ABSTRACT
Complete ammonia oxidizers (comammox Nitrospira) are ubiquitous in coastal wetland sediments and play an important role in nitrification. Our study examined the impact of habitat modifications on comammox Nitrospira communities in coastal wetland sediments across tropical and subtropical regions of southeastern China. Samples were collected from 21 coastal wetlands in five provinces where native mudflats were invaded by Spartina alterniflora and subsequently converted to aquaculture ponds. The results showed that comammox Nitrospira abundances were mainly influenced by sediment grain size rather than by habitat modifications. Compared to S. alterniflora marshes and native mudflats, aquaculture pond sediments had lower comammox Nitrospira diversity, lower clade A.1 abundance, and higher clade A.2 abundance. Sulfate concentration was the most important factor controlling the diversity of comammox Nitrospira. The response of comammox Nitrospira community to habitat change varied significantly by location, and environmental variables accounted for only 11.2% of the variations in community structure across all sites. In all three habitat types, dispersal limitation largely controlled the comammox Nitrospira community assembly process, indicating the stochastic nature of these sediment communities in coastal wetlands.
IMPORTANCE
Comammox Nitrospira have recently gained attention for their potential role in nitrification and nitrous oxide (N2O) emissions in soil and sediment. However, their distribution and assembly in impacted coastal wetland are poorly understood, particularly on a large spatial scale. Our study provides novel evidence that the effects of habitat modification on comammox Nitrospira communities are dependent on the location of the wetland. We also found that the assembly of comammox Nitrospira communities in coastal wetlands was mainly governed by stochastic processes. Nevertheless, sediment grain size and sulfate concentration were identified as key variables affecting comammox Nitrospira abundance and diversity in coastal sediments. These findings are significant as they advance our understanding of the environmental adaptation of comammox Nitrospira and how future landscape modifications may impact their abundance and diversity in coastal wetlands.
KEYWORDS: comammox Nitrospira, habitat change, aquaculture reclamation, coastal wetlands, nitrification
INTRODUCTION
Nitrification was traditionally thought to be performed sequentially by two distinct groups of prokaryotes, ammonia-oxidizers (AOA or AOB) and nitrite-oxidizing bacteria (NOB). However, this understanding has been revised due to the recent discovery of complete ammonia oxidizers (comammox Nitrospira), which can carry out the entire process of nitrification within a single cell (1, 2). Although attempts to isolate and cultivate comammox Nitrospira from field samples have had limited success due to their low growth rates and interference from other co-occurring nitrifiers, comammox Nitrospira were identified and classified into two monophyletic sister clades A and B (3), with clade A further divided into A.1, A.2, and A.3 using PCR assays of functional genes (4). Comammox Nitrospira have been detected in various habitats, including farmlands (5, 6), forests (7), wetlands (8, 9), riparian zones (10), and engineered systems (11, 12). While canonical AOB and AOA produce the greenhouse gas nitrous oxide (N2O) as a byproduct (13), contributing to climate warming, comammox Nitrospira appear to have the necessary functional genes and enzymes to produce N2O (14), but their overall contribution to N2O emission remains uncertain (9, 14, 15).
Coastal wetlands play a critical role in the global nitrogen cycle (16, 17), and are inhabited by a vast diversity of microorganisms, including comammox Nitrospira (18 – 20). It has been reported that comammox Nitrospira thrived in extremely high-salinity sediments (18) and harbored genes that enable them to adapt to low oxygen environment (21). Sun et al. (9) demonstrated that comammox Nitrospira played a key role in nitrification and N2O production in a coastal wetland. However, coastal wetland ecosystems in China are experiencing increasing impact from invasive species and land-use changes, which can modify physicochemical conditions (22, 23) and affect comammox Nitrospira in sediment. For example, in the subtropical Shanyutan Wetland, tidal mudflat areas dominated by different native and invasive plant species exhibit distinct comammox Nitrospira abundances and diversity (24). Both clades A and B of comammox Nitrospira are present in coastal tidal mudflats across the tropical-subtropical gradient in China (19), many of which have been invaded by Spartina alterniflora and some of the S. alterniflora marshes have been cleared to construct aquaculture ponds (25, 26). These habitat modifications have resulted in significant changes in sediment carbon content (27), carbon mineralization (27), and N2O production potential (28), but their corresponding effects on comammox Nitrospira have not been thoroughly examined.
Microbial community assembly can be influenced by deterministic or stochastic processes, and understanding their relative importance is fundamental to comprehending the characteristics and functions of the microbial ecosystem (29, 30). Previous research has investigated community assembly processes in various groups of microorganisms, including protists (31), fungi (32), bacteria (33), archaea (34), and diazotrophs (35). Comammox Nitrospira, which has not yet been reported in marine ecosystems (14), is likely introduced into coastal wetlands from adjacent terrestrial or freshwater habitats through stochastic processes. However, a recent study demonstrated that deterministic processes were more important in influencing comammox Nitrospira community assembly in a riverine estuary due to the strong selection by environmental factors (36). Therefore, it remains an open question as to which process plays a more prominent role in the comammox Nitrospira community assembly in coastal wetlands, especially those impacted by plant invasion and land-use change.
We collected sediment samples from 21 coastal wetland sites across southeastern China, including native mudflats (MFs), adjacent S. alterniflora marshes (SAs), and aquaculture ponds (APs), and analyzed their sediment properties and comammox Nitrospira communities. Our study aimed to (i) examine the effects of habitat modification on comammox Nitrospira communities in coastal wetlands across a large spatial scale; (ii) assess the critical factors that influence the abundance, diversity, and community composition of comammox Nitrospira in coastal wetlands; and (iii) explore the assembly mechanism of comammox Nitrospira communities in different habitats. We hypothesized that the invasion of mudflats by S. alterniflora would increase the abundance, and diversity of comammox Nitrospira due to the rhizospheric supply of oxygen and substrates, but the subsequent clearing of S. alterniflora marshes to create aquaculture ponds would reverse this trend.
RESULTS
Sediment physicochemical properties in different habitat types
Sediment samples were collected from 21 coastal wetlands in five provinces across subtropical and tropical climate zones (Fig. 1). Habitat modifications have a substantial impact on many sediment physicochemical properties in these coastal wetlands (Table S1). S. alterniflora invasion significantly increased soil organic carbon (SOC), NH4+−N, NO3−−N, and microbial biomass nitrogen (MBN) content, while the conversion of S. alterniflora marsh into aquaculture ponds significantly increased SO42− content but reduced SOC, NH4+−N, NO3−−N, and MBN content. The SO42− concentration was 8.90 and 9.13 mg L−1 in mudflats and S. alterniflora marsh, respectively, while aquaculture ponds significantly increased the value to 17.5 mg L−1. Interestingly, both S. alterniflora invasion and conversion of S. alterniflora marsh into aquaculture ponds did not affect pH, Cl−, salinity, grain size (proportions of clay, silt and sand), C/N, or microbial biomass carbon (MBC).
Fig 1.
The location of the study area and 21 sampling sites across the coastal regions in southeastern China.
