Abstract
The COVID-19 pandemic has been met with an unprecedented response from the scientific community, leading to the development, investigation, and authorization of vaccines and antivirals, ultimately reducing the impact of SARS-CoV-2 on global public health. However, SARS-CoV-2 is far from being eradicated, continues to evolve, and causes substantial health and economic burdens. In this narrative review, we posit essential points on SARS-CoV-2 and its responsible management during the transition from the acute phase of the COVID-19 pandemic. As discussed, despite Omicron (sub)variant(s) causing clinically milder infections, SARS-CoV-2 is far from being a negligible pathogen. It requires continued genomic surveillance, particularly if one considers that its future (sub)lineages do not necessarily have to be milder. Antivirals and vaccines remain the essential elements in COVID-19 management. However, the former could benefit from further development and improvements in dosing, while the seasonal administration of the latter requires simplification to increase interest and tackle vaccine hesitancy. It is also essential to ensure the accessibility of COVID-19 pharmaceuticals and vaccines in low-income countries and improve the understanding of their use in the context of the long-term goals of SARS-CoV-2 management. Regardless of location, the primary role of COVID-19 awareness and education must be played by healthcare workers, who directly communicate with patients and serve as role models for healthy behaviors.
Keywords: SARS-CoV-2, vaccination, antivirals, viral evolution, infectious diseases
1. Introduction
The coronavirus disease (COVID-19), caused by SARS-CoV-2 and first reported by the Chinese authorities in late 2019, rapidly became an emerging, evolving situation, spreading inevitably to other Asian countries and continents. The World Health Organization (WHO) first declared a Public Health Emergency of International Concern (PHEIC) on 30 January 2020 and considered COVID-19 as a pandemic on 11 March 2020 [1,2,3]. On 5 May 2023, it was announced that COVID-19 no longer had PHEIC status [4]. Within the three years, three months, and five days that passed in between, over 765 million SARS-CoV-2 infections were confirmed, with nearly 7 million deaths due to COVID-19 [5]. However, the true toll of the pandemic is likely a few-fold higher due to underdiagnosis and underreporting [6]. In addition, a range of symptoms can persist or emerge following acute SARS-CoV-2 infection, a condition known as post-COVID-19 syndrome, post-acute sequelae of SARS-CoV-2, or long-COVID [7], which also causes a considerable burden if one considers that its global prevalence has been estimated at 43% in the general population [8] and 25% in children and adolescents [9], although its accurate estimations are challenging due to both under- and overdiagnosis [10].
The COVID-19 crisis has led to the implementation of a hygiene regime, face masking, pursuing diagnostic testing daily, and imposing temporary school closures and national lockdown measures. Therefore, it has also had a broad societal impact, exacerbated pre-existing deep-rooted structural inequalities, caused numerous changes in different strata of life, and resulted in economic losses [11,12,13,14,15,16].
The emergence of SARS-CoV-2 has also led to an unprecedented scientific response encompassing essential research on diagnostic methods and studies of COVID-19 immunology, viral pathogenicity, and potential therapeutic targets [17,18]. Various pharmaceuticals (e.g., arbidol hydroxychloroquine, darunavir, lopinavir, favipiravir, remdesivir, ribavirin, ritonavir, interferons, dexamethasone, and tocilizumab) have been repurposed for COVID-19 treatment, with mixed effectiveness results [19,20,21,22]. The use of convalescent plasma was eventually abandoned due to the lack of clinical benefits observed in severely ill patients [23,24], while the effectiveness of different monoclonal antibodies has been dramatically impacted by SARS-CoV-2’s evolution [25,26]. The development and authorization of the first-generation anti-SARS-CoV-2 oral drugs (nirmatrelvir/ritonavir and molnupiravir) brought hope in 2022 that they may represent a turning point due to the possibility of their use outside the clinical setting [27]. However, the relatively high price of these pharmaceuticals and interactions with other drugs have been limiting factors in their use [27].
In 2020, great efforts also focused on developing vaccines to circumvent the need for social distancing and personal protective equipment [28]. This eventually led to their authorization in late 2020/early 2021 and the massive global vaccination campaigns pursued amid an influx of misinformation, fake news, and anti-vaccine propaganda [29]. It is estimated that COVID-19 vaccines prevented 19.8 million deaths in 2021 alone [30]. When the WHO announced that COVID-19 was no longer a PHEIC in May 2023, over 5.5 billion individuals had received at least one vaccine dose. Despite the high effectiveness of vaccines against severe disease and death [31,32], it soon became evident that due to a gradual decrease in serum antibodies, vaccination did not offer long-term protection from SARS-CoV-2 infection, leading to the recommendation of subsequent booster doses. In addition, viral evolution has led to the emergence of lineages, such as Omicron and its descendants, characterized by an increased ability to escape humoral responses [33,34,35]. Although the primary goal of COVID-19 vaccination is to decrease the rates of hospitalization, admission to intensive care units, and deaths [36] and is often achievable due to the extended duration of vaccine-induced cellular immunity and its lower susceptibility to viral mutations [37,38,39], some individuals may still experience severe COVID-19 due to a worse response to immunization because of age-related immunosenescence, primary or secondary immune deficiencies, and various lifestyle factors [40,41,42,43].
All in all, SARS-CoV-2 is far from being eradicated in the near future. It remains, as also emphasized by the WHO, a global health threat [4]. According to the official data, nearly 11 thousand COVID-19 deaths, with 64% in high-income countries, were reported in May 2023 since the WHO rescinded the PHEIC status. It is most likely that COVID-19 will become endemic, meaning that it will remain consistently present at predictable spread and occurrence rates. In this context, endemic does not necessarily imply that infection rates are low or that the disease is mild. For example, malaria is regarded as endemic in selected world regions, with its incidence rate and mortality remaining relatively stable since 2015, with approximately 600,000 deaths annually [44]. The endemic phase of COVID-19 will also require an appropriate management strategy and preparedness to decrease the disease burden systematically and by no means should it be equated with safe infections [45].
Therefore, in this narrative review, we highlight the essential issues regarding the benefits and future of COVID-19 vaccination, SARS-CoV-2’s evolution and its impact on the clinical significance of the disease, and the continuous need to pursue various control measures when exiting the acute phase of the COVID-19 pandemic but still existing with the virus, which can have profound effects on public health. The topics discussed here are pivotal to limiting the overall burden of SARS-CoV-2 and decreasing the effect that its infection may have on the most vulnerable risk groups during long-term co-existence with the viral pathogen. This discussion is particularly pivotal in guiding health policymakers and authorities when the COVID-19 threat is no longer heavily covered by the media and may be perceived by the general public as less important.
2. SARS-CoV-2 Is Here to Stay and Will Continue to Evolve
The priority of vaccinology has always been to decrease the clinical severity of infection. Preventing infection (whether symptomatic or asymptomatic) has been a secondary goal. The eradication of the pathogen is the most challenging task. As of today, smallpox remains the only human disease successfully eradicated due to vaccination campaigns [46]. SARS-CoV-2 will continue circulating in the human population, primarily because of the short-lived immune response following natural infection and vaccination and due to viral evolution. SARS-CoV-2 belongs to the RNA viruses, which exhibit higher rates of spontaneous mutation than DNA viruses [47]. The primary mechanism behind this phenomenon lies in the lower replication fidelity of the polymerase enzyme, ultimately leading to point mutations. Frequently, they do not affect virus biology or are deleterious to its further replication. A small minority of such mutations will provide fitness advantages and impact different aspects of virus biology, e.g., pathogenicity, infectivity, transmissibility, and antigenicity. The SARS-CoV-2 mutation rate has been estimated at 1 × 10−6–2 × 10−6 mutations per nucleotide per replication cycle [45,48], which is lower than the rates of various other RNA viruses, such as influenza viruses (3 × 10−5), human immunodeficiency virus (10−4 to 10−5), and hepatitis C virus (3.5 × 10−5 to 1.2 × 10−4) [49,50,51]. This is because SARS-CoV-2’s polymerase, similar to that of other coronaviruses, utilizes a proofreading 3′-to-5′ exoribonuclease of the nonstructural protein 14, a mechanism ensuring higher fidelity of replication, which is not present in the majority of RNA viruses. Nevertheless, the accumulation of point mutations in SARS-CoV-2 gives rise to novel lineages and sublineages that are competitive regarding transmissibility. An example of such a mutation is D614G in the spike protein, which emerged in late January/early February 2020 and increased SARS-CoV-2’s infectivity and soon became widespread [52].
The other process that can drive SARS-CoV-2’s adaptation is recombination. It results from the co-infection of the host cell with two genetically distinct viruses that, when recombined, produce viable hybrid progeny [53]. The odds of this process playing a more profound role in SARS-CoV-2’s evolution have increased over time due to the emergence and subsequent co-circulation of genetically divergent viral (sub)lineages, a phenomenon particularly evident in the Omicron era [45]. An example of such a SARS-CoV-2 recombinant that gained global relevance is the XBB that emerged from the recombination of the BA.2.10.1 and BA.2.75 sublineages [54]. The further accumulation of point mutations within this recombinant lineage gave rise to XBB.1.5, which became dominant in various world regions in 2023 [55].
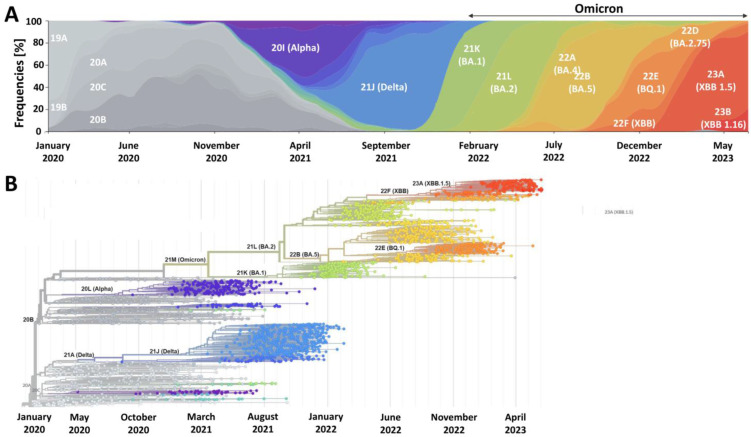
SARS-CoV-2 will continue to evolve by accumulating advantageous point mutations and recombination events (Figure 1). It is pivotal to monitor its evolution and understand the key biological and clinical features of the emerging (sub)variants. One should note that the virus can become more adapted through various processes that depend on ecological and epidemiological factors. For example, mutations leading to the enhanced evasion of humoral immunity are more likely to be subject to higher selective pressure when the population immunity levels gradually increase. Such vaccine-bypassing and antibody-resistant mutations are observed in Omicron, and it can be expected that they might become a dominating pathway of SARS-CoV-2’s evolution when most of the world is either infected or vaccinated [56]. However, this does not imply the entire loss of the effectiveness of COVID-19 vaccines, since a vital role in the antiviral response is played by the vaccine-induced adaptive cellular immunity [57], which is less prone to evasion through viral mutations, as also evidenced in the case of various Omicron sublineages [58,59]. Nevertheless, it indicates that managing the SARS-CoV-2 burden will require the systematic administration of booster doses and consideration of updated variant-adapted doses, particularly if one aims to increase protection from symptomatic infection. In parallel, the implementation of novel vaccination strategies is needed to utilize other antigens than the spike protein due to a high number of mutations in its gene (second to the gene encoding nonstructural protein 3) [60]. In response to this need, approaches based on multiple antigen-targeted cell-mediated immunity have been suggested to overcome waning antibody responses and attenuate infectious breakthrough events and the disease severity of future SARS-CoV-2 variants [61].
Figure 1.
The emergence of SARS-CoV-2 variants over the course of the COVID-19 pandemic (A) and their phylogeny (B). The data and graphs were retrieved from Nextstrain.org [62].
3. Omicron Lineage Is Milder but Not Negligible
The SARS-CoV-2 variant belonging to the Omicron lineage was identified for the first time in November 2021 in Africa. It has been characterized by a large number of sense mutations, exceeding 30 in the gene encoding the spike protein, including 10 in the receptor-binding domain [63]. Its high transmissibility soon led to its global distribution, a rise in novel sublineages, and the replacement and ultimate extinction of previous viral variants (Figure 1). Numerous studies have consistently shown that the enhanced transmissibility of Omicron is due to its ability to better evade the humoral immunity of the vaccinated and individuals with a history of SARS-CoV-2 infection [64,65]. At the same time, there is mounting evidence that the Omicron lineage causes milder infections in humans. Firstly, experimental studies demonstrated its less efficient membrane fusion kinetics than previous SARS-CoV-2 lineages, preferential endocytic cell entry, and faster replication in the human bronchus, while less efficient in lung cells [66,67,68]. All of these features translate into decreased severity of infection. This has been clearly reflected in in vivo studies employing naïve animals, including rodents and non-human primates [69,70,71,72,73]. Epidemiological analyses of various human populations confirmed that Omicron infections are characterized by decreased lower respiratory tract involvement, reduced odds of hospitalization due to severe COVID-19, and less mortality [74,75,76,77,78]. Despite the emergence of subsequent Omicron sublineages, such as BA.4/BA.5 and XBB, the hospitalization and death risk remained lower compared to previous SARS-CoV-2 variants, such as Delta [79,80,81,82].
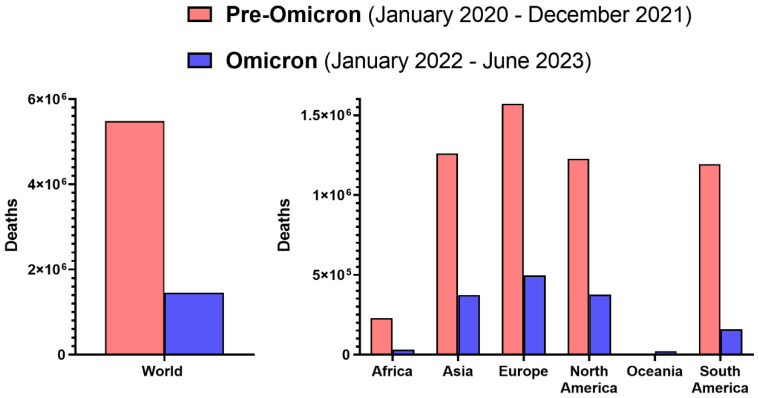
Although Omicron causes statistically milder infections with a better clinical prognosis, contrary to some opinions, it should not be regarded as a negligible pathogen (Figure 2). Since the beginning of 2022, when Omicron became dominant in most world regions (Figure 1), until the first half of 2023, approximately 1.45 million deaths of COVID-19 patients were confirmed [5]. The mean monthly death rate in the pre-Omicron period amounted to 228 thousand, while, during the Omicron era, it fell over 2.5-fold to 86 thousand, which still was substantial and resulted in larger mortality than in the case of seasonal influenza, whose annual toll is estimated globally at 290,000–650,000 deaths [83]. This was also reflected by the results of a comparative study in patients requiring hospitalization, which demonstrated that Omicron infection is associated with an approximately 1.5-fold higher risk of in-hospital all-cause mortality than seasonal influenza viruses [84]. Moreover, due to high transmissibility and the ability to infect many individuals in a given period, the rising rates of hospitalization due to this variant in some countries were even higher than during the Delta wave [85]. Further, within the first 1.5 years of Omicron’s dominance, more than 478 million cases of infection were officially reported, representing 165% of cases in the first two years since early 2020 [5]. This indicates that Omicron, even if its infections are more frequently mild, can substantially affect absenteeism from work and school due to illness. Lastly, individuals infected with Omicron can also report, similarly to other SARS-CoV-2 variants, a range of persisting symptoms, collectively known as post-COVID-19 syndrome, post-acute sequelae of SARS-CoV-2, or long-COVID [7]. Although the odds of this condition with Omicron were reported to be significantly reduced compared to the Delta variant [86], its estimated rate in the post-acute cohort (6–15 months from infection) was 17%, most often characterized by chronic fatigue, but the range of symptoms encompassed systemic, cardiac, dermatologic, ocular, otologic, gastrointestinal, metabolic, musculoskeletal, neurologic, psychiatric and respiratory, and urinary [87].
Figure 2.
The global death toll of SARS-CoV-2 Omicron during the first 1.5 years of its dominance compared to the pre-Omicron period. Graphs were prepared based on data collected by Our World in Data [5].
Omicron is not a “natural solution” to the COVID-19 problem, as some have suggested [88], and should not be, along with various sublineages, regarded as a negligible pathogen. Despite a milder course of disease, it continues to cause substantial health and economic burdens, the management of which requires appropriate awareness, preparedness, and resources.
