Abstract
Background
Glycaemic control of Type 1 Diabetes Mellitus (T1DM) remains a challenge due to hypoglycaemic episodes and the burden of insulin self-management. Advancements have been made with the development of automated insulin delivery (AID) devices, yet, previous reviews have only assessed the use of AID over days or weeks, and potential benefits with longer time of AID use in this population remain unclear.
Methods
We performed a systematic review and meta-analysis of randomised controlled trials comparing AID (hybrid and fully closed-loop systems) to usual care (sensor augmented pumps, multiple daily insulin injections, continuous glucose monitoring and predictive low-glucose suspend) for adults and children with T1DM with a minimum duration of 3 months. We searched PubMed, Embase, Cochrane Central, and Clinicaltrials.gov for studies published up until April 4, 2023. Main outcomes included time in range 70–180 mg/dL as the primary outcome, and change in HbA1c (%, mmol/mol), glucose variability, and psychosocial impact (diabetes distress, treatment satisfaction and fear of hypoglycaemia) as secondary outcomes. Adverse events included diabetic ketoacidosis (DKA) and severe hypoglycaemia. Statistical analyses were conducted using mean differences and odds ratios. Sensitivity analyses were performed according to age, study duration and type of AID device. The protocol was registered in PROSPERO, CRD42022366710.
Results
We identified 25 comparisons from 22 studies (six crossover and 16 parallel designs) including a total of 2376 participants (721 in adult studies, 621 in paediatric studies, and 1034 in combined studies) which were eligible for analysis. Use of AID devices ranged from 12 to 96 weeks. Patients using AID had 10.87% higher time in range [95% CI 9.38 to 12.37; p < 0.0001, I2 = 87%) and 0.37% (4.77 mmol/mol) lower HbA1c (95% CI − 0.49% (− 6.39 mmol/mol) to – 0.26 (− 3.14 mmol/mol); p < 0·0001, I2 = 77%]. AID systems decreased night hypoglycaemia, time in hypoglycaemia and hyperglycaemia and improved patient distress, with no increase in the risk of DKA or severe hypoglycaemia. No difference was found regarding treatment satisfaction or fear of hypoglycaemia. Among children, there was no difference in glucose variability or time spent in hypoglycaemia between the use of AID systems or usual care. In sensitivity analyses, results remained consistent with the overall analysis favouring AID.
Conclusion
The use of AID systems over 12 weeks, regardless of technical or clinical differences, improved glycaemic outcomes and diabetes distress without increasing the risk of adverse events in adults and children with T1DM.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13098-023-01144-4.
Keywords: Closed-loop, Automated insulin delivery, HbA1c, TIR, Time in range, Hypoglycaemia, Glucose control, Diabetes technology, Type 1 diabetes, T1DM
Background
Type 1 diabetes mellitus (T1DM) is a chronic autoimmune disease, characterised by the progressive destruction of pancreatic beta cells [1, 2]. Intensive insulin treatment is the current standard of care for T1DM. Unfortunately, the proportion of patients achieving a controlled HbA1c and their time in range (TIR) glycaemic level is low. A large proportion of individuals with type 1 diabetes are unable to meet recommended glycaemic targets [3, 4] and severe hypoglycaemia is a recurrent problem [5].
Since the 1960s, several automated insulin delivery (AID) systems have been developed. The goal of such devices is to achieve better glycaemic control, reduce glucose variability, and decrease the risk of micro and macrovascular complications as well as treatment distress [6]. An AID system consists of three components: a continuous glucose monitor (CGM), a pump able to continuously deliver insulin, and a computer algorithm controlling insulin delivery through glucose-responsive feedback [7]. In the last 15 years, multiple closed-loop (CL) systems were developed, such as predictive low-glucose suspend (PLGS) systems, hybrid closed-loop (HCL) systems, and fully closed-loop (FCL) systems, however, their long-term impact on clinical and functional outcomes is still unclear. Previous randomised controlled trials (RCTs) have obtained variable conclusions. While some showed no significant difference in mean overnight blood glucose when comparing CL and Sensor-augmented Insulin Pump (SAP) in adults [8], adolescents [9], and children [10], others showed no difference in time spent in hypoglycaemia [11]. Recent trials using more advanced AID systems have demonstrated better therapeutic efficacy regarding HbA1c levels and TIR [12].
During the last decade, several meta-analyses of RCTs have been reported and show encouraging results on the effectiveness of AID devices in optimising glycaemic control, but assessments have only focused on studies with limited time of AID use, mostly hours or days [13]. To our knowledge, only one published meta-analysis with 11 RCTs has discussed the potential of these devices up to 8 weeks of use [14]. However, no previous meta-analysis has exclusively assessed studies with over 12 weeks of AID use, which is a more appropriate period of time to properly detect changes in HbA1c levels [15]. Furthermore, we did not find any meta-analyses assessing the longer use of AID systems according to different age groups compared to usual care (UC), which currently represents the use of multiple daily insulin injections (MDII), SAP, CGM or PLGS. Lastly, severe adverse events (AEs) and psychosocial outcomes, which can influence clinical decisions, have not yet been assessed in the setting of longer and continuous use of AID systems.
In this updated systematic review and meta-analysis, our objective was to investigate the impact of AID systems compared to UC on glucose control, as well as treatment satisfaction and distress based on the evidence from RCTs with a duration above 12 weeks. We aimed to determine whether the use of AID systems improved TIR, HbA1c, and glycaemic variability, reduced AEs, and impacted psychosocial outcomes from a functional perspective.
Methods
This review was performed in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) Statement and recommendations of the Cochrane Collaboration Handbook for Systematic Reviews of Interventions [16]. The protocol of this meta-analysis was registered on PROSPERO on October 22, 2022 (ID CRD42022366710).
Search strategy
We systematically searched PubMed, EMBASE, Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov databases up to April 4, 2023, using terms such as: ‘Type 1 Diabetes’, ‘T1DM’, ‘closed-loop’, ‘automated insulin delivery’, ‘AID’, ‘randomized’ and ‘RCT’. The complete search strategy is available in Supplementary Appendix A. No filters or language restrictions were applied in our search. Grey literature was not searched. We also utilised a technique of backward snowballing, searching for additional eligible studies through a review of the references from prior publications [17]. Three authors performed the literature search independently (AG, AM, and LH) following predefined search criteria. Eventual conflicts were resolved by consensus among the authors.
Study selection
The research question was defined according to the PICOTT framework and studies were included in the systematic review if they met the following eligibility criteria: (1) enrolling adult or paediatric patient population with T1DM; (2) comparing CL systems with UC; (3) assessing any of the outcomes of interest; (4) RCTs with parallel or crossover designs; and (5) with a minimum duration of at least 12 weeks. We included both hybrid-loop and fully CL systems in our analysis. UC was considered to include SAP, MDII, CGM, or PLGS. A full description of the current insulin devices can be found in Additional file 1: Table S1.
We excluded studies with overlapping patient populations, understood as derived from overlapping institutions, patients and recruitment periods, and clinical trials with no results after contacting the primary investigator. Additionally, crossover studies with less than 12 weeks of washout periods were excluded from the analysis of change in HbA1c (%), unless outcomes from each phase of the study were reported. In this case, only phase 1 results were included in our HbA1c analysis. If two or more studies with overlapping populations reported different outcomes of interest, they were included if these could be analysed in a non-overlapping manner.
Data collection and extraction
Two authors (AG and EMHP) extracted outcome data independently using a standardised document and disagreements were resolved by consensus. Four corresponding authors were contacted for additional data (one provided the information). Furthermore, three independent authors (IRM, VCSM and ACS) extracted additional baseline data for individual studies, including study and patient characteristics (Tables 1, 2). Participant-level data was not requested.
Table 1.
