Abstract
The rise in antibiotic-resistant strains of clinically important pathogens is a major threat to global health. The World Health Organization (WHO) has recognized the urgent need to develop alternative treatments to address the growing list of priority pathogens. Antimicrobial peptides (AMPs) rank among the suggested options with proven activity and high potential to be developed into effective drugs. Many AMPs are naturally produced by living organisms protecting the host against pathogens as a part of their innate immunity. Mechanisms associated with AMP actions include cell membrane disruption, cell wall weakening, protein synthesis inhibition, and interference in nucleic acid dynamics, inducing apoptosis and necrosis. Acinetobacter baumannii is a critical pathogen, as severe clinical implications have developed from isolates resistant to current antibiotic treatments and conventional control procedures, such as UV light, disinfectants, and drying. Here, we review the natural AMPs representing primary candidates for new anti-A. baumannii drugs in post-antibiotic-era and present computational tools to develop the next generation of AMPs with greater microbicidal activity and reduced toxicity.
Keywords: AMP, Acinetobacter baumannii, resistance, mechanism of action, mechanism of action
1. Introduction
The rise of antibiotic resistance is a major aid to global mortality statistics and represents a challenge for societies, including healthcare providers, governmental agencies, and the pharmaceutical industry. The inability to develop new antibiotics to interfere with drug-resistant pathogens suggests the world is heading toward a post-antibiotic era [1,2]. For bacteria, three types of antimicrobial resistance have been described: intrinsic, acquired, and adaptive; the last is known as resistance due to changes in bacterial phenotype [3,4,5,6,7,8,9,10,11]. The main mechanisms of antimicrobial resistance are target modification or mutation, efflux pumps, permeability reduction, hydrolysis or enzymatic inactivation, metabolic enhancement or auxotrophy, community cooperative resistance, target protective protein (TPPs), changes in cell morphology, and self-repair systems (Table 1). While many mechanisms lead to resistance, the exposure of microbes to inadequate doses of antimicrobial drugs can trigger their evolution, contributing to the selection of antimicrobial resistance [12,13].
According to Magiorakos et al. (2012), a multidrug-resistant (MDR) strain shows resistance to at least one antimicrobial in more than three classes of antimicrobials; an extensively drug-resistant (XDR) strain displays resistance to at least one antimicrobial among all classes of antimicrobials, while a pan drug-resistant (PDR) strain is resistant to all antimicrobial agents [14]. In hospital settings, ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, A. baumannii, Pseudomonas aeruginosa, and Enterobacter species) comprise the main opportunistic pathogens in nosocomial infections, posing a global health threat due to their ability to evade traditional antibiotics used in conventional therapies, accounting for increased morbidity and mortality in healthcare systems [15,16,17].
A. baumannii has long been associated with human disease [18] and has globally emerged as a concerning hospital-related pathogen, frequently presenting MDR, XDR, and PDR phenotypes. The Acinetobacter calcoaceticus–A. baumannii complex (Acb) belongs to the Moraxellaceae family [19], comprising the following species: A. calcoaceticus, A. baumannii, A. pittii, A. nosocomialis, A. seifertii, and A. lactucae (a later heterotypic synonym of A. dijkshoorniae) [20,21]. Acb species differ in epidemiology, pathogenicity, and antimicrobial resistance [22]. While their genetic and physiological relatedness makes them difficult to distinguish phenotypically using standard laboratory methods [23], A. baumannii is the most widespread in hospitals, causing wounds, skin and urinary tracts infections, and also diseases such as pneumonia, meningitis, and bacteremia [24,25]. All contribute to longer hospital stays, higher treatment costs, and increased morbidity and mortality risks [26].
Treatment options have proven limited for A. baumannii due to its extended virolome and resistome, evasion of host immune effectors, survival under extreme environmental conditions, growth in biofilms, and latent growth on a minimal metabolic rate [27,28].
Table 1.
Bacterial resistance mechanisms against antibiotics.
| Antibiotic Resistance Mechanism | Characteristics | Example | Ref. |
|---|---|---|---|
| Target modification or mutation | Mutation or modification of bacterial site will interfere with target matching, thus affecting the effect of antibiotics | Modifying PBPs in MRSA, production of β-lactamases or carbapenemases in genus Klebsiella; fluoroquinolone-resistant S. aureus Mycobacterium tuberculosis resistance to rifampicin is mainly caused by the mutation of the rpoB gene and vancomycin-resistant Enterococcus (VRE) |
[29] |
| Reduced permeability | Deletion or damage of Omps is a source of bacterial resistance | Loss of porin D2 from outer cell wall in imipenem-resistant P. aeruginosa | [30] |
| Inactivating enzymes | Inactivating enzymes produced by bacteria, such as antibiotic hydrolases or similar enzymes, can hydrolyze or modify antibiotics inside the cell, rendering their inactivation before reaching the target site | Production of penicillin-inactivating β-lactamase by penicillin-resistant S. aureus, Haemophilus influenzae, and Escherichia coli bacteria, gentamicin-resistant enterococci via enzymatic inactivation of aminoglycosides and carbapenem-producing Enterobacteriaceae | [31] |
| Efflux pumps | Pumping of harmful molecules out of the bacterial cell | Increased efflux of tetracycline, macrolides, clindamycin, or fluoroquinolones in S. aureus | [32] |
| Metabolic enhancement or auxotrophy | Core genome mutations change metabolic pathways and induce antibiotic resistance | The genome of clinically pathogenic E. coli | [33,34] |
| Community cooperative resistance | Most bacteria coexist in communities, collectively resisting antibiotic effects; bacterial biofilms are efficiently protective of biofilm-forming bacterial species | P. aeruginosa, S. aureus, S maltophilia, and other bacteria | [35] |
| Target protective proteins (TPPs) | Bacterial synthetic protein protects antibiotic targets from antibiotics, eliminating their bacteriostatic effects | Clinically isolated S.aureus and other staphylococcus resistance to fusidic acid due to the level acquisition of genes encoding the FusB-type protein | [36] |
| Cell morphology changes | Modulating the body’s relative area via absorption efficiency changes can lead to the dilution of antibiotics entering the bacterial cell | Cells of the commonly used model organism Caulobacter crescentus | [37] |
| Self-repair systems | The multiple antibiotic resistance operon of enteric bacteria manipulates DNA repair and outer membrane integrity, enhancing antibiotic resistance | E. coli multiple antibiotic resistance (mar) loci was recognized as a determinant for cross-resistance to tetracyclines, quinolones, and β-lactams | [38] |
AR, antibiotic resistance; Ref., reference; MLSB = macrolide, lincoside, streptogramin; PB, penicillin-binding.
Colistin is, currently, the main therapeutic option against resistant strains of A. baumannii. Unfortunately, since its reintroduction, reports on A. baumannii colistin resistance mechanisms have been reported, including the complete loss of LPS, modifications of the LPS target or plasmid-encoded MCR genes, and colistin efflux from the cell [39].
The World Health Organization (WHO) recently highlighted the resistance of A. baumannii to carbapenems (CRAb) [40,41], which classifies the species as a “priority for research and development of new antibiotic treatments.” CRAb is a “critical” pathogen [42]. Antimicrobial peptides (AMPs) have a high potential for use in the research and development of anti-Acinetobacter drugs [43,44].
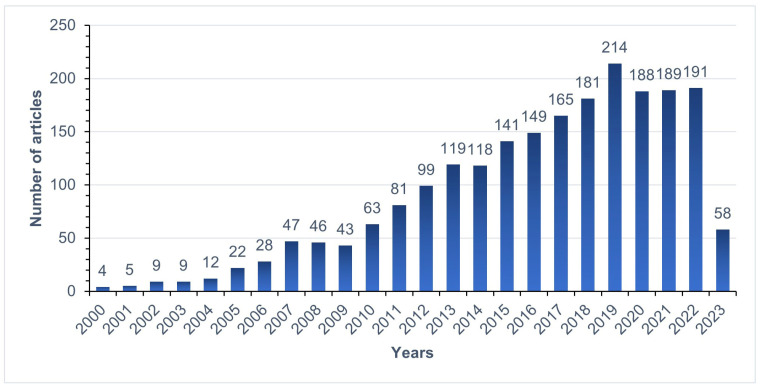
In this review, from January 2000 to April 2023, an extensive literature search was carried out at PubMed to update current knowledge about the activity of antimicrobial peptides (AMPs), combining keywords related to Acinetobacter baumannii and antimicrobial peptides (Figure 1) and finding several AMPs capable of acting against MDR A. baumannii. According to our search criteria, no previous publication on this topic was found.
Figure 1.
Number of articles selected according to the year of publication.
2. Antimicrobial Peptides
Antimicrobial peptides, also known as host defense peptides, are produced naturally by living organisms as a part of their innate immune system against pathogens. AMPs are amphipathic molecules of varying molecular weights containing 11–50 amino acids with an overall positive electric charge [45,46], classified as α-helical, β-sheet, or extended peptides [47,48,49]. AMPs are essential in regulating immune processes such as inflammation, activating and recruiting immune system cells [45]. In addition, they can inhibit protein and nucleic acid synthesis, occasionally leading to apoptosis and necrosis [50,51].
AMP activities begin on cell membranes through electrostatic interactions. As polycationic peptides, their multiple positive amino acids drive electrostatic interactions with lipid membranes that are also influenced by hydrophobic interactions (Figure 2, Table 2). Due to inherent differences between bacterial and mammalian cell surfaces, there would be preferences when AMPs associate with a cell surface, leading to an accumulation at the surface and self-assembly reaching a particular concentration [52,53]. At this stage, several models have been proposed to describe the mechanism of action (MOA) of AMPs.
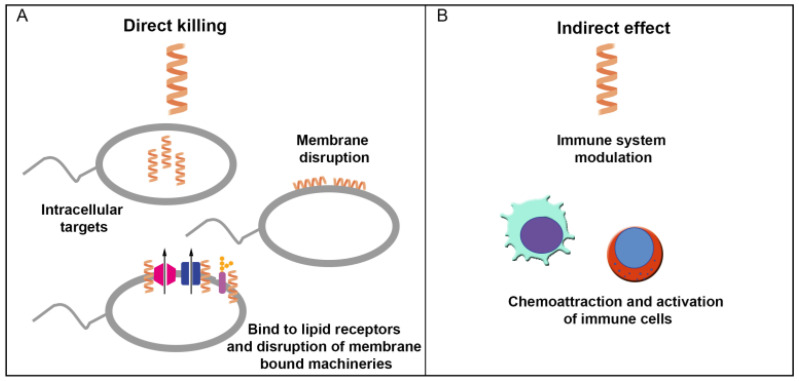
Figure 2.
Antimicrobial peptide (AMP) mechanisms on bacterial cells: (A) AMPs directly affect bacterial membrane and intracellular targets and disrupt lipid receptors and membrane-bound machinery. (B) AMPs indirectly trigger the activation and chemoattraction of immune cells.
Table 2.
General mechanism of AMP actions: direct killing by inhibiting membranes, bacterial lysis, and immune modulation.
| Mechanism of AMP | Mode of Action | Reference |
|---|---|---|
| Direct killing: Membrane target |
Electrostatic interactions and hydrophobic interactions (peptide and bacterial cell surface), membrane rupture-bound types of machinery and bacterial lysis—bilayer disruption | [54,55,56,57,58,59] |
| Direct killing: Non-membrane target | Action on the bacterial cell wall, activation of autolysin, intracellular targets: inhibition of protein/nucleic acid synthesis, disruption of enzymatic activities and bacterial lysis | [54,55,56,57,60] |
| Immune modulation | Chemotaxis, activation of immunocytes, microbial killing; anti-endotoxin activity, suppression of toll-like receptors (TLRs) and/or cytokine-mediated production of proinflammatory cytokines and preventing excessive and harmful proinflammatory responses, controls the inflammation | [57,61,62] |
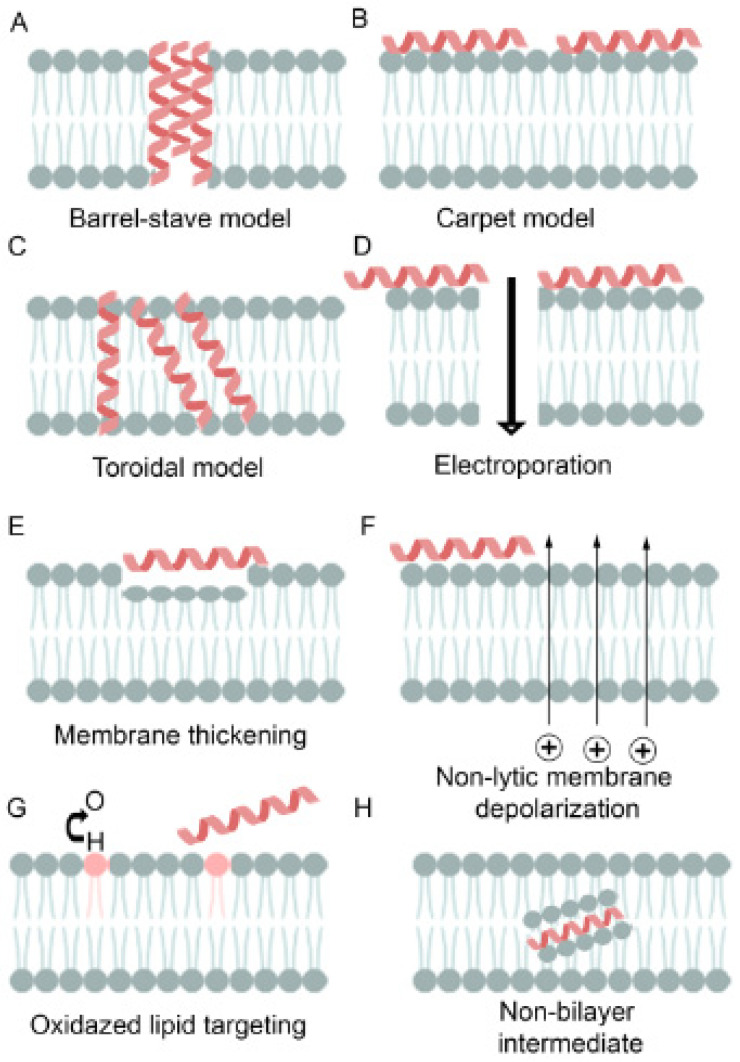
Multiple modes of action have been proposed for AMPs during interactions with bacterial cell surfaces, commonly known as transmembrane pore and non-pore models (Figure 3). The pore model presents differentiated forms such as barrel-stave and toroidal, reflecting the bilayer’s net arrangement. The barrel-stave shape preserves bilayer organization and begins as AMPs are parallel to the surface before perpendicularly inserting into the lipid bilayer [63]. The amphipathic structure of α and/or β sheet peptides permits lateral peptide–peptide interactions between hydrophilic amino acids to form the lumen, as well as the hydrophobic regions’ interaction with bilayer lipids [64,65], such as organizing and resembling a protein ion channel (Figure 3A). A minimum length of 22 residues in an α-helical structure or eight residues in a β sheet is needed to span a lipid bilayer. Only a subset of known AMPs, such as alamethicin [66], pardaxin [67,68], and protegrins [63], have been shown to form barrel-stave channels.
Figure 3.
AMP mechanisms of action on bacterial membranes: (A) In the barrel-staved model, the accumulation of AMPs inserted into the membrane bilayer forms a pore. (B) In the carpet model, AMPs accumulate on the surface until a critical concentration displays detergent behavior to form micelles. (C) Accumulated AMPs inserted in vertical and bent orientations form a pore in the toroidal pore model. (D) Positively charged AMPs interact with negatively charged cell membranes adsorbing, leading to electroporation. (E) AMP interaction can interfere with membrane thickening, making the membrane more fragile. (F) Non-lytic membrane depolarization. (G) AMP oxidizes membrane lipids, leading to reactive oxygen species and increased lysis and permeability. (H) AMP generation of the non-bilayer intermediate that interacts with the membrane.
Toroidal pores also result from the perpendicular insertion of AMPs into the lipid bilayer but do not display lateral peptide–peptide interactions [66]. Rather, peptides disrupt the hydrophobic/hydrophilic arrangement of the bilayer and induce a local curvature in the lipid bilayer (Figure 3B). Pores are formed from a dynamic interaction between the inserted peptides and phospholipid head groups, creating a transient lipid–peptide supramolecule. In toroidal pores, the disruption in the hydrophobic and hydrophilic arrangement of the bills is temporary. Upon disintegration, some peptides are translocated to the inner cytoplasmic leaflet, allowing cell entry to target intracellular components [69]. Several AMPs, such as magainin 2 [70], lacticin Q [70], aurein 2.2 [71], and melittin [66,70], have been shown to form toroidal pores. For aurein 2.2, lipid composition and thickness have been shown to influence pore formation [72,73]. In a 1:1 mixture of 1-palmitoyl-2-oleoyl-sn-glycerol-3-phospho-(1-rac-glycerol) with 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine, toroidal pores are formed. In a membrane model of 1:1 1,2-dimyristoyl-sn-glycero-3-phospho-(1-rac-glycerol) with 1,2-dimyristoyl-sn-glyce rol-3-phosphocholine, aurein 2.2 does not form discrete pores. Other features of toroidal pores include ion and size selectivity [74]. Both toroidal and barrel-stave pores ultimately lead to membrane depolarization and cell death.
The carpet model describes AMPs that do not insert into the lipid bilayer to form pores [70,74,75,76]; peptides adsorb to the cell surface (Figure 3C). Upon reaching a threshold concentration, membrane integrity is compromised by a detergent-like effect that leads to the formation of micelles (Figure 3D). As the results in the carpet model are not dependent on specific amino acid compositions, lengths, or interactions, they can describe the MOA of several AMPs at high concentrations due to their amphiphilic nature, such as cecropin [77], indolicidin [78], aurein 1.2 [76], and LL-37 [75]. It has been suggested that the carpet-like mechanism is a prerequisite step for the toroidal pore model [71]. Other models have been proposed, including interfacial activity, electroporation, and Shai–Huang–Matsazuki models [71]. However, in most cases, the studies used the results from model membrane systems. Only a few AMPs have been studied in whole bacterial cells to define their MOAs [79,80], suggesting that the results from model membranes describing potential MOAs may need to explain their actions against the full pathogen.
Many AMPs are currently being studied to describe their therapeutic efficacy against A. baumannii strains. We have curated the online antimicrobial peptide database, APD3, to list the many examples of AMPs under study (Table 3 and Table 4). These include both peptides produced by living organisms and novel peptides inspired by their activities.
Table 3.
AMPs produced by living organisms demonstrating anti-A. baumannii activity.