4. Future Viral Variants May Not Always Cause Milder Disease
It is challenging to predict the future clinical relevance of SARS-CoV-2. However, it is not certain that its further evolution will lead to a decrease in infection severity. As demonstrated by the recent study, the directions of change in intrinsic case severity across successive SARS-CoV-2 variant waves have been inconsistent. It has increased continuously from the early lineages, through the Alpha variant to the Delta lineage, to decrease substantially in the case of BA.1 Omicron and even further when BA.2 emerged [89]. This contradicts the notion that SARS-CoV-2’s transmissibility can only be enhanced at the expense of its pathogenicity, since the Delta variant, infection with which was characterized by increased severity, was significantly more transmissible than preceding lineages [90]. This advantage in spread was gained predominantly by higher viral loads [90,91]. In turn, the Omicron variant does not cause elevated viral loads in the respiratory tract compared to those observed for the Delta variant, while some studies have reported that these loads might even be lower [92,93,94]. In addition, it does not reveal a higher affinity to the angiotensin-converting enzyme 2 receptor and demonstrates attenuated fusogenicity due to decreased use of the cellular protease TMPRSS2, resulting in the greater utilization of the endocytic pathway during cell entry [67,95,96]. Its enhanced transmissibility is due to the efficient escape from humoral immunity in individuals with a history of SARS-CoV-2 infection and those who are vaccinated. SARS-CoV-2 may continue to evolve to develop greater escape from infection- and vaccination-acquired immunity. This could lead to its high transmissibility without a considerable increase in severity, particularly if the immune escape mostly concerns humoral and not cellular responses. However, considering that SARS-CoV-2 is most transmissible prior to symptom onset and at the beginning of the symptomatic phase [97,98,99], the mutation-enhancing viral loads could also lead to superior transmission yet be potentially accompanied by more severe infections due to a higher risk of hyperinflammation and disease severity under such a scenario [100].
Moreover, the viral evolution may lead to a gradual increase in fusogenicity, which is known to impact the disease severity [101]. This process has already been reported for more newly emerging Omicron subvariants such as BA.4/BA.5 and XBB, which demonstrate higher fusogenicity of the spike protein compared to early BA.1 and BA.2 SARS-CoV-2 [102,103,104]. Experimental studies have shown a close relationship between enhanced viral fusogenicity and pathogenicity [102,105]. Moreover, there is evidence that further Omicron subvariants, such as BA.5, reveal higher efficiency in infecting lungs than an early BA.1 subvariant [106]. Although epidemiological studies reveal some differences in clinical severity between the original and later Omicron subvariants, they consistently indicate that it remains reduced compared to the Delta lineage [107,108,109]. It is plausible that a history of immunization, be it SARS-CoV-2 infection, COVID-19 vaccination, or both, plays a protective role in attenuating the increased severity in the human population that would otherwise be expected.
When considering the future of SARS-CoV-2 evolution, one should note that it can also infect non-human hosts, including wild animals and livestock [110,111,112,113,114], and potentially return to the human population through contact with these species. In addition, Omicron can likely utilize a broader range of host species than other SARS-CoV-2 variants, while the risk of cross-species infection is higher due to increased human mobility than in the case of the pre-Omicron era, when various sanitary restrictions were imposed [115]. The clinical consequences of such retransmission to the human population are challenging to predict since mutation-driven adaptations to a new host may lead to decreased adaptation to the human environment but also to the better evasion of acquired immunity, including the cellular response, and thus higher susceptibility to severe disease [116,117,118,119].
In conclusion, predictions of the exact clinical trajectories of future SARS-CoV-2 (sub)variants should be made cautiously to avoid communication disregarding the relevance of this pathogen but also fear-promoting messages. SARS-CoV-2 requires continuous genomic surveillance conducted globally with data sharing in the open domain and accompanied by in vitro and in vivo studies on viral biology, pathogenicity, and the evasion of acquired immunity. This approach is essential for the timely implementation or modification of safety measures, including vaccines.
5. Vaccines Remain a Key Component of Primary COVID-19 Prevention
The benefits of COVID-19 vaccination are well documented. According to a mathematical modeling study, their administration prevented 19.8 million deaths in 2021 alone [30]. Numerous analyses encompassing a period preceding the dominance of the Omicron demonstrate the public health impact of COVID-19 vaccines in different world regions regarding averted deaths, hospitalizations, and infection [120,121,122,123,124,125,126,127,128]. According to a meta-analysis that included real-world studies conducted before Omicron’s emergence, the overall COVID-19 vaccine effectiveness against SARS-CoV-2 infection, COVID-19-related hospitalization, admission to the intensive care unit, and death was 89.1, 97.2, 97.4, and 99.0%, respectively, with better effectiveness against infection observed for mRNA vaccines [31]. Further, the majority of conducted studies have shown that vaccination reduces the risk of long-COVID [129,130,131,132,133,134,135,136].
However, vaccine effectiveness against infection decreased when the Omicron lineage emerged and became widespread due to its enhanced ability to escape humoral responses [32,137,138,139]. According to a meta-analysis, booster dose administration improved to some extent protection against symptomatic Omicron infection, reaching 57% within three months from administration but decreasing to 33% after six months [140]. However, COVID-19 vaccines remained highly effective in protecting against severe COVID-19 and death in the era of Omicron, and this effect was further demonstrated to be improved/restored by booster vaccinations [141,142]. As indicated in the meta-analysis, the real-world effectiveness of booster doses against severe disease caused by Omicron infection was 86% [140]. Another meta-analysis estimated the effectiveness of booster doses against Omicron infection and hospitalization at 70% and 89%, respectively, decreasing to 43% and 71% at 112 days or later [143]. In children and adolescent populations, the pooled effectiveness of two COVID-19 vaccine doses against symptomatic Omicron infection was 51 and 61%, respectively, with the pooled effectiveness against hospitalization of 70% [144]. As calculated in the UK, a booster dose program in autumn–winter 2021 averted 12.8 million cases, 1.1 million hospitalizations, and 290,000 deaths during the first three months of Omicron’s dominance in 2022 [145]. This clearly shows that although the authorized COVID-19 vaccines are still not optimal, they save lives, protect health, and decrease the economic losses caused by SARS-CoV-2.
In response to the emergence of the Omicron lineage, novel bivalent booster mRNA vaccines were developed and authorized in the second half of 2022. Their administration provided additional protection against symptomatic SARS-CoV-2 in immunocompetent persons who had previously received monovalent vaccines only [146]. Early estimates show that in adults aged 18–49 years, the effectiveness of a bivalent mRNA booster dose (with mRNA encoding primary spike protein antigen and BA.4/BA.5 spike protein) given 2–3 months earlier compared to no bivalent booster was 52% against symptomatic infection with the BA.5 Omicron subvariant and 48% against infection with XBB/XBB.1.5 [147]. A retrospective cohort study conducted in Israel confirmed that bivalent mRNA vaccines reduced hospitalization and mortality in individuals aged ≥65 years [148]. However, one should note that the effectiveness of these bivalent booster doses against infection with the Omicron variant was not as high as could be expected. This phenomenon may be due to immunological imprinting, according to which the immune system of those already vaccinated with monovalent vaccines was primed to respond to the ancestral strain of SARS-CoV-2. As a result, the administration of bivalent vaccines revoked the response to epitopes shared by Omicron (BA.4/BA.5) and the ancestral strain, rather than to unique epitopes of Omicron, as also directly demonstrated by the lack of BA.5-specific antibodies in the serum of individuals boosted with bivalent COVID-19 vaccines [149,150,151,152]. Therefore, future booster doses are likely to be monovalent and lack the index-virus antigen, also because they have been adapted to lineages currently considered extinct [153].
Importantly, studies conducted during Omicron’s dominance also show that vaccines continue to decrease the risk of long-COVID [154]. As estimated, the booster dose administration in autumn–winter 2021 resulted in a 68% reduction in newly diagnosed long-COVID cases in the first quarter of 2022, when Omicron was the dominant SARS-CoV-2 lineage [145].
In summary, the available evidence consistently demonstrates that all individuals should stay up to date with recommended COVID-19 vaccines, including receiving updated doses. COVID-19 vaccination reduces the overall burden of SARS-CoV-2 regardless of the dominating lineage, including the clinically milder Omicron. Nevertheless, it requires booster doses, including those based on updated antigens. Importantly, the mRNA platform enables the rapid manufacturing of novel versions of COVID-19 vaccines if such a need arises [155].
At the same time, there is a need to pursue efforts to develop vaccine candidates that could confer more durable protection against SARS-CoV-2 infection and be less prone to mutations in genes encoding the spike protein. One approach in this regard is focusing on the use of self-amplified mRNA vaccine candidates that aim to induce multiple antigen-targeted cell-mediated immunity in addition to neutralizing humoral responses in order to bypass waning antibody concentrations and attenuate the infectious breakthrough and disease severity of future SARS-CoV-2 variants [61]. Preclinical data show that using dual-antigen mRNA vaccines, encoding viral nucleocapsid and spike proteins, is superior in controlling SARS-CoV-2 (including the Omicron variant) in the lower and upper respiratory tracts than immunization with mRNA encoding exclusively the spike protein [156].
In addition, further efforts to develop effective intranasal COVID-19 vaccines are necessary as this route of administration may offer several advantages. Contrary to intramuscular vaccines, it can induce a mucosal immunity that plays a role in host defense in the upper respiratory airway, a primary entry site of viruses such as SARS-CoV-2, ultimately preventing virus infection, replication, shedding, transmission, and disease development and progression [157,158]. Secondly, the components of these vaccines can be absorbed through the mucosa, leading to systemic immunity [159,160]. Lastly, the intranasal route of administration is less invasive and painless and may translate into lower vaccine fears and improved acceptance [161,162]. Thus far, the development of safe and efficacious intranasal COVID-19 vaccines remains a challenge [163]. The intranasal COVID-19 adenoviral vaccine candidate failed to induce robust mucosal and systemic immunity in a phase 1 clinical trial [164], despite encouraging preclinical data [165]. In turn, the intranasal administration of lipid nanoparticles employed to encapsulate mRNA led to lung inflammatory responses and resulted in a high mortality rate [166]. These findings indicate the need for better preclinical models for mucosal immunity in humans and to develop strategies for the safe delivery of some vaccines, i.e., based on the mRNA platform.
6. Simplifying COVID-19 Booster Vaccination Will Improve Vaccine Acceptance and Intake
Given that COVID-19 vaccines will remain a primary strategy to decrease the health burden caused by SARS-CoV-2, it is essential to simplify the vaccination protocols, particularly regarding booster administration. Various observations demonstrate a significant decline in vaccination-induced antibodies within six months from the previous dose and indicate that biannual boosting with mRNA vaccines (most frequently used for this purpose) will induce the highest level of protection against infection [167,168,169,170]. Under such an approach, the risk of breakthrough infection over six years was estimated at 7–11%. In comparison, annual boosting would also substantially reduce the 6-year risk to 25–31%. In turn, delaying boosting beyond two years yielded cumulative risks of future infection nearly as high as foregoing boosting entirely [170].
However, one should bear in mind that interest in COVID-19 vaccines decreases with subsequent boosting doses. For example, by June 2023, 76% of the population had received at least one dose of the COVID-19 vaccine in the European Economic Area, the primary course of vaccination was completed by 73%, the first booster was received by 55%, the second one by 14%, while a third one only by 2% [171]. This trend has a multifactorial basis, including low perceived benefits of receiving a booster vaccine, a low subjective risk of severe COVID-19, disappointment in vaccines due to experiencing a breakthrough infection or adverse effects after the previous vaccine dose, and a loss of trust in health authorities during the pandemic [172,173,174,175]. All of these factors are more or less rooted in inappropriate communication on the role of COVID-19 vaccination in decreasing the overall SARS-CoV-2 burden on public and individual health as well as the economy. They may also arise from the various unknown factors regarding COVID-19 vaccines that existed when they were introduced (e.g., regarding the durability of immunity) and confusion about shifting public health guidelines regarding vaccine safety, changing the interval between doses, mixing particular vaccine brands, and introducing subsequent booster doses without knowing whether and when additional ones will be required [176]. These issues are currently clarified, allowing for the simplification of COVID-19 booster strategies, translating into lower vaccine hesitancy and better acceptance.
SARS-CoV-2 reveals a seasonal behavior, which is generally in line with that seen for other respiratory viruses, such as influenza viruses and respiratory syncytial virus [177,178,179]. For example, in Europe and the United States, the highest SARS-CoV-2 burden, i.e., infections, emergency visits, hospitalizations, and deaths, can be expected between autumn and early spring [179,180,181]. This strongly supports the notion that the administration of seasonal booster vaccines should be performed in a similar timeframe as in the case of influenza [179]. This timeframe also coincides with when RSV vaccines (currently gaining authorization for use in particular groups) will be recommended [182]. This creates an opportunity, being comfortable and less time-consuming from the perspective of those interested in vaccination, to offer seasonal booster COVID-19 vaccines simultaneously during the same visit as those against influenza and RSV. As recently shown, concurrently administering COVID-19 and influenza vaccines was not associated with additional safety risks and remained immunogenic, although marginally lower anti-S antibody levels were observed compared to booster COVID-19 vaccination alone [183]. Ultimately, the future may lie in combined vaccines. Some multicomponent mRNA vaccine candidates against COVID-19, influenza, and RSV have already entered clinical phases of testing (e.g., mRNA-1230; NCT05585632). The mRNA platform enables the development of updated COVID-19 boosters and seasonal influenza vaccines based on antigen selection approximately three months before an increased number of infections is expected [155]. This approach should increase the protection levels, not only against severe disease but also symptomatic infection, during a period when respiratory diseases are the most overwhelming for healthcare systems. A decision to select a novel version of the SARS-CoV-2 antigen should be made based on genomic surveillance data and the genetic divergence between currently and previously dominating viral sublineages.
Seasonal COVID-19 booster campaigns should preferentially target those at the highest risk of severe disease, including the elderly and individuals with comorbidities and immunodeficiencies, but also pregnant women and healthcare workers. This approach has also been recommended recently in a joint statement by the European Centre for Disease Prevention and Control and the European Medicines Agency [184]. We posit that these groups should be prioritized for reimbursed vaccines by local authorities. However, seasonal COVID-19 vaccines should also be made available for other eligible groups, e.g., through commercial distribution in a similar fashion to how influenza vaccines are offered in different world regions. Their administration also to those at lower risk of severe disease would decrease the risk of experiencing mild symptomatic infection and its consequences, such as long-COVID and being forced to abstain from work temporarily. It seems reasonable to recommend seasonal booster COVID-19 vaccinations, preferentially pursued at the same time as immunization against other respiratory viruses such as influenza and RSV.
7. Antivirals Represent a Strategy to Adapt to Long-Term Co-Existence with SARS-CoV-2
COVID-19 treatment depends on the severity of the infection and the presence of risk factors in infected patients. Pharmaceuticals targeting SARS-CoV-2 aim to inhibit viral replication and prevent disease progression to a more severe form [27]. For this purpose, they need to be applied in the early symptomatic phase [27]. However, ensuring that the targeted site is not subject to frequent mutations is critical. Otherwise, the effectiveness of such pharmaceuticals may soon decrease due to viral evolution. Such an effect has been observed in the case of various monoclonal antibodies either used to treat infection or as preexposure prophylaxis [185].
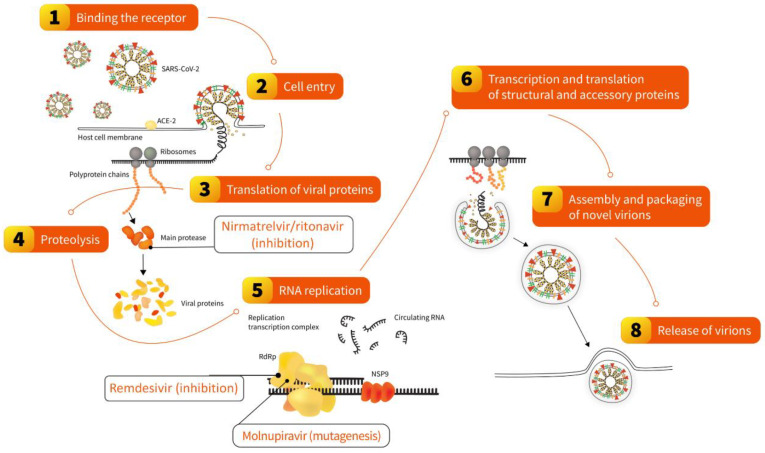
In 2022, two oral antivirals specifically targeting the SARS-CoV-2 replication cycle were recommended for use in different world regions: nirmatrelvir/ritonavir and molnupiravir [27] (Figure 3). Both require 5-day treatment initiated no later than five days from symptom onset. The former inhibits the main SARS-CoV-2 protease, pivotal to processing polyprotein precursors, ultimately leading to the inability of the virus to replicate. This mechanism is ensured by nirmatrelvir, which is extended in the presence of a low dose of ritonavir, acting as an inhibitor of CYP3A-mediated metabolism. The pivotal phase 2–3 double-blind, randomized, controlled trial on nirmatrelvir/ritonavir, conducted in symptomatic, unvaccinated, nonhospitalized adults at high risk for progression to severe COVID-19, reported a reduction in hospital admission or death by 97% relative to a placebo. Experimental studies demonstrated that it remains effective against various Omicron subvariants [185], observations that were further confirmed by clinical trials and real-world studies conducted in different populations and reporting reduced hospitalization and mortality in treated patients [186,187,188]. In addition, a large cohort study found that treatment with nirmatrelvir/ritonavir during the period dominated by the Omicron variant was associated with a reduced risk of long-COVID regardless of vaccination status and prior infection [189].
