Abstract
Prior studies have reported improvements in population-level risk factor burden and cardiovascular disease (CVD) outcomes using polypills for CVD risk reduction. However, a comprehensive assessment of the impact of polypills on CVD outcomes, mortality, adherence, and side effects across different settings has not previously been reported. We performed a systematic review and meta-analysis of randomized controlled trials examining the association between polypill therapy and CVD outcomes published before February 2021. The primary outcome of interest was the risk of major adverse CVD events (MACE). Risk ratios for dichotomous outcomes were converted to log RR and pooled using a generic inverse variance weighted random-effects model. Data for continuous outcomes were pooled using random-effects modeling and presented as mean differences with 95% CIs. Eight studies representing 25,584 patients were included for analysis. In the overall pooled analysis, the use of polypills was associated with a non-significant reduction in the risk of MACE (RR: 0.85; 95% CI: 0.70–1.02) and significant reductions in the risk of all-cause mortality (RR: 0.90; 95% CI: 0.81–1.00). The reductions in the risk of MACE with polypill use varied by baseline risk and nature of the study population (primary prevention vs. secondary prevention), with the most significant risk reduction among lower-risk cohorts, including within primary prevention populations [RR 0.70 (0.62, 0.79)]. Among measures of CVD risk factors, modest but significant reductions were observed for systolic and diastolic blood pressure [systolic: mean difference 1.99 mmHg (95% CI: −3.07 to −0.91); diastolic: mean difference 1.30 mmHg (95% CI: −2.42 to −0.19), but not for levels of total or low-density lipoprotein-cholesterol. Use of the polypill strategy significantly improved drug adherence (RR: 1.31; 95% CI: 1.11–1.55) with no association between polypill use and rates of adverse events or drug discontinuation. The use of polypill formulations is associated with significant reductions in CVD risk factors and the risk of all-cause mortality and MACE, particularly in the low-risk and primary prevention population.
Keywords: Polypill, Major adverse cardiovascular events, Prevention, Heart disease
INTRODUCTION
Cardiovascular disease (CVD) represents a major cause of morbidity and mortality globally, resulting in 18 million deaths in 2019.1 Moreover, CVD prevalence is accelerating, with cases increasing from 271 million to 523 million between 1990 and 2019, and disability-adjusted life years lost doubling in the same period. The increasing burden of CVD can be linked to increasing levels of modifiable CVD risk factors, including hypertension and hyperlipidemia, that are ubiquitous in high- and low- and middle-income countries.2 Despite existing guidance and widely available therapies for reducing CVD risk, most at-risk adults are not prescribed evidence-based treatments or are non-adherent to recommended regimens.3, 4 Adherence has additionally been observed to decline with increasing numbers of preventive therapies prescribed and with the duration of therapy, complicating efforts to stem comorbid risk factors.3, 5, 6 As a result, novel approaches to risk factor modification, particularly for low- and middle-income countries (LMIC), are needed and have the potential to shift the paradigm of CVD management in the 21st century.
Polypills, which combine multiple CVD risk-reduction agents in a single pill, were proposed two decades ago as a strategy to achieve universal risk factor reduction and improved medication adherence in at-risk populations.7 This strategy is based on a population approach to disease, by which modest and incremental risk factor modifications in large populations are theorized to yield substantial reductions in the global burden of CVD outcomes, including myocardial infarction (MI) and stroke.8 In 2003, Wald and Law anticipated a dramatic 88% reduction in ischemic heart disease and 80% reduction in strokes if all adults were provided a polypill combination of antihypertensive agents, lipid-lowering therapies, and aspirin.7 Although results of subsequent trials to evaluate the efficacy of polypills have been largely promising, their benefits have not realized the anticipated impacts, and consensus regarding the appropriate role of polypills as part of a global CVD risk-reduction strategy is lacking. Substantial gaps in the polypill literature remain, moreover, with critics citing inadequate evidence of benefit in reducing CVD events, incomplete characterization of the safety of the approach, insufficient proof of the impact of polypills on adherence, and inconsistency in the ideal composition of the polypill as shortcomings of the existing literature base.
A comprehensive assessment of the impact of polypill implementation on CVD outcomes and risk factor levels in light of the publication of several recent large-scale outcome trials of the polypill is currently lacking. This represents a critical knowledge gap for characterizing the potential benefit and harm of the polypill strategy. Therefore, in this systematic review and meta-analysis, we conducted a comprehensive review of the polypill literature and assessed the efficacy of polypills on CVD outcomes in addition to intermediate CVD risk markers such as hypertension and hyperlipidemia. We also evaluated the impact of polypills on markers of adherence and drug discontinuation and the prevalence of reported adverse effects to inform the future study of the polypill and approaches to global CVD reduction.
METHODS
Literature Search Strategy
This systematic review and meta-analysis was conducted following the established methods recommended by the Preferred Reporting Items for Systematic review and Meta-Analyses (PRISMA) and Cochrane guidelines.9, 10 Approval from the institutional review board was not required as data used in this study was publicly available. We systematically searched two databases (Medline and Scopus) for all randomized controlled trials (RCTs) that examined the associations between polypill therapy and CVD outcomes and all-cause mortality in February 2021, without any time or language restrictions. We additionally performed manual searches through reference lists of original publications, pertinent editorials, and review articles. Mesh terms and Boolean operators were used to produce a search strategy for each database (Supplemental Table 1).
