Abstract
We have designed a new class of modified antisense oligodeoxyribonucleotides (ODN) consisting of a central contiguous stretch of 6–8 unmodified nucleotides flanked by 3′- and 5′-regions containing several nucleotides joined by cationic internucleoside linkages. The positive charge results from modification of the internucleoside linkages as N,N-diethylethylenediamine phosphoramidates. These zwitterionic compounds show improved antisense activity in both Xenopus oocytes and embryos compared to our previously described chimeric oligonucleotides possessing neutral terminal internucleoside linkages. Using the localized maternal mRNA An2 as a target, we have shown that chimeric oligonucleotides with terminal positive charges are very effective in the sequence-specific elimination of maternal messages present in both oocytes and embryos. In addition, using the embryonic mRNA GS17 as a target, we have shown that these oligonucleotides can direct RNase H-mediated cleavage of messages produced at the onset of zygotic transcription, after the mid-blastula stage. These new compounds should be useful in attenuating embryonic gene expression to study the role of specific proteins in early vertebrate development.
INTRODUCTION
The elimination of mRNAs by antisense oligonucleotides (ODNs) is a useful technique for determining the role of specific gene products in a variety of cellular processes. Antisense ODNs have been demonstrated to be effective in vivo and in cell culture and tissue explant systems (1–4). Oocytes and embryos of the frog Xenopus laevis have proven to be particularly useful systems to test the efficacy of antisense ODNs (5–7). A major reason for this success is the ability to directly microinject the compounds, thereby avoiding the complex and poorly understood process of ODN internalization. Detailed kinetic studies of ODN action are made possible when variables associated with ODN cellular uptake are eliminated. Antisense ODNs have also proven to be useful in Xenopus embryos in determining the role of specific maternal mRNAs in early embryogenesis (5,8). To our knowledge, however, there have been no previous reports describing the depletion of mRNAs produced by the embryo later in development.
Investigators have used two general antisense strategies to alter gene expression following the transcription of a target mRNA. Both approaches begin with the hybridization of an ODN to the complementary target message. Whether the target message will be inactivated by a RNase H-dependent or RNase H-independent pathway depends upon the nature of the ODN strand present in the DNA:RNA heteroduplex. ODNs possessing predominantly anionic internucleoside linkages (either phosphodiesters or phosphorothioates) form heteroduplexes that are substrates for the enzyme RNase H (9–11). RNase H cleaves the RNA component of the heteroduplex rendering the message untranslatable. The two RNA cleavage fragments produced from this reaction are unstable in the X.laevis oocytes and embryos and are rapidly degraded (5). The ODN, however, is unaffected by this reaction and will dissociate from the cleaved RNA and bind to another target message. Therefore, the potential exists for a few oligonucleotide molecules to completely eliminate a large pool of target messages.
RNase H-independent activation is seen when the DNA:RNA heteroduplex is not a substrate for intracellular RNase H. This occurs when the heteroduplex contains an ODN with no anionic region, i.e. a fully modified oligonucleotide (12,13). Translation of the target message is inhibited by physically preventing the ribosome from binding to the message or by blocking passage of the ribosomal complex through the region of the heteroduplex. Because no intrinsic enzymatic activity is required for this type of inhibition, there is no requirement for the ODN to possess any degree of native structure. Studies using the methylphosphonate-modified ODNs developed by Miller and Ts’o (14) have shown that steric inhibition of translation can occur (12,15). The inhibition reported, however, was seen only at relatively high concentrations of ODN. In a direct comparison of anionic and non-ionic ODNs, ODN-directed cleavage of a message by RNase H was shown to be the most effective means of antisense inhibition (10). In general, it appears that ODNs that do not act through an RNase H-dependent mechanism are protected from nuclease degradation, but are rather ineffective antisense agents. An exception to this general rule may be the recently developed morpholino-modified ODNs described by Summerton and Weller that are reported to effectively inhibit translation, at relatively low concentrations, in a specific manner (16).
