Abstract
Metastatic neuroendocrine prostate cancer (NEPC) is a rare, aggressive disease with limited data on second-line treatment. In this retrospective, multi-institutional study, clinical and treatment data were collected for 58 patients with NEPC who received second-line systemic therapy after first-line platinum. Treatments were highly varied but uniformly poor in improving survival. Further study and consensus are needed for second-line NEPC treatment.
Background:
De novo neuroendocrine prostate cancer (NEPC) and treatment-emergent neuroendocrine prostate cancer (T-NEPC) are rare diseases with a poor prognosis. After first-line platinum chemotherapy, there is no consensus on second-line treatments.
Patients and Methods:
Patients with a pathologic diagnosis of de novo NEPC or T-NEPC between 2000 and 2020 who received first-line platinum and any second-line systemic therapy were selected and standardized clinical data was collected via the electronic health record at each institution. The primary endpoint was overall survival (OS) based on second-line therapy. Secondary endpoints included objective response rate (ORR) to second-line therapy, PSA response, and time on treatment.
Results:
Fifty-eight patients (32 de novo NEPC, 26 T-NEPC) from 8 institutions were included. At de novo NEPC or T-NEPC diagnosis, the overall cohort had a median age of 65.0 years (IQR 59.2–70.3) and median PSA of 3.0 ng/dL (IQR 0.6–17.9). Following first-line platinum chemotherapy, 21 patients (36.2%) received platinum chemotherapy, 10 (17.2%) taxane monotherapy, 11 (19.0%) immunotherapy, 10 (17.2%) other chemotherapy, and 6 (16.2%) other systemic therapy. Among 41 evaluable patients, the ORR was 23.5%. The mOS after start of second-line therapy was 7.4 months (95% CI 6.1–11.9).
Conclusions:
In this retrospective study, patients with de novo NEPC or T-NEPC who received second-line therapy were treated with wide variety of treatment regimens, reflecting the lack of consensus in this setting. Most patients received chemotherapy-based treatments. Overall prognosis was poor and ORR was low in the second line regardless of treatment choice.
Keywords: Small cell, Chemotherapy
Background
Neuroendocrine prostate cancer (NEPC), also referred to as small cell prostate carcinoma, is a rare but lethal disease with a poor prognosis.1 NEPC is a varied clinical entity that can present as de novo disease or develop during treatment of prostate adenocarcinoma in the metastatic castrate-resistant prostate cancer (mCRPC) setting.
NEPC can be diagnosed as pure small cell neuroendocrine carcinoma in the prostate gland or as focal neuroendocrine differentiation admixed with prostate adenocarcinoma.2–4 NEPC is morpho-logically similar to the oat cell or intermediate cell appearance small cell lung cancer, and nearly 90% of cases are positive for a neuroendocrine marker. NEPC has historically been diagnosed based on classic morphologic and immunohistochemical profiles.5 However, advances in molecular analysis such as single-cell sequencing has demonstrated early development of neuroendocrine features in CRPC cells.6, 7 Improved understanding of the genomics and pathogenesis of NEPC reflects the changing standards for how the disease is defined.5 For our study, we subcategorized histologically-diagnosed NEPC into 2 clinical groups: de novo NEPC and treatment-emergent NEPC (T-NEPC).
De novo NEPC is an uncommon histologic subtype (estimated < 1% of prostate cancer cases) often presenting with a low PSA and metastatic disease at diagnosis.8, 9 De novo NEPC is also a clinically aggressive disease with a survival rate of 14.3% at 5 years and median OS of less than 1 year.8 T-NEPC occurs with relative frequency (17%−30%) over the course of treatment with androgen deprivation therapy (ADT) and is associated with shortened survival.2, 10 T-NEPC cells terminally differentiate via lineage plasticity to lack expression for the androgen receptor (AR), conferring resistance to ADT and AR targeted therapy.10–12
The mainstay of NEPC treatment is platinum-based chemotherapy, similar to small cell lung cancer (SCLC) regimens.13 However, the Advanced Prostate Cancer Consensus Conference (APCCC) 2017 illustrates the lack of agreement regarding first-line therapy with 58% favoring standard mCRPC treatment and 42% favoring platinum-based chemotherapy.14 For metastatic NEPC, patients often receive a combination of ADT and platinum chemotherapy in the first line. Similar to SCLC, NEPC is often responsive to initial treatment. However, most patients relapse with resistant disease and short survival. In the second-line setting, there are no current standard treatments for NEPC and treatment is often extrapolated from those used for SCLC.
NEPC patients are often excluded from clinical trials for SCLC and prostate adenocarcinoma. As such, treatment decisions rely on individual clinician preference and prior experiences in the treatment of SCLC. In this retrospective study, we aim to gather real-world data on clinical practice patterns among oncologists and evaluate the treatment and outcomes of patients in the second-line setting with de novo NEPC and T-NEPC.