Baseline qualitative characteristics of included studies
| Study | NCT ID | Country | Intervention | Control | Population | Primary outcome | Outcomes measureda |
|---|---|---|---|---|---|---|---|
| Abraham [26] | ACTRN12616000753459 | Australia | MiniMed 670G | MDI/SAP therapy | Adults and Children | TIR 70–180 mg/dL | Clinical and functional |
| ADAPT (Choudhary [27]) | NCT04235504 | France, Germany And UK | MiniMed 670G | MDI therapy | Adults | Change in HbA1c | Clinical and functional |
| APCam11 (Tauschmann [28]) | NCT02523131 | USA and UK | Modified FlorenceM | SAP therapy | Adults and children | TIR 70–180 mg/dL | Clinical and functional |
| AP@home (Thabit [29]) | NCT01778348 + NCT01961622 | UK, Germany and Austria | FlorenceD2A and FlorenceD2W | SAP therapy | Adults and children | TIR 70–180 mg/dL for adults and TIR 70–145 mg/dL for children and adolescents | Clinical |
| Boughton [30] | NCT04025762 | UK and Austria | CamAPS FX | SAP therapy | Adults | TIR 70–180 mg/dL | Clinical |
| Brown [31] | NCT03591354 | USA | t:slim X2 with control-IQ | PLGS therapy | Adults | TIR 70–180 mg/dL | Clinical |
| Burnside [32] | ACTRN12620000034932 | New Zealand | AndroidAPS 2.8 | SAP | Adults and children | TIR 70–180 mg/dL | Clinical |
| DAN05 (Ware [33],Hood [46]) | NCT02925299 | UK and USA | FlorenceM or CamAPS FX | SAP therapy | Children | Change in HbA1c | Clinical and functional |
| DAN06 (Boughton [34]) | NCT02871089 | UK | FlorenceM/CamAPS FX | MDI therapy | Children and adolescents | AUC for the plasma C-peptide | Clinical |
| DCPL3 (Brown [35], Kudva [47]) | NCT03563313 | USA | t:slim X2 with Control-IQ | SAP therapy | Adults and children | TIR 70–180 mg/dL | Clinical and functional |
| DCLP4 (Pinsker [36]) | NCT04436796 | USA | Interoperable artificial pancreas system (iAPS) | SAP/PLGS therapy | Adults | TIR 70–180 mg/dL | Clinical and functional |
| DCLP5 (Breton [37], Cobry [48]) | NCT03844789 | USA | t:slim X2 with control-IQ | SAP/PLGS therapy | Children | TIR 70–180 mg/dL | Clinical and functional |
| DIABELOOP WP7 (Benhamou [38]) | NCT02987556 | France | Diabeloop Generation 1 (DBLG1) | SAP therapy | Adults | TIR 70–180 mg/dL | Clinical and functional |
| iDCL (Kovatchev [39]) | NCT02985866 | USA | Control-IQ | SAP therapy | Adults | Time below 70 mg/dL and above 180 mg/dL | Clinical |
| Garg [40] | NCT02748018 | USA and Canada | MiniMed 670G hybrid closed loop | CSII | Children, adolescents and adults |
Group 1: change in HbA1c Group 2: reducing %TBR < 70 mg/dL |
Clinical and functional |
| Matejko [7] | NCT04616391 | Poland | MiniMed 780G | MDI therapy | Adults | TIR 70–180 mg/dL | Clinical and functional |
| McAuley [41] | ACTRN12617000520336 | Australia | MiniMed 670G | MDI/SAP therapy | Adults | TIR 70–180 mg/dL | Clinical and functional |
| ORACL (McAuley [42]) | ACTRN126190000516190 | Australia | MiniMed 670G | SAP therapy | Adults | TIR 70–180 mg/dL | Clinical and functional |
| PEDAP trial (Wadwa) [43]) | NCT04796779 | USA | T:slim X2 with Control-IQ | MDI/SAP therapy | Children | TIR 70–180 mg/dL | Clinical |
| Reiss [44] | NCT03428932 | USA | MiniMed 670G | MDI/SAP therapy | Children | Metrics of gray matter | Clinical and functional |
| Russell [11] | NCT04200313 | USA | iLet device | PLGS/SAP/MDI therapy | Adults and children | Change in HbA1c | Clinical |
| Ware [45] | NCT03784027 | Austria, Germany, Luxembourg and UK | CamAPS FX | SAP therapy | Children | TIR 70–180 mg/dL | Clinical |
CGM Continuous glucose monitor, TIR Time in Range, MDI Multiple Daily Injections, SAP sensor augmented pump, AUC Area under the curve, PLGS, Predictive low-glucose suspend system, HbA1c Glycated Hemoglobin, UK United Kingdom, USA United States of America
aFunctional outcomes include participant-reported questionnaires/patients reported outcomes
Table 2.
Baseline quantitative characteristics of included studies
| Study | Patient No, I/C | Female %, I/C | Age, years, I/Cd | Duration of assessment, wks | Study design | Baseline HbA1c, % (mmol/mol)d | Duration of diabetes, yearsd | BMI, kg/m2d | Baseline daily insulin dose, units/kgd |
|---|---|---|---|---|---|---|---|---|---|
| Abraham [26] | 67/68 | 55/57 | 15.2 ± 3.3/15.4 ± 3.0 | 24 | Parallel | 8.0 ± 1.0 (64 ± 10)/7.9 ± 1.0 (63 ± 11) | 7.9 ± 4.2/7.6 ± 3.4 | 0.7 ± 0.8/0.7 ± 0.7c | 0.8 ± 0.2/0.9 ± 1.2 |
| ADAPT (Choudhary [27]) | 41/41 | 54/39 | 41.5 ± 11.63/39.7 ± 13.12 | 24 | Parallel | 9.0 ± 1.0 (75.7 ± 7.83)/9.1 ± 0.7 (74.9 ± 10.64) | 18.8 ± 11.4/18.1 ± 10.0 | 27.0 ± 4.4/25.8 ± 4.9 | 54.3 ± 25.9/53.3 ± 22.3e |
| APCam11 (Tauschmann [28]) | 46/40 | 48/55 | 22 (13–36)/21 (11–36)a | 12 | Parallel | 8.3 ± 0.6(68 ± 7)/8.2 ± 0.5 (66 ± 5) | 13 (7–20)/10 (7–19)a | 28 ± 4)/27 ± 3 | 0.76 ± 0.25/0.69 ± 0.8 |
| AP@home (Thabit [29])b | 33/ 25 | 45/44 | 40.0 ± 9.4/12.0 ± 3.4 | 12 | Crossover | 8.5 ± 0.7 (69 ± 7)/8.1 ± 0.9 (65 ± 10) | 20.9 ± 9.3/4.7 ± 2.6 | 25.5 ± 4.4/18.9 ± 3.5 | 0.62 ± 0.15/0.89 ± 0.24 |
| Boughton [30] | 20/17 | 40/47 | 68(63–70)/67 (62–70)a | 16 | Crossover | 7.5 ± 1.0 (57 ± 10)/7.4 ± 0.9 (58 ± 10) | 38 (32–48)/38 (32–48)a | 28.2 (25.4–31.7)/27.4 (24.9–38.5)a | 45·8 (38.3–51.1)/40·0 (35·4–62·4)e |
| Brown [31] | 54/55 | 52/45 | 32 ± 14/34 ± 17 | 13 | Parallel | 7.0 ± 0.8 (54 ± 8.5)/7.1 ± 0.8 (54 ± 8.4) | 18 ± 8.3/16 ± 7.3 | 26 (23, 30)/25 (23, 29)a | 0.59 (0.49, 0.86)/0·68 (0.46, 0.93)a |
| Burnside [32] | 44/53 | 52/48 | 26.59 ± 14.33/23.29 ± 17.51 | 24 | Parallel | 7.55 (60.0 ± 13.7)/7.65 (62.1 ± 9.1)f | 15.20 ± 13.42/12.33 ± 11.82 | 24.40 ± 5.86/23.67 ± 6.42 | 44.6 ± 16.17/43.01 ± 17.36 |
| DAN05 (Ware [33], Hood [46] | 65/68 | 57/57 | 13.1 ± 2.6/12.8 ± 2.9 | 24 | Parallel | 8.2 ± 0.7 (66 ± 8)/8.3 ± 0.8 (67 ± 8)c | 6.3 ± 3.3/6.6 ± 3.1 | 0.35 ± 0.86/0.58 ± 0.89c | 0.93 ± 0.23/0.95 ± 0.24 |
| DAN06 (Boughton [34]) | 51/46 | 49/39 | 12 ± 2/12 ± 2 | 96 | Parallel | 10.7 ± 1.8 (93 ± 18)/10.5 ± 1.6 (94 ± 20) | NA | 53 ± 29/51 ± 34g | 0.87 ± 0.33/0.82 ± 0.38 |
| DCPL3 (Brown [35], Kudva [47]) | 112/56 | 48/54 | 33 ± 16/33 ± 17 | 24 | Parallel | 7.4 ± 1.0/7.4 ± 0.8 | 17 (8, 28)/15 (7, 23)a | 25 (23, 29)/25 (22, 28)a | 46 (31, 62)/45 (35, 61)a, e |
| DCLP4 (Pinsker 2022) [36]) | 18/16 | 36·8/56·3 | 41 ± 16/37 ± 15 | 13 | Crossover | 6.9 ± 1.0 | 18 (12, 29)a | 28 ± 5 | NA |
| DCLP5 (Breton [37], Cobry [48]) | 78/23 | 49/52 | 11.3 ± 2.0/10.8 ± 2.4 | 16 | Parallel | 7.7 ± 1.1/8.0 ± 1.1 | 5.0 ± 2.8/6.0 ± 2.8 | 0.4 ± 1·0/0.5 ± 1·0c | 0.89 ± 0.24/0.94 ± 0.24 |
| DIABELOOP WP7 (Benhamou [38]) | 32/31 | 62 | 48.2 ± 13.4 | 12 | Crossover | 7.6 ± 0.9 (59.4 ± 9.8) | 28.0 ± 13.6 | 24.8 ± 3.5 | 36.3 ± 8.9e |
| iDCL (Kovatchev [39]) | 65/62 | 49/45 | 33 ± 16/32 ± 14 | 12 | Parallel | 7.4 ± 0.9 (57 ± 9.8)/7.4 ± 0.8 (57 ± 8.7) | 19 (7, 27)/16 (11, 27)a | 27 (24, 31)/25 (23, 29)a | 0.73 ± 0.22/0.68 ± 0.25 |
| Garg [40] | 151/151 | 47/62 | 39.9 ± 19.8/35.7 ± 18.4 | 24 | Parallel | 8.2 ± 1.3/8.1 ± 1.2 | 21.5 ± 13.6/19.6 ± 13.1 | 26.8 ± 5.8/27.0 ± 6.9 | NA |
| Matejko [7] | 20/17 | 40/47·1 | 39.8 ± 8.3/40.9 ± 7.8 | 12 | Parallel | 7.05 ± 0.8 (54 ± 9)/7.4 ± 1.2 (57 ± 13) | 17.1 ± 12.2/17.6 ± 12.2 | 24.5 ± 3.3/25.6 ± 2.64 | NA |
| McAuley 2020 [41] | 61/59 | 54/53 | 43.7 ± 11.7/44.7 ± 11.8 | 26 | Parallel | 7.4 ± 0.9 (62 ± 12)/7.5 ± 0·8 (61 ± 10) | 24.0 ± 12.0/24.1 ± 12.5 | 26.8 ± 5.3/26.0 ± 4.0 | 0.51 (0.41, 0.63)/0.54 (0.45, 0.66)a |
| ORACL (McAuley [42]) | 15/15 | 63 | 67 ± 5 | 16 | Crossover | 7.6 ± 0.9 (58 ± 7) | 38 (20–47)a | 27.6 (26.4–31.0)a | 0.55 (0.41–0.66)a |
| PEDAP trial (Wadwa [43]) | 68/34 | 49/56 | 3.84 ± 1.23/4.06 ± 1.25 | 13 | Parallel | 7.5 ± 1.2/7.7 ± 0.9 | 1.04 (0.71·1.85)/1.40 (0.91,2·11) | 81 (57,94)/77 (56,9)g | 0.66 ± 0.17/0.66 ± 0.23 |
| Reiss [44] | 21/21 | 40/47 | 14–17 | 24 | Parallel | 8.7/ 8.45f | NA | NA | NA |
| Russell [11] | 147/72 | 49/38 | 28 ± 19/28 ± 20 | 13 | Parallel | 7.9 ± 1.2/7.7 ± 1·1 | 16 ± 14/18 ± 15 | 28.9 ± 5.5/29.1 ± 6.9 | 0.75 (0.57, 1.00)/0.75 (0.56, 0.94)a |
| Ware [45] | 39/35 | 54/29 | 5.6 ± 1.4/5.6 ± 1.7 | 16 | Crossover | 7.3 ± 0.7 (56.3 ± 7.4)/7.4 ± 0.6 (57 ± 7.1) | 2.5 ± 1.7/2.7 ± 1.9 | 67.3 ± 23.2/71.1 ± 24.6g | 0.76 (0.67–0.83)/0.77 (0.69–0.86)a |
NA not available, I/C Intervention/Control, HbA1c, Glycated Hemoglobin
aMedian (IQR)
bData are reported as Adults/Children and Adolescents
cReported as Body-mass index Z score
dReported values as mean ± SD unless specified
eValues are reported in units per day, not units/kg
fNo SD available
gAge and sex-adjusted BMI percentile
For studies reporting data for paediatric and adult patients separately, we planned to analyse these as separate comparisons. For crossover studies, we planned a priori to analyse group means and standard deviations, assuming no correlation between groups (as parallel study designs). The bias introduced with this assumption is generally conservative [18]. For missing means data, we used the formula proposed by Wan et al. [19] using medians and interquartile ranges as recommended by the Cochrane Collaboration [18]. We collected adjusted mean differences (MD) as originally reported in each study when available.
Outcome measurements
Our main outcomes were TIR 70-180 mg/dL as the primary outcome and HbA1c (%) change. Secondary outcomes of interest included coefficient of glucose variability (CV), % time < 70 mg/dL, % time < 54 mg/dL, nocturnal hypoglycaemia (< 70 mg/dL), and %time > 250 mg/dL. We assessed the following psychosocial outcomes: Hypoglycaemia Fear Survey (HFS) [20]; Diabetes Treatment Satisfaction Questionnaire (DTSQ) [21]; treatment distress measured by the scales Diabetes Distress Scale (DDS) [22] and Problem Areas in Diabetes (PAID) [23]. Safety endpoints included diabetic ketoacidosis (DKA) and severe hypoglycaemia.
Quality assessment
Each included study was appraised using the Cochrane Risk of Bias Assessment Tool (RoB-2) for RCTs [24] by at least two independent investigators (AG, CH, IS, and CG). Further, the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) tool was employed by two independent authors (IAM and IRM) using the GRADEpro Guideline Development Tool [25] to evaluate the level of certainty of the evidence in this meta-analysis, with categorizations ranging from high to very low [26]. Any disagreements were discussed and resolved through a consensus.
Statistical analysis
Binary adverse outcomes were summarised using the Mantel–Haenszel test, with an odds ratio (OR) and 95% confidence interval (CI) as a measure of effect size. Continuous outcomes were compared with weighted and standardised MDs. Statistical heterogeneity was assessed by I2 and sources of heterogeneity were sought if I2 was greater than 50%. When low heterogeneity was identified (I2 < 25%), a fixed-effects model was used. We performed sensitivity analyses using the leave-one-out strategy as well as Baujat plots. We further investigated causes of heterogeneity by performing subgroup analyses according to type of AID device.
In addition, a random effect meta-regression analysis was performed to assess the impact of baseline HbA1c and study duration on overall MD. Publication bias was assessed for HbA1c and TIR 70–180 mg/dL through the generation of a funnel plot and Egger’s test, where a p-value less than 0.05 indicates the presence of publication bias. Review Manager 5.4.1 software (Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) and RStudio version 4.1.2 (R Foundation for Statistical Computing) were used for the statistical analysis.
Role of the funding source
There was no funding source for this study. AG and EMHP had full access to all the data in the study and all authors had responsibility for the final publication.
Results
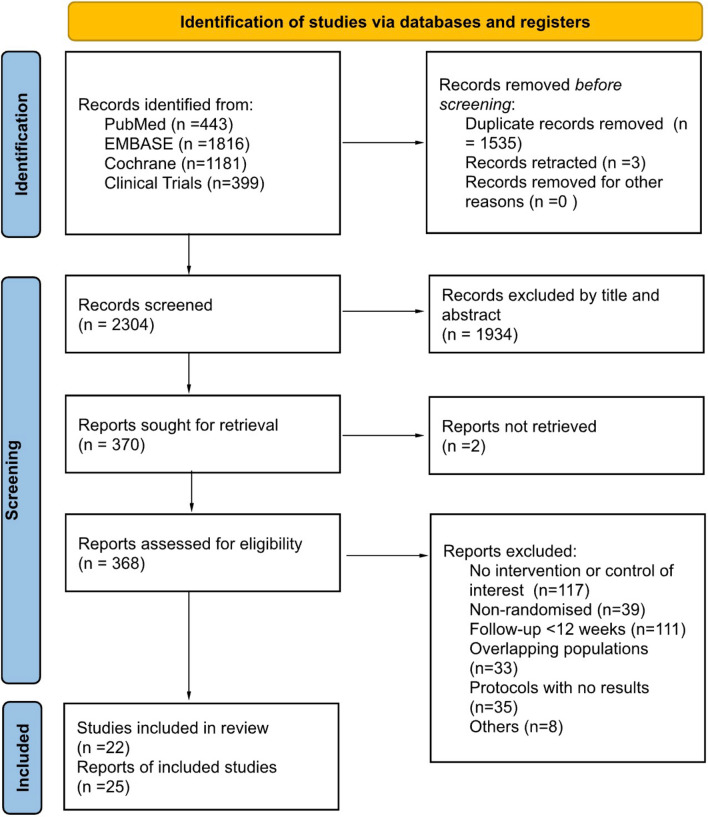
Our search identified a total of 3839 unique studies, of which 25 reports from 22 RCTs, including 2376 randomised participants, fulfilled the study eligibility criteria (Fig. 1) [27]. Of the 25 reports identified, 22 assessed primarily clinical outcomes [8, 12, 28–47], while 3 studies [48–50] assessed solely patient-reported outcomes.
Fig. 1.
PRISMA flow of study selection
Characteristics of included studies
Characteristics of studies contributing data to this meta-analysis are presented in Tables 1 and 2. The trials were conducted across eight countries spanning three continents. Seventeen studies had a parallel-group design, while five were crossover studies. Most RCTs included only adults (n = 9), while a similar number included only children (n = 6) or mixed both adults and children (n = 7). Females comprised 46.6% (n = 1068) of the included population. The mean age of adult participants ranged from 32 to 68 years, and of paediatric participants ranged from 3.8 to 15.4 years. The mean duration of T1DM ranged from 1 to 38 years, with a mean Body Mass Index ranging from 18.9 to 29.1 kg/m2, and a baseline HbA1c ranging from 6.9 to 10.7%. Among the 19 included trials, four (n = 380) assessed the use of CamAPS FX (CamDiab) [31, 36, 38, 46]; five (n = 636) assessed MiniMed 670G (Medtronic) [28, 34, 43, 44, 46]; five (n = 605) assessed t:slim X2 with Control IQ (Tandem) [33, 35, 36, 38, 45]; two (n = 144) assessed Modified Florence [30, 31]; two (n = 119) assessed MiniMed 780G (Medtronic) [8, 29]; two assessed openAPS (n = 129) [34, 41]; one (n = 63) assessed DBLG1 (Dbl-diabetes) [40]; and one (n = 219) assessed iLet Bionic Pancreas (Beta Bionics) [12]. Duration of CL or UC use ranged from 12 to 96 weeks.
