Abstract
Objective:
Traumatic spinal cord injury (TSCI) is a life altering event most often causing permanent physical disability. Little is known about the risk of developing Alzheimer’s disease and related dementia (ADRD) among middle-aged and older adults living with TSCI. Time to diagnosis of and adjusted hazard for ADRD was assessed.
Design:
Cohort Study
Setting:
Using 2007–2017 claims data from the Optum Clinformatics Data Mart, we identified adults (45+) with diagnosis of TSCI (n=7,019). Adults without TSCI diagnosis were included as comparators (n=916,516). Using age, sex, race/ethnicity, cardiometabolic, psychologic, and musculoskeletal chronic conditions, U.S. Census Division, and socioeconomic variables, we propensity score matched persons with and without TSCI (n=6,083). Incidence estimates of ADRD was compared at 4-years of enrollment. Survival models were used to quantify unadjusted, fully adjusted, and propensity-matched unadjusted and adjusted hazard ratios for incident ADRD.
Participants:
Adults with and without TSCI.
Intervention:
Not applicable.
Main Outcomes Measures:
Diagnosis of ADRD
Results:
Both middle-aged and older adults with TSCI had higher incident ADRD compared to those without TSCI [(0.5% vs. 0.2% and 11.7% vs. 3.3% among 45–64 and 65+ years old unmatched cohorts, respectively) (0.5% vs. 0.3% and 10.6% vs. 6.2% among 45–64 and 65+ years old matched cohorts, respectively)]. Fully adjusted survival models indicated that adults with TSCI had a greater hazard for ADRD (among 45–64 years old: unmatched HR: 3.19 (95% CI: 2.30–4.44), matched HR: 1.93 (95% CI:1.06–3.51); among 65+ years old: unmatched HR: 1.90 (95% CI: 1.77, 2.04), matched HR: 1.77 (1.55, 2.02)).
Conclusions:
Adults with TSCI are at a heightened risk for ADRD. Improved clinical screening and early interventions aiming to preserve cognitive function are of paramount importance for this patient cohort.
Keywords: Alzheimer’s Disease, Dementia, Traumatic, Spinal Cord Injury, Optum Claims
Introduction
Traumatic spinal cord injury (TSCI) is associated with life-long debilitating consequences that negatively impact physical functioning and secondary morbidities.1,2 Some evidence suggests that TSCI may exacerbate certain biological mechanisms due to neuronal damage that elevate the risk for Alzheimer’s disease and related dementia (ADRD).3,4 It is important to identify risk factors for ADRD because early identification of ADRD risk factors may not only elucidate better understanding of pathophysiology of ADRD but also help to identify potential preventative mechanisms or targeted treatment.5,6
Following a TSCI, a higher level of tau protein may accumulate in the cerebrospinal fluid.7 Furthermore, TSCI causes chronic inflammation,8 which triggers the accumulation of amyloid precursor protein (APP) throughout the spinal cord. This may lead to increased production and subsequent transmission of beta amyloid to different regions of the brain not only causing further inflammation but also resulting in neuronal degeneration.9 Moreover, adults with TSCI have higher prevalence of cardiometabolic10 and psychological11 conditions; all of which are considered risk factors for ADRD. To date, no large-scale study in the U.S. has examined the association between TSCI and ADRD.
In this study, we examined a national administrative claims database to quantify time to diagnosis of and adjusted hazard ratios for ADRD after TSCI. Our study underscores an urgent need for understanding the risk factors for ADRD among people with TSCI. Our main hypothesis is that adults with TSCI have shorter ADRD-free survival compared with their matched cohort of adults without TSCI.
Methods
Data Source
We used national, private administrative claims from the Clinformatics DataMart Database (OptumInsight, Eden Prairie, MN). OptumInsight is a de-identified claims database capturing inpatient, outpatient, and emergency department use of over 80 million privately insured people throughout their enrollment. This study was deemed exempt by the University of Michigan Institutional Review Board.
Sample Selection
All adults aged 45 years or older at the time of enrollment starting from 2007 to 2017 were deemed potentially eligible for this analysis. We excluded individuals with less than 12 months of continuous enrollment such that there was a sufficient history of service utilization for comorbidity history. The schematic flow diagram is presented in Supplement Figure 1.
Identification of Patients with a TSCI
Our cut-off date among our cohort of people with TSCI was January 2014 to ensure at least 4 years of follow up. Thus, all enrolled members with a diagnosis of TSCI were identified using International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) (Supplementary Table S1). To allow sufficient longitudinal follow up for individuals with TSCI, we retained those with four or more continuous years of enrollment following their first diagnosis of TSCI.
We identified a comparison cohort of controls without TSCI using the same aforementioned inclusion criteria. The additional exclusion criteria for identifying the control cohort included removal of any individual with other physically disabling neurological disorders. Among remaining individuals without TSCI, a 20% simple random sample of members was selected to represent the control group. Post-hoc analyses of demographic characteristics were compared between the 20% random sample of controls and all controls to ensure no unintentional bias in the sampled control cohort attributable to random selection.
Outcome
The primary outcome was time in days to first diagnosis of ADRD following index TSCI. Due to inappropriate coding practice in administrative data, underdiagnosis for the disease, and misdiagnosis of the specific type of dementia, we combined ADRD into one umbrella term.12–14 ADRD was identified in the follow-up period using ICD-9-CM or ICD-10-CM diagnosis codes on any single claim (Supplemental Table 2). If multiple claims were identified, the first claims after TSCI was considered the incident date of ADRD.
