Abstract
The introduction of pollutants, such as thiacloprid and benzo[a]pyrene (B[a]P), into the waters of urbanized coastal and estuarine areas through fossil fuel spills, domestic and industrial waste discharges, atmospheric inputs, and continental runoff poses a major threat to the fauna and flora of the aquatic environment and can have a significant impact on the internal defense system of invertebrates such as mussels. Using monoclonal and polyclonal anti-Toll-like receptor 2 (TLR2) and anti-inducible nitric oxide synthetase (iNOS) antibodies for the first time, this work aims to examine hemocytes in the mantle and gills of M. galloprovincialis as biomarkers of thiacloprid and B[a]P pollution and analyze their potential synergistic effect. To pursue this objective, samples were exposed to the pollutants, both individually and simultaneously. Subsequently, oxidative stress biomarkers were evaluated by enzymatic analysis, while tissue changes and the number of hemocytes in the different contaminated groups were assessed via histomorphological and immunohistochemical analyses. Our findings revealed that in comparison to a single exposure, the two pollutants together significantly elevated oxidative stress. Moreover, our data may potentially enhance knowledge on how TLR2 and iNOS work as part of the internal defense system of bivalves. This would help in creating new technologies and strategies, such as biosensors, that are more suitable for managing water pollution, and garnering new details on the condition of the marine ecosystem.
Keywords: hemocytes, pollution, biomarker, internal defense system
1. Introduction
Industrialization and new techniques of intensive agriculture have resulted in a steady and exponential increase in the production of waste, which is often discharged or accumulated in water systems eventually flowing into rivers and seas [1]. Water pollution represents an alteration of its original characteristics through the introduction of anthropogenic contaminants, i.e., various chemical and toxic substances, such as biocides, pesticides, cosmetics, and pharmaceuticals, altering its use for human nutrition and/or sustenance of biotic communities [2]. Moreover, many of these substances, slowly degrading, remain for long periods and can also bioaccumulate, thus enhancing their dangerousness in the aquatic environment [3,4,5,6].
Thiacloprid [(Z)-3-(6-chloro-3-pyridylmethyl)-1,3-thiazolidin-2-ylidencianamide], a type of neonicotinoid insecticide, is highly selective in action and has excellent systemic activity against sucking and biting insects such as whiteflies and aphids [7]. As a potent agonist of insect nicotinic acetylcholine receptors, thiacloprid can bind to gamma-aminobutyric acid (GABA) receptors on the postsynaptic membrane. In addition, it can disrupt signal transduction in the insect central nervous system [8,9]. Neonicotinoids are currently the most widely used and sold insecticides in the world and provide effective pest control [10,11]. The massive use of these pesticides first contaminates the soil of the treated crops before transferring residues to the aquatic environment. Moreover, recent monitoring studies in numerous nations have shown that these pollutants have contaminated streams, rivers, and lakes all across the world, with residue levels in the low g/L (ppb) range [10,12].
Polycyclic aromatic hydrocarbons (PAHs) are highly detected environmental pollutants and widely distributed in the marine environment, introduced into the aquatic systems of urbanized coastal and estuarine areas through fossil fuel spills, domestic and industrial waste discharges, atmospheric inputs, and continental runoff [13].
Benzo[a]pyrene (B[a]P) is an isomer belonging to the (PAHs) and is characterized by a pyrene condensed with a phenyl group (C20H12). Among the members of its class, it is certainly the most studied compound for its hepatotoxic, carcinogenic, mutagenic, teratogenic, and immunosuppressive effects, which can affect wild and farmed aquatic animals through the trophic chain [14]. Aquatic organisms are widely used as models for the study of contaminants in marine and freshwater ecosystems because they can assimilate pollutants in numerous ways, including ingestion or filtration of particles suspended in water, ion exchange of dissolved heavy metals through lipophilic membranes (gills), and surface adsorption tissues [15,16]. Histopathological changes are used as biomarkers to assess the overall health status of aquatic animals exposed to pollutants [2]. Bivalve mollusks, particularly mussels, are considered good bioindicators of water pollution. First, this is due to their wide distribution, sedentary life mode, and high abundance, and second, because of their filter-feeding mode, ability to accumulate high levels of substances in their organs and high tolerance to abiotic changes [17]. Mussels also attract attention concerning the assessment of human health risks associated with water deterioration [18]. Several studies highlighted a vast amount of toxic effects described in aquatic animal models following exposure to benzo[a]pyrene (B[a]P), including oxidative stress, cytotoxicity, mutagenicity, tumor formation, and immune repression [19,20]. In addition, B[a]P is highly genotoxic to both marine and freshwater species, following the IARC hazard range, being classified in Group 1 for its extreme health risk [21], although its genotoxic susceptibility has been studied mainly in freshwater species [22]. Several studies have investigated the presence of B[a]P in aquatic organisms as biomarkers of toxicity [23,24]. In addition, B[a]P has also been found in bivalves (Mytilus galloprovincialis, Lamarck 1819), cephalopods (Todarodes sagittatus, Lamarck 1798), crustaceans (Nephrops norvegicus, Linnaeus 1758), and fish (Mullus barbatus Linnaeus 1758, Scomber scombrus Linnaeus 1758, Micromesistius poutassou Risso 1827, Merluccius merluccius Linnaeus 1758) in several basins from the central Adriatic Sea [25].
The internal defense system of mussels, as of all invertebrates, lacks the highly specific adaptive immunity of jawed vertebrates characterized by lymphocytes and the production of immunoglobulin antibodies. However, they protect themselves from microbes, parasites, and environmental threats by circulating blood cells responsible for the production of reactive oxygen and nitrogen species and phagocytosis, i.e., highly conserved processes [26]. These defense mechanisms include pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs), NOD-like receptors (NLRs) [27], and phagocytosis [28]. Phagocytic cells observed in invertebrates bear different names (amoebocytes, coelomocytes, or hemocytes) depending on the host species, but exhibit morphology attributable to vertebrate macrophages with relatively comparable functions [29]. Several studies have analyzed defense cells as biomarkers in fish [30,31,32] and in invertebrates [33].
This study aims to evaluate hemocytes in the mantle and gills of M. galloprovincialis as biomarkers of thiacloprid and B[a]P pollution and investigate their possible synergistic effect using mono- and polyclonal anti-TLR2 and anti-iNOS antibodies for the first time.
2. Materials and Methods
2.1. Contaminants
Thiacloprid (purity 99.9%) was purchased as a powder from Sigma-Aldrich (Prague, Czech Republic). Distilled water was used as a solvent to prepare a stock solution (1 mg L−1), and from this, dilutions were made to obtain the concentration of 4.5 μg/L−1. B[a]P was purchased from Sigma-Aldrich (Saint Louis, MO, USA) and was put into solution according to the manufacturer’s instructions and was brought to a concentration of 1 μg/L−1.
2.2. Selection of Concentrations
This study aims to assess the potential synergic effect of the combination of two of the contaminants most found in the aquatic environment at concentrations likely to overlap with those already detected. Based on previous studies [34], we selected a concentration of 4.5 μg/L−1 thiacloprid as it is similar to that found in the environment, and similarly, we selected a 1 μg/L concentration of B[a]P as reported from previous studies [35,36].
2.3. Animal Preparation
In the present study, M. galloprovincialis was used as a model organism by which to assess the combined effect of a chemical mixture of thiacloprid and B[a]P. Mussels were purchased from a local farm of bivalves at Faro Lake (Company Faro SRL, Frutti di Mare, Messina, Italy). A total of 320 mussels (mean length = 6.21 ± 0.14 cm) were housed in the laboratory for acclimation for 10 days before conducting the experiments.
2.4. Experimental Design
Healthy mussels were randomly split into four 40 L experimental tanks. The groups were divided as follows: one control group (n = 40) with only fresh seawater; one group (n = 40) exposed to thiacloprid 4.5 μg/L−1; one group (n = 40) exposed to B[a]P 1 μg/L−1; and one group (n = 40) exposed to both thiacloprid 4.5 μg/L−1 and B[a]P 1 μg/L−1. The organisms were maintained under controlled conditions (aeration, temperature 23.03 ± 0.6 °C, pH 7.54 ± 0.16, salinity 31.79 ± 1.37, 1100 m Osm kg−1, 12 h light:12 h dark) for 7 days, and the experiment was repeated two times. This assessment of toxicity was performed using a semi-static system with natural seawater. Seawater and stock solutions were refreshed every 48 h to guarantee a constant supply of fresh brackish food and prevent famine while exposed for an extended period. Twenty mussels from each experimental group were randomly collected on the 7th day of exposure. No mortality was found in the control group and the group exposed to thiacloprid 4.5 μg/L−1. Both thiacloprid 4.5 μg/L−1 and the B[a]P 1 μg/L single concentration exposure group showed no statistically relevant mortality (<3% and 4%, respectively); on the contrary, thiacloprid 4.5 μg/L−1 and B[a]P 1 μg/L−1 displayed a mortality rate of 8%.