Study Selection
All articles retrieved from the systematic search were exported to the Endnote Reference Library (Version X8.1; Clarivate Analytics, Philadelphia, PA, USA), where duplicates were removed. The remaining articles were assessed based on title and abstract by two independent reviewers (M.S.K and T.J.S). Finally, full texts were evaluated for relevance. Discrepancies were resolved by discussion with a third investigator (A.P). The search was restricted to RCTs of humans reporting major adverse CVD events (MACE) and all-cause mortality as a primary or secondary endpoint. Polypill interventions were required to include at least one lipid-lowering and one blood-pressure lowering component versus placebo, usual care, or active drug comparator for any treatment duration. Studies reporting fewer than ten combined MACE events between the intervention and control arm were excluded from the analysis. Our study population included participants ≥18 years of age with no restriction for the absence or presence of pre-existing atherosclerotic CVD. Two trials, TIPS-2 and the TEMPUS trial, compared full-dose polypill with low-dose polypill and morning polypill dose with evening polypill dose, respectively, and therefore were not included in our analysis.
Data Extraction
Two investigators (M.S.K and T.J.S) independently abstracted data from the short-listed studies using pre-specified collection forms. In addition to participants and trial characteristics, data on primary and secondary outcomes were extracted. The primary outcome of interest for this analysis was the risk of MACE, which included death from CVD causes, acute coronary syndrome, stroke, heart failure, MI, cardiac arrest, arterial revascularization, or angina. Secondary endpoints included all-cause mortality, surrogate CVD endpoints, including changes in systolic blood pressure (BP; SBP) and diastolic BP (DBP), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and change in EQ-5D health state, adverse therapy effects (including myalgia, cough, dyspepsia/gastrointestinal irritation), and drug adherence and discontinuation. The EQ-5D represents a standardized instrument for measuring health status by evaluating health in five domains (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) and incorporating a visual analog scale by which respondents evaluate their overall health.11 Two un-blinded investigators (M.S.K. and T.J.S) independently appraised the potential risk of bias of the RCTs using the Cochrane Risk of Bias Tool. Any disagreements were resolved through discussion with a third investigator (A.P).
Study Quality Assessment
Study quality was assessed using the Jadad Scale12 and the Cochrane Risk of Bias tool.13 A visual inspection of the funnel plot was used to evaluate the publication bias. Studies were assigned positive indicators in the Cochrane tool for randomized controlled study design, providing descriptions of treatment allocation concealment and blinding, reporting on loss to follow-up, and providing endpoint data on those not included in the final analysis. “High” quality is indicated by a Jadad score ≥ 3 across five metrics, including randomization, appropriateness of randomization scheme, double-blind design, appropriateness of blinding scheme, and description of dropouts and withdrawals.
Statistical Analysis
Statistical analysis was performed using the Open Meta-Analyst (V10.10 CEBM @ Brown, New Jersey, USA) and Review Manager (V.5.3 Cochrane Collaboration, London, United Kingdom). For dichotomous outcomes, risk ratios (RR) and 95% confidence intervals (CI) from individual RCTs were converted to log RRs and corresponding standard errors, which were then pooled using a generic inverse variance weighted random-effects model. For continuous outcomes, data were pooled using a random-effects model, and results were presented as mean differences with 95% CIs. Annualized event rates (AERs), defined as the number of patients having an outcome as a proportion of patients at risk divided by patient-years follow-up (reported per 1000 patient-years), were calculated for the MACE and all-cause mortality outcomes. Subgroup analyses were conducted for MACE stratifying trials based on the inclusion of a primary vs. secondary prevention population and by high (AER ≥ median value) vs. low (AER < median value) AERs in the comparator group. Sensitivity analyses were performed based on the year of publication, comparing early (before 2015) and late (after 2015) trials. Meta-regression was carried for the primary outcome by the proportion of females included in each trial, mean population age, the proportion of patients with baseline coronary heart disease (CHD) in the control arm, and AER in the control group to evaluate the influence of specific population risk characteristics on efficacy of the polypill. We assessed for heterogeneity across trials using the I2 test (I2=25%−50% was considered mild, 50%−75% moderate, and >75% severe heterogeneity). A p-value of <0.05 was considered significant in all cases.