Covariates and Comorbidities
Independent variables included age (split into two categories: 45–64, 65 or older), sex, race/ethnicity, Elixhauser comorbidity index, U.S. Census Divisions, educational attainment, and net worth. We also identified psychological, cardiometabolic, and musculoskeletal diseases that were prevalent in the one-year lookback period before index TSCI diagnosis to develop a matched cohort. The aforementioned conditions were identified based on a single healthcare encounter of at least one of the pertinent ICD-9 codes (Supplement Table 3). For psychological morbidity, we included: (1) insomnia, (2) adjustment disorders, (3) anxiety disorders and post-traumatic stress disorder (PTSD), (4) impulse control disorders, (5) mood disorders, (6) personality disorders, (7) alcohol-related disorders, (8) substance-related disorders, and (9) central pain syndrome. Cardiometabolic morbidity included: (1) cardiac dysrhythmias, (2) heart failure, (3) peripheral and visceral atherosclerosis, (4) non-alcoholic fatty liver disease, (5) chronic kidney disease, (6) type 2 diabetes, (7) hypercholesterolemia, and (8) hypertension. Musculoskeletal morbidity included: (1) rheumatoid arthritis and related disease, (2) osteoarthritis, (3) osteoporosis, (4) pathological fracture, (5) other connective tissue disease, (6) muscle atrophy weakness, and (7) myalgia.
Statistical Analysis
Bivariate analyses of baseline demographic characteristics between TSCI patients and general population controls were examined for differences between groups. For categorical variables, column percentages were compared between both groups using effect size calculations.15 For large sample studies such as those using administrative claims, the effect size calculation is used since these studies are typically statistically overpowered and do not provide clinically meaningful differences in proportions between groups. For continuous variables, means and standard deviations were calculated. Standardized mean differences were calculated for continuous variables to ascertain clinically meaningful differences between groups.
For individuals with TSCI, we captured full comorbidity history in the one year of enrollment on their insurance plan prior to the index diagnosis. Individuals with TSCI must be adults aged 45 years or older at the time of the start of their first diagnosis. For randomly sampled controls, all patients with sufficient continuous enrollment within the study period of five years were randomly assigned a time zero to begin follow-up. The selection of the randomly assigned date required one year of enrollment to collect comorbidity history, and four years of post-index date follow-up to measure the first diagnosis of TSCI. The random assignment of the dates was determined assuming a uniform distribution across a specified interval of candidate dates that meet pre- and post-index date criteria and age criteria. This approach was used to address potential bias of selecting patients who were systematically younger during their enrollment on the plan for the sampled control group.
To examine disease-free ADRD survival of TSCI patients compared to general population controls, those patients that have no evidence of ADRD in each group in the one-year lookback period were graphed with Kaplan-Meier product limit survival curves for a four-year period post-index SCI diagnosis. To establish incidence in claims, we used a one-year lookback period from the index date in each group to obtain evidence of any service utilization with a diagnosis of any ADRD. Patients with ADRD in the one-year lookback period were excluded from the product-limit survival curves and other subsequent analyses.
To estimate the risk of incident ADRD, we constructed three analytic approaches. First, we used a bivariate regression to estimate the crude, unadjusted hazard ratio for ADRD comparing TSCI to the general population representing the exposure variable. Second, to estimate the adjusted hazard ratio, we performed multivariable regression to adjust for demographics, socioeconomic, and composite Elixhauser comorbidity index (Supplement Table 4). Since age is the most significant predictor of ADRD, we included an interaction term between TSCI (binary variable) and age (categorical variable) to examine the risk of TSCI between those 45–64 years of age and those 65 or older.
Finally, in order to account for selection bias attributable to TSCI patients, we performed propensity score matching. To achieve these ends, we estimated the propensity to be in the case (TSCI) group using multivariable logistic regression adjusting for demographics, individual comorbidities, and socioeconomic variables. We used a one-to-one caliper matching algorithm with caliper size of 0.0001 without replacement. To assess covariate balance, post-matched analyses of effect sizes were compared between the case (TSCI) and control groups. In order to quantify the risk of ADRD in the follow-up period, bivariate and multivariable Weibull regression were used to calculate the hazard ratio (Supplement Table 5). All patients were right censored if they did not experience ADRD in the follow-up period or disenrolled from the plan.
All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC). All hazard ratios included a calculation of the 95% confidence interval. Statistical testing was two-tailed with a significance level of 0.05 and effect sizes used a 0.2 meaningful difference cutoff. Interaction term analysis was conducted using PROC LIFETEST and LSMEANS for marginal hazard ratios.
Sensitivity Analysis
Since incident TSCI is usually discovered in the hospital setting, we conducted a sensitivity analysis that restricted our cohort to the first TSCI diagnosed in the inpatient setting. This was due to baseline differences being sufficiently noteworthy between those diagnosed with SCI in any setting compared to the general population. Therefore, after restricting the analysis to those whose inpatient facility and professional claims indicate a diagnosis for a hospitalization with SCI, we found baseline differences to be similar between the case and the general population. Therefore, our results reflect the full cohort of SCI patients discovered in any setting at the index diagnosis.
Due to large incident of traumatic brain injury (TBI) at the time of TSCI and high prevalence of cardiovascular disease among TSCI patients, we conducted a sensitivity analysis that included fitting models that assess within-group effects (marginal hazard ratios) for incident ADRD by interacting TSCI and TBI as well as TSCI and cardiovascular disease, respectively. The predicted marginal hazard ratios for our unmatched and matched cohorts are reported in Supplemental Tables 6 and 7, respectively. The results did not confer increased risk of ADRD. While literature has suggested that mild TBI might be associated with increased risk of ADRD, due to low prevalence of TBI (3.12% among cases and 0.18% among controls), further investigation on this topic is warranted.
