Abstract
Objective
To conduct a systematic review and meta-analysis evaluating the diagnostic value of folate receptor-positive (FR+) circulating tumour cells (CTCs) as a potential tumour marker for lung cancer diagnosis.
Methods
The PubMed, Embase, and Web of Science databases were searched for relevant articles published between database inception and November 2022. Eligible studies were selected based on inclusion and exclusion criteria. Sensitivity, specificity, positive and negative likelihood ratios, diagnostic odds ratio, and area under the curve (AUC) were pooled with 95% confidence intervals (CI), using RevMan 5.4 and STATA 17.0 software to assess the diagnostic value of FR+CTC for lung cancer.
Results
After screening, 11 studies involving 3469 subjects were eligible for inclusion. The pooled sensitivity and specificity were 0.79 (95% CI 0.76, 0.82) and 0.84 (95% CI 0.81, 0.96), respectively, and the pooled positive and negative likelihood ratios were 4.90 (95% CI 4.25, 5.65) and 0.25 (95% CI 0.22, 0.29), respectively. The pooled diagnostic odds ratio was 19.70 (95% CI 16.06, 24.16). The AUC of the pooled summary receiver operating characteristic curve was 0.89 (95% CI 0.85, 0.91). Sensitivity analysis showed that this result was stable after one-by-one study elimination.
Conclusion
Folate receptor-positive CTCs may have good diagnostic value in lung cancer.
Keywords: Lung cancer, biomarker, folate receptor, circulating tumour cell, FR+CTC, diagnostic marker
Introduction
Lung cancer is one of the most common types of malignant tumour, with the highest incidence worldwide. 1 Many patients are diagnosed at an advanced stage, 2 leading to poor prognosis and a high mortality rate. With improvements in technology, attention is focused on the early diagnosis of lung cancer. However, some patients are still misdiagnosed with lung cancer and undergo non-essential surgery, which increases the medical burden of the population.
Current techniques used for lung cancer diagnosis include imaging techniques, such as X-ray and computed tomography, and non-imaging techniques, such as pathologic evaluation via biopsy and tumour markers. 3 Disadvantages associated with imaging techniques include high false-positive rates and radiation exposure. 4 Pathological biopsy depends on the nodule's location and size, and the pathologist's subjective experience may influence the results. In addition, tumour markers show different diagnostic efficacy depending on their specificity, and need to be combined with other methods to produce convincing results. Due to the current situation, there is an urgent need to identify new biomarkers to find effective methods for early diagnosis.
A meta-analysis investigating the role of circulating tumour cells (CTCs) in lung cancer diagnosis included one abstract and four complete studies that analysed the diagnostic accuracy of ligand-targeted (LT)-polymerase chain reaction (PCR) for detecting folate receptor-positive (FR+) CTCs in lung cancer. 5 The pooled sensitivity and specificity were 0.77 (95% confidence interval [CI] 0.75, 0.79) and 0.87 (95% CI 0.85, 0.89), respectively, with a summary receiver operating characteristic (SROC) area under the curve (AUC) of 0.84. The results suggest that CTCs have a high clinical utility in diagnosing lung cancer and may be a potential biomarker to differentiate lung cancer from controls. Therefore, the aim of the present meta-analysis was to assess the diagnostic value of FR+CTCs in lung cancer.
Materials and methods
The authors applied to register the study with PROSPERO in October 2022, but received a message after 30 days stating that there is a substantial delay due to submission backlogs, and all except COVID-19 articles are being processed automatically if they have been waiting beyond 30 days (with various automatic rejection criteria). No further correspondence was received.
Data sources and search strategy
Two researchers (FC and YN) independently searched the PubMed, Embase, and Web of Science online databases for relevant studies published between the database creation and 1 November 2022. A systematic search was conducted using the main search terms “lung cancer,” “folate receptor,” and “circulating tumour cells” and the combined key term “lung cancer.” Because of the limited search results, terms such as “diagnosis” and “markers” were not used.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) study participants were patients with lung cancer diagnosed by pathology; (2) detection of FR + CTCs in the participants' blood; and (3) the ability to present a complete list of true-positive, true-negative, false-positive, and false-negative sample sizes from the study, or to calculate these values using the sample size combined with sensitivity, specificity, and area under the ROC curve (AUC).
