Abstract
Objective:
Evaluate the association between prefrailty and the risk of heart failure (HF) among older adults.
Design, setting, and participants:
This prospective, community-based cohort study included participants from the ARIC study who underwent detailed frailty assessment using Fried Criteria and physical function assessment using the Short Performance Physical Battery [SPPB] score. Individuals with prevalent HF and frailty were excluded.
Main outcomes and measures:
Adjusted association between prefrailty (vs. robust) and physical function measures (SPPB score, grip strength, and gait speed) and incident HF (overall and HF subtypes, HF with reduced [HFrEF, EF<50%] and preserved ejection fraction [HFpEF]) were assessed using Cox proportional hazards models.
Results:
Among 5,210 participants (mean age 75 years, 58% women), 2565(49.2%) were identified as prefrail. In cross-sectional analysis, prefrail individuals had higher burden of chronic myocardial injury (troponin, Std β = 0.08[0.05 – 0.11]) and neurohormonal stress (NT-ProBNP, Std β = 0.04[0.02 – 0.05]) after adjustment for potential confounders. Over a median follow-up of 4.6 years, there were 232(4.5%) HF events (HFrEF: 102; HFpEF: 97). Prefrailty was associated with an increased risk of HF after adjusting for potential clinical confounders and cardiac biomarkers [aHR(95% CI) = 1.65(1.24–2.20)]. Among HF subtypes, prefrailty was associated with an increased risk of HFpEF but not HFrEF [aHR(95% CI) = 1.73(1.11–2.70) and 1.38(0.90–2.10), respectively]. A lower SPPB score was also associated with an increased risk of overall HF and HFpEF, but not HFrEF. Among individual components, increased grip strength and gait speed were associated with a decreased risk of HFpEF, but not HFrEF.
Conclusions and relevance:
Subtle abnormalities in physiological reserve (prefrailty) and impairment in physical function (SPPB) were both significantly associated with a higher risk of incident HF, particularly HFpEF. These findings highlight the potential role of routine assessment of geriatrics syndromes for early identification of HF risk.
Keywords: prefrailty, physical function, heart failure, heart failure with preserved ejection fraction
INTRODUCTION
Heart failure with preserved ejection fraction (HFpEF) is the most common phenotype of heart failure (HF) encountered in older individuals. HFpEF is characterized by exercise intolerance and often clinical volume overload despite preserved left ventricular ejection fraction.1–3 In addition to a high risk of mortality and hospitalization, HFpEF is also associated with functional impairment and poor quality-of-life.4, 5 Unlike heart failure with reduced ejection fraction (HFrEF), HFpEF continues to be refractory to available medical therapies highlighting the need for novel approaches to its prevention.6–8
An important first step to prevention is identifying intermediate phenotypes that may underlie the progression from at-risk stage to clinical HFpEF. Frailty is a state of reduced physiologic reserve with increased vulnerability and poor resolution of homeostasis following stress that predisposes individuals to an increased risk of adverse outcomes.9 Prior studies have identified key similarities in the pathophysiology of HFpEF and frailty and implicated frailty as an important factor in the development and progression of HFpEF.10–12 The transition from a robust state to frailty is often encountered among older individuals and is most likely occurs through the subclinical accumulation of several metabolic and physiologic impairments.13–15 Prefrailty, an intermediate stage that precedes frailty, represents an early stage of physiologic impairment that may be potentially reversible with effective multimodality interventions.16–19 The association of pre-frailty and other measures of functional impairments with the risk of HF and its subtypes, HFpEF and HFrEF, among community-dwelling adults is not well-established. A better understanding of the contribution of geriatric syndromes such as pre-frailty and frailty to development of HFpEF may facilitate primary care physicians and geriatricians to identify these older high-risk patients and target them with preventive strategies aimed at reducing progression to frailty and downstream HF.4, 20, 21 Accordingly, in this study, we evaluated the association of the presence of prefrailty and other objective measures of functional impairment with the risk of incident HF and its subtypes among community-dwelling older adults.
METHODS
Study Population
This study used deidentified, publicly available data from the Atherosclerotic Risk in Communities (ARIC) study obtained from the National Institute of Health Biologic Specimen and Data Repository Coordinating Center (BioLINCC). Details of the ARIC study have been previously described.22 In brief, ARIC is a community-based cohort study that enrolled 15,792 participants in 1987–1989 from four US communities (Jackson, MI; Forsyth County, NC; Washington County, MD; and selected suburbs of Minneapolis, MN). For the present analysis, participants from visit 5, conducted between 2011–2013, where participants underwent detailed assessment of frailty and functional impairment, were used as baseline (N = 5953). While 6538 participants attended visit 5, 585 did not consent to data release in the BioLINCC cohort. As the present study focused on the associations of prefrailty and risk of HF outcomes, we further excluded participants with baseline frailty (N = 335), HF (N = 400), or unknown CVD or HF status (N = 8) at the time of visit 5 (baseline). The final study population included 5210 participants (Supplemental Figure 1). All ARIC participants gave their written and informed consent, and the study was approved by the institutional review of the coordinating center.
Outcomes of Interest
Our primary outcome of interest was physician adjudicated incident HF hospitalization event. A detailed description of HF event adjudication is described in the Supplemental Materials. Briefly, potential HF hospitalization events were ascertained through either annual cohort follow-up, death registries, or hospital surveillance and adjudicated by physician reviewers as previously described.23, 24 Among HF subtypes, HFpEF was defined by incident HF hospitalization event with LVEF ≥50%.25 HFrEF was identified by LVEF < 50% at the time of HF hospitalization. All-cause mortality events were confirmed using the National Death Index.26 All events were adjudicated by the event adjudication committee.