Results
Table 1 represents unadjusted characteristics of individuals with and without TSCI within our unmatched and matched cohorts during the 1-year lookback period. Average number of enrollment years for those with and without TSCI was 10.9 (standard deviation (SD)=3.4) and 8.7 (SD=3.3) years, respectively. Majority of people with TSCI, 4,545 (64.8%), were 65 years or older, which represent primarily Medicare Advantage Part C patients. Females represent 59.3% (n=4,163) and 53.6% (n=491,029) of adults with and without TSCI, respectively. There were no significant differences in race/ethnicity between individuals with and without TSCI, with White being the majority in both groups (case=4,505 (64.2%); control=586,624 (64.0%)). We did not find any significant differences between our case and control groups in their educational attainment and net worth. For example, about 54.8% (n=3,847) of those with TSCI vs. 53.2% (n=487,526) of those without had fewer than 4-year college degree; and, 9.9% (n=694) of people with TSCI vs. 10.5% (n=96,323) of those without had a net worth of $150K–$249K. There was no difference in basic demographic characteristics between case and control groups after matching.
Table 1.
Descriptive Characteristics of Adults with (Case) and Without (Control) Traumatic SCI
| PREMATCHED | POSTMATCHED | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Spinal Cord Injury | Case | Control | ES | Case | Control | ES |
|
| ||||||
| Overall | 7019 (100%) | 916516 (100%) | 6083 (100%) | 6083 (100%) | ||
|
| ||||||
| Full Enrollment Length | ||||||
| Mean (StD) | 10.9 (3.4) | 8.7 (3.3) | 10.9 (3.4) | 8.6 (3.3) | ||
| Median (Q1-Q3) | 10.6 (8.0–13.2) | 7.8 (6.0–10.7) | 10.5 (8.0–13.1) | 7.5 (6.0–10.6) | ||
| Years Post Eligibility Start Date† | ||||||
| Mean (StD) | 6.1 (1.7) | 5.3 (1.5) | 6.1 (1.7) | 5.3 (1.5) | ||
| Median (Q1-Q3) | 5.6 (4.7–7.0) | 4.8 (4.2–5.9) | 5.6 (4.7–7.1) | 4.7 (4.2–5.8) | ||
| Age Group | ||||||
| 45–64 | 2474 (35.2%) | 511120 (55.8%) | 0.42 | 2317 (38.1%) | 2227 (36.6%) | 0.03 |
| 65 or Older | 4545 (64.8%) | 405396 (44.2%) | 0.42 | 3766 (61.9%) | 3856 (63.4%) | 0.03 |
| Gender | ||||||
| Female | 4163 (59.3%) | 491029 (53.6%) | 0.12 | 3479 (57.2%) | 3514 (57.8%) | 0.01 |
| Male | 2856 (40.7%) | 425487 (46.4%) | 0.12 | 2604 (42.8%) | 2569 (42.2%) | 0.01 |
| Race/Ethnicity | ||||||
| Asian | 206 (2.9%) | 28114 (3.1%) | 0.01 | 170 (2.8%) | 169 (2.8%) | 0.00 |
| Black | 494 (7.0%) | 73822 (8.1%) | 0.04 | 447 (7.3%) | 423 (7.0%) | 0.01 |
| Hispanic | 542 (7.7%) | 72777 (7.9%) | 0.01 | 471 (7.7%) | 458 (7.5%) | 0.01 |
| Unknown | 1272 (18.1%) | 155179 (16.9%) | 0.03 | 1070 (17.6%) | 1052 (17.3%) | 0.01 |
| White | 4505 (64.2%) | 586624 (64.0%) | 0.00 | 3925 (64.5%) | 3981 (65.4%) | 0.02 |
| Education | ||||||
| Less than 12th Grade | 48 (0.7%) | 5376 (0.6%) | 0.01 | 42 (0.7%) | 42 (0.7%) | 0.00 |
| High School Diploma | 1873 (26.7%) | 231118 (25.2%) | 0.03 | 1621 (26.6%) | 1632 (26.8%) | 0.00 |
| Less than Bachelor | 3847 (54.8%) | 487526 (53.2%) | 0.03 | 3339 (54.9%) | 3362 (55.3%) | 0.01 |
| Bachelor Degree Plus | 1091 (15.5%) | 156476 (17.1%) | 0.04 | 942 (15.5%) | 923 (15.2%) | 0.01 |
| Unknown | 160 (2.3%) | 36020 (3.9%) | 0.09 | 139 (2.3%) | 124 (2.0%) | 0.02 |
| Net Worth | ||||||
| Unknown | 1095 (15.6%) | 135560 (14.8%) | 0.02 | 926 (15.2%) | 918 (15.1%) | 0.00 |
| <$25K | 1119 (15.9%) | 120560 (13.2%) | 0.08 | 968 (15.9%) | 988 (16.2%) | 0.01 |
| $25K–$149K | 1247 (17.8%) | 147804 (16.1%) | 0.05 | 1073 (17.6%) | 1080 (17.8%) | 0.01 |
| $150K–$249K | 694 (9.9%) | 96323 (10.5%) | 0.02 | 608 (10.0%) | 628 (10.3%) | 0.01 |
| $250K–$499K | 1070 (15.2%) | 167313 (18.3%) | 0.08 | 939 (15.4%) | 925 (15.2%) | 0.01 |
| $500K+ | 1794 (25.6%) | 248956 (27.2%) | 0.04 | 1569 (25.8%) | 1544 (25.4%) | 0.01 |
Source: The 2007–2017 OptumInsight
Abbreviations: StD: Standard Deviation; ES: Effect Size; Q1-Q3: Quartile 1 and Quartile 3.
Effect size equal to or greater than 0.20 considered significant.
In Table 2, we compared a set of psychological, cardiometabolic, and musculoskeletal conditions between adults with and without TSCI. In our unmatched cohorts, prevalence of all conditions including any psychological (36.0% vs. 16.4%), cardiometabolic (77.0% vs. 53.1%), and musculoskeletal (70.1% vs. 33.6%) conditions were substantially higher among people with TSCI compared with those without. There was no significant difference in listed chronic conditions between case and control groups after matching.