| Peptide | Source | Sequence (nº Amino Acid) | Structure | MIC against A. baumannii (μg/mL) | Ref. | |
|---|---|---|---|---|---|---|
| ATBS | MDR | |||||
| Agelaia-MPI | Agelaia pallipes pallipes | INWLKLGKAIIDAL (14aa) | AH | 6.25 | 12.5–25 | [81] |
| aHylin a1-15K | Hypsiboas albopunctatus (American frog) | IAKAILPLALKALKKLIK-NH2 (19aa) | AH | 1–2 * | 1–2 * | [82] |
| Alytesirin-1c | Frog skin peptide | GLKEIFKAGLGSLVKGIAAHVAS-NH2 (23aa) | AH | — | 11.3–22.6 | [83] |
| α-Helical-26 (A12L/A20L) | D- and L-diastereomeric peptides | Ac KWKSFLKTFKSLKKTVLHTL LKAISS-NH2 (26aa) |
AH | — | 0.5–1.0 | [84] |
| AM-CATH21 | GLFKKLRRKIKKGFKKIFKRL (21aa) | AH | 42 | 10 | [85] | |
| AM-CATH28 | American alligator | KIKKGFKKIFKRLPPIGVGVSIPLAGKR (28aa) | AH | 28 | 10 | |
| AM-CATH36 | GLFKKLRRKIKKGFKKIFKRLPPIG VGVSIPLAGKR (36aa) | AH | 5.2 | 5.2 | ||
| Am23SK | Alligator mississippiensis | SCRFSGGYCIWNWERCRSGHFLVALCPFRKRCCK (34aa) | AH | — | 2 | [86] |
| Artlysin Art-175 | Pseudomonas aeruginosa bacteriophage | Comprises a modified variant of endolysin KZ144 with an N-terminal fusion to SMAP-29 | NF | — | 4–20 | [87] |
| Aurein 1,2 | Frog skin peptide | GLFDIIKKIAESF (13aa) | AH | 16 | — | [88] |
| Bactenecin | Bovine neutrophil granules, Caprine | LCRIVVIRVCR (12aa) | B-turn structure Ciclyc | 64 | — | [89,90,91] |
| Bicarinalin (YRTX-Tb1a) | Tetramorium bicarinatum venom | KIKIPWGKVKDFLVGGMKAV (20aa) | AH | — | 4 | [92] |
| BMAP-27 | Bovine myeloid | GRFKRFRKKFKKLFKKLSPVIPLLHLG (27aa) | AH | 8–16 | 4–16 | [93] |
| BmKn1 | Heterometrus petersii (Scorpion venom gland) | FIGAVAGLLSKIF (13aa) | AH | >40 | — | [94] |
| BmKn2 | Mesobuthus martensii Karsch (Scorpion) | FIGAIARLLSKIF-NH2 (13aa) | AH | 10 | 5–10 | [95] |
| B2RP-Era | Frog skin peptide | GVIKSVLKGVAKTVALGML-NH2 (19aa) | AH | 8–32 | 8–64 | [96,97] |
| Brevinina 2 (B2RP) | Frog skin peptide | GIWDTIKSMGKVFAGKILQNL-NH2 (21aa) | AH | 29 | 7–13.9 | [98] |
| BR003-cecropin A | Aedes aegypti | GGLKKLGKKLEGAGKRVFNAAEK ALPVVAGAKALRK (36aa) | AH | 5 | 5 | [99] |
| Buforin II | Frog skin peptide | TRSSRAGLQFPVGRVHRLLRK (21aa) | AH | 8–19.5 | 0.25–39 | [100,101,102,103] |
| Caerin 1.1 | GLLSVLGSVAKHVLPHVVPVIAEHL-NH2 (25aa) | AH | 7.5 | — | [104] | |
| Caerin 1.9 | Australian tree frog | GLFGVLGSIAKHVLPHVVPVIAEKL-NH2 | 3.75 | — | ||
| Caerin 1.1 + Caerin 1.9 | GLLSVLGSVAKHVLPHVVPVIAEHL-NH2+ GLFGVLGSIAKHVLPHVVPVIAEKL-NH2 | 0.9375–1.875 | — | |||
| CATH-BF derivative (Cath-A and OH- |
Bungarus fasciatus (Snake venom) |
KFFRKLKKSVKKRAKEFFKKPRVI GVSIPF(30aa) | AH | — | 8–32 | [105] |
| Cecropin A | Hyalophora cecropia (Cecropia moth) | KWKLFKKIEKVGQNIRDGIIKAGP AVAVVGQATQIAK (37aa) | AH | 32 | 0.5–32 | [106,107] |
| Cecropin P1 |
Ascaris suum (Pig) |
SWLSKTAKKLENSAKKRISEGIAIA IQGGPR (31aa) | AH | 1.6 | — | [106,108] |
| Citropin 1.1. |
Litora genus (Australian tree frog) |
GLFDVIKKVASVIGGL-NH2 (16aa) | AH | 16 | — | [88] |
| CL defensin | Cimex Lectularius (Bedbug) | ATCDLFSFQSKWVTPNHAACAAHCTARGNRGGRCKKAVCHCRK (43aa) | AH, antiparallel BS; N-terminal loop | — | — | [109] |
| Colistin (Polymyxin E) | Bacillus colistinus | C52H98N16O13 (cyclic compound) | BS n and B-tur | Antibiofilm, side effects | — | [110] |
| Con10 |
Opisthacanthus cayaporum (Scorpion venoms) |
FWSFLVKAASKILPSLIGGGDDNKSSS (27aa) | AH | 12.5 | 12.5 | [81] |
| CPF-AM1 | Frog skin peptide | GLGSVLGKALKIGANLL (19aa) | AH | 16–128 | 4–128 | [96,111,112] |
| CPF-B1 | Frog skin peptide | GLGSLLGKAFKIGLKTVGKMMGGAPREQ (28aa) | AH | — | 11.4–22.8 | [113] |
| CPF-C1 | Frog skin peptide | GFGSLLGKALRLGANVL (17aa) | AH | 5 | — | [112] |
| Ctriporin | Heterometrus petersii (Scorpion venom gland) | FLWGLIPGAISAVTSLIKK (19aa) | AH | 20 | 20–40 | [94] |
| Cy02 (cyclotide) | Viola odorata | GIPCGESCVWIPCISSAIGCSCKSKVCYRN (30aa) | BSs | — | 15 * | [114] |
| Danalexin |
American bulfrog (Rana catesbeiana) |
LGGLIKIVPAMICAVTKKC (19aa) | AH | — | 4–16 | [115] |
| DCD-1 L | Eccrine sweat glands | SSLLEKGLDGAKKAVGGLGKLGKDAVEDLESVGKGAVHDVKDVLD SVL (48aa) | AH | 16 | — | [116,117] |
| D-150-177C, HBcARD derivative peptide | Hepatitis B virus | RRRGRSPRRRTPSPRRRRSQSPRR RRSC (28aa) | AH | 16 | 16–32 | [118] |
| Delfibactin A | Gram-negative bactéria Delfia spp. |
C40H68N14O18 | NF | — | 16 | [119] |
| [D4K] B2RP | Frog skin peptide | GIWKTIKSMGKVFAGKILQNL-NH2 (21aa) | AH | 4–16 | 4–16 | [96,120] |
| D-Myrtoxin-Mp 1a (Mp1a) |
Myrmecia pilosula (Venom) |
IDWKKVDWKKVSKKTCKVMXKACKEL-NH2 (26aa) | AH | 0.025 * | — | [121] |
| DOH-CATH30 | King cobra (Snake venon) |
KFFKKLKNSVKKRAKKFFKKPRVIGVSIPF (30aa) | AH | — | 1.56–12.5 | [122] |
| [E4k] Alytesirin-1c | Frog skin peptide | GLKEIFKAGLGSLVKGIAAHVAS-NH2 (23aa) | AH | 4–16 | 4–16 | [96,120] |
| [E6k,D9k] Hymenochirin-1B | Frog skin peptide | LKLSPKTKDTLKKVLKGAIKGAIA IASMA-NH2 (29aa) | AH | — | 4.9 | [123] |
| Epi-122–42 | Epinephelus coioides (Orange-spotted grouper) | GFIFHIIKGLFHAGKMIHGLV (21aa) | NF | — | 4–32 | [124] |
| Epsilon-poly L-lysine (EPL)-catechol | Streptomyces albulus derived | Complex | NF | — | Reducing bacterial burden in vivo | [125] |
| Esc(1-21) | Frog-skin | GIFSKLAGKKIKNLLISGLKG-NH2 (21aa) | AH | — | 17.5–35 | [126] |
| Esc(1-21) + Colistina | GIFSKLAGKKIKNLLISGLKG-NH2 (21aa) + Colistina | AH | — | 1.1–4.4 | ||
| FLIP 7 | Calliphora vicina (Medicinal Maggots) | ATCDLLSGTGANHSACAAHCLLRGNRGGYCNGKAVCVCRN (40aa) | AH | — | 125–416 biofilm bactéria sensitivity | [127] |
| [G4K] XT7 | Frog skin peptide | GLLGPLLKIAAKVGSNLL-NH2 (18aa) | AH | 4–32 | 4–64 | [96,128] |
| Glatiramer acetate | Homo sapiens | EAYKAAEKAYAAKEAAKEAAKAKAEKKAAYAKAKAAKYEKKAKKAAAEYKKK (52aa) |
NF | Reduct viable cells | Reduct viable cells | [129] |
| HBD-2 | The epithelial lining of the respiratory/urinary tracts | GIGDPVTCLKSGAICHPVFCPRRYKQIGTCGLPGTKCCKKP (41aa) | Beta | 3.90–9.35 | 3.25–4.5 | [130] |
| HBD-3 | The epithelial lining of the respiratory/urinary tracts | GIINTLQKYYCRVRGGRCAVLSCLPKEEQIGKCSTRGRKCCRRKK (45aa) | AH + BS | 4 | 4 | [131] |
| HBD-3 | Epithelial cells | GIINTLQKYYCRVRGGRCAVLSCLPKEEQIGKCSTRGRKCCRRKK (45aa) | AH + BS | — | 4–16 | [124] |
| HD5d5 |
Homo sapiens (Polymorphonuclear neutrophil) |
ARARCRRGRAARRRRLRGVCRIRGRLRRLAAR (32aa) | AH | 40 | 40 | [132] |
| Histatin-8 | Homo sapiens | KFHEKHHSHRGY (12aa) | AH | 8 | — | [133] |
| HNP-1 |
Homo sapiens (Polymorphonuclear |
ACYCRIPACIAGERRYGTCIYQGRLWAFCC (30aa) | AH | 50 | — | [106] |
| HNP-2 | (neutrophil) | CYCRIPACIAGERRYGTCIYQGRLWAFCC (29aa) | 50 | — | [106] | |
| Hp1404 | Heterometrus petersii (Scorpion venom gland) | GILGKLWEGVKSIF (14aa) | AH | 5 | 5–10 | [94,134] |
| Hp1404 analogs | GILGKLWEGVKSIF (14aa) analogs | 3.13–25 * | — | |||
| Hp l404 analogs (A, K, V, L, I, W) | Heterometrus petersii (Scorpion venom gland) | GILGKLWEGVKSIF-NH2 (14aa) | AH | 3.13–12.5 | 3.13–16.25 | [134,135] |
| Hylin a1 | Hypsiboas albopunctatus (American frog) | IFGAILPLALGALKNLIK-NH2 (18aa) | AH | 2 * | 2–8 * | [82] |
| Hylin a1-11K | IAKAILPLALKALKNLIK-NH2 (19aa) | 1–2* | 1–2 * | |||
| Hymenochirin-1 Pa | Frog skin peptide | LKLSPKTKDTLKKVLKGAIKGAIAIASMA-NH2 (29aa) | AH | — | — | [136] |
| Im4 | Heterometrus petersii (Scorpion venom gland) | FIGMIPGLIGGLISAIK (17aa) | AH | >40 | — | [94] |
| Im5 | FLGSLFSIGSKLLPGVIKLFQRKKQ (25aa) | AH | 2.5 | 2.5–10 | ||
| Indolicidin | Cytoplasmic granules of the bovine neutrophils | LPWKWPWWPWRR-NH2 (13aa) | Other structure | 4 | 2–64 | [89,106,133] |
| KS-12 | KRIVQRIKDFLR (12aa) | AH | 256 | 64–256 | [137] | |
| KR-20 | Homo sapiens | KRIVQRIKDFLRNLVPRTES (20aa) | 64 | 16–32 | ||
| KR-30 | KSKEKIGKEFKRIVQRIKDFLRNLV PRTES (30aa) | 16 | 8–16 | |||
| Lactoferrin (Lf) | Camel (Colostrum milk) |
Large protein | complex | — | Significant clearance of A. baumannii | [138] |
| Lactoperoxidase (Lpo) | rates in lung | |||||
| Latarcin 2a | Pleuronectes americanos (Winter flounder) | H-GLFGKLIKKFGRKAISYA VKKARGKH-OH (26aa) |
AH | 16 | 8–64 | [93] |
| LL-37 | Homo sapiens | LLGDFFRKSKEKIGKEFKRIVQRIK DFLRNLVPRTES (37aa) | AH | 32 | 16–32 | [137,139] |
| LS-AMP-E1 |
Lycosa sinensis (Chinese wolf spider) |
AGMKNIIDAIKKKLGGKL (18aa) | AH | — | 25–100 * | [140] |
| LS-AMP-F1 | TGLGKIGYLMKKLLSKAKV (19aa) |
AH | — | 3.1–12.5 * | [141] | |
| LS-sarcotoxin | Lucilla serricata | GWLKKIGKKIERVGQHTRDATIQTIGVAQQAANVAATLK-NH2 (39aa) | AH | 4 | 4–8 | [141] |
| LS-stomoxyn | GFRKRFNKLVKKVKHTIKETANVSKDVAIVAGSGVAVGAAM-NH2 (41aa) | AH | 8 | 4–16 | ||
| Lynronne-1 | Bovine rumen microbiome | LPRRNRWSKIWKKVVTVFS (19aa) | AH | 4 | — | [142] |
| Magainin-1 | Frog skin peptide | GIGKFLHSAGKFGKAFVGEIMKS (23aa) | AH | — | 256 | [100,101] |
| Magainin-2 | Frog skin peptide | GIGKFLHSAKKFGKAFVGEIMNS (23aa) | AH | 9.8–64 | 4.9–64 | [100,101,143] |
| Mastoparan |
Vespula lewisii (Hornet venom) |
INLKALAALAKKIL (14aa) | AH | 4 | — | [106,144,145] |
| Mastoparan-AF (EMP-AF) |
Vespa affinis (Hornet venom) |
INLKAIAALAKKLF-NH2 (14aa) | AH | 2–16 | 2–16 | [146] |
| Mastoparan-Chitosan Nanoconstruct |
Vespula lewissi (Wasp venom) |
INLKALAALAKKIL-NH2 (14aa) | AH | — | 2–4 | [147] |
| Maximin H2 | Oreochromis niloticus (Nile Tilapia) | ILGPVLSMVGSALGGLIKKI-NH2 (20aa) | AH | 64 | 16–128 | [93] |
| Mdc | Housefly larvae | GWLKKIGKKIERVGQHTRDATIQ TIGVAQQANAVAATLKG (40aa) | D-helix | 4 | 4 | [148] |
| Melittin | Apis mellifera (European honeybee) | GIGAVLKVLTTGLPALISWIKRKRQQ (26aa) | AH | 0.25–4 | 0.25–25 | [106,149,150] |
| Melittin with colistin (COL) |
Apis mellifera (European honeybee) |
GIGAVLKVLTTGLPALISWIKRKR QQ (26aa) + COL | AH | 0.37–0.5 | 0.19–0.37 | [151] |
| Melittin with imipenem (IPM) | GIGAVLKVLTTGLPALISWIKRKR QQ (26aa) + IPM | AH | 0.31–0.37 | 0.12–0.25 | ||
| Mini-ChBac7.5 Nα |
Capra hircus (Domestic goat) |
RRLRPRRPRLPRPRPRPRPRPR (22aa) | AH | — | 2 * | [152] |
| Mini-ChBac7.5 Nβ | RRLRPRRPRLPRPRPRPRPRP (21aa) | AH | — | 4 * | ||
| Myxinidin 2 | Myxine glutinosa L (Atlantic hagfish) | KIKWILKYWKWS (12aa) | AH | — | 12.5 | [153] |
| Myxinidin 3 | RIRWILRYWRWS (12aa) | BS | — | 6.3 | ||
| N10 | Blood biopanning | ACKDVNTSMCGGK (13aa) | AH | 500 | 500 | [154] |
| NA-CATH |
Naja atra (Snake venom) |
KRFKKFFKKLKNSVKKRAKKFFKK PKVIGVTFPF (34aa) | AH | 10 | 10 | [85] |
| NB2 | Biofilm biopanning | ACERSIRTVCGGK (13aa) | AH | 500 | 500 | [154] |
| NDBP5.8 | Opisthacanthus cayaporum (Scorpion venoms) | GILGKIWEGVKSLI (14aa) | AH | >25 | >25 | [81] |
| Nisin |
Lactococcus lactis (Probiotic bacterium) |
MSTKDFNLDLVSVSKKDSGASPRITSISLCTPGGKTGALNGCNMKTATCHCSIHVSK (34aa) | NF | 128 | 64–128 | [155] |
| Nisin + P10 combined |
Lactococcus lactis (Probiotic bacterium) + Synthetic derivated |
MSTKDFNLDLVSVSKKDSGASPRITSISLCTPGGKTGALNGCNMKTATCHCSIHVSK (34aa) + LAREYKKIVEKLKRWLRQVLRTLR (24aa) | NF | 32 | 16–32 | |
| Nodule-specific cysteine-rich (NCR) peptide and its derivatives | Medicago trunculata | RNGCIVDPRCPYQQCRRPLYCRRR (24aa) | AH | 1.6–25 MBC | — | [156,157] |
| NRC12 | Flatfish Genes | GWKKWFNRAKKVGKTVGGLAVDHYL-NH2 (25aa) | AH | 16 | 8–32 | [93] |
| Nuripep 1653 | Derived from the P54 nutrient reservoir protein (aa 271–292) pea protein from Pisum sativum | VRGLAPKKSLWPFGGPFKSPFN (22aa) | AH | — | 12 | [158] |
| OH-CATH30 | King cobra (Snake venom) |
KFFKKLKNSVKKRAKKFFKKPRVI GVSIPF(30aa) | AH | 10 | 10 | [122] |
| Pexiganan | Frog skin peptide | GIGKFLKKAKKFGKAFVKILKK (22aa) | AH | 1–8 | 1–8 | [88,159,160] |
| PGLa-AM1 | Frog skin peptide | GMASKAGSVLGKVAKVALKAAL-NH2 (22aa) | AH | 16–128 | 16–128 | [96,161] |
| Pilosulin | Ant venom (toxin pilosulin) | GLGSVFGRLARILGRVIPKV-NH2 (20aa) | AH | 16 | 8–16 | [93] |
| Pleurocidin | Pleuronectes americanus (Winter flounder) | GWGSFFKKAAHVGKHVGKAALTHYL-NH2 (25aa) | AH | 16 | 8–32 | [93] |
| Polydin-I |
Polybia dimorpha (Social wasp) |
AVAGEKLWLLPHLLKMLLTPTP (22aa) | AH | >25 | >25 | [81] |
| Polybia-MPII | Pseudopolybia vespiceps testacea | INWLKLGKMVIDAL (14aa) | AH | 12.5 | 25 | [81] |
| Protegrin-1 | Cimex lectularius | RGGRLCYCRRRFCVCVGR-NH2 (18aa) | AH | — | 2–8 | [162] |
| P307SQ-8C | Hepatitis B virus | NAKDYKGAAAEFPKWNKAGGRV LAGLVKRRKSQSRESQC (39aa) | NF | 125 | 62.5–125 | [163] |
| Ranalexin | Rana catesbeiana (American bulfrog) | LGGLIKIVPAMICAVTKKC (19aa) | AH | — | 4–18 | [115] |
| SAAP-148 | Homo sapiens | LKRVWKRVFKLLKRYWRQLKKPVR (24aa) | AH | — | 6 | [164] |
| Spiniferin | Heterometrus petersii (Scorpion venom gland) | ILGEIWKGIKDIL (13aa) | AH | >40 | — | [94] |
| [S7K, G11K] Alytesirrin-2a | Frog skin peptide | ILGKLLKTAAKLLSNL-NH2 (16aa) | AH | — | 8 | [165] |
| SMAP29 | Sheep myeloid | RGLRRLGRKIAHGVKKYGPTVLRIIRIAG (29aa) | AH | 8 | 4–32 | [93] |
| Tachyplesin III |
Tachypleus gigas and Carcinoscorpius rotundicauda
(Horseshoe crabs) |
KWCFRVCYRGICYRKCR-NH2 (17aa) | BS 2 dissulfite bridges | — | 8–16 | [120] |
| Temporin A | Rana temporaria (European red frog) | FLPLIGRVLSGIL-NH2 (13aa) | AH | 128 | — | [88] |
| TP4 | Oreochromis niloticus (Nile tilapia) | FIHHIIGGLFSAGKAIHRLIRRRRR (25aa) | AH | 16 | 8–32 | [93] |
| Venon cocktail proteins |
Leiurus quinquestriatus (Scorpion venom) |
Cocktail | NF | — | 50.6% of inhibition (20 mg/mL of venom) | [166] |
| VsCT1 | Heterometrus petersii (Scorpion venom gland) | FLKGIIDTVSNWL (13aa) | AH | >40 | — | [94] |
| WAM-1 | Macropus eugenii (Tammar wallaby) | KRGFGKKLRKRLKKFRNSIK KRLKNFNVVIPIPLPG (36aa) | AH | 8.12 | 4–64 | [89,167] |
| WLBU2- arginine-rich amphiphilic peptide | Skin wounds | RRWVRRVRRWVRRVVRVVRRWVRR (24aa) | NF | ~7.484 | ~7.484 | [168] |
| ZY4 cathelicidin-BF-15 derived | Bungarus fasciatus (Snake venom) | VCKRWKKWKR KWKKWCV-NH2 (17aa) | Cyclic SH-bridge | — | 4.6–9.4 | [169] |
AH, α-helical; BS, β-sheet; *, result in µM; aa, amino acid; ~, mean of; NF, not found; NPs, nanoparticles; ATBS, antibiotic-susceptible; Ref., reference.
Table 4.
Synthetic AMPs point out anti-A. baumannii activity.