Abundances of comammox Nitrospira in different habitat types
The abundance of comammox Nitrospira clade A was estimated to be 5.71 × 106 copies g‒1 sediment in the mudflats (Fig. 2A). Neither S. alterniflora invasion nor subsequent conversion of S. alterniflora marsh into aquaculture ponds significantly altered the abundance of clade A. Random forest analysis revealed that the proportions of clay and silt particles in the sediments were the strongest predictors of clade A abundance across all three habitat types (Fig. 2B). Spearman correlation analysis showed a positive correlation between the abundance of clade A and the proportions of clay and silt particles, while sand particles exhibited a negative correlation (Fig. 2C). Additionally, clade A abundance was positively correlated with sediment water content and organic carbon content but negatively with bulk density (Fig. 2C).
Fig 2.
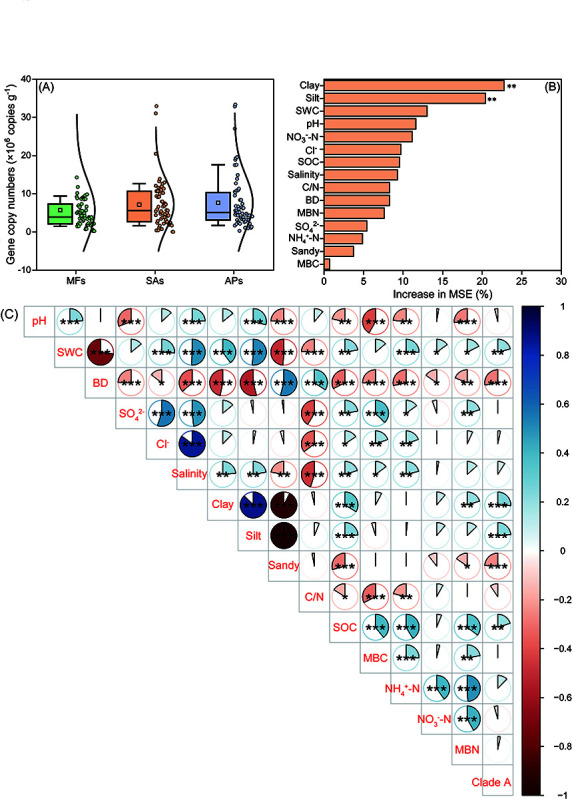
Box-normal plots showing the distribution of the abundance of comammox Nitrospira clade A in the three wetland habitat types (A). Random forest analysis showing the importance of environmental factors in influencing the abundance of comammox Nitrospira clade A (B). Spearman correlations among environmental variables and the abundance of comammox Nitrospira clade A (C). Color of the circle indicates the direction of correlation (blue = positive; red = negative). Size of the circle is proportional to the r2 value. Asterisks indicate levels of significance (*P < 0.05; **P < 0.01; ***P < 0.001). MFs, SAs, and APs represent mud flats, S. alterniflora marshes, and aquaculture ponds, respectively. BD, bulk density; MBC, microbial biomass carbon; MBN, microbial biomass nitrogen; SOC, soil organic carbon; SWC, sediment water content.
Diversity and community structures of comammox Nitrospira
After quality filtering, 3,921,886 sequences were retrieved from 184 sediment samples (five samples were discarded due to negative PCR results), with 10,172–43,868 sequences per sample. The Shannon diversity, Simpson diversity, Chao1 richness, and Observed species of comammox Nitrospira were significantly lower in aquaculture ponds, with the average values of 1.32, 0.53, 116, and 62.6, respectively (Fig. 3A, C, E, and G), while no significant difference was found between mudflats and S. alterniflora marshes. The Shannon diversity, Simpson diversity, Chao1 richness, and Observed species of comammox Nitrospira were most strongly (Fig. 3B, D, F, and H) and negatively (Fig. S1) affected by SO42− and salinity. Grain size (proportions of clay, silt, and sand), Cl−, and C/N were also significant factors.
Fig 3.
Box-normal plots showing the distribution of the Shannon diversity (A), Simpson diversity (C), Chao1 richness (E), and Observed species (G) of comammox Nitrospira in the three wetland habitat types. Random forest analysis showing the importance of environmental factors in influencing the Shannon diversity (B), Simpson diversity (D), Chao1 richness (F), and Observed species (H) of comammox Nitrospira. Different lowercase letters above the boxes indicate significant differences between wetland habitat types (P < 0.05). Asterisks indicate levels of significance (*P < 0.05; **P < 0.01). BD, bulk density; MBC, microbial biomass carbon; MBN, microbial biomass nitrogen; SOC, soil organic carbon; SWC, sediment water content.
Principal coordinate analysis (PCoA) indicated that the community structures of comammox Nitrospira in all three habitat types clustered together (Fig. 4A). However, permutational multivariate analysis of variance (PERMANOVA), analysis of similarities (ANOSIM), and multiple-response permutation procedure (MRPP) analyses showed that comammox Nitrospira community structure in aquaculture ponds significantly differed from both mudflats and Spartina marshes, whereas the latter two showed no significant differences (Table S1). Notably, sampling sites and their interactions with habitat change explained 33% and 24% of the variations in the community structure of comammox Nitrospira, respectively, while habitat change alone explained only 2% (Fig. 4A). Mantel test revealed that sediment water content, bulk density, SO42−, Cl−, salinity, silt and sand fractions, and C/N were critical factors influencing the community structure of comammox Nitrospira (Fig. 4; Fig. S2). Canonical correspondence analysis (CCA) showed that all the environmental factors together explained ~11% of the variation in comammox Nitrospira community structure among all samples (Fig. S2).
Fig 4.

Principal coordinate analysis (PCoA) of comammox Nitrospira communities the three wetland habitat types (A). Mantel test showing the relationships between the comammox Nitrospira community structure and environmental factors the three wetland habitat types (B). The numbers in PCoA plot indicate the R2 values, while asterisks *** represent statistically significant at 0.001 probability level as revealed by PERMANOVA. Edge width of plot B is proportional to Mantel’s R value, and the edge colors indicate statistical significance. Pairwise correlations coefficients of environmental factors are shown with color gradients. BD, bulk density; MBC, microbial biomass carbon; MBN, microbial biomass nitrogen; SOC, soil organic carbon; SWC, sediment water content.
Phylogenetic analysis revealed that all comammox Nitrospira sequences belonged to clades A.1, A.2, or B but not clade A.3 (Fig. 5; Table S3). Generally, the relative abundance of clade A.1 was determined to be 81.1% and 78.5% in mudflats and S. alterniflora marshes, respectively, with no significant difference, but it was significantly reduced to 65.3% in aquaculture ponds. In contrast, Clade A.2 OTU1524 and OTU26605 were notably more abundant in aquaculture ponds (Fig. 5). Overall, the invasion of mudflats by S. alterniflora did not significantly change the relative abundances of clades A.1, A.2, and B. However, subsequent conversion to aquaculture ponds significantly decreased the relative abundance of clade A.1 and increased the relative abundances of clades A.2 and B (Fig. 6).
Fig 5.
Neighbor-Joining tree showing the relationships among representative sequences in this study (Top 100) and the reference sequences from GenBank. The bar chart showing the relative abundance of each OTU in the three wetland habitat types. The colored ranges indicate different clades of comammox Nitrospira. Bootstrap values of >50% based on 1,000 replicates are shown next to the branches. AP, aquaculture pond; MF, mud flat; SA, S. alterniflora marsh.
Fig 6.
