Abstract
Kudzu root (Pueraria lobate (Willd.) Ohwi, KR) is an edible plant with rich nutritional and medicinal values. Over the past few decades, an ample variety of biological effects of Pueraria isoflavone have been evaluated. Evidence has shown that Pueraria isoflavone can play an active role in antioxidant, anti-inflammatory, anti-cancer, neuroprotection, and cardiovascular protection. Over 50 isoflavones in kudzu root have been identified, including puerarin, daidzein, daidzin, 3′-hydroxy puerarin, and genistein, each with unambiguous structures. However, the application of these isoflavones in the development of functional food and health food still depends on the extraction, purification and identification technology of Pueraria isoflavone. In recent years, many green and novel extraction, purification, and identification techniques have been developed for the preparation of Pueraria isoflavone. This review provides an updated overview of these techniques, specifically for isoflavones in KR since 2018, and also discusses and compares the advantages and disadvantages of these techniques in depth. The intention is to provide a research basis for the green and efficient extraction, purification, and identification of Pueraria isoflavone and offers investigators a valuable reference for future studies on the KR.
Keywords: puerarin isoflavone, extraction, purification, analysis techniques
1. Introduction
Kudzu root (Pueraria lobate (Willd.) Ohwi, KR), a legume taxon indigenous to Southeast Asia, boasts a rich variety of species and can be found across China [1], including in Hebei, Guangxi, Anhui, Liaoning, and Shandong. In fact, it has become one of the most prevalent twining shrub plants in urban forests. In 2022, the Ministry of Health of China officially declared KR as a “medicine and food homology” plant, thereby expanding its use in health food.
Kudzu root is a traditional Chinese food and medicinal herb. It was commonly used in fresh food, stir-fried vegetables, soup, and tea. Pueraria-series products have developed in recent years due to their abundance of nutrients like starch, isoflavones, and dietary fiber, both domestically and internationally. At present, there are many explorations on the eating methods of KR. In addition to being used in meal replacement powders for brewing and eating, KR noodles, biscuits, cakes, steamed bread, and other flour products can also be made by combining KR with other botanical raw materials [2,3]. KR can be used to create a variety of tasty beverages [4,5,6]. Most importantly, as an excellent natural material for the development of new health foods, KR is extensively used in the food and health product industries and has great market potential. By searching the website of the China State Administration for Market Regulation using the keyword “Kudzu root”, 613 health food products containing KR with clear approval numbers were collected. There were 386 examples of KR health food in the form of capsules. KR health food was produced into 27 different types of tea, 9 different types of powder, and 6 different types of beverages.
The common ingredients of KR include flavonoids, starch, cellulose fiber, protein, etc. Among them, the best-effect ingredients are isoflavones. Isoflavones are part of a large family of secondary plant metabolites called flavonoids. They are dietary phytoestrogens occurring naturally in legumes. A reliable source of isoflavones is Pueraria lobata, according to pertinent research studies. The mass fraction of puerarin can range from 1.58% to 7.68%, whereas the overall mass fraction of isoflavones in Pueraria lobata can range from 6.2% to 17.0% [7,8]. Currently, it has been revealed that more than 50 different types of puerarin isoflavone have unambiguous structures, such as Puerarin [9], Daidzein, Daidzin, Puerarinxyloside [10], 3′-Hydroxy Puerarin, Genistein, Genistin, Biochanin A, Formononetin [11], 6″-O-Malonylgenistin [12], 6″-O-α-d-Glucopyranosylpuerarin [13], Calycosin [14], 4′-Hydroxy-7-Hydroxymethyl-6-Methoxyisoflavone [15], 4′,6-Dimethoxy-8-Hydroxy-7-Hydroxymethyl Isoflavone [16], 3′-MethoxypuerarinA, 3′-MethoxypuerarinB [17], etc.
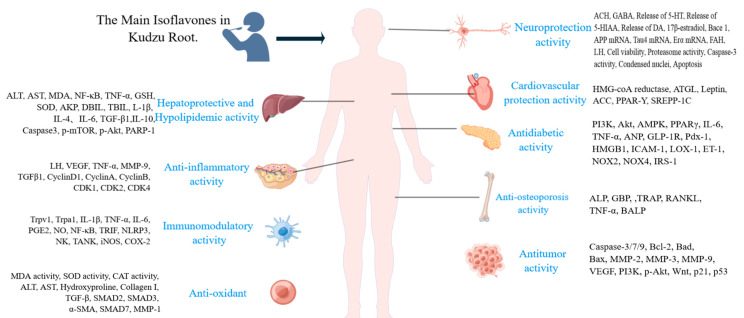
The main isoflavones in KR are displayed in Table 1. The biological activity and mechanism studies of PR isoflavone are shown in Figure 1. They have demonstrated positive effects, including hepatoprotective [18,19], anti-inflammatory [20], anti-cancer [21,22,23], anti-osteoporosis [24,25,26], and protection for the heart and brain [27,28]. Puerarin can be used to reduce cardiac hypertrophy and arrhythmia [27,28,29,30] and prevent atherosclerosis [31,32]. Research has revealed that puerarin isoflavone has positive effects on common health issues like diabetes, hypertension, and hyperlipidemia. It has been established that puerarin, genistein, daidzein and daidzin are efficient treatments for hypertension [33,34,35,36,37,38]. Pueraria extract, according to Li et al., can lower blood pressure in mice fed a high-salt diet by modulating gut flora [37]. According to Yang et al., pueraria is effective in treating physical illnesses that co-occur with depression [38]. It covers post-stroke depression as well as depression caused by coexisting conditions, such as diabetes, coronary heart disease, migraines, Parkinson’s disease, and others. KR has the potential for an alcohol intake reduction [39] and, thus, in the long-term, prevention of alcohol addiction [40]. Zhou et al. gave alcoholic male Wistar rats puerarin, daidzein, and puerarin extract [41]. The findings demonstrated that daidzin and puerarin were able to dramatically raise the levels of two types of ghrelin, which in turn decreased alcohol consumption.
Table 1.
The main isoflavones in kudzu root [42].
| Name | Chemical Formula | Structural Formula | Content (μg/g) |
|---|---|---|---|
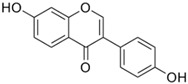
| Puerarin | C21H20O9 |
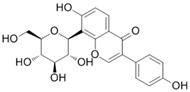
|
4.28–76.10 |
| Daidzein | C15H19O4 |
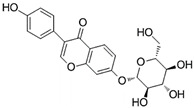
|
0.36–16.48 |
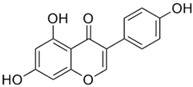
| Daidzin | C21H12O9 |
|
0.05–6.74 |
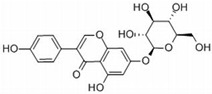
| 3′ hydroxyPuerarin | C21H20O10 |
|
0.20–20.61 |
| Genistein | C15H10O5 |
|
- |
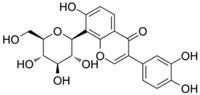
| Genistin | C21H20O10 |
|
7.63–51.43 |
| Formononetin | C16H12O4 |
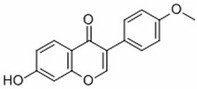
|
- |
| 6″-O-Malonylgenistin | C24H22O13 |
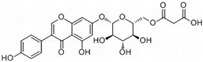
|
- |
-: Not reported.
Figure 1.
Bioactivities of main isoflavones in kudzu root and their underlying mechanisms.
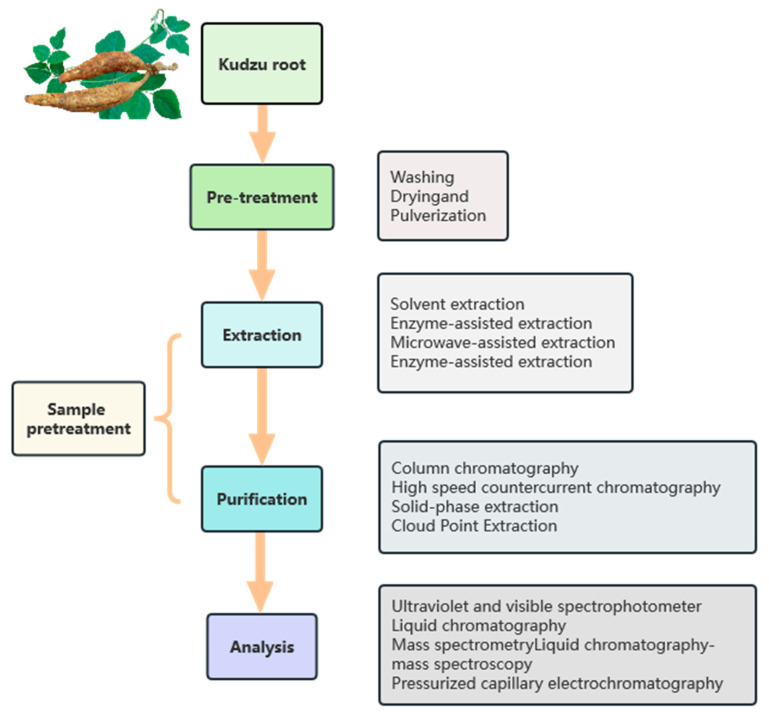
Effective and adequate procedures are required for the extraction, purification, and analysis of puerarin isoflavone. In recent years, a variety of methods for extracting flavonoids have been developed, such as organic solvent extraction, ultrasonic-assisted extraction, microwave extraction, and supercritical fluid extraction. Advances in science and technology have made it possible to identify puerarin isoflavone using more sophisticated instruments and multi-instrument combination detection methods, such as high-resolution mass spectrometry (HRMS), LC-MS, GC-MS, etc. This review provides an updated overview of these techniques for isoflavones in KR. A general outline of a sample flow chart covering the preparation, extraction, purification, and analysis process is represented in Figure 2.
Figure 2.
The procedure of extraction, purification, and structural analysis of isoflavones in kudzu root.
2. Sample Pretreatment Methods
Sample pretreatment refers to the separation of components from complex systems by enzymatic hydrolysis, extraction, enrichment, purification, and concentration. It is a process of removing impurity interference, increasing the concentration of the component to be tested, and facilitating the qualitative and quantitative detection of the instrument. The primary goal of the enzymatic hydrolysis process is to dissolve, suspend, or glue the intracellular components into the solvent in order to achieve the goal of extraction. This is achieved by using one or more enzyme solutions to dissolve the plant’s cell wall and break macromolecular chains like those of cellulose and pectin through hydrolysis. The removal of impurity interference and a rise in the concentration of the material under testing can be accomplished through purification, which facilitates qualitative and quantitative detection by the equipment. Effective pretreatment technology may increase target separation, minimize loss, and boost the extraction rate [43,44,45]. The methods for preparing and analyzing KR samples to identify isoflavone components are presented in Table 2.
Table 2.
Summary of the various techniques used to prepare analytical kudzu root for the determination of their isoflavones.