Frailty and Functional Status Assessment
The frailty phenotype was defined using the Cardiovascular Health Study components as previously described by Fried et al.21, 27 Briefly, frailty phenotype was identified using five criteria as detailed in Supplemental Table 1 and Supplemental Materials. Individuals with three or more components were categorized as frail, one or two defined as prefrail, and none defined as robust. As this study excluded participants classified as frail, only participants categorized as prefrail or robust were included. The Short Physical Performance Battery (SPPB) score, a standardized objective screening tool for primary lower-extremity functional impairment, was also calculated for each individual.28 SPPB comprises three components: standing balance, timed repeated chair rise, and gait speed. Each component is scaled and scored from 0 to 4 based on specific cut points (Supplemental Table 2). The components are then summed for a total score ranging from 0 to 12.
Clinical Covariates and Cardiac Biomarkers
Demographic, anthropometric, laboratory, and clinical characteristics were assessed at the time of the fifth visit. Criteria to identify comorbidities such as hypertension, HF, CVD, diabetes are detailed in the Supplemental Materials. High-sensitivity cardiac troponin-T (hs-cTnT) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) concentrations were measured as previously described using standard assays and described in the Supplemental Materials.29 As all covariates had < 10% missingness, missing data were imputed using random forest imputation.30
Statistical Analysis
Baseline characteristics were compared across categories of frailty. Differences across groups were assessed using one-way ANOVA for continuous and χ2-test for categorical variables. Exposure variables of interest for this analysis were prefrailty (vs. robust), SBBP score, grip strength, and gait speed. Cross-sectional associations between exposure variables of interest and levels of hs-cTnT and NT-proBNP were assessed using multivariable linear regression models. Owing to the skewed distribution, hs-cTnT and NT-proBNP levels were log-transformed for the regression analysis. Separate models were constructed for each exposure variable and the biomarker outcome of interest with adjustment for the following covariates (Model 1): age, sex, race, education level, income, systolic BP, body mass index, hypertension, smoking status, cardiovascular disease, diabetes status, statin medication, eGFR, HDL-c, and HbA1c levels.
Unadjusted cumulative incidence of overall HF and HF subtypes were assessed across frailty groups using time-to-event analysis with the log-rank test. Multivariable Cox proportional hazard were used to evaluate the association of frailty measures and risk of incident HF. Separate models were constructed for each exposure variable of interest described above with adjustment for the following potential confounders based on biological plausibility and prior literature: Model 1: as above; Model 2: Model 1 + hs-cTnT + NT-proBNP. Similar adjusted Cox models were also constructed to evaluate the association of prefrailty and functional status measures with risk HFpEF and HFrEF separately with all-cause mortality and the other HF subtype treated as a censoring event. Multivariable adjusted restricted cubic splines were constructed to evaluate the association of continuous SPPB score with risk of HF subtypes. Multivariable Cox models were also used to assess the risk of incident HF across increasing Fried scores (0 vs. 1 vs. 2) with the same adjustments as the primary analysis. To account for competing risks (death and other HF subtype), multivariable Fine-Gray proportional subdistribution hazards models were fitted with the same adjustments as the primary analysis.31 Sensitivity analyses were performed to address the potential for reverse causation in the observed association between HF and frailty by 1) excluding participants with a prior history of CVD, 2) excluding participants with an HF event within 12 months of baseline, and 3) stratifying participants with NT-proBNP ≥ 125pg/mL. Finally, we assessed the improvement in C-index and continuous net reclassification index (NRI) between the ARIC HF biomarkers risk score with and without the exposure variable (either prefrailty, SPPB score, grip strength, or 4M walk time). Improvement in C-index was assessed using the DeLong’s test.29, 32 Analyses were performed using R version 3.6.3 (R Foundation, Vienna, Austria) with P < 0.05 indicating significance.
RESULTS
The study included 5210 participants (mean age 75 ± 5 years, 58% women, 20% African American), of which 2565 (49.2%) were identified as prefrail. Compared to robust participants, prefrail individuals were more likely to be older, women, and of the self-identified Black race. Prefrail individuals also had a significantly higher prevalence of traditional CV risk factors such as hypertension, diabetes, and hyperlipidemia. Prefrail individuals also had a higher burden of chronic myocardial injury, as assessed by hs-cTn levels, and neurohormonal stress, as set by the NT-ProBNP levels, as compared with the robust individuals. Among measures of functional impairment, pre-frail individuals had lower SPPB scores, worse grip strength, and slower 4M walk time
Over a median follow-up of 4.6 (interquartile range: 4.1–5.1) years from visit 5, the primary outcome of incident HF occurred in 232 (4.5%) participants, of which 102 (44.0%) were HFrEF, 97 (41.8%) were HFpEF, and 33 were undetermined.
Prefrailty, measures of physical function, and biomarkers of chronic myocardial injury and neurohormonal stress
We evaluated the association of frailty categorization, SPPB score, grip strength, and gait speed (4m walk time) and levels of biomarkers of chronic myocardial injury (hs-TnT) and neurohormonal stress (NT-ProBNP). Compared to robust individuals, prefrail (vs. robust) individuals had higher levels of hs-cTnT (Std β = 0.08[0.05–0.10], P< 0.001) and NT-ProBNP (Std β = 0.03[0.02–0.05], P< 0.001) after adjustment for potential confounders (Supplemental Table 3). Similarly, lower SPPB score, indicating worse functional status, was also significantly associated with higher hs-cTn (Std β per 1-unit lower SPPB = 0.04[0.04–0.05], P< 0.001) and NT-proBNP (Std β per 1-unit lower SPPB = 0.01[0.01–0.02], P< 0.001) levels. Among individual components of physical function, lower grip strength and slower 4M walk time were also significantly associated with higher levels of hs-cTnT and NT-proBNP (Supplemental Table 3).