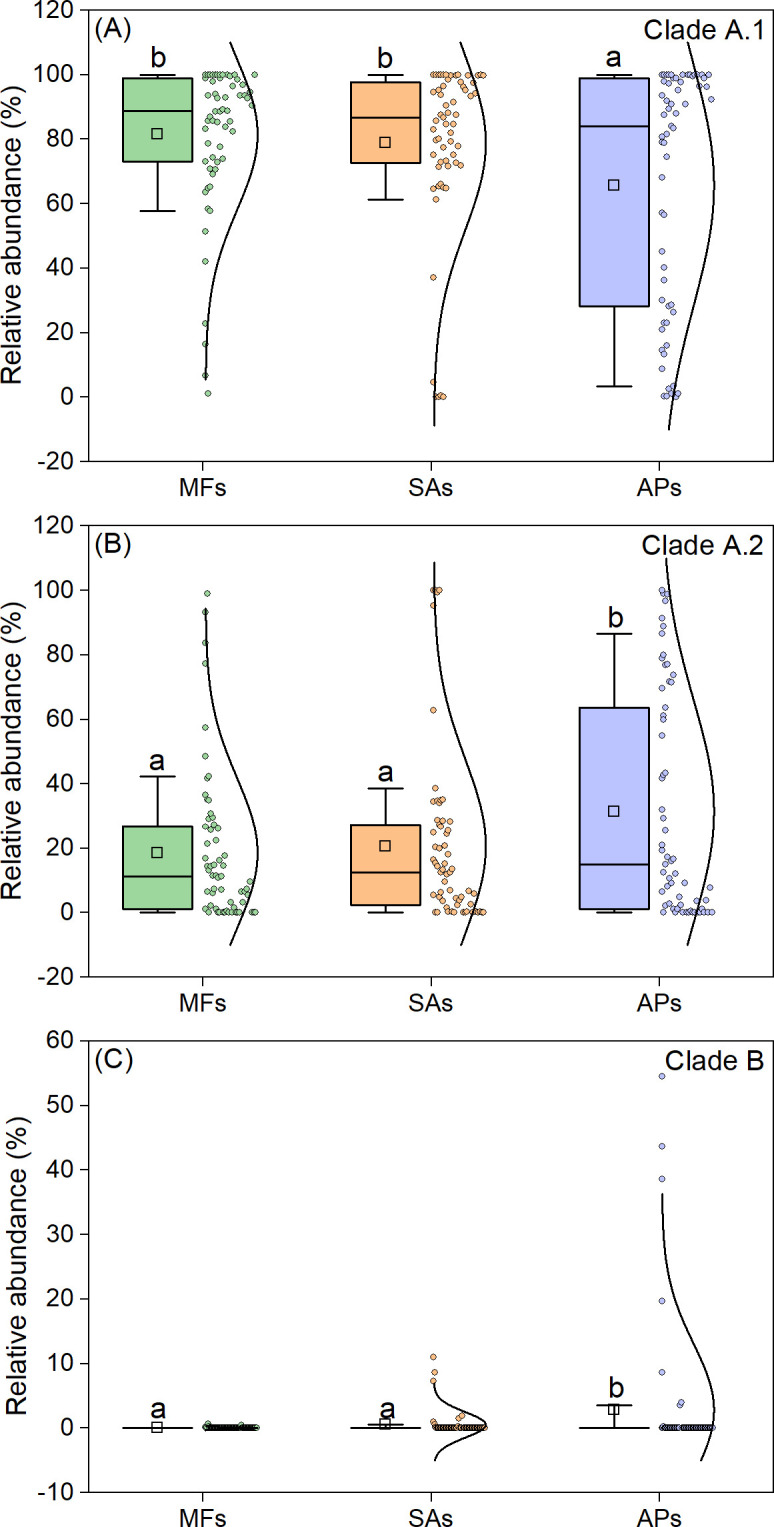
Box-normal plots showing relative abundance of each clade of comammox Nitrospira in the three wetland habitat types. Different lowercase letters above the boxes indicate significant differences between wetland habitat types (P < 0.05). APs, aquaculture ponds; MFs, mudflats; SA, S. alterniflora marshes.
Assembly processes of comammox Nitrospira community
Our results revealed that dispersal limitation, homogenous selection, and heterogeneous selection were the key processes driving the community assembly of comammox Nitrospira across all three habitat types (Fig. 7). Dispersal limitation played a dominant role, accounting for >60% of the relative contributions, while homogenous selection and heterogeneous selection contributed 19%–27% and 11%–13% of the relative contributions, respectively. The bootstrapping test results indicated that habitat modification did not significantly alter the relative importance of these different processes in comammox Nitrospira community assembly.
Fig 7.
Summary of the relative importance of each process driving the community assembly of comammox Nitrospira based on iCAMP analysis. MFs, SAs, and APs represent mudflats, S. alterniflora marshes, and aquaculture ponds, respectively.
DISCUSSION
Transformation of coastal wetlands due to invasive plants and aquaculture may drastically alter sediment properties and comammox Nitrospira communities, but studies in the literature have yielded conflicting results. For example, Lin et al. (24) reported a significant shift in comammox Nitrospira community structure between mudflats and S. alterniflora marshes in the Min River estuary, whereas Liu et al. (18) found a very similar comammox Nitrospira community structure between these two habitats in the Zhang River estuary. These discrepancies suggest that the response of comammox Nitrospira communities to habitat change may vary depending on location. In our study, by analyzing samples from three habitat types (mudflats, S. alterniflora marshes, and aquaculture ponds) in 21 wetland sites across a broad geographical range, we found that the comammox Nitrospira community structure was more influenced by wetland locations and their interactions with landscape modification than by landscape change alone (Fig. 4A).
Our data showed that the invasion of mudflats by S. alterniflora increased SOC, ammonium, and nitrate contents of the sediment but had minor effects on comammox Nitrospira diversity (Fig. 3B) or community structure (Fig. 4B). These findings are consistent with previous observations in an estuarine wetland (24). In contrast, the conversion of S. alterniflora marshes into aquaculture ponds reduced comammox Nitrospira diversity, likely due to the elevated SO42− concentration in the pond sediments (Table S1). This finding is surprising since microbial diversity was found to be higher in aquaculture ponds than natural wetlands (37) and comammox Nitrospira species were firstly enriched and characterized in aquaculture systems (2). It might be possibly due to that the continuous flooding in aquaculture ponds reduced the oxygen penetration into sediments (22), making the conditions more unfavorable for some comammox Nitrospira species than other microorganisms. Furthermore, some members of comammox Nitrospira are sensitive to sulfate (38), and this was supported by our Random Forest (Fig. 3) and Spearman’s correlation (Fig. S1) analyses. Thus, sulfate concentration might be a good indicator to predict the diversity of comammox Nitrospira in coastal wetlands during habitat modification in future.
Soil texture plays a critical role in determining soil microbial community structure (39, 40). However, the importance of sediment grain sizes has been often overlooked in previous studies of comammox Nitrospira (20, 41, 42). Sessitsch et al. (43) suggested that finer particles were associated with greater bacterial diversity due to higher nutrient availability and better protection from predators. Similarly, Boey et al. (44) reported that the transcripts of AOA and AOB were more abundant in muds than in sands in an estuarine wetland. Consistent with these findings, our data demonstrated a strong and positive correlation between the abundance of comammox Nitrospira clade A with the proportions of clay and silt particles, but a negative correlation with sand content (Fig. 2C). Since the sediment grain size distribution did not differ significantly between habitat types (Table S1), the overall abundance of comammox Nitrospira clade A did not vary (Fig. 2A). Nevertheless, we did observe some differences when we examined the subdivisions of the clade.