Figure 3.
Steps of the SARS-CoV-2 replication cycle in the human cell disrupted by oral antivirals nirmatrelvir/ritonavir and molnupiravir and intravenously administrated remdesivir. The scheme was used and modified with permission [27].
Molnupiravir is a small-molecule ribonucleoside pro-drug of N-hydroxycytidine [190], which was tested prior to the COVID-19 pandemic for potential use against SARS-CoV-1 and MERS-CoV [191,192]. Its mechanism of action is based on so-called lethal mutagenesis, the process in which viral RNA-dependent RNA polymerase is misdirected to induce transition mutations throughout the genome during viral replication, ultimately leading to errors deleterious for the virus. A double-blind, randomized, placebo-controlled phase 3 clinical trial in symptomatic, unvaccinated, nonhospitalized adults showed that the risk of death was 89% lower in the group receiving molnupiravir for five days [193]. However, no clinical benefit was found in the clinical trial involving hospitalized patients [194]. These studies were conducted during the dominance of viral variants other than Omicron. Nevertheless, experimental in vitro studies demonstrated that molnupiravir remains efficacious against this variant [185], further confirmed in clinical trials and real-world studies also involving hospitalized COVID-19 patients [186,195,196]. Moreover, molnupiravir use was also associated with a reduced risk of long-COVID regardless of vaccination status and history of previous SARS-CoV-2 infection [197].
Apart from oral antivirals, which can be used in outpatient and inpatient settings, an important treatment option in patients hospitalized with COVID-19 includes remdesivir, an intravenously administrated non-canonical nucleotide developed prior to the COVID-19 pandemic (Figure 3). It acts as an inhibitor of the RNA-dependent RNA polymerase of RNA viruses of several families, including Paramyxoviridae, Filoviridae, and Coronaviridae. Based on evidence from clinical studies, remdesivir was authorized in 2020 by various health authorities to treat COVID-19 in adults and adolescents (>12 years with weight ≥ 40 kg) who require oxygen therapy. It can also be used in adults who do not require oxygen supplementation but represent a high-risk group for severe COVID-19 [198]. Experimental in vitro studies have shown that it remains efficacious against Omicron’s sublineages, including BQ.1.1 and XBB [185]. This was also confirmed in the real-world analysis in which remdesivir use in patients hospitalized during Omicron’s dominance was an independent predictor of lower mortality, similar to the period dominated by the Delta lineage [199].
In summary, antivirals such as nirmatrelvir/ritonavir, molnupiravir, and remdesivir retain their effectiveness against the novel SARS-CoV-2 sublineages and continue to be important elements of COVID-19 therapy. It is pivotal to ensure their availability, particularly when an increased number of SARS-CoV-2 infections can be expected (e.g., during the autumn–winter season in the temperate zone). In this regard, oral antivirals are the pharmaceuticals of choice as they reduce healthcare costs through decreased hospitalization rates [200,201,202]. At the same time, it is important to pursue research on the potential benefits arising from therapies based on the combination of antivirals, assessed mostly as case reports [203] or in vivo rodent studies [204]. Such combinations may slow the emergence of resistance mutations, as already evidenced for other pathogens [27,205,206]. This may be particularly of interest in the case of immunocompromised patients since they are often characterized by an extended time of viral elimination, even when treated with available antivirals [203,207,208,209]. The high-dosing regimen recommended for nirmatrelvir/ritonavir (three tablets administrated twice a day for five days) and molnupiravir (four capsules every 12 h for five days) also represents a challenge due to the risk of missing a dose or inappropriate adherence, which is not possible to directly control outside the clinical setting. Moreover, nirmatrelvir/ritonavir tablets and molnupiravir capsules are relatively large (8.5 × 17.5 mm and 7.6 × 21.8 mm, respectively [210,211]) and cannot be chewed or crushed and may be difficult to swallow by selected patients, including the elderly, for whom this issue has been particularly recognized [212,213]. Improved formulations, requiring reduced dosing and based on a smaller size of swallowed tablets/capsules, would be ultimately desired. Simultaneously, continued efforts to increase the portfolio of anti-SARS-CoV-2 pharmaceuticals, preferentially administrated orally and acting on different viral targets, are highly encouraged [27].
8. Leaving No Country Behind: Low-Income Regions Require Better Access to COVID-19 Vaccines and Antivirals
Considering that COVID-19 vaccines and SARS-CoV-2 antivirals remain essential tools during the transition from the acute phase of the pandemic, it is pivotal to pursue efforts to increase their availability and willingness to use in low-income countries. Although the case–fatality ratio for various low-income areas, e.g., the African continent, remains below that observed globally [214], this does not imply that SARS-CoV-2 is a negligible pathogen. This is also because the reasons behind such epidemiological phenomena are unclear, with various hypotheses put forward, including cross-protection from other infections or younger populations than in other world regions [215,216,217]. However, low-income countries likely have the highest rates of underreported COVID-19 cases (including severely diseased) and COVID-19 mortality [218,219]. At the same time, they are represented by low vaccination rates, with approximately 25% of the population of low-income countries having completed an initial vaccination protocol [5]. This is due to several factors, including insufficient supply, limited local vaccine production, inequitable distribution, weak healthcare systems, a low perceived risk, and high vaccine hesitancy [220].
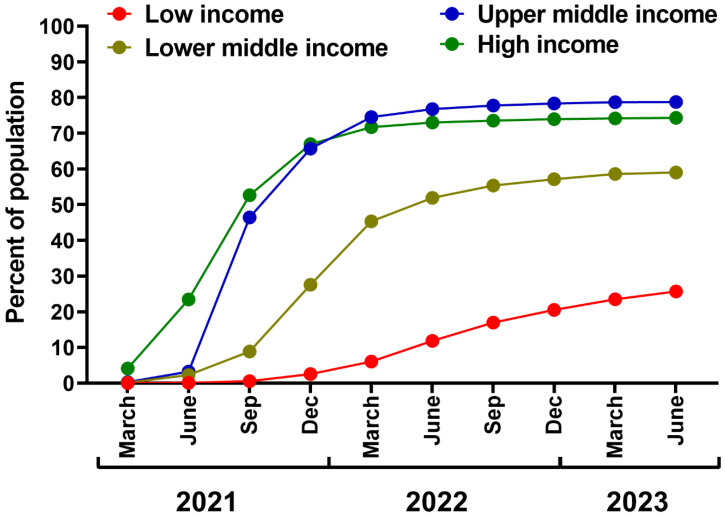
The efforts should be continued to improve vaccine equity in low-income countries through better support of humanitarian initiatives from high-income countries, such as the COVID-19 Vaccines Global Access (COVAX) initiative. As of early 2023, it has delivered 1.88 billion doses [221], despite its initial target to deliver 2 billion doses in 2021 [222]. This results in great discrepancies in vaccination rates in the world, i.e., 2.5 years after COVID-19 vaccine authorization (June 2023), the percentage of the population with a completed initial vaccination protocol in low-income countries was similar to that already reached by high-income regions after six months of the campaign in 2021 (Figure 4). This failure resulted mainly from the subdued efforts of wealthy regions, vaccine nationalism, and trade between high-income countries [36,223,224], summarized by the WHO’s Director-General as a “handful of rich countries gobbling up the anticipated supply as manufacturers sell to the highest bidder, while the rest of the world scrambles for the scraps” [225]. Notably, the most considerable benefits of COVID-19 vaccination, in terms of averted deaths, have been demonstrated for high-income and upper–middle-income regions, likely due to better logistics, swift rollout, and improved access to highly efficient mRNA vaccines [30]. These findings also underline the need for vaccine aid and support in regions of lower income (Figure 4). As estimated, universal vaccination in low-income and lower–middle-income countries with three doses of a mRNA vaccine would prevent as many as 1.5 million COVID-19-related deaths in a period already dominated by the Omicron lineage [226].
Figure 4.
The percentage of the population with completed initial COVID-19 vaccination protocol by economic group. Prepared based on data retrieved from Our World in Data [5].
One should also note that prior to Omicron’s emergence, researchers continuously warned that vaccine inequity during the COVID-19 pandemic not only reflected a moral crisis but also increased the odds of the emergence of novel, problematic SARS-CoV-2 variants [36]. Although the exact origins of the Omicron lineage remain unknown, it is suggested that it may arise during the infection of immunocompromised individuals (e.g., HIV/AIDS patients) or even cross-infection between a group of them due to extended viral replication and the selection of neutralization resistance mutations in such subjects [227,228]. In addition, a study conducted before the emergence of Omicron has shown that the mutation frequency positively correlates with the percentage of unvaccinated individuals in a population, with the highest frequency found for regions with vaccination rates below 10–20%. In turn, the rate of individuals who completed a primary vaccination course in Africa, which has the highest population of people living with HIV (predominantly in the Sub-Saharan area), was approximately 5% at the time of Omicron’s identification (compared to nearly 55% in the USA, 65% in the European Union, and 50% in Oceania). Although infections with Omicron are milder compared to the SARS-CoV-2 lineages preceding it, a lesson must be learned, particularly if one considers that viral genomic surveillance in low-income countries is limited [229]. When various health authorities issue novel recommendations regarding COVID-19 vaccination [153], there is no reason to shape them differently for low- and high-income regions, since COVID-19 remains a global issue and should be treated equally regardless of one’s origin or ethnicity [224]. Importantly, it is crucial to ensure that low-income regions continue to move away from aid dependence through various mechanisms enabling the local production of vaccines, including those based on innovative technologies such as mRNA platforms. This could be done by building on the existing capacity, developing sustainable financing mechanisms and quality control systems, prioritizing research funding and regional integration, and collaboration conceptions based on technology co-creation and co-ownership, as discussed elsewhere [155]. The improvement of the manufacturing and supply of vaccines based on technologies such as mRNA would also be necessary outside the COVID-19 realm if one considers their potential to deliver preventive tools against other infectious diseases [155,230], some of which are particularly burdensome in low-income countries and have zoonotic origins [231,232].
Simultaneously to vaccine equity, it is essential to improve accessibility to SARS-CoV-2 antivirals in low-income countries, particularly those available in the oral form [233]. The first-generation oral antivirals are relatively expensive [234], highlighting the need for aid in delivering these pharmaceuticals to low-income regions, developing generic versions of these drugs, and pursuing efforts to produce them locally [27]. This is particularly important since these antivirals can substantially reduce the risk that infected patients will require specialized healthcare, access to which is limited under low-income resources.
All of these efforts require integration with improved education and awareness campaigns to fight vaccine hesitancy, educate on infectious diseases, including COVID-19, and build trust in local authorities and vaccine manufacturers. These goals will also likely require external support, bringing together experience from vaccination campaigns in developed regions and local specificity. The improvement of accessibility to pharmaceuticals and their acceptance in low-income areas should be an integral part of a strategy for pathogen management in high-income countries if one considers that, in an increasingly connected modern world, the risks arising from infectious disease can be globally shared [235].
9. Healthcare Workers Play a Crucial Role in Maintaining Public COVID-19 Awareness
Healthcare workers are a pivotal part of health communication as they interact directly with patients, including those at high risk of various diseases such as severe COVID-19. Moreover, they serve as role models of healthy behaviors, including vaccination decisions [236]. In fact, their role in general COVID-19 awareness may even be more influential during the transition from the acute phase of the pandemic. This is because, earlier, regular communication with patients in this regard was likely curbed due to the allocation of healthcare resources to fight COVID-19, social distancing, closures of primary care units, and increased stress experienced by healthcare workers [237,238,239,240]. At the same time, COVID-19 received high media coverage, with various information on preventive measures often reported daily [241]. Vaccination campaigns, the lifting of sanitary restrictions, the spread of the clinically milder Omicron lineage, and the emergence of other issues of public importance (e.g., a war in Ukraine) translated into a decreased interest in COVID-19 in traditional and social media. This may have led to the false assumption that COVID-19 is no longer a threat requiring any preventive measures, e.g., seasonal vaccinations.
Therefore, it is pivotal to ensure that healthcare workers continue their efforts to communicate the risks for particular groups of patients, follow the recommendations on vaccination, and communicate them further in an understandable manner. As recently noted in the joint statement by the European Centre for Disease Prevention and Control and the European Medicines Agency, seasonal COVID-19 vaccination of healthcare workers should be considered because they have a higher risk of exposure to SARS-CoV-2 while playing a key role in the functioning of the healthcare system [184]. However, their decision to vaccinate is also likely to be influential for their patients [242]. Therefore, ensuring an appropriate education level on COVID-19 vaccination among healthcare workers, including primary physicians, is crucial. As shown in a study led by the WHO/Europe, healthcare workers are more confident in recommending COVID-19 vaccines to their patients if they undergo dedicated online training on how to communicate with patients regarding the vaccination [243]. They will also likely be more confident in discussing COVID-19’s risks with particular groups of patients after completing the training course, updating them on current SARS-CoV-2 sublineages in circulation and their clinical relevance. Moreover, it is essential to continue efforts to raise awareness of the effectiveness of non-pharmacological measures, such as face masks and hand hygiene, in limiting the spread of SARS-CoV-2, as well as other respiratory infections, particularly when feeling unwell or when in contact with elderly or immunosuppressed patients during periods of increased transmission of SARS-CoV-2.
One should note that apart from physicians, an increasingly important role in vaccination is played by pharmacists [244,245,246]. A study conducted in the US demonstrated that one in four people who refused to receive influenza and pneumococcal vaccines could eventually decide to receive a vaccination after consultation with a pharmacist in a pharmacy [247]. As calculated in other analyses, including pharmacists in consultation services among seniors for influenza vaccination is cost-effective and improves vaccination rates in this group [248]. Therefore, political and organizational barriers should not limit pharmacists’ participation in COVID-19 vaccination campaigns. Therefore, the local authorities should consider increasing the rights of pharmacists in qualifying, prescribing, and vaccinating patients against COVID-19, ultimately simplifying seasonal vaccination campaigns and likely translating into higher vaccination rates.
10. Conclusions
The acute phase of the COVID-19 pandemic may be over, as reflected by the WHO’s decision to rescind its PHEIC status in May 2023, but SARS-CoV-2 continues to spread, evolve, and cause economic and health burdens. There are still many uncertainties regarding the future of COVID-19, its severity, and its global impact in the long-term perspective. Therefore, as highlighted in the present paper, it requires sustained genomic surveillance and the promotion of prevention strategies that are as simplified as possible, continuously supported by the healthcare community, and accessible also in low-income regions. All of these elements should be a part of the strategy to adapt to long-term co-existence with SARS-CoV-2 in a manner that prevents healthcare systems from being overwhelmed.
Author Contributions
P.R.: conceptualization, project administration, resources, writing—original draft. M.P.-Ś.: writing—review and editing. T.J.: writing—review and editing. E.K.: writing—review and editing. A.N.-O.: writing—review and editing. M.P.: writing—review and editing. M.B.: writing—review and editing. J.J.: writing—review and editing. L.S.: writing—review and editing. J.W.: writing—review and editing. R.F.: conceptualization, supervision. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
All authors were members of the Expert Committee on Prevention and Treatment of Infectious Diseases. P.R., T.J. and R.F. report consultation and lecture fees from Moderna and Pfizer. E.K. reports consultation and lecture fees from AstraZeneca, GSK, Moderna, Novartis, Novavax, GSK, Pfizer, and Sanofi. A.N.-S. and J.W. report consultation and lecture fees from Astra Zeneca, GSK, Moderna, and Pfizer. J.J. reports consultation and lecture fees from Gilead, Moderna, and Pfizer. L.S. reports grants and lecture fees from GSK and Pfizer.
Funding Statement
Pfizer organized the meeting of the Expert Committee on Prevention and Treatment of Infectious Diseases and funded editorial support to prepare this manuscript, but it had no effect on its content, which remains the sole intellectual property of the authors.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Jiang S., Xia S., Ying T., Lu L. A Novel Coronavirus (2019-NCoV) Causing Pneumonia-Associated Respiratory Syndrome. Cell. Mol. Immunol. 2020;17:554. doi: 10.1038/s41423-020-0372-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sohrabi C., Alsafi Z., O’Neill N., Khan M., Kerwan A., Al-Jabir A., Iosifidis C., Agha R. World Health Organization Declares Global Emergency: A Review of the 2019 Novel Coronavirus (COVID-19) Int. J. Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cucinotta D., Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Statement on the Fifteenth Meeting of the IHR (2005) Emergency Committee on the COVID-19 Pandemic. [(accessed on 7 June 2023)]. Available online: https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(COVID-19)-pandemic.