Prefrailty and risk of incident HF and its subtypes
In unadjusted analysis, the cumulative incidence of HF was higher among pre-frail vs. robust individuals (8.3% vs. 3.3%, log-rank p<0.001, Supplemental Figure 2). In adjusted analyses, prefrail (vs. robust) individuals had a significantly higher risk of incident HF that remained significant after further adjustments for measures of biomarkers of chronic myocardial injury (hs-TnT) and neurohormonal stress (NT-ProBNP) [HR (95% CI) = 1.65(1.24–2.20); model 2, Table 2]. A graded increase in HF risk was observed for increasing Fried scores from 2.7% with scores of 0 to 6.6% with scores of 2 (Supplemental Figure 3). In most adjusted analyses, a 1-unit increase in Fried score was associated with 33% higher risk of HF [HR (95% CI) = 1.33(1.01–1.74); model 2; Supplemental Table 4].
Table 2.
Multivariable adjusted associations of pre-frailty status and physical function measures and risk of incident heart failure.
| Covariate | Model 1 | Model 2 | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Prefrailty (ref: robust) | 1.73 (1.30, 2.31) | <0.001 | 1.65 (1.24, 2.20) | <0.001 |
| SPPB (per 1-unit decrease) | 1.12 (1.06, 1.18) | <0.001 | 1.11 (1.05, 1.16) | <0.001 |
| Grip strength (per 1SD decrease) | 1.33 (1.10, 1.60) | 0.002 | 1.26 (1.04, 1.53) | 0.02 |
| 4M Walk time (per 1SD increase) | 1.14 (1.03, 1.25) | 0.005 | 1.12 (1.01, 1.23) | 0.03 |
Baseline covariates = age, sex, race, education level, income, systolic blood pressure, body mass index, hypertension, smoking status, cardiovascular disease, diabetes status, statin medication, eGFR, HDL-c, and HbA1c levels.
Model 1 = baseline covariates + exposure variable of interest (grip strength, 4M walk time, prefrailty, or SPPB score each in a separate model)
Model 2 = baseline covariates + hs-cTn + NT-proBNP + exposure variable of interest (grip strength, 4M walk time, prefrailty, or SPPB score each in a separate model)
Abbreviations:
CI, confidence interval; HF, heart failure; HR, hazard ratio; hs-cTn, high-sensitivity cardiac troponin; NT-proBNP, N-terminal pro-hormone B-type natriuretic peptide; SD, standard deviation; SPPB, Short Physical Performance Battery
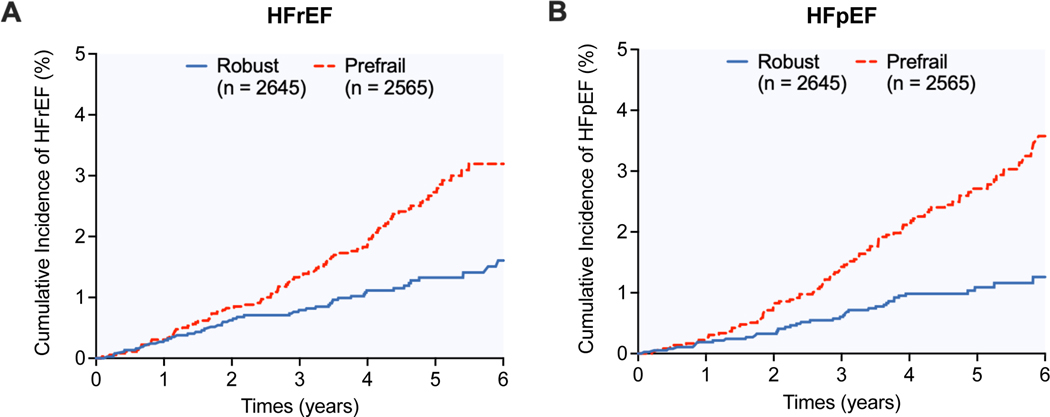
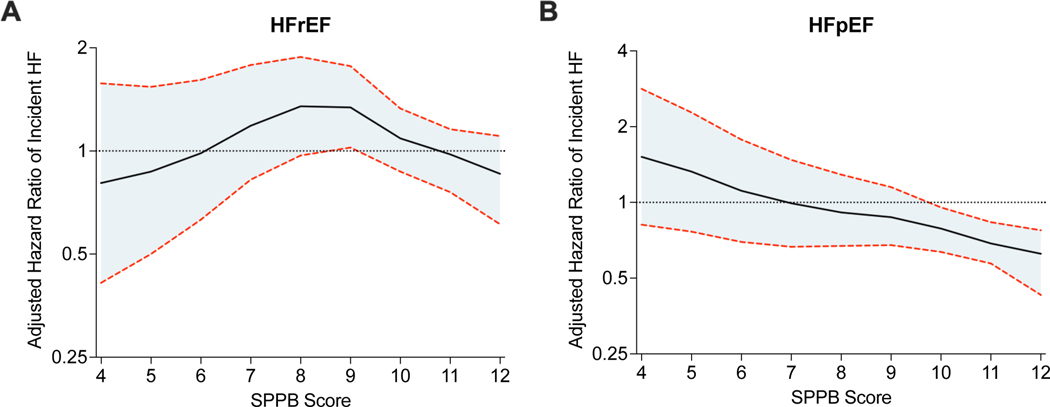
Among HF subtypes, prefrail individuals had a higher cumulative incidence of HFpEF and HFrEF events (log-rank p<0.001 for both, Figure 1A and 1B). In adjusted analysis, prefrail (vs. robust) individuals had a significantly higher risk of HFpEF in the most adjusted model [HR (95% CI): 1.73(1.11–2.70); model 2, Table 3 and Figure 2A]. In contrast, prefrailty was significantly associated with the risk of HFrEF in partially (model 1) adjusted model but this association was attenuated and not significant in the most adjusted model (model 2) (Figure 2B). Consistent patterns of associations were also observed in a sensitivity analysis excluding participants with a prior history of CVD, excluding individuals with an incident HF event within 12 months, or in accounting for the competing risk of death and other HF subtype (Supplemental Tables 5–7). In stratified analysis by NT-ProBNP levels, individuals in the higher NT-proBNP strata (≥ 125pg/mL) had a significantly higher risk of overall HF, HFpEF, and HFrEF (Supplemental Table 8). However, the association between prefrail (vs. robust) status and higher risk of HF, HFpEF, and HFpEF was consistent across both high and low NT-ProBNP level strata (Supplemental Figure 4, Supplemental Table 9).