| Analytes | Extraction Method | Extraction Solvent | Condition | Separation and Purification | Analysis Method | Productive Rate (%) |
References |
|---|---|---|---|---|---|---|---|
| Isoflavones | Sonication extract (PLs) and reflux extract (PLr) | Methanol | Ultrasonic bath (frequency of 40 kHz; Power Sonic 520 W, at room temperature for 2 h.) | Semi-preparative reversed-phase HPLC | HPLC | 7.99–10.57 | [46] |
| Puerarin, daidzin, daidzein | Refluxing extraction | 80% ethanol | Refluxed in a water bath at 80 °C for 2 h | MSPE Magnetic solid-phase extraction |
ZIF-8-pressurized capillary electrochromatography (pCEC) | - | [47] |
| Puerarin and total flavonoids | Immersion method | 30% ethanol | Water bath (70 °C) | - | UPLC-MS, NIR, and UV-Vis portable | - | [48] |
| Nine isoflavones | Ultrasonic-assisted extraction | Methanol | Sonicated at 40 °C for 20 min | - | HPLC | - | [8] |
| puerarin | SPE | 80% methanol | Sonicated for 30 min | SPE | LC-MS/MS | - | [49] |
| Six isoflavones | Refluxing extraction and ultrasonic-assisted extraction | 50% methanol | Ultrasonic bath (frequency of 40 kHz; Power Sonic 120 W) | - | UPLC | - | [50] |
| Isoflavones | Ultrasonic-assisted extraction based on NADES | NADESs: choline chloride and citric acid at a 1:2 molar ratio | Ultrasonic bath (frequency of 37 kHz; Power Sonic 580 W, at 60 °C for 3 h.) | Reversed stationary phase column | HPLC-DAD | 1.09 ± 0.006 | [51] |
| Isoflavone | Ultrasonic-assisted extraction | Water or ethanol 65° | Amplitude = 65% nominal power, cycle = 1, 40 °C ± 1 °C | - | HPLC-PDA | - | [52] |
| Puerarin and daidzein | Ultrasonic-assisted extraction | Methanol-glacial acetic acid (100:1) | Ultrasonic bath (frequency of 40 kHz; Power Sonic 100 W) | - | Non-aqueous capillary electrophoresis (NCAE) | - | [53] |
| Puerarin | Ultrasonic-assisted extraction | 70% ethanol | Ultrasonic bath (Power Sonic 350 W at 60 °C for 3 h.) | Acid hydrolysis | HPLC-IR | - | [54] |
| Puerarin and daidzein | Ultrasonic-assisted extraction | Water | - | - | HPLC | - | [55] |
| Total flavonoids | Ultrasonic-assisted extraction | 35% ethanol | Ultrasonic bath (frequency of 53 kHz; Power Sonic 200 W) | - | UV | 2.76 | [56] |
| Flavonoids | Microwave-assisted extraction | 60% ethanol, | Microwave power 340 W for 4 min | - | UV | - | [57] |
| Six isoflavones | Refluxing extraction | 30% ethanol | - | - | HPLC | - | [58] |
| Puerarin | Ultrasonic-assisted extraction | 70% ethanol | Ultrasonic bath (frequency of 42 kHz; Power Sonic 70 W) | - | UV | - | [59] |
| Flavonoids | Ultrasonic-assisted extraction | 75% ethanol | Ultrasonic extraction 30 min | - | UV | - | [60] |
| Nine isoflavones | SPE | 20% ethanol solution (containing 0.1% formic acid) | - | - | pCEC | - | [61] |
| Puerarin and daidzein | Ultrasonic-assisted extraction | Ethanol | - | - | Differential pulse voltammetry | - | [62] |
| flavonoids | Refluxing extraction | 60% ethanol | Reflux extraction 1.5 h | - | UV-Vis | - | [63] |
| Flavonoids | Microwave-assisted extraction | 42% ethanol | Microwave power 828 W for 23 min | - | UV | 11.74 | [64] |
| Puerarin | Ultrasonic-assisted extraction | 58% ethanol | Ultrasonic bath at 70 °C for 32 min | - | UV-Vis | - | [65] |
| Puerarin | Microwave-assisted extraction | 70% ethanol | Mrowave 9.7 min | column C18 | HPLC | - | [66] |
| flavonoids | Immersion method | 40% ethanol | Water bath at 80 °C for 2 h | - | UV | 3.06 | [67] |
| Four isoflavones | Ultrasonic-assisted extraction | 30% ethanol | Ultrasonic extraction 1 h | - | HPLC | - | [68] |
| Three isoflavones | Ultrasonic-assisted extraction | 50% ethanol | Ultrasonic extraction 40 min | - | HPLC | - | [69] |
| Six isoflavones | Ultrasonic-assisted extraction | 70% methanol | Ultrasonic bath (frequency of 40 kHz; Power Sonic 250 W, for 3 h.) | - | HPLC | - | [70] |
| Five isoflavones | Ultrasonic-assisted extraction | 70% methanol | Ultrasonic extraction 1 h | - | HPLC | - | [71] |
| Puerarin | Ultrasonic-assisted extraction | 0.6 mg/mL β-CD | Ultrasonic extraction at 40 °C for 1 h | - | Three-dimensional fluorescence spectrum | - | [72] |
| Flavonoids | Ultrasonic-assisted extraction | 40% ethanol | Ultrasonic bath (Power Sonic 300 W for 20 min) | - | GC/MS | - | [73] |
| Puerarin and daidzein | Refluxing extraction | 80% ethanol | - | - | HPLC | - | [74] |
| Puerarin | Microwave-assisted ionic liquid extraction | 1.0 mol/L ionic liquids | Microwave power 400 W for 8 min | - | UV | - | [75] |
| Four isoflavones | Immersion method | 30% ethanol | - | - | HPLC | - | [76] |
| Total flavonoids and puerarin | Refluxing extraction | Methanol | Heat reflux extraction 1 h | - | UV-Vis and HPLC | - | [77] |
| Puerarin | Microwave-assisted enzymatic extraction technology | Cellulose dose 190 U/g | Microwave power 450 W for 7 s | - | UV | 8.87 | [78] |
-: Not reported.
2.1. Extraction Methods of Pueraria Isoflavone
The current research demonstrated that extraction is the basis of the research and application of KR isoflavones; research about the extraction methods mainly focuses on the extraction rate. It has always been the focus of researchers to improve the extraction efficiency of isoflavones from KR. From the perspectives of extraction rate, industrial applicability, and environmental protection, the extraction methods of KR isoflavones can be classified into traditional extraction methods and modern extraction methods. Solvent extraction is a commonly used traditional isoflavone extraction method, and the solvents include supercritical fluids and ionic liquids. Modern extraction techniques include microwave extraction, ultrasonic extraction, enzyme-assisted extraction, high-speed countercurrent chromatography, solid-phase extraction, and cloud point extraction.
2.1.1. Solvent Extraction
According to the extract’s temperature, the solvent extraction process is often split into cold and hot categories. Percolation and impregnation are the two primary components of the cold extraction process. Decoction, continuous reflux, sometimes known as Soxhlet extraction, and reflux extraction are the three basic techniques used in hot extraction [79]. The most commonly used method for extracting isoflavones from KR is organic solvent extraction, followed by solvent removal. The secret to increasing extraction efficiency is by selecting the right extraction solvent. Ethanol, methanol, and water are usually used to extract isoflavones from KR. Because of its great extraction efficiency and low toxicity, ethanol is preferred. Xu [48] established a dual-spectral technique based on near-infrared (NIR) and ultraviolet-visible (UV-Vis) spectroscopy for the analyses of puerarin and total flavonoids by 30% ethanol (400 mL) to extract the puerarin and total flavonoids from KR in a water bath (70 °C) for 120 min. Huang’s [50] investigation examined the extraction of several active components using reflux extraction, ultrasound-assisted extraction, and various extraction solvents (30%, 50%, 80% methanol, and 30%, 50%, and 80% ethanol). The findings revealed no discernible difference between reflux and ultrasound, with the best extraction effect occurring when 50% methanol was utilized as the extraction solvent.
More and more green solvents are emerging to replace conventional volatile harmful solvents as science and technology advance. For instance, the extraction of Pueraria isoflavone using ILS, non-ionic surfactant micelle solution, and NADES. The application of novel green solvents in flavonoid extraction is presented in Table 3. Deep eutectic solvents (DES) are a class of green solvents commonly associated with ionic liquids because of their common properties, such as high thermal stability, low volatility, and low vapor pressure [80]. NADESs are composed of natural compounds produced by cell metabolism, and they have similar characteristics to DES. For the extraction of puerarin isoflavones, Saied A [81] created NADESs with a molar ratio of choline chloride to citric acid of 1:2. A high-performance liquid chromatography (HPLC)-diode array detector (DAD) coupled with high-resolution (HR) mass spectrometry (MS) was used to detect the extract of KR, and 10 isoflavones were detected. The amount of isoflavones in KR was measured to be 1.09 ± 0.006% overall. Puerarin was extracted from KR by Fan [82] using ultrasonic-aided extraction with IL as the extraction solvent using the response surface optimization approach. The quality of KR, the type and concentration of IL, the strength and duration of ultrasonic extraction, and all of these factors were optimized. Finally, 0.43 g of KR raw materials was added to 10 mL of 1.06 mol/L 1-butyl-3-methylimidazolium bromide aqueous solution. The best extraction effect could be reached by extracting for 27.43 min while using 480 W of ultrasonic power.
Table 3.
Application of novel green solvents in flavonoid extraction.
| Title Compounds |
Species | Composition | Auxiliary Extraction |
Extraction Effect | References |
|---|---|---|---|---|---|
| Daidzein, genistein, puerarin | NADESs | ChCl/citric acid | - | NADESs extract had higher antioxidant activity than methanol extract and significantly reduced the degradation of isoflavones. | [83] |
| Puerarin | NADESs | L-Pro/malic acid | - | The extraction amount of NADESs was 2.2 times higher than that of water and also significantly higher than that of methanol. The bioavailability of the extract was 323% of the aqueous extract. | [84] |
| Puerarin | IL | 1-normal-butyl-3- methylimidazolium chloride |
MAE | The extraction rate of puerarin was 4.201%, which was three times higher than that of the traditional extraction method. | [75,85] |
| Flavonoids | IL | 1-Butyl-3-methylimidazolium bromide | UAE | The extraction amount of pueraria flavonoids was 774.95 mg/g. | [86] |
| Puerarin isoflavones |
NADESs | Choline chloride to citric acid of 1:2 | - | The amount of isoflavones in KR was measured to be 1.09 ± 0.006% overall. | [81] |
| Puerarin | IL | 1-butyl-3-methylimidazolium bromide aqueous solution | UAE | The proposed ILUAE offered shorter extraction time and remarkably higher efficiencies | [82] |
-: Not reported.
Regarding other unique isoflavones in non-KR, various research is also very extensive. Methanol, acetone, ethanol, and water were utilized by Yoshiara [87] as solvents to extract soybean isoflavones. Fifteen groups of mixed solvents were set up to extract soybean isoflavones using the simplex-centroid design method. The outcomes demonstrated that various soybean isoflavone types had various extraction efficiencies as a result of various structures. The glycoside isoflavones were effectively extracted using a combination of water, acetone, and acetonitrile. The extraction of isoflavones in the form of malonyl glucoside was positively impacted by the mixture of water, acetone, and ethanol. Jurga [88] promoted the extraction of daidzein and genistein from red clover blossoms using excipients in conjunction with ultrasound-assisted, hot reflux, and impregnation procedures. Finally, it was found that a vinylpyrrolidone-vinyl acetate copolymer facilitated the solubilization and availability of the active components of a plant extract.
2.1.2. Microwave-Assisted Extraction (MAE)
The microwave-assisted extraction method, which is frequently used for trace analysis of organic compounds in solid and liquid samples [89], builds on solvent extraction. MEA utilizes the internal heating effect of the microwave (thermal stress breaks the cells, the solvent and material molecules constantly rub with the change in alternating electromagnetic fields, and the degree of cell breakage increases). It benefits from low liquid consumption, high extraction efficiency, strong selectivity, low pollution, and consistent heating, which prevents the gelatinization and agglomeration of the medicinal components. However, because of the rapid local temperature rise, it may quickly denature and inactivate the heat-sensitive material, reducing the effectiveness of the extraction. Liu [64] used response surface methodology to investigate the effects of various factors on the extraction efficiency of total flavonoids from PuerariaeLobatae Radix. The results showed that the effect of various factors on the extraction efficiency was a specific gravity liquid–solid ratio > ethanol concentration > time > power. When 42% ethanol solution was used, the liquid–solid ratio was 43:1, the microwave power was 828 W, the extraction time was 23 min, and the best extraction effect was obtained. Hu [57] optimized the process conditions, such as the volume fraction of the extraction agent, extraction time, microwave power and solid-liquid ratio after the cleaning, drying, crushing, and sieving of KR. Finally, the extraction was carried out under the conditions of a 60% ethanol volume fraction, 1:50 solid–liquid ratio, 4 min extraction time and 340 W microwave power. Shi [90] used the orthogonal test method to optimize the extraction process of Pueraria isoflavone with the extraction amount of five Pueraria isoflavones as the evaluation index. The optimum extraction process of KR was as follows: 60% ethanol reflux extraction three times, each time adding 15 times the amount of solvent, and extracting for 1 h. The results showed that the contents of 3′-hydroxy puerarin, 3′-methoxy puerarin, puerarin, daidzin, and daidzein were more than 90% of the total isoflavone content.
Cai [91] studied the microwave-assisted extraction technology of genistein and genistin from Flemingia Macrophylla with uniform design and orthogonal design. The result showed that the optimal extraction process was set as follows: microwave power of 700 W, extraction temperature of 80 °C, analytical agent ratio of 7.1 mL/g, microwave time of 120 s, liquid/solid ratio of 35 mL/g, and an extraction time of 50 min. Under the optimum conditions, the yield of genistein and genistin was 1.3804 mg/g. Li [92] used methanol reflux extraction to determine calycoisoflavones and formononetin by HPLC. The optimal microwave conditions were as follows: low-temperature heating for 5 min, thickening for 2 cm, calycoflavone 3.984 μg/g, and formononetin 42.314 μg/g.
2.1.3. Ultrasound-Assisted Extraction (UAE)
The cavitation effect caused by an ultrasonic wave is used to create a cavitation gas cannon in the extraction solution in the ultrasonic-aided extraction technique, which is based on the solution extraction method. With the eruption of the shock wave, the cavitation gas cannon bursts, destroying the plant’s cell wall and increasing the solubility of the target component [93]. In contrast to other instrument-assisted extraction methods, it does not need heating, making it safe and considerate of chemicals that are sensitive to heat. Its limitation by ultrasonic attenuation factors is a drawback. For instance, the tank is too big to create an ultrasonic blank region, which negatively impacts the effectiveness of the extraction process. Punam [46] employed several extraction techniques and methanol as the extraction solvent to extract isoflavones from KR flowers. Comparisons were made between the methanol ultrasonic extract (PLs) and the methanol reflux extract (PLr). In PLs and PLr, the percentage of Pueraria isoflavone was 7.99% and 10.57%, respectively. Zhou [70] utilized 50 mL of 70% methanol as the extraction solution at 40 °C and 0.2 g of PuerariaeLobatae Radix and PuerariaeThomsonii Radix sample powder. HPLC was utilized to establish the content detection technique of 3′hydroxy puerarin, puerarin, 3′-methoxy puerarin, daidzin, puerarinapioside, and puerarin-6″-O-xyloside in KR and Pueraria thomsonii under the conditions of 250 W, 40 kHz, and ultrasonic extraction for 30 min. For the extraction of puerarin, Wu [94] utilized ethanol with a concentration of 71.35%, an extraction period of 49.08 min, and a solvent-to-material ratio of 21.72. Li [95] used single-factor and Box–Behnken experiments to improve the extraction conditions of flavonoids from “Gange 2” and KR. Two different types of Pueraria flavonoids produced yields of 3.66 mg/g and 4.08 mg/g under ideal circumstances.
