors in muscle cells, and activating signaling pathways involved in protein synthesis [126]. Decreased estrogen-associated signaling impairs mitochondrial function leading to muscle atrophy [127]. Estrogens can also indirectly affect muscle growth by modulating the production and activity of other hormones, such as growth hormone and insulin-like growth factor 1 (IGF-1), which are important for muscle development. Estrogens may regulate the release of these hormones from the pituitary gland and the liver, thereby influencing muscle growth [128]. Moreover, estrogens can influence muscle mass indirectly through their impact on body composition. Estrogens regulate fat distribution, and higher body fat levels have been associated with lower muscle mass. By influencing body composition, estrogens can indirectly affect muscle growth and maintenance [126]. Estrogen decreases in menopause may contribute to accelerated muscle loss and sarcopenia in females [129,130,131]. It is important to note that the role of estrogens in muscle growth is complex and can vary depending on factors such as age, sex, hormone levels, and other individual characteristics. Additionally, the research on this topic is still evolving, and different studies may have differing findings and conclusions.
2.3.6. Androgens
Testosterone is a potent anabolic stimulus primarily through improvement in the re-utilization of amino acids released from protein degradation [132] (see also the above paragraph), and this will be further discussed below. Testosterone and progesterone, but not estradiol, stimulated muscle protein synthesis in postmenopausal women [133].
2.4. Exercise
Exercise is a powerful stimulus to promote skeletal muscle protein synthesis and net protein anabolism, involving specific metabolic and morphological adaptations in muscle [79], also interacting with substrates [80,134]. Exercise produces diverse changes in amino acid metabolism and protein turnover in muscle, according to the exercise phase. Acute changes in amino acid metabolism during exercise are primarily catabolic (i.e., increased amino acid oxidation), yet exercise does not cause muscle wasting. This is because both immediate and later post-exercise phases are anabolic. Thus, regular exercise is essential for optimizing muscle growth and hypertrophy.
The type of exercise also determines the magnitudes of these processes, and exercise requires a sequence of metabolic adjustments from the catabolic period of the ongoing exercise to the anabolic period of recovery. Two primary exercise types commonly associated with muscle growth are resistance and endurance. Resistance exercise, such as weightlifting, involves using external resistance to challenge the muscles. This type of exercise is a primary intervention used to develop strength and stimulate muscle hypertrophy. Muscle mass increases constitute key components of conditioning in the outcome of various sports due to the correlation between cross-sectional muscle area and muscle strength [135,136].
2.4.1. Resistance Exercise Can Be Further Classified into Two Main Categories:
High-Intensity Resistance Exercise: This exercise type typically involves lifting heavy weights for a relatively low number of repetitions. It primarily targets fast-twitch muscle fibers, which have a higher potential for muscle growth. High-intensity resistance exercise promotes muscle hypertrophy by acutely causing mechanical tension and muscle damage, subsequently triggering muscle protein synthesis and adaptation [137]. High-intensity resistance exercise is a potent stimulus for skeletal MPS and hypertrophy in young adults during high-intensity exercise and post-exercise recovery [138,139,140,141,142,143].
Moderate-Intensity Resistance Exercise: This exercise type involves using moderate weights for more repetitions. While the hypertrophic response may be less pronounced than high-intensity resistance exercise, moderate-intensity resistance exercise still contributes to muscle growth and can benefit endurance and functional capacity [137].
Endurance exercise, or aerobic exercise, involves continuous rhythmic movements that challenge the cardiovascular system and improve endurance. Endurance exercises include running, cycling, swimming, and brisk walking. While endurance exercise primarily focuses on cardiovascular fitness and endurance, it can also impact muscle growth, particularly in prolonged training. Endurance exercise can enhance the oxidative capacity of muscles, improve energy efficiency, and increase the density of blood vessels within the muscle tissue. These changes can contribute to improved muscle function and endurance, but the hypertrophic response regarding muscle size may be relatively limited compared to resistance exercise [144].
2.4.2. Effects of Exercise in Conjunction with Nutrient Intake
The specific adaptation to each type of exercise can vary depending on factors such as exercise intensity, volume, frequency, and individual characteristics. Combining resistance and endurance exercises in a well-designed training program can provide comprehensive benefits for muscle growth, strength, endurance, and overall fitness. It has been shown that combined strength and endurance training in the evening may lead to larger gains in muscle mass [145]. Additionally, other factors such as nutrition, rest, and recovery play crucial roles in maximizing the benefits of exercise and promoting muscle growth. Adequate protein intake, overall caloric balance, and appropriate recovery periods are important factors to be considered when optimizing muscle growth in response to exercise.
During the acute phase, the energy needs to stimulate amino acid oxidation/catabolism (together with that of glucose and fat), thus depleting intracellular amino acid pools. In contrast, in the recovery phase, the depleted amino acid pools and energy need to be reconstituted. Since the response of muscle protein metabolism to a resistance exercise bout lasts up to 5 h [55], such an ample post-exercise recovery phase allows a comfortable, positive anabolic interaction with food (protein) intake. Immediately following exercise, muscle protein turnover, i.e., protein synthesis, breakdown, and amino acid transport, are accelerated. However, the net protein balance remains negative (i.e., catabolic or not above zero) unless food is ingested [80]. Therefore, food intake associated with exercise is necessary to bring muscle protein balance to be positive. Nevertheless, as it is commonly experienced, it generally takes weeks to months before training-induced changes in skeletal muscle mass become apparent. The prolonged time course for hypertrophy reflects the slow turnover rate of muscle proteins, which is about 1% per day for contractile proteins [37,146].
The molecular mechanism driving the anabolic effect of exercise in muscle recognizes the mammalian target of rapamycin complex 1 (mTORC1) activation as a central, although not exclusive, mechanism regulating muscle cell size and growth [147,148,149]. The same mTORC1 mechanism stimulates human skeletal muscle protein synthesis by essential amino acids [150]. Provision of amino acids, whether in free form [150,151,152,153,154] or as intact protein [155], in association with resistance exercise, increases muscle protein synthesis and net protein accretion resulting in a positive net muscle protein balance.
While dietary protein supplementation can augment the effects of resistance exercise on the increase in skeletal muscle mass and strength, it also can preserve skeletal muscle mass during periods of diet-induced energy restriction [156]. The time relationships between nutrient intake and exercise have been the object of detailed investigations.
Both pre- and/or post-exercise nutritional interventions (i.e., by carbohydrate + protein or protein alone) effectively increase body strength and improve body composition [157]. However, the size and timing of a pre-exercise meal may also affect the delay after which the post-exercise protein feeding is optimal. The post-exercise ingestion (from immediate to 2 h after) of high-quality protein sources stimulates robust increments in MPS. A mixed meal intake immediately after resistance exercise may effectively suppress MPB (muscle protein breakdown) in the morning in young volunteers [158]
Without exercise, meal timing and administration frequency exhibited little effect on body composition and body weight, whereas meal frequency can favorably improve appetite and satiety.
2.4.3. Exercise–Insulin Interaction
Insulin and exercise can positively interact in the stimulation of protein anabolism, in a complex fashion. Both protein synthesis and degradation rates are ≥1-fold greater following the post-exercise recovery than at rest [159]. The effects of insulin on muscle protein synthesis and degradation, either without or with exercise, are complex. Insulin-stimulated protein synthesis at rest, but not in the recovery post-exercise phase. In contrast, insulin decreased protein degradation following exercise as opposed to no effect at rest. Thus, the post-exercise phase can be viewed as a (transient) insulin-resistant condition as regards protein synthesis that, in terms of the effects on net protein balance, is however overwhelmed by a greater insulin-mediated suppression of proteolysis.
3. Part 2. The Pathophysiology of Sarcopenia in Ageing: Metabolic Hormonal, Cardiovascular, and Functional Changes in the Elderly and the Effects of Nutrition, Exercise, and Other Factors
The study of the mechanism(s) leading to ageing sarcopenia captures a fast-growing interest in both the basic and clinical–experimental sciences. While basic sciences rely on a variety of in-depth molecular, in vitro, and in vivo approaches, in vivo human studies are unique in that they translate the investigation in the human model, in turn, hampered by objective ethical/technical/investigational limitations, as outlined above.
Sarcopenia in ageing is associated with many metabolic, hormonal, cardiovascular, and functional changes. [160] The metabolic rate tends to decrease with age, resulting in a slower rate of energy expenditure, favoring weight gain and obesity with its related conditions. A reduction in muscle mass is observed, which affects mobility and overall physical function and is a major cause of diminished energy expenditure. Bone density tends to decrease with age, making older individuals more prone to fractures and osteoporosis. A decreased elasticity of blood vessels and an increased risk of hypertension and cardiovascular diseases are usually observed. Hormone levels, such as estrogen and testosterone, decrease, with effects on reproductive function, sexual health, and the gut–brain axis interplay. The immune system’s efficiency can decline, resulting in a higher susceptibility to infections and a diminished response to vaccinations. Cognitive abilities may change with age as well as vision and hearing. Digestive functions can be affected by reduced acid production by the stomach and changes in bowel motility. Older individuals may also experience changes in sleep patterns. Lung capacity and respiratory function may decrease, affecting lung health and physical endurance.
3.1. The Molecular Mechanisms behind Anabolic Resistance with Ageing
Anabolic resistance refers to the reduced ability of skeletal muscle tissue to respond to common anabolic stimuli, such as dietary protein and exercise, by increasing muscle protein synthesis rates (MPS). In healthy individuals aged ~18 to 50 who are not sedentary and eat sufficient daily amounts of protein and energy, skeletal muscle mass remains relatively unchanged throughout daily life [161].
3.1.1. Intracellular Signaling
mTOR Kinase
Basal mTOR activation is elevated during ageing and may contribute to anabolic resistance disrupting metabolic signaling pathways [162]. Markofki and colleagues aimed to investigate the effect of age on basal muscle protein synthesis and the signaling pathways involved in muscle protein synthesis, specifically the mechanistic target of the rapamycin complex 1 (mTORC1) pathway. They recruited a large cohort of young (18–30 y) and older (65–80 y) men and women to assess potential age-related differences and employed stable isotope tracer techniques and muscle biopsies to measure muscle protein synthesis rates and examine mTORC1 signaling at the basal level and in response to feeding. The study’s findings demonstrated that muscle protein synthesis rates were lower in older than young individuals. Additionally, the basal activation of mTORC1 was higher but attenuated in the older group in response to feeding. These findings suggest that age-related declines in muscle protein synthesis may be partly attributed to impairments in the mTORC1 signaling pathway. Moreover, there might be age-related differences in mTOR signaling in response to resistance exercise. Compared to their younger counterparts, older individuals experience delayed phosphorylation of mTORC1 up to 24 h after exercise and food intake, which is necessary for promoting muscle protein synthesis [162,163].
AKT Kinase
Studies suggest that anabolic resistance in ageing muscle may involve dysregulation of Akt signaling and downstream pathways [164]. Some key points regarding Akt and anabolic resistance in older people include impaired Akt activation, blunted protein synthesis response, and insulin resistance [165]. Studies have shown a reduced Akt activation in response to anabolic stimuli, such as resistance exercise and nutrient intake, in older adults compared to younger individuals [163]. Anabolic resistance in ageing muscle is characterized by a diminished muscle protein synthesis response to anabolic stimuli, which can be attributed, at least in part, to impaired Akt signaling. Age-related insulin resistance can impair Akt signaling and anabolic resistance in skeletal muscle [166].
3.1.2. Extracellular Signaling
2a. IGF-1: The role of IGF-1 in anabolic resistance, particularly in ageing and age-related muscle loss, is a topic of ongoing research. While IGF-1 is typically associated with anabolic processes and muscle growth, anabolic resistance refers to the reduced responsiveness of skeletal muscles to anabolic stimuli, leading to impaired muscle protein synthesis and loss. Ageing is associated with a decline in circulating IGF-1 levels, which can contribute to anabolic resistance. Reduced IGF-1 availability may impair the anabolic response to exercise and nutrient intake [141]. Anabolic resistance in older adults may involve dysregulation of the IGF-1 signaling pathway, including reduced activation of the IGF-1 receptor and downstream signaling molecules, such as the Akt/mTOR pathway, leading to diminished muscle protein synthesis [20]. Insulin resistance, commonly observed in older adults, can impact IGF-1 signaling and contribute to anabolic resistance [167].
3a. hGH: Several studies have explored the potential role of hGH in anabolic resistance, shedding light on its potential role in combating age-related muscle loss. One review by Velloso (2008) highlighted the interactions between hGH, insulin-like growth factor 1 (IGF-1), and muscle mass regulation, proposing that GH may play a role in anabolic resistance and the decline in muscle mass observed with ageing [168]. Older men receiving rhGH (recombinant GH) demonstrated greater muscle strength and size gains than in the placebo group. The findings suggest that rhGH supplementation in combination with resistance exercise may have potential benefits for improving muscle strength in elderly individuals [169,170]. In a study by Cuneo et al. (1991), hGH treatment was administered to elderly individuals to investigate its effects on skeletal muscle and found that GH supplementation improved muscle protein synthesis and increased lean body mass, indicating a potential role for GH in overcoming anabolic resistance in older people [171]. Finally, hGH treatment for 10 y in Growth Hormone-Deficient (GHD) adults resulted in increased lean body and muscle mass, a less atherogenic lipid profile, reduced carotid intima-media thickness, and improved psychological well-being [172]. Clinical trials investigating the efficacy of hGH for sarcopenia have produced mixed findings. While some studies have shown positive effects on muscle mass and function [173], others have yielded limited or inconsistent results. Safety concerns associated with hGH supplementation also contribute to its limited use. Potential side effects such as fluid retention, joint pain, insulin resistance, and increased risk of diseases like diabetes and cardiovascular disorders raise caution about the long-term use of hGH, particularly in older populations [174]. The cost and accessibility of recombinant hGH pose additional challenges. GH therapy is an expensive intervention, and its high cost may limit its widespread use in clinical settings, making it less feasible as a routine treatment option for preventing or curing sarcopenia [175]. In conclusion, while recombinant GH has shown potential benefits for sarcopenia in some studies, its use in clinical practice for this condition is limited. Mixed findings, safety concerns, cost considerations, and the lack of consensus guidelines contribute to the limited use of hGH in sarcopenia management. As a result, a comprehensive approach that addresses multiple aspects of sarcopenia is preferred. Future research may provide further insights into GH therapy’s potential benefits and safety profile for sarcopenia.
A study reported that serum levels of IGF-1 were increased in elderly women but not in men and correlated with frailty but not with their amount of muscle mass [176]. In elderly men, myostatin, a member of the transforming growth factor β (TGF-β) superfamily and an endogenous inhibitor of myogenesis, was increased [176]. Other studies have reported that IGF-1, myostatin, and insulin resistance were correlated with sarcopenia in elderly patients (both males and females) undergoing hemodialysis [177].
So far, it has not been precisely determined which genes are directly related to the possibility of developing sarcopenia. The studies that have been carried out so far have qualified genes predisposed to such properties that showed a correlation with muscle mass, muscle building, or impact on their functionality, to any extent. From a review that analyzed fifty-four studies on genes related to sarcopenia, twenty-six genes were identified and studied. The ACTN3, ACE, and VDR genes were the most frequently studied, although the IGF1/IGFBP3, TNFα, APOE, CNTF/R, and UCP2/3 genes were also shown to be significantly associated with muscle phenotypes in two or more studies [178].
The expression of the Mechano Growth Factor (MGF), a member of the IGF-1 (insulin-like Growth Factor 1) super family, has been shown to be both exercise and age dependent. MGF, also called IGF-1Ec, has a unique E domain with a 49bp insert in humans (52bp in rodents; IGF-1Eb), which results in a reading frame shift during the IGF-1 gene splicing to produce a distinct mature isoform. It has been administered on human cell cultures, and it has been concluded that the MGF-24aa-E peptide alone has a marked ability to enhance satellite cell activation, proliferation, and fusion for muscle repair and maintenance and could provide a new strategy to combat age-related sarcopenia without the oncogenic side effects observed for IGF1 [118]. In the context of ageing, MGF transcript production diminishes; however, when introduced to myoblast cultures derived from muscle biopsies of elderly individuals, these cells continue to exhibit replicative ability [119].
4a Anabolic Steroids: Several studies have investigated the relationship between testosterone and anabolic resistance. Bhasin et al. (2003) examined the impact of testosterone supplementation on muscle protein synthesis in older men and found that testosterone administration effectively restored muscle protein synthesis rates in older individuals, overcoming the anabolic resistance typically observed with ageing [179]. In another study, older men with low testosterone levels who received testosterone replacement therapy showed significant improvement in muscle protein synthesis rates after resistance exercise [180]. Another study investigated the effects of short-term testosterone administration on skeletal muscle protein synthesis in older men with low testosterone levels. They reported that testosterone replacement therapy improved muscle protein synthesis rates, indicating that testosterone plays a crucial role in overcoming anabolic resistance in ageing muscle; testosterone coupled with resistance exercise is an effective short-term intervention to improve muscle mass/function in older non-hypogonadal men [180,181]. Collectively, these studies may highlight the role of testosterone in combating anabolic resistance. Testosterone supplementation in individuals with low testosterone levels can restore muscle protein synthesis rates and enhance the anabolic response to exercise, counteracting the detrimental effects of anabolic resistance. Nevertheless, the use of high-testosterone doses in the elderly may be hampered by the fear of accelerating prostate cancer [182]. It is important to note that anabolic resistance is a complex phenomenon influenced by various factors, and testosterone is just one aspect of the overall picture. Other factors, such as nutritional status, physical activity levels, and other hormonal interactions, contribute to anabolic resistance. Therefore, a comprehensive approach that addresses multiple factors is necessary to manage individuals’ anabolic resistance effectively.
3.2. Anabolic Resistance in Ageing: Human Studies
Elderly subjects exhibit many abnormalities in protein metabolism in respect to younger subjects, here concisely summarized:
An increased splanchnic “trapping” of the ingested substrates, henceforth reducing amino acid delivery to peripheral tissues, such as skeletal muscle.
A decreased amino acid utilization by muscle, and/or the requirement for a greater AA load/delivery to stimulate appropriately PS in muscle, compatible with an anabolic-resistant state. In other words, the skeletal muscle in ageing might be less sensitive to lower (normal) levels of amino acids than that in young adults, and may thus require more protein to acutely stimulate muscle protein synthesis above rest, to achieve the required accretion of muscle proteins.
A decrease in energy production otherwise required to sustain the energy-expensive PS.
Altered protein digestion.
A decrease in transluminal AA transport.
An intestinal microbiota different from that of younger people.
Any of the above-listed potential factors could explain why elderly people would require, and/or are recommended to assume, at least ≈ 50% more protein than either young or mature subjects [66]. In the following sections, we report the literature data on protein turnover in ageing, both in basal and “post-absorptive” conditions, and following nutrition, exercise, and response to hormones.