Effects on glucose control
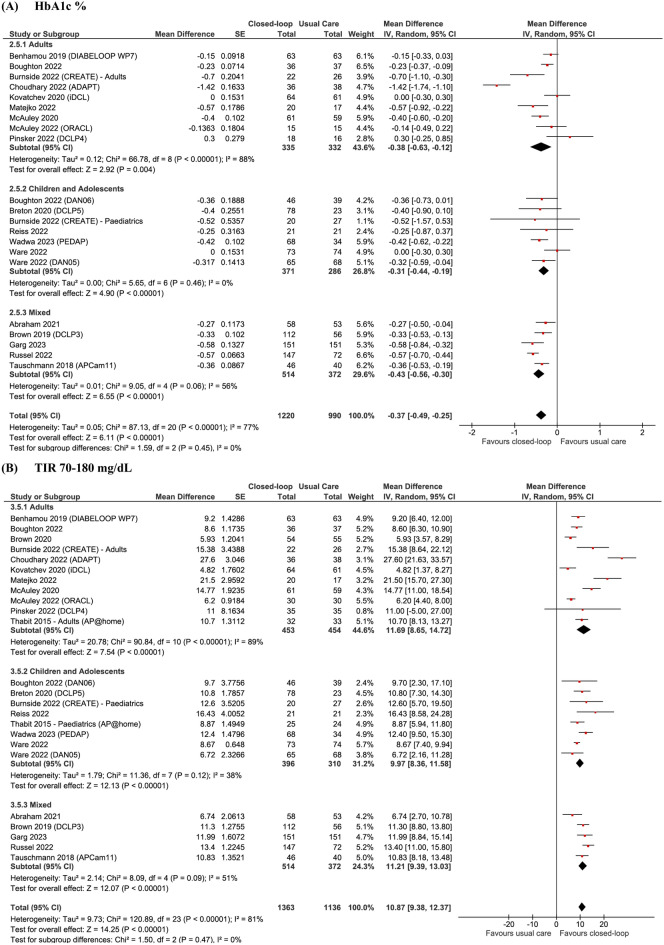
In a pooled analysis of 19 studies (n = 2210) for the primary outcome displayed in Fig. 2A and Table 3, treatment with CL systems led to a significant decrease in HbA1c % (MD – 0.37; 95% CI − 0.49 to − 0.26; p < 0.0001) and mmol/mol (MD − 4.77; 95% CI − 6.39 to − 3.14;p < 0.001), for adults (MD − 0.38; 95% CI − 0.63 to − 0.12; p = 0.004), children (MD − 0.31; 95% CI − 0.44 to − 0.19; p < 0.001) and mixed populations (MD − 0.46; 95% CI − 0.56 to − 0.30; p < 0.0001). There was a high statistical heterogeneity for the overall (I2 = 77%), adult (I2 = 88%), and mixed analyses (I2 = 62%), but not for children (I2 = 0%). In the overall analysis of 22 studies (n = 2499), there was also a significant 10.87% increase in TIR 70–180 mg/dL for the CL group when compared to UC (95% CI 9.38 to 12.37; p < 0.0001; Fig. 2B), which was similarly seen in adults (MD 11.69; 95% CI 8.65 to 14.62; p < 0.0001), children (MD.9·97; 95% CI 8.36 to 11.58; p < 0.0001) and mixed populations (MD 11.21; 95% CI.9·39 to 13.03; p < 0.0001). Statistical heterogeneity was high (I2 = 81%), but decreased in the children subgroup (I2 = 38%).
Fig. 2.
Forest plots for (A) HbA1c % and (B) TIR 70–180 mg/dL
Table 3.
Summary results of overall meta-analysis for each outcome and according to age subgroups
| Outcome | No of patients (no of comparisons) | Pooled Result (CI 95%) | P value | Heterogeneity |
|---|---|---|---|---|
| HbA1c (%)a | ||||
| Overall | 2210 (20) | − 0.37 (− 0.49 to − 0.26) | < 0.001 | 77% |
| Adults | 667 (9) | − 0.38 (− 0.63 to − 0.12) | 0.004 | 88% |
| Pediatric | 657 (7) | − 0.31 (− 0.44 to − 0.19) | < 0.001 | 0% |
| Mixed | 886 (4) | − 0.46 (− 0.56 to − 0.30) | < 0.001 | 62% |
| HbA1c (mmol/mol)a | ||||
| Overall | 1229 (13) | − 4.77 (− 6.39 to − 3.14) | < 0.001 | 85% |
| Adults | 596 (7) | − 4.87 (− 7.66 to − 2.97) | 0.001 | 91% |
| Pediatric | 327 (3) | − 6.77 (− 11·90 to − 1·64) | 0.010 | 83% |
| Mixed | 306 (6) | − 3.70 (− 5.00 to − 2.39) | < 0.001 | 0% |
| TIRa | ||||
| Overall | 2499 (24) | 10.87 (9.38 to 12.37) | < 0.001 | 81% |
| Adults | 907 (11) | 11.69 (8.65 to 14.72) | < 0.001 | 89% |
| Pediatric | 706 (8) | 9.97 (8.36 to 11.58) | < 0.001 | 38% |
| Mixed | 886 (5) | 11.21 (9.39 to 13.03) | < 0.001 | 51% |
| DKAb | ||||
| Overall | 2413 (22) | 1.62 (0.64 to 4.12) | 0.31 | 0% |
| Adults | 1020 (11) | 0.85 (0.22 to 3·25) | 0.81 | 0% |
| Pediatric | 669 (7) | 3.06 (0.63 to 14.85) | 0.17 | 0% |
| Mixed | 724 (4) | 2.67 (0.11 to 67.40) | 0.55 | NA |
| Severe hypoglycaemiab | ||||
| Overall | 2244 (22) | 1.29 (0.78 to 2.15) | 0.32 | 0% |
| Adults | 915 (11) | 1.24 (0.64 to 2.38) | 0.53 | 0% |
| Pediatric | 773 (8) | 2.35 (0.89 to 6.20) | 0.08 | 0% |
| Mixed | 556 (3) | 0.11 (0.01 to 2.03) | Not estimable | NA |
| Pro | ||||
| Distressc | 763 (7) | − 0.18 (− 0.34 to − 0.03) | 0.02 | 8% |
| FOHb | 403 (5) | − 2·35(− 5·21 to 0·51) | 0.11 | 45% |
| Satisfactionª | 569 (6) | 0.00 (− 3.10 to 3.10) | 0.83 | 79% |
| % Time (< 54 mg/dl)a | ||||
| Overall | 1917 (19) | − 0.14 (− 0.22 to − 0.07) | < 0.001 | 77% |
| Adults | 842 (10) | − 0.23 (− 0·37 to − 0·10) | < 0.001 | 85% |
| Pediatric | 577 (6) | − 0·03 (− 0·09 to − 0·03) | 0.32 | 0% |
| Mixed | 498(3) | − 0.15 (− 0.31 to 0.02) | 0.08 | 83% |
| % Time (< 70 mg/dl)a | ||||
| Overall | 2499 (24) | − 0.65 (− 1.05 to − 0.26) | 0.001 | 95% |
| Adults | 907 (11) | − 0.82 (− 1·43 to − 0·21) | 0.008 | 96% |
| Pediatric | 706 (8) | − 0.14 (− 0.41 to 0.68) | 0.62 | 83% |
| Mixed | 886 (5) | − 1.39 (− 2·17 to − 0·60) | < 0.001 | 92% |
| % Time (> 250 mg/dl)a | ||||
| Overall | 1731 (16) | − 4.46 (− 5.79 to − 3.14) | < 0.001 | 93% |
| Adults | 769 (9) | − 3.51 (− 4.97 to − 2.05) | < 0.001 | 90% |
| Pediatric | 464 (4) | − 6.63 (− 8.14 to − 4.92) | 0.009 | 32% |
| Mixed | 498 (3) | − 4.24 (− 9.16 to 0.67) | 0.09 | 97% |
| Nocturnal hypoglycaemiaa | ||||
| Overall | 1661 (17) | − 1.28 (− 1.76 to − 0.79) | < 0.001 | 84% |
| Adults | 717 (9) | − 1.03 (− 1.70 to − 0.36) | 0.003 | 87% |
| Pediatric | 474 (6) | − 1.18 (− 1.91 to − 0·45) | 0.002 | 55% |
| Mixed | 470 (2) | − 3.17 (− 7.37 to 1.03) | 0.14 | 96% |
| CVa | ||||
| Overall | 2197 (23) | − 1.09 (− 1.80 to − 0.39) | < 0.001 | 81% |
| Adults | 907 (11) | − 1.74 (− 2.79 to − 0.70) | 0.002 | 83% |
| Pediatric | 706 (8) | 0.33 (− 0.88 to 1.55) | 0.88 | 77% |
| Mixed | 584 (4) | − 1.81 (− 3.38 to − 0·25) | 0.02 | 82% |
TIR time in range, PRO Patients-Reported Outcomes, FOH Fear of hypoglycaemia, HP hyperglycemia, CV coefficient of variation
aMean difference, bOdds ratio, cStandardized mean difference
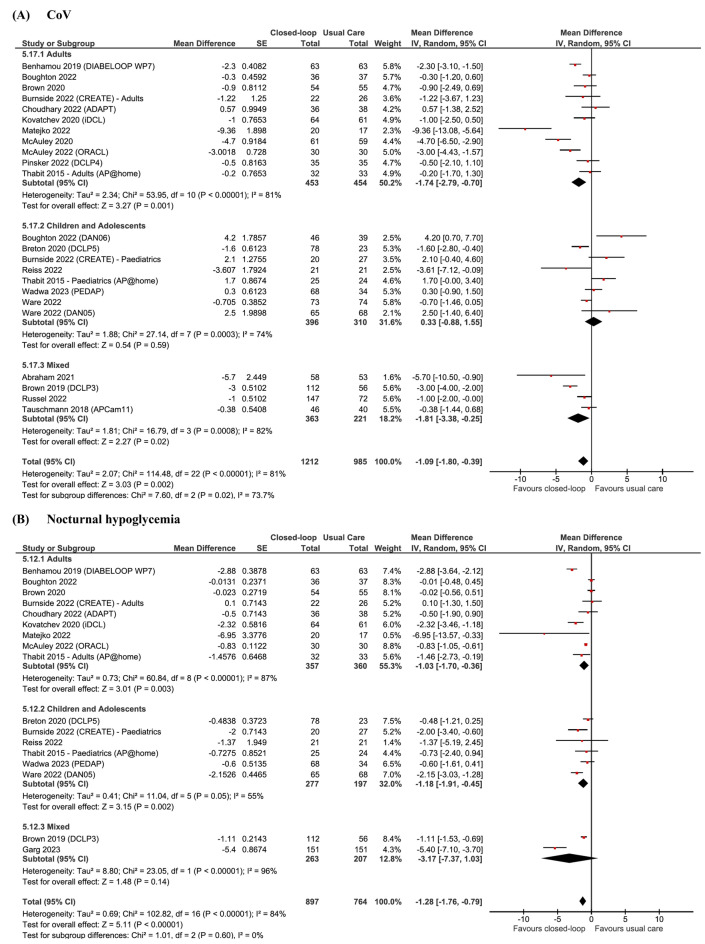
Further analyses for glycaemic control significantly favoured the use of CL systems for endpoints of CV (MD − 1.09; 95% CI − 1.80 to − 0.39; p = 0.0007; Fig. 3A), % time < 70 mg/dL (MD − 0.65; 95% CI − 1.05 to − 0.26; p = 0.009), % time < 54 mg/dL (MD − 0.14; 95% CI − 0.22 to − 0.07; p < 0.0001), % time > 250 mg/dL (MD − 4.46; 95% CI − 5.79 to − 3.14; p < 0.0001), and nocturnal hypoglycaemia (MD − 1.28; 95% CI − 1.76 to − 0.79; p < 0.0001; Fig. 3B). No significant differences were found for the use of CL in children for % time < 54 mg/dL (p = 0.32); < 70 mg/dL (p = 0.62); and CV (p = 0.88) when compared to UC. Further detailed findings for age subgroups can be seen in Table 3. As shown in Fig. 4, the rate of episodes of DKA (Additional file 1: Figure S1A) and severe hypoglycaemia (Additional file 1: Figure S1B) was not significantly different between groups (p = 0.31 and p = 0.32, respectively).