| Peptide | Source | Sequence (nº Amino Acid) | Structure | MIC against A. baumannii (μg/mL) | Ref. | |
|---|---|---|---|---|---|---|
| ATBS | MDR | |||||
| AS-CATH8 | Synthetic peptide | KRVNWAKVGRTALKLLPYIFG (21aa) | AH | 0.6 | — | [86] |
| BmKn2-7 | FIKRIARLLRKIF-NH2 (13aa) | AH | 5 | 5–10 | [95] | |
| BmKn2-7R | Synthetic peptide | FIRRIARLLRRIF-NH2 (13aa) | AH | 2.5 | 2.5–5 | |
| BmKn2-7K | FIKKIAKLLKKIF-NH2 (13aa) | AH | 2.5 | 2.5–5 | ||
| BP100 | KKLFKKILKYL (11aa) | AH | — | 4 | [170] | |
| BP214 | Hybrid peptide | KKLFKKILRYL (11aa) | AH | 2 | — | |
| BP214 analogs | KKLFKKILRYL (11aa) analogs | AH | >64 | — | ||
| CA(1–8)-ME(1–12) (CAME) | Chimeric peptide | KWKLFKKIGIGAVLKVLTTG-NH2 (20aa) | AH | 3.12 | 3.12–12.5 | [171] |
| CA(1–8)-MA(1–12) (CAMA) | KWKLFKKIGIGKFLHSAKKF-NH2 (20aa) | AH | 12.5 | 3.12–12.5 | ||
| Cecropin-4 | Synthetic peptide | GWLKKIGKKIERVGQNTRDATIQ AIGVAQQAANVAATLKG (40aa) | AH | 4 | 4 | [172,173] |
| Cecropin A (1–8) melittin (1–10) (CAME) | Hybrid peptide | KWKLFKKIGIGAVLKVLTTG-NH2 (20aa) | AH | 32 | 8–32 | [93] |
| Ceragenins; CSA-192; CSA-131; D-150-177C; HBcARDderivative | Cholic acid synthetic mimics | Steroids compounds | NF | — | — | [174] |
| Chex1-Arg20 amide (ARV-1502) | NA | RPNKPRPYLPRPRPPRPVR-NH2 (19aa) | NF | — | Reduction of bacterial load | [175] |
| D-AP19 | Hybrid peptide | RLFRRVKKVAGKIAKRIWK-NH2 (19aa) | NF | 7.81 | 3.91–15.63 | [176] |
| DGL 13K | Synthetic derived D-enantiomers of GL13K derived from the salivary protein BPIFA2 |
GKIIKLKASLKLL-NH2 (13aa) | NF | — | 8–32 | [177] |
| D-Mt6 | Synthetic peptide | KFKKTAKWLIKSAWLLLKSLALKMK (25aa) | AH | 8 | — | [178] |
| DP7 | Computationally designed | VQWRIRVAVIRK (12aa) | AH | — | 4–16 | [179,180,181] |
| ECPep-D | Synthetic peptide | RPFTRAQWFAIQHISPRTIAMRAINNYRWR (30aa) | NF | 37.57 | — | [182] |
| ECPep-2D-Orn | Synthetic peptide | OPFTOAQWFAIQHISPOTIAMOAINNYOWO (30aa) | NF | 17.53 | — | [182] |
| GW-A2 | GAKYAKIIYNYLKKIANALW (20aa) | AH | 32 | 8–32 | [93] | |
| GW-H1a | Synthetic peptide | GYNYAKKLANLAKKFANALW-NH2 (20aa) | AH | 32 | 8–32 | |
| GW-Q6 | GIKIAKKAITIAKKIAKIYW (20aa) | AH | 16 | 8–16 | ||
| HP(2–9)-MA(1–12) (HPMA) | Chimeric peptide | AKKVFKRLGIGKFLHSAKKF-NH2 (20aa) | AH | 6.25 | 3.12–6.25 | [171] |
| HP(2–9)-ME(1–12) (HPME) | AKKVFKRLGIGAVLKVLTTG (20aa) | AH | 6.25 | 3.12–12.5 | ||
| I16K-piscidin-1 and analogs | Hybrid striped bass Morone saxatilis x M. chrysops | FFHHIFRGIVHVGKTIHRLVTG (22aa) | NF | — | 3.1 | [183] |
| IKR18 | Computationally designed | IKRQYKRFFKLFKWFLKK (18aa) | AH | 1 | — | [184] |
| LJ-hep2(66–86) | Synthetic peptide | IKCKFCCGCCTPGVCGVCCRF (21aa) | NF | — | 1.5–3 | [185] |
| LyeTx I-bPEG | Synthetic peptide | WLTALKFLGKNLGKLAKQQCAKL (PEG) (24aa) |
AH | — | — | [186] |
| mCM11, cecropin–melittin 11 | Synthetic peptide | NH2-WRLFRRILRVL-NH2 (11aa) | AH | 32 | <4–>512 | [187] |
| MSI-78 | Synthetic peptide, magainin analog | GIGLPLLLALLPGLAPVLILLL-NH2 (22aa) | AH | — | 5 | [188] |
| Mt6 | Synthetic peptide | KKFKKTAKWLIKSAWLLLKSLALKMK (26aa) | AH | 8 | — | [178] |
| NCR169C and its substitution derivatives | Synthetic peptide | KSKKPLFKIWKCVENVCVLWYK | AH | 1.6–12.5 MBC | — | [189] |
| Octominin, Octominin-CNPs | Synthetic derived, defensin 3 of Octopus minor | GWLIRGAIHAGKAIHGLIHRRRH (23aa) | AH | — | 5 | [190,191] |
| Octopromycin | Synthetic peptide | N-RRLIRTDTGPIIYDYFKDQLLKKGMVI LRESMKNLKGM-C (38aa) |
AH | — | 50 | [192] |
| OG1410 | ApoE-based synthetic peptide | acetyl-ASAib-LRKL-Aib-KRLL-amide | AH | 16 | 16 | [193] |
| Omega 76-shuft1 | Computationally designed | AFLLKKKKGIIFFEKAKKGK (20aa) | AH | — | 4–16 | [194] |
| Omiganan | Synthetic peptide | ILRWPWWPWRRK-NH2 (12aa) | AH | 32 | — | [88] |
| r-Omiganan | KRRWPWWPWRLI-NH2 (12aa) | AH | 16 | — | ||
| OMN6 | Synthetic peptide | H-M-C-KWKLFKKIEKVGQNIRDGIIKA-GP-AVAVVGQATQIAK-C-NH2 (40aa) | AH | 8 | 4–8 | [195,196] |
| ′Ω17 family peptides | Computationally designed | RKKAIKLVKKLVKKLKKALK (20aa) | AH | 2 | 1–8 | [194] |
| ′Ω76 family peptides | FLKAIKKFGKEFKKIGAKLK (20aa) | AH | 4 | 2–8 | ||
| P10 | Synthetic derivated | LAREYKKIVEKLKRWLRQVLRTLR (24aa) | NF | 4 | 8–32 | [155] |
| P10 + Nisin combined | Synthetic derivated + Lactococcus lactis (Probiotic bacterium) | LAREYKKIVEKLKRWLRQVLRTLR (24aa) + MSTKDFNLDLVSVSKKDSGASPRITSISLCTPGGKTGALNGCNMKTATCHCSIHVSK (34aa) | NF | 1 | 4–16 | |
| PapMA | Hybrid peptide | RWKIFKKIPKFLHSAKKF-NH2 (18aa) | AH | 32 | 16–32 | [197] |
| pepD2 | Computationally designed |
WKKLKKLLKKLKKL-NH2 (14aa) | AH | 8 | - | [198] |
| PLP-3 | Synthetic peptides derived from the innate immune system of vertebrates | ~RRPVCVVPLPRVPCLRRR~ | B- hairpin | 1–2 | 1–2 | [199] |
| PNA (RXR)4 XB | Peptide nucleic acid conjugated to (RXR)4 Phosphorodiamidate Morpholino Oligomers | RXRRXRRXRRXRXB (14aa) | NF | — | 1.25 * | [200] |
| Pro9-3 | Computationally designed |
RLWLAIWRR-NH2 (9aa) | AH | 16 | 8–64 | [201] |
| Pro9-3D | RLWLAIWRR-NH2 (9aa) | AH | 8 | 4–16 | ||
| RR | Computationally designed | WLRRIKAWLRR (11aa) | AH | — | 25–99 | [202,203] |
| RR2 | WIRRIKKWIRRVHK (14aa) | AH | — | 3–6 | ||
| RR-4 | WLRRIKAWLRRIKA (14aa) | AH | — | 3–6 | ||
| R-Pro9-3 | Computationally designed | RRWIALWLR-NH2 (9aa) | AH | 16 | 8–32 | [201] |
| R-Pro9-3D | RRWIALWLR-NH2 (9aa) | AH | 8 | 4–16 | ||
| S4A | NA | IOWAGOLFOLFO-NH2 (12aa) | AH | 100 | 50 | [204] |
| SAAP-148 NPs | Synthetic peptide | LKRVWKRVFKLLKRYWRQLKKPVR (24aa) + NPs | AH | — | — | [205] |
| Scolopendin A2 | Synthetic peptide | AGLQFKVGRIGRLLRK (16aa) | NF | — | 16 | [206] |
| SPO | NA | NINONWNANGNONLNFNONLNFNO-NH2 (22aa) | AH | 100 | 50 | [204] |
| Stapled AMP Mag (i + 4)1, 15(A9 K, B21A, N22 K, S23 K) | NA, based on magainin two structure | Mag(i + 4)1,15(A9K,B21A,N22K,S23K) | complex | — | — | [207] |
| TAT-RasGAP317–326 anticancer peptide | Chimeric (cell penetrating sequence + Src homology sequence) |
G48RKKRRQRRR57 + W317MWVTNLRTD326 | AH | Growth inhibitory effect | — | [208,209] |
| TAT-RasGAP317–326 | Chimeric peptide | G48RKKRRQRRR57 (10aa) | NF | — | — | [210] |
| TP2-5 | Computationally designed | KKCIAKAILKKAKKLLKKLVNP (22aa) | AH | 3.125 | 1.56–3.125 | [211] |
| TP2-6 | KKCIAKAILKKAKKLLKDLVNP (22aa) | AH | 3.125 | 3.125–12.5 | ||
| Trichogin analogs | Synthetic peptide | 1-Oct-Aib-Gly-Leu-Aib-Gly-Gly-Leu-Aib-Gly-Ile-Lol | >128 | — | [212] | |
| zp3 | Synthetic peptide | GIIAGIIIKIKK-NH2 (12aa) | AH | 4 | — | [213] |
AH, α-helical; BS, β-sheet; NA, unavailable; *, result in µM; aa, amino acid; >, bigger then; <, less than; NF, not found; NPs, nanoparticles; Ref., reference.
2.1. Cathelicidins
Cathelicidins are a group of more than 30 cationic AMPs (CAMPs) identified from the immune system of several vertebrates. Their structure comprises two domains involved in antimicrobial activity [214,215]. Cathelicidins have shown good activity compared to broad-spectrum carbapenems (imipenem and meropenem), antibiotics of choice to treat MDR A. baumannii (MIC = 16–32 mg/L) [216].
2.1.1. Humans
The human cathelicidin LL-37 has an α-helical structure and is produced as a component of the innate immune response. It exhibits broad-spectrum microbicidal activity against Gram-positive and Gram-negative bacteria, observed as plasma–membrane disruptions [217]. It also neutralizes lipopolysaccharide (LPS) and modulates the immune response through cellular activation, inflammation regulation, chemotaxis, and wound healing [139,218,219,220,221]. LL-37 and its fragments KS-30 and KR-12 showed activity against four susceptible MDR A. baumannii clinical isolates [137]. LL-37 inhibited those five isolates at concentrations between 16 and 32 μg/mL; meanwhile, the minimum inhibitory concentration (MIC) for KS-30 and KR-12 was 8–16 and 128–256 μg/mL, respectively. In biofilms, LL-37 and KS-30 fragments significantly inhibited and dispersed A. baumannii on abiotic surfaces at 32 and 64 μg/mL, respectively [137].
LL-37-based synthetic peptides showed potent microbicidal activity against ESKAPE pathogens (P. aeruginosa, A. baumannii, and S. aureus) without selecting for resistance. They could eliminate persistent cells and biofilms at micromolar concentrations [164]. SAAP-148 is an α-helical AMP that is able to inhibit A. baumannii MDR growth and prevent biofilm formation at a concentration of 6 μg/mL. An ex vivo human skin infection model and an in vivo murine skin infection model eliminated acute and biofilm-related infections at concentrations of ~5% [164]. Its antibiofilm activity improved when incorporated into nanoparticles of Poly(lactic-co-glycolic) (PLGA) that gradually increased over time, suggesting a sustained local release of the peptide based on the dose–effect in vitro profile [105].
P10, a synthetic derivative of LL-37, is cationic, showing stronger activity than LL-37 [155,222]. The de novo pepD2, also LL-37-based, was designed as a trigonal distribution of positive charges in its helical structure. It displayed an 8 µg/mL MIC against the A. baumannii ATCC-type strain. WLBU2 (also called PLG0206) is an engineered cationic amphipathic α-helix, derived from LL-37 peptide, that can be inserted into bacterial membranes, leading to cell death as well as potent antibacterial effects against the biofilms of MDR A. baumannii and K. pneumoniae [168]. MIC values for WLBU2 were reported to be 1.5–3.2 μM for an XDR A. baumannii [223], 7.484 μM for clinical isolates [92], and 7.943 μM for K. pneumoniae.
2.1.2. Snake
A large number of cathelicidins have been identified from snakes. Cathelicidin-BF (Cath-BF) was isolated from the venous glands of a banded krait (Bungatus fasciatus) [224]. It is one of the best-known cathelicidins, presenting an α-helical structure. Two mechanisms are attributed to its antimicrobial activity: disrupting bacteria membranes and directly pointing intracellular targets [224]. It has been proven to be highly active against the drug-resistant clinical isolates of A. baumannii and can inhibit growth at 12.8 μg/mL [105]. ZY4, a disulfide bridge, stabilized the cyclic peptide derivative of Cath-BF and displayed activity against clinical MDR isolates of A. baumannii, with MIC values ranging between 4.6 and 9.4 μg/mL. ZY4 killed bacteria via membrane permeabilization and inhibited biofilm formation [169]. With a half-life of 1.8 h in vivo, ZY4 displayed good stability and a low tendency to induce resistance. NA-CATH has an N-terminal α-helical structure with an unstructured C-terminal [85]. Identified from the Chinese cobra (Naja atra) [225], it can inhibit the growth of drug-resistant strains of A. baumannii at 10 µg/mL [225]. Its antimicrobial MOA appears to occur through membrane deformation and the formation of transient pores [226]. OH-CATH was identified from the king cobra (Ophiophagus hannah) [122]. Its analog, DOH-CATH30, exhibits microbicidal activity against MDR A. baumannii (1.56 to 12.5 μg/mL MIC).
2.1.3. Alligator
The antibacterial activity of American alligator (Alligator mississippiensis) serum can be attributed to the presence of CAMPs, and several have been identified [227]. AM-CATH36 inhibited the growth of both drug-resistant and susceptible A. baumannii at 2.5 µg/mL, while its two fragments, AM-CATH28 and AM-CATH21, inhibited at 10 µg/mL [85]. All three appear to permeabilize bacterial membranes. MDR clinical isolates seemed more susceptible to the fragments than the full-length peptide. The recently identified As-CATH8 displayed in vitro activity profiles similar to the last-resort vancomycin and polymyxin B antibiotics. In a murine abscess model of high-density bacterial infections, As-CATH8 showed good activity against A. baumannii (MIC = 0.6 µg/mL) and S. aureus [86].
2.1.4. Wallaby
WAM-1 is a cathelicidin in marsupial milk that is isolated from the Tammar wallaby (Macropus eugenii) mammary gland [167,228]. It inhibited biofilm formation in clinical isolates and dispersed the 24-hour-old biofilms of tested isolates, including MDR strains [89]. In comparison to LL-37, WAM-1 shows several desirable properties. WAM-1 in vitro activity was 12 to 80 times more effective than LL-37 at eliminating the clinical isolates of A. baumannii, and its activity as a peptide is not reduced in the presence of total serum or high NaCl concentrations. Although its MOA is unknown, it does not lead to hemolysis and has the potential for in vivo applications [89].
2.1.5. Hoofed Animals
Domesticated animals have yielded several cathelicidins. Bovine neutrophils cytoplasmic granules contain indolicidin, a short tryptophan-rich cationic peptide that displaces divalent cations on the surface of cell membranes, forms pores, and can inhibit DNA-processing enzymes [90,229,230,231,232]. Indolicidin showed potent anti-A. baumannii activity (4–32 μg/mL MIC) on susceptible clinical isolates and 16 μg/mL against colistin-resistant strains [106]. In an in vitro combination with antimicrobial agents, indolicidin MIC was tested against 12 MDR clinical isolates and was reported to be between 2 and 64 μg/mL [90]. Bactenecin is a cyclic, arginine-rich cationic AMP isolated from cows, sheep, and goats with a type I β-turn structure and a disulfide bond between cysteines at positions 3 and 11 [90,133]. Bactenecin can make cell membranes more permeable and inhibit RNA and protein synthesis: 16 and 64 µg/mL MIC against susceptible and colistin-resistant A. baumannii, respectively [79,106]. Other studies of cathelicidins include bovine BMAP-27, sheep SMAP29, and goat minibactenecins [152], which have been shown to inhibit the growth of clinical MDR A. baumannii [93].
2.2. Defensins
Animals, plants, and fungi produce an ancient class of AMPs called defensins that contain six to eight conserved cysteine residues. Their MOA includes binding cell membranes, forming pores, and, consequently, killing pathogens [233]. Defensins have been categorized into α, β, and θ-defensins subfamilies [234].
2.2.1. Human α-Defensins
The CAMPs HNP-1 and HNP-2 are α-defensins that are produced in human neutrophils that differ in their N-terminal amino acid. They are components of human neutrophil peptides in polymorphonuclear neutrophil granules released via secretion upon microbes’ activation [235]. A. baumannii ATCC 19606 was affected by 50 μg/mL MIC, while a colistin-resistant strain appeared more susceptible (MIC = 3.25 μg/mL) [106]. HD5, another human defensin, had little effect on A. baumannii (MIC = 320 μg/mL). However, its derivative HD5d5 showed a lower MIC (40 μg/mL) through cell membrane damage and cell entry, reducing superoxide dismutase and catalase activities [132,236].
2.2.2. β-Defensins
HBD-2 and HBD-3 are human β-defensins found on the epithelial lining of respiratory and urinary tracts. Interestingly, they appear more effective against MDR clinical isolates [237]. The other β-defensin, HBD-3, combines an α-helical segment with a β strand and can kill non-MDR and MDR A. baumannii isolates in serum-free conditions [238]. HBD-3 also showed wound-healing properties and a potential application in wound dressings [131,239]. In A. mississippiensis, the AM23sk isoform of HBD-3 β-Defensin showed in vitro antibacterial activity against A. baumannii (MIC = 2 μg/mL) [86].
2.2.3. Insect Defensins
The insect defensin, CL-defensin, can partially permeabilize A. baumannii and, different from others, is predicted to have an N-terminal loop, an α-helix segment, and an antiparallel β-sheet according to circular dichroism spectroscopy [109].
2.3. Frog AMP
2.3.1. Magainin and Pexiganan
The skin of the African clawed frog (Xenopus laevis) has two α-helical cationic amphipathic AMPs, Magainin-1 and Magainin-2 [240]. Their primary MOA against microbes is pore formation [101,241]. Magainin-2 shows higher activity against MDR A. baumannii (4.9–64 μg/mL) and can inhibit isolates and eliminate biofilms [101,106]. It also offers greater stability in physiological conditions and low hemolytic activity. Magainin-2 shows anticancer potential and low toxicity against non-cancerous mammalian cells [85]. A synthetic analog of Magainin-2, Pexiganan, or MSI-78 also displays a broad potent action against the formation of toroidal pores [242,243,244]. Pexiganan can inhibit the growth of clinical MDR A. baumannii at 1–8 μg/mL [159,160,245]. Studies testing ATCC 196060, a reference strain of A. baumannii, confirmed pexiganan’s antimicrobial and antibiofilm activity [88].
2.3.2. Brevinin-2 Related Peptide
Skin secretions from both mink frog (Rana septentrionalis) and carpenter frog (R. virgatipes) contain B2RP, a brevinin-related AMP with an α-helical structure that affects bacterial membrane organization [246,247]. B2RP can inhibit susceptible A. baumannii (29 μg/mL) and MDR isolates (7–13.9 μg/mL) [98]. However, its hemolytic properties limit its potential use [248]. Three analogs of B2RP (D4K, K16A, and L18K) showed reduced red blood cell toxicity and a two-fold increase in activity against A. baumannii growth [96,98]. The D4K substitution also showed activity against colistin-resistant and XDR A. baumannii clinical isolates [120]. B2RP-ERa is a smaller cationic peptide that is structurally similar to B2RP found in the skin of Asian frogs (Hylarana erythraea) [97,249]. It can inhibit susceptible Acinetobacter growth 8–32 μg/mL and drug resistant (8–64 μg/mL) [96]. B2RP-ERa shows anti-inflammatory characteristics without toxicity on peripheral blood mononuclear cells or red blood cells [249,250].
2.3.3. Alyteserins
Alyteserin-1c and Alyteserin-2a are two cationic AMPs that show that anti-A. baumannii activity is released from the skin secretions of midwife toads (Alytes obstetricans) following norepinephrine stimulation [83,165,251]. Alyteserin-1c inhibited MDR A. baumannii growth and caused death between 11.3 and 22.6 μg/mL, showing low hemolytic activity [83]. The substitution E4K further reduced the effects on red blood cells while improving growth inhibition of colistin-resistant and XDR A. baumannii isolates [120]. Structural changes of Alyteserin-2a also resulted in an analog with 4–8-fold greater antimicrobial activity and less hemolytic effects [95].
2.3.4. Peptide Glycine–Leucine-Amide
The volcano-clawed frog (Xenopus amieti) produces PGLa-AM1, peptide glycine–leucine-amide, an AMP with anti-Acinetobacter activity. PGLa-AM1 can kill susceptible and colistin-resistant A. baumannii (16–128 μg/mL) [161]. Due to its low hemolytic activity, it is also active against other ESKAPE pathogens, including E. coli and S. aureus [81,111,161].
2.3.5. Caerulein Precursor Fragment
Also isolated from the volcano-clawed frog, the caerulein precursor fragment (CPF-AM1) is a cationic AMP that binds bacterial LPS [97,120]. CPF-AM1 inhibits the growth of susceptible and colistin-resistant isolates, showing minimal fibroblast toxicity and hemolytic activity [136]. CPF-B1 was isolated from a Marsabit clawed frog (Xenopus borealis), displaying anti-A. baumannii activity at concentrations between 11.4 and 22.8 μg/mL and low hemolysis [113]. From the Peracca clawed frog (Xenopus clivii), CPF-C1 is another member of the caerulein family of peptides with proven activity against A. baumannii, including inhibitory activity as low as 5 μg/mL concentration [112].
2.3.6. Hymenochirins
Hymenochirin-1B was isolated from a Zaire Dwarf clawed frog (Hymenochirus boettgeri) and is the first member of the hymenochirins class of AMPs of their host defense system [252,253]. Hymenochirin-1B is an α-helical cationic peptide able to inhibit the growth of MDR A. baumannii at 19.1 μg/mL MIC [123]. In addition to its antimicrobial activity, it displays anticancer and immunomodulatory properties. Hymenochirin-1B, generated by E6K and D9K substitutions, showed a nearly 4-fold increase in activity against MDR and XDR A. baumannii isolates and reduced toxicity to human erythrocytes [123]. Hymenochirin-1Pa was isolated from Merlin’s dwarf gray frog (Pseudhymenochirus merlini) and was able to inhibit the growth of XDR A. baumannii between 7.5 and 15 μg/mL; however, it showed moderate hemolytic activity [136,253].
2.3.7. XT-7
Skin norepinephrine stimulation allows secretion of XT-7 from Western clawed frog (Xenopus tropicalis) [254], an AMP with anti-Acinetobacter activity against the Euroclone I NM8 strain at 22.2 μg/mL MIC [112]. A G4K substitution increased XT-7 therapeutical index [128], inhibiting susceptible and drug-resistant A. baumannii by as low as 4 μg/mL [161].
2.3.8. Buforins
The stomach of an Asian toad (Bufo gargarizans) yielded Burfoin I [255]. Its derivative, Buforin II, is a potent antimicrobial peptide that kills bacteria by crossing the membrane to bind intracellular targets, including DNA and RNA, inhibiting cellular activities [102]. Buforin II can hinder the growth of susceptible and resistant Acinetobacter isolates between 0.25 and 39 μg/mL [100,101]. By itself, or in combination with antibiotic treatments, Buforin II demonstrated good potential when tested in an A. baumannii rat sepsis model [96].
2.3.9. Caerin 1.1 and 1.9
The host defense peptides caerin 1.1 and caerin 1.9 from an Australian tree frog (Litoria caerulea) were isolated from skin secretion. They are α-helical cationic amphipathic AMPs with antiviral, antitumor, antimicrobial, and neuropeptide-type activities [256]. Each displayed anti-A. baumannii growth activity was more effective when in combination [104].
2.3.10. Hylin a1
Hylin a1 is an α-helical cationic amphipathic AMP that was isolated from the skin secretion of a white spotted tree frog (Hypsiboas albopunctatus) [257]. Its antimicrobial activity has been attributed to its action on bacterial membranes. However, it also displays a strong hemolytic activity. Two analogs, Hylin a1-11K and Hylin a1-15K, showed good antimicrobial activity against carbapenem-resistant A. baumannii clinical isolates at 1–2 µM without changes in hemolytic activity [82].
2.4. Fish Piscins
Fish possess a strong innate immune system as a first-line defense against various pathogens [258]. Several antimicrobial components can be found within the epidermal mucus, including AMPs, lysozyme, proteases, and lectins [259]. Piscidins are cationic AMPs expressed by fish mast cells [260], which comprise a family of structurally related mature peptides between 21 and 44 residues. They are made of an amphipathic α-helical structure, which suggests that piscins have bactericidal activities against microorganisms [261]. The piscidin AMP family includes pleurocidin, moronecidin, chrysophsin, dicentracin, epinecidin-1, and myxinidin [262].
Pleurocidin is an amphipathic α-helical cationic peptide found in the gills, gut, and on the skin of winter flounder (Pseudopleuronectes americanus) [263], which is genetically similar to piscidin [264]. It displays broad-spectrum activity against pathogenic bacteria and fungi such as K. pneumoniae, S. aureus, P. aeruginosa, and the opportunistic oral pathogen C. albicans [263,265]. Against MDR A. baumannii isolates, pleurocidin inhibits growth between 8 and 32 μg/mL [93]. Its MOA appears to be caused by membrane disruption due to its binding [266]; however, it shows lower hemolysis when compared to other natural peptides using in vitro toxicity studies [267].