Specifically altering gene expression during vertebrate embryogenesis is a goal of many developmental biologists. The transgenic approach to interrupt gene expression has proven useful in the mouse and, more recently, the zebrafish. The elimination of specific mRNAs in developing X.laevis embryos, however, has been elusive. The rate of degradation of unmodified ODNs in embryos is too rapid for substantial efficacy at non-toxic concentrations (5). Chimeric ODNs containing neutral terminal phosphoramidate linkages were developed to block the active single-stranded exonucleases present (6). The central region of these compounds is left unmodified and, upon hybridization, serves as a substrate for cellular RNase H. These 2-methoxyethyl phosphoramidate-modified compounds, possessing several neutral internucleoside linkages, have been repeatedly used in the elimination of maternally derived messages in developing Xenopus embryos (5,8). Unfortunately, these first generation chimeric ODNs have not proven to be effective antisense agents against messages produced by zygotic transcription (i.e. those produced by the embryo after the mid-blastula transition). This report describes the development of chimeric antisense ODNs in which several terminal internucleoside linkages are modified as N,N-diethylethylenediamine (DEED) phosphoramidates to produce a positive charge under physiological conditions (17). These zwitterionic ODNs are more effective as antisense agents than are their neutral counterparts in both Xenopus oocytes and embryos. In addition, these new compounds can effectively direct the specific degradation of target messages resulting from later, zygotic transcription.
MATERIALS AND METHODS
Oligonucleotide synthesis
Modified ODNs were synthesized on an ABI model 391 DNA synthesizer, as described previously, using hydrogen phosphonate chemistry (5,18). All reagents used for automated DNA synthesis were obtained from Glen Research (Sterling, VA). To generate unmodified phosphodiester bonds, hydrogen phosphonate diesters were oxidized for 4 min with freshly prepared 5% iodine in tetrahydrofuran (THF):pyridine:water (15:2:2) and then for 3 min with the same solution diluted 1:1 with 8% triethylamine in THF:water (43:3). Oxidative amidation of hydrogen phosphonate diesters was performed manually using a 10% solution of either 2-methoxyethylamine or N,N-diethylethylenediamine (Aldrich, Milwaukee, WI) in anhydrous CCl4 as previously described (5,17). Briefly, ODNs containing both phosphodiester and phosphoramidate linkages were synthesized in blocks. The desired number of 3′ residues were first coupled and then either oxidized or oxidatively amidated. The next blocks of residues were then individually condensed and subsequently oxidized or oxidatively amidated. Further processing and purification of oligonucleotides were performed as previously described (5,6). Following Sephadex G-25 column chromatography, oligonucleotides were dissolved in sterile water and quantitated by UV spectroscopy. Prior to in vivo use, ODNs were first analyzed for purity by analytical reversed phase HPLC. All ODNs demonstrated a single, symmetrical peak. Molecular masses of model ODNs containing the same phosphoramidate linkages described here were obtained by mass spectrometry and were consistent with the predicted masses (data not shown).
Microinjection of oocytes and embryos
State VI oocytes were obtained from mature female frogs and maintained as previously described (5). ODNs, in a total volume of 10–20 nl, were injected into the cytoplasm of oocytes as described by Colman (19).
Eggs were obtained from hormonally treated females and fertilized as previously described (20). The embryos were injected with ODNs, in a total volume of 10–20 nl, as previously described (5). All manipulations of oocytes and embryos were performed at ambient temperature.
Analysis of RNA
Oocytes and embryos were flash frozen in dry ice and stored at –70°C prior to processing. Total RNA was extracted from defined numbers of oocytes and embryos as previously described (21). RNA was then separated by electrophoresis through a formaldehyde–agarose (1.2%) gel prior to transfer to a Nytran membrane (Schleicher & Schuell) (20). RNA was covalently crosslinked to the membrane using a Stratalinker (Stratagene, La Jolla, CA). All probes were 32P-labeled by random or specific priming of either complete cDNAs or cDNA fragments. Radioactive dATP used in these reactions was purchased from Amersham Pharmacia Biotech (Uppsala, Sweden). The An2 probe was a BglII fragment isolated from the An2 cDNA. The GS17 probe used was a SmaI–EcoRI fragment isolated from the GS17 cDNA (a gift from Paul Krieg, Tucson, AZ). The An3 probe was generated from the full-length cDNA. The histone probe was from a cDNA fragment corresponding to nt 70–287. The 18S rRNA probe was a 5′-end-labeled oligonucleotide, complementary to nt 1031–1060 of the Xenopus 18S rRNA. After overnight hybridization and washing until background signal was minimal, filters were exposed at –70°C to Kodak X-OMAT AR film using an intensifying screen.
