Abstract
Background:
Patient-centric measures of hospital performance for transcatheter aortic valve replacement (TAVR) are needed.
Objectives:
Evaluate 30-day risk-adjusted home time as a hospital performance metric for patients undergoing TAVR.
Methods:
We identified 160,792 Medicare beneficiaries who underwent elective TAVR from 2015 to 2019. Home time was calculated for each patient as the number of days alive and spent outside a hospital, skilled nursing (SNF), and long-term acute care facilities for 30 days after the TAVR procedure date. Correlations between risk-adjusted 30-day home time and other metrics (30-day risk-adjusted readmission rate (RSRR), 30-day risk-adjusted mortality rate (RSMR), and annual TAVR volume) were estimated using Pearson correlation. Meaningful upward or downward reclassification (≥2 quartile rank) in hospital performance based on quartiles of risk-adjusted 30-day home time compared with quartiles of other measures were assessed.
Results:
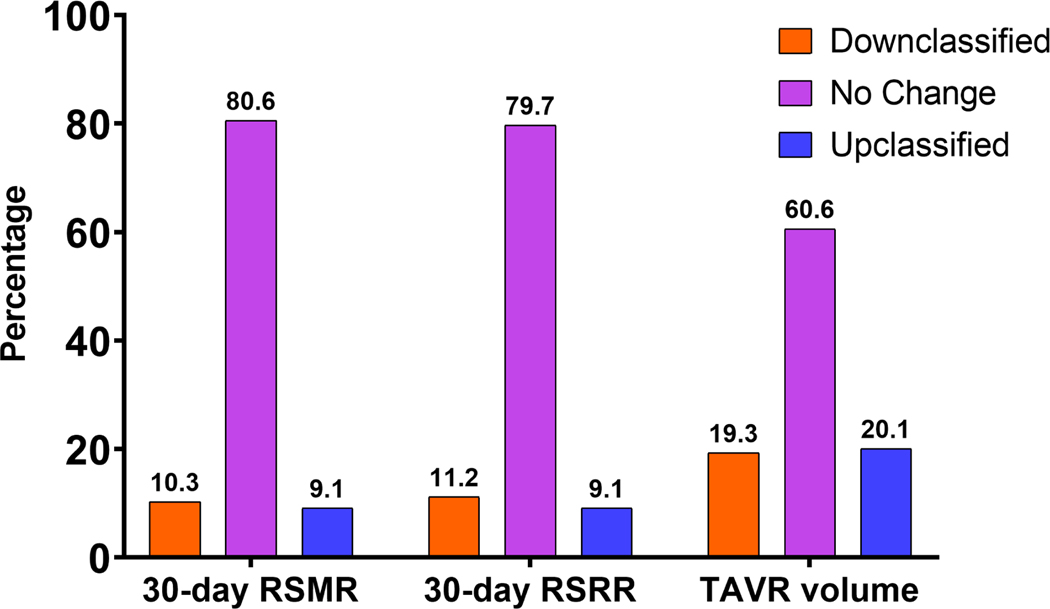
Median risk-adjusted 30-day home time was 27.5 days (IQR:26.3–28.5). The largest proportion of days lost from 30-day home time was hospital stay after TAVR and SNF stay. An inverse correlation was observed between hospital-level risk-adjusted 30-day home time and 30-RSRR (r:−0.465, P<0.001) 30-RSMR (r:−0.399, P<0.001). The use of the 30-day risk-adjusted home time was associated with reclassification in the hospital performance rank hospitals compared with other metrics (9.1% up-classified; 11.2% down-classified vs. RSRR; 9.1% up-classified; 10.3% down-classified vs. RSMR, and 0.394 vs. annual TAVR volume, 20.1% up-classified; 19.3% down-classified vs. TAVR volume).
Conclusion:
Risk-adjusted 30-day home time represents a novel patient-centered performance metric for TAVR hospitals that may provide a complimentary assessment to currently used metrics.
Keywords: Transcatheter aortic valve replacement, performance, outcomes
CONDENSED ABSTRACT
We evaluated 30-day risk-adjusted home time as a performance metric for hospital performance among Medicare beneficiaries who underwent transcatheter aortic valve replacement (TAVR). The median risk-adjusted 30-day home time was 27.5 days (IQR 26.3–28.5) days. Top performing hospitals based on risk-adjusted 30-day home time had lower in-hospital and 1-year mortality, 30-day RSRR, and 30-day RSMR. There was a significant inverse correlation between risk-adjusted 30-day home time and 30-RSRR and with 30-day RSMR. There was meaningful reclassification in almost half of the hospitals based on 30-day risk-adjusted home time quartiles compared with hospital annual TAVR volume.
INTRODUCTION
Transcatheter aortic valve replacement (TAVR) has rapidly become the first-line therapy for a majority of patients with aortic stenosis (AS), based on evidence from large multicenter randomized controlled trials.(1–4) Since the US Food and Drug Administration (FDA) approved TAVR in 2012, TAVR centers have expanded rapidly across the US.(5) To ensure the quality and safety of the TAVR procedure, the Center for Medicare and Medicaid Services (CMS) has established certain criteria to ensure that adequate infrastructure is present at hospitals before starting a new TAVR program. These include the on-site availability of cardiac surgery and interventional cardiology expertise, adequate expertise in post-operative care in patients with open heart procedures, and at least 50 open heart surgeries in the preceding year.(6) The CMS requires that TAVR programs perform at least 20 TAVR procedures per year to maintain their status.(6)
Prior studies have shown an association between hospital TAVR volume and short-term outcomes, including 30-day mortality.(7,8) Hospital TAVR volume is a structural performance metric representing hospital resources and operator skill quality. There is evidence that the relationship between procedure volume and clinical outcomes plateaus after the initial learning curve.(9) Accordingly, recent studies have focused on developing hospital performance measures based on clinical outcomes such as the 30-day risk-adjusted mortality rate (RSMR), and 30-day risk-adjusted readmission rate (RSRR), and the Transcatheter Valve Therapy registry (TVT) composite clinical score.(10–12) While these clinical outcome metrics capture patient-level risk, case-mix, and post-procedural care quality, they may not account entirely for the care quality variation in patient-centered outcomes in the post-discharge period. This is particularly relevant considering the high burden of functional impairment, frailty, and difficulty in self-care among older patients who undergo TAVR.(13,14) Thus, there is an unmet need for complimentary patient-centered performance metrics for TAVR performing hospitals.