Figure 1.
Cumulative incidence of (A) heart failure with reduced ejection fraction and (B) heart failure with preserved ejection fraction among robust vs. prefail individuals
Abbreviations: HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction
Table 3.
Multivariable adjusted associations of pre-frailty status and physical function measures and risk of incident heart failure subtypes: heart failure with preserved ejection fraction (HFpEF) and heart failure with reduced ejection fraction (HFrEF).
| HFrEF (N = 102 events) | HFpEF (N = 97 events) | |||||||
|---|---|---|---|---|---|---|---|---|
| Covariate | Model 1 | Model 2 | Model 1 | Model 2 | ||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Prefrailty (ref: robust) | 1.45 (0.95, 2.21) | 0.09 | 1.38 (0.90, 2.10) | 0.14 | 1.81 (1.16, 2.83) | 0.009 | 1.73 (1.11, 2.70) | 0.02 |
| SPPB (per 1-unit decrease) | 1.09 (1.01, 1.18) | 0.03 | 1.08 (0.99, 1.70) | 0.07 | 1.13 (1.04, 1.22) | 0.003 | 1.12 (1.03, 1.21) | 0.006 |
| Grip strength (per 1SD decrease) | 1.39 (1.05, 1.83) | 0.02 | 1.32 (0.99, 1.74) | 0.06 | 1.37 (1.01, 1.85) | 0.04 | 1.32 (0.97, 1.79) | 0.08 |
| 4M Walk time (per 1SD increase) | 1.04 (0.86, 1.25) | 0.68 | 1.01 (0.83, 1.23) | 0.91 | 1.20 (1.05, 1.38) | 0.008 | 1.19 (1.04, 1.36) | 0.01 |
Baseline covariates = age, sex, race, education level, income, systolic blood pressure, body mass index, hypertension, smoking status, cardiovascular disease, diabetes status, statin medication, eGFR, HDL-c, and HbA1c levels.
Model 1 = baseline covariates + exposure variable of interest (grip strength, 4M walk time, prefrailty, or SPPB score each in a separate model)
Model 2 = baseline covariates + hs-cTn + NT-proBNP + exposure variable of interest (grip strength, 4M walk time, prefrailty, or SPPB score each in a separate model)
Abbreviations:
CI, confidence interval; HR, hazard ratio; hs-cTn, high-sensitivity cardiac troponin; NT-proBNP, N-terminal pro-hormone B-type natriuretic peptide; SD, standard deviation; SPPB, Short Physical Performance Battery
Figure 2.
Continuous association between SPPB score and risk of (A) heart failure with reduced ejection fraction and (B) heart failure with preserved ejection fraction. Restricted cubic spline showing the continuous adjusted association between SPPB score and risk of incident HF. The shaded area shows the 95% confidence interval. The model was adjusted for age, sex, race, education level, income, systolic blood pressure, body mass index, hypertension, smoking status, cardiovascular disease, diabetes status, statin medication, estimated glomerular filtration rate, high density lipoprotein-cholesterol, hemoglobin A1c, high-sensitivity troponin, and natriuretic peptide levels.
Abbreviations: HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; SPPB, Short Physical Performance Battery
Measures of physical function and risk of incident HF and its subtypes
As assessed by SPPB, worse physical function was significantly associated with a higher risk of HF after adjustment for potential confounders. Specifically, a 1-unit lower SPPB score was associated with a 10% higher risk of HF in the most adjusted model [HR (95% CI) = 1.11(1.05–1.16); model 2, Table 2]. Similarly, both lower grip strength and slower 4m walk time were also significantly associated with a higher risk of HF that remained significant after further adjustments for cardiac biomarkers [HR (95% CI) per 1-standard deviation (SD) = 1.26(1.04–1.53) and 1.12(1.01–1.23), respectively; model 2, Table 2].
Among HF subtypes, lower SPPB was significantly associated with risk of HFpEF but not HFrEF in the most adjusted analysis [HFpEF: HR (95% CI) per 1-unit lower SPPB = 1.12(1.03–1.21); HFrEF: HR (95% CI) per 1-unit lower SPPB = 1.08(0.99–1.70); model 2, Table 3]. Similar patterns of associations were noted between individual measures of physical function (grip strength and 4m walk time) and risk of HF subtypes (Table 3). A similar pattern of association was observed in multiple sensitivity analyses (Supplemental Tables 5–7,9).
Prognostic utility of pre-frailty and measures of functional status
The C-index for predicting incident HF risk using the ARIC HF biomarker risk score was 0.76. When prefrailty status was added to the risk score, there was a significant improvement in model discrimination (C-index = 0.79, P by DeLong test = 0.004) (Supplemental Table 10). There was a trend towards significance with the addition of SPPB score (C-index = 0.78, P by DeLong test = 0.06) to the ARIC HF risk model. The addition of both prefrailty status and SPPB score also resulted in a significant increase in continuous NRI (Supplemental Table 10). Conversely, there was no improvement in model performance with the addition of grip strength or gait speed (P by DeLong test = 0.16 and 0.09, respectively).