RESULTS
Characteristics of Included Studies
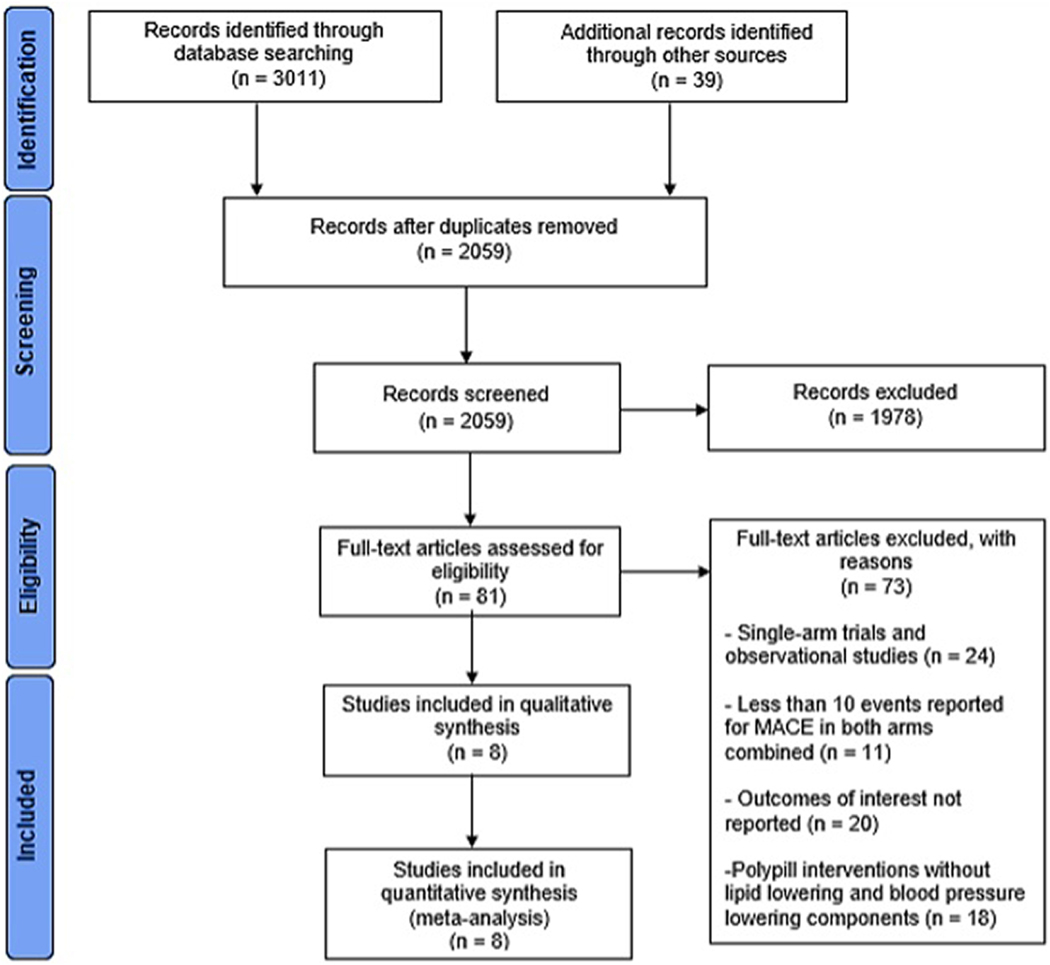
Results from the literature search and study selection process are summarized in Figure 1. We included 8 RCTs enrolling 25584 participants [mean age: 63.53 years (SD=1.23), 56% male] with a median follow-up of 36.75 (interquartile range: 12.75–58.20) months.8, 14–20 Baseline characteristics of included trials are summarized in Table 1. Included trials were published between 2013 and 2020 and set in predominantly LMIC settings, though seven trials included at least one high-income country. Four trials included a secondary prevention population only, three included primary prevention populations, and one trial, the PolyIran study, included both a primary and secondary prevention cohort. Six studies evaluated an aspirin-containing polypill that additionally included a statin and between one and three antihypertensive agents. One trial (TIPS-3) included a no-aspirin arm with a polypill containing statin and three antihypertensive agents without aspirin. One trial (HOPE-3) evaluated a ’polypill-style’ strategy involving treatment with two fixed-dose antihypertensive agents and a statin, administered in a separate pill. In four trials, the comparator arm received placebo (or non-pharmacological intervention only); in three trials, comparators received usual care; and in one trial, participants in the control arm received the same agents as the polypill arm administered as separate pill formulations. Among trials that reported baseline cardiovascular risk factors, hypertension prevalence ranged from 28% to 84% [mean 56.5%], and diabetes prevalence ranged from 6% to 37% [mean 27%]. Smoking prevalence was reported in four of eight trials and ranged from 5 to 15%. Additional detailed characteristics of included trials are included in Supplemental Table 2.
Figure 1: PRISMA flow chart summarizing the results of the literature search.
Eight trials were selected from the initial 2,059 potential articles.
MACE, major adverse cardiovascular event
Table 1:
Baseline characteristics of studies included in the meta-analysis.
| Study | Year | Country | Participants, n | Male, % | Mean age, y | HTN, % | DM, % | Smokers, % | Follow-Up, m | MACE outcomes observed, n | |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Experimental | Control | ||||||||||
| UMPIRE | 2013 | India, England, Ireland and Netherlands | 1002 | 1002 | 81.9 | 61.8 | N/A | 28.1 | N/A | 15.0 | 85 |
| FOCUS | 2014 | Argentina, Italy, Paraguay, Spain |
340 | 345 | 80.26 | 64.01 | 28.42 | 26.16 | 15.06 | 9.0 | 18 |
| IMPACT | 2014 | New Zealand | 256 | 257 | 26.9 | 62.0 | N/A | 44 | N/A | 12.0 | 34 |
| Kanyini GAP | 2014 | Australia | 311 | 312 | 63.0 | 63.4 | N/A | N/A | N/A | 20.7 | 48 |
| HOPE 3 | 2016 | 21 countries including China, India, Colombia, Argentina and Canada |
3180 | 3168 | 53.8 | 65.7 | 37.9 | 5.8 | N/A | 67.2 | 352 |
| PolyIran | 2019 | Iran | 3421 | 3417 | 49.7 | N/A | 49.3 | 15.0 | 4.7 | 60.0 | 503 |
| TIPS 3 | 2020 | India, Colombia, Bangladesh, Canada, Malaysia, Indonesia, Tunisia, Tanzania and the Philippines | 2861 | 2852 | 47.1 | 63.9 | 83.8 | 36.6 | 8.9 | 52.8 | 296 |
| TIPS ASA | 2020 | India, Colombia, Bangladesh, Canada, Malaysia, Indonesia, Tunisia, Tanzania and the Philippines | 1429 | 1421 | 47.3 | 63.9 | 83.2 | 36.8 | 8.8 | 52.8 | 147 |
UMPIRE, Use of a Multidrug Pill In Reducing cardiovascular Events; FOCUS, Fixed-Dose Combination Drug for Secondary Cardiovascular Prevention; IMPACT, IMProving adherence using Combination Therapy; Kanyini GAP, Kanyini Guidelines Adherence with the Polypill; HOPE-3, Heart Outcomes Prevention Evaluation-3; TIPS 3, The International Polycap Study-3; TIPS ASA, The International Polycap Study Acetylsalicylic Acid; HTN, hypertension; DM, diabetes mellitus; MACE, major adverse cardiovascular events; y, years; m, months.
