Abstract
Lates calcarifer, also known as Barramundi or Asian seabass, is a highly productive and fast-growing species that is well suited to large-scale aquaculture due to its attractive harvestable yields (premium fish). This fish has been envisioned as having the potential to be the “Salmon of Tropics”. Cultivating Lates calcarifer in aquaculture poses challenges, as the dense populations that make such aquaculture commercially viable facilitate the rapid spread of infectious diseases, which in turn significantly impact yield. Hence, the immunization of juveniles is necessary, and the development of new immunization agents enhances the efficiency of aquaculture and improves food security. In our study, we characterize seven novel strains of the bacterial pathogen Streptococcus iniae that were collected from commercial fish farms in Singapore and Australia. We find that the capsular operon in our strains is highly conserved and identify a number of major surface antigens previously described in Streptococcus. A genome analysis indicates that the present strains are closely related but form distinct strains within the S. iniae species. We then proceed to demonstrate that inoculation with the inactivated strain P3SAB cross-protects Lates calcarifer against S. iniae infections in vivo from a variety of strains found in both Singapore and Australia.
Keywords: aquaculture, bacterial pathogens, vaccination, Lates calcarifer, Streptococcus iniae
1. Introduction
According to the United Nations Food and Agriculture Organization (FAO), current aquaculture produces an approximately equal share of global seafood production as wild catch. Significant growth in the aquaculture market suggests that aquaculture will surpass wild catch as a source of fish in the coming years [1]. A separate FAO report indicates that the consumption of fish accounts for approximately 20% of daily protein intake globally, making fish, and therefore aquaculture, a key backbone of human nutrition [2] and global food security. Over 90% of cultured fish by mass and approximately 85% of cultured fish by value is cultivated in the Asia-Pacific region [3].
Lates calcarifer, also known as Barramundi or Asian seabass [4], is a fish of major commercial interest with annual aquaculture production in excess of 30,000 metric tons. Lates calcarifer is found in costal water and freshwater from the Indian coast to the southwestern Pacific, generally in warmer waters [5]. This fish can be cultured in seawater, brackish water, and freshwater but spawning generally occurs in brackish water near the mouths of rivers. The fish is fast growing and popular for human consumption, which explains the interest in this species for aquaculture enterprises. Initially, farming of Lates calcarifer started in Thailand and has since expanded throughout Southeast Asia and to northern Australia [5]. Dense stocking in aquaculture enclosures increases the likelihood and impact of infectious diseases. Lates calcarifer is susceptible to several emerging viral (Megalocytivirus, Lates calcarifer herpesvirus, and Lates calcarifer Birnavirus) and bacterial infections (Tenacibaculum maritimum, Vibrio harveyi, and Streptococcus iniae) [6,7,8,9,10]. Infection by Streptococcus bacteria can cause high mortality rates over a period of 3–7 days, but chronic outbreaks lasting weeks with continuing mortality are possible in both marine and freshwater fish. High water temperatures in tropical and subtropical regions are particularly conducive to the spread of this Gram-positive bacterium. Specifically, in 1999 Bromage et al. [10] identified S. iniae as a significant pathogen threatening Lates calcarifer populations in Australian aquaculture. The isolated strains were shown to induce mortality in 60–80% of sample populations. Clinical signs in infected fish include unilateral or bilateral exophthalmia, corneal opacity, hemorrhage at the base of fins, skin discoloration, tail rot, and erratic swimming behavior. The bacterium can be isolated from the brains of surviving fish, indicating that a reservoir of S. iniae persists even after an outbreak and the removal of deceased fish. Furthermore, S. iniae has also been isolated in the aftermath of major disease-induced mortality from other commercially relevant fish species, e.g., tilapia or rabbit fish. Hence, the prevalence of S. iniae constitutes a major threat to the successful cultivation of Lates calcarifer.
In addition to the threat, S. iniae poses some concern for human health as well. While uncommon, cases of S. iniae infections have been documented in humans, starting in 1995 in Canada [11] with more recent cases also occurring in Asia, e.g., in Hong Kong [12] and Singapore [13]. Symptoms from S. iniae infection in humans can include bacteremia cellulitis, arthritis, meningitis, and endocarditis [13].