Home time—defined as the number of days spent alive at home outside of a hospital or a skilled nursing facility— is associated with self-reported health, functional status, and clinical outcomes in hospitalized patients with acute cardiovascular conditions.(15–18) Hospital-level risk-adjusted 30-day home time is strongly associated with mortality and readmission rates for heart failure and acute myocardial infarction.(15,16) The potential utility of home time as a hospital-level performance metric for patients undergoing TAVR has not been assessed previously. Accordingly, we evaluated the performance of risk-adjusted 30-day home time as a hospital performance metric for TAVR. We hypothesize that hospitals with higher risk-adjusted 30-day home time for patients undergoing TAVR will have lower short- and long-term risk of mortality, readmission, and adverse composite clinical endpoint including in-hospital and 30-day stroke, major bleeding, and acute kidney injury requiring dialysis.
METHODS
Study cohort
We identified Medicare beneficiaries who underwent TAVR from 01/01/2015 to 11/30/2019 utilizing International Classification of Diseases (ICD) procedure codes (35.05, 35.06, 02RF37Z, 02RF38Z, 02RF3JZ, 02RF3KZ, 02RF37H, 02RF38H, 02RF3JH, and 02RF3KH) from Medicare Provider Analysis and Review (MedPar) Part A 100% Files. MedPar Files include all admissions for Medicare beneficiaries enrolled in Fee for Services (FFS) to hospitals, skilled nursing facilities (SNF), and long-term acute care facilities (LTAC). If a patient had more than one TAVR procedure, only the first procedure was included. We excluded patients who had urgent/emergent TAVR, valve-in-valve TAVR, and patients younger than 66 years at the time of the procedure or were enrolled for <1 year in FFS before the TAVR procedure. We also excluded patients admitted from SNF or LTAC to undergo TAVR, discharged to hospice, left against medical advice, or received palliative care within 30 days before the TAVR procedure, and those released after 11/30/2019 to allow for complete 30 days of home time calculations. Patients’ demographics, including age, sex, race, and enrollment information, were derived from Medicare Beneficiary Summary Files. Patients’ comorbidities were ascertained using diagnosis, and procedure ICD codes from inpatient claims one year before the TAVR procedure. Hospital characteristics such as teaching affiliation, bed count, rural versus urban location, availability of cardiac rehabilitation, ownership, participation in bundled payment programs, and census region were derived from the American Hospital Association data for 2016. We excluded hospitals that performed less than 10 TAVR procedures each year. The institutional review boards at the University of Iowa and at the Cleveland Clinic approved this study with a waiver of patient informed consent.
Risk-adjusted 30-day home time, 30-day RSRR, and 30-day RSMR
The primary outcome of our study was 30-day home time, which was calculated as the number of days within 30 days after the TAVR procedure date spent alive at home. The Day after TAVR was considered day 0, and then for each patient, we calculated the number of days spent in a facility (SNF, LTAC, or an acute care hospital) within the first 30 days and subtracted them from the 30-day home time. Part of a day spent in the facility was counted against home-time (admission and discharge days). If a patient died within 30-days from the procedure date, days lost due to death were also subtracted from home time. For 30-day RSRR, readmissions to short-term acute care hospitals only were included per CMS definitions. Calculation of readmission included patients discharged alive from the hospital after undergoing TAVR.
30-day mortality was calculated from the day of TAVR and included patients who died in the hospital. Since the focus of the metric is to evaluate the care quality of the TAVR performing hospital, for patients transferred to another facility after the index TAVR procedure (N = 493, 0.25% of the study cohort), home time calculations, 30-day RSRR, and 30-day RSMR were calculated as above and attributed to the TAVR performing hospital. We also calculated the hospital annual TAVR volume by dividing the total number of TAVR procedures done in each hospital by the number of years it was active during the study period. Data on mortality were available through 8/2020, and other clinical outcomes were available through 12/2019.
Calculation of 30-day risk-adjusted home time, mortality rate, and readmission rate
Generalized linear mixed models were used to calculate risk-adjusted rates of 30-day home time, mortality, and readmission to account for differences in case-mix across study hospitals. Candidate variables for risk adjustment included patients’ age, sex, and the 259 chronic conditions defined using published CMS risk software/models, cardiac conditions defined by ICD codes, and essential components of a prior validated frailty score TAVR patients(e-Table 1).(2) As per the CMS approach,(19) neither race or socioeconomic status were included in the adjustment model. The study cohort was divided into two halves— a derivation and a validation cohort. The risk adjustment models were constructed using a log link and Poisson distribution and estimated using maximum likelihood with 30-day home time as the outcome with hospital site as a random effect and patient variables as fixed effects. These models were performed on 100 bootstrap samples from the derivation cohort. The final risk adjustment model included candidate variables that were significant (P-value <0.01) in >80% of the models. After determining the final model, predicted home time and expected home time were calculated with and without linear unbiased prediction modeling to estimate random effects. As per the CMS models of hospital performance,(19) the predicted home time is the predicted 30-day home time for a hospital based on its performance for its specific case mix (that is, the hospital specific effect on the home time). The expected home time is based on the 30-day home time at an average hospital with a patient case mix like that hospital’s (that is, if patients with the same characteristics had been treated at an average hospital, rather than at that hospital). The predicted/expected home time ratio multiplied by the overall crude home time was used to determine the risk-adjusted 30-day home time.(20,21) 30-day RSRR and 30-day RSMR were calculated using a similar approach, with 30-day readmission and 30-day mortality as the dependent variable, respectively. The final risk adjustment models were tested in the validation and derivation cohorts, and model fit statistics (R-squared for generalized linear mixed model, and area under the curve and C-statistics for logistic regression) were calculated. Final risk adjustment models for 30-day home-time, 30-day RSRR, and 30-day RSMR are shown in e-Tables 2, 3, and 4. The risk-adjusted models demonstrated adequate discrimination in the derivation and validation cohorts, as shown in e-Table 5.
Statistical analysis
Hospitals were stratified into 30-day risk-adjusted home time quartiles, with quartile 1 (Q1) representing lowest performing and the quartile 4 representing highest performing hospitals. We compared hospital-level patient and hospital characteristics across hospital quartiles of 30-day risk-adjusted home time. Patient characteristics were reported for each study group (quartiles) as the median (25th and 75th percentile) of the hospital-level proportions of patients (for categorical variables) and hospital-level means (for continuous variables) and compared across the 2 groups using the Kruskal-Wallis test. Hospital characteristics were also reported as median (25th and 75th percentile) for continuous variables and proportion for categorical variables and compared using the Kruskal-Wallis test for continuous variables, and the Chi-square test for categorical variables. Distribution of hospital-level risk-adjusted 30-day home time, 30-day RSRR, 30-day RSMR, and TAVR volume across hospitals was examined using histogram plots. The proportion of 30-day home time lost to SNF, LTAC, hospital readmission, and death was calculated by summing total days for each category and dividing by the total lost days of home time. The in-hospital mortality, 30-day readmission, 30-day mortality, 1-year mortality, 1-year all-cause readmissions, and hospital TAVR volumes among patients across the 30-day home time–based hospital groups were compared with an ANOVA test. The rate of hospital readmissions over one year was also calculated as follows: The total number of admissions in one year after TAVR discharge date / time patient was alive in months up to one year after TAVR discharge. We also calculated a composite clinical endpoint including in-hospital and 30-day stroke, major bleeding, and acute kidney injury requiring dialysis. Correlation between risk-adjusted 30-day home time, 30-day RSRR, 30-day RSMR, rate of hospital readmissions over one year, and hospital annual TAVR volume was examined using Pearson correlation coefficient. We also tested the correlation between annual TAVR volume and 30-day RSRR, and 30-day RSMR. As a sensitivity analysis, we also repeated the study analysis using unadjusted 30-day home time.