2.1.4. Enzyme-Assisted Extraction (EAE)
The structure of the plant’s cell wall and intercellular material will obstruct the diffusion of the target compounds into the extraction solvent during the extraction of plant isoflavones. The addition of enzymes will destroy the normal structure of cell walls and intercellular substances [96]. Common enzymes employed in the extraction of the plant’s active components include cellulase, pectinase, amylase, and protease. Single-enzyme extraction technology is being used in the actual extraction process less and less. The combination of composite enzyme methods, enzyme-assisted methods, and ultrasonic-assisted methods is being used increasingly [97]. The benefits of EAE include gentle reaction conditions, eco-friendly extraction solvents, and minimal active substance loss. However, because the enzyme is environment-sensitive, it will become inactive at high temperatures and at low pH levels, the extraction cost will increase, and the extraction efficiency will be reduced. By using the response surface approach, Liu [78] adjusted the extraction parameters for the enzyme-microwave-assisted synergistic extraction of puerarin. They looked into the three variables of cellulose dosage, microwave power, and microwave treatment time. By using the response surface method, the ideal procedure was discovered: a cellulose dosage of 190 U/g, microwave power of 450 W, and microwave treatment period of 7 s. The extraction rate for puerarin under these circumstances was 8.87 mg per 100 g. The method parameters for the enzymatic-ultrasonic extraction of puerarin from KR were improved by Huang [98]. According to the findings, the ideal conditions for extracting puerarin from KR were as follows: 0.4% cellulase addition, 70 min of enzymatic hydrolysis, a 30:1 (mL/g) liquid–solid ratio, 52% volume of ethanol, 31 min ultrasonic extraction, and 64 °C for the ultrasonic temperature. The yield of puerarin in these circumstances was 8.78 mg/g.
The extraction of soybean isoflavones has always been the focus of research. In order to increase the extraction rate of soybean isoflavones, Guo [99] selected Trichoderma viride cellulose from ten cellulases and optimized the amount of enzyme added, as well as the enzymatic hydrolysis temperature, enzymatic hydrolysis time, substrate concentration, reaction system pH, and other parameters. The findings demonstrated that more cost-effective and efficient parameters included substrate concentrations of 0.8 to 2.0 mg/mL, enzyme dosages of 7% to 11%, a reaction system pH of 5.0, an enzymatic hydrolysis temperature of 55 °C, and shaking reaction times of 5 to 6 h. The overall rate of aglycone conversion and the total rate of hydrolysis of soybean isoflavone glycosides, respectively, were as high as 93.21% and 59.25%.
2.1.5. Extraction Techniques Summary
The conventional solvent extraction technique is ineffective, and using more solvents increases the possibility of environmental damage. In addition to being a popular topic in current research, the emergence of NADESs offers a new option for the extraction of Pueraria isoflavones and other plant-active components. Technology for modern instrument-assisted extraction has been continuously improved. Although Pueraria isoflavone extraction efficiency and time can be increased and decreased with the use of ultrasonic, microwave, and enzyme-assisted technologies, there are still certain drawbacks. For instance, some isoflavones that readily degrade and are not heat-resistant cannot be extracted with MAE. Ultrasonic blank areas and high noise are drawbacks of UAE. The expense and easily influenced enzyme activity by the environment are the two drawbacks of EAE. As a result, the composite extraction approach is the main area of interest and development right now. A current issue is how to use it for the commercial extraction of Pueraria isoflavones.
2.2. Purification Techniques for Preparing Pueraria Isoflavone
In order to further obtain high-purity isoflavones, it is often necessary to separate and purify the extracted isoflavones through a complex process.
2.2.1. Column Chromatography
Column chromatography is a typical technique that utilizes the various partition coefficients of various components in the stationary phase and the mobile phase. It is often used to separate and purify flavonoids from plant-active substances. C18 reverse-phase fillers, silica gel, macroporous resin, etc., are examples of fillers that are frequently employed. Its major benefits are high efficiency, low cost, and ease of operation. Yang’s method [100] for enriching puerarin and daidzein in PuerariaeLobatae Radix utilized the HPD800 macroporous adsorption resin. The purity of puerarin increased from 8.62% to 35.26% after macroporous adsorption resin enrichment, and the purity of daidzein grew from 0.276% to 10.17%. The ability of AB-8, NKA, and X-5 macroporous adsorption resins to adsorb the total flavonoids from KR was examined by Wang [101]. The yield of the total flavonoids was 76.22%, and the results indicated that AB-8 resin was the optimum adsorbent for separation and purification. The extracting solution of KR was combined with the 0.1 M Ni2+ solution by Pan [102], separated using the dry silica gel column technique, and eluted using a gradient of chloroform and methanol. Finally, more than 95% of genistein and daidzein were pure. The ultimate extraction rate was 2.09% and was achieved by Yang [66] using a 70% ethanol solution microwave-assisted extraction, C18 chromatographic column separation and purification, and diode array detection. Additionally, semi-preparative HPLC is a quick and efficient separation technique for high-purity puerarin isoflavones. Using semi-preparative reverse-phase HPLC, Punam [46] isolated and purified isoflavones from the flowers of KR. When isoflavones from KR flowers were extracted using various techniques, the concentration ranged from 7.99% to 10.57%. An innovative technique for extracting and separating isoflavones from KR was created and confirmed by Li [103]. The separation of isoflavones occurred using countercurrent chromatography and semi-preparative liquid chromatography, as well as ultrasound-assisted extraction of the isoflavones based on ionic liquids. In the end, 500 g of KR yielded daidzein-4′, 7-diglucoside (42.2 mg), puerarin 6′-O-xyloside (88.3 mg), and 3′-hydroxypuerarin (48.5 mg) with purities of 98.2%, 96.3%, and 97.1%, respectively.
2.2.2. High-Speed Countercurrent Chromatography (HSCCC)
HSCCC is a countercurrent chromatography-based separation method that primarily enhances the resolution, injection, and separation time of CCC. It uses chromatography with liquid–liquid separation [54,104]. It combines the benefits of liquid–liquid extraction and distribution chromatography, replaces the solid stationary phase in the conventional separation method with liquid, and can be extensively employed in the separation and purification of various components in natural mixtures. It has recently become one of the hotspots in the study of effective separation techniques [54,105]. High-speed countercurrent chromatography can be used to separate natural antioxidants because traditional solid–liquid separation cannot prevent the irreversible adsorption loss of the solid carrier matrix utilized in the chromatographic column. The monomer compounds were extracted by Liu [106] using a two-phase solvent system of ethyl acetate, ethanol, and water (4.0:0.5:3.0, v/v/v). The monomer compounds were then separated and purified by high-speed countercurrent chromatography and assessed by high-performance liquid chromatography. In the end, three compounds were obtained: puerarin (90.60%), genistin (99.00%), and irisflorentin (91.73%). The puerarin extract was chosen as the research object by He [107], who used the HSCCC ethyl acetate: n-butanol: water (2:1:3, v/v/v) solvent system at a speed of 900 r/min with a flow rate of 1.5 mL/min and a separation temperature of 25 °C under the conditions of separation and purification of high-purity puerarin samples by ultraviolet absorption spectroscopy, finally obtaining a purity of more than 98%, with an expanded uncertainty of less than 1% of the puerarin standard sample.
Calycosin and formononetin were successfully isolated from Astragalus membranaceus by Pan [108] and purified using high-speed countercurrent chromatography. Calycosin was purified using a two-phase solvent system of n-hexane, ethyl acetate, ethanol, and water (3:5:3:5), while formononetin was purified using a two-phase solvent system of n-hexane, ethyl acetate, ethanol, and water (4:5:4:5). The analysis revealed that formononetin 2.0 mg had a purity of 98.9% and calycosin 1.3 mg had a purity of 95.8%.
2.2.3. Solid-Phase Extraction (SPE)
The goal of solid-phase extraction (SPE) technology is to separate and enrich the component(s) to be tested by using solid adsorbents to adsorb the component(s) to be tested in liquid samples and then eluted with eluent to achieve the separation and enrichment of the components to be tested. For the extraction and separation of Pueraria isoflavone, SPE columns such as the C18 column and HLB column are frequently utilized. To extract isoflavones from KR, Zhu [47] developed a magnetic ZIF-8 pressurized capillary electrochromatography (pCEC) method. This method was used to separate and detect the isoflavones puerarin, daidzin, and daidzein in KR. The limit of detection (LOD) of puerarin, daidzein, and daidzein was 0.02, 0.03, and 0.03 μg/mL, respectively. The average recovery was in the range of 98.5–100.3% and an RSD of < 4.0%. Using 36 mL of a 50% ethanol solution and 4 mL of hydrochloric acid, Hou [109] applied 0.5 g of TBHQ to 0.1 g of the KR samples while employing nitrogen protection and a water bath heating reflux for 120 min. The extract was extracted using an HLB solid-phase extraction column, followed by an elution step using 5 mL of a 20% methanol solution and 10 mL of methanol. Eleven flavonoid aglycones were extracted and purified at a flow rate of 1–2 mL/min.
Qiao [110] separated the material on a Diamonsil C18 (250 mm 4.6 mm, 5 m) column with methanol-0.01% KH2PO4 (28:72) after treating it with a Hyper Sep C18 solid-phase extraction column. The column temperature was 40 °C, the volume flow rate was 1.0 mL/min, and the detection wavelength was 250 nm for the mobile phase. The SPE method is the foundation of dispersive solid-phase extraction (d-SPE). After removing the interference with complete oscillation or centrifugation, the solid adsorbent is introduced directly to the solvent. Due to their ease of use and sparing use of solvents, these two techniques have garnered considerable interest.
2.2.4. Cloud Point Extraction (CPE)
The new technique, cloud point extraction (CPE), also known as micellar-mediated extraction (MMe), is used to separate chemicals that are lipophilic from those that are water-soluble. It is mostly accomplished by adding surfactants to the solution that needs to be separated, followed by adjustments to the electrolyte, pH, temperature, and other variables [111]. Jiang [112] employed a Phenomenex C18 column, non-ionic surfactant Triton X-114 as an extractant, methanol-0.25% glacial acetic acid solution as the mobile phase, gradient elution, and a UV detector for detection at a wavelength of 250 nm. The content approach was used to calculate the amounts of the five isoflavones, puerarin, daidzin, genistin, daidzein, and genistein, in KR. Chi [113] investigated the ideal extraction conditions for an aqueous two-phase extraction of Pueraria isoflavone using the CRE method. The outcomes demonstrated that the optimal conditions for the separation and purification of the total flavonoids from KR were an aqueous two-phase system with an ethanol mass fraction of 50.14% and a dipotassium hydrogen phosphate mass fraction of 22.67% in a specific range of mass concentrations. The distribution coefficient of the total flavonoids in KR in the aqueous two-phase system increases with increasing mass concentration of the total flavonoids in KR. When the Pueraria crude extract mass concentration was greater than 0.014 g/mL, the variance coefficient declined. The extraction rate is mostly unaffected by the changes in mass concentration under ideal extraction circumstances. The extraction rate reached 99.23%, and the distribution coefficient of the system’s total flavonoids was 35.99. A technique for the simultaneous separation and determination of puerarin and daidzin was developed by Qu [51]. By combining CPE and the concentration with an HPLC technique, genistein, daidzein, genistin, and formononetin were identified in Pueraria. The optimal extraction conditions were as follows: a surfactant Triton X-100 concentration of 0.07 g/mL, NaCl addition of 0.6 g, a liquid–solid ratio of 80:1 (mL/g), and an equilibrium time of 40 min. Under the optimal conditions, the total maximum extraction amount of six isoflavones reached 8.92 mg/g. The CPE method can avoid the use of a large number of organic solvents and does not require the use of large and complex instruments; rather, it only needs to be completed in the plugged centrifuge tube. However, the separation principle has requirements for both the matrix and the target compound, which limits the application range of the CPE method.
2.2.5. Purification Techniques Summary
Because it is inexpensive and simple to use, column chromatography can be widely employed in industrial production; however, it has drawback-low purifying effectiveness. SPE and QuEChERS have developed quickly in recent years, with one of the main development paths being the study and use of novel materials for the effective extraction of KR. HSCCC has effective separation skills and can decrease the usage of organic solvents; however, the price of the necessary equipment is too costly and makes it unsuitable for promotion. Although the CPE approach does not require the use of big or complicated apparatus and can reduce the amount of organic solvents used, its range of applications is limited. Therefore, it is important to think about ways to increase separation efficiency while lowering the extraction and separation costs. This supports the expansion of the use of Pueraria isoflavones in the food and pharmaceutical industries.
