Abstract
Lower respiratory tract infections (LRIs) are a significant cause of disability-adjusted life-years (DALYs) across all age groups, especially in children under 9 years of age, and adults over 75. The main causative agents are viruses, such as influenza and respiratory syncytial virus (RSV). Viral LRIs in adults have historically received less attention. This study investigated the incidence of RSV and influenza in adult patients admitted to a referral hospital, as well as the clinical profile of these infections. Molecular testing was conducted on nasopharyngeal samples taken from a respiratory surveillance cohort comprising adult (15–59 years) and elderly (60+ years) hospitalized patients who tested negative for SARS-CoV-2, to determine the prevalence for influenza and RSV. Influenza was found to be less frequent among the elderly. The main symptoms of RSV infections were cough, fever, dyspnea, malaise, and respiratory distress, while headache, nasal congestion, a sore throat, and myalgia were most frequent in influenza. Elderly patients with RSV were not found to have more severe illness than adults under age 60, underscoring the importance of providing the same care to adults with this viral infection.
Keywords: influenza A virus, influenza B virus, respiratory syncytial virus, severe acute respiratory syndrome
1. Introduction
In 2019, lower respiratory tract infections (LRIs) were the fourth-leading cause of disability-adjusted life-years (DALYs) across all age groups, with the greatest impact on children aged 0–9 years, followed by adults over the age of 75. Historical data reveal similar trends, with the strongest impact on children and the elderly [1]. Furthermore, an estimated 176,740 people died from LRIs in South America in 2019 alone, and 3,872,414 life-years were lost [2].
Several viral infections cause LRIs [as reviewed in [3]], but three stand out: respiratory syncytial virus (RSV) [4], and influenza A (IAV) and B (IBV) [5], due to their case numbers, and the consequent burden on health systems due to the increased hospitalization and morbidity rates, especially among children under five years of age [4,5]. Moreover, the accurate etiological diagnosis of LRIs presents a further challenge when viral infections present overlapping symptoms [6]. Viral respiratory infections, including RSV and influenza, may manifest as various respiratory syndromes, such as bronchiolitis, wheezing, asthma exacerbation, croup, pneumonia, and pneumonitis [7].
Up until now, the research on LRIs has focused on children under five and the elderly; the host’s age is a major concern, as early severe disease develops in these groups [8]. Within this context, viral LRIs in non-elderly adults have received little attention. With the emergence of SARS-CoV-2, the unprecedented pandemic, and its devastating effects worldwide, even less attention was paid to other non-SARS-CoV-2 pathogens. Brazil had reported 484,323 cases of severe acute respiratory syndrome (SARS) by epidemiological week 47 of 2022 (ending 26 November). Of these cases, 2.2% were caused by influenza, 3.0% by other respiratory viruses, and 42.4% by undefined agents or etiologies. Among them, the age group most frequently affected was the elderly, with 216,706 cases (50.11%), with 4177 being due to influenza, and 1503 caused by other respiratory viruses [9].
To assess the circulation of respiratory viruses other than SARS-CoV-2 in adults during the COVID-19 pandemic, between December 2021 and April 2022, this study investigated the incidence of RSV and influenza in patients admitted to a referral hospital. We also evaluated the clinical characteristics of infections caused by different viral agents.
2. Materials and Methods
2.1. Description of the Cohort
This study analyzed data from a respiratory surveillance hospital cohort in São José do Rio Preto, São Paulo, Brazil, comprising enrolled adults (individuals aged 15–59 years were considered adults) and elderly patients (60+ years) who were hospitalized at the Hospital de Base de São José do Rio Preto with respiratory symptoms. Nasopharyngeal swab samples were obtained from patients presenting respiratory tract infection symptoms between December 2021 and April 2022.
2.2. Sample Preparation
Total RNA was extracted from 100 µL of nasopharyngeal swab samples using an Extracta Kit Fast DNA and RNA Viral Testing kit (MVXA-P096 FAST), according to the manufacturer’s instructions for the Extracta 96 DNA/RNA extractor and purifier (Loccus, Cotia, Brazil). A one-step real-time polymerase chain reaction (RT-qPCR) was performed, using primers and probes targeting the RNA-dependent RNA polymerase (RdRp), envelope (E), and nucleocapsid (N) of the SARS-CoV-2 genome and human RNAse P designed using the GeneFinder COVID-19 PLUS RealAmp Kit. (OSANG Healthcare, Anyang, Republic of Korea).
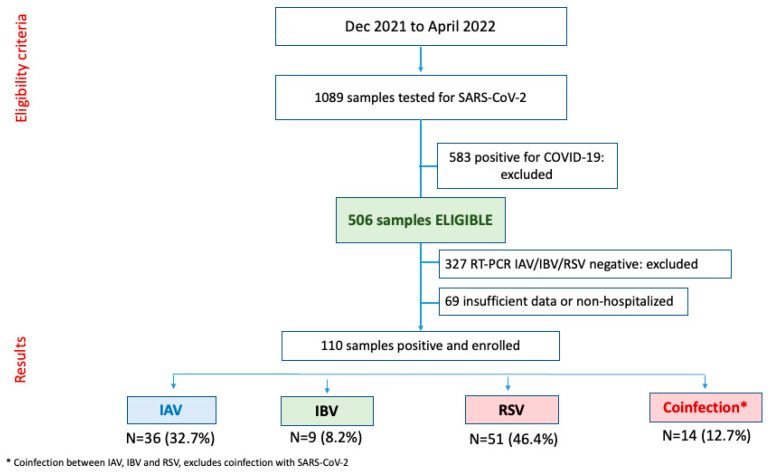
Samples that yielded negative results for COVID-19 were tested for three different respiratory viruses (IAV, IBV, and RSV), using an Allplex SARS-CoV-2/IAV/IBV/RSV assay (Seegene Inc., Seoul, Republic of Korea), according to the manufacturer’s instructions. All samples that tested positive for COVID-19 were excluded from the study. RT-qPCR was conducted with a QuantStudio 3 Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA, USA). The results were interpreted based on the cycle threshold (Ct), with samples presenting Cq ≤ 40 considered positive. Positive and negative controls included in the GeneFinder COVID-19 Plus RealAmp kit and the Allplex SARS-CoV-2/IAV/IBV/RSV assay were used, in addition to positive samples included as a positive control. All samples that tested negative for SARS-CoV-2, but positive for other viruses, were included (Figure 1).
