Abstract
Objective:
Evaluate the association between postoperative opioid prescribing and new persistent opioid use.
Summary Background Data:
Opioid-naïve patients who develop new persistent opioid use after surgery are at increased risk of opioid-related morbidity and mortality. However, the extent to which postoperative opioid prescribing is associated with persistent postoperative opioid use is unclear.
Methods:
Retrospective study of opioid-naïve adults undergoing surgery in Michigan from 1/1/2017-10/31/2019. Postoperative opioid prescriptions were identified using a statewide clinical registry and prescription fills were identified using Michigan’s prescription drug monitoring program. The primary outcome was new persistent opioid use, defined as filling at least one opioid prescription between post-discharge days 4-90 and filling at least one opioid prescription between post-discharge days 91-180.
Results:
37,654 patients underwent surgery with a mean age of 52.2 (16.7) years and 20,923 (55.6%) female patients. 31,920 (84.8%) patients were prescribed opioids at discharge. 622 (1.7%) patients developed new persistent opioid use after surgery. Being prescribed an opioid at discharge was not associated with new persistent opioid use (aOR 0.88 [95% CI 0.71-1.09]). However, among patients prescribed an opioid, patients prescribed the second largest (12 [IQR 3] pills) and largest (20 [IQR 7] pills) quartiles of prescription size had higher odds of new persistent opioid use compared to patients prescribed the smallest quartile (7 [IQR 1] pills) of prescription size (aOR 1.39 [95% CI 1.04-1.86]) and aOR 1.97 [95% CI 1.44-2.70], respectively).
Conclusions:
In a cohort of opioid-naïve patients undergoing common surgical procedures, the risk of new persistent opioid use increased with the size of the prescription. This suggests that while opioid prescriptions in and of themselves may not place patients at risk of long-term opioid use, excessive prescribing does. Consequently, these findings support ongoing efforts to mitigate excessive opioid prescribing after surgery to reduce opioid-related harms.
Mini-Abstract
In this retrospective study of 37,654 opioid-naïve patients undergoing common surgical procedures, being prescribed an opioid postoperatively was not associated with new persistent opioid use. However, among patients prescribed opioids, larger prescriptions were associated with 1.97 higher odds of new persistent opioid use compared to smaller prescriptions, a result that was statistically significant.
Introduction
New persistent opioid use after surgery occurs when an opioid-naïve individual is prescribed an opioid for short-term postoperative pain control, but then continues to fill opioid prescriptions beyond the period in which their acute pain is expected to have resolved.1 Roughly 7% of patients develop new persistent opioid use after surgery, making it a critical mechanism by which routine surgical care contributes to opioid-related harms in the United States.2,3 Specifically, long-term opioid use places patients at increased risk of complications, mortality, higher healthcare utilization, and higher spending, predisposing individuals who develop new persistent opioid use after surgery to worse health outcomes in the future.3-5 Given the potential risks of developing persistent opioid use after surgery, it is critical to understand its drivers and risk factors.
Currently, the association between postoperative opioid prescribing and new persistent opioid use is unclear. To date, studies of new persistent opioid use after surgery have examined the association between a patient filling a postoperative opioid prescription and becoming a new persistent opioid user.6-12 While these studies have convincingly demonstrated that patients who fill an opioid prescription after surgery are at increased risk of persistent opioid use, they fail to address whether prescribing opioids to a patient increases that risk. This is a critical and often underappreciated distinction. First, virtually all efforts to address opioid-related morbidity after surgery target prescribing itself, either in the form of prescribing recommendations or legislative restrictions of postoperative opioid prescribing.13,14 Therefore, it is essential to understand the relationship between prescribing practice itself and persistent use in order to understand the effectiveness of these measures. Second, patients who are prescribed but do not immediately fill opioids are excluded from claims-based analyses of prescription fills, even though they may also be at risk of persistent use given the availability of an opioid prescription.15 Third, virtually all prior studies of persistent postoperative opioid use rely on claims of a single insurance type, thereby limiting their generalizability to more heterogeneous populations with different types of insurance. This is a crucial limitation, as the incidence of persistent opioid use has been found to vary by payor.16 Insofar as surgeons and healthcare systems are motivated to address opioid-related morbidity after surgery, understanding how prescribing practice itself is associated with long-term opioid use can inform efforts to improve practice and protect patients.
Therefore, we conducted the following study to evaluate the association between postoperative opioid prescriptions and persistent opioid use after surgery. To do so, we created a novel linkage between two data sources. Using a clinically rich, multi-payer, statewide surgical registry, we identified opioid-naïve patients undergoing surgery and determined whether they were prescribed an opioid upon discharge. Importantly, in contrast to claims-based data, this allowed us to identify the actual prescription associated with the surgical episode, regardless of whether the patient immediately filled that prescription. We then analyzed data from Michigan’s statewide prescription drug monitoring program (PDMP), which contains all controlled substance prescription fills, to determine the incidence of persistent opioid use up to 6 months after surgery. Combining prescribing data directly from patients’ medical records with universal prescription records provides a unique opportunity to precisely investigate the relationship between provider practice and patient outcomes.
Methods
Data Sources and Cohort Selection
This study used two data sources corresponding to its exposure and outcome. First, a clinical registry that abstracts data directly from patients’ medical records was used to ascertain the primary exposure: being prescribed an opioid after surgery. Second, a statewide PDMP that captures any opioid prescription fill was used to ascertain the primary outcome: new persistent opioid use after surgery.