3. Analytical Methods
In order to study the content and biochemical properties of Pueraria isoflavone, it is necessary to identify and quantitatively analyze the extracted and purified compounds. The various techniques for detecting isoflavones in KR and its products are summarized in Table 4. The most commonly used methods for the detection of Pueraria isoflavone are thin-layer chromatography (TLC) [114], LC [115], capillary electrophoresis (CE) [116], UV, MS, etc. In order to improve the sensitivity and accuracy of the detection results, liquid chromatography is often used in combination with other technologies, including LC-UV/Vis, LC-MS, LC-MS/MS, etc.
Table 4.
Various analytical methods for the determination of isoflavones in kudzu root.
| Sample Matrix/Source | Analytes | Instrument Type | Stationary Phase | Mobile Phase | Flow Rate; Injection | Determination | LOD/LOQ | Recovery (%) |
RSD (%) |
References |
|---|---|---|---|---|---|---|---|---|---|---|
| PuerariaeFlos | Isoflavones | HPLC | C18-column-column (4.6 × 250 mm, 5 μm) | A:water B:acetonitrile |
1 mL/min; 20 µL | 245 nm | LOD:0.0014 mg/600 µL LOQ:0.0036 mg/600 µL |
98.41 | 0.79 | [46] |
| Pueraria lobata | Puerarin, daidzin, daidzein | pCEC | EP-100–20/45–3-C18 capillary column | A:45%methanol B:55% 17.5 mM sodium dihydrogen phosphate (pH 4.0) |
0.08 mL/min | 250 nm | LOD:0.02–0.03 µg/mL | 98.5–100.3 | <4.0 | [47] |
| PuerariaeLobatae Radix | Nine isoflavones | HPLC | ZORBAX Eclipse XDB-C18 column (4.6 mm × 250 mm, 5 µm) |
A:0.1% formic acid-water B:acetonitrile |
1 mL/min; 10 µL | 100.3–101.1 | 0.33–1.3 | [8] | ||
| Kudzu food | Puerarin | LC-MS/MS | Cadenza CL-C18 column (3 × 100 mm, 3 µm) | A:ammonium formate in 0.2% formic acid B:2 mM ammonium formate and 0.2% formic acid in 95% acetonitrile |
0.5 mL/min; 5 µL | LOD:0.013 µg/mL LOQ:0.063 µg/mL |
83.3–108.2 | [49] | ||
| PuerariaeLobatae Radix | Six isoflavones | UPLC | BEH C18 column (2.1 mm × 50 mm, 1.7 µm) | A:acetonitrile B:0.05% formic acid |
0.2 mL/min; 2 µL | 250 nm | 97.78–99.63 | 1.0–2.3 | [50] | |
| Kudzu roots (KR) | Isoflavones | HPLC-DAD | Poroshell 120 EC-C18-column (3.0 mm × 100 mm, 2.7 µm) | A:containing 0.1% (v/v) acetic acid in the water B:containing 0.1% acetic acid (v/v) |
0.7 mL/min; 5 µL | 245 nm | [51] | |||
| Pueraria lobata | Puerarin and daidzein | NCAE | Uncoated fused silica capillary column 50 cm × 75 µm ID | 90 mmol/L-1 sodium cholate-3.0% acetic acid-15% acetonitrile in methanol | Injection pressure: 50 mbar Injection time: 5 s |
254 nm | LOD:0.4 µg/mL LOQ:0.2 µg/mL |
96.72; 97.26 | 1.94; 2.17 | [53] |
| Pueraria lobata | Puerarin | HPLC-IR | Pgrandsil C18 column (5 µm, 4.6 mm × 250 mm) | Methanol: 36% acetic acid:water = 25:3:72 (v/v) | 1.0 mL/min; 10 µL | 250 nm | [54] | |||
| Pueraria lobata | Puerarin and daidzein | HPLC | Spherigel C18 column (250 mm × 4.6 mm, 5 µm) | A:methanol B:wate |
1.0 mL/min; 10 mL | 250 nm | 99.87; 100.32 | 0.70; 1.80 | [55] | |
| Pueraria lobata and Pueraria thomsonii | Six isoflavones | HPLC | C18 column (250 mm × 4.6 mm, 3.5 µm), | A:methanol B:0.2% acetic acid |
0.8 mL/min, 10 µL | 250 nm | 98.0–103.4 | [58] | ||
| Pueraria lobata | Puerarin | UV | A:phosphoric acid-water B:acetonitrile |
99.56 | 1.14 | [59] | ||||
| Pueraria lobata and Pueraria thomsonii | Flavonoids | UV | 250 nm | 103.29 | 2.90 | [60] | ||||
| Kudzu food | Nine isoflavones | pCEC | C18 column (100 µm × 45 cm, 3 μm); | Acetonitrile-15 mmol/L buffer solution of potassium phosphate (15:85, v/v) | 40 µL | 230 nm | LOD:0.5~1.0 mg/kg LOQ:2.0~5.0 mg/kg |
86.2~98.6 | 1.9~5.3 | [61] |
| Pueraria lobata | Puerarin and daidzein | Differential pulse voltammetry | Ep = 0.600 V | 75.31–87.24 | [62] | |||||
| Roots, stems, leaves and flowers of pueraria lobata | Flavonoids | UV-Vis | 250 nm | 99.96 | 0.89 | [63] | ||||
| Pueraria lobata | Puerarin | HPLC | C18 column (150 mm × 4.6 mm × 5 µm) | A:methanol B:water |
1 mL/min | 250 nm | 97.3–99.2 | 3.72–6.21 | [66] | |
| Pueraria lobata and Pueraria thomsonii | Four isoflavones | HPLC | YMC-Pack ODS-A C18-column (250 mm × 4.6 mm, 5 µm) | A:water B:methanol |
1.0 mL/min; 10 µL | 254 nm | 96.52–100.22 | 0.75–1.84 | [68] | |
| Pueraria lobata | Three isoflavones | HPLC | Symmetry C18 column (4.6 mm × 250 mm, 5 µm) | A:methanol B:water |
0.7 mL/min | 250 nm | 99.0–109.1 | 0.99–1.93 | [69] | |
| Pueraria lobata and Pueraria thomsonii | Six isoflavones | HPLC | Poroshell 120 EC-C18 (150 mm × 4.6 mm, 4 µm) | A:0.1% phosphoric acid B:acetonitrile |
1.0 mL/min; 10 µL | 250 nm | 97.40~101.93 | 1.40~2.85 | [70] | |
| Pueraria lobata | Five isoflavones | HPLC | Phenomenex C18 column (250 mm × 4.6 mm, 5 µm) | A:methanol B:water |
1.0 mL/min | 260 nm | 96.0–100.1 | 0.68–1.17 | [71] | |
| Kudzu food | Puerarin | Three-dimensional fluorescence spectrum | λex/λem = 340 nm/466 nm | LOD: 1.27 × 10−3 µg/mL | 95.82~100.33 | [72] | ||||
| Pueraria lobata | Flavonoids | GC/MS | HP-5 (30 m × 0.25 mm × 0.25 µm) | Helium gas | 1.0 mL/min; 1 µL | 510 nm | 98.60 | 1.86 | [73] | |
| Pueraria lobata | Puerarin and daidzein | HPLC | Agilent chromatographic column (4.6 mm × 250 mm) | A:phosphoric acid-water B:acetonitrile |
1 mL/min; 10 µL | 203 nm | 99.56–100.96 | 1.98–2.13 | [74] | |
| Pueraria lobata | Four isoflavones | HPLC | C18 column (250 mm × 4.6 mm, 5 µm) | A:acetonitrile B:0.1% formic acid-water |
0.8 mL/min; 10 µL | 254 nm | 97.6~103.6 | <2.0 | [76] | |
| Pueraria lobata | Total flavonoids and puerarin | UV-Vis and HPLC | Methanol-water (25:75) | 1.0 mL/min | 250 nm | 94.16–98.4 | 1.42–2.38 | [77] |
3.1. LC Coupled with UV
In order to assess a substance qualitatively and quantitatively, UV/Vis uses the substance’s molecules or ions to absorb light in a certain wavelength range. For online quantitative monitoring, Xu [48] integrated near-infrared (NIR) and ultraviolet-visible (UV-Vis) spectroscopy. The liquid material from the MAE extraction was separated by Hu [57] using a high-speed centrifuge. The constituent parts of the extract were separated using thin-layer chromatography, and qualitative and quantitative analyses of the extract were carried out using UV/Vis. Preliminarily, it may be determined that the mother nucleus structure of the Pueraria flavonoids in the Dabie Mountains is primarily composed of isoflavone, dihydroflavone, and dihydroflavonols. This conclusion is based on the peak form of the UV-visible methanol spectrum of the KR extract. Although UV/Vis testing is quick and inexpensive, it can only detect substances that absorb in the ultraviolet and visible ranges, and it is easily interfered with by impurities. As a result, the combination’s influence on detection is minimal. By utilizing the separation properties of LC, the combination with LC can increase the accuracy of the detection results. The UV and LC information of Pueraria isoflavones is shown in Table 5. High-performance liquid chromatography was employed by Li [77] to quantify the puerarin content of KR. The stationary phase was the C18 column, the mobile phase was methanol–water (25:75), the flow rate was 1.0 mL/min, and the detection wavelength was 250 nm. Five batches from four different manufacturing regions had a puerarin content that ranged from 2.13% to 5.61%. Huang [50] employed a C18 column as the stationary phase, acetonitrile-0.05% formic acid as the mobile phase, with a flow rate of 0.2 mL/min, a column temperature of 30 °C, a sample volume of 2 L, gradient elution, and a detection wavelength of 250 nm. Principal component analysis and fingerprint library identification were performed. Pueraria glycoside was among the six major differential components identified. It was demonstrated that environmental influences from various production areas might influence the accumulation of secondary metabolites in KR by the discovery of soybean saponin and soyasapogenol in KR.
Table 5.
The UV and LC information of Pueraria isoflavones [115].
| Name | Detecting Wavelength (nm) |
Retention Time (min) |
|---|---|---|
| Puerarin | 222.8 | 39.790 |
| Daidzein | 206.3 | 58.684 |
| Daidzin | 226.3 | 52.262 |
| 3′ hydroxyPuerarin | 222.8 | 29.208 |
| Genistein | 229.8 | 115.292 |
| Genistin | 226.3 | 76.748 |
| Formononetin | 253.5 | 112.505 |
Li [117] established a high-performance liquid chromatography method for the simultaneous determination of soyasapogenol, genistein, formononetin, and biochanin A in red clover extract. The gradient elution was carried out using the Bridge C18 chromatographic column, with methanol (A) and 10 mmoL disodium hydrogen phosphate solution (B) serving as the mobile phases. The flow rate was 1 mL/min, and the sample volume was 10 L. A 270 nm detecting wavelength was chosen. The recovery rate was finally 96–100.10%. Lin [118] used ethanol–water (3:1, v/v) to extract from soybean, separated by the Acquity UPLC ® HSS T3 column, using methanol and 0.1% formic acid solution as the mobile phase for gradient elution. The contents of genistein, daidzein, and glycitein in soybeans were determined by UPLC-MS/MS. The LODs were 0.5, 0.4, and 0.1 mg/kg, respectively. The average recovery was 79.3–107%, and the RSD was 1.3–6.8%.
3.2. LC Coupled with MS
When combining two separation and detection methods, its sensitivity is higher than that of liquid chromatography alone, and its selectivity and specificity are also superior. It also overcomes the lack of standards in liquid chromatography. The MS information of Pueraria isoflavones is shown in Table 6. In 45 different food types, including 22 different liquid and 23 different solid processed food types, Ahn [49] reported a range of plant-active components. The stationary phase was a Cadenza CL-C18-column (3100 mm, 3 m). The mobile phase was composed of a 95% acetonitrile solution containing 2 mM ammonium formate and 0.2% formic acid and a formic acid solution containing 0.2% ammonium formate. Additionally, a flow rate of 0.5 mL per minute was used for the gradient elution. Finally, the food had a puerarin level of 13.6–23.9 mg/g. Hou [109] used LC-MS/MS to determine the content of 11 flavonoid aglycones in the SPE extract of KR. The RP-18 column was used as the stationary phase, and the methanol–water system (daidzin, daidzein, formononetin) or methanol-0.15% formic acid solution system (the other eight flavonoid glycosides and 11 flavonoid aglycones) was used as the mobile phase for gradient elution. The recovery rate of 11 flavonoid aglycones was 75.0–94.0%, and the RSD was 3.5–12%. Shen [119] established the LC-MS/MS qualitative analysis method and HPLC analysis method for the content of puerarin in cold-clearing heat granules. The average recovery of puerarin was 100.09%. Wu [120] used Q-ExactiveLC-MS to collect data from Tongmai Jiangtang capsules (TJC). The 15 batches of TJC samples were detected, and the fingerprints were established. The average recovery of puerarin was 95.30–103.56%, and the mass fraction was 5.374–8.805 mg/g.
Table 6.