To assess reclassification of hospital performance based on 30-day risk-adjusted home time, we compared the hospital performance categories based on the quartile of 30-day home time (quartile 1–4, lowest to highest performing) with their categories based on the 30-day RSRR and 30-day RSMR (quartile 1–4, highest to lowest performing). Hospitals that moved up from a lower to a higher performing quartile by at least two quartiles were considered up-classified. In comparison, hospitals that moved down from a higher to a lower performing quartile by at least two quartiles were considered down-classified. Net reclassification index (NRI) was also calculated to determine the reclassification in hospital performance using home time vs. 30-day RSRR and RSMR. The analysis was performed using SAS version 9.4 (SAS Institute, Inc., Cary, North Carolina) and GraphPad Prism version 8.
RESULTS
Distribution of Hospital-level Performance Metrics
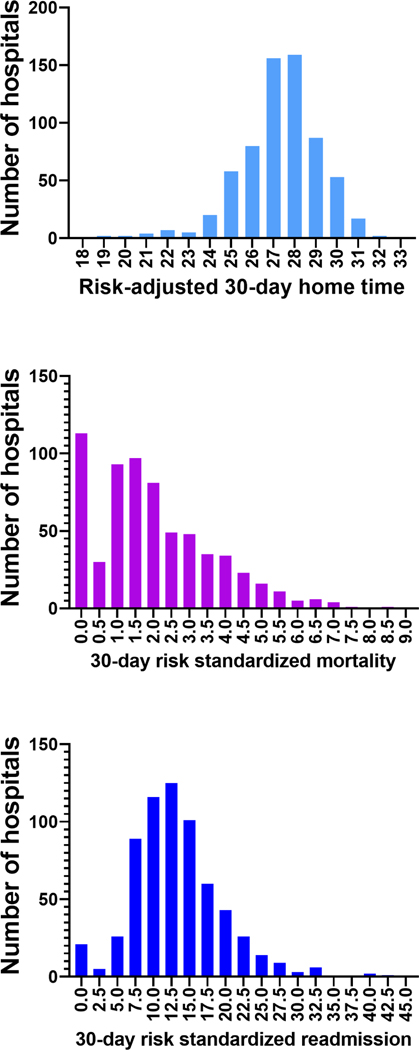
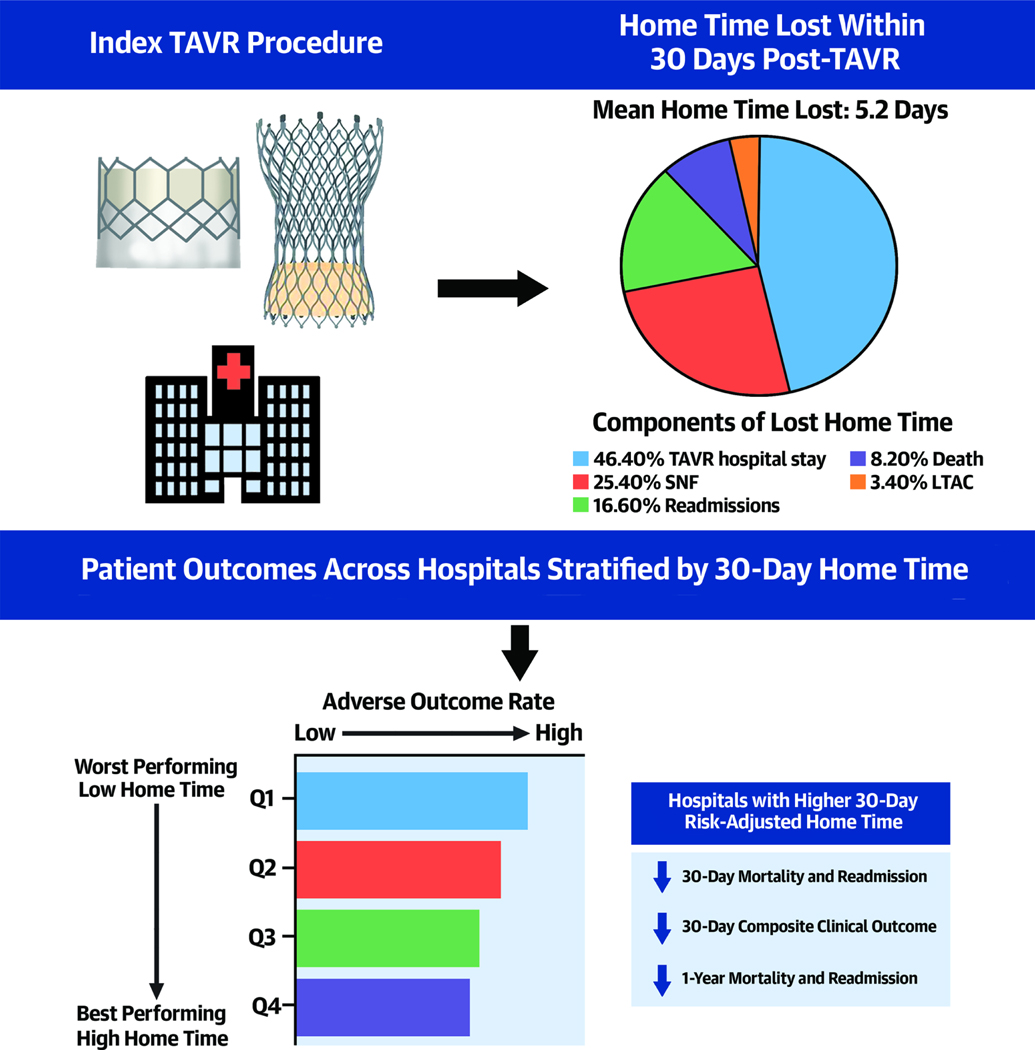
The final study cohort for home time and mortality analysis included 160,792 patients from 652 TAVR centers and 158,992 patients for readmission analysis (e-Figure 1). Study patients who underwent TAVR had a mean age was 81.9±6.8 years and included 53.5% men, 90.8% patients of the self-reported White race, and 3.1% of the Black race. Median hospital-level risk-adjusted 30-day home time was 27.4 days (IQR 26.3–28.5) days, while the median 30-day RSMR was 1.7% (IQR 0.9%−2.9%) and median 30-day RSRR was 12.6% (IQR 9.3%−16.5%). The median annual TAVR volume was 56 procedures (IQR 34– 92 procedures). There was substantial variation in risk-adjusted 30-day home-time, 30-day RSRR, 30-day RSMR, and annual TAVR volume (Figure 1, e-Figure 2). In the overall cohort, at the patient level, the largest proportion of days lost from a perfect 30-day home time was due to length of hospital stay after TAVR procedure (46.4%, mean: 2.2±3.2 days), followed by SNF stays (25.4%, mean: 1.2±4.6 days), hospital readmissions (16.6%, mean: 0.8±2.7 days), death (8.2%, mean: 0.38±3.1 days), and LTAC stays (3.4%, mean: 0.16±1.5 days),
Figure 1.