The first data source used in this study was the Michigan Surgical Quality Collaborative (MSQC) clinical registry. The MSQC is a well-known collaborative quality improvement network funded by Blue Cross Blue Shield of Michigan and made up of 70 hospitals in Michigan.17-21 The MSQC maintains a registry of prospectively collected data on patients undergoing surgery that includes patient demographics, patient clinical characteristics, perioperative processes, and 30-day outcomes.22 Data are abstracted directly from medical records by trained Surgical Clinical Quality Reviewers. A sampling algorithm is used to minimize selection bias, and interrater reliability assessments and data audits are performed regularly to ensure accuracy.23
Using the MSQC clinical registry, we identified adult patients (18 years and older) who underwent one of the following surgical procedures between January 1, 2017, and October 31, 2019 and had valid discharge prescription data: laparoscopic appendectomy, laparoscopic cholecystectomy, colon/small bowel procedures, inguinal/femoral hernia repair, ventral/incisional hernia repair, laparoscopic hysterectomy, vaginal hysterectomy, total abdominal hysterectomy, and thyroidectomy. Patients were excluded if they died, underwent reoperation within 30 days of surgery, or were discharged to a destination other than home.
The second data source used in this study was the Michigan Automated Prescription System (MAPS). MAPS is Michigan’s PDMP which tracks all prescription fills for controlled substances (schedules 2-5) in Michigan.24 Because the state requires pharmacies to electronically report all controlled substance prescriptions to MAPS, prescription fills include all payor types including self-pay. An independent third-party data broker linked prescription fills from MAPS to individual patients in the MSQC clinical registry via an encrypted, de-identified database for analysis.
We excluded patients who were not Michigan residents, as MAPS only contains prescriptions for Michigan residents. Patients were also excluded if they had more than one match in MAPS. Finally, patients with an opioid prescription fill in MAPS between preoperative days −365 to −1 were also excluded so that only opioid-naïve patients were included in our cohort (Supplemental Figure 1).
The Institutional Review Board of the University of Michigan determined this study to be exempt from regulation and the need for informed consent given that it was a secondary analysis of deidentified data. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines25 and a preregistered study protocol available at https://dx.doi.org/10.17504/protocols.io.buyenxte.
Outcomes and Explanatory Variables
The primary outcome of this study was new persistent opioid use, defined as 1) filling at least one opioid prescription in MAPS between post-discharge days 4-90, and then 2) filling at least one additional opioid prescription in MAPS between post-discharge days 91-180.11 This definition specifically requires two separate opioid prescription fills over two sequential time periods in order to identify persistent use as opposed to one-off prescription fills.26 We also characterized immediate postoperative prescription fills, defined as filling an opioid prescription in MAPS between post-discharge days 0-3.
The primary explanatory variable of this study was being prescribed an opioid after surgery. This is distinct from prior claims-based studies that use immediate postoperative prescription fills as the main exposure since opioid prescriptions were identified from the MSQC clinical registry, which abstracts opioid prescriptions at the time of discharge from the medical record.27 Among patients who received an opioid prescription, the secondary explanatory variable of interest was opioid prescription size, which was standardized to milligrams of oral morphine equivalents (OME) to adjust for the varying potencies of different types of opioids. For example, a 5 mg tablet of hydrocodone is equivalent to 5 mg OME whereas a 5 mg tablet of oxycodone is equivalent to 7.5 mg OME. Conversion to OME allows for standardized comparison regardless of medication type. The amount of mg OME was then categorized into quartiles.28
The MSQC clinical registry also provided relevant patient and procedural characteristics, including demographics (age, sex, race/ethnicity, insurance type), patient characteristics (American Society of Anesthesiologists [ASA] classification, obesity, history of cancer, cigarette use in the 12 months prior to surgery, diabetes, functional status, chronic obstructive pulmonary disease, congestive heart failure, hypertension, chronic steroid use, and dialysis dependence), and clinical characteristics (admission status, surgical priority, surgical approach, procedure, and year of surgery). Clinical adverse events within 30 days of discharge included postoperative complications (e.g., pneumonia, urinary tract infection, surgical site infection), emergency department (ED) visits, readmission, and length of stay greater than 14 days. Patients met a composite endpoint of a “30-day adverse event” if they had a complication, ED visit, or readmission within 30 days of discharge.
Statistical Analysis
Descriptive statistics were calculated for demographic variables, patient characteristics, clinical characteristics, and 30-day clinical adverse events. Univariate statistics were used to assess baseline differences between patients who were and were not prescribed an opioid at discharge. A multivariable logistic regression model was used to estimate the association between all explanatory variables and new persistent opioid use among all patients. A second multivariable logistic regression model was then used to estimate the association between all explanatory variables and new persistent opioid use among the subset of patients who received an opioid prescription.
Although the main analysis controlled for postoperative adverse events, we performed an additional sensitivity analysis in which we analyzed factors associated with new persistent opioid use separately for patients who did and did not experience any 30-day adverse events. Adverse events such as complications and ED visits have been found to increase the risk of persistent opioid use after surgery.29 This sensitivity analysis consisted of separate multivariable logistic regression models for each group, with being prescribed an opioid at discharge and opioid prescription size as the covariates of interest. All statistical tests were performed using Stata version 15.1 (StataCorp). All statistical tests were 2-sided and significance was set at P < 0.05.
Results
A total of 37,654 patients met inclusion criteria and were included in the analysis. The mean (SD) age of the cohort was 52.2 (16.7) years and 20,923 (55.6%) patients were female (Table 1). In general, most patients were of white race (80.9%) and had private insurance (54.7%). The most common procedures were laparoscopic cholecystectomy (26.9%), inguinal and femoral hernia repair (18.9%), ventral and incisional hernia repair (13.7%), and laparoscopic appendectomy (12.1%). Most patients (84.8%) were prescribed an opioid upon discharge. Mean prescription size was 101.2 (77.0) mg OME, equivalent to 13.5 (10.3) tablets of oxycodone 5 mg. Filling a prescription between post-discharge days 0-3 was more common among patients who received a postoperative opioid prescription compared to patients who did not (24,711 [77.4%] vs. 997 patients [17.4%], P<0.001).