The MS information of Pueraria isoflavones.
| Name |
Retention Time (min) |
Theoretical Molecular Weight | Precise Molecular Weight | Fragment Ions |
|---|---|---|---|---|
| Puerarin | 11.71 | 547.1437 | 547.1437 | 415.1007, 325.0706, 295.0600, 267.0662, 233.3677, 189.0642 |
| Daidzein | 19.83 | 253.0506 | 253.0510 | 224.0472, 208.0538, 196.0535, 135.0113 241.0444, 217.0496 |
| Daidzin | 9.59 | 415.1034 | 415.1023 | 295.0602, 227.0499, 267.0659 |
| 3′ hydroxyPuerarin | 9.18 | 431.0983 | 431.0968 | 331.0552, 269.0448 |
| Genistein | 20.715 | 269.0455 | 269.0457 | 241.0497, 213.0521, 199.0390, 197.0624, 185.0614, 141.0770, 181.0660, 169.0658 |
| Genistin | 15.42 | 431.0983 | 431.0971 | 311.0564, 269.0439, 241.0495 |
3.3. Pressurized Capillary Electrochromatography (pCEC)
Pressurized capillary electrochromatography (pCEC) is a new micro-separation technology combining high-performance liquid chromatography (HPLC) and capillary electrophoresis (CE). It not only has a high column efficiency and selectivity [121] but also has the advantages of low consumption with the mobile phase and sample, less environmental pollution, and a high performance–price ratio. It is especially suitable for the analysis of trace, multi-component, and complex-matrix components [122]. By using pressurized capillary electrochromatography, Zhang [61] developed a method for the detection of nine isoflavones in KR foods. He then used this method to investigate the isoflavone content of various KR foods. Nine isoflavones were isolated from KR food using a 20% ethanol solution that contained 0.1% formic acid and was purified using a PRiME HLB column. The voltage intensity was set at +2 kV, and the mobile phase was composed of acetonitrile-15 mmol/L potassium phosphate buffer (15:85, v/v). In the concentration range of 10.0–200.0 g/mL, the linearity of the nine isoflavone standard solutions was positive. The detection limit was 0.5–1.0 mg/kg, the limit of quantitation was 2.0–5.0 mg/kg, and the recovery rate was 86.2–98.6%. For the separation and detection of three isoflavones (puerarin, daidzin, and daidzein) in KR, Zhu [47] developed a magnetic ZIF-8-pressure capillary electrochromatography (pCEC) method. The results showed that the components recovered at a rate of between 98.5% and 100.3%, with a 4.0% RSD.
3.4. Analytical Methods Summary
In the current detection method, TLC has little personnel and equipment needs. It is inaccurate and requires lengthy experimental processes. The sensitivity and speed of ELISA are advantages; however, the cross-contamination reaction is a disadvantage. A novel method known as pCEC combines the benefits of capillary electrophoresis with the great selectivity of HPLC. In recent years, it has become one of chromatography’s hot sites. However, it is still in the development stage, with few applications, and there are problems, such as a small sample load and difficult cleaning. LC-UV/Vis and LC-MS are widely used in the detection and analysis of Pueraria isoflavones. The sensitivity and selectivity of MS have been improved as a result of the development of HRMS, TOF MS, ion-trap MS, and orbital trap. Improved detection techniques can also help with standardization, reliability, and food quality control, as well as the chemical analysis of natural goods.
4. Outlook
4.1. Application Value and Health Benefits of KR
The nutritional profile of KR is extensive and diverse, encompassing flavonoids, dietary fiber, starch, and various trace elements [123]. The primary constituent of KR is starch, with its levels reaching approximately 40% [124]. Of the functional components, kudzu starch is frequently utilized as a functional food for the management of type 2 diabetes mellitus (T2DM) [125]. Studies have demonstrated that there are variations in the thermodynamic properties, pasting properties, solubility, swelling, and structural characteristics of the starches extracted from different KR varieties [126]. KR is recognized for its high fiber content, which includes both soluble dietary fiber (SDF) and insoluble dietary fiber (IDF), with respective contents of 30% and 10%. In addition to dietary starch and fiber, KR is a source of isoflavones, particularly puerarin (comprising approximately 60% of the total isoflavones) and, to a lesser extent, daidzein and daidzin [127,128,129,130]. Pueraria isoflavone has been shown to confer numerous health benefits on various bodily systems, including the brain, liver, heart, kidney, bone, stomach, muscle, skin, and reproductive system [131]. Zhou et al.’s estimates indicate that puerarin and daidzein exhibit comparable antioxidant activity [132]. Liu et al.’s findings suggest that puerarin mitigates depression-like behavior in ovariectomized rats by activating the cAMP-CREB-BDNF signaling pathway [133]. Nrf2 serves as a critical regulator of cellular defense against diverse forms of oxidative damage. Yu and Wang’s research demonstrates that KR can counteract CD-induced Nrf2 inhibition, thereby preventing autophagy suppression and NLRP3 inflammasome activation [134,135]. GUO found that puerarin can inhibit inflammation and apoptosis through HDAC1/HDAC3 signaling, thereby alleviating streptozotocin (STZ)-induced osteoporosis in rats [136]. There are also rich, grand elements, such as Ca and Mg, and profitable elements, such as the trace elements Zn, Cu, and Fe, in KR [137]. These elements have many benefits for the brain, liver, heart, kidneys, stomach, etc.
4.2. Current Trends and Future Perspectives
Many researchers have now experimented with various techniques for extracting, purifying, and analyzing Pueraria isoflavone. The development of health foods using Pueraria as the primary raw material, as well as the use of Pueraria isoflavone and the plant’s major active element, have both benefited from these studies. In terms of extraction, some researchers combine new techniques to increase the extraction efficiency of Pueraria isoflavone. These techniques include enzyme extraction, UAE, MAE, and heating reflux. More and more green solvents, such as ILS, NADES, etc., have been used to extract Pueraria isoflavone, with promising results, in an effort to limit the usage of organic solvents. This will also become one of the primary research paths in the future. Column chromatography is commonly used for the separation and purification of Pueraria isoflavone. One of the recent research areas for more effective separation is HSCCC. It can separate and purify the different components found in natural mixtures and has the advantages of partition chromatography and liquid–liquid extraction. It has a promising future for applications. In terms of analysis and detection, UV/Vis is the most common. In order to improve the accuracy of the detection results, UV/Vis is generally combined with LC to overcome its disadvantage of being easily impacted by impurities. To improve sensitivity and the capacity for quantitative analysis, several studies also use LC-MS or LC-MS/MS to detect Pueraria isoflavone. However, the majority of these projects take place in laboratories. More research and guidance are still required to adapt it to actual factory production.
Future studies on the separation, purification, analysis, and detection of Pueraria isoflavone may focus on the following aspects:
For the extraction and purification processes, it is particularly important to develop novel, eco-friendly green solvents and auxiliary extraction instruments. The creation and production of high-purity puerarin isoflavone may play a vital role in the subsequent stage of research and health food manufacturing.
The demand for the detection of puerarin isoflavone in health foods is steadily increasing day by day with product development and manufacturing. Next, attention should be given to the development of pretreatment techniques for various health food substrates. New spectral, chromatographic, and mass spectrometry methods are also being developed to meet the demands of fast detection of Pueraria isoflavone contents in a variety of substrates. The combined use of multiple analytical instruments can help to increase the sensitivity and accuracy of detection.
5. Conclusions
KR’s potential as a nutrition source and its economic value cannot be undermined. Pueraria isoflavone is the main active constituent of the functional food KR and has a wide range of biological activities. The cornerstone for Pueraria isoflavones’ use in functional foods and in-depth research is the technology for extraction, purification, and detection. Research in recent years has shown that the purity and extraction efficiencies of Pueraria isoflavone are being improved by using more efficient extraction and separation methods as well as environmentally friendly extraction solvents. The precision and accuracy of the method for the determination of isoflavone contents in KR are continuously improving. The detection procedure is also developing in the direction of being rapid, simple, of high sensitivity, strong specificity, and low cost. We hope that this review can enhance people’s interest in the use of isoflavones in KR as a nutritional food.
Author Contributions
T.X. and Y.L.: Writing—original draft preparation; R.L. and S.L.: writing—review and editing; J.H. and X.B.: formal analysis; R.F. and J.W.: supervision and conceptualization. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the Scientific Research Project of the Department of Science and Technology of Liaoning Province (2022-MS-407), Liaoning Province Innovation Capacity Promotion Joint Fund (2022-NLTS-14-05), Scientific Research Project of the Department of Education of Liaoning Province (SYYX202013), National Natural Science Foundation of China (81903793) and Innovation Training Program for college students at Shenyang Medical College (S202210164006).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Maciejewska-Turska M., Pecio Ł., Zgórka G. Isolation of Mirificin and Other Bioactive Isoflavone Glycosides from the Kudzu Root Lyophilisate Using Centrifugal Partition and Flash Chromatographic Techniques. Molecules. 2022;27:6227. doi: 10.3390/molecules27196227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Su G., He D., Xie W. Response Surface Optimization of Radix Pueraria and Yam Noodle Production Process. Food Ind. 2020;41:117–121. [Google Scholar]
- 3.Zhu D. Study on the preparation of puerarin enzyme by yeast fermentation. Anhui Agric. Sci. Bull. 2021:82–87. doi: 10.13386/j.issn1002-0306.2020.12.013. [DOI] [Google Scholar]
- 4.Qiao D., Chen Y., Duan X., Chen C. Preparation and Antioxidant Properties of Pueraria Lobata Juice and Pueraria Beverage. Farm Prod. Process. 2018;8:1–6. doi: 10.16693/j.cnki.1671-9646(X).2018.04.029. [DOI] [Google Scholar]
- 5.Xu L., Liu J. Research of Ribes nigrum and Pueraria Drinks and Its Anti-fatigue Function. Food Res. Dev. 2017;38:132–137. doi: 10.3969/j.issn.1005-6521.2017.22.027. [DOI] [Google Scholar]
- 6.Zhu Z., Luo Y., Xue J., Guo M., Wang X. Acute Toxicity Test and Sober and Hepatoproctive Efficacy Evaluation of Radix Puerariae Functional Beverage. Food Res. Dev. 2016;37:160–163. doi: 10.3969/j.issn.1005-6521.2016.21.037. [DOI] [Google Scholar]