A number of proteins were previously identified for their suitability as vaccine candidates. In particular, the protein Sip11 has been shown to be protective against S. iniae infection in Japanese flounder [14]. Formalin-killed bacteria have also been explored as a vaccine candidate in rainbow trout and have proven effective in reducing mortality [15].
In this study, we investigate the genetics of seven independent isolates of S. iniae obtained from infected Lates calcarifer and Eleutheronema tetradactylum cultured in Singapore and Australia. We used long-read nanopore sequencing [16] to obtain and assemble the individual S. iniae genomes and analyze major differences and similarities, in particular in the capsular operon of S. iniae, which is a key protective antigen and host defense mechanism [17] and is important for virulence [18].
Subsequently, we demonstrate that broadly neutralizing vaccines can be obtained from the inactivated P3SAB strain of S. iniae that shows high cross-protection against both Singaporean and Australian strains in Lates calcarifer.
2. Materials and Methods
2.1. Isolation of S. iniae Strains
Seven S. iniae strains were isolated during disease outbreaks from various farms in Singapore and Australia (Table 1). Out of the seven strains, six were isolated from Asian seabass (Lates calcarifer) and one strain was isolated from a threadfin fish (E. tetradactylum).
Table 1.
Summary of S. iniae isolates.
| Strains | Origin | Species | Year Isolated |
|---|---|---|---|
| P3SAB | Singapore | Asian seabass | 2016 |
| Si 1-19 | Singapore | Asian seabass | 2019 |
| Si 4-21 | Singapore | Asian seabass | 2021 |
| Si 7-21 | Singapore | Asian seabass | 2021 |
| Si 8-21 | Singapore | Asian seabass | 2021 |
| Si 6-21 | Singapore | Threadfin | 2021 |
| CB Si-1 | Australia | Asian seabass | 2018 |
S. iniae strains were isolated on Columbia Agar plates with 5% sheep blood (63784, Bio Rad, Hercules, CA, USA). Aseptically, swab samples were collected from the brain, liver, kidney, and eyeball of the diseased fish showing clinical symptoms. Agar plates were incubated at 30 °C for 48–72 h. Colonies were checked for Streptococci morphology (cocci in chains) under microscope.
2.2. Identification of S. iniae by PCR
PCR identification of S. iniae was performed using specific primers based on the lactate oxidase-encoding (lox) gene [14]. Briefly, colonies were sub-cultured in tryptic soy broth (TSB) medium (22092, Sigma-Aldrich, St. Louis, MO, USA) and incubated overnight at 30 °C. TSB culture was boiled for 5 min at 95 °C and centrifuged to collect supernatant for PCR analysis. An 870 bp fragment of the lox gene was amplified in a PCR reaction containing 1 μM of each primer (LOX-1: 5′ AAGGGGAAATCGCAAGTGCC-3′and LOX-2: 5′-ATATCTGATTGGGCCGTCTAA-3′), 0.2 mM of each dNTP, 2.5 U DNA polymerase (PL1204, Vivantis, Shah Alam, Malaysia), and 1× reaction buffer along with the boiled suspension as the template. Thermocycling was carried out using an initial denaturation step at 95 °C for 1 min followed by 35 cycles of denaturation at 92 °C for 1 min, annealing at 60 °C for 1 min, extension at 72 °C for 2 min, and a final extension at 72 °C for 5 min. Positive and negative controls were included in each PCR reaction. PCR products were detected using 1% agarose gel electrophoresis containing 1× safe green dye (SD0101, Vivantis, Shah Alam, Malaysia). Bands were visualized under blue light transilluminator (TT-BLT-470, Hercuvan, Shah Alam, Malaysia). Post confirmation, S. iniae strains were stored in 20% glycerol at −80 °C for later use.
2.3. Genomic DNA Extraction
S. iniae isolates were cultured for approximately 7–8 h in 50 mL TSB until mid-log phase (OD600 = 0.5). The culture stock (8 mL) pellets were used to carry out gDNA extraction by phenol chloroform isoamyl alcohol (PCI) method with modification to remove capsular polysaccharides. [19] Briefly, the aqueous phase containing DNA was treated with water-saturated diethyl ether (296082, Sigma-Aldrich, St. Louis, MO, USA) and 5M sodium chloride (S-5150, Sigma-Aldrich, St. Louis, MO, USA) before precipitation with ethanol. DNA pellets were dissolved in 10 mM Tris buffer (pH 8.0) and stored at 4 °C before sequencing.