2.5. Tissue Preparation
Following the procedures for sample preparation for light microscopy, M. galloprovincialis samples were processed. After fixation in Immunofix, the samples were washed in crescent alcohol (up to 100% alcohol) to remove water from the tissues and allow better penetration of paraffin. The samples were washed with xylol, which is solvent for paraffin, to clarify the tissues. Finally, they were included, via inclusion unit, in bioplast [37]. On each slide, 5 μm thick sections were placed that had been cut using a rotary microtome (LEICA 2065 Supercut, Nussloch, Germany). Following deparaffinization in xylene, slides were rehydrated in alcohol solutions ranging from 100% to 30% alcohol, followed by distilled water.
2.6. Histological Analysis
The sections were stained using Mallory Trichrome (04–020802, BioOptica Milano S.p.A., Milan, Italy), Masson Trichrome (04–010802, BioOptica Milano S.p.A., Milan, Italy), and Alcian blue/Periodic Acid Schiff (AB/PAS) (04–163802 BioOptica Milano S.p.A., Milan, Italy) [38,39].
2.7. Confocal Microscopy
Slices received treatment in a 2.5% solution of bovine serum albumin (BSA) after deparaffinization and rehydration. After that, primary anti-TLR2 and anti-iNOS antibodies were incubated in the sections. The incubation of secondary antibodies was carried out after one night of exposition to the primary antibodies. To prevent photobleaching, the slices were mounted using Fluoromount [40,41]. As a negative control, experiments were carried out without the primary antibodies. Rat gut tissues were used as a positive control to ensure the immunopositivity of the primary antibodies (data not shown). The slices were examined using a confocal laser scanning microscope with a META module (Zeiss LSM DUO, Carl Zeiss MicroImaging GmbH, Jena, Germany). The fluorescence was detected using argon (458 nm) and helium–neon (543 nm) lasers. The images were captured and enhanced using Zen 2011 (LSM 700 Zeiss software, Oberkochen, Germany). To prevent photodegradation, each picture was snapped as rapidly as possible. The digital pictures were added to a figure composite using Adobe Photoshop CC version 2019 (Adobe Systems, San Jose, CA, USA). The fluorescence intensity curves were then evaluated using Zen 2011 “Display profile” feature. Information about the antibodies is provided in Table 1.
Table 1.
Antibodies data.
| Antibody | Supplier | Product Number | Dilution | Animal Source |
|---|---|---|---|---|
| TLR2 | Active Motif, La Hulpe, Belgium | 40981 | 1:125 | rabbit |
| iNOS | Santa Cruz Biotechnology, Inc., Dallas, TX, USA | Sc-7271 | 1:200 | mouse |
| Alexa Fluor 488 Donkey anti-Mouse IgG FITC conjugated | Molecular Probes, Invitrogen, Eugene, OR, USA | A21202 | 1:300 | donkey |
| Alexa Fluor 594 Donkey anti-Rabbit IgG TRITC conjugated | Molecular Probes, Invitrogen, Eugene, OR, USA | A21207 | 1:300 | donkey |
2.8. Statistical Analysis
Ten sections and twenty fields per sample were examined to collect data for the quantitative analysis. ImageJ 1.53e was used to evaluate the amount and positivity of the cells. SigmaPlot version 14.0 (Systat Software, San Jose, CA, USA) was used to count the number of hemocytes that were TLR2- and iNOS-positive [42]. One-way ANOVA and the Student’s t-test were employed to analyze the normally distributed data. The mean values and standard deviations (SD) of hemocytes detected are presented as follows: ** p ≤ 0.01, * p ≤ 0.05.
2.9. Oxidative Stress Biomarkers
Mantle and gills tissues (n = 10 for each experimental group) were defrosted and weighed separately. Briefly, the samples were homogenized on ice, with 180 μL of ice-cold physiological saline. The supernatant was obtained by centrifuging the homogenate at 4000× g for 15 min at 4 °C. According to previous studies, the content of superoxide dismutase (SOD), catalase (CAT), and malondialdehyde (MDA) in the supernatant was measured using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) [43,44]. Lipid peroxidation products (measured as MDA) were investigated via the thiobarbituric acid (TBARS) method, and the MDA concentration was expressed as nanomoles per mg protein as seen in a previous study [45]. One unit of SOD activity was defined as the amount of enzyme required to inhibit the oxidation reaction by 50% and was expressed as U/mg protein. One unit of CAT activity was defined as the amount of enzyme required to consume 1 μmol H2O2 in 1 s and was expressed as U/mg protein. Protein concentration was assayed using the Bradford assay using bovine serum albumin as a standard.
2.10. Data Analysis
All values in the figures and text are expressed as the mean ± standard deviation (SD) of N number of experiments. The results were analyzed via one-way ANOVA followed by a Bonferroni post hoc test for multiple comparisons. The data were tested for normal distribution with Bartlett’s test, and they were represented as mean ± standard deviation (SD), (p value of <0.0001). Statistical analysis was performed using Graphpad Prism 9 (Graphpad, Boston, MA, USA).
3. Results
3.1. Histological Analysis
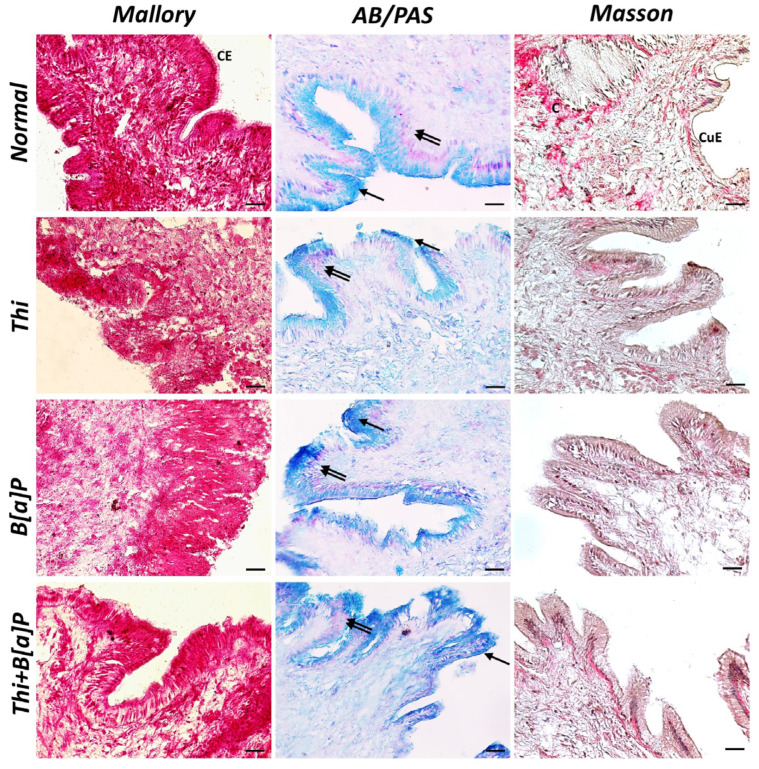
Histological analysis of the mantle of the control group showed a cuboidal epithelium that becomes columnar in some sections. Most of the mantle edges showed the presence of collagen and muscle. AB/PAS histochemical staining revealed sulfated mucosubstances distributed among the collagen fibers of the mantle margin in the form of agglomerates. Mucosal cells containing acidic (light blue stained) and neutral (magenta purple) mucopolysaccharides were observed in the epithelium. In the treated groups, there is an increase in acidic mucopolysaccharides compared to neutral ones. Mucosal cells with purple secretion, indicating a mixture of acidic and neutral mucopolysaccharides, are evident in the epithelia of pollutant-exposed specimens. The epithelial cells appear less organized, and the connective tissue is looser, especially in the group exposed to both pollutants. There is also a decrease in the presence of cilia in sensory function cells. Infiltrates of hemocytes appear evident in the exposed groups (Figure 1).
Figure 1.
Cross section of mantle of M. galloprovincialis (40×, scale bar: 10 µm). Mallory trichrome and Masson trichrome morphological staining revealed a homogeneous, well-organized connective tissue (C) and an epithelium with columnar (CE) and cuboidal (CuE) cells in control animals. AB/PAS histochemical staining revealed the presence of acidic (arrow) and neutral (double arrow) mucopolysaccharides. In groups exposed to pollutants, the epithelium was more disorganized, with looser connective tissue and an overproduction of acidic mucopolysaccharides.