The exclusion criteria were: (1) duplicate studies; (2) reviews, abstracts, case reports, editorials, letters, or editorial articles; (3) not a study of lung cancer with FR + CTC; (4) not a diagnostic study; (5) not human study subject; (6) data incomplete or calculated to be inconsistent with the original article; and/or (7) study not published in Chinese or English language.
Quality assessment
The quality of included studies was assessed by two independent researchers (FC and YN) using the Quality Assessment of Diagnostic Accuracy Study-2 (QUADAS-2) diagnostic criteria scale (https://www.bristol.ac.uk/population-health-sciences/projects/quadas/quadas-2). Another researcher (JZ) resolved any conflicting findings. The scale assesses the quality of included studies in four domains: patient selection, index testing, reference standards, and flow and timing, and lists 14 specific items that are evaluated with ‘Yes’, ‘No’, or ‘Unclear’ for assessment.
Data extraction
Data extracted from each article included the following: the first author's name, year of publication, sample size, the mean age of the sample, sex of the sample, and the threshold value of CTC in each study. Moreover, the true positive, true negative, false positive, and false negative sample sizes in each study were extracted directly or calculated using the exact sample size combined with sensitivity, specificity, and AUC values from the ROC curve.
Statistical analysis
A bivariate mixed-effects binary regression modelling framework was used to generate pooled sensitivities, combined specificities, positive likelihood ratios (PLR), negative likelihood ratios (NLR), diagnostic odds ratios (DOR) and corresponding 95% confidence intervals (CIs), and the confidence and prediction contours in SROC curves.
In addition, Fagan's line plot was used to analyse the clinical value of FR+CTC in diagnosing lung cancer. Cochran's Q and χ2-test were used for heterogeneity assessment, Spearman’s correlation coefficient was used to test for heterogeneity caused by threshold effects, and I2 > 50% was defined as greater heterogeneity. P-values < 0.05 were considered statistically significant. The source of heterogeneity was determined by meta-regression and subgroup analysis. In addition, sensitivity analysis was conducted to exclude each included study to determine whether the final results were stable. Finally, publication bias in the enrolled studies was evaluated by Deek’s funnel plot asymmetry test. Data were analysed with Stata software, version 17.0 (Stata Corporation, College Station, TX, USA); RevMan, version 5.4.1 (Cochrane Collaboration Network, 2020, Copenhagen, Denmark); and MetaDiSc, version 1.4 (Clinical Biostatistics Team, The Ramón y Cajal Hospital, Madrid, Spain) for meta-analysis.