2.4. Library Preparation
An amount of 1.5 to 2 µg of isolated DNA was sheared to a target size of 30 kb on the Megaruptor 3 (Diagenode) according to the manufacturer’s instructions. Sheared DNA size was assessed using the Genomic Screentape on the Agilent 4200 Tapestation (Aglient Technologies, Santa Clara, CA, USA) based on the manufacturer’s instructions. An additional 0.4× Ampure XP (Beckman Coulter) size selection was performed on selected samples exhibiting increased proportion of DNA sizes <4 kb on the genomic screentape profile to aid in removal of DNA fragments <2 kb.
An amount of 500 ng of sheared and size-selected DNA was input to the PCR-free multiplexed whole-genome sequencing (WGS) library using the ligation sequencing kit (SQK-LSK109) in combination with native barcoding expansion kits (EXP-NBD104 and 114), according to the manufacturer’s instructions (Oxford Nanopore Technologies, ONT, Oxford, UK). The final barcoded library pool was loaded on a FLO-MIN106D, R9.4.1 flow cell and sequenced on the GridION (Oxford Nanopore Technologies) with super accurate (SUP) live basecalling on Guppy 5.0.12, MinKNOW release 21.05.12. Demultiplexing of basecalled, pass-filter fastq was completed using qcat (Oxford Nanopore Technologies). Two sequencing runs were conducted for sample Si 1-19-P3SAB (flow cell ID FAQ65824) and sample Si 6-21-CB Si-1 (flow cell ID FAQ94395). Sequencing parameters and results are given in Table 2.
Table 2.
Sequencing, assembly, and annotation data.
| Strain ID | Si 1-19 | Si 4-21 | P3SAB | Si 6-21 | Si 7-21 | Si 8-21 | CB Si-1 |
|---|---|---|---|---|---|---|---|
| Origin | Singapore | Singapore | Singapore | Singapore | Singapore | Singapore | Australia |
| Accession | CP129326 | CP129327 | CP129328 | CP129329 | CP129330 | CP129331 | CP129332 |
| Host | Lates calcarifer | Lates calcarifer | Lates calcarifer | E. Tetradactylum | Lates calcarifer | Lates calcarifer | Lates calcarifer |
| Sequencing Date | 2 September 2021 | 2 September 2021 | 2 September 2021 | 27 December 2021 | 27 December 2021 | 27 December 2021 | 27 December 2021 |
| Total Read length | 764 Mb | 1063 Mb | 808 Mb | 3675 Mb | 5940 Mb | 3754 Mb | 2413 Mb |
| Reads N50 | 13,145 | 14,745 | 15,134 | 15,820 | 14,895 | 15,212 | 16,608 |
| Reads N90 | 8188 | 8575 | 8368 | 9480 | 8832 | 8755 | 9937 |
| Assembly Length | 2,099,239 | 2,103,151 | 2,109,823 | 2,100,739 | 2,102,756 | 2,102,750 | 2,119,653 |
| Average Coverage | 351 | 492 | 375 | 1699 | 2761 | 1752 | 1109 |
| Number of Genes | 2013 | 2021 | 2033 | 2074 | 2086 | 2088 | 2092 |
| CDSs | 1923 | 1931 | 1943 | 1984 | 1996 | 1998 | 2002 |
| rRNA | 18 | 18 | 18 | 18 | 18 | 18 | 18 |
| tRNA | 68 | 68 | 68 | 68 | 68 | 68 | 68 |
| Protein-Coding Genes | 1524 | 1546 | 1531 | 1445 | 1448 | 1471 | 1476 |