Fig. 3.
Forest plots for (A) CV and (B) nocturnal hypoglycaemia
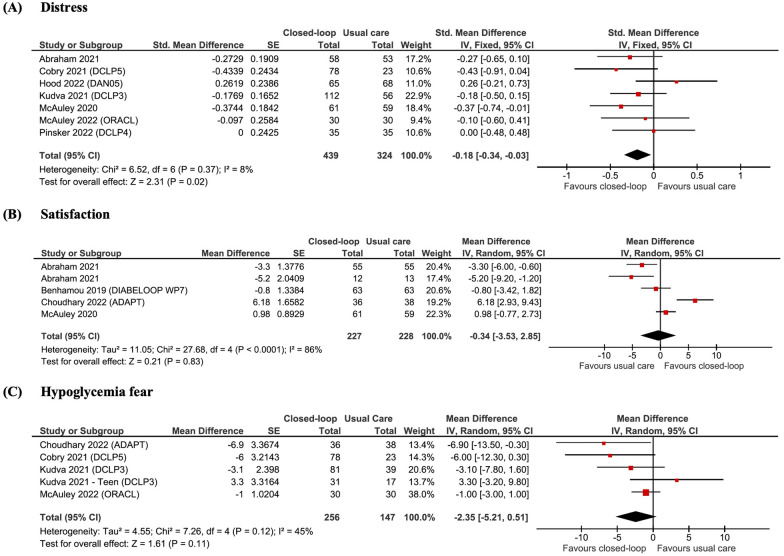
Fig. 4.
Meta-analysis of patient-reported outcomes of (A) diabetes distress measured by Diabetes Distress Survey (DDS) and Problem Areas in Diabetes (PAID), B Diabetes Treatment Satisfaction Questionnaire (DTSQ), and (C) Hypoglycaemia Fear Scale (HFS)
Effects on psychosocial outcomes
The pooled analysis for patient-reported outcomes found decreased diabetes distress for the CL group (SMD − 0.18; 95% CI − 0.34 to − 0.03; p = 0.02; Fig. 4A), but no significant differences for fear of hypoglycaemia (p = 0.11, Fig. 4B) and treatment satisfaction (p = 0.83, Fig. 4C).
Risk of bias in included studies
The risk of bias assessment of each RCT is provided in the Additional file 1: Appendix A for clinical (Additional file 1: Figure S3) and functional (Additional file 1: Figure S4) outcomes. For clinical outcomes, three were rated as “some concerns'' due to missing outcome data [7] and deviations from the protocol (machine errors) [35, 40], and seven were rated as “high risk” due to lack of laboratory-measured HbA1c assessment [44, 46] or due to insufficient washout time [36, 38, 43, 47] in crossover studies. All trials were open-label but used adequate methods for allocating participants and objective measurements of clinical outcomes. For patient-reported outcomes, trials were assessed as “some concerns'' due to the subjective nature of the assessment (Additional file 1: Figure S4).
GRADE assessment and publication bias
Following the GRADE criteria (Additional file 1: Table S3), there was moderate certainty of evidence for HbA1c reduction in the mixed and paediatric populations, and for TIR 70–180 mg/dL in the paediatric population. In contrast, there was low certainty of evidence for HbA1c reduction in the adult population, for TIR 70–180 mg/dL in the mixed and adult populations, and for CV and night hypoglycaemia. Funnel plots for HbA1c showed no indication of publication bias visually (Additional file 1: Figure S5) or based on Egger’s regression test (p = 0.93; Additional file 1: Figure S6A), yet a significant value was found for TIR (p = 0.02; Additional file 1: Figure S6B).
Sensitivity analyses
We explored the consistency of treatment effects using the leave-one-out strategy (Additional file 1: Figure S7), which revealed that Choudhary 2022 [29] was the study responsible for driving the heterogeneity from 58 to 77%, also confirmed by the Baujat plot (Additional file 1: Figure S8). Yet, results remained statistically significant to favour CL systems even when each individual study was removed from the analysis (Additional file 1: Figure S7). To further investigate reasons for the observed heterogeneity of effect for glycaemic control endpoints, we stratified our analyses by type of AID machines (Additional file 1: Table S2). As seen in Fig. 5, heterogeneity decreased substantially for most machine subgroups and findings remained mostly consistent with the overall analysis, favouring CL systems over UC. Nonetheless, the openAPS subgroup revealed no significant differences between CL and UC for change in HbA1c. MiniMed 780G and iLet Pancreas were found to be most effective to improve HbA1c and TIR outcomes (Fig. 5), MiniMed 670G was most effective to improve CV (Additional file 1: Figure S1), and openAPS was most effective at preventing nocturnal hypoglycaemia when compared to other machines (Additional file 1: Table S2). In addition, we performed a meta-regression based on follow-up duration and baseline HbA1c (Additional file 1: Figure S5). Although the results showed no significant association between the study duration and the mean differences for change in HbA1c (p = 0.57; Additional file 1: Figure S9), higher baseline HbA1c was significantly associated with greater change scores (p = 0.02; Additional file 1: Figure S10).
Fig. 5.
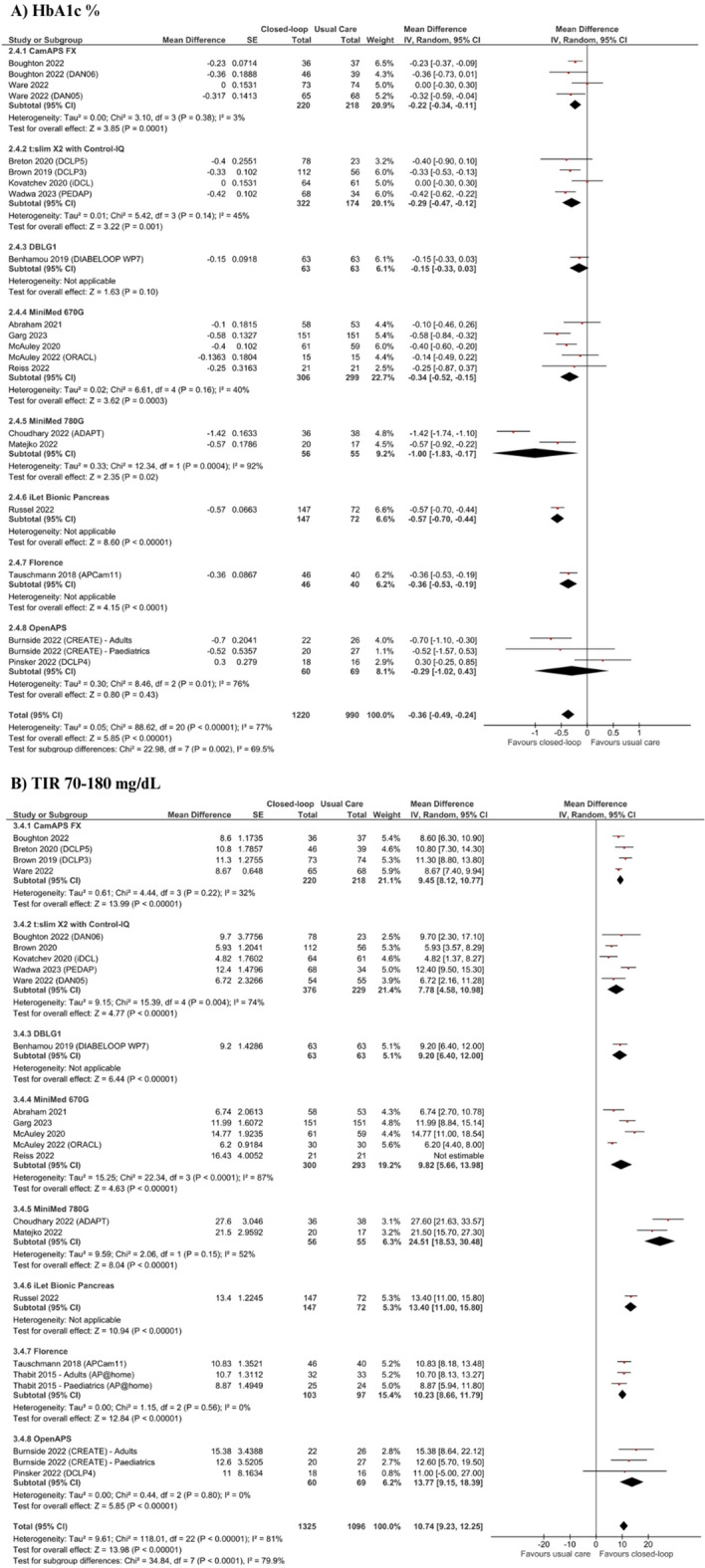
Subgroup analysis based on CL systems for (A) HbA1c % and (B) TIR 70–180 mg/dL
Discussion
In this systematic review and meta-analysis of 22 RCTs and 2376 patients, we compared the use of AID devices versus UC during a period of 12 to 96 weeks. Our main findings were: (1) A significantly improved HbA1c level, % TIR 70–180 mg/dL, CV, % time < 54 mg/dL, < 70 mg/dL, < 250 mg/dL and risk of nocturnal hypoglycaemia, with the use of AID devices; (2) a significant improvement in diabetes distress in the CL group; (3) no significant difference in the risk of DKA or severe hypoglycaemia between groups; (4) no significant reduction in % time < 54 mg/dL, < 70 mg/dL, and CV observed between paediatric groups, and (5) no significant improvement in fear of hypoglycaemia and treatment satisfaction.