Figure 1.
Selection flow of samples and distribution of respiratory agents in the hospital cohort.
Demographic and clinical data, such as sex, ethnicity, age, education level, comorbidities, signs/symptoms, and severity were obtained from the participants’ electronic medical records or local reporting forms and subjected to descriptive and inferential analysis. The chi-square test or Fisher’s exact test was used to compare the categorical variables for the groups diagnosed with IAV, IBV, and RSV, with p < 0.05 considered statistically significant. All statical analyses were performed using the SPSS Statistics software package (version 28.0.1.1; IBM Corporation, Armonk, NY, USA).
3. Results
During the specified study period, 110 samples were considered eligible for the study (Figure 1 and Table 1): 36 (32.73%) were positive for the IAV virus, and 9 (8.18%) for IBV, and RSV was most prevalent, with 51 (46.36%; p < 0.001). Although they were not included in the final analysis of this study, coinfection with the three viruses of interest was observed in 14 samples (12.73%).
Table 1.
Epidemiological data from 110 patients with samples showing infection with influenza A, influenza B, and respiratory syncytial virus in the respiratory surveillance cohort.
| Total Samples (N = 110) | ||||||
|---|---|---|---|---|---|---|
| N Response | N Positive or Mean | % or s.d. | p-Value | X 2 | df | |
| Agent | ||||||
| RSV | 110 | 51 | 46.36% | <0.001 | 41.782 | 3 |
| Influenza A | 110 | 36 | 32.73% | |||
| Influenza B | 110 | 9 | 8.18% | |||
| Coinfection | 110 | 14 | 12.73% | |||
| Sex | ||||||
| Female | 110 | 52 | 47.27% | 0.567 | 0.327 | 1 |
| Male | 110 | 58 | 52.73% | |||
| Age group | ||||||
| Mean age | 110 | 50.38 | 20.42 | - | - | - |
| Adult | 110 | 71 | 64.55% | 0.002 | 9.309 | 1 |
| Elderly | 110 | 39 | 35.45% | |||
| Ethnicity | ||||||
| White | 104 | 94 | 90.38% | <0.001 | 152.558 | 2 |
| Black | 104 | 7 | 6.73% | |||
| Mixed race | 104 | 3 | 2.88% | |||
| Education | ||||||
| Primary incomplete | 93 | 40 | 43.01% | 0.115 | 4.323 | 2 |
| Primary | 93 | 0 | 0.00% | |||
| High school | 93 | 24 | 25.81% | |||
| College | 93 | 29 | 31.18% | |||
| Comorbidities | 97 | 62 | 63.92% | 0.006 | 7.515 | 1 |
| Cardiopathy | 97 | 16 | 16.49% | <0.001 | 43.557 | 1 |
| Blood pressure | 97 | 34 | 35.05% | 0.003 | 8.670 | 1 |
| Hematological disease | 97 | 3 | 3.09% | <0.001 | 85.371 | 1 |
| Down syndrome | 97 | 2 | 2.06% | <0.001 | 89.165 | 1 |
| Liver disease | 97 | 6 | 6.19% | <0.001 | 74.485 | 1 |
| Asthma | 97 | 1 | 1.03% | <0.001 | 93.041 | 1 |
| Diabetes | 97 | 20 | 20.62% | <0.001 | 33.495 | 1 |
| Neurovascular disease | 97 | 7 | 7.22% | <0.001 | 71.021 | 1 |
| Neurological disease | 97 | 5 | 5.15% | <0.001 | 78.031 | 1 |
| Pneumopathy | 97 | 7 | 7.22% | <0.001 | 71.021 | 1 |
| Immunosuppression | 97 | 1 | 1.03% | <0.001 | 93.041 | 1 |
| Kidney disease | 97 | 7 | 7.22% | <0.001 | 71.021 | 1 |
| Obesity | 97 | 5 | 5.15% | <0.001 | 78.031 | 1 |
| Smoker | 85 | 16 | 18.82% | <0.001 | 33.047 | 1 |
| Cancer | 97 | 9 | 9.28% | <0.001 | 64.340 | 1 |
df: degrees of freedom.
The patients infected with IAV were predominantly adults (83.33%; p < 0.001), and white (87.88%; p < 0.001), with a mean age of 38.56 years (±19.51). No significant statistical differences were observed with respect to sex, education, or the presence of comorbidities. For patients infected with IBV, no significant differences were noted, although they were predominantly female (77.78%), white (100%) adults (77.78%), with a mean age of 42.89 years (±18.19), and education up through high school (57.14%). No age group or sex was predominant in the RSV patient group, but white ethnicity (90.20%; p < 0.001), education up to elementary school (56.52%; p = 0.004), and the presence of comorbidities (84.78%; p < 0.001) were more prevalent; the mean age was 59.20 years (±18.21) (Supplementary Table S1).
The clinical data were subdivided according to the diagnosis of influenza A/B (Flu) or RSV, as shown in Table 2 and complemented by Supplementary Table S2. Influenza appeared less frequently in the elderly participants. We found a significant difference in the mean ages: RSV-infected patients were older (59.2 years vs. 39.42 years for patients with influenza). No significant differences were found for sex or ethnicity.
Table 2.
Clinical characteristics of 96 patients infected with influenza and respiratory syncytial virus in the respiratory surveillance cohort.