2.5. Genome Assembly and Annotation
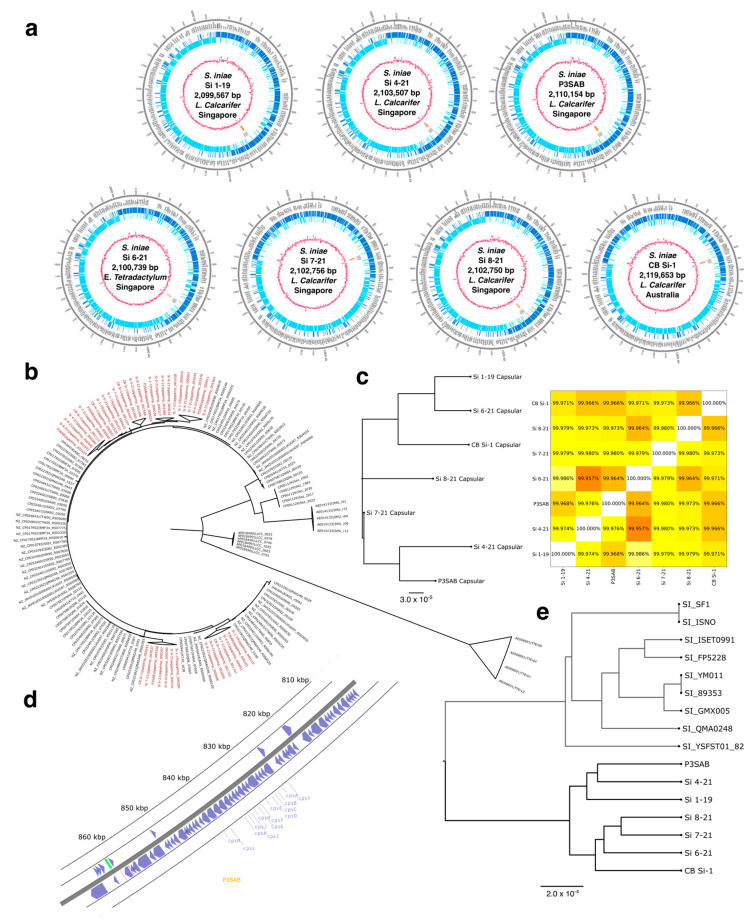
Genomes were assembled using the Flye Pipeline [20,21] specifying a circular genome. For all 7 samples, the largest fragment obtained was 2.1 Mb in length, which is consistent with previous assemblies of S. iniae [22]. Mean coverage ranged from 351× to 2749×. Assembly statistics are summarized in Table 2. After assembly, medaka (https://github.com/nanoporetech/medaka (accessed on 23 April 2023)) was used for genome polishing as currently recommended for Oxford Nanopore assemblies. Subsequent to assembly we used the NCBI Prokaryotic Genome Annotation Pipeline (PGAP) [23] to identify genes, which returned approximately 2000 genes per assembly, of which about 1500 genes were identified as protein-coding genes. These numbers are also consistent with previously obtained S. iniae annotations. Details on number and type of genes identified are shown in Table 2. A general overview of annotated genes is given in Figure 1a, with forward-strand genes highlighted in dark blue and reverse-strand genes highlighted in light blue. GC content was approximately 37% with local peaks of up to 65%. Local GC content is shown as a magenta trace in Figure 1.
Figure 1.
Characterization of 7 S. iniae strains. (a) Schematic view of S. iniae genome assemblies. All samples assembled consistently to circular genomes of 2.1 Mb. Forward-strand genes are indicated in dark blue, reverse-strand located genes are indicated in light blue, and the capsular operon location is indicated in orange. The central magenta line indicates local GC content in a 1 kb window. (b) Phylogenetic tree of S. iniae and related Streptococcus species 16S rRNA. It can be seen that the 7 strains characterized here fit within S. iniae but form a distinct clade. (d) Detailed view of the capsular operon in the vaccine strain P3SAB. All capsular proteins are present in series. (e) Phylogenetic tree derived from protein orthogroups. The geographic association is preserved, showing strains Si 1-19 -P3SAB originating from Singapore as a single clade with low internal genetic diversity, whereas the Australian strains Si 6-21-CB Si-1 show some genetic variation at the protein level. (c) Phylogenetic tree and sequence identity matrix of S. iniae capsular operon. The sequences of the capsular operon are highly conserved with an average sequence identity of well over 99%. It is evident that the clade segregation is not associated with origin geography, unlike the pattern observed for all proteins. This indicates a high degree of genomic stability in this region and the association of the capsular genes with virality and immunogenicity make these conserved antigenic regions highly attractive for vaccine design.