Tilapia piscidin 2 (TP2) is an inactive antibacterial peptide found in Nile tilapia (Oreochromis niloticus) [268], which was modified to develop peptides TP2-5 and TP2-6, improving cationic and amphipathic balance [269]. Such changes resulted in a significant improvement in their antimicrobial potential in normal media against A. baumannii wild-type (MIC = 3.1 μg/mL) and MDR isolates (MIC = 1.6–12.5 μg/mL) [211]. Another AMP from Nile tilapia (TP4) displayed antimicrobial activity against susceptible and MDR A. baumannii between 16 and 32 μg/mL MIC [97].
2.5. Hepcidin
First identified from human ultrafiltrate blood and urine samples and called a liver-expressed antimicrobial peptide (LEAP-1) [270,271], hepcidin is a cationic amphipathic peptide that functions in many vertebrates. Hepcidin reportedly involves iron metabolism, inflammation, and clearance of invading pathogens [272]. Since the first fish hepcidin was reported in hybrid striped bass in 2002 [273], many isoforms have been identified across numerous fish species. Unlike a single gene in humans, many teleost fish have more than two hepcidin genes, most notably among Perciformes and Pleuronectiformes [274]. Fish hepcidin isoforms are currently phylogenetically classified into HAMP1-type and HAMP2-type [275,276,277]. From Japanese seabass (Lateolabrax japonicus), LJ-hep2 peptide has been investigated using its recombinant precursor protein (rLJ-hep2), which is expressed in Pichia pastoris and is a chemically synthesized mature peptide LJ-hep2 (66–86), with LJ-hep2 (66–86) displaying stronger antimicrobial activity against clinically isolated MDR A. baumannii (MIC = 1.5–3 μg/mL) [179].
2.6. Melittin
The cationic amphipathic α-helical AMP melittin was isolated from European honeybee (Apis mellifera) venom, comprising nearly half its dry weight [278]. Numerous melittin properties have been reported, including antibacterial [278], antiparasitic [279], and antifungal [280], along with anticancer and antiviral properties [281]. Its primary MOA is a carpet-like interaction with membranes, leading to pore formation and lysis [282]. Melittin displays potent antimicrobial activity against clinical MDR and XDR Acinetobacter at concentrations as low as 0.125 μg/mL [149,150]. In a mouse model of third-degree burns, the topical application of melittin at 16 µg/mL eliminated 93.3% of an XDR A. baumannii [149]. Importantly, the injured derma and surrounding tissue, including red blood cells, showed no toxicity. Brazilian clinical studies confirmed melittin activity against most Acinetobacter strains except for one PDR [283].
Trypsin Modulating Oostatic Factor (AeaTMOF) is a proline-rich amphipathic decapeptide that is analogous to PrAMP, which was first reported in honeybees [284]. AeaTMOF (5 mM) was very effective against A. baumannii, inhibiting cell growth during 15 h incubation [285].
2.7. Cecropins
Cecropin describes a class of AMPs in which primary MOA is attributed to membrane lysis [286]. The founding compound, cecropin A, was isolated from giant silk moth (Hyalophora cecropia) hemolymph [287]. Initial results showed in vitro antibacterial and anticancer activity [288]. Viability studies performed in the Caenorhabditis elegans model on A. baumannii infections demonstrated that 15 cecropin or cecropin-like peptides displayed antimicrobial activity and improved survival [99]. Several other studies have further defined the growth inhibition of individual peptides, including cecropin A against colistin-resistant MDR clinical isolates [106,107], BR003-cecropin A (from Aedes aegypti) against MDR A. baumannii [99], Musca domestica cecropin (Mdc) from housefly (Musca domestica) larvae against standard and MDR isolates [148], cecropin-4 from houseflies against MDR and XDR clinical isolates [172,173,205], and cecropin P1 from pig roundworms (Ascaris suum) against colistin-susceptible A. baumannii [106]. Many cecropins also display antibiofilm activity, such as myxinidin isolated from hagfish (Myxine glutinosa) [153] and the AMP complex Fly Larvae Immune Peptides 7 (FLIP7) in blowfly (Calliphora vicina) larvae [127].
The fusion of cecropin A to endolysin ST01 has been shown to have increased bactericidal activity against ESKAPE pathogens, with A. baumannii (ATCC 17978) being eliminated at a concentration of 0.25 [289]. Another hybrid of cecropin with melittin, CAMEL, rapidly kills A. baumannii [88]. OMN6 is a 40-amino acid synthetic cyclic peptide based on cecropin A that displays increased stability and a significant decrease in proteolytic degradation and low cytotoxicity against eukaryotic cells. This peptide exerts a rapid bactericidal effect causing a selective bacterial membrane disruption [195], which is effective in A. baumannii laboratory (MIC = 8 μg/mL) and clinical isolates (MIC = 4–8 μg/mL), suggesting a low likelihood for resistance development [195].
2.8. Mastoparan
Mastoparan was isolated from hornet (Vespula lewisii) venom [236]. While it displays good activity against wild-type, colistin-resistant, and PDR clinical A. baumannii [106,290], it also shows high hemolytic activity, which would prevent its therapeutic application [291]. Action against clinical MDR A. baumannii (2–16 μg/mL MIC) was observed for mastoparan-AF isolated from Vespa affinis [148]. Improvements in serum stability (24 h) have been achieved for mastoparan analogs, resulting in the growth inhibition of XDR clinical isolates [106]. Higher therapeutic efficiency against MDR clinical isolates has been acquired by conjugating mastoparan to chitin, resulting in nanoconstructs (Afreenish hassan). Improvements in hemolytic toxicity have not been reported.
2.9. Histatins
Histatins are a family of low-molecular-weight, histidine-rich cationic peptides isolated from salivary glands, which display antimicrobial activity through membrane disruption [240]. The only member that affects A. baumannii is histatin-8, a hemagglutination-inhibiting peptide [248]. It inhibited the growth of colistin-susceptible and colistin-resistant isolates at 32 μg/mL [106].
2.10. Dermcidin
The dcd gene in humans encodes dermcidin, a two-region anionic AMP produced and secreted by eccrine sweat glands and transported to the skin surface [116,292]. The N-terminal peptide is involved with neuronal cell survival in response to oxidative stress [116]; meanwhile, the C-terminal fragment shows anti-Acinetobacter activity [293]. With a net charge of -2, DCD-1L can interact with negatively charged bacterial phospholipids. Clinical PDR A. baumannii shows a two-fold increase in susceptibility compared to XDR isolates and the standard ATCC 19606 strain [117]. DCD-1L can also inhibit bacterial attachment and biofilm formation, which could affect infection initiation [117].
2.11. Tachyplesin III
The hemolymph of Southeast Asian horseshoe crabs (Tachypleu gigas and Carcinoscorpius rotundicauda) contains tachyplesin III and 17 amino acids AMP. As opposed to an α-helical structure, this peptide presents a cyclic β-sheet with two disulfide bridges. Against an XDR clinical A. baumannii, tachyplesin III had 8–16 μg/mL MIC and could fully eliminate the bacteria at twice the MIC concentration [294]. However, it also displays high toxicity against mammalian cells, preventing therapeutic applications [294].
2.12. Spider Peptides
Several AMPs have been isolated from spider venom. Lycosin-I is a 23-amino acid peptide from a Chinese wolf spider (Lycosa singoriensis) venom, resulting in 8–32 µg/mL MIC against MDR A. baumannii [140,295]. Ant spider venom (Lachesana tarabaevi) and latarcins 2a also displayed potent antimicrobial activity against clinical MDR A. baumannii (8–64 μg/mL) [93]. Like Lycosin-I, LS-AMP-E1, and LS-AMP-F1, those from burrowing wolf spiders (Lycosa sinensis) had different inhibitory activity against other clinical drug-resistant bacteria and could effectively inhibit the formation of biofilms with no obvious hemolytic effects. Among ESKAPE pathogens, LS-AMP-F1 was the most effective against A. baumannii, with the lowest being 3.1 µM MIC [140]. LyeTx I was isolated from a wolf spider from Brazil (Lycosa erythrognatha) and showed inhibitory activity against several MDR bacteria. However, it also showed hemolytic and cytotoxic effects. Conjugating a derivative, LyeTx I-b, to PEG could eliminate these contradictory effects while maintaining MIC values against A. baumannii, such as antibiofilm formation, and did not induce resistance [186].
2.13. Scorpion
Many AMPs have been identified from scorpion venom, displaying antimicrobial activity against A. baumannii, such as Hp1404, ctriporin, and Im5 [94]. Notably, these peptides also show harmful effects, such as hemolysis, requiring sequence alterations to fix. Hp1404 was isolated from the venomous gland of a giant forest scorpion (Heterometrus petersii) and is an amphipathic α-helical peptide that exhibits antimicrobial activity against methicillin-resistant S. aureus along with cytotoxicity. Many Hp1404 analogs showed lower cytotoxic activity against MDR A. baumannii [134]. BmKn2 is another naturally occurring cationic α-helical AMP in the Chinese scorpion (Mesobuthus martensii Karsch), showing antimicrobial and strong hemolytic activity. It only shows activity against Gram-positive bacteria, such as S. aureus. Its mutant BmKn2-7 has lower hemolytic activity and presents a broad antimicrobial spectrum [296]. Another analog, BmKn2-7K, is non-toxic at antimicrobial dosages and exhibits potent antimicrobial activity via a membrane-lytic mechanism against antibiotic-resistant ESKAPE pathogens. For MDR A. baumannii, BmKn2-7K and BmKn2-7R (MIC = 2.5–5 µg/mL) showed potent and improved antimicrobial activity compared to BmKn2-7 (MIC = 5–10 µg/mL) [95].
2.14. Lynronne-1
Lynronne-1 is an α-helical cationic amphipathic peptide identified through the metagenomic investigation of bovine rumen microbiome to discover novel AMPs. Although Lynronne-1 in vivo activity was lower than conventional antibiotics, it showed selectivity for bacterial cells, low hemolytic activity, and minimal cytotoxicity against mammalian cells [297]. Against most common Gram-positive and Gram-negative pathogenic bacteria, Lynronne-1 displayed broad-spectrum activity, including methicillin-resistant S. aureus (MRSA) 8–32 μg/mL MIC and A. baumannii (4 μg/mL MIC) [297].
2.15. Hybrid Peptides
The combination of different AMPs offers a rational approach to developing non-natural AMPs. PapMA peptide consists of 18 amino acids, combining the first eight amino acids from papiliocin, a 37-residue AMP purified from the larvae of a swallowtail butterfly (Papilio xuthus) with resides 4–12 of magainin 2, and a 23-residue AMP purified from African clawed frog (Xenopus laevis) skin. A proline hinge joined the two fragments. While PapMA showed high antimicrobial activity, it was cytotoxic to mammalian cells [298]. The hybrid peptides P7A3 and A3P7 that combined cathelicidin (P7) and aurein (A3) were obtained using the flipping technique [299]. The serial truncation of the C-terminal led to an optimal candidate, AP19, that was stable against proteolytic enzymes via a D-amino acid substitution (D-AP19). The final peptide rapidly killed A. baumannii ATCC 19606 (MIC = 7.81 µg/mL) via membrane disruption and showed a low tendency to induce bacterial resistance. It also exhibited potent antibacterial activity against MDR and XDR A. baumannii (MIC = 3.91–15.63) [176]. BP214 is a cationic amphipathic all-D decapeptide developed from a short cecropin A-melittin hybrid peptide BP100 [300], which showed excellent activity against colistin-resistant A. baumannii and modest hemolytic properties [301].
3. Resistance to AMPS
Resistance to AMPs can be acquired through their degradation, sequestration, and impedance by exopolymers or biofilm matrix molecules, as well as through the alteration of membrane lipid composition and exporting mechanisms [53,302,303,304,305,306,307] (Table 5). Following its long-term clinical use, colistin resistance has been documented for A. baumannii [308,309]. Resistance was also observed after inactivating one of the genes involved in LPS biosynthesis. As colistin is a last-resource drug to treat MDR nosocomial pathogens, resistance is an important clinical issue [309,310,311]. Several nanocarriers have been developed to overcome low bioavailability, proteolysis, and toxicity associated with AMPs [312,313]. Changes in molecular structure, biochemical modifications, and their combination with common antibiotics have been reported to minimize AMP resistance [303].
Table 5.
Gram-negative bacterial resistance mechanisms against AMP.
| Mechanism | Gram-Negative Bacteria | Reference |
|---|---|---|
| Degradation or sequestration by secreted proteins | Proteolytic degradation | [314,315,316] |
| Impedance by exopolymers or biofilm matrix molecules | Alginate, polysialic acid | [304,317,318] |
| Cytoplasmic outer membrane alteration | Increased IM rigidity by PG acylation | [319] |
| Surface modification | Repulsion by lipid A phosphate modification increased OM rigidity by lipid A acylation. O-antigen of LPS |
[320,321] |
| Multidrug efflux pump | Export via efflux pumps (RND family) | [322,323] |
4. Conclusions
Among ESKAPE pathogens, A. baumannii is of major concern for nosocomial and community-acquired infections. Due to its high ability to acquire resistance and biofilm formation, there has been an alarming loss of antibiotic efficacy and a rise in MDR isolates worldwide. The shortage of new antibiotic treatments shows the need to transition to a “post-antibiotic era” by developing new alternative therapeutical approaches. AMPs have emerged as excellent candidates due to the broadness of natural peptides found as part of innate immune systems, demonstrating activity against many A. baumannii, including clinical MDR and XDR isolates. While many AMPs display undesirable effects, such as hemolysis and host toxicity, studies have demonstrated the ability to modify their sequences to improve performance. AMPs isolated from natural sources have attracted significant interest in recent years as promising pharmacological substitutes for conventional antibiotics; moreover, extensive research has been undertaken on the discovery, production, and optimization of peptide drugs. Future advances in bioinformatics and studies on peptide sequence/structure/function could be able to develop synthetic AMPs to address major health concerns. Our review of AMPs highlighted common characteristics, such as cationic, α-helical structure, interactions with bacterial membranes, bilipid pore formation, and intracellular component targeting. Many possibilities for performance improvement combined with traditional treatments and their use as bioconjugates encourage future applications. Peptide drugs currently represent a significant proportion of the pharmaceutical market. Considering their therapeutic potential, market prospects, and economic values, antimicrobial peptides are expected to attract investments and research efforts, achieving success in the medium to long term. In addition to their antimicrobial properties, many AMPs have demonstrated other beneficial activities such as anticancer, antioxidant, wound healing, and angiogenesis that further support additional research.
Author Contributions
Conceptualization, S.G.D.S. and K.R.; methodology, K.R., G.C.L. and S.G.D.S.; writing—original draft preparation, K.R.; review and editing, K.R., S.G.D.S. and D.W.P.J.; funding acquisition, C.M.M. and S.G.D.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Carlos Chagas Filho Foundation for Research Support of the State of Rio de Janeiro/FAPERJ (#110.198-13) and the Brazilian Council for Scientific Research (CNPq, #467.488/2014-2 and 301744/2019-0). Funding was also provided by FAPERJ (#210.003/2018) through the National Institutes of Science and Technology Program (INCT) to Carlos M. Morel (INCT-IDPN).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.O’Neill J. Tackling Drug-Resistance Infections Globally: Final Report and Recommendations. The Review on Antimicrobial Resistance; Government of the United Kingdom; London, UK: 2016. 84p [Google Scholar]
- 2.Tacconelli E., Carrara E., Savoldi A., Harbarth S., Mendelson M., Monnet D.L., Pulcini C., Kahlmeter G., Kluytmans J., Carmeli Y., et al. Discovery, research, and development of new. antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018;18:318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 3.Lewis K. Persister cells, dormancy, and infectious disease. Nat. Rev. Microbiol. 2007;5:48–56. doi: 10.1038/nrmicro1557. [DOI] [PubMed] [Google Scholar]
- 4.Fisher R.A., Gollan B., Helaine S. Persistent bacterial infections and persister cells. Nat. Rev. Microbiol. 2017;15:453–464. doi: 10.1038/nrmicro.2017.42. [DOI] [PubMed] [Google Scholar]
- 5.Fernández L., Breidenstein E.B.M., Hancock R.E.W. Importance of adaptive and stepwise changes in the rise and spread of antimicrobial resistance. In: Keen P., Monforts M., editors. Antimicrobial Resistance in the Environment. Wiley-Blackwell; Hoboken, NJ, USA: 2011. pp. 43–71. [Google Scholar]
- 6.Olivares J., Bernardini A., Garcia-Leon G., Corona F., Sanchez M.B., Martinez J.L. The intrinsic resistome of bacterial pathogens. Front. Microbiol. 2013;30:103. doi: 10.3389/fmicb.2013.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis K., Shan Y. Persister awakening. Mol. Cell. 2016;63:3–4. doi: 10.1016/j.molcel.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 8.Conlon B.P., Rowe S.E., Gandt A.B., Nuxoll A.S., Donegan N.P., Zalis E.A., Clair G., Adkins J.N., Cheung A.L., Lewis K. Persister formation in Staphylococcus aureus is associated with ATP depletion. Nat. Microbiol. 2016;1:16051. doi: 10.1038/nmicrobiol.2016.51. [DOI] [PubMed] [Google Scholar]
- 9.Shan Y., Brown Gandt A., Rowe S.E., Deisinger J.P., Conlon B.P., Lewis K. ATP-dependent persister formation in Escherichia coli. mBio. 2017;8:e02267-16. doi: 10.1128/mBio.02267-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magana M., Sereti C., Ioannidis A., Mitchell C.A., Ball A.R., Magiorkinis E., Chatzipanagiotou S., Hamblin M.R., Hadjifrangiskou M., Tegos G.P., et al. Options and limitations in clinical investigation of bacterial biofilms. Clin. Microbiol. Rev. 2018;31:e00084-16. doi: 10.1128/CMR.00084-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cameron D.R., Shan Y., Zalis E.A., Isabella V., Lewis K. A genetic determinant of persister cell formation in bacterial pathogens. J. Bacteriol. 2018;200:e00303–e00318. doi: 10.1128/JB.00303-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prestinaci F., Pezzotti P., Pantosti A. Antimicrobial resistance: A global, multifaceted phenomenon. Pathog. Glob. Health. 2015;109:309–318. doi: 10.1179/2047773215Y.0000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmes A.H., Moore L.S.P., Sundsfjord A., Steinbakk M., Regmi S., Karkey A., Guerin P.J., Piddock L.J. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet. 2016;387:176–187. doi: 10.1016/S0140-6736(15)00473-0. [DOI] [PubMed] [Google Scholar]
- 14.Magiorakos A.P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G., Harbarth S., Hindler J.F., Kahlmeter G., Olsson-Liljequist B., et al. Multidrug-resistant, extensively drug-resistant and pan drug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 15.Rice L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J. Infect. Dis. 2008;197:1079–1081. doi: 10.1086/533452. [DOI] [PubMed] [Google Scholar]
- 16.Friedman N.D., Temkin E., Carmeli Y. The negative impact of antibiotic resistance. Clin. Microbiol. Infect. 2016;22:416. doi: 10.1016/j.cmi.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Xie R., Zhang X.D., Zhao Q., Peng B., Zheng J. Analysis of global prevalence of antibiotic resistance in Acinetobacter baumannii infections disclosed a faster increase in OECD countries. Emerg. Microbes Infect. 2018;7:1–10. doi: 10.1038/s41426-018-0038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vijayakumar S., Biswas I., Veeraraghavan B. Accurate identification of clinically important Acinetobacter spp.: An update. Future Sci. OA. 2019;5:FSO395. doi: 10.2144/fsoa-2018-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eze E.C., Chenia H.Y., El Zowalaty M.E. Acinetobacter baumannii biofilms: Effects of physicochemical factors, virulence, antibiotic resistance determinants, gene regulation, and future antimicrobial treatments. Infect. Drug Resist. 2018;11:2277–2299. doi: 10.2147/IDR.S169894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cosgaya C., Mari-Almirall M., van Assche A., Fernandez-Orth D., Mosqueda N., Telli M., Huys G., Higgins P.G., Seifert H., Lievens B., et al. Acinetobacter dijkshoorniae sp. nov., a member of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex mainly recovered from clinical samples in different countries. Int. J. Syst. Evol. Microbiol. 2016;66:4105–4111. doi: 10.1099/ijsem.0.001318. [DOI] [PubMed] [Google Scholar]
- 21.Nemec A., Krizova L., Maixnerova M., Sedo O., Brisse S., Higgins P.G. Acinetobacter seifertii sp. nov., a member of the Acinetobacter calcoaceticus–Acinetobacter baumannii complex isolated from human clinical specimens. Int. J. Syst. Evol. Microbiol. 2015;63:934–942. doi: 10.1099/ijs.0.000043. [DOI] [PubMed] [Google Scholar]
- 22.Chen T.L., Lee Y.T., Kuo S.C., Yang S.P., Fung C.P., Lee S.D. Rapid identification of Acinetobacter baumannii, Acinetobacter nosocomialis, and Acinetobacter pittii with a multiplex PCR assay. J. Med. Microbiol. 2014;63:1154–1159. doi: 10.1099/jmm.0.071712-0. [DOI] [PubMed] [Google Scholar]
- 23.Marí-Almirall M., Cosgaya C., Higgins P.G., Van Assche A., Telli M., Huys G., Lievens B., Seifert H., Dijkshoorn L., Roca I., et al. MALDI-TOF/MS identification of species from the Acinetobacter baumannii (ab) group revisited: Inclusion of the novel A. seifertii and A. dijkshoorniae species. Clin. Microbiol. Infect. 2017;23 doi: 10.1016/j.cmi.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 24.Dijkshoorn L., Nemec A., Seifert H. An increasing threat in hospitals: Multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 2007;5:939–951. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- 25.Garnacho-Montero J., Timsit J.F. Managing Acinetobacter baumannii infections. Curr. Opin. Infect. Dis. 2019;32:69–76. doi: 10.1097/QCO.0000000000000518. [DOI] [PubMed] [Google Scholar]
- 26.Peleg A.Y., Seifert H., Paterson D.L. Acinetobacter baumannii: Emergence of a successful pathogen. Clin. Microbiol. Rev. 2008;21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willyard C. The drug-resistant bacteria that pose the greatest health threats. Nature. 2017;543:15. doi: 10.1038/nature.2017.21550. [DOI] [PubMed] [Google Scholar]
- 28.Barth V.C.J., Rodrigues B.Á., Bonatto G.D., Gallo S.W., Pagnussatti V.E., Ferreira C.A.S., de Oliveira S.D. Heterogeneous persister cells formation in Acinetobacter baumannii. PLoS ONE. 2013;8:e84361. doi: 10.1371/journal.pone.0084361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davies J. Origins and evolution of antibiotic resistance. Microbiologia. 1996;12:9–16. doi: 10.1128/MMBR.00016-10. [DOI] [PubMed] [Google Scholar]
- 30.McPhee J.B., Tamber S., Brazas M.D., Lewenza S., Hancock R.E.W. Antibiotic Resistance Due to Reduced Uptake. In: Mayers D.L., editor. Antimicrobial Drug Resistance: Mechanisms of Drug Resistance. Humana Press; Totowa, NJ, USA: 2009. pp. 97–110. [Google Scholar]
- 31.Tooke C.L., Hinchliffe P., Bragginton E.C., Colenso C.K., Hirvonen V.H.A., Takebayashi Y., Spencer J. β-Lactamases and β-Lactamase Inhibitors in the 21st Century. J. Mol. Biol. 2019;431:3472–3500. doi: 10.1016/j.jmb.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amaral L., Martins A., Spengler G., Molnar J. Efflux pumps of Gram-negative bacteria: What they do, how they do it, with what and how to deal with them. Front. Pharmacol. 2014;4:168. doi: 10.3389/fphar.2013.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zampieri M. The genetic underground of antibiotic resistance. Science. 2021;371:783–784. doi: 10.1126/science.abf7922. [DOI] [PubMed] [Google Scholar]
- 34.Mee M.T., Collins J.J., Church G.M., Wang H.H. Syntrophic exchange in synthetic microbial communities. Proc. Natl. Acad. Sci. USA. 2014;111:E2149–E2156. doi: 10.1073/pnas.1405641111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Høiby N., Bjarnsholt T., Givskov M., Molin S., Ciofu O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents. 2010;35:322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 36.Sharkey L.K., Edwards T.A., O’Neill A.J. ABC-F proteins mediate antibiotic resistance through ribosomal protection. mBio. 2016;7:e01975. doi: 10.1128/mBio.01975-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mickiewicz K.M., Kawai Y., Drage L., Gomes M.C., Davison F., Pickard R., Hall J., Mostowy S., Aldridge P.D., Errington J. Possible role of L-form switching in recurrent urinary tract infection. Nat. Commun. 2019;10:4379. doi: 10.1038/s41467-019-12359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hao Z., Lou H., Zhu R., Zhu J., Zhang D., Zhao B.S., Zeng S., Chen X., Chan J., He C., et al. The multiple antibiotic resistance regulator MarR is a copper sensor in Escherichia coli. Nat. Chem. Biol. 2014;10:21–28. doi: 10.1038/nchembio.1380. [DOI] [PubMed] [Google Scholar]
- 39.Novović K., Jovčić B. Colistin resistance in Acinetobacter baumannii: Molecular mechanisms and epidemiology. Antibiotics. 2023;12:516. doi: 10.3390/antibiotics12030516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lukovic B., Gajic I., Dimkic I., Kekic D., Zornic S., Pozder T., Radisavljevic S., Opavski N., Kojic M., Ranin L. The first nationwide multicenter study of Acinetobacter baumannii recovered in Serbia: Emergence of OXA-72, OXA-23 and NDM-1-producing isolates. Antimicrob. Resist. Infect. Control. 2020;9:101. doi: 10.1186/s13756-020-00769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Isler B., Doi Y., Bonomo R.A., Paterson D.L. New treatment options against carbapenem-resistant Acinetobacter baumannii infections. Antimicrob. Agents Chemother. 2019;63:e01110–e01118. doi: 10.1128/AAC.01110-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.World Health Organization (WHO) Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. [(accessed on 20 June 2023)]; Available online: https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf.