RESULTS
Elimination of maternal messages
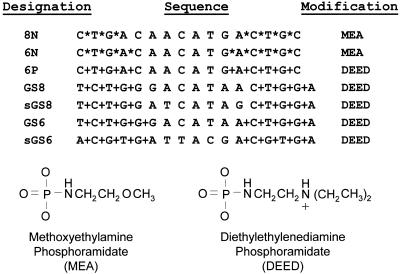
Seven ODNs were synthesized for these experiments (Fig. 1). The sequences of the ODNs, as well as the modified phosphoramidate linkages, are shown. We view an antisense ODN as a cofactor for the enzyme RNase H that is required for substrate (target mRNA) recognition. These compounds are therefore designed to possess two different regions, each serving a distinct function. The terminal flanking regions contain internucleoside linkages which are modified as either 2-methoxyethylamine (MEA) or DEED phosphoramidates to impart protection from single-stranded exonucleases. The extensive modification of the ODN is also predicted to reduce degradation by intracellular endonuclease activities. In addition, with the DEED modification, the tertiary amine group of each modified linkage will be protonated under physiological conditions, altering the electrostatic characteristics of the entire ODN. It should be noted that each phosphoramidate linkage added to an ODN introduces a chiral center. The studies presented here used ODNs representing a racemic mixture of all possible stereoisomers. A central region of 6–8 unmodified nucleotide linkages is required for the resulting heteroduplex to be a substrate for RNase H. More heavily modified ODNs, possessing fewer than six unmodified linkages, do not effectively reduce the level of target mRNA (6). The chimeric structure of these ODNs creates a limited RNase H cleavage site that is only part of the recognition/binding site for the target message. By decreasing the RNase H active site to the absolute minimum, one would predict an increase in antisense specificity as base pair mismatches in that narrowed critical region may preclude enzymatic activity.
Figure 1.
ODN sequences. ODNs 8N, 6N and 6P are complementary to the initiation codon region of the maternal Xenopus mRNA An2. ODNs GS8 and GS6 are complementary to the initiation codon region of the zygotic Xenopus mRNA GS17. ODNs sGS8 and sGS6 possess the same nucleotide composition, but the sequence is scrambled to differ from that of the GS17 ODNs. Asterisks (*) represent MEA internucleoside linkages, while plus signs (+) represent DEED-modified linkages. The structure of both modified linkages are shown below the ODN sequences.
The antisense efficacy of ODNs possessing terminal cationic internucleoside linkages was first tested by microinjection into X.laevis oocytes. The localized mRNA An2, which encodes the α-subunit of the mitochondrial ATPase, was chosen as a target maternal message because of its relatively high abundance (22). ODNs 6N, 6N′, 8N and 6P were injected into the cytoplasm of mature Xenopus oocytes. ODN 6N′ represents a second, separate preparation of compound 6N. After a 3 h incubation, total RNA was isolated and subjected to northern analysis. A representative autoradiograph (Fig. 2) demonstrates that the cationic ODN 6P was more effective at eliminating An2 mRNA than any of the neutral ODNs 6N, 6N′ or 8N. Importantly, none of the injected ODNs caused a significant reduction in the level of an unrelated control mRNA (An3), which demonstrates the specificity of this process. In addition, ODNs 6N and 6N′ had virtually no effect on either target (An2) or control (An3) mRNA levels in Xenopus oocytes. This finding suggests that the reduction in An2 mRNA seen after injection with 8N or 6P is not the result of a non-specific effect following ODN injection.
Figure 2.
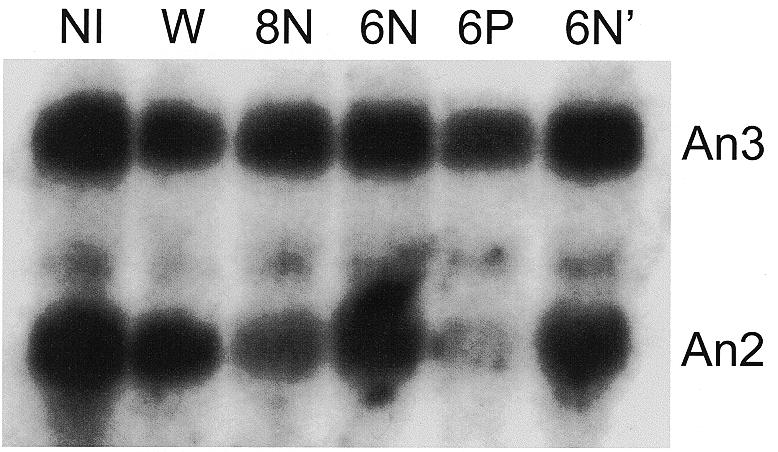
Antisense activity of DEED-modified ODNs in oocytes. Mature X.laevis oocytes were injected with 12 ng of either 6P, 6N, 6N′, 8N or water (W). After 3 h, 10 oocytes were harvested and levels of An2 and An3 (unrelated control) mRNA were determined by northern analysis. NI, non-injected.