- 5.Mathieu E., Ritchie H., Rodés-Guirao L., Appel C., Giattino C., Hasell J., Macdonald B., Dattani S., Beltekian D., Ortiz-Ospina E., et al. Coronavirus Pandemic (COVID-19). Our World Data 2020. [(accessed on 10 August 2023)]. Available online: https://ourworldindata.org/coronavirus.
- 6.Wang H., Paulson K.R., Pease S.A., Watson S., Comfort H., Zheng P., Aravkin A.Y., Bisignano C., Barber R.M., Alam T., et al. Estimating Excess Mortality Due to the COVID-19 Pandemic: A Systematic Analysis of COVID-19-Related Mortality, 2020–2021. Lancet. 2022;399:1513–1536. doi: 10.1016/S0140-6736(21)02796-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., Cook J.R., Nordvig A.S., Shalev D., Sehrawat T.S., et al. Post-Acute COVID-19 Syndrome. Nat. Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C., Haupert S.R., Zimmermann L., Shi X., Fritsche L.G., Mukherjee B. Global Prevalence of Post-Coronavirus Disease 2019 (COVID-19) Condition or Long COVID: A Meta-Analysis and Systematic Review. J. Infect. Dis. 2022;226:1593–1607. doi: 10.1093/infdis/jiac136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez-Leon S., Wegman-Ostrosky T., Ayuzo Del Valle N.C., Perelman C., Sepulveda R., Rebolledo P.A., Cuapio A., Villapol S. Long-COVID in Children and Adolescents: A Systematic Review and Meta-Analyses. Sci. Rep. 2022;12:9950. doi: 10.1038/s41598-022-13495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO Post COVID-19 Condition (Long COVID) [(accessed on 19 May 2023)]. Available online: https://www.who.int/europe/news-room/fact-sheets/item/post-COVID-19-condition.
- 11.Schnitzler L., Janssen L.M.M., Evers S.M.A.A., Jackson L.J., Paulus A.T.G., Roberts T.E., Pokhilenko I. The Broader Societal Impacts of COVID-19 and the Growing Importance of Capturing These in Health Economic Analyses. Int. J. Technol. Assess. Health Care. 2021;37:e43. doi: 10.1017/S0266462321000155. [DOI] [PubMed] [Google Scholar]
- 12.Shang Y., Li H., Zhang R. Effects of Pandemic Outbreak on Economies: Evidence from Business History Context. Front. Public Health. 2021;9:632043. doi: 10.3389/fpubh.2021.632043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller I.F., Becker A.D., Grenfell B.T., Metcalf C.J.E. Disease and Healthcare Burden of COVID-19 in the United States. Nat. Med. 2020;26:1212–1217. doi: 10.1038/s41591-020-0952-y. [DOI] [PubMed] [Google Scholar]
- 14.Lenzen M., Li M., Malik A., Pomponi F., Sun Y.-Y., Wiedmann T., Faturay F., Fry J., Gallego B., Geschke A., et al. Global Socio-Economic Losses and Environmental Gains from the Coronavirus Pandemic. PLoS ONE. 2020;15:e0235654. doi: 10.1371/journal.pone.0235654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sidor A., Rzymski P. Dietary Choices and Habits during COVID-19 Lockdown: Experience from Poland. Nutrients. 2020;12:1657. doi: 10.3390/nu12061657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onyeaka H., Anumudu C.K., Al-Sharify Z.T., Egele-Godswill E., Mbaegbu P. COVID-19 Pandemic: A Review of the Global Lockdown and Its Far-Reaching Effects. Sci. Prog. 2021;104:368504211019854. doi: 10.1177/00368504211019854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nowakowska J., Sobocińska J., Lewicki M., Lemańska Ż., Rzymski P. When Science Goes Viral: The Research Response during Three Months of the COVID-19 Outbreak. Biomed. Pharmacother. 2020;129:110451. doi: 10.1016/j.biopha.2020.110451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghebreyesus T.A., Swaminathan S. Scientists Are Sprinting to Outpace the Novel Coronavirus. Lancet. 2020;395:762–764. doi: 10.1016/S0140-6736(20)30420-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cusinato J., Cau Y., Calvani A.M., Mori M. Repurposing Drugs for the Management of COVID-19. Expert Opin. Ther. Pat. 2021;31:295–307. doi: 10.1080/13543776.2021.1861248. [DOI] [PubMed] [Google Scholar]
- 20.Flisiak R., Zarębska-Michaluk D., Berkan-Kawińska A., Tudrujek-Zdunek M., Rogalska M., Piekarska A., Kozielewicz D., Kłos K., Rorat M., Bolewska B., et al. Remdesivir-Based Therapy Improved the Recovery of Patients with COVID-19 in the Multicenter, Real-World SARSTer Study. Pol. Arch. Intern. Med. 2021;131:103–110. doi: 10.20452/pamw.15735. [DOI] [PubMed] [Google Scholar]
- 21.Zarębska-Michaluk D., Jaroszewicz J., Rogalska M., Martonik D., Pabjan P., Berkan-Kawińska A., Bolewska B., Oczko-Grzesik B., Kozielewicz D., Tudrujek-Zdunek M., et al. Effectiveness of Tocilizumab with and without Dexamethasone in Patients with Severe COVID-19: A Retrospective Study. J. Inflamm. Res. 2021;14:3359–3366. doi: 10.2147/JIR.S322645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flisiak R., Flisiak-Jackiewicz M., Rzymski P., Zarębska-Michaluk D. Tocilizumab for the Treatment of COVID-19. Expert Rev. Anti-Infect. Ther. 2023;21:791–797. doi: 10.1080/14787210.2023.2226867. [DOI] [PubMed] [Google Scholar]
- 23.Moniuszko-Malinowska A., Czupryna P., Zarębska-Michaluk D., Tomasiewicz K., Pancewicz S., Rorat M., Dworzańska A., Sikorska K., Bolewska B., Lorenc B., et al. Convalescent Plasma Transfusion for the Treatment of COVID-19-Experience from Poland: A Multicenter Study. J. Clin. Med. 2020;10:28. doi: 10.3390/jcm10010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simonovich V.A., Burgos Pratx L.D., Scibona P., Beruto M.V., Vallone M.G., Vázquez C., Savoy N., Giunta D.H., Pérez L.G., Sánchez M.D.L., et al. A Randomized Trial of Convalescent Plasma in COVID-19 Severe Pneumonia. N. Engl. J. Med. 2021;384:619–629. doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang S., Hillyer C., Du L. Neutralizing Antibodies against SARS-CoV-2 and Other Human Coronaviruses. Trends Immunol. 2020;41:355–359. doi: 10.1016/j.it.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brobst B., Borger J. Benefits and Risks of Administering Monoclonal Antibody Therapy for Coronavirus (COVID-19). StatPearls Publishing. [(accessed on 10 August 2023)];2023 Available online: https://www.ncbi.nlm.nih.gov/books/NBK574507. [PubMed]
- 27.Rahmah L., Abarikwu S.O., Arero A.G., Jibril A.T., Fal A., Flisiak R., Makuku R., Marquez L., Mohamed K., Ndow L., et al. Oral Antiviral Treatments for COVID-19: Opportunities and Challenges. Pharmacol. Rep. 2022;74:1255–1278. doi: 10.1007/s43440-022-00388-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thanh Le T., Andreadakis Z., Kumar A., Gómez Román R., Tollefsen S., Saville M., Mayhew S. The COVID-19 Vaccine Development Landscape. Nat. Rev. Drug Discov. 2020;19:305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- 29.Rzymski P., Borkowski L., Drąg M., Flisiak R., Jemielity J., Krajewski J., Mastalerz-Migas A., Matyja A., Pyrć K., Simon K., et al. The Strategies to Support the COVID-19 Vaccination with Evidence-Based Communication and Tackling Misinformation. Vaccines. 2021;9:109. doi: 10.3390/vaccines9020109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watson O.J., Barnsley G., Toor J., Hogan A.B., Winskill P., Ghani A.C. Global Impact of the First Year of COVID-19 Vaccination: A Mathematical Modelling Study. Lancet Infect. Dis. 2022;22:1293–1302. doi: 10.1016/S1473-3099(22)00320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng C., Shao W., Chen X., Zhang B., Wang G., Zhang W. Real-World Effectiveness of COVID-19 Vaccines: A Literature Review and Meta-Analysis. Int. J. Infect. Dis. 2022;114:252–260. doi: 10.1016/j.ijid.2021.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rzymski P., Kasianchuk N., Sikora D., Poniedziałek B. COVID-19 Vaccinations and Rates of Infections, Hospitalizations, ICU Admissions, and Deaths in Europe during SARS-CoV-2 Omicron Wave in the First Quarter of 2022. J. Med. Virol. 2022;95:e28131. doi: 10.1002/jmv.28131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ao D., He X., Hong W., Wei X. The Rapid Rise of SARS-CoV-2 Omicron Subvariants with Immune Evasion Properties: XBB.1.5 and BQ.1.1 Subvariants. MedComm. 2023;4:e239. doi: 10.1002/mco2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willett B.J., Grove J., MacLean O.A., Wilkie C., De Lorenzo G., Furnon W., Cantoni D., Scott S., Logan N., Ashraf S., et al. SARS-CoV-2 Omicron Is an Immune Escape Variant with an Altered Cell Entry Pathway. Nat. Microbiol. 2022;7:1161–1179. doi: 10.1038/s41564-022-01143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qu P., Faraone J.N., Evans J.P., Zheng Y.-M., Carlin C., Anghelina M., Stevens P., Fernandez S., Jones D., Panchal A.R., et al. Enhanced Evasion of Neutralizing Antibody Response by Omicron XBB.1.5, CH.1.1, and CA.3.1 Variants. Cell Rep. 2023;42:112443. doi: 10.1016/j.celrep.2023.112443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rzymski P., Camargo C.A., Fal A., Flisiak R., Gwenzi W., Kelishadi R., Leemans A., Nieto J.J., Ozen A., Perc M., et al. COVID-19 Vaccine Boosters: The Good, the Bad, and the Ugly. Vaccines. 2021;9:1299. doi: 10.3390/vaccines9111299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woldemeskel B.A., Garliss C.C., Blankson J.N. MRNA Vaccine-Elicited SARS-CoV-2-Specific T Cells Persist at 6 Months and Recognize the Delta Variant. Clin. Infect. Dis. 2021;75:e898–e901. doi: 10.1093/cid/ciab915. [DOI] [PubMed] [Google Scholar]
- 38.Jordan S.C., Shin B.-H., Gadsden T.-A.M., Chu M., Petrosyan A., Le C.N., Zabner R., Oft J., Pedraza I., Cheng S., et al. T Cell Immune Responses to SARS-CoV-2 and Variants of Concern (Alpha and Delta) in Infected and Vaccinated Individuals. Cell. Mol. Immunol. 2021;18:2554–2556. doi: 10.1038/s41423-021-00767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jergovic M., Coplen C.P., Uhrlaub J.L., Beitel S.C., Burgess J.L., Lutrick K., Ellingson K.D., Watanabe M., Nikolich-Žugich J. Resilient T Cell Responses to B.1.1.529 (Omicron) SARS-CoV-2 Variant. medRxiv. 2022 doi: 10.1101/2022.01.16.22269361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collier D.A., Ferreira I.A.T.M., Kotagiri P., Datir R.P., Lim E.Y., Touizer E., Meng B., Abdullahi A., CITIID-NIHR BioResource COVID-19 Collaboration. Elmer A., et al. Age-Related Immune Response Heterogeneity to SARS-CoV-2 Vaccine BNT162b2. Nature. 2021;596:417–422. doi: 10.1038/s41586-021-03739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brosh-Nissimov T., Orenbuch-Harroch E., Chowers M., Elbaz M., Nesher L., Stein M., Maor Y., Cohen R., Hussein K., Weinberger M., et al. BNT162b2 Vaccine Breakthrough: Clinical Characteristics of 152 Fully Vaccinated Hospitalized COVID-19 Patients in Israel. Clin. Microbiol. Infect. 2021;27:1652–1657. doi: 10.1016/j.cmi.2021.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hallam J., Jones T., Alley J., Kohut M.L. Exercise after Influenza or COVID-19 Vaccination Increases Serum Antibody without an Increase in Side Effects. Brain Behav. Immun. 2022;102:1–10. doi: 10.1016/j.bbi.2022.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rzymski P., Pazgan-Simon M., Kamerys J., Moniuszko-Malinowska A., Sikorska K., Wernik J., Zarębska-Michaluk D., Supronowicz Ł., Sobala-Szczygieł B., Skrzat-Klapaczyńska A., et al. Severe Breakthrough COVID-19 Cases during Six Months of Delta Variant (B.1.617.2) Domination in Poland. Vaccines. 2022;10:557. doi: 10.3390/vaccines10040557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.WHO. [(accessed on 10 August 2023)]. Available online: https://apps.who.int/iris/rest/bitstreams/1484818/retrieve.