3.2.1. Basal Skeletal Muscle Protein Turnover in Ageing
It was initially reported that muscle protein breakdown is elevated, by as much as ≈ 50%, in the elderly compared to younger adults [183] and that muscle net protein balance is negative [184]. Similarly, a decrease in the fractional synthesis rate (FSR) of skeletal muscle proteins was initially reported in elderly subjects [142,185,186,187,188,189,190]. A −20 to 30% decrease in myosin heavy chain (MHC) FSR was observed even in middle age [191]. In respect to MHC isoform expression, that of MHC-1 did not change with age, whereas the expression of MHC-IIa isoform was decreased by ≈35% in middle age (≈54 y) as compared to young subjects, further decreasing (by ≈50%) at older ages [191]. A ≈ 50% reduction in mitochondrial protein FSR was also demonstrated in middle age, not further decreasing at ≈75 y. In addition, a decrease in the activities of mitochondrial enzymes was observed in muscle homogenates from aged people, consistent with a decreased muscle oxidative capacity [186]. Mitochondrial ATP production measured using various substrate combinations was also lower in the elderly than in young subjects [192]. Moreover, genes and proteins related to mitochondrial shaping proteins decreased in sedentary elderly [35]. Pooled data from ≈150 healthy subjects of both sexes aged 18–89 y demonstrated a progressive decrease in mitochondrial DNA and mRNA abundance and mitochondrial ATP production with advancing age [190].
However, at variance with these earlier reports, more recent studies consistently failed to confirm the earlier findings of decreased basal muscle protein synthesis in elderly subjects, showing little or no differences between young and old adults [60,138,193,194,195,196]. These opposite, contrasting results could have been due to several previously unaccounted factors, such as the experimental methods themselves, the sample populations studied (particularly regarding the general health status, diet, habitual physical activity, or else), or other unappreciated causes, underlying the complexity, as well as the limitations, of the in vivo investigations. On the other hand, should basal daily muscle protein synthesis rates in the aged subjects be lower (by ≈20 to 30% or more), than that of younger people, as originally reported, these figures would end up in a more marked, unrealistic muscle wasting than what is typically observed (estimated as 0.5–1.5% per year between 50 and 80 y old subjects, or 3–8% per decade), as well as into complete muscle loss [197], or even into negative numbers, when projected over years.
It should, however, be recognized that basal muscle protein turnover could particularly be altered in frail, elderly subjects, being potentially associated also with a chronic, subtle, systemic inflammatory state and/or other co-morbidities [198,199]. Indeed, inflammatory mediators, such as cytokines, particularly TNFα, may impair skeletal muscle protein FSR, by interfering with the phosphorylation of the mammalian target of the rapamycin (mTOR) pathway [150], critically involved in the regulation of mRNA translation, muscle protein synthesis, and growth [200]. Therefore, it is currently accepted that basal skeletal muscle protein turnover is near-normal in healthy elderly subjects. In contrast, a different, complex picture can emerge when studying the response in ageing of skeletal muscle protein turnover to anabolic stimuli, such as substrates (i.e., mixed meals, protein, amino acid mixtures, other metabolites), exercise, anabolic hormones, or their combinations.
3.2.2. Skeletal Muscle Protein Turnover in Ageing in Response to Nutrition and Exercise
Elderly subjects exhibit some peculiarities in the handling of oral feeding. Using the essential amino acid leucine as a tracer, a greater first-pass splanchnic uptake of ingested amino acid(s) was reported in the elderly rather than in young people, suggesting that a lower proportion of ingested amino acid reached the peripheral circulation [195]. Such a greater splanchnic extraction could limit the amino-acid-mediated stimulation of muscle protein synthesis in peripheral tissues such as skeletal muscle. Consequently, sustaining splanchnic vs. muscle protein synthesis could indicate a “metabolic priority” during recovery from metabolic stress in healthy elderly persons. Such a mechanism might become more relevant in older individuals suffering from chronic diseases and/or subjected to poly-medications [201]. However, in contrast with the above report, it was also reported that oral amino acids stimulated muscle protein anabolism in the elderly, despite the higher first-pass splanchnic extraction [202].
A protein pulse rather than a spread (or continued) oral feeding stimulated the best protein accretion in the elderly [203], but not in young women, in whom the protein feeding pattern did not affect protein accretion. In older men, rapidly absorbed whey protein stimulated postprandial muscle protein accretion more efficiently than the “slow protein” casein hydrolysate [82]. The whey protein effect was superior to that of casein hydrolysate too, likely due to the combination of the whey protein’s fast digestion and absorption with its greater leucine content. Conversely, in younger subjects, a slowly digested protein (casein) achieves a better anabolic effect than a rapidly digested one [77]. Thus, fast-absorbable protein (or protein supplements), rich in well-balanced EAA, could be ideal and specific in the nutrition of aged people [204,205]. The cooking mode is also important. Well-done, more-digestible meat is assimilated better than rare meat in the elderly [206].
Other protein types, such as the vegetal wheat protein (administered in a 35 g dose) increased muscle protein synthesis rates in healthy older men, which was nearly as much as 35 g whey protein hydrolysate over 2–4 h. [207].
Generally speaking, dietary protein supplementation can augment the gains in skeletal muscle mass and strength mediated by resistance exercise, and can preserve skeletal muscle mass during periods of diet-induced energy restriction [208]. A bout of aerobic exercise increased the anabolic effect of nutrient intake in older adults [209]. Such an effect could have been driven, in addition to the protein themselves, through an exercise-induced augmentation of nutrient-stimulated vasodilation, resulting in increased nutrient delivery to muscle, rather than through improved insulin signaling. Conversely, others did not show any additional effects of protein over that induced by exercise itself, on skeletal muscle hypertrophy. Verdijk et al. compared the increment(s) in skeletal muscle mass and strength following 3 months of resistance exercise training, with or without protein ingestion, either prior to or immediately after each exercise session in elderly males who habitually consumed about 1.0 g protein/kg per day [210]. They concluded that timed protein supplementation prior to and after each exercise bout did not further increase skeletal muscle hypertrophy.
3.2.3. Anabolic Response in Skeletal Muscles of Aged People
The studies aiming at detecting the existence of resistance to anabolic stimuli in ageing (i.e., of “anabolic resistance”) are complex and sometimes inconclusive, often due to the variability of the experimental setting. Several factors are to be considered, such as the amount, the quality, and the administration pattern of the protein, the energy content of the meal, etc. The same considerations should apply to the effects of amino acid mixtures. Furthermore, when exercise is combined with nutrition, the anabolic response can vary with the exercise intensity, whether it is applied to the whole body or to a single leg, with the timing of food administration, etc. Therefore, any conclusion drawn from individual studies should be balanced considering many concurrent variables. A summary of available data is reported in Table 2.
Data consistent with a normal anabolic response (= no resistance).
Several studies reported a normal response of MPS to oral administration of either protein or free amino acids in the elderly. In a dose–response study performed by feeding frequent small boluses of liquid meals to healthy > 60 y old subjects, Welle and Thornton [211] reported that a comparable stimulation of MPS was attained independently from both the protein load and the protein contribution to total energy, i.e., from low (7% corresponding to 0.6 g protein/kg/day) to moderate (14% total energy, i.e., 1.2 g protein/kg/day) or high (28% total energy, i.e., 2.4 g protein /kg/day). They concluded that in older subjects, there was no dose-dependent effect of the amount of protein intake on MPS; therefore, the “lower” protein dose was sufficient to stimulate MPS maximally. The lower dose tested in this study was comparable to that found in young subjects of other studies (Table 2).
Similarly, the administration of an EAA drink (with an amino acid composition approximating their distribution in muscle protein) to both young and elderly subjects stimulated muscle protein synthesis to the same extent in both groups [58], although the response in the elderly was retarded albeit still sustained, as opposed to the faster, short-lived one of the young.
No impairment of muscle protein synthesis to protein intake was detected in elderly subjects after ingestion of large amounts of carbohydrates and proteins [211], or of either moderate (≈115 g) [212] or large (≈300 g) amounts of beef [213]. By comparing sarcopenic (≈80 y) and healthy (≈70 y) older men, the ingestion of ≈20 g of a leucine-enriched whey protein load, increased muscle protein synthesis rates to the same extent in both groups [214].
In older adults, following the ingestion of mixed meals containing combinations of animal (beef) and vegetal proteins, the whole-body anabolic response linearly increased with increasing protein intake, primarily due to the suppression of protein breakdown. Notably, muscle protein synthesis (i.e., one factor contributing to the net protein accretion, or balance, together with the suppression of protein degradation) was further stimulated by a protein dose (70 g) above that previously considered as “optimal” in the elderly (≈35 g/kg BW) [215], yet attaining the same maximal response as that of young subjects. However, at the 40 g dose, the stimulation of muscle protein synthesis in the elderly was lower than that observed in the young volunteers, therefore compatible with anabolic resistance in the former group at a “lower” (i.e., 40 g) mixed protein dose (see also below). The above-reported protein dose(s) stimulating MPS in older subjects were, however, greater than the 20 g high-quality, whey protein dose that produced the maximal effect on MPS in young people, either exercising or not [72]. The muscle protein synthetic response to the combined ingestion of protein and carbohydrate (i.e., an additional energy source) was not impaired in healthy older men [216]. Similarly, the co-ingestion of carbohydrates with protein and free leucine stimulated muscle protein synthesis to the same extent in young and elderly lean men [217].
Table 2.
Effects of protein-rich food ingestion on Muscle Protein Synthesis (MPS). According to the study type, the data are grouped as Dose–response studies in the elderly (n = 1 study). Sarcopenic vs. healthy elderly subjects (n = 1 study). Studies reporting similar responses in old and young subjects (=no anabolic resistance) (n = 7 studies). Studies reported a decreased/blunted/delayed response (=anabolic resistance) in old subjects compared to young controls (n = 10 studies).
| Type of Food/Protein Tested | Subjects | Dose A: Either the Threshold or the Lowest Dose Increasing MPS | Dose B: Either the Highest Dose Tested or that Maximally Stimulating MPS | Exercise Status | Comment | Reference |
|---|---|---|---|---|---|---|
| Dose–response study | ||||||
| Liquid meals (recalculated from original data to weight and total protein intake over the test) | Healthy (62–75 y) males and females | 29 g | 115 g | −/+Ex | Max effect obtained at the lowest dose. MPS increases greater after exercise. |
[211] |
| Sarcopenic vs. healthy elderly | ||||||
| Leucine-enriched whey protein | Sarcopenic (81 y) males | 21 g | / | −Ex | Similar increase in MPS in both groups | [214] |
| Healthy (69 y) males | ||||||
| Similar response to controls (= no resistance) | ||||||
| Whey protein isolate | Old (71 y) males | 10 g | 40 g | −/+Ex | Exercise enhanced max effect at 40 g protein | [218] |
| Beef (beef contains −22% of weight as protein) | O (68–70 y) vs. Y (34–41 y) |
113 g | 340 g | −Ex | [212,213] | |
| CHO + WP + Leu (Data recalculated for the subjects’ average weight (75 kg). The table reports the total of the EAA+Leu administered, which were fractionated in 6 doses every hour) | O (75 y) vs. Y (20 y) males | / | 72 g WP + 13.5 g Leu | +Ex | 30 min of moderate-intensity physical activity | [213] |
| EAA drink | Elderly (67 y) males and females | 15 g | / | −Ex | Retarded albeit still sustained response in the elderly; faster, short-lived one in the young over 3 h | [58] |
| Mixed animal (beef) and vegetal foods | Elderly (69 y) males and females | 35 g protein | 70 g protein | −Ex | ≈5x greater response of MPS at the higher protein dose. At the 35 g dose, the response in O was > 5x lower than that in Y (see below) | [216] |
| Mixed animal (beef) and vegetal foods | Y (31 y) males and females | 40–44 g protein | 66–70 g prot | −/+Ex | At the 36 g dose, the response in O was > 5x less than that in Y | [57] |
| Protein and CHO | O (75 y) males | 20 g | −Ex | [217] | ||
| Y (21 y) males | ||||||
| Decreased/Blunted/Delayed response (= anabolic resistance) | ||||||
| Combined analysis of Whey Protein (n = 5 studies) Egg (n = 1 study) (original data recalculated to body weight) |
Elderly (≈71 y) males | 8 g | ≈32 g | −Ex | Delayed response in the older group | [73] |
| Young (≈22 y) males | 8 g | ≈20g | −Ex | |||
| Intact whey protein | O (≥70 y) | 5–20 g | ≥20 to 40 g | −/+Ex | In the resting elderly, the response of MPS plateaus at 20 g, at a lower value than in Y (40 g). Resistance exercise can increase MPS only in O but at greater protein intake. |
[72,218] |
| Y (> 23 y) | 5–20 g | 20 g | −/+Ex | |||
| Crystalline EAA | Y (28) vs. O (70 y) (Similar response of MPS in the two groups) |
2.5 g (?) | 20–40 g (the highest dose (40 g) was tested only in the older group) | −/+Ex | [60] | |
| AA + Leucine | O (68–70 y) males and females | 15 (Published data are reported as 0.35 g/FFM (Free Fat Mass). Here, they have been recalculated per mean subject assuming the FFM individuals’ LBM (Lean Body Mass).) | / | −/+Ex −/+insuin |
MPD = pre and post ex, with or without insulin | [59] |
| Leucine-enriched EAA | O (67) vs. Y (29) | 6.7 g EAA | / | −Ex | A higher leucine dose (41%) was necessary to match the increase in MPS of the O to that of the Y | [195] |
Abbreviations used in the Table: EAA: Essential Amino Acid(s); Ex: Exercise; O: Old; Y: Young; y: years; WP: Whey protein.
Data consistent with an impaired response (=anabolic resistance)
Other studies are, however, compatible with the existence of some degree(s) of anabolic resistance in the skeletal muscle of the elderly. Resistance in the elderly can be demonstrated following the administration of either protein or amino acid, as well as after hormone, exercise, or their combinations.
In the elderly, the dose of high-quality dietary protein administered as a single bolus (0.40 g protein/kg BW/meal, corresponding to ≈ 32 g protein/meal in an 80 kg person), required to stimulate myofibrillar protein synthesis maximally, was greater than that required in young controls, (0.24 g protein) [73].
When crystalline EAA were administered, the literature data were somehow contrasting. It was initially reported that 2.5 g crystalline EAA were sufficient to elicit an increase above basal of MPS in both young and elderly subjects, whereas resistance of MPS in the elderly was apparent only after 10–20 g EAAs [60]. However, in subsequent studies using intact whey protein, it was shown that the lower dose capable of eliciting a response of MPS in the elderly is greater than that in the young, and that the ageing muscle responds maximally at 20 g EAAs, a dose ≈ 2× lower than that in the young (40 g EAAs) [60]. In the elderly, however, MPS could be further increased with a 35–40 g intact whey protein intake. Thus, in the elderly, the dose of high-quality whey protein required to attain a maximal stimulation of skeletal muscle protein synthesis was about onefold greater than that of younger subjects [72].
A ten-day administration of a supplement containing fast-digestive proteins (soluble milk proteins compared to casein alone) could overcome muscle anabolic resistance in the elderly [205]. Furthermore, oral EAA ingestion exhibited a retarded albeit still sustained response of MPS in the elderly as compared to young subjects [58].
In older men, skeletal muscle was also less responsive to the anabolic effects of leucine in the postprandial phase than in the young controls [219]. Also, a higher proportion of leucine within an essential amino acid mixture was required for the optimal stimulation of muscle protein synthesis in the elderly, rather than in young subjects [195].
3.2.4. Insulin Resistance
Insulin resistance on muscle protein synthesis in ageing was reported in another study showing that leg phenylalanine net balance, although not different between young and old subjects at baseline, was significantly increased in both groups with hyperinsulinemia, but to a greater extent in the young [220].
Nevertheless, increasing insulin availability through a local insulin infusion increased amino acid uptake but did not enhance muscle protein synthesis rates in healthy young and older men following the administration of a casein-based protein drink [221]. Thus, in the post-prandial phase, skeletal muscle appears to be in an insulin-resistant state to the stimulation of protein synthesis, without difference between young and elderly subjects (see also above).
3.2.5. Resistance to the Anabolic Effects of Exercise
Resistance to exercise-induced protein anabolic effects could be another factor favoring sarcopenia. Multiple pieces of evidence exist supporting this view. Following a single bout of resistance exercise in the post-absorptive state, the dose–response curve of the stimulation of protein synthesis by resistance exercise intensity reached the maximum at 60–90% 1 RM, but was shifted to the right/low and blunted in the elderly compared to young controls, up to 1–4 h post-exercise [138]. At the molecular level, the phosphorylation of p70s6K and 4EBP1 at 60–90% 1 RM was decreased in the older group. Phosphorylation of p70s6K 1h post-exercise at 60–90% 1 RM predicted the rate of MPS at 1–2 h post-exercise in the young but not in the old. These data indicate that older men exhibit resistance to anabolic exercise of both MPS and intracellular mediators. In another study, although the acute MPS response to combined resistance exercise and EAA ingestion was comparable in young and older men, such a response was delayed with ageing, and it was associated with unresponsiveness of ERK1/2 signaling and of AMPK activation [222]. Similarly, in post-absorptive elderly subjects, following a single bout of resistance exercise, MPS increased to a lesser extent in the recovery phase than that observed in young controls [163].
At variance with the above data, however, other reports show that aerobic exercise could overcome age-related (insulin) resistance of muscle protein anabolism following a leucine-enriched essential amino acid–carbohydrate mixture, through an improvement of the endothelial function and of the Akt/mammalian target of rapamycin signaling [223]. Thus, exercise combined with EAA administration could effectively counteract age-associated sarcopenia and other conditions leading to muscle wasting. Conversely, no additional effect of heavy resistance exercise on myofibrillar protein synthesis was detected in elderly men after milk protein and carbohydrate ingestion [224].
3.2.6. Deleterious Effects of Bed Rest in the Elderly
A condition opposite to exercise is bed rest, an undesired situation very common in hospitalized older subjects, as well as in those old persons in whom, for a variety of factors, physical activity is restrained and/or abolished, either voluntarily or not [156,225,226,227]. In older adults, bed rest significantly restrained the EAA-induced increase in MPS, through a reduction in mTORC1 signaling and amino acid transport [228]. EAA supplementation above RDA may help to preserve muscle function in the elderly during inactivity [229].
3.2.7. Effect of the Inflammatory State
Acute and chronic inflammation retain undesired effects on skeletal muscle mass and protein metabolism. Thus, age-associated inflammation (either subtle or overt) may negatively affect the anabolic sensitivity of skeletal muscle in the elderly. Toth et al. reported a strong relationship exists between MPS and circulating concentrations of several markers of immune activation [199]. At the mechanistic level, cytokines, in particularly, TNF-α, may impair MPS by blunting the phosphorylation of proteins in the mammalian target of the rapamycin (mTOR) intracellular signaling pathway [200].
3.2.8. Role of Blood Perfusion of Skeletal Muscle
Effective blood perfusion is another variable conditioning the delivery of anabolic substrates and hormones to muscle. Basal leg blood flow was greater in young than in elderly subjects, and it was stimulated in response to physiologic hyperinsulinemia in the young but not in the elderly [220]. Furthermore, a positive relationship was found between the insulin-induced changes in blood flow and the increase in muscle protein synthesis. Conversely, the stimulation of muscle protein FSR responded similarly to exogenous amino acids in healthy younger and older adults under conditions of comparable NO-induced hyperemia, and the maintenance of an adequate blood flow perfusion may have a favorable impact on protein synthesis in ageing skeletal muscle [230].
3.3. Strategies to Counteract Anabolic Resistance in Ageing
A number of strategies can be applied in the effort to optimize muscle protein anabolism and/or to combat anabolic resistance in the elderly. They can exploit the effects of nutrition, exercise, or both, as well as that of other specific interventions.