Patients and Methods
Study Design and Patients
This retrospective, multi-institutional study collected secondary-use clinical data. Following local scientific review and institutional review board (IRB) approval at the University of Colorado Cancer Center, the protocol was distributed to participating partner institutions for approval by their respective IRBs. At each site, institutional electronic health records (EHR) and databases were queried for patients with metastatic disease and a histologically confirmed diagnosis of small cell prostate cancer, neuroendocrine prostate cancer, or prostate cancer with neuroendocrine differentiation.
Male patients aged 18 years or older with de novo NEPC were defined as having histologically confirmed metastatic neuroendocrine prostate cancer at diagnosis. Molecular confirmation was not required. Patients could have pure small cell neuroendocrine carcinoma of the prostate or prostate adenocarcinoma admixed with any component of neuroendocrine differentiation. Patients with T-NEPC were defined as those with mCRPC who have biopsy-proven neuroendocrine differentiation after a prior diagnosis of prostate adenocarcinoma. Prior chemotherapy for prostate adenocarcinoma was allowed, but not required. Patients in both the de novo NEPC and T-NEPC cohorts must have received at least first-line and second-line systemic therapy for de novo NEPC or T-NEPC. First-line systemic therapy must be platinum-based chemotherapy, meaning cisplatin plus etoposide, carboplatin plus etoposide, carboplatin plus docetaxel, or carboplatin plus cabazitaxel.13 Consecutive patients were diagnosed with de novo NEPC or T-NEPC between January 1, 2000 to December 31, 2020.
Demographic, clinical, pathologic, molecular, radiographic, laboratory, treatment, and outcomes data were extracted by investigators from the EHR at each institution. Standardized data collection templates were used to minimize inter-observer variation. Only de-identified data was shared.
Outcomes
Patients were evaluated in the overall cohort and stratified by diagnosis of de novo NEPC or T-NEPC. The primary endpoint of this study was OS from start of second-line therapy to death from any cause or censoring at date of last follow-up. For all participating sites, data for response, and survival were collected using a cutoff date of June 30, 2021.
Secondary endpoints of this study include objective response rate (ORR) to second-line therapy, PSA response ≥50%, and time on treatment. ORR was based on local physician assessment, guided by modified Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 principles. Time on treatment was defined as time from initiation of second-line therapy to discontinuation for any reason including progressive disease, toxicity, patient preference, or death.
Statistical Analysis
Descriptive statistics are presented for baseline characteristics of the entire patient population, by cohort, and by second-line therapy. For continuous variables, the median and interquartile range (IQR) were reported, and nonparametric Kruskal-Wallis tests were conducted to compare baseline characteristics among stratification groups. For categorical variables, the frequencies and the percentages were calculated, and Fisher’s exact tests were conducted to compare baseline characteristics among stratification groups. For OS, the survival probability (Kaplan-Meier survival curve) was calculated by the Kaplan-Meier method, and the median survival time was reported with the corresponding 2-sided 95% Brookmeyer-Crowley’s Confidence Interval (CI) if feasible. The median and IQR were calculated for time on treatment. The cumulative frequencies, estimated proportions for each second-line treatment therapy, were calculated for ORR, PSA response, or best responses. The median time and IQR were reported for the time on second-line therapy. The analyses for OS, ORR, PSA response, time on second-line therapy, and best response were conducted for aggregated population and by cohorts if feasible. All statistical analyses were performed by an independent statistician to ensure unbiased data review. Statistical analyses were run on R version 4.1.0.
Results
A total of 58 patients, 32 patients with de novo NEPC and 26 patients with T-NEPC, from 8 institutions were included. At de novo NEPC or T-NEPC diagnosis, the overall cohort had a median age of 65.0 years (IQR 59.2–70.3) and a median PSA of 2.6 ng/dL (IQR 0.6–17.9) (Table 1). Median PSA was significantly higher in patients with de novo NEPC at 3.6 ng/dL (IQR 1.2–21.7) compared to 0.8 ng/dL (IQR 0.1–8.2) for patients with T-NEPC (P = .030). The patients were predominantly white (49 patients, 84.5%) and non-Hispanic (54 patients, 93.1%). Patients with de novo NEPC were more likely than patients with T-NEPC to have mixed neuroendocrine and adenocarcinoma histology (71.0% vs. 30.4%, respectively; P = 0.007), although the amount of mixed and pure neuroendocrine histology was roughly equal in the overall cohort. The most common sites of metastasis at diagnosis were lymph node (75.6%), bone (48.3%), and liver (44.8%). Nearly half (28 patients, 48.3%) received treatment to the prostate most often with surgery (9 patients, 15.5%) or radiation (14 patients, 24.1%).
Table 1.