Distribution of different hospital-level performance metrics across TAVR hospitals.
Distribution of hospital-level A) risk-adjusted 30-day home time, B) 30-day RSMR, and C) 30-day RSRR, presented as histogram plots.
Abbreviations: RSMR: Risk adjusted mortality rate; RSRR: Risk adjusted readmission rate; TAVR: Transcatheter aortic valve repair
Patients and Hospitals Characteristics
Patient characteristics across hospital quartiles of risk-adjusted 30-day home time (Q1- lowest performing, Q4- highest performing) are shown in Table 1. Patient characteristics at the hospital level across quartiles of risk-adjusted home time were not meaningfully different except for a lower burden of ischemic heart disease and a higher burden of heart failure in the high vs. low performing hospitals. Patients in high performing hospitals were more likely to be discharged home and less likely to be discharged to SNF or long-term acute care facilities. The hospital-level characteristics across quartiles of risk-adjusted 30-day home time are shown in Table 2. High performing hospitals had a modestly lower number of beds compared with the low performing hospitals. There was no significant difference between high and low performing hospitals in teaching affiliation status, ownership, or annual TAVR volume. High performing hospitals were more likely to be in the West and less likely in the Northeast region.
Table 1:
Hospital-level clinical characteristics of patients receiving TAVR across 30-day home time-based hospital groups
| Variable | Low Performing → High Performing | P Value | |||
|---|---|---|---|---|---|
| Quartile 1 (N = 163) | Quartile 2 (N=163) | Quartile 3 (N=163) | Quartile 4 (N=163) | ||
| No. of patients | 36,898 | 45,001 | 44,049 | 34,844 | |
| Age, years | 81.9 (81.2–82.8) | 81.7 (81.1–82.6) | 81.5 (80.9–82.6) | 81.3 (80.4–82.4) | <0.001 |
| Men | 52.7 (49.3–55.7) | 53.5 (51–56.6) | 53.4 (50.3–56.6) | 54.5 (50–58.1) | 0.2 |
|
Race
Non-Hispanic White Black Asian Hispanic |
93.9 (87.6–96.8) 1.6 (0.3–4.2) 1.8 (0.3–5.2) 0.3 (0–0.8) |
93.3 (87.8–96.8) 2 (0.4–4.4) 1.3 (0.4–3.7) 0.2 (0–0.8) |
92.5 (86.8–96.4) 1.7 (0.5–5.3) 1.4 (0–4.5) 0.3 (0–1.1) |
93.6 (84.4–97.2) (0–3.4) 1.5 (0–5.4) 0.2 (0–1.8) |
0.05 |
| Hypertension | 93.6 (91.1–96.3) | 93.7 (91.1–95.6) | 94 (90.3–96.2) | 93.4 (90.1–96.6) | 0.9 |
| Diabetes | 42.4 (38.3–47.9) | 41.9 (37.5–46.6) | 41.1 (37.3–45.9) | 41.7 (36.8–47.7) | 0.5 |
| Heart failure | 64 (53.4–77.5) | 72.8 (58.8–88) | 75.8 (60–90.7) | 77.2 (52.9–92.2) | <0.001 |
| CKD | 38.6 (33.3–43.5) | 38.2 (32.5–45.2) | 39.3 (34.4–44.5) | 37.5 (31.4–44.2) | 0.4 |
| Liver and biliary disease | 6.5 (4.5–8.3) | 6 (4.5–7.6) | 6.1 (4.4–7.6) | 6.5 (4.3–9.1) | 0.6 |
| COPD | 27.2 (22.8–31.9) | 27.2 (22.8–31) | 26.9 (22.4–31.4) | 25.4 (20.2–31.2) | 0.3 |
| Prior stroke | 13.9 (11–17.7) | 13.9 (9.9–17.3) | 13.7 (10.7–17.4) | 12.5 (8.9–17.5) | 0.1 |
| Prior MI | 7.7 (5.9–9.8) | 6.9 (5.3–8.8) | 7.4 (5.7–10.8) | 6.8 (5–9.9) | 0.03 |
| Prior PCI | 28.7 (24.1–34.5) | 27.7 (24.2–33.3) | 28.1 (23.7–34.1) | 27 (21.6–34) | 0.2 |
| Prior CABG | 19.9 (17.2–23.9) | 20.1 (17.3–23) | 19.2 (15.8–22.7) | 17.4 (13.5–21.4) | <0.001 |
| Apical TAVR | 0.8 (0–2.5) | 0.9 (0–2.5) | 0.8 (0–2.1) | 0.4 (0–1.7) | 0.02 |
| Discharged SNF or LTAC | 10.5 (7.7–15.2) | 8.1 (5.6–10.1) | 6.1 (3.9–8.3) | 3.4 (1.1–5.9) | <0.001 |
| Discharged home | 64.9 (53.9–72.8) | 71.9 (60.1–79.9) | 77.1 (67.7–83.1) | 84.3 (74.8–89.9) | <0.001 |
| Days spent home | 24.2 (23.4–24.8) | 25.3 (24.9–25.8) | 26 (25.6–26.3) | 26.9 (26.5–27.7) | <0.001 |