Association Between Polypill Therapy and Risk of MACE
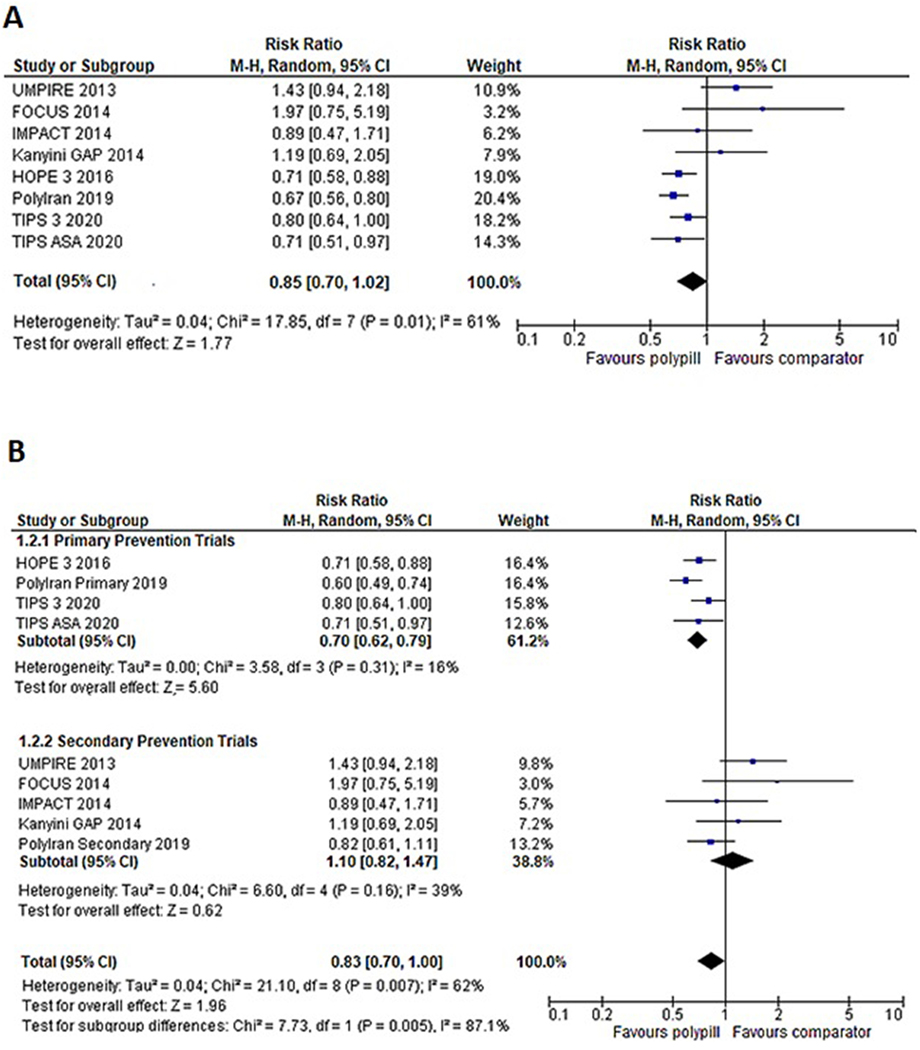
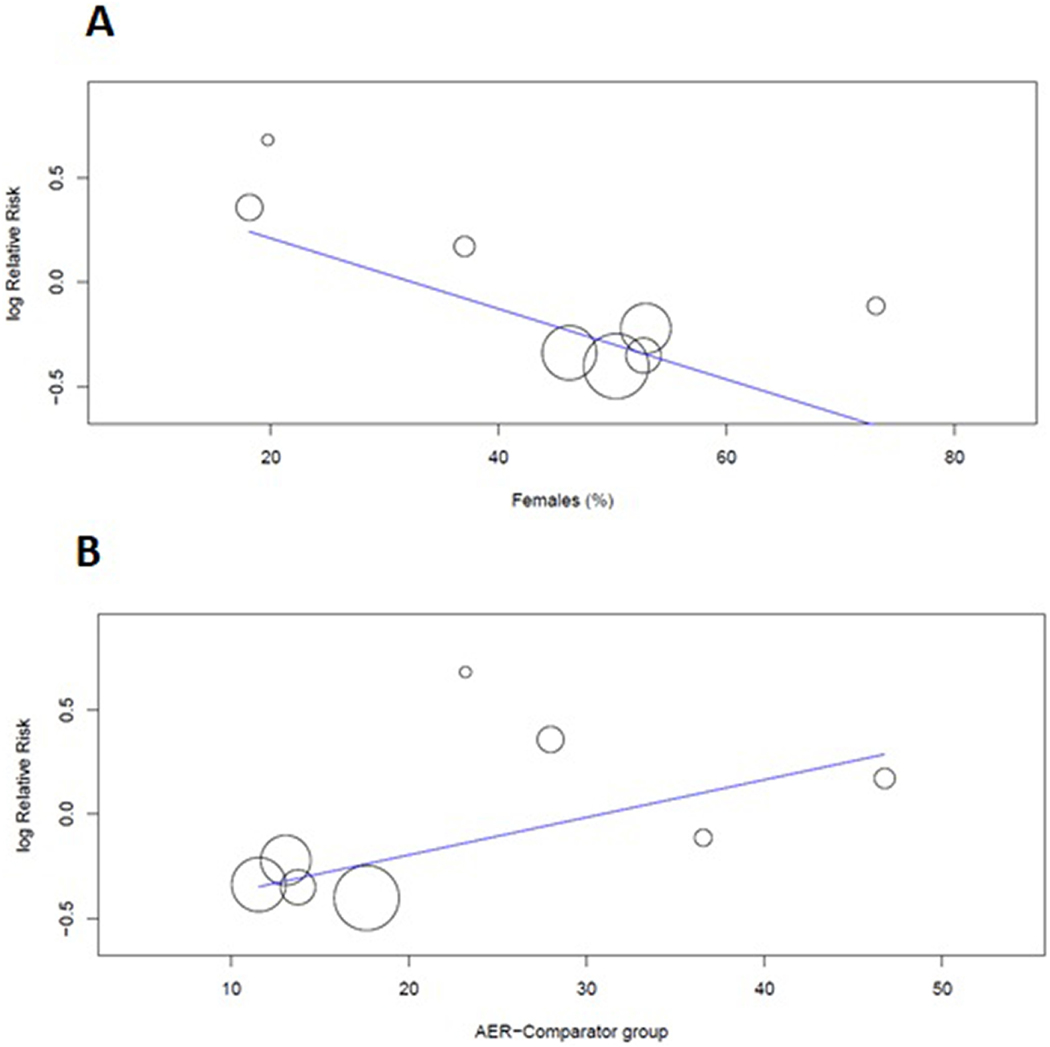
Figure 2A shows individual and pooled RR estimates for the polypill compared with the control group. There were a total of 1483 MACE events observed among the 25584 participants across eight studies. Use of the polypill reduced the relative risk of MACE by 15% [RR 0.85 (0.70–1.02)] compared to controls, though this result was not statistically significant. Subgroup analyses by risk cohort demonstrated a significant 30% reduction in the risk of MACE among primary prevention participants [n=21012; RR 0.70 (0.62, 0.79)], with no significant difference in risk among secondary prevention participants [n=4572; RR 1.10 (0.82, 1.47)] (Figure 2B). This finding was additionally demonstrated in a sensitivity analysis by AER, in which we observed a significant 30% reduction in the risk of MACE among low-risk cohorts (with low AER), but not among high-risk cohorts [RR 0.70 (0.62, 0.79) among low AER trials] (Supplemental Figure 1). Meta-regression by cohort characteristics additionally demonstrated that low-risk features, including a higher proportion of women and lower AER, were associated with a greater risk reduction associated with the polypill [female gender coefficient −0.017 (p=0.002); AER coefficient 0.018 (p=0.012)] (Figure 3). Additional meta-regression performed by baseline CHD prevalence at baseline in the control arm was not statistically significant but suggested a greater benefit for the polypill in studies with a lower baseline prevalence of CHD (Supplemental Figure 2). In sensitivity analysis by publication date, we observed a significant 28% reduction in the risk of MACE among “late” trials (published after 2015) [RR 0.71 (0.64, 0.80)], which comprised the majority of participants and events in the overall cohort (n=21749) (Supplemental Figure 3).
Figure 2:
A) Forest plot comparing major adverse cardiovascular events (MACE) between polypill and comparator group.