Achieving glycaemic control of T1DM while also avoiding hypoglycaemia is a challenge for patients [51, 52]. A high cognitive load for T1DM patients and care team is required and previous studies show distress or depressive symptoms in up to 40% of patients [53]. Although HbA1c is currently the metric of choice by most endocrinology and diabetes societies [54, 55], TIR and HbA1c should be used as complementary parameters to guide care [56] and allow evaluation in clinical research [57].
To our knowledge, our study is the most comprehensive meta-analysis of use of AID for 12–96 weeks. Our analysis integrated data from 25 reports and 2376 participants, a population that almost tripled compared to a previous meta-analysis [14]. Furthermore, this is the first analysis with studies over 12 weeks of duration, stratified by age groups and type of AID device used. Our findings augment the certainty about the beneficial effects of the continuous use of CL systems on HbA1c, TIR, hypoglycaemia, and distress of patients, without increasing the risk of AEs. Given that glycaemic variability has been linked to chronic diabetic complications [58], respective reductions of 0·37% (4.77 mmol/mol) in HbA1c levels and 1·09% in CV have important implications for patient care. As the mean baseline HbA1c in our population was 7·73% (61 mmol/mol), our findings present a conservative and safe strategy to avoid the risk of hypoglycaemia commonly associated with large changes in HbA1c [59]. Furthermore, an increase of 10% TIR has been correlated with an HbA1c reduction of 0·5–0·8% [60], which is slightly higher compared to our TIR and HbA1c assessment. Our analyses also show that higher HbA1c levels at baseline are correlated with greater changes in HbA1c after the use of such devices, which may lead to further benefits to certain patient groups. Our findings are similar to the analyses by Weinsman and colleagues [13], although our results for reduction of time in hypoglycaemia are much smaller. The longer periodicity of the studies included provides a pragmatic setting for assessment, where greater variables and confounding factors reflect a better real-life picture of treatment impact.
In addition, our meta-analysis provides a unique framework for comparing 7 permutations of different technologies. The breadth of these findings provides estimates of treatment effects with particular relevance to clinical decision-making and cost-effectiveness analyses. The application of our results may be illustrated through an approach to device selection. For example, some devices appeared to offer the greatest potential for improved glycaemia compared to other systems in our sensitivity analyses, although no definite conclusions can be as no head-to-head comparisons were performed. Furthermore, there is a growing body of literature assessing the use of openAPS, or “do-it-yourself” (DIY) devices which are remotely controlled by open-source algorithms [61]. Given the limited knowledge about DIY systems [62], our analysis provides insight into the potential benefit of openAPS.
Most studies in our analysis did not assess fully automated systems [8, 29, 31–38, 40–46], which still require manual input from the user [63]. Therefore, the use of such devices in children and adolescents remains a challenge. Previous meta-analyses on paediatric populations, such as a recent one by Michou and colleagues [64], have shown a reduced risk of hypoglycaemia when assessing RCTs of mostly less than 12 weeks duration. Nonetheless, our analysis with RCTs of 12 to 96 weeks duration did not show a significantly reduced risk of hypoglycaemia nor coefficient of variation for the paediatric population, which could have been due to several reasons. For instance, children are more likely to experience hypoglycaemia due to increased physical activity, hormonal changes, varied eating habits and lifestyle, and inability to communicate symptoms appropriately [65]. Furthermore, considerable proportion of RCTs included have reported system errors and malfunctioning during the longer duration of the trials, potentially having important impacts for children and adolescents who are at a higher risk of hypoglycaemia or those not achieving target control [4]. These findings have important implications to the design of future paediatric trials, which should consider placing significant focus on patient education, device functioning and type of system used.
Finally, this was the first meta-analysis to assess how long-term use of AID impacts patient-reported outcomes with a considerable number of studies. Although our findings show significantly improved diabetes distress and a tendency for reduced fear of hypoglycaemia, no benefits were seen for treatment satisfaction. The high cost of AID devices, connectivity problems, automation-related errors, pump glitches, and other issues associated with insulin pumps have been perceived as drawbacks by T1DM patients [5]. Moreover, most studies included in our analyses use CL algorithms that still require manual bolus input. Further improvements towards fully AID may result in improved quality of life and treatment satisfaction. Lastly, psychosocial measures varied between trials, limiting the populations of our analyses. Given that such outcomes have been recently receiving increased attention [5], future studies may consider using more consistent and widely used measures to aid interpretation of psychosocial impact.
Our study has important limitations. The lack of blinding in the studies, as it is potentially unfeasible to blind patients in such RCTs, reduced the certainty of evidence for our findings. It is important to note that heterogeneity was high for most glycaemic outcomes, especially in the adult and mixed populations. However, this finding was expected given the highly variable clinical and technical factors involved in studies performed in real-life conditions without supervision. Subgroup analyses of different machines and meta-regression were performed to minimise and interpret such heterogeneities. Furthermore, we did not search the grey literature, which can increase the risk of publication bias. However, we believe that restricting our research to peer-reviewed sources minimised other sources of bias ensuring a more rigorous evaluation. Unfortunately, no study used outcomes such as mortality or macrovascular and microvascular complications as outcomes. Therefore, our study relies on surrogate measures for patient-oriented outcomes. Finally, recent bihormonal CL systems were not included as the RCTs on these devices only had a short follow-up period.
Conclusion
This systematic review and meta-analysis confirms previous findings in the literature of short-duration studies, showing that the prolonged use of AID devices under pragmatic settings results in a small, but important 0·37% (4.77 mmol/mol) reduction in HbA1c levels and may lead to a large 10·87% increase in TIR. Findings also suggest reductions in nocturnal and daily hypoglycaemia as well as patient distress without increasing the risk of DKA and severe hypoglycaemia. This estimate is beneficial in planning future long-term clinical trials assessing the use of fully automated and bihormonal AID devices. The synthesis of all system subgroups emphasises the potential benefits of certain CL systems, although this finding requires head-to-head comparisons before definitive conclusions can be made. Our results show that use of CL technology between 12 and 96 weeks has considerable benefits in a variety of clinical settings. Ultimately, it will be at the discretion of clinicians and patients to understand the potential benefits associated with different CL systems and decide on the most optimal insulin delivery method to improve patient outcomes.
Supplementary Information
Additional file 1: Appendix A. Search Strategy. Table S1. Descriptions of current insulin delivery devices. Table S2. Additional glycemic outcomes based on CL machines. Table S3. GRADE assessment. Figure S1. Forest plots for (A) DKA and (B) severe hypoglycaemia. Figure S2. Subgroup analysis based on closed-loop system devices for the outcomes of (A) CV and (B) nocturnal hypoglycemia. Figure S3. Critical appraisal according to the Cochrane Collaboration’s tool for assessing risk of bias in randomised trials for clinical outcomes. Figure S4. Critical appraisal according to the Cochrane Collaboration’s tool for assessing risk of bias in randomised trials for functional outcomes. Figure S5. Funnel plots for (A) HbA1c % and (B) TIR 70-180 mg/dL show no evidence of publication bias. Figure S6. Egger’s regression test does not suggest significant publication bias for (A) HbA1c (%) endpoint; but suggests significant publication bias for (B) % TIR 70-180 mg/dL endpoint. Figure S7. Leave-one-out sensitivity analysis for the outcome of HbA1c (%). Figure S8. Baujat plot for the outcome of HbA1c (%). Figure S9. Meta-regression exploring the association between mean differences of HbA1c level (%) and duration of follow-up (weeks). Figure S10. Meta-regression exploring the association between mean differences of HbA1c level (%) and baseline HbA1c (%).
Acknowledgements
The authors thank Dr. Rhanderson Cardoso, Brigham and Women’s Hospital, for his excellent technical assistance and analytical contributions in the preparation of this manuscript.
Abbreviations
- AE
Adverse event
- AID
Automated insulin delivery
- CGM
Continuous glucose monitoring
- CI
Confidence interval
- CL
Closed-loop
- CV
Coefficient of glucose variability
- DDS
Diabetes distress scale
- DIY
Do It Yourself
- DKA
Diabetic ketoacidosis
- DTSQ
Diabetes Treatment Satisfaction Questionnaire
- HCL
Hybrid Closed-loop
- HFS
Hypoglycaemia Fear Survey
- MDII
Multiple daily insulin injections
- OR
Odds ratio
- PLGS
Predictive low-glucose suspend
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analysis
- PAID
Problem areas in diabetes
- RCT
Randomised controlled trial
- SAP
Sensor-augmented Insulin Pump
- TIR
Time in range
- T1DM
Type 1 diabetes mellitus
- UC
Usual care
Author contributions
EMHP, AM, IRM and AG wrote the study protocol and designed the statistical analyses. AG, AM, and LCH. assessed the eligibility of studies for inclusion in this analysis. IA.FS, CG, CH, and AG assessed the risk of bias, and IAM. and IRM. performed the GRADE assessment. AG and EMHP had access to, and verified, the underlying data from all original research articles, and conducted statistical analyses. AG, IRM, JERLJ and JRS wrote the first draft of the report. All authors were involved in data interpretation, manuscript writing, and manuscript editing. JRS provided senior supervision. All authors critically revised the report for important intellectual content, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
No funding was provided for this study.