Demographic and Clinical Characteristics Stratified by de novo NEPC and T-NEPC Cohort
| Characteristics | De novo NEPC | T-NEPC | Total | P | |
|---|---|---|---|---|---|
| Age at NEPC Diagnosis | Median (IQR) | 65.0 (58.8–70.5) | 67.0 (60.8–70.3) | 65.0 (59.2–70.3) | .467 |
| Sex | Male | 32 (100) | 26 (100) | 58 (100.0) | |
| Race | Asian | 2 (6.2) | 0 (0) | 2 (3.4) | .283 |
| Black | 2 (6.2) | 3 (11.5) | 5 (8.6) | ||
| Other | 2 (6.2) | 0 (0.0) | 2 (3.4) | ||
| White | 26 (81.2) | 23 (88.5) | 49 (84.5) | ||
| Ethnicity | Hispanic | 3 (9.4) | 1 (3.8) | 4 (6.9) | .760 |
| Non-Hispanic | 29 (90.6) | 25 (96.2) | 54 (93.1) | ||
| Histology | Mixed | 22 (71.0) | 7 (30.4) | 29 (53.7) | .007 |
| Pure | 9 (29.0) | 16 (69.6) | 25 (46.3) | ||
| PSA at NEPC diagnosis (ng/dL) | Median (IQR) | 3.6 (1.2–21.7) | 0.8 (0.1–8.2) | 2.6 (0.6–17.9) | .030 |
| Metastasis: lymph node | No | 5 (15.6) | 9 (34.6) | 14 (24.1) | .170 |
| Yes | 27 (84.4) | 17 (65.4) | 44 (75.9) | ||
| Metastasis: bone | No | 17 (53.1) | 13 (50.0) | 30 (51.7) | 1.000 |
| Yes | 15 (46.9) | 13 (50.0) | 28 (48.3) | ||
| Metastasis: liver | No | 21 (65.6) | 11 (42.3) | 32 (55.2) | .131 |
| Yes | 11 (34.4) | 15 (57.7) | 26 (44.8) | ||
| Metastasis: brain | No | 32 (100) | 26 (100) | 58 (100.0) | |
| Metastasis: lung | No | 27 (84.4) | 20 (76.9) | 47 (81.0) | .702 |
| Yes | 5 (15.6) | 6 (23.1) | 11 (19.0) | ||
| Metastasis: other | No | 28 (87.5) | 16 (61.5) | 44 (75.9) | .047 |
| Yes | 4 (12.5) | 10 (38.5) | 14 (24.1) | ||
| Treatment to primary | None | 20 (62.5) | 10 (38.5) | 30 (51.7) | .022 |
| Other | 0 (0) | 1 (3.8) | 1 (1.7) | ||
| Radiation | 10 (31.2) | 4 (15.4) | 14 (24.1) | ||
| Radiation, cryotherapy | 0 (0) | 1 (3.8) | 1 (1.7) | ||
| Surgery | 2 (6.2) | 7 (26.9) | 9 (15.5) | ||
| Surgery, radiation | 0 (0) | 3 (11.5) | 3 (5.2) | ||
| First line systemic therapy | Platinum | 29 (90.6) | 24 (92.3) | 53 (91.4) | 1.000 |
| Platinum + immunotherapy | 3 (9.4) | 2 (7.7) | 5 (8.6) | ||
| Concurrent ADT in first line therapy | Yes | 20 (62.5) | 23 (88.5) | 43 (74.1) | .052 |
| No | 12 (37.5) | 3 (11.5) | 15 (25.9) | ||
| PSA response (≥50%) to first line systemic therapy | Yes | 16 (64.0) | 7 (36.8) | 23 (52.3) | .138 |
| No | 9 (36.0) | 12 (63.2) | 21 (47.7) | ||
| Best response to first line systemic therapy | CR | 3 (9.4) | 1 (3.8) | 4 (6.9) | .160 |
| PR | 15 (46.9) | 19 (73.1) | 34 (58.6) | ||
| SD | 6 (18.8) | 1 (3.8) | 7 (12.1) | ||
| PD | 8 (25.0) | 5 (19.2) | 13 (22.4) | ||
| Time on first line therapy in month | Median (IQR) | 4.0 (3.4 to 7.8) | 3.5 (2.3 to 4.6) | 3.8 (3.0 to 4.9) | .118 |
| ECOG just before second line systemic therapy | 0 | 2 (8.0) | 0 (0) | 2 (4.3) | .458 |
| 1 | 13 (52.0) | 9 (42.9) | 22 (47.8) | ||
| 2 | 9 (36.0) | 11 (52.4) | 20 (43.5) | ||
| 3 | 1 (4.0) | 1 (4.8) | 2 (4.3) | ||
| Second line systemic therapy | Platinum-based | 11 (34.4) | 10 (38.5) | 21 (36.2) | .291 |
| Taxane monotherapy | 4 (12.5) | 6 (23.1) | 10 (17.2) | ||
| Immunotherapy | 7 (21.9) | 4 (15.4) | 11 (19.0) | ||
| Other chemo | 8 (25.0) | 2 (7.7) | 10 (17.2) | ||
| Other | 2 (6.2) | 4 (15.4) | 6 (10.3) |
During first-line treatment with platinum-based chemotherapy, 5 patients (8.6%) also received immunotherapy and 43 patients (74.1%) received concurrent ADT. Thirty-eight patients (65.5%) had a CR or PR to first-line therapy and 52.3% achieved a PSA response ≥50%. Patients remained on first-line chemotherapy for a median of 3.8 months (IQR 3.0–4.9).