Table 1 –
Cohort Characteristics
| Characteristic | Overall (N = 37654) |
No Prescription (N = 5734) |
Prescription (N = 31920) |
P |
|---|---|---|---|---|
| Age | 52.2 (16.7) | 57.7 (17.6) | 51.3 (16.4) | <.001 |
| Sex | ||||
| Male | 16731 (44.4) | 2405 (41.9) | 14326 (44.9) | <.001 |
| Female | 20923 (55.6) | 3329 (58.1) | 17594 (55.1) | |
| Race | ||||
| White, non-Hispanic | 30453 (80.9) | 4860 (84.8) | 25593 (80.2) | <.001 |
| Black, non-Hispanic | 3408 (9.1) | 370 (6.5) | 3038 (9.5) | |
| Hispanic | 1116 (3.0) | 128 (2.2) | 988 (3.1) | |
| Other or Unknown | 2677 (7.1) | 376 (6.6) | 2301 (7.2) | |
| Insurance Type | ||||
| Private | 20612 (54.7) | 2555 (44.6) | 18057 (56.6) | <.001 |
| Medicare | 9376 (24.9) | 2185 (38.1) | 7191 (22.5) | |
| Medicaid | 5777 (15.3) | 669 (11.7) | 5108 (16.0) | |
| Dual Medicare/Medicaid | 585 (1.6) | 116 (2.0) | 469 (1.5) | |
| No Insurance | 771 (2.1) | 132 (2.3) | 639 (2.0) | |
| Other | 533 (1.4) | 77 (1.3) | 456 (1.4) | |
| ASA Classification | ||||
| Class 1 | 3968 (10.5) | 455 (7.9) | 3513 (11.0) | <.001 |
| Class 2 | 21941 (58.3) | 2945 (51.4) | 18996 (59.5) | |
| Class 3 | 11117 (29.5) | 2167 (37.8) | 8950 (28.0) | |
| Class 4-5 | 603 (1.6) | 161 (2.8) | 442 (1.4) | |
| Unknown | 25 (0.1) | 6 (0.1) | 19 (0.1) | |
| Obesity | ||||
| Yes | 16876 (44.8) | 2385 (41.6) | 14491 (45.4) | <.001 |
| No | 20673 (54.9) | 3337 (58.2) | 17336 (54.3) | |
| Unknown | 105 (0.3) | 12 (0.2) | 93 (0.3) | |
| Cancer | 2257 (6.0) | 577 (10.1) | 1680 (5.3) | <.001 |
| Cigarette Use | 7367 (19.6) | 930 (16.2) | 6437 (20.2) | <.001 |
| Diabetes | 4073 (10.8) | 779 (13.6) | 3294 (10.3) | <.001 |
| Functional Status | ||||
| Yes | 208 (0.6) | 83 (1.5) | 125 (0.4) | <.001 |
| No | 37360 (99.2) | 5637 (98.3) | 31723 (99.4) | |
| Unknown | 86 (0.2) | 14 (0.2) | 72 (0.2) | |
| COPD | 1293 (3.4) | 281 (4.9) | 1012 (3.2) | <.001 |
| CHF | 85 (0.2) | 26 (0.5) | 59 (0.2) | <.001 |
| Hypertension | 13431 (35.7) | 2403 (41.9) | 11028 (34.6) | <.001 |
| Chronic Steroid Use | 728 (1.9) | 131 (2.3) | 597 (1.9) | 0.036 |
| Dialysis | 74 (0.2) | 28 (0.5) | 46 (0.1) | <.001 |
| Admission Status | ||||
| Inpatient | 19345 (51.4) | 3990 (69.6) | 15355 (48.1) | <.001 |
| Outpatient | 18303 (48.6) | 1742 (30.4) | 16561 (51.9) | |
| Unknown | 6 (<0.1) | 2 (<0.1) | 4 (0.01) | |
| Non-Elective Surgery | 10195 (27.1) | 2242 (39.1) | 7953 (24.9) | <.001 |
| Surgical Approach | ||||
| Minimally Invasive | 26875 (71.4) | 3947 (68.8) | 22928 (71.8) | <.001 |
| Open | 10682 (28.4) | 1776 (31.0) | 8906 (27.9) | |
| Unknown | 97 (0.3) | 11 (0.2) | 86 (0.3) | |
| Procedure | ||||
| Laparoscopic Appendectomy | 4546 (12.1) | 772 (13.5) | 3774 (11.8) | <.001 |
| Laparoscopic Cholecystectomy | 10116 (26.9) | 1539 (26.8) | 8577 (26.9) | |
| Colon/Small Bowel | 3471 (9.2) | 1157 (20.2) | 2314 (7.3) | |
| Inguinal/Femoral Hernia Repair | 7121 (18.9) | 672 (11.7) | 6449 (20.2) | |
| Ventral/Incisional Hernia Repair | 5158 (13.7) | 538 (9.4) | 4620 (14.5) | |
| Laparoscopic Hysterectomy | 3337 (8.9) | 376 (6.6) | 2961 (9.3) | |
| Vaginal Hysterectomy | 1779 (4.7) | 277 (4.8) | 1502 (4.7) | |
| Total Abdominal Hysterectomy | 1175 (3.1) | 111 (1.9) | 1064 (3.3) | |
| Thyroidectomy | 951 (2.5) | 292 (5.1) | 659 (2.1) | |
| Year of Surgery | ||||
| 2017 | 5119 (13.6) | 795 (13.9) | 4324 (13.6) | 0.763 |
| 2018 | 11982 (31.8) | 1808 (31.5) | 10174 (31.9) | |
| 2019 | 20553 (54.6) | 3131 (54.6) | 17422 (54.6) | |
| 30-Day Adverse Events | ||||
| Composite | 3943 (10.5) | 663 (11.6) | 3280 (10.3) | 0.003 |
| Postoperative Complication | 1061 (2.8) | 239 (4.2) | 822 (2.6) | <.001 |
| Emergency Department Visit | 2612 (6.9) | 376 (6.6) | 2236 (7.0) | 0.219 |
| Readmission | 1045 (2.8) | 221 (3.9) | 824 (2.6) | <.001 |
| Length of Stay > 14 days | 246 (0.7) | 91 (1.6) | 155 (0.5) | <.001 |
All values represented as N (%) except for age which is represented as mean (SD). Composite 30-day adverse events = composite of complication, emergency department visit, or readmission within 30 days of discharge.