The use of polypill reduced the incidence of MACE by 15%, but this association was not significant (p=0.077).
B) Subgroup analysis comparing MACE outcomes between polypill and comparator groups in primary prevention and secondary prevention trials.
Polypill was significantly associated with a 30% decrease in the risk of MACE in primary prevention trials (p<0.001), while no favorable effect of the polypill was noted in secondary prevention trials (p=0.538).
M-H, Mantel Haenszel; CI, confidence interval; FOCUS, Fixed-Dose Combination Drug for Secondary Cardiovascular Prevention; IMPACT, IMProving adherence using Combination Therapy; Kanyini GAP, Kanyini Guidelines Adherence with the Polypill; UMPIRE, Use of a Multidrug Pill In Reducing cardiovascular Events; HOPE-3, Heart Outcomes Prevention Evaluation-3; TIPS 3, The International Polycap Study-3; TIPS ASA, The International Polycap Study Acetylsalicylic Acid
Figure 3: Meta-regression plots assessing the effect of A) female gender and B) annualized event rate per 1,000 person-years (AER) on the risk ratio associated with polypill for the MACE outcome.
An increasing proportion of women (slope= −0.017, p=0.002) and lower AER (slope= 0.018, p=0.012) were each associated with greater risk reduction for MACE with the use of polypill. AER, annualized event rate
Association Between Polypill and Risk of All-Cause Mortality
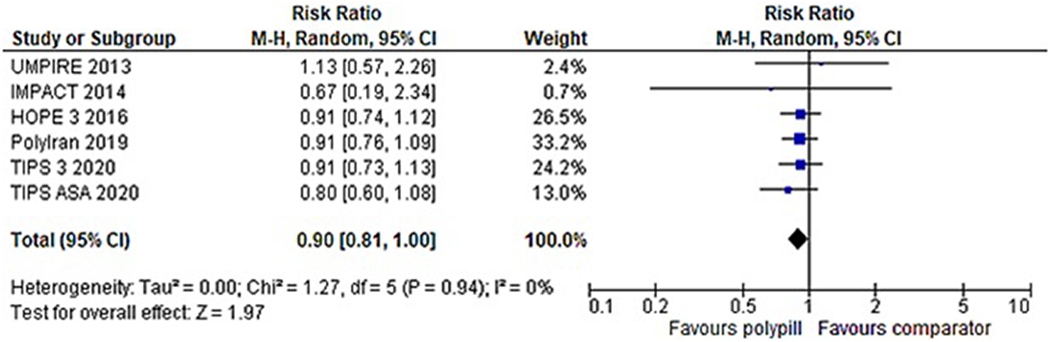
Six studies reported outcomes for all-cause mortality (1,287 events in 24,266 participants). The forest plot in Figure 4 demonstrates individual and pooled risk ratios. Use of the polypill resulted in a significantly reduced risk of death [RR 0.90 (0.81, 1.00)], with low levels of study heterogeneity for this outcome (I2=0%, p=0.94).
Figure 4: Forest plot comparing all-cause mortality between polypill and comparator group among six studies reporting this outcome.
Use of the polypill was associated with an 11% reduction in the risk of all-cause mortality (p=0.048).