The antisense activities of compounds 6N and 6P were next tested in X.laevis embryos. Thirty minutes after injection of ODN into single cell embryos, total RNA was isolated and subjected to northern analysis. A representative autoradiograph (Fig. 3) demonstrates that ODN 6P is more effective at reducing An2 mRNA levels than 6N in developing embryos. Neither ODN altered the levels of control RNAs (histone H4 mRNA and 18S rRNA). The improved antisense efficacy of 6P in embryos is consistent with the data presented in Figure 2. In contrast to the results obtained in oocytes, however, ODN 6N directs the degradation of a large fraction of An2 mRNA in Xenopus embryos during the 30 min incubation. We have repeatedly seen this increase in antisense activity in embryos compared to oocytes after injecting the same amount of the same ODN, supporting the hypothesis that a difference exists in either the localization or the activity of the RNase H molecules present in these two cell types.
Figure 3.
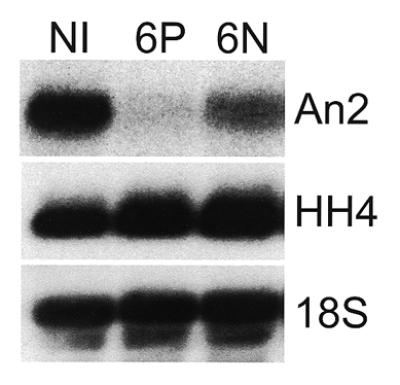
Antisense activity of DEED-modified ODNs in embryos. Single-cell X.laevis embryos were injected with 2 ng of ODN 6P or 6N. After 30 min, 10 embryos were harvested and levels of An2 (target) and histone H4 (control) mRNAs, as well as 18S rRNA, were determined by northern analysis. NI, non-injected.
Elimination of zygotic messages
Xenopus embryos contain two types of mRNAs. Maternal messages are those present in the egg prior to fertilization. These mRNAs encode proteins that drive development during the first several hours after fertilization, when the embryo is undergoing rapid replication but is transcriptionally inactive. Approximately 7–8 h after fertilization (around the 4000 cell stage) the embryo enters the mid-blastula transition, at which time the cell cycle slows dramatically and zygotic transcription commences (23,24). These zygotic messages encode proteins that direct further embryonic differentiation and organogenesis. Eliminating maternal messages in developing Xenopus embryos has been accomplished using antisense ODNs possessing neutral internucleoside linkages (5,8). Although these techniques have proven useful in examining very early development, our ultimate goal has been to attenuate the levels of the later zygotic messages. This would allow the routine manipulation of developmentally interesting molecules not present in the embryo until after the onset of zygotic transcription.
GS17, one of the first messages produced by the embryo after the mid-blastula transition, was chosen as the model zygotic transcript for antisense elimination (25). Although the GS17 message possesses an open reading frame encoding 147 amino acids, GS17 protein has never been demonstrated. In addition, no indication of the possible function of this putative protein has been deduced from the amino acid sequence. We tested the antisense efficacy of two ODNs, GS6 and GS8, for their abilities to target specific degradation of the GS17 mRNA. Two nanograms of ODN GS6 or GS8, as well as their scrambled sequence controls (sGS6 and sGS8), were injected into single-cell Xenopus embryos. Ten hours later embryos were harvested and total RNA was isolated and analyzed. Both GS6 and GS8 showed efficacy in attenuating GS17 mRNA levels (Fig. 4). We have consistently seen this pattern of antisense activity, particularly the increased efficacy of GS6 compared to GS8. Additionally, the non-specific decrease in GS17 mRNA seen with the sGS8 oligonucleotide has never been seen with the sGS6 compound. This non-specific activity may have been reduced by eliminating the complementarity between GS17 mRNA and the control ODNs in the modified 3′- and 5′-terminal regions of sGS6 compared to sGS8. There was no effect of any injected ODN on the levels of control transcripts, histone H4 mRNA and 18S rRNA. The levels of GS17 mRNA in both the water-injected and the sGS6 ODN-injected embryos are consistently lower than in the uninjected embryos. This difference may represent a non-specific effect resulting from manipulation associated with injection.