| Flu | RSV | ||||||
|---|---|---|---|---|---|---|---|
| N Response | N Positive or Mean | % or s.d. | N Response | N Positive or Mean | % or s.d. | p-Value | |
| Age group | |||||||
| Adult (15–59 years) | 45 | 37 | 82.22% | 51 | 23 | 45.10% | <0.001 |
| Elderly (60+ years) | 45 | 8 | 17.78% | 51 | 28 | 54.90% | |
| Mean age (years) | 45 | 39.42 | 19.13 | 51 | 59.20 | 18.21 | <0.001 |
| Sex | |||||||
| Female | 45 | 21 | 46.67% | 51 | 26 | 50.98% | 0.673 |
| Male | 45 | 24 | 53.33% | 51 | 25 | 49.02% | |
| Ethnicity | |||||||
| White | 41 | 37 | 90.24% | 51 | 46 | 90.20% | 0.894 |
| Black | 41 | 3 | 7.32% | 51 | 3 | 5.88% | |
| Mixed race | 41 | 1 | 2.44% | 51 | 2 | 3.92% | |
| Education | |||||||
| Primary incomplete | 38 | 7 | 18.42% | 46 | 26 | 56.52% | 0.002 |
| Primary | 38 | 0 | 0.00% | 46 | 0 | 0.00% | |
| High school | 38 | 15 | 39.47% | 46 | 9 | 19.57% | |
| College | 38 | 16 | 42.11% | 46 | 11 | 23.91% | |
| Comorbidities | 38 | 18 | 47.37% | 49 | 39 | 79.59% | <0.001 |
| Cardiopathy | 38 | 1 | 2.63% | 49 | 14 | 28.57% | <0.001 |
| Blood pressure | 38 | 7 | 18.42% | 49 | 19 | 38.78% | 0.024 |
| Hematological disease | 38 | 0 | 0.00% | 49 | 2 | 4.08% | 0.499 |
| Down syndrome | 38 | 0 | 0.00% | 49 | 2 | 4.08% | 0.499 |
| Liver disease | 38 | 0 | 0.00% | 49 | 6 | 12.24% | 0.300 |
| Asthma | 38 | 1 | 2.63% | 49 | 0 | 0.00% | 0.452 |
| Diabetes | 38 | 4 | 10.53% | 49 | 13 | 26.53% | 0.044 |
| Neurovascular disease | 38 | 0 | 0.00% | 49 | 6 | 12.24% | 0.030 |
| Neurological disease | 38 | 0 | 0.00% | 49 | 5 | 10.20% | 0.061 |
| Pneumopathy | 38 | 1 | 2.63% | 49 | 5 | 10.20% | 0.215 |
| Immunosuppression | 38 | 0 | 0.00% | 48 | 1 | 2.08% | 1 |
| Kidney disease | 38 | 0 | 0.00% | 49 | 6 | 12.24% | 0.030 |
| Obesity | 38 | 1 | 2.63% | 49 | 3 | 6.12% | 0.623 |
| Smoker | 35 | 6 | 17.14% | 41 | 7 | 17.07% | 1 |
| Cancer | 38 | 4 | 10.53% | 49 | 5 | 10.20% | 1 |
| Signs and symptoms | |||||||
| Fever | 37 | 22 | 59.46% | 42 | 16 | 38.10% | 0.058 |
| Headache | 37 | 17 | 45.95% | 42 | 9 | 21.43% | 0.021 |
| Myalgia | 37 | 19 | 51.35% | 42 | 8 | 19.05% | 0.003 |
| Nasal congestion | 37 | 21 | 56.76% | 42 | 10 | 23.81% | 0.003 |
| Cough | 37 | 29 | 78.38% | 42 | 30 | 71.43% | 0.478 |
| Sore throat | 37 | 15 | 40.54% | 42 | 4 | 9.52% | <0.001 |
| Dyspnea | 37 | 4 | 10.81% | 42 | 16 | 38.10% | 0.005 |
| Respiratory distress | 37 | 1 | 2.70% | 42 | 15 | 35.71% | <0.001 |
| Diarrhea | 37 | 2 | 5.41% | 42 | 4 | 9.52% | 0.679 |
| Vomiting | 37 | 0 | 0.00% | 42 | 1 | 2.38% | 1 |
| Abdominal pain | 37 | 3 | 8.11% | 42 | 2 | 4.76% | 0.661 |
| Malaise | 37 | 8 | 21.62% | 42 | 15 | 35.71% | 0.169 |
| Loss of smell | 37 | 1 | 2.70% | 42 | 0 | 0.00% | 0.468 |
| Loss of taste | 37 | 1 | 2.70% | 42 | 0 | 0.00% | 0.468 |
| Hospitalization | 45 | 45 | 100.00% | 51 | 51 | 100.00% | NA |
| ICU | 42 | 1 | 2.38% | 51 | 5 | 9.80% | 0.217 |
| Ventilatory support | 42 | 4 | 9.52% | 50 | 23 | 46.00% | <0.001 |
| Death | 43 | 8 | 18.60% | 46 | 15 | 32.61% | 0.132 |
Comorbidities were more frequently present in RSV-infected patients (79.59%) than in other groups (p < 0.001). Of these, high blood pressure was most common in both groups (flu = 18.42%; RSV = 38.78%), but less frequent in the patients with influenza (p = 0.024). An additional statistically significant comorbidity was cardiopathy, which was observed more frequently in patients with RSV (p = 0.004). The frequency of comorbidities by infection group is shown in Table 2.
The main symptoms of influenza were (in descending order) coughing (78.38%), fever (59.46%), nasal congestion (56.76%), myalgia (51.35%), and headache (45.95%). In RSV infections, coughing (71.43%), fever (38.1%), dyspnea (38.1%), malaise (35.71%), and respiratory distress (35.71%) were predominant. Group analysis indicated that headache (p = 0.021), nasal congestion (p = 0.003), sore throat (p > 0.001), and myalgia (p = 0.003) were more frequent in the flu group, while potentially severe symptoms, such as respiratory distress (p < 0.001) and dyspnea (p = 0.005), were more frequent in the RSV group, as shown in Table 2. In terms of severity, no significant difference was seen in deaths or the need for intensive care, but ventilatory support was more frequently required in patients with RSV (p < 0.001). These variables also appear in Table 2.
Our subsequent analysis only examined the hospitalized adults; the most frequently identified respiratory agent in this group was IAV (42.25%), followed by RSV (32.39%), coinfections (15.49%), and IBV (9.86%) (p < 0.001) (Table 3). At this stage, the coinfection group was excluded, as it was impossible to define which agent influenced the variables. No differences were observed with regard to sex or ethnicity, while adults with an incomplete primary school education were predominant in the flu group (p = 0.037). Comorbidities were generally more frequent in the RSV group (p = 0.042) (Table 3).
Table 3.