M-H, Mantel Haenszel; CI, confidence interval; UMPIRE, Use of a Multidrug Pill In Reducing cardiovascular Events; IMPACT, IMProving adherence using Combination Therapy; HOPE-3, Heart Outcomes Prevention Evaluation-3; TIPS 3, The International Polycap Study-3; TIPS ASA, The International Polycap Study Acetylsalicylic Acid
Association Between Polypill and Changes in CVD Risk Factors
Table 2 shows the pooled mean difference for five surrogate CVD endpoints, including SBP, DBP, TC, LDL-C, and EQ-5D score, which reflects self-reported overall health. Reductions in SBP and DBP were modest, but statistically significant [SBP MD −1.99 mmHg (−3.07, −0.91); DBP MD −1.30 mmHg (−2.42, −0.19)]. Among three trials assessing TC and five trials evaluating LDL-C levels between baseline and follow-up, no significant reduction was observed. Three trials reported EQ-5D scores, with a modest and non-significant improvement observed using the polypill.
Table 2:
Effect of polypill on cardiovascular risk factors as noted in the pooled analysis.
| Surrogate outcome | Studies, n | Pooled mean difference (95% CI) | I2, % | P for Heterogeneity |
|---|---|---|---|---|
| Systolic blood pressure | 4 | −1.99 (−3.07, −0.91) | 0 | 0.716 |
| Diastolic blood pressure | 4 | −1.30 (−2.42, −0.19) | 60.8 | 0.05 |
| Total cholesterol | 3 | −0.01 (−0.21, 0.20) | 62.1 | 0.072 |
| LDL-cholesterol | 5 | −0.31 (−0.73, 0.11) | 94.4 | P<0.001 |
| EQ-5D | 3 | 0.01 (0, 0.02) | 0 | 0.697 |
LDL, low density lipoprotein; CI, confidence interval
Association Between Use of the Polypill and Drug Adherence, Discontinuation, and Adverse Events
We evaluated the impact of the polypill on drug adherence and discontinuation rates (Table 3). Across five trials reporting on adherence (n=10183), adherence was significantly increased among polypill recipients [RR 1.34 (1.11, 1.55)]. No significant differences in rates of drug discontinuation were observed [RR 0.99 (0.93, 1.04)]. The pooled risk of three primary adverse events reported by included studies is additionally shown in Table 3. Four studies reported the risk of myalgia (n=7544) and dyspepsia (n=8853), with no significant difference observed in the risk of these events using the polypill. Three studies reported on the risk of cough (n=9258) and demonstrated a mild and non-significant increase in the risk of this outcome with the use of the polypill [RR 1.47 (0.96, 2.26)].
Table 3:
Association of polypill with adherence, drug discontinuation, and individual therapy-associated adverse events reported by included studies.
| Outcome of Interest | Studies, n | Pooled relative risk (95% CI) | I2, % | P for Heterogeneity |
|---|---|---|---|---|
| Adherence | 5 | 1.31 (1.11, 1.55) | 95.5 | <0.001 |
| Drug Discontinuation | 4 | 0.99 (0.94, 1.04) | 23 | 0.27 |
| Myalgia | 4 | 0.93 (0.60, 1.46) | 0 | 0.471 |
| Cough | 3 | 1.47 (0.96, 2.26) | 0 | 0.470 |
| Dyspepsia/GI irritation | 4 | 1.14 (0.73, 1.78) | 0 | 0.896 |
GI, gastrointestinal; CI, confidence interval
Study Quality and Publication Bias
Seven of the eight included trials achieved a “high” quality rating based on Jadad score ≥ 3 and qualitative assessment (Supplemental Table 3). One trial (FOCUS) did not meet the high-quality criteria rating due to lack of blinding and incomplete outcome data. Asymmetrical funnel plots for both MACE (Supplemental Figure 4) and all-cause mortality (Supplemental Figure 5) were observed.
DISCUSSION
In this meta-analysis of randomized clinical trials evaluating the impact of the polypill on CVD events and CVD risk factors, we observed several novel findings. First, the use of a polypill strategy resulted in substantial overall reductions in MACE and all-cause mortality risk. Risk reduction for MACE was more pronounced among low-risk participants, including primary prevention populations and cohorts with a greater proportion of women and lower event rates. Second, polypills resulted in modest but significant reductions in surrogate CVD metrics, including BP, with no significant increase in the risk of adverse events associated with therapy. Finally, polypill use resulted in significant improvements in adherence to treatment with no effect on drug discontinuation, demonstrating both the biological and practical impacts of the polypill strategy.
Two prior meta-analyses have evaluated the impact of polypills on CVD and mortality outcomes, reporting an uncertain effect on both due to limited availability of outcomes trials and low event rates in existing literature at the time of publication.21, 22 By comparison, after the inclusion of recently published large outcomes trials, we observed a non-significant 15% reduction in MACE using the polypill with substantial heterogeneity in treatment effects across different risk cohorts and a statistically significant 10% reduction in the risk of all-cause mortality. Specifically, patients representing the lowest risk profiles for MACE achieved the most significant benefits from polypill therapy. In contrast, no significant effect of the polypill was observed in higher-risk cohorts, including secondary prevention participants, and in cohorts with higher baseline event rates and greater representation of men, who traditionally bear a higher burden of CVD risk.23 This pattern is consistent with prior observations regarding the benefits of intensive glycemic control or lipid management for CVD risk reduction in diabetes and, including risk modification among low-risk individuals with statin therapy. Our study findings support the notion of a “prevention paradox” in population health, which posits that small reductions in the entire distribution of risk are likely to achieve greater benefits than large reductions at the extremes of risk, where the risk of outcomes may be less modifiable.24–26 The relatively modest decreases in CVD risk factor burden (such as BP and TC levels) observed in the current study suggest that the benefits of the polypill are driven by small changes in risk factors among low-risk populations. The high-risk populations likely require more targeted and aggressive risk factor modification to achieve comparable gains.