Figure 4.
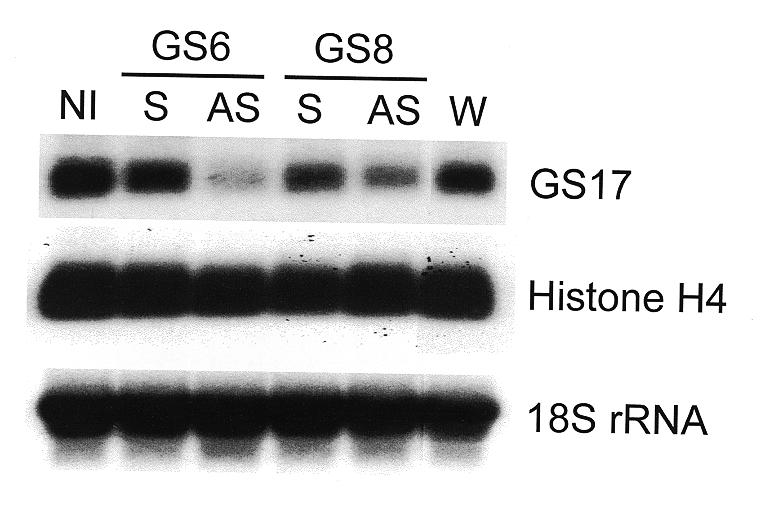
Specific depletion of a zygotic transcript using DEED-modified ODNs. Single-cell X.laevis embryos were injected with either water (W) or 2 ng of ODN GS6 (AS), GS8 (AS), sGS6 (S) or sGS8 (S). After 10 h, 10 embryos were harvested and levels of GS17 (target) and histone H4 (control) mRNAs, as well as 18S rRNA, were determined by northern analysis. NI, non-injected.
A sequence-specific depletion of the zygotic GS17 transcript using antisense ODNs has been demonstrated (Fig. 4). When studying development in Xenopus embryos using ODNs, however, it is critical to demonstrate the specificity of ODN activity since non-specific abnormalities associated with microinjection are well described (26). An alternative explanation for the decreased GS17 mRNA seen above is that the antisense (but not the sense) ODNs induced a developmental arrest preventing the embryos from reaching the mid-blastula transition, the stage at which GS17 is normally expressed. The fact that the external morphology of the embryos injected with water, antisense ODN or scrambled ODN was identical (data not shown) argues against a non-specific, toxic effect of the antisense ODNs. An investigation into the possibility that a non-specific developmental arrest is responsible for the decreased GS17 mRNA seen following injection of ODN GS6 or GS8 is shown in the following experiment. Single cell embryos were injected as described above. Embryos were harvested 10–10.5 h after fertilization and total RNA was isolated. Two populations of embryos were harvested following each ODN injection: a subset clearly possessing blastopore lips (+) and a subset that had no external evidence of blastopore formation (–). The blastopore is a structure formed by invagination of the blastula and marks the beginning of gastrulation. Embryos possessing blastopore lips are clearly gastrulating and would therefore, by definition, have passed the mid-blastula stage. The embryos not possessing blastopore lips should have passed the mid-blastula stage based on the time following fertilization, however, no external features are evident to prove this. As predicted, however, stage-matched siblings of embryos not demonstrating blastopore lips at the time of harvest did indeed show evidence of gastrulation shortly after harvest of the siblings. Both sets of embryos (+ and –), from both GS6 and GS8 injections, showed a decrease in GS17 mRNA compared to both uninjected embryos and those injected with either water or control ODN (Fig. 5). ODN injection had no effect on the level of 18S rRNA. If a developmental arrest was responsible for the decreased levels of GS17 mRNA (i.e. the ODN prevented progress through the mid-blastula transition), then one would expect that no antisense ODN-injected embryos would proceed further to gastrulation. This is obviously not the case, as embryos began gastrulation whether uninjected or injected with antisense ODN, control ODN or water. These experiments demonstrate that a sequence-specific, ODN-mediated process, not an artifactual developmental arrest, explains the reduced levels of GS17 mRNA following antisense ODN injection. The data presented here demonstrate a new class of ODN that can effectively mediate the degradation of targeted messages produced after the mid-blastula transition in X.laevis embryos.