- 7.Zeng W., Huang D., Xie S., Du B., Li P. Research Progress on the Composition, Structure and Efficacy Mechanism of Pueraria Isoflavones. Food Sci. 2023;44:353–361. [Google Scholar]
- 8.Mu P., An Q., Zhang Y., Guo L., Guo M., Zheng Y., Zhang D. The content of 9 isoflavone components in Kudzu herbs was determined by one test and multiple evaluations. China J. Chin. Mater. Medica. 2019;44:4888–4895. doi: 10.19540/j.cnki.cjcmm.20190812.202. [DOI] [PubMed] [Google Scholar]
- 9.Song W., Li Y., Qiao X., Qian Y., Ye M. Chemistry of the Chinese herbal medicine Puerariae Radix (Ge-Gen): A review. J. Chin. Pharm. Sci. 2014;23:347–360. [Google Scholar]
- 10.Kinjo J., Furusawa J., Baba J., Takeshita T., Yamasaki M., Nohara T. Studies on the Constituents of Pueraria Lobata. III. Isoflavonoids and Related Compounds in the Roots and the Voluble Stems. Chem. Pharm. Bull. 1987;35:4846–4850. doi: 10.1248/cpb.35.4846. [DOI] [Google Scholar]
- 11.Ji P., Zhang L., Li M. Study on the Chemical Constituents in Puerarialobata. China Pharm. 2020;23:1184–1188. [Google Scholar]
- 12.Prasain J.K., Reppert A., Jones K., Moore D.R., Barnes S., Lila M.A. Identification of Isoflavone Glycosides in Pueraria Lobata Cultures by Tandem Mass Spectrometry. Phytochem. Anal. PCA. 2007;18:50–59. doi: 10.1002/pca.951. [DOI] [PubMed] [Google Scholar]
- 13.Kayano S., Matsumura Y., Kitagawa Y., Kobayashi M., Nagayama A., Kawabata N., Kikuzaki H., Kitada Y. Isoflavone C-Glycosides Isolated from the Root of Kudzu (Pueraria Lobata) and Their Estrogenic Activities. Food Chem. 2012;134:282–287. doi: 10.1016/j.foodchem.2012.02.137. [DOI] [Google Scholar]
- 14.Seong S.H., Roy A., Jung H.A., Jung H.J., Choi J.S. Protein Tyrosine Phosphatase 1B and α-Glucosidase Inhibitory Activities of Pueraria Lobata Root and Its Constituents. J. Ethnopharmacol. 2016;194:706–716. doi: 10.1016/j.jep.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Li J., Mi Q., Zhang F., Yang Y., Chen J., Li Y., Zhang C., Yang G., Hu F., Liu Z. Two New Isoflavones from Pueraria Lobata and Their Bioactivities. Chem. Nat. Compd. 2018;54:851–855. doi: 10.1007/s10600-018-2497-6. [DOI] [Google Scholar]
- 16.Zhang F.M., Jing L., Mi Q.L., Tang S.Y., Li X.M. Three New Isoflavones from the Root of Pueraria Lobata and Their Bioactivities. Heterocycles Int. J. Rev. Commun. Heterocycl. Chem. 2017;94:1582–1588. doi: 10.3987/COM-17-13739. [DOI] [Google Scholar]
- 17.Hu Q., Xiang H., Shan J., Jiao Q., Lou H. Two Pairs of Diastereoisomeric Isoflavone Glucosides from the Roots of Pueraria Lobata. Fitoterapia. 2020;144:104594. doi: 10.1016/j.fitote.2020.104594. [DOI] [PubMed] [Google Scholar]
- 18.Zhao Y.Z., Zhang L., Gupta P.K., Tian F.R., Mao K.L., Qiu K.Y., Yang W., Lv C.Z., Lu C.T. Using PG-Liposome-Based System to Enhance Puerarin Liver-Targeted Therapy for Alcohol-Induced Liver Disease. AAPS PharmSciTech. 2016;17:1376–1382. doi: 10.1208/s12249-015-0427-5. [DOI] [PubMed] [Google Scholar]
- 19.Liu C.-M., Ma J.-Q., Liu S.-S., Feng Z.-J., Wang A.-M. Puerarin Protects Mouse Liver against Nickel-Induced Oxidative Stress and Inflammation Associated with the TLR4/P38/CREB Pathway. Chem. Biol. Interact. 2016;243:29–34. doi: 10.1016/j.cbi.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 20.Chen X., Huang C., Sun H., Hong H., Jin J., Bei C., Lu Z., Zhang X. Puerarin Suppresses Inflammation and ECM Degradation through Nrf2/HO-1 Axis in Chondrocytes and Alleviates Pain Symptom in Osteoarthritic Mice. Food Funct. 2021;12:2075–2089. doi: 10.1039/d0fo03076g. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y., Ma Y., Zheng Y., Song J., Yang X., Bi C., Zhang D., Zhang Q. In Vitro and in Vivo Anticancer Activity of a Novel Puerarin Nanosuspension against Colon Cancer, with High Efficacy and Low Toxicity. Int. J. Pharm. 2013;441:728–735. doi: 10.1016/j.ijpharm.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 22.Jia L., Hu Y., Yang G., Li P. Puerarin Suppresses Cell Growth and Migration in HPVpositive Cervical Cancer Cells by Inhibiting the PI3K/MTOR Signaling Pathway. Exp. Ther. Med. 2019;18:543–549. doi: 10.3892/etm.2019.7589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu Z., Li W. Induction of Apoptosis by Puerarin in Colon Cancer HT-29 Cells. Cancer Lett. 2006;238:53–60. doi: 10.1016/j.canlet.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 24.Zhang G., Wang Y., Tang G., Ma Y. Puerarin Inhibits the Osteoclastogenesis by Inhibiting RANKL-Dependent and -Independent Autophagic Responses. BMC Complement. Altern. Med. 2019;19:269. doi: 10.1186/s12906-019-2691-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu L.J., Liu L.Q., Bo T., Li S.J., Zhu Z., Cui R.R., Mao D.A. Puerarin Suppress Apoptosis of Human Osteoblasts via ERK Signaling Pathway. Int. J. Endocrinol. 2013;2013:786574. doi: 10.1155/2013/786574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Z., Shangguan Z., Liu Y., Wang J., Li X., Yang S., Liu S. Puerarin Protects Pancreatic β-Cell Survival via PI3K/Akt Signaling Pathway. J. Mol. Endocrinol. 2014;53:71–79. doi: 10.1530/JME-13-0302. [DOI] [PubMed] [Google Scholar]
- 27.Tang L., Liu D., Yi X., Xu T., Liu Y., Luo Y., Yin D., He M. The Protective Effects of Puerarin in Cardiomyocytes from Anoxia/Reoxygenation Injury Are Mediated by PKC. Cell Biochem. Funct. 2014;32:378–386. doi: 10.1002/cbf.3026. [DOI] [PubMed] [Google Scholar]
- 28.Wan H., Zhu H., Tian M., Hu X., Yang J., Zhao C., Zhang H. Protective Effect of Chuanxiongzine-Puerarin in a Rat Model of Transient Middle Cerebral Artery Occlusion-Induced Focal Cerebral Ischemia. Nucl. Med. Commun. 2008;29:1113. doi: 10.1097/MNM.0b013e3283108995. [DOI] [PubMed] [Google Scholar]
- 29.Zhao G., Ning H., Cai S.A., Liu X.W., Li A.Q., Cheng C.F., Yin H., Li L.R., Mai Y.P., Liu S.M. Contributions of Nrf2 to Puerarin Prevention of Cardiac Hypertrophy and Its Metabolic Enzymes Expression in Rats. J. Pharmacol. Exp. Ther. 2018;366:458–469. doi: 10.1124/jpet.118.248369. [DOI] [PubMed] [Google Scholar]
- 30.Lin Y.H., Ni X.B., Zhang J.W., Ou C.W., Chen M.S. Effect of Puerarin on Action Potential and Sodium Channel Activation in Human Hypertrophic Cardiomyocytes. Biosci. Rep. 2020;40:BSR20193369. doi: 10.1042/BSR20193369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deng Y., Lei T., Li H., Mo X., Wang Z., Ou H. ERK5/KLF2 Activation Is Involved in the Reducing Effects of Puerarin on Monocyte Adhesion to Endothelial Cells and Atherosclerotic Lesion in Apolipoprotein E-Deficient Mice. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864:2590. doi: 10.1016/j.bbadis.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 32.Li C.H., Gong D., Chen L.Y., Zhang M., Tang C.K. Puerarin Promotes ABCA1-Mediated Cholesterol Efflux and Decreases Cellular Lipid Accumulation in THP-1 Macrophages. Eur. J. Pharmacol. 2017;811:74. doi: 10.1016/j.ejphar.2017.05.055. [DOI] [PubMed] [Google Scholar]
- 33.Li X., Lin Y., Zhou H., Yao L., Zhou M.S. Puerarin Protects against Endothelial Dysfunction and End-Organ Damage in Ang II-Induced Hypertension. Clin. Exp. Hypertens. 2017;39:58–64. doi: 10.1080/10641963.2016.1200603. [DOI] [PubMed] [Google Scholar]
- 34.Shi W., Yuan R., Chen X., Xin Q., Wang Y., Shang X., Cong W., Chen K. Puerarin Reduces Blood Pressure in Spontaneously Hypertensive Rats by Targeting ENOS. Am. J. Chin. Med. 2019;47:19–38. doi: 10.1142/S0192415X19500022. [DOI] [PubMed] [Google Scholar]
- 35.Jin F., Qi H. Protective effect of genistein on monocrotaline induced pulmonary hypertension in rats by inhibiting oxidative stress. Drug Eval. Res. 2020;43:695–699. [Google Scholar]
- 36.Beavers D.P., Beavers K.M., Miller M., Stamey J., Messina M.J. Exposure to Isoflavone-Containing Soy Products and Endothelial Function: A Bayesian Meta-Analysis of Randomized Controlled Trials. Nutr. Metab. Cardiovasc. Dis. 2012;22:182–191. doi: 10.1016/j.numecd.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 37.Li X., Min E., Wang B., Sun L. Effects of Pueraria mirifica extract on blood pressure and intestinal flora in mice on a high-salt diet. Acta Nutr. Sin. 2020;42:491–496, 504. doi: 10.13325/j.cnki.acta.nutr.sin.20201027.002. [DOI] [Google Scholar]
- 38.Yang Q., Fu Y., Ren J., Wang C., Gao L., Ding L. Research progress on application and mechanisms of Puerariae Lobatae Radix in treatment of physical disease comorbid with depression. Chung Ts′ao Yao. 2023;54:4701–4712. [Google Scholar]
- 39.Szulc M., Kujawski R., Baraniak J., Kania-Dobrowolska M., Kamińska E., Gryszczyńska A., Czora-Poczwardowska K., Winiarska H., Mikołajczak P.Ł. Differential Influence of Pueraria Lobata Root Extract and Its Main Isoflavones on Ghrelin Levels in Alcohol-Treated Rats. Pharmaceuticals. 2021;15:25. doi: 10.3390/ph15010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen K., Wei P., Shi L. Research progress on the pharmacological effects of pueraria isoflavones. Drug Eval. Res. 2022;45:2602–2610. [Google Scholar]
- 41.Zhou B.-G., Zhao H.-M., Lu X.-Y., Zhou W., Liu F.-C., Liu X.-K., Liu D.-Y. Effect of Puerarin Regulated MTOR Signaling Pathway in Experimental Liver Injury. Front. Pharmacol. 2018;9:1165. doi: 10.3389/fphar.2018.01165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fu X., Ye Y., Zhao X., Sun X. Research Progress into Multiple Uses for Puerariae lobatae and Its Application in Food. Food Res. Dev. 2021;42:197–205. [Google Scholar]
- 43.Wathon M.H., Beaumont N., Rayner C.M., Blackburn R.S. Extraction of Anthocyanins from Aronia Melanocarpa Skin Waste as A Sustainable Source of Natural Colorants; Proceedings of the BIOColours Conference 2018; Breda, The Netherlands. 28 May 2018. [Google Scholar]
- 44.Zhou Y., Staniszewska I., Liu Z.L., Zielinska D., Zielińska M. Microwave-Vacuum-Assisted Drying of Pretreated Cranberries: Drying Kinetics, Bioactive Compounds and Antioxidant Activity. LWT-Food Sci. Technol. 2021;146:111464. doi: 10.1016/j.lwt.2021.111464. [DOI] [Google Scholar]
- 45.Álvarez A., Terreros S., Cocero M.J., Mato R.B. Microwave Pretreatment for the Extraction of Anthocyanins from Saffron Flowers: Assessment of Product Quality. Antioxidants. 2021;10:1054. doi: 10.3390/antiox10071054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thapa P., Kim H.M., Hong J.P., Kim R., Paudel S.B., Choi H., Jang D.S., Nam J.W. Absolute Quantification of Isoflavones in the Flowers of by QHNMR. Plants. 2022;11:548. doi: 10.3390/plants11040548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu J., Cheng H., Zhou M., Li S., Tang T., Feng J. Determining Three Isoflavones from Pueraria Lobata Using Magnetic ZIF-8 Nanoparticle-Based Solid-Phase Extraction and Pressurized Capillary Electrochromatography. J. Pharm. Biomed. Anal. 2022;212:114592. doi: 10.1016/j.jpba.2022.114592. [DOI] [PubMed] [Google Scholar]
- 48.Xu Z., Talpur Z.H., Yang W., Xiong Y., Wu T., Zhang Y., Shen X., Du Y. Dual-Spectrum Online Monitoring of Puerarin and Total Flavonoids Contents during the Extraction Process of Pueraria Lobata. Talanta. 2022;248:123608. doi: 10.1016/j.talanta.2022.123608. [DOI] [PubMed] [Google Scholar]
- 49.Ahn S.-J., Kim H.J., Lee A., Min S.-S., In S., Kim E. Determination of 12 Herbal Compounds for Estimating the Presence of Angelica Gigas Root, Cornus Fruit, Licorice Root, Pueraria Root, and Schisandra Fruit in Foods by LC-MS/MS. Food Addit. Contam. Part Chem. Anal. Control Expo. Risk Assess. 2020;37:1437–1448. doi: 10.1080/19440049.2020.1778187. [DOI] [PubMed] [Google Scholar]