Second-line therapies were subcategorized as platinum-based chemotherapy, taxane monotherapy, immunotherapy, other chemotherapy, or other systemic therapy. Platinum-based chemotherapy was the most used treatment in the second line for all patients and within the individual de novo NEPC and T-NEPC cohorts (Table 2). Half of patients (10 total patients) retreated with platinum-based chemotherapy had a treatment-free interval of ≥6 months after the first line (8 of 11 patients with de novo NEPC, 2 of 9 patients with T-NEPC). Of the 41 total patients evaluable for response, the ORR was 23.5%. A PSA response ≥50% occurred in 6 of 37 patients (16.2%). Patients treated with further platinum-based chemotherapy were more likely to have an objective response (9 patients, 50.0%, P=.016). Only 1 patient who received platinum-based chemotherapy had a CR. Second-line therapy was primarily discontinued due to PD (43 patients, 75.4%).
Table 2.
Clinical Outcomes of Overall NEPC Cohort Stratified by Second-Line Therapy
| Characteristics | Platinum-Based | Taxane Monotherapy | Immunotherapy | Other Chemo | Other | Total | P | |
|---|---|---|---|---|---|---|---|---|
| De novo NEPC | 11 (52.4) | 4 (40.0) | 7 (63.6) | 8 (80.0) | 2 (33.3) | 32 (55.2) | .291 | |
| T-NEPC | 10 (47.6) | 6 (60.0) | 4 (36.4) | 2 (20.0) | 4 (66.7) | 26 (44.8) | ||
| PSA response (≥50%) to second line systemic therapy | Yes | 2 (14.3) | 1 (11.1) | 0 (0) | 1 (25.0) | 2 (40.0) | 6 (16.2) | .480 |
| No | 12 (85.7) | 8 (88.9) | 5 (100.0) | 3 (75.0) | 3 (60.0) | 31 (83.8) | ||
| Best response to second line systemic therapy | CR | 1 (5.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.0) | .233 |
| PR | 8 (44.4) | 0 (0) | 1 (11.1) | 2 (20.0) | 0 (0) | 11 (21.6) | ||
| SD | 1 (5.6) | 2 (22.2) | 0 (0.0) | 1 (10.0) | 1 (20.0) | 5 (9.8) | ||
| PD | 8 (44.4) | 7 (77.8) | 8 (88.9) | 7 (70.0) | 4 (80.0) | 34 (66.7) | ||
| Objective response to second line systemic therapy | Yes | 9 (50.0) | 0 (0) | 1 (11.1) | 2 (20.0) | 0 (0) | 12 (23.5) | .016 |
| No | 9 (50.0) | 9 (100) | 8 (88.9) | 8 (80.0) | 5 (100) | 39 (76.5) | ||
| Time on second line therapy in months | Median (IQR) | 2.8 (1.6–4.7) | 1.7 (0.7–2.4) | 1.4 (1.0–2.2) | 2.0 (1.5–3.9) | 1.8 (1.5–6.2) | 2.0 (1.0–3.9) | .232 |
| Reason for discontinuation of second line therapy | PD | 14 (70.0) | 7 (70.0) | 9 (81.8) | 8 (80.0) | 5 (83.3) | 43 (75.4) | .283 |
| Toxicity | 1 (5.0) | 3 (30.0) | 0 (0) | 0 (0) | 0 (0) | 4 (7.0) | ||
| Patient preference | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Death | 3 (15.0) | 0 (0) | 2 (18.2) | 1 (10) | 1 (16.7) | 7 (12.3) | ||
| Other | 2 (10) | 0 (0) | 0 (0.0) | 1 (10) | 0 (0) | 3 (5.3) | ||
| Survival status at last follow-up | Dead | 18 (85.7) | 6 (60.0) | 10 (90.9) | 9 (90.0) | 5 (83.3) | 48 (82.8) | .326 |
| Censored | 3 (14.3) | 4 (40) | 1 (9.1) | 1 (10) | 1 (16.7) | 10 (17.2) | ||
| Follow-up time i n months | Median (IQR) | 7.1 (4.7–10.7) | 5.9 (4.7–9.4) | 2.4 (1.8–7.9) | 12.4 (6.8–17.2) | 3.2 (2.2–19.9) | 6.9 (3.2–11.8) | .065 |
At last follow-up, 48 patients were dead, 6 patients were alive, and 4 patients were censored. The mOS was 7.4 months (95% CI 6.1–11.9) and median follow-up time was 6.9 months (Figures 1 and 2). Median OS was shortest for patients treated with immunotherapy at 2.4 months (95% CI, 1.8-NR) (Table 3). The overall 1-year survival probability was 31.9% (95% CI, 21.2–47.9). Median OS was 10.0 months (95% CI 7.3–15.1) for patients with de novo SCPC and 5.8 months (95% CI, 2.4–15.0) for patients with T-NEPC with a HR 5.6 (1.1–27.6, P=.034).
Figure 1.