Results
Literature search
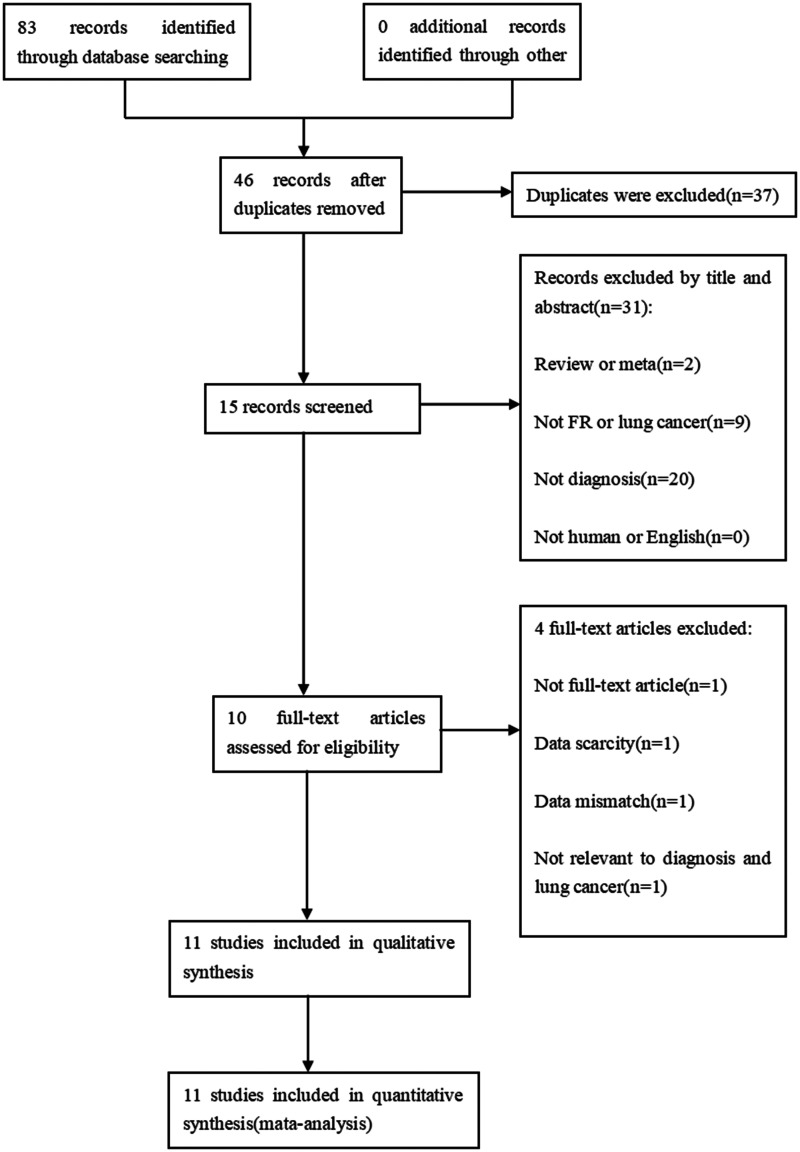
A systematic search of the above databases resulted in the identification of 83 records relevant to the purpose of the study. Following removal of 37 duplicate entries, the titles and abstracts were screened according to the inclusion and exclusion criteria, resulting in a further 31 articles being excluded. The remaining 15 articles were downloaded for full-text review, resulting in the exclusion of four further articles due to the unavailability of diagnosis-related data or articles with abstracts only. This left a total of 10 articles included in the study (Figure 1).6–15 One of the articles (Chen et al., 2015), 6 included two studies (one with a benign lung disease control group and one with a control group comprising healthy individuals plus patients with benign lung disease), so a total of 11 studies were included.
Figure 1.
Flow chart of the study selection process.
To note, the age range of the included study subjects was approximately 45–65 years, so another study, 16 with a population older than 80 years (sample size of 63), was excluded. Moreover, the control group in this excluded study was normal, 16 which was inconsistent with the studies included in the present meta-analysis that comprised control groups with either benign lung disease or a combination of healthy individuals and those with benign disease–taking into account the clinical reality. The study by Li et al. 16 differs from all the included studies in two ways, which may improve the true positive and reduce the true negative. After reading the complete text, the two outcomes (true positive and true negative) were found to be 0.957 and 0.65. So, based on these reasons, the study was finally excluded.
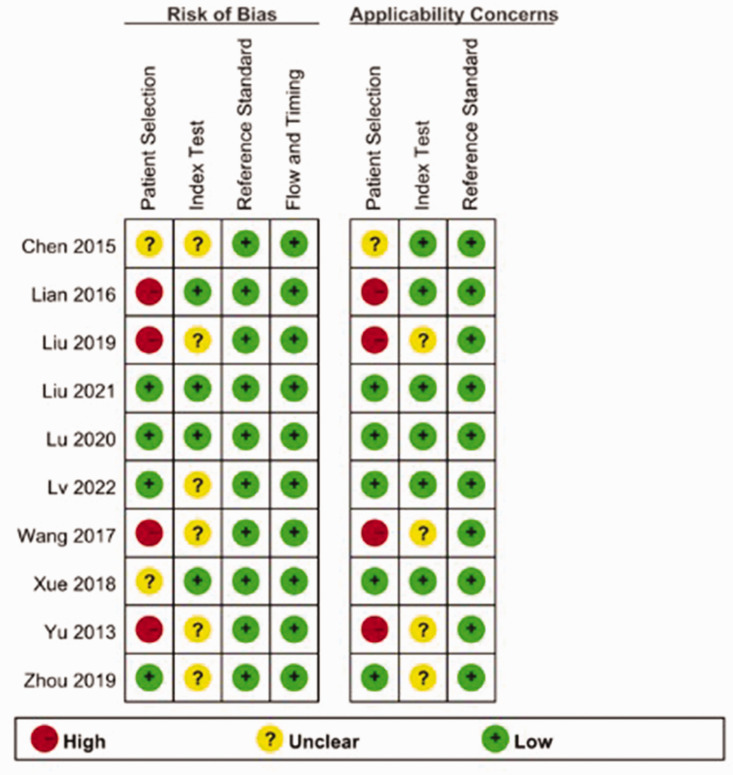
Results of quality analysis of the included studies are shown in Figure 2. The 11 included studies all comprised Asian participants from China, and were published between 2013 and 2022. A total of 3469 subjects, including 2197 cases and 1272 controls, were included in the present meta-analysis. All included studies detected FR + CTC expression by PCR using the CytoploRare reagent (GenoSaber, Nantong, China). Related data extracted from the primary studies, including true positive (TP), false positive (FP), false negative (FN), and true negative (TN) results, were extracted and are summarised in Table 1.6–15
Figure 2.