- 50.Huang Z.-Y., Shen Q.-N., Li P., Su L.-L., Chen L.-H., Lu T.-L., Mao C.-Q. Quality research of Puerariae Lobatae Radix from different habitats with UPLC fingerprint and determination of multi-component content. Zhongguo Zhong Yao Za Zhi Zhongguo Zhongyao Zazhi China J. Chin. Mater. Medica. 2019;44:2051–2058. doi: 10.19540/j.cnki.cjcmm.20190116.002. [DOI] [PubMed] [Google Scholar]
- 51.Qu L., Song K., Zhang Q., Guo J., Huang J. Puerariae Lobatae Simultaneous Determination of Six Isoflavones from Radix by CPE-HPLC and Effect of Puerarin on Tyrosinase Activity. Molecules. 2020;25:344. doi: 10.3390/molecules25020344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mocan A., Carradori S., Locatelli M., Secci D., Cesa S., Mollica A., Riga S., Angeli A., Supuran C.T., Celia C., et al. Bioactive Isoflavones from Pueraria Lobata Root and Starch: Different Extraction Techniques and Carbonic Anhydrase Inhibition. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018;112:441–447. doi: 10.1016/j.fct.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 53.Zhang S., Zhang Y. Determination of Puerarin and Daidzein in Puerariae Radix by Non-aqueous Capillary Electrophoresis. China Pharm. 2020;1632:588–590. doi: 10.1016/j.chroma.2020.461602. [DOI] [Google Scholar]
- 54.Zhao L., Zhang W., Wang J., Liu Q., Chen Q. Extraction and Bioactivity Analysis of Puerarin. Food Res. Dev. 2022;43:107–112. [Google Scholar]
- 55.Cui Y., Liu X., Cai F. Study on the Ultrasonic Extraction Process of Puerarin and Daidzein in Puerariae Lobatae Radix. China Pharm. 2018;27:7–9. [Google Scholar]
- 56.Wang R., Ou T., Li Z. Study on the Technology of Ultrasonic-Assisted Total Flavonoids Extraction of Pueraria lobata. J. Zhaotong Univ. 2019;41:19–22. [Google Scholar]
- 57.Hu Y., Shan Y., Yao Z., Li G., Zhong Y. Microwave-assisted Extraction of Flavonoids from Radix Pueraria in Dabie Mountains. Guangzhou Chem. Ind. 2019;47:92–95. [Google Scholar]
- 58.Liu Y., Yin M., Xu T., Duan H., Cheng M. Comparison of the Content of Six Isoflavones in the Mealy-like Roots and the Wooden-like Roots of Pueraria lobata from Anhui Province, China. J. Anhui Univ. Chin. Med. 2019;38:77–81. doi: 10.3969/j.issn.2095-7246.2019.05.019. [DOI] [Google Scholar]
- 59.Wang L., Guo S., Zou H., Song Y., Xie C. Content Determination of Puerarin in Kudzu. Guangzhou Chem. Ind. 2021;49:73–75. doi: 10.3969/j.issn.1001-9677.2021.22.025. [DOI] [Google Scholar]
- 60.Tan Z., Wu F., Zhang M., Shang X., Yan H., Wang Y., Xiao L. Determination of Germplasm Resources Total Flavonoids in Pueraria Lobata and P. Thomsonii from Different Areas of Guangxi. Guangdong Chem. Ind. 2021;48:159–161. [Google Scholar]
- 61.Zhang N., Wang Y., Zhao Y. Determination of nine isoflavones in pueraria foods by pressurized capillary electrochromatography. China Food Addit. 2022;33:204–210. doi: 10.19804/j.issn1006-2513.2022.04.026. [DOI] [Google Scholar]
- 62.Liu X., Wang Z., Ling X. Application of Polydopamine Graphene Oxide Modified Electrode in the Analysis of Puerarin and Daidzein in Pueraria. J. Hubei Univ. Med. 2021;40:446–453. doi: 10.13819/j.issn.2096-708X.2021.05.002. [DOI] [Google Scholar]
- 63.Zeng H., Li J., Chen C., He X., Chen X., Yu J., Wang X. Optimization of Extraction Technology and Content Determination of Total Flavonoids in Aerial Parts of Three Kinds of Pueraria Lobate from Jiangxi Province. Pract. Clin. J. Integr. Tradit. Chin. West. Med. 2019;19:173–176. doi: 10.13638/j.issn.1671-4040.2019.08.090. [DOI] [Google Scholar]
- 64.Liu Y., Liu J., Zhang C., Wang X., Liu X., Liu H., Ye Q., Guo L. Study on microwave extraction process of total flavonoids from Pueraria lobata and its antioxidant activity. Lishizhen Med. Mater. Medica Res. 2020;31:68–72. [Google Scholar]
- 65.Yu W., Zhao N., Zhang Y. Determination the extraction process of puerarin from pueraria iobata by response surface methodology. Cereals Oils. 2021;34:108–111, 116. doi: 10.3969/j.issn.1008-9578.2021.12.026. [DOI] [Google Scholar]
- 66.Yang J., Nie Y., Chen S., Li P., Li Z., Li X. Determination and Extraction of Puerarin in Gegen by Microwave-Assisted Extraction Process Optimized by Response Surface Methodology. Contemp. Chem. Ind. 2018;47:64–67. doi: 10.13840/j.cnki.cn21-1457/tq.2018.01.017. [DOI] [Google Scholar]
- 67.Gong P., Zhai P., Zhang M., Chen X., Wang X., Chen F. Optimization of extraction process of total flavonoids from Pueraria lobata and its antioxidant activity. China Brew. 2022;41:172–176. [Google Scholar]
- 68.Xu W., Du C., Xu S., Li Q., Su L., Yang G., Jiang H. Quantitative determination of 4 isoflavones of Pueraria lobata and Pueraria thomsonii from Zitong in Sichuan province by HPLC. Hubei Agric. Sci. 2019;5:114–116, 167. doi: 10.14088/j.cnki.issn0439-8114.2019.09.027. [DOI] [Google Scholar]
- 69.Zhu Z., Zhao Y., Che Y., Wang Z. Simultaneous determination of isoflavonoids in Radix Puerariae Lobatae from different habitats and different drying methods by HPLC. J. Yunnan Minzu Univ. Sci. Ed. 2022;31:157–161. [Google Scholar]
- 70.Zhou L., Pan X., Qiu W., Wang Y., Zou L., Li C., Lu M. Determination of 6 isoflavones in Puerariae lobatae Radix and Puerariae thomsonii Radix by HPLC. J. Chin. Med. Mater. 2021;44:2374–2378. doi: 10.13863/j.issn1001-4454.2021.10.023. [DOI] [Google Scholar]
- 71.Xu G., Xiong X., Wu C., Zhang Z., Sha J. Simultaneous determination of 5 isoflavones in Radix puerariae by HPLC. Northwest Pharm. J. 2020;35:29–32. doi: 10.3969/j.issn.1004-2407.2020.01.007. [DOI] [Google Scholar]
- 72.Wang S., Wen Z., Chen Q., Chen L., Zheng Z. β-cyclodextrin-sensitized Three-dimensional Fluorescence Spectra of Puerarin and Its Application to Determination of Puerarin in Radix Puerariae Foods. J. Chin. Inst. Food Sci. Technol. Hydrolys. Soybean Isoflavones Catalyzed Cell. 2018;18:219–224. doi: 10.16429/j.1009-7848.2018.12.028. [DOI] [Google Scholar]
- 73.Tang T., Zhu J., Zhou M., Huang F., Huang H., Cheng H. Component Analysis of Volatile Oils from Pueraria lobata Stems and Study on Extraction Technology of Total Flavonoids from Pueraria lobata Roots. China Condiment. 2022;47:182–187. doi: 10.3969/j.issn.1000-9973.2022.06.034. [DOI] [Google Scholar]
- 74.Bu D., Zhang D., Wang C. Optimization of the Extraction Process of Pueraria Lobata and Rhizoma Chuanxiong by Response Surface Methodology. J. Liaoning Univ. Tradit. Chin. Med. 2019;21:54–57, 225. doi: 10.13194/j.issn.1673-842x.2019.04.015. [DOI] [Google Scholar]
- 75.Li L., Yu D., Su S., Wang G. Process of Microwave-Assisted Ionic Liquid Extraction of Puerarin from Pueraria lobata Radix. Food Ind. 2020;41:131–135. [Google Scholar]
- 76.Wang X., Li S., Song A., Han F. Determination of four kinds of Isoflavonoids in Puerariae Radix. Yunnan Chem. Technol. 2021;48:61–63. [Google Scholar]
- 77.Li H., Yang C. Determination of Total Flavonoids and Puerarin in Pueraria Lobata from Four Main Producing Areas. J. Shaanxi Univ. Chin. Med. 2018;41:61–64. doi: 10.13424/j.cnki.jsctcm.2018.03.021. [DOI] [Google Scholar]
- 78.Liu X., Liu Y., Yang Y., Xiao J., Zhou H. Optimization of Microwave-assisted Enzymatic Extraction Technology of Puerarin from Radix Puerariae by Response Surface Methodology. Food Ferment. Sci. Technol. 2018;54:13–17. [Google Scholar]
- 79.Tan H. Extraction Method of Effective Components of Pueraria. Foreign Med. Pharm. 1975;1:55. [Google Scholar]
- 80.Hansen B.B., Spittle S., Chen B., Poe D., Zhang Y., Klein J.M., Horton A., Adhikari L., Zelovich T., Doherty B.W., et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021;121:1232–1285. doi: 10.1021/acs.chemrev.0c00385. [DOI] [PubMed] [Google Scholar]
- 81.Aboushanab S.A., Shevyrin V.A., Slesarev G.P., Melekhin V.V., Shcheglova A.V., Makeev O.G., Kovaleva E.G., Kim K.H. Antioxidant and Cytotoxic Activities of Kudzu Roots and Soy Molasses against Pediatric Tumors and Phytochemical Analysis of Isoflavones Using HPLC-DAD-ESI-HRMS. Plants. 2022;11:741. doi: 10.3390/plants11060741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fan J.-P., Cao J., Zhang X.-H., Huang J.-Z., Kong T., Tong S., Tian Z.-Y., Xie Y.-L., Xu R., Zhu J.-H. Optimization of Ionic Liquid Based Ultrasonic Assisted Extraction of Puerarin from Radix Puerariae Lobatae by Response Surface Methodology. Food Chem. 2012;135:2299–2306. doi: 10.1016/j.foodchem.2012.07.038. [DOI] [PubMed] [Google Scholar]
- 83.Duru K.C., Slesarev G.P., Aboushanab S.A., Kovalev I.S., Zeidler D.M., Kovaleva E.G., Bhat R. An Eco-Friendly Approach to Enhance the Extraction and Recovery Efficiency of Isoflavones from Kudzu Roots and Soy Molasses Wastes Using Ultrasound-Assisted Extraction with Natural Deep Eutectic Solvents (NADES) Ind. Crops Prod. 2022;182:114886. doi: 10.1016/j.indcrop.2022.114886. [DOI] [Google Scholar]
- 84.Huang Y., Yang J., Zhao Y., Yu L., He Y., Wan H., Li C. Screening, Optimization, and Bioavailability Research of Natural Deep Eutectic Solvent Extracts from Radix Pueraria. Molecules. 2021;26:729. doi: 10.3390/molecules26030729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pan J., Kuang M., Tian H., Wang H., Zhang T., Yang F., Hou Z., Li Z. Optimization of Puerarin Extraction Process in Pueraria and Antioxidant Activity of Its Extract. Chin. Tradit. Pat. Med. 2018;40:2430–2436. [Google Scholar]
- 86.Song Y., Guan H., Liu B., Gong D., Zhang N. Study on Ionic Liquid-Ultrasound-Assisted Extraction of Pueraria Flavonoids and Antioxidant Activity. Food Res. Dev. 2020;41:97–100, 107. [Google Scholar]
- 87.Yoshiara L.Y., Madeira T.B., Delaroza F., da Silva J.B., Ida E.I. Optimization of Soy Isoflavone Extraction with Different Solvents Using the Simplex-Centroid Mixture Design. Int. J. Food Sci. Nutr. 2012;63:978–986. doi: 10.3109/09637486.2012.690026. [DOI] [PubMed] [Google Scholar]
- 88.Kazlauskaite J.A., Ivanauskas L., Bernatoniene J. Novel Extraction Method Using Excipients to Enhance Yield of Genistein and Daidzein in Trifolium Pratensis L. Pharmaceutics. 2021;13:777. doi: 10.3390/pharmaceutics13060777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eskilsson C.S., Björklund E. Analytical-Scale Microwave-Assisted Extraction. J. Chromatogr. A. 2000;902:227–250. doi: 10.1016/S0021-9673(00)00921-3. [DOI] [PubMed] [Google Scholar]
- 90.Shi X., He Z., Gu J., Wang J., Tan R. Applying the Grading Methods of Five Kudzu Root Isoflavones on Optimizing the Extraction Process. Res. Pract. Chin. Med. 2016;30:49–52. [Google Scholar]
- 91.Cai J., Zhou X., Xiong J., Sun S., Liang X., Zho C., Wei K. Study on the Microwave-Assisted Extraction Technology of Genistein and Genistin from Flemingia Macrophylla with Uniform Design and Orthogonal Design. Appl. Chem. Ind. 2016;45:402–405, 411. doi: 10.16581/j.cnki.issn1671-3206.20160115.042. [DOI] [Google Scholar]
- 92.Li Y., Niu J., Cao R., Yan X. Optimization of Microwave Processing Method for Hedysari by Orthogonal Test. Chin. J. Hosp. Pharm. 2017;37:1475–1478. [Google Scholar]
- 93.Liu X., Liao Y., Wang Y., Tian Q., Xie K., Ou H. Research progress on application of ultrasound hyphenated technique in extraction of active phytochemicals. Food Ferment. Ind. 2022;48:319–327. doi: 10.13995/j.cnki.11-1802/ts.029505. [DOI] [Google Scholar]