3.3.1. The Effect of Complex Nutritional Supplements
Although natural foods rich in high-quality protein (dairy products, meat, and egg) can adequately stimulate protein anabolism as efficiently as that of protein-rich supplements and/or of specifically designed amino acid mixtures, the advantage of using specific nutritional supplements remains questionable [231]. In older women, the intake of 3 g of a leucine-enriched EAA supplement (LEAA) (containing 1.2 g leucine) stimulated MPS as much as 20 g WP (containing 2 g leucine). Thus, the composition of EAA (rich in leucine) rather than the AA/protein amount seems to be crucial to stimulating skeletal muscle anabolism [232]. When comparing the effects of a soy and milk blend with whey protein alone, on post-exercise muscle protein synthesis, the anabolic effects were similar in older subjects [233], at variance with what was observed in the young, in whom the soy and milk blend helped to prolong the stimulatory effect on MPS from 2 h (with whey protein alone) to 4 h. The co-ingestion of carbohydrate and fat with an isonitrogenous nutritional supplement (21 g of leucine-enriched whey protein) did not affect nor further improve postprandial muscle protein synthesis in older men, showing that the provision of extra energy does not modulate muscle protein synthesis [234]. The ingestion of casein added to a milk matrix, modulated dietary protein digestion and absorption kinetics, however, without affecting postprandial muscle protein synthesis in older men [208].
In a clinical trial of a 3-month supplementation period with 15 g EAA vs. placebo in older women (≈70 y), the acute anabolic response of skeletal muscle FSR to EAA supplementation was maintained over the observation period; however, LBM increased only in the supplemented ones but not in the controls, whereas muscle strength was unchanged in both groups [235]. Thus, EAA supplementation may help in improving LBM and combat, with yet uncertain mechanism(s), the debilitating effects of sarcopenia. In another study, EAA supplementation with exercise in sarcopenic older women improved speed gait but not lean mass or strength. In elderly subjects supplemented with 15 g EAAs three times daily for ten days of bed rest, functional parameters were improved following inactivity [229].
The supplementation of either protein (whey and soy), leucine, or creatine did not improve the training-induced adaptations in pre-frail and frail elderly, regardless of sex. Therefore, these findings would not support using supplements to expand the effects of resistance exercise to counteract frailty-related muscle wasting dynapenia [236].
At variance with some of the above-listed reports, even at low EAA ingestion, healthy older adults trained for 12 w with progressive bouts of resistance exercise, exhibited a normal increase in muscle strength, cross-sectional area, and mixed muscle protein FSR [237], via the stimulation of the mTORC1 complex, thus showing that a healthy, exercise-conditioned condition may help to maintain normal sensitivity to the anabolic effects of EAA and muscle protein accretion.
On the whole, many variables may account for the inter-study variability and the end-point message(s), which could appear somewhat confusing from the reported studies. This might be due to the specific outcomes selected, the doses and the duration of EAA supplementation, or the general, health-related conditions of the enrolled subjects, habitual physical activity, etc. Also, some of the chosen endpoints (such as the increase in MPS) may not really reflect a true improvement in muscle function.
In addition, it should be considered that the administration of nutritional supplements in elderly subjects at nutritional risk may be offset by a simultaneous reduction in voluntary food intake [238,239].
3.3.2. Specific Effects of Leucine Addition
The addition of leucine to other nutritional products may be a valid supplement to be used in the elderly, although with somehow limited effects [240]. Older subjects consuming a leucine supplement showed a greater increase in MPS rates from baseline than the controls, but not in lean body mass or muscle function [61]. In a recent study in older men (74 y), co-ingestion of 2.5 g leucine with 20 g casein resulted in a 22% higher muscle protein synthetic rate compared with ingestion of casein alone [241].
The leucine effect seems to be dose-dependent. Leucine added as a supplement (3–5 g) to either whey protein or EAA solutions in either younger or older men, showed comparable increments in skeletal muscle myofibrillar protein synthesis (MyoPS), at variance with no sustained stimulation observed in control subjects receiving only 1.8 g leucine [242,243]. However, the leucine effect in skeletal muscle of aged people might be impaired, as mTORC1 activation is defective, and sensitivity and responsiveness of muscle protein synthesis to amino acids decreased [50]. Conversely, the basal activation of mTOR seems to be higher. A combined effect of age-related impairment of muscle signaling and insufficient availability/delivery of nutrients and growth factors to the muscle might contribute to sarcopenia. Thus, whether ageing per se affects mTORC1 signaling is yet uncertain, because of the common association between poor protein assumption, reduced/absent physical activity, and concurrent diseases. In studies in which habitual protein intake exceeded 1.0–1.1 g/kg/day, and included a moderate/high proportion of dairy protein, prolonged leucine supplementation (2.5 g daily for six months) did not increase muscle mass or strength in healthy type 2 diabetic older adults [244].
3.3.3. Exercise Strategies
The primary target(s) of sarcopenia prevention/amelioration is the reduction in the risk of falls and fractures and the maintenance of independence in everyday tasks. The interventions applied to attain these targets look very similar to those aimed at the amelioration of general health status with ageing, such as improvements in muscle mass and strength, bone density, cardiovascular fitness, maximal oxygen consumption, endurance, and energy metabolism, in addition to the reduction in insulin resistance and the associated risk of diabetes mellitus, coronary heart disease, hypertension, and obesity [245]. In other words, the benefits of regular exercise translate into an improvement in the quality of life of elderly populations. Practicing regular exercise is a prerequisite to attain and maintain these benefits over time. In order to attain these key objectives, much attention has to be devoted to the implementation of specific exercise programs, aiming at increasing the awareness of their safety, required constancy, and compliance.
Following regular training, older subjects can increase muscle strength as much as that of younger control subjects, even by threefold, over a few months. This result is initially accomplished by neural adaptation(s) and greater muscle fiber recruitment, while more prolonged resistance training leads to an increase in muscle mass/size too, (partially) restoring the loss of the cross-sectional area of type II muscle fibers occurring with ageing [246]. Resistance training contributes to improvement in the functional capacity of the levels of physical activity and allows participation in daily living activities to be maintained, thus being motivationally efficient too. Specific protocols for the implementation of exercise to combat sarcopenia in ageing, in the old [247], as well as the oldest-old (80–85 y) [248] and the physically frail [249], have been proposed.
Both resistance/strength/weight exercise and endurance/aerobic training of skeletal muscles may be useful in the prevention and treatment of sarcopenia. Strenght training can positively affect the neuromuscular system, and increase hormone concentrations and the MPS rate [250]; however, from a recent meta-analysis, the benefit of combining dietary supplements and exercise is not so straightforward among different populations [251].
Exercise training has been consistently shown to be highly effective in slowing down and possibly preventing the insurgence of sarcopenia in older people, counteracting the loss of muscle mass, and strength, and an increase in intramuscular fat infiltrations. While stressing the importance of a personalized approach, general physical activity guidelines suggest that a combination of aerobic exercise, progressive resistance training, and balance training might be the ideal intervention for a sarcopenic population. Of note in a mixed training involving aerobic, strength, and balance exercises was effective in improving or preserving motoneuronal health and neuromuscular junction (NMJ) stability, together with muscle mass, strength, and functionality in an old, sarcopenic population [252]. These sarcopenic subjects were trained three times per week for 2 years with a mix of aerobic, strength, and balance exercises matched with nutritional advice [252]. The same group of researchers followed older dancers for 6 months, reporting a major stability in neuromuscular junctions and a superior functional performance despite no differences in muscle size [253].
3.3.4. Steps for Adaptation to Exercise
First, a physical assessment of the functional status of the elderly is mandatory. Elderly adults are commonly more susceptible to orthopedic traumas and cardiovascular complications. Therefore, an accurate evaluation of their actual independence with respect to daily life activities (ADL), and of cardiovascular tolerance and conditions, should be performed. Patients with already compromised ADLs should be tested more specifically for sarcopenia. The evaluation of the risk of falls, and their prevention, should be part of the initial treatment strategy. In the case of resistance training programs, the elderly should be tested for their CV fitness as well as properly instructed on safe lifting techniques.
A proper warm-up step should precede the onset of resistance training. Since a loss in elasticity of muscle and connective tissue, and the subsequent increase in stiffness, are common with advancing age, so a preliminary step would reduce the risk of injuries in older subjects. Resistance exercise should start at a low intensity, and then gradually increase over time. Strength training should begin with relatively light weights, being comfortably lifted through a full range of joint rotation/motion while maintaining a proper posture and mechanics. Individuals should avoid holding and/or deeply changing their breath frequency during force production (i.e., perform a Valsalva maneuver). The lifted weight should be gradually increased paralleling the improvement in strength, with the goal to meet the chosen, relatively high-intensity resistance training program, which has proven to be the most effective in older populations [254].
The exercise intensity is usually targeted to a percentage of the individual’s one-repetition maximum (1RM is the maximum weight that can be safely lifted one time). Exercise intensity is calibrated to a weight allowing one set of 8 to 15 repetitions to be completed leading to volitional fatigue (i.e., between 8- and 15RM), approximately corresponding to 60 to 80% of 1RM. This intensity leads to significant improvements in muscle strength and mass. [255]. Although higher exercise intensities (up to 85 to 100% of 1RM) may be associated with greater improvements in strength, they may increase the risk of musculoskeletal injuries in older subjects. When an increase in muscle endurance is pursued, a lower-intensity weight that can be lifted for more repetitions is recommended. [245].
The duration (i.e., the volume) of resistance training necessary to produce the maximum benefits in muscle strength is currently under debate. Usually, multiple sets (2 to 3 sets) of 8- to 15RM are recommended. Conversely, others suggest that a single set is as efficient as multiple sets for strength gains in adults. [255]. Notably, increased compliance to a single set of exercises may allow the completion of the resistance training program in a shorter period of time, thus reducing the rate of dropouts. [256,257]. Albeit a single set seems to be sufficient to produce significant gains in strength, multiple sets can be employed when greater strength gains are desired.
Improvements in muscle strength are usually observed with a training frequency of 2 to 3 days/week in the elderly. While increasing the frequency to 4 to 5 days/week may induce further strength gains, a decrease in compliance may however ensue. Thus, a frequency of 2 to 3 days/week would be recommended for elderly individuals beginning a resistance training program [245].
Ideally, resistance training is directed at the large muscle groups of the arms/shoulders, chest, back, hips, and legs that support everyday activities. Each repetition should be performed slowly through the full option of motions, with sufficient time to lift the weight (i.e., concentric exercise) as well as to lower the weight (i.e., eccentric exercise). Thus, appropriate, variable-resistance machines using weight stacks should be employed in this population, as they reduce the risk of injuries to extremities, and decrease the risk of lower back injuries and that of exercised-induced hypertensive spikes, because the weights can be progressively titrated in small increments, and resistance can be applied through an ample range of motion. [245].
3.3.5. Caution and Contraindications to Exercise in the Elderly
While healthy older adults should safely engage in some mild to moderate physical activity, caution should be taken about the planning of more vigorous exercise in these subjects. The American College of Cardiology and American Heart Association recommends a test of exercise stress in yet asymptomatic men older than 40 years, as well as in women older than 50 years, before the initiation of vigorous exercise programs [258,259,260].
Specifically, in older adults with existing disease, the risks associated with regular exercise may outweigh its potential benefits. As a matter of fact, exercise may elicit/aggravate pre-existing diseases and conditions common to older adults, such as arthritis, osteoporosis, angina, and hypertension at advanced stages. An accurate collection of their medical history, a thorough physical examination and risk stratification, and a close supervision and education performed by trained personnel can minimize the potential risks and hazards of exercise in the elderly [258,259,260,261].
3.3.6. Other Treatments
Although the positive effects of testosterone replacement in elderly (hypogonadal) men have been outlined above, only modest increments in muscle mass and strength were produced, and not observed in all studies [182]. The potential for an acceleration of prostate cancer should be taken into account too. Similarly, the replacement of hGH in elderly subjects did not increase muscle strength and did not expand strength gains following resistance training [182]. The pharmacological stimulation of the GHRH-IGF-I axis including its major circulating binding protein (IGF-I/IGFBP-3) is yet to be fully tested and investigated. The potential of myostatin inhibition in the treatment of sarcopenia associated with chronic kidney disease is under consideration [262][]. New exercise technologies, and supplementation by antioxidants, vitamin D, eicosapentaenoic acid, or ursolic acid, as well as activation of peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α), may be considered too [263].
3.3.7. Optimizing Nutrition–Exercise Interaction in the Stimulation of Skeletal Muscle Anabolism in Ageing
The magnitude of the anabolic response to nutrients combined with exercise may be influenced by factors other than just the amount of a nutrient ingested or the magnitude of exercise. The daily distribution and timing of protein ingestion, the co-ingestion of different nutrients, and the type/quality of the protein or of the amino acid mixture ingested may all influence protein accretion (Table 3).
Table 3.
Nutritional indications of protein intake to optimize skeletal muscle mass and function, and combat anabolic resistance in ageing.
| 1. Consume a balanced diet rich in natural foods providing sufficient amounts of high-quality protein, to sustain (skeletal) muscle protein accretion; |
| 2. Keep/increase protein intake (preferentially of high quality) at ≥1.5 g/kg/day; |
| 3. Provide ≥ 30 g protein at each main meal, equally spread into the meals, to promote an optimal per meal stimulation of MPS; |
| 4. Prefer protein-rich natural foods over protein-rich supplements; |
| 5. Avoid as far as possible continuous 24 h nutrition; |
| 6. Provide sufficient energy; |
| 7. When adding supplements: |
| a. Use them only in specific cases; |
| b. Use EAA supplements preferentially rich in the BCAA; |
| c. Add leucine either to natural protein foods or to the AA supplements; |
| d. Consider that intake of protein supplements can proportionally lead to an involuntary reduction in the intake of protein-rich natural foods, thus partially offsetting their anabolic effect; |
| 8. When combined with exercise, assume nutrition taking into account: |
| a. The timing of administration: |
| i. pre-exercise; |
| ii. at the beginning (t = 0′) (preferred); |
| iii. or following exercise… |
| b. Amount and type of nutrition? |
| i. Natural protein-rich foods? And/or: |
| ii. Supplements; |
| 9. Consider other non-protein supplements: |
| 1. Creatine, PUFA (Polyunsaturated fatty acids). |
A number of reports have also investigated the optimal daily distribution of food protein, as well as the timing of the administration with respect to exercise performance (see also above).
In regard to food protein distribution over the three main daily meals, it was reported that non-frail elderly subjects consume dietary proteins more evenly than pre-frail and frail subjects, thus suggesting that the daily protein load should be consumed as ≈30 g protein (in a representative 75 kg subject) at each of the three daily mealtimes [264]. Conversely, in a subsequent randomized-controlled trial, the pattern of meal-protein intake (i.e., even vs. not even) over the day did not affect lean body mass, muscle strength, or other functional outcomes, as well as whole-body protein kinetics and MPS over 8 weeks in older adults [265].
With respect to the timing of food protein food administration with exercise (i.e., before, at the beginning, i.e., at t = 0′, or after exercise performance), in the only published study in the elderly, the early intake of a protein supplement immediately before (at t = 0′) each bout of resistance-type exercise in a 12-week intervention study in the elderly sustained skeletal muscle hypertrophy, as opposed to having no effect with the supplement intake taken 2 h after exercise [266]. Notably, protein ingestion in the evening/night before sleep increased Muscle Protein Synthesis throughout the night in healthy older men [267].
A list and the quantities of common foods providing ≈ 30 g of protein are reported in Table 4. (data compiled from ref. [268]) The quantities of animal foods are lower than those of vegetal foods (except for soybeans and quinoa), because of their balanced content of essential amino acids. The reported quantities are only indicative, because most of these foods have not been specifically tested in controlled studies of stimulation of skeletal MPS with exercise in the elderly.
Table 4.
List and quantities of common animal and vegetal foods providing ≈ 30 g protein, and their caloric content, useful to improve muscle anabolism in ageing with exercise.
| Animal Foods | g | Calories |
| Cow whole milk | 909 | 582 |
| Ricotta from cow milk | 340 | 498 |
| Ricotta from buffalo milk | 286 | 606 |
| Low-fat, fresh cheese | 98 | 446 |
| High-fat, aged cheese | 89 | 347 |
| Chicken egg (n = 4.4) | 242 | 322 |
| Beef meat | 136 | 151 |
| Pork meat | 183 | 268 |
| Chicken breast | 131 | 131 |
| Bresaola | 94 | 142 |
| Turkey breast | 125 | 134 |
| Seabass (filets) | 141 | 210 |
| Fresh salmon | 163 | 302 |
| Vegetal Foods | g | Calories |
| Soybeans | 100 | 404 |
| Wheat meal | 273 | 927 |
| Corn meal | 345 | 1248 |
| Beans | 566 | 752 |
| Quinoa | 234 | 863 |
| Oatmeal | 238 | 917 |
| Rice | 450 | 1487 |
| Lentils | 475 | 436 |
| Chickpeas | 429 | 514 |
Besides protein or amino acid supplements, other substances could be considered to promote skeletal muscle accretion [269].
3.3.8. Muscle and Bones
The idea of a bone–muscle unit has emerged in the past decades. Different studies have provided valuable insights into the intricate communication between muscle and bone during anabolic resistance [270,271,272]. These investigations have revealed that muscle and bone are tightly interconnected tissues, with reciprocal interactions and shared regulatory mechanisms. Mechanistically, it has been established that mechanical loading, generated by muscle contractions during physical exercise, plays a pivotal role in preserving bone health. By applying forces to the bone, mechanical loading stimulates bone remodeling and fosters the maintenance of bone density and strength [273]. Conversely, disrupting this mechanical loading during periods of muscle disuse, such as immobilization or inactivity, can result in bone loss and increased susceptibility to osteoporosis [274]. Moreover, molecular mediators released by muscle cells, including growth factors, hormones, and cytokines, have emerged as crucial signaling molecules facilitating the bidirectional crosstalk between muscle and bone. These molecular messengers transmit signals and exert regulatory effects on bone cell activity, ultimately influencing bone turnover and remodeling processes. From the muscle can be released osteoinducer (IGF-1, FGF-2, IL-15, OGN, FAM5C, Tmem119, and osteoactivin) and osteoinhibitor (IL-6 and myostatin), among different stimuli [275]. Consequently, interventions targeting anabolic resistance and aiming to preserve or enhance muscle mass and function, such as resistance exercise and optimizing protein intake, hold considerable potential to positively impact both muscle and bone health [276]. A comprehensive understanding of the intricate communication between muscle and bone during anabolic resistance is of paramount importance for the development of effective strategies to combat age-related muscle and bone disorders.
4. Conclusions
Recent research has produced a huge amount of data about the molecular and nutritional, as well as lifestyle- and health-related factors that can affect the development and the worsening of sarcopenia in the elderly. These data could well be exploited for the design of interventions aimed at optimizing skeletal muscle accretion and function, and combat sarcopenia in the elderly. Further research is needed to expand our knowledge about the mechanisms and the possible treatments that can maintain muscle function and prevent age-related loss of function and falls.