Epidemiological data from 71 adult patients infected with influenza A, influenza B, and respiratory syncytial virus in the respiratory surveillance cohort.
| Samples from Hospitalized Adults | ||||||
|---|---|---|---|---|---|---|
| N Response | N Positive or Mean | % or s.d. | p-Value | X 2 | df | |
| Agent | ||||||
| RSV | 71 | 23 | 32.39% | <0.001 | 19 | 3 |
| Influenza A | 71 | 30 | 42.25% | |||
| Influenza B | 71 | 7 | 9.86% | |||
| Coinfection | 71 | 11 | 15.49% | |||
| Sex | ||||||
| Female | 71 | 31 | 43.66% | 0.285 | 1.141 | 1 |
| Male | 71 | 40 | 56.34% | |||
| Age group | ||||||
| Mean age | 71 | 38 | 13.20 | - | - | - |
| Ethnicity | ||||||
| White | 67 | 58 | 86.57% | <0.001 | 85.642 | 2 |
| Black | 67 | 6 | 8.96% | |||
| Mixed race | 67 | 3 | 4.48% | |||
| Education | ||||||
| Primary incomplete | 63 | 16 | 25.40% | 0.692 | 0.737 | 2 |
| Primary | 63 | 0 | 0.00% | |||
| High school | 63 | 20 | 31.75% | |||
| College | 63 | 21 | 33.33% | |||
| Comorbidities | 63 | 28 | 44.44% | <0.001 | 0.778 | 1 |
| Cardiopathy | 63 | 3 | 4.76% | <0.001 | 51.571 | 1 |
| Blood pressure | 63 | 15 | 23.81% | <0.001 | 17.286 | 1 |
| Hematological disease | 63 | 2 | 3.17% | <0.001 | 55.254 | 1 |
| Down syndrome | 63 | 2 | 3.17% | <0.001 | 55.524 | 1 |
| Liver disease | 63 | 4 | 6.35% | <0.001 | 48.016 | 1 |
| Asthma | 63 | 0 | 0.00% | |||
| Diabetes | 63 | 9 | 14.29% | <0.001 | 32.143 | 1 |
| Neurovascular disease | 63 | 1 | 1.59% | <0.001 | 59.063 | 1 |
| Neurological disease | 63 | 0 | 0.00% | |||
| Pneumopathy | 63 | 3 | 4.76% | <0.001 | 51.571 | 1 |
| Immunosuppression | 63 | 1 | 1.59% | <0.001 | 59.063 | 1 |
| Kidney disease | 63 | 4 | 6.35% | <0.001 | 48.016 | 1 |
| Obesity | 63 | 3 | 4.76% | <0.001 | 51.571 | 1 |
| Smoker | 53 | 10 | 18.87% | <0.001 | 20.547 | 1 |
| Cancer | 63 | 4 | 6.35% | <0.001 | 48.016 | 1 |
| Symptoms | ||||||
| Fever | 60 | 31 | 51.67% | 0.796 | 0.067 | 1 |
| Headache | 60 | 25 | 41.67% | 0.197 | 1.667 | 1 |
| Myalgia | 60 | 36 | 60.00% | 0.302 | 1.067 | 1 |
| Nasal congestion | 60 | 30 | 50.00% | 1 | 0 | 1 |
| Cough | 60 | 48 | 80.00% | <0.001 | 21.6 | 1 |
| Sore throat | 60 | 20 | 33.33% | 0.010 | 6.667 | 1 |
| Dyspnea | 60 | 12 | 20.00% | <0.001 | 21.6 | 1 |
| Respiratory distress | 60 | 7 | 11.67% | <0.001 | 35.267 | 1 |
| Diarrhea | 60 | 5 | 8.33% | <0.001 | 41.667 | 1 |
| Vomiting | 60 | 0 | 0.00% | NA | NA | NA |
| Abdominal pain | 60 | 4 | 6.67% | <0.001 | 45.067 | 1 |
| Malaise | 60 | 11 | 18.33% | <0.001 | 24.067 | 1 |
| Loss of smell | 60 | 2 | 3.33% | <0.001 | 52.267 | 1 |
| Loss of taste | 60 | 2 | 3.33% | <0.001 | 52.267 | 1 |
| Hospitalization | 71 | 71 | 100.00% | NA | NA | NA |
| ICU | 70 | 5 | 7.14% | <0.001 | 51.429 | 1 |
| Ventilatory support | 69 | 12 | 17.39% | <0.001 | 29.348 | 1 |
| Death | 57 | 3 | 5.26% | <0.001 | 18.284 | 1 |
When symptoms were associated with the viral agent, fever (62.50%; p = 0.012), headache (53.13%; p = 0.008), and myalgia (56.25%; p = 0.005) were seen to be more frequent in the flu group, while dyspnea (42.11%; p = 0.003) and respiratory distress (31.58%; p = 0.002) were, again, associated with RSV infection. Ventilatory support was more frequently required in RSV-infected patients (26.09%; p = 0.009), consistent with the higher frequency of dyspnea and respiratory distress (Table 4).
Table 4.
Clinical and epidemiological data from 60 adult patients infected with influenza and respiratory syncytial virus in the respiratory surveillance cohort.