2.6. Genome Analysis
The assembled genomes were analyzed using OrthoFinder [24,25] to identify orthologous genes and derive a phylogenetic tree considering the entire coding genome. OrthoFinder uses BLAST [26] to group orthologous genes into so-called orthogroups and subsequently provides a sequence alignment, a variety of statistics, and phylogenetic inference using the STAG approach [27]. The capsular proteins were identified by sequence alignment against the 21 kb reference capsular operon identified by Millard et al. [18] (GenBank AY904444) using MAFFT [28].
2.7. Vaccine Preparation
The vaccine for S. iniae strain P3SAB was prepared as ready-to-use water-in-oil emulsions for intraperitoneal injection. To begin with, the S. iniae P3SAB strain was cultured in TSB media (50 mL) and incubated overnight at 28 °C with gentle agitation (150 rpm). The culture was inactivated using formalin (252549, Sigma Aldrich, St. Louis, MO, USA) with a final concentration of 0.4% (v/v) for at least 48 h at 4–8 °C. Inactivated cultures were further washed and diluted with sterile saline, then emulsified at a 30:70 ratio using MONTANIDE ISA (Seppic) oil adjuvant. Sterility of the vaccine was verified by standard procedures.
2.8. Experimental Fish and Husbandry
One hundred and twelve healthy Barramundi (Lates calcarifer) fingerlings of approximately 12 g were obtained from a fingerling supplier in Australia and were acclimatized for 2 weeks with aeration. Fish were fed to satiation twice daily with a commercial feed (Lucky Star 4, Lucky Star Holdings, Singapore). The water temperature was maintained at 30 ± 3 °C and salinity at 10 ppt. Water quality was regulated using a hang-on filter (with 3 filtering effects: physical, chemical, and biological) and daily cleaning. Ammonia, pH, and nitrate were checked regularly and water changes (10–20%) were performed as required. Fish were screened for general pathogens before the experiment. All the experimental procedures were performed according to National Advisory Committee for Laboratory Animal Research (NACLAR) guidelines and approved by the Institutional Animal Care and Use Committee (IACUC), approval number U-2022/F/01.
2.9. Vaccination Challenge and Sampling
A total of 112 fingerlings were randomly distributed into 7 groups (n = 16) according to Table 3. Corresponding to each streptococcal strain there was one vaccinated and one non-vaccinated group. Vaccinated group fingerlings were intraperitoneally (IP) injected with 50 µL of the P3SAB strain vaccine. Two weeks post vaccination, fish were challenged with 100 µL of 1 × 105 CFU/mL of respective S. iniae strains, as shown in Table 3.
Table 3.
The experimental groups for animal trials.
| Groups | Treatment | Vaccine | Challenge Strain | Serum Collected |
|---|---|---|---|---|
| 1 | Negative control | PBS | PBS | Yes |
| 2 | Group A | S. iniae P3SAB | S. iniae P3SAB | Yes |
| 3 | Group A positive control | PBS | S. iniae P3SAB | - |
| 4 | Group B | S. iniae P3SAB | S. iniae Si 6-21 | Yes |
| 5 | Group B positive control | PBS | S. iniae Si 6-21 | - |
| 6 | Group C | S. iniae P3SAB | S. iniae CB Si-1 | Yes |
| 7 | Group C positive control | PBS | S. iniae CB Si-1 | Yes |
Challenge inoculum was prepared by culturing S. iniae strains in 50 mL TSB up to an optical density of 0.5 at 600 nm. Cells were washed twice and resuspended in PBS to an OD600 = 1, which corresponds to 1 × 108 CFU/mL.
Fish were monitored continuously post challenge for 14 days for signs of infection, such as unilateral or bilateral exophthalmia, eye opacity, disorientation, loss of equilibrium, hemorrhages at the base of the fins and in internal organs, darkening of the skin, pale livers, and enlarged spleens. Ten days post challenge samples were collected for bacteriology and serum analysis. Relative percent of survival (RPS) was calculated according to the following formula: RPS = 1 − (% mortality in vaccinated/% mortality in control) × 100.