Quality assessment of 10 articles comprising 11 studies of folate receptor-positive circulating tumour cells in the diagnosis of lung cancer, assessed using Quality Assessment of Diagnostic Accuracy Study-2.
Table 1.
Characteristics of 11 studies of folate receptor-positive circulating tumour cells in the diagnosis of lung cancer, included in the meta-analysis.
| Author | Year | Country | Ethnicity | Age (range), years |
Patients/Controls | Control group | Method | Cut-off (U/ml) | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls | Patients | ||||||||||
| Chen1 6 | 2015 | China | Asian | 50 (15–85) | 59 (23–83) | 473/283 | Healthy/BLD | PCR | 8.93 | 74.4 | 86.6 |
| Chen2 6 | 2015 | China | Asian | 49 (15–85) | 59 (23–83) | 473/227 | BLD | PCR | 8.93 | 74.2 | 85 |
| Lian 7 | 2016 | China | Asian | 51 (37–66) | 61 (38–72) | 97/18 | BLD | PCR | 8.7 | 82.5 | 72.2 |
| Liu 8 | 2019 | China | Asian | 61 (34–80) | 47 (22–77) | 75/71 | Healthy/BLD | PCR | 7.9 | 78.7 | 81.7 |
| Liu 9 | 2021 | China | Asian | NA | 58 (46–70) | 184/39 | BLD | PCR | 8.7 | 89.1 | 92.3 |
| Lu 10 | 2020 | China | Asian | 56 (37–68) | 56 (42–77) | 22/15 | BLD | PCR | 8.7 | 72.7 | 93.3 |
| Lv 11 | 2022 | China | Asian | 49 (35–63) | 61 (50–71) | 282/256 | BLD | PCR | 8.9 | 81.9 | 80.9 |
| Wang 12 | 2017 | China | Asian | 44 (19–77) | 60 (21–79) | 197/171 | Healthy/BLD | PCR | 8.7 | 77.7 | 89.5 |
| Xue 13 | 2018 | China | Asian | 52 (27–65) | 61 (43–88) | 72/26 | Healthy/BLD | PCR | 8.7 | 81.94 | 73.08 |