Overall, 622 (1.7%) patients met criteria for new persistent opioid use after surgery. The incidence of new persistent opioid use was 1.6% (499 patients) among patients who were prescribed opioids postoperatively and 2.1% (123 patients) among patients who were not prescribed opioids postoperatively. In a multivariable logistic regression model, being prescribed an opioid at discharge was not associated with new persistent opioid use (aOR 0.88 [95% CI 0.71-1.09]) (Table 2). There were, however, a number of patient factors associated with new persistent opioid use including Black race (aOR 1.42 [95% CI 1.12-1.79]), Medicaid (aOR 1.53 [95% CI 1.22-1.90]) and dual Medicare/Medicaid insurance (aOR 2.08 [95% CI 1.37-3.15]), and several comorbidities. Patients who experienced a composite endpoint of 30-day adverse events had 2.08 (95% CI 1.71-2.53) higher odds of new persistent opioid use. Finally, the incidence of new persistent opioid use varied by procedure (Supplemental Table 1). Compared to laparoscopic cholecystectomy, colon/small bowel procedures were associated with 1.49 (95% CI 1.07-2.08) higher odds of persistent use while laparoscopic appendectomy was associated lower odds of persistent use (aOR 0.57 [95% CI 0.40-0.82]).
Table 2 –
Multivariable logistic regression model of new persistent opioid use after surgery.
| Characteristic | Odds Ratio (95% CI) | P |
|---|---|---|
| Prescribed Opioid at Discharge | 0.88 (0.71-1.09) | 0.235 |
| Age | 1.00 (0.99-1.00) | 0.374 |
| Male (ref: Female) | 0.94 (0.78-1.15) | 0.555 |
| Race (ref: White, non-Hispanic) | ||
| Black, non-Hispanic | 1.42 (1.12-1.79) | 0.004 |
| Hispanic | 1.09 (0.68-1.74) | 0.732 |
| Other or Unknown | 0.92 (0.65-1.31) | 0.644 |
| Insurance Type (ref: Private) | ||
| Medicare | 1.10 (0.86-1.41) | 0.451 |
| Medicaid | 1.53 (1.22-1.90) | <.001 |
| Dual Medicare/Medicaid | 2.08 (1.37-3.15) | 0.001 |
| No Insurance | 0.91 (0.47-1.74) | 0.77 |
| Other | 1.15 (0.56-2.35) | 0.702 |
| ASA Classification (ref: Class 1) | ||
| Class 2 | 1.23 (0.83-1.83) | 0.309 |
| Class 3 | 2.04 (1.33-3.11) | 0.001 |
| Class 4-5 | 2.87 (1.60-5.14) | <.001 |
| Obesity | 1.18 (0.99-1.40) | 0.07 |
| Cancer | 1.55 (1.16-2.05) | 0.003 |
| Cigarette Use | 1.83 (1.52-2.20) | <.001 |
| Diabetes | 1.20 (0.95-1.51) | 0.126 |
| Functional Status (ref: Independent) | ||
| Non-Independent | 1.14 (0.56-2.32) | 0.728 |
| Unknown | 0.76 (0.11-5.54) | 0.791 |
| Chronic Obstructive Pulmonary Disease | 1.20 (0.87-1.66) | 0.262 |
| Congestive Heart Failure | 1.08 (0.37-3.14) | 0.888 |
| Hypertension | 1.13 (0.93-1.37) | 0.223 |
| Chronic Steroid Use | 1.71 (1.14-2.56) | 0.009 |
| Dialysis | 0.63 (0.15-2.68) | 0.534 |
| Inpatient Admission | 1.07 (0.84-1.35) | 0.592 |
| Nonelective Surgery | 1.51 (1.18-1.94) | 0.001 |
| Surgical Approach (ref: Minimally Invasive) | ||
| Open | 1.25 (0.98-1.60) | 0.071 |
| Unknown | 2.57 (0.80-8.28) | 0.115 |
| Procedure (ref: Laparoscopic Cholecystectomy) | ||
| Laparoscopic Appendectomy | 0.57 (0.40-0.82) | 0.003 |
| Colon/Small Bowel | 1.49 (1.07-2.08) | 0.018 |
| Inguinal/Femoral Hernia Repair | 1.15 (0.81-1.63) | 0.430 |
| Ventral/Incisional Hernia Repair | 1.15 (0.82-1.61) | 0.425 |
| Laparoscopic Hysterectomy | 1.22 (0.86-1.74) | 0.262 |
| Vaginal Hysterectomy | 1.07 (0.66-1.73) | 0.787 |
| Total Abdominal Hysterectomy | 0.99 (0.57-1.72) | 0.979 |
| Thyroidectomy | 0.55 (0.27-1.14) | 0.107 |
| Year of Surgery (ref: 2017) | ||
| 2018 | 1.55 (1.19-2.02) | 0.001 |
| 2019 | 1.02 (0.79-1.33) | 0.872 |
| 30-Day Adverse Event | 2.08 (1.71-2.53) | <.001 |
| Length of Stay > 14 Days | 1.34 (0.72-2.49) | 0.361 |
30-day adverse event = composite of complications, emergency department visit, or readmission within 30 days of discharge.
