Abstract
Curcumin, the active component of the rhizome of Curcuma longa, is a safe substance whose applications are extensively used in medicinal, biological, pharmacological activities, and food cosmetic additives. In the field of medicine, curcuminoids have a greater impact; they have been associated with the suppression of neuropathic pain, depression, angiogenesis, tumorigenesis, diabetes, and diseases of the liver, skin, and pulmonary systems, as well as cardiovascular and nervous systems. These are in high demand and have high market potential and inflated costs. For the aforementioned uses, as well as for basic research, it is crucial to get pure curcumin from plant sources. There is a need for effective extraction and purification techniques that adhere to standards for process efficiency, environmental friendliness, and safety. Scope: This account offers an accurate and thorough explanation of the many techniques used to extract and purify curcumin from plant sources, as well as a look at its various roles in the pharmaceutical, cosmetic, medical, and other industries. Curcumin’s prospective and commercial roles are also discussed. Key findings: Curcuminoids have been extracted and purified by using a broad range of techniques that are utilized extensively across the world. Extraction of curcuminoids includes both traditional and contemporary approaches, of which a handful include Soxhlet extraction, maceration, solvent extraction, ultrasound-assisted extraction, microwave-assisted extraction, enzyme-assisted extraction, and supercritical liquid extraction. The other process called purification can be performed alone or in combination with techniques. The use of column chromatography and semipreparative high-performance liquid chromatography are examples of traditional purification procedures, and other innovative methods include high-speed counter-current chromatography and supercritical fluid chromatography.
1. Introduction
Curcumin is the main active ingredient in Curcuma longa L (Turmeric), a yellow Indian spice (native to Southeast Asia) obtained from the ginger family (Zingiberaceae); it is also known as diferuloylmethane. With its dried and powdered form, it is used all over the world as a spice and coloring agent (in textiles, pharmaceuticals, confectionery, and cosmetics).1,2 The genus Curcuma contains the species C. longa, Curcuma alismatifolia, Curcuma amarissima, Curcuma cesia, and Curcuma prakasha. The species C. longa, sometimes known as turmeric, is the most prominent. In addition to curcumin, demethoxycurcumin, bisdemethoxycurcumin, diarylheptanoid curcuminoids, and curcuminoids are secondary components of turmeric. The percentage composition of curcuminoids derivatives is around 3–5% comprising three derivatives including curcumin (75%), demethoxycurcumin (10–20%), and bisdemethoxycurcumin (5%).3,4
Numerous research studies have been done on curcumin because of its broad range of biological activities. Curcumin has been found to have anti-inflammatory, antibacterial, antidepressant, antioxidant, anticancer, and depressive effects.5−7 It’s antioxidant properties through the removal of free radicals,8 anti-inflammatory properties through the inhibition of NF-kB and AP-1 activation,9 anticancer properties through the suppression of angiogenesis, induction of apoptosis, and inhibition of the expression of antiapoptotic proteins, immune system defence,10,11 and antibacterial, antifungal, antiviral activities.12,13 Curcumin has been shown to be effective in the treatment of cancer, diabetes, obesity, Alzheimer’s disease, and stroke.
Curcumin may be obtained in two ways: synthetically and by extraction from plants. Numerous publications have explained how to make synthetic curcumin.14,15 However, extracting curcumin from plants, which are already present naturally, still represents the most cost-effective method of producing curcumin. Soxhlet extraction, maceration, and solvent extraction are examples of typical extraction methods4,16,17 On the other hand, sophisticated extraction technique examples include supercritical liquid extraction, enzyme-assisted extraction, ultrasound-assisted extraction, microwave-assisted extraction, etc.4,13,18−21 have been described for the effective extraction of curcumin from plant sources. In order to purify curcumin from curcumin extracts, several alternative purification procedures, alone or in combination, have been used. It has been possible to separate and purify curcumin using both traditional purification methods, such as column chromatography and semipreparative high-performance liquid chromatography, and additional cutting-edge methods, such as high-speed counter-current chromatography and supercritical fluid chromatography.
The choice of an appropriate solvent is one of the factors that impact the extraction process and has a significant impact on the yield and composition of the extracts that are generated.22 Traditional organic solvents are often utilized in several sectors to prepare bioactive components from natural product sources. It is extremely hard to extract all metabolites from biomass in a single step using a single solvent due to the diverse polarity and physical characteristics of natural chemicals. Polyphenols have previously been extracted from the plant matrix using a variety of solvents, including alcohols, ethyl acetate, and chloroform.23 For basic research as well as for actual use in the realms of food, cosmetics, and medicine, obtaining curcumin from plant sources is crucial. Nevertheless, its usage has been complicated, by a few of its properties, including chemical instability, low water liquefaction, photo transformation, a disproportionately rapid pace of metabolic breakdown, and restricted bioaccessibility. As a result, various researchers have made an effort to use techniques like encapsulation to solve these particular problems.24−26
2. Extraction Techniques
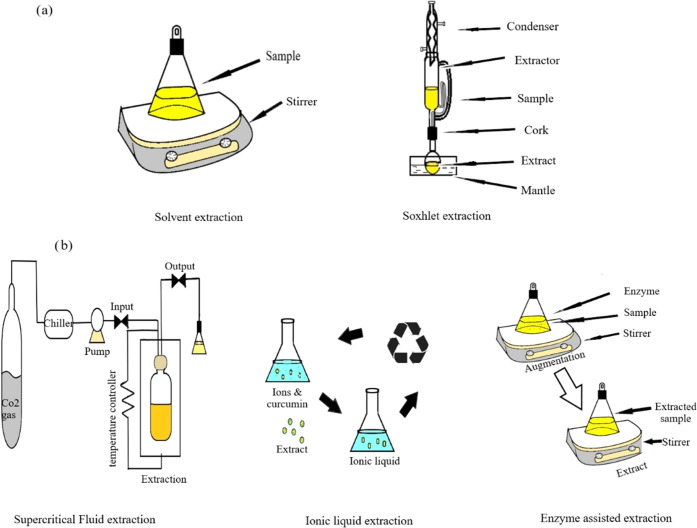
Historically, curcuminoids have been extracted using methods such as conventional solid–liquid extraction, sonication, Soxhlet extraction, and similar processes. But during these conventional extraction processes, curcuminoids are degraded by exposure to light, elevated temperatures, and oxygen.27 Traditional extraction methods can be replaced by pressurized liquid extraction (PLE), which is more effective and often uses less solvent.28 PLE may be utilized to speed up extraction and retain the solvent in the compressed liquid zone throughout the pressures between 3.5 and 35 MPa and temperatures that lie between 313 and 473 K.29 In order to address various difficulties, novel extraction methods have been designed. These methods include MAE, UAE, EAE, and PLE. These state-of-the-art techniques demonstrate the potential to extract curcumin from plant sources and are considered ecologically friendly green technology.
2.1. Conventional Methods for Extraction of Curcumin
The ideas behind sample extraction have existed since the beginning of human history. Throughout these ages, key natural chemicals from medicinal and other economically important plants were routinely subjected to percolation through soaking. In fact, to speed up the mass transfer of the target compounds into the organic phase, the necessary products were sometimes leached with the proper solvent while being heated and/or stirred.30 Up until the invention of the Soxhlet extractor at the end of the 17th century, maceration and its associated problems, such as ineffective extraction and excessive time consumption, remained common. In fact, a German scientist named Franz Ritter von Soxhlet (January 12, 1848-May 5, 1926) created extraction equipment in 1879 that is now known as the Soxhlet extractor. For the solid–liquid extraction of bioactive chemicals from plants, the Soxhlet extraction method is considered the gold standard and the method of reference. Several publications have reported the utilization of a Soxhlet extractor to extract curcumin from plants. When Shirsath et al. examined the curcumin extraction yield from the UAE, Soxhlet extraction, and solid–liquid extraction, they discovered that Curcumin extracted using the Soxhlet method had a greater yield than either the solid–liquid extraction method or the UAE.4 In contrast to the UAE, Soxhlet extraction was conducted at a considerably greater temperature for a much longer period of time. The effectiveness of Soxhlet extraction has been improved by the development of microwave, automated, ultrasound, high-pressure assisted, and other forms of Soxhlet extraction.