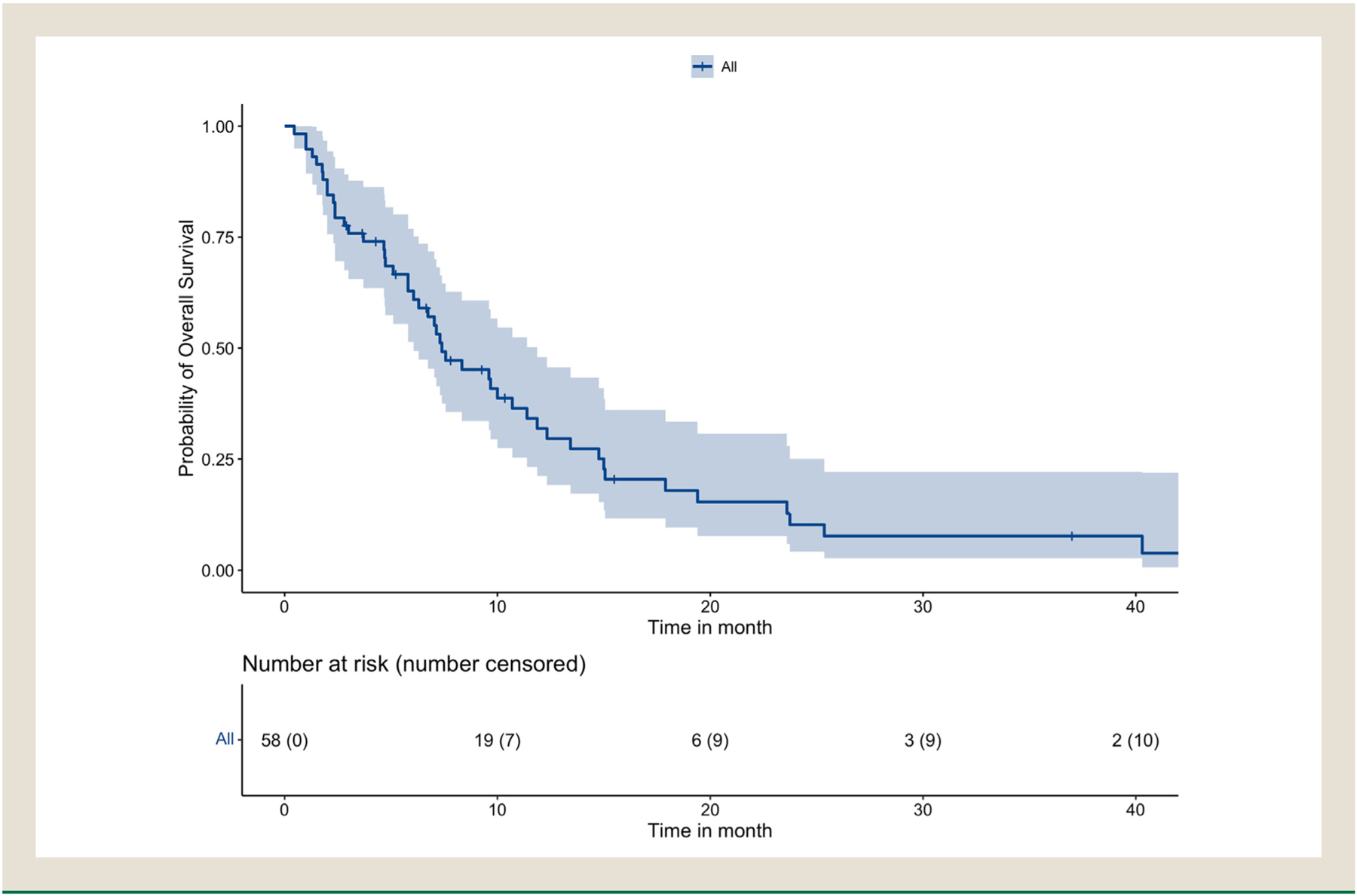
Kaplan-Meier survival curve of overall patient cohort.
Figure 2.