Overall, reductions in blood pressure and lipid parameters compared with controls in the current study were modest, and like atherosclerotic CVD (ASCVD) events, underestimate the theorized benefits of the polypill anticipated by Wald and Law.2, 7 There are several potential explanations for this discrepancy, including overestimation of the impact of aspirin on risk reduction for primary prevention and larger anticipated impacts on BP and lipid reduction using polypill in Wald and Law’s initial analysis. Recent and larger-scale trials appear to demonstrate the smaller net benefit of the polypill in CVD risk factor levels, moreover, when compared with early trials.8 A 2012 meta-analysis incorporating six early polypill trials illustrates this pattern, observingand observed a pooled SBP/DBP reduction of 9 mmHg and 5 mmHg, respectively, compared with mean differences of 5 mmHg SBP and 1.30 mmHg DBP in the current study.3 A similar pattern is observed for reductions in total and LDL-C, for which Elley et al. observed substantially greater reductions than are observed in the current analysis.3
Two phenomena likely underlie this observation. First, estimates by Wald and Law were based on the assumption of an untreated comparator, and rates of treatment with any antihypertensive or statin agents in the control arm of early trials were likely similarly low. However, as prevention strategies have improved globally, treatment rates among usual care recipients have increased, impacting the estimated added benefit of a polypill and resulting in smaller reductions in BP and lipid parameters in trials evaluating a usual care comparator. This is evidenced in the UMPIRE trial, where despite lower levels of adherence, 71% of usual care participants were prescribed at least two antihypertensive agents, and 87.6% were prescribed statin therapy.17 Second, polypills included in the current study varied widely in composition and dosing. It is plausible that those incorporating more aggressive dosing of antihypertensive agents and more potent statin therapy may have the potential to drive further reductions in CVD risk factors and, in turn, CVD events. Exploratory analyses reported in the TIPS-3 trial support this hypothesis, finding that centers achieving the greatest reductions in CVD risk factors also reported the greatest reductions in CVD events on follow-up.8 Still, the observation of a net reduction in ASCVD events despite only modest improvements in CVD risk factors ultimately demonstrates the potency of the polypill population-risk approach. It also supports the hypothesis that even minor shifts in population risk may result in meaningful reductions in outcomes.
As theorized, we observed significant improvements in therapy adherence among polypill recipients. This impact of the polypill strategy is substantial given that most trials included participants with low healthcare contact, often in LMIC, where non-adherence rates to CVD preventive therapies have historically been highest.27 Moreover, although other interventions, including mobile messaging and patient counseling and education, have shown promise in improving medication adherence for chronic medical conditions, few have been as rigorously and consistently shown to impact adherence as the polypill strategy, highlighting the strength of this approach.28, 29 This observation is further strengthened by finding no differences in adverse event rates associated with the polypill across trials and no differences in drug discontinuation rates in the current study. Findings of higher rates of drug discontinuation among polypill recipients in the TIPS-3 trial recently raised concern that delays in drug distribution and resupply due to health infrastructure-related limitations in LMIC may limit the long-term success of any primary or secondary prevention strategy.8 However, we did not observe similar trends across other large randomized controlled trials. Longer-term evaluations focused on the reasons for drug discontinuation and strategies to improve care delivery at the health system level are needed to determine the long-term feasibility of the polypill strategy.
Our study findings have several important implications for public health. Polypill strategies have long been theorized to have the potential to meaningfully impact global CVD prevalence, though large-scale trial data has lagged behind the theorized benefits. The present study highlights the potential for polypills to impact CVD risk factor burden and drive substantial and meaningful reductions in ASCVD events and mortality. This is of enormous public interest, as polypill formulations are currently available at low cost in many low- and middle-income countries. These findings may help to guide policymakers and physicians in modifying risk prevention strategies to achieve maximal population benefits, particularly where resources are limited. Our observation of greater benefits associated with the polypill among low-risk populations additionally highlights its value as a population-based strategy for CVD risk reduction. It also demonstrates the potential for impacting event rates with modest modifications in individual CVD risk factors.
There are several strengths of our study approach. First, we present the most comprehensive review of polypill trials to date with a large, pooled sample size and several studies with longterm follow-up, enhancing our ability to detect differences in CVD outcomes not previously appreciated. Second, we include several sensitivity and subgroup analyses in the present report confirming the robustness of our findings and allowing us to evaluate the efficacy of the polypill across care settings, risk populations, and geographic boundaries. Third, we observed low heterogeneity for all-cause mortality and adverse event reporting and moderate heterogeneity for the evaluation of the primary ASCVD outcome, strengthening our findings.
However, the current study is not without limitations. First, we cannot exclude the effects of unmeasured and residual confounding in this meta-analysis as we did not access patient-level data for the current analysis. Second, substantial heterogeneity observed for several of our secondary outcomes, including in the evaluation of CVD risk factor levels, adherence, and in subgroup and sensitivity analyses, is reflective of heterogeneity in study design and care settings. In particular, adherence and drug discontinuation assessments are impacted by a lack of gold-standard measures. Third, diversity in formulations of the polypill used across trials limits our ability to speak to the strengths or weaknesses of particular formulations, dosing regimens, or distribution strategies, all of which have important implications for applications of a polypill strategy in real-world settings.
In conclusion, in this meta-analysis, we observed reductions in the risk of MACE events that are most significant among low-risk populations, significant reductions in the risk of all-cause mortality, and modest decreases in individual CVD risk factors. The polypill was additionally associated with significant improvements in therapy adherence with no increase in the risk of adverse events or drug discontinuation. Further research is needed to clarify ideal dosing and strategies for implementing a polypill approach at the population level.
Supplementary Material
Disclosures:
Dr. Pandey received grant funding outside the present study from Applied Therapeutics; has received honoraria outside of the present study as an advisor/consultant for Tricog Health Inc and Lilly, USA, and has received nonfinancial support from Pfizer and Merck.Dr. Pandey is supported by the Gilead Sciences Research Scholar Program, the National Institute of Aging GEMSSTAR Grant (1R03AG067960-01), and grant support from Applied Therapeutics.