2.10. Antibody Response by ELISA
Antibody response post challenge was measured by whole-cell ELISA using sera from experimental fish [27]. Briefly, high-binding 96-well ELISA plates (3590, Costar, Washington, DC, USA) were coated overnight at 4 °C with inactivated P3SAB cells (100 uL/well) resuspended in carbonate-bicarbonate coating buffer (pH 9.6) to an OD600 = 1.0. The next day, plates were washed with PBS containing 0.05% Tween 20 (PBST) before blocking with conjugate buffer (1% Bovine serum albumin in PBST) at 22 °C for 2 h. Diluted sera (1:10) from surviving fish were applied to the wells and incubated at 22 °C for 2 h before washing with PBST. The secondary antibody was an anti-Asian seabass IgM monoclonal antibody (AquaMab F-02, Aquatic Diagnostics, Scotland, UK). Plates were incubated with secondary antibody for 2 h at 22 °C. After washing, Horseradish peroxidase-conjugated tertiary antibody (Goat anti-mouse IgG; A16066, Invitrogen, Waltham, WA, USA) diluted 1:10,000 in conjugate buffer was applied for 1 h. Color was developed for 5 min using 3,3′,5,5′–Tetramethylbenzidine (TMB; T0440, Sigma-Aldrich, St. Louis, MO, USA) and OD was measured at 450 nm with a Hercuvan NS-100 microplate reader. Antisera from 8 fish per vaccine group were analyzed individually and the results were expressed as a mean OD ± standard error.
3. Results
3.1. Sequence Analysis
The genomic properties of all the strains are consistent with previous assemblies of S. iniae strains, including QMA0248 [22], SF1 [29], ISET0901 [30], and ISNO [31]. Detailed properties are shown in Table 2. Circular plots of all genomes are shown in Figure 1a. All strain assemblies contain approximately 2000 coding sequences, 18 rRNAs (6 each of 5S, 16S, and 23S), as well as 68 tRNAs. The number of protein-coding genes is approximately 1500 per strain. PGAP was able to assign a function to most coding regions with only 5–7% of regions annotated as hypothetical proteins, which is consistent and even exceeds in some cases the annotation rates for the high-quality QMA0248 assembly [22]. All assemblies contain the chaperonin-family protein GroEL, which has been shown to be specific to S. iniae [32].
To assess the genetic distinctness of our strains we compared the 16S rRNAs with previously reported S. iniae strains found in the NCBI nucleotide database. We identified 43 distinct assemblies of S. iniae and included the related bacteria Thermoanaerobacter tengcongensis (AE008691), Streptococcus equi (CP001129), Streptococcus mutans (AE014133), and Lactococcus lactis (AP018499) in our analysis to gauge the genetic distance within the S. iniae species relative to related Streptococcus species. All strains of S. iniae include multiple copies of 16S rRNA, and this pattern holds true for other Streptococcus species. The limited genetic divergence of individual copies compared to the overall divergence within S. iniae indicate that these duplication events precede the species formation of S. iniae. The resulting phylogeny clearly situates our strains within the S. iniae clade, but also shows that the reported strains are distinct from previously sequenced strains of S. iniae, as they form a distinct clade within S. iniae (Figure 1b).
Orthofinder [25] was then used to categorize protein-coding genes and analyze the homology of protein-coding genes between strains. Of all genes, 99.1% were assigned to orthogroups, and just 20 genes out of a total of 10,370 protein-coding genes across all seven strains could not be associated with orthologous genes in other strains. A total of 1706 orthogroups were identified, with on average about 1250 protein-coding genes shared between each pair of strains. On average, 1240 of these orthologous mappings are one-to-one, indicating on one hand low levels of gene duplication throughout the S. iniae genome and at the same time high consistency between strains from geographically different regions, namely Singapore and Australia, as well as across host species. Using the STAG method [27] as implemented in OrthoFinder, we constructed a phylogenetic tree of all seven strains discussed here and nine fully annotated strains available in the NCBI database. We again found that our strains form a distinct subgroup that shares a more distant ancestor with previously described S. iniae strains.