Although there was no significant difference in the relative abundances of clades A.1, A.2, and B between mudflats and S. alterniflora, conversion of S. alterniflora marshes to aquaculture ponds decreased the relative abundance of clade A.1 and increased that of clades A.2 and B (Fig. 6). Previous studies have shown that clade A.1 is often dominant in coastal wetlands (45, 46), whereas clade A.2 is more abundant in agricultural soils (4, 47). Our findings support these results and suggest that natural substrates may be more favorable for clade A.1, whereas cultivated substrates in farms and aquaculture ponds may be more favorable for clades A.2 and B. Nevertheless, clades A.1 and A.2 were highly diverse containing many different operational taxonomic units (OTUs), indicating that each clade may respond to habitat change differently (Fig. 5). Since clade A.1 was found to be the most active comammox Nitrospira communities in nitrification in coastal wetlands (9), the lower relative abundance of clade A.1 and diversity of comammox Nitrospira in aquaculture ponds might result in a lower potential activity of comammox Nitrospira communities. However, direct evidence was needed to verify this in future study.
Despite the broad range of geographical conditions among the 21 wetland sites that we studied, environmental variables only explained 11.2% of the variations in comammox Nitrospira community structure (Fig. S2), and homogeneous and heterogeneous selections together contributed to only about one-third of the comammox Nitrospira community assembly process (Fig. 7). These findings seem to contrast with those of Liu et al. (36) who suggested that selection by local environmental factors was the most important in a riverine estuary, and environmental factors explained 60.2% of the comammox Nitrospira community variations in their study. Our results, on the other hand, showed that the assembly of the comammox Nitrospira community was predominantly shaped by dispersal limitation (Fig. 7). There is no known source of comammox Nitrospira from the sea (14). Meanwhile, unlike a river-dominated estuary, the wetlands that we studied had very restricted freshwater flow, which would greatly limit dispersal rate. This was even more evident in the case of the isolated aquaculture ponds, where the importance of dispersal limitation increased to nearly 70% in the community assembly process (Fig. 7). Furthermore, in tidally influenced wetlands, fluctuations in environmental conditions could induce stresses and frequently disrupt the microbial community structure (48, 49). Similarly, the common aquaculture practice of drying out the pond sediments and conditioning them with lime between farming seasons would significantly disrupt the sediment microbial community (50, 51), adding stochasticity to the community assembly process. In addition, we observed that habitat modification did not significantly influence the comammox Nitrospira community assembly processes. This is counter-intuitive since there are large ecological differences between the three habitats (52 – 54). This finding may be due to the fact that sampling sites explained more variance in comammox Nitrospira community structure than habitats, and the comammox Nitrospira community assembly in coastal wetlands is mainly governed by stochastic processes at a large spatial scale, which weakened the role of habitat modification in influencing community assembly.
In conclusion, our study found that comammox Nitrospira was present across the 21 coastal wetlands in the tropical and subtropical zones of China. By comparing samples from different habitat types, we demonstrated that habitat modification affected comammox Nitrospira diversity in the sediment but did not impact the abundance of comammox Nitrospira clade A. Despite the wide geographical range covered in our study, environmental variables explained only a small fraction of the variations in comammox Nitrospira community structure, of which the response to habitat modification appeared to be dependent on wetland location. Additionally, we found that the community assembly process was predominantly influenced by dispersal limitation. The location-dependent response to habitat change and the stochasticity of the community assembly process would make it difficult to predict how comammox Nitrospira community and activity change on different spatial and temporal scales without in situ measurements. Nevertheless, our study identified key variables, such as sediment grain size, salinity, and sulfate concentration, that affect comammox Nitrospira abundance and diversity, which can be used to make an initial assessment of how future landscape modification may impact comammox Nitrospira in coastal sediments.
MATERIALS AND METHODS
Study sites and sampling
Sediment samples were collected from 21 coastal wetland sites in southeastern China (20°42′ N to 31°51′ N; 109°11′ E to 122°11′ E) across the subtropical and tropical climate zones (Fig. 1). The latitude and longitude of each site can be found in Table S3. These sites were located in five provinces: two in Shanghai (SH), six in Zhejiang (ZJ), nine in Fujian (FJ), three in Guangdong (GD), and one in Guangxi (GX) (Fig. 1). The annual average temperature ranged from 11.0°C to 23.0°C and the annual average precipitation ranged from 100 to 220 cm across the five provinces. In 2014, the total area of coastal wetlands in these five provinces was approximately 2.58 × 106 ha, accounting for 44.5% of the total coastal wetlands in China (55). Some areas of the mudflats in these coastal wetlands have been converted into marshes by the invasive S. alterniflora, and parts of these marshes have been reclaimed and converted into aquaculture ponds. These aquaculture ponds were created about 12–16 years ago by complete removal of original marsh vegetations. The distance between sampled habitats at each site was generally <500 m. In total, S. alterniflora marshes cover 3.34 × 104 ha (56) or 61.2% of the total area of S. alterniflora marshes in China, whereas coastal aquaculture ponds cover 5.31 × 105 ha (57) or 36.9% of the total area of aquaculture ponds in China.
Surface sediments in the top 20 cm were collected with a steel corer (1.5 m long, 5 cm internal diameter). Sediment samples were collected in triplicates from all three habitat types at each site: native MF, SA, and AP, between December 2019 and January 2020, resulting in a total of 189 sediment samples. All sediment samples were transferred into zip-lock bags and transported to the laboratory in a constant temperature box containing ice. The soil samples were divided into two parts. One part was stored at 4°C in the dark for no more than 2 weeks to determine soil physicochemical variables, while the other part was stored at −80°C for subsequent DNA extraction.
Physicochemical analysis
Plant residues and stones were removed from the sediment samples before analysis. Grain size was measured using a Master Sizer 2000 Laser Particle Size Analyzer (Malvern Scientific Instruments, Suffolk, UK). Sediment water content and bulk density were determined after drying fresh samples at 105°C for 48 hours (58, 59). Samples were diluted with deionized water in a 1:2.5 ratio to measure pH using an Orion 868 pH meter (USA). Salinity was measured by diluting samples in a 1:5 ratio and using a Eutech Instruments‐Salt6 salinity meter (USA). Porewater NO3−−N and NH4+−N were extracted in 2 M KCl solution (60, 61) and measured using a flow injection analyzer (Skalar Analytical SAN++, Netherlands). Concentrations of SO42− and Cl− were measured following the methods of Chen and Sun (62) using an ion chromatograph (Dionex 2100, USA). Sediment MBN and MBC contents were determined using the fumigation-extraction method (63).
DNA extraction and quantitative PCR
DNA was extracted from 0.5 g of each freeze-dried sediment sample using the FastDNA Spin Kit for Soil (MP Biomedicals, CA, USA) following the manufacturers’ protocols. The quality and quantity of DNA were determined by gel electrophoresis and a spectrophotometer (NanoDrop Technologies, Wilmington, USA).