| Days lost to death | 0.53 (0.29–0.73) | 0.44 (0.27–0.6) | 0.32 (0.2–0.49) | 0.17 (0–0.33) | <0.001 |
| Days lost to readmissions | 0.88 (0.66–1.25) | 0.82 (0.62–1) | 0.75 (0.56–0.91) | 0.56 (0.37–0.71) | <0.001 |
| Days lost to LTAC | 0.13 (0–0.38) | 0.08 (0–0.32) | 0.06 (0–0.22) | 0 (0–0.09) | <0.001 |
| Days lost to SNF | 1.81 (1.21–2.36) | 1.18 (0.84–1.48) | 0.85 (0.57–1.13) | 0.42 (0–0.69) | <0.001 |
| Days lost to hospital stay starting the day after TAVR procedure | 2.3 (1.97–2.69) | 2.09 (1.8–2.46) | 1.9 (1.65–2.28) | 1.63 (1.38–1.94) | <0.001 |
The patient characteristics are presented as the median (25th – 75th Percentile) of hospital-level proportions (for categorical patient characteristics) and the median (25th – 75th Percentile) of hospital-level means (for continuous patient characteristics)
CKD: Chronic kidney disease, COPD: Chronic obstructive pulmonary disease, PCI: Percutaneous coronary intervention, CABG: Coronary artery bypass grafting, MI: Myocardial infarction, LTAC: Long-term acute care, SNF: Skilled nursing facility, TAVR: Transcatheter aortic valve replacement
Table 2:
Hospital characteristics across quartiles of risk-adjusted 30-day home following the TAVR procedure
| Variable | Low Performing → High Performing | P Value | |||
|---|---|---|---|---|---|
| Quartile 1 (N=163) | Quartile 2 (N=163) | Quartile 3 (N=163) | Quartile 4 (N=163) | ||
| Number of beds Median (IQR) | 423 (322–632) | 414 (286–615) | 440 (292–630) | 383 (266–520) | 0.04 |
|
Teaching affiliation (%)
Major Minor Non |
27.6 57.7 14.7 |
30.3 58.0 11.7 |
30.9 56.2 13.0 |
24.7 58.0 17.3 |
0.8 |
| Bundled payment (%) | 43.2 | 50.0 | 43.0 | 50.7 | 0.4 |
| Cardiac rehabilitation (%) | 90.7 | 95.3 | 96.1 | 87.2 | 0.01 |
|
Ownership (%)
Government Investor Not for profit |
7.4 19.6 73.0 |
8.6 13.0 78.4 |
9.9 14.2 75.9 |
5.6 9.3 85.2 |
0.1 |
|
Urban location Rural location |
98.2 1.8 |
97.5 2.5 |
97.5 2.5 |
97.5 2.5 |
0.9 |
|
Region (%)
Midwest Northeast Southeast Southwest West |
33.3 22.2 24.1 12.4 8.0 |
24.7 19.1 29.6 10.5 16.1 |
20.1 20.1 32.7 8.2 18.9 |
22.2 10.8 23.4 10.1 33.5 |
<0.001 |
| Length of hospital stay day after TAVR procedure Median (IQR), days | 2.30 (2.0–2.7) | 2.09 (1.8–2.5) | 1.90 (1.7–2.3) | 1.64 (1.4–2.0) | <0.001 |
| 30-day home time Median (IQR), days | 25.3 (24.5–25.9) | 26.9 (26.7–27.2) | 27.9 (27.7–28.2) | 29.4 (28.9–29.9) | <0.001 |
| 30-RSRR Median (IQR) (%) | 16.0 (12.1–20.7) | 14.0 (10.8–18.3) | 12.6 (9.3–15.3) | 9.2 (6.7–11.7) | <0.001 |
| 30-RSMR Median (IQR) (%) | 2.7 (1.5–4.0) | 2.2 (1.3–3.5) | 1.6 (0.9–1.5) | 0.9 (0.0–1.6) | <0.001 |
| Annual TAVR Volume median (IQR) | 54 (34–87) | 66 (36–104) | 59 (38–108) | 43 (28–71) | 0.05 |
| Rate of admissions per 100 person-months at 1 year * Median (IQR) | 11.9 (9.5–14.9) | 11.4 (9.6–14.5) | 10.1 (8.0–12.6) | 8.9 (6.8–10.5) | <0.001 |
Restricted to TAVR patients before 01/01/2019 to allow one year of follow up data (N=113,978 in 599 TAVR centers)
TAVR: Transcatheter aortic valve replacement; IQR: Interquartile range; RSMR: Risk-adjusted mortality rate; RSRR: Risk adjusted readmission rate
Association of 30-day Risk-adjusted Home Time with Clinical Outcomes on follow up
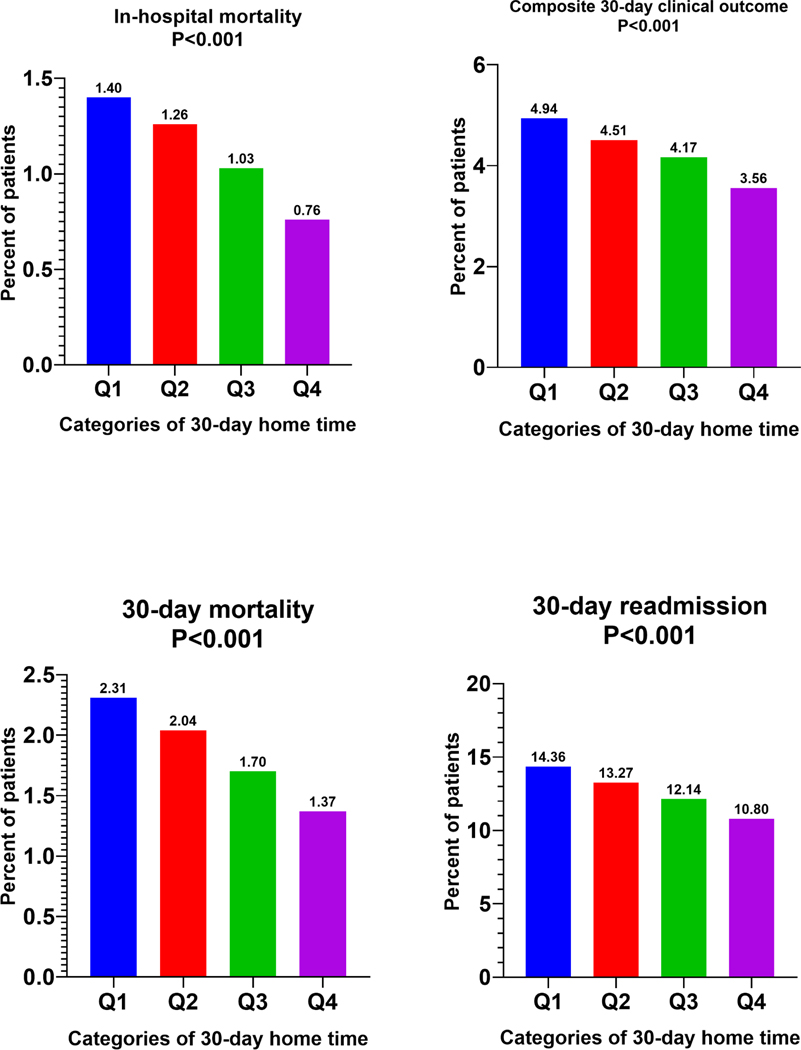
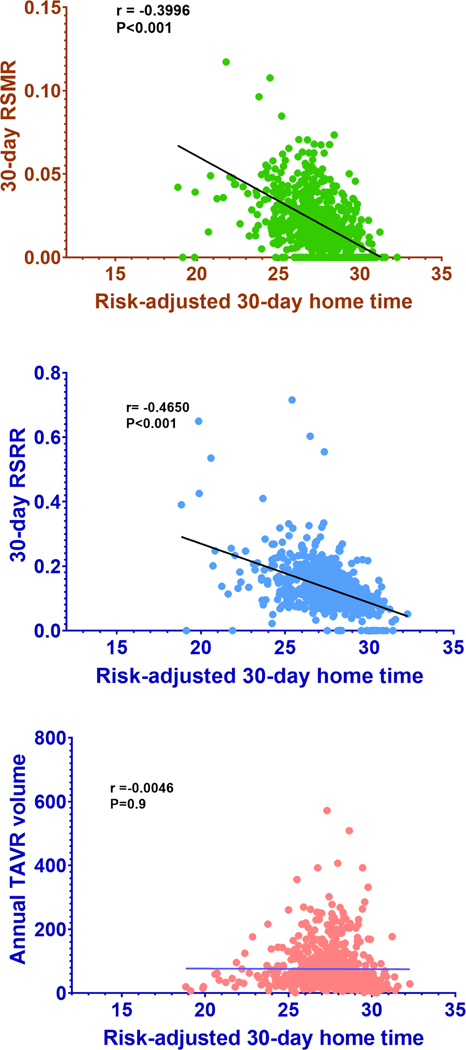