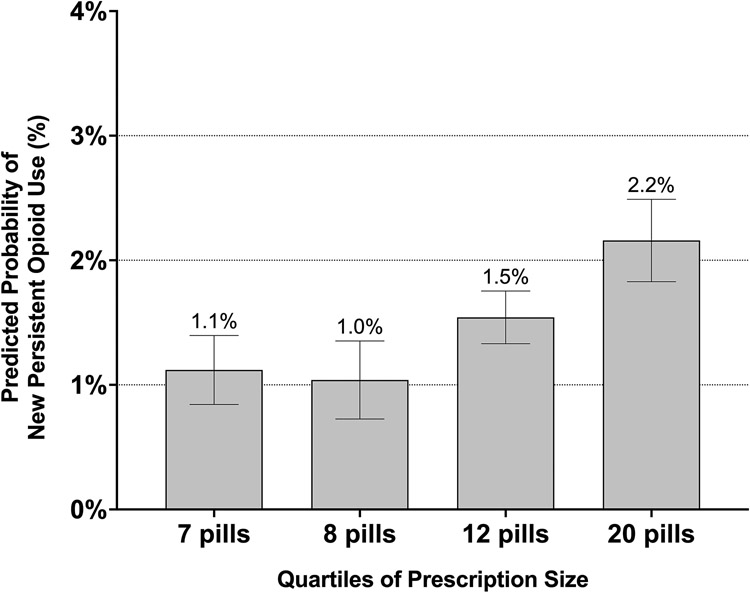
Among the 31,920 patients prescribed opioids at discharge, the risk of new persistent opioid use increased with initial prescription size (Figure 1). Specifically, compared to the smallest quartile of prescription size, patients prescribed the second largest (aOR 1.39 [95% CI 1.04-1.86]) and largest quartiles (aOR 1.97 [95% CI 1.44-2.70]) of initial prescription size had higher odds of new persistent opioid use after surgery (Table 3).
Figure 1 – Predicted Probability of New Persistent Opioid Use by Quartile of Prescription Size Among Patients Prescribed Opioids at Discharge.
Prescription size quartiles are the median prescription size in equivalent tablets of oxycodone 5 mg for each quartile, rounded to the nearest whole number of pills. Median and interquartile range (IQR) for each quartile was: 1st quartile 7 (IQR 1) pills, 2nd quartile 8 (IQR 0) pills, 3rd quartile 12 (IQR 3) pills, 4th quartile 20 (IQR 7) pills.
Table 3 -.
Multivariable logistic regression model of new persistent opioid use after surgery among 31,920 patients prescribed opioids at discharge.
| Characteristic | Odds Ratio (95% CI) | P |
|---|---|---|
| Opioid Prescription Size (ref: 1st Quartile (Smallest)) | ||
| 2nd Quartile | 0.93 (0.62-1.37) | 0.704 |
| 3rd Quartile | 1.39 (1.04-1.86) | 0.026 |
| 4th Quartile (Largest) | 1.97 (1.44-2.70) | <.001 |
| Age | 1.00 (0.99-1.01) | 0.931 |
| Male (ref: Female) | 0.93 (0.75-1.17) | 0.552 |
| Race (ref: White, non-Hispanic) | ||
| Black, non-Hispanic | 1.36 (1.05-1.77) | 0.020 |
| Hispanic | 1.19 (0.72-1.97) | 0.495 |
| Other or Unknown | 0.97 (0.66-1.42) | 0.880 |
| Insurance Type (ref: Private) | ||
| Medicare | 1.05 (0.79-1.39) | 0.735 |
| Medicaid | 1.43 (1.12-1.83) | 0.004 |
| Dual Medicare/Medicaid | 2.02 (1.26-3.22) | 0.003 |
| No Insurance | 0.90 (0.43-1.85) | 0.767 |
| Other | 1.50 (0.73-3.08) | 0.268 |
| ASA Classification (ref: Class 1) | ||
| Class 2 | 1.11 (0.72-1.69) | 0.637 |
| Class 3 | 1.69 (1.07-2.67) | 0.024 |
| Class 4-5 | 2.35 (1.22-4.53) | 0.011 |
| Obesity | 1.21 (0.99-1.48) | 0.056 |
| Cancer | 1.41 (1.01-1.97) | 0.046 |
| Cigarette Use | 1.85 (1.51-2.27) | <.001 |
| Diabetes | 1.28 (0.99-1.65) | 0.065 |
| Functional Status (ref: Independent) | ||
| Non-Independent | 1.27 (0.50-3.25) | 0.615 |
| Chronic Obstructive Pulmonary Disease | 1.23 (0.85-1.79) | 0.269 |
| Congestive Heart Failure | 1.88 (0.63-5.68) | 0.260 |
| Hypertension | 1.15 (0.92-1.42) | 0.221 |
| Chronic Steroid Use | 1.50 (0.93-2.42) | 0.100 |
| Dialysis | 1.18 (0.27-5.13) | 0.828 |
| Inpatient Admission | 0.98 (0.75-1.28) | 0.897 |
| Nonelective Surgery | 1.74 (1.31-2.32) | <.001 |
| Surgical Approach (ref: Minimally Invasive) | ||
| Open | 1.34 (1.02-1.76) | 0.034 |
| Unknown | 1.54 (0.37-6.42) | 0.555 |
| Procedure (ref: Laparoscopic Cholecystectomy) | ||
| Laparoscopic Appendectomy | 0.55 (0.36-0.84) | 0.005 |
| Colon/Small Bowel | 1.76 (1.20-2.58) | 0.003 |
| Inguinal/Femoral Hernia Repair | 1.11 (0.75-1.64) | 0.596 |
| Ventral/Incisional Hernia Repair | 1.11 (0.77-1.62) | 0.568 |
| Laparoscopic Hysterectomy | 1.27 (0.86-1.88) | 0.224 |
| Vaginal Hysterectomy | 1.19 (0.71-2.00) | 0.507 |
| Total Abdominal Hysterectomy | 0.73 (0.39-1.37) | 0.327 |
| Thyroidectomy | 0.70 (0.31-1.57) | 0.385 |
| Year of Surgery (ref: 2017) | ||
| 2018 | 1.85 (1.35-2.54) | <.001 |
| 2019 | 1.60 (1.16-2.2) | 0.004 |
| 30-Day Adverse Event | 2.15 (1.73-2.68) | <.001 |
| Length of Stay > 14 Days | 0.90 (0.35-2.32) | 0.826 |
30-day adverse event = composite of complications, emergency department visit, or readmission within 30 days of discharge.