Table 2.
Incident of Chronic Conditions among Adults with (Case) and Without (Control) Traumatic SCI
| PREMATCHED | POSTMATCHED | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Spinal Cord Injury | Case | Control | ES | Case | Control | ES |
|
| ||||||
| Overall | 7019 (100%) | 916516 (100%) | 6083 (100%) | 6083 (100%) | ||
|
| ||||||
| Psychological | ||||||
| Any Psychological | 2530 (36.0%) | 149925 (16.4%) | 0.45 | 1985 (32.6%) | 1899 (31.2%) | 0.03 |
| Insomnia | 425 (6.1%) | 29587 (3.2%) | 0.14 | 336 (5.5%) | 334 (5.5%) | 0.00 |
| Adjustment disorders | 144 (2.1%) | 11357 (1.2%) | 0.07 | 117 (1.9%) | 102 (1.7%) | 0.02 |
| Anxiety disorders | 892 (12.7%) | 56461 (6.2%) | 0.23 | 690 (11.3%) | 664 (10.9%) | 0.01 |
| PTSD | 27 (0.4%) | 1894 (0.2%) | 0.04 | 23 (0.4%) | 19 (0.3%) | 0.02 |
| Impulse control | 3 (0.0%) | 217 (0.0%) | 0.00 | 2 (0.0%) | 3 (0.0%) | 0.00 |
| Mood disorders | 1242 (17.7%) | 72191 (7.9%) | 0.30 | 948 (15.6%) | 951 (15.6%) | 0.00 |
| Personality disorders | 21 (0.3%) | 883 (0.1%) | 0.05 | 15 (0.2%) | 17 (0.3%) | 0.02 |
| Alcohol-related dis. | 206 (2.9%) | 6869 (0.7%) | 0.17 | 155 (2.5%) | 141 (2.3%) | 0.01 |
| Substance-related dis. | 202 (2.9%) | 5455 (0.6%) | 0.19 | 132 (2.2%) | 110 (1.8%) | 0.03 |
| Central Pain | 901 (12.8%) | 22276 (2.4%) | 0.42 | 605 (9.9%) | 590 (9.7%) | 0.01 |
| Cardiometabolic | ||||||
| Any Cardiometabolic | 5405 (77.0%) | 487055 (53.1%) | 0.45 | 4539 (74.6%) | 4540 (74.6%) | 0.00 |
| Cardiac dysrhythmias | 1606 (22.9%) | 88072 (9.6%) | 0.44 | 1228 (20.2%) | 1263 (20.8%) | 0.01 |
| Heart Failure | 671 (9.6%) | 26163 (2.9%) | 0.42 | 483 (7.9%) | 457 (7.5%) | 0.02 |
| Peripheral and visceral atherosclerosis | 818 (11.7%) | 40467 (4.4%) | 0.44 | 605 (9.9%) | 626 (10.3%) | 0.01 |
| Liver; Non-Alcohol | 120 (1.7%) | 9506 (1.0%) | 0.06 | 98 (1.6%) | 77 (1.3%) | 0.03 |
| Chronic kidney disease | 682 (9.7%) | 37242 (4.1%) | 0.31 | 537 (8.8%) | 542 (8.9%) | 0.00 |
| Diabetes Type II | 1731 (24.7%) | 150414 (16.4%) | 0.21 | 1481 (24.3%) | 1442 (23.7%) | 0.01 |
| Hypercholesterolemia | 1703 (24.3%) | 152123 (16.6%) | 0.18 | 1401 (23.0%) | 1435 (23.6%) | 0.01 |
| Hypertension | 4561 (65.0%) | 395374 (43.1%) | 0.44 | 3791 (62.3%) | 3871 (63.6%) | 0.03 |
| Musculoskeletal | ||||||
| Any Musculoskeletal | 4923 (70.1%) | 307891 (33.6%) | 0.73 | 3990 (65.6%) | 3986 (65.5%) | 0.00 |
| Rheumatoid arthritis | 311 (4.4%) | 15546 (1.7%) | 0.19 | 236 (3.9%) | 193 (3.2%) | 0.04 |
| Osteoarthritis | 2146 (30.6%) | 119632 (13.1%) | 0.48 | 1687 (27.7%) | 1753 (28.8%) | 0.02 |
| Osteoporosis | 1500 (21.4%) | 49193 (5.4%) | 0.63 | 986 (16.2%) | 1011 (16.6%) | 0.01 |
| Pathological fracture | 1213 (17.3%) | 4088 (0.4%) | 0.87 | 401 (6.6%) | 371 (6.1%) | 0.02 |
| Other connective tissue | 3750 (53.4%) | 228676 (25.0%) | 0.66 | 3042 (50.0%) | 3099 (50.9%) | 0.02 |
| Muscle Atrophy | 679 (9.7%) | 13505 (1.5%) | 0.69 | 445 (7.3%) | 420 (6.9%) | 0.02 |
| Myalgia | 610 (8.7%) | 36268 (4.0%) | 0.23 | 469 (7.7%) | 456 (7.5%) | 0.01 |
Source: The 2007–2017 OptumInsight
Abbreviations: StD: Standard Deviation; ES: Effect Size.
Effect size equal to or greater than 0.20 considered significant.