Across hospitals groups with increasing risk-adjusted 30-day home time, patients undergoing TAVR had a graded decline in the in-hospital mortality (Q1=1.40% vs. Q4=0.76%; P<0.01), 30-day mortality (Q1=2.31% vs. Q4=1.37%; P<0.01), 30-day readmission (Q1=14.36% vs. Q4=10.80%; P<0.01) and 30-day composite clinical outcome (Q1=4.94%, Q4=3.56%; P<0.01) (Figure 2). A significant inverse correlation was observed between hospital-level risk-adjusted 30-day home time and 30-RSRR (r= −0.465; P<0.001) as well as 30-day RSMR (r= 0.3996; P<0.001) in the study cohort (Figure 3). There was no correlation between risk-adjusted 30-day home time and a hospital’s annual TAVR volume (r= −0.0046; P=0.9, Figure 3). Similarly, there was no correlation between the hospital’s annual TAVR volume and 30-day RSRR or 30-day RSMR (e-Figure 3).
Figure 2.
Short-term outcomes across hospitals categories stratified by 30-day home-time.
In-hospital, 30-day mortality, 30-day readmissions and 30-day composite clinical outcome across hospitals quartiles stratified by risk-adjusted 30-day home time (Q1 worst performing → Q4 best performing).
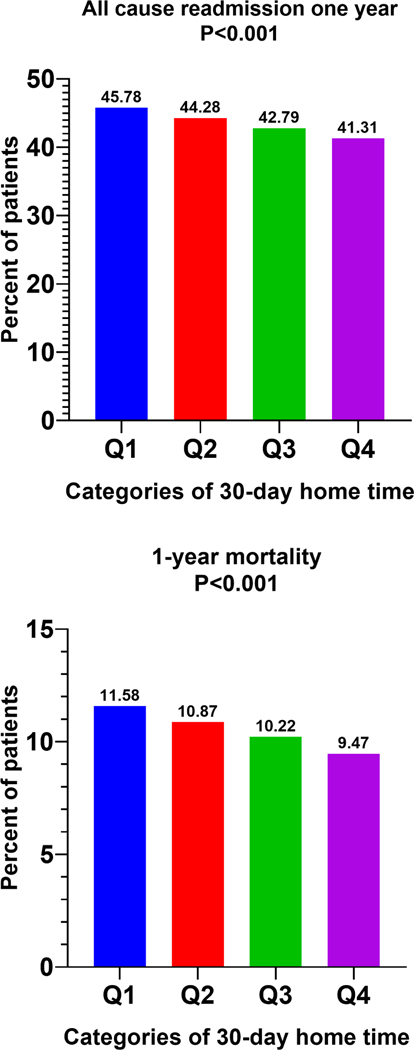
Figure 3.
Long-term outcomes across hospitals categories stratified by 30-day home-time.
One-year readmission and 1-year mortality across hospitals quartiles stratified by risk-adjusted 30-day home time (Q1 worst performing → Q4 best performing).
For 1-year mortality outcome, cohort was restricted for TAVR procedures performed before September 2019 and for 1-year readmissions, cohort was restricted for TAVR procedures performed before January 2019.
Like 30-day outcomes, 1-year mortality (Q1=11.58% vs. Q4 = 9.47%; P<0.001) and 1-year all-cause readmissions (Q1=45.78% vs. Q4=41.31%; P<0.001) among patients undergoing TAVR also decreased in a graded fashion across hospital groups of increasing 30-day risk-adjusted home time (Figure-4). A significant inverse correlation was observed between hospital-level risk-adjusted 30-day home time rate of 1-year admissions per patient-month time (r= −0.3011; P<0.001) in the study cohort (eFigure-4).
Figure 4.
Correlation between hospital-level risk-adjusted 30-day home time and other performance metrics.
Pearson correlation between risk-adjusted 30-day home time and A) 30-day RSMR, B) 30-day RSRR, and C) TAVR hospital volume. Hospital-level risk-adjusted 30-day home time was calculated as predicted/expected multiplied by crude home time for that hospital. As some hospitals had the ratio of predicted/expected >1, this could make the risk adjusted home time >30.
In sensitivity analysis stratifying hospitals by unadjusted 30-day home time following TAVR, the pattern of associations between 30-day home time and other hospital-level performance metrics was similar to that observed in the primary analysis. Thus, higher unadjusted 30-day home time was associated with lower length of stay, lower 30-day RSRR, and lower 30-day RSMR (eTable-6)
Reclassification in Hospitals Performance by risk-adjusted 30-day home time
Compared with annual TAVR hospital volume, 30-day risk-adjusted home time meaningfully up-classified 20.1% of hospitals to a higher performing, and down-classified 19.3% to lower performing strata with a NRI of 0.394 (P<0.001). Hospitals that were up classified had significantly lower 30-day and 1-year mortality than those down classified (30-day mortality 1.26% versus 1.97%, P<0.001, and 1-year mortality 9.5% vs. 10.6%, P=0.002). Compared with the 30-day RSRR, 30-day home time was associated with a meaningful reclassification in the performance status of 20.3% of the hospitals (9.1% up-classified; 11.2% down-classified, NRI 0.203, P<0.001) (Figure-5). Similarly, 19.3% of the hospitals were reclassified based on risk-adjusted 30-day home time compared with 30-day RSMR (9.1% up-classified; 10.3% down-classified, NRI 0.193, P<0.001).