ABBREVIATIONS
- AER
Annualized event rates
- ASCVD
Atherosclerotic cardiovascular disease
- BP
Blood pressure
- CHD
Coronary heart disease
- CI
Confidence Interval
- CVD
Cardiovascular disease
- DBP
Diastolic blood pressure
- LDL-C
Low-density lipoprotein cholesterol
- LMIC
Low- and middle-income countries
- MACE
Major adverse cardiovascular events
- MI
Myocardial Infarction
- PRISMA
Preferred Reporting Items for Systematic review and Meta-Analyses
- RCT
Randomized controlled trials
- RR
Relative risk
- SBP
systolic blood pressure
- TC
Total cholesterol
Footnotes
COI:
Other authors have no relevant disclosures
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Roth GA, Mensah GA, Johnson CO, et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J Am Coll Cardiol. 2020;76:2982–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lonn E, Bosch J, Teo KK, Pais P, Xavier D, Yusuf S. The polypill in the prevention of cardiovascular diseases: key concepts, current status, challenges, and future directions. Circulation. 2010;122:2078–2088. [DOI] [PubMed] [Google Scholar]
- 3.Elley CR, Gupta AK, Webster R, et al. The efficacy and tolerability of ‘polypills’: meta-analysis of randomised controlled trials. PLoS One. 2012;7:e52145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selak V, Webster R, Stepien S, et al. Reaching cardiovascular prevention guideline targets with a polypill-based approach: a meta-analysis of randomised clinical trials. Heart. 2019;105:42–48. [DOI] [PubMed] [Google Scholar]
- 5.Chapman RH, Benner JS, Petrilla AA, et al. Predictors of adherence with antihypertensive and lipid-lowering therapy. Arch Intern Med. 2005;165:1147–1152. [DOI] [PubMed] [Google Scholar]
- 6.WHO. Adherence to Long-term Therapies: Evidence for Action2003. [PubMed] [Google Scholar]
- 7.Wald NJ, Law MR. A strategy to reduce cardiovascular disease by more than 80%. BMJ. 2003;326:1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yusuf S, Joseph P, Dans A, et al. Polypill with or without Aspirin in Persons without Cardiovascular Disease. N Engl J Med. 2021;384:216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. [DOI] [PubMed] [Google Scholar]
- 10.Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devlin NJ, Brooks R. EQ-5D and the EuroQol Group: Past, Present and Future. Appl Health Econ Health Policy. 2017;15:127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ. 2001;323:42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 14.Castellano JM, Sanz G, Penalvo JL, et al. A polypill strategy to improve adherence: results from the FOCUS project. J Am Coll Cardiol. 2014;64:2071–2082. [DOI] [PubMed] [Google Scholar]
- 15.Selak V, Elley CR, Bullen C, et al. Effect of fixed dose combination treatment on adherence and risk factor control among patients at high risk of cardiovascular disease: randomised controlled trial in primary care. BMJ. 2014;348:g3318. [DOI] [PubMed] [Google Scholar]
- 16.Indian Polycap S, Yusuf S, Pais P, et al. Effects of a polypill (Polycap) on risk factors in middle-aged individuals without cardiovascular disease (TIPS): a phase II, double-blind, randomised trial. Lancet. 2009;373:1341–1351. [DOI] [PubMed] [Google Scholar]
- 17.Thom S, Poulter N, Field J, et al. Effects of a fixed-dose combination strategy on adherence and risk factors in patients with or at high risk of CVD: the UMPIRE randomized clinical trial. JAMA. 2013;310:918–929. [DOI] [PubMed] [Google Scholar]
- 18.Roshandel G, Khoshnia M, Poustchi H, et al. Effectiveness of polypill for primary and secondary prevention of cardiovascular diseases (PolyIran): a pragmatic, cluster-randomised trial. Lancet. 2019;394:672–683. [DOI] [PubMed] [Google Scholar]
- 19.Patel A, Cass A, Peiris D, et al. A pragmatic randomized trial of a polypill-based strategy to improve use of indicated preventive treatments in people at high cardiovascular disease risk. Eur J Prev Cardiol. 2015;22:920–930. [DOI] [PubMed] [Google Scholar]
- 20.Yusuf S, Lonn E, Pais P, et al. Blood-Pressure and Cholesterol Lowering in Persons without Cardiovascular Disease. N Engl J Med. 2016;374:2032–2043. [DOI] [PubMed] [Google Scholar]
- 21.Bahiru E, de Cates AN, Farr MR, et al. Fixed-dose combination therapy for the prevention of atherosclerotic cardiovascular diseases. Cochrane Database Syst Rev. 2017;3:CD009868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Cates AN, Farr MR, Wright N, et al. Fixed-dose combination therapy for the prevention of cardiovascular disease. Cochrane Database Syst Rev. 2014:CD009868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mosca L, Barrett-Connor E, Wenger NK. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation. 2011;124:2145–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Segar MW, Patel KV, Vaduganathan M, et al. Development and validation of optimal phenomapping methods to estimate long-term atherosclerotic cardiovascular disease risk in patients with type 2 diabetes. Diabetologia. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cholesterol Treatment Trialists C, Mihaylova B, Emberson J, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rose G. Sick individuals and sick populations. Int J Epidemiol. 1985;14:32–38. [DOI] [PubMed] [Google Scholar]
- 27.Kolandaivelu K, Leiden BB, O’Gara PT, Bhatt DL. Non-adherence to cardiovascular medications. Eur Heart J. 2014;35:3267–3276. [DOI] [PubMed] [Google Scholar]
- 28.Santo K, Kirkendall S, Laba TL, et al. Interventions to improve medication adherence in coronary disease patients: A systematic review and meta-analysis of randomised controlled trials. Eur J Prev Cardiol. 2016;23:1065–1076. [DOI] [PubMed] [Google Scholar]
- 29.Thakkar J, Kurup R, Laba TL, et al. Mobile Telephone Text Messaging for Medication Adherence in Chronic Disease: A Meta-analysis. JAMA Intern Med. 2016;176:340–349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.