We measured the abundances of comammox Nitrospira amoA genes using quantitative PCR (qPCR) on a CFX384 Optical Real-Time Detection System (Bio-Rad Laboratories Inc., Hercules, CA, USA) according to Lin et al. (24). We used the primer sets CA377f/C576r and CB377f/C576r to amplify the amoA genes of comammox Nitrospira clades A and B, respectively (64). The qPCR reaction mixture contained 5 µL SYBR qPCR mix, 0.2 µL of optimized concentration of forward and reverse primers, 1 µL of DNA template, and 3.6 µL of sterile distilled water. The thermal conditions were 95°C for the initial 3 minutes, followed by 38 cycles of 95°C for 30 seconds, 55°C for 25 seconds, and 72°C for 20 seconds. A tenfold serial dilution of plasmid DNA containing each target gene was used to generate standard curves. The amplification efficiency was 105.3%, and R2 was 0.997 for clade A. However, clade B amplification was characterized by strong non-specific amplification despite efforts to optimize thermal conditions and change primer pairs. Consequently, data on clade B abundances were excluded from further analysis.
High-throughput amplicon sequencing and bioinformatics analysis
The amplification of comammox Nitrospira amoA genes was conducted using a nested PCR approach (65). Primer sets A189Y/C576r and CA209f/C576r were used for the first and second rounds of PCR amplifications, respectively. A unique sample identifying barcode was added to the CA209f primer for the second round. The resultant PCR products were purified with a Qiagen Gel Extraction Kit (Qiagen, Germany), and libraries were constructed using a NEXTFLEX Rapid DNA-Seq Kit (Bioo Scientific, USA). Paired-end sequencing (2 × 300 bp) was carried out on an Illumina MiSeq platform.
The raw paired-end reads of each sample were merged using FLASH (Version 1.2.7) and quality controlled by QIIME (66), as previously described (67). Chimeric sequences were subsequently checked and removed using the UCHIME algorithm within the USEARCH package (68). Since there is no established species-level cut-off value for the amoA gene of comammox Nitrospira (69), high-quality sequences were grouped into OTUs at a 97% similarity level. Singleton and doubleton OTUs were eliminated to reduce the rate of spurious OTUs generated by PCR and sequencing errors (70). Representative sequence of each remaining OTU was subsequently selected and compared to NCBI database, and only those sequences that were affiliated with comammox Nitrospira were retained for downstream analysis. A neighbor-joining phylogenetic tree was constructed using representative sequences and reference sequences retrieved from NCBI, using the MEGA-X software (71). The phylogenetic tree was visualized using the Interactive Tree of Life (http://itol.embl.de) (72).
Community assembly analysis
A phylogenetic bin-based null model framework (iCAMP) was used to evaluate the relative importance of different community assembly processes (73), using OTU table, phylogenetic tree, treatment file, and taxonomic table. This procedure was conducted in iCAMP (v 1.3.2) with default settings using a pipeline on the Galaxy platform (http://ieg3.rccc.ou.edu:8080). The significance of difference in the relative importance of each assembly process among different habitat types was assessed using bootstrapping with 1,000 replications on the Galaxy platform.
Statistical analyses
To investigate the effect of habitat changes on sediment and porewater physiochemical properties, the abundance and diversity of comammox Nitrospira, and the relative abundance of comammox Nitrospira clades, one-way ANOVA was performed with LSD test using the SPSS Statistics 20 (IBM, USA). Alpha diversity indexes including Shannon, Simpson, Chao1 and Observed species were calculated using the diversity and estimateR functions in the “vegan” package in R. Random forest analysis was conducted to evaluate the importance of environmental factors in influencing the abundance and diversity of comammox Nitrospira using the “randomForest” package in R (v 4.2.1). Spearman correlation analysis was used to test for the associations among the abundance and diversity of comammox Nitrospira, and the relative abundance of comammox Nitrospira clades, and environmental factors, using the “corrplot” and “Hmisc” packages in R. The abundance data of OTU matrices were standardized using the Hellinger transformation in the vegan package in R. Principal coordinate analysis (PCoA) was performed using the pcoa function in the “ape” package. Mantel test and CCA were carried out to assess the effects of environmental factors on comammox Nitrospira community composition. Environmental factors with variance inflation factors (VIF) <20 were selected for the CCA analysis. PERMANOVA, ANOSIM, and MRPP were performed to determine the effects of habitat change on the community structure of comammox Nitrospira, using the adonis, anosim, and mrpp functions, respectively, in the vegan package.
ACKNOWLEDGMENTS
This work was financially supported by the National Natural Science Foundation of China (41930756, 42077041) and Natural Resources Science and Technology Innovation Project of Fujian Province (KY-090000–04-2022-012).
The authors declare that they have no conflict of interest
Contributor Information
Ping Yang, Email: yangping528@sina.cn.
Ji-Zheng He, Email: jzhe@fjnu.edu.cn.
Isaac Cann, University of Illinois Urbana-Champaign, Urbana, Illinois, USA .
DATA AVAILABILITY
Raw sequences have been submitted to DNA Data Bank of Japan (DDBJ) database with the accession number of DRA015269.
DISCLOSURES
The authors declare that they have no conflict of interest
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/aem.00807-23.