Solid–liquid extraction, usually referred to as “maceration” or “soaking”, is a routinely used and well-known technique for solvent extraction of solid materials. Curcumin has been extracted from plants using a wide variety of solvents, such as nonpolar organic solvents and mixtures of organic solvents and water.16,3 The solvents used for the extraction of curcumin from C. longa L by Popuri and Pagala comprise acetone, ethyl acetone, ethanol, methanol, and isopropanol. The outcomes demonstrated that ethanol extraction at 30 °C for 1 h with a sample-to-solid ratio of 8:1 generated the highest yield. In accordance with it, ethanol was the most favored organic solvent for extracting curcumin.4,16,31 Additionally, the solvent mixture’s ethanol content is a significant factor in the extraction efficiency.3
Another common extraction technique for bioactive substances and essential oils is hydro/steam distillation. To obtain flavor-free curcumin, it has been used to extract the essential oil from raw turmeric powder or turmeric oleoresin. A good yield of deodorized turmeric was produced following hydro-distillation in research by Silva et al.17 Deodorized turmeric contained the same amount of curcuminoids as the control sample and a sample that had undergone extraction using hexane for deodorization. These outcomes demonstrated the efficacy of the hydro-distillation technique in creating curcuminoids or turmeric powder while retaining little to no turmeric taste and no color.
The traditional liquid–liquid extractions technique is a strong and effective separation method that has been widely applied in a variety of applications, such as the purification of antibiotics, natural products, precious metals, proteins, and hydrocarbons, among others.32 This method is quite similar to the traditional Soxhlet extraction method in terms of its operating restrictions.
2.2. Novel Methods for Extraction of Curcumin
The basis of ultrasound is the simultaneous compression and expansion of sound waves with a frequency range of 20 kHz to 100 MHz. Intense shear forces, shock waves, macroturbulences, micromixing, and acoustic streaming are produced as a result of the cavitation phenomena, which are caused by ultrasonic waves. Motion energy is transformed into heat during the sonication process, acting as the activation energy for several physicochemical processes, which facilitate the conveyance of mass.33,34 Hence, ultrasound-assisted extraction is noted as one of the novel methods for extracting curcumin. Operating parameters, including the rate, environment, and duration of application, both the qualities of the plant’s material and the parameters of the solvent, are significant determinants of curcumin output in ultrasound-assisted extraction; in order to recover curcumin, it is therefore wise to optimize the UAE process first.
Shirsath et al. determined the ideal operating parameters after examining the impact of operating factors on the extraction yield using the UAE, which include:
35 °C temperature,
solid/ethanol ratio of 1:25 (g/mL),
an hour for the extraction
250 W of ultrasonic power and
a 22 kHz ultrasonic frequency.
The yield that was achieved was 72%, ten percent more than using solvent extraction.4 Higher extraction yields than those obtained with high-frequency ultrasound were made possible by low-frequency ultrasound. UAE showed that process intensification was possible by requiring less time, less heating, and increased extraction yield.79 Microwave-assisted extraction is dependable, automated, environmentally friendly, and employs microwave radiation to extract bioactive chemicals. Electromagnetic waves play a crucial part with frequencies between 300 MHz and 300 GHz, which are substantially higher than in the UAE. 2.45 GHz electromagnetic waves engaging directly with polar solvent molecules through ionic conduction and dipole rotation underlie microwave (MW) heating.34 Notably, MAE has several benefits over conventional procedures, such as the combined decrease of extraction time, solvent, and energy consumption, and it yields a higher amount of material.35 Furthermore, industrial scale-up systems have been developed due to their low cost and ease of operation. In their Taguchi L9 orthogonal design, Mandal et al. made use of the solvent-sample duo-heating synergism and microwaves for the extraction of curcumin from C. longa L.36 Ideal circumstances were as follows:
140 W (20%) of microwave power,
4 min of exposure,
20-mesh screening of the particles,
solid-to-acetone ratio of 1:20.
This produced a yield of curcumin that was greater than that obtained after Soxhlet extraction, maceration, and stirring extraction, at 4.98%. The aforementioned instances demonstrated that as compared to traditional methods, curcumin extraction by MAE had superior accuracy, a higher yield, and a noticeably shorter extraction time.
There are several techniques, including ionic liquid-based extraction, pressurized liquid extraction, supercritical fluid extraction, and ultrasonic and supercritical carbon dioxide-aided extraction.
In order to reduce environmental contamination, “green solvents” have recently displaced traditional organic solvents as the preferred choice. Ionic liquids have drawn a lot of attention in this respect because of their distinctive chemical and physical characteristics, including their low volatility, high-temperature stability, adjustable viscosity, and capacity to preserve biological activity.37,38Table 1 summarizes the various extraction processes of curcuminoids.
Table 1. Summary on Various Extractions of Curcuminoids.
| treatment | solid/solvent ratio | yield (%) | conclusion |
|---|---|---|---|
| soxhlet extraction | |||
| 65 °C, 2 days | solid/ethanol—1:4 | 1.25 | the extracts also included secondary metabolite components in addition to curcumin.44 |
| 78 °C, 8 h | solid/ethanol—1:25 | 1.28 | high temperature and time-consuming process.4 |
| 78 °C, 14 h | solid/ethanol—1:30 | 3.22 | when comparing the outcomes of traditional batch extraction versus ultrasound-assisted extraction (UAE), this yield (100%) was taken into consideration as the highest yield possible.79 |
| solvent extraction | |||
| 30–80 °C, 12–24 h, agitation speed 30–70 rpm | solid/ethanol—1:8 | 1.8 | a time-consuming process, solvent also played a crucial role.16 |
| 30 °C, 1 h | solid/acetone, ethyl acetone, ethanol, methanol, or isopropanol—1:8 | 2.4 | ethanol extraction produced the best yield.30 |
| ultrasound-assisted extraction (UAE) | |||
| 25–55 °C, 1 h, particle size 0.09–0.85 mm, 250 W, 22 kHz | solid/ethanol, methanol, acetone, and ethyl acetate—1:15–1:55 | 0.92 | when compared to sequential extraction, the yield produced under optimal circumstances was substantially greater.4 |
| 10 min, 50–70% amplitude, 1–3 s pulsed duration time | solid/ethanol—1:200 | 1.03 | pulsed UAE had better efficiency.45 |
| four particle sizes as 0.09, 0.10, 0.21, and 0.85 mm 30 to 60 °C, 22 kHz, 240 W | solid/methanol, ethanol, ethyl acetate, acetone, and acetonitrile—1:10–1:50 | 3.22 | although this tendency might not be widespread, the extraction yield rose with power.79 |
| microwave-assisted extraction (MAE) | |||
| 4 min, 140–420 W, particles screened through a sieve 10–40, 2450 MHz | solid/acetone—1:20 | 4.98 | when compared to traditional extraction techniques, it demonstrated a considerable time reduction with substantially improved accuracy.36 |
| 0.5–10 min, 40–120 W, 2450 MHz | solid/ethanol—1:200 | 1.01 | MAE had better efficiency comparatively.45 |
| enzyme-assisted extraction (EAE) | |||
| particle size 0.180–0.425 mm, 1–5% mixture of α-amylase and amyloglucosidase, 2–8 h of incubation (65 °C), 2–24 h of extraction (15–45 °C) | solid/carbamate ionic liquid—1:10–1:50 | 5.73 | pretreating turmeric with a combination of α-amylase and amyloglucosidase enzymes significantly increased the extraction yield by 2%.20 |
| 0–5% α-amylase, glucoamylase, a mixture of cellulase and xylanase, pH 4–6, 2.5–10 h of incubation, 4–16 h of extraction | solid/acetone—1:8 | 4.1 | under ideal circumstances, glucoamylase and α-amylase boosted curcumin yields by 26.04% and 31.83%, respectively.41 |
| accelerated solvent extraction (ASE) | |||
| 393.15–433.15 K, 1–2.5 MPa, 0.6–2 mm–sized particulates. 6–22 min | solvent-free | 3.8 | the negative effects of harmful organic solvents are reduced.43 |
| 90–250 °C, 5.0 MPa, pH 1–5.5 | solvent-free | 1.61 | extraction yields using phosphate buffer at 200 °C and pH 1.5 is highest.46 |
| supercritical fluid extraction (SFE) | |||
| 323.15 K, 300 bar, 5 mL/min CO2 rate of circulation, and 1Hr of static time, 300 min dynamic time | solid/ethanol, acetone—1:3 | 1.46 | high extraction recovery at minimum extraction time was obtained with MAE.13 |
| 313–333 K, 10–35 MPa, CO2 flow rate of 8.6 × 10–3 kg/min | solid/CO2 solvent—12:1 | 4.3 | the outcomes show that the extraction temperature and pressure had the greatest impact on the curcuminoid extraction.42 |
| Ionic liquids-based extraction | |||
| room temperature, 10–90 min, UAE (100–250 W) | solid/[OMIM]Br—1:30 | 6.14 | these optimized conditions produced total curcuminoids 6.1773 g/100 g.39 |
| room temperature, 10–90 min, UAE (100–250 W) | solid/carbamate ionic liquid—1:10–1:50 | 5.73 | the curcuminoids yield in carbamate ionic liquid-based EAE was higher.47 |
| 30–80 °C, 2–12 min, MAE (300 W, 2455 MHz) | solid/ [OMIM]Br—1:15–1:60 | 5.3 | [OMIM][Br] had a comparable impact as ethanol and methanol.48 |
Ionic liquids have been utilized in conjunction with various extraction techniques, such as UAE, MAE, and EAE, to extract curcumin, and encouraging findings showed that these techniques, which are based on ionic liquids, not only have a lower environmental effect but also have a higher extraction efficiency. When the ionic liquid concentration achieves its CMC, which is a feature of micelle solubilization, the solubility of curcumin substantially increases. Curcumin’s solubility is getting more soluble as ionic liquid hydrophobic chain grows.71 Hydrophilic ionic liquids [Bmim]Br, [Him]Br, [Omim]Br, and [Omin][BF4] were studied by Xu et al. as solvents for the UAE of curcuminoids from turmeric.39
Room temperature,
Time 10 min to 1 h 30 min,
[OMIM]Br to solid ratio of 30:1.