| Yu 14 | 2013 | China | Asian | 45 (23–77) | 59 (25–85) | 153/113 | Healthy/BLD | PCR | 8.64 | 73.2 | 84.1 |
| Zhou 15 | 2019 | China | Asian | NA | NA | 169/53 | BLD | PCR | 8.3 | 79.88 | 75.47 |
Data presented as mean (range) or number of patients.
BLD, benign lung disease; PCR, polymerase chain reaction; NA, not available.
Meta-analysis
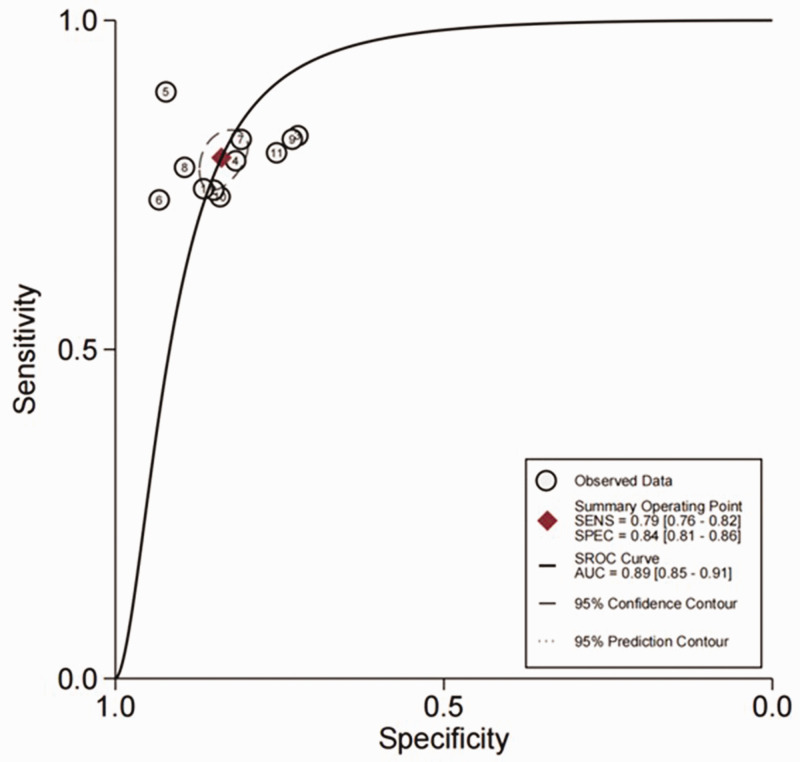
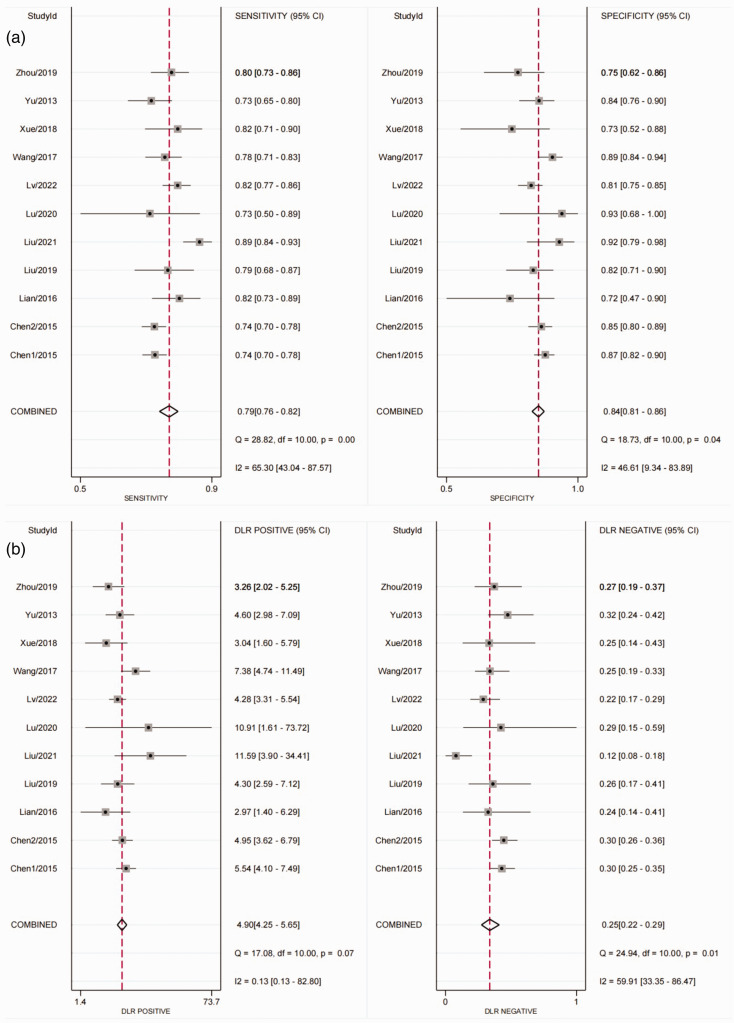
The AUC of the SROC for FR + CTC (Figure 3) was 0.89 (95% CI 0.85, 0.91). The pooled results for the diagnostic performance of FR + CTC in lung cancer detection revealed a sensitivity of 0.79 (95% CI 0.76, 0.82) and specificity of 0.84 (95% CI 0.81, 0.96), summarised in Figure 4a, and the following PLR and NLR combinations: 4.90 (95% CI 4.25, 5.65) and 0.25 (95% CI 0.22, 0.29), respectively (Figure 4b). The pooled diagnostic odds ratio was 19.70 (95% CI 16.06, 24.16).
Figure 3.
Summary receiver operating characteristic curve (SROC) for 11 studies of folate receptor-positive circulating tumour cells in the diagnosis of lung cancer. SENS, sensitivity; SPEC, specificity; AUC, area under the curve.
Figure 4.
Forest plots showing analysis of: (a) sensitivity and specificity for folate receptor-positive circulating tumour cells (FR + CTC) in the diagnosis of lung cancer; and (b) positive likelihood ratios and negative likelihood ratios for FR + CTC in the diagnosis of lung cancer. DLR, diagnostic likelihood ratio.