Supporting data.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Daims H, Lebedeva EV, Pjevac P, Han P, Herbold C, Albertsen M, Jehmlich N, Palatinszky M, Vierheilig J, Bulaev A, Kirkegaard RH, von Bergen M, Rattei T, Bendinger B, Nielsen PH, Wagner M. 2015. Complete nitrification by Nitrospira bacteria. Nature 528:504–509. doi: 10.1038/nature16461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Kessel M, Speth DR, Albertsen M, Nielsen PH, Op den Camp HJM, Kartal B, Jetten MSM, Lücker S. 2015. Complete nitrification by a single microorganism. Nature 528:555–559. doi: 10.1038/nature16459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koch H, van Kessel M, Lücker S. 2019. Complete nitrification: insights into the ecophysiology of comammox Nitrospira. Appl Microbiol Biotechnol 103:177–189. doi: 10.1007/s00253-018-9486-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li C, He ZY, Hu HW, He JZ. 2023. Niche specialization of comammox Nitrospira in terrestrial ecosystems: oligotrophic or copiotrophic?. Crit Rev Env Sci Tec 53:161–176. doi: 10.1080/10643389.2022.2049578 [DOI] [Google Scholar]
- 5. Orellana LH, Chee-Sanford JC, Sanford RA, Löffler FE, Konstantinidis KT. 2018. Year-round shotgun metagenomes reveal stable microbial communities in agricultural soils and novel ammonia oxidizers responding to fertilization. Appl Environ Microbiol 84:e01646-17. doi: 10.1128/AEM.01646-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. He ZY, Sun A, Jiao XY, Ge AH, Hu HW, Jin S, Liu X, Lin Y, He JZ. 2022. Fertilization has a greater effect than rhizosphere on community structures of comammox Nitrospira in an alkaline agricultural soil. Applied Soil Ecology 175:104456. doi: 10.1016/j.apsoil.2022.104456 [DOI] [Google Scholar]
- 7. Li C, Hu HW, Chen QL, Yan ZZ, Thi Nguyen BA, Chen D, He JZ. 2021. Niche specialization of comammox Nitrospira clade A in terrestrial ecosystems. Soil Biology and Biochemistry 156:108231. doi: 10.1016/j.soilbio.2021.108231 [DOI] [Google Scholar]
- 8. Liu S, Wang H, Chen L, Wang J, Zheng M, Liu S, Chen Q, Ni J. 2020a. Comammox Nitrospira within the Yangtze River continuum: community, biogeography, and ecological drivers. ISME J 14:2488–2504. doi: 10.1038/s41396-020-0701-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sun D, Tang X, Li J, Liu M, Hou L, Yin G, Chen C, Zhao Q, Klümper U, Han P. 2022. Chlorate as a comammox Nitrospira specific inhibitor reveals nitrification and N2O production activity in coastal wetland. Soil Biology and Biochemistry 173:108782. doi: 10.1016/j.soilbio.2022.108782 [DOI] [Google Scholar]
- 10. Wang S, Wang X, Jiang Y, Han C, Jetten MSM, Schwark L, Zhu G. 2021b. Abundance and functional importance of complete ammonia oxidizers and other nitrifiers in a riparian ecosystem. Environ Sci Technol 55:4573–4584. doi: 10.1021/acs.est.0c00915 [DOI] [PubMed] [Google Scholar]
- 11. Roots P, Wang Y, Rosenthal AF, Griffin JS, Sabba F, Petrovich M, Yang F, Kozak JA, Zhang H, Wells GF. 2019. Comammox Nitrospira are the dominant ammonia oxidizers in a mainstream low dissolved oxygen nitrification reactor. Water Res 157:396–405. doi: 10.1016/j.watres.2019.03.060 [DOI] [PubMed] [Google Scholar]
- 12. Koike K, Smith GJ, Yamamoto-Ikemoto R, Lücker S, Matsuura N. 2022. Distinct comammox Nitrospira catalyze ammonia oxidation in a full-scale groundwater treatment bioreactor under copper limited conditions. Water Res 210:117986. doi: 10.1016/j.watres.2021.117986 [DOI] [PubMed] [Google Scholar]
- 13. Hu HW, Chen D, He JZ. 2015. Microbial regulation of terrestrial nitrous oxide formation: understanding the biological pathways for prediction of emission rates. FEMS Microbiol Rev 39:729–749. doi: 10.1093/femsre/fuv021 [DOI] [PubMed] [Google Scholar]
- 14. Zhu G, Wang X, Wang S, Yu L, Armanbek G, Yu J, Jiang L, Yuan D, Guo Z, Zhang H, Zheng L, Schwark L, Jetten MSM, Yadav AK, Zhu Y-G. 2022. Towards a more labor-saving way in microbial ammonium oxidation: a review on complete ammonia oxidization (comammox). Sci Total Environ 829:154590. doi: 10.1016/j.scitotenv.2022.154590 [DOI] [PubMed] [Google Scholar]
- 15. Kits KD, Jung MY, Vierheilig J, Pjevac P, Sedlacek CJ, Liu S, Herbold C, Stein LY, Richter A, Wissel H, Brüggemann N, Wagner M, Daims H. 2019. Low yield and abiotic origin of N2O formed by the complete nitrifier Nitrospira inopinata. Nat Commun 10:1836. doi: 10.1038/s41467-019-09790-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang H, Gilbert JA, Zhu Y, Yang X. 2018. Salinity is a key factor driving the nitrogen cycling in the mangrove sediment. Sci Total Environ 631–632:1342–1349. doi: 10.1016/j.scitotenv.2018.03.102 [DOI] [PubMed] [Google Scholar]
- 17. Adame MF, Roberts ME, Hamilton DP, Ndehedehe CE, Reis V, Lu J, Griffiths M, Curwen G, Ronan M. 2019. Tropical coastal wetlands ameliorate nitrogen export during floods. Front Mar Sci 6:671. doi: 10.3389/fmars.2019.00671 [DOI] [Google Scholar]
- 18. Liu Z, Zhang C, Wei Q, Zhang S, Quan Z, Li M. 2020b. Temperature and salinity drive comammox community composition in mangrove ecosystems across southeastern China. Sci Total Environ 742:140456. doi: 10.1016/j.scitotenv.2020.140456 [DOI] [PubMed] [Google Scholar]
- 19. Sun D, Tang X, Zhao M, Zhang Z, Hou L, Liu M, Wang B, Klümper U, Han P. 2020. Distribution and diversity of comammox Nitrospira in coastal wetlands of China. Front Microbiol 11:589268. doi: 10.3389/fmicb.2020.589268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang D-Q, Zhou C-H, Nie M, Gu J-D, Quan Z-X. 2021a. Abundance and niche specificity of different types of complete ammonia oxidizers (comammox) in salt marshes covered by different plants. Sci Total Environ 768:144993. doi: 10.1016/j.scitotenv.2021.144993 [DOI] [PubMed] [Google Scholar]
- 21. Palomo A, Pedersen AG, Fowler SJ, Dechesne A, Sicheritz-Pontén T, Smets BF. 2018. Comparative genomics sheds light on niche differentiation and the evolutionary history of comammox Nitrospira. ISME J 12:1779–1793. doi: 10.1038/s41396-018-0083-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang P, Bastviken D, Lai DYF, Jin BS, Mou XJ, Tong C, Yao YC. 2017. Effects of coastal marsh conversion to shrimp aquaculture ponds on CH4 and N2O emissions. Estuar Coastal Shelf S 199:125–131. doi: 10.1016/j.ecss.2017.09.023 [DOI] [Google Scholar]
- 23. Tan L, Ge Z, Zhou X, Li S, Li X, Tang J. 2020. Conversion of coastal wetlands, riparian wetlands, and peatlands increases greenhouse gas emissions: a global meta-analysis. Glob Chang Biol 26:1638–1653. doi: 10.1111/gcb.14933 [DOI] [PubMed] [Google Scholar]
- 24. Lin Y, Ye G, Hu H-W, Yang P, Wan S, Feng M, He Z-Y, He J-Z. 2023. Plant species-driven distribution of individual clades of comammox Nitrospira in a subtropical estuarine wetland. Microb Ecol 85:209–220. doi: 10.1007/s00248-021-01940-3 [DOI] [PubMed] [Google Scholar]