The capsular operon plays In important rIlI in immune evasion for S. iniae. Hence, we identified the genomic location of the capsular genes and extracted the sequences from our assemblies. Capsular gene locations are indicated by orange blocks in Figure 1a. A detailed view of the capsular operon in the proposed vaccine strain P3SAB is shown in Figure 1d. The capsular genes appear highly conserved throughout all strains, with the sequence identity across the capsular operon exceeding 99.9%, as shown in Figure 1c. In addition, the phylogenetic relations do not coincide with geographic regions or the general phylogenetic patterns observed for the full genome. This appears to indicate a highly stable region that is an effective antigen and hence a good candidate for vaccine-induced immunity.
Manzer et al. [33] identified a number of prominent surface antigens in different species of the Streptococcus genus. We extracted the reference sequences for the antigens SpaP (P23504), SspA (Q54185), SspB (Q54186), BspA (Q8E589), AspA (Q48S75), Pas (Q9KW51), SpaA (Q53414), PAaA (Q9LBG3), and PAh (Q59HN9) from the Uniprot database and used these sequences as a blast query against blast databases built on our S. iniae strains. A summary of hits for the P3SAB strain is given in Table 4. The results for all strains including the exact E-values are included as a supplementary data file. We were able to identify candidate genes for AspA, BspA, PAh, Pas, SpaA, and SpaP consistently and PAaA in four of the seven genomes. We were unable to identify orthologues to SspA or SspB in any of our strains.
Table 4.
Antigen candidate genes in S. iniae strain P3SAB.
| Antigen | Gene Locus | Gene Tag | Gene Description |
|---|---|---|---|
| AspA | [1,912,471:1,912,948] (−) | pgaptmp_001861 | LPXTG cell wall anchor domain-containing protein |
| AspA | [1,976,370:1,977,459] (−) | pgaptmp_001909 | YSIRK-type signal peptide-containing protein |
| BspA | [480,510:483,732] (+) | pgaptmp_000491 | hypothetical protein |
| PAh | [155,147:156,713] (+) | pgaptmp_000195 | LPXTG cell wall anchor domain-containing protein |
| PAh | [1,912,471:1,912,948] (−) | pgaptmp_001861 | LPXTG cell wall anchor domain-containing protein |
| Pas | [220,766:221,828] (+) | pgaptmp_000261 | HAMP domain-containing histidine kinase |
| SpaA | [155,147:156,713] (+) | pgaptmp_000195 | LPXTG cell wall anchor domain-containing protein |
| SpaA | [480,510:483,732] (+) | extdb:pgaptmp_000491 | hypothetical protein |
| SpaA | [1,912,471:1,912,948] (−) | extdb:pgaptmp_001861 | LPXTG cell wall anchor domain-containing protein |
| SpaP | [155,147:156,713] (+) | extdb:pgaptmp_000195 | LPXTG cell wall anchor domain-containing protein |
| AspA | [1,912,471:1,912,948] (−) | extdb:pgaptmp_001861 | LPXTG cell wall anchor domain-containing protein |
3.2. Vaccination and RPS
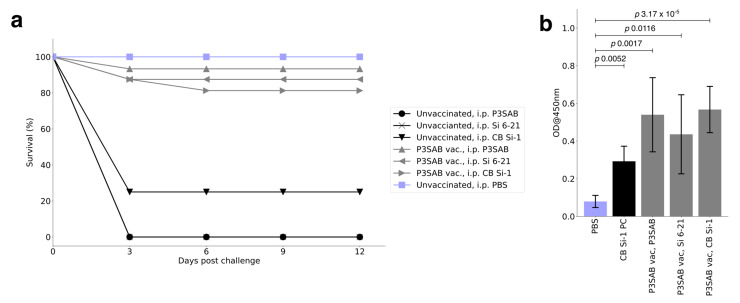
The fingerlings in both vaccinated and non-vaccinated groups were exposed to respective S. iniae strains via IP injections (Table 3), while one group served as negative control and was injected with 0.1 mL PBS. From day 3 onward post challenge, the fish in positive control groups started to show classical S. iniae clinical signs with accumulated mortality of 75–100% (P3SAB and Si 6-21 strains showed 0% and CB Si-1 a 25% survival rate). However, all the vaccine groups were healthy with no observed clinical signs, and the survival percentages in these vaccinated fish were above 80% (Figure 2a).
Figure 2.