| Flu (A and B) | RSV | ||||||
|---|---|---|---|---|---|---|---|
| N Response | N Positive or Mean | % or s.d. | N Response | N Positive or Mean | % or s.d. | p-Value | |
| Mean age (years) | 37 | 31.97 | 10.72 | 23 | 43.26 | 13.29 | 0.001 |
| Sex | |||||||
| Female | 37 | 17 | 45.95% | 23 | 11 | 47.83% | 0.887 |
| Male | 37 | 20 | 54.05% | 23 | 12 | 52.17% | |
| Ethnicity | |||||||
| White | 34 | 30 | 88.24% | 23 | 19 | 82.61% | 0.633 |
| Black | 34 | 3 | 8.82% | 23 | 2 | 8.70% | |
| Mixed race | 34 | 1 | 2.94% | 23 | 2 | 8.70% | |
| Education | |||||||
| Primary incomplete | 30 | 3 | 10.00% | 20 | 8 | 40.00% | 0.037 |
| Primary | 30 | 0 | 0.00% | 20 | 0 | 0.00% | |
| High school | 30 | 13 | 43.33% | 20 | 7 | 35.00% | |
| College | 30 | 14 | 46.67% | 20 | 5 | 25.00% | |
| Comorbidities | 33 | 10 | 30.30% | 20 | 13 | 65.00% | 0.013 |
| Cardiopathy | 33 | 0 | 0.00% | 20 | 3 | 15.00% | 0.049 |
| Blood pressure | 33 | 5 | 15.15% | 20 | 5 | 25.00% | 0.475 |
| Hematological disease | 33 | 0 | 0.00% | 20 | 1 | 5.00% | 0.377 |
| Down syndrome | 33 | 0 | 0.00% | 20 | 2 | 10.00% | 0.138 |
| Liver disease | 33 | 0 | 0.00% | 20 | 4 | 20.00% | 0.017 |
| Asthma | 33 | 0 | 0.00% | 20 | 0 | 0.00% | NA |
| Diabetes | 33 | 1 | 3.03% | 20 | 5 | 25.00% | 0.024 |
| Neurovascular disease | 33 | 0 | 0.00% | 20 | 1 | 5.00% | 0.377 |
| Neurological disease | 33 | 0 | 0.00% | 20 | 0 | 0.00% | NA |
| Pneumopathy | 33 | 0 | 0.00% | 20 | 2 | 10.00% | 0.138 |
| Immunosuppression | 33 | 0 | 0.00% | 20 | 1 | 5.00% | 0.377 |
| Kidney disease | 33 | 0 | 0.00% | 20 | 3 | 15.00% | 0.049 |
| Obesity | 33 | 1 | 3.03% | 20 | 1 | 5.00% | 1 |
| Smoker | 28 | 4 | 14.29% | 17 | 3 | 17.65% | 1 |
| Cancer | 33 | 3 | 9.09% | 20 | 1 | 5.00% | 1 |
| Symptoms | |||||||
| Fever | 32 | 20 | 62.50% | 19 | 5 | 26,32% | 0.012 |
| Headache | 32 | 17 | 53.13% | 19 | 3 | 15.79% | 0.008 |
| Myalgia | 32 | 18 | 56.25% | 19 | 3 | 15.79% | 0.005 |
| Nasal congestion | 32 | 19 | 59.38% | 19 | 5 | 26.32% | 0.022 |
| Cough | 32 | 24 | 75.00% | 19 | 17 | 89.47% | 0.287 |
| Sore throat | 32 | 13 | 40.63% | 19 | 3 | 15.79% | 0.065 |
| Dyspnea | 32 | 2 | 6.25% | 19 | 8 | 42.11% | 0.003 |
| Respiratory distress | 32 | 0 | 0.00% | 19 | 6 | 31.58% | 0.002 |
| Diarrhea | 32 | 1 | 3.13% | 19 | 4 | 21.05% | 0.058 |
| Vomiting | 32 | 0 | 0.00% | 19 | 0 | 0.00% | NA |
| Abdominal pain | 32 | 3 | 9.38% | 19 | 1 | 5.26% | 1 |
| Malaise | 32 | 6 | 18.75% | 19 | 4 | 21.05% | 1 |
| Loss of smell | 32 | 1 | 3.13% | 19 | 0 | 0.00% | 1 |
| Loss of taste | 32 | 1 | 3.13% | 19 | 0 | 0.00% | 1 |
| Hospitalization | 37 | 37 | 100.00% | 23 | 23 | 100.00% | NA |
| ICU | 36 | 1 | 2.78% | 23 | 3 | 13.04% | 0.289 |
| Ventilatory support | 36 | 2 | 5.56% | 23 | 6 | 26.09% | 0.009 |
| Death | 36 | 6 | 16.67% | 20 | 8 | 40.00% | 0.053 |
A final analysis compared the clinical manifestations of RSV infection between adults and elderly patients (Table 5). No differences were observed in sex or ethnicity, while elderly patients with an incomplete primary school education were most prevalent (69.23%; p = 0.049), as was the presence of comorbidities (p = 0.001), most notably cardiopathy (p = 0.046) and high blood pressure (p = 0.049). The most common symptoms in adults (in descending order) were coughing (89.47%), dyspnea (42.11%), respiratory distress (31.78%), fever (26.32%), and nasal congestion (26.32%). The elderly patients were more likely to have a cough (56.42%), fever (47.83%), malaise (47.83%), respiratory distress (39.13%), and dyspnea (34.78%). Differences were only observed for coughing (p = 0.037) and diarrhea (p = 0.035), which were more common in the adults. Interestingly, no differences were observed for dyspnea, respiratory distress, ventilatory support, or death.
Table 5.
Clinical manifestations of RSV in hospitalized adult and elderly patients.
| Adult | Elderly | ||||||
|---|---|---|---|---|---|---|---|
| N Response | N Positive or Mean | % or s.d. | N Response | N Positive or Mean | % or s.d. | p-Value | |
| Mean age (years) | 23 | 43.26 | 13.29 | 28 | 72.29 | 8.74 | <0.001 |
| Sex | |||||||
| Female | 23 | 11 | 47.83% | 28 | 15 | 53.57% | 0.683 |
| Male | 23 | 12 | 52.17% | 28 | 13 | 46.43% | |
| Ethnicity | |||||||
| White | 23 | 19 | 82.61% | 28 | 27 | 96.43% | 0.195 |
| Black | 23 | 2 | 8.70% | 28 | 1 | 3.57% | |
| Mixed race | 23 | 2 | 8.70% | 28 | 0 | 0.00% | |
| Education | |||||||
| Primary incomplete | 20 | 8 | 40.00% | 26 | 18 | 69.23% | 0.049 |
| Primary | 20 | 0 | 0.00% | 26 | 0 | 0.00% | |
| High school | 20 | 7 | 35.00% | 26 | 2 | 7.69% | |
| College | 20 | 5 | 25.00% | 26 | 6 | 23.08% | |
| Comorbidities | 20 | 13 | 65.00% | 26 | 26 | 100.00% | 0.001 |
| Cardiopathy | 20 | 3 | 15.00% | 26 | 11 | 42.31% | 0.046 |
| Blood pressure | 20 | 5 | 25.00% | 26 | 14 | 53.85% | 0.049 |
| Hematological disease | 20 | 1 | 5.00% | 26 | 1 | 3.85% | 1 |
| Down syndrome | 20 | 2 | 10.00% | 26 | 0 | 0.00% | 0.184 |
| Liver disease | 20 | 4 | 20.00% | 26 | 2 | 7.69% | 0.380 |
| Asthma | 20 | 0 | 0.00% | 26 | 0 | 0.00% | NA |
| Diabetes | 20 | 5 | 25.00% | 26 | 8 | 30.77% | 0.667 |
| Neurovascular disease | 20 | 1 | 5.00% | 26 | 5 | 19.23% | 0.212 |
| Neurological disease | 20 | 0 | 0.00% | 26 | 5 | 19.23% | 0.059 |
| Pneumopathy | 20 | 2 | 10.00% | 26 | 3 | 11.54% | 1 |
| Immunosuppression | 20 | 1 | 5.00% | 26 | 0 | 0.00% | 0.435 |
| Kidney disease | 20 | 3 | 15.00% | 26 | 3 | 11.54% | 1 |