Availability of data and materials
All data are publicly available in the relevant primary and secondary papers from relevant trials as listed in the References.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
We declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Thrower SL, Bingley PJ. Prevention of type 1 diabetes. Br Med Bull. 2011;99:73–88. doi: 10.1093/bmb/ldr020. [DOI] [PubMed] [Google Scholar]
- 2..International Diabetes Federation. Diabetes atlas. 8th edn. 2017. 10.1093/bmb/ldr020
- 3.Foster NC, Beck RW, Miller KM, Clements MA, Rickels MR, DiMeglio LA, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016–2018. Diabetes Technol Ther. 2019;21:66–72. doi: 10.1089/dia.2018.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kellee MM, Nicole CF, Roy WB, Richard MB, Stephanie ND, Linda AD, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D exchange clinic registry. Diabetes Care. 2015;38(6):971–978. doi: 10.2337/dc15-0078. [DOI] [PubMed] [Google Scholar]
- 5.Pettus JH, Zhou FL, Shepherd L, Preblick R, Hunt PR, Paranjape S, et al. Incidences of severe hypoglycaemia and diabetic ketoacidosis and prevalence of microvascular complications stratified by age and glycaemic control in U.S. adult patients with type 1 diabetes: a real-world study. Diabetes Care. 2019;42:2220–2227. doi: 10.2337/dc19-0830. [DOI] [PubMed] [Google Scholar]
- 6.Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/nejm199309303291401. [DOI] [PubMed] [Google Scholar]
- 7.Ware J, Hovorka R. Closed-loop insulin delivery: update on the state of the field and emerging technologies. Expert Rev Med Devices. 2022;27:1–7. doi: 10.1080/17434440.2022.2142556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matejko B, Juza A, Kieć-Wilk B, Cyranka K, Krzyżowska S, Chen X, Cohen O, Da Silva J, Malecki MT, Klupa T. Transitioning of people with type 1 diabetes from multiple daily injections and self-monitoring of blood glucose directly to minimed 780G advanced hybrid closed-loop system: a two-center, randomized. Controll Study Diabetes Care. 2022;45(11):2628–2635. doi: 10.2337/dc22-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hovorka R, Elleri D, Thabit H, et al. Overnight closed-loop insulin delivery in young people with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care. 2014;37(5):1204e11. doi: 10.2337/dc13-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dauber A, Corcia L, Safer J, et al. Closed-loop insulin therapy improves glycemic control in children aged <7 years: a randomized controlled trial. Diabetes Care. 2013;36(2):222e7. doi: 10.2337/dc12-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Messer LH, Buckingham BA, Cogen F, Daniels M, Forlenza G, Jafri RZ, et al. Positive impact of the bionic pancreas on diabetes control in youth 6–17 years old with type 1 diabetes: a multicenter randomized trial. Diabetes Technol Ther. 2022;24(10):712–725. doi: 10.1089/dia.2022.0201.pub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bionic Pancreas Research G. Russell SJ, Beck RW, Damiano ER, El-Khatib FH, Ruedy KJ, et al. Multicenter, randomized Trial of a bionic pancreas in type 1 diabetes. N Engl J Med. 2022;387(13):1161–1172. doi: 10.1056/NEJMoa2205225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weisman A, Bai JW, Cardinez M, Kramer CK, Perkins BA. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5(7):501–512. doi: 10.1016/S2213-8587(17)30167-5. [DOI] [PubMed] [Google Scholar]
- 14.Jiao X, Shen Y, Chen Y. Better TIR, HbA1c, and less hypoglycaemia in closed-loop insulin system in patients with type 1 diabetes: a meta-analysis. BMJ Open Diabetes Res Care. 2022;10(2):e002633. doi: 10.1136/bmjdrc-2021-002633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Diabetes Association 6 Glycemic targets: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(1):S73–84. doi: 10.2337/dc21-S006. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane handbook for systematic reviews of interventions. Chichester: John Wiley & Sons; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mourão E, Kalinowski M, Murta L, Mendes E, Wohlin C. Investigating the use of a hybrid search strategy for systematic reviews. In2017 ACM/IEEE International Symposium on Empirical Software Engineering and Measurement (ESEM) 2017 (pp. 193–8). IEEE. 10.1109/ESEM.2017.30
- 18.Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page M, Welch V. Meta-analysis of change scores cochrane handbook for systematic reviews of interventions. London: John Wiley & Sons; 2011. [Google Scholar]
- 19.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:1–3. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonder-Frederick L, Nyer M, Shepard JA, Vajda K, Clarke W. Assessing fear of hypoglycaemia in children with type 1 diabetes and their parents. Diabetes Manag. 2011;1(6):627. doi: 10.2217/DMT.11.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bradley CL. The diabetes treatment satisfaction questionnaire: DTSQ. Handbook of psychology and diabetes: a guide to psychological measurement in diabetes research and practice. London: Harwood Academic Publishers; 1994. [Google Scholar]
- 22.Polonsky WH, Fisher L, Earles J, Dudl RJ, Lees J, Mullan J, Jackson RA. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28(3):626–631. doi: 10.2337/diacare.28.3.626. [DOI] [PubMed] [Google Scholar]
- 23.Evans MA, Weil LE, Shapiro JB, Anderson LM, Vesco AT, Rychlik K, et al. Psychometric properties of the parent and child problem areas in diabetes measures. J Pediatr Psychol. 2019;44(6):703–713. doi: 10.1093/jpepsy/jsz018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 25.GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University and Evidence Prime. 2022. www.gradepro.org. Accessed 3 May 2023.
- 26.Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group. 2013. www.guidelinedevelopment.org/handbook. Accessed 3 May 2023.
- 27.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abraham MB, de Bock M, Smith GJ, Dart J, Fairchild JM, King BR, Ambler GR, Cameron FJ, McAuley SA, Keech AC, Jenkins A. Effect of a hybrid closed-loop system on glycemic and psychosocial outcomes in children and adolescents with type 1 diabetes: a randomized clinical trial. JAMA Pediatr. 2021;175(12):1227–1235. doi: 10.1001/jamapediatrics.2021.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choudhary P, Kolassa R, Keuthage W, Kroeger J, Thivolet C, Evans M, Ré R, de Portu S, Vorrink L, Shin J, Habteab A. Advanced hybrid closed loop therapy versus conventional treatment in adults with type 1 diabetes (ADAPT): a randomised controlled study. Lancet Diabetes Endocrinol. 2022;10(10):720–731. doi: 10.1016/S2213-8587(22)00212-1. [DOI] [PubMed] [Google Scholar]
- 30.Tauschmann M, Thabit H, Bally L, Allen JM, Hartnell S, Wilinska ME, Ruan Y, Sibayan J, Kollman C, Cheng P, Beck RW. Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet. 2018;392(10155):1321–1329. doi: 10.1016/S0140-6736(18)31947-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thabit H, Tauschmann M, Allen JM, Leelarathna L, Hartnell S, Wilinska ME, et al. Home use of an artificial beta cell in type 1 diabetes. N Engl J Med. 2015;373(22):2129–2140. doi: 10.1056/NEJMoa1509351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boughton CK, Hartnell S, Thabit H, Mubita WM, Draxlbauer K, Poettler T, et al. Hybrid closed-loop glucose control compared with sensor augmented pump therapy in older adults with type 1 diabetes: an open-label multicentre, multinational, randomised, crossover study. Lancet Healthy Longev. 2022;3(3):e135–e142. doi: 10.1016/S2666-7568(22)00005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown SA, Beck RW, Raghinaru D, Buckingham BA, Laffel LM, Wadwa RP, Kudva YC, Levy CJ, Pinsker JE, Dassau E, Doyle FJ., III Glycemic outcomes of use of CLC versus PLGS in type 1 diabetes: a randomized controlled trial. Diabetes Care. 2020;43(8):1822–1828. doi: 10.2337/dc20-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burnside MJ, Lewis DM, Crocket HR, Meier RA, Williman JA, Sanders OJ, Jefferies CA, Faherty AM, Paul RG, Lever CS, Price SK. Open-source automated insulin delivery in type 1 diabetes. N Engl J Med. 2022;387(10):869–881. doi: 10.1056/NEJMoa2203913. [DOI] [PubMed] [Google Scholar]
- 35.Ware J, Boughton CK, Allen JM, Wilinska ME, Tauschmann M, Denvir L, et al. Cambridge hybrid closed-loop algorithm in children and adolescents with type 1 diabetes: a multicentre 6-month randomised controlled trial. Lancet Digit Health. 2022;4(4):e245–e255. doi: 10.1016/S2589-7500(22)00020-6. [DOI] [PubMed] [Google Scholar]
- 36.Boughton CK, Allen JM, Ware J, Wilinska ME, Hartnell S, Thankamony A, Randell T, Ghatak A, Besser RE, Elleri D, Trevelyan N. Closed-loop therapy and preservation of C-peptide secretion in type 1 diabetes. N Engl J Med. 2022;387(10):882–893. doi: 10.1056/NEJMoa2203496. [DOI] [PubMed] [Google Scholar]