The yield of curcuminoids in the [OMIM]Br-based UAE was 6.14%, above the yields of the heat-reflux extraction using 85% ethanol (4.40%) and 85% ethanol (5.12%).39 A C18 column (250 mm, 4.6 mm, 5 mm Welch materials) running at 30 °C was used to separate the analytes chromatographically and evaluate them. As mobile phases, acetonitrile/water (60:40, v/v) and glacial acetic acid (0.5%) solution was employed. The injection volume was 20 L; the flow rate was set at 1.0 mL/min 1, and the detection wavelength was 430 nm. The mass ratio of curcuminoids in the extracting solution to the turmeric powder is used to quantify extraction efficiency.71 In comparison to its solubility in ethanol (4.9 mg/mL), curcumin’s aqueous solubility might reach almost 15 mg/mL with imidazole Ionic Liquids and 55 mg/mL with quaternary ammonium Ionic Liquids.72 Curcumin–ionic liquid interactions are most likely to include hydrogen bonds, hydrophobic interactions, and π–π interactions based on their structural similarities.73 In order to further investigate the effects of the hydrophilic headgroup, Jinghang Li’s study focused on five solubilizers with the same hydrophobic chain. The ranking of solubilization is as follows: [N22212] Br > [C12Tr] Br > [N11112] [C12mim] Br > Dodecyl sulfate of sodium > Br.
In contrast to imidazole ionic liquids, it was discovered that curcumin is more soluble with quaternary ammonium ionic liquids, suggesting that the π–π interaction is not a significant issue in this case. Furthermore, [N222 12] Br’s larger headgroup makes it more soluble than [N111 12] Br, resulting in a looser arrangement of surfactant molecules in micelles.74,75 Additionally, imidazole ionic liquids exhibit superior extraction capabilities compared to other ionic liquids, which may be explained by their stronger hydrogen bonds and enhanced capacity to dissociate and degrade cellulose in the turmeric cell wall.
Studies have shown that the electric charge of the surfactant headgroup has a substantial impact on the electrostatic interaction between curcumin and the surfactant headgroup. Compared to cationic surfactants, anionic surfactants exhibit a poorer affinity for curcumin.76,77 Gökdemir’s research group employed an alternative sustainable solvent, the ionic liquid [BMIM][Tf2N] (1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl) imide). Response surface methodology (RSM)-based face-centered central composite design (FCCCD) was used to analyze the effects of a few factors on curcumin extraction. The experimental yield ranged from 0.76 to 2.94%.78
In recent work, Isabelle S. developed and used novel aqueous two-phase micellar systems (ATPMS) for the separation and purification of curcumin. These systems included Pluronic F68, a triblock amphiphilic copolymer. In copolymer (1.0–10.0 wt %) and Ionic Liquids (0.1–3.0 M) aqueous solutions, curcumin is stable for up to 24 h.66
The creation of an energy-saving extraction method with quick curcumin dissolution and little negative environmental consequences is made possible by the use of ionic liquids.
The complex structural polysaccharides that make up plant cell walls give cells stability and resistance to the removal of intracellular components. Specialized hydrolytic enzymes are required to break down the matrix of the plant cell wall in order to access the bioactive elements located inside the cytosolic spaces, as well as those bound to the cellular walls. Curcumin is extracted using the EAE technique using enzymes such as glucoamylase, amyloglucosidase, and α-amylase.20,40,41 It is significant to remember that a variety of variables, including temperature, time, pH, and enzyme concentration, have an impact on the specificity and selectivity of enzymes. Therefore, in order to achieve the highest level of hydrolytic activity, the utilization of optimal enzyme reaction conditions is required.
In the study by Sahne et al., under optimal conditions, the application of α-amylase and Glucan 1,4-a-glucosidase upon sample yielded a 4.1% curcumin yield utilizing enzyme-assisted extraction. They also optimized the process parameters to obtain the highest yield of curcumin. The optimum conditions include
15–45 °C
2 h to 1-day extraction time
A solid-to-carbamate-ionic liquid proportion ranging from 1:10–1:50
Accelerated solvent extraction is an appealing green technology that extracts bioactive chemicals from liquid solvents at high temperature–pressure circumstances below their critical point at temperatures above their atmospheric boiling points.42 This environmentally friendly method is referred to as subcritical water extraction, pressurized liquid extraction, or pressurized hot water extraction if H2O is used as the extracting solvent. Figure 1 shows the schematic diagram of (a) conventional and (b) novel extraction systems.
Figure 1.
Schematic diagram of (a) conventional and (b) novel extraction systems.
The lowered dielectric constant of subcritical water is comparable to that of organic solvents, making it an efficient extraction solvent for a variety of medium- and low-polarity chemicals. In a study by Kiamahalleh et al., SWE conditions at optimum conditions investigated the curcumin yield from unflavored turmeric43
particle size of 0.6–2 mm,
120–160 °C extraction temperature,
Pressure at 10–25 bar, and
retention time of 6–22 min.
Under ideal circumstances, the scientists were able to extract 3.8 wt % of curcumin from C. longa L.
There are several techniques, including ionic liquid-based extraction, pressurized liquid extraction, supercritical fluid extraction, and ultrasonic and supercritical carbon dioxide-aided extraction. The results of several research articles are presented in Table 1, together with the yield and optimum conditions.
3. Purification
Curcumin is subjected to further separation and purification steps following a successful extraction using various techniques. A number of purifying techniques, such as counter-current chromatography (HSCCC), thin-layer column chromatography, supercritical fluid chromatography, and crystallization methods, were designed for purifying based on variables like polarity, ligand interaction, and solubility. A single chemical component can be isolated from a mixture by using the chromatography technique known as column chromatography in chemistry. Based on the differential adsorption of chemicals to the adsorbent, chromatography may separate substances into fractions by letting the compounds pass through the column at various speeds. Since a variety of adsorbents (normal phase, reversed phase, or other) may be utilized with a variety of solvents, the process is broadly adaptable. On ranges ranging from micrograms to kilograms, the approach may be applied. The fundamental benefit of column chromatography is that the stationary phase, which is utilized in the procedure, is relatively inexpensive and easily disposed of. With the latter, recycling-induced stationary phase deterioration and cross-contamination are avoided. Quite different methoxy substitution patterns on the aromatic ring result in distinct polarities in curcumin, DMC, and BDMC. Due to the variations in how curcuminoids interact with the stationary phase, curcumin and crude curcumin can be distinguished from one another. Figure 2 shows the chemical structure of curcuminoid.
Figure 2.
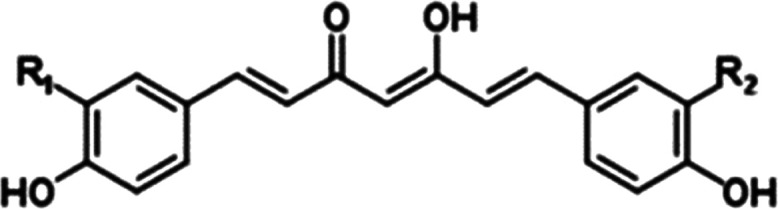
Curcuminoid structure.