A sensitivity analysis was performed to separately analyze patients who experienced 30-day adverse events and those who did not (Supplemental Tables 2-3). In both groups, there was no association between being prescribed opioids at discharge and new persistent opioid use (aOR 0.84 [95% CI 0.66-1.07] for patients without 30-day adverse events; aOR 1.03 [95% CI 0.65-1.62] for patients with 30-day adverse events) and associations of other factors with persistent opioid use were similar to the overall cohort. Among patients prescribed opioids at discharge, the risk of new persistent opioid use increased with initial prescription size among patients who did not experience 30-day adverse events (second largest quartile aOR 1.70 [95% CI 1.20-2.40]; largest quartile aOR 2.38 [95% CI 1.63-3.45]), however there was no association between prescription size and new persistent opioid use among patients who experienced 30-day adverse events.
Discussion
In this large cohort of opioid-naïve patients undergoing common surgical procedures, there was no association between being prescribed an opioid at discharge and developing new persistent opioid use. However, among patients who were prescribed an opioid after surgery, larger prescriptions were associated with increased risk of new persistent opioid use compared to smaller prescriptions. Specifically, patients who received the largest prescriptions had roughly twice the risk of new persistent opioid use as patients who received the smallest prescriptions. This suggests that while initial postoperative opioid prescriptions in and of themselves may not increase the risk of long-term opioid use, excessive opioid prescribing does increase this risk. Understanding the relationship between prescribing practice, patient characteristics, and persistent opioid use is critical to inform ongoing efforts to minimize the risks of opioid exposure at the time of surgery.
While prior work has established an association between filling an opioid prescription immediately after surgery and developing persistent opioid use, to our knowledge this is the first study to demonstrate that simply providing a postoperative opioid prescription does not increase the risk of long-term use, but that providing a larger opioid prescription does.6 Whereas several studies have shown that patients who fill an opioid prescription are at increased risk of persistent opioid use, surgeons and policymakers may have little to no control over whether a patient decides to fill their prescription. They do, however, decide whether to prescribe opioids after surgery and how much. Moreover, virtually all efforts to mitigate opioid-related morbidity after surgery have taken the form of prescribing recommendations or legislative prescribing restrictions. Therefore, the results of this study have practical implications for prescribing practice.
First, the association between larger prescriptions and increased risk of new persistent opioid use suggests that efforts to reduce excessive postoperative opioid prescribing could reduce the risk of long-term use. Several strategies have been implemented to minimize postoperative opioid prescribing, including standardized prescribing guidelines based on patient-reported use, “opioid-sparing” recovery pathways, and patient-centered decision-making models.30-32 These initiatives have achieved equal or superior levels of pain control and patient satisfaction compared to usual care.27,33,34 Given the association between large prescriptions and persistent opioid use in the current study, these opioid-sparing efforts should continue to be adopted as the standard of care for postoperative recovery. Nevertheless, additional work is needed to understand the extent to which these practice changes will affect the incidence of persistent use after surgery. Barth et al., for example, recently found that even among patients receiving guideline-directed prescribing, 12.2% of patients still filled an opioid prescription between postoperative months 6-12.35 Moreover, these prescriptions coincided with new diagnoses, suggesting that some proportion of new opioid use may be unrelated to the initial postoperative prescription. Although the higher incidence in that study is likely due to a more lenient definition of long-term opioid use as well as inclusion of additional surgical procedures, it nevertheless suggests that a non-significant proportion of patients may continue using opioids long after their operation.
This study also identified several patient-level factors associated with new persistent opioid use. Specifically, factors such as Black race, Medicaid insurance, and increased baseline comorbidity burden – all of which identify subgroups of patients who face social strain, social vulnerability, and increased barriers to accessing care – suggest that patients’ social determinants of health may play a role in their risk of persistent opioid use after surgery.36 These associations may help inform tailored postoperative engagement based on individual risk factors. Moreover, clinical characteristics such as non-elective surgery, cancer diagnosis, and undergoing colon/small bowel surgery were also associated with increased odds of persistent opioid use after surgery. The higher prevalence of these features among patients who did not receive an opioid prescription likely underlies the higher unadjusted rate of persistent opioid use in that group. Additional work that examines these mediators and moderators could help uncover modifiable factors to optimize postoperative pain management.
Integrating a state’s PDMP data with a surgical registry represents an important complement to previous claims-bases analyses. Others have investigated persistent opioid use with PDMP data to examine long-term opioid prescriptions after surgery and emergency room visits.37,38 In this cohort, the integration of clinical registry data alongside PDMP data allowed us to more precisely assess the relationship between prescribing and long-term fills, an approach that could be replicated in other settings. Currently, 49 states have operational PDMPs and most are accompanied by legislation mandating their use.39,40 Their ability to capture prescription fills at scale, including fills for other potentiating medications, is a valuable resource. Moreover, use of non-payer-based data from PDMPs may provide a more representative picture of trends in long-term postoperative opioid use.