Subgroup analysis
Sources of heterogeneity were investigated by analysis of subgroups (Figure 5a–5d) stratified according to the following features: sample size (≥ 200 versus < 200); period of publication (during or before 2018 versus after 2018); type of control group (benign disease versus benign disease and normal), threshold settings (set in advance according to the manufacturer's recommended value versus the optimal solution based on the subject's ROC curve). The results showed that the heterogeneity was not due to the above reasons (all P > 0.05 and all I2 < 25%).
Figure 5.
Forrest plots showing the analysis of folate receptor-positive circulating tumour cells (FR + CTC) in the diagnosis of lung cancer in studies grouped according to: (a) sample size (≥ 200 versus < 200); (b) period of publication (during or before 2018 versus after 2018); (c) type of control group (healthy and benign lung disease [BLD] versus BLD alone); and (d) threshold settings (receiver operating characteristic [ROC] curve versus the manual [test-manufacturer’s recommended threshold value]).
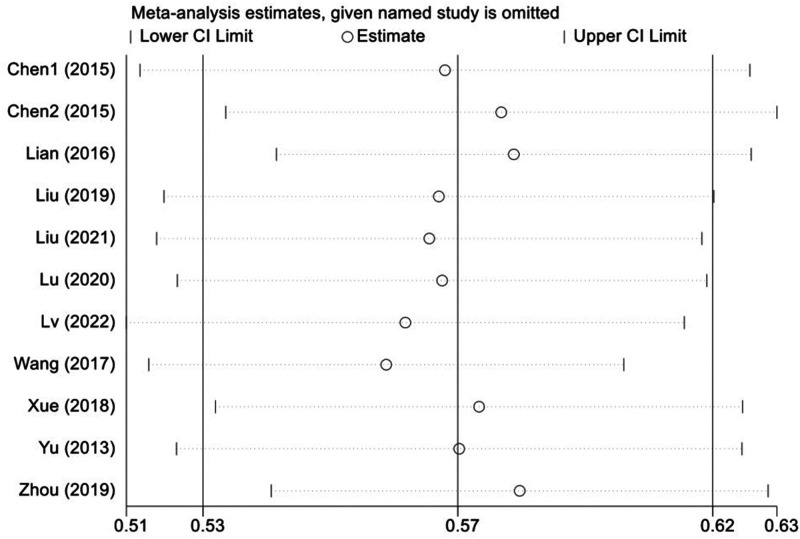
Sensitivity analysis
Sensitivity analysis was performed to identify whether the results of the meta-analysis were reliable. The results were shown to be stable after one-by-one exclusion of the included studies, indicating that the pooled results were reliable (Figure 6).
Figure 6.
Plot showing results of sensitivity analysis via one-by-one exclusion of 11 studies of folate receptor-positive circulating tumour cells in the diagnosis of lung cancer.
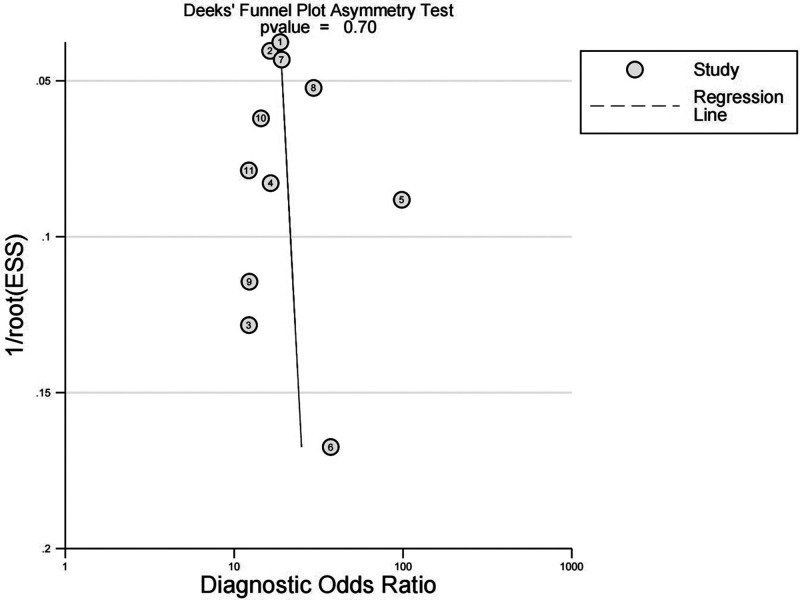
Publication bias
Publication bias was assessed by Deek’s funnel plot asymmetry test. The funnel plot did not show asymmetry, and the slope value was not statistically significant, indicating no evidence of publication bias (Figure 7).