DISCUSSION
In this community-based cohort study, we observed several significant findings. First, impairment in physiological reserve, as identified by the presence of prefrailty, was common and prevalent in up to 50% of individuals. Second, prefrailty was significantly associated with a higher burden of chronic myocardial injury, neurohormonal stress, and a higher risk of incident HF hospitalization. Third, among HF subtypes, the presence of prefrailty categorization was more strongly and consistently associated with risk of HFpEF but not HFrEF hospitalization after adjustment for potential confounders. Furthermore, the higher risk of HFpEF hospitalization among prefrail individuals was independent of the burden of chronic myocardial injury and stress. Fourth, among physical function measures, grip strength, gait speed, and SPPB were each significantly associated with a higher risk of HFpEF hospitalization but not HFrEF. Finally, the physiological reserve measures, when added to the well-established HF risk scores, significantly improved HF risk prediction. Our study findings suggest that early and modest impairment in physiologic reserve and physical function may predispose older individuals to a higher risk of HF hospitalization, particularly HFpEF in older adults.
Frailty, prefrailty, and risk of adverse cardiovascular outcomes
Frailty is a state of increased vulnerability to endogenous and exogenous stress factors, resulting from decreased physiological reserves and dysfunction and dysregulation of multiple systems, which interfere with homeostasis and response to stress.21, 33 Importantly, it is a continuum characterized by an earlier potentially reversible state of prefrailty.20 Among non-fatal CV outcomes, previous studies have demonstrated a significant association between the presence of frailty and risk of HF and ASCVD.13, 14, 27 However, frailty represents an advanced stage of impairment in physiologic reserve, and it remains unclear if it can be modified or reversed with therapeutic interventions. In contrast, prefrailty is an earlier potentially reversible stage of decline in physiologic reserve. Prior studies have demonstrated a delay in the functional decline among pre-frail individuals with effective therapies such as exercise training.34, 35 Studies evaluating the association between prefrailty and risk of HF and its subtypes are lacking. This is particularly relevant considering the growing burden of frailty and HF among older adults. In the Health ABC cohort, Khan et al. provided evidence that frailty is independently associated with an increased risk of incident HF.36 Similarly, in a study from the Pro-VA cohort, Sergi et al. demonstrated the association between prefrailty and an increased risk of CVD.37 Our study findings have extended these observations by evaluating the association between prefrailty and risk of HF subtypes observing a more consistent and stronger risk for HFpEF than HFrEF among prefrail individuals.
Contribution of prefrailty to HFpEF
We observed that the risk of HFpEF associated with prefrailty and functional impairment was independent of traditional CV risk factors and markers of chronic myocardial injury and stress. These observations highlight the systemic nature of HFpEF and the potential contribution of non-cardiac pathways towards its development. An increase in inflammatory markers originating in the fatty tissue in the setting of multi-morbidity, physical inactivity, and aging leads to loss of capillarity, sarcopenia, mitochondrial dysfunction, and endothelial dysfunction. This high-stress environment can also lead to multi-organ dysfunction, frailty, and cardiac and skeletal muscle myopathy and collectively contributes to the development and clinical manifestation of HFpEF with a high burden of functional impairment, poor quality-of-life, and increased risk of hospitalization and mortality.38–40
Contribution of impairment in physical function to HFpEF
Similar to prefrailty, we also observed a significant association between individual and composite physical function measures, such as grip strength, gait speed, SPPB score, and risk of HFpEF. In contrast, impairment in these physical function measures was not associated with the risk of HFrEF after adjustment for potential confounders. Previous studies have evaluated the association of SPPB and mortality and shown SPPB score is an independent predictor of all-cause mortality among patients discharged from acute care hospitals.41 Further studies by Bellettiere et al. reported strong linear inverse associations between SPPB and incident CVD.42 In the Health ABC cohort, worse performance on a physical function assessment battery was associated with a higher risk for HF.36 Prior analyses have also demonstrated grip strength and gait speed as an independent predictor of adverse cardiovascular outcomes, including the risk of HF.43, 44 In UK Biobank data, higher grip strength was associated with a lower incidence of HF risk and a similar association was found for gait speed in the Pro-VA study.37, 45 Our study findings add to the existing literature by demonstrating the unique contribution of impairment in physical function toward the development of HFpEF.
Clinical implications
Our study findings have important clinical implications. HFpEF is increasing in prevalence among older adults and is often managed in the outpatient setting by primary care physicians and geriatricians.4 Early identification and implementation of multimodality intervention strategies that modify the progression of prefrailty to frailty or lower the risk of HF, such as exercise training or improved physical activity, may be key to stemming the rising burden of HFpEF among older adults.39, 47 Findings from our study suggest that early decline in physiologic reserve, as identified by prefrailty, and impairment in physical function may be important and independent contributors to the development of HFpEF in older adults. Similarly, we observed that the addition of physiologic reserve measures, as identified by prefrailty, significantly improves the performance of a well-established HF risk prediction model. These findings suggest that routine assessment of physical function and frailty assessment among older individuals by the primary care providers and geriatricians may facilitate early identification of individuals at high risk of developing HFpEF. Once identified, these individuals could be targeted to improve physical function, exercise endurance, and cardiovascular reserve. Future studies are needed to determine if such interventions may modify the risk of HF hospitalizations in older, prefrail individuals.
Limitations
Our study is not without limitations. First, consistent with the study’s observational nature, our findings may be subject to selection bias and unmeasured confounding. However, our study included over 90% of the HF free participants from the ARIC visit 5, and we adjusted for several biologically plausible confounders, including measures of cardiac biomarkers. Second, limitations to the frailty instrument used in ARIC have been reported previously, including lack of “unintentional” weight loss assessment based on the estimated 10% weight loss between visit 4 and 5, and use of Becke’s questionnaire for physical activity assessment, unlike prior frailty instruments developed in Women’s Health Initiative and Cardiovascular Health Study.27 Third, owing to the lack of availability of data on dietary habits, we were unable to adjust for potential dietary risk factors such as salt intake in the models evaluating the association of pre-frailty/functional status and risk of HF. Fourth, the low number of HFrEF and HFpEF may provide imprecision around the hazard ratio estimates. However, additional competing risk analysis using Fine-Gray subdistribution hazard models showed a similar significant association between prefrailty and risk of incident HFpEF but not HFrEF events. Fourth, there is a potential for reverse causation in the observed association between pre-frailty/low SPPB score and high risk of HF. It is plausible that subclinical HF may contribute to poor functional status and pre-frailty at baseline before diagnosis of clinical HF. However, a consistent pattern of significant association between pre-frailty, functional impairment, and risk of HF, particularly HFpEF in in sensitivity analysis excluding individuals with CVD, landmarking at 12 months, and across strata of individuals with low vs. high NT-ProBNP suggest that the observed associations are not completely related to reverse causation. Furthermore, the reverse causation and unmeasured confounding would be expected to confound the prefrailty association with HF subtypes. The consistent independent association of prefrailty and other physical function measures with the risk of HFpEF but not HFrEF hospitalization highlights the robustness of our study findings. Finally, we did not adjust for multiple comparisons in our study, and the empirical findings should be considered hypothesis-generating and need to be validated in other independent cohort studies.