- 37.Brown SA, Kovatchev BP, Raghinaru D, Lum JW, Buckingham BA, Kudva YC, Laffel LM, Levy CJ, Pinsker JE, Wadwa RP, Dassau E. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med. 2019;381(18):1707–1717. doi: 10.1056/NEJMoa1907863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pinsker JE, Dassau E, Deshpande S, Raghinaru D, Buckingham BA, Kudva YC, et al. Outpatient randomized crossover comparison of zone model predictive control automated insulin delivery with weekly data driven adaptation versus sensor-augmented pump: results from the International diabetes closed-loop trial 4. Diabetes Technol Ther. 2022;24(9):635–642. doi: 10.1089/dia.2022.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Breton MD, Kanapka LG, Beck RW, Ekhlaspour L, Forlenza GP, Cengiz E, Schoelwer M, et al. A randomized trial of closed-loop control in children with type 1 diabetes. N Engl J Med. 2020;383(9):836–845. doi: 10.1056/NEJMoa2004736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benhamou PY, Franc S, Reznik Y, Thivolet C, Schaepelynck P, Renard E, Guerci B, Chaillous L, Lukas-Croisier C, Jeandidier N, Hanaire H. Closed-loop insulin delivery in adults with type 1 diabetes in real-life conditions: a 12-week multicentre, open-label randomised controlled crossover trial. Lancet Digit Health. 2019;1(1):e17–25. doi: 10.1016/S2589-7500(19)30003-2. [DOI] [PubMed] [Google Scholar]
- 41.Kovatchev B, Anderson SM, Raghinaru D, Kudva YC, Laffel LM, Levy C, et al. Randomized controlled trial of mobile closed-loop control. Diabetes Care. 2020;43(3):607–615. doi: 10.2337/dc19-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garg SK, Grunberger G, Weinstock R, Lawson ML, Hirsch IB, DiMeglio LA, et al. Improved glycaemia with hybrid closed-loop versus continuous subcutaneous insulin infusion therapy: results from a randomized controlled trial. Diabetes Technol Ther. 2023;25(1):1–2. doi: 10.1089/dia.2022.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McAuley SA, Lee MH, Paldus B, Vogrin S, De Bock MI, Abraham MB, et al. Six months of hybrid closed-loop versus manual insulin delivery with fingerprick blood glucose monitoring in adults with type 1 diabetes: a randomized, controlled trial. Diabetes Care. 2020;43(12):3024–3033. doi: 10.2337/dc20-1447. [DOI] [PubMed] [Google Scholar]
- 44.McAuley SA, Trawley S, Vogrin S, Ward GM, Fourlanos S, Grills CA, et al. Closed-loop insulin delivery versus sensor-augmented pump therapy in older adults with type 1 diabetes (ORACL): a randomized, crossover trial. Diabetes Care. 2022;45(2):381–390. doi: 10.2337/dc21-1667. [DOI] [PubMed] [Google Scholar]
- 45.Wadwa RP, Reed ZW, Buckingham BA, DeBoer MD, Ekhlaspour L, Forlenza GP, et al. Trial of hybrid closed-loop control in young children with type 1 diabetes. N Engl J Med. 2023;388(11):991–1001. doi: 10.1056/NEJMoa2210834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reiss AL, Jo B, Arbelaez AM, Tsalikian E, Buckingham B, Weinzimer SA, et al. A Pilot randomized trial to examine effects of a hybrid closed-loop insulin delivery system on neurodevelopmental and cognitive outcomes in adolescents with type 1 diabetes. Nat Commun. 2022;13(1):4940. doi: 10.1038/s41467-022-32289-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ware J, Allen JM, Boughton CK, Wilinska ME, Hartnell S, Thankamony A, et al. Randomized trial of closed-loop control in very young children with type 1 diabetes. N Engl J Med. 2022;386(3):209–219. doi: 10.1056/NEJMoa2111673. [DOI] [PubMed] [Google Scholar]
- 48.Hood KK, Garcia-Willingham N, Hanes S, Tanenbaum ML, Ware J, et al. Lived experience of CamAPS FX closed loop system in youth with type 1 diabetes and their parents. Diabetes Obes Metab. 2022;24(12):2309–2318. doi: 10.1111/dom.14815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kudva YC, Laffel LM, Brown SA, Raghinaru D, Pinsker JE, Ekhlaspour L, et al. Patient-reported outcomes in a randomized trial of closed-loop control: the pivotal international diabetes closed-loop trial. Diabetes Technol Ther. 2021;23(10):673–683. doi: 10.1089/dia.2021.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cobry EC, Kanapka LG, Cengiz E, Carria L, Ekhlaspour L, Buckingham BA, et al. Health-related quality of life and treatment satisfaction in parents and children with type 1 diabetes using closed-loop control. Diabetes Technol Ther. 2021;23(6):401–409. doi: 10.1089/dia.2020.0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Ridder F, den Brinker M, De Block C. The road from intermittently scanned continuous glucose monitoring to hybrid closed-loop systems Part B: results from randomized controlled trials. Ther Adv Endocrinol Metab. 2019;10:2042018819871903. doi: 10.1177/2042018819871903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wood JR, Miller KM, Maahs DM, Beck RW, DiMeglio LA, Libman IM, et al. Most youth with type 1 diabetes in the T1D exchange clinic registry do not meet American diabetes association or international society for pediatric and adolescent diabetes clinical guidelines. Diabetes Care. 2013;36(7):2035–2037. doi: 10.2337/dc12-1959/-/DC1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buchberger B, Huppertz H, Krabbe L, Lux B, Mattivi JT, Siafarikas A. Symptoms of depression and anxiety in youth with type 1 diabetes: a systematic review and meta-analysis. Psychoneuroendocrinology. 2016;1(70):70–84. doi: 10.1016/j.psyneuen.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 54.Runge AS, Kennedy L, Brown AS, et al. Does time-in-range matter? Perspectives from people with diabetes on the success of current therapies and the drivers of improved outcomes. Clin Diabetes. 2018;36(2):112–119. doi: 10.2337/cd17-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data INTERPRETATION: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593–1603. doi: 10.2337/dci19-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beck RW, Bergenstal RM, Riddlesworth TD, Kollman C, Li Z, Brown AS, Close KL. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42(3):400–405. doi: 10.2337/dc18-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Battelino T, Alexander CM, Amiel SA, Arreaza-Rubin G, Beck RW, Bergenstal RM, et al. Continuous glucose monitoring and metrics for clinical trials: an international consensus statement. Lancet Diabetes Endocrinol. 2023;11(1):42–57. doi: 10.1016/S2213-8587(22)00319-9. [DOI] [PubMed] [Google Scholar]
- 58.Gorst C, Kwok CS, Aslam S, Buchan I, Kontopantelis E, Myint PK, et al. Long-term glycemic variability and risk of adverse outcomes: a systematic review and meta-analysis. Diabetes Care. 2015;38(12):2354–2369. doi: 10.2337/dc15-1188. [DOI] [PubMed] [Google Scholar]
- 59.Benhalima K, Standl E, Mathieu C. The importance of glycemic control: how low should we go with HbA1c? Start early, go safe, go low. J Diabetes Complicat. 2011;25(3):202–207. doi: 10.1016/j.jdiacomp.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 60.Vigersky RA, McMahon C. The relationship of hemoglobin A1C to time-in-range in patients with diabetes. Diabetes Technol Ther. 2019;21(2):81–85. doi: 10.1089/dia.2018.0310. [DOI] [PubMed] [Google Scholar]
- 61.Kesavadev J, Srinivasan S, Saboo B, Krishna BM, Krishnan G. The do-it-yourself artificial pancreas: a comprehensive review. Diabetes Ther. 2020;11:1217–1235. doi: 10.1007/s13300-020-00823-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crabtree TS, Choudhary P, Hammond P, Lumb A, McLay A, Wilmot EG. Health-care professional opinions of DIY artificial pancreas systems in the UK. Lancet Diabetes Endocrinol. 2020;8(3):186–187. doi: 10.1016/S2213-8587(19)30417-6. [DOI] [PubMed] [Google Scholar]
- 63.Quintal A, Messier V, Rabasa-Lhoret R, Racine E. A critical review and analysis of ethical issues associated with the artificial pancreas. Diabetes Metab. 2019;45(1):1. doi: 10.1016/j.diabet.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Michou P, Gkiourtzis N, Christoforidis A, Kotanidou EP, Galli-Tsinopoulou A. The efficacy of automated insulin delivery systems in children and adolescents with type 1 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2023 doi: 10.1016/j.diabres.2023.110678. [DOI] [PubMed] [Google Scholar]
- 65.Urakami T. The advanced diabetes technologies for reduction of the frequency of hypoglycemia and minimizing the occurrence of severe hypoglycemia in children and adolescents with type 1 diabetes. J Clin Med. 2023;12(3):781. doi: 10.3390/jcm12030781. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Appendix A. Search Strategy. Table S1. Descriptions of current insulin delivery devices. Table S2. Additional glycemic outcomes based on CL machines. Table S3. GRADE assessment. Figure S1. Forest plots for (A) DKA and (B) severe hypoglycaemia. Figure S2. Subgroup analysis based on closed-loop system devices for the outcomes of (A) CV and (B) nocturnal hypoglycemia. Figure S3. Critical appraisal according to the Cochrane Collaboration’s tool for assessing risk of bias in randomised trials for clinical outcomes. Figure S4. Critical appraisal according to the Cochrane Collaboration’s tool for assessing risk of bias in randomised trials for functional outcomes. Figure S5. Funnel plots for (A) HbA1c % and (B) TIR 70-180 mg/dL show no evidence of publication bias. Figure S6. Egger’s regression test does not suggest significant publication bias for (A) HbA1c (%) endpoint; but suggests significant publication bias for (B) % TIR 70-180 mg/dL endpoint. Figure S7. Leave-one-out sensitivity analysis for the outcome of HbA1c (%). Figure S8. Baujat plot for the outcome of HbA1c (%). Figure S9. Meta-regression exploring the association between mean differences of HbA1c level (%) and duration of follow-up (weeks). Figure S10. Meta-regression exploring the association between mean differences of HbA1c level (%) and baseline HbA1c (%).
Data Availability Statement
All data are publicly available in the relevant primary and secondary papers from relevant trials as listed in the References.