| Obesity | 20 | 1 | 5.00% | 26 | 2 | 7.69% | 1 |
| Smoker | 17 | 3 | 17.65% | 26 | 4 | 15.38% | 1 |
| Cancer | 20 | 1 | 5.00% | 26 | 4 | 15.38% | 0.369 |
| Signs and Symptoms | |||||||
| Fever | 19 | 5 | 26.32% | 23 | 11 | 47.83% | 0.153 |
| Headache | 19 | 3 | 15.79% | 23 | 6 | 26.09% | 0.477 |
| Myalgia | 19 | 3 | 15.79% | 23 | 5 | 21.74% | 0.709 |
| Nasal congestion | 19 | 5 | 26.32% | 23 | 5 | 21.74% | 1 |
| Cough | 19 | 17 | 89.47% | 23 | 13 | 56.52% | 0.037 |
| Sore throat | 19 | 3 | 15.79% | 23 | 1 | 4.35% | 0.313 |
| Dyspnea | 19 | 8 | 42.11% | 23 | 8 | 34.78% | 0.627 |
| Respiratory distress | 19 | 6 | 31.58% | 23 | 9 | 39.13% | 0.611 |
| Diarrhea | 19 | 4 | 21.05% | 23 | 0 | 0.00% | 0.035 |
| Vomiting | 19 | 0 | 0.00% | 23 | 1 | 4.35% | 1 |
| Abdominal pain | 19 | 1 | 5.26% | 23 | 1 | 4.35% | 1 |
| Malaise | 19 | 4 | 21.05% | 23 | 11 | 47.83% | 0.071 |
| Loss of smell | 19 | 0 | 0.00% | 23 | 0 | 0.00% | NA |
| Loss of taste | 19 | 0 | 0.00% | 23 | 0 | 0.00% | NA |
| Hospitalization | 23 | 23 | 100.00% | 28 | 28 | 100.00% | NA |
| ICU | 23 | 3 | 13.04% | 28 | 2 | 7.14% | 0.647 |
| Ventilatory support | 23 | 6 | 26.09% | 27 | 15 | 55.56% | 0.142 |
| Death | 20 | 8 | 40.00% | 26 | 7 | 26.92% | 0.348 |
4. Discussion
This study reports the frequency of IAV, IBV, and RSV as the cause of LRIs in patients hospitalized between December 2021 and April 2022. This is not the typical season for such respiratory infections in the Southern Hemisphere, and the COVID-19 pandemic was still underway in Brazil. We found RSV to be the main causative agent among the respiratory viruses for which we tested, associated with dyspnea, respiratory distress, and the need for ventilator support, while influenza caused disease characterized by milder symptoms. Most notably, no significant differences were reported in how RSV infections manifested among adult and elderly patients.
RSV is recognized as an important cause of respiratory infection, particularly in children and the elderly. In children, RSV may manifest as an upper respiratory tract infection, characterized by nasal congestion, coughing, fever, malaise, poor appetite, and dehydration. About one-third of infants may develop LRIs that cause pneumonia, bronchiolitis, and laryngotracheitis [10]. These patients may present with tachypnea, wheezing, noisy breathing, and even apnea, progressing to respiratory failure and death [10,11]. The presence of fever is not imperative to identifying suspected cases in RSV, nor in influenza, as proposed by the WHO, as it is absent in around 50% of cases in children and elderly patients [12]. RSV is also associated with long-term sequelae, such as asthma, recurrent wheezing, atopy, and allergies, as well as abnormal respiratory function due to airway remodeling [10]. A review of the costs associated with pediatric hospitalizations in the United States from 2014 to 2021 determined that the average cost per RSV hospitalization ranged from USD 10,214 to USD 57,406, depending on the age group [13].
The literature highlights the involvement of RSVs in children, as well as in elderly people and adults with comorbidities, particularly immunosuppression, asthma, chronic obstructive pulmonary disease, and congestive heart failure [14], in whom this virus may represent an important concern. In these individuals, RSV infection may manifest as nasal congestion and coughing, wheezing, ear pain, sinusitis, crackles, infiltrates on chest radiographs, pneumonia, and respiratory distress. As in children, longer-term complications may be observed, such as a permanent decline in the respiratory function [10]. Falsey et al. observed an annual incidence of RSV ranging from 4 to 10% in high-risk adults, with a higher utilization of medical care; in contrast, the annual frequency in the elderly population was 3–7% [15]. A study in a Thai hospital cohort of adults aged 15 years and above reported 69 cases of RSV infection [16]. These patients were mostly above the age of 50 (87%), and all had at least one comorbidity. The most common clinical presentation was community-acquired pneumonia (82.6%), followed by asthma or chronic obstructive pulmonary disease exacerbation, and acute bronchitis. An analysis of the costs of RSV-associated hospitalization in the elderly estimated an average between USD 8241 and USD 16,034 [17,18]. In adults, the average cost was USD 11,124 [18].
In our study, RSV was identified in over 40% of the samples from non-COVID-19-SARS cases, with similar frequencies in the adult and elderly populations (45.1% vs. 54.9%). Dyspnea was observed in around 38%, and respiratory distress in around 35%, highlighting the clinical impacts of the disease. Comorbidities in general were more frequent in the elderly, as expected. Immunosuppression and pulmonary or cardiac disease are also more common in the elderly, and could represent a risk factor for severe RSV infection. However, the analysis of the clinical manifestation and outcomes of RSV infection did not reveal differences in the occurrence of dyspnea, respiratory distress, or the need for ventilatory support between the adult and elderly groups. Even when comorbidities that could potentially raise the risk for severe disease were present, RSV infection manifested with the same potential severity in both adult and elderly patients, causing dyspnea, respiratory distress, and requiring ventilatory support.