Vaccine efficacy in vivo. (a) Percentage survival of control and vaccinated fish during the trial. Overall, unvaccinated groups showed higher mortality percentage (75–100%) compared to vaccinated groups. (b) Graph showing serum antibody responses between non-vaccinated and vaccinated fish (mean ± SE). Vaccinated group showed significantly higher antibody titers compared to non-vaccinated groups.
3.3. ELISA
In order to determine the cross-protective antibody responses in vaccinated fish, serum was collected and analyzed by whole-cell ELISA. Compared to control group fish, significantly higher serum antibody titers were observed in the vaccinated groups (Figure 2c). Antibody responses were also observed in the non-vaccinated group, which did not provide cross-protection against S. iniae infection.
4. Discussion
In this study, we characterized seven distinct strains of S. iniae, a bacterial pathogen that is highly lethal to Asian seabass. The strains originate from aquaculture operations in Australia and Singapore and thus span a wide geographic area in Southeast Asia and were collected from distinct species. We found that the strains are closely related genetically, with sequence identities around 95%. The capsular genes, which have previously been identified as important surface antigens [18], show minimal variability (Figure 1c). Additionally, we were able to identify several candidate genes previously identified as important antigens in other species of the Streptococcus genus [33].
In addition, we show that inoculation with the P3SAB strain was universally protective against various strains of S. iniae infection. Survival rates without inoculation were 0% for infection with P3SAB and Si 6-21 and 25% with CB Si-1, consistent with the expected high mortality (Figure 2a). When inoculated with the P3SAB strain vaccine, a significant increase in survival to above 80% against all tested strains was observed. The presence of the serum of antibodies that are reactive with different S. iniae strains as shown by ELISA experiments is further evidence of successful vaccination. However, these preliminary data are based on small-scale experimental trials. In the future, additional large-scale trials are required to corroborate the results.
Our work demonstrates that the P3SAB-based inactivated vaccine is clearly effective in protecting populations of Lates calcarifer and potentially E. tetradactylum from S. iniae infections, and thus provides enhanced protection to fish grown in aquaculture. These findings are consistent with earlier findings of Wang et al. [34] demonstrating the effectiveness and cross-protection of tilapia species using formalin-killed-cell-based vaccines based on S. iniae and S. agalacitae. The vaccine is relatively straightforward and economical to produce and thus holds great promise to improve yields in seabass aquaculture throughout Southeast Asia and Australia. This work also demonstrates a viable strategy for the rapid development of vaccines for new and emerging bacterial pathogens in aquaculture operations. Previous efforts have been made to use protein-based vaccines derived from α-enolase. Liu et al. [35] show that pure recombinant α-enolase shows comparatively low effectiveness against S. iniae infection in tilapia, with survival rates of approximately 31%, but demonstrate that these rates can be improved using a carbon-nanotube-based carrier system to about 69%. Wang et al. [36] also demonstrate the effectiveness of α-enolase-based vaccines against S. iniae infection in channel catfish, with survival rates of about 45%. These studies provide some indication that effective protein-based vaccines against S. iniae are technically viable. Our identification of conserved antigens might enable a future vaccination strategy based on other recombinant proteins or mRNA that might be broadly protective against a variety of related S. iniae infections, but it is unclear at this point if such a strategy can be economically employed in aquaculture.
Acknowledgments
The authors would like to thank Markus Schrittwieser for the collection of samples from diseased fish and Rose Davis from UVAXX for maintenance and support of the animal facility.
Author Contributions
Conceptualization, S.A. and R.G.H.; methodology, S.A.; software, R.G.H.; validation, S.A., S.M. and R.G.H.; formal analysis, S.A. and R.G.H.; investigation, S.A., S.M. and R.G.H.; resources, S.A. and R.G.H.; data curation, R.G.H.; writing—original draft preparation, S.A., S.M. and R.G.H.; writing—review and editing, S.A. and R.G.H.; visualization, R.G.H.; supervision, S.A. and R.G.H.; project administration, S.A. and R.G.H.; funding acquisition, S.A. and R.G.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
All the experimental procedures were performed according to National Advisory Committee for Laboratory Animal Research (NACLAR) guidelines and approved by the Institutional Animal Care and Use Committee (IACUC), approval number U-2022/F/01.
Informed Consent Statement
Not applicable.
Data Availability Statement
The genome assemblies of all strains are deposited in the NCBