Abstract
Plasmid DNA incubated in interphase Xenopus egg extracts is normally assembled into chromatin and then into synthetic nuclei which undergo one round of regulated replication. During a study of restriction endonuclease cut plasmid replication intermediates (RIs) by the Brewer–Fangman 2D gel electrophoresis technique, we have observed the formation of a strong spike of X-shaped DNA molecules in extracts that otherwise yield very little or no RIs. Formation of these joint molecules is also efficiently induced in replication-competent extracts upon inhibition of replication fork progression by aphidicolin. Although their electrophoretic properties are quite similar to those of Holliday junctions, 2D gels of doubly cut plasmids show that these junctions can link two plasmid molecules at any pair of DNA sequences, with no regard for sequence homology at the branch points. Neutral–neutral–alkaline 3D gels show that the junctions only contain single strands of parental size and no recombinant strands. A hemicatenane, in which one strand of a duplex is wound around one strand of another duplex, is the most likely structure to account for these observations. The mechanism of formation of these novel joint DNA molecules and their biological implications are discussed.
INTRODUCTION
Xenopus egg extracts are a powerful system to analyse eukaryotic DNA replication. Any plasmid DNA incubated in these egg extracts is assembled into chromatin and then into synthetic nuclei (1,2). The minichromosomes in the nuclei undergo one round of semiconservative DNA replication under cell cycle control. This experimental system closely mimics replication of early embryonic nuclei. We have previously studied plasmid replication in this system (3–5) using a two-dimensional (2D) gel electrophoresis technique which allows the separation of linear from branched DNA restriction fragments (Fig. 1). This technique was originally devised by Bell and Byers (6) to identify X-shaped branched DNA molecules joined by a single or multiple Holliday junctions in DNA from yeast cells in pachytene of meiosis I (7). The Bell and Byers technique was further developed by Brewer and Fangman (8) to identify branched replication intermediates (RIs) of different shapes. Using this technique, it has been demonstrated that replication initiates at a single, randomly located position on small plasmids (<15 kb) incubated in Xenopus egg extracts (3–5,9). Here, we report the formation of X-shaped DNA molecules in extracts unable to support DNA replication.
Figure 1.
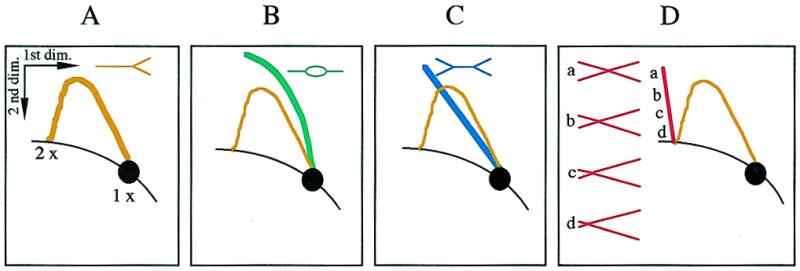
Schematic diagrams of 2D gel patterns obtained by analysing a restriction fragment. The 1× spot at the lower right corner of each panel depicts the position at which non-replicating monomer length molecules migrate. The diagonal curve intersecting the 1× spot depicts the line formed by all linear molecules in the population. The shape of three classes of RIs, simple Ys (A), bubbles (B) and double Ys (C), and the arc they form on 2D gels are shown on separate panels. The vertical spike and the drawings in (D) depict the migration behaviour of X-shaped DNA molecules according to the position (a–d) of the branch point.
Spikes of X-shaped DNA molecules have been found by 2D gel analyses of single copy loci in DNA from meiotic, but not mitotic, Saccharomyces cerevisiae cells (10–13). Based on their single strand composition and sensitivity to Holliday junction resolving endonucleases, these joint molecules (JMs) appear to consist of double Holliday junctions (12). They form frequently between homologues (IHJM) but rarely between sister chromatids (ISJM) and do not form between homologous chromosomes (10,11). Formation of IHJM and ISJM depends on the meiosis-specific RecA homologue Dmc1 and the mitotic RecA homologues Rad51, Rad55 and Rad57. IHJM additionally depend on the meiosis-specific protein Red1 (13). More recently, spikes of X-shaped molecules (designated xDNA) have been observed by 2D gel analysis of the rDNA locus in mitotically growing yeast cells (14). xDNA appears to consist of Holliday junctions. In wild-type cells, xDNA is only detected at S phase. The level of xDNA is elevated in mutants defective in DNA polymerases α and δ but not ɛ. The stimulation of xDNA in mutants defective in polymerase δ is dependent on RAD52 but not RAD51, RAD55 or RAD57. The authors suggested that xDNA occurs when recombination between sister chromatids is stimulated to repair replication-related lesions during S phase. Recent genetic data in Escherichia coli suggest that the recombination protein complex RuvAB acts at arrested replication forks to promote the formation of a Holliday junction by reannealing of the two nascent strands. Subsequent processing by the RecBCD complex may result in replication fork rescue without actual DNA breakage (15). A similar mechanism may explain the observation of xDNA in the rDNA of mitotically growing yeast cells (for further discussions see 16,17).
We report here that spikes of X-shaped DNA molecules are detected by 2D gel electrophoretic analysis of plasmid DNA in Xenopus egg extracts that are unable to support efficient DNA replication, as well as in replication-competent extracts treated with the DNA polymerase inhibitor aphidicolin. In contrast to the previous observations in yeast, these X-shaped DNA molecules are not Holliday junctions. They can form between any part of two plasmid molecules regardless of the homology of DNA sequences at the branch point and they are only composed of single strands of parental size. We suggest that these X-shaped molecules are hemicatenanes. We discuss their possible mechanism of formation and biological role.