Figure 5.
Hospital performance reclassification by risk-adjusted home time vs. other metrics.
Meaningful upward and downward reclassification of TAVR hospitals performance based on risk-adjusted 30-day home time compared to 30-day RSRR, 30-day RSMR, and hospital TAVR volume.
DISCUSSION
In this hospital-level analysis of Medicare beneficiaries undergoing TAVR between 2015–2019, we observed that 30-day risk-adjusted home time could be measured as a hospital-level performance metric using administrative claims data. Second, there was a considerable variation in hospital performance based on 30-day risk-adjusted home time. Third, the 30-day risk-adjusted home time was significantly correlated with other clinically meaningful hospital performance measures such as 30-day RSRR and 30-day RSMR, 30-day composite clinical outcome, and 1-year outcomes of all-cause readmission and mortality (Central Illustration). Finally, there was reclassification in 40% of the hospitals based on 30-day risk-adjusted home time vs. hospital annual TAVR volume. Reclassification in performance was also observed in up to one-fifth of the hospitals using 30-day home time vs. 30-day RSMR and 30-day RSRR.
Central Illustration.
Risk-adjusted home time as a hospital-level performance metric for TAVR
30-day home time can be estimated in patients following a TAVR procedure using claims data. Most of the home time lost over 30-day follow up following a TAVR procedure were related to in-hospital stay during the index procedure followed by days spent in skilled nursing facility and hospital stay related to readmission. Hospitals with higher 30-day risk adjusted home time demonstrated lower 30-day and 1-year mortality and readmission rates and better clinical outcomes.
In recent years, hospital TAVR volume has gained prominence as a measure of hospital TAVR quality and payors and policymakers, based on the previous observations of lower risk for adverse clinical outcomes at higher volume centers.(7) However, the association of TAVR volume— a structural performance measure— with clinical outcomes is subject to a ceiling effect such that after a certain learning curve threshold, the relationship between TAVR volume and clinical outcomes plateaus.(9) Furthermore, TAVR volume may not capture care-related variations in patients-centered outcomes post-discharge, such as functional status, independence in self-care, and quality of life. More recently, there has been an increased impetus on developing clinical outcome-based hospital performance metrics for TAVR. Desai et al. have used data from the STS/TVT registry and developed a 30-day composite risk model which focuses on the risk of procedural complications in a hierarchical order of severity.(12) The authors observed significant site-level variability in the quality of TAVR care and were able to stratify hospitals based on the peri-procedural complication risk based on this score. The 30-day risk-adjusted home time may complement the existing structural and procedural outcome-based performance metrics and provide a more comprehensive and patient-centered measure of hospital performance and care quality.
The most significant contributors to loss of home-time days among TAVR patients were days spent in the hospital and SNFs after the TAVR procedure. This is likely reflective of the high burden of comorbidities, poor functional status, and increased frailty burden among patients undergoing TAVR, which increases the need for SNF care post-discharge.(13,14) SNFs and LTACs have substantial variability in care quality and post-discharge outcomes, and contribute significantly to the healthcare costs among older adults with chronic conditions.(22) Implementing 30-day home time as a hospital-level performance metric would potentially incentivize TAVR centers to be more judicious in utilizing SNFs/LTACs and partner with higher performing SNFs/LTACs that provide better care quality. The 30-day home time-based performance metric may also promote greater use of home health care facilities among patients. Finally, the use of a comprehensive metric such as the home time that accounts for both in-hospital and post-discharge care would promote shared accountability between the TAVR performing hospital as well as the SNF and LTACs involved in the post-discharge care.
There were modest differences in hospital characteristics stratified by risk-adjusted 30-day home. Hospitals with higher home time were slightly smaller in size and had modestly lower TAVR volume. However, these differences were relatively small and suggest that 30-day home time-based hospital performance assessment may be less dependent on the structural characteristics of the hospital. These findings highlight the potentially complementary nature of home-time to existing structural measures of care quality such as TAVR volume.
There are several strengths to the use of 30-day home time as a hospital performance metric for TAVR. Prior studies have demonstrated that 30-day home time is associated with functional status, quality of life in other conditions.(17,18,23) Among patients with TAVR, 30-day home time captured variations in post-discharge health care utilization such as SNF and LTAC stays. This is particularly relevant considering the high burden of frailty, poor self-care, and poor functional status in patients undergoing TAVR.(13,14) Furthermore, the significant associations of higher 30-day risk-adjusted home time with 30-day and longer-term clinical outcomes of readmission, mortality, and adverse composite events highlights its clinical prognostic utility in ranking hospital performance. Taken together, 30-day home time provides a comprehensive measure of hospital performance that accounts for the overall burden of healthcare utilization and is associated with short-term and long-term outcomes. Furthermore, it is easily understandable for patients, families, and providers to compare different hospitals offering the procedure. Future studies are needed to determine if the hospital-level 30-day home time may also predict changes in quality of life and functional status in patients discharged following a TAVR procedure.
The use of home-time as a hospital-level performance metric should be considered in the context of some other important potential implications. In contrast with the home time estimation for acute myocardial infarction and HF,(15,16) the 30-day home time metric for TAVR was calculated from the day after the procedure to account for hospital stay during the index procedure. Length of hospital stay following TAVR may be driven by the procedural success and care provided during the peri-procedural period and thus, was incorporated in the home time estimation. It is theoretically plausible that home time as a performance metric may incentivize hospitals to perform TAVR preferentially in lower risk, less frail patients to avoid loss of home time from downstream use of LTAC or SNFs. This may be mitigated using comprehensive risk adjustment models derived from electronic medical records or existing clinical registries. Also, home time metric could potentially disincentivize hospitals to use SNF/LTAC in patients who may benefit from these post-acute care facilities. However, such practices to improve home time would be counterproductive and may lead to higher downstream readmission or mortality — outcomes that are accounted for in-home time assessment— in patients who would have benefited from SNF/LTAC use post-TAVR.
Our study has some important limitations. First, we included only Medicare beneficiaries, which makes our results not generalizable to younger patients. However, Medicare is the payer for >90% of TAVR procedures done in the US.(24) Second, we utilized administrative claims in our risk adjustment models, and thus, our analysis was prone to ICD diagnosis coding errors. Third, we lacked important information on clinical data such as ejection fraction, functional status, and medications. Fourth, we lacked information on emergency department visits, social support, home health utilization— factors that could impact home time. Finally, the risk-adjustment model derived from MedPAR inpatient claims data did not include potentially relevant outpatient clinical data.