Existing research revealed that column adsorption chromatography is a useful technique for producing individual curcuminoids at a large scale with high purity.47,49−50,51 For instance, Revathy et al. reported using a silica gel-based adsorption chromatography technique using chloroform/methanol as the mobile phase to separate curcumin, DMC, and BDMC as shown in Table 2: Curcumin, DMC, and BDMC structures in comparison with Curcuminoid. Using this technique, 5 g of extracts yielded 906.4 mg of curcumin, 597.5 mg of DMC, and 390.5 mg of BDMC, with purity levels of 84, 86, and 80.6%, respectively.50 Using silica and diol columns in series with methanol/chloroform as the mobile phase, Jayaprakasha et al. devised a false two-dimensional (2D) liquid flash chromatography to segregate curcumin, DMC, bisdemethoxycurcumin, and DHBDMC from turmeric oleoresin. The combined quantitative approach (qNMR) was used to assess the purity of separated curcuminoids, which was found to be between 92.4 and 95.45%.49
Table 2. Curcumin, DMC, and BDMC Structures Were Compared with Curcuminoid.
| curcuminoid | CUR | DMC | BDMC |
|---|---|---|---|
| R1 | –OCH3 | –H | –H |
| R2 | –OCH3 | –OCH3 | –H |
One of the most crucial procedures for separation and purification in chemical engineering processing sectors, such as the food and pharmaceutical sectors, is crystallization. In general, there are four ways to crystallize substances: antisolvent addition, chilling, evaporation, and consolidation. Sometimes combining these methods will yield better results. To distinguish curcumin from unprocessed curcumin (a complex consisting of curcumin, DMC, and BDMC) and suppress unconstrained nucleation of undesirable components, it is imperative to select solvents with a relatively high solubility for DMC and BDMC and a low solubility for curcumin.52,53 Curcumin purification from commercially available crude curcumin by three- or four-stage consecutive cooling crystallization has been examined in research by Ukrainczyk et al. It was discovered that the purification of curcumin is enhanced greatly by gradual cooling and seeded crystallization, particularly with the metastable Form II seed. Additionally, by adjusting the crystallization settings, it is possible to minimize the number of crystallization stages necessary to achieve a particular purity, which considerably enhances the total curcumin yield.53
When it comes to the separation and purification of natural compounds, medicines, and other items, HPLC is one of the most effective and well-accepted methods available. Although it is more difficult for operators to use and somewhat more expensive than Conventional liquid chromatography, this method’s benefits include high-efficiency separation, continuous detection, self-governing, greater yield, and strong repeatability.54,55 Recycling prep HPLC (up to five cycles) was utilized to achieve pure curcumin at high sample loading concentrations, and greater purity (>99.0%) of curcumin was obtained when the sample concentration was up to 6 mg. Numerous studies indicated that the semiprep HPLC approach is both practical and efficient since high-purity curcumin could be achieved.56−58
The preparative separation of numerous active chemicals from natural products has been successfully accomplished using a variety of techniques, including preparative supercritical fluid chromatography, high-speed counter-current chromatography, gas chromatography, and many more, while others praise it for being highly effective and environment-friendly. As high-purity curcumin, DMC, and BDMC can be quickly produced, these are among the most effective tools for separating curcumin from curcuminoids.
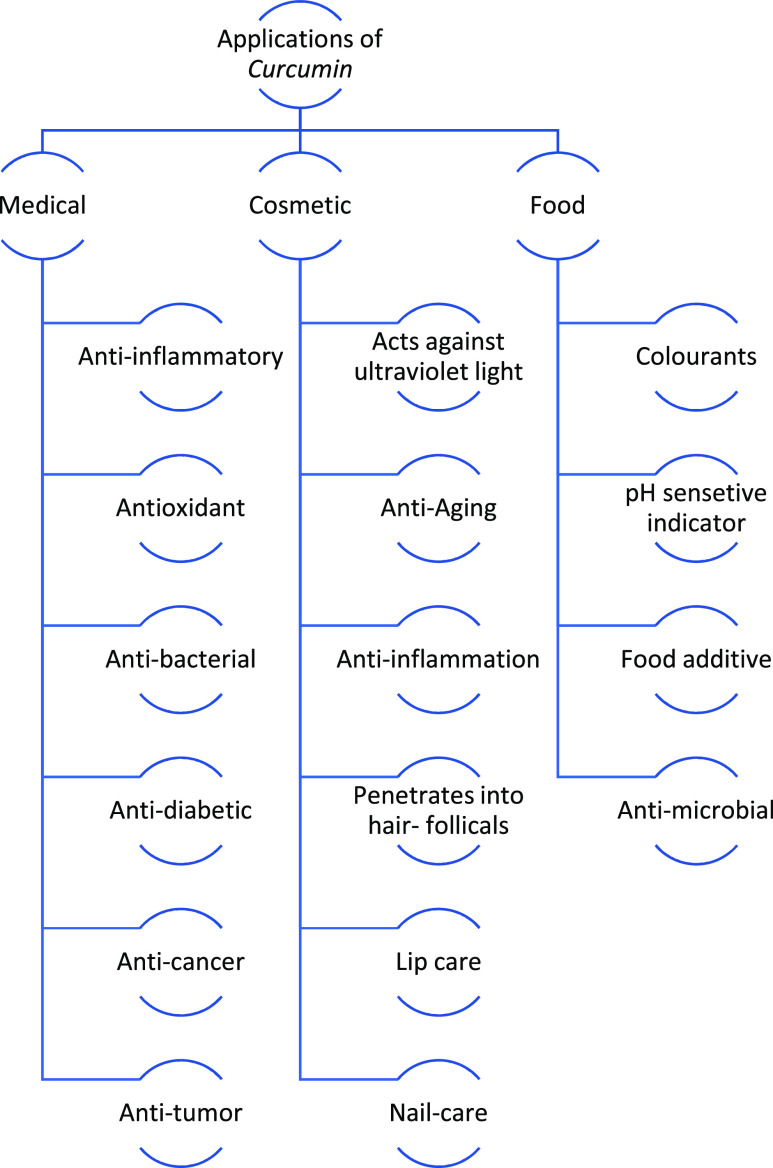
4. Applications of Curcumin
Ayurvedic and traditional Chinese medicine have long used turmeric, which comes from the rhizome of the Curcuma longa plant, as a natural remedy for skin issues, wound healing, and respiratory, digestive, and joint health. Recent studies have demonstrated the anti-inflammatory, neuroprotective, cardioprotective, anticancer, and antioxidant properties of curcumin. Curcumin’s uses in food, medicine, cosmetics, and health goods have been the subject of several research.59−61
4.1. Curcumin for Medicinal Purposes
Curcumin has been shown to have antiproliferative effects in a number of cancers by inhibiting the transcription factor nuclear factor kappa-B and downstream gene products (including Cyclin D1, c-myc, NOS, Bcl-2, TNF-, MMP-9, COX-2, and interleukins). Curcumin also affects a number of cell adhesion molecules and growth factor receptors implicated in tumor angiogenesis, metastasis, and tumor growth.62 Numerous cancers, including brain, melanoma, head, colorectal, bone, lung, neck, pancreatic, prostate, and colon tumors, can be treated with curcumin.3,23 Moreover, research on safety evaluations shows that curcumin is well tolerated at a high dose without any negative side effects.63 Additionally, curcumin is useful in the prevention and treatment of cardiovascular illnesses such as myocardial infarction, cardiomyopathy, and oxidative heart damage.23
The majority of its modes of action are connected to its anti-inflammatory properties. By exerting antioxidant, anti-inflammatory, and anti-protein aggregation activities, additionally, curcumin has anti-inflammatory properties that can treat and prevent damage to the nervous system, particularly brain disorders, it is also associated with this important organ, such as Alzheimer’s and Parkinson’s diseases.23,64 In addition to producing novel derivatives, the most typical experimental research on curcumin focuses on its antioxidant properties. The biological categorization of curcumin as a pro-oxidant and an antioxidant as well as a scavenger of free radicals, a reducing agent, and an inhibitor of DNA damage, particularly in the presence of Cu or Fe ions, has been shown in studies. It has been noted that curcumin’s ability to bind to Fe, Mn, and Cu can modify its antioxidant capabilities.23 The biochemical properties of curcumin, such as its anti-inflammatory, anti-infectious, and antioxidant activities, have been reported to contribute to tissue remodelling, granulation tissue development, and collagen deposition, which are all involved in enhancing wound healing.65
4.2. Curcumin in Other Industries
Due to its antioxidant and anti-inflammatory properties, curcumin has been utilized in the beauty industry for many years. With positive effects against ultraviolet (UV) light, aging, inflammation, hair loss, lip care, and nail care, it has shown progress in far-ranging of cosmetic treatments such as skin, nails, hair, lips, and face.61,67 Curcumin’s continual presence improves skincare and esthetics. In addition, curcumin is recommended for the treatment of acne vulgaris as an antibacterial and anti-inflammatory agent. Curcumin in vehicles with a concentration of 0.43 g/mL greatly reduced P. acnes growth. Confocal laser scanning microscopy proved that the curcumin-loaded vehicles created a curcumin reservoir in the skin.12 Additionally, curcumin-loaded formulations can be utilized to administer topical curcumin deep into and through the hair follicles, successfully penetrating there.70
Curcumin has historically been used as a natural food coloring ingredient since it has a pleasing bright yellow-orange hue and may be used in place of synthetic food colorings. It is frequently used to enhance the appearance of dishes including rice, beef, mustard, pastries, dairy items, and canned fish.60 Curcumin can also function as a natural preservative to lengthen the shelf life of the food. Escherichia coli, Staphylococcus aureus, Salmonella typhimurium, and Listeria monocytogenes are just a few of the microorganisms that it effectively combats.68 Due to curcumin’s great sensitivity to acid–base reactions, it is also suggested that curcumin be used as a pH-sensitive indicator or sensor for monitoring and providing producers, retailers, or consumers with information about the quality of food. The color of the sensor may be immediately observed during food spoiling through eye inspection, and it can also be quantitatively quantified after canning with color analysis software, hence a food analyte.69Figure 3 displays the application of curcumin in different industries.