- 43.Domalaon R., Zhanel G.G., Schweizer F. Short antimicrobial peptides and peptide scaffolds as promising antibacterial agents. Curr. Top. Med. Chem. 2016;16:1217–1230. doi: 10.2174/1568026615666150915112459. [DOI] [PubMed] [Google Scholar]
- 44.Vrancianu C.O., Gheorghe I., Czobor I.B., Chifiriuc M.C. Antibiotic resistance profiles, molecular mechanisms and innovative treatment strategies of Acinetobacter baumannii. Microorganisms. 2020;8:935. doi: 10.3390/microorganisms8060935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fan L., Sun J., Zhou M., Zhou J., Lao X., Zheng H., Xu H. DRAMP: A comprehensive data repository of antimicrobial peptides. Sci. Rep. 2016;6:24482. doi: 10.1038/srep24482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar P., Kizhakkedathu J.N., Straus S.K. Antimicrobial peptides: Diversity, mechanism of action and strategies to improve the activity and biocompatibility in vivo. Biomolecules. 2018;8:4. doi: 10.3390/biom8010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang L.J., Gallo R.L. Antimicrobial peptides. Curr. Biol. 2016;26:R14–R19. doi: 10.1016/j.cub.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 48.Zhang G., Sunkara L.T. Avian antimicrobial host defense peptides: From biology to therapeutic applications. Pharmaceuticals. 2014;7:220. doi: 10.3390/ph7030220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cruz J., Ortiz C., Guzman F., Fernandez-Lafuente R., Torres R. Antimicrobial peptides: Promising compounds against pathogenic microorganisms. Curr. Med. Chem. 2014;21:2299. doi: 10.2174/0929867321666140217110155. [DOI] [PubMed] [Google Scholar]
- 50.Govender T., Dawood A., Esterhuyse A.J., Katerere D.R. Antimicrobial properties of the skin secretions of frogs. S. Afr. J. Sci. 2012;108:25–30. doi: 10.4102/sajs.v108i5/6.795. [DOI] [Google Scholar]
- 51.Pfalzgraff A., Brandenburg K., Weindl G. Antimicrobial peptides and their therapeutic potential for bacterial skin infections and wounds. Front. Pharmacol. 2018;9:281. doi: 10.3389/fphar.2018.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Epand R.M., Walker C., Epand R.F., Magarvey N.A. Molecular mechanisms of membrane targeting antibiotics. Biochim. Biophys. Acta. 2016;1858:980–987. doi: 10.1016/j.bbamem.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 53.Andersson D.I., Hughes D., Kubicek-Sutherland J.Z. Mechanisms and consequences of bacterial resistance to antimicrobial peptides. Drug Resist. Updates. 2016;26:43–57. doi: 10.1016/j.drup.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 54.Bobone S., Roversi D., Giordano L., De Zotti M., Formaggio F., Toniolo C., Park Y., Stella L. The lipid dependence of antimicrobial peptide activity is an unreliable experimental test for different pore models. Biochemistry. 2012;51:10124–10126. doi: 10.1021/bi3015086. [DOI] [PubMed] [Google Scholar]
- 55.Roversi D., Luca V., Aureli S., Park Y., Mangoni M.L., Stella L. How many antimicrobial peptide molecules kill a bacterium? The case of PMAP-23. ACS Chem. Biol. 2014;9:2003–2007. doi: 10.1021/cb500426r. [DOI] [PubMed] [Google Scholar]
- 56.Huang H.W. Action of antimicrobial peptides: Two-state model. Biochemistry. 2000;39:8347–8352. doi: 10.1021/bi000946l. [DOI] [PubMed] [Google Scholar]
- 57.Parchebafi A., Tamanaee F., Ehteram H., Ahmad E., Nikzad H., Haddad Kashani H. The dual interaction of antimicrobial peptides on bacteria and cancer cells; mechanism of action and therapeutic strategies of nanostructures. Microb. Cell Fact. 2022;21:118. doi: 10.1186/s12934-022-01848-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brogden K.A., Ackermann M., Huttner K.M. Small, anionic, and charge-neutralizing propeptide fragments of zymogens are antimicrobial. Antimicrob. Agents Chemother. 1997;41:1615–1617. doi: 10.1128/AAC.41.7.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Subbalakshmi C., Sitaram N. Mechanism of antimicrobial action of indolicidin. FEMS Microbiol. Lett. 1998;160:91–96. doi: 10.1111/j.1574-6968.1998.tb12896.x. [DOI] [PubMed] [Google Scholar]
- 60.Sass V., Pag U., Tossi A., Bierbaum G., Sahl H.G. Mode of action of human beta-defensin 3 against Staphylococcus aureus and transcriptional analysis of responses to defensin challenge. Int. J. Med. Microbiol. 2008;298:619–633. doi: 10.1016/j.ijmm.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 61.Soehnlein O., Kai-Larsen Y., Frithiof R., Sorensen O.E., Kenne E., Scharffetter-Kochanek K., Eriksson E.E., Herwald H., Agerberth B., Lindbom L. Neutrophil primary granule proteins HBP and HNP1-3 boost bacterial phagocytosis by human and murine macrophages. J. Clin. Investig. 2008;118:3491–3502. doi: 10.1172/JCI35740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Funderburg N., Lederman M.M., Feng Z., Drage M.G., Jadlowsky J., Harding C.V., Weinberg A., Sieg S.F. Human -defensin-3 activates professional antigen-presenting cells via Toll-like receptors 1 and 2. Proc. Natl. Acad. Sci. USA. 2007;104:18631–18635. doi: 10.1073/pnas.0702130104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ehrenstein G., Lecar H. Electrically gated ionic channels in lipid bilayers. Q. Rev. Biophys. 1977;10:1–34. doi: 10.1017/S0033583500000123. [DOI] [PubMed] [Google Scholar]
- 64.Brogden K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 65.Breukink E., de Kruijff B. The lantibiotic nisin, a special case or not? Biochim. Biophys. Acta. 1999;1462:223–234. doi: 10.1016/S0005-2736(99)00208-4. [DOI] [PubMed] [Google Scholar]
- 66.Wimley W.C. Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chem. Biol. 2010;5:905–917. doi: 10.1021/cb1001558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rapaport D., Shai Y. Interaction of fluorescently labeled pardaxin and its analogs with lipid bilayers. J. Biol. Chem. 1991;266:23769–23775. doi: 10.1016/S0021-9258(18)54349-0. [DOI] [PubMed] [Google Scholar]
- 68.Shai Y., Bach D., Yanovsky A. Channel formation properties of synthetic pardaxin and analogs. J. Biol. Chem. 1990;265:20202–20209. doi: 10.1016/S0021-9258(17)30490-8. [DOI] [PubMed] [Google Scholar]
- 69.Uematsu N., Matsuzaki K. Polar angle as a determinant of amphipathic α-helix-lipid interactions: A model peptide study. Biophys. J. 2000;79:2075–2083. doi: 10.1016/S0006-3495(00)76455-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee T.-H., Hall K.N., Aguilar M.-I. Antimicrobial peptide structure and mechanism of action: A focus on the role of membrane structure. Curr. Top. Med. Chem. 2016;16:25–39. doi: 10.2174/1568026615666150703121700. [DOI] [PubMed] [Google Scholar]
- 71.Cheng J.T.J., Hale J.D., Elliot M., Hancock R.E.W., Straus S.K. Effect of membrane composition on antimicrobial peptides aurein 2.2 and 2.3 from Australian southern bell frogs. Biophys. J. 2009;96:552–565. doi: 10.1016/j.bpj.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sparr E., Ash W.L., Nazarov P.V., Rijkers D.T.S., Hemminga M.A., Tieleman D.P., Killian J.A. Self-association of transmembrane-helices in model membranes. J. Biol. Chem. 2005;280:39324–39331. doi: 10.1074/jbc.M502810200. [DOI] [PubMed] [Google Scholar]
- 73.Cheng J.T.J., Hale J.D., Elliott M., Hancock R.E.W., Straus S.K. The importance of bacterial membrane composition in the structure and function of aurein 2.2 and selected variants. Biochim. Biophys. Acta Biomembr. 2011;1808:622–633. doi: 10.1016/j.bbamem.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 74.Yeaman M.R., Yount N.Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003;55:27–55. doi: 10.1124/pr.55.1.2. [DOI] [PubMed] [Google Scholar]
- 75.Shai Y. Mode of action of membrane-active antimicrobial peptides. Biopolymers. 2002;66:236–248. doi: 10.1002/bip.10260. [DOI] [PubMed] [Google Scholar]
- 76.Fernandez D.I., Le Brun A.P., Whitwell T.C., Sani M.-A., James M., Separovic F. The antimicrobial peptide aurein 1.2 disrupts model membranes via the carpet mechanism. Phys. Chem. Chem. Phys. 2012;14:15739. doi: 10.1039/c2cp43099a. [DOI] [PubMed] [Google Scholar]
- 77.Sitaram N., Nagaraj R. Interaction of antimicrobial peptides with biological and model membranes: Structural and charge requirements for activity. Biochim. Biophys. Acta. 1999;1462:29–54. doi: 10.1016/S0005-2736(99)00199-6. [DOI] [PubMed] [Google Scholar]
- 78.Rozek A., Friedrich C.L., Hancock R.E. Structure of the bovine antimicrobial peptide indolicidin bound to dodecyl phosphocholine and sodium dodecyl sulfate micelles. Biochemistry. 2000;39:15765–15774. doi: 10.1021/bi000714m. [DOI] [PubMed] [Google Scholar]
- 79.Gee M.L., Burton M., Grevis-James A., Hossain M.A., McArthur S., Palombo E.A., Wade J.D., Clayton A.H. Imaging the action of antimicrobial peptides on living bacterial cells. Sci. Rep. 2013;3:1557. doi: 10.1038/srep01557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Choi H., Rangarajan N., Weisshaar J.C. Lights, camera, action! Antimicrobial peptide mechanisms imaging in space and time. Trends Microbiol. 2016;24:111–122. doi: 10.1016/j.tim.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.das Neves R.C., Mortari M.R., Schwartz E.F., Kipnis A., Junqueira-Kipnis A.P. Antimicrobial and antibiofilm effects of peptides from venom of social wasp and scorpion on multidrug-resistant Acinetobacter baumannii. Toxins. 2019;11:216. doi: 10.3390/toxins11040216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Park H.J., Kang H.K., Park E., Kim M.K., Park Y. Bactericidal activities and action mechanism of the novel antimicrobial peptide Hylin a1 and its analog peptides against Acinetobacter baumannii infection. Eur. J. Pharm. Sci. 2022;175:106205. doi: 10.1016/j.ejps.2022.106205. [DOI] [PubMed] [Google Scholar]
- 83.Conlon J.M., Ahmed E., Pal T., Sonnevend A. Potent and rapid bactericidal action of alyteserin-1c and its [E4K] analog against multidrug-resistant strains of Acinetobacter baumannii. Peptides. 2010;31:1806–1810. doi: 10.1016/j.peptides.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 84.Mant C., Jiang Z., Gera L., Davis T., Nelson K.L., Bevers S., Hodges R.S. De novo designed amphipathic α-helical antimicrobial peptides incorporating dab and dap residues on the polar face to treat the gram-negative pathogen, Acinetobacter baumannii. J. Med. Chem. 2019;62:3354–3366. doi: 10.1021/acs.jmedchem.8b01785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barksdale S.M., Hrifko E.J., van Hoek M.L. Cathelicidin antimicrobial peptide from Alligator mississippiensis has antibacterial activity against multi-drug resistant Acinetobacter baumanii and Klebsiella pneumonia. Dev. Comp. Immunol. 2017;70:135–144. doi: 10.1016/j.dci.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 86.Santana F.L., Arenas I., Haney E.F., Estrada K., Hancock R.E.W., Corzo G. Identification of a crocodylian β-defensin variant from Alligator mississippiensis with antimicrobial and antibiofilm activity. Peptides. 2021;141:170549. doi: 10.1016/j.peptides.2021.170549. [DOI] [PubMed] [Google Scholar]
- 87.Defraine V., Schuermans J., Grymonprez B., Govers S.K., Aertsen A., Fauvart M., Michiels J., Lavigne R., Briers Y. Efficacy of artilysin art-175 against resistant and persistent Acinetobacter baumannii. Antimicrob. Agents Chemother. 2016;60:3480–3488. doi: 10.1128/AAC.00285-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jaśkiewicz M., Neubauer D., Kazor K., Bartoszewska S., Kamysz W. Antimicrobial activity of selected antimicrobial peptides against planktonic culture and biofilm of Acinetobacter baumannii. Probiotics Antimicrob. Proteins. 2019;11:317–324. doi: 10.1007/s12602-018-9444-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Spencer J.J., Pitts R.E., Pearson R.A., King L.B. The effects of antimicrobial peptides WAM-1 and LL-37 on multidrug-resistant Acinetobacter baumannii. Pathog. Dis. 2018;76:fty007. doi: 10.1093/femspd/fty007. [DOI] [PubMed] [Google Scholar]
- 90.Falla T.J., Karunaratne D.N., Hancock R.E. Mode of action of the antimicrobial peptide indolicidin. J. Biol. Chem. 1996;271:19298–19303. doi: 10.1074/jbc.271.32.19298. [DOI] [PubMed] [Google Scholar]
- 91.Wu M., Hancock R.E. Interaction of the cyclic antimicrobial cationic peptide bactenecin with the outer and cytoplasmic membrane. J. Biol. Chem. 1999;274:29–35. doi: 10.1074/jbc.274.1.29. [DOI] [PubMed] [Google Scholar]
- 92.Eales M.G., Ferrari E., Goddard A.D., Lancaster L., Sanderson P., Miller C. Mechanistic and phenotypic studies of bicarinalin, BP100 and colistin action on Acinetobacter baumannii. Res. Microbiol. 2018;169:296–302. doi: 10.1016/j.resmic.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 93.Jung C.-J., Liao Y.-D., Hsu C.-C., Huang T.-Y., Chuang Y.-C., Chen J.-W., Kuo Y.-M., Chia J.-S. Identification of potential therapeutic antimicrobial peptides against Acinetobacter baumannii in a mouse model of pneumonia. Sci. Rep. 2021;11:7318. doi: 10.1038/s41598-021-86844-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Luo X., Ye X., Ding L., Zhu W., Zhao Z., Luo D., Liu N., Sun L., Chen Z. Identification of the scorpion venom-derived antimicrobial peptide Hp1404 as a new antimicrobial agent against carbapenem-resistant Acinetobacter baumannii. Microb. Pathog. 2021;157:104960. doi: 10.1016/j.micpath.2021.104960. [DOI] [PubMed] [Google Scholar]
- 95.Sahoo A., Swain S.S., Behera A., Sahoo G., Mahapatra P.K., Panda S.K. Antimicrobial peptides derived from insects offer a novel therapeutic option to combat biofilm: A review. Front. Microbiol. 2021;10:661195. doi: 10.3389/fmicb.2021.661195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Conlon J.M., Sonnevend A., Pál T., Vila-Farrés X. Efficacy of six frog skin-derived antimicrobial peptides against colistin-resistant strains of the Acinetobacter baumannii group. Int. J. Antimicrob. Agents. 2012;39:317–320. doi: 10.1016/j.ijantimicag.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 97.Al-Ghaferi N., Kolodziejek J., Nowotny N., Coquet L., Jouenne T., Leprince J., Vaudry H., King J.D., Conlon J.M. Antimicrobial peptides from the skin secretions of the South-East Asian frog Hylarana erythraea (Ranidae) Peptides. 2010;31:548–554. doi: 10.1016/j.peptides.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 98.Conlon J.M., Ahmed E., Condamine E. Antimicrobial properties of brevinin-2-related peptide and its analogs: Efficacy against multidrug-resistant Acinetobacter baumannii. Chem. Biol. Drug Des. 2009;74:488–493. doi: 10.1111/j.1747-0285.2009.00882.x. [DOI] [PubMed] [Google Scholar]
- 99.Jayamani E., Rajamuthiah R., Larkins-Ford J., Fuchs B.B., Conery A.L., Vilcinskas A., Ausubel F.M., Mylonakis E. Insect-derived cecropins display activity against Acinetobacter baumannii in a whole-animal high-throughput Caenorhabditis elegans model. Antimicrob. Agents Chemother. 2015;59:1728–1737. doi: 10.1128/AAC.04198-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Giacometti A., Cirioni O., Del Prete M.S., Barchiesi F., Paggi A.M., Petrelli E., Scalise G. Comparative activities of polycationic peptides and clinically used antimicrobial agents against multidrug-resistant nosocomial isolates of Acinetobacter baumannii. J. Antimicrob. Chemother. 2000;46:807–810. doi: 10.1093/jac/46.5.807. [DOI] [PubMed] [Google Scholar]
- 101.Kim M.K., Kang N., Ko S.J., Park J., Park E., Shin D.W., Kim S.H., Lee S.A., Lee J.I., Lee S.H., et al. Antibacterial and antibiofilm activity and mode of action of Magainin 2 against drug-resistant Acinetobacter baumannii. Int. J. Mol. Sci. 2018;19:3041. doi: 10.3390/ijms19103041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Park C.B., Kim H.S., Kim S.C. Mechanism of action of the antimicrobial peptide buforin II: Buforin II kills microorganisms by penetrating the cell membrane and inhibiting cellular functions. Biochem. Biophys. Res. Commun. 1998;244:253–257. doi: 10.1006/bbrc.1998.8159. [DOI] [PubMed] [Google Scholar]
- 103.Cirioni O., Silvestri C., Ghiselli R., Orlando F., Riva A., Gabrielli E., Mocchegiani F., Cianforlini N., Trombettoni M.M., Saba V., et al. Therapeutic efficacy of buforin II and rifampin in a rat model of Acinetobacter baumannii sepsis. Crit. Care Med. 2009;37:1403–1407. doi: 10.1097/CCM.0b013e31819c3e22. [DOI] [PubMed] [Google Scholar]
- 104.Chen S., Zhang P., Xiao L., Liu Y., Wu K., Ni G., Li H., Wang T., Wu X., Chen G., et al. Caerin 1.1 and 1.9 peptides from australian tree frog inhibit antibiotic-resistant bacteria growth in a murine skin infection model. Microbiol. Spectr. 2021;9:e0005121. doi: 10.1128/Spectrum.00051-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tajbakhsh M., Akhavan M.M., Fallah F., Karimi A. A recombinant snake cathelicidin derivative peptide: Antibiofilm properties and expression in Escherichia coli. Biomolecules. 2018;8:118. doi: 10.3390/biom8040118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vila-Farres X., De La Maria C.G., López-Rojas R., Pachón J., Giralt E., Vila J. In vitro activity of several antimicrobial peptides against colistin-susceptible and colistin-resistant Acinetobacter baumannii. Clin. Microbiol. Infect. 2012;18:383–387. doi: 10.1111/j.1469-0691.2011.03581.x. [DOI] [PubMed] [Google Scholar]
- 107.Giacometti A., Cirioni O., Kamysz W., D’Amato G., Silvestri C., Del Prete M.S., Łukasiak J., Scalise G. Comparative activities of cecropin A, melittin, and cecropin A-melittin peptide CA(1-7)M(2-9)NH2 against multidrug-resistant nosocomial isolates of Acinetobacter baumannii. Peptides. 2003;24:1315–1318. doi: 10.1016/j.peptides.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 108.Boman H.G., Agerberth B., Boman A. Mechanisms of action on Escherichia coli of cecropin P1 and PR-39, two antibacterial peptides from pig intestine. Infect. Immun. 1993;61:2978–2984. doi: 10.1128/iai.61.7.2978-2984.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kaushal A., Gupta K., Van Hoek M.L. Characterization of Cimex lectularius (bedbug) defensin peptide and its antimicrobial activity against human skin microflora. Biochem. Biophys. Res. Commun. 2016;470:955–960. doi: 10.1016/j.bbrc.2016.01.100. [DOI] [PubMed] [Google Scholar]
- 110.Cai Y., Wang R., Liang B.B., An M.M. In-vitro bactericidal activity of colistin against biofilm-associated Pseudomonas aeruginosa and Acinetobacter baumannii. J. Hosp. Infect. 2009;72:368–370. doi: 10.1016/j.jhin.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 111.Conlon J.M., Al-Ghaferi N., Ahmed E., Meetani M.A., Leprince J., Nielsen P.F. Orthologs of magainin, PGLa, procaerulein-derived, and proxenopsin-derived peptides from skin secretions of the octoploid frog Xenopus amieti (Pipidae) Peptides. 2010;31:989–994. doi: 10.1016/j.peptides.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 112.Conlon J.M., Mechkarska M., Ahmed E., Leprince J., Vaudry H., King J.D., Takada K. Purification and properties of antimicrobial peptides from skin secretions of the Eritrea clawed frog Xenopus clivii (Pipidae) Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011;153:350–354. doi: 10.1016/j.cbpc.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 113.Mechkarska M., Ahmed E., Coquet L., Leprince J., Jouenne T., Vaudry H., King J.D., Conlon J.M. Antimicrobial peptides with therapeutic potential from skin secretions of the Marsabit clawed frog Xenopus borealis (Pipidae) Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010;152:467–472. doi: 10.1016/j.cbpc.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 114.Kirkpatrick C.L., Broberg C.A., McCool E.M., Lee W.J., Chao A., McConnell E.W., Pritchard D.A., Hebert M., Fleeman R., Adams J., et al. The “PepSAVI-MS” pipeline for natural product bioactive peptide discovery. Anal. Chem. 2017;89:1194–1201. doi: 10.1021/acs.analchem.6b03625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Domhan C., Uhl P., Kleist C., Zimmermann S., Umstätter F., Leotta K., Mier W., Wink M. Replacement of L-amino acids by d-amino acids in the antimicrobial peptide ranalexin and its consequences for antimicrobial activity and biodistribution. Molecules. 2019;24:2987. doi: 10.3390/molecules24162987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sağmak Tartar A., Özer Balin Ş., Akbulut A., Yardim M., Aydin S. Roles of dermcidin, salusin-α, salusin-β and TNF-α in the pathogenesis of human brucellosis. Iran. J. Immunol. 2019;16:182–189. doi: 10.22034/IJI.2019.80261. [DOI] [PubMed] [Google Scholar]