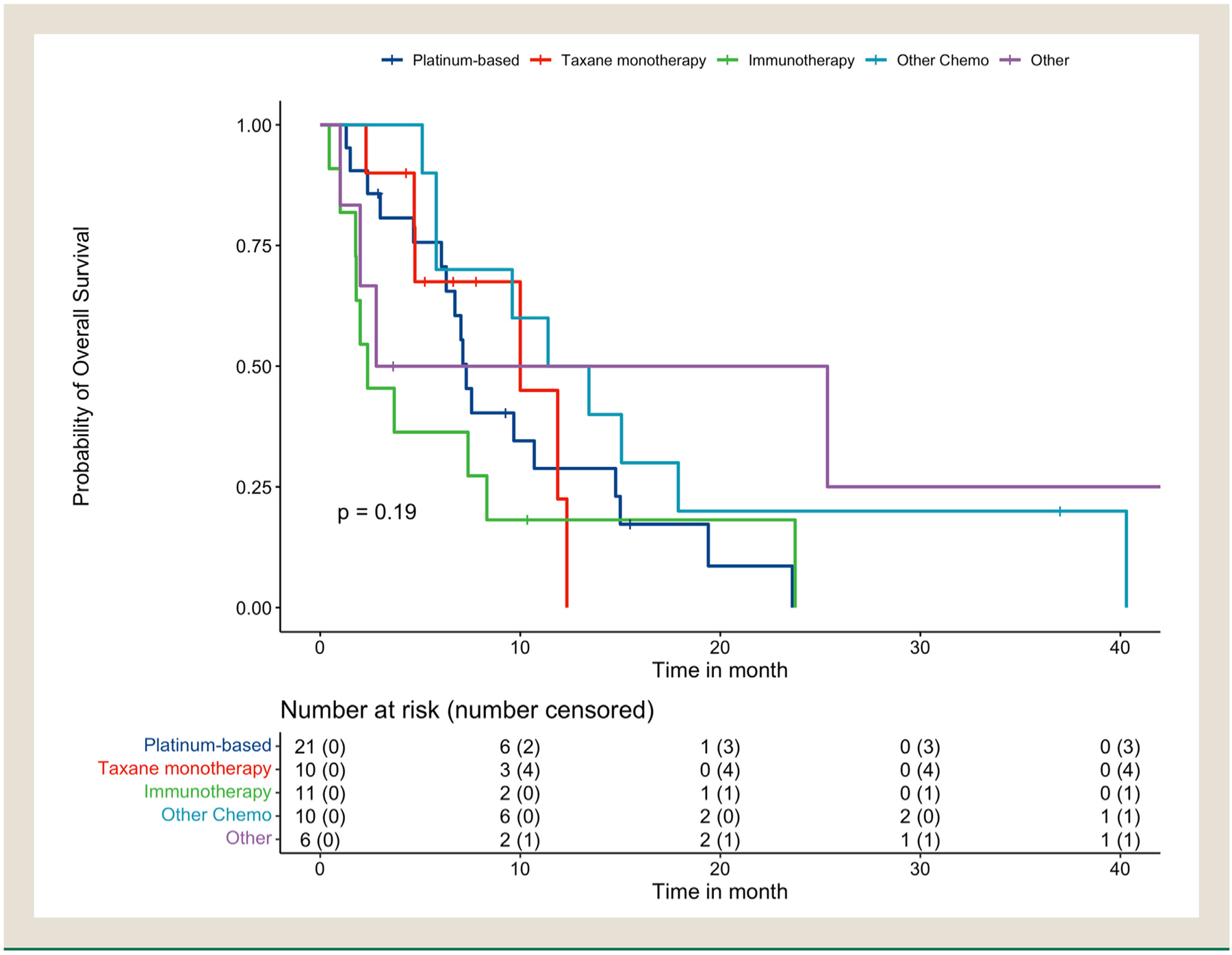
Kaplan-Meier survival curves of overall cohort stratified by second-line therapy.
Table 3.
Median overall survival and 1-year survival by second-line therapy.
| Overall Survival | One-year Survival | |||||||
|---|---|---|---|---|---|---|---|---|
| N | Events | Months | 95% CI | N at Risk | Events | Percent (%) | 95% CI | |
| Platinum-based | 21 | 18 | 7.3 | 6.3–15.0 | 5 | 14 | 28.8 | 14.2–58.6 |
| Taxane monotherapy | 10 | 6 | 10.0 | 4.7-NR | 1 | 5 | 22.5 | 4.3–100.0 |
| Immunotherapy | 11 | 10 | 2.4 | 1.8-NR | 1 | 9 | 18.2 | 5.2–63.7 |
| Other chemo | 10 | 9 | 12.4 | 5.8-NR | 5 | 5 | 50.0 | 26.9–92.9 |
| Other | 6 | 5 | 14.1 | 2-NR | 2 | 3 | 50.0 | 22.5–100.0 |
The log-rank test suggests that the overall survival is not significantly different by second-line therapy (Chi-squared statistic = 6.11, df = 4, P-value =.191). CI = confidence interval, NR = not reached.
Discussion
Using clinical and treatment data from 8 North American cancer centers, this study examines outcomes of second-line therapy in one of the largest cohorts of patients with histologically defined NEPC.15 Median OS for patients with NEPC remained poor at 7.39 months with heterogenous second-line treatment choices by clinicians and no clear benefit for 1 option over another. Consistent with survey data from the APCCC 2017, platinum chemotherapy plus etoposide or a taxane (docetaxel or cabazitaxel) were commonly used second-line treatments by oncologists.14
While prior studies have shown platinum-based chemotherapy and docetaxel to be modestly effective treatments for NEPC, there is a dearth of information related to the comparative effectiveness of treatment regimens, particularly in the second line. Taxane chemotherapy is a standard of care treatment for mCRPC, and docetaxel has activity in SCLC.16, 17 The addition to carboplatin plus docetaxel has been shown to improve clinical efficacy in treating mCRPC.18 The use of platinum-based chemotherapy in NEPC is based on data from early phase clinical trials with varied definitions of the disease. In a phase II trial of 40 patients with mCRPC with neuroendocrine features, carboplatin plus etoposide was given as a second-line therapy after docetaxel.19 The median PFS was 2.1 months, and median OS was 19 months. The phase II French Genito-Urinary Tumor Group (GETUG) P01 trial also studied the carboplatin plus etoposide combination in 60 patients with mCRPC with visceral metastasis or elevated neuron-specific enolase (NSE) and/or chromogranin A.20 Median PFS was 2.9 months, and median OS was 9.6 months. Another phase II trial evaluated the combination of carboplatin and docetaxel for 4 cycles, followed by salvage cisplatin plus etoposide, in 120 mCRPC patients with clinically aggressive variant prostate cancer.21 Median PFS was 2.9 months, and median OS was 9.6 months. Overall survival rates from these studies were similar to ours, and the mPFS rates were comparable to the median TOT of 2.0 months (IQR 1.0–3.9) in our cohort.