- 94.Wu Y., Wang X., Fan E. Optimisation of Ultrasound-Assisted Extraction of Puerarin and Total Isoflavones from Puerariae Lobatae Radix (Pueraria Lobata (Wild.) Ohwi) with Response Surface Methodology. Phytochem. Anal. PCA. 2012;23:513–519. doi: 10.1002/pca.2349. [DOI] [PubMed] [Google Scholar]
- 95.Li Z., Wang M., Yang Y., Zhao J. The Response Surface Method Optimized the Extraction Process and Antioxidant Activity of Two Pueraria Lobata Flavonoids. Liquor Mak. 2023;50:43–51. [Google Scholar]
- 96.Ismail B., Hayes K. β-Glycosidase Activity toward Different Glycosidic Forms of Isoflavones. J. Agric. Food Chem. 2005;53:4918–4924. doi: 10.1021/jf0404694. [DOI] [PubMed] [Google Scholar]
- 97.Dong Y., Lin H., Miao S., Lu X. Advances in Enzymatic Extraction of Polysaccharides. Sci. Technol. Food Ind. 2021;42:351–358. doi: 10.13386/j.issn1002-0306.2020050016. [DOI] [Google Scholar]
- 98.Huang J., Liang Y., Wei H., Ruan B. Optimizeation of Enzymatic-Ultrasonic Extraction Technique of Puerarin from Pueraria Lobata Using Plackett-Burman Combined with Response Surface Methodology. Storage Process. 2023;23:37–44. [Google Scholar]
- 99.Guo M., Yang X., Duan Z. Investigation on the Hydrolysis of Soybean Isoflavones Catalyzed by Cellulase. China Oils Fats. 2023;48:110–115, 139. doi: 10.19902/j.cnki.zgyz.1003-7969.220009. [DOI] [Google Scholar]
- 100.Yang Z., Wang S., Gao J., Feng C., Chen Y., Yong X., Zhou J., Liu X., Xie X., Zheng T. Study on Separation and Purification Process of Puerarin and Daidzein. Jiangsu Agric. Sci. 2016;44:297–300. [Google Scholar]
- 101.Wang H., Zhang H. Influence of Macroporous Adsorption Resin on Purification of Total Flavonoids from GeGen. West. J. Tradit. Chin. Med. 2015;28:27–29. doi: 10.15806/j.issn.2311-8571.2015.0020. [DOI] [Google Scholar]
- 102.Pan J., Xu T. Coordination Chromatography Separates Genistein and Soy Glycoside Monomers. Food Sci. 2005;26:353–355. doi: 10.3321/j.issn:1002-6630.2005.09.089. [DOI] [Google Scholar]
- 103.Li S., Li Y., Wang Y., Li R., Niu H., Liu C., Zhang Y. Ionic-Liquid-Based Ultrasound-Assisted Extraction Combined with Countercurrent Chromatography and Semipreparative LC for the Preparation of Monoamine Oxidase B Inhibitors from Pueraria Thomsonii. J. Sep. Sci. 2022;45:1116–1127. doi: 10.1002/jssc.202100799. [DOI] [PubMed] [Google Scholar]
- 104.Ito Y., Sandlin J., Bowers W.G. High-Speed Preparative Counter-Current Chromatography with a Coil Planet Centrifuge. J. Chromatogr. A. 1982;244:247–258. doi: 10.1016/S0021-9673(00)85688-5. [DOI] [Google Scholar]
- 105.Yang Y., Muhammad K.B., Zhang X., Zhao Y., Kit-Leong C., Liu Y. Advances in Separation and Purification of Bioactive Polysaccharides through High-Speed Counter-Current Chromatography. J. Chromatogr. Sci. 2020;58:992–1000. doi: 10.1093/chromsci/bmaa063. [DOI] [PubMed] [Google Scholar]
- 106.Wu T., Liu C., Huang Y., Li S., Wang Y. Simultaneous Screening and Isolation of Activated Constituents from Puerariae Flos by Ultrafiltration with Liquid Chromatography and Mass Spectrometry Combined with High-Speed Counter-Current Chromatography. J. Sep. Sci. 2018;41:4458–4468. doi: 10.1002/jssc.201800691. [DOI] [PubMed] [Google Scholar]
- 107.He T., Wang W., Xu S., Du N., Xi X., Lan T. Development of Puerarin Reference Material. Anal. Instrum. 2018;217:82–89. [Google Scholar]
- 108.Pan C., Wang H., Shan H., Lü H. Preparative Isolation and Purification of Calycosin and Formononetin from Astragali Radix Using Hydrolytic Extraction Combined with High Speed Countercurrent Chromatography. J. Chromatogr. Sci. 2021;59:412–418. doi: 10.1093/chromsci/bmab021. [DOI] [PubMed] [Google Scholar]
- 109.Hou J., Xie W., Shi Y., Li J., Wang P., Mao R., Zhang W. The Contents of 11 Flavone Glycosides in Kudzu Were Simultaneously Determined by Solid Phase Extraction-Liquid Chromatography-Tandem Mass Spectrometry. Phys. Test. Chem. Anal. BChemical Anal. 2021;57:865–872. [Google Scholar]
- 110.Qiao C., Yang X., Gao X. Determination of Puerarin in Qinggan Jiangya Capsule by Solid Phase Extraction-High Performance Liquid Chromatography. Chin. Med. Mod. Distance Educ. China. 2021;19:143–145. [Google Scholar]
- 111.Yan L., Mao Q., Shi H., Zhu Y., Li X., Chen Y. Overview of sample pretreatment technology in high performance liquid chromatography. Chem. Anal. Meterage. 2022;31:93–99. [Google Scholar]
- 112.Jiang L., Zhou G., Li Y. Simultaneous Determination of Isoflavonoids in Kudzu Powder by High Performance Liquid Chromatography Coupled with Micelle-Mediated Extraction. Food Sci. 2011;32:186–190. [Google Scholar]
- 113.Chi R., Zhan S., Zhang Y., Chen Z., Liu Z., Li Y. Extraction of Pueraria Total Flavonoids in Ethanol-Inorganic Salt Aqueous Systems. J. Wuhan Inst. Technol. 2014;36:1–7. [Google Scholar]
- 114.Xu T. Simplified Quantification of Representative Bioactives in Food Through TLC Image Analysis. Food Anal. Methods. 2019;12:2886–2894. doi: 10.1007/s12161-019-01645-x. [DOI] [Google Scholar]
- 115.Hu L., Zhou C., Huang Y., Wang Y., Wei G., Liang Z., Zhou C. HPLC Coupled with Electrospray Ionization Multi-stage Tandem MS and TLC Analysis of Flavones-C-glycosides and Bibenzyl of Dendrobium Hercoglossum. J. Sep. Sci. 2020;43:3885–3901. doi: 10.1002/jssc.202000647. [DOI] [PubMed] [Google Scholar]
- 116.Fang C., Wan X., Tan H., Jiang C. Separation and Determination of Isoflavonoids in Several Kudzu Samples by High-Performance Capillary Electrophoresis (HPCE) Ann. Chim. 2006;96:117–124. doi: 10.1002/adic.200690002. [DOI] [PubMed] [Google Scholar]
- 117.Li Y., Zheng N., Wang J., Zhao S. Determination of Four Isoflavones in Red Clover Extract by High-Performance Liquid Chromatography. China Feed. 2022;24:45–51. doi: 10.15906/j.cnki.cn11-2975/s.2022030054-11. [DOI] [Google Scholar]
- 118.Lin S.-J., Qiu F.-B., Xue R.-X., Huang C., Lu L.-M., Liu G.-P., Huang Y.-S., Chen H.-Y. Determination for 3 Kinds of Isoflavones in Soybean by Ultra Performance Liquid Chromatography-Tandem Mass Spectrometry. J. Food Saf. Qual. 2020;11:6006–6011. [Google Scholar]
- 119.Shen Q., Shen X., Liu X. Qualitative Analysis and Determination of Four Components in Ganmao Qingre Granules by LC-MS/MS and HPLC. Chin. J. Mod. Appl. Pharm. 2021;38:2245–2249. [Google Scholar]
- 120.Wu Y., Wan H., Li C., Wan H., Yang J. Composition Analysis and Fingerprint Establishment of Tongmai Jiangtang Capsule Based on HPLC-Q-Exactive-MS and HPLC. Chin. Tradit. Herb. Drugs. 2022;53:6686–6697. doi: 10.7501/j.issn.0253-2670.2022.21.005. [DOI] [Google Scholar]
- 121.Wang B., Cheng F., Gao S., Ge W., Zhang M. Double Enzymatic Hydrolysis Preparation of Heme from Goose Blood and Microencapsulation to Promote Its Stability and Absorption. Food Chem. 2017;217:699–704. doi: 10.1016/j.foodchem.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 122.Hao J.W., Liu X.Q., Zang Y.J., Chen N.D., Shi M.Z. Simultaneous Determination of 16 Important Biologically Active Phytohormones in Dendrobium Huoshanese by Pressurized Capillary Electrochromatography. J. Chromatogr. B. 2021;1171:122612. doi: 10.1016/j.jchromb.2021.122612. [DOI] [PubMed] [Google Scholar]
- 123.Liu D., Ma L., Zhou Z., Liang Q., Xie Q., Ou K., Liu Y., Su Y. Starch and Mineral Element Accumulation during Root Tuber Expansion Period of Pueraria Thomsonii Benth. Food Chem. 2021;343:128445. doi: 10.1016/j.foodchem.2020.128445. [DOI] [PubMed] [Google Scholar]
- 124.Xu X., Guo Y., Chen S., Ma W., Xu X., Hu S., Jin L., Sun J., Mao J., Shen C. The Positive Influence of Polyphenols Extracted From Pueraria Lobata Root on the Gut Microbiota and Its Antioxidant Capability. Front. Nutr. 2022;9:868188. doi: 10.3389/fnut.2022.868188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Song X., Dong H., Zang Z., Wu W., Zhu W., Zhang H., Guan Y. Kudzu Resistant Starch: An Effective Regulator of Type 2 Diabetes Mellitus. Oxid. Med. Cell Longev. 2021;2021:4448048. doi: 10.1155/2021/4448048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Duan X., Guan Y., Dong H., Yang M., Chen L., Zhang H., Naeem A., Zhu W. Study on Structural Characteristics and Physicochemical Properties of Starches Extracted from Three Varieties of Kudzu Root (Pueraria Lobata Starch) J. Food Sci. 2023;88:1048–1059. doi: 10.1111/1750-3841.16472. [DOI] [PubMed] [Google Scholar]
- 127.Bihlet A.R., Byrjalsen I., Andersen J.R., Simonsen S.F., Mundbjerg K., Helmer B., Riis B.J., Karsdal M.A., Christiansen C. The Efficacy and Safety of Multiple Dose Regimens of Kudzu (Pueraria Lobata) Root Extract on Bone and Cartilage Turnover and Menopausal Symptoms. Front. Pharmacol. 2021;12:760629. doi: 10.3389/fphar.2021.760629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yuan Y., Zong J., Zhou H., Bian Z.-Y., Deng W., Dai J., Gan H.-W., Yang Z., Li H., Tang Q.-Z. Puerarin Attenuates Pressure Overload-Induced Cardiac Hypertrophy. J. Cardiol. 2014;63:73–81. doi: 10.1016/j.jjcc.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 129.Martínez-Riera R., Pérez-Mañá C., Papaseit E., Fonseca F., de la Torre R., Pizarro N., Torrens M., Farré M. Soy Isoflavone Extract Does Not Increase the Intoxicating Effects of Acute Alcohol Ingestion in Human Volunteers. Front. Pharmacol. 2019;10:131. doi: 10.3389/fphar.2019.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang X.-L., Cao X.-Y., Lai R.-C., Xie M.-X., Zeng W.-A. Puerarin Relieves Paclitaxel-Induced Neuropathic Pain: The Role of Nav1.8 Β1 Subunit of Sensory Neurons. Front. Pharmacol. 2018;9:1510. doi: 10.3389/fphar.2018.01510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang S., Zhang S., Wang S., Gao P., Dai L. A Comprehensive Review on Pueraria: Insights on Its Chemistry and Medicinal Value. Biomed. Pharmacother. 2020;131:110734. doi: 10.1016/j.biopha.2020.110734. [DOI] [PubMed] [Google Scholar]
- 132.Zhou H., Li X., Shang Y., Chen K. Radical Scavenging Activity of Puerarin: A Theoretical Study. Antioxidants. 2019;8:590. doi: 10.3390/antiox8120590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu R., Li Y., Wang Z., Li J., Liang Y., Li M. Puerarin Mitigates Symptoms of Depression in Ovariectomized Female Rats by Regulating Hippocampal CAMP-CREB-BDNF Signaling Pathway. Trop. J. Pharm. Res. 2021;20:1403–1409. doi: 10.4314/tjpr.v20i7.12. [DOI] [Google Scholar]
- 134.Yu Z.-M., Wan X.-M., Xiao M., Zheng C., Zhou X.-L. Puerarin Induces Nrf2 as a Cytoprotective Mechanism to Prevent Cadmium-Induced Autophagy Inhibition and NLRP3 Inflammasome Activation in AML12 Hepatic Cells. J. Inorg. Biochem. 2021;217:111389. doi: 10.1016/j.jinorgbio.2021.111389. [DOI] [PubMed] [Google Scholar]
- 135.Wang L.-Y., Fan R.-F., Yang D.-B., Zhang D., Wang L. Puerarin Reverses Cadmium-Induced Lysosomal Dysfunction in Primary Rat Proximal Tubular Cells via Inhibiting Nrf2 Pathway. Biochem. Pharmacol. 2019;162:132–141. doi: 10.1016/j.bcp.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 136.Guo C.-J., Xie J.-J., Hong R.-H., Pan H.-S., Zhang F.-G., Liang Y.-M. Puerarin Alleviates Streptozotocin (STZ)-Induced Osteoporosis in Rats through Suppressing Inflammation and Apoptosis via HDAC1/HDAC3 Signaling. Biomed. Pharmacother. Biomed. Pharmacother. 2019;115:108570. doi: 10.1016/j.biopha.2019.01.031. [DOI] [PubMed] [Google Scholar]
- 137.Han P., Liu L., Liu J., Zhang H., Wu Y., Wu Y., Yu F., Wu D. Determination of copper, zinc, iron, calcium and magnesium in Pueraria lobata ohwi by FAAS. Guang Pu Xue Yu Guang Pu Fen Xi Guang Pu. 2005;25:1507–1509. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.