Conclusion
In conclusion, among older participants in the ARIC community-based study, we observed that subtle abnormalities in physiological reserve, as identified by the presence of prefrailty, and impairment in physical function, as assessed by SPPB, were both significantly associated with a higher risk of incident HF, particularly HFpEF. These findings highlight the potential role of routine assessment for geriatric syndromes, such as frailty and functional impairment, as an effective strategy for early identification of older individuals who may be at an increased risk for HFpEF. Future trials are needed to test if physical rehabilitation interventions to reverse prefrailty and improve physical function can decrease the risk of HF, especially HFpEF.
Supplementary Material
Supplemental Figure 1: CONSORT diagram
Supplemental Figure 2: Cumulative incident of HF across frailty measures
Supplemental Figure 3: Rates of HF by Fried criteria
Supplemental Figure 4: Rates of HF by natriuretic peptide levels and HF subtypes
Supplemental Methods: Outcomes and clinical covariates
Supplemental Table 1: Frailty phenotype criteria
Supplemental Table 2: SPPB score criteria
Supplemental Table 3: Association of cardiac stress with frailty measures
Supplemental Table 4: Association of Fried scores and incident HF
Supplemental Table 5: Subgroup analyses excluding participants with CVD and HF within 12 months
Supplemental Table 6: Subgroup analyses across HF subtypes
Supplemental Table 7: Competing risks analysis across HF subtypes
Supplemental Table 8: Event rates across natriuretic peptide level strata
Supplemental Table 9: Association of frailty with HF across natriuretic peptide strata
Supplemental Table 10: Predictive performance of frailty measures
Table 1.
Baseline characteristics of participants stratified by robust vs. prefrail status
| Robust (N = 2645) | Prefrail (N = 2565) | P-value | |
|---|---|---|---|
| Age, years | 74.5 (4.7) | 76.5 (5.3) | <0.001 |
| Male, % | 1195 (45.2) | 1012 (39.5) | <0.001 |
| White race, % | 2157 (81.6) | 1997 (77.9) | 0.001 |
| SBP, mmHg | 130.3 (17.6) | 130.6 (18.3) | 0.48 |
| DBP, mmHg | 67.6 (10.4) | 65.5 (10.8) | <0.001 |
| HR, bpm | 64.5 (10.5) | 65.6 (11.3) | <0.001 |
| BMI, kg/m2 | 28.4 (5.1) | 28.8 (5.9) | 0.007 |
| Current smoker, % | 131 (5.3) | 156 (6.4) | 0.13 |
| Alcohol use | <0.001 | ||
| Current | 1421 (57.7) | 1130 (46.2) | |
| Former | 610 (24.8) | 744 (30.4) | |
| Never | 432 (17.5) | 574 (23.4) | |
| Education level, % | <0.001 | ||
| High school or less | 264 (10.0) | 380 (14.8) | |
| Some college | 1326 (50.1) | 1057 (41.2) | |
| College graduate | 1055 (39.9) | 1128 (44.0) | |
| Household income, $ | 50774 (28313) | 42634 (26915) | <0.001 |
| Cardiovascular disease, % | 291 (11.2) | 362 (14.4) | <0.001 |
| Diabetes, % | 565 (22.1) | 816 (32.7) | <0.001 |
| Diabetes medication use, % | 383 (14.6) | 594 (23.3) | <0.001 |
| Hypertension, % | 1653 (63.2) | 1745 (69.3) | <0.001 |
| Anemia*, % | 232 (9.4) | 370 (14.9) | <0.001 |
| COPD, % | 97 (3.7) | 177 (6.9) | <0.001 |
| CKD†, % | 620 (23.4) | 756 (29.5) | <0.001 |
| Cancer, % | 79 (3.0) | 94 (3.7) | 0.20 |
| Mild cognitive impairment, % | 447 (16.9) | 601 (23.4) | <0.001 |
| Dementia, % | 57 (2.2) | 143 (5.6) | <0.001 |
| Hypertension medication use, % | 1634 (62.5) | 1732 (68.7) | <0.001 |
| Statin use, % | 1355 (51.5) | 1358 (53.2) | 0.22 |
| HbA1c, % | 5.9 (0.7) | 6.0 (0.9) | <0.001 |
| eGFR, mL/min/1.73 m2 | 71.4 (15.7) | 69.1 (17.6) | <0.001 |
| HDL-c, mg/dL | 52.4 (13.6) | 52.0 (14.2) | 0.34 |
| Triglycerides, mg/dL | 126.2 (60.9) | 126.8 (66.9) | 0.75 |
| LDL-c, mg/dL | 106.4 (33.5) | 102.7 (35.4) | <0.001 |
| NT-proBNP, pg/mL | 204.9 (628.1) | 295.4 (770.5) | <0.001 |
| hs-TnT, mcg/L | 12.1 (9.9) | 14.0 (10.8) | <0.001 |
| Fat mass, kg | 27.8 (10.1) | 27.8 (11.6) | 0.94 |
| Lean body mass, kg | 51.6 (11.2) | 50.2 (10.7) | <0.001 |
Defined as hemoglobin < 13.0 g/dL for men or 11.2 g/dL for women
Defined as eGFR < 60 mL/min/1.73m2
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; hs-cTnT, high-sensitivity cardiac troponin T; HR, heart rate; NT-proBNP, N-terminal pro-B-type natriuretic peptide; SBP, systolic blood pressure
Key Points.