Figure 3.
Application of curcumin in different industries.
5. Results and Discussion
In the Ginithillawala research, the influence of the high-pressure homogenization (HPH) pretreatment phase on the turmeric rhizome was assessed, along with the effect of various extraction and drying processes on the recovery of curcumin. Both HPH-treated and control (non-HPH) samples underwent Soxhlet extraction (SE) and ultrasound-assisted extraction (UAE), followed by drying. The lowest particle sizes in the turmeric suspensions and the maximum curcumin concentration in the aqueous supernatant were seen under an HPH treatment setting of 100 MPa for 10 cycles. The release of curcumin from freeze-dried turmeric extracts derived from Soxhlet extraction and UAE was similarly observed to be enhanced by HPH treatment (+76.2 and +57.5%, respectively).80
In order to compare the outcomes of ultrasound-assisted extraction (UAE), a yield of 4.409 mg curcumin/g of C. aromatica powder was obtained through Soxhlet extraction, and this yield was regarded as the highest yield (100%).79 Highly wet, untreated turmeric was utilized to extract curcumin and antioxidants using liquefied dimethyl ether. Liquid dimethyl ether was used to extract more curcumin (7.94 mg/g of dry weight (DW)) than ethanol (6.77 mg/g DW). In addition, microscopic and spectroscopic examinations showed that neither cellulose extraction nor cell wall apoptosis was caused by liquefied dimethyl ether. Liquefied dimethyl ether, however, demonstrated a somewhat poorer antioxidant efficacy and extraction capacity for total phenolic components than ethanol.81
Degot’s group extraction studies in the region of greatest solubility revealed that a 1:10 powder-to-solvent ratio produced the best extraction yield in terms of extraction effectiveness and sustainability. Additional research revealed that the solubility is not a linear function of the total extraction efficiency for any of the three curcuminoids. Regarding the total curcuminoid content, extraction studies in pure EtOAc, the solvent composition of the critical point, and the point of greatest solubility produced findings that were quite comparable. As an adjuvant to ethanol/triacetin mixes, a choline chloride + lactic acid (1:1) natural deep eutectic solvent (NADES) is employed to solubilize and extract curcumin from Curcuma longa. A decent extraction with a yield of about 90% requires a powder-to-solvent ratio of 1:8. According to the green chemistry principles, increasing the powder-to-solvent ratio further would just result in the solvent being wasted and would not considerably improve the extraction yield. However, compared to the powder-to-solvent ratio (1:4) in the binary combination of EtOH/TriA (4:6) as utilized by Degot et al., this solvent, which included NADES in place of water, increased the extraction yield by around 12%. Since Degot et al. discovered a remarkable solubility synergy in the binary system EtOH/TriA, they employed a water/EtOH/TriA system to extract curcumin.82−84
6. Conclusions
Over the past few decades, a large amount of research on the extraction and purification of curcumin from plant sources has been published. This study’s main objective is to give researchers and industry experts a comprehensive understanding of the various processes for extracting and purifying curcumin from plant sources so that they may obtain effective curcumin. Curcumin extraction techniques should use safe solvents, generate a high yield, and be energy efficient. Although traditional extraction methods have been demonstrated to be time-consuming, notably with Soxhlet, it has been established in several studies that they compare favorably to more modern extraction methods. Some of the main advantages of the conventional extraction method are its ease of use, minimal operational expenses, and, therefore, reasonable price. The results also demonstrate that the temperature and pressure of the extraction had the greatest effects on the curcuminoid extraction. To improve extraction efficiency, reduce the use of dangerous solvents, shorten processing times, and use less energy, new extraction techniques have been researched. These include UAE, MAE, EAE, PLE, SWE, SFE, and ionic liquid-based extraction.
The purifying procedure is essential for the generation of curcumin. Both conventional (such as column chromatography and HPLC) and advanced techniques (such as counter-current chromatography and thin-layer column chromatography, crystallization, etc.) have been thoroughly studied. The extra innovative methods have exceptional accuracy and provide high-quality yields quickly and affordably. Numerous research on the extracting and purifying of curcumin has focused on the possible implementation of curcumin in various industries, including pharmaceuticals, cosmetics, and food. The biological effects of free and encapsulated curcumin as well as its potential industrial applications have been extensively studied in the literature.
Acknowledgments
The authors are thankful to the Vice Chancellor and Dean Academic of KLEF University for allowing us to take up this important investigation and granting permission to publish this article. The authors are also indebted to the Chief Editor, ACS Omega, and the anonymous learned reviewer for thoroughly evaluating the manuscript and suggesting modifications, which improved the quality of the manuscript.
The authors declare no competing financial interest.