- 117.Farshadzadeh Z., Pourhajibagher M., Taheri B., Ekrami A., Modarressi M.H., Azimzadeh M., Bahador A. Antimicrobial and anti-biofilm potencies of dermcidin-derived peptide DCD-1L against Acinetobacter baumannii: An in vivo wound healing model. BMC Microbiol. 2022;22:25. doi: 10.1186/s12866-022-02439-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 118.Chen H.L., Su P.Y., Kuo S.C., Lauderdale T.Y., Shih C. Adding a c-terminal cysteine (ctc) can enhance the bactericidal activity of three different antimicrobial peptides. Front. Microbiol. 2018;9:1440. doi: 10.3389/fmicb.2018.01440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tejman-Yarden N., Robinson A., Davidov Y., Shulman A., Varvak A., Reyes F., Rahav G., Nissan I. Delftibactin-A, a non-ribosomal peptide with broad antimicrobial activity. Front. Microbiol. 2019;10:2377. doi: 10.3389/fmicb.2019.02377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu C.B., Shan B., Bai H.M., Tang J., Yan L.Z., Ma Y.B. Hydrophilic/hydrophobic characters of antimicrobial peptides derived from animals and their effects on multidrug resistant clinical isolates. Dongwuxue Yanjiu. 2015;36:41–47. doi: 10.13918/j.issn.2095-8137.2015.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dekan Z., Headey S.J., Scanlon M., Baldo B.A., Lee T.-H., Aguilar M.-I., Deuis J.R., Vetter I., Elliott A.G., Amado M., et al. ∆-Myrtoxin-Mp1a is a helical heterodimer from the venom of the jack jumper ant that has antimicrobial, membrane-disrupting, and nociceptive activities. Angew. Chem. Int. Ed. Engl. 2017;56:8495–8499. doi: 10.1002/anie.201703360. [DOI] [PubMed] [Google Scholar]
- 122.Zhao F., Lan X.Q., Du Y., Chen P.Y., Zhao J., Zhao F., Lee W.-H., Zhang Y. King cobra peptide OH-CATH30 as a potential candidate drug through clinic drug-resistant isolates. Zool. Res. 2018;9:87–96. doi: 10.24272/j.issn.2095-8137.2018.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mechkarska M., Prajeep M., Radosavljevic G.D., Jovanovic I.P., Al Baloushi A., Sonnevend A., Lukic M.L., Conlon J.M. An analog of the host-defense peptide hymenochirin-1B with potent broad-spectrum activity against multidrug-resistant bacteria and immunomodulatory properties. Peptides. 2013;50:153–159. doi: 10.1016/j.peptides.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 124.Bolatchiev A. Antimicrobial peptides Epinecidin-1 and Beta-Defesin-3 are effective against a broad spectrum of antibiotic-resistant bacterial isolates and increase survival rate in experimental sepsis. Antibiotics. 2022;11:76. doi: 10.3390/antibiotics11010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Khan A., Xu M., Wang T., You C., Wang X., Ren H., Zhou H., Khan A., Han C., Li P. Catechol cross-linked antimicrobial peptide hydrogels prevent multidrug-resistant Acinetobacter baumannii infection in burn wounds. Biosci. Rep. 2019;39:BSR20190504. doi: 10.1042/BSR20190504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sacco F., Bitossi C., Casciaro B., Loffredo M.R., Fabiano G., Torrini L., Raponi F., Raponi G., Mangoni M.L. The antimicrobial peptide Esc(1-21) synergizes with colistin in inhibiting the growth and in killing multidrug resistant Acinetobacter baumannii strains. Antibiotics. 2022;11:234. doi: 10.3390/antibiotics11020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gordya N., Yakovlev A., Kruglikova A., Tulin D., Potolitsina E., Suborova T., Bordo D., Rosano C., Chernysh S. Natural antimicrobial peptide complexes in the fighting of antibiotic resistant biofilms: Calliphora vicina medicinal maggots. PLoS ONE. 2017;12:e0173559. doi: 10.1371/journal.pone.0173559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Conlon J.M., Galadari S., Raza H., Condamine E. Design of potent, non-toxic antimicrobial agents based upon the naturally occurring frog skin peptides, ascaphin-8 and peptide XT-7. Chem. Biol. Drug Des. 2008;72:58–64. doi: 10.1111/j.1747-0285.2008.00671.x. [DOI] [PubMed] [Google Scholar]
- 129.Christiansen S.H., Murphy R.A., Juul-Madsen K., Fredborg M., Hvam M.L., Axelgaard E., Skovdal S.M., Meyer R.L., Sørensen U.B.S., Möller A., et al. The immunomodulatory drug glatiramer acetate is also an effective antimicrobial agent that kills gram-negative bacteria. Sci. Rep. 2017;7:15653. doi: 10.1038/s41598-017-15969-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Routsias J.G., Karagounis P., Parvulesku G., Legakis N.J., Tsakris A. In vitro bactericidal activity of human β-defensin 2 against nosocomial strains. Peptides. 2010;31:1654–1660. doi: 10.1016/j.peptides.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 131.Maisetta G., Batoni G., Esin S., Florio W., Bottai D., Favilli F., Campa M. In vitro bactericidal activity of human beta-defensin 3 against multidrug-resistant nosocomial strains. Antimicrob. Agents Chemother. 2006;50:806–809. doi: 10.1128/AAC.50.2.806-809.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang C., Zhao G., Wang S., Chen Y., Gong Y., Chen S., Xu Y., Hu M., Wang X., Zeng H., et al. A simplified derivative of human defensin 5 with potent and efficient activity against multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2018;62:e01504–e01517. doi: 10.1128/AAC.01504-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Romeo D., Skerlavaj B., Bolognesi M., Gennaro R. Structure and bactericidal activity of an antibiotic dodecapeptide purified from bovine neutrophils. J. Biol. Chem. 1988;263:9573–9575. doi: 10.1016/S0021-9258(19)81553-3. [DOI] [PubMed] [Google Scholar]
- 134.Hong M.J., Kim M.K., Park Y. Comparative antimicrobial activity of Hp404 peptide and its analogs against Acinetobacter baumannii. Int. J. Mol. Sci. 2021;22:5540. doi: 10.3390/ijms22115540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Neshani A., Sedighian H., Mirhosseini S.A., Ghazvini K., Zare H., Jahangiri A. Antimicrobial peptides as a promising treatment option against Acinetobacter baumannii infections. Microb. Pathog. 2020;146:104238. doi: 10.1016/j.micpath.2020.104238. [DOI] [PubMed] [Google Scholar]
- 136.Serra I., Scorciapino M.A., Manzo G., Casu M., Rinaldi A.C., Attoub S., Mechkarska M., Conlon J.M. Conformational analysis and cytotoxic activities of the frog skin host-defense peptide, hymenochirin-1Pa. Peptides. 2014;61:114–121. doi: 10.1016/j.peptides.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 137.Feng X., Sambanthamoorthy K., Palys T., Paranavitana C. The human antimicrobial peptide LL-37 and its fragments possess both antimicrobial and antibiofilm activities against multidrug-resistant Acinetobacter baumannii. Peptides. 2013;49:131–137. doi: 10.1016/j.peptides.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 138.Mahdi L., Mahdi N., Al-Kakei S., Musafer H., Al-Joofy I., Essa R., Zwain L., Salman I., Mater H., Al-Alak S., et al. Treatment strategy by lactoperoxidase and lactoferrin combination: Immunomodulatory and antibacterial activity against multidrug-resistant Acinetobacter baumannii. Microb. Pathog. 2018;114:147–152. doi: 10.1016/j.micpath.2017.10.056. [DOI] [PubMed] [Google Scholar]
- 139.Neshani A., Zare H., Akbari Eidgahi M.R., Chichaklu A.H., Movaqar A., Ghazvini K. Review of antimicrobial peptides with anti-helicobacter pylori activity. Helicobacter. 2019;24:e12555. doi: 10.1111/hel.12555. [DOI] [PubMed] [Google Scholar]
- 140.Tan H., Wang J., Song Y., Liu S., Lu Z., Luo H., Tang X. Antibacterial potential analysis of novel α-helix peptides in the chinese wolf spider Lycosa sinensis. Pharmaceutics. 2022;14:2540. doi: 10.3390/pharmaceutics14112540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hirsch R., Wiesner J., Marker A., Pfeifer Y., Bauer A., Hammann P.E., Vilcinskas A. Profiling antimicrobial peptides from the medical maggot Lucilia sericata as potential antibiotics for MDR gram-negative bacteria. J. Antimicrob. Chemother. 2019;74:96–107. doi: 10.1093/jac/dky386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jayawant E.S., Hutchinson J., Gašparíková D., Lockey C., Pruñonosa Lara L., Guy C., Brooks R.L., Dixon A.M. Molecular basis of selectivity and activity for the antimicrobial peptide lynronne-1 informs rational design of peptide with improved activity. Chembiochem. 2021;22:2430–2439. doi: 10.1002/cbic.202100151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: Isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc. Natl. Acad. Sci. USA. 1987;84:5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vila-Farrés X., López-Rojas R., Pachón-Ibáñez M.E., Teixidó M., Pachón J., Vila J., Giralt E. Sequence-activity relationship, and mechanism of action of mastoparan analogues against extended-drug resistant Acinetobacter baumannii. Eur. J. Med. Chem. 2015;101:34–40. doi: 10.1016/j.ejmech.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 145.Al-Khafaji Z., Al-Samaree M. Design of synthetic antimicrobial peptides against resistant Acinetobacter baumannii using computational approach. Int. J. Pharmaceut Sci. Res. 2017;8:2033–2039255. doi: 10.13040/IJPSR.0975-8232.8(5).2033-39. [DOI] [Google Scholar]
- 146.Lin C.H., Lee M.C., Tzen J.T.C., Lee H.M., Chang S.M., Tu W.C., Lin C.F. Efficacy of Mastoparan-AF alone and in combination with clinically used antibiotics on nosocomial multidrug-resistant Acinetobacter baumannii. Saudi J. Biol. Sci. 2017;24:1023–1029. doi: 10.1016/j.sjbs.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hassan A., Ikram A., Raza A., Saeed S., Zafar Paracha R., Younas Z., Khadim M.T. Therapeutic potential of novel mastoparan-chitosan nanoconstructs against clinical MDR Acinetobacter baumannii: In silico, in vitro and in vivo studies. Int. J. Nanomed. 2021;16:3755–3773. doi: 10.2147/IJN.S296717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gui S., Li R., Feng Y., Wang S. Transmission electron microscopic morphological study and flow cytometric viability assessment of Acinetobacter baumannii susceptible to Musca domestica cecropin. ScientificWorldJournal. 2014;2014:657536. doi: 10.1155/2014/657536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pashaei F., Bevalian P., Akbari R., Pooshang Bagheri K. Single dose eradication of extensively drug resistant Acinetobacter spp. In a mouse model of burn infection by melittin antimicrobial peptide. Microb. Pathog. 2019;127:60–69. doi: 10.1016/j.micpath.2018.11.055. [DOI] [PubMed] [Google Scholar]
- 150.Akbar R., Hakemi-Vala M., Pashaie F., Bevalian P., Hashemi A., Pooshang Bagheri K. Highly synergistic effects of melittin with conventional antibiotics against multidrug-resistant isolates of Acinetobacter baumannii and Pseudomonas aeruginosa. Microb. Drug Resist. 2019;25:193–202. doi: 10.1089/mdr.2018.0016. [DOI] [PubMed] [Google Scholar]
- 151.Bardbari A.M., Arabestani M.R., Karami M., Keramat F., Aghazadeh H., Alikhani M.Y., Bagheri K.P. Highly synergistic activity of melittin with imipenem and colistin in biofilm inhibition against multidrug-resistant strong biofilm producer strains of Acinetobacter baumannii. Eur. J. Clin. Microbiol. Infect. Dis. 2018;37:443–454. doi: 10.1007/s10096-018-3189-7. [DOI] [PubMed] [Google Scholar]
- 152.Shamova O.V., Orlov D.S., Zharkova M.S., Balandin S.V., Yamschikova E.V., Knappe D., Hoffmann R., Kokryakov V.N., Ovchinnikova T.V. Minibactenecins ChBac7.Nα and ChBac7. Nβ—Antimicrobial peptides from leukocytes of the goat Capra hircus. Acta Naturae. 2016;8:136–146. doi: 10.32607/20758251-2016-8-3-136-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Han H.M., Ko S., Cheong M.J., Bang J.K., Seo C.H., Luchian T., Park Y. Myxinidin2 and myxinidin3 suppress inflammatory responses through STAT3 and MAPKs to promote wound healing. Oncotarget. 2017;8:87582–87597. doi: 10.18632/oncotarget.20908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Irani N., Basardeh E., Samiee F., Fateh A., Shooraj F., Rahimi A., Shahcheraghi F., Vaziri F., Masoumi M., Pazhouhandeh M., et al. The inhibitory effect of the combination of two new peptides on biofilm formation by Acinetobacter baumannii. Microb. Pathog. 2018;121:310–317. doi: 10.1016/j.micpath.2018.05.051. [DOI] [PubMed] [Google Scholar]
- 155.Jahangiri A., Neshani A., Mirhosseini S.A., Ghazvini K., Zare H., Sedighian H. Synergistic effect of two antimicrobial peptides, Nisin and P10 with conventional antibiotics against extensively drug-resistant Acinetobacter baumannii and colistin-resistant Pseudomonas aeruginosa isolates. Microb. Pathog. 2021;150:104700. doi: 10.1016/j.micpath.2020.104700. [DOI] [PubMed] [Google Scholar]
- 156.Jenei S., Tiricz H., Szolomájer J., Tímár E., Klement É., Al Bouni M.A., Lima R.M., Kata D., Harmati M., Buzás K., et al. Potent chimeric antimicrobial derivatives of the medicago truncatula ncr247 symbiotic peptide. Front. Microbiol. 2020;11:270. doi: 10.3389/fmicb.2020.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lima R.M., Rathod B.B., Tiricz H., Howan D.H.O., Al Bouni M.A., Jenei S., Tímár E., Endre G., Tóth G.K., Kondorosi É. legume plant peptides as sources of novel antimicrobial molecules against human pathogens. Front. Mol. Biosci. 2022;9:870460. doi: 10.3389/fmolb.2022.870460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mohan N.M., Zorgani A., Jalowicki G., Kerr A., Khaldi N., Martins M. Unlocking NuriPep 1653 from common pea protein: A potent antimicrobial peptide to tackle a pan-drug resistant Acinetobacter baumannii. Front Microbiol. 2019;18:2086. doi: 10.3389/fmicb.2019.02086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Flamm R.K., Rhomberg P.R., Simpson K.M., Farrell D.J., Sader H.S., Jones R.N. In vitro spectrum of pexiganan activity when tested against pathogens from diabetic foot infections and with selected resistance mechanisms. Antimicrob. Agents Chemother. 2015;59:1751–1754. doi: 10.1128/AAC.04773-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ge Y., MacDonald D.L., Holroyd K.J., Thornsberry C., Wexler H., Zasloff M. In vitro antibacterial properties of pexiganan, an analog of magainin. Antimicrob. Agents Chemother. 1999;43:782–788. doi: 10.1128/AAC.43.4.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.McLean D.T., McCrudden M.T., Linden G.J., Irwin C.R., Conlon J.M., Lundy F.T. Antimicrobial and immunomodulatory properties of PGLa-AM1, CPF-AM1, and magainin-AM1: Potent activity against oral pathogens. Regul. Pept. 2014;194–195:63–68. doi: 10.1016/j.regpep.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 162.Morroni G., Simonetti O., Brenciani A., Brescini L., Kamysz W., Kamysz E., Neubauer D., Caffarini M., Orciani M., Giovanetti E., et al. In vitro activity of Protegrin-1, alone and in combination with clinically useful antibiotics, against Acinetobacter baumannii strains isolated from surgical wounds. Med. Microbiol. Immunol. 2019;208:877–883. doi: 10.1007/s00430-019-00624-7. [DOI] [PubMed] [Google Scholar]
- 163.Thandar M., Lood R., Winer B.Y., Deutsch D.R., Euler C.W., Fischetti V.A. novel engineered peptides of a phage lysin as effective antimicrobials against multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2016;60:671–679. doi: 10.1128/AAC.02972-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.De Breij A., Riool M., Cordfunke R.A., Malanovic N., De Boer L., Koning R.I., Ravensbergen E., Franken M., van der Heijde T., Boekema B.K., et al. The antimicrobial peptide SAAP-148 combats drug-resistant bacteria and biofilms. Sci. Transl. Med. 2018;10:eaan4044. doi: 10.1126/scitranslmed.aan4044. [DOI] [PubMed] [Google Scholar]
- 165.Conlon J.M., Mechkarska M., Arafat K., Attoub S., Sonnevend A. Analogues of the frog skin peptide alyteserin-2a with enhanced antimicrobial activities against Gram-negative bacteria. J. Pept. Sci. 2012;18:270–275. doi: 10.1002/psc.2397. [DOI] [PubMed] [Google Scholar]
- 166.Al-Asmari A.K., Alamri M.A., Almasoudi A.S., Abbasmanthiri R., Mahfoud M. Evaluation of the in vitro antimicrobial activity of selected Saudi scorpion venoms tested against multidrug-resistant micro-organisms. J. Glob. Antimicrob. Resist. 2017;10:14–18. doi: 10.1016/j.jgar.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 167.Wang J., Wong E.S.W., Whitley J.C., Li J., Stringer J.M., Short K.R., Renfree M.B., Belov K., Cocks B.G. Ancient antimicrobial peptides kill antibiotic-resistant pathogens: Australian mammals provide new options. PLoS ONE. 2011;6:e24030. doi: 10.1371/journal.pone.0024030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Swedan S., Shubair Z., Almaaytah A. Synergism of cationic antimicrobial peptide WLBU2 with antibacterial agents against biofilms of multi-drug resistant Acinetobacter baumannii and Klebsiella pneumoniae. Infect. Drug Resist. 2019;12:2019–2030. doi: 10.2147/IDR.S215084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mwangi J., Yin Y., Wang G., Yang M., Li Y., Zhang Z., Lai R. The antimicrobial peptide ZY4 combats multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii infection. Proc. Natl. Acad. Sci. USA. 2019;116:26516–26522. doi: 10.1073/pnas.1909585117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Andersen I.K.L., Thomsen T.T., Rashid J., Bobak T.R., Oddo A., Franzyk H., Løbner-Olesen A., Hansen P.R. C-locked analogs of the antimicrobial peptide BP214. Antibiotics. 2022;11:1080. doi: 10.3390/antibiotics11081080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gopal R., Kim Y.G., Lee J.H., Lee S.K., Chae J.D., Son B.K., Seo C.H., Park Y. Synergistic effects and antibiofilm properties of chimeric peptides against multidrug-resistant Acinetobacter baumannii strains. Antimicrob. Agents Chemother. 2014;58:1622–1629. doi: 10.1128/AAC.02473-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Peng J., Long H., Liu W., Wu Z., Wang T., Zeng Z., Guo G., Wu J. Antibacterial mechanism of peptide Cec4 against Acinetobacter baumannii. Infect. Drug Resist. 2019;12:417–2428. doi: 10.2147/IDR.S214057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Peng J., Wang Y., Wu Z., Mao C., Li L., Cao H., Qiu Z., Guo G., Liang G., Shen F. Antimicrobial peptide Cec4 eradicates multidrug-resistant Acinetobacter baumannii in vitro and in vivo. Drug Des. Dev. Ther. 2023;30:977–992. doi: 10.2147/DDDT.S405579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hacioglu M., Oyardi O., Bozkurt-Guzel C., Savage P.B. Antibiofilm activities of ceragenins and antimicrobial peptides against fungal-bacterial mono and multispecies biofilms. J. Antibiot. 2020;73:455–462. doi: 10.1038/s41429-020-0299-0. [DOI] [PubMed] [Google Scholar]
- 175.Ostorhazi E., Hoffmann R., Herth N., Wade J.D., Kraus C.N., Otvos L., Jr. Advantage of a narrow spectrum host defense (antimicrobial) peptide over a broad spectrum analog in preclinical drug development. Front. Chem. 2018;6:359. doi: 10.3389/fchem.2018.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Jariyarattanarach P., Klubthawee N., Wongchai M., Roytrakul S., Aunpad R. Novel D-form of hybrid peptide (D-AP19) rapidly kills Acinetobacter baumannii while tolerating proteolytic enzymes. Sci. Rep. 2022;12:15852. doi: 10.1038/s41598-022-20236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gorr S.U., Brigman H.V., Anderson J.C., Hirsch E.B. The antimicrobial peptide DGL13K is active against drug-resistant gram-negative bacteria and sub-inhibitory concentrations stimulate bacterial growth without causing resistance. PLoS ONE. 2022;17:e0273504. doi: 10.1371/journal.pone.0273504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kong D., Hua X., Zhou R., Cui J., Wang T., Kong F., You H., Liu X., Adu-Amankwaah J., Guo G., et al. Antimicrobial and anti-inflammatory activities of MAF-1-derived antimicrobial peptide Mt6 and its D-enantiomer D-Mt6 against Acinetobacter baumannii by targeting cell membranes and lipopolysaccharide interaction. Microbiol. Spectr. 2022;10:e0131222. doi: 10.1128/spectrum.01312-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wu X., Wang Z., Li X., Fan Y., He G., Wan Y., Yu C., Tang J., Li M., Zhang X., et al. In vitro and in vivo activities of antimicrobial peptides developed using an amino acid-based activity prediction method. Antimicrob. Agents Chemother. 2014;58:5342–5349. doi: 10.1128/AAC.02823-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wu X., Li Z., Li X., Tian Y., Fan Y., Yu C., Zhou B., Liu Y., Xiang R., Yang L. Synergistic effects of antimicrobial peptide DP7 combined with antibiotics against multidrug-resistant bacteria. Drug Des. Devel Ther. 2017;11:939–946. doi: 10.2147/DDDT.S107195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhang R., Wang Z., Tian Y., Yin Q., Cheng X., Lian M., Zhou B., Zhang X., Yang L. Efficacy of antimicrobial peptide DP7, designed by machine-learning method, against methicillin-resistant Staphylococcus aureus. Front. Microbiol. 2019;10:1175. doi: 10.3389/fmicb.2019.01175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Li J., Prats-Ejarque G., Torrent M., Andreu D., Brandenburg K., Fernández-Millán P., Boix E. In vivo evaluation of ecp peptide analogues for the treatment of Acinetobacter baumannii infection. Biomedicines. 2022;10:386. doi: 10.3390/biomedicines10020386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Taheri B., Mohammadi M., Momenzadeh N., Farshadzadeh Z., Roozbehani M., Dehghani P., Hajian S., Darvishi S., Shamseddin J. Substitution of lysine for isoleucine at the center of the nonpolar face of the antimicrobial peptide, piscidin-1, leads to an increase in the rapidity of bactericidal activity and a reduction in toxicity. Infect. Drug Resist. 2019;12:1629–1647. doi: 10.2147/IDR.S195872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ramalho S.R., de Sardi J.C.O., Júnior E.C., Marchetto R., Wender H., Vargas L.F.P., de Miranda A., Almeida C.V., de Oliveira Almeida L.H., de Oliveira C.F.R., et al. The synthetic antimicrobial peptide IKR18 displays anti-infectious properties in Galleria mellonella in vivo model. Biochim. Biophys. Acta Gen. Subj. 2022;1866:130244. doi: 10.1016/j.bbagen.2022.130244. [DOI] [PubMed] [Google Scholar]