Among older adults, impairments in physiological reserve (prefrailty) and physical function (SPPB score) are significantly associated with a higher risk of incident heart failure.
Prefrailty and impaired physical function are associated with an increased risk of HFpEF, but not HFrEF.
Why Does This Paper Matter?
Prefrailty may be implicated in the development of heart failure, especially HFpEF, in older adults.
Acknowledgments
Sponsor’s Role
No funding source or sponsor had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript, and decision to submit the manuscript for publication.
Funding and Disclosures
Dr. Pandey has served on the advisory board of Roche Diagnostics and is supported Texas Health Resources Clinical Scholarship, Gilead Sciences Research Scholar Program, and the National Institute of Aging GEMSSTAR Grant (1R03AG067960-01).
Dr. Maurer is supported by a K24AG036778 award from NIA.
Dr. Goyal is supported by K76AG064428 from NIA and 20CDA35310455 from AHA
Dr. Hummel is supported by NIH grants R01AG062582 and R01HL139813, and CARA-009-16F9050.
Dr. Forman is funded by NIH grants R01AG060499, R01AG058883, R01AG051376, and P30AG024827.
Dr. Butler has been a Consultant to Abbott, Adrenomed, Arena Pharma, Array, Amgen, Applied Therapeutics, Astra Zeneca, Bayer, Boehringer Ingelheim, Cardior, CVRx, Eli Lilly, G3 Pharma, Imbria, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, Roche, Sequana Medical, V-Wave Limited, and Vifor.
The remaining authors have nothing to disclose.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- [1].Shah SJ, Borlaug BA, Kitzman DW, et al. Research Priorities for Heart Failure With Preserved Ejection Fraction: National Heart, Lung, and Blood Institute Working Group Summary. Circulation. 2020;141: 1001–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Shah SJ, Kitzman DW, Borlaug BA, et al. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation. 2016;134: 73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kitzman DW, Gardin JM, Gottdiener JS, et al. Importance of heart failure with preserved systolic function in patients > or = 65 years of age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol. 2001;87: 413–419. [DOI] [PubMed] [Google Scholar]
- [4].Upadhya B, Kitzman DW. Heart failure with preserved ejection fraction: New approaches to diagnosis and management. Clin Cardiol. 2020;43: 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Upadhya B, Pisani B, Kitzman DW. Evolution of a Geriatric Syndrome: Pathophysiology and Treatment of Heart Failure with Preserved Ejection Fraction. J Am Geriatr Soc. 2017;65: 2431–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Del Buono MG, Iannaccone G, Scacciavillani R, et al. Heart failure with preserved ejection fraction diagnosis and treatment: An updated review of the evidence. Prog Cardiovasc Dis. 2020. [DOI] [PubMed] [Google Scholar]
- [7].Parikh KS, Sharma K, Fiuzat M, et al. Heart Failure With Preserved Ejection Fraction Expert Panel Report: Current Controversies and Implications for Clinical Trials. JACC Heart Fail. 2018;6: 619–632. [DOI] [PubMed] [Google Scholar]
- [8].Upadhya B, Haykowsky MJ, Kitzman DW. Therapy for heart failure with preserved ejection fraction: current status, unique challenges, and future directions. Heart Fail Rev. 2018;23: 609–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381: 752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pandey A, Kitzman D, Reeves G. Frailty Is Intertwined With Heart Failure: Mechanisms, Prevalence, Prognosis, Assessment, and Management. JACC Heart Fail. 2019;7: 1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kinugasa Y, Yamamoto K. The challenge of frailty and sarcopenia in heart failure with preserved ejection fraction. Heart. 2017;103: 184–189. [DOI] [PubMed] [Google Scholar]
- [12].Woo J, Yang X, Tin Lui L, et al. Utility of the FRAIL Questionnaire in Detecting Heart Failure with Preserved Ejection Fraction. J Nutr Health Aging. 2019;23: 373–377. [DOI] [PubMed] [Google Scholar]
- [13].Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol. 2014;63: 747–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Afilalo J, Karunananthan S, Eisenberg MJ, Alexander KP, Bergman H. Role of frailty in patients with cardiovascular disease. Am J Cardiol. 2009;103: 1616–1621. [DOI] [PubMed] [Google Scholar]
- [15].Walston J, Bandeen-Roche K, Buta B, et al. Moving Frailty Toward Clinical Practice: NIA Intramural Frailty Science Symposium Summary. J Am Geriatr Soc. 2019;67: 1559–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Binder EF, Schechtman KB, Ehsani AA, et al. Effects of exercise training on frailty in community-dwelling older adults: results of a randomized, controlled trial. J Am Geriatr Soc. 2002;50: 1921–1928. [DOI] [PubMed] [Google Scholar]
- [17].Serra-Prat M, Sist X, Domenich R, et al. Effectiveness of an intervention to prevent frailty in pre-frail community-dwelling older people consulting in primary care: a randomised controlled trial. Age Ageing. 2017;46: 401–407. [DOI] [PubMed] [Google Scholar]
- [18].Watanabe Y, Yamada Y, Yoshida T, et al. Comprehensive geriatric intervention in community-dwelling older adults: a cluster-randomized controlled trial. J Cachexia Sarcopenia Muscle. 2020;11: 26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yu R, Tong C, Ho F, Woo J. Effects of a Multicomponent Frailty Prevention Program in Prefrail Community-Dwelling Older Persons: A Randomized Controlled Trial. J Am Med Dir Assoc. 2020;21: 294 e291–294 e210. [DOI] [PubMed] [Google Scholar]
- [20].Lang PO, Michel JP, Zekry D. Frailty syndrome: a transitional state in a dynamic process. Gerontology. 2009;55: 539–549. [DOI] [PubMed] [Google Scholar]
- [21].Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56: M146–156. [DOI] [PubMed] [Google Scholar]
- [22].Investigators ARIC. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129: 687–702. [PubMed] [Google Scholar]