Abbreviations
AA: amino acid; ADL: Daily Living Activity; EAA: essential amino acids; HMB: β-hydroxyl, β-methylbutyrate; hGH: human Growth Hormone; MPB: muscle protein breakdown; MPS: muscle protein synthesis; PD: protein degradation; PS: protein synthesis; RP: repetition maximum; WP: whey protein.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
AIRC id 23527: AFM telethon 22982, PNRR.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Rosenberg I.H. Sarcopenia: Origins and Clinical Relevance. Clin. Geriatr. Med. 2011;27:337–339. doi: 10.1016/j.cger.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Dent E., Morley J.E., Cruz-Jentoft A.J., Arai H., Kritchevsky S.B., Guralnik J., Bauer J.M., Pahor M., Clark B.C., Cesari M., et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): Screening, Diagnosis and Management. J. Nutr. Health Aging. 2018;22:1148–1161. doi: 10.1007/s12603-018-1139-9. [DOI] [PubMed] [Google Scholar]
- 3.Bhasin S., Travison T.G., Manini T.M., Patel S., Pencina K.M., Fielding R.A., Magaziner J.M., Newman A.B., Kiel D.P., Cooper C., et al. Sarcopenia Definition: The Position Statements of the Sarcopenia Definition and Outcomes Consortium. J. Am. Geriatr. Soc. 2020;68:1410–1418. doi: 10.1111/jgs.16372. [DOI] [PubMed] [Google Scholar]
- 4.Ackermans L.L.G.C., Rabou J., Basrai M., Schweinlin A., Bischoff S.C., Cussenot O., Cancel-Tassin G., Renken R.J., Gómez E., Sánchez-González P., et al. Screening, Diagnosis and Monitoring of Sarcopenia: When to Use Which Tool? Clin. Nutr. ESPEN. 2022;48:36–44. doi: 10.1016/j.clnesp.2022.01.027. [DOI] [PubMed] [Google Scholar]
- 5.Shafiee G., Keshtkar A., Soltani A., Ahadi Z., Larijani B., Heshmat R. Prevalence of Sarcopenia in the World: A Systematic Review and Meta- Analysis of General Population Studies. J. Diabetes Metab. Disord. 2017;16:21. doi: 10.1186/s40200-017-0302-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deschenes M.R. Effects of Aging on Muscle Fibre Type and Size. Sports Med. 2004;34:809–824. doi: 10.2165/00007256-200434120-00002. [DOI] [PubMed] [Google Scholar]
- 7.Nilwik R., Snijders T., Leenders M., Groen B.B.L., van Kranenburg J., Verdijk L.B., van Loon L.J.C. The Decline in Skeletal Muscle Mass with Aging Is Mainly Attributed to a Reduction in Type II Muscle Fiber Size. Exp. Gerontol. 2013;48:492–498. doi: 10.1016/j.exger.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Brunner F., Schmid A., Sheikhzadeh A., Nordin M., Yoon J., Frankel V. Effects of Aging on Type II Muscle Fibers: A Systematic Review of the Literature. J. Aging Phys. Act. 2007;15:336–348. doi: 10.1123/japa.15.3.336. [DOI] [PubMed] [Google Scholar]
- 9.Cruz-Jentoft A.J., Sayer A.A. Sarcopenia. Lancet. 2019;393:2636–2646. doi: 10.1016/S0140-6736(19)31138-9. [DOI] [PubMed] [Google Scholar]
- 10.Ferrucci L., Fabbri E. Inflammageing: Chronic Inflammation in Ageing, Cardiovascular Disease, and Frailty. Nat. Rev. Cardiol. 2018;15:505–522. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan S., Larsson S.C. Epidemiology of Sarcopenia: Prevalence, Risk Factors, and Consequences. Metabolism. 2023;144:155533. doi: 10.1016/j.metabol.2023.155533. [DOI] [PubMed] [Google Scholar]
- 12.Sundberg C.W., Prost R.W., Fitts R.H., Hunter S.K. Bioenergetic Basis for the Increased Fatigability with Ageing. J. Physiol. 2019;597:4943–4957. doi: 10.1113/JP277803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshida T., Delafontaine P. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells. 2020;9:1970. doi: 10.3390/cells9091970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drummond M.J., Fry C.S., Glynn E.L., Dreyer H.C., Dhanani S., Timmerman K.L., Volpi E., Rasmussen B.B. Rapamycin Administration in Humans Blocks the Contraction-Induced Increase in Skeletal Muscle Protein Synthesis: Rapamycin Blocks Protein Synthesis in Human Muscle. J. Physiol. 2009;587:1535–1546. doi: 10.1113/jphysiol.2008.163816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francaux M., Demeulder B., Naslain D., Fortin R., Lutz O., Caty G., Deldicque L. Aging Reduces the Activation of the MTORC1 Pathway after Resistance Exercise and Protein Intake in Human Skeletal Muscle: Potential Role of REDD1 and Impaired Anabolic Sensitivity. Nutrients. 2016;8:47. doi: 10.3390/nu8010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Léger B., Cartoni R., Praz M., Lamon S., Dériaz O., Crettenand A., Gobelet C., Rohmer P., Konzelmann M., Luthi F., et al. Akt Signalling through GSK-3beta, MTOR and Foxo1 Is Involved in Human Skeletal Muscle Hypertrophy and Atrophy. J. Physiol. 2006;576:923–933. doi: 10.1113/jphysiol.2006.116715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Camillo B., Eduati F., Nair S.K., Avogaro A., Toffolo G.M. Leucine Modulates Dynamic Phosphorylation Events in Insulin Signaling Pathway and Enhances Insulin-Dependent Glycogen Synthesis in Human Skeletal Muscle Cells. BMC Cell. Biol. 2014;15:9. doi: 10.1186/1471-2121-15-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kakigi R., Yoshihara T., Ozaki H., Ogura Y., Ichinoseki-Sekine N., Kobayashi H., Naito H. Whey Protein Intake after Resistance Exercise Activates MTOR Signaling in a Dose-Dependent Manner in Human Skeletal Muscle. Eur. J Appl. Physiol. 2014;114:735–742. doi: 10.1007/s00421-013-2812-7. [DOI] [PubMed] [Google Scholar]
- 19.Jacquemin V., Furling D., Bigot A., Butler-Browne G.S., Mouly V. IGF-1 Induces Human Myotube Hypertrophy by Increasing Cell Recruitment. Exp. Cell Res. 2004;299:148–158. doi: 10.1016/j.yexcr.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 20.Barclay R.D., Burd N.A., Tyler C., Tillin N.A., Mackenzie R.W. The Role of the IGF-1 Signaling Cascade in Muscle Protein Synthesis and Anabolic Resistance in Aging Skeletal Muscle. Front. Nutr. 2019;6:146. doi: 10.3389/fnut.2019.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen M., Koskinen S.O., Petersen S.G., Doessing S., Frystyk J., Flyvbjerg A., Westh E., Magnusson S.P., Kjaer M., Langberg H. Ethinyl Oestradiol Administration in Women Suppresses Synthesis of Collagen in Tendon in Response to Exercise: Oral Contraceptives and Tendon Collagen. J. Physiol. 2008;586:3005–3016. doi: 10.1113/jphysiol.2007.147348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perrini S., Laviola L., Carreira M.C., Cignarelli A., Natalicchio A., Giorgino F. The GH/IGF1 Axis and Signaling Pathways in the Muscle and Bone: Mechanisms Underlying Age-Related Skeletal Muscle Wasting and Osteoporosis. J. Endocrinol. 2010;205:201–210. doi: 10.1677/JOE-09-0431. [DOI] [PubMed] [Google Scholar]
- 23.Chikani V., Ho K.K.Y. Action of GH on Skeletal Muscle Function: Molecular and Metabolic Mechanisms. J. Mol. Endocrinol. 2014;52:R107–R123. doi: 10.1530/JME-13-0208. [DOI] [PubMed] [Google Scholar]
- 24.West D.W.D., Phillips S.M. Anabolic Processes in Human Skeletal Muscle: Restoring the Identities of Growth Hormone and Testosterone. Physician Sports Med. 2010;38:97–104. doi: 10.3810/psm.2010.10.1814. [DOI] [PubMed] [Google Scholar]
- 25.Florini J.R. Effects of Testosterone on Qualitative Pattern of Protein Synthesis in Skeletal Muscle. Biochemistry. 1970;9:909–912. doi: 10.1021/bi00806a027. [DOI] [PubMed] [Google Scholar]
- 26.Mauras N., Hayes V., Welch S., Rini A., Helgeson K., Dokler M., Veldhuis J.D., Urban R.J. Testosterone Deficiency in Young Men: Marked Alterations in Whole Body Protein Kinetics, Strength, and Adiposity. J. Clin. Endocrinol. Metab. 1998;83:1886–1892. doi: 10.1210/jc.83.6.1886. [DOI] [PubMed] [Google Scholar]
- 27.Demling R.H., Orgill D.P. The Anticatabolic and Wound Healing Effects of the Testosterone Analog Oxandrolone after Severe Burn Injury. J. Crit. Care. 2000;15:12–17. doi: 10.1053/jcrc.2000.0150012. [DOI] [PubMed] [Google Scholar]
- 28.Vingren J.L., Kraemer W.J., Ratamess N.A., Anderson J.M., Volek J.S., Maresh C.M. Testosterone Physiology in Resistance Exercise and Training: The Up-Stream Regulatory Elements. Sports Med. 2010;40:1037–1053. doi: 10.2165/11536910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 29.Vierck J. Satellite Cell Regulation Following Myotrauma Caused by Resistance Exercise. Cell Biol. Int. 2000;24:263–272. doi: 10.1006/cbir.2000.0499. [DOI] [PubMed] [Google Scholar]
- 30.Bhasin S., Woodhouse L., Casaburi R., Singh A.B., Bhasin D., Berman N., Chen X., Yarasheski K.E., Magliano L., Dzekov C., et al. Testosterone Dose-Response Relationships in Healthy Young Men. Am. J. Physiol. Endocrinol. Metab. 2001;281:E1172–E1181. doi: 10.1152/ajpendo.2001.281.6.E1172. [DOI] [PubMed] [Google Scholar]
- 31.Plotkin D.L., Delcastillo K., Van Every D.W., Tipton K.D., Aragon A.A., Schoenfeld B.J. Isolated Leucine and Branched-Chain Amino Acid Supplementation for Enhancing Muscular Strength and Hypertrophy: A Narrative Review. Int. J. Sport Nutr. Exerc. Metab. 2021;31:292–301. doi: 10.1123/ijsnem.2020-0356. [DOI] [PubMed] [Google Scholar]
- 32.Tezze C., Amendolagine F.I., Nogara L., Baraldo M., Ciciliot S., Arcidiacono D., Zaramella A., Masiero G., Ferrarese G., Realdon S., et al. A Combination of Metformin and Galantamine Exhibits Synergistic Benefits in the Treatment of Sarcopenia. JCI Insight. 2023;8:e168787. doi: 10.1172/jci.insight.168787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malena A., Pennuto M., Tezze C., Querin G., D’Ascenzo C., Silani V., Cenacchi G., Scaramozza A., Romito S., Morandi L., et al. Androgen-Dependent Impairment of Myogenesis in Spinal and Bulbar Muscular Atrophy. Acta Neuropathol. 2013;126:109–121. doi: 10.1007/s00401-013-1122-9. [DOI] [PubMed] [Google Scholar]
- 34.Tezze C., Romanello V., Sandri M. FGF21 as Modulator of Metabolism in Health and Disease. Front. Physiol. 2019;10:419. doi: 10.3389/fphys.2019.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tezze C., Romanello V., Desbats M.A., Fadini G.P., Albiero M., Favaro G., Ciciliot S., Soriano M.E., Morbidoni V., Cerqua C., et al. Age-Associated Loss of OPA1 in Muscle Impacts Muscle Mass, Metabolic Homeostasis, Systemic Inflammation, and Epithelial Senescence. Cell Metab. 2017;25:1374–1389. doi: 10.1016/j.cmet.2017.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Solagna F., Tezze C., Lindenmeyer M.T., Lu S., Wu G., Liu S., Zhao Y., Mitchell R., Meyer C., Omairi S., et al. Pro-Cachectic Factors Link Experimental and Human Chronic Kidney Disease to Skeletal Muscle Wasting Programs. J. Clin. Investig. 2021;131:e135821. doi: 10.1172/JCI135821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith K., Rennie M.J. The Measurement of Tissue Protein Turnover. Baillière’s Clin. Endocrinol. Metab. 1996;10:469–495. doi: 10.1016/S0950-351X(96)80651-3. [DOI] [PubMed] [Google Scholar]
- 38.Gorissen S.H.M., Rémond D., Van Loon L.J.C. The Muscle Protein Synthetic Response to Food Ingestion. Meat Sci. 2015;109:96–100. doi: 10.1016/j.meatsci.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 39.Wolfe R.R. Regulation of Muscle Protein by Amino Acids. J. Nutr. 2002;132:3219S–3224S. doi: 10.1093/jn/131.10.3219S. [DOI] [PubMed] [Google Scholar]
- 40.Tang J.E., Moore D.R., Kujbida G.W., Tarnopolsky M.A., Phillips S.M. Ingestion of Whey Hydrolysate, Casein, or Soy Protein Isolate: Effects on Mixed Muscle Protein Synthesis at Rest and Following Resistance Exercise in Young Men. J. Appl. Physiol. 2009;107:987–992. doi: 10.1152/japplphysiol.00076.2009. [DOI] [PubMed] [Google Scholar]
- 41.Bennet W.M., Connacher A.A., Scrimgeour C.M., Rennie M.J. The Effect of Amino Acid Infusion on Leg Protein Turnover Assessed by L-[15N]Phenylalanine and L-[1-13C]Leucine Exchange. Eur. J. Clin. Investig. 2008;20:41–50. doi: 10.1111/j.1365-2362.1990.tb01789.x. [DOI] [PubMed] [Google Scholar]
- 42.Tessari P., Barazzoni R., Zanetti M., Kiwanuka E., Tiengo A. The Role of Substrates in the Regulation of Protein Metabolism. Baillière’s Clin. Endocrinol. Metab. 1996;10:511–532. doi: 10.1016/S0950-351X(96)80681-1. [DOI] [PubMed] [Google Scholar]
- 43.Nygren J., Nair K.S. Differential Regulation of Protein Dynamics in Splanchnic and Skeletal Muscle Beds by Insulin and Amino Acids in Healthy Human Subjects. Diabetes. 2003;52:1377–1385. doi: 10.2337/diabetes.52.6.1377. [DOI] [PubMed] [Google Scholar]
- 44.Volpi E., Kobayashi H., Sheffield-Moore M., Mittendorfer B., Wolfe R.R. Essential Amino Acids Are Primarily Responsible for the Amino Acid Stimulation of Muscle Protein Anabolism in Healthy Elderly Adults. Am. J. Clin. Nutr. 2003;78:250–258. doi: 10.1093/ajcn/78.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kimball S.R., Jefferson L.S. Regulation of Global and Specific MRNA Translation by Oral Administration of Branched-Chain Amino Acids. Biochem. Biophys. Res. Commun. 2004;313:423–427. doi: 10.1016/j.bbrc.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 46.Ham D.J., Caldow M.K., Lynch G.S., Koopman R. Leucine as a Treatment for Muscle Wasting: A Critical Review. Clin. Nutr. 2014;33:937–945. doi: 10.1016/j.clnu.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 47.May M.E., Buse M.G. Effects of Branched-Chain Amino Acids on Protein Turnover. Diabetes Metab. Rev. 1989;5:227–245. doi: 10.1002/dmr.5610050303. [DOI] [PubMed] [Google Scholar]
- 48.Anthony J.C., Anthony T.G., Kimball S.R., Jefferson L.S. Signaling Pathways Involved in Translational Control of Protein Synthesis in Skeletal Muscle by Leucine. J. Nutr. 2001;131:856S–860S. doi: 10.1093/jn/131.3.856S. [DOI] [PubMed] [Google Scholar]
- 49.Buse M.G., Reid S.S. Leucine. A Possible Regulator of Protein Turnover in Muscle. J. Clin. Investig. 1975;56:1250–1261. doi: 10.1172/JCI108201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Bandt J.-P. Leucine and Mammalian Target of Rapamycin–Dependent Activation of Muscle Protein Synthesis in Aging. J. Nutr. 2016;146:2616S–2624S. doi: 10.3945/jn.116.234518. [DOI] [PubMed] [Google Scholar]
- 51.Van Loon L.J.C. Leucine as a Pharmaconutrient in Health and Disease. Curr. Opin. Clin. Nutr. Metab. Care. 2012;15:71–77. doi: 10.1097/MCO.0b013e32834d617a. [DOI] [PubMed] [Google Scholar]
- 52.Tessari P., Zanetti M., Barazzoni R., Vettore M., Michielan F. Mechanisms of Postprandial Protein Accretion in Human Skeletal Muscle. Insight from Leucine and Phenylalanine Forearm Kinetics. J. Clin. Investig. 1996;98:1361–1372. doi: 10.1172/JCI118923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gwin J.A., Church D.D., Wolfe R.R., Ferrando A.A., Pasiakos S.M. Muscle Protein Synthesis and Whole-Body Protein Turnover Responses to Ingesting Essential Amino Acids, Intact Protein, and Protein-Containing Mixed Meals with Considerations for Energy Deficit. Nutrients. 2020;12:2457. doi: 10.3390/nu12082457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Witard O.C., Jackman S.R., Breen L., Smith K., Selby A., Tipton K.D. Myofibrillar Muscle Protein Synthesis Rates Subsequent to a Meal in Response to Increasing Doses of Whey Protein at Rest and after Resistance Exercise. Am. J. Clin. Nutr. 2014;99:86–95. doi: 10.3945/ajcn.112.055517. [DOI] [PubMed] [Google Scholar]
- 55.Moore D.R., Tang J.E., Burd N.A., Rerecich T., Tarnopolsky M.A., Phillips S.M. Differential Stimulation of Myofibrillar and Sarcoplasmic Protein Synthesis with Protein Ingestion at Rest and after Resistance Exercise. J. Physiol. 2009;587:897–904. doi: 10.1113/jphysiol.2008.164087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robinson M.J., Burd N.A., Breen L., Rerecich T., Yang Y., Hector A.J., Baker S.K., Phillips S.M. Dose-Dependent Responses of Myofibrillar Protein Synthesis with Beef Ingestion Are Enhanced with Resistance Exercise in Middle-Aged Men. Appl. Physiol. Nutr. Metab. 2013;38:120–125. doi: 10.1139/apnm-2012-0092. [DOI] [PubMed] [Google Scholar]
- 57.Kim I.-Y., Schutzler S., Schrader A., Spencer H.J., Azhar G., Ferrando A.A., Wolfe R.R. The Anabolic Response to a Meal Containing Different Amounts of Protein Is Not Limited by the Maximal Stimulation of Protein Synthesis in Healthy Young Adults. Am. J. Physiol. -Endocrinol. Metab. 2016;310:E73–E80. doi: 10.1152/ajpendo.00365.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paddon-Jones D., Sheffield-Moore M., Zhang X.-J., Volpi E., Wolf S.E., Aarsland A., Ferrando A.A., Wolfe R.R. Amino Acid Ingestion Improves Muscle Protein Synthesis in the Young and Elderly. Am. J. Physiol. Endocrinol. Metab. 2004;286:E321–E328. doi: 10.1152/ajpendo.00368.2003. [DOI] [PubMed] [Google Scholar]