- 185.Gong R., An Z., Zhang W., Chen F., Wang K.J. The antimicrobial peptide lj-hep2 from Lateolabrax japonicus exerting activities against multiple pathogenic bacteria and immune protection in vivo. Mar. Drugs. 2022;20:651. doi: 10.3390/md20100651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Brito J.C.M., Willliam G.L., Resende J.M., Assis D.C.S., Boff D., Cardoso V.N., Amaral F.A., Souza-Fagundes E.M., Fernandes S.O.A., Lima M.E. Pegylated LyeTx I-b peptide is effective against carbapenem-resistant Acinetobacter baumannii in an in vivo model of pneumonia and shows reduced toxicity. Int. J. Pharm. 2021;609:121156. doi: 10.1016/j.ijpharm.2021.121156. [DOI] [PubMed] [Google Scholar]
- 187.Eshtiaghi S., Nazari R., Fasihi-Ramandi M. Molecular docking, anti-biofilm & antibacterial activities and therapeutic index of mCM11 peptide on Acinetobacter baumannii strains. Curr. Microbiol. 2023;80:191. doi: 10.1007/s00284-023-03217-z. [DOI] [PubMed] [Google Scholar]
- 188.Denardi L.B., de Arruda Trindade P., Weiblen C., Ianiski L.B., Stibbe P.C., Pinto S.C., Santurio J.M. In vitro activity of the antimicrobial peptides h-Lf1-11, MSI-78, LL-37, fengycin 2B, and magainin-2 against clinically important bacteria. Braz. J. Microbiol. 2022;53:171–177. doi: 10.1007/s42770-021-00645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Howan D.H.O., Jenei S., Szolomajer J., Endre G., Kondorosi É., Tóth G.K. Enhanced antibacterial activity of substituted derivatives of NCR169C peptide. Int. J. Mol. Sci. 2023;24:2694. doi: 10.3390/ijms24032694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Jayathilaka E.H.T.T., Rajapaksha D.C., Nikapitiya C., De Zoysa M., Whang I. Antimicrobial and anti-biofilm peptide octominin for controlling multidrug-resistant Acinetobacter baumannii. Int. J. Mol. Sci. 2021;22:5353. doi: 10.3390/ijms22105353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jayathilaka E.H.T.T., Nikapitiya C., De Zoysa M., Whang I. Antimicrobial peptide octominin-encapsulated chitosan nanoparticles enhanced antifungal and antibacterial activities. Int. J. Mol. Sci. 2022;23:15882. doi: 10.3390/ijms232415882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rajapaksha D.C., Jayathilaka E.H.T.T., Edirisinghe S.L., Nikapitiya C., Lee J., Whang I., De Zoysa M. Octopromycin: Antibacterial and antibiofilm functions of a novel peptide derived from Octopus minor against multidrug-resistant Acinetobacter baumannii. Fish Shellfish Immunol. 2021;117:82–94. doi: 10.1016/j.fsi.2021.07.019. [DOI] [PubMed] [Google Scholar]
- 193.Wang B., Zhang F.W., Wang W.X., Zhao Y.Y., Sun S.Y., Yu J.H., Vitek M.P., Li G.F., Ma R., Wang S., et al. Apolipoprotein E mimetic peptide COG1410 combats pandrug-resistant Acinetobacter baumannii. Front. Microbiol. 2022;13:934765. doi: 10.3389/fmicb.2022.934765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Nagarajan D., Roy N., Kulkarni O., Nanajkar N., Datey A., Ravichandran S., Thakur C.T.S., Aprameya I.V., Sarma S.P., Chakravortty D., et al. Ω76: A designed antimicrobial peptide to combat carbapenem- and tigecycline-resistant Acinetobacter baumannii. Sci. Adv. 2019;5:eaax 1946. doi: 10.1126/sciadv.aax1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mandel S., Michaeli J., Nur N., Erbetti I., Zazoun J., Ferrari L., Felici A., Cohen-Kutner M., Bachnoff N. OMN6 a novel bioengineered peptide for the treatment of multidrug resistant Gram-negative bacteria. Sci. Rep. 2021;11:6603. doi: 10.1038/s41598-021-86155-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Michaeli J., Mandel S., Maximov S., Zazoun J., Savoia P., Kothari N., Valmont T., Ferrari L., Duncan L.R., Hawser S., et al. In vitro and in vivo antimicrobial activity of the novel peptide OMN6 against multidrug-resistant Acinetobacter baumannii. Antibiotics. 2022;11:1201. doi: 10.3390/antibiotics11091201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Choi J., Jang A., Yoon Y.K., Kim Y. development of novel peptides for the antimicrobial combination therapy against carbapenem-resistant Acinetobacter baumannii Infection. Pharmaceutics. 2021;13:1800. doi: 10.3390/pharmaceutics13111800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Chen S.P., Chen E.H., Yang S.Y., Kuo P.S., Jan H.M., Yang T.C., Hsieh M.Y., Lee K.T., Lin C.H., Chen R.P. A systematic study of the stability, safety, and efficacy of the de novo designed antimicrobial peptide PepD2 and its modified derivatives against Acinetobacter baumannii. Front. Microbiol. 2021;12:678330. doi: 10.3389/fmicb.2021.678330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Moreno-Morales J., Guardiola S., Ballesté-Delpierre C., Giralt E., Vila J. A new synthetic protegrin as a promising peptide with antibacterial activity against MDR Gram-negative pathogens. J. Antimicrob. Chemother. 2022;77:3077–3085. doi: 10.1093/jac/dkac284. [DOI] [PubMed] [Google Scholar]
- 200.Rose M., Lapuebla A., Landman D., Quale J. In vitro and in vivo activity of a novel antisense peptide nucleic acid compound against multidrug-resistant Acinetobacter baumannii. Microb. Drug Resist. 2019;25:961–965. doi: 10.1089/mdr.2018.0179. [DOI] [PubMed] [Google Scholar]
- 201.Krishnan M., Choi J., Jang A., Yoon Y.K., Kim Y. Antiseptic 9-Meric peptide with potency against carbapenem-resistant Acinetobacter baumannii infection. Int. J. Mol. Sci. 2021;22:12520. doi: 10.3390/ijms222212520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Mohamed M.F., Hamed M.I., Panitch A., Seleem M.N. Targeting methicillin-resistant Staphylococcus aureus with short salt-resistant synthetic peptides. Antimicrob. Agents Chemother. 2014;58:4113–4122. doi: 10.1128/AAC.02578-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mohamed M.F., Brezden A., Mohammad H., Chmielewski J., Seleem M.N. A short D-enantiomeric antimicrobial peptide with potent immunomodulatory and antibiofilm activity against multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Sci. Rep. 2017;7:6953. doi: 10.1038/s41598-017-07440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sharma D., Choudhary M., Vashistt J., Shrivastava R., Bisht G.S. Cationic antimicrobial peptide and its poly-N-substituted glycine congener: Antibacterial and antibiofilm potential against A. baumannii. Biochem. Biophys. Res. Commun. 2019;518:472–478. doi: 10.1016/j.bbrc.2019.08.062. [DOI] [PubMed] [Google Scholar]
- 205.Ali M., van Gent M.E., de Waal A.M., van Doodewaerd B.R., Bos E., Koning R.I., Cordfunke R.A., Drijfhout J.W., Nibbering P.H. Physical and functional characterization of plga nanoparticles containing the antimicrobial peptide SAAP-148. Int. J. Mol. Sci. 2023;24:2867. doi: 10.3390/ijms24032867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Farzi N., Oloomi M., Bahramali G., Siadat S.D., Bouzari S. Antibacterial properties and efficacy of LL-37 fragment GF-17D3 and scolopendin A2 peptides against resistant clinical strains of Staphylococcus aureus, Pseudomonas aeruginosa, and Acinetobacter baumannii in vitro and in vivo model studies. Probiotics Antimicrob. Proteins. 2023. ahead-of-print . [DOI] [PubMed]
- 207.Mourtada R., Herce H.D., Yin D.J., Moroco J.A., Wales T.E., Engen J.R., Walensky L.D. Design of stapled antimicrobial peptides that are stable, nontoxic and kill antibiotic-resistant bacteria in mice. Nat. Biotechnol. 2019;37:1186–1197. doi: 10.1038/s41587-019-0222-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Heulot M., Jacquier N., Aeby S., Le Roy D., Roger T., Trofimenko E., Barras D., Greub G., Widmann C. The anticancer peptide TAT-RasGAP317-326 exerts broad antimicrobial activity. Front. Microbiol. 2017;8:994. doi: 10.3389/fmicb.2017.00994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Vizzarro G., Jacquier N. In vitro synergistic action of TAT-RasGAP317-326 peptide with antibiotics against Gram-negative pathogens. J. Glob. Antimicrob. Resist. 2022;31:295–303. doi: 10.1016/j.jgar.2022.10.003. [DOI] [PubMed] [Google Scholar]
- 210.Heinonen T., Hargraves S., Georgieva M., Widmann C., Jacquier N. The antimicrobial peptide TAT-RasGAP317-326 inhibits the formation and expansion of bacterial biofilms in vitro. J. Glob. Antimicrob. Resist. 2021;25:227–231. doi: 10.1016/j.jgar.2021.03.022. [DOI] [PubMed] [Google Scholar]
- 211.Hazam P.K., Cheng C.C., Hsieh C.Y., Lin W.C., Hsu P.H., Chen T.L., Lee Y.T., Chen J.Y. Development of bactericidal peptides against multidrug-resistant Acinetobacter baumannii with enhanced stability and low toxicity. Int. J. Mol. Sci. 2022;23:2191. doi: 10.3390/ijms23042191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Dalla Torre C., Sannio F., Battistella M., Docquier J.D., De Zotti M. Peptaibol analogs show potent antibacterial activity against multidrug resistant opportunistic pathogens. Int. J. Mol. Sci. 2023;24:7997. doi: 10.3390/ijms24097997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zeng P., Yi L., Xu J., Gao W., Xu C., Chen S., Chan K.F., Wong K.Y. Investigation of antibiofilm activity, antibacterial activity, and mechanistic studies of an amphiphilic peptide against Acinetobacter baumannii. Biochim. Biophys. Acta Biomembr. 2021;1863:183600. doi: 10.1016/j.bbamem.2021.183600. [DOI] [PubMed] [Google Scholar]
- 214.Wang X., Duan H., Li M., Xu W., Wei L. Characterization and mechanism of action of amphibian-derived wound-healing-promoting peptides. Front. Cell Dev. Biol. 2023;11:1219427. doi: 10.3389/fcell.2023.1219427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kościuczuk E.M., Lisowski P., Jarczak J., Strzałkowska N., Jóźwik A., Horbańczuk J., Krzyżewski J., Zwierzchowski L., Bagnicka E. Cathelicidins: Family of antimicrobial peptides. A review. Mol. Biol. Rep. 2012;39:10957–10970. doi: 10.1007/s11033-012-1997-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Dowzicky M.J., Chmelařová E. Antimicrobial susceptibility of gram-negative and gram-positive bacteria collected from eastern Europe: Results from the tigecycline evaluation and surveillance trial (T.E.S.T.), 2011–2016. J. Glob. Antimicrob. Resist. 2019;17:44–52. doi: 10.1016/j.jgar.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 217.Björstad A., Askarieh G., Brown K.L., Christenson K., Forsman H., Onnheim K., Li H.N., Teneberg S., Maier O., Hoekstra D., et al. The host defense peptide LL-37 selectively permeabilizes apoptotic leukocytes. Antimicrob. Agents Chemother. 2009;53:1027–1038. doi: 10.1128/AAC.01310-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.De Y., Chen Q., Schmidt A.P., Anderson G.M., Wang J.M., Wooters J., Oppenheim J.J., Chertov O. LL-37 the neutrophil granule-and epithelial cell-derived cathelicidin utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils monocytes and T cells. J. Exp. Med. 2000;192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Nijnik A., Hancock R.E.W. Host defense peptides: Antimicrobial and immunomodulatory activity and potential applications for tackling antibiotic-resistant infections. Emerg. Health Threats J. 2009;2:e1. doi: 10.3134/ehtj.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Neshani A., Zare H., Eidgahi M.R.A., Kakhki R.K., Safdari H., Khaledi A., Ghazvini K. LL-37: A review of antimicrobial profile against sensitive and antibiotic-resistant human bacterial pathogens. Gene Rep. 2019;17:100519. doi: 10.1016/j.genrep.2019.100519. [DOI] [Google Scholar]
- 221.Esfandiyari R., Halabian R., Behzadi E., Sedighian H., Jafari R., Fooladi A.A.I. Performance evaluation of antimicrobial peptide ll-37 and hepcidin and β-defensin-2 secreted by mesenchymal stem cells. Heliyon. 2019;5:e02652. doi: 10.1016/j.heliyon.2019.e02652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Haisma E.M., de Breij A., Chan H., van Dissel J.T., Drijfhout J.W., Hiemstra P.S., El Ghalbzouri A., Nibbering P.H. LL-37-derived peptides eradicate multidrug-resistant Staphylococcus aureus from thermally wounded human skin equivalents. Antimicrob. Agents Chemother. 2014;58:4411–4419. doi: 10.1128/AAC.02554-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Deslouches B., Steckbeck J.D., Craigo J.K., Doi Y., Mietzner T.A., Montelaro R.C. Rational design of engineered cationic antimicrobial peptides consisting exclusively of arginine and tryptophan, and their activity against multidrug-resistant pathogens. Antimicrob. Agents Chemother. 2013;57:2511–2521. doi: 10.1128/AAC.02218-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Liu C., Shan B., Qi J., Ma Y. Systemic responses of multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii following exposure to the antimicrobial peptide cathelicidin-BF imply multiple intracellular targets. Front. Cell Infect. Microbiol. 2017;7:466. doi: 10.3389/fcimb.2017.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhao H., Gan T.X., Liu X.D., Jin Y., Lee W.H., Shen J.H., Zhang Y. Identification and characterization of novel reptile cathelicidins from elapid snakes. Peptides. 2008;29:1685–1691. doi: 10.1016/j.peptides.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 226.Du H., Samuel R.L., Massiah M.A., Gillmor S.D. The structure and behavior of the NA-CATH antimicrobial peptide with liposomes. Biochim. Biophy Acta. 2015;1848:2394–2405. doi: 10.1016/j.bbamem.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 227.Bishop B.M., Juba M.L., Devine M.C., Barksdale S.M., Rodriguez C.A., Chung M.C., Russo P.S., Vliet K.A., Schnur J.M., van Hoek M.L. Bioprospecting the American alligator (Alligator mississippiensis) host defense peptidome. PLoS ONE. 2015;10:e0117394. doi: 10.1371/journal.pone.0117394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Daly K.A., Digby M.R., Lefévre C., Nicholas K.R., Deane E.M., Williamson P. Identification characterization and expression of cathelicidin in the pouch young of Tammar wallaby (Macropus eugenii) Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008;149:524–533. doi: 10.1016/j.cbpb.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 229.Selsted M.E., Novotny M.J., Morris W.L., Tang Y.Q., Smith W., Cullor J.S. Indolicidin is a novel bactericidal tridecapeptide amide from neutrophils. J. Biol. Chem. 1992;267:4292–4295. doi: 10.1016/S0021-9258(18)42830-X. [DOI] [PubMed] [Google Scholar]
- 230.Végh A.G., Nagy K., Bálint Z., Kerényi A., Rákhely G., Váró G., Szegletes Z. Effect of antimicrobial peptide-amide: Indolicidin on biological membranes. J. Biomed. Biotechnol. 2011;2011:670589. doi: 10.1155/2011/670589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Hsu C.H., Chen C., Jou M.L., Lee A.Y.L., Lin Y.C., Yu Y.P., Huang W.T., Wu S.H. Structural and DNA-binding studies on the bovine antimicrobial peptide indolicidin: Evidence for multiple conformations involved in binding to membranes and DNA. Nucleic Acids Res. 2005;33:4053–4064. doi: 10.1093/nar/gki725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Marchand C., Krajewski K., Lee H.F., Antony S., Johnson A.A., Amin R., Kvaratskhelia M., Pommier Y. Covalent binding of the natural antimicrobial peptide indolicidin to DNA abasic sites. Nucleic Acids Res. 2006;34:5157–5165. doi: 10.1093/nar/gkl667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Knutelski S., Awad M., Łukasz N., Bukowski M., Śmiałek J., Suder P., Dubin G., Mak P. Isolation, identification, and bioinformatic analysis of antibacterial proteins and peptides from immunized hemolymph of red palm weevil Rhynchophorus ferrugineus. Biomolecules. 2021;11:83. doi: 10.3390/biom11010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Schneider J.J., Unholzer A., Schaller M., Schäfer-Korting M., Korting H.C. Human defensins. J. Mol. Med. 2005;83:587–595. doi: 10.1007/s00109-005-0657-1. [DOI] [PubMed] [Google Scholar]
- 235.Lehrer R.I., Lu W. α-Defensins in human innate immunity. Immunol. Rev. 2012;45:84–112. doi: 10.1111/j.1600-065X.2011.01082.x. [DOI] [PubMed] [Google Scholar]
- 236.Wanniarachchi Y.A., Kaczmarek P., Wan A., Nolan E.M. Human defensin 5 disulfide array mutants: Disulfide bond deletion attenuates antibacterial activity against Staphylococcus aureus. Biochemistry. 2011;37:8005–8017. doi: 10.1021/bi201043j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Marcelino-Pérez G., Ruiz-Medrano R., Gallardo-Hernández S., Xoconostle-Cázares B. Adsorption of recombinant human β-defensin 2 and two mutants on mesoporous silica nanoparticles and its effect against Clavibacter michiganensis subsp. Michiganensis. Nanomaterials. 2021;11:2144. doi: 10.3390/nano11082144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Schibli D.J., Hunter H.N., Aseyev V., Starner T.D., Wiencek J.M., McCray P.B., Tack B.F., Vogel H.J. The solution structures of the human beta-defensins lead to a better understanding of the potent bactericidal activity of HBD3 against Staphylococcus aureus. J. Biol. Chem. 2002;277:8279–8289. doi: 10.1074/jbc.M108830200. [DOI] [PubMed] [Google Scholar]
- 239.Hirsch T., Spielmann M., Zuhaili B., Fossum M., Metzig M., Koehler T., Steinau H.-U., Yao F., Onderdonk A.B., Steinstraesser L., et al. Human beta defensin-3 promotes wound healing in infected diabetic wounds. J. Gene Med. 2009;11:220–228. doi: 10.1002/jgm.1287. [DOI] [PubMed] [Google Scholar]
- 240.Zerweck J., Strandberg E., Kukharenko O., Reichert J., Bürck J., Wadhwani P., Ulrich A.S. Molecular mechanism of synergy between the antimicrobial peptides PGLa and magainin 2. Sci. Rep. 2017;7:13153. doi: 10.1038/s41598-017-12599-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Tamba Y., Yamazaki M. Magainin 2-induced pore formation in the lipid membranes depends on its concentration in the membrane interface. J. Phys. Chem. B. 2009;113:486–4852. doi: 10.1021/jp8109622. [DOI] [PubMed] [Google Scholar]
- 242.Maloy W.L., Kari U.P. Structure-activity studies on magainins and other host defense peptides. Biopolymers. 1995;37:105–122. doi: 10.1002/bip.360370206. [DOI] [PubMed] [Google Scholar]
- 243.Gottler L.M., Ramamoorthy A. Structure membrane orientation mechanism and function of pexiganan-a highly potent antimicrobial peptide designed from magainin. Biochim. Biophys. Acta. 2009;1788:1680–1686. doi: 10.1016/j.bbamem.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ramamoorthy A., Thennarasu S., Lee D.K., Tan A., Maloy L. Solid-state NMR investigation of the membrane-disrupting mechanism of antimicrobial peptides MSI-78 and MSI-594 derived from magainin 2 and melittin. Biophys. J. 2006;91:206–216. doi: 10.1529/biophysj.105.073890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Fuchs P.C., Barry A.L., Brown S.D. In vitro antimicrobial activity of MSI-78 a magainin analog. Antimicrob. Agents Chemother. 1998;42:1213–1216. doi: 10.1128/AAC.42.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Bevier C.R., Sonnevend A., Kolodziejek J., Nowotny N., Nielsen P.F., Conlon J.M. Purification and characterization of antimicrobial peptides from the skin secretions of the mink frog (Rana septentrionalis) Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2004;139:31–38. doi: 10.1016/j.cca.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 247.Conlon J.M., Abraham B., Sonnevend A., Jouenne T., Cosette P., Leprince J., Vaudry H., Bevier C.R. Purification and characterization of antimicrobial peptides from the skin secretions of the carpenter frog Rana virsgatipes (Ranidae, Aquarana) Reg. Peptides. 2005;13:38–45. doi: 10.1016/j.regpep.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 248.Savelyeva A., Ghavami S., Davoodpour P., Asoodeh A., Los M.J. An overview of Brevinin superfamily: Structure function and clinical perspectives. Adv. Exp. Med. Biol. 2014;818:197–212. doi: 10.1007/978-1-4471-6458-6_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Popovic S., Urbán E., Lukic M., Conlon J.M. Peptides with antimicrobial and anti-inflammatory activities that have therapeutic potential for treatment of acne vulgaris. Peptides. 2012;34:275–282. doi: 10.1016/j.peptides.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 250.Popovic S., Djurdjevic P., Zaric M., Mijailovic Z., Avramovic D., Baskic D. Effects of host defense peptides B2RP Brevinin-2GU D-Lys-Temporin Lys-XT-7 and DLys-Ascaphin-8 on peripheral blood mononuclear cells: Preliminary study. Period. Biol. 2017;119:113–118. doi: 10.18054/pb.v119i2.4781. [DOI] [Google Scholar]