While the strengths of this study include its large size, use of granular prescribing data from a multi-institution registry, and use of a statewide PDMP, it has limitations. First, this study lacks information regarding the indication for prescription fills and is unable to differentiate between opioid prescription fills due to new persistent use versus a new pain-related diagnosis or surgical procedure. Despite this limitation, the incidence of persistent opioid use in this study aligns with prior work.11 Second, selection bias may be present in this retrospective non-randomized design, though our analysis accounts for relevant chronic health conditions and excludes patients with preoperative opioid use. Third, factors at the patient, provider, and procedure level contribute to the decision to prescribe opioids, which are partially mitigated by covariates included in our multivariable models. Fourth, peri- or postoperative pain levels as well as inpatient opioid use were not included, which may also be associated with persistent opioid use.41 Understanding the relationship between acute surgical pain and chronic opioid use will further help surgeons tailor prescribing practice. Lastly, the lack of an association between prescription size and new persistent opioid use among patients who experienced 30-day adverse events may be due to type II error given the relatively small size of that subgroup.
Conclusion
In a cohort of opioid-naïve patients undergoing common surgical procedures, patients who were prescribed an opioid after surgery were not at increased risk of new persistent opioid use. However, among patients who were prescribed an opioid, the risk of new persistent opioid use increased with the size of the prescription. This suggests that while opioid prescriptions in and of themselves may not place patients at risk of long-term opioid use, excessive prescribing likely does. Consequently, these findings support ongoing efforts to mitigate excessive opioid prescribing after surgery as a way to reduce patients’ risk of persistent opioid use and its associated morbidity.
Supplementary Material
Supplemental Figure 1 – Cohort selection diagram.
Disclosures:
RH receives funding for research from Blue Cross Blue Shield of Michigan Foundation and the National Institute of Diabetes and Digestive and Kidney Diseases (5T32DK108740-05). CSB is supported by the Ruth L. Kirschstein Postdoctoral Research Fellowship Award administered by the National Institute on Drug Abuse (F32-DA050416). YL and VG have no disclosures. CB, ME, JW, and MB receive funding from the Michigan Department of Health and Human Services and the National Institute on Drug Abuse (R01DA042859). Dr. Bicket reports past consultation with Axial Healthcare and Alosa Health not related to this work. Dr. Brummett is a consultant for Heron Therapeutics, Vertex Pharmaceuticals, Alosa Health and the Benter Foundation, not related to this work. No funder or sponsor had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. YL and VG had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Hah JM, Bateman BT, Ratliff J, Curtin C, Sun E. Chronic Opioid Use After Surgery: Implications for Perioperative Management in the Face of the Opioid Epidemic. Anesth Analg. 2017;125(5):1733–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waljee JF, Li L, Brummett CM, Englesbe MJ. Iatrogenic Opioid Dependence in the United States: Are Surgeons the Gatekeepers? Ann Surg. 2017;265(4):728–730. [DOI] [PubMed] [Google Scholar]
- 3.Lawal OD, Gold J, Murthy A, et al. Rate and Risk Factors Associated With Prolonged Opioid Use After Surgery: A Systematic Review and Meta-analysis. JAMA Netw Open. 2020;3(6):e207367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JS, Vu JV, Edelman AL, et al. Health Care Spending and New Persistent Opioid Use After Surgery. Ann Surg. 2020;272(1):99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson DB, Cata JP, Niu J, et al. Persistent opioid use is associated with worse survival after lobectomy for stage I non-small cell lung cancer. Pain. 2019;160(10):2365–2373. [DOI] [PubMed] [Google Scholar]
- 6.Brummett CM, Waljee JF, Goesling J, et al. New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surg. 2017;152(6):e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JS, Hu HM, Edelman AL, et al. New Persistent Opioid Use Among Patients With Cancer After Curative-Intent Surgery. J Clin Oncol. 2017;35(36):4042–4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harbaugh CM, Lee JS, Hu HM, et al. Persistent Opioid Use Among Pediatric Patients After Surgery. Pediatrics. 2018;141(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brescia AA, Waljee JF, Hu HM, et al. Impact of Prescribing on New Persistent Opioid Use After Cardiothoracic Surgery. Ann Thorac Surg. 2019;108(4):1107–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown CR, Chen Z, Khurshan F, Groeneveld PW, Desai ND. Development of Persistent Opioid Use After Cardiac Surgery. JAMA Cardiol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howard R, Gunaseelan V, Brummett C, Waljee J, Englesbe M, Telem D. New Persistent Opioid Use After Inguinal Hernia Repair. Ann Surg. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santosa KB, Hu HM, Brummett CM, et al. New persistent opioid use among older patients following surgery: A Medicare claims analysis. Surgery. 2020;167(4):732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vu JV, Howard RA, Gunaseelan V, Brummett CM, Waljee JF, Englesbe MJ. Statewide Implementation of Postoperative Opioid Prescribing Guidelines. N Engl J Med. 2019;381(7):680–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chua KP, Kimmel L, Brummett CM. Disappointing Early Results From Opioid Prescribing Limits for Acute Pain. JAMA Surg. 2020. [DOI] [PubMed] [Google Scholar]