MATERIALS AND METHODS
Replication of plasmid DNA in Xenopus egg extract
pBR322 DNA was prepared from the Rec+ BL21 strain using a Qiagen kit or by standard alkaline lysis and caesium chloride gradient centrifugation. Extracts from unfertilised Xenopus eggs (1) were prepared as described (3,18). Where indicated, 20 mg/ml aphidicolin dissolved in DMSO (both from Sigma) or an equivalent amount of DMSO alone (control) were added to the extract to the indicated concentration at the same time as DNA. After incubation in the extract, plasmid DNA was isolated by digestion with RNase A and proteinase K, phenol/chloroform extraction and ethanol precipitation and digested with restriction enzymes as described (3).
Two-dimensional agarose gel electrophoresis
The first electrophoresis was in a 0.4% (w/v) agarose gel in TBE buffer (89 mM Tris–borate, 2 mM EDTA) at 1 V/cm at room temperature for 18 h for the EcoRI-digested pBR322 DNA or 16 h for the AlwNI–BamHI-digested pBR322 DNA. The gel was stained with 0.3 µg/ml ethidium bromide. The second dimension was in 1.1% agarose for the EcoRI-digested pBR322 DNA or 1.3% agarose for the AlwNI–BamHI-digested pBR322 DNA. The second electrophoresis was in TBE buffer with 0.3 µg/ml ethidium bromide at 5 V/cm for 7 h in a 4°C cold room, with buffer recirculation.
Three-dimensional agarose gel electrophoresis
The first and second (neutral–neutral) electrophoreses were as described above. The 2D gel was soaked in 0.04 N NaOH, 1 mM EDTA five times for 1 h each at room temperature. The third (alkaline) electrophoresis was performed in the same direction as the first one, in 0.04 N NaOH, 1 mM EDTA buffer at 1 V/cm for 18 h at room temperature, with buffer recirculation.
Southern transfer and hybridisation
After electrophoresis, the gels were blotted in 0.4 N NaOH onto Hybond-N+ (Amersham). The membranes were probed with either the full-length EcoRI-digested pBR322 (Fig. 2) or with gel-purified A (2.5 kb) or B (1.8 kb) AlwNI–BamHI fragments of pBR322 (Figs 3 and 5–7). Probes were labelled with [α-32P]dATP using a Megaprime DNA labelling kit (Amersham). The membranes were exposed to Fuji BAS MP 2040S imaging plates and the images were read on a Fuji BAS 1000 phosphoimager using the TINA software. Quantifications were made with TINA or MAC BAS v.2.2 software. For image display, each file was transferred to Photoshop 3 and the upper and lower cut-off values were adjusted to the range of pixel intensity in the region of interest (excluding the intense signals from the 1× spots). The relationship between signal and image intensity was linear over this range.
Figure 2.
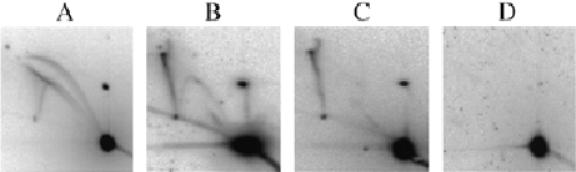
Four types of Xenopus egg extracts. pBR322 DNA was incubated in egg extracts for 90 min. DNA was purified, cut with EcoRI and analysed by 2D gel electrophoresis. After Southern blotting, the membranes were hybridised with radioactively labelled pBR322 DNA.
Figure 3.
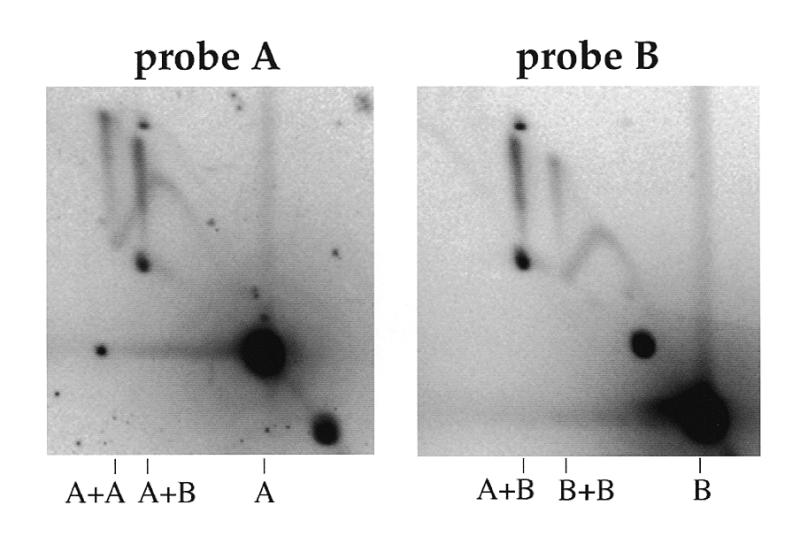
Lack of sequence homology at the branch point of the junctions. pBR322 was incubated in Xenopus egg extracts. DNA was purified, cut with AlwNI and BamHI and analysed by 2D gel electrophoresis. After Southern blotting, the membrane was sequentially hybridised with probes A and B. Probes A and B are the 2.5 and 1.8 kb AlwNI–BamHI fragments of pBR322 DNA, respectively.
Figure 5.