- 45.Markov P.V., Ghafari M., Beer M., Lythgoe K., Simmonds P., Stilianakis N.I., Katzourakis A. The Evolution of SARS-CoV-2. Nat. Rev. Microbiol. 2023;21:361–379. doi: 10.1038/s41579-023-00878-2. [DOI] [PubMed] [Google Scholar]
- 46.Strassburg M.A. The Global Eradication of Smallpox. Am. J. Infect. Control. 1982;10:53–59. doi: 10.1016/0196-6553(82)90003-7. [DOI] [PubMed] [Google Scholar]
- 47.Combe M., Sanjuán R. Variation in RNA Virus Mutation Rates across Host Cells. PLoS Pathog. 2014;10:e1003855. doi: 10.1371/journal.ppat.1003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amicone M., Borges V., Alves M.J., Isidro J., Zé-Zé L., Duarte S., Vieira L., Guiomar R., Gomes J.P., Gordo I. Mutation Rate of SARS-CoV-2 and Emergence of Mutators during Experimental Evolution. Evol. Med. Public Health. 2022;10:142–155. doi: 10.1093/emph/eoac010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manzanares-Meza L.D., Medina-Contreras O. SARS-CoV-2 and Influenza: A Comparative Overview and Treatment Implications. Bol. Med. Hosp. Infant. Mex. 2020;77:262–273. doi: 10.24875/BMHIM.20000183. [DOI] [PubMed] [Google Scholar]
- 50.Rawson J.M.O., Landman S.R., Reilly C.S., Mansky L.M. HIV-1 and HIV-2 Exhibit Similar Mutation Frequencies and Spectra in the Absence of G-to-A Hypermutation. Retrovirology. 2015;12:60. doi: 10.1186/s12977-015-0180-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakamura F., Takeda H., Ueda Y., Takai A., Takahashi K., Eso Y., Arasawa S., Iguchi E., Shimizu T., Mishima M., et al. Mutational Spectrum of Hepatitis C Virus in Patients with Chronic Hepatitis C Determined by Single Molecule Real-Time Sequencing. Sci. Rep. 2022;12:7083. doi: 10.1038/s41598-022-11151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Korber B., Fischer W.M., Gnanakaran S., Yoon H., Theiler J., Abfalterer W., Hengartner N., Giorgi E.E., Bhattacharya T., Foley B., et al. Tracking Changes in SARS-CoV-2 Spike: Evidence That D614G Increases Infectivity of the COVID-19 Virus. Cell. 2020;182:812–827.e19. doi: 10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jackson B., Boni M.F., Bull M.J., Colleran A., Colquhoun R.M., Darby A.C., Haldenby S., Hill V., Lucaci A., McCrone J.T., et al. Generation and Transmission of Interlineage Recombinants in the SARS-CoV-2 Pandemic. Cell. 2021;184:5179–5188.e8. doi: 10.1016/j.cell.2021.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chakraborty C., Bhattacharya M., Chopra H., Islam M.A., Saikumar G., Dhama K. The SARS-CoV-2 Omicron Recombinant Subvariants XBB, XBB.1, and XBB.1.5 Are Expanding Rapidly with Unique Mutations, Antibody Evasion, and Immune Escape Properties—An Alarming Global Threat of a Surge in COVID-19 Cases Again? Int. J. Surg. 2023;109:1041–1043. doi: 10.1097/JS9.0000000000000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parums D.V. Editorial: The XBB.1.5 (‘Kraken’) Subvariant of Omicron SARS-CoV-2 and Its Rapid Global Spread. Med. Sci. Monit. 2023;29:e939580. doi: 10.12659/MSM.939580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang R., Chen J., Wei G.-W. Mechanisms of SARS-CoV-2 Evolution Revealing Vaccine-Resistant Mutations in Europe and America. J. Phys. Chem. Lett. 2021;12:11850–11857. doi: 10.1021/acs.jpclett.1c03380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sadarangani M., Marchant A., Kollmann T.R. Immunological Mechanisms of Vaccine-Induced Protection against COVID-19 in Humans. Nat. Rev. Immunol. 2021;21:475–484. doi: 10.1038/s41577-021-00578-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lasrado N., Collier A.-R.Y., Miller J., Hachmann N.P., Liu J., Sciacca M., Wu C., Anand T., Bondzie E.A., Fisher J.L., et al. Waning Immunity against XBB.1.5 Following Bivalent MRNA Boosters. bioRxiv. 2023 doi: 10.1101/2023.01.22.525079. [DOI] [Google Scholar]
- 59.Muik A., Lui B.G., Diao H., Fu Y., Bacher M., Toker A., Grosser J., Ozhelvaci O., Grikscheit K., Hoehl S., et al. Progressive Loss of Conserved Spike Protein Neutralizing Antibody Sites in Omicron Sublineages Is Balanced by Preserved T Cell Immunity. Cell Rep. 2023;42:112888. doi: 10.1016/j.celrep.2023.112888. [DOI] [PubMed] [Google Scholar]
- 60.Abbasian M.H., Mahmanzar M., Rahimian K., Mahdavi B., Tokhanbigli S., Moradi B., Sisakht M.M., Deng Y. Global Landscape of SARS-CoV-2 Mutations and Conserved Regions. J. Transl. Med. 2023;21:152. doi: 10.1186/s12967-023-03996-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McCafferty S., Haque A.K.M.A., Vandierendonck A., Weidensee B., Plovyt M., Stuchlíková M., François N., Valembois S., Heyndrickx L., Michiels J., et al. A Dual-Antigen Self-Amplifying RNA SARS-CoV-2 Vaccine Induces Potent Humoral and Cellular Immune Responses and Protects against SARS-CoV-2 Variants through T Cell-Mediated Immunity. Mol. Ther. 2022;30:2968–2983. doi: 10.1016/j.ymthe.2022.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nextstrain Genomic Epidemiology of SARS-CoV-2 with Subsampling Focused Globally since Pandemic Start. [(accessed on 14 June 2023)]. Available online: https://nextstrain.org/ncov/
- 63.Viana R., Moyo S., Amoako D.G., Tegally H., Scheepers C., Althaus C.L., Anyaneji U.J., Bester P.A., Boni M.F., Chand M., et al. Rapid Epidemic Expansion of the SARS-CoV-2 Omicron Variant in Southern Africa. Nature. 2022;603:679–686. doi: 10.1038/s41586-022-04411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arora P., Zhang L., Rocha C., Sidarovich A., Kempf A., Schulz S., Cossmann A., Manger B., Baier E., Tampe B., et al. Comparable Neutralisation Evasion of SARS-CoV-2 Omicron Subvariants BA.1, BA.2, and BA.3. Lancet Infect. Dis. 2022;22:766–767. doi: 10.1016/S1473-3099(22)00224-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu L., Iketani S., Guo Y., Chan J.F.-W., Wang M., Liu L., Luo Y., Chu H., Huang Y., Nair M.S., et al. Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2. Nature. 2021;602:676–681. doi: 10.1038/s41586-021-04388-0. [DOI] [PubMed] [Google Scholar]
- 66.Hui K.P.Y., Ho J.C.W., Cheung M.-C., Ng K.-C., Ching R.H.H., Lai K.-L., Kam T.T., Gu H., Sit K.-Y., Hsin M.K.Y., et al. SARS-CoV-2 Omicron Variant Replication in Human Bronchus and Lung Ex Vivo. Nature. 2022;603:715–720. doi: 10.1038/s41586-022-04479-6. [DOI] [PubMed] [Google Scholar]
- 67.Suzuki R., Yamasoba D., Kimura I., Wang L., Kishimoto M., Ito J., Morioka Y., Nao N., Nasser H., Uriu K., et al. Attenuated Fusogenicity and Pathogenicity of SARS-CoV-2 Omicron Variant. Nature. 2022;603:700–705. doi: 10.1038/s41586-022-04462-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang X.-J., Yao L., Zhang H.-Y., Zhu K.-L., Zhao J., Zhan B.-D., Li Y.-K., He X.-J., Huang C., Wang Z.-Y., et al. Neutralization Sensitivity, Fusogenicity, and Infectivity of Omicron Subvariants. Genome Med. 2022;14:146. doi: 10.1186/s13073-022-01151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Halfmann P.J., Iida S., Iwatsuki-Horimoto K., Maemura T., Kiso M., Scheaffer S.M., Darling T.L., Joshi A., Loeber S., Singh G., et al. SARS-CoV-2 Omicron Virus Causes Attenuated Disease in Mice and Hamsters. Nature. 2022;603:687–692. doi: 10.1038/s41586-022-04441-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abdelnabi R., Foo C.S., Zhang X., Lemmens V., Maes P., Slechten B., Raymenants J., André E., Weynand B., Dallmeier K., et al. The Omicron (B.1.1.529) SARS-CoV-2 Variant of Concern Does Not Readily Infect Syrian Hamsters. Antivir. Res. 2022;198:105253. doi: 10.1016/j.antiviral.2022.105253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McMahan K., Giffin V., Tostanoski L.H., Chung B., Siamatu M., Suthar M.S., Halfmann P., Kawaoka Y., Piedra-Mora C., Jain N., et al. Reduced Pathogenicity of the SARS-CoV-2 Omicron Variant in Hamsters. Med. 2022;3:262–268.e4. doi: 10.1016/j.medj.2022.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chan J.F.-W., Chu H. Pathogenicity of SARS-CoV-2 Omicron BA.1.1 in Hamsters. EBioMedicine. 2022;80:104035. doi: 10.1016/j.ebiom.2022.104035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu W., Wang J., Yang Y., Tang C., Yang C., Li B., Wang H., Zhou Y., Huang Q., Yang H., et al. SARS-CoV-2 Omicron (B.1.1.529) Infection in Rhesus Macaques, Hamsters, and BALB/c Mice with Severe Lung Histopathological Damage. J. Med. Virol. 2023;95:e28846. doi: 10.1002/jmv.28846. [DOI] [PubMed] [Google Scholar]
- 74.Menni C., Valdes A.M., Polidori L., Antonelli M., Penamakuri S., Nogal A., Louca P., May A., Figueiredo J.C., Hu C., et al. Symptom Prevalence, Duration, and Risk of Hospital Admission in Individuals Infected with SARS-CoV-2 during Periods of Omicron and Delta Variant Dominance: A Prospective Observational Study from the ZOE COVID Study. Lancet. 2022;399:1618–1624. doi: 10.1016/S0140-6736(22)00327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Flisiak R., Rzymski P., Zarębska-Michaluk D., Ciechanowski P., Dobrowolska K., Rogalska M., Jaroszewicz J., Szymanek-Pasternak A., Rorat M., Kozielewicz D., et al. Variability in the Clinical Course of COVID-19 in a Retrospective Analysis of a Large Real-World Database. Viruses. 2023;15:149. doi: 10.3390/v15010149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Consolazio D., Murtas R., Tunesi S., Lamberti A., Senatore S., Faccini M., Russo A.G. A Comparison between Omicron and Earlier COVID-19 Variants’ Disease Severity in the Milan Area, Italy. Front. Epidemiol. 2022;2:891162. doi: 10.3389/fepid.2022.891162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nyberg T., Ferguson N.M., Nash S.G., Webster H.H., Flaxman S., Andrews N., Hinsley W., Bernal J.L., Kall M., Bhatt S., et al. Comparative Analysis of the Risks of Hospitalisation and Death Associated with SARS-CoV-2 Omicron (B.1.1.529) and Delta (B.1.617.2) Variants in England: A Cohort Study. Lancet. 2022;399:1303–1312. doi: 10.1016/S0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bager P., Wohlfahrt J., Bhatt S., Stegger M., Legarth R., Møller C.H., Skov R.L., Valentiner-Branth P., Voldstedlund M., Fischer T.K., et al. Risk of Hospitalisation Associated with Infection with SARS-CoV-2 Omicron Variant versus Delta Variant in Denmark: An Observational Cohort Study. Lancet Infect. Dis. 2022;22:967–976. doi: 10.1016/S1473-3099(22)00154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jassat W., Abdool Karim S.S., Ozougwu L., Welch R., Mudara C., Masha M., Rousseau P., Wolmarans M., Selikow A., Govender N., et al. Trends in Cases, Hospitalizations, and Mortality Related to the Omicron BA.4/BA.5 Subvariants in South Africa. Clin. Infect. Dis. 2023;76:1468–1475. doi: 10.1093/cid/ciac921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pung R., Kong X.P., Cui L., Chae S.-R., Chen M.I.-C., Lee V.J., Marc Ho Z.J. Severity of SARS-CoV-2 Omicron XBB Subvariants in Singapore. Lancet Reg. Health—West. Pac. 2023;37:100849. doi: 10.1016/j.lanwpc.2023.100849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Karyakarte R.P., Das R., Rajmane M.V., Dudhate S., Agarasen J., Pillai P., Chandankhede P.M., Labhshetwar R.S., Gadiyal Y., Kulkarni P.P., et al. Chasing SARS-CoV-2 XBB.1.16 Recombinant Lineage in India and the Clinical Profile of XBB.1.16 Cases in Maharashtra, India. Cureus. 2023;15:e39816. doi: 10.7759/cureus.39816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Flisiak R., Zarębska-Michaluk D., Dobrowolska K., Rorat M., Rogalska M., Kryńska J.A., Moniuszko-Malinowska A., Czupryna P., Kozielewicz D., Jaroszewicz J., et al. Change in the Clinical Picture of Hospitalized Patients with COVID-19 between the Early and Late Period of Dominance of the Omicron SARS-CoV-2 Variant. J. Clin. Med. 2023;12:5572. doi: 10.3390/jcm12175572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.World Health Organization Influenza. [(accessed on 14 June 2023)]. Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal)
- 84.Portmann L., de Kraker M.E.A., Fröhlich G., Thiabaud A., Roelens M., Schreiber P.W., Troillet N., Iten A., Widmer A., Harbarth S., et al. Hospital Outcomes of Community-Acquired SARS-CoV-2 Omicron Variant Infection Compared with Influenza Infection in Switzerland. JAMA Netw. Open. 2023;6:e2255599. doi: 10.1001/jamanetworkopen.2022.55599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Taylor C.A., Whitaker M., Anglin O., Milucky J., Patel K., Pham H., Chai S.J., Alden N.B., Yousey-Hindes K., Anderson E.J., et al. COVID-19-Associated Hospitalizations among Adults during SARS-CoV-2 Delta and Omicron Variant Predominance, by Race/Ethnicity and Vaccination Status—COVID-NET, 14 States, July 2021–January 2022. MMWR Morb. Mortal. Wkly. Rep. 2022;71:466–473. doi: 10.15585/mmwr.mm7112e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Antonelli M., Pujol J.C., Spector T.D., Ourselin S., Steves C.J. Risk of Long COVID Associated with Delta versus Omicron Variants of SARS-CoV-2. Lancet. 2022;399:2263–2264. doi: 10.1016/S0140-6736(22)00941-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thaweethai T., Jolley S.E., Karlson E.W., Levitan E.B., Levy B., McComsey G.A., McCorkell L., Nadkarni G.N., Parthasarathy S., Singh U., et al. Development of a Definition of Postacute Sequelae of SARS-CoV-2 Infection. JAMA. 2023;329:1934–1946. doi: 10.1001/jama.2023.8823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li Wan Po A. Omicron Variant as Nature’s Solution to the COVID-19 Pandemic. J. Clin. Pharm. Ther. 2022;47:3–5. doi: 10.1111/jcpt.13614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pascall D.J., Vink E., Blacow R., Bulteel N., Campbell A., Campbell R., Clifford S., Davis C., da Silva Filipe A., El Sakka N., et al. Directions of Change in Intrinsic Case Severity across Successive SARS-CoV-2 Variant Waves Have Been Inconsistent. J. Infect. 2023;87:128–135. doi: 10.1016/j.jinf.2023.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Earnest R., Uddin R., Matluk N., Renzette N., Turbett S.E., Siddle K.J., Loreth C., Adams G., Tomkins-Tinch C.H., Petrone M.E., et al. Comparative Transmissibility of SARS-CoV-2 Variants Delta and Alpha in New England, USA. Cell Rep. Med. 2022;3:100583. doi: 10.1016/j.xcrm.2022.100583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.King K.L., Wilson S., Napolitano J.M., Sell K.J., Rennert L., Parkinson C.L., Dean D. SARS-CoV-2 Variants of Concern Alpha and Delta Show Increased Viral Load in Saliva. PLoS ONE. 2022;17:e0267750. doi: 10.1371/journal.pone.0267750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yuasa S., Nakajima J., Takatsuki Y., Takahashi Y., Tani-Sassa C., Iwasaki Y., Nagano K., Sonobe K., Yoshimoto T., Nukui Y., et al. Viral Load of SARS-CoV-2 Omicron Is Not High despite Its High Infectivity. J. Med. Virol. 2022;94:5543–5546. doi: 10.1002/jmv.27974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Laitman A.M., Lieberman J.A., Hoffman N.G., Roychoudhury P., Mathias P.C., Greninger A.L. The SARS-CoV-2 Omicron Variant Does Not Have Higher Nasal Viral Loads Compared to the Delta Variant in Symptomatic and Asymptomatic Individuals. J. Clin. Microbiol. 2022;60:e0013922. doi: 10.1128/jcm.00139-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Puhach O., Adea K., Hulo N., Sattonnet P., Genecand C., Iten A., Jacquérioz F., Kaiser L., Vetter P., Eckerle I., et al. Infectious Viral Load in Unvaccinated and Vaccinated Individuals Infected with Ancestral, Delta or Omicron SARS-CoV-2. Nat. Med. 2022;28:1491–1500. doi: 10.1038/s41591-022-01816-0. [DOI] [PubMed] [Google Scholar]
- 95.Wu L., Zhou L., Mo M., Liu T., Wu C., Gong C., Lu K., Gong L., Zhu W., Xu Z. SARS-CoV-2 Omicron RBD Shows Weaker Binding Affinity than the Currently Dominant Delta Variant to Human ACE2. Signal Transduct. Target. Ther. 2022;7:8. doi: 10.1038/s41392-021-00863-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Meng B., Abdullahi A., Ferreira I.A.T.M., Goonawardane N., Saito A., Kimura I., Yamasoba D., Gerber P.P., Fatihi S., Rathore S., et al. Altered TMPRSS2 Usage by SARS-CoV-2 Omicron Impacts Infectivity and Fusogenicity. Nature. 2022;603:706–714. doi: 10.1038/s41586-022-04474-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.He X., Lau E.H.Y., Wu P., Deng X., Wang J., Hao X., Lau Y.C., Wong J.Y., Guan Y., Tan X., et al. Temporal Dynamics in Viral Shedding and Transmissibility of COVID-19. Nat. Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 98.Manica M., De Bellis A., Guzzetta G., Mancuso P., Vicentini M., Venturelli F., Zerbini A., Bisaccia E., Litvinova M., Menegale F., et al. Intrinsic Generation Time of the SARS-CoV-2 Omicron Variant: An Observational Study of Household Transmission. Lancet Reg. Health Eur. 2022;19:100446. doi: 10.1016/j.lanepe.2022.100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jiang L., Tang L., Zhu L., Zhu Y., Yang S., Chen W., Fan Y., Yang X., Yang S., Zheng Y., et al. Viral Dynamics during SARS-CoV-2 Omicron Infection Highlight Presymptomatic and Asymptomatic Infectiousness. J. Infect. 2023;86:537–539. doi: 10.1016/j.jinf.2022.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fajnzylber J., Regan J., Coxen K., Corry H., Wong C., Rosenthal A., Worrall D., Giguel F., Piechocka-Trocha A., Atyeo C., et al. SARS-CoV-2 Viral Load Is Associated with Increased Disease Severity and Mortality. Nat. Commun. 2020;11:5493. doi: 10.1038/s41467-020-19057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Saito A., Irie T., Suzuki R., Maemura T., Nasser H., Uriu K., Kosugi Y., Shirakawa K., Sadamasu K., Kimura I., et al. Enhanced Fusogenicity and Pathogenicity of SARS-CoV-2 Delta P681R Mutation. Nature. 2022;602:300–306. doi: 10.1038/s41586-021-04266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kimura I., Yamasoba D., Tamura T., Nao N., Suzuki T., Oda Y., Mitoma S., Ito J., Nasser H., Zahradnik J., et al. Virological Characteristics of the SARS-CoV-2 Omicron BA.2 Subvariants, Including BA.4 and BA.5. Cell. 2022;185:3992–4007.e16. doi: 10.1016/j.cell.2022.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xia S., Wang L., Jiao F., Yu X., Xu W., Huang Z., Li X., Wang Q., Zhu Y., Man Q., et al. SARS-CoV-2 Omicron Subvariants Exhibit Distinct Fusogenicity, but Similar Sensitivity, to Pan-CoV Fusion Inhibitors. Emerg. Microbes Infect. 2023;12:2178241. doi: 10.1080/22221751.2023.2178241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xia S., Jiao F., Wang L., Yu X., Lu T., Fu Y., Huang Z., Li X., Huang J., Wang Q., et al. SARS-CoV-2 Omicron XBB Subvariants Exhibit Enhanced Fusogenicity and Substantial Immune Evasion in Elderly Population, but High Sensitivity to Pan-Coronavirus Fusion Inhibitors. J. Med. Virol. 2023;95:e28641. doi: 10.1002/jmv.28641. [DOI] [PubMed] [Google Scholar]