- 15.Bicket MC, Long JJ, Pronovost PJ, Alexander GC, Wu CL. Prescription Opioid Analgesics Commonly Unused After Surgery: A Systematic Review. JAMA Surg. 2017;152(11):1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chua KP, Hu HM, Waljee JF, Nalliah RP, Brummett CM. Persistent Opioid Use Associated With Dental Opioid Prescriptions Among Publicly and Privately Insured US Patients, 2014 to 2018. JAMA Netw Open. 2021;4(4):e216464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Share DA, Campbell DA, Birkmeyer N, et al. How a regional collaborative of hospitals and physicians in Michigan cut costs and improved the quality of care. Health Aff (Millwood). 2011;30(4):636–645. [DOI] [PubMed] [Google Scholar]
- 18.Birkmeyer NJ, Share D, Campbell DA Jr., Prager RL, Moscucci M, Birkmeyer JD. Partnering with payers to improve surgical quality: the Michigan plan. Surgery. 2005;138(5):815–820. [DOI] [PubMed] [Google Scholar]
- 19.Campbell DA Jr., Kubus JJ, Henke PK, Hutton M, Englesbe MJ. The Michigan Surgical Quality Collaborative: a legacy of Shukri Khuri. Am J Surg. 2009;198(5 Suppl):S49–55. [DOI] [PubMed] [Google Scholar]
- 20.Englesbe MJ, Dimick JB, Sonnenday CJ, Share DA, Campbell DA Jr. The Michigan surgical quality collaborative: will a statewide quality improvement initiative pay for itself? Ann Surg. 2007;246(6):1100–1103. [DOI] [PubMed] [Google Scholar]
- 21.Campbell DA Jr., Henderson WG, Englesbe MJ, et al. Surgical site infection prevention: the importance of operative duration and blood transfusion--results of the first American College of Surgeons-National Surgical Quality Improvement Program Best Practices Initiative. J Am Coll Surg. 2008;207(6):810–820. [DOI] [PubMed] [Google Scholar]
- 22.Campbell DA Jr., Englesbe MJ, Kubus JJ, et al. Accelerating the pace of surgical quality improvement: the power of hospital collaboration. Arch Surg. 2010;145(10):985–991. [DOI] [PubMed] [Google Scholar]
- 23.Healy MA, Regenbogen SE, Kanters AE, et al. Surgeon Variation in Complications With Minimally Invasive and Open Colectomy: Results From the Michigan Surgical Quality Collaborative. JAMA Surg. 2017;152(9):860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LARA - MI Automated Prescription System (MAPS). Michigan.gov. Published 2021. Accessed September 10, 2021. https://www.michigan.gov/lara/0,4601,7-154-89334_72600_72603_55478---,00.html.
- 25.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. [DOI] [PubMed] [Google Scholar]
- 26.Jivraj NK, Raghavji F, Bethell J, et al. Persistent Postoperative Opioid Use: A Systematic Literature Search of Definitions and Population-based Cohort Study. Anesthesiology. 2020;132(6):1528–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howard R, Brown CS, Lai YL, et al. The Association of Postoperative Opioid Prescriptions with Patient Outcomes. Ann Surg. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gammaitoni AR, Fine P, Alvarez N, McPherson ML, Bergmark S. Clinical application of opioid equianalgesic data. Clin J Pain. 2003;19(5):286–297. [DOI] [PubMed] [Google Scholar]
- 29.Brummett CM, Evans-Shields J, England C, et al. Increased health care costs associated with new persistent opioid use after major surgery in opioid-naive patients. J Manag Care Spec Pharm. 2021;27(6):760–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howard R, Waljee J, Brummett C, Englesbe M, Lee J. Reduction in Opioid Prescribing Through Evidence-Based Prescribing Guidelines. JAMA Surg. 2018;153(3):285–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Overton HN, Hanna MN, Bruhn WE, et al. Opioid-Prescribing Guidelines for Common Surgical Procedures: An Expert Panel Consensus. J Am Coll Surg. 2018;227(4):411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prescribing Recommendations. Michigan OPEN. https://michigan-open.org/prescribing-recommendations/. Published 2020. Accessed September 12, 2021.
- 33.Anderson M, Hallway A, Brummett C, Waljee J, Englesbe M, Howard R. Patient-Reported Outcomes After Opioid-Sparing Surgery Compared With Standard of Care. JAMA Surg. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hallway A, Vu J, Lee J, et al. Patient Satisfaction and Pain Control Using an Opioid-Sparing Postoperative Pathway. J Am Coll Surg. 2019;229(3):316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barth RJJ, Porter ED, Kelly JL, et al. Reasons for Long-term Opioid Prescriptions After Guideline-directed Opioid Prescribing and Excess Opioid Pill Disposal. Annals of Surgery. 2021. [DOI] [PubMed] [Google Scholar]
- 36.Cheung PT, Wiler JL, Lowe RA, Ginde AA. National study of barriers to timely primary care and emergency department utilization among Medicaid beneficiaries. Ann Emerg Med. 2012;60(1):4–10 e12. [DOI] [PubMed] [Google Scholar]
- 37.Tan TL, Rondon AJ, Wilt Z, et al. Understanding Opioid Use After Total Hip Arthroplasty: A Comprehensive Analysis of a Mandatory Prescription Drug Monitoring Program. J Am Acad Orthop Surg. 2020;28(20):e917–e922. [DOI] [PubMed] [Google Scholar]
- 38.Meisel ZF, Lupulescu-Mann N, Charlesworth CJ, Kim H, Sun BC. Conversion to Persistent or High-Risk Opioid Use After a New Prescription From the Emergency Department: Evidence From Washington Medicaid Beneficiaries. Ann Emerg Med. 2019;74(5):611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Substance Abuse and Mental Health Services Administration. (2017). Prescription Drug Monitoring Programs: A Guide for Healthcare Providers. In Brief, Volume 10, Issue 1. [Google Scholar]
- 40.Haffajee RL. Prescription Drug Monitoring Programs - Friend or Folly in Addressing the Opioid-Overdose Crisis? N Engl J Med. 2019;381(8):699–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hill MV, Stucke RS, Billmeier SE, Kelly JL, Barth RJ Jr. Guideline for Discharge Opioid Prescriptions after Inpatient General Surgical Procedures. J Am Coll Surg. 2018;226(6):996–1003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 – Cohort selection diagram.