- 59.Fujita S., Volpi R. Nutrition and sarcopenia of ageing. Nutr. Res. Rev. 2004;17:69–76. doi: 10.1079/NRR200481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cuthbertson D., Smith K., Babraj J., Leese G., Waddell T., Atherton P., Wackerhage H., Taylor P.M., Rennie M.J. Anabolic Signaling Deficits Underlie Amino Acid Resistance of Wasting, Aging Muscle. FASEB J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 61.Xu Z., Tan Z., Zhang Q., Gui Q., Yang Y. The Effectiveness of Leucine on Muscle Protein Synthesis, Lean Body Mass and Leg Lean Mass Accretion in Older People: A Systematic Review and Meta-Analysis. Br. J. Nutr. 2015;113:25–34. doi: 10.1017/S0007114514002475. [DOI] [PubMed] [Google Scholar]
- 62.Nakamura K., Kihata A., Naraba H., Kanda N., Takahashi Y., Sonoo T., Hashimoto H., Morimura N. Β-Hydroxy-β-methylbutyrate, Arginine, and Glutamine Complex on Muscle Volume Loss in Critically Ill Patients: A Randomized Control Trial. J. Parenter. Enter. Nutr. 2020;44:205–212. doi: 10.1002/jpen.1607. [DOI] [PubMed] [Google Scholar]
- 63.Xi P. Regulation of Protein Metabolism by Glutamine: Implications for Nutrition and Health. Front. Biosci. 2011;16:578. doi: 10.2741/3707. [DOI] [PubMed] [Google Scholar]
- 64.Svanberg E., Möller-Loswick A.-C., Matthews D.E., Körner U., Lundholm K. The Effect of Glutamine on Protein Balance and Amino Acid Flux across Arm and Leg Tissues in Healthy Volunteers: Glutamine and Muscle Protein Anabolism. Clin. Physiol. 2001;21:478–489. doi: 10.1046/j.1365-2281.2001.00346.x. [DOI] [PubMed] [Google Scholar]
- 65.Zachwieja J.J., Witt T.L., Yarasheski K.E. Intravenous Glutamine Does Not Stimulate Mixed Muscle Protein Synthesis in Healthy Young Men and Women. Metabolism. 2000;49:1555–1560. doi: 10.1053/meta.2000.18524. [DOI] [PubMed] [Google Scholar]
- 66.Deutz N.E.P., Bauer J.M., Barazzoni R., Biolo G., Boirie Y., Bosy-Westphal A., Cederholm T., Cruz-Jentoft A., Krznariç Z., Nair K.S., et al. Protein Intake and Exercise for Optimal Muscle Function with Aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014;33:929–936. doi: 10.1016/j.clnu.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boirie Y., Dangin M., Gachon P., Vasson M.-P., Maubois J.-L., Beaufrère B. Slow and Fast Dietary Proteins Differently Modulate Postprandial Protein Accretion. Proc. Natl. Acad. Sci. USA. 1997;94:14930–14935. doi: 10.1073/pnas.94.26.14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burd N.A., Yang Y., Moore D.R., Tang J.E., Tarnopolsky M.A., Phillips S.M. Greater Stimulation of Myofibrillar Protein Synthesis with Ingestion of Whey Protein Isolate v. Micellar Casein at Rest and after Resistance Exercise in Elderly Men. Br. J. Nutr. 2012;108:958–962. doi: 10.1017/S0007114511006271. [DOI] [PubMed] [Google Scholar]
- 69.Van Vliet S., Beals J.W., Holwerda A.M., Emmons R.S., Goessens J.P., Paluska S.A., De Lisio M., Van Loon L.J.C., Burd N.A. Time-Dependent Regulation of Postprandial Muscle Protein Synthesis Rates after Milk Protein Ingestion in Young Men. J. Appl. Physiol. 2019;127:1792–1801. doi: 10.1152/japplphysiol.00608.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Monteyne A.J., Dunlop M.V., Machin D.J., Coelho M.O.C., Pavis G.F., Porter C., Murton A.J., Abdelrahman D.R., Dirks M.L., Stephens F.B., et al. A Mycoprotein-Based High-Protein Vegan Diet Supports Equivalent Daily Myofibrillar Protein Synthesis Rates Compared with an Isonitrogenous Omnivorous Diet in Older Adults: A Randomised Controlled Trial. Br. J. Nutr. 2021;126:674–684. doi: 10.1017/S0007114520004481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Macnaughton L.S., Wardle S.L., Witard O.C., McGlory C., Hamilton D.L., Jeromson S., Lawrence C.E., Wallis G.A., Tipton K.D. The Response of Muscle Protein Synthesis Following Whole-Body Resistance Exercise Is Greater Following 40 g than 20 g of Ingested Whey Protein. Physiol. Rep. 2016;4:e12893. doi: 10.14814/phy2.12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Breen L., Phillips S.M. Skeletal Muscle Protein Metabolism in the Elderly: Interventions to Counteract the “anabolic Resistance” of Ageing. Nutr. Metab. 2011;8:68. doi: 10.1186/1743-7075-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moore D.R., Churchward-Venne T.A., Witard O., Breen L., Burd N.A., Tipton K.D., Phillips S.M. Protein Ingestion to Stimulate Myofibrillar Protein Synthesis Requires Greater Relative Protein Intakes in Healthy Older Versus Younger Men. J. Gerontol. Ser. A. 2015;70:57–62. doi: 10.1093/gerona/glu103. [DOI] [PubMed] [Google Scholar]
- 74.Churchward-Venne T.A., Pinckaers P.J.M., Smeets J.S.J., Betz M.W., Senden J.M., Goessens J.P.B., Gijsen A.P., Rollo I., Verdijk L.B., van Loon L.J.C. Dose-Response Effects of Dietary Protein on Muscle Protein Synthesis during Recovery from Endurance Exercise in Young Men: A Double-Blind Randomized Trial. Am. J. Clin. Nutr. 2020;112:303–317. doi: 10.1093/ajcn/nqaa073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fujita S., Dreyer H.C., Drummond M.J., Glynn E.L., Cadenas J.G., Yoshizawa F., Volpi E., Rasmussen B.B. Nutrient Signalling in the Regulation of Human Muscle Protein Synthesis: Nutrient Signalling and Muscle Protein Synthesis. J. Physiol. 2007;582:813–823. doi: 10.1113/jphysiol.2007.134593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.West S., Monteyne A.J., Whelehan G., Abdelrahman D.R., Murton A.J., Finnigan T.J.A., Blackwell J.R., Stephens F.B., Wall B.T. Mycoprotein Ingestion within or without Its Wholefood Matrix Results in Equivalent Stimulation of Myofibrillar Protein Synthesis Rates in Resting and Exercised Muscle of Young Men. Br. J. Nutr. 2023;130:20–32. doi: 10.1017/S0007114522003087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dangin M., Boirie Y., Garcia-Rodenas C., Gachon P., Fauquant J., Callier P., Ballèvre O., Beaufrère B. The Digestion Rate of Protein Is an Independent Regulating Factor of Postprandial Protein Retention. Am. J. Physiol. -Endocrinol. Metab. 2001;280:E340–E348. doi: 10.1152/ajpendo.2001.280.2.E340. [DOI] [PubMed] [Google Scholar]
- 78.Reidy P.T., Walker D.K., Dickinson J.M., Gundermann D.M., Drummond M.J., Timmerman K.L., Cope M.B., Mukherjea R., Jennings K., Volpi E., et al. Soy-Dairy Protein Blend and Whey Protein Ingestion after Resistance Exercise Increases Amino Acid Transport and Transporter Expression in Human Skeletal Muscle. J. Appl. Physiol. 2014;116:1353–1364. doi: 10.1152/japplphysiol.01093.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tipton K.D., Wolfe R.R. Exercise, Protein Metabolism, and Muscle Growth. Int. J. Sport Nutr. Exerc. Metab. 2001;11:109–132. doi: 10.1123/ijsnem.11.1.109. [DOI] [PubMed] [Google Scholar]
- 80.Biolo G., Tipton K.D., Klein S., Wolfe R.R. An Abundant Supply of Amino Acids Enhances the Metabolic Effect of Exercise on Muscle Protein. Am. J. Physiol. -Endocrinol. Metab. 1997;273:E122–E129. doi: 10.1152/ajpendo.1997.273.1.E122. [DOI] [PubMed] [Google Scholar]
- 81.Churchward-Venne T.A., Murphy C.H., Longland T.M., Phillips S.M. Role of Protein and Amino Acids in Promoting Lean Mass Accretion with Resistance Exercise and Attenuating Lean Mass Loss during Energy Deficit in Humans. Amino Acids. 2013;45:231–240. doi: 10.1007/s00726-013-1506-0. [DOI] [PubMed] [Google Scholar]
- 82.Pennings B., Boirie Y., Senden J.M., Gijsen A.P., Kuipers H., Van Loon L.J. Whey Protein Stimulates Postprandial Muscle Protein Accretion More Effectively than Do Casein and Casein Hydrolysate in Older Men. Am. J. Clin. Nutr. 2011;93:997–1005. doi: 10.3945/ajcn.110.008102. [DOI] [PubMed] [Google Scholar]
- 83.Hall W.L., Millward D.J., Long S.J., Morgan L.M. Casein and Whey Exert Different Effects on Plasma Amino Acid Profiles, Gastrointestinal Hormone Secretion and Appetite. Br. J. Nutr. 2003;89:239–248. doi: 10.1079/BJN2002760. [DOI] [PubMed] [Google Scholar]
- 84.Rasmussen B.B., Tipton K.D., Miller S.L., Wolf S.E., Wolfe R.R. An Oral Essential Amino Acid-Carbohydrate Supplement Enhances Muscle Protein Anabolism after Resistance Exercise. J. Appl. Physiol. 2000;88:386–392. doi: 10.1152/jappl.2000.88.2.386. [DOI] [PubMed] [Google Scholar]
- 85.Tipton K.D., Elliott T.A., Cree M.G., Aarsland A.A., Sanford A.P., Wolfe R.R. Stimulation of Net Muscle Protein Synthesis by Whey Protein Ingestion before and after Exercise. Am. J. Physiol. Endocrinol. Metab. 2007;292:E71–E76. doi: 10.1152/ajpendo.00166.2006. [DOI] [PubMed] [Google Scholar]
- 86.Reeds P.J., Burrin D.G., Davis T.A., Stoll B. Amino Acid Metabolism and the Energetics of Growth. Arch. Anim. Nutr. 1998;51:187–197. doi: 10.1080/17450399809381918. [DOI] [PubMed] [Google Scholar]
- 87.Smiles W.J., Hawley J.A., Camera D.M. Effects of Skeletal Muscle Energy Availability on Protein Turnover Responses to Exercise. J. Exp. Biol. 2016;219:214–225. doi: 10.1242/jeb.125104. [DOI] [PubMed] [Google Scholar]
- 88.Hedden M.P., Buse M.G. Effects of Glucose, Pyruvate, Lactate, and Amino Acids on Muscle Protein Synthesis. Am. J. Physiol. 1982;242:E184–E192. doi: 10.1152/ajpendo.1982.242.3.E184. [DOI] [PubMed] [Google Scholar]
- 89.Goichon A., Coëffier M., Claeyssens S., Lecleire S., Cailleux A.-F., Bôle-Feysot C., Chan P., Donnadieu N., Lerebours E., Lavoinne A., et al. Effects of an Enteral Glucose Supply on Protein Synthesis, Proteolytic Pathways, and Proteome in Human Duodenal Mucosa. Am. J. Clin. Nutr. 2011;94:784–794. doi: 10.3945/ajcn.110.009738. [DOI] [PubMed] [Google Scholar]
- 90.Bowtell J.L., Leese G.P., Smith K., Watt P.W., Nevill A., Rooyackers O., Wagenmakers A.J., Rennie M.J. Effect of Oral Glucose on Leucine Turnover in Human Subjects at Rest and during Exercise at Two Levels of Dietary Protein. Pt 1J. Physiol. 2000;525:271–281. doi: 10.1111/j.1469-7793.2000.00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Staples A.W., Burd N.A., West D.W.D., Currie K.D., Atherton P.J., Moore D.R., Rennie M.J., Macdonald M.J., Baker S.K., Phillips S.M. Carbohydrate Does Not Augment Exercise-Induced Protein Accretion versus Protein Alone. Med. Sci. Sports Exerc. 2011;43:1154–1161. doi: 10.1249/MSS.0b013e31820751cb. [DOI] [PubMed] [Google Scholar]
- 92.Koopman R., Beelen M., Stellingwerff T., Pennings B., Saris W.H.M., Kies A.K., Kuipers H., Van Loon L.J.C. Coingestion of Carbohydrate with Protein Does Not Further Augment Postexercise Muscle Protein Synthesis. Am. J. Physiol. -Endocrinol. Metab. 2007;293:E833–E842. doi: 10.1152/ajpendo.00135.2007. [DOI] [PubMed] [Google Scholar]
- 93.Robert J.-J., Bier D., Schoeller D., Wolfe R., Matthews D.E., Munro H.N., Young V.R. Effects of Intravenous Glucose on Whole Body Leucine Dynamics, Studied With 1-13C-Leucine, in Healthy Young and Elderly Adults. J. Gerontol. 1984;39:673–681. doi: 10.1093/geronj/39.6.673. [DOI] [PubMed] [Google Scholar]
- 94.Heiling V.J., Campbell P.J., Gottesman I.S., Tsalikian E., Beaufrere B., Gerich J.E., Haymond M.W. Differential Effects of Hyperglycemia and Hyperinsulinemia on Leucine Rate of Appearance in Normal Humans. J. Clin. Endocrinol. Metab. 1993;76:203–206. doi: 10.1210/jcem.76.1.8093620. [DOI] [PubMed] [Google Scholar]
- 95.Howarth K.R., Phillips S.M., MacDonald M.J., Richards D., Moreau N.A., Gibala M.J. Effect of Glycogen Availability on Human Skeletal Muscle Protein Turnover during Exercise and Recovery. J. Appl. Physiol. 2010;109:431–438. doi: 10.1152/japplphysiol.00108.2009. [DOI] [PubMed] [Google Scholar]
- 96.Thomsen H.H., Rittig N., Johannsen M., Møller A.B., Jørgensen J.O., Jessen N., Møller N. Effects of 3-Hydroxybutyrate and Free Fatty Acids on Muscle Protein Kinetics and Signaling during LPS-Induced Inflammation in Humans: Anticatabolic Impact of Ketone Bodies. Am. J. Clin. Nutr. 2018;108:857–867. doi: 10.1093/ajcn/nqy170. [DOI] [PubMed] [Google Scholar]
- 97.Rodriguez N., Schwenk W.F., Beaufrere B., Miles J.M., Haymond M.W. Trioctanoin Infusion Increases in Vivo Leucine Oxidation: A Lesson in Isotope Modeling. Am. J. Physiol. -Endocrinol. Metab. 1986;251:E343–E348. doi: 10.1152/ajpendo.1986.251.3.E343. [DOI] [PubMed] [Google Scholar]
- 98.Haymond M.W., Tessari P., Beaufrere B., Rodriguez N., Bailey J., Miles J.M. Effects of Parenteral Lipid on Leucine Metabolism: Dependence of Fatty Acid Chain Length. JPEN J. Parenter. Enter. Nutr. 1988;12:94S–97S. doi: 10.1177/014860718801200613. [DOI] [PubMed] [Google Scholar]
- 99.Lang C.H. Elevated Plasma Free Fatty Acids Decrease Basal Protein Synthesis, but Not the Anabolic Effect of Leucine, in Skeletal Muscle. Am. J. Physiol. -Endocrinol. Metab. 2006;291:E666–E674. doi: 10.1152/ajpendo.00065.2006. [DOI] [PubMed] [Google Scholar]
- 100.Tsintzas K., Jones R., Pabla P., Mallinson J., Barrett D.A., Kim D.-H., Cooper S., Davies A., Taylor T., Chee C., et al. Effect of Acute and Short-Term Dietary Fat Ingestion on Postprandial Skeletal Muscle Protein Synthesis Rates in Middle-Aged, Overweight, and Obese Men. Am. J. Physiol. -Endocrinol. Metab. 2020;318:E417–E429. doi: 10.1152/ajpendo.00344.2019. [DOI] [PubMed] [Google Scholar]
- 101.Nair K.S., Welle S.L., Halliday D., Campbell R.G. Effect of Beta-Hydroxybutyrate on Whole-Body Leucine Kinetics and Fractional Mixed Skeletal Muscle Protein Synthesis in Humans. J. Clin. Investig. 1988;82:198–205. doi: 10.1172/JCI113570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Holeček M. Beta-Hydroxy-Beta-Methylbutyrate Supplementation and Skeletal Muscle in Healthy and Muscle-Wasting Conditions: HMB Supplementation and Muscle. J. Cachexia Sarcopenia Muscle. 2017;8:529–541. doi: 10.1002/jcsm.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wilkinson D.J., Hossain T., Hill D.S., Phillips B.E., Crossland H., Williams J., Loughna P., Churchward-Venne T.A., Breen L., Phillips S.M., et al. Effects of Leucine and Its Metabolite Β-hydroxy-β-methylbutyrate on Human Skeletal Muscle Protein Metabolism. J. Physiol. 2013;591:2911–2923. doi: 10.1113/jphysiol.2013.253203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Furst T., Massaro A., Miller C., Williams B.T., LaMacchia Z.M., Horvath P.J. β-Alanine Supplementation Increased Physical Performance and Improved Executive Function Following Endurance Exercise in Middle Aged Individuals. J. Int. Soc. Sports Nutr. 2018;15:32. doi: 10.1186/s12970-018-0238-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Artioli G.G., Gualano B., Smith A., Stout J., Lancha A.H. Role of β-Alanine Supplementation on Muscle Carnosine and Exercise Performance. Med. Sci. Sports Exerc. 2010;42:1162–1173. doi: 10.1249/MSS.0b013e3181c74e38. [DOI] [PubMed] [Google Scholar]
- 106.Parise G., Mihic S., MacLennan D., Yarasheski K.E., Tarnopolsky M.A. Effects of Acute Creatine Monohydrate Supplementation on Leucine Kinetics and Mixed-Muscle Protein Synthesis. J. Appl. Physiol. 2001;91:1041–1047. doi: 10.1152/jappl.2001.91.3.1041. [DOI] [PubMed] [Google Scholar]
- 107.Tipton K.D., Ferrando A.A. Improving Muscle Mass: Response of Muscle Metabolism to Exercise, Nutrition and Anabolic Agents. Essays Biochem. 2008;44:85–98. doi: 10.1042/bse0440085. [DOI] [PubMed] [Google Scholar]
- 108.Tessari P., Inchiostro S., Biolo G., Vincenti E., Sabadin L. Effects of Acute Systemic Hyperinsulinemia on Forearm Muscle Proteolysis in Healthy Man. J. Clin. Investig. 1991;88:27–33. doi: 10.1172/JCI115287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Biolo G., Declan Fleming R.Y., Wolfe R.R. Physiologic Hyperinsulinemia Stimulates Protein Synthesis and Enhances Transport of Selected Amino Acids in Human Skeletal Muscle. J. Clin. Investig. 1995;95:811–819. doi: 10.1172/JCI117731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Timmerman K.L., Lee J.L., Dreyer H.C., Dhanani S., Glynn E.L., Fry C.S., Drummond M.J., Sheffield-Moore M., Rasmussen B.B., Volpi E. Insulin Stimulates Human Skeletal Muscle Protein Synthesis via an Indirect Mechanism Involving Endothelial-Dependent Vasodilation and Mammalian Target of Rapamycin Complex 1 Signaling. J. Clin. Endocrinol. Metab. 2010;95:3848–3857. doi: 10.1210/jc.2009-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tessari P., Inchiostro S., Biolo G., Marescotti M.C., Fantin G., Boscarato M.T., Merola G., Mantero F., Tiengo A. Leucine Kinetics and the Effects of Hyperinsulinemia in Patients With Cushing’s Syndrome. J. Clin. Endocrinol. Metab. 1989;68:256–262. doi: 10.1210/jcem-68-2-256. [DOI] [PubMed] [Google Scholar]