- 105.Yuan S., Ye Z.-W., Liang R., Tang K., Zhang A.J., Lu G., Ong C.P., Man Poon V.K., Chan C.C.-S., Mok B.W.-Y., et al. Pathogenicity, Transmissibility, and Fitness of SARS-CoV-2 Omicron in Syrian Hamsters. Science. 2022;377:428–433. doi: 10.1126/science.abn8939. [DOI] [PubMed] [Google Scholar]
- 106.Hoffmann M., Wong L.-Y.R., Arora P., Zhang L., Rocha C., Odle A., Nehlmeier I., Kempf A., Richter A., Halwe N.J., et al. Omicron Subvariant BA.5 Efficiently Infects Lung Cells. Nat. Commun. 2023;14:3500. doi: 10.1038/s41467-023-39147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wolter N., Jassat W., Walaza S., Welch R., Moultrie H., Groome M.J., Amoako D.G., Everatt J., Bhiman J.N., Scheepers C., et al. Clinical Severity of SARS-CoV-2 Omicron BA.4 and BA.5 Lineages Compared to BA.1 and Delta in South Africa. Nat. Commun. 2022;13:5860. doi: 10.1038/s41467-022-33614-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Robertson C., Kerr S., Sheikh A. Severity of Omicron BA.5 Variant and Protective Effect of Vaccination: National Cohort and Matched Analyses in Scotland. Lancet Reg. Health Eur. 2023;28:100638. doi: 10.1016/j.lanepe.2023.100638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ciuffreda L., Lorenzo-Salazar J.M., García-Martínez de Artola D., Gil-Campesino H., Alcoba-Florez J., Rodríguez-Pérez H., Íñigo-Campos A., Salas-Hernández J., Rodríguez-Nuñez J., Muñoz-Barrera A., et al. Reinfection Rate and Disease Severity of the BA.5 Omicron SARS-CoV-2 Lineage Compared to Previously Circulating Variants of Concern in the Canary Islands (Spain) Emerg. Microbes Infect. 2023;12:2202281. doi: 10.1080/22221751.2023.2202281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Oude Munnink B.B., Sikkema R.S., Nieuwenhuijse D.F., Molenaar R.J., Munger E., Molenkamp R., van der Spek A., Tolsma P., Rietveld A., Brouwer M., et al. Transmission of SARS-CoV-2 on Mink Farms between Humans and Mink and Back to Humans. Science. 2021;371:172–177. doi: 10.1126/science.abe5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hoffmann M., Zhang L., Krüger N., Graichen L., Kleine-Weber H., Hofmann-Winkler H., Kempf A., Nessler S., Riggert J., Winkler M.S., et al. SARS-CoV-2 Mutations Acquired in Mink Reduce Antibody-Mediated Neutralization. Cell Rep. 2021;35:109017. doi: 10.1016/j.celrep.2021.109017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Domańska-Blicharz K., Orłowska A., Smreczak M., Niemczuk K., Iwan E., Bomba A., Lisowska A., Opolska J., Trębas P., Potyrało P., et al. Mink SARS-CoV-2 Infection in Poland—Short Communication. J. Vet. Res. 2021;65:1–5. doi: 10.2478/jvetres-2021-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Palmer M.V., Martins M., Falkenberg S., Buckley A., Caserta L.C., Mitchell P.K., Cassmann E.D., Rollins A., Zylich N.C., Renshaw R.W., et al. Susceptibility of White-Tailed Deer (Odocoileus Virginianus) to SARS-CoV-2. J. Virol. 2021;95:e00083-2. doi: 10.1128/JVI.00083-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chandler J.C., Bevins S.N., Ellis J.W., Linder T.J., Tell R.M., Jenkins-Moore M., Root J.J., Lenoch J.B., Robbe-Austerman S., DeLiberto T.J., et al. SARS-CoV-2 Exposure in Wild White-Tailed Deer (Odocoileus Virginianus) Proc. Natl. Acad. Sci. USA. 2021;118:e2114828118. doi: 10.1073/pnas.2114828118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li L., Han P., Huang B., Xie Y., Li W., Zhang D., Han P., Xu Z., Bai B., Zhou J., et al. Broader-Species Receptor Binding and Structural Bases of Omicron SARS-CoV-2 to Both Mouse and Palm-Civet ACE2s. Cell Discov. 2022;8:65. doi: 10.1038/s41421-022-00431-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Konishi T. SARS-CoV-2 Mutations among Minks Show Reduced Lethality and Infectivity to Humans. PLoS ONE. 2021;16:e0247626. doi: 10.1371/journal.pone.0247626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Devaux C.A., Pinault L., Delerce J., Raoult D., Levasseur A., Frutos R. Spread of Mink SARS-CoV-2 Variants in Humans: A Model of Sarbecovirus Interspecies Evolution. Front. Microbiol. 2021;12:675528. doi: 10.3389/fmicb.2021.675528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Willgert K., Didelot X., Surendran-Nair M., Kuchipudi S.V., Ruden R.M., Yon M., Nissly R.H., Vandegrift K.J., Nelli R.K., Li L., et al. Transmission History of SARS-CoV-2 in Humans and White-Tailed Deer. Sci. Rep. 2022;12:12094. doi: 10.1038/s41598-022-16071-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kuchipudi S.V., Surendran-Nair M., Ruden R.M., Yon M., Nissly R.H., Vandegrift K.J., Nelli R.K., Li L., Jayarao B.M., Maranas C.D., et al. Multiple Spillovers from Humans and Onward Transmission of SARS-CoV-2 in White-Tailed Deer. Proc. Natl. Acad. Sci. USA. 2022;119:e2121644119. doi: 10.1073/pnas.2121644119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gupta S., Cantor J., Simon K.I., Bento A.I., Wing C., Whaley C.M. Vaccinations against COVID-19 May Have Averted up to 140,000 Deaths in the United States. Health Aff. 2021;40:1465–1472. doi: 10.1377/hlthaff.2021.00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kayano T., Sasanami M., Kobayashi T., Ko Y.K., Otani K., Suzuki M., Nishiura H. Number of Averted COVID-19 Cases and Deaths Attributable to Reduced Risk in Vaccinated Individuals in Japan. Lancet Reg. Health West. Pac. 2022;28:100571. doi: 10.1016/j.lanwpc.2022.100571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mattiuzzi C., Henry B.M., Lippi G. COVID-19 Vaccination Uptake Strongly Predicts Averted Deaths of Older People across Europe. Biomed. J. 2022;45:961–962. doi: 10.1016/j.bj.2022.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sikora D., Rzymski P. COVID-19 Vaccination and Rates of Infections, Hospitalizations, ICU Admissions, and Deaths in the European Economic Area during Autumn 2021 Wave of SARS-CoV-2. Vaccines. 2022;10:437. doi: 10.3390/vaccines10030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yi S., Choe Y.J., Lim D.S., Lee H.R., Kim J., Kim Y.-Y., Kim R.K., Jang E.J., Lee S., Park E., et al. Impact of National COVID-19 Vaccination Campaign, South Korea. Vaccine. 2022;40:3670–3675. doi: 10.1016/j.vaccine.2022.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McCarthy C.V., O’Mara O., van Leeuwen E., CMMID COVID-19 Working Group. Jit M., Sandmann F. The Impact of COVID-19 Vaccination in Prisons in England and Wales: A Metapopulation Model. BMC Public Health. 2022;22:1003. doi: 10.1186/s12889-022-13219-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Santos C.V.B.D., de Noronha T.G., Werneck G.L., Struchiner C.J., Villela D.A.M. Estimated COVID-19 Severe Cases and Deaths Averted in the First Year of the Vaccination Campaign in Brazil: A Retrospective Observational Study. Lancet Reg. Health Am. 2023;17:100418. doi: 10.1016/j.lana.2022.100418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Haas E.J., McLaughlin J.M., Khan F., Angulo F.J., Anis E., Lipsitch M., Singer S.R., Mircus G., Brooks N., Smaja M., et al. Infections, Hospitalisations, and Deaths Averted via a Nationwide Vaccination Campaign Using the Pfizer-BioNTech BNT162b2 MRNA COVID-19 Vaccine in Israel: A Retrospective Surveillance Study. Lancet Infect. Dis. 2022;22:357–366. doi: 10.1016/S1473-3099(21)00566-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sacco C., Mateo-Urdiales A., Petrone D., Spuri M., Fabiani M., Vescio M.F., Bressi M., Riccardo F., Del Manso M., Bella A., et al. Estimating Averted COVID-19 Cases, Hospitalisations, Intensive Care Unit Admissions and Deaths by COVID-19 Vaccination, Italy, January-September 2021. Eurosurveillance. 2021;26:2101001. doi: 10.2807/1560-7917.ES.2021.26.47.2101001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Brannock M.D., Chew R.F., Preiss A.J., Hadley E.C., Redfield S., McMurry J.A., Leese P.J., Girvin A.T., Crosskey M., Zhou A.G., et al. Long COVID Risk and Pre-COVID Vaccination in an EHR-Based Cohort Study from the RECOVER Program. Nat. Commun. 2023;14:2914. doi: 10.1038/s41467-023-38388-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Simon M.A., Luginbuhl R.D., Parker R. Reduced Incidence of Long-COVID Symptoms Related to Administration of COVID-19 Vaccines Both before COVID-19 Diagnosis and up to 12 Weeks After. medRxiv. 2021 doi: 10.1101/2021.11.17.21263608. [DOI] [Google Scholar]
- 131.Antonelli M., Penfold R.S., Merino J., Sudre C.H., Molteni E., Berry S., Canas L.S., Graham M.S., Klaser K., Modat M., et al. Risk Factors and Disease Profile of Post-Vaccination SARS-CoV-2 Infection in UK Users of the COVID Symptom Study App: A Prospective, Community-Based, Nested, Case-Control Study. Lancet Infect. Dis. 2022;22:43–55. doi: 10.1016/S1473-3099(21)00460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Senjam S.S., Balhara Y.P.S., Kumar P., Nichal N., Manna S., Madan K., Ahmed N.H., Gupta N., Sharma R., Gupta Y., et al. Assessment of Post COVID-19 Health Problems and Its Determinants in North India: A Descriptive Cross Section Study. medRxiv. 2021 doi: 10.1101/2021.10.03.21264490. [DOI] [Google Scholar]
- 133.Ayoubkhani D., Bosworth M.L., King S., Pouwels K.B., Glickman M., Nafilyan V., Zaccardi F., Khunti K., Alwan N.A., Walker A.S. Risk of Long COVID in People Infected with Severe Acute Respiratory Syndrome Coronavirus 2 after 2 Doses of a Coronavirus Disease 2019 Vaccine: Community-Based, Matched Cohort Study. Open Forum Infect. Dis. 2022;9:ofac464. doi: 10.1093/ofid/ofac464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Al-Aly Z., Bowe B., Xie Y. Long COVID after Breakthrough SARS-CoV-2 Infection. Nat. Med. 2022;28:1461–1467. doi: 10.1038/s41591-022-01840-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Taquet M., Dercon Q., Harrison P.J. Six-Month Sequelae of Post-Vaccination SARS-CoV-2 Infection: A Retrospective Cohort Study of 10,024 Breakthrough Infections. Brain Behav. Immun. 2022;103:154–162. doi: 10.1016/j.bbi.2022.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Notarte K.I., Catahay J.A., Velasco J.V., Pastrana A., Ver A.T., Pangilinan F.C., Peligro P.J., Casimiro M., Guerrero J.J., Gellaco M.M.L., et al. Impact of COVID-19 Vaccination on the Risk of Developing Long-COVID and on Existing Long-COVID Symptoms: A Systematic Review. EClinicalMedicine. 2022;53:101624. doi: 10.1016/j.eclinm.2022.101624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yu X., Wei D., Xu W., Li Y., Li X., Zhang X., Qu J., Yang Z., Chen E. Reduced Sensitivity of SARS-CoV-2 Omicron Variant to Antibody Neutralization Elicited by Booster Vaccination. Cell Discov. 2022;8:4. doi: 10.1038/s41421-022-00375-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yu J., Collier A.-R.Y., Rowe M., Mardas F., Ventura J.D., Wan H., Miller J., Powers O., Chung B., Siamatu M., et al. Neutralization of the SARS-CoV-2 Omicron BA.1 and BA.2 Variants. N. Engl. J. Med. 2022;386:1579–1580. doi: 10.1056/NEJMc2201849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lau J.J., Cheng S.M.S., Leung K., Lee C.K., Hachim A., Tsang L.C.H., Yam K.W.H., Chaothai S., Kwan K.K.H., Chai Z.Y.H., et al. Real-World COVID-19 Vaccine Effectiveness against the Omicron BA.2 Variant in a SARS-CoV-2 Infection-Naive Population. Nat. Med. 2023;29:348–357. doi: 10.1038/s41591-023-02219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mohammed H., Pham-Tran D.D., Yeoh Z.Y.M., Wang B., McMillan M., Andraweera P.H., Marshall H.S. A Systematic Review and Meta-Analysis on the Real-World Effectiveness of COVID-19 Vaccines against Infection, Symptomatic and Severe COVID-19 Disease Caused by the Omicron Variant (B.1.1.529) Vaccines. 2023;11:224. doi: 10.3390/vaccines11020224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zarębska-Michaluk D., Hu C., Brzdęk M., Flisiak R., Rzymski P. COVID-19 Vaccine Booster Strategies for Omicron SARS-CoV-2 Variant: Effectiveness and Future Prospects. Vaccines. 2022;10:1223. doi: 10.3390/vaccines10081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Solante R., Alvarez-Moreno C., Burhan E., Chariyalertsak S., Chiu N.-C., Chuenkitmongkol S., Dung D.V., Hwang K.-P., Ortiz Ibarra J., Kiertiburanakul S., et al. Expert Review of Global Real-World Data on COVID-19 Vaccine Booster Effectiveness and Safety during the Omicron-Dominant Phase of the Pandemic. Expert Rev. Vaccines. 2023;22:1–16. doi: 10.1080/14760584.2023.2143347. [DOI] [PubMed] [Google Scholar]
- 143.Wu N., Joyal-Desmarais K., Ribeiro P.A.B., Vieira A.M., Stojanovic J., Sanuade C., Yip D., Bacon S.L. Long-Term Effectiveness of COVID-19 Vaccines against Infections, Hospitalisations, and Mortality in Adults: Findings from a Rapid Living Systematic Evidence Synthesis and Meta-Analysis up to December, 2022. Lancet Respir. Med. 2023;11:439–452. doi: 10.1016/S2213-2600(23)00015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li Y., Liang H., Ding X., Cao Y., Yang D., Duan Y. Effectiveness of COVID-19 Vaccine in Children and Adolescents with the Omicron Variant: A Systematic Review and Meta-Analysis. J. Infect. 2023;86:e64–e66. doi: 10.1016/j.jinf.2023.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mendes D., Chapman R., Aruffo E., Gal P., Nguyen J.L., Hamson L., Di Fusco M., Czudek C., Yang J. Public Health Impact of UK COVID-19 Booster Vaccination Programs during Omicron Predominance. Expert Rev. Vaccines. 2023;22:90–103. doi: 10.1080/14760584.2023.2158816. [DOI] [PubMed] [Google Scholar]
- 146.Link-Gelles R., Ciesla A.A., Fleming-Dutra K.E., Smith Z.R., Britton A., Wiegand R.E., Miller J.D., Accorsi E.K., Schrag S.J., Verani J.R., et al. Effectiveness of Bivalent MRNA Vaccines in Preventing Symptomatic SARS-CoV-2 Infection—Increasing Community Access to Testing Program, United States, September-November 2022. MMWR Morb. Mortal. Wkly. Rep. 2022;71:1526–1530. doi: 10.15585/mmwr.mm7148e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Link-Gelles R., Ciesla A.A., Roper L.E., Scobie H.M., Ali A.R., Miller J.D., Wiegand R.E., Accorsi E.K., Verani J.R., Shang N., et al. Early Estimates of Bivalent MRNA Booster Dose Vaccine Effectiveness in Preventing Symptomatic SARS-CoV-2 Infection Attributable to Omicron BA.5- and XBB/XBB.1.5-Related Sublineages among Immunocompetent Adults—Increasing Community Access to Testing Program, United States, December 2022-January 2023. MMWR Morb. Mortal. Wkly. Rep. 2023;72:119–124. doi: 10.15585/mmwr.mm7205e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Arbel R., Peretz A., Sergienko R., Friger M., Beckenstein T., Duskin-Bitan H., Yaron S., Hammerman A., Bilenko N., Netzer D. Effectiveness of a Bivalent MRNA Vaccine Booster Dose to Prevent Severe COVID-19 Outcomes: A Retrospective Cohort Study. Lancet Infect. Dis. 2023;23:914–921. doi: 10.1016/S1473-3099(23)00122-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Collier A.-R.Y., Miller J., Hachmann N.P., McMahan K., Liu J., Bondzie E.A., Gallup L., Rowe M., Schonberg E., Thai S., et al. Immunogenicity of BA.5 Bivalent MRNA Vaccine Boosters. N. Engl. J. Med. 2023;388:565–567. doi: 10.1056/NEJMc2213948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Offit P.A. Bivalent COVID-19 Vaccines—A Cautionary Tale. N. Engl. J. Med. 2023;388:481–483. doi: 10.1056/NEJMp2215780. [DOI] [PubMed] [Google Scholar]
- 151.Wang Q., Bowen A., Valdez R., Gherasim C., Gordon A., Liu L., Ho D.D. Antibody Response to Omicron BA.4-BA.5 Bivalent Booster. N. Engl. J. Med. 2023;388:567–569. doi: 10.1056/NEJMc2213907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Carreño J.M., Singh G., Simon V., Krammer F., PVI Study Group Bivalent COVID-19 Booster Vaccines and the Absence of BA.5-Specific Antibodies. Lancet Microbe. 2023;4:e569. doi: 10.1016/S2666-5247(23)00118-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.WHO Statement on the Antigen Composition of COVID-19 Vaccines. [(accessed on 18 June 2023)]. Available online: https://www.who.int/news/item/18-05-2023-statement-on-the-antigen-composition-of-COVID-19-vaccines.