In our cohort, re-treatment with platinum-based chemotherapy yielded the highest ORR of 50.0%. Taxane monotherapy had no objective responses in the second line yet had a slightly higher but not significant mOS of 10.0 months compared to a mOS of 7.3 months with platinum-based chemotherapy. Immunotherapy as second-line treatment had the lowest mOS of 2.37 months. Previous sensitivity to a platinum or taxane chemotherapy in mCRPC or the first line likely would promote retreatment with these agents. However, only 2 patients who received a second-line taxane monotherapy had been treated with a taxane prior to the development of T-NEPC. Additionally, the ORR to first-line platinum-based chemotherapy was 66.7% in patients retreated with a platinum regimen in the second line. This is similar to the ORR of 65.5% to first-line platinum-based chemotherapy in the overall cohort. Nonetheless, patients may not be candidates for second-line platinum chemotherapy and taxanes given the risk of cumulative and overlapping toxicities. In the first line, patients with de novo NEPC or T-NEPC were unlikely to receive immunotherapy in addition to platinum-based chemotherapy. However, the improvement in OS and PFS with the addition of atezolizumab to carboplatin plus etoposide in extensive-stage SCLC was more recently described and is not included in the current NCCN Guidelines for prostate cancer.13, 22 Combination immunotherapy has previously been shown to have activity in NEPC and is an area of ongoing study.23
Between the de novo NEPC and T-NEPC cohorts, more patients with T-NEPC underwent prior locoregional treatment to the prostate. By definition, T-NEPC arises during treatment of prostate adenocarcinoma, which can include localized or low-volume disease. Despite an increased frequency of mixed histology with an adenocarcinoma component, patients with de novo NEPC received ADT less often than those with T-NEPC. The role of ADT in de novo NEPC has never been tested. The frequency of mixed histology, however, presents the rationale that components of AR driven disease may exist such that the addition of ADT could confer benefit to patients with a minimal increase in toxicity. Meanwhile, patients with T-NEPC have already received treatment for mHSPC and mCRPC, which includes ADT. The longer mOS of de novo NEPC on second-line therapy may in part be due to the longer treatment history of T-NEPC with its cumulative toxicity.
Limitations
As a retrospective study of rare disease subtypes, this study is limited by a small sample size, no central pathology review, lack of molecular biomarkers, and by confounding due to unquantified variables. Lack of clinical trials and clear treatment guidelines in this space translate into a heterogenous group of systemic treatments that were categorized in broad subgroups. Disease response or progression was not confirmed via central review, relying instead on physician interpretation locally. Patients who did not receive second-line therapy were excluded, potentially biasing results in favor of more chemotherapy-sensitive disease in a clinically robust patient population. Nevertheless, this study provides valuable insight into the real-world decisions of clinicians as they navigate treatment in the absence of large, randomized controlled trials.
Conclusions
In this multi-institutional, retrospective study, the various second-line therapies used by clinicians to treat patients with de novo NEPC or T-NEPC were unified in their limited benefit. NEPC-specific clinical trials are ongoing to evaluate second- and subsequent-line treatment options for de novo NEPC and T-NEPC after platinum-based chemotherapy, including various immune checkpoint inhibitors as well as antibody-drug conjugates, novel enzymatic pathway inhibitors, and conventional chemotherapy.1 As the understanding of NEPC continues to evolve, improved diagnostic strategies and a clearer definition of the disease would allow more patients with NEPC to be identified and, in turn, promote drug and clinical trial development for this disease. This study illustrates the need for further clinical trials of novel agents and novel combinations in the second-line as current practices are disparate and the prognosis for de novo NEPC and T-NEPC remains very poor.
Clinical Practice Points.
Neuroendocrine prostate cancer (NEPC) is a rare and aggressive variant of prostate cancer, which can present as de novo disease or more commonly during treatment for castrate-resistant prostate cancer.
After first-line platinum-based chemotherapy for metastatic NEPC, there is limited data and no consensus for second-line treatment.
In this retrospective, multi-institutional study, the clinical characteristics, treatment data, and outcomes were collected for 58 patients with NEPC who received second-line systemic therapy after first-line platinum chemotherapy.
Second-line treatments were heterogenous but uniformly poor in improving overall survival.
Platinum chemotherapy rechallenge had the highest overall response rate of 50.0%.
Median overall survival for patients with NEPC was poor at 7.4 months (95% CI, 6.1–11.9) from start of second-line therapy.