Figure 7.
Deek’s Funnel plot showing that there was no publication bias in the 11 studies of folate receptor-positive circulating tumour cells in the diagnosis of lung cancer.
Discussion
Many studies have suggested that CTCs may be used in the diagnosis of solid tumours, such as lung cancer and breast cancer, under the ‘liquid biopsy’ approach.17,18 In addition, CTC results may easily be noninvasively obtained, making it more acceptable to patients than pathological diagnosis, the gold standard for lung cancer diagnosis. 19 The CTC test is reproducible, may dynamically monitor changes, and, more importantly, CTCs can be detected in blood samples from most patients with various solid tumours but rarely in samples from healthy individuals.20,21 In contrast, the FR may be expressed in non-blood-derived circulating cells, which provides a theoretical basis for using FR + CTC for lung cancer diagnosis. Several works have explored the role of FR + CTC in lung cancer, which may be broad: for example, FR + CTC may be used for diagnosis of lung cancer, prediction of prognosis after surgery, prediction of prognosis after drug therapy, and target of drug therapy.15,22–24
The overall accuracy of FR + CTCs in lung cancer diagnosis may be highly heterogeneous. Therefore, sources of heterogeneity were further assessed in the present study by conducting subgroup and sensitivity analyses for various parameters, such as year of publication, sample size, and type of control group. Since different thresholds were used in the included studies, Spearman's correlation coefficient was used to analyse the threshold effect. The result showed that heterogeneity did not show a threshold effect, suggesting that heterogeneity may be caused by other factors. Sensitivity analyses showed that the results were stable after one-by-one exclusion of studies, indicating that the present pooled results were reliable.
The results of the present meta-analysis may be limited by several factors. First, because of the small number of included studies and the absence of sensitivity and specificity results for tumour stage, pathological tumour type, and age classification of subjects in some of the studies, no subgroup analysis of these aspects was performed. Secondly, all included studies were Chinese, so the present findings may not be generalizable. Thirdly, this meta-analysis was based on published studies, and the unavailability of non-published data is usually associated with an overestimation of the actual effect, which may lead to publication bias. Finally, due to the limited data, the primary source of heterogeneity remains to be identified.
Conclusions
The results of this meta-analysis suggest that FR + CTCs may have a high diagnostic value for lung cancer. Future large-scale prospective studies are required to validate and assess the present findings in terms of their clinical value.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605231199763 for The value of folate receptor-positive circulating tumour cells as a diagnostic biomarker for lung cancer: a systematic review and meta-analysis by Fangjun Chen, Yihong Ni, Jin Zhang, Yang Hao, Zhoujunyi Tian and Guangliang Qiang in Journal of International Medical Research
Footnotes
The authors declare that there is no conflict of interest.
Funding: This study was supported by the Clinical Key Project of Peking University Third Hospital (BYSYRCYJ2023001).