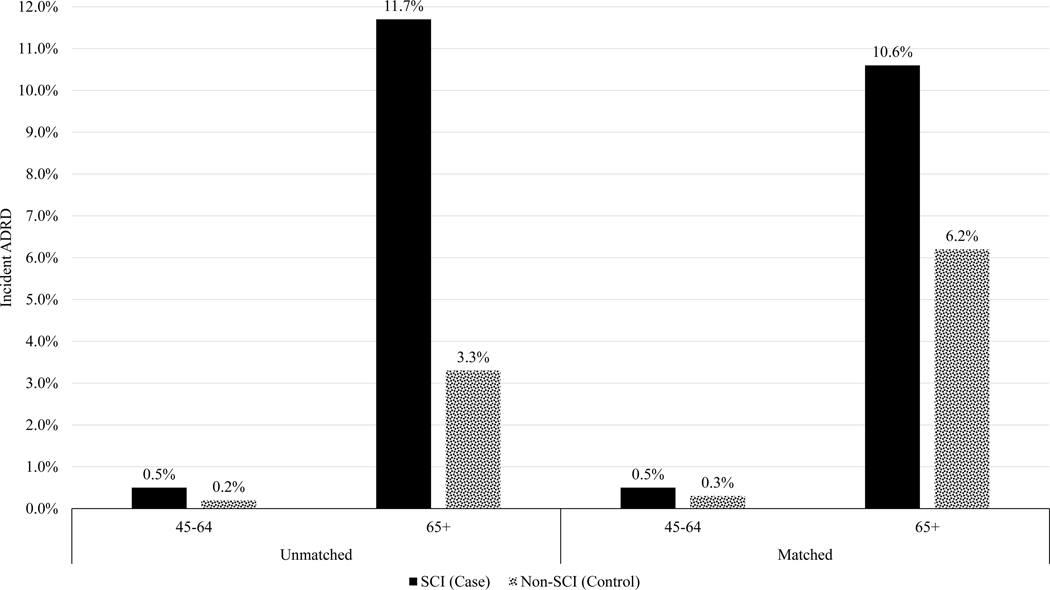
Unadjusted and unmatched average incidences of ADRD among individuals with and without TSCI were 0.5% (n=36) and 0.2% (n=1,837) among the 45–64 age group and 11.7% (n=819) and 3.3% (n=30,226) among the 65+ age group, respectively (Figure 1). After matching, average incident ADRD among persons with and without TSCI were 0.5% (n=33) and 0.2% (n==16) among 45–64 age group and 10.6% (n=644) and 6.2% (n=375) among 65+ age group, respectively.
Figure 1.
Unmatched and matched average incidences of ADRD among individuals with and without TSCI
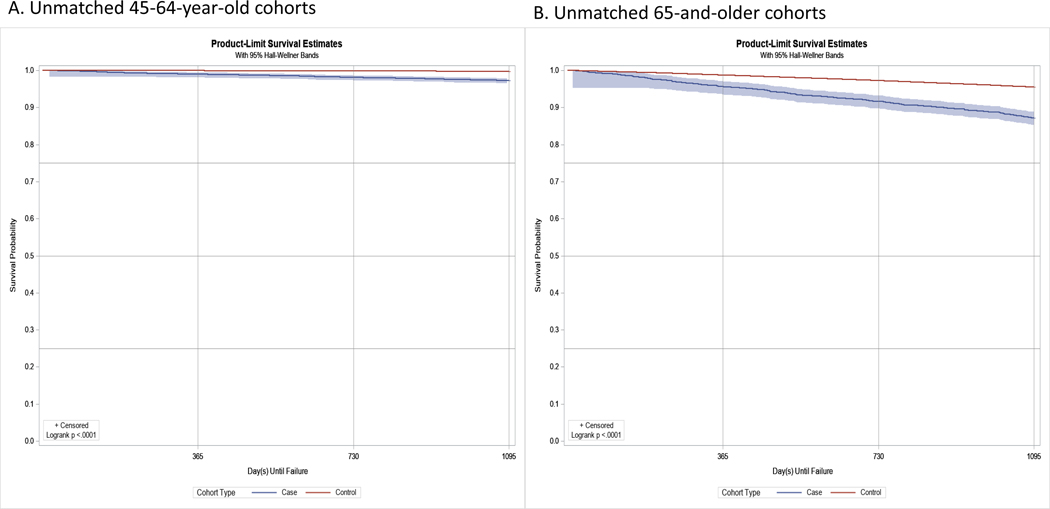
Figure 2 displays the Kaplan-Meier curve of the unmatched cohorts of adults with (case) and without (control) TSCI for ADRD-free survival. Among the unmatched cohorts of adults, at the end of a 4-year period, 13% of adults with TSCI compared with 4% of those without had ADRD. Among the matched cohorts, the difference between the two groups reduced to about 5% (12% of adults with TSCI compared with 7% of those without had incident ADRD).
Figure 2.
Unmatched and matched Kaplan-Meier product-limit survival curves (4-year) for adults with and without TSCI
Table 3 represents various hazard ratios between unmatched and matched cohorts. Adjusted unmatched and matched hazard ratios (HR) of TSCI for incident ADRD among 45–64 age group were HR=3.19 (95% CI: 2.30, 4.44; P < 0.001) and HR=1.93 (95% CI: 1.06, 3.51), respectively; and, adjusted unmatched and matched hazard ratios (HR) of TSCI for incident ADRD among 65+ age group were HR=1.90 (95% CI: 1.77, 2.04; P < 0.001) and HR=1.77 (95% CI: 1.55, 2.02), respectively (regression results are presented in Supplemental Tables 4 and 5).
Table 3.
Hazard Ratios of SCI for ADRD
| Unmatched Cohort | Matched Cohort | |||
|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | |
| Among those 45 to 64 years of age | 4.08 (2.93, 5.67) *** | 3.19 (2.30, 4.44) *** | 1.99 (1.09, 3.62) *** | 1.93 (1.06, 3.51) *** |
| Among those 65+ years of age | 2.51 (2.34, 2.70) *** | 1.90 (1.77, 2.04) *** | 1.79 (1.56, 2.04) *** | 1.77 (1.55, 2.02) *** |
Note: As with incidence estimates (Tables 1 and 2), all survival models used case (SCI) and control cohorts, which required a one-year clean period with no evidence of the ADRD. To estimate the hazard ratios (HRs) of SCI among each age group, we interacted categorical age group (45–64 and 65+) with SCI.