- [23].Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol. 2008;101: 1016–1022. [DOI] [PubMed] [Google Scholar]
- [24].Rosamond WD, Chang PP, Baggett C, et al. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5: 152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Butler J, Anker SD, Packer M. Redefining Heart Failure With a Reduced Ejection Fraction. JAMA. 2019. [DOI] [PubMed] [Google Scholar]
- [26].White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49: 223–233. [DOI] [PubMed] [Google Scholar]
- [27].Kucharska-Newton AM, Palta P, Burgard S, et al. Operationalizing Frailty in the Atherosclerosis Risk in Communities Study Cohort. J Gerontol A Biol Sci Med Sci. 2017;72: 382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. The New England journal of medicine. 1995;332: 556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Nambi V, Liu X, Chambless LE, et al. Troponin T and N-terminal pro-B-type natriuretic peptide: a biomarker approach to predict heart failure risk--the atherosclerosis risk in communities study. Clin Chem. 2013;59: 1802–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Stekhoven DJ, Buhlmann P. MissForest--non-parametric missing value imputation for mixed-type data. Bioinformatics. 2012;28: 112–118. [DOI] [PubMed] [Google Scholar]
- [31].Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94: 496–509. [Google Scholar]
- [32].DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44: 837–845. [PubMed] [Google Scholar]
- [33].Warraich HJ, Kitzman DW, Whellan DJ, et al. Physical Function, Frailty, Cognition, Depression, and Quality of Life in Hospitalized Adults >/=60 Years With Acute Decompensated Heart Failure With Preserved Versus Reduced Ejection Fraction. Circ Heart Fail. 2018;11: e005254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Apóstolo J, Cooke R, Bobrowicz-Campos E, et al. Effectiveness of interventions to prevent pre-frailty and frailty progression in older adults: a systematic review. JBI Database System Rev Implement Rep. 2018;16: 140–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fried LP, Tangen CM, Walston J, et al. Frailty in Older Adults: Evidence for a Phenotype. The Journals of Gerontology: Series A. 2001;56: M146–M157. [DOI] [PubMed] [Google Scholar]
- [36].Khan H, Kalogeropoulos AP, Georgiopoulou VV, et al. Frailty and risk for heart failure in older adults: the health, aging, and body composition study. Am Heart J. 2013;166: 887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sergi G, Veronese N, Fontana L, et al. Pre-frailty and risk of cardiovascular disease in elderly men and women: the Pro.V.A. study. J Am Coll Cardiol. 2015;65: 976–983. [DOI] [PubMed] [Google Scholar]
- [38].Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J, Kitzman DW. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;60: 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kitzman DW, Brubaker P, Morgan T, et al. Effect of Caloric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA. 2016;315: 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kalogeropoulos A, Georgiopoulou V, Psaty BM, et al. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. Journal of the American College of Cardiology. 2010;55: 2129–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Corsonello A, Lattanzio F, Pedone C, et al. Prognostic significance of the short physical performance battery in older patients discharged from acute care hospitals. Rejuvenation Res. 2012;15: 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bellettiere J, Lamonte MJ, Unkart J, et al. Short Physical Performance Battery and Incident Cardiovascular Events Among Older Women. Journal of the American Heart Association. 2020;9: e016845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Leong DP, Teo KK, Rangarajan S, et al. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. The Lancet. 2015;386: 266–273. [DOI] [PubMed] [Google Scholar]
- [44].Pavasini R, Serenelli M, Celis-Morales CA, et al. Grip strength predicts cardiac adverse events in patients with cardiac disorders: an individual patient pooled meta-analysis. Heart. 2019;105: 834–841. [DOI] [PubMed] [Google Scholar]
- [45].Sillars A, Celis-Morales CA, Ho FK, et al. Association of Fitness and Grip Strength With Heart Failure: Findings From the UK Biobank Population-Based Study. Mayo Clin Proc. 2019;94: 2230–2240. [DOI] [PubMed] [Google Scholar]
- [46].Kalogeropoulos AP, Georgiopoulou VV, deFilippi CR, Gottdiener JS, Butler J, Cardiovascular Health S. Echocardiography, natriuretic peptides, and risk for incident heart failure in older adults: the Cardiovascular Health Study. JACC Cardiovasc Imaging. 2012;5: 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Pandey A, Kitzman DW. Searching for the Optimal Exercise Training Regimen in Heart Failure With Preserved Ejection Fraction. JAMA. 2021;325: 537–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: CONSORT diagram
Supplemental Figure 2: Cumulative incident of HF across frailty measures
Supplemental Figure 3: Rates of HF by Fried criteria
Supplemental Figure 4: Rates of HF by natriuretic peptide levels and HF subtypes
Supplemental Methods: Outcomes and clinical covariates
Supplemental Table 1: Frailty phenotype criteria
Supplemental Table 2: SPPB score criteria
Supplemental Table 3: Association of cardiac stress with frailty measures
Supplemental Table 4: Association of Fried scores and incident HF
Supplemental Table 5: Subgroup analyses excluding participants with CVD and HF within 12 months
Supplemental Table 6: Subgroup analyses across HF subtypes
Supplemental Table 7: Competing risks analysis across HF subtypes
Supplemental Table 8: Event rates across natriuretic peptide level strata
Supplemental Table 9: Association of frailty with HF across natriuretic peptide strata
Supplemental Table 10: Predictive performance of frailty measures