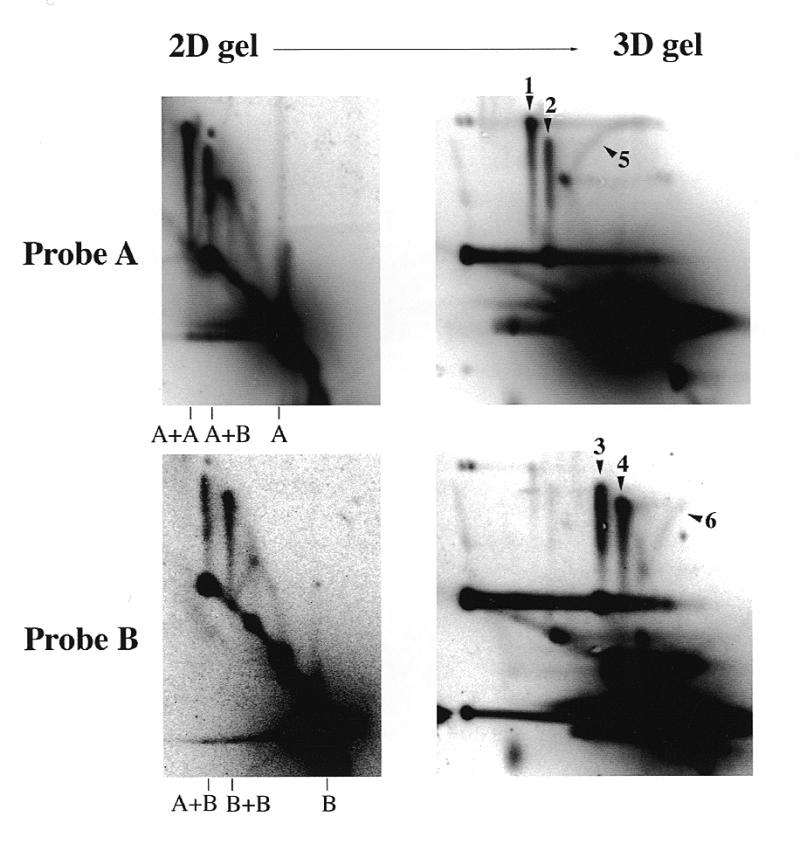
2D (neutral–neutral) and 3D (neutral–neutral–alkaline) gel analysis of the junction component single DNA strands. pBR322 was incubated in Xenopus egg extracts for 90 min. DNA was purified and cut with AlwNI and BamHI and loaded on two duplicate 2D gels. One of the 2D gels was subjected to a third electrophoresis in alkali in the same direction as the first electrophoresis. After Southern blotting, the membranes were sequentially hybridised with the radioactively labelled A and B probes. Signals labelled 1–6 are described in the text.
Figure 7.
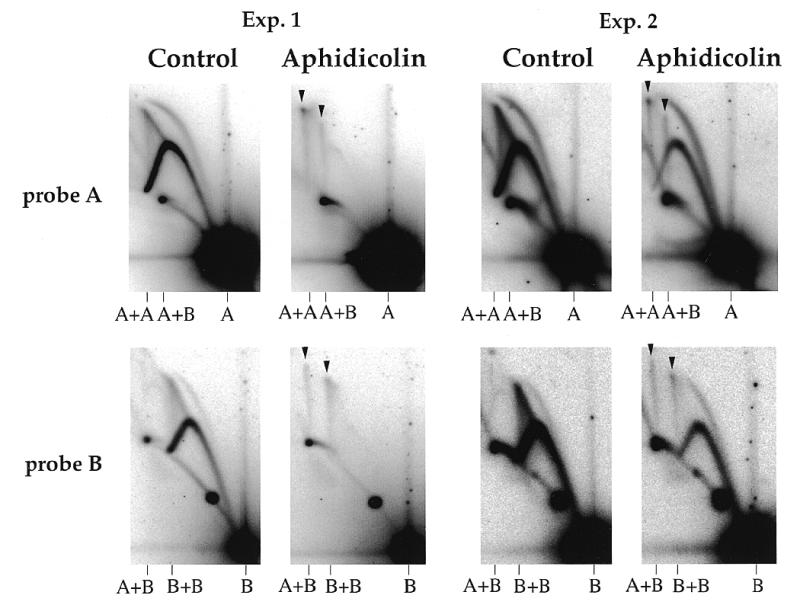
Inhibition of replication elongation induces formation of the junctions. pBR322 DNA was incubated in type A egg extracts (two independent experiments) for 90 min in the presence of DMSO alone (control) or of 45 µg/ml aphidicolin in DMSO. DNA was purified, cut with AlwNI and BamHI and analysed by 2D gel electrophoresis. After Southern blotting, the membranes were sequentially hybridised with the radioactively labelled A and B probes. Spikes of A+A, A+B and B+B junctions are indicated by arrowheads.
RESULTS
Novel joint DNA molecules form in egg extracts partly deficient for replication
pBR322 DNA was incubated for 90 min in different batches of Xenopus egg extracts. The DNA was purified, cut with EcoRI, run on 2D gels and probed with a full-length pBR322 probe. Four different types of extract were distinguished based on the resulting 2D gel profile (Fig. 2). Type A extracts showed the standard pattern expected of a replication-competent extract: in addition to the spot of linear molecules, complete bubble, simple Y and double Y arcs were clearly detected (Fig. 2A). Some extracts (Fig. 2D) yielded no detectable RIs. In some other extracts (Fig. 2B and C), RIs were detectable at only a low (Fig. 2B) or very low (Fig. 2C) level. In contrast to type D extracts, which appear completely incapable of any synthesis, type B and C extracts can support a low level of replication. Importantly, a strong spike extending upward from the arc of linear fragments at mass 2× was observed in type B and C extracts.
The variability in the amount of bubbles, simple Ys and double Ys was not surprising since it is generally held that the ability of an extract to replicate DNA is unpredictable and largely dependent on conditions of preparation of the extract (egg collection and activation and separation of egg contents). However, the strong spike observed in type B and C extracts was unexpected. This spike migrated like X-shaped DNA molecules observed in other systems (Fig. 1). The alternative hypothesis, that such molecules are very late double Y RIs, is excluded by the experiments described below and by their high abundance in replication-defective extracts. Because EcoRI introduced a single cut in each pBR322 molecule, the X-shaped molecules must consist of two full-length linear pBR322 molecules with no replication fork. These X-shaped molecules were not observed to form to a detectable level in extracts with a high replicative efficiency. With type B extracts, a low level of X-shaped molecules was sometimes detected after direct labelling of replication products by addition of [α-32P]dATP to the extract. However, when the same blot was subsequently hybridised with the plasmid probe, the ratio of X-shaped molecules to RIs was found to be higher than by direct labelling (data not shown). We conclude that in type B extracts, X-shaped molecules can form between unreplicated as well as replicated plasmids.
In order to characterise the replication defect of the extracts, we examined their ability to assemble nuclei using sperm chromatin as template and their ability to synthesise the complementary strand of single-stranded M13 templates, a reaction which does not require nuclear assembly. All extracts tested were capable of complementary strand synthesis, arguing against a primase or polymerase defect (data not shown). Nevertheless, we show below that polymerase inhibition by aphidicolin can trigger the formation of X-shaped DNA in type A extracts. One type D extract was capable of nuclear assembly and its replication defect has not been determined. However, nuclear assembly was totally blocked in most type D extracts and was slowed down, though not abolished, in type B/C extracts (data not shown). This result could explain the complete lack or low amount of RIs observed in type D and type B/C extracts, respectively. It is also consistent with the kinetic analysis shown in Figure 6 (see below), which demonstrates that in type B extracts the first RIs appear after a longer time than in type A extracts.
Figure 6.
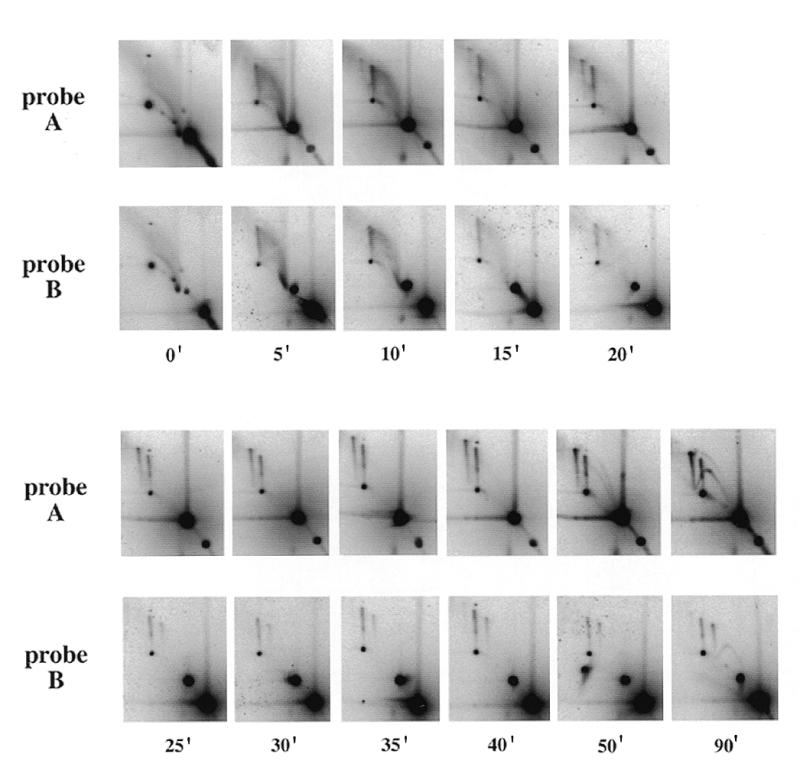
Time course analysis of junction formation in egg extracts. pBR322 was incubated in a type B extract and samples were removed at the indicated times. DNA was purified, cut with AlwNI and BamHI and separated by 2D gel electrophoresis. After Southern blotting, the membranes were sequentially hybridised with the radioactively labelled A and B probes.
The junctions are not homologous junctions
The electrophoretic properties of the X-shaped DNA molecules were reminiscent of those of Holliday junctions. In order to determine whether the junctions were formed between homologous regions, plasmid DNA purified from a type B extract was digested with BamHI and AlwNI, which generates two fragments of 2.5 kb (A) and 1.8 kb (B), and analysed on 2D gels (Fig. 3). If the junctions were homologous junctions, only A+A and B+B spikes should be observed. However, three spikes were observed, with apparent masses consistent with the three possible types of junctions: A+A (5 kb), A+B (4.3 kb) and B+B (3.6 kb). The relative intensities of the three spikes were quantified. The signal ratios were 1.8:2.6:1.0, respectively. The ratios predicted if the three types of junction were formed at random and in proportion to the respective size of the two fragments, taking into account the amount of hybridisable sequence, were 1.93:1.39:1.0. These results show that the junctions could form at any pair of DNA sequences, with no regard for potential homology at the branch point. Therefore they are not Holliday junctions. The higher than expected amount of the A+B junctions will be discussed later.
The junctions do not contain recombined single strands
The junctions must be DNA–DNA junctions since they were resistant to RNase and proteinase K treatment followed by phenol/chloroform extraction. Two possible structures could account for junction of two plasmids at any pair of DNA sequences (Fig. 4). The two plasmids could be linked by a hemicatenane, in which one strand of a duplex is wound around one strand of another duplex without base pairing at the branch point (Fig. 4A). This type of junction should only contain single strands of parental size. Alternatively, two plasmids with a single-strand break or gap could be joined by an aberrant repair event (Fig. 4B). In this case, the junctions should contain two single strands of parental size and two recombined strands of variable size (from a few bases to the sum of the two parental single strands).
Figure 4.
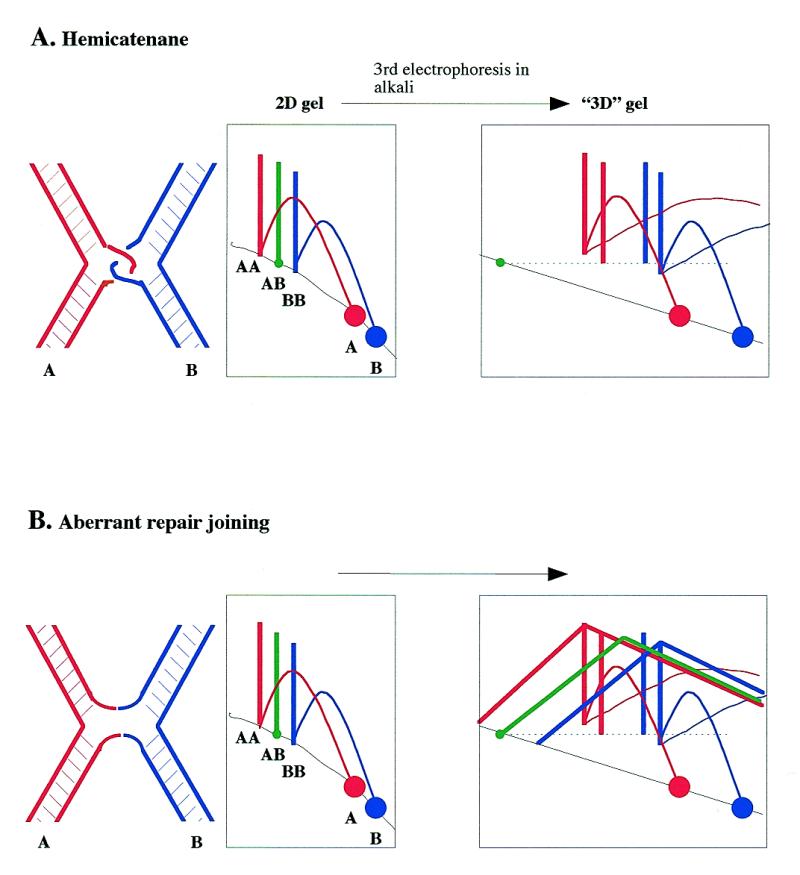
Two models of the structure of the junctions and predicted behaviour on 3D gels. (A) Hemicatenane; (B) aberrant repair joining. The predicted migration behaviour of component single strands of A+A, B+B and A+B junctions after a third electrophoresis in alkali is shown. Molecular species detected by probe A are shown in red, by probe B in blue and by both probes in green.
In order to analyse the single strand composition of the junctions, we devised a three-dimensional (3D) neutral–neutral–alkaline gel electrophoresis method (Fig. 4), which combines features of previously described 3D gel (19) and ‘stop-and-go-alkaline’ (20) techniques. Two duplicate 2D gels of BamHI–AlwNI-cut pBR322 DNA are prepared. One of the 2D gels is soaked in alkali to denature DNA and the resulting single strands are separated by alkaline electrophoresis in the same direction as the first dimension. Single strands run as novel spikes or arcs according to their masses and are detected by two successive probings with probes A and B. If the junction is a hemicatenane, only spikes corresponding to single strands of parental sizes should be observed. The A+A and B+B spikes will each give rise on the 3D gel to a single spike of single strands of size A and B, respectively. The A+B spike will give rise to two spikes of single strands, one of size A and one of size B. The A probe will therefore detect two spikes of A single strands, one coming from the A+A junction and the other from the A+B junction. Similarly, the B probe will detect two spikes of B single strands, one coming from the A+B junction and the other from the B+B junction. In the hypothesis of aberrant repair joining, additional arcs corresponding to recombined strands should be observed in amounts similar to the spikes. The size of the two recombined strands should vary according to the position of the branch point. Recombined strands should both have the size of the parental strands when the branch point is at the middle of the X (top of the spike). When the branch point is asymmetrically located on the X (bottom of the spike), one recombined strand will be smaller and the other will be larger. The resulting shape expected for these arcs is shown in Figure 4.
When the experiment was performed (Fig. 5) the 3D gels clearly showed the four predicted spikes corresponding to single strands of parental size A (arrowheads 1 and 2) and B (arrowheads 3 and 4). However, no arcs corresponding to single strands of recombinant size were detected. Thus, there was no evidence for aberrant strand recombination at the branch point. We detected two minor arcs (arrowheads 5 and 6) starting from the bottom of the A+A and B+B spikes and rising upwards and rightwards to the level of the top of each spike. No similar arc was associated with the A+B spike. These minor arcs did not have the shape predicted for recombined strands. The size of the strand was close to full-length A or B single strands for molecules at the bottom of the spike but was about half the size near the top of the spike. Therefore these arcs had the shape and intensity predicted if a minor fraction of parental single strands in spikes A+A and B+B had a nick or a gap at a position close to the branch point. We conclude that most of the material in the A+A, A+B and B+B spikes are hemicatenanes, whereas a minor fraction of the molecules in the A+A and B+B spikes carry a nick near the branch point and may have a different structure.
Kinetic analysis of junction formation
A replication time course of junction formation in a type B egg extract is shown in Figure 6. No junction was detectable in the input DNA (time 0 min). A smear spreading upwards and leftwards from the 1× spot in the input DNA disappeared after 15–20 min incubation in the extract. This smear was no longer detectable when plasmid DNA was purified by CsCl gradient centrifugation (data not shown) and may represent degraded plasmid molecules rapidly processed by the extract. After only 5 min incubation, an A+B spike was observed. However, the A+A and B+B spikes were first detected only after 15–20 min. A stable level of the three spikes A+A, B+B and A+B was reached after 25 min and persisted at least until 90 min. In other experiments, the spikes were still detected after 240 min (data not shown). The first RIs were only detected after 50 min in this extract and their amount was still low after 90 min. In efficient replication extracts, RIs are first detected after 25–30 min and reach a high level after only 50 min (not shown, but see fig. 1 in 5). This extract was able to replicate DNA, but with reduced efficiency and slower kinetics.
The fact that only A+B junctions could be detected between 5 and 15 min was surprising. One possible explanation is that the DNA concentration is initially low in the extract, only allowing the formation of intramolecular junctions, and that during subsequent condensation associated with nuclear formation the resulting increase in local DNA concentration allows the formation of intermolecular junctions. The relative intensities of the three spikes were quantified for the 25–90 min time points. The signal ratios were 1.5:2.0:1.0 (25 min), 1.4:1.8:1.0 (30 min), 1.8:3.2:1.0 (35 min), 1.8:2.6:1.0 (40 min), 2.0:1.7:1.0 (50 min) and 1.9:1.2:1.0 (90 min), while the ratios predicted by a random intermolecular mechanism were 1.93:1.39:1.0. In summary, the ratio of A+A to B+B spikes was not very different from predicted, while the initial excess of the A+B spike progressively diminished and returned to the predicted value after 90 min. These results could be explained if the A+B spike initially contained mostly intramolecular A+B junctions and if intermolecular junctions became predominant with time.
Inhibition of replication forks stimulates the formation of the junctions
So far the X-shaped molecules were only observed to form in extracts with an impaired replicative efficiency (type B/C). We then used aphidicolin, an inhibitor of DNA polymerases, to directly examine the role of replication inhibition on the formation of junctions in extracts with high replicative efficiencies. pBR322 DNA was incubated for 90 min in type A egg extracts with or without 45 µg/ml aphidicolin, purified, cut with BamHI–AlwNI and run on 2D gels (Fig. 7). In the control samples, the arcs of RIs were intense and complete, as expected for a replication-competent extract, and no spikes were detectable. In the presence of aphidicolin, the RIs disappeared (experiment 1) or decreased (experiment 2), which may reflect a variable extract sensitivity to aphidicolin. The lack of detectable arcs of RIs in experiment 1 indicated that forks were arrested by aphidicolin very close to the initiation site. In experiment 2, bubbles and early Ys were easily detected but late Ys and double Ys were much less abundant in the presence of aphidicolin, indicating that forks also stalled, but only after having progressed on average over longer distances (up to ~2 kb) than in experiment 1. In both cases, aphidicolin induced the appearance of the three spikes of A+A, B+B and A+B junctions, in parallel with the inhibition of replication. The proportions of the three spikes were 1.3:0.6:1.0 (experiment 1) and 1.2:0.8:1.0 (experiment 2), consistent with the formation of predominantly intermolecular junctions within replicating nuclei. These results demonstrate that inhibition of replication elongation, either partial or complete, stimulates formation of the junctions.
DISCUSSION
A novel type of DNA–DNA junction in Xenopus egg extracts
We describe here the formation of a novel type of DNA–DNA junction between pBR322 plasmid molecules incubated in interphase Xenopus egg extracts. These junctions are not Holliday junctions. They can link any part of two plasmids, without change in the size of single strands and with no regard for sequence homology at the branch point. They are most likely hemicatenane junctions. Some hemicatenanes may also form within a single plasmid molecule, at least at early times in the extract. The formation of these junctions occurs when the extract is unable to support efficient replication or when replication is artificially blocked with aphidicolin.
Homologous and illegitimate recombination have been extensively studied in Xenopus oocytes and eggs (21,22). Two types of recombination have been observed in Xenopus eggs and oocytes: non-conservative single strand annealing homologous recombination and end-joining recombination. Neither of these two processes predicts the formation of the novel X-shaped molecules we observe.
A hemicatenane is the most probable structure of the joint molecules
Using a 3D gel electrophoresis technique, we show that the joint molecules only contain single strands of parental size. A simple structure to account for the single strand composition, the stability and the lack of sequence homology at the branch point is a hemicatenane, in which one strand of a duplex is wound around one strand of another duplex. We cannot determine from the data shown here if the two linked single strands are wound once or several times around each other. The 3D gels also reveal that a minority of A+A and B+B junctions carry a single strand nick near the branch point. It is possible that some A+A and B+B hemicatenanes occasionally isomerise into true double Holliday junctions. The observed broken parental strands may be transient intermediates in the resolution of these hypothetical double Holliday junctions.
Possible hemicatenane structures have been previously observed in two different circumstances. In an electron microscopy analysis of psoralen crosslinked replicating SV40 minichromosomes, dimers of fully replicated SV40 molecules connected by a single-stranded bridge located within 500 bp of the termination region have been observed (23). It could not be determined whether these dimers represent hemicatenanes or two circles joined by a Holliday junction, although the former hypothesis was favoured (24). The authors suggested that these dimers are very late RIs engaged in strand segregation (23,24). In a second study, it was reported that hemicatenation of plasmid DNA can be catalysed in vitro by the Rec1 protein and a topoisomerase from the fungus Ustilago maydis (25). This reaction appears to be promoted by Z-DNA sequences. The structures reported in these two studies were found to exclusively form between homologous regions. Here, we find that hemicatenanes can form between unreplicated as well as replicated plasmid molecules and without sequence homology at the branch point. Therefore the hemicatenane structures reported in these two studies do not seem to be related to our observations.
Possible mechanism for hemicatenation
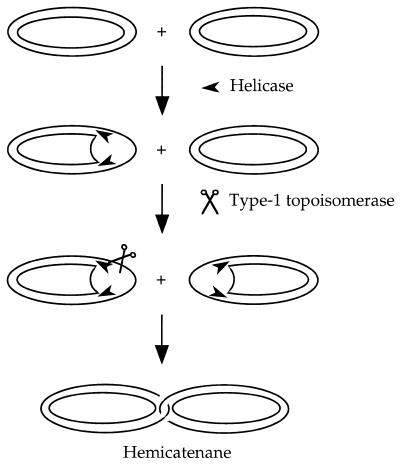
We propose that the association of a helicase and a type 1 topoisomerase could be implicated in hemicatenane formation. Helicases and topoisomerases play multiple roles in replication, recombination, repair and segregation of chromosomes. Type 1 topoisomerases are able to catenate or decatenate duplex DNA circles if a single strand discontinuity is present on one of the duplexes (26,27). In the yeast S.cerevisiae, mutations in the SGS1 gene, which encodes a RecQ-like helicase, suppress the slow growth phenotype associated with top3 mutations and lead to a hyper-recombination phenotype on their own. It has been proposed that Sgs1 helicase activity would provide a single-stranded DNA region necessary for the action of topoisomerase III (a type 1 topoisomerase) (25,28). In human, the BLM and WRN genes implicated in Bloom’s and Werner’s syndromes also code for a RecQ-like helicase and their deficiency results in replication defects and pronounced genetic instability (29,30). rqh1+, a Schizosaccharomyces pombe homologue of these genes, is implicated in reversible S phase arrest in response to drugs that inhibit DNA synthesis (31). Recently, it has been shown that the E.coli or S.cerevisiae topoisomerase III, in association with the RecQ helicase from E.coli, is capable of catenating fully duplex plasmid DNA in vitro (32). The authors proposed a model for catenation in which RecQ unwinds a covalently closed DNA molecule to yield single-stranded DNA, a preferential DNA binding site for topoisomerase III. Two concerted strand passage reactions then produce the equivalent of a double-stranded DNA passage. Here, we suggest that the combined action of a helicase and a type 1 topoisomerase could also catalyse the formation of hemicatenanes if two plasmids are each unwound by a helicase and then linked by a single strand passage event (Fig. 8).
Figure 8.
Model for hemicatenation promoted by the combined action of a helicase and a type 1 topoisomerase. This model is adapted from studies on full catenation of duplex DNAs by RecQ and topo III (32). Helicases (possibly RecQ-like) unwind two plasmid DNAs to allow the action of a type 1 topoisomerase (possibly topoisomerase III) on single-stranded DNA. One single strand DNA passage event forms a hemicatenane.
Possible biological roles for the formation of hemicatenes
Hemicatenanes form very quickly and persist thereafter in extracts that support low or very low replication (type B/C). Hemicatenanes also form when plasmid replication is blocked with aphidicolin in replication-competent extracts (type A). These results argue that the formation of hemicatenanes is not a particular feature of deficient extracts but is triggered in response to some defect in DNA replication. In the replication-competent extracts, the signal for hemicatenation could be activated after initiation of replication by the presence of stalled replication forks. The signal triggering hemicatenation in replication-deficient extracts remains to be determined. As these extracts do not appear deficient in elongation of single-stranded templates, this could indicate a different mechanism leading to hemicatenanes.
DNA replication and recombination are linked by multiple pathways. It has been shown that stalled replication forks are unstable and stimulate recombination events in their vicinity, either in E.coli (15,33) or in yeast (34). In E.coli, recombination proteins act at arrested replication forks to promote the formation of a Holliday junction (15). Hemicatenation could be one of a large number of events triggered by checkpoint pathways in response to malfunction of DNA synthesis and could be involved in recombinational repair of stalled replication forks. One speculative role of hemicatenanes could be to hold broken DNA fragments together, which could facilitate subsequent repair. Another speculation is that hemicatenation could facilitate homology searching. The search for homologous sequences between two DNA segments in a 3D space would be time consuming, while if a hemicatenane forms and allows linked DNA segments to slide relative to each other, the search could proceed in a one-dimensional space, which should be much faster. Once sufficient base pairing potential has been found, a hemicatenane could simply isomerize into a pair of Holliday junctions. This idea is consistent with the evidence for introduction of nicks near some of the A+A and B+B junctions, but not the A+B junction.
Hemicatenation in other organisms
The formation of hemicatenanes is probably not peculiar to Xenopus. Spikes of X-shaped DNA molecules have often been detected by 2D gel electrophoresis in other organisms and some of these may be more easily accounted for by hemicatenanes rather than by Holliday junctions because they extend from the arc of linear fragments at a mass different from 2× that of the linear fragment probed. One example can be found in a study of the replication of the extrachromosomal circular rDNA plasmids of the protozoan parasite Entamoeba histolytica (35). Another example is found in a study of the effect of topoisomerase mutations on the replication of the S.cerevisiae 2 µm plasmid (36). 2D gel analysis of doubly cut plasmids replicated in the absence of topoisomerase II and probed for a single fragment show two spikes of so-called ‘aberrant’ X-shaped molecules migrating as described here for hemicatenanes. Finally, we have preliminary evidence for hemicatenation in E.coli (data not shown).
The observations reported here and the additional examples of possible hemicatenanes were all obtained using circular DNA. Because hemicatenation seems to form between any DNA fragments without regard for sequence homology at the branch point, they would be more difficult to detect in a whole genome. The discrete spikes reported here could be detected due to the small number of restriction fragments generated from circular DNA. This may explain why hemicatenation has not been detected so far in complex genomes.
Acknowledgments
ACKNOWLEDGEMENTS
We thank G. Pierron for his comments on the manuscript. This work was supported by the ATIPE-CNRS, the Ligue Nationale Française Contre le Cancer and the Association pour la Recherche sur le Cancer. I.L. was supported by fellowships from the MENESR and the Association pour la Recherche sur le Cancer.
REFERENCES
- 1.Blow J.J. and Laskey,R.A. (1986) Cell, 47, 577–587. [DOI] [PubMed] [Google Scholar]
- 2.Blow J.J. and Sleeman,A.M. (1990) J. Cell Sci., 95, 383–391. [DOI] [PubMed] [Google Scholar]
- 3.Hyrien O. and Méchali,M. (1992) Nucleic Acids Res., 20, 1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hyrien O. and Méchali,M. (1993) EMBO J., 12, 4511–4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lucas I., Chevrier-Miller,M., Sogo,J.M. and Hyrien,O. (2000) J. Mol. Biol., 296, 769–786. [DOI] [PubMed] [Google Scholar]
- 6.Bell L. and Byers,B. (1983) Anal. Biochem., 130, 527–535. [DOI] [PubMed] [Google Scholar]
- 7.Bell L.R. and Byers,B. (1983) Cold Spring Harbor Symp. Quant. Biol., 47, 829–840. [DOI] [PubMed] [Google Scholar]
- 8.Brewer B.J. and Fangman,W.L. (1987) Cell, 51, 463–471. [DOI] [PubMed] [Google Scholar]
- 9.Mahbubani H.M., Paull,T., Elder,J.K. and Blow,J.J. (1992) Nucleic Acids Res., 20, 1457–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins I. and Newlon,C.S. (1994) Cell, 76, 65–75. [DOI] [PubMed] [Google Scholar]
- 11.Schwacha A. and Kleckner,N. (1994) Cell, 76, 51–63. [DOI] [PubMed] [Google Scholar]
- 12.Schwacha A. and Kleckner,N. (1995) Cell, 83, 783–791. [DOI] [PubMed] [Google Scholar]
- 13.Schwacha A. and Kleckner,N. (1997) Cell, 90, 1123–1135. [DOI] [PubMed] [Google Scholar]
- 14.Zou H. and Rothstein,R. (1997) Cell, 90, 87–96. [DOI] [PubMed] [Google Scholar]
- 15.Seigneur M., Bidnenko,V., Ehrlich,S.D. and Michel,B. (1998) Cell, 95, 419–430. [DOI] [PubMed] [Google Scholar]
- 16.Rothstein R. and Gangloff,S. (1999) Nature Genet., 22, 4–6. [DOI] [PubMed] [Google Scholar]
- 17.Hyrien O. (2000) Biochimie, 82, 5–17. [DOI] [PubMed] [Google Scholar]
- 18.Martin-Parras L., Lucas,I., Martinez-Robles,M.L., Hernandez,P., Krimer,D.B., Hyrien,O. and Schvartzman,J.B. (1998) Nucleic Acids Res., 26, 3424–3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang C. and Gerbi,S.A. (1994) Mol. Cell. Biol., 14, 1520–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalejta R.F., Lin,H.B., Dijkwel,P.A. and Hamlin,J.L. (1996) Mol. Cell. Biol., 16, 4923–4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehman C.W., Clemens,M., Worthylake,D.K., Trautman,J.K. and Carroll,D. (1993) Mol. Cell. Biol., 13, 6897–6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carroll D. and Lehman,C.W. (1991) Methods Cell Biol., 36, 467–486. [DOI] [PubMed] [Google Scholar]
- 23.Sogo J.M., Stahl,H., Koller,T. and Knippers,R. (1986) J. Mol. Biol., 189, 189–204. [DOI] [PubMed] [Google Scholar]
- 24.Laurie B., Katritch,V., Sogo,J., Koller,T., Dubochet,J. and Stasiak,A. (1998) Biophys. J., 74, 2815–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kmiec E.B. and Holloman,W.K. (1986) Cell, 44, 545–554. [DOI] [PubMed] [Google Scholar]
- 26.Brown P.O. and Cozzarelli,N.R. (1981) Proc. Natl Acad. Sci. USA, 78, 843–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DiGate R.J. and Marians,K.J. (1988) J. Biol. Chem., 263, 13366–13373. [PubMed] [Google Scholar]
- 28.Gangloff S., McDonald,J.P., Bendixen,C., Arthur,L. and Rothstein,R. (1994) Mol. Cell. Biol., 14, 8391–8398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellis N.A., Groden,J., Ye,T.Z., Straughen,J., Lennon,D.J., Ciocci,S., Proytcheva,M. and German,J. (1995) Cell, 83, 655–666. [DOI] [PubMed] [Google Scholar]
- 30.Yu C.E., Oshima,J., Fu,Y.H., Wijsman,E.M., Hisama,F., Alisch,R., Matthews,S., Nakura,J., Miki,T., Ouais,S., Martin,G.M., Mulligan,J. and Schellenberg,G.D. (1996) Science, 272, 258–262. [DOI] [PubMed] [Google Scholar]
- 31.Stewart E., Chapman,C.R., Al-Khodairy,F., Carr,A.M. and Enoch,T. (1997) EMBO J., 16, 2682–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harmon F.G., DiGate,R.J. and Kowalczykowski,S.C. (1999) Mol. Cell, 3, 611–620. [DOI] [PubMed] [Google Scholar]
- 33.Michel B., Ehrlich,S.D. and Uzest,M. (1997) EMBO J., 16, 430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobayashi T., Heck,D.J., Nomura,M. and Horiuchi,T. (1998) Genes Dev., 12, 3821–3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dhar S.K., Choudhury,N.R., Mittal,V., Bhattacharya,A. and Bhattacharya,S. (1996) Mol. Cell. Biol., 16, 2314–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levac P. and Moss,T. (1996) Chromosoma, 105, 250–260. [DOI] [PubMed] [Google Scholar]