Regression models for fully adjusted unmatched and matched cohorts are reported in Supplementary Tables 4 and 5.
P-value < 0.001
Discussion
This study examined the risk of TSCI on incident ADRD, comparing unmatched and matched cohorts of adults with and without TSCI among two different age groups of 45–64 and 65+. Three main findings emerge: (1) risk of incident ADRD is substantially and significantly higher among persons with TSCI compared to those without; (2) TSCI approximately doubles the risk of early onset ADRD (among people 45–64 years old); (3) secondary comorbid conditions that are more prevalent among people with TSCI may also increase the risk of incident ADRD. These findings are of prominent importance because they denote the need for early detection and targeted interventions using preventative, rehabilitative, and therapeutic services that may preserve cognitive function among persons with TSCI.
Our findings are in concordance with other emerging evidence indicative of association between TSCI and ADRD.3,10,16–18 There is a neurodegenerative mechanism that manifest after sustaining TSCI.8 This may enable biological mechanisms related to neural dysfunction, neuroinflammation, and toxicity which are potential risk factors driving incident ADRD.7,9,19 For example, prior clinical studies showed that 40–60% of TSCI patients have some cognitive and emotional deficits.20 Roth et al. stated that TSCI patients show declines in attention span, concentration, and memory function.21 These evidence suggests that potential neurodegenerative consequences of TSCI may evolve over time and affect both the spine and brain.20
Furthermore, our findings suggest that TSCI increases the risk of early-onset ADRD more than it increases the risk among older people. Hazard of early onset ADRD is approximately twice among middle-aged persons with TSCI (45–64 years old) compared to their counterparts without TSCI. This plausibly can be explained by the risk associated directly with aforementioned pathophysiologic impact of TSCI on spine and brain.
Moreover, higher levels of anxiety, depression, and other chronic conditions among people with TSCI may further raise their risk for cognitive decline and ADRD.22–24 People with TSCI compared with adults without disability have a more sedentary lifestyle and lower level of physical and social activities.25,26 Living with TSCI places individuals at a greater risk of chronic comorbidities.1,17,27,28,29 After matching our case and control groups in psychological, cardiometabolic, and musculoskeletal conditions, risk of TSCI for incident ADRD declined. This suggest that portion of this additional risk may go back to higher prevalence of secondary conditions among people with TSCI and thus is potentially preventable.
ADRD is one of the most devastating neurodegenerative conditions.30 Appropriate preventative and rehabilitative services are central to improvement in neurological recovery.31–34 Further research on healthy lifestyle emphasizing on cognitive leisure activities,35 social engagement and community living,36 and use of preventative services that may reduce the risk of ADRD and other chronic conditions among this patient population is imperative.
Study Limitations
This study had several limitations. First, using claims data we were unable to distinguish between incident and prevalent TSCI. Although we used a one-year lookback period to ensure no prior SCI, it is plausible that diagnosis of SCI has not been recorded over the prior year’s healthcare encounters. Accordingly, with only limited enrollment periods on an insurance plan, we did not have visibility to the length of time a person has lived with TSCI since they could have acquired it before our one-year lookback period. To address this limitation, knowing that certain chronic conditions that are also risk factors for ADRD had much higher prevalence among people with SCI, we matched our SCI population to those without SCI on all our observable confounders. Although matching revealed a reduction in the risk difference between SCI and non-SCI individuals for ADRD, the risk remained substantially higher. Second, due to errors in administrative claims’ diagnostic codes, confirmatory identification of TSCI may not always be accurate. While our estimates of TSCI and other reported chronic conditions may not be 100% accurate, prior research indicates that claim-based estimates have high sensitivity and specificity.37,38 Having said this, it is also well known that for a broad set of reasons, certain conditions such as ADRD are underdiagnosed.12,39 Thus, it is conceivable and likely that our measures for incident ADRD diagnosis is underestimated. Third, using diagnosis codes to define TSCI, we had no measure of the neurological classification. Inclusion of various functional measures in future studies on this topic will serve as an appropriate adjunctive to existing data and will enrich and inform severity of injury and other unobserved factors related to the TSCI patient which may influence incident ADRD. Fourth, while literature has suggested that TBI might be associated with increased risk of ADRD,40due to potential under-coding of TBI using administrative claims,41 our sensitivity analysis did not show incremental risk. Finally, although we adjusted/matched for some socioeconomic variables, we had no information on lifestyle choices and level of cognitive engagements.
The strengths of our study can be captured in two primary aspects. First, this is the largest study to date examining the trajectory course of ADRD among privately insured people with TSCI in the U.S. Second, while randomized clinical trials may be pondered as the gold standard in clinical research, large observational studies are increasingly proving to be practical and valuable in research.42
Conclusions
To summarize, this study used large national private claims data to examine the hazard of TSCI in association with incident ADRD. Adults with TSCI are at a heightened risk for incident ADRD. Further efforts are required to develop recommendations for healthy life choices, leisure cognitive activities and clinical guidelines with emphasis on preserving cognitive function.