References
- Dai Y.; Verpoorte R.; Choi Y. H. Natural deep eutectic solvents providing enhanced stability of natural colorants from safflower (Carthamus tinctorius). Food Chem. 2014, 159, 116–121. 10.1016/j.foodchem.2014.02.155. [DOI] [PubMed] [Google Scholar]
- de los Ángeles Fernández M.; Espino M.; Gomez F. J. V.; Silva M. F. Novel approaches mediated by tailor-made green solvents for the extraction of phenolic compounds from agro-food industrial by-products. Food Chem. 2018, 239, 671–678. 10.1016/j.foodchem.2017.06.150. [DOI] [PubMed] [Google Scholar]
- Rafiee Z.; Nejatian M.; Daeihamed M.; Jafari S. M. Application of curcumin loaded nanocarriers for food, drug, and cosmetic purposes. Trends Food Sci. Technol. 2019, 88, 445–458. 10.1016/j.tifs.2019.04.017. [DOI] [Google Scholar]
- Shirsath S. R.; Sable S. S.; Gaikwad S. G.; Sonawane S. H.; Saini D. R.; Gogate P. R. Intensification of extraction of curcumin from Curcuma amada using ultrasound assisted approach: Effect of different operating parameters. J. Ultrason. Sonochem. 2017, 38, 437–445. 10.1016/j.ultsonch.2017.03.040. [DOI] [PubMed] [Google Scholar]
- Bener M.; Ozyürek M.; Güçlü K.; Apak R. Optimization of microwave assisted extraction of curcumin from Curcuma longa L. (turmeric) and evaluation of antioxidant activity in multi-test systems. Rec. Nat. Prod. 2016, 10 (5), 542–554. [Google Scholar]
- Farhood B.; Mortezaee K.; Goradel N. H.; Khanlarkhani N.; Salehi E.; Nashtaei M. S.; Najafi M.; Sahebkar A. Curcumin as an anti-inflammatory agent: Implications to radiotherapy and chemotherapy. J. Cell. Physiol. 2019, 234 (5), 5728–5740. 10.1002/jcp.27442. [DOI] [PubMed] [Google Scholar]
- Naeini M. B.; Momtazi A. A.; Jaafari M. R.; Johnston T. P.; Barreto G.; Banach M.; Sahebkar A. Antitumor effects of curcumin: A lipid perspective. J. Cell. Physiol. 2019, 234 (9), 14743–14758. 10.1002/jcp.28262. [DOI] [PubMed] [Google Scholar]
- Meghana K.; Sanjeev G.; Ramesh B. Curcumin prevents streptozotocin induced islet damage by scavenging free radicals: A prophylactic and protective role. Eur. J. Pharmacol. 2007, 577 (1–3), 183–191. 10.1016/j.ejphar.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Kang G.; Kong P.-J.; Yuh Y.-J.; Lim S.-Y.; Yim S.-V.; Chun W.; Kim S. S. Curcumin suppresses lipopolysaccharide-induced cyclooxygenase-2 expression by inhibiting activator protein 1 and nuclear factor κB bindings in BV2 microglial cells. J. Pharmacol. Sci. 2004, 94 (3), 325–328. 10.1254/jphs.94.325. [DOI] [PubMed] [Google Scholar]
- Dorai T.; Cao Y.-C.; Dorai B.; Buttyan R.; Katz A. E. Therapeutic potential of curcumin in human prostate cancer. III. Curcumin inhibits proliferation, induces apoptosis, and inhibits angiogenesis of LNCaP prostate cancer cells in vivo. Prostate 2001, 47 (4), 293–303. 10.1002/pros.1074. [DOI] [PubMed] [Google Scholar]
- Sa G.; Das T. Anti-cancer effects of curcumin: Cycle of life and death. Cell Div. 2008, 3 (1), 14 10.1186/1747-1028-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.-H.; Huang H.-Y. In vitro anti-Propionibacterium activity by curcumin containing vesicle system. Chem. Pharm. Bull. 2013, 61 (4), 419–425. 10.1248/cpb.c12-01043. [DOI] [PubMed] [Google Scholar]
- Wakte P. S.; Sachin B. S.; Patil A. A.; Mohato D. M.; Band T. H.; Shinde D. B. Optimization of microwave, ultra-sonic and supercritical carbon dioxide assisted extraction techniques for curcumin from Curcuma longa. Sep. Purif. Technol. 2011, 79 (1), 50–55. 10.1016/j.seppur.2011.03.010. [DOI] [Google Scholar]
- Pabon H. J. J. A synthesis of curcumin and related compounds. Recl. Trav. Chim. Pays-Bas 1964, 83 (4), 379–386. 10.1002/recl.19640830407. [DOI] [Google Scholar]
- Rodrigues J. L.; Araújo R. G.; Prather K. L. J.; Kluskens L. D.; Rodrigues L. R. Production of curcuminoids from tyrosine by a metabolically engineered Escherichia coli using caffeic acid as an intermediate. Biotechnol. J. 2015, 10 (4), 599–609. 10.1002/biot.201400637. [DOI] [PubMed] [Google Scholar]
- Paulucci V. P.; Couto R. O.; Teixeira C. C.; Freitas L. A. P. Optimization of the extraction of curcumin from Curcuma longa rhizomes. Rev. Bras. Farmacogn. 2013, 23 (1), 94–100. 10.1590/S0102-695X2012005000117. [DOI] [Google Scholar]
- Silva L. V.; Nelson D. L.; Drummond M. F. B.; Dufoss′e L.; Gloria M. B. A. Comparison of hydrodistillation methods for the deodorization of turmeric. Food Res. Int. 2005, 38 (8–9), 1087–1096. 10.1016/j.foodres.2005.02.025. [DOI] [Google Scholar]
- Gökdemir B.; Baylan N.; Çehreli S. Application of a novel ionic liquid as an alternative green solvent for the extraction of curcumin from turmeric with response surface methodology: Determination and optimization study. Anal. Lett. 2020, 53, 2111–2121. 10.1080/00032719.2020.1730394. [DOI] [Google Scholar]
- Kwon H.-L.; Chung M.-S. Pilot-scale subcritical solvent extraction of curcuminoids from Curcuma long L. Food Chem. 2015, 185, 58–64. 10.1016/j.foodchem.2015.03.114. [DOI] [PubMed] [Google Scholar]
- Sahne F.; Mohammadi M.; Najafpour G. D.; Moghadamnia A. A. Extraction of bioactive compound curcumin from turmeric (Curcuma longa L.) via different routes: A comparative study. Pak. J. Biotechnol. 2016, 13 (3), 173–180. [Google Scholar]
- Bakirtzi C.; Triantafyllidou K.; Makris D. P. Novel lactic acid-based natural deep eutectic solvents: Efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from common native Greek medicinal plants. J. Appl. Res. Med. Aromat. Plants 2016, 3 (3), 120–127. 10.1016/j.jarmap.2016.03.003. [DOI] [Google Scholar]
- Dai Y.; Witkamp G.-J.; Verpoorte R.; Choi Y. H. Natural deep eutectic solvents as a new extraction media for phenolic metabolites in Carthamus tinctorius L. Anal. Chem. 2013, 85 (13), 6272–6278. 10.1021/ac400432p. [DOI] [PubMed] [Google Scholar]
- Noorafshan A.; Ashkani-Esfahani S. A review of therapeutic effects of curcumin. Curr. Pharm. Des. 2013, 19 (11), 2032–2046. 10.2174/1381612811319110006. [DOI] [PubMed] [Google Scholar]
- Jiang T.; Liao W.; Charcosset C. Recent advances in encapsulation of curcumin in nanoemulsions: A review of encapsulation technologies, bioaccessibility and applications. Food Res. Int. 2020, 132, 109035 10.1016/j.foodres.2020.109035. [DOI] [PubMed] [Google Scholar]
- Araiza-Calahorra A.; Akhtar M.; Sarkar A. Recent advances in emulsion-based delivery approaches for curcumin: From encapsulation to bioaccessibility. Trends Food Sci. Technol. 2018, 71, 155–169. 10.1016/j.tifs.2017.11.009. [DOI] [Google Scholar]
- Suresh D.; Manjunatha H.; Srinivasan K. Effect of heat processing of spices on the concentrations of their bioactive principles: Turmeric (Curcuma longa), red pepper (Capsicum annuum) and black pepper (Piper nigrum). J. Food Compos. Anal. 2007, 20, 346–351. 10.1016/j.jfca.2006.10.002. [DOI] [Google Scholar]
- Santos D. T.; Veggi P. C.; Meireles M. A. A. Optimization and economic evaluation of pressurized liquid extraction of phenolic compounds from jabuticaba skins. J. Food Eng. 2012, 108, 444–452. 10.1016/j.jfoodeng.2011.08.022. [DOI] [Google Scholar]
- Teo C. C.; Tan S. N.; Yong J. W. H.; Hew C. S.; Ong E. S. Pressurized hot water extraction (PHWE). J. Chromatogr. A 2010, 1217, 2484–2494. 10.1016/j.chroma.2009.12.050. [DOI] [PubMed] [Google Scholar]