Meanwhile, influenza accounted for 40.91% of cases in this study. Adults were more affected than the elderly, especially by IAV. It is important to note that Brazil’s national immunization program recommends flu vaccination for at-risk groups, including the elderly and adults with comorbidities, but not for healthy adults [19]. The mean age of the patients with IAV and IBV was also lower than that of the RSV patients, and the frequency of comorbidities was consistent with age. The clinical symptom data showed that influenza produced milder disease than RSV, with headaches, myalgia, nasal congestion, and sore throat seen most frequently; these symptoms are usually associated with the upper respiratory tract. In contrast, RSV was associated with a higher frequency of dyspnea and respiratory distress. Our data reinforce that RSV represents a higher potential severity than influenza, a finding corroborated by the more frequent need for ventilatory support.
Influenza was the most common infection when only adult patients were analyzed. The infection was also milder compared to RSV, with fever, headache, myalgia, and nasal congestion most frequently manifesting. In general, IAV and IBV infections may be mostly asymptomatic, with some upper respiratory tract symptoms, such as fever, chills, myalgia, malaise, a dry cough, a sore throat, and nasal discharge [20,21]. The US Centers for Disease Control and Prevention (CDC) estimates the rates of hospitalization and death in symptomatic infections at 1% and 0.1%, respectively [22]. Severe influenza may include respiratory complications such as bronchiolitis, bronchitis, pneumonia, bacterial coinfections, respiratory failure, and acute respiratory distress syndrome, or non-respiratory developments, including heart involvement, myositis, aseptic meningitis, encephalomyelitis, and Guillain–Barré syndrome [20,21]. A four-year prospective hospital cohort involving RSV and influenza revealed similar mortality rates in both diseases: 8% and 7%, respectively [15]. Although we found higher rates, we did not find a statistically significant difference between these viruses. Even though influenza may have the potential for severe disease, we found a higher frequency of this outcome in cases of RSV infection (p < 0.001 for respiratory distress, dyspnea, and ventilatory support). Even in adults, 6.25% of infected patients reported dyspnea, 5.56% required ventilatory support, and 2.78% needed intensive care.
Seasonal outbreaks of influenza and RSV tend to occur in both tropical and temperate countries in winter [23,24], but summertime cases, such as the ones seen in our cohort, do not represent an unexplained event, as even influenza and RSV are common in that season. Non-pharmaceutical interventions to control the spread of SARS-CoV-2, such as masking, also effectively limited the transmission of other respiratory agents, such as RSV and influenza. Cases of viral respiratory illnesses rebounded as control measures loosened, even out of season; this effect was also observed in other countries, including Australia, New Zealand, and South Africa [25]. Similarly, a flu outbreak occurred in Rio de Janeiro in November 2021, when lower mean temperatures were observed, alongside a discrepancy between flu vaccine strains and the circulating virus [23].
5. Conclusions
Our findings demonstrate the importance of differential diagnostics for respiratory infections, even during outbreaks and epidemics. Although the literature has adequately described the effects of respiratory agents in children and the elderly, in our cohort, we did not observe different clinical manifestations of RSV infections in adult and elderly patients, suggesting that this disease may have a significant clinical impact in adults in general, and not just in known risk groups. This observation underscores the need to define the agents that cause moderate and severe respiratory infections, especially as preventive and therapeutic options emerge. Previous studies have been supporting clinical trials in newborns and elderly (for example, clinicaltrials.gov: NCT04908683; NCT04908683; NCT05559476; NCT04732871), but they have not been focusing on adults, although these comprise a potential severity group that should be targeted through future vaccination programs. This is highlighted in this study through our demonstration of how dyspnea, respiratory distress, and the need for ventilatory support occurred at similar frequencies in adults and the elderly.
Acknowledgments
We acknowledge colleagues from Hospital de Base de São José do Rio Preto for support during sample and data collection.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v15091848/s1, Table S1: General characteristics of each group in the respiratory surveillance cohort, by infectious agent.; Table S2: Clinical characteristics of patients infected by influenza A and B and respiratory syncytial virus in the respiratory surveillance cohort, December 2021–April 2022.
Author Contributions
Conceptualization: C.F.E. and M.L.N.; Methodology: C.F.E., C.A.B., L.S., B.d.C.M. and M.L.N.; Investigation: C.F.E., A.T.V., C.A.B., F.A.G., B.F.d.S., L.S. and B.d.C.M.; Funding acquisition: C.F.E., N.V. and M.L.N.; Project administration: C.F.E. and M.L.N.; Supervision: N.V. and M.L.N.; Writing—original draft: C.F.E., A.T.V., F.A.G., B.F.d.S., N.V. and M.L.N.; Writing—review and editing: C.F.E., N.V. and M.L.N. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and conducted on retrospective samples, with approval by the Institutional Review Board of the School of Medicine of São José do Rio Preto (FAMERP) (protocol code process 31588920.0.0000.5415, approved on 14 May 2020). The FAMERP IRB granted approval for the study, and the consent terms were waived. Confidentiality was ensured through the de-identification of all samples before data entry and analysis.
Informed Consent Statement
The consent terms were waived by the IRB.