Supplementary Material
Supplemental Figure S1. Schematic flow diagram
Supplemental Table S1. Diagnostic codes for traumatic spinal cord injury
Supplemental Table S2. Diagnostic codes for Alzheimer’s disease and related dementia
Supplemental Table S3. Diagnostic codes for comorbid conditions
Supplemental Table S4. Regression results for unmatched cohorts of adults with and without TSCI
Supplemental Table S5. Regression results for matched cohorts of adults with and without TSCI Supplemental
Table S6. Adjusted predicted incremental hazards of traumatic brain injury and cardiovascular disease for ADRD after traumatic spinal cord injury among unmatched cohorts of adults
Supplemental Table S7. Adjusted predicted incremental hazards of traumatic brain injury and cardiovascular disease for ADRD after traumatic spinal cord injury among matched cohorts of adults
Funding:
The National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR #90RTHF0001-01-00).
Abbreviations:
- ADRD
Alzheimer’s Disease and Related Dementia
- APP
Amyloid Precursor Protein
- AIC
Akaiki Information Criterion
- CI
Confidence Interval
- ICD
International Classification of Diseases
- HR
Hazard Ratio
- PTSD
post-traumatic stress disorder
- TSCI
Traumatic Spinal Cord Injury
Footnotes
Disclosure: Mr. Kamdar has a financial relationship with Lucent Surgical, outside the submitted work.
References
- 1.Fann JR, Bombardier CH, Richards JS, et al. Depression after spinal cord injury: comorbidities, mental health service use, and adequacy of treatment. Archives of Physical medicine and Rehabilitation. 2011;92(3):352–360. [DOI] [PubMed] [Google Scholar]
- 2.Krause JS, Saunders LL. Health, secondary conditions, and life expectancy after spinal cord injury. Archives of physical medicine and rehabilitation. 2011;92(11):1770–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeh T-S, Ho Y-C, Hsu C-L, Pan S-L. Spinal cord injury and Alzheimer’s disease risk: a population-based, retrospective cohort study. Spinal cord. 2018;56(2):151–157. [DOI] [PubMed] [Google Scholar]
- 4.Huang S-W, Wang W-T, Chou L-C, Liou T-H, Lin H-W. Risk of dementia in patients with spinal cord injury: a nationwide population-based cohort study. Journal of neurotrauma. 2017;34(3):615–622. [DOI] [PubMed] [Google Scholar]
- 5.Alzheimer’s Association. 2017 Alzheimer’s disease facts and figures. Alzheimer’s and Dementia. 2017;13(4):325–373. [Google Scholar]
- 6.Delacourte A, Buée L. Tau pathology: a marker of neurodegenerative disorders. Current opinion in neurology. 2000;13(4):371–376. [DOI] [PubMed] [Google Scholar]
- 7.Kwon BK, Stammers AM, Belanger LM, et al. Cerebrospinal fluid inflammatory cytokines and biomarkers of injury severity in acute human spinal cord injury. Journal of neurotrauma. 2010;27(4):669–682. [DOI] [PubMed] [Google Scholar]
- 8.Allison D, Ditor D. Immune dysfunction and chronic inflammation following spinal cord injury. Spinal cord. 2015;53(1):14–18. [DOI] [PubMed] [Google Scholar]
- 9.Song HL, Shim S, Kim DH, et al. β-Amyloid is transmitted via neuronal connections along axonal membranes. Annals of neurology. 2014;75(1):88–97. [DOI] [PubMed] [Google Scholar]
- 10.Schmitz N, Deschênes SS, Burns RJ, et al. Cardiometabolic dysregulation and cognitive decline: potential role of depressive symptoms. The British Journal of Psychiatry. 2018;212(2):96–102. [DOI] [PubMed] [Google Scholar]
- 11.Tsuno N, Homma A. What is the association between depression and Alzheimer’s disease? Expert review of neurotherapeutics. 2009;9(11):1667–1676. [DOI] [PubMed] [Google Scholar]
- 12.Amjad H, Roth DL, Sheehan OC, Lyketsos CG, Wolff JL, Samus QM. Underdiagnosis of dementia: an observational study of patterns in diagnosis and awareness in US older adults. Journal of general internal medicine. 2018;33(7):1131–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turner RS, Stubbs T, Davies DA, Albensi BC. Potential new approaches for diagnosis of alzheimer’s disease and related dementias. Frontiers in neurology. 2020;11:496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fillit H, Geldmacher DS, Welter RT, Maslow K, Fraser M. Optimizing coding and reimbursement to improve management of Alzheimer’s disease and related dementias. Journal of the American Geriatrics Society. 2002;50(11):1871–1878. [DOI] [PubMed] [Google Scholar]
- 15.Bezeau S, Graves R. Statistical power and effect sizes of clinical neuropsychology research. Journal of clinical and experimental neuropsychology. 2001;23(3):399–406. [DOI] [PubMed] [Google Scholar]
- 16.Sachdeva R, Gao F, Chan CC, Krassioukov AV. Cognitive function after spinal cord injury: A systematic review. Neurology. 2018;91(13):611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterson MD, Kamdar N, Chiodo A, Tate DG. Psychological morbidity and chronic disease among adults with traumatic spinal cord injuries: a longitudinal cohort study of privately insured beneficiaries. Paper presented at: Mayo Clinic Proceedings 2020. [DOI] [PubMed] [Google Scholar]
- 18.Peterson M, Kamdar N, Tate D . Mental Health Disorders And Chronic Disease Among Adults With Spinal Cord Injury. Archives of Physical Medicine and Rehabilitation. 2019;100(10):e97. [Google Scholar]
- 19.Le MN, Kim W, Lee S, McKee AC, Hall GF. Multiple mechanisms of extracellular tau spreading in a non-transgenic tauopathy model. American journal of neurodegenerative disease. 2012;1(3):316. [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Cao T, Ritzel RM, He J, Faden AI, Wu J. Dementia, Depression, and Associated Brain Inflammatory Mechanisms after Spinal Cord Injury. Cells. 2020;9(6):1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roth E, Davidoff G, Thomas P, et al. A controlled study of neuropsychological deficits in acute spinal cord injury patients. Spinal Cord. 1989;27(6):480–489. [DOI] [PubMed] [Google Scholar]