Figure 5.
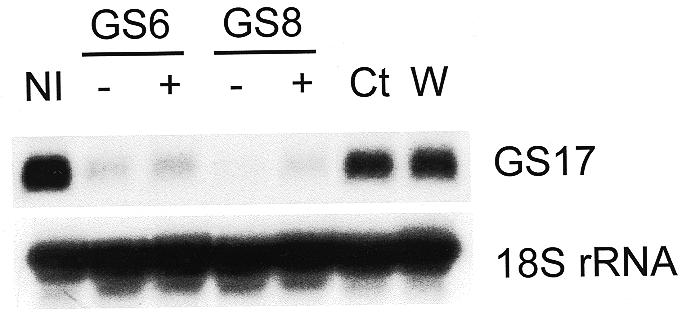
Decreased GS17 mRNA levels are not a result of developmental arrest. Single-cell X.laevis embryos were injected with either water (W) or 2 ng of ODN GS6 or GS8. After 10–10.5 h, embryos were separated into groups possessing blastopore lips (+) and not possessing blastopore lips (–). Total RNA was isolated and levels of GS17 mRNA and 18S rRNA were determined by northern analysis. Ct, control ODN; NI, non-injected.
DISCUSSION
Extensive modification of ODNs is crucial for their antisense activity in Xenopus embryos. Although imparting nuclease resistance is critical, other factors seem to affect the in vivo efficacy of antisense ODNs. As shown here, the introduction of several terminal DEED phosphoramidate linkages increases the efficacy of antisense ODNs. The structure of this modified linkage is similar to that of the previously described MEA phosphoramidate (5) in that they both possess a substituted ethylamine moiety in a phosphoramidate linkage. The improved antisense activity seen with the DEED compounds, therefore, most likely results from the presence of the terminal tertiary amine. Protonated amino moieties impart a more positive charge to the ODN and may improve antisense activity in several unrelated ways. In the first place, as a result of electrostatic interactions, the ODN with cationic termini may bind more strongly to the target message than compounds containing the neutral phosphoramidate modification. An increase in the strength of association would result in greater biological activity at lower concentrations. We have found that the melting temperature of ODN 6P with a complementary DNA strand in 150 mM NaCl and 10 mM sodium phosphate (pH 7.3) is 53.1°C, while that of ODN 6N is 46.4°C. Therefore, ODN 6P would be predicted to bind more strongly than ODN 6N to an RNA complement in vivo. Secondly, the DEED-modified ODNs may be more stable than those containing neutral modifications. It is possible that these zwitterionic compounds interact less favorably with the single-stranded nucleases present in Xenopus embryos, perhaps by electrostatic repulsion of positively charged amino acids at the recognition or active sites. Additionally, the change in net charge could affect localization of the modified ODNs, possibly favoring nuclear transport and subsequent accumulation. Finally, the heteroduplex consisting of the DEED-modified ODN and the target message may assume a unique conformation (different from that seen with the heteroduplex containing the neutral phosphate modifications) that results in a better substrate for Xenopus RNase H activity. Any combination of the above speculations may explain the enhanced activity of the DEED-modified antisense ODNs over those previously reported. ODNs possessing both positively charged and negatively charged linkages have been previously described; however, these compounds contained alternating linkages and would not be expected to form heteroduplexes that could direct mRNA degradation by RNase H (27,28).
In summary, we now have three different classes of ODN available for different types of antisense studies in Xenopus oocytes and embryos. Unmodified ODNs are effective in oocytes, but have little antisense activity in developing embryos. ODNs possessing terminal neutral modifications are effective antisense agents when used to eliminate maternal messages in either oocytes or developing embryos. Finally, the ODNs described here, possessing terminal cationic internucleoside linkages, have a broad spectrum of antisense activity that includes both maternal messages and those produced by embryos later in development. These new compounds should prove useful in studying the roles of specific proteins in Xenopus embryogenesis. In addition, they may also be useful in studies involving other systems in which more traditional antisense ODNs have proved ineffective.
Acknowledgments
ACKNOWLEDGEMENTS
This work was supported in part by National Institutes of Health grants HD 27748 (J.M.D.) and HL62178 (D.L.W.).