- 154.Ballouz T., Menges D., Kaufmann M., Amati R., Frei A., von Wyl V., Fehr J.S., Albanese E., Puhan M.A. Post COVID-19 Condition after Wildtype, Delta, and Omicron SARS-CoV-2 Infection and Prior Vaccination: Pooled Analysis of Two Population-Based Cohorts. PLoS ONE. 2023;18:e0281429. doi: 10.1371/journal.pone.0281429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rzymski P., Szuster-Ciesielska A., Dzieciątkowski T., Gwenzi W., Fal A. MRNA Vaccines: The Future of Prevention of Viral Infections? J. Med. Virol. 2023;95:e28572. doi: 10.1002/jmv.28572. [DOI] [PubMed] [Google Scholar]
- 156.Hajnik R.L., Plante J.A., Liang Y., Alameh M.-G., Tang J., Bonam S.R., Zhong C., Adam A., Scharton D., Rafael G.H., et al. Dual Spike and Nucleocapsid MRNA Vaccination Confer Protection against SARS-CoV-2 Omicron and Delta Variants in Preclinical Models. Sci. Transl. Med. 2022;14:eabq1945. doi: 10.1126/scitranslmed.abq1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Alu A., Chen L., Lei H., Wei Y., Tian X., Wei X. Intranasal COVID-19 Vaccines: From Bench to Bed. EBioMedicine. 2022;76:103841. doi: 10.1016/j.ebiom.2022.103841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ramvikas M., Arumugam M., Chakrabarti S.R., Jaganathan K.S. Micro and Nanotechnology in Vaccine Development. Elsevier; Amsterdam, The Netherlands: 2017. Nasal Vaccine Delivery; pp. 279–301. [Google Scholar]
- 159.Sengupta A., Azharuddin M., Cardona M.E., Devito C., von Castelmur E., Wehlin A., Pietras Z., Sunnerhagen M., Selegård R., Aili D., et al. Intranasal Coronavirus SARS-CoV-2 Immunization with Lipid Adjuvants Provides Systemic and Mucosal Immune Response against SARS-CoV-2 S1 Spike and Nucleocapsid Protein. Vaccines. 2022;10:504. doi: 10.3390/vaccines10040504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shim S., Soh S.H., Im Y.B., Ahn C., Park H.-T., Park H.-E., Park W.B., Kim S., Yoo H.S. Induction of Systemic Immunity through Nasal-Associated Lymphoid Tissue (NALT) of Mice Intranasally Immunized with Brucella Abortus Malate Dehydrogenase-Loaded Chitosan Nanoparticles. PLoS ONE. 2020;15:e0228463. doi: 10.1371/journal.pone.0228463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.McLenon J., Rogers M.A.M. The Fear of Needles: A Systematic Review and Meta-Analysis. J. Adv. Nurs. 2019;75:30–42. doi: 10.1111/jan.13818. [DOI] [PubMed] [Google Scholar]
- 162.Oladoye M.J. Intranasal Vaccines: A Panacea to Vaccine Hesitancy? Med. Res. J. 2022;7:274–275. doi: 10.5603/MRJ.a2022.0043. [DOI] [Google Scholar]
- 163.Dhama K., Dhawan M., Tiwari R., Emran T.B., Mitra S., Rabaan A.A., Alhumaid S., Alawi Z.A., Al Mutair A. COVID-19 Intranasal Vaccines: Current Progress, Advantages, Prospects, and Challenges. Hum. Vaccin. Immunother. 2022;18:2045853. doi: 10.1080/21645515.2022.2045853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Madhavan M., Ritchie A.J., Aboagye J., Jenkin D., Provstgaad-Morys S., Tarbet I., Woods D., Davies S., Baker M., Platt A., et al. Tolerability and Immunogenicity of an Intranasally-Administered Adenovirus-Vectored COVID-19 Vaccine: An Open-Label Partially-Randomised Ascending Dose Phase I Trial. EBioMedicine. 2022;85:104298. doi: 10.1016/j.ebiom.2022.104298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.van Doremalen N., Purushotham J.N., Schulz J.E., Holbrook M.G., Bushmaker T., Carmody A., Port J.R., Yinda C.K., Okumura A., Saturday G., et al. Intranasal ChAdOx1 NCoV-19/AZD1222 Vaccination Reduces Viral Shedding after SARS-CoV-2 D614G Challenge in Preclinical Models. Sci. Transl. Med. 2021;13:eabh0755. doi: 10.1126/scitranslmed.abh0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ndeupen S., Qin Z., Jacobsen S., Bouteau A., Estanbouli H., Igyártó B.Z. The MRNA-LNP Platform’s Lipid Nanoparticle Component Used in Preclinical Vaccine Studies Is Highly Inflammatory. iScience. 2021;24:103479. doi: 10.1016/j.isci.2021.103479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Đaković Rode O., Bodulić K., Zember S., Cetinić Balent N., Novokmet A., Čulo M., Rašić Ž., Mikulić R., Markotić A. Decline of Anti-SARS-CoV-2 IgG Antibody Levels 6 Months after Complete BNT162b2 Vaccination in Healthcare Workers to Levels Observed Following the First Vaccine Dose. Vaccines. 2022;10:153. doi: 10.3390/vaccines10020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hosseinian S., de Assis R., Khalil G., Luu M.K., Jain A., Horvath P., Nakajima R., Palma A.M., Hoang A., Razzak E., et al. Analysis and Comparison of SARS-CoV-2 Variant Antibodies and Neutralizing Activity for 6 Months after a Booster MRNA Vaccine in a Healthcare Worker Population. Front. Immunol. 2023;14:1166261. doi: 10.3389/fimmu.2023.1166261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Silva M.F.S., Pinto A.C.M.D., de Oliveira F.d.C.E., Caetano L.F., Araújo F.M.d.C., Fonseca M.H.G. Antibody Response 6 Months after the Booster Dose of Pfizer in Previous Recipients of CoronaVac. J. Med. Virol. 2023;95:e28169. doi: 10.1002/jmv.28169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Townsend J.P., Hassler H.B., Dornburg A. Infection by SARS-CoV-2 with Alternate Frequencies of MRNA Vaccine Boosting. J. Med. Virol. 2023;95:e28461. doi: 10.1002/jmv.28461. [DOI] [PubMed] [Google Scholar]
- 171.European Centre for Disease Prevention and Control COVID-19 Vaccine Tracker. [(accessed on 31 January 2022)]. Available online: https://vaccinetracker.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html.
- 172.Fieselmann J., Annac K., Erdsiek F., Yilmaz-Aslan Y., Brzoska P. What Are the Reasons for Refusing a COVID-19 Vaccine? A Qualitative Analysis of Social Media in Germany. BMC Public Health. 2022;22:846. doi: 10.1186/s12889-022-13265-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rzymski P., Poniedziałek B., Fal A. Willingness to Receive the Booster COVID-19 Vaccine Dose in Poland. Vaccines. 2021;9:1286. doi: 10.3390/vaccines9111286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rzymski P., Sikora D., Zeyland J., Poniedziałek B., Kiedik D., Falfushynska H., Fal A. Frequency and Nuisance Level of Adverse Events in Individuals Receiving Homologous and Heterologous COVID-19 Booster Vaccine. Vaccines. 2022;10:754. doi: 10.3390/vaccines10050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sobierajski T., Rzymski P., Wanke-Rytt M. Impact of the COVID-19 Pandemic on Attitudes toward Vaccination: Representative Study of Polish Society. Vaccines. 2023;11:1069. doi: 10.3390/vaccines11061069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Capurro G., Tustin J., Jardine C.G., Driedger S.M. When Good Messages Go Wrong: Perspectives on COVID-19 Vaccines and Vaccine Communication from Generally Vaccine Accepting Individuals in Canada. Hum. Vaccin. Immunother. 2022;18:2145822. doi: 10.1080/21645515.2022.2145822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hernandez N., Caetano-Anollés G. Worldwide Correlations Support COVID-19 Seasonal Behavior and Impact of Global Change. Evol. Bioinform. Online. 2023;19:11769343231169376. doi: 10.1177/11769343231169377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.D’Amico F., Marmiere M., Righetti B., Scquizzato T., Zangrillo A., Puglisi R., Landoni G. COVID-19 Seasonality in Temperate Countries. Environ. Res. 2022;206:112614. doi: 10.1016/j.envres.2021.112614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wiemken T.L., Khan F., Puzniak L., Yang W., Simmering J., Polgreen P., Nguyen J.L., Jodar L., McLaughlin J.M. Seasonal Trends in COVID-19 Cases, Hospitalizations, and Mortality in the United States and Europe. Sci. Rep. 2023;13:3886. doi: 10.1038/s41598-023-31057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gavenčiak T., Monrad J.T., Leech G., Sharma M., Mindermann S., Bhatt S., Brauner J., Kulveit J. Seasonal Variation in SARS-CoV-2 Transmission in Temperate Climates: A Bayesian Modelling Study in 143 European Regions. PLoS Comput. Biol. 2022;18:e1010435. doi: 10.1371/journal.pcbi.1010435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Centers for Disease Control and Prevention National Emergency Department Visits for COVID-19, Influenza, and Respiratory Syncytial Virus. [(accessed on 14 June 2023)]; Available online: https://www.cdc.gov/ncird/surveillance/respiratory-illnesses/index.html.
- 182.Harris E. FDA Clears RSV Vaccine for Adults Aged 60 Years or Older. JAMA. 2023;329:1817. doi: 10.1001/jama.2023.8657. [DOI] [PubMed] [Google Scholar]
- 183.Dulfer E.A., Geckin B., Taks E.J.M., GeurtsvanKessel C.H., Dijkstra H., van Emst L., van der Gaast-de Jongh C.E., van Mourik D., Koopmans P.C., Domínguez-Andrés J., et al. Timing and Sequence of Vaccination against COVID-19 and Influenza (TACTIC): A Single-Blind, Placebo-Controlled Randomized Clinical Trial. Lancet Reg. Health Eur. 2023;29:100628. doi: 10.1016/j.lanepe.2023.100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.ECDC-EMA Statement on Updating COVID-19 Vaccines Composition for New SARS-CoV-2 Virus Variants. [(accessed on 14 June 2023)]. Available online: https://www.ecdc.europa.eu/en/news-events/ecdc-ema-statement-updating-COVID-19-vaccines-composition-new-sars-cov-2-virus-variants.
- 185.Imai M., Ito M., Kiso M., Yamayoshi S., Uraki R., Fukushi S., Watanabe S., Suzuki T., Maeda K., Sakai-Tagawa Y., et al. Efficacy of Antiviral Agents against Omicron Subvariants BQ.1.1 and XBB. N. Engl. J. Med. 2023;388:89–91. doi: 10.1056/NEJMc2214302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wan E.Y.F., Yan V.K.C., Mok A.H.Y., Wang B., Xu W., Cheng F.W.T., Lai F.T.T., Chui C.S.L., Li X., Wong C.K.H., et al. Effectiveness of Molnupiravir and Nirmatrelvir-Ritonavir in Hospitalized Patients with COVID-19: A Target Trial Emulation Study. Ann. Intern. Med. 2023;176:505–514. doi: 10.7326/M22-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Weng C., Xie R., Han G., Yuan Y., Li S., Wang C., Wang X., Jiang W., Jiang L. Safety and Efficacy of Paxlovid against Omicron Variants of Coronavirus Disease 2019 in Elderly Patients. Infect. Dis. Ther. 2023;12:649–662. doi: 10.1007/s40121-023-00760-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Schwartz K.L., Wang J., Tadrous M., Langford B.J., Daneman N., Leung V., Gomes T., Friedman L., Daley P., Brown K.A. Population-Based Evaluation of the Effectiveness of Nirmatrelvir-Ritonavir for Reducing Hospital Admissions and Mortality from COVID-19. CMAJ. 2023;195:E220–E226. doi: 10.1503/cmaj.221608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Xie Y., Choi T., Al-Aly Z. Association of Treatment with Nirmatrelvir and the Risk of Post-COVID-19 Condition. JAMA Intern. Med. 2023;183:554–564. doi: 10.1001/jamainternmed.2023.0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Imran M., Kumar Arora M., Asdaq S.M.B., Khan S.A., Alaqel S.I., Alshammari M.K., Alshehri M.M., Alshrari A.S., Mateq Ali A., Al-Shammeri A.M., et al. Discovery, Development, and Patent Trends on Molnupiravir: A Prospective Oral Treatment for COVID-19. Molecules. 2021;26:5795. doi: 10.3390/molecules26195795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Agostini M.L., Pruijssers A.J., Chappell J.D., Gribble J., Lu X., Andres E.L., Bluemling G.R., Lockwood M.A., Sheahan T.P., Sims A.C., et al. Small-Molecule Antiviral β-d-N 4-Hydroxycytidine Inhibits a Proofreading-Intact Coronavirus with a High Genetic Barrier to Resistance. J. Virol. 2019;93:e01348-19. doi: 10.1128/JVI.01348-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Barnard D.L., Hubbard V.D., Burton J., Smee D.F., Morrey J.D., Otto M.J., Sidwell R.W. Inhibition of Severe Acute Respiratory Syndrome-Associated Coronavirus (SARSCoV) by Calpain Inhibitors and Beta-D-N4-Hydroxycytidine. Antivir. Chem. Chemother. 2004;15:15–22. doi: 10.1177/095632020401500102. [DOI] [PubMed] [Google Scholar]
- 193.Jayk Bernal A., Gomes da Silva M.M., Musungaie D.B., Kovalchuk E., Gonzalez A., Delos Reyes V., Martín-Quirós A., Caraco Y., Williams-Diaz A., Brown M.L., et al. Molnupiravir for Oral Treatment of COVID-19 in Nonhospitalized Patients. N. Engl. J. Med. 2022;386:509–520. doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Arribas J.R., Bhagani S., Lobo S.M., Khaertynova I., Mateu L., Fishchuk R., Park W.Y., Hussein K., Kim S.W., Ghosn J., et al. Randomized Trial of Molnupiravir or Placebo in Patients Hospitalized with COVID-19. NEJM Evid. 2022;1:EVIDoa2100044. doi: 10.1056/EVIDoa2100044. [DOI] [PubMed] [Google Scholar]
- 195.Wong C.K.H., Au I.C.H., Lau K.T.K., Lau E.H.Y., Cowling B.J., Leung G.M. Real-World Effectiveness of Early Molnupiravir or Nirmatrelvir-Ritonavir in Hospitalised Patients with COVID-19 without Supplemental Oxygen Requirement on Admission during Hong Kong’s Omicron BA.2 Wave: A Retrospective Cohort Study. Lancet Infect. Dis. 2022;22:1681–1693. doi: 10.1016/S1473-3099(22)00507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Flisiak R., Zarębska-Michaluk D., Rogalska M., Kryńska J.A., Kowalska J., Dutkiewicz E., Dobrowolska K., Jaroszewicz J., Moniuszko-Malinowska A., Rorat M., et al. Real-World Experience with Molnupiravir during the Period of SARS-CoV-2 Omicron Variant Dominance. Pharmacol. Rep. 2022;74:1279–1285. doi: 10.1007/s43440-022-00408-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Xie Y., Choi T., Al-Aly Z. Molnupiravir and Risk of Post-Acute Sequelae of COVID-19: Cohort Study. BMJ. 2023;381:e074572. doi: 10.1136/bmj-2022-074572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.EMA Veklury. [(accessed on 22 January 2023)]. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/veklury.