Data Availability Statement
De-identified data are available from the authors upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was supported by the São Paulo Research Foundation (FAPESP) via grant 2013/21719-3 for M.L.N., and 2022/09229-0 for C.F.E., and by INCT Dengue Program grant 465425/2014-3 (M.L.N.). M.L.N. is a Brazilian National Council for Scientific and Technological Development (CNPq) Research Fellow. M.L.N. and N.V. are partly funded by the Centers for Research in Emerging Infectious Diseases (CREID), “The Coordinating Research on Emerging Arboviral Threats Encompassing the Neotropics (CREATE-NEO)” grant U01AI151807 (to N.V.) from the National Institutes of Health (NIH/USA).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Collaborators G.R.T.C. Global, regional, and national burden of respiratory tract cancers and associated risk factors from 1990 to 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Respir. Med. 2021;9:1030–1049. doi: 10.1016/S2213-2600(21)00164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzalez Mosegui G.B., Antoñanzas Villar F., De Mello Vianna C.M. Burden of disease attributed to acute respiratory infections in South America. J. Infect. Dev. Ctries. 2022;16:1614–1622. doi: 10.3855/jidc.17009. [DOI] [PubMed] [Google Scholar]
- 3.Cilloniz C., Luna C.M., Hurtado J.C., Marcos M., Torres A. Respiratory viruses: Their importance and lessons learned from COVID-19. Eur. Respir. Rev. 2022;31:220051. doi: 10.1183/16000617.0051-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi T., McAllister D.A., O’Brien K.L., Simoes E.A.F., Madhi S.A., Gessner B.D., Polack F.P., Balsells E., Acacio S., Aguayo C., et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet. 2017;390:946–958. doi: 10.1016/S0140-6736(17)30938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nair H., Brooks W.A., Katz M., Roca A., Berkley J.A., Madhi S.A., Simmerman J.M., Gordon A., Sato M., Howie S., et al. Global burden of respiratory infections due to seasonal influenza in young children: A systematic review and meta-analysis. Lancet. 2011;378:1917–1930. doi: 10.1016/S0140-6736(11)61051-9. [DOI] [PubMed] [Google Scholar]
- 6.Simoes E.A. Overlap between respiratory syncytial virus infection and influenza. Lancet. 2001;358:1382–1383. doi: 10.1016/S0140-6736(01)06488-1. [DOI] [PubMed] [Google Scholar]
- 7.Pavia A.T. Viral infections of the lower respiratory tract: Old viruses, new viruses, and the role of diagnosis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2011;52((Suppl. S4)):S284–S289. doi: 10.1093/cid/cir043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denny F.W. The clinical impact of human respiratory virus infections. Pt 2Am. J. Respir. Crit. Care Med. 1995;152:S4–S12. doi: 10.1164/ajrccm/152.4_Pt_2.S4. [DOI] [PubMed] [Google Scholar]
- 9.Brazilian Ministry of Health . Doença pelo Novo Coronavírus—COVID-19. Semana Epidemiológica 38; Ministério da Saúde; Brasília, Brazil: 2022. Boletim Epidemiológico Especial.113p [Google Scholar]
- 10.Jha A., Jarvis H., Fraser C., Openshaw P. Respiratory syncytial virus. In: Hui D.S., Rossi R.A., Johnston S.L., editors. SARS, MERS and Other Viral Lung Infection. European Respiratory Society; Scheffield, UK: 2016. [PubMed] [Google Scholar]
- 11.Domachowske J., Halczyn J., Bonville C.A. Preventing Pediatric Respiratory Syncytial Virus Infection. Pediatr. Ann. 2018;47:e371–e376. doi: 10.3928/19382359-20180816-01. [DOI] [PubMed] [Google Scholar]
- 12.WHO RSV Surveillance Case Definitions. [(accessed on 9 August 2023)]. Available online: https://www.who.int/teams/global-influenza-programme/global-respiratory-syncytial-virus-surveillance/case-definitions.
- 13.Bowser D.M., Rowlands K.R., Hariharan D., Gervasio R.M., Buckley L., Halasa-Rappel Y., Glaser E.L., Nelson C.B., Shepard D.S. Cost of Respiratory Syncytial Virus Infections in US Infants: Systematic Literature Review and Analysis. J. Infect. Dis. 2022;226((Suppl. S2)):S225–S235. doi: 10.1093/infdis/jiac172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CDC Respiratory Syncytial Virus Infection (RSV) [(accessed on 5 November 2022)]; Available online: https://www.cdc.gov/rsv/clinical/index.html.
- 15.Falsey A.R., Hennessey P.A., Formica M.A., Cox C., Walsh E.E. Respiratory syncytial virus infection in elderly and high-risk adults. New Engl. J. Med. 2005;352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 16.Chuaychoo B., Ngamwongwan S., Kaewnaphan B., Athipanyasilp N., Horthongkham N., Kantakamalakul W., Muangman N. Clinical manifestations and outcomes of respiratory syncytial virus infection in adult hospitalized patients. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2019;117:103–108. doi: 10.1016/j.jcv.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ackerson B., An J., Sy L.S., Solano Z., Slezak J., Tseng H.F. Cost of Hospitalization Associated With Respiratory Syncytial Virus Infection Versus Influenza Infection in Hospitalized Older Adults. J. Infect. Dis. 2020;222:962–966. doi: 10.1093/infdis/jiaa183. [DOI] [PubMed] [Google Scholar]
- 18.Choi Y., Hill-Ricciuti A., Branche A.R., Sieling W.D., Saiman L., Walsh E.E., Phillips M., Falsey A.R., Finelli L. Cost determinants among adults hospitalized with respiratory syncytial virus in the United States, 2017–2019. Influenza Other Respir. Viruses. 2022;16:151–158. doi: 10.1111/irv.12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brazilian Ministry of Health . 23a Campanha Nacional de Vacinação Contra Influenza. Ministério da Saúde; Brasília, Brazil: 2021. Informe Técnico.29p [Google Scholar]
- 20.Uyeki T.M., Hui D.S., Zambon M., Wentworth D.E., Monto A.S. Influenza. Lancet. 2022;400:693–706. doi: 10.1016/S0140-6736(22)00982-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Javanian M., Barary M., Ghebrehewet S., Koppolu V., Vasigala V., Ebrahimpour S. A brief review of influenza virus infection. J. Med. Virol. 2021;93:4638–4646. doi: 10.1002/jmv.26990. [DOI] [PubMed] [Google Scholar]
- 22.CDC Disease Burden of Flu. [(accessed on 25 February 2023)]; Available online: https://www.cdc.gov/flu/about/burden/index.html.
- 23.Nott R., Fuller T.L., Brasil P., Nielsen-Saines K. Out-of-Season Influenza during a COVID-19 Void in the State of Rio de Janeiro, Brazil: Temperature Matters. Vaccines. 2022;10:821. doi: 10.3390/vaccines10050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alonso W.J., Viboud C., Simonsen L., Hirano E.W., Daufenbach L.Z., Miller M.A. Seasonality of influenza in Brazil: A traveling wave from the Amazon to the subtropics. Am. J. Epidemiol. 2007;165:1434–1442. doi: 10.1093/aje/kwm012. [DOI] [PubMed] [Google Scholar]
- 25.Eden J.S., Sikazwe C., Xie R., Deng Y.M., Sullivan S.G., Michie A., Levy A., Cutmore E., Blyth C.C., Britton P.N., et al. Off-season RSV epidemics in Australia after easing of COVID-19 restrictions. Nat. Commun. 2022;13:2884. doi: 10.1038/s41467-022-30485-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified data are available from the authors upon request.