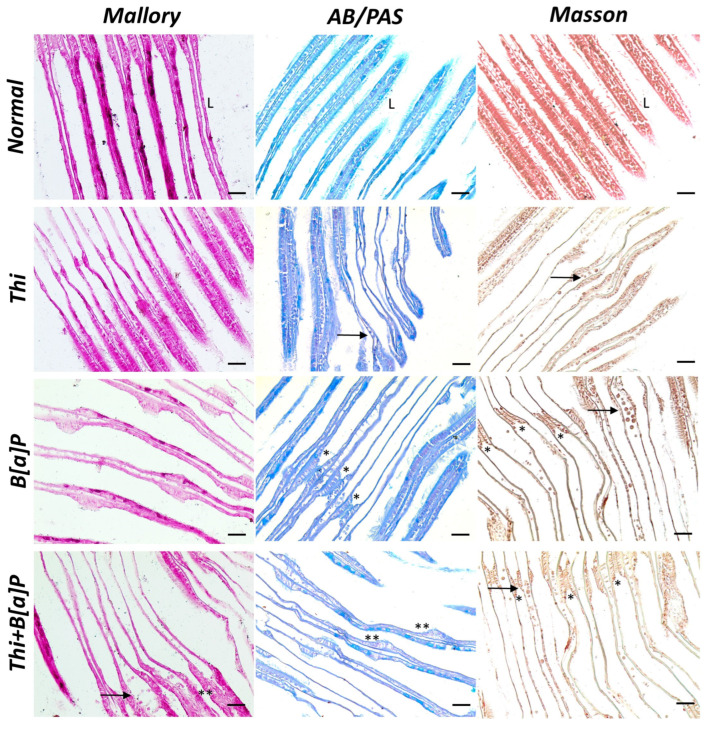
The gills of the control specimens show normal morphology, and the presence of well-organized lamellae, with no changes in the epithelia. In contrast, histology of the gills of pollutant-exposed mussels showed a cartilaginous core, lamellar damage, and epithelial changes, sometimes with lamellae fusion. In addition, some epithelial cells were hypertrophic and hyperplastic. Tissue damage appears evident in all exposed groups but is greater in the group exposed to both pollutants, where there is progressive congestion of the lamellae. Moreover, hemocyte infiltrates appear evident in the exposed groups (Figure 2).
Figure 2.
Cross section of gills of M. galloprovincialis (40×, scale bar: 10 µm). Control specimens showed well-organized, linear gills with homogeneous lamellae (L) and compact epithelium. In the exposed groups, the lamellae appear disorganized, and the epithelial tissue appear inhomogeneous. Fusion of lamellae (*) with hyperplastic and hypertrophic cells (**) is also evident in some places. In addition, aggregates of hemocytes are evident in the pollutant-exposed groups (arrow).
3.2. Immunohistochemical Analysis
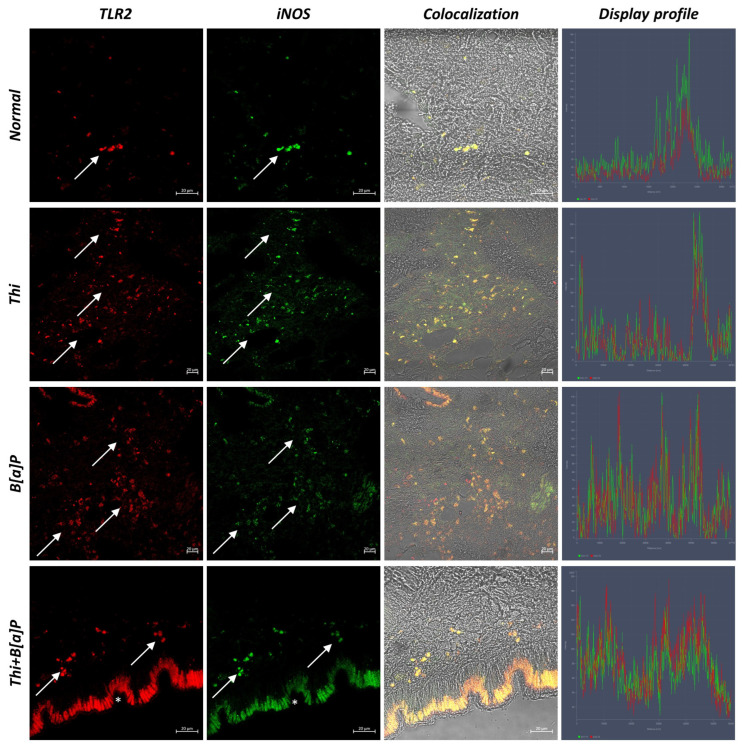
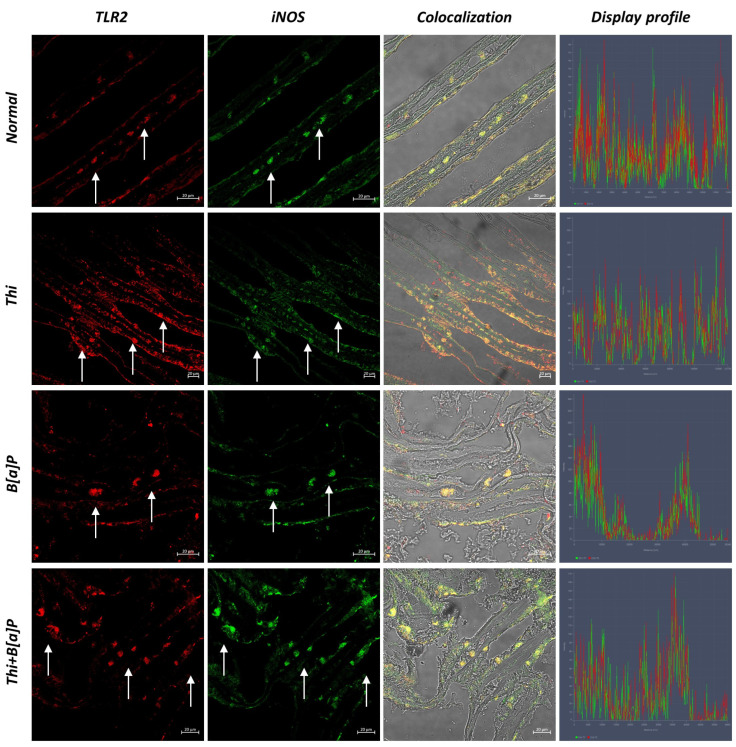
Confocal microscopic investigation revealed hemocytes immunoreactive to TLR2 and iNOS in all groups. Moreover, hemocytes immunoreactive to these molecules, which colocalize conspicuously, are visible in both the epithelium and subepithelium. However, there is a noticeable increase in the number of hemocytes in the exposed groups, especially in the one exposed to both pollutants. Furthermore, markedly positive epithelial cells to both antibodies are noted, especially in the group exposed to B[a]P and thiacloprid. Interestingly, not all hemocytes colocalized for the antibodies tested, suggesting a difference in the function of these cells (Figure 3 and Figure 4).
Figure 3.
Cross section of mantle of M. galloprovincialis (40×, scale bar: 20 µm). Confocal microscopy analysis shows hemocytes positive for TLR2 (red) and iNOS (green) (arrow). Not all hemocytes colocalize for the antibodies tested. Colocalized hemocytes can be seen in yellow. In groups exposed to pollutants, an increase in the number of immunoreactive hemocytes is observed. Strongly antibody-reactive epithelial cells are observed in the exposed groups (*). Use of the “display profile” function confirms the colocalization of antibodies.
Figure 4.
Cross section of gills of M. galloprovincialis (40×, scale bar: 20 µm) Immunofluorescence. Hemocytes with TLR2 (red) and iNOS (green) positivity were identified using confocal microscopy (arrow). For the tested antibodies, not all hemocytes colocalize. There is an increase in immunoreactive hemocytes among populations that have been exposed to pollutants. The “display profile” feature’s use validates the colocalization of antibodies.
Statistical analysis revealed an increasing number of immunoreactive hemocytes to TLR2 and iNOS, especially in the group exposed to the synergic effect of thiacloprid and B[a]P (Table 2).
Table 2.
Statistical results (mean values ± standard deviations; n = 3).
| Normal | Thiacloprid | B[a]P | Thiacloprid + B[a]P | |
|---|---|---|---|---|
| Mantle | ||||
| TLR2 | 345.72 ± 68.92 * | 469.82 ± 64.21 ** | 471.69 ± 31.64 ** | 604.57 ± 63.07 * |
| iNOS | 273.48 ± 65.38 * | 436.45 ± 43.41 * | 443.54 ± 53.87 ** | 592.35 ± 92.31 ** |
| TLR2 + iNOS | 212.30 ± 49.34 ** | 401.03 ± 37.24 * | 416.24 ± 44.59 * | 532.18 ± 83.12 ** |
| Gills | ||||
| TLR2 | 312.31 ± 54.23 ** | 451.29 ± 61.94 * | 458.46 ± 37.14 ** | 622.98 ± 63.42 * |
| iNOS | 251.78 ± 35.29 ** | 423.37 ± 51.27 * | 432.43 ± 20.83 * | 598.23 ± 96.12 ** |
| TLR2 + iNOS | 201.02 ± 17.03 * | 398.39 ± 47.91 ** | 406.90 ± 23.74 ** | 542.71 ± 45.82 * |
** p ≤ 0.01, * p ≤ 0.05.
3.3. Oxidative Stress Biomarkers and Lipid Peroxidation
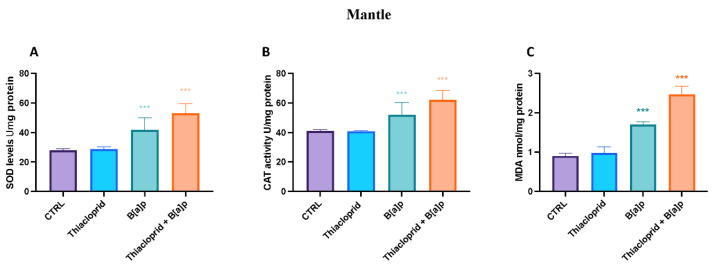
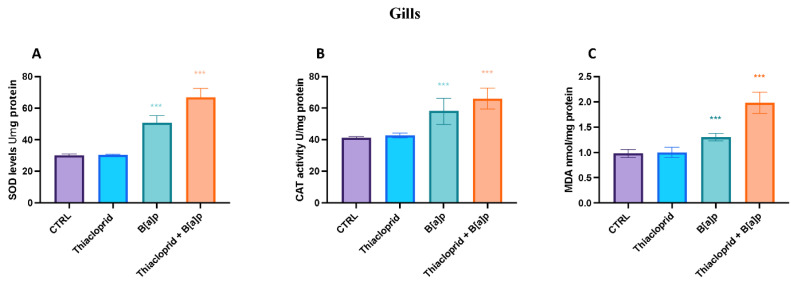
SOD, CAT, and MDA activity was not found to be considerably impacted by the thiacloprid 4.5 μg/L−1 exposure group in the current investigation (Figure 5 and Figure 6). On the contrary, the B[a]P 1 μg/L−1 group presented an increase in SOD, CAT, and MDA activity in both mantle and gills after 7 days of exposition compared to the control group (Figure 5 and Figure 6). Finally, the thiacloprid 4.5 μg/L−1 and B[a]P 1 μg/L−1 mixture group showed a significant rise in the activity of SOD and CAT in the both gills and mantle compared to the single-exposure groups and the control (Figure 5 and Figure 6).