- 112.Horber F.F., Haymond M.W. Human Growth Hormone Prevents the Protein Catabolic Side Effects of Prednisone in Humans. J. Clin. Investig. 1990;86:265–272. doi: 10.1172/JCI114694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schakman O., Kalista S., Barbé C., Loumaye A., Thissen J.P. Glucocorticoid-Induced Skeletal Muscle Atrophy. Int. J. Biochem. Cell Biol. 2013;45:2163–2172. doi: 10.1016/j.biocel.2013.05.036. [DOI] [PubMed] [Google Scholar]
- 114.Louard R.J., Bhushan R., Gelfand R.A., Barrett E.J., Sherwin R.S. Glucocorticoids Antagonize Insulin’s Antiproteolytic Action on Skeletal Muscle in Humans. J. Clin. Endocrinol. Metab. 1994;79:278–284. doi: 10.1210/jcem.79.1.8027242. [DOI] [PubMed] [Google Scholar]
- 115.Fryburg D.A., Gelfand R.A., Barrett E.J. Growth Hormone Acutely Stimulates Forearm Muscle Protein Synthesis in Normal Humans. Am. J. Physiol. -Endocrinol. Metab. 1991;260:E499–E504. doi: 10.1152/ajpendo.1991.260.3.E499. [DOI] [PubMed] [Google Scholar]
- 116.Haymond M.W., Horber F., De Feo P., Kahn S.E., Mauras N. Effect of Human Growth Hormone and Insulin-Like Growth Factor I on Whole-Body Leucine and Estimates of Protein Metabolism. Horm. Res. 1993;40:92–94. doi: 10.1159/000183773. [DOI] [PubMed] [Google Scholar]
- 117.Kandalla P.K., Goldspink G., Butler-Browne G., Mouly V. Mechano Growth Factor E Peptide (MGF-E), Derived from an Isoform of IGF-1, Activates Human Muscle Progenitor Cells and Induces an Increase in Their Fusion Potential at Different Ages. Mech. Ageing Dev. 2011;132:154–162. doi: 10.1016/j.mad.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 118.Górecki D.C., Beręsewicz M., Zabłocka B. Neuroprotective Effects of Short Peptides Derived from the Insulin-like Growth Factor 1. Neurochem. Int. 2007;51:451–458. doi: 10.1016/j.neuint.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 119.Qin L.-L., Li X.-K., Xu J., Mo D.-L., Tong X., Pan Z.-C., Li J.-Q., Chen Y.-S., Zhang Z., Wang C., et al. Mechano Growth Factor (MGF) Promotes Proliferation and Inhibits Differentiation of Porcine Satellite Cells (PSCs) by down-Regulation of Key Myogenic Transcriptional Factors. Mol. Cell. Biochem. 2012;370:221–230. doi: 10.1007/s11010-012-1413-9. [DOI] [PubMed] [Google Scholar]
- 120.Matthews D.E., Pesola G., Campbell R.G. Effect of epinephrine on amino acid and energy metabolism in humans. Pt 1Am. J. Physiol. 1990;258:E948–E956. doi: 10.1152/ajpendo.1990.258.6.E948. [DOI] [PubMed] [Google Scholar]
- 121.Schiefermeier M., Ratheiser K., Zauner C., Roth E., Eichler H., Matthews D. Epinephrine Does Not Impair Utilization of Exogenous Amino Acids in Humans. Am. J. Clin. Nutr. 1997;65:1765–1773. doi: 10.1093/ajcn/65.6.1765. [DOI] [PubMed] [Google Scholar]
- 122.Kraenzlin M.E., Keller U., Keller A., Thélin A., Arnaud M.J., Stauffacher W. Elevation of Plasma Epinephrine Concentrations Inhibits Proteolysis and Leucine Oxidation in Man via Beta-Adrenergic Mechanisms. J. Clin. Investig. 1989;84:388–393. doi: 10.1172/JCI114178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fryburg D.A., Gelfand R.A., Jahn L.A., Oliveras D., Sherwin R.S., Sacca L., Barrett E.J. Effects of Epinephrine on Human Muscle Glucose and Protein Metabolism. Am. J. Physiol. -Endocrinol. Metab. 1995;268:E55–E59. doi: 10.1152/ajpendo.1995.268.1.E55. [DOI] [PubMed] [Google Scholar]
- 124.Choo J.J., Horan M.A., Little R.A., Rothwell N.J. Anabolic Effects of Clenbuterol on Skeletal Muscle Are Mediated by Beta 2-Adrenoceptor Activation. Am. J. Physiol. -Endocrinol. Metab. 1992;263:E50–E56. doi: 10.1152/ajpendo.1992.263.1.E50. [DOI] [PubMed] [Google Scholar]
- 125.Ryall J.G., Lynch G.S. The Potential and the Pitfalls of β-Adrenoceptor Agonists for the Management of Skeletal Muscle Wasting. Pharmacol. Ther. 2008;120:219–232. doi: 10.1016/j.pharmthera.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 126.Yoh K., Ikeda K., Horie K., Inoue S. Roles of Estrogen, Estrogen Receptors, and Estrogen-Related Receptors in Skeletal Muscle: Regulation of Mitochondrial Function. Int. J. Mol. Sci. 2023;24:1853. doi: 10.3390/ijms24031853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Boland R., Vasconsuelo A., Milanesi L., Ronda A.C., de Boland A.R. 17beta-Estradiol Signaling in Skeletal Muscle Cells and Its Relationship to Apoptosis. Steroids. 2008;73:859–863. doi: 10.1016/j.steroids.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 128.Tsai W.-J.A., McCormick K.M., Brazeau D.A., Brazeau G.A. Estrogen Effects on Skeletal Muscle Insulin-like Growth Factor 1 and Myostatin in Ovariectomized Rats. Exp. Biol. Med. 2007;232:1314–1325. doi: 10.3181/0704-RM-92. [DOI] [PubMed] [Google Scholar]
- 129.Khadilkar S.S. Musculoskeletal Disorders and Menopause. J. Obstet. Gynaecol. India. 2019;69:99–103. doi: 10.1007/s13224-019-01213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hansen M. Female Hormones: Do They Influence Muscle and Tendon Protein Metabolism? Proc. Nutr. Soc. 2018;77:32–41. doi: 10.1017/S0029665117001951. [DOI] [PubMed] [Google Scholar]
- 131.Aloia J.F., McGowan D.M., Vaswani A.N., Ross P., Cohn S.H. Relationship of Menopause to Skeletal and Muscle Mass. Am. J. Clin. Nutr. 1991;53:1378–1383. doi: 10.1093/ajcn/53.6.1378. [DOI] [PubMed] [Google Scholar]
- 132.Cho E.-J., Choi Y., Jung S.-J., Kwak H.-B. Role of Exercise in Estrogen Deficiency-Induced Sarcopenia. J. Exerc. Rehabil. 2022;18:2–9. doi: 10.12965/jer.2244004.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Smith G.I., Yoshino J., Reeds D.N., Bradley D., Burrows R.E., Heisey H.D., Moseley A.C., Mittendorfer B. Testosterone and Progesterone, But Not Estradiol, Stimulate Muscle Protein Synthesis in Postmenopausal Women. J. Clin. Endocrinol. Metab. 2014;99:256–265. doi: 10.1210/jc.2013-2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Norton L.E., Layman D.K. Leucine Regulates Translation Initiation of Protein Synthesis in Skeletal Muscle after Exercise. J. Nutr. 2006;136:533S–537S. doi: 10.1093/jn/136.2.533S. [DOI] [PubMed] [Google Scholar]
- 135.Herman J.R., Rana S.R., Chleboun G.S., Gilders R.M., Hageman F.C., Hikida R.S., Kushnick M.R., Ragg K.E., Staron R.S., Toma K. Correlation Between Muscle Fiber Cross-Sectional Area And Strength Gain Using Three Different Resistance-Training Programs In College-Aged Women. J. Strength Cond. Res. 2010;24:1. doi: 10.1097/01.JSC.0000367128.04768.0a. [DOI] [PubMed] [Google Scholar]
- 136.Jones E.J., Bishop P.A., Woods A.K., Green J.M. Cross-Sectional Area and Muscular Strength: A Brief Review. Sports Med. 2008;38:987–994. doi: 10.2165/00007256-200838120-00003. [DOI] [PubMed] [Google Scholar]
- 137.Schoenfeld B.J. The Mechanisms of Muscle Hypertrophy and Their Application to Resistance Training. J. Strength Cond. Res. 2010;24:2857–2872. doi: 10.1519/JSC.0b013e3181e840f3. [DOI] [PubMed] [Google Scholar]
- 138.Kumar V., Selby A., Rankin D., Patel R., Atherton P., Hildebrandt W., Williams J., Smith K., Seynnes O., Hiscock N., et al. Age-Related Differences in the Dose-Response Relationship of Muscle Protein Synthesis to Resistance Exercise in Young and Old Men: Age-Related Effects of Exercise on Muscle Anabolism. J. Physiol. 2009;587:211–217. doi: 10.1113/jphysiol.2008.164483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mayhew D.L., Kim J., Cross J.M., Ferrando A.A., Bamman M.M. Translational Signaling Responses Preceding Resistance Training-Mediated Myofiber Hypertrophy in Young and Old Humans. J. Appl. Physiol. 2009;107:1655–1662. doi: 10.1152/japplphysiol.91234.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Phillips S.M., Tipton K.D., Aarsland A., Wolf S.E., Wolfe R.R. Mixed Muscle Protein Synthesis and Breakdown after Resistance Exercise in Humans. Am. J. Physiol. -Endocrinol. Metab. 1997;273:E99–E107. doi: 10.1152/ajpendo.1997.273.1.E99. [DOI] [PubMed] [Google Scholar]
- 141.Yarasheski K.E., Pak-Loduca J., Hasten D.L., Obert K.A., Brown M.B., Sinacore D.R. Resistance Exercise Training Increases Mixed Muscle Protein Synthesis Rate in Frail Women and Men >/=76 Yr Old. Am. J. Physiol. 1999;277:E118–E125. doi: 10.1152/ajpendo.1999.277.1.E118. [DOI] [PubMed] [Google Scholar]
- 142.Yarasheski K.E., Zachwieja J.J., Bier D.M. Acute Effects of Resistance Exercise on Muscle Protein Synthesis Rate in Young and Elderly Men and Women. Am. J. Physiol. 1993;265:E210–E214. doi: 10.1152/ajpendo.1993.265.2.E210. [DOI] [PubMed] [Google Scholar]
- 143.Sheffield-Moore M., Paddon-Jones D., Sanford A.P., Rosenblatt J.I., Matlock A.G., Cree M.G., Wolfe R.R. Mixed Muscle and Hepatic Derived Plasma Protein Metabolism Is Differentially Regulated in Older and Younger Men Following Resistance Exercise. Am. J. Physiol. -Endocrinol. Metab. 2005;288:E922–E929. doi: 10.1152/ajpendo.00358.2004. [DOI] [PubMed] [Google Scholar]
- 144.Konopka A.R., Harber M.P. Skeletal Muscle Hypertrophy After Aerobic Exercise Training. Exerc. Sport Sci. Rev. 2014;42:53–61. doi: 10.1249/JES.0000000000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Küüsmaa M., Schumann M., Sedliak M., Kraemer W.J., Newton R.U., Malinen J.-P., Nyman K., Häkkinen A., Häkkinen K. Effects of Morning versus Evening Combined Strength and Endurance Training on Physical Performance, Muscle Hypertrophy, and Serum Hormone Concentrations. Appl. Physiol. Nutr. Metab. 2016;41:1285–1294. doi: 10.1139/apnm-2016-0271. [DOI] [PubMed] [Google Scholar]
- 146.Kuang J., McGinley C., Lee M.J.-C., Saner N.J., Garnham A., Bishop D.J. Interpretation of Exercise-Induced Changes in Human Skeletal Muscle MRNA Expression Depends on the Timing of the Post-Exercise Biopsies. Peer J. 2022;10:e12856. doi: 10.7717/peerj.12856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wackerhage H., Schoenfeld B.J., Hamilton D.L., Lehti M., Hulmi J.J. Stimuli and Sensors That Initiate Skeletal Muscle Hypertrophy Following Resistance Exercise. J. Appl. Physiol. 2019;126:30–43. doi: 10.1152/japplphysiol.00685.2018. [DOI] [PubMed] [Google Scholar]
- 148.Bodine S.C., Stitt T.N., Gonzalez M., Kline W.O., Stover G.L., Bauerlein R., Zlotchenko E., Scrimgeour A., Lawrence J.C., Glass D.J., et al. Akt/MTOR Pathway Is a Crucial Regulator of Skeletal Muscle Hypertrophy and Can Prevent Muscle Atrophy in Vivo. Nat. Cell Biol. 2001;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 149.Schiaffino S., Reggiani C., Akimoto T., Blaauw B. Molecular Mechanisms of Skeletal Muscle Hypertrophy. JND. 2021;8:169–183. doi: 10.3233/JND-200568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dickinson J.M., Fry C.S., Drummond M.J., Gundermann D.M., Walker D.K., Glynn E.L., Timmerman K.L., Dhanani S., Volpi E., Rasmussen B.B. Mammalian Target of Rapamycin Complex 1 Activation Is Required for the Stimulation of Human Skeletal Muscle Protein Synthesis by Essential Amino Acids. J. Nutr. 2011;141:856–862. doi: 10.3945/jn.111.139485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Børsheim E., Tipton K.D., Wolf S.E., Wolfe R.R. Essential Amino Acids and Muscle Protein Recovery from Resistance Exercise. Am. J. Physiol. Endocrinol. Metab. 2002;283:E648–E657. doi: 10.1152/ajpendo.00466.2001. [DOI] [PubMed] [Google Scholar]
- 152.Miller S.L., Tipton K.D., Chinkes D.L., Wolf S.E., Wolfe R.R. Independent and Combined Effects of Amino Acids and Glucose after Resistance Exercise. Med. Sci. Sports Exerc. 2003;35:449–455. doi: 10.1249/01.MSS.0000053910.63105.45. [DOI] [PubMed] [Google Scholar]
- 153.Tipton K.D., Borsheim E., Wolf S.E., Sanford A.P., Wolfe R.R. Acute Response of Net Muscle Protein Balance Reflects 24-h Balance after Exercise and Amino Acid Ingestion. Am. J. Physiol. Endocrinol. Metab. 2003;284:E76–E89. doi: 10.1152/ajpendo.00234.2002. [DOI] [PubMed] [Google Scholar]
- 154.Tipton K.D., Ferrando A.A., Phillips S.M., Doyle D., Wolfe R.R. Postexercise Net Protein Synthesis in Human Muscle from Orally Administered Amino Acids. Am. J. Physiol. 1999;276:E628–E634. doi: 10.1152/ajpendo.1999.276.4.E628. [DOI] [PubMed] [Google Scholar]
- 155.Tipton K.D., Elliott T.A., Cree M.G., Wolf S.E., Sanford A.P., Wolfe R.R. Ingestion of Casein and Whey Proteins Result in Muscle Anabolism after Resistance Exercise. Med. Sci. Sports Exerc. 2004;36:2073–2081. doi: 10.1249/01.MSS.0000147582.99810.C5. [DOI] [PubMed] [Google Scholar]
- 156.English K.L., Paddon-Jones D. Protecting Muscle Mass and Function in Older Adults during Bed Rest. Curr. Opin. Clin. Nutr. Metab. Care. 2010;13:34–39. doi: 10.1097/MCO.0b013e328333aa66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kerksick C.M., Arent S., Schoenfeld B.J., Stout J.R., Campbell B., Wilborn C.D., Taylor L., Kalman D., Smith-Ryan A.E., Kreider R.B., et al. International Society of Sports Nutrition Position Stand: Nutrient Timing. J. Int. Soc. Sports Nutr. 2017;14:33. doi: 10.1186/s12970-017-0189-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kume W., Yasuda J., Hashimoto T. Acute Effect of the Timing of Resistance Exercise and Nutrient Intake on Muscle Protein Breakdown. Nutrients. 2020;12:1177. doi: 10.3390/nu12041177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Biolo G., Williams B.D., Fleming R.Y., Wolfe R.R. Insulin Action on Muscle Protein Kinetics and Amino Acid Transport during Recovery after Resistance Exercise. Diabetes. 1999;48:949–957. doi: 10.2337/diabetes.48.5.949. [DOI] [PubMed] [Google Scholar]
- 160.Michel J.-P., Beattie B.L., Martin F.C., Walston J. Oxford Textbook of Geriatric Medicine. 3rd ed. Oxford University Press; Oxford, UK: 2018. 1392p. [Google Scholar]
- 161.Janssen I. The Epidemiology of Sarcopenia. Clin. Geriatr. Med. 2011;27:355–363. doi: 10.1016/j.cger.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 162.Markofski M.M., Dickinson J.M., Drummond M.J., Fry C.S., Fujita S., Gundermann D.M., Glynn E.L., Jennings K., Paddon-Jones D., Reidy P.T., et al. Effect of Age on Basal Muscle Protein Synthesis and MTORC1 Signaling in a Large Cohort of Young and Older Men and Women. Exp. Gerontol. 2015;65:1–7. doi: 10.1016/j.exger.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fry C.S., Drummond M.J., Glynn E.L., Dickinson J.M., Gundermann D.M., Timmerman K.L., Walker D.K., Dhanani S., Volpi E., Rasmussen B.B. Aging Impairs Contraction-Induced Human Skeletal Muscle MTORC1 Signaling and Protein Synthesis. Skelet. Muscle. 2011;1:11. doi: 10.1186/2044-5040-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Drummond M.J., Dreyer H.C., Fry C.S., Glynn E.L., Rasmussen B.B. Nutritional and Contractile Regulation of Human Skeletal Muscle Protein Synthesis and MTORC1 Signaling. J. Appl. Physiol. 2009;106:1374–1384. doi: 10.1152/japplphysiol.91397.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Funai K., Parkington J.D., Carambula S., Fielding R.A. Age-associated decrease in contraction-induced activation of downstream targets of Akt/mTor signaling in skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R1080–R1086. doi: 10.1152/ajpregu.00277.2005. [DOI] [PubMed] [Google Scholar]
- 166.Rivas D.A., Lessard S.J., Rice N.P., Lustgarten M.S., So K., Goodyear L.J., Parnell L.D., Fielding R.A. Diminished skeletal muscle microRNA expression with aging is associated with attenuated muscle plasticity and inhibition of IGF-1 signaling. FASEB J. 2014;28:4133–4147. doi: 10.1096/fj.14-254490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li M., Chi X., Wang Y., Setrerrahmane S., Xie W., Xu H. Trends in Insulin Resistance: Insights into Mechanisms and Therapeutic Strategy. Signal Transduct. Target. Ther. 2022;7:216. doi: 10.1038/s41392-022-01073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Velloso C.P. Regulation of Muscle Mass by Growth Hormone and IGF-I. Br. J. Pharmacol. 2008;154:557–568. doi: 10.1038/bjp.2008.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zachwieja J.J., Yarasheski K.E. Does Growth Hormone Therapy in Conjunction With Resistance Exercise Increase Muscle Force Production and Muscle Mass in Men and Women Aged 60 Years or Older? Phys. Ther. 1999;79:76–82. doi: 10.1093/ptj/79.1.76. [DOI] [PubMed] [Google Scholar]