- 199.Dobrowolska K., Zarębska-Michaluk D., Brzdęk M., Rzymski P., Rogalska M., Moniuszko-Malinowska A., Kozielewicz D., Hawro M., Rorat M., Sikorska K., et al. Retrospective Analysis of the Effectiveness of Remdesivir in COVID-19 Treatment during Periods Dominated by Delta and Omicron SARS-CoV-2 Variants in Clinical Settings. J. Clin. Med. 2023;12:2371. doi: 10.3390/jcm12062371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sandin R., Harrison C., Draica F., Wiemken T.L., Ma C., Fusco M.D., Markson L., Dzingina M. Estimated Impact of Oral Nirmatrelvir;Ritonavir on Reductions in Hospitalizations and Associated Costs within High-Risk COVID-19 Patients in the US. Res. Sq. 2022 doi: 10.21203/rs.3.rs-2191067/v1. [DOI] [Google Scholar]
- 201.Savinkina A., Paltiel A.D., Ross J.S., Gonsalves G. Population-Level Strategies for Nirmatrelvir/Ritonavir Prescribing-A Cost-Effectiveness Analysis. Open Forum Infect. Dis. 2022;9:ofac637. doi: 10.1093/ofid/ofac637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wai A.K.-C., Chan C.Y., Cheung A.W.-L., Wang K., Chan S.C.-L., Lee T.T.-L., Luk L.Y.-F., Yip E.T.-F., Ho J.W.-K., Tsui O.W.-K., et al. Association of Molnupiravir and Nirmatrelvir-Ritonavir with Preventable Mortality, Hospital Admissions and Related Avoidable Healthcare System Cost among High-Risk Patients with Mild to Moderate COVID-19. Lancet Reg. Health West. Pac. 2023;30:100602. doi: 10.1016/j.lanwpc.2022.100602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Marangoni D., Antonello R.M., Coppi M., Palazzo M., Nassi L., Streva N., Povolo L., Malentacchi F., Zammarchi L., Rossolini G.M., et al. Combination Regimen of Nirmatrelvir/Ritonavir and Molnupiravir for the Treatment of Persistent SARS-CoV-2 Infection: A Case Report and a Scoping Review of the Literature. Int. J. Infect. Dis. 2023;133:53–56. doi: 10.1016/j.ijid.2023.04.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jeong J.H., Chokkakula S., Min S.C., Kim B.K., Choi W.-S., Oh S., Yun Y.S., Kang D.H., Lee O.-J., Kim E.-G., et al. Combination Therapy with Nirmatrelvir and Molnupiravir Improves the Survival of SARS-CoV-2 Infected Mice. Antivir. Res. 2022;208:105430. doi: 10.1016/j.antiviral.2022.105430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hill J.A., Cowen L.E. Using Combination Therapy to Thwart Drug Resistance. Future Microbiol. 2015;10:1719–1726. doi: 10.2217/fmb.15.68. [DOI] [PubMed] [Google Scholar]
- 206.Moreno S., Perno C.F., Mallon P.W., Behrens G., Corbeau P., Routy J.-P., Darcis G. Two-Drug vs. Three-Drug Combinations for HIV-1: Do We Have Enough Data to Make the Switch? HIV Med. 2019;20((Suppl. 4)):2–12. doi: 10.1111/hiv.12716. [DOI] [PubMed] [Google Scholar]
- 207.Sun F., Lin Y., Wang X., Gao Y., Ye S. Paxlovid in Patients Who Are Immunocompromised and Hospitalised with SARS-CoV-2 Infection. Lancet Infect. Dis. 2022;22:1279. doi: 10.1016/S1473-3099(22)00430-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Helleberg M., Niemann C.U., Moestrup K.S., Kirk O., Lebech A.-M., Lane C., Lundgren J. Persistent COVID-19 in an Immunocompromised Patient Temporarily Responsive to Two Courses of Remdesivir Therapy. J. Infect. Dis. 2020;222:1103–1107. doi: 10.1093/infdis/jiaa446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Camprubí D., Gaya A., Marcos M.A., Martí-Soler H., Soriano A., Mosquera M.D.M., Oliver A., Santos M., Muñoz J., García-Vidal C. Persistent Replication of SARS-CoV-2 in a Severely Immunocompromised Patient Treated with Several Courses of Remdesivir. Int. J. Infect. Dis. 2021;104:379–381. doi: 10.1016/j.ijid.2020.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.European Medicines Agency Use of Molnupiravir for the Treatment of COVID-19. [(accessed on 14 June 2023)]. Available online: https://www.ema.europa.eu/en/documents/referral/lagevrio-also-known-molnupiravir-mk-4482-COVID-19-article-53-procedure-assessment-report_en.pdf.
- 211.European Medicines Agency Paxlovid Assessment Report. [(accessed on 14 June 2023)]. Available online: https://www.ema.europa.eu/en/documents/assessment-report/paxlovid-epar-public-assessment-report_en.pdf.
- 212.Stegemann S., Gosch M., Breitkreutz J. Swallowing Dysfunction and Dysphagia Is an Unrecognized Challenge for Oral Drug Therapy. Int. J. Pharm. 2012;430:197–206. doi: 10.1016/j.ijpharm.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 213.Hummler H., Stillhart C., Meilicke L., Grimm M., Krause E., Mannaa M., Gollasch M., Weitschies W., Page S. Impact of Tablet Size and Shape on the Swallowability in Older Adults. Pharmaceutics. 2023;15:1042. doi: 10.3390/pharmaceutics15041042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lawal Y. Africa’s Low COVID-19 Mortality Rate: A Paradox? Int. J. Infect. Dis. 2021;102:118–122. doi: 10.1016/j.ijid.2020.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Osei S.A., Biney R.P., Anning A.S., Nortey L.N., Ghartey-Kwansah G. Low Incidence of COVID-19 Case Severity and Mortality in Africa; Could Malaria Co-Infection Provide the Missing Link? BMC Infect. Dis. 2022;22:78. doi: 10.1186/s12879-022-07064-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ashworth J., Mathie D., Scott F., Mahendran Y., Woolhouse M., Stoevesandt O., Mduluza T., Mutapi F. Peptide Microarray IgM and IgG Screening of Pre-SARS-CoV-2 Human Serum Samples from Zimbabwe for Reactivity with Peptides from All Seven Human Coronaviruses: A Cross-Sectional Study. Lancet Microbe. 2023;4:e215–e227. doi: 10.1016/S2666-5247(22)00295-6. [DOI] [Google Scholar]
- 217.Diop B.Z., Ngom M., Pougué Biyong C., Pougué Biyong J.N. The Relatively Young and Rural Population May Limit the Spread and Severity of COVID-19 in Africa: A Modelling Study. BMJ Glob. Health. 2020;5:e002699. doi: 10.1136/bmjgh-2020-002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Gill C.J., Mwananyanda L., MacLeod W.B., Kwenda G., Pieciak R.C., Etter L., Bridges D., Chikoti C., Chirwa S., Chimoga C., et al. What Is the Prevalence of COVID-19 Detection by PCR among Deceased Individuals in Lusaka, Zambia? A Postmortem Surveillance Study. BMJ Open. 2022;12:e066763. doi: 10.1136/bmjopen-2022-066763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Levin A.T., Owusu-Boaitey N., Pugh S., Fosdick B.K., Zwi A.B., Malani A., Soman S., Besançon L., Kashnitsky I., Ganesh S., et al. Assessing the Burden of COVID-19 in Developing Countries: Systematic Review, Meta-Analysis and Public Policy Implications. BMJ Glob. Health. 2022;7:e008477. doi: 10.1136/bmjgh-2022-008477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kunyenje C.A., Chirwa G.C., Mboma S.M., Ng’ambi W., Mnjowe E., Nkhoma D., Ngwira L.G., Chawani M.S., Chilima B., Mitambo C., et al. COVID-19 Vaccine Inequity in African Low-Income Countries. Front. Public Health. 2023;11:1087662. doi: 10.3389/fpubh.2023.1087662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Member State Briefing Update on Global COVID-19 Vaccination. [(accessed on 18 June 2023)]. Available online: https://apps.who.int/gb/COVID-19/pdf_files/2023/05_01/Item1.pdf.
- 222.U.S International COVID-19 Vaccine Donations Tracker. [(accessed on 11 June 2023)]. Available online: https://www.kff.org/global-health-policy/issue-brief/u-s-international-COVID-19-vaccine-donations-tracker/
- 223.Hassan F., Yamey G., Abbasi K. Profiteering from Vaccine Inequity: A Crime against Humanity? BMJ. 2021;374:n2027. doi: 10.1136/bmj.n2027. [DOI] [PubMed] [Google Scholar]
- 224.Rzymski P., Szuster-Ciesielska A. The COVID-19 Vaccination Still Matters: Omicron Variant Is a Final Wake-up Call for the Rich to Help the Poor. Vaccines. 2022;10:1070. doi: 10.3390/vaccines10071070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.WHO COVAX Allocation. [(accessed on 15 May 2022)]. Available online: https://www.who.int/initiatives/act-accelerator/covax/allocation.
- 226.Savinkina A., Bilinski A., Fitzpatrick M., Paltiel A.D., Rizvi Z., Salomon J., Thornhill T., Gonsalves G. Estimating Deaths Averted and Cost per Life Saved by Scaling up MRNA COVID-19 Vaccination in Low-Income and Lower-Middle-Income Countries in the COVID-19 Omicron Variant Era: A Modelling Study. BMJ Open. 2022;12:e061752. doi: 10.1136/bmjopen-2022-061752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hoffman S.A., Costales C., Sahoo M.K., Palanisamy S., Yamamoto F., Huang C., Verghese M., Solis D.A., Sibai M., Subramanian A., et al. SARS-CoV-2 Neutralization Resistance Mutations in Patient with HIV/AIDS, California, USA. Emerg. Infect. Dis. 2021;27:2720–2723. doi: 10.3201/eid2710.211461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Cele S., Karim F., Lustig G., San J.E., Hermanus T., Tegally H., Snyman J., Moyo-Gwete T., Wilkinson E., Bernstein M., et al. SARS-CoV-2 Prolonged Infection during Advanced HIV Disease Evolves Extensive Immune Escape. Cell Host Microbe. 2022;30:154–162.e5. doi: 10.1016/j.chom.2022.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Gonzalez-Reiche A.S. SARS-CoV-2 in Low-Income Countries: The Need for Sustained Genomic Surveillance. Lancet Glob. Health. 2023;11:e815–e816. doi: 10.1016/S2214-109X(23)00197-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Le T., Sun C., Chang J., Zhang G., Yin X. MRNA Vaccine Development for Emerging Animal and Zoonotic Diseases. Viruses. 2022;14:401. doi: 10.3390/v14020401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Chauhan R.P., Dessie Z.G., Noreddin A., El Zowalaty M.E. Systematic Review of Important Viral Diseases in Africa in Light of the “One Health” Concept. Pathogens. 2020;9:301. doi: 10.3390/pathogens9040301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Gwenzi W., Skirmuntt E.C., Musvuugwa T., Teta C., Halabowski D., Rzymski P. Grappling with (Re)-Emerging Infectious Zoonoses: Risk Assessment, Mitigation Framework, and Future Directions. Int. J. Disaster Risk Reduct. 2022;82:103350. doi: 10.1016/j.ijdrr.2022.103350. [DOI] [Google Scholar]
- 233.Iacobucci G. COVID-19: “Grotesque Inequity” That Only a Quarter of Paxlovid Courses Go to Poorer Countries. BMJ. 2022;379:o2795. doi: 10.1136/bmj.o2795. [DOI] [PubMed] [Google Scholar]
- 234.Beasley D. Price of COVID Treatments from Pfizer, Merck, GSK Align with Patient Benefits-Report. Reuters 2022. [(accessed on 10 August 2023)]. Available online: https://www.reuters.com/business/healthcare-pharmaceuticals/price-covid-treatments-pfizer-merck-gsk-align-with-patient-benefits-report-2022-02-03/
- 235.Baker R.E., Mahmud A.S., Miller I.F., Rajeev M., Rasambainarivo F., Rice B.L., Takahashi S., Tatem A.J., Wagner C.E., Wang L.-F., et al. Infectious Disease in an Era of Global Change. Nat. Rev. Microbiol. 2022;20:193–205. doi: 10.1038/s41579-021-00639-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Watts M.S. Physicians as Role Models in Society. West. J. Med. 1990;152:292. [PMC free article] [PubMed] [Google Scholar]
- 237.Betancourt J.A., Rosenberg M.A., Zevallos A., Brown J.R., Mileski M. The Impact of COVID-19 on Telemedicine Utilization Across Multiple Service Lines in the United States. Healthcare. 2020;8:380. doi: 10.3390/healthcare8040380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Bazan D., Nowicki M., Rzymski P. Medical Students as the Volunteer Workforce during the COVID-19 Pandemic: Polish Experience. Int. J. Disaster Risk Reduct. 2021;55:102109. doi: 10.1016/j.ijdrr.2021.102109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Benfante A., Di Tella M., Romeo A., Castelli L. Traumatic Stress in Healthcare Workers during COVID-19 Pandemic: A Review of the Immediate Impact. Front. Psychol. 2020;11:569935. doi: 10.3389/fpsyg.2020.569935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Wittenberg E., Goldsmith J.V., Chen C., Prince-Paul M., Johnson R.R. Opportunities to Improve COVID-19 Provider Communication Resources: A Systematic Review. Patient Educ. Couns. 2021;104:438–451. doi: 10.1016/j.pec.2020.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Mach K.J., Salas Reyes R., Pentz B., Taylor J., Costa C.A., Cruz S.G., Thomas K.E., Arnott J.C., Donald R., Jagannathan K., et al. News Media Coverage of COVID-19 Public Health and Policy Information. Humanit. Soc. Sci. Commun. 2021;8:220. doi: 10.1057/s41599-021-00900-z. [DOI] [Google Scholar]
- 242.Fotiadis K., Dadouli K., Avakian I., Bogogiannidou Z., Mouchtouri V.A., Gogosis K., Speletas M., Koureas M., Lagoudaki E., Kokkini S., et al. Factors Associated with Healthcare Workers’ (HCWs) Acceptance of COVID-19 Vaccinations and Indications of a Role Model towards Population Vaccinations from a Cross-Sectional Survey in Greece, May 2021. Int. J. Environ. Res. Public Health. 2021;18:10558. doi: 10.3390/ijerph181910558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.World Health Organization . Evaluation Report for the Training Module “Communicating with Patients about COVID-19 Vaccination”: Greece. World Health Organization; Geneva, Switzerland: 2023. [Google Scholar]
- 244.Burson R.C., Buttenheim A.M., Armstrong A., Feemster K.A. Community Pharmacies as Sites of Adult Vaccination: A Systematic Review. Hum. Vaccines Immunother. 2016;12:3146–3159. doi: 10.1080/21645515.2016.1215393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Sobierajski T., Rzymski P., Wanke-Rytt M. The Influence of Recommendation of Medical and Non-Medical Authorities on the Decision to Vaccinate against Influenza from a Social Vaccinology Perspective: Cross-Sectional, Representative Study of Polish Society. Vaccines. 2023;11:994. doi: 10.3390/vaccines11050994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Paudyal V., Fialová D., Henman M.C., Hazen A., Okuyan B., Lutters M., Cadogan C., da Costa F.A., Galfrascoli E., Pudritz Y.M., et al. Pharmacists’ Involvement in COVID-19 Vaccination across Europe: A Situational Analysis of Current Practice and Policy. Int. J. Clin. Pharm. 2021;43:1139–1148. doi: 10.1007/s11096-021-01301-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Queeno B.V. Evaluation of Inpatient Influenza and Pneumococcal Vaccination Acceptance Rates with Pharmacist Education. J. Pharm. Pract. 2017;30:202–208. doi: 10.1177/0897190016628963. [DOI] [PubMed] [Google Scholar]
- 248.Pullagura G.R., Waite N.M., Houle S.K.D., Violette R., Wong W.W.L. Cost-Utility Analysis of Offering a Novel Remunerated Community Pharmacist Consultation Service on Influenza Vaccination for Seniors in Ontario, Canada. J. Am. Pharm. Assoc. 2019;59:489–497.e1. doi: 10.1016/j.japh.2019.02.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.