In conclusion, hospital-level 30-day risk-adjusted home time is a meaningful patient-centered hospital performance metric for TAVR that can be estimated from administrative claims data. 30-day home time is associated with important short-term and 1-year clinical outcomes and meaningfully reclassifies hospital performance compared with other well-established hospital performance and care quality metrics.
Supplementary Material
Perspectives.
Competency in Systems-Based Practice:
Risk-adjusted home time following TAVR is a valid patient-centered hospital quality measure.
Translational Outlook:
Further research is needed to correlate home time and patient-level quality of life measures and determine the relationship of home time with utilization of post-discharge resources such as skilled nursing and long-term care facilities.
Disclosures:
Dr. Mack has received consulting fees from Gore; has served as a trial co-primary investigator for Edwards Lifesciences and Abbott; and has served as a study chair for Medtronic. Dr. Desai is a consultant for Medtronic, Myokardia, and Bristo Myers Squibb, is on the executive steering committee of a trial sponsored by Bristol Myers Squibb, and is the Haslam Family Endowed Chair in cardiovascular medicine. Dr. Pandey has served on the advisory board of Roche Diagnostics, has received non-financial support from Pfizer and Merck, has received research support from the Texas Health Resources Clinical Scholarship, the Gilead Sciences Research Scholar Program, the National Institute of Aging GEMSSTAR Grant (1R03AG067960-01), and Applied Therapeutics.
ABBREVIATIONS
- AS
Aortic stenosis
- CMS
Center for Medicare and Medicaid Services
- LTAC
Long term acute care facility
- RSRR
Risk standardized readmission rate
- RSMR
Risk standardized mortality rate
- SNF
Skilled nursing facility
- TAVR
Transcatheter aortic valve replacement
Footnotes
All remaining authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Leon MB, Smith CR, Mack MJ et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2016;374:1609–20. [DOI] [PubMed] [Google Scholar]
- 2.Kundi H, Popma JJ, Reynolds MR et al. Frailty and related outcomes in patients undergoing transcatheter valve therapies in a nationwide cohort. Eur Heart J 2019;40:2231–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mack MJ, Leon MB, Thourani VH et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med 2019;380:1695–1705. [DOI] [PubMed] [Google Scholar]
- 4.Makkar RR, Fontana GP, Jilaihawi H et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med 2012;366:1696–704. [DOI] [PubMed] [Google Scholar]
- 5.Mentias A, Sarrazin MV, Desai M, Kapadia S, Cram P, Girotra S. Expansion of transcatheter aortic valve replacement in the United States. Am Heart J 2021;234:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CMS. Proposed Decision Memo for Transcatheter Aortic Valve Replacement (TAVR). 2019. [Google Scholar]
- 7.Vemulapalli S, Carroll JD, Mack MJ et al. Procedural Volume and Outcomes for Transcatheter Aortic-Valve Replacement. N Engl J Med 2019;380:2541–2550. [DOI] [PubMed] [Google Scholar]
- 8.Khera S, Kolte D, Gupta T et al. Association Between Hospital Volume and 30-Day Readmissions Following Transcatheter Aortic Valve Replacement. JAMA Cardiol 2017;2:732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russo MJ, McCabe JM, Thourani VH et al. Case Volume and Outcomes After TAVR With Balloon-Expandable Prostheses: Insights From TVT Registry. J Am Coll Cardiol 2019;73:427–440. [DOI] [PubMed] [Google Scholar]
- 10.Dodson JA, Williams MR, Cohen DJ et al. Hospital Practice of Direct-Home Discharge and 30-Day Readmission After Transcatheter Aortic Valve Replacement in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (STS/ACC TVT) Registry. J Am Heart Assoc 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirji S, McGurk S, Kiehm S et al. Utility of 90-Day Mortality vs 30-Day Mortality as a Quality Metric for Transcatheter and Surgical Aortic Valve Replacement Outcomes. JAMA Cardiol 2020;5:156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desai ND, O’Brien SM, Cohen DJ et al. A Composite Metric for Benchmarking Site Performance in TAVR: Results from the STS/ACC TVT Registry. Circulation 2021. [DOI] [PubMed] [Google Scholar]
- 13.Afilalo J, Lauck S, Kim DH et al. Frailty in Older Adults Undergoing Aortic Valve Replacement: The FRAILTY-AVR Study. J Am Coll Cardiol 2017;70:689–700. [DOI] [PubMed] [Google Scholar]
- 14.Kiani S, Stebbins A, Thourani VH et al. The Effect and Relationship of Frailty Indices on Survival After Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv 2020;13:219–231. [DOI] [PubMed] [Google Scholar]
- 15.Pandey A, Keshvani N, Vaughan-Sarrazin MS et al. Evaluation of Risk-Adjusted Home Time After Hospitalization for Heart Failure as a Potential Hospital Performance Metric. JAMA Cardiol 2021;6:169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandey A, Keshvani N, Vaughan-Sarrazin MS, Gao Y, Girotra S. Evaluation of Risk-Adjusted Home Time After Acute Myocardial Infarction as a Novel Hospital-Level Performance Metric for Medicare Beneficiaries. Circulation 2020;142:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quinn TJ, Dawson J, Lees JS et al. Time spent at home poststroke: “home-time” a meaningful and robust outcome measure for stroke trials. Stroke 2008;39:231–3. [DOI] [PubMed] [Google Scholar]
- 18.Lee H, Shi SM, Kim DH. Home Time as a Patient-Centered Outcome in Administrative Claims Data. J Am Geriatr Soc 2019;67:347–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.https://qualitynet.cms.gov/inpatient/hrrp/measures. Date of Access: 10/13/2021.
- 20.Krumholz HM, Lin Z, Drye EE et al. An administrative claims measure suitable for profiling hospital performance based on 30-day all-cause readmission rates among patients with acute myocardial infarction. Circ Cardiovasc Qual Outcomes 2011;4:243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krumholz HM, Wang Y, Mattera JA et al. An administrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with an acute myocardial infarction. Circulation 2006;113:1683–92. [DOI] [PubMed] [Google Scholar]
- 22.Neuman MD, Wirtalla C, Werner RM. Association between skilled nursing facility quality indicators and hospital readmissions. JAMA 2014;312:1542–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nair R, Gao Y, Vaughan-Sarrazin MS, Perencevich E, Girotra S, Pandey A. Risk-Standardized Home Time as a Novel Hospital Performance Metric for Pneumonia Hospitalization Among Medicare Beneficiaries: a Retrospective Cohort Study. J Gen Intern Med 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel N, Doshi R, Kalra R, Bajaj NS, Arora G, Arora P. Costs of Transcatheter Aortic Valve Replacement: Implications of Proposed Medicare Cuts. JACC Cardiovasc Interv 2018;11:610–612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.