- 25. Mao D, Liu M, Wang Z, Li L, Man W, Jia M, Zhang Y. 2019. Rapid invasion of Spartina alterniflora in the coastal zone of mainland China: spatiotemporal patterns and human prevention. Sensors 19:2308. doi: 10.3390/s19102308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Duan Y, Tian B, Li X, Liu D, Sengupta D, Wang Y, Peng Y. 2021. Tracking changes in aquaculture ponds on the China coast using 30 years of Landsat images. Int J Appl Earth Obs 102:102383. doi: 10.1016/j.jag.2021.102383 [DOI] [Google Scholar]
- 27. Yang P, Zhang LH, Lai DYF, Yang H, Tan LS, Luo LJ, Tong C, Hong Y, Zhu WY, Tang KW. 2022b. Landscape change affects soil organic carbon mineralization and greenhouse gas production in coastal wetlands. Global Biogeochem Cy 36. doi: 10.1029/2022GB007469 [DOI] [Google Scholar]
- 28. Yang P, Tang KW, Tong C, Lai DYF, Zhang L, Lin X, Yang H, Tan L, Zhang Y, Hong Y, Tang C, Lin Y. 2022a. Conversion of coastal wetland to aquaculture ponds decreased N2O emission: evidence from a multi-year field study. Water Res 227:119326. doi: 10.1016/j.watres.2022.119326 [DOI] [PubMed] [Google Scholar]
- 29. Stegen JC, Lin X, Fredrickson JK, Chen X, Kennedy DW, Murray CJ, Rockhold ML, Konopka A. 2013. Quantifying community assembly processes and identifying features that impose them. ISME J 7:2069–2079. doi: 10.1038/ismej.2013.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhou J, Ning D. 2017. Stochastic community assembly: does it matter in microbial ecology? Microbiol Mol Biol R 81:e00002-17. doi: 10.1128/MMBR.00002-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ceja-Navarro JA, Wang Y, Ning D, Arellano A, Ramanculova L, Yuan MM, Byer A, Craven KD, Saha MC, Brodie EL, Pett-Ridge J, Firestone MK. 2021. Protist diversity and community complexity in the rhizosphere of switchgrass are dynamic as plants develop. Microbiome 9:96. doi: 10.1186/s40168-021-01042-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guo J, Ling N, Chen Z, Xue C, Li L, Liu L, Gao L, Wang M, Ruan J, Guo S, Vandenkoornhuyse P, Shen Q. 2020. Soil fungal assemblage complexity is dependent on soil fertility and dominated by deterministic processes. New Phytol 226:232–243. doi: 10.1111/nph.16345 [DOI] [PubMed] [Google Scholar]
- 33. Tripathi BM, Stegen JC, Kim M, Dong K, Adams JM, Lee YK. 2018. Soil pH mediates the balance between stochastic and deterministic assembly of bacteria. ISME J 12:1072–1083. doi: 10.1038/s41396-018-0082-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gao G-F, Peng D, Wu D, Zhang Y, Chu H, Atomi H. 2021. Increasing inundation frequencies enhance the stochastic process and network complexity of the soil archaeal community in coastal wetlands. Appl Environ Microbiol 87:e02560-20. doi: 10.1128/AEM.02560-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Feng M, Adams JM, Fan K, Shi Y, Sun R, Wang D, Guo X, Chu H. 2018. Long-term fertilization influences community assembly processes of soil diazotrophs. Soil Biol Biochem 126:151–158. doi: 10.1016/j.soilbio.2018.08.021 [DOI] [Google Scholar]
- 36. Liu Z, Wei Q, Zou D, Zhang S, Zhang C, Quan Z, Li M. 2022. Deterministic factors determine the comammox community composition in the Pearl River Estuary ecosystem. Microbiol Spectr 10:e0101622. doi: 10.1128/spectrum.01016-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. She Y, Qi X, Xin X, He Y, Wang W, Li Z. 2023. Insights into microbial interactive mechanism regulating dissimilatory nitrate reduction processes in riparian freshwater aquaculture sediments. Environ Res 216:114593. doi: 10.1016/j.envres.2022.114593 [DOI] [PubMed] [Google Scholar]
- 38. Fowler SJ, Palomo A, Dechesne A, Mines PD, Smets BF. 2018. Comammox Nitrospira are abundant ammonia oxidizers in diverse groundwater‐fed rapid sand filter communities. Environ Microbiol 20:1002–1015. doi: 10.1111/1462-2920.14033 [DOI] [PubMed] [Google Scholar]
- 39. Karimi B, Terrat S, Dequiedt S, Saby NPA, Horrigue W, Lelièvre M, Nowak V, Jolivet C, Arrouays D, Wincker P, Cruaud C, Bispo A, Maron P-A, Bouré NCP, Ranjard L. 2018. Biogeography of soil bacteria and archaea across France. Sci Adv 4:eaat1808. doi: 10.1126/sciadv.aat1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xia Q, Rufty T, Shi W. 2020. Soil microbial diversity and composition: links to soil texture and associated properties. Soil Biol Biochem 149:107953. doi: 10.1016/j.soilbio.2020.107953 [DOI] [Google Scholar]
- 41. Bernhard AE, Beltz J, Giblin AE, Roberts BJ. 2021. Biogeography of ammonia oxidizers in New England and Gulf of Mexico salt marshes and the potential importance of comammox. ISME Commun 1:1. doi: 10.1038/s43705-021-00008-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sun D, Zhao M, Tang X, Liu M, Hou L, Zhao Q, Li J, Gu JD, Han P. 2021b. Niche adaptation strategies of different clades of comammox Nitrospira in the Yangtze Estuary. Int Biodeter Biodegr 164:105286. doi: 10.1016/j.ibiod.2021.105286 [DOI] [Google Scholar]
- 43. Sessitsch A, Weilharter A, Gerzabek MH, Kirchmann H, Kandeler E. 2001. Microbial population structures in soil particle size fractions of a long-term fertilizer field experiment. Appl Environ Microbiol 67:4215–4224. doi: 10.1128/AEM.67.9.4215-4224.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Boey JS, Mortimer R, Couturier A, Worrallo K, Handley KM. 2022. Estuarine microbial diversity and nitrogen cycling increase along sand–mud gradients independent of salinity and distance. Environ Microbiol 24:50–65. doi: 10.1111/1462-2920.15550 [DOI] [PubMed] [Google Scholar]
- 45. Wang X, Lu L, Zhou X, Tang X, Kuang L, Chen J, Shan J, Lu H, Qin H, Adams J, Wang B. 2020c. Niche differentiation of comammox Nitrospira in the mudflat and reclaimed agricultural soils along the north branch of Yangtze River Estuary. Front Microbiol 11:618287. doi: 10.3389/fmicb.2020.618287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhao M, Tang X, Sun D, Hou L, Liu M, Zhao Q, Klümper U, Quan Z, Gu J-D, Han P. 2021. Salinity gradients shape the nitrifier community composition in Nanliu River Estuary sediments and the ecophysiology of comammox Nitrospira inopinata. Sci Total Environ 795:148768. doi: 10.1016/j.scitotenv.2021.148768 [DOI] [PubMed] [Google Scholar]
- 47. Lin Y, Fan J, Hu HW, Duan C, Ye G, Wan S, He ZY, Zheng Y, He JZ. 2022. Differentiation of individual clusters of comammox Nitrospira in an acidic Ultisol following long-term fertilization. Applied Soil Ecology 170:104267. doi: 10.1016/j.apsoil.2021.104267 [DOI] [Google Scholar]