- 251.Conlon J.M., Demandt A., Nielsen P.F., Leprince J., Vaudry H., Woodhams D.C. The alyteserins: Two families of antimicrobial peptides from the skin secretions of the midwife toad Alytes obstetricans (Alytidae) Peptides. 2009;30:1069–1073. doi: 10.1016/j.peptides.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 252.Wang G., Li X., Wang Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2015;44:D1087–D1093. doi: 10.1093/nar/gkv1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Conlon J.M., Prajeep M., Mechkarska M., Coquet L., Leprince J., Jouenne T., Vaudry H., King J.D. Characterization of the host-defense peptides from skin secretions of Merlin’s clawed frog Pseudhymenochirus merlini: Insights into phylogenetic relationships among the Pipidae. Comp. Biochem. Physiol. Part. D Genom. Proteonomics. 2013;8:352–357. doi: 10.1016/j.cbd.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 254.Ali M.F., Soto A., Knoop F.C., Conlon J.M. Antimicrobial peptides isolated from skin secretions of the diploid frog Xenopus tropicalis (Pipidae) Biochim. Biophys. Acta. 2001;1550:81–89. doi: 10.1016/S0167-4838(01)00272-2. [DOI] [PubMed] [Google Scholar]
- 255.Park C.B., Kim M.S., Kim S.C. A novel antimicrobial peptide from Bufo bufo gargarizans. Biochem. Biophy Res. Commun. 1996;218:408–413. doi: 10.1006/bbrc.1996.0071. [DOI] [PubMed] [Google Scholar]
- 256.Apponyi M.A., Pukala T.L., Brinkworth C.S., Maselli V.M., Bowie J.H., Tyler M.J., Booker G.W., Wallace J.C., Carver J.A., Separovic F. Host-defence peptides of Australian anurans: Structure, mechanism of action and evolutionary significance. Peptides. 2004;25:1035–1054. doi: 10.1016/j.peptides.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 257.Castro M.S., Ferreira T.C.G., Cilli E.M., Crusca JR E., Mendes-Giannini M.J.S., Sebben A., Ricart C.A.O., Sousa M.V., Fontes W. Hylin a1, the first cytolytic peptide isolated from the arboreal South American frog Hypsiboas albopunctatus (“spotted treefrog”) Peptides. 2009;30:291–296. doi: 10.1016/j.peptides.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 258.Subramanian S., Ross N.W., Mackinnon S.L. Comparison of the biochemical composition of normal epidermal mucus and extruded slime of hagfish (Myxine glutinosa L.) Fish. Shellfish. Immunol. 2008;25:625–632. doi: 10.1016/j.fsi.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 259.Ellis A.E. Innate host defense mechanisms of fish against viruses and bacteria. Dev. Comp. Immunol. 2001;25:827–839. doi: 10.1016/S0145-305X(01)00038-6. [DOI] [PubMed] [Google Scholar]
- 260.Silphaduang U., Noga E.J. Peptide antibiotics in mast cells of fish. Nature. 2001;414:268–269. doi: 10.1038/35104690. [DOI] [PubMed] [Google Scholar]
- 261.Noga E.J., Silphaduang U., Park N.G., Seo J.K., Stephenson J., Kozlowicz S. Piscidin 4, a novel member of the piscidin family of antimicrobial peptides. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2009;152:299–305. doi: 10.1016/j.cbpb.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 262.Peng K.C., Lee S.H., Hour A.L., Pan C.Y., Lee L.H., Chen J.Y. Five diferent piscidins from Nile tilapia, Oreochromis niloticus: Analysis of their expressions and biological functions. PLoS ONE. 2012;7:e50263. doi: 10.1371/journal.pone.0050263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Cole A.M., Weis P., Diamond G. Isolation and characterization of pleurocidin, an antimicrobial peptide in the skin secretions of winter flounder. J. Biol. Chem. 1997;272:12008–12013. doi: 10.1074/jbc.272.18.12008. [DOI] [PubMed] [Google Scholar]
- 264.Tao R., Tong Z., Lin Y., Xue Y., Wang W., Kuang R., Wang P., Tian Y., Ni L. Antimicrobial and antibiofilm activity of pleurocidin against cariogenic microorganisms. Peptides. 2011;32:1748–1754. doi: 10.1016/j.peptides.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 265.Lee J., Lee D.G. Structure–antimicrobial activity relationship between pleurocidin and its enantiomer. Exp. Mol. Med. 2008;40:370–376. doi: 10.3858/emm.2008.40.4.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Patrzykat A., Friedrich C.L., Zhang L., Mendoza V., Hancock R.E.W. Sublethal concentrations of pleurocidin-derived antimicrobial peptides inhibit macromolecular synthesis in Escherichia coli. Antimicrob. Agents Chemother. 2002;46:605–614. doi: 10.1128/AAC.46.3.605-614.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Lee J., Lee D.G. Influence of the hydrophobic amino acids in the N- and Cterminal regions of pleurocidin on antifungal activity. J. Microbiol. Biotechnol. 2010;20:1192–1195. doi: 10.4014/jmb.1004.04041. [DOI] [PubMed] [Google Scholar]
- 268.Hazam P.K., Cheng C.C., Lin W.C., Hsieh C.Y., Chen J.Y. Strategic modification of low-active natural antimicrobial peptides confers ability to neutralize pathogens in vitro and in vivo. Eur. J. Med. Chem. 2022:unpublished. doi: 10.1016/j.ejmech.2023.115131. [DOI] [PubMed] [Google Scholar]
- 269.Hazam P.K., Chen J.Y. Therapeutic utility of the antimicrobial peptide Tilapia Piscidin 4 (TP4) Aquac. Rep. 2020;17:100409. doi: 10.1016/j.aqrep.2020.100409. [DOI] [Google Scholar]
- 270.Krause A., Neitz S., Mägert H.J., Schulz A., Forssmann W.G., Schulz-Knappe P., Adermann K. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000;480:147–150. doi: 10.1016/S0014-5793(00)01920-7. [DOI] [PubMed] [Google Scholar]
- 271.Park C.H., Valore E.V., Waring A.J., Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J. Biol. Chem. 2001;276:7806–7810. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 272.Barton J.C., Acton R.T. Hepcidin, iron, and bacterial infection. Vitam. Horm. 2019;110:223–242. doi: 10.1016/bs.vh.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 273.Shike H., Lauth X., Westerman M.E., Ostland V.E., Carlberg J.M., Van Olst J.C., Shimizu C., Bulet P., Burns J.C. Bass hepcidin is a novel antimicrobial peptide induced by bacterial challenge. Eur. J. Biochem. 2002;269:2232–2237. doi: 10.1046/j.1432-1033.2002.02881.x. [DOI] [PubMed] [Google Scholar]
- 274.Padhi A., Verghese B. Evidence for positive Darwinian selection on the hepcidin gene of Perciform and Pleuronectiform fishes. Mol. Divers. 2007;11:119–130. doi: 10.1007/s11030-007-9066-4. [DOI] [PubMed] [Google Scholar]
- 275.Hilton K.B., Lambert L.A. Molecular evolution and characterization of hepcidin gene products in vertebrates. Gene. 2008;415:40–48. doi: 10.1016/j.gene.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 276.Wang K.J., Cai J.J., Cai L., Qu H.D., Yang M., Zhang M. Cloning and expression of a hepcidin gene from a marine fish (Pseudosciaena crocea) and the antimicrobial activity of its synthetic peptide. Peptides. 2009;30:638–646. doi: 10.1016/j.peptides.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 277.Neves J.V., Caldas C., Vieira I., Ramos M.F., Rodrigues P.N. Multiple hepcidins in a teleost fish, Dicentrarchus labrax: Different hepcidins for differen roles. J. Immunol. 2015;195:2696–2709. doi: 10.4049/jimmunol.1501153. [DOI] [PubMed] [Google Scholar]
- 278.Fennell J.F., Shipman W.H., Cole L.J. Antibacterial action of melittin, a polypeptide from bee venom. Proceed Soc. Exp. Biol. Med. 1968;127:707–710. doi: 10.3181/00379727-127-32779. [DOI] [PubMed] [Google Scholar]
- 279.Pereira A.V., de Barros G., Pinto E.G., Tempone A.G., Orsi R.O., Dos Santos L.D., Calvi S., Ferreira R.S., Jr., Pimenta D.C., Barraviera B. Melittin induces in vitro death of Leishmania (Leishmania) infantum by triggering the cellular innate immune response. J. Venom. Anim. Toxins Trop. Dis. 2016;22:1. doi: 10.1186/s40409-016-0055-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Park J., Kwon O., An H.J., Park K.K. Antifungal effects of bee venom components on Trichophyton rubrum: A novel approach of bee venom study for possible emerging antifungal agent. Ann. Dermatol. 2018;30:202–210. doi: 10.5021/ad.2018.30.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Kim Y.W., Chaturvedi P.K., Chun S.N., Lee Y.G., Ahn W.S. Honeybee venom possesses anticancer and antiviral effects by differential inhibition of HPV E6 and E7 expression on cervical cancer cell line. Oncol. Rep. 2015;33:1675–1682. doi: 10.3892/or.2015.3760. [DOI] [PubMed] [Google Scholar]
- 282.Van den Bogaart G., Guzman J.V., Mika J.T., Poolman B. On the mechanism of pore formation by melittin. J. Biol. Chem. 2008;283:33854–33857. doi: 10.1074/jbc.M805171200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Rangel K., Lechuga G.C., Almeida Souza A.L., Carvalho J.P.R.S., Villas-Bôas M.H.S., De Simone S.G. Pan-drug resistant Acinetobacter baumannii but not other strains are resistant to the bee venom peptide melittin. Antibiotics. 2020;149:178. doi: 10.3390/antibiotics9040178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Casteels P., Ampe C., Jacobs F., Vaeck M., Tempst P. Apidaecins: Antibacterial peptides from honeybees. EMBO J. 1989;8:2387–2391. doi: 10.1002/j.1460-2075.1989.tb08368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Borovsky D., Rougé P., Shatters R.G., Jr. Bactericidal properties of proline-rich Aedes aegypti trypsin modulating oostatic factor (AeaTMOF) Life. 2022;13:19. doi: 10.3390/life13010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Wu Q., Patočka J., Kuča K. Insect Antimicrobial peptides, a mini review. Toxins. 2018;10:461. doi: 10.3390/toxins10110461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Steiner H., Hultmark D., Engström Å., Bennich H., Boman H.G. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature. 1981;292:246. doi: 10.1038/292246a0. [DOI] [PubMed] [Google Scholar]
- 288.Hui L., Leung K., Chen H.M. The combined effects of antibacterial peptide cecropin a and anticancer agents on leukemia cells. Anticancer Res. 2002;22:2811–2816. [PubMed] [Google Scholar]
- 289.Lim J., Hong J., Jung Y., Ha J., Kim H., Myung H., Song M.J. Bactericidal effect of Cecropin A fused endolysin on drug-resistant gram-negative pathogens. Microbiol. Biotechnol. 2022;32:816–823. doi: 10.4014/jmb.2205.05009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Moreno M., Giralt E. Three valuable peptides from bee and wasp venoms for therapeutic and biotechnological use: Melittin apamin and mastoparan. Toxins. 2015;7:1126–1150. doi: 10.3390/toxins7041126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Chen X., Zhang L., Wu Y., Wang L., Ma C., Xi X., Bininda-Emonds O.R.P., Shaw C., Chen T., Zhou M. Evaluation of the bioactivity of a mastoparan peptide from wasp venom and of its analogues designed through targeted engineering. Int. J. Biol. Sci. 2018;14:599–607. doi: 10.7150/ijbs.23419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Burian M., Schittek B. The secrets of dermcidin action. Int. J. Med. Microbiol. 2015;305:283–286. doi: 10.1016/j.ijmm.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 293.Zeth K., Méndez-Vilas A. Structure and Mechanism of Human Antimicrobial Peptide Dermcidin and Its Antimicrobial Potential; Formatex Research Center: Badajoz, Spain, 2013; Volume 2. pp. :1333–1342. [Google Scholar]
- 294.Liu C., Qi J., Shan B., Ma Y. Tachyplesin causes membrane instability that kills multidrug-resistant bacteria by inhibiting the 3-ketoacyl carrier protein reductase FabG. Front. Microbiol. 2018;9:825. doi: 10.3389/fmicb.2018.00825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Wang L., Wang Y.J., Liu Y.Y., Li H., Guo L.X., Liu Z.H., Shi X.L., Hu M. In vitro potential of Lycosin-I as an alternative antimicrobial drug for treatment of multidrug-resistant Acinetobacter baumannii infections. Antimicrob. Agents Chemother. 2014;58:6999–7002. doi: 10.1128/AAC.03279-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Cao L., Dai C., Li Z., Fan Z., Song Y., Wu Y., Cao Z., Li W. Antibacterial activity and mechanism of a scorpion venom peptide derivative in vitro and in vivo. PLoS ONE. 2012;7:e40135. doi: 10.1371/journal.pone.0040135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Oyama L.B., Girdwood S.E., Cookson A.R., Fernandez-Fuentes N., Privé F., Vallin H.E., Wilkinson T.J., Golyshin P.N., Golyshina O.V., Mikut R., et al. The rumen microbiome: An underexplored resource for novel antimicrobial discovery. NPJ Biofilms Microbiomes. 2017;3:33. doi: 10.1038/s41522-017-0042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Shin A., Lee E., Jeon D., Park Y.G., Bang J.K., Park Y.S., Shin S.Y., Kim Y. Peptoid-substituted hybrid antimicrobial peptide derived from papiliocin and magainin 2 with enhanced bacterial selectivity and anti-inflammatory activity. Biochemistry. 2015;54:3921–3931. doi: 10.1021/acs.biochem.5b00392. [DOI] [PubMed] [Google Scholar]
- 299.Klubthawee N., Adisakwattana P., Hanpithakpong W., Somsri S., Aunpad R. A novel, rationally designed, hybrid antimicrobial peptide, inspired by cathelicidin and aurein, exhibits membrane-active mechanisms against Pseudomonas aeruginosa. Sci. Rep. 2021;10:9117. doi: 10.1038/s41598-020-65688-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Ferre R., Melo M.N., Correia A.D., Feliu L., Bardaji E., Planas M., Castanho M. Synergistic effects of the membrane actions of cecropin-melittin antimicrobial hybrid peptide BP100. Biophys. J. 2009;96:1815–1827. doi: 10.1016/j.bpj.2008.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Oddo A., Thomsen T.T., Kjelstrup S., Gorey C., Franzyk H., Frimodt-Møller N., Løbner-Olesen A., Hansen P.R. An all-D amphipathic undecapeptide shows promising activity against colistin-resistant strains of Acinetobacter baumannii and a dual mode of action. Antimicrob. Agents Chemother. 2016;60:592–599. doi: 10.1128/AAC.01966-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Ageitos J.M., Sánchez-Pérez A., Calo-Mata P., Villa T.G. Antimicrobial peptides (AMPs): Ancient compounds that represent novel weapons in the fight against bacteria. Biochem. Pharmacol. 2017;133:117. doi: 10.1016/j.bcp.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 303.Moravej H., Moravej Z., Yazdanparast M., Heiat M., Mirhosseini A., Moghaddam M.M., Mirnejad R. Antimicrobial peptides: Features action and their resistance mechanisms in bacteria. Microb. Drug Resist. 2018;24:747. doi: 10.1089/mdr.2017.0392. [DOI] [PubMed] [Google Scholar]
- 304.Joo H.S., Fu C.I., Otto M. Bacterial strategies of resistance to antimicrobial peptides. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016;371:20150292. doi: 10.1098/rstb.2015.0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Omardien S., Brul S., Zaat S.A.J. Antimicrobial activity of cationic antimicrobial peptides against gram-positive: Current progress made in understanding the mode of action and the response of bacteria. Front. Cell Dev. Biol. 2016;4:111. doi: 10.3389/fcell.2016.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Morita Y., Tomida J., Kawamura Y. MexXY multidrug efflux system of Pseudomonas aeruginosa. Front. Microbiol. 2012;3:408. doi: 10.3389/fmicb.2012.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Bechinger B., Gorr S.U. Antimicrobial peptides: Mechanisms of action and resistance. J. Dent. Res. 2017;96:254–260. doi: 10.1177/0022034516679973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Jeannot K., Bolard A., Plésiat P. Resistance to polymyxins in gram-negative organisms. Int. J. Antimicrob. Agents. 2017;49:526–535. doi: 10.1016/j.ijantimicag.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 309.Liu Y.Y., Wang Y., Walsh T.R., Yi L.X., Zhang R., Spencer J., Doi Y., Tian G., Dong B., Huang X., et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016;16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 310.Paterson D.L., Harris P.N.A. Colistin resistance: A major breach in our last line of defense. Lancet Infect. Dis. 2016;16:132–133. doi: 10.1016/S1473-3099(15)00463-6. [DOI] [PubMed] [Google Scholar]
- 311.Macnair C.R., Stokes J.M., Carfrae L.A., Fiebig-Comyn A.A., Coombes B.K., Mulvey M.R., Brown E.D. Overcoming mcr-1 mediated colistin resistance with colistin in combination with other antibiotics. Nat. Commun. 2018;9:458. doi: 10.1038/s41467-018-02875-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Gheorghe I., Saviuc C., Ciubuca B., Lazar V., Chifiriuc M.C. Chapter 8—Nano drug delivery. In: Grumezescu A.M., editor. Nanomaterials for Drug Delivery and Therapy. William Andrew Publishing; Norwich, NY, USA: 2019. pp. 225–244. [Google Scholar]
- 313.Sun B., Wibowo D., Middelberg A.P.J., Zhao C.X. Cost-effective downstream processing of recombinantly produced pexiganan peptide and its antimicrobial activity. AMB Express. 2018;8:6. doi: 10.1186/s13568-018-0541-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Falciani C., Lozzi L., Pollini S., Luca V., Carnicelli V., Brunetti J., Lelli B., Bindi S., Scali S., Di Giulio A., et al. Isomerization of an antimicrobial peptide broadens antimicrobial spectrum to gram-positive bacterial pathogens. PLoS ONE. 2012;7:e46259. doi: 10.1371/journal.pone.0046259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Hirt H., Gorr S.U. Antimicrobial peptide GL13K is effective in reducing biofilms of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2013;57:4903–4910. doi: 10.1128/AAC.00311-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.de la Fuente-Núñez C., Cardoso M.H., de Souza Cândido E., Franco O.L., Hancock R.E. Synthetic antibiofilm peptides. Biochim. Biophys. Acta. 2016;1858:1061–1069. doi: 10.1016/j.bbamem.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Kraus D., Peschel A. Molecular mechanisms of bacterial resistance to antimicrobial peptides. Curr. Top. Microbiol. Immunol. 2006;306:231–250. doi: 10.1007/3-540-29916-5_9. [DOI] [PubMed] [Google Scholar]
- 318.Verma-Gaur J., Qu Y., Harrison P.F., Lo T.L., Quenault T., Dagley M.J., Bellousoff M., Powell D.R., Beilharz T.H., Traven A. Integration of posttranscriptional gene networks into metabolic adaptation and biofilm maturation in Candida albicans. PLoS Genet. 2015;11:e1005590. doi: 10.1371/journal.pgen.1005590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Rhee S.H. Lipopolysaccharide: Basic biochemistry, intracellular signaling, and physiological impacts in the gut. Intest. Res. 2014;12:90–95. doi: 10.5217/ir.2014.12.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Carvalho F., Atilano M.L., Pombinho R., Covas G., Gallo R.L., Filipe S.R., Sousa S., Cabanes D. L-Rhamnosylation of Listeria monocytogenes wall teichoic acids promotes resistance to antimicrobial peptides by delaying interaction with the membrane. PLoS Pathog. 2015;11:e1004919. doi: 10.1371/journal.ppat.1004919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Pelletier M.R., Casella L.G., Jones J.W., Adams M.D., Zurawski D.V., Hazlett K.R., Doi Y., Ernst R.K. Unique structural modifications are present in the lipopolysaccharide from colistin-resistant strains of Acinetobacter baumannii. Antimicrob. Agents Chemother. 2013;57:4831–4840. doi: 10.1128/AAC.00865-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Alvarez-Ortega C., Olivares J., Martínez J.L. RND multidrug efflux pumps: What are they good for? Front. Microbiol. 2013;4:7. doi: 10.3389/fmicb.2013.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Delmar J.A., Su C.C., Yu E.W. Bacterial multidrug efflux transporters. Annu. Rev. Biophys. 2014;43:93–117. doi: 10.1146/annurev-biophys-051013-022855. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.