- de Castro M. D. L.; Priego-Capote F. Soxhlet extraction versus accelerated solvent extraction. Compre. Sampling Sample Prep. 2012, 55, 83–103. 10.1016/B978-0-12-381373-2.00038-7. [DOI] [Google Scholar]
- Popuri A. K.; Pagala B. Extraction of curcumin from turmeric roots. Int. J. Innovative Res. Stud. 2013, 2 (5), 289–299. [Google Scholar]
- Sogi D. S.; Sharma S.; Oberoi D. P. S.; Wani I. A. Effect of extraction parameters on curcumin yield from turmeric. J. Food Sci. Technol. 2010, 47 (3), 300–304. 10.1007/s13197-010-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement R.; Hao C.. Liquid–Liquid Extraction: Basic Principles and Automation. In Comprehensive Sampling and Sample Preparation: Analytical Techniques for Scientists; Elsevier, 2012; pp 51–63. [Google Scholar]
- Zhang R.; Li S.; Zhu Z.; He J. Recent advances in valorization of chaenomeles fruit: A review of botanical profile, phytochemistry, advanced extraction technologies and bioactivities. Trends Food Sci. Technol. 2019, 91, 467–482. 10.1016/j.tifs.2019.07.012. [DOI] [Google Scholar]
- Garavand F.; Rahaee S.; Vahedikia N.; Jafari S. M. Different techniques for extraction and micro/nanoencapsulation of saffron bioactive ingredients. Trends Food Sci. Technol. 2019, 89, 26–44. 10.1016/j.tifs.2019.05.005. [DOI] [Google Scholar]
- Mena-García A.; Ruiz-Matute A. I.; Soria A. C.; Sanz M. L. Green techniques for extraction of bioactive carbohydrates. TrAC, Trends Anal. Chem. 2019, 119, 115612 10.1016/j.trac.2019.07.023. [DOI] [Google Scholar]
- Mandal V.; Mohan Y.; Hemalatha S. Microwave assisted extraction of curcumin by sample–solvent dual heating mechanism using Taguchi L9 orthogonal design. J. Pharm. Biomed. Anal. 2008, 46 (2), 322–327. 10.1016/j.jpba.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Pandey S. Analytical applications of room-temperature ionic liquids: A review of recent efforts. Anal. Chim. Acta 2006, 556 (1), 38–45. 10.1016/j.aca.2005.06.038. [DOI] [PubMed] [Google Scholar]
- Passos H.; Freire M. G.; Coutinho J. A. P. Ionic liquid solutions as extractive solvents for value-added compounds from biomass. Green Chem. 2014, 16 (12), 4786–4815. 10.1039/C4GC00236A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J.; Wang W.; Liang H.; Zhang Q.; Li Q. Optimization of ionic liquid based ultrasonic assisted extraction of antioxidant compounds from Curcuma longa L. using response surface methodology. Ind. Crops Prod. 2015, 76, 487–493. 10.1016/j.indcrop.2015.07.025. [DOI] [Google Scholar]
- Kurmudle N. N.; Bankar S. B.; Bajaj I. B.; Bule M. V.; Singhal R. S. Enzyme assisted three phase partitioning: A novel approach for extraction of turmeric oleoresin. Process Biochem. 2011, 46 (1), 423–426. 10.1016/j.procbio.2010.09.010. [DOI] [Google Scholar]
- Kurmudle N.; Kagliwal L. D.; Bankar S. B.; Singhal R. S. Enzyme-assisted extraction for enhanced yields of turmeric oleoresin and its constituents. Food Biosci. 2013, 3, 36–41. 10.1016/j.fbio.2013.06.001. [DOI] [Google Scholar]
- Osorio-Tobón J. F.; Carvalho P. I. N.; Rostagno M. A.; Meireles M. A. A. Process integration for turmeric products extraction using supercritical fluids and pressurized liquids: Economic evaluation. Food Bioprod. Process. 2016, 98, 227–235. 10.1016/j.fbp.2016.02.001. [DOI] [Google Scholar]
- Valizadeh Kiamahalleh M.; Najafpour-Darzi G.; Rahimnejad M.; Moghadamnia A. A.; Kiamahalleh M. V. High performance curcumin subcritical water extraction from turmeric (Curcuma longa L.). J. Chromatogr. B 2016, 1022, 191–198. 10.1016/j.jchromb.2016.04.021. [DOI] [PubMed] [Google Scholar]
- Dutta B. Study of secondary metabolite constituents and curcumin contents of six different species of genus (Curcuma). J. Med. Plants Stud. 2015, 3 (5), 116–119. [Google Scholar]
- Coradini K.; Lima F. O.; Oliveira C. M.; Chaves P. S.; Athayde M. L.; Carvalho L. M.; Beck R. C. R. Co-encapsulation of resveratrol and curcumin in lipid-core nanocapsules improves their in vitro antioxidant effects. Eur. J. Pharm. Biopharm. 2014, 88 (1), 178–185. 10.1016/j.ejpb.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Euterpio M. A.; Cavaliere C.; Capriotti A. L.; Crescenzi C. Extending the applicability of pressurized hot water extraction to compounds exhibiting limited water solubility by pH control: Curcumin from the turmeric rhizome. Anal. Bioanal. Chem. 2011, 401 (9), 2977–2985. 10.1007/s00216-011-5383-7. [DOI] [PubMed] [Google Scholar]
- Sahne F.; Mohammadi M.; Najafpour G. D.; Moghadamnia A. A. Enzyme assisted ionic liquid extraction of bioactive compound from turmeric (Curcuma longa L.): Isolation, purification and analysis of curcumin. Ind. Crops Prod. 2017, 95, 686–694. 10.1016/j.indcrop.2016.11.037. [DOI] [Google Scholar]
- Liang H.; Wang W.; Xu J.; Zhang Q.; Shen Z.; Zeng Z.; Li Q. Optimization of ionic liquid-based microwave-assisted extraction technique for curcuminoids from Curcuma longa L. Food Bioprod. Process. 2017, 104, 57–65. 10.1016/j.fbp.2017.04.003. [DOI] [Google Scholar]
- Jayaprakasha G. K.; Rao L. J M.; Sakariah K. K. Improved HPLC method for the determination of curcumin, demethoxycurcumin, and bisdemethoxycurcumin. J. Agric. Food Chem. 2002, 50 (13), 3668–3672. 10.1021/jf025506a. [DOI] [PubMed] [Google Scholar]
- Revathy S.; Elumalai S.; Benny M.; Antony B. Isolation, purification, and identification of curcuminoids from turmeric (Curcuma longa L.) by column chromatography. J. Exp. Sci. 2011, 2, 21–25. [Google Scholar]
- Yang Z.; Zheng S.; Rui Z.; Fang Y.; Ji H. Enhanced separation and purification of curcuminoids on polyamide column via noncovalent interactions. Sep. Purif. Technol. 2015, 152, 155–159. 10.1016/j.seppur.2015.07.068. [DOI] [Google Scholar]
- Horosanskaia E.; Yuan L.; Seidel-Morgenstern A.; Lorenz H. Purification of curcumin from ternary extract-similar mixtures of curcuminoids in a single crystallization step. Crystals 2020, 10 (3), 206 10.3390/cryst10030206. [DOI] [Google Scholar]
- Ukrainczyk M.; Hodnett B. K.; Rasmuson Å. C. Process parameters in the purification of curcumin by cooling crystallization. Org. Process Res. Dev. 2016, 20 (9), 1593–1602. 10.1021/acs.oprd.6b00153. [DOI] [Google Scholar]
- Feng J.; Xiao Y.; Guo Z.; Yu D.; Jin Y.; Liang X. Purification of compounds from Lignum Dalbergia Odorifera using two-dimensional preparative chromatography with Click oligo (ethylene glycol) and C18 column. J. Sep. Sci. 2011, 34 (3), 299–307. 10.1002/jssc.201000609. [DOI] [PubMed] [Google Scholar]
- Yao S.; Luo J.; Huang X.; Kong L. Application of preparative high-speed counter-current chromatography/preparative high-performance liquid chromatography mode in rapid separation of saponins. J. Chromatogr. B 2008, 864 (1–2), 69–77. 10.1016/j.jchromb.2008.01.047. [DOI] [PubMed] [Google Scholar]
- Abu-Lafi S.; Akkawi M.; Abu-Remeleh Q.; Qutob M.; Lutgen P. Curcumin, a natural isolate from Curcuma longa (turmeric) with high β-hematin inhibitory potential. Pharm. Pharmacol. Int. J. 2019, 7 (1), 22–26. 10.15406/ppij.2019.07.00228. [DOI] [Google Scholar]
- Mollayi S.; Tamhidi S.; Hashempour H.; Ghassempour A. Recycling preparative high performance liquid chromatography for the separation of curcumin from curcuminoids in Curcuma longa L. Acta Chromatogr. 2015, 27 (2), 387–398. 10.1556/AChrom.27.2015.2.13. [DOI] [Google Scholar]