- 48. Kearns PJ, Angell JH, Howard EM, Deegan LA, Stanley RHR, Bowen JL. 2016. Nutrient enrichment induces dormancy and decreases diversity of active bacteria in salt marsh sediments. Nat Commun 7:1–9. doi: 10.1038/ncomms12881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nemergut DR, Schmidt SK, Fukami T, O’Neill SP, Bilinski TM, Stanish LF, Knelman JE, Darcy JL, Lynch RC, Wickey P, Ferrenberg S. 2013. Patterns and processes of microbial community assembly. Microbiol Mol Biol Rev 77:342–356. doi: 10.1128/MMBR.00051-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rurangwa E, Verdegem MCJ. 2015. Microorganisms in recirculating aquaculture systems and their management. Rev Aquacult 7:117–130. doi: 10.1111/raq.12057 [DOI] [Google Scholar]
- 51. Xu M, Xu R-Z, Shen X-X, Gao P, Xue Z-X, Huang D-C, Jin G-Q, Li C, Cao J-S. 2022. The response of sediment microbial communities to temporal and site-specific variations of pollution in interconnected aquaculture pond and ditch systems. Sci Total Environ 806:150498. doi: 10.1016/j.scitotenv.2021.150498 [DOI] [PubMed] [Google Scholar]
- 52. Hong Y, Zhang L, Yang P, Tong C, Lin Y, Lai DYF, Yang H, Tian Y, Zhu W, Tang KW. 2023. Responses of coastal sediment organic and inorganic carbon to habitat modification across a wide latitudinal range in southeastern China. Catena 225:107034. doi: 10.1016/j.catena.2023.107034 [DOI] [Google Scholar]
- 53. Yang P, Lai DYF, Yang H, Lin Y, Tong C, Hong Y, Tian Y, Tang C, Tang KW. 2022. Large increase in CH4 emission following conversion of coastal marsh to aquaculture ponds caused by changing gas transport pathways. Water Res 222:118882. doi: 10.1016/j.watres.2022.118882 [DOI] [PubMed] [Google Scholar]
- 54. Ye G, Chen J, Yang P, Hu HW, He ZY, Wang D, Cao D, Zhang W, Wu B, Wu Y, Wei X, Lin Y. 2023. Non-native plant species invasion increases the importance of deterministic processes in fungal community assembly in a coastal wetland. Microb Ecol 86:1120–1131. doi: 10.1007/s00248-022-02144-z [DOI] [PubMed] [Google Scholar]
- 55. Sun Z, Sun W, Tong C, Zeng C, Yu X, Mou X. 2015. China's coastal wetlands: conservation history, implementation efforts, existing issues and strategies for future improvement. Environ Int 79:25–41. doi: 10.1016/j.envint.2015.02.017 [DOI] [PubMed] [Google Scholar]
- 56. Liu MY, Mao DH, Wang ZM, Li L, Man WD, Jia MM, Ren CY, Zhang YZ. 2018. Rapid invasion of Spartina alterniflora in the coastal zone of mainland China: new observations from landsat OLI images. Remote Sens 10:1933. doi: 10.3390/rs10121933 [DOI] [Google Scholar]
- 57. Duan Y, Li X, Zhang L, Chen D, Liu S, Ji H. 2020. Mapping national-scale aquaculture ponds based on the google earth engine in the Chinese coastal zone. Aquaculture 520:734666. doi: 10.1016/j.aquaculture.2019.734666 [DOI] [Google Scholar]
- 58. Percival J, Lindsay P. 1997. Measurement of physical properties of sediments, p 7–38. In Mudrock A, Azcue JM, Mudrock P (ed), Manual of physico-chemical analysis of aquatic sediments. CRC Press, New York, USA. [Google Scholar]
- 59. Yin S, Bai JH, Wang W, Zhang GL, Jia J, Cui BS, Liu X. 2019. Effects of soil moisture on carbon mineralization in floodplain wetlands with different flooding frequencies. J Hydrol 574:1074–1084. doi: 10.1016/j.jhydrol.2019.05.007 [DOI] [Google Scholar]
- 60. Gao D, Liu M, Hou L, Derrick YFL, Wang W, Li X, Zeng A, Zheng Y, Han P, Yang Y, Yin G. 2019. Effects of shrimp-aquaculture reclamation on sediment nitrate dissimilatory reduction processes in a coastal wetland of southeastern China. Environ Pollut 255:113219. doi: 10.1016/j.envpol.2019.113219 [DOI] [PubMed] [Google Scholar]
- 61. Yin G, Hou L, Liu M, Li X, Zheng Y, Gao J, Jiang X, Wang R, Yu C, Lin X. 2017. DNRA in intertidal sediments of the Yangtze Estuary. J Geophys Res Biogeosci 122:1988–1998. doi: 10.1002/2017JG003766 [DOI] [Google Scholar]
- 62. Chen BB, Sun ZG. 2020. Effects of nitrogen enrichment on variations of sulfur in plant-soil system of Suaeda salsa in coastal marsh of the Yellow River estuary. China. Ecol Indic 109:105797. doi: 10.1016/j.ecolind.2019.105797 [DOI] [Google Scholar]
- 63. Templer P, Findlay S, Lovett G. 2003. Soil microbial biomass and nitrogen transformations among five tree species of the Catskill Mountains, New York, USA. Soil Biol Biochem 35:607–613. doi: 10.1016/S0038-0717(03)00006-3 [DOI] [Google Scholar]
- 64. Jiang R, Wang J-G, Zhu T, Zou B, Wang D-Q, Rhee S-K, An D, Ji Z-Y, Quan Z-X, Stams AJM. 2020. Use of newly designed primers for quantification of complete ammonia-oxidizing (comammox) bacterial clades and strict nitrite oxidizers in the genus Nitrospira. Appl Environ Microbiol 86:e01775-20. doi: 10.1128/AEM.01775-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Xia F, Wang J-G, Zhu T, Zou B, Rhee S-K, Quan Z-X. 2018. Ubiquity and diversity of complete ammonia oxidizers (comammox). Appl Environ Microbiol 84:e01390-18. doi: 10.1128/AEM.01390-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. doi: 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lin Y, Ye G, Ding W, Hu HW, Zheng Y, Fan J, Wan S, Duan C, He JZ. 2020. Niche differentiation of comammox Nitrospira and canonical ammonia oxidizers in soil aggregate fractions following 27-year fertilizations. Agric Ecosyst Environ 304:107147. doi: 10.1016/j.agee.2020.107147 [DOI] [Google Scholar]
- 68. Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. 2011. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27:2194–2200. doi: 10.1093/bioinformatics/btr381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pjevac P, Schauberger C, Poghosyan L, Herbold CW, van Kessel M, Daebeler A, Steinberger M, Jetten MSM, Lücker S, Wagner M, Daims H. 2017. AmoA-targeted polymerase chain reaction primers for the specific detection and quantification of comammox Nitrospira in the environment. Front Microbiol 8:1508. doi: 10.3389/fmicb.2017.01508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mamet SD, Helgason BL, Lamb EG, McGillivray A, Stanley KG, Robinson SJ, Aziz SU, Vail S, Siciliano SD. 2022. Phenology-dependent root bacteria enhance yield of Brassica Napus. Soil Biol Biochem 166:108468. doi: 10.1016/j.soilbio.2021.108468 [DOI] [Google Scholar]
- 71. Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. doi: 10.1093/molbev/msy096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Letunic I, Bork P. 2019. Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucleic Acids Res 47:W256–W259. doi: 10.1093/nar/gkz239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ning D, Yuan M, Wu L, Zhang Y, Guo X, Zhou X, Yang Y, Arkin AP, Firestone MK, Zhou J. 2020. A quantitative framework reveals ecological drivers of grassland microbial community assembly in response to warming. Nat Commun 11:4717. doi: 10.1038/s41467-020-18560-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting data.
Data Availability Statement
Raw sequences have been submitted to DNA Data Bank of Japan (DDBJ) database with the accession number of DRA015269.