Figure 5.
Antioxidant biomarkers (A,B) and lipid peroxidation (C) in the mantle of M. galloprovincialis exposed to thiacloprid, B[a]P and thiacloprid + B[a]P. The values are presented as the means ± SD; n = 10; The asterisk denotes a statistically significant difference when compared with the CTRL: *** p< 0.0001 versus control.
Figure 6.
Antioxidant biomarkers (A,B) and lipid peroxidation (C) in gills of M. galloprovincialis exposed to thiacloprid, B[a]P and thiacloprid + B[a]P. The values are presented as the means ± SD; n = 10; The asterisk denotes a statistically significant difference when compared with the CTRL: *** p< 0.0001 versus control.
4. Discussion
Environmental pollution, as well as population expansion and resource scarcity, has become a potential risk to humanity. Chemical pollutants, including heavy metals, microplastics, and persistent organic pollutants, enter living organisms through ingestion, inhalation, and contact [46]. Sewage discharge, agriculture, and industrial activities are some of the main sources of contamination in the aquatic environment [47]. As the demand for vegetables, grains, and fruits has increased due to the continued expansion of the world’s population, the use of pesticides to protect crops from disease or predators has also increased [48]. Often, these substances reach freshwater ecosystems, affecting the organisms that inhabit them. Pesticides or insecticides flowing into rivers can affect the morphology, function, and health of organisms [49]. An aquatic ecosystem can be contaminated by a combination of different pesticides and other pollutants, so organisms such as mussels may bioaccumulate more than one substance [50]. Thiacloprid is a neuroactive insecticide belonging to the class of neonicotinoids, which as pesticides similar in structure to nicotine. Neonicotinoids are competitors of acetylcholine, and their mechanism of action is disruption of signal transmission [51,52].
Mussels are sessile bivalve mollusks widely distributed in the world’s oceans and important members of many coastal ecosystems. As filter feeders, they can accumulate and concentrate contaminants from their surroundings, making them effective bioindicators of environmental quality [53]. Hemocytes, the circulating immune cells of mussels, have been proposed as potential biomarkers of environmental stressors due to their sensitivity to changes in the surrounding environment and their role in the immune response. These defense cells are involved in several physiological and immunological processes, including phagocytosis, encapsulation, and production of reactive oxygen species and nitric oxide [54]. These processes can be affected by exposure to pollutants, pathogens, and other environmental stressors. Therefore, changes in hemocyte morphology, function, and gene expression can provide valuable information about the health and condition of mussels and their environment [55].
In this study, we evaluated for the first time the effect of thiacloprid and B[a]P, individually and combined, on hemocytes in the mantle and gills of M. galloprovincialis using anti-TLR2 and anti-iNOS antibodies, confirming the ecological role of these cells as environmental biomarkers.
The mantle, gills, and hemocytes of M. galloprovincialis play crucial roles in mussel survival, growth, and reproduction. The mantle is a tissue that surrounds the body of the mussel and secretes the shell. It is an important organ for mussel growth, shell formation, and protection from predators. It also plays a role in the uptake and accumulation of pollutants in the body of the mussel [56]. Several studies have shown how environmental contaminants carry a significant increase in peroxide dismutase, catalase, and glutathione peroxidase in the mantle [57,58]. Gills are an important organ for respiration and exchange of gases and nutrients in the body of the mussel. They are also an important site for the uptake and accumulation of pollutants in the mussel’s body. Wang (2018) analyzed the effects of cadmium on the gills of M. galloprovincialis, showing that cadmium exposure led to a significant decrease in the activity of antioxidant enzymes in the gills, inducing oxidative stress [59].
Our study showed tissue changes in the mantle and gills as evidenced by histological data. Physiologically, the mantle is composed of an epithelium-rich columnar in cells with secretory activity, with a well-organized connective underneath [60]. The gills normally consist of rows of gill filaments formed by epithelial tissue, aligned to create a homogeneous trabecular structure, typical of gill lamellae, with apical ciliated cells that facilitate water flow, oxygen uptake, and food collection [61]. The epithelia of animals in the pollutant-exposed groups were disorganized and non-homogeneous, with several congested cells and structures. Hemocytes are specialized cells in the body of mussels that play important roles in immune function, wound healing, and nutrient uptake and transport [62]. Stara et al. (2021) analyzed the effects of long-term exposure of M. galloprovincialis to thiacloprid, suggesting altered hemocyte and hemolymph biochemical parameters, antioxidants, and ROS production and induced histological alterations in mussel tissues [34]. Hoer (2015) analyzed the effects of exposure to polycyclic aromatic hydrocarbon (PAH) fluoranthene on hemocytes of the blue mussel Mytilus trossulus (Gould, 1850). The study found that PAH exposure caused an increase in the number of circulating hemocytes [63]. A study by Karagiannis et al. (2011) analyzed the effects of exposure to the herbicide atrazine on gene expression in hemocytes of the blue mussel M. galloprovincialis, finding that exposure to atrazine causes changes in the expression of genes involved in oxidative stress, DNA damage, and apoptosis [64]. Hemocytes also play an ecological role by being involved in the uptake, transport, detoxification, and elimination of pollutants and their metabolites.
B[a]P can be taken up by hemocytes through receptor-mediated endocytosis and can then be transported throughout the bivalve’s circulatory system. This process can lead to the accumulation of B[a]P in various tissues and organs of the bivalve, including the gills and mantle [59]. Frenzilli et al. (2009) analyzed the effects of B[a]P on hemocytes of the clam Chamelea gallina (Linnaeus, 1758). The study found that B[a]P exposure resulted in increased hemocyte numbers and production of oxygen and nitrogen free radicals [65]. In addition to B[a]P uptake and transport, hemocytes also play a role in the detoxification and elimination of the pollutant. However, B[a]P metabolites can be more toxic than the parent compound and can induce cell damage and dysfunction in hemocytes [66].
Toll-like receptors (TLRs) are a class of phylogenetically highly conserved transmembrane proteins that play an important role in the innate immune system by recognizing pathogen-associated molecular patterns (PAMPs) and initiating immune responses [67,68,69,70]. We previously demonstrated TLR2 expression in the defense cells of invertebrates [71,72], protochordates [73,74], and several aquatic vertebrates such as myxins [74,75], cartilaginous fish [76], and bony fish [77,78,79]. Inducible nitric oxide synthase (iNOS) is an enzyme that catalyzes the production of nitric oxide (NO) in response to various stimuli, such as pathogens and inflammatory cytokines. iNOS is expressed in a variety of immune cells, including the hemocytes of bivalves such as mussels [80]. In previous studies, we evaluated the expression of iNOS in the defense cells of invertebrates [72] and vertebrates [81,82]. iNOS is also involved in the response to environmental stressors in mussels. A study by Dailianis (2009) analyzed the expression of iNOS in hemocytes of the mussel M. galloprovincialis in response to cadmium exposure, finding that iNOS was overexpressed in the hemocytes of mussels exposed to the contaminant [83]. A study by Shi et al. (2012) analyzed the role of iNOS in hemocyte proliferation and metabolism in the scallop Chlamys farreri (Müller, 1776), showing that iNOS expression was upregulated in the hemocytes of scallops subjected to immune challenge, and that iNOS inhibition resulted in a significant decrease in hemocyte proliferation and metabolic activity. This suggested that iNOS plays a role in the regulation of cellular metabolism in these organisms [84]. Consistent with these data, we found hemocytes immunoreactive to TLR2 and iNOS in all groups tested. Quantitative analysis showed that the number of hemocytes increased in the group exposed to B[a]P and in the group exposed to thiacloprid. In the group exposed to both pollutants, a significant increase in hemocytes was noted, suggesting a synergistic effect of the two compounds on defense cells. Moreover, interestingly, not all hemocytes colocalized for the antibodies tested, suggesting that hemocytes positive for iNOS might also have a cytotoxic function, while those positive only for TLR2 might perform a mostly phagocytic function, as reported in previous studies [71,72]. Furthermore, TLR2- and iNOS-positive epithelial cells were found in pollutant-exposed groups, suggesting an involvement of these cells in oxidative stress-induced responses. Although the antibodies used are produced in mammals, we could see marked cross-reactivity, also evidenced by the “display profile” function of the confocal microscope. In addition, an interesting response of hemocytes to environmental pollutants emerges from our data on how these cells are able to increase in number and even overexpress recognition molecules implicated in defense systems, thus showing an evolutionary conservation of host defense mechanisms. However, several genomics studies have shown the presence of human- or mammalian-produced immune molecules in invertebrate defense cells [85,86,87,88]. Moreover, cross-reactive immune molecules such as TLRs of human and mammalian origin have been tested and found in several invertebrates [89,90,91].