- 170.Taaffe D.R., Pruitt L., Reim J., Hintz R.L., Butterfield G., Hoffman A.R., Marcus R. Effect of Recombinant Human Growth Hormone on the Muscle Strength Response to Resistance Exercise in Elderly Men. J. Clin. Endocrinol. Metab. 1994;79:1361–1366. doi: 10.1210/jcem.79.5.7525633. [DOI] [PubMed] [Google Scholar]
- 171.Cuneo R.C., Salomon F., Wiles C.M., Hesp R., Sönksen P.H. Growth Hormone Treatment in Growth Hormone-Deficient Adults. I. Effects on Muscle Mass and Strength. J. Appl. Physiol. 1991;70:688–694. doi: 10.1152/jappl.1991.70.2.688. [DOI] [PubMed] [Google Scholar]
- 172.Gibney J., Wallace J.D., Spinks T., Schnorr L., Ranicar A., Cuneo R.C., Lockhart S., Burnand K.G., Salomon F., Sonksen P.H., et al. The Effects of 10 Years of Recombinant Human Growth Hormone (GH) in Adult GH-Deficient Patients. J. Clin. Endocrinol. Metab. 1999;84:2596–2602. doi: 10.1210/jcem.84.8.5916. [DOI] [PubMed] [Google Scholar]
- 173.Fielding R.A., Vellas B., Evans W.J., Bhasin S., Morley J.E., Newman A.B., Abellan van Kan G., Andrieu S., Bauer J., Breuille D., et al. Sarcopenia: An Undiagnosed Condition in Older Adults. Current Consensus Definition: Prevalence, Etiology, and Consequences. International Working Group on Sarcopenia. J. Am. Med. Dir. Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Papadakis M.A. Growth Hormone Replacement in Healthy Older Men Improves Body Composition but Not Functional Ability. Ann. Intern. Med. 1996;124:708. doi: 10.7326/0003-4819-124-8-199604150-00002. [DOI] [PubMed] [Google Scholar]
- 175.Foo J., Maghnie M., Colao A., Vlachaki I., Colombo G. Cost-Consequence Analysis for Human Recombinant Growth Hormone (r-HGH) Treatment Administered via Different Devices in Children with Growth Hormone Deficiency in Italy. CEOR. 2019;11:525–537. doi: 10.2147/CEOR.S195265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chew J., Tay L., Lim J.P., Leung B.P., Yeo A., Yew S., Ding Y.Y., Lim W.S. Serum Myostatin and IGF-1 as Gender-Specific Biomarkers of Frailty and Low Muscle Mass in Community-Dwelling Older Adults. J. Nutr. Health Aging. 2019;23:979–986. doi: 10.1007/s12603-019-1255-1. [DOI] [PubMed] [Google Scholar]
- 177.Widajanti N., Soelistijo S., Hadi U., Thaha M., Aditiawardana, Widodo, Firdausi H., Nurina Y., Asikin M., Srinowati H., et al. Association between Sarcopenia and Insulin-Like Growth Factor-1, Myostatin, and Insulin Resistance in Elderly Patients Undergoing Hemodialysis. J. Aging Res. 2022;2022:1327332. doi: 10.1155/2022/1327332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pratt J., Boreham C., Ennis S., Ryan A.W., De Vito G. Genetic Associations with Aging Muscle: A Systematic Review. Cells. 2019;9:12. doi: 10.3390/cells9010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bhasin S. Testosterone Supplementation for Aging-Associated Sarcopenia. J. Gerontol. Ser. A. 2003;58:M1002–M1008. doi: 10.1093/gerona/58.11.M1002. [DOI] [PubMed] [Google Scholar]
- 180.Gharahdaghi N., Rudrappa S., Brook M.S., Idris I., Crossland H., Hamrock C., Abdul Aziz M.H., Kadi F., Tarum J., Greenhaff P.L., et al. Testosterone Therapy Induces Molecular Programming Augmenting Physiological Adaptations to Resistance Exercise in Older Men. J. Cachexia Sarcopenia Muscle. 2019;10:1276–1294. doi: 10.1002/jcsm.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gharahdaghi N., Rudrappa S., Brook M.S., Farrash W., Idris I., Aziz M.H.A., Kadi F., Papaioannou K., Phillips B.E., Sian T., et al. Pharmacological Hypogonadism Impairs Molecular Transducers of Exercise-induced Muscle Growth in Humans. J. Cachexia Sarcopenia Muscle. 2022;13:1134–1150. doi: 10.1002/jcsm.12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Borst S.E. Interventions for Sarcopenia and Muscle Weakness in Older People. Age Ageing. 2004;33:548–555. doi: 10.1093/ageing/afh201. [DOI] [PubMed] [Google Scholar]
- 183.Trappe T., Williams R., Carrithers J., Raue U., Esmarck B., Kjaer M., Hickner R. Influence of Age and Resistance Exercise on Human Skeletal Muscle Proteolysis: A Microdialysis Approach. J. Physiol. 2004;554:803–813. doi: 10.1113/jphysiol.2003.051755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Welle S., Thornton C., Jozefowicz R., Statt M. Myofibrillar Protein Synthesis in Young and Old Men. Am. J. Physiol. 1993;264:E693–E698. doi: 10.1152/ajpendo.1993.264.5.E693. [DOI] [PubMed] [Google Scholar]
- 185.Welle S., Thornton C., Statt M. Myofibrillar Protein Synthesis in Young and Old Human Subjects after Three Months of Resistance Training. Am. J. Physiol. 1995;268:E422–E427. doi: 10.1152/ajpendo.1995.268.3.E422. [DOI] [PubMed] [Google Scholar]
- 186.Rooyackers O.E., Adey D.B., Ades P.A., Nair K.S. Effect of Age on in Vivo Rates of Mitochondrial Protein Synthesis in Human Skeletal Muscle. Proc. Natl. Acad. Sci. USA. 1996;93:15364–15369. doi: 10.1073/pnas.93.26.15364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Balagopal P., Rooyackers O.E., Adey D.B., Ades P.A., Nair K.S. Effects of Aging on in Vivo Synthesis of Skeletal Muscle Myosin Heavy-Chain and Sarcoplasmic Protein in Humans. Am. J. Physiol. 1997;273:E790–E800. doi: 10.1152/ajpendo.1997.273.4.E790. [DOI] [PubMed] [Google Scholar]
- 188.Hasten D.L., Pak-Loduca J., Obert K.A., Yarasheski K.E. Resistance Exercise Acutely Increases MHC and Mixed Muscle Protein Synthesis Rates in 78-84 and 23-32 Yr Olds. Am. J. Physiol. Endocrinol. Metab. 2000;278:E620–E626. doi: 10.1152/ajpendo.2000.278.4.E620. [DOI] [PubMed] [Google Scholar]
- 189.Yarasheski K.E., Welle S., Nair K.S. Muscle Protein Synthesis in Younger and Older Men. JAMA. 2002;287:317–318. doi: 10.1001/jama.287.3.317. [DOI] [PubMed] [Google Scholar]
- 190.Short K.R., Vittone J.L., Bigelow M.L., Proctor D.N., Nair K.S. Age and Aerobic Exercise Training Effects on Whole Body and Muscle Protein Metabolism. Am. J. Physiol. Endocrinol. Metab. 2004;286:E92–E101. doi: 10.1152/ajpendo.00366.2003. [DOI] [PubMed] [Google Scholar]
- 191.Balagopal P., Schimke J.C., Ades P., Adey D., Nair K.S. Age Effect on Transcript Levels and Synthesis Rate of Muscle MHC and Response to Resistance Exercise. Am. J. Physiol. Endocrinol. Metab. 2001;280:E203–E208. doi: 10.1152/ajpendo.2001.280.2.E203. [DOI] [PubMed] [Google Scholar]
- 192.Karakelides H., Irving B.A., Short K.R., O’Brien P., Nair K.S. Age, Obesity, and Sex Effects on Insulin Sensitivity and Skeletal Muscle Mitochondrial Function. Diabetes. 2010;59:89–97. doi: 10.2337/db09-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Volpi E., Mittendorfer B., Rasmussen B.B., Wolfe R.R. The Response of Muscle Protein Anabolism to Combined Hyperaminoacidemia and Glucose-Induced Hyperinsulinemia Is Impaired in the Elderly. J. Clin. Endocrinol. Metab. 2000;85:4481–4490. doi: 10.1210/jc.85.12.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Volpi E., Sheffield-Moore M., Rasmussen B.B., Wolfe R.R. Basal Muscle Amino Acid Kinetics and Protein Synthesis in Healthy Young and Older Men. JAMA. 2001;286:1206–1212. doi: 10.1001/jama.286.10.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Katsanos C.S., Kobayashi H., Sheffield-Moore M., Aarsland A., Wolfe R.R. A High Proportion of Leucine Is Required for Optimal Stimulation of the Rate of Muscle Protein Synthesis by Essential Amino Acids in the Elderly. Am. J. Physiol. Endocrinol. Metab. 2006;291:E381–E387. doi: 10.1152/ajpendo.00488.2005. [DOI] [PubMed] [Google Scholar]
- 196.Katsanos C.S., Kobayashi H., Sheffield-Moore M., Aarsland A., Wolfe R.R. Aging Is Associated with Diminished Accretion of Muscle Proteins after the Ingestion of a Small Bolus of Essential Amino Acids. Am. J. Clin. Nutr. 2005;82:1065–1073. doi: 10.1093/ajcn/82.5.1065. [DOI] [PubMed] [Google Scholar]
- 197.Hughes V.A., Frontera W.R., Wood M., Evans W.J., Dallal G.E., Roubenoff R., Fiatarone Singh M.A. Longitudinal Muscle Strength Changes in Older Adults: Influence of Muscle Mass, Physical Activity, and Health. J. Gerontol. A Biol. Sci. Med. Sci. 2001;56:B209–B217. doi: 10.1093/gerona/56.5.B209. [DOI] [PubMed] [Google Scholar]
- 198.Degens H. The Role of Systemic Inflammation in Age-Related Muscle Weakness and Wasting. Scand. J. Med. Sci. Sports. 2010;20:28–38. doi: 10.1111/j.1600-0838.2009.01018.x. [DOI] [PubMed] [Google Scholar]
- 199.Toth M.J., Matthews D.E., Tracy R.P., Previs M.J. Age-Related Differences in Skeletal Muscle Protein Synthesis: Relation to Markers of Immune Activation. Am. J. Physiol. Endocrinol. Metab. 2005;288:E883–E891. doi: 10.1152/ajpendo.00353.2004. [DOI] [PubMed] [Google Scholar]
- 200.Lang C.H., Frost R.A., Nairn A.C., MacLean D.A., Vary T.C. TNF-Alpha Impairs Heart and Skeletal Muscle Protein Synthesis by Altering Translation Initiation. Am. J. Physiol. Endocrinol. Metab. 2002;282:E336–E347. doi: 10.1152/ajpendo.00366.2001. [DOI] [PubMed] [Google Scholar]
- 201.Moreau K., Walrand S., Boirie Y. Protein Redistribution From Skeletal Muscle to Splanchnic Tissue on Fasting and Refeeding in Young and Older Healthy Individuals. J. Am. Med. Dir. Assoc. 2013;14:696–704. doi: 10.1016/j.jamda.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 202.Volpi E., Mittendorfer B., Wolf S.E., Wolfe R.R. Oral Amino Acids Stimulate Muscle Protein Anabolism in the Elderly despite Higher First-Pass Splanchnic Extraction. Am. J. Physiol. 1999;277:E513–E520. doi: 10.1152/ajpendo.1999.277.3.E513. [DOI] [PubMed] [Google Scholar]
- 203.Arnal M.A., Mosoni L., Boirie Y., Houlier M.L., Morin L., Verdier E., Ritz P., Antoine J.M., Prugnaud J., Beaufrère B., et al. Protein Pulse Feeding Improves Protein Retention in Elderly Women. Am. J. Clin. Nutr. 1999;69:1202–1208. doi: 10.1093/ajcn/69.6.1202. [DOI] [PubMed] [Google Scholar]
- 204.Gryson C., Walrand S., Giraudet C., Rousset P., Migné C., Bonhomme C., Le Ruyet P., Boirie Y. “Fast Proteins” with a Unique Essential Amino Acid Content as an Optimal Nutrition in the Elderly: Growing Evidence. Clin. Nutr. 2014;33:642–648. doi: 10.1016/j.clnu.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 205.Walrand S., Gryson C., Salles J., Giraudet C., Migné C., Bonhomme C., Le Ruyet P., Boirie Y. Fast-Digestive Protein Supplement for Ten Days Overcomes Muscle Anabolic Resistance in Healthy Elderly Men. Clin. Nutr. 2016;35:660–668. doi: 10.1016/j.clnu.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 206.Buffière C., Gaudichon C., Hafnaoui N., Migné C., Scislowsky V., Khodorova N., Mosoni L., Blot A., Boirie Y., Dardevet D., et al. In the Elderly, Meat Protein Assimilation from Rare Meat Is Lower than That from Meat That Is Well Done. Am. J. Clin. Nutr. 2017;106:1257–1266. doi: 10.3945/ajcn.117.158113. [DOI] [PubMed] [Google Scholar]
- 207.Gorissen S.H., Horstman A.M., Franssen R., Crombag J.J., Langer H., Bierau J., Respondek F., Van Loon L.J. Ingestion of Wheat Protein Increases In Vivo Muscle Protein Synthesis Rates in Healthy Older Men in a Randomized Trial. J. Nutr. 2016;146:1651–1659. doi: 10.3945/jn.116.231340. [DOI] [PubMed] [Google Scholar]
- 208.Churchward-Venne T.A., Snijders T., Linkens A.M., Hamer H.M., Van Kranenburg J., Van Loon L.J. Ingestion of Casein in a Milk Matrix Modulates Dietary Protein Digestion and Absorption Kinetics but Does Not Modulate Postprandial Muscle Protein Synthesis in Older Men. J. Nutr. 2015;145:1438–1445. doi: 10.3945/jn.115.213710. [DOI] [PubMed] [Google Scholar]
- 209.Timmerman K.L., Dhanani S., Glynn E.L., Fry C.S., Drummond M.J., Jennings K., Rasmussen B.B., Volpi E. A Moderate Acute Increase in Physical Activity Enhances Nutritive Flow and the Muscle Protein Anabolic Response to Mixed Nutrient Intake in Older Adults. Am. J. Clin. Nutr. 2012;95:1403–1412. doi: 10.3945/ajcn.111.020800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Verdijk L.B., Jonkers R.A.M., Gleeson B.G., Beelen M., Meijer K., Savelberg H.H.C.M., Wodzig W.K.W.H., Dendale P., van Loon L.J.C. Protein Supplementation before and after Exercise Does Not Further Augment Skeletal Muscle Hypertrophy after Resistance Training in Elderly Men. Am. J. Clin. Nutr. 2009;89:608–616. doi: 10.3945/ajcn.2008.26626. [DOI] [PubMed] [Google Scholar]
- 211.Welle S., Thornton C.A. High-Protein Meals Do Not Enhance Myofibrillar Synthesis after Resistance Exercise in 62- to 75-Yr-Old Men and Women. Am. J. Physiol. 1998;274:E677–E683. doi: 10.1152/ajpendo.1998.274.4.E677. [DOI] [PubMed] [Google Scholar]
- 212.Symons T.B., Schutzler S.E., Cocke T.L., Chinkes D.L., Wolfe R.R., Paddon-Jones D. Aging Does Not Impair the Anabolic Response to a Protein-Rich Meal. Am. J. Clin. Nutr. 2007;86:451–456. doi: 10.1093/ajcn/86.2.451. [DOI] [PubMed] [Google Scholar]
- 213.Symons T.B., Sheffield-Moore M., Wolfe R.R., Paddon-Jones D. A Moderate Serving of High-Quality Protein Maximally Stimulates Skeletal Muscle Protein Synthesis in Young and Elderly Subjects. J. Am. Dietic Assoc. 2009;109:1582–1586. doi: 10.1016/j.jada.2009.06.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kramer I.F., Verdijk L.B., Hamer H.M., Verlaan S., Luiking Y.C., Kouw I.W.K., Senden J.M., Van Kranenburg J., Gijsen A.P., Bierau J., et al. Both Basal and Post-Prandial Muscle Protein Synthesis Rates, Following the Ingestion of a Leucine-Enriched Whey Protein Supplement, Are Not Impaired in Sarcopenic Older Males. Clin. Nutr. 2017;36:1440–1449. doi: 10.1016/j.clnu.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 215.Park S., Jang J., Choi M.D., Shin Y.-A., Schutzler S., Azhar G., Ferrando A.A., Wolfe R.R., Kim I.-Y. The Anabolic Response to Dietary Protein Is Not Limited by the Maximal Stimulation of Protein Synthesis in Healthy Older Adults: A Randomized Crossover Trial. Nutrients. 2020;12:3276. doi: 10.3390/nu12113276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kiskini A., Hamer H.M., Wall B.T., Groen B.B.L., de Lange A., Bakker J.A., Senden J.M.G., Verdijk L.B., van Loon L.J.C. The Muscle Protein Synthetic Response to the Combined Ingestion of Protein and Carbohydrate Is Not Impaired in Healthy Older Men. Age (Dordr) 2013;35:2389–2398. doi: 10.1007/s11357-013-9522-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Koopman R., Verdijk L., Manders R.J., Gijsen A.P., Gorselink M., Pijpers E., Wagenmakers A.J., Van Loon L.J. Co-Ingestion of Protein and Leucine Stimulates Muscle Protein Synthesis Rates to the Same Extent in Young and Elderly Lean Men. Am. J. Clin. Nutr. 2006;84:623–632. doi: 10.1093/ajcn/84.3.623. [DOI] [PubMed] [Google Scholar]
- 218.Yang Y., Breen L., Burd N.A., Hector A.J., Churchward-Venne T.A., Josse A.R., Tarnopolsky M.A., Phillips S.M. Resistance Exercise Enhances Myofibrillar Protein Synthesis with Graded Intakes of Whey Protein in Older Men. Br. J. Nutr. 2012;108:1780–1788. doi: 10.1017/S0007114511007422. [DOI] [PubMed] [Google Scholar]
- 219.Mitchell W.K., Phillips B.E., Hill I., Greenhaff P., Lund J.N., Williams J.P., Rankin D., Wilkinson D.J., Smith K., Atherton P.J. Human Skeletal Muscle Is Refractory to the Anabolic Effects of Leucine during the Postprandial Muscle-Full Period in Older Men. Clin. Sci. 2017;131:2643–2653. doi: 10.1042/CS20171230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Rasmussen B.B., Fujita S., Wolfe R.R., Mittendorfer B., Roy M., Rowe V.L., Volpi E. Insulin Resistance of Muscle Protein Metabolism in Aging. FASEB J. 2006;20:768–769. doi: 10.1096/fj.05-4607fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Groen B.B.L., Horstman A.M.H., Hamer H.M., De Haan M., Van Kranenburg J., Bierau J., Poeze M., Wodzig W.K.W.H., Rasmussen B.B., Van Loon L.J.C. Increasing Insulin Availability Does Not Augment Postprandial Muscle Protein Synthesis Rates in Healthy Young and Older Men. J. Clin. Endocrinol. Metab. 2016;101:3978–3988. doi: 10.1210/jc.2016-1436. [DOI] [PubMed] [Google Scholar]
- 222.Drummond M.J., Dreyer H.C., Pennings B., Fry C.S., Dhanani S., Dillon E.L., Sheffield-Moore M., Volpi E., Rasmussen B.B. Skeletal Muscle Protein Anabolic Response to Resistance Exercise and Essential Amino Acids Is Delayed with Aging. J. Appl. Physiol. 2008;104:1452–1461. doi: 10.1152/japplphysiol.00021.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Fujita S., Rasmussen B.B., Cadenas J.R., Drummond M.J., Glynn E.L., Sattler F.R., Volpi E. Aerobic Exercise Overcomes the Age-Related Insulin Resistance of Muscle Protein Metabolism by Improving Endothelial Function and Akt/Mammalian Target of Rapamycin Signaling. Diabetes. 2007;56:1615–1622. doi: 10.2337/db06-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Reitelseder S., Dideriksen K., Agergaard J., Malmgaard-Clausen N.M., Bechshoeft R.L., Petersen R.K., Serena A., Mikkelsen U.R., Holm L. Even Effect of Milk Protein and Carbohydrate Intake but No Further Effect of Heavy Resistance Exercise on Myofibrillar Protein Synthesis in Older Men. Eur. J. Nutr. 2019;58:583–595. doi: 10.1007/s00394-018-1641-1. [DOI] [PubMed] [Google Scholar]