These findings demonstrate the ongoing lack of consensus for NEPC treatment in the second line and illustrate the need for further clinical trials of novel agents and novel combinations.
Footnotes
Disclosure
The authors have stated that they have no conflicts of interest.
References
- 1.Spetsieris N, Boukovala M, Patsakis G, Alafis I, Efstathiou E. Neuroendocrine and aggressive-variant prostate cancer. Cancers (Basel). 2020;12:3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aggarwal R, Zhang T, Small EJ, Armstrong AJ. Neuroendocrine prostate cancer: subtypes, biology, and clinical outcomes. J Natl Compr Canc Netw. 2014;12:719–726. [DOI] [PubMed] [Google Scholar]
- 3.Conteduca V, Oromendia C, Eng KW, et al. Clinical features of neuroendocrine prostate cancer. Eur J Cancer. 2019;121:7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epstein JI, Amin MB, Beltran H, et al. Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. Am J Surg Pathol. 2014;38:756–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beltran H, Tomlins S, Aparicio A, et al. Aggressive variants of castration-resistant prostate cancer. Clin Cancer Res. 2014;20:2846–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng Q, Butler W, Zhou Y, et al. Pre-existing castration-resistant prostate cancer–like cells in primary prostate cancer promote resistance to hormonal therapy. Eur Urol. 2022;81:446–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brady NJ, Bagadion AM, Singh R, et al. Temporal evolution of cellular heterogeneity during the progression to advanced AR-negative prostate cancer. Nat Commun. 2021;12:3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deorah S, Rao MB, Raman R, Gaitonde K, Donovan JF. Survival of patients with small cell carcinoma of the prostate during 1973–2003: a population-based study. BJU Int. 2012;109:824–830. [DOI] [PubMed] [Google Scholar]
- 9.Mucci NR, Akdas G, Manely S, Rubin MA. Neuroendocrine expression in metastatic prostate cancer: evaluation of high throughput tissue microarrays to detect heterogeneous protein expression. Hum Pathol. 2000;31:406–414. [DOI] [PubMed] [Google Scholar]
- 10.Aggarwal R, Huang J, Alumkal JJ, et al. Clinical and genomic characterization of treatment-emergent small-cell neuroendocrine prostate cancer: a multi-institutional prospective study. J Clin Oncol. 2018;36:2492–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonkhoff H Neuroendocrine differentiation in human prostate cancer. Morpho-genesis, proliferation and androgen receptor status. Ann Oncol. 2001;12(suppl 2):S141–S144. [DOI] [PubMed] [Google Scholar]
- 12.Beltran H, Hruszkewycz A, Scher HI, et al. The role of lineage plasticity in prostate cancer therapy resistance. Clin Cancer Res. 2019;25:6916–6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Comprehensive Cancer Network. Prostate Cancer (Version 4.2022).
- 14.Gillessen S, Attard G, Beer TM, et al. Management of patients with advanced prostate cancer: the report of the advanced prostate cancer consensus conference APCCC 2017. Eur Urol. 2018;73:178–211. [DOI] [PubMed] [Google Scholar]
- 15.Nadal R, Schweizer M, Kryvenko ON, Epstein JI, Eisenberger MA. Small cell carcinoma of the prostate. Nat Rev Urol. 2014;11:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smyth JF, Smith IE, Sessa C, et al. Activity of docetaxel (Taxotere) in small cell lung cancer. The early clinical trials group of the EORTC. Eur J Cancer. 1994;30A:1058–1060. [DOI] [PubMed] [Google Scholar]
- 17.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. [DOI] [PubMed] [Google Scholar]
- 18.Corn PG, Heath EI, Zurita A, et al. Cabazitaxel plus carboplatin for the treatment of men with metastatic castration-resistant prostate cancers: a randomised, open-label, phase 1–2 trial. Lancet Oncol. 2019;20:1432–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loriot Y, Massard C, Gross-Goupil M, et al. Combining carboplatin and etoposide in docetaxel-pretreated patients with castration-resistant prostate cancer: a prospective study evaluating also neuroendocrine features. Ann Oncol. 2009;20:703–708. [DOI] [PubMed] [Google Scholar]
- 20.Flechon A, Pouessel D, Ferlay C, et al. Phase II study of carboplatin and etoposide in patients with anaplastic progressive metastatic castration-resistant prostate cancer (mCRPC) with or without neuroendocrine differentiation: results of the French Genito-Urinary Tumor Group (GETUG) P01 trial. Ann Oncol. 2011;22:2476–2481. [DOI] [PubMed] [Google Scholar]
- 21.Aparicio AM, Harzstark AL, Corn PG, et al. Platinum-based chemotherapy for variant castrate-resistant prostate cancer. Clin Cancer Res. 2013;19:3621–3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horn L, Mansfield AS, Szczesna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379:2220–2229. [DOI] [PubMed] [Google Scholar]
- 23.McGregor BA, Campbell MT, Xie W, et al. Results of a multicenter, phase 2 study of nivolumab and ipilimumab for patients with advanced rare genitourinary malignancies. Cancer. 2021;127:840–849. [DOI] [PubMed] [Google Scholar]