The use of hemocytes as biomarkers of environmental stressors in mussels has several advantages over traditional biomarkers, such as enzyme activity and tissue analysis. Hemocytes are a non-destructive and minimally invasive sampling method because they can be collected without harming the organism. In addition, hemocytes are highly sensitive to changes in the environment, making them effective indicators of short-term exposure to stressors. However, there are also limitations to the use of hemocytes as biomarkers. Hemocyte responses to stressors can be highly variable, and changes in their morphology and function can be influenced by factors such as age, sex, and reproductive status. Furthermore, the interpretation of hemocyte responses can be complex, as changes in gene expression may reflect both direct effects of stressors and secondary effects of immune activation. Despite these limitations, the use of hemocytes as biomarkers of environmental stressors in mussels is promising. The activity of enzymes involved in the antioxidants system was also herein investigated. Environmental contaminants-induced oxidative stress is thought to be caused by two main mechanisms: an increase in ROS production and a decrease in cellular antioxidant defenses [92,93]. Oxidative stress is primarily brought on by reactive oxygen species (ROS). Since oxygen free radicals can react excessively with SOD and CAT, disrupting the body’s antioxidant defense system, excessive ROS generation in vivo leads to oxidative stress [94]. SOD is an anti-oxidant enzyme that can shield the body from environmental oxidative damage, eliminating ROS and stopping lipid peroxidation. Data collected showed an imbalance in the antioxidant defense system with an evident rise in the levels of SOD and CAT in both the mantle and gills of mussels after 7 days of exposition. Moreover, the levels of antioxidant enzyme were more consistent in the co-exposure group (thiacloprid 4.5 μg/L−1 and B[a]P 1 μg/L−1) compared to the single exposure groups of thiacloprid and B[a]P.
5. Conclusions
Our results showed that the combination of the two contaminants increased oxidative stress strongly compared to the single exposition. Further research is needed in order to standardize sampling protocols and identify the most effective hemocyte-based biomarkers for different types of stressors. Moreover, the use of multi-biomarker approaches, which combine hemocyte-based biomarkers with other traditional biomarkers, may provide a more comprehensive assessment of environmental quality. However, our data may provide useful insights to improve the understanding of the roles of TLR2 and INOS in the internal defense system of bivalves, and their involvement in the management of environmental pollutants. This could enable the development of new strategies and technologies such as the creation of biosensors suitable for improving water pollution management and providing ever-new details on the health of the marine ecosystem.
Author Contributions
Conceptualization, A.A. and D.D.P.; formal analysis, A.A., D.D.P. and A.F.; investigation, A.A., D.D.P., A.F., S.M., C.D., S.F., M.A., N.S. and E.R.L.; data curation, A.A., D.D.P., A.F., S.M., C.D., S.F., M.A., N.S. and E.R.L.; writing—original draft preparation, A.A. and D.D.P.; writing—review and editing, A.A., D.D.P., N.S. and E.R.L.; visualization, A.A., D.D.P. and A.F.; supervision, N.S. and E.R.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work has been partially funded by the European Union (NextGeneration EU) through the MUR-PNRR project SAMOTHRACE (ECS00000022).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Khan M.S., Javed M., Rehman M.T., Urooj M., Ahmad M.I. Heavy Metal Pollution and Risk Assessment by the Battery of Toxicity Tests. Sci. Rep. 2020;10:16593. doi: 10.1038/s41598-020-73468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alesci A., Cicero N., Fumia A., Petrarca C., Mangifesta R., Nava V., Lo Cascio P., Gangemi S., Di Gioacchino M., Lauriano E.R. Histological and Chemical Analysis of Heavy Metals in Kidney and Gills of Boops Boops: Melanomacrophages Centers and Rodlet Cells as Environmental Biomarkers. Toxics. 2022;10:218. doi: 10.3390/toxics10050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ware G.W., editor. Reviews of Environmental Contamination and Toxicology. Volume 143. Springer; New York, NY, USA: 1995. [Google Scholar]
- 4.Vinodhini R., Narayanan M. Bioaccumulation of Heavy Metals in Organs of Fresh Water Fish Cyprinus carpio (Common Carp) Int. J. Environ. Sci. Technol. 2008;5:179–182. doi: 10.1007/BF03326011. [DOI] [Google Scholar]
- 5.Gupta S., Gupta K. Bioaccumulation of Pesticides and Its Impact on Biological Systems. In: Srivastava P.K., Singh V.P., Singh A., Tripathi D.K., Singh S., Prasad S.M., Chauhan D.K., editors. Pesticides in Crop Production. Wiley; New York, NY, USA: 2020. pp. 55–67. [Google Scholar]
- 6.Zenker A., Cicero M.R., Prestinaci F., Bottoni P., Carere M. Bioaccumulation and Biomagnification Potential of Pharmaceuticals with a Focus to the Aquatic Environment. J. Environ. Manag. 2014;133:378–387. doi: 10.1016/j.jenvman.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 7.Santacroce M.P., Pastore A.S., Tinelli A., Colamonaco M., Crescenzo G. Implications for Chronic Toxicity of Benzo[a]Pyrene in Sea Bream Cultured Hepatocytes: Cytotoxicity, Inflammation, and Cancerogenesis. Environ. Toxicol. 2015;30:1045–1062. doi: 10.1002/tox.21978. [DOI] [PubMed] [Google Scholar]
- 8.Kabir H., El-Aty A.A., Rahman M., Kim S.-W., Choi J.-H., Lee Y.-J., Truong L.T.B., Lee K.-B., Kim M.-R., Shin H.-C., et al. The Disappearance Rate and Risk Assessment of Thiacloprid Residues in Asian Pear Using Liquid Chromatography Confirmed with Tandem Mass Spectrometry: Thiacloprid Residues in Asian Pear. Biomed. Chromatogr. 2017;31:e3861. doi: 10.1002/bmc.3861. [DOI] [PubMed] [Google Scholar]
- 9.Wang X., Anadón A., Wu Q., Qiao F., Ares I., Martínez-Larrañaga M.-R., Yuan Z., Martínez M.-A. Mechanism of Neonicotinoid Toxicity: Impact on Oxidative Stress and Metabolism. Annu. Rev. Pharmacol. Toxicol. 2018;58:471–507. doi: 10.1146/annurev-pharmtox-010617-052429. [DOI] [PubMed] [Google Scholar]
- 10.Gomez S.D., Bustos P.S., Sánchez V.G., Ortega M.G., Guiñazú N. Trophoblast Toxicity of the Neonicotinoid Insecticide Acetamiprid and an Acetamiprid-Based Formulation. Toxicology. 2020;431:152363. doi: 10.1016/j.tox.2020.152363. [DOI] [PubMed] [Google Scholar]
- 11.Kaur S., Khera K.S., Kondal J.K. Heavy Metal Induced Histopathological Alterations in Liver, Muscle and Kidney of Freshwater Cyprinid, Labeo rohita (Hamilton) J. Entomol. Zool. Stud. 2018;6:2137–2144. [Google Scholar]
- 12.Di Paola D., Iaria C., Capparucci F., Arangia A., Crupi R., Cuzzocrea S., Spanò N., Gugliandolo E., Peritore A.F. Impact of Mycotoxin Contaminations on Aquatic Organisms: Toxic Effect of Aflatoxin B1 and Fumonisin B1 Mixture. Toxins. 2022;14:518. doi: 10.3390/toxins14080518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Georgieva E., Antal L., Stoyanova S., Arnaudova D., Velcheva I., Iliev I., Vasileva T., Bivolarski V., Mitkovska V., Chassovnikarova T., et al. Biomarkers for Pollution in Caged Mussels from Three Reservoirs in Bulgaria: A Pilot Study. Heliyon. 2022;8:e09069. doi: 10.1016/j.heliyon.2022.e09069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Cauwenberghe L., Janssen C.R. Microplastics in Bivalves Cultured for Human Consumption. Environ. Pollut. 2014;193:65–70. doi: 10.1016/j.envpol.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 15.Ikenaka Y., Sakamoto M., Nagata T., Takahashi H., Miyabara Y., Hanazato T., Ishizuka M., Isobe T., Kim J.-W., Chang K.-H. Effects of Polycyclic Aromatic Hydrocarbons (PAHs) on an Aquatic Ecosystem: Acute Toxicity and Community-Level Toxic Impact Tests of Benzo[a]Pyrene Using Lake Zooplankton Community. J. Toxicol. Sci. 2013;38:131–136. doi: 10.2131/jts.38.131. [DOI] [PubMed] [Google Scholar]
- 16.Reynaud S., Raveton M., Ravanel P. Interactions between Immune and Biotransformation Systems in Fish: A Review. Aquat. Toxicol. 2008;87:139–145. doi: 10.1016/j.aquatox.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 17.Wang L., Camus A.C., Dong W., Thornton C., Willett K.L. Expression of CYP1C1 and CYP1A in Fundulus heteroclitus during PAH-Induced Carcinogenesis. Aquat. Toxicol. 2010;99:439–447. doi: 10.1016/j.aquatox.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Chemical Agents and Related Occupations. IARC Monogr. Eval. Carcinog. Risks Hum. 2012;100:9–562. [PMC free article] [PubMed] [Google Scholar]
- 19.Almeida J.R., Gravato C., Guilhermino L. Challenges in Assessing the Toxic Effects of Polycyclic Aromatic Hydrocarbons to Marine Organisms: A Case Study on the Acute Toxicity of Pyrene to the European Seabass (Dicentrarchus Labrax L.) Chemosphere. 2012;86:926–937. doi: 10.1016/j.chemosphere.2011.10.059. [DOI] [PubMed] [Google Scholar]
- 20.Carlson E.A., Li Y., Zelikoff J.T. The Japanese Medaka (Oryzias latipes) Model: Applicability for Investigating the Immunosuppressive Effects of the Aquatic Pollutant Benzo[a]Pyrene (BaP) Mar. Environ. Res. 2002;54:565–568. doi: 10.1016/S0141-1136(02)00175-7. [DOI] [PubMed] [Google Scholar]
- 21.Cao X., Huo S., Zhang H., Zhao X., Pang C., Ma C., Zheng J., Wu F. Intensive Land-Based Activities Increase the Potential Risk of Benzo[α]Pyrene (BaP) to Aquatic Ecosystems and Human Health in Coastal Areas of China. J. Clean. Prod. 2022;371:133571. doi: 10.1016/j.jclepro.2022.133571. [DOI] [Google Scholar]
- 22.Jung D., Matson C.W., Collins L.B., Laban G., Stapleton H.M., Bickham J.W., Swenberg J.A., Di Giulio R.T. Genotoxicity in Atlantic Killifish (Fundulus heteroclitus) from a PAH-Contaminated Superfund Site on the Elizabeth River, Virginia. Ecotoxicology. 2011;20:1890–1899. doi: 10.1007/s10646-011-0727-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cardellicchio N., Buccolieri A., Giandomenico S., Lopez L., Pizzulli F., Spada L. Organic Pollutants (PAHs, PCBs) in Sediments from the Mar Piccolo in Taranto (Ionian Sea, Southern Italy) Mar. Pollut. Bull. 2007;55:451–458. doi: 10.1016/j.marpolbul.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Solé M., Manzanera M., Bartolomé A., Tort L., Caixach J. Persistent Organic Pollutants (POPs) in Sediments from Fishing Grounds in the NW Mediterranean: Ecotoxicological Implications for the Benthic Fish Solea sp. Mar. Pollut. Bull. 2013;67:158–165. doi: 10.1016/j.marpolbul.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 25.Zacchino V., Centoducati G., Narracci M., Selvaggi M., Santacroce M.P. Effects of Benzo[a]Pyrene on Gilthead Sea Bream (Sparus aurata L.) Hepatocytes Exposed In Vitro to Short and Long Term Trials. Ital. J. Anim. Sci. 2013;12:e17. doi: 10.4081/ijas.2013.e17. [DOI] [Google Scholar]
- 26.Perugini M., Visciano P., Giammarino A., Manera M., Di Nardo W., Amorena M. Polycyclic Aromatic Hydrocarbons in Marine Organisms from the Adriatic Sea, Italy. Chemosphere. 2007;66:1904–1910. doi: 10.1016/j.chemosphere.2006.07.079. [DOI] [PubMed] [Google Scholar]
- 27.Thunnissen N.W., Geurts K.A.G., Hoeks S., Hendriks A.J. The Impact of Imidacloprid and Thiacloprid on the Mean Species Abundance in Aquatic Ecosystems. Sci. Total Environ. 2022;822:153626. doi: 10.1016/j.scitotenv.2022.153626. [DOI] [PubMed] [Google Scholar]
- 28.Wang W., Chen H., Gao D., Long J., Long H., Zhang Y. Dissipation and Dietary Risk Assessment of Thiacloprid and Tolfenpyrad in Tea in China. Agronomy. 2022;12:3166. doi: 10.3390/agronomy12123166. [DOI] [Google Scholar]
- 29.Litman G.W., Cooper M.D. Why Study the Evolution of Immunity? Nat. Immunol. 2007;8:547–548. doi: 10.1038/ni0607-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Traylor-Knowles N., Vandepas L.E., Browne W.E. Still Enigmatic: Innate Immunity in the Ctenophore Mnemiopsis leidyi. Integr. Comp. Biol. 2019;59:811–818. doi: 10.1093/icb/icz116. [DOI] [PubMed] [Google Scholar]
- 31.Elvington M., Liszewski M.K., Atkinson J.P. Evolution of the Complement System: From Defense of the Single Cell to Guardian of the Intravascular Space. Immunol. Rev. 2016;274:9–15. doi: 10.1111/imr.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buchmann K. Evolution of Innate Immunity: Clues from Invertebrates via Fish to Mammals. Front. Immunol. 2014;5:459. doi: 10.3389/fimmu.2014.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sayed A., Younes H.A.M. Melanomacrophage Centers in Clarias gariepinus as an Immunological Biomarker for Toxicity of Silver Nanoparticles. J. Microsc. Ultrastruct. 2017;5:97. doi: 10.1016/j.jmau.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naguib M., Mahmoud U.M., Mekkawy I.A., Sayed A.E.-D.H. Hepatotoxic Effects of Silver Nanoparticles on Clarias gariepinus; Biochemical, Histopathological, and Histochemical Studies. Toxicol. Rep. 2020;7:133–141. doi: 10.1016/j.toxrep.2020.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dang M., Nowell C., Nguyen T., Bach L., Sonne C., Nørregaard R., Stride M., Nowak B. Characterisation and 3D Structure of Melanomacrophage Centers in Shorthorn Sculpins (Myoxocephalus scorpius) Tissue Cell. 2019;57:34–41. doi: 10.1016/j.tice.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Impellitteri F., Curpăn A.-S., Plăvan G., Ciobica A., Faggio C. Hemocytes: A Useful Tool for Assessing the Toxicity of Microplastics, Heavy Metals, and Pesticides on Aquatic Invertebrates. Int. J. Environ. Res. Public Health. 2022;19:16830. doi: 10.3390/ijerph192416830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stara A., Pagano M., Albano M., Savoca S., Di Bella G., Albergamo A., Koutkova Z., Sandova M., Velisek J., Fabrello J., et al. Effects of Long-Term Exposure of Mytilus galloprovincialis to Thiacloprid: A Multibiomarker Approach. Environ. Pollut. 2021;289:117892. doi: 10.1016/j.envpol.2021.117892. [DOI] [PubMed] [Google Scholar]
- 38.González-Soto N., Hatfield J., Katsumiti A., Duroudier N., Lacave J.M., Bilbao E., Orbea A., Navarro E., Cajaraville M.P. Impacts of Dietary Exposure to Different Sized Polystyrene Microplastics Alone and with Sorbed Benzo[a]Pyrene on Biomarkers and Whole Organism Responses in Mussels Mytilus galloprovincialis. Sci. Total Environ. 2019;684:548–566. doi: 10.1016/j.scitotenv.2019.05.161. [DOI] [PubMed] [Google Scholar]
- 39.Beiras R., Verdejo E., Campoy-López P., Vidal-Liñán L. Aquatic Toxicity of Chemically Defined Microplastics Can Be Explained by Functional Additives. J. Hazard. Mater. 2021;406:124338. doi: 10.1016/j.jhazmat.2020.124338. [DOI] [PubMed] [Google Scholar]
- 40.Alesci A., Messina E., Zuwala K., Fumia A., Miller A., D’Angelo R., Kuciel M., Albano M., Savoca S., Capillo G. Mechanosensory Cells in Annelid Oligochaete Lumbricus terrestris (Linnaeus, 1758): A New Insight on Worm Evolution. Acta Zool. 2023 doi: 10.1111/azo.12475. [DOI] [Google Scholar]
- 41.Alesci A., Pergolizzi S., Mokhtar D.M., Fumia A., Aragona M., Lombardo G.P., Messina E., D’Angelo R., Cascio P.L., Sayed R.K. Morpho-Structural Adaptations of the Integument in Different Aquatic Organisms. Acta Histochem. 2023;125:152031. doi: 10.1016/j.acthis.2023.152031. [DOI] [PubMed] [Google Scholar]
- 42.Lauriano E.R., Żuwała K., Kuciel M., Budzik K.A., Capillo G., Alesci A., Pergolizzi S., Dugo G., Zaccone G. Confocal Immunohistochemistry of the Dermal Glands and Evolutionary Considerations in the Caecilian, Typhlonectes natans (Amphibia: Gymnophiona) Acta Zool. 2016;97:154–164. doi: 10.1111/azo.12112. [DOI] [Google Scholar]
- 43.Lauriano E.R., Icardo J.M., Zaccone D., Kuciel M., Satora L., Alesci A., Alfa M., Zaccone G. Expression Patterns and Quantitative Assessment of Neurochemical Markers in the Lung of the Gray Bichir, Polypterus senegalus (Cuvier, 1829) Acta Histochem. 2015;117:738–746. doi: 10.1016/j.acthis.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 44.Pergolizzi S., Rizzo G., Favaloro A., Alesci A., Pallio S., Melita G., Cutroneo G., Lauriano E.R. Expression of VAChT and 5-HT in Ulcerative Colitis Dendritic Cells. Acta Histochem. 2021;123:151715. doi: 10.1016/j.acthis.2021.151715. [DOI] [PubMed] [Google Scholar]
- 45.Liu Y., Wang J., Wei Y., Zhang H., Xu M., Dai J. Induction of Time-Dependent Oxidative Stress and Related Transcriptional Effects of Perfluorododecanoic Acid in Zebrafish Liver. Aquat. Toxicol. 2008;89:242–250. doi: 10.1016/j.aquatox.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 46.Di Paola D., Iaria C., Capparucci F., Cordaro M., Crupi R., Siracusa R., D’Amico R., Fusco R., Impellizzeri D., Cuzzocrea S., et al. Aflatoxin B1 Toxicity in Zebrafish Larva (Danio rerio): Protective Role of Hericium erinaceus. Toxins. 2021;13:710. doi: 10.3390/toxins13100710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fusco R., Cordaro M., Siracusa R., Peritore A.F., Gugliandolo E., Genovese T., D’Amico R., Crupi R., Smeriglio A., Mandalari G., et al. Consumption of Anacardium occidentale L. (Cashew Nuts) Inhibits Oxidative Stress through Modulation of the Nrf2/HO−1 and NF-KB Pathways. Molecules. 2020;25:4426. doi: 10.3390/molecules25194426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou X., Gao S., Yue M., Zhu S., Liu Q., Zhao X.-E. Recent Advances in Analytical Methods of Oxidative Stress Biomarkers Induced by Environmental Pollutant Exposure. TrAC Trends Anal. Chem. 2023;160:116978. doi: 10.1016/j.trac.2023.116978. [DOI] [Google Scholar]
- 49.Gheorghe S., Stoica C., Vasile G.G., Nita-Lazar M., Stanescu E., Lucaciu I.E. Metals Toxic Effects in Aquatic Ecosystems: Modulators of Water Quality. In: Tutu H., editor. Water Quality. InTechOpen Limited; London, UK: 2017. [Google Scholar]
- 50.Tresnakova N., Famulari S., Zicarelli G., Impellitteri F., Pagano M., Presti G., Filice M., Caferro A., Gulotta E., Salvatore G., et al. Multi-Characteristic Toxicity of Enantioselective Chiral Fungicide Tebuconazole to a Model Organism Mediterranean Mussel Mytilus galloprovincialis Lamarck, 1819 (Bivalve: Mytilidae) Sci. Total Environ. 2023;862:160874. doi: 10.1016/j.scitotenv.2022.160874. [DOI] [PubMed] [Google Scholar]
- 51.Moreau P., Burgeot T., Renault T. Pacific Oyster (Crassostrea gigas) Hemocyte Are Not Affected by a Mixture of Pesticides in Short-Term In Vitro Assays. Environ. Sci. Pollut. Res. 2014;21:4940–4949. doi: 10.1007/s11356-013-1931-3. [DOI] [PubMed] [Google Scholar]
- 52.Hong Y., Liao W., Yan Z., Bai Y., Feng C., Xu Z., Xu D. Progress in the Research of the Toxicity Effect Mechanisms of Heavy Metals on Freshwater Organisms and Their Water Quality Criteria in China. J. Chem. 2020;2020:9010348. doi: 10.1155/2020/9010348. [DOI] [Google Scholar]
- 53.Bado-Nilles A., Gagnaire B., Thomas-Guyon H., Le Floch S., Renault T. Effects of 16 Pure Hydrocarbons and Two Oils on Haemocyte and Haemolymphatic Parameters in the Pacific Oyster, Crassostrea gigas (Thunberg) Toxicol. Vitr. 2008;22:1610–1617. doi: 10.1016/j.tiv.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 54.Vagi M.C., Petsas A.S., Kostopoulou M.N. Potential Effects of Persistent Organic Contaminants on Marine Biota: A Review on Recent Research. Water. 2021;13:2488. doi: 10.3390/w13182488. [DOI] [Google Scholar]
- 55.Inoue K., Onitsuka Y., Koito T. Mussel Biology: From the Byssus to Ecology and Physiology, Including Microplastic Ingestion and Deep-Sea Adaptations. Fish Sci. 2021;87:761–771. doi: 10.1007/s12562-021-01550-5. [DOI] [Google Scholar]
- 56.De La Ballina N.R., Maresca F., Cao A., Villalba A. Bivalve Haemocyte Subpopulations: A Review. Front. Immunol. 2022;13:826255. doi: 10.3389/fimmu.2022.826255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perez D.G., Fontanetti C.S. Hemocitical Responses to Environmental Stress in Invertebrates: A Review. Environ. Monit. Assess. 2011;177:437–447. doi: 10.1007/s10661-010-1645-7. [DOI] [PubMed] [Google Scholar]
- 58.Salas C., Bueno-Pérez J.D.D., López-Téllez J.F., Checa A.G. Form and Function of the Mantle Edge in Protobranchia (Mollusca: Bivalvia) Zoology. 2022;153:126027. doi: 10.1016/j.zool.2022.126027. [DOI] [PubMed] [Google Scholar]
- 59.Dobal V., Suárez P., Ruiz Y., García-Martín O., San Juan F. Activity of Antioxidant Enzymes in Mytilus galloprovincialis Exposed to Tar: Integrated Response of Different Organs as Pollution Biomarker in Aquaculture Areas. Aquaculture. 2022;548:737638. doi: 10.1016/j.aquaculture.2021.737638. [DOI] [Google Scholar]
- 60.Capolupo M., Franzellitti S., Valbonesi P., Lanzas C.S., Fabbri E. Uptake and Transcriptional Effects of Polystyrene Microplastics in Larval Stages of the Mediterranean Mussel Mytilus galloprovincialis. Environ. Pollut. 2018;241:1038–1047. doi: 10.1016/j.envpol.2018.06.035. [DOI] [PubMed] [Google Scholar]
- 61.Wang J., Deng W., Zhou T., Bai B., Chang A.K., Ying X. Cadmium-Induced Oxidative Stress in Meretrix meretrix Gills Leads to Mitochondria-Mediated Apoptosis. Ecotoxicology. 2021;30:2011–2023. doi: 10.1007/s10646-021-02465-8. [DOI] [PubMed] [Google Scholar]
- 62.Rocha T.L., Gomes T., Cardoso C., Letendre J., Pinheiro J.P., Sousa V.S., Teixeira M.R., Bebianno M.J. Immunocytotoxicity, Cytogenotoxicity and Genotoxicity of Cadmium-Based Quantum Dots in the Marine Mussel Mytilus galloprovincialis. Mar. Environ. Res. 2014;101:29–37. doi: 10.1016/j.marenvres.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 63.Höher N., Turja R., Köhler A., Lehtonen K.K., Broeg K. Immunological Responses in the Mussel Mytilus trossulus Transplanted at the Coastline of the Northern Baltic Sea. Mar. Environ. Res. 2015;112:113–121. doi: 10.1016/j.marenvres.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 64.Karagiannis D., Vatsos I.N., Angelidis P. Effects of Atrazine on the Viability and the Formation of Byssus of the Mussel Mytilus galloprovincialis. Aquac. Int. 2011;19:103–110. doi: 10.1007/s10499-010-9344-5. [DOI] [Google Scholar]
- 65.Frenzilli G., Nigro M., Lyons B. The Comet Assay for the Evaluation of Genotoxic Impact in Aquatic Environments. Mutat. Res. Rev. Mutat. Res. 2009;681:80–92. doi: 10.1016/j.mrrev.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 66.Guo B., Feng D., Xu Z., Qi P., Yan X. Acute Benzo[a]Pyrene Exposure Induced Oxidative Stress, Neurotoxicity and Epigenetic Change in Blood Clam Tegillarca granosa. Sci. Rep. 2021;11:18744. doi: 10.1038/s41598-021-98354-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pergolizzi S., Fumia A., D’Angelo R., Mangano A., Lombardo G.P., Giliberti A., Messina E., Alesci A., Lauriano E.R. Expression and Function of Toll-like Receptor 2 in Vertebrate. Acta Histochem. 2023;125:152028. doi: 10.1016/j.acthis.2023.152028. [DOI] [PubMed] [Google Scholar]
- 68.Alesci A., Lauriano E.R., Fumia A., Irrera N., Mastrantonio E., Vaccaro M., Gangemi S., Santini A., Cicero N., Pergolizzi S. Relationship between Immune Cells, Depression, Stress, and Psoriasis: Could the Use of Natural Products Be Helpful? Molecules. 2022;27:1953. doi: 10.3390/molecules27061953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alesci A., Gitto M., Kotańska M., Lo Cascio P., Miller A., Nicosia N., Fumia A., Pergolizzi S. Immunogenicity, Effectiveness, Safety and Psychological Impact of COVID-19 MRNA Vaccines. Hum. Immunol. 2022;83:755–767. doi: 10.1016/j.humimm.2022.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alesci A., Pergolizzi S., Fumia A., Miller A., Cernigliaro C., Zaccone M., Salamone V., Mastrantonio E., Gangemi S., Pioggia G., et al. Immune System and Psychological State of Pregnant Women during COVID-19 Pandemic: Are Micronutrients Able to Support Pregnancy? Nutrients. 2022;14:2534. doi: 10.3390/nu14122534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alesci A., Capillo G., Fumia A., Albano M., Messina E., Spanò N., Pergolizzi S., Lauriano E.R. Coelomocytes of the Oligochaeta Earthworm Lumbricus terrestris (Linnaeus, 1758) as Evolutionary Key of Defense: A Morphological Study. Zool. Lett. 2023;9:5. doi: 10.1186/s40851-023-00203-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alesci A., Fumia A., Albano M., Messina E., D’Angelo R., Mangano A., Miller A., Spanò N., Savoca S., Capillo G. Investigating the Internal System of Defense of Gastropoda Aplysia depilans (Gmelin, 1791): Focus on Hemocytes. Fish Shellfish. Immunol. 2023;137:108791. doi: 10.1016/j.fsi.2023.108791. [DOI] [PubMed] [Google Scholar]
- 73.Alesci A., Pergolizzi S., Lo Cascio P., Capillo G., Lauriano E.R. Localization of Vasoactive Intestinal Peptide and Toll-like Receptor 2 Immunoreactive Cells in Endostyle of Urochordate Styela plicata (Lesueur, 1823) Microsc. Res Tech. 2022;85:2651–2658. doi: 10.1002/jemt.24119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lauriano E.R., Aragona M., Alesci A., Lo Cascio P., Pergolizzi S. Toll-like Receptor 2 and α-Smooth Muscle Actin Expressed in the Tunica of a Urochordate, Styela Plicata. Tissue Cell. 2021;71:101584. doi: 10.1016/j.tice.2021.101584. [DOI] [PubMed] [Google Scholar]
- 75.Alesci A., Pergolizzi S., Savoca S., Fumia A., Mangano A., Albano M., Messina E., Aragona M., Lo Cascio P., Capillo G. Detecting Intestinal Goblet Cells of the Broadgilled Hagfish Eptatretus cirrhatus (Forster, 1801): A Confocal Microscopy Evaluation. Biology. 2022;11:1366. doi: 10.3390/biology11091366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alesci A., Capillo G., Fumia A., Messina E., Albano M., Aragona M., Lo Cascio P., Spanò N., Pergolizzi S., Lauriano E.R. Confocal Characterization of Intestinal Dendritic Cells from Myxines to Teleosts. Biology. 2022;11:1045. doi: 10.3390/biology11071045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lauriano E.R., Alesci A., Aragona M., Pergolizzi S., Miller A., Zuwala K., Kuciel M., Zaccone G., Germanà A., Guerrera M.C. Immunohistochemistry of the Gut-Associated Lymphoid Tissue (GALT) in African Bonytongue (Heterotis niloticus, Cuvier 1829) Int. J. Mol. Sci. 2023;24:2316. doi: 10.3390/ijms24032316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alesci A., Capillo G., Mokhtar D.M., Fumia A., D’Angelo R., Lo Cascio P., Albano M., Guerrera M.C., Sayed R.K.A., Spanò N., et al. Expression of Antimicrobic Peptide Piscidin1 in Gills Mast Cells of Giant Mudskipper Periophthalmodon schlosseri (Pallas, 1770) Int. J. Mol. Sci. 2022;23:13707. doi: 10.3390/ijms232213707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alesci A., Albano M., Savoca S., Mokhtar D.M., Fumia A., Aragona M., Lo Cascio P., Hussein M.M., Capillo G., Pergolizzi S. Confocal Identification of Immune Molecules in Skin Club Cells of Zebrafish (Danio rerio, Hamilton 1882) and Their Possible Role in Immunity. Biology. 2022;11:1653. doi: 10.3390/biology11111653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Donaghy L., Hong H.-K., Jauzein C., Choi K.-S. The Known and Unknown Sources of Reactive Oxygen and Nitrogen Species in Haemocytes of Marine Bivalve Molluscs. Fish Shellfish. Immunol. 2015;42:91–97. doi: 10.1016/j.fsi.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 81.Alesci A., Pergolizzi S., Capillo G., Lo Cascio P., Lauriano E.R. Rodlet Cells in Kidney of Goldfish (Carassius auratus, Linnaeus 1758): A Light and Confocal Microscopy Study. Acta Histochem. 2022;124:151876. doi: 10.1016/j.acthis.2022.151876. [DOI] [PubMed] [Google Scholar]
- 82.Alesci A., Pergolizzi S., Fumia A., Calabrò C., Lo Cascio P., Lauriano E.R. Mast Cells in Goldfish (Carassius auratus) Gut: Immunohistochemical Characterization. Acta Zool. 2022;104:366–379. doi: 10.1111/azo.12417. [DOI] [Google Scholar]
- 83.Dailianis S. Production of Superoxides and Nitric Oxide Generation in Haemocytes of Mussel Mytilus galloprovincialis (Lmk.) after Exposure to Cadmium: A Possible Involvement of Na+/H+ Exchanger in the Induction of Cadmium Toxic Effects. Fish Shellfish. Immunol. 2009;27:446–453. doi: 10.1016/j.fsi.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 84.Shi X., Wang L., Zhou Z., Yang C., Gao Y., Wang L., Song L. The Arginine Kinase in Zhikong Scallop Chlamys Farreri Is Involved in Immunomodulation. Dev. Comp. Immunol. 2012;37:270–278. doi: 10.1016/j.dci.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 85.Kron N.S. In Search of the Aplysia Immunome: An In Silico Study. BMC Genom. 2022;23:543. doi: 10.1186/s12864-022-08780-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Caurcel C., Laetsch D.R., Challis R., Kumar S., Gharbi K., Blaxter M. MolluscDB: A Genome and Transcriptome Database for Molluscs. Phil. Trans. R. Soc. B. 2021;376:20200157. doi: 10.1098/rstb.2020.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zou Y., Xu X., Xiao X., Wang Y., Yang H., Zhang Z. Genome-Wide Identification and Characterization of Toll-like Receptors (TLR) Genes in Haliotis discus hannai, H. rufescens, and H. laevigata. Fish Shellfish. Immunol. 2023;137:108728. doi: 10.1016/j.fsi.2023.108728. [DOI] [PubMed] [Google Scholar]
- 88.Davison A., Neiman M. Mobilizing Molluscan Models and Genomes in Biology. Phil. Trans. R. Soc. B. 2021;376:20200163. doi: 10.1098/rstb.2020.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Blanco G.A.C., Escalada A.M., Alvarez E., Hajos S. LPS-Induced Stimulation of Phagocytosis in the Sipunculan Worm Themiste petricola: Possible Involvement of Human CD14, CD11B and CD11C Cross-Reactive Molecules. Dev. Comp. Immunol. 1997;21:349–362. doi: 10.1016/S0145-305X(97)00007-4. [DOI] [PubMed] [Google Scholar]
- 90.Cossarizza A., Cooper E.L., Suzuki M.M., Salvioli S., Capri M., Gri G., Quaglino D., Franceschi C. Earthworm Leukocytes That Are Not Phagocytic and Cross-React with Several Human Epitopes Can Kill Human Tumor Cell Lines. Exp. Cell Res. 1996;224:174–182. doi: 10.1006/excr.1996.0125. [DOI] [PubMed] [Google Scholar]
- 91.Engelmann P., Pál J., Berki T., Cooper E.L., Németh P. Earthworm Leukocytes React with Different Mammalian Antigen-Specific Monoclonal Antibodies. Zoology. 2002;105:257–265. doi: 10.1078/0944-2006-00068. [DOI] [PubMed] [Google Scholar]
- 92.Xie Z., Lu G., Zhou R., Ma Y. Thiacloprid-Induced Hepatotoxicity in Zebrafish: Activation of the Extrinsic and Intrinsic Apoptosis Pathways Regulated by P53 Signaling Pathway. Aquat. Toxicol. 2022;246:106147. doi: 10.1016/j.aquatox.2022.106147. [DOI] [PubMed] [Google Scholar]
- 93.Lin S., Ren A., Wang L., Huang Y., Wang Y., Wang C., Greene N.D. Oxidative Stress and Apoptosis in Benzo[a]Pyrene-Induced Neural Tube Defects. Free. Radic. Biol. Med. 2018;116:149–158. doi: 10.1016/j.freeradbiomed.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wijesinghe W.A.J.P., Jeon Y.J., Ramasamy P., Wahid M.E.A., Vairappan C.S. Anticancer Activity and Mediation of Apoptosis in Human HL-60 Leukaemia Cells by Edible Sea Cucumber (Holothuria edulis) Extract. Food Chem. 2013;139:326–331. doi: 10.1016/j.foodchem.2013.01.058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.