REFERENCES
- 1.Driver S., Robinson,G., Flanagan,J., Shen,W., Smith,L., Thomas,D. and Roberts,P. (1999) Nature Biotechnol., 17, 1184–1187. [DOI] [PubMed] [Google Scholar]
- 2.Runyan R., Wendler,C., Romano,L., Boyer,A., Dagle,J. and Weeks,D. (1999) Methods Companion Methods Enzymol., 18, 316–321. [DOI] [PubMed] [Google Scholar]
- 3.Veal G., Agrawal,S. and Byrn,R. (1998) Nucleic Acids Res., 26, 5670–5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Potts J., Dagle,J., Walder,J., Weeks,D. and Runyan,R. (1991) Proc. Natl Acad. Sci. USA, 88, 1516–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dagle J., Weeks,D. and Walder,J. (1990) Nucleic Acids Res., 18, 4751–4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dagle J., Weeks,D. and Walder,J. (1991) Antisense Res. Dev., 1, 11–20. [DOI] [PubMed] [Google Scholar]
- 7.Woolf T., Jennings,C., Rebagliata,M. and Melton,D. (1990) Nucleic Acids Res., 18, 1763–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weeks D., Walder,J. and Dagle,J. (1991) Development, 111, 1173–1178. [DOI] [PubMed] [Google Scholar]
- 9.Stein C., Subasinghe,C., Shinozuka,K. and Cohen,J. (1988) Nucleic Acids Res., 16, 3209–3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walder R. and Walder,J. (1988) Proc. Natl Acad. Sci. USA, 85, 5011–5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shuttleworth J. and Colman,A. (1988) EMBO J., 7, 427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maher L. and Dolnick,B. (1988) Nucleic Acids Res., 16, 3341–3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agrawal S., Mayrand,S., Zamecnik,P. and Pederson,T. (1990) Proc. Natl Acad. Sci. USA, 87, 1401–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller P., Fang,K., Kondo,N. and Ts’o,P.O.P. (1971) J. Am. Chem. Soc., 93, 6657–6665. [DOI] [PubMed] [Google Scholar]
- 15.Blake K., Murakami,A., Spitz,S., Glave,S., Reddy,M., Ts’o,P.O.P. and Miller,P. (1985) Biochemistry, 24, 6139–6145. [DOI] [PubMed] [Google Scholar]
- 16.Summerton J. and Weller,D. (1997) Antisense Nucleic Acid Drug Dev., 7, 187–195. [DOI] [PubMed] [Google Scholar]
- 17.Dagle J. and Weeks,D. (1996) Nucleic Acids Res., 24, 2143–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Froehler B., Ng,P. and Matteucci,M. (1986) Nucleic Acids Res., 14, 5399–5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colman A. (1984) In Hames,D. and Higgins,S. (eds), Transcription and Translation: A Practical Approach. IRL Press, Oxford, UK, pp. 271–302.
- 20.Rebagliati M., Weeks,D., Harvey,R. and Melton,D. (1985) Cell, 42, 769–777. [DOI] [PubMed] [Google Scholar]
- 21.Chomczynski P. and Sacchi,N. (1987) Anal. Biochem., 162, 156–159. [DOI] [PubMed] [Google Scholar]
- 22.Weeks D. and Melton,D. (1987) Proc. Natl Acad. Sci. USA, 84, 2798–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newport J. and Kirschner,M. (1982) Cell, 30, 675–686. [DOI] [PubMed] [Google Scholar]
- 24.Newport J. and Kirschner,M. (1982) Cell, 30, 687–696. [DOI] [PubMed] [Google Scholar]
- 25.Krieg P. and Melton,D. (1985) EMBO J., 4, 3463–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vize P.D., Hemmati-Brivanlou,A., Harland,R. and Melton,D.A. (1991) In Kay,B.K. and Peng,H.B. (eds), Methods in Cell Biology, Vol. 36, Xenopus laevis: Practical Uses in Cell and Molecular Biology. Academic Press Inc., FL, pp. 367–387.
- 27.Letsinger R., Singman,C., Histland,G. and Salunkhe,M. (1988) J. Am. Chem. Soc., 110, 4470–4471. [Google Scholar]
- 28.Horn T., Chaturvedi,S., Balasubramaniam,T. and Letsinger,R. (1996) Tetrahedron Lett., 37, 743–746. [Google Scholar]