- 225.Coker R.H., Wolfe R.R. Bedrest and Sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care. 2012;15:7–11. doi: 10.1097/MCO.0b013e32834da629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Walker D.K., Dickinson J.M., Timmerman K.L., Drummond M.J., Reidy P.T., Fry C.S., Gundermann D.M., Rasmussen B.B. Exercise, Amino Acids, and Aging in the Control of Human Muscle Protein Synthesis. Med. Sci. Sports Exerc. 2011;43:2249–2258. doi: 10.1249/MSS.0b013e318223b037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Bowden Davies K.A., Pickles S., Sprung V.S., Kemp G.J., Alam U., Moore D.R., Tahrani A.A., Cuthbertson D.J. Reduced Physical Activity in Young and Older Adults: Metabolic and Musculoskeletal Implications. Ther. Adv. Endocrinol. 2019;10:204201881988882. doi: 10.1177/2042018819888824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Drummond M.J., Dickinson J.M., Fry C.S., Walker D.K., Gundermann D.M., Reidy P.T., Timmerman K.L., Markofski M.M., Paddon-Jones D., Rasmussen B.B., et al. Bed Rest Impairs Skeletal Muscle Amino Acid Transporter Expression, MTORC1 Signaling, and Protein Synthesis in Response to Essential Amino Acids in Older Adults. Am. J. Physiol. -Endocrinol. Metab. 2012;302:E1113–E1122. doi: 10.1152/ajpendo.00603.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ferrando A.A., Paddon-Jones D., Hays N.P., Kortebein P., Ronsen O., Williams R.H., McComb A., Symons T.B., Wolfe R.R., Evans W. EAA Supplementation to Increase Nitrogen Intake Improves Muscle Function during Bed Rest in the Elderly. Clin. Nutr. 2010;29:18–23. doi: 10.1016/j.clnu.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 230.Dillon E.L., Casperson S.L., Durham W.J., Randolph K.M., Urban R.J., Volpi E., Ahmad M., Kinsky M.P., Sheffield-Moore M. Muscle Protein Metabolism Responds Similarly to Exogenous Amino Acids in Healthy Younger and Older Adults during NO-Induced Hyperemia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;301:R1408–R1417. doi: 10.1152/ajpregu.00211.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Gielen E., Beckwée D., Delaere A., De Breucker S., Vandewoude M., Bautmans I. Sarcopenia Guidelines Development Group of the Belgian Society of Gerontology and Geriatrics (BSGG) Nutritional Interventions to Improve Muscle Mass, Muscle Strength, and Physical Performance in Older People: An Umbrella Review of Systematic Reviews and Meta-Analyses. Nutr. Rev. 2021;79:121–147. doi: 10.1093/nutrit/nuaa011. [DOI] [PubMed] [Google Scholar]
- 232.Bukhari S.S.I., Phillips B.E., Wilkinson D.J., Limb M.C., Rankin D., Mitchell W.K., Kobayashi H., Greenhaff P.L., Smith K., Atherton P.J. Intake of Low-Dose Leucine-Rich Essential Amino Acids Stimulates Muscle Anabolism Equivalently to Bolus Whey Protein in Older Women at Rest and after Exercise. Am. J. Physiol. -Endocrinol. Metab. 2015;308:E1056–E1065. doi: 10.1152/ajpendo.00481.2014. [DOI] [PubMed] [Google Scholar]
- 233.Borack M.S., Reidy P.T., Husaini S.H., Markofski M.M., Deer R.R., Richison A.B., Lambert B.S., Cope M.B., Mukherjea R., Jennings K., et al. Soy-Dairy Protein Blend or Whey Protein Isolate Ingestion Induces Similar Postexercise Muscle Mechanistic Target of Rapamycin Complex 1 Signaling and Protein Synthesis Responses in Older Men. J. Nutr. 2016;146:2468–2475. doi: 10.3945/jn.116.231159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kramer I.F., Verdijk L.B., Hamer H.M., Verlaan S., Luiking Y., Kouw I.W.K., Senden J.M., van Kranenburg J., Gijsen A.P., Poeze M., et al. Impact of the Macronutrient Composition of a Nutritional Supplement on Muscle Protein Synthesis Rates in Older Men: A Randomized, Double Blind, Controlled Trial. J. Clin. Endocrinol. Metab. 2015;100:4124–4132. doi: 10.1210/jc.2015-2352. [DOI] [PubMed] [Google Scholar]
- 235.Dillon E.L., Sheffield-Moore M., Paddon-Jones D., Gilkison C., Sanford A.P., Casperson S.L., Jiang J., Chinkes D.L., Urban R.J. Amino Acid Supplementation Increases Lean Body Mass, Basal Muscle Protein Synthesis, and Insulin-Like Growth Factor-I Expression in Older Women. J. Clin. Endocrinol. Metab. 2009;94:1630–1637. doi: 10.1210/jc.2008-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Roschel H., Hayashi A.P., Fernandes A.L., Jambassi-Filho J.C., Hevia-Larraín V., De Capitani M., Santana D.A., Gonçalves L.S., De Sá-Pinto A.L., Lima F.R., et al. Supplement-Based Nutritional Strategies to Tackle Frailty: A Multifactorial, Double-Blind, Randomized Placebo-Controlled Trial. Clin. Nutr. 2021;40:4849–4858. doi: 10.1016/j.clnu.2021.06.024. [DOI] [PubMed] [Google Scholar]
- 237.Moro T., Brightwell C.R., Deer R.R., Graber T.G., Galvan E., Fry C.S., Volpi E., Rasmussen B.B. Muscle Protein Anabolic Resistance to Essential Amino Acids Does Not Occur in Healthy Older Adults before or after Resistance Exercise Training. J. Nutr. 2018;148:900–909. doi: 10.1093/jn/nxy064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Timmerman K.L., Volpi E. Amino Acid Metabolism and Regulatory Effects in Aging. Curr. Opin. Clin. Nutr. Metab. Care. 2008;11:45–49. doi: 10.1097/MCO.0b013e3282f2a592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Fiatarone Singh M.A., Bernstein M.A., Ryan A.D., O’Neill E.F., Clements K.M., Evans W.J. The effect of oral nutritional supplements on habitual dietary quality and quantity in frail elders. J. Nutr. Health Aging. 2000;4:5–12. [PubMed] [Google Scholar]
- 240.Conde Maldonado E., Marqués-Jiménez D., Casas-Agustench P., Bach-Faig A. Effect of Supplementation with Leucine Alone, with Other Nutrients or with Physical Exercise in Older People with Sarcopenia: A Systematic Review. Endocrinol. Diabetes Nutr. (Engl. Ed.) 2022;69:601–613. doi: 10.1016/j.endien.2022.11.012. [DOI] [PubMed] [Google Scholar]
- 241.Wall B.T., Hamer H.M., De Lange A., Kiskini A., Groen B.B.L., Senden J.M.G., Gijsen A.P., Verdijk L.B., Van Loon L.J.C. Leucine Co-Ingestion Improves Post-Prandial Muscle Protein Accretion in Elderly Men. Clin. Nutr. 2013;32:412–419. doi: 10.1016/j.clnu.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 242.Churchward-Venne T.A., Breen L., Di Donato D.M., Hector A.J., Mitchell C.J., Moore D.R., Stellingwerff T., Breuille D., Offord E.A., Baker S.K., et al. Leucine Supplementation of a Low-Protein Mixed Macronutrient Beverage Enhances Myofibrillar Protein Synthesis in Young Men: A Double-Blind, Randomized Trial. Am. J. Clin. Nutr. 2014;99:276–286. doi: 10.3945/ajcn.113.068775. [DOI] [PubMed] [Google Scholar]
- 243.Dickinson J.M., Gundermann D.M., Walker D.K., Reidy P.T., Borack M.S., Drummond M.J., Arora M., Volpi E., Rasmussen B.B. Leucine-Enriched Amino Acid Ingestion after Resistance Exercise Prolongs Myofibrillar Protein Synthesis and Amino Acid Transporter Expression in Older Men. J. Nutr. 2014;144:1694–1702. doi: 10.3945/jn.114.198671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Leenders M., Verdijk L.B., Van Der Hoeven L., Van Kranenburg J., Hartgens F., Wodzig W.K.W.H., Saris W.H.M., Van Loon L.J.C. Prolonged Leucine Supplementation Does Not Augment Muscle Mass or Affect Glycemic Control in Elderly Type 2 Diabetic Men. J. Nutr. 2011;141:1070–1076. doi: 10.3945/jn.111.138495. [DOI] [PubMed] [Google Scholar]
- 245.Mazzeo R.S., Tanaka H. Exercise Prescription for the Elderly: Current Recommendations. Sports Med. 2001;31:809–818. doi: 10.2165/00007256-200131110-00003. [DOI] [PubMed] [Google Scholar]
- 246.Lexell J., Taylor C.C. Variability in Muscle Fibre Areas in Whole Human Quadriceps Muscle: Effects of Increasing Age. J. Anat. 1991;174:239–249. [PMC free article] [PubMed] [Google Scholar]
- 247.Dhillon R.J.S., Hasni S. Pathogenesis and Management of Sarcopenia. Clin. Geriatr. Med. 2017;33:17–26. doi: 10.1016/j.cger.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Valenzuela P.L., Castillo-García A., Morales J.S., Izquierdo M., Serra-Rexach J.A., Santos-Lozano A., Lucia A. Physical Exercise in the Old. In: Terjung R., editor. Comprehensive Physiology. Wiley; Hoboken, NJ, USA: 2019. pp. 1281–1304. [DOI] [PubMed] [Google Scholar]
- 249.Billot M., Calvani R., Urtamo A., Sánchez-Sánchez J.L., Ciccolari-Micaldi C., Chang M., Roller-Wirnsberger R., Wirnsberger G., Sinclair A., Vaquero-Pinto M.N., et al. Preserving Mobility in Older Adults with Physical Frailty and Sarcopenia: Opportunities, Challenges, and Recommendations for Physical Activity Interventions. CIA. 2020;15:1675–1690. doi: 10.2147/CIA.S253535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Roth S.M., Ferrell R.F., Hurley B.F. Strength Training for the Prevention and Treatment of Sarcopenia. J. Nutr. Health Aging. 2000;4:143–155. [PubMed] [Google Scholar]
- 251.Denison H.J., Cooper C., Sayer A.A., Robinson S.M. Prevention and Optimal Management of Sarcopenia: A Review of Combined Exercise and Nutrition Interventions to Improve Muscle Outcomes in Older People. Clin. Interv. Aging. 2015;10:859–869. doi: 10.2147/CIA.S55842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Monti E., Tagliaferri S., Zampieri S., Sarto F., Sirago G., Franchi M.V., Ticinesi A., Longobucco Y., Adorni E., Lauretani F., et al. Effects of a 2-year Exercise Training on Neuromuscular System Health in Older Individuals with Low Muscle Function. J. Cachexia Sarcopenia Muscle. 2023;14:794–804. doi: 10.1002/jcsm.13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Franchi M.V., Badiali F., Sarto F., Müller P., Müller N.G., Rehfeld K., Monti E., Rankin D., Longo S., Lund J., et al. Neuromuscular Aging: A Case for the Neuroprotective Effects of Dancing. Gerontology. 2023;69:73–81. doi: 10.1159/000524843. [DOI] [PubMed] [Google Scholar]
- 254.Chodzko-Zajko W.J., Proctor D.N., Fiatarone Singh M.A., Minson C.T., Nigg C.R., Salem G.J., Skinner J.S. Exercise and Physical Activity for Older Adults. Med. Sci. Sports Exerc. 2009;41:1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- 255.Evans W.J. Exercise Training Guidelines for the Elderly. Med. Sci. Sports Exerc. 1999;31:12–17. doi: 10.1097/00005768-199901000-00004. [DOI] [PubMed] [Google Scholar]
- 256.Feigenbaum M.S., Pollock M.L. Prescription of Resistance Training for Health and Disease. Med. Sci. Sports Exerc. 1999;31:38–45. doi: 10.1097/00005768-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 257.Feigenbaum M.S., Pollock M.L. Strength Training: Rationale for Current Guidelines for Adult Fitness Programs. Phys. Sportsmed. 1997;25:44–63. doi: 10.3810/psm.1997.02.1137. [DOI] [PubMed] [Google Scholar]
- 258.Messier S.P., Dill M.E. Alterations in Strength and Maximal Oxygen Uptake Consequent to Nautilus Circuit Weight Training: Research Quarterly for Exercise and Sport: Vol 56, No 4. [(accessed on 24 August 2023)]. Available online: https://www.tandfonline.com/doi/abs/10.1080/02701367.1985.10605339.
- 259.Gibbons R.J., Balady G.J., Beasley J.W., Bricker J.T., Duvernoy W.F., Froelicher V.F., Mark D.B., Marwick T.H., McCallister B.D., Thompson P.D., et al. ACC/AHA Guidelines for Exercise Testing. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing) J. Am. Coll. Cardiol. 1997;30:260–311. doi: 10.1016/s0735-1097(97)00150-2. [DOI] [PubMed] [Google Scholar]
- 260.Fletcher G.F., Ades P.A., Kligfield P., Arena R., Balady G.J., Bittner V.A., Coke L.A., Fleg J.L., Forman D.E., Gerber T.C., et al. Exercise Standards for Testing and Training: A Scientific Statement from the American Heart Association. Circulation. 2013;128:873–934. doi: 10.1161/CIR.0b013e31829b5b44. [DOI] [PubMed] [Google Scholar]
- 261.Heath E.H. ACSM’s Guidelines for Exercise Testing and Prescription, 7th Edition. Med. Sci. Sports Exerc. 2005;37:2018. doi: 10.1249/01.mss.0000189073.33400.04. [DOI] [Google Scholar]
- 262.Verzola D., Barisione C., Picciotto D., Garibotto G., Koppe L. Emerging role of myostatin and its inhibition in the setting of chronic kidney disease. Kidney Int. 2019;95:506–517. doi: 10.1016/j.kint.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 263.Meynial-Denis D., Guerin O., Schneider S.M., Volkert D., Sieber C.C. New Strategies to Fight against Sarcopenia at Old Age. J. Aging Res. 2012;2012:676042. doi: 10.1155/2012/676042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Bollwein J., Diekmann R., Kaiser M.J., Bauer J.M., Uter W., Sieber C.C., Volkert D. Dietary Quality Is Related to Frailty in Community-Dwelling Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2013;68:483–489. doi: 10.1093/gerona/gls204. [DOI] [PubMed] [Google Scholar]
- 265.Kim I.-Y., Schutzler S., Schrader A.M., Spencer H.J., Azhar G., Wolfe R.R., Ferrando A.A. Protein Intake Distribution Pattern Does Not Affect Anabolic Response, Lean Body Mass, Muscle Strength or Function over 8 Weeks in Older Adults: A Randomized-Controlled Trial. Clin. Nutr. 2018;37:488–493. doi: 10.1016/j.clnu.2017.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Esmarck B., Andersen J.L., Olsen S., Richter E.A., Mizuno M., Kjaer M. Timing of Postexercise Protein Intake Is Important for Muscle Hypertrophy with Resistance Training in Elderly Humans. J. Physiol. 2001;535:301–311. doi: 10.1111/j.1469-7793.2001.00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kouw I.W., Holwerda A.M., Trommelen J., Kramer I.F., Bastiaanse J., Halson S.L., Wodzig W.K., Verdijk L.B., Van Loon L.J. Protein Ingestion before Sleep Increases Overnight Muscle Protein Synthesis Rates in Healthy Older Men: A Randomized Controlled Trial. J. Nutr. 2017;147:2252–2261. doi: 10.3945/jn.117.254532. [DOI] [PubMed] [Google Scholar]
- 268.CREA Food Composition Tables (Tabelle di Composizione Degli Alimenti) 2019. [(accessed on 1 August 2023)]. National Researc Institute For foods and Nutrition (Istituto Nazionale di Ricerca per gli Alimenti e la Nutrizione, INRAN). Rome (Italy) Available online: http://sapermangiare.mobi/tabelle_alimenti.html.
- 269.McKendry J., Currier B.S., Lim C., Mcleod J.C., Thomas A.C.Q., Phillips S.M. Nutritional Supplements to Support Resistance Exercise in Countering the Sarcopenia of Aging. Nutrients. 2020;12:2057. doi: 10.3390/nu12072057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Miyakoshi N., Hongo M., Mizutani Y., Shimada Y. Prevalence of Sarcopenia in Japanese Women with Osteopenia and Osteoporosis. J. Bone Miner. Metab. 2013;31:556–561. doi: 10.1007/s00774-013-0443-z. [DOI] [PubMed] [Google Scholar]
- 271.Bonewald L.F., Kiel D., Clemens T., Esser K., Orwoll E., O’Keefe R., Fielding R. Forum on Bone and Skeletal Muscle Interactions: Summary of the Proceedings of an ASBMR Workshop. J. Bone Miner. Res. 2013;28:1857–1865. doi: 10.1002/jbmr.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Verschueren S., Gielen E., O’Neill T.W., Pye S.R., Adams J.E., Ward K.A., Wu F.C., Szulc P., Laurent M., Claessens F., et al. Sarcopenia and Its Relationship with Bone Mineral Density in Middle-Aged and Elderly European Men. Osteoporos. Int. 2013;24:87–98. doi: 10.1007/s00198-012-2057-z. [DOI] [PubMed] [Google Scholar]
- 273.Kaji H. Interaction between Muscle and Bone. J. Bone Metab. 2014;21:29–40. doi: 10.11005/jbm.2014.21.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Nunes E.A., Stokes T., McKendry J., Currier B.S., Phillips S.M. Disuse-Induced Skeletal Muscle Atrophy in Disease and Nondisease States in Humans: Mechanisms, Prevention, and Recovery Strategies. Am. J. Physiol. -Cell Physiol. 2022;322:C1068–C1084. doi: 10.1152/ajpcell.00425.2021. [DOI] [PubMed] [Google Scholar]
- 275.Tagliaferri C., Wittrant Y., Davicco M.-J., Walrand S., Coxam V. Muscle and Bone, Two Interconnected Tissues. Ageing Res. Rev. 2015;21:55–70. doi: 10.1016/j.arr.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 276.Paulussen K.J.M., McKenna C.F., Beals J.W., Wilund K.R., Salvador A.F., Burd N.A. Anabolic Resistance of Muscle Protein Turnover Comes in Various Shapes and Sizes. Front. Nutr. 2021;8:615849. doi: 10.3389/fnut.2021.615849. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.