- 22.Barnard ND, Bush AI, Ceccarelli A, et al. Dietary and lifestyle guidelines for the prevention of Alzheimer’s disease. Neurobiology of aging. 2014;35:S74–S78. [DOI] [PubMed] [Google Scholar]
- 23.Bhat NR. Linking cardiometabolic disorders to sporadic Alzheimer’s disease: a perspective on potential mechanisms and mediators. Journal of neurochemistry. 2010;115(3):551–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Speck CE, Kukull WA, Brenner DE, et al. History of depression as a risk factor for Alzheimer’s disease. Epidemiology. 1995:366–369. [DOI] [PubMed] [Google Scholar]
- 25.Verschuren O, Dekker B, van Koppenhagen C, Post M. Sedentary behavior in people with spinal cord injury. Archives of physical medicine and rehabilitation. 2016;97(1):173. [DOI] [PubMed] [Google Scholar]
- 26.Vissers M, Van den Berg-Emons R, Sluis T, Bergen M, Stam H, Bussmann H. Barriers to and facilitators of everyday physical activity in persons with a spinal cord injury after discharge from the rehabilitation centre. Journal of Rehabilitation Medicine. 2008;40(6):461–467. [DOI] [PubMed] [Google Scholar]
- 27.Jensen MP, Molton IR, Groah SL, et al. Secondary health conditions in individuals aging with SCI: terminology, concepts and analytic approaches. Spinal Cord. 2012;50(5):373–378. [DOI] [PubMed] [Google Scholar]
- 28.Hall OT, McGrath RP, Peterson MD, et al. The Burden of Traumatic Spinal Cord Injury in the United States: Disability-Adjusted Life Years. Arch Phys Med Rehabil. 2019;100(1):95–100. [DOI] [PubMed] [Google Scholar]
- 29.Groah SL, Nash MS, Ward EA, et al. Cardiometabolic risk in community-dwelling persons with chronic spinal cord injury. J Cardiopulm Rehabil Prev. 2011;31(2):73–80. [DOI] [PubMed] [Google Scholar]
- 30.Barnes DE, Yaffe KJTLN. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. 2011;10(9):819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whiteneck G, Meade MA, Dijkers M, Tate DG, Bushnik T, Forchheimer MB. Environmental factors and their role in participation and life satisfaction after spinal cord injury. Archives of physical medicine and rehabilitation. 2004;85(11):1793–1803. [DOI] [PubMed] [Google Scholar]
- 32.Burns AS, Ditunno JF. Establishing prognosis and maximizing functional outcomes after spinal cord injury: a review of current and future directions in rehabilitation management. Spine. 2001;26(24S):S137–S145. [DOI] [PubMed] [Google Scholar]
- 33.Hammell KW. Experience of rehabilitation following spinal cord injury: a meta-synthesis of qualitative findings. Spinal Cord. 2007;45(4):260–274. [DOI] [PubMed] [Google Scholar]
- 34.Marino RJ, Ditunno JF Jr, Donovan WH, Maynard F Jr . Neurologic recovery after traumatic spinal cord injury: data from the Model Spinal Cord Injury Systems. Archives of physical medicine and rehabilitation. 1999;80(11):1391–1396. [DOI] [PubMed] [Google Scholar]
- 35.Stern C, Munn Z. Cognitive leisure activities and their role in preventing dementia: a systematic review. International Journal of Evidence-Based Healthcare. 2010;8(1):2–17. [DOI] [PubMed] [Google Scholar]
- 36.Flicker L. Modifiable lifestyle risk factors for Alzheimer’s disease. Journal of Alzheimer’s disease. 2010;20(3):803–811. [DOI] [PubMed] [Google Scholar]
- 37.Reeves S, Garcia E, Kleyn M, et al. Identifying sickle cell disease cases using administrative claims. Academic pediatrics. 2014;14(5):S61–S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leslie W, Lix L, Yogendran M. Validation of a case definition for osteoporosis disease surveillance. Osteoporosis international. 2011;22(1):37–46. [DOI] [PubMed] [Google Scholar]
- 39.Lin P-J, Kaufer DI, Maciejewski ML, Ganguly R, Paul JE, Biddle AK. An examination of Alzheimer’s disease case definitions using Medicare claims and survey data. Alzheimer’s & Dementia. 2010;6(4):334–341. [DOI] [PubMed] [Google Scholar]
- 40.Nordström A, Nordström P. Traumatic brain injury and the risk of dementia diagnosis: A nationwide cohort study. PLoS medicine. 2018;15(1):e1002496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lasry O, Dendukuri N, Marcoux J, Buckeridge DL. Accuracy of administrative health data for surveillance of traumatic brain injury: a Bayesian latent class analysis. Epidemiology. 2018;29(6):876–884. [DOI] [PubMed] [Google Scholar]
- 42.Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. New England Journal of Medicine. 2000;342(25):1878–1886. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. Schematic flow diagram
Supplemental Table S1. Diagnostic codes for traumatic spinal cord injury
Supplemental Table S2. Diagnostic codes for Alzheimer’s disease and related dementia
Supplemental Table S3. Diagnostic codes for comorbid conditions
Supplemental Table S4. Regression results for unmatched cohorts of adults with and without TSCI
Supplemental Table S5. Regression results for matched cohorts of adults with and without TSCI Supplemental
Table S6. Adjusted predicted incremental hazards of traumatic brain injury and cardiovascular disease for ADRD after traumatic spinal cord injury among unmatched cohorts of adults
Supplemental Table S7. Adjusted predicted incremental hazards of traumatic brain injury and cardiovascular disease for ADRD after traumatic spinal cord injury among matched cohorts of adults