ORCID iDs: Fangjun Chen https://orcid.org/0000-0003-3509-6308
Guangliang Qiang https://orcid.org/0000-0002-7809-1892
References
- 1.Thai AA, Solomon BJ, Sequist LVet al. Lung cancer. Lancet 2021; 398: 535–554. [DOI] [PubMed] [Google Scholar]
- 2.Li MY, Liu LZ, Dong M. Progress on pivotal role and application of exosome in lung cancer carcinogenesis, diagnosis, therapy and prognosis. Mol Cancer 2021; 20: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nooreldeen R, Bach H. Current and future development in lung cancer diagnosis. Int J Mol Sci 2021; 22: 8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li W, Liu JB, Hou LKet al. Liquid biopsy in lung cancer: significance in diagnostics, prediction, and treatment monitoring. Mol Cancer 2022; 21: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Illahi Y, Siddiqui N, Nadeem M. WITHDRAWN: Diagnostic accuracy of folate receptor-positive circulating tumor cells detected by ligand-targeted polymerase chain reaction in patients with non-small-cell lung cancer: A meta-analysis. Hematol Oncol Stem Cell Ther 2020; S1658-3876: 30035–30032. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Zhou F, Li Xet al. Folate receptor-positive circulating tumor cell detected by LT-PCR-based method as a diagnostic biomarker for non-small-cell lung cancer. J Thorac Oncol 2015; 10: 1163–1171. [DOI] [PubMed] [Google Scholar]
- 7.Lian H, Ding Z, Yuan Det al. Diagnostic value of folate receptor-positive circulating tumor cell in lung cancer: a pilot study. Zhongguo Fei Ai Za Zhi 2016; 19: 813–820 [In Chinese, English abstract]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J, Han M, Huang H. Validation of the diagnostic efficiency of folate receptor-positive circulating tumor cells in lung cancers: a prospective observational study. Transl Cancer Res 2019; 8: 1242–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu D, Mao Y, Ma H. Value of circulating tumor cells in the diagnosis and treatment of solitary pulmonary nodules. Ann Transl Med 2021; 9: 501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu G, Wang R, Tian Xet al. Diagnostic value of folate receptor-positive circulating tumor cell detection in subcentimeter pulmonary nodules. Cancer Research and Clinic 2020; 32: 1–5. [Google Scholar]
- 11.Lv X, Wu S, Xu Xet al. The combination of folate receptor-positive circulating tumor cells and serum tumor markers suggests a histological diagnosis of lung cancer. J Thorac Dis 2022; 14: 158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Wu C, Qiao Let al. Clinical significance of folate receptor-positive circulating tumor cells detected by ligand-targeted polymerase chain reaction in lung cancer. J Cancer 2017; 8: 104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xue Y, Cong W, Xie Set al. Folate-receptor-positive circulating tumor cells as an efficacious biomarker for the diagnosis of small pulmonary nodules. J Cancer Res Ther 2018; 14: 1620–1626. [DOI] [PubMed] [Google Scholar]
- 14.Yu Y, Chen Z, Dong Jet al. Folate receptor-positive circulating tumor cells as a novel diagnostic biomarker in non-small cell lung cancer. Transl Oncol 2013; 6: 697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Q, Geng Q, Wang Let al. Value of folate receptor-positive circulating tumour cells in the clinical management of indeterminate lung nodules: a non-invasive biomarker for predicting malignancy and tumour invasiveness. EBioMedicine 2019; 41: 236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li N, Zhong D, Chen Het al. The utility of folate receptor-positive circulating tumor cell in cancer diagnosis in the elderly population. Cancer Manag Res 2019; 11: 4097–4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tellez-Gabriel M, Knutsen E, Perander M. Current status of circulating tumor cells, circulating tumor DNA, and exosomes in breast cancer liquid biopsies. Int J Mol Sci 2020; 21: 9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinzani P, D'Argenio V, Del Re Met al. Updates on liquid biopsy: current trends and future perspectives for clinical application in solid tumors. Clin Chem Lab Med 2021; 59: 1181–1200. [DOI] [PubMed] [Google Scholar]
- 19.Zhao Q, Yuan Z, Wang Het al. Role of circulating tumor cells in diagnosis of lung cancer: a systematic review and meta-analysis. J Int Med Res 2021; 49: 300060521994926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyu M, Zhou J, Ning Ket al. The diagnostic value of circulating tumor cells and ctDNA for gene mutations in lung cancer. Onco Targets Ther 2019; 12: 2539–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maly V, Maly O, Kolostova Ket al. Circulating tumor cells in diagnosis and treatment of lung cancer. In Vivo 2019; 33: 1027–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Li B, Pan Yet al. Preoperative folate receptor-positive circulating tumor cell level is a prognostic factor of long term outcome in non-small cell lung cancer patients. Front Oncol 2021; 10: 621435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tie Y, Zheng H, He Zet al. Targeting folate receptor β positive tumor-associated macrophages in lung cancer with a folate-modified liposomal complex. Signal Transduct Target Ther 2020; 5: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X, Zhou F, Li Xet al. Folate receptor-positive circulating tumor cells as a predictive biomarker for the efficacy of first-line pemetrexed-based chemotherapy in patients with non-squamous non-small cell lung cancer. Ann Transl Med 2020; 8: 631. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605231199763 for The value of folate receptor-positive circulating tumour cells as a diagnostic biomarker for lung cancer: a systematic review and meta-analysis by Fangjun Chen, Yihong Ni, Jin Zhang, Yang Hao, Zhoujunyi Tian and Guangliang Qiang in Journal of International Medical Research