- Xu G.; Hao C.; Tian S.; Gao F.; Sun W.; Sun R. A method for the preparation of curcumin by ultrasonic-assisted ammonium sulfate/ethanol aqueous two phase extraction. J. Chromatogr. B 2017, 1041–1042, 167–174. 10.1016/j.jchromb.2016.12.029. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay I.; Biswas K.; Bandyopadhyay U.; Banerjee R. K. Turmeric and curcumin: Biological actions and medicinal applications. Curr. Sci. 2004, 87 (1), 44–53. [Google Scholar]
- Esatbeyoglu T.; Huebbe P.; Ernst I. M. A.; Chin D.; Wagner A. E.; Rimbach G. Curcumin-from molecule to biological function. Angew. Chem., Int. Ed. 2012, 51 (22), 5308–5332. 10.1002/anie.201107724. [DOI] [PubMed] [Google Scholar]
- Friedrich R. B.; Kann B.; Coradini K.; Offerhaus H. L.; Beck R. C. R.; Windbergs M. Skin penetration behavior of lipid-core nanocapsules for simultaneous delivery of resveratrol and curcumin. Eur. J. Pharm. Sci. 2015, 78, 204–213. 10.1016/j.ejps.2015.07.018. [DOI] [PubMed] [Google Scholar]
- Wilken R.; Veena M. S.; Wang M. B.; Srivatsan E. S. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol. Cancer 2011, 10, 12 10.1186/1476-4598-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: A component of turmeric (Curcuma longa). J. Altern. Complementary Med. 2003, 9 (1), 161–168. 10.1089/107555303321223035. [DOI] [PubMed] [Google Scholar]
- Vajragupta O. Manganese complexes of curcumin and its derivatives: Evaluation for the radical scavenging ability and neuroprotective activity. Free Radical Biol. Med. 2003, 35 (12), 1632–1644. 10.1016/j.freeradbiomed.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Mahmood K.; Zia K. M.; Zuber M.; Salman M.; Anjum M. N. Recent developments in curcumin and curcumin based polymeric materials for biomedical applications: A review. Int. J. Biol. Macromol. 2015, 81, 877–890. 10.1016/j.ijbiomac.2015.09.026. [DOI] [PubMed] [Google Scholar]
- Kurnik I. S.; Noronha M. A.; Câmara M. C. C.; Mazzola P. G.; Vicente A. A.; Pereira J. F. B.; Lopes A. M. Separation and purification of curcumin using novel aqueous two-phase micellar systems composed of amphiphilic copolymer and cholinium ionic liquids. Sep. Purif. Technol. 2020, 250, 117262 10.1016/j.seppur.2020.117262. [DOI] [Google Scholar]
- Kaur C. D.; Saraf S. Topical vesicular formulations of Curcuma longa extract on recuperating the ultraviolet radiation-damaged skin: Vesicular herbal formulations improving skin properties. J. Cosmet. Dermatol. 2011, 10 (4), 260–265. 10.1111/j.1473-2165.2011.00586.x. [DOI] [PubMed] [Google Scholar]
- Sandikci Altunatmaz S.; Aksu F. Y.; Issa G.; Kahraman B. B.; Altiner D. D.; Buyukunal S. Antimicrobial effects of curcumin against L. monocytogenes, S. aureus, S. Typhimurium and E. coli O157:H7 pathogens in minced meat. Vet. Med. 2016, 61, 256–262. 10.17221/8880-VETMED. [DOI] [Google Scholar]
- Kuswandi B.; Jayus; Larasati T. S.; Abdullah A.; Heng L. Y. Real-time monitoring of shrimp spoilage using on-package sticker sensor based on natural dye of curcumin. Food Analytical. Methods 2012, 5 (4), 881–889. 10.1007/s12161-011-9326-x. [DOI] [Google Scholar]
- Jung S.; Otberg N.; Thiede G.; Richter H.; Sterry W.; Panzner S.; Lademann J. Innovative liposomes as a transfollicular drug delivery system: Penetration into porcine hair follicles. J. Invest. Dermatol. 2006, 126 (8), 1728–1732. 10.1038/sj.jid.5700323. [DOI] [PubMed] [Google Scholar]
- Li J.; Wang Z.; Yao S.; Song H. Aqueous solubilization and extraction of curcumin enhanced by imidazolium, quaternary ammonium, and tropine ionic liquids, and insight of ionic liquids-curcumin interaction. J. Mol. Liq. 2020, 317, 113906 10.1016/j.molliq.2020.113906. [DOI] [Google Scholar]
- Shen Y.; Farajtabar A.; Xu J.; Wang J.; Xia Y.; Zhao H.; Xu R. Thermodynamic solubility modeling, solvent effect and preferential solvation of curcumin in aqueous co-solvent mixtures of ethanol, n-propanol, isopropanol and propylene glycol. J. Chem. Thermodyn. 2019, 131, 410–419. 10.1016/j.jct.2018.11.022. [DOI] [Google Scholar]
- Nozaki Y.; Yamaguchi K.; Tomida K.; Taniguchi N.; Hara H.; Takikawa Y.; Fukao K.; et al. Phase Transition and Dynamics in Imidazolium-Based Ionic Liquid Crystals through a Metastable Highly Ordered Smectic Phase. J. Phys. Chem. B 2016, 120 (23), 5291–5300. 10.1021/acs.jpcb.6b03804. [DOI] [PubMed] [Google Scholar]
- Klevens H. B. Effect of Electrolytes Upon the Solubilization of Hydrocarbons and Polar Compounds. J. Am. Chem. Soc. 1950, 72 (8), 3780–3785. 10.1021/ja01164a124. [DOI] [Google Scholar]
- Schott H. Solubilization of a water-insoluble dye. II. J. Phys. Chem. A 1967, 71 (11), 3611–3617. 10.1021/j100870a041. [DOI] [Google Scholar]
- Ghatak C.; Rao V. G.; Mandal S.; Ghosh S.; Sarkar N. An Understanding of the Modulation of Photophysical Properties of Curcumin inside a Micelle Formed by an Ionic Liquid: A New Possibility of Tunable Drug Delivery System. J. Phys. Chem. B 2012, 116 (10), 3369–3379. 10.1021/jp211242c. [DOI] [PubMed] [Google Scholar]
- Aboudiab B.; Tehrani-Bagha A. R.; Patra D. Curcumin Degradation Kinetics in Micellar Solutions: Enhanced Stability in the Presence of Cationic Surfactants. Colloids Surf., A 2020, 592, 124602 10.1016/j.colsurfa.2020.124602. [DOI] [Google Scholar]
- Gökdemir B.; Baylan N.; Çehreli S. Application of a Novel Ionic Liquid as an Alternative Green Solvent for the Extraction of Curcumin from Turmeric with Response Surface Methodology: Determination and Optimization Study. Anal. Lett. 2020, 53 (13), 2111–2121. 10.1080/00032719.2020.1730394. [DOI] [Google Scholar]
- Shirsath S. R.; Sable S. S.; Gaikwad S. G.; Gogate P. R. Ultrasound assisted curcumin recovery from Curcuma aromatica: Understanding the effect of different operating parameters. Chem. Eng. Process.- Process Intensif. 2021, 169, 108604 10.1016/j.cep.2021.108604. [DOI] [Google Scholar]
- Vichakshana G. A. D.; Foo S. C.; Choo W. S. Impact of high-pressure homogenization pretreatment on recovery of curcumin from turmeric by different combinations of extraction and drying methods. Innovative Food Sci. Emerging Technol. 2023, 83, 103249 10.1016/j.ifset.2022.103249. [DOI] [Google Scholar]
- Kanda H.; Zhu L.; Zhu W.; Wang T. Ethanol-free extraction of curcumin and antioxidant activity of components from wet Curcuma longa L. by liquefied dimethyl ether. Arabian J. Chem. 2023, 16 (4), 104585 10.1016/j.arabjc.2023.104585. [DOI] [Google Scholar]
- Degot P.; Huber V.; El Maangar A.; Gramüller J.; Rohr L.; Touraud D.; Kunz W.; et al. Triple role of sodium salicylate in solubilization, extraction, and stabilization of curcumin from Curcuma longa. J. Mol. Liq. 2021, 329, 115538 10.1016/j.molliq.2021.115538. [DOI] [Google Scholar]
- Huber V.; Muller L.; Degot P.; Touraud D.; Kunz W. NADES-based surfactant-free microemulsions for solubilization and extraction of curcumin from Curcuma Longa. Food Chem. 2021, 355, 129624 10.1016/j.foodchem.2021.129624. [DOI] [PubMed] [Google Scholar]
- Degot P.; Huber V.; Hofmann E.; Hahn M.; Touraud D.; Kunz W. Solubilization and extraction of curcumin from Curcuma Longa using green, sustainable, and food-approved surfactant-free microemulsions. Food Chem. 2021, 336, 127660 10.1016/j.foodchem.2020.127660. [DOI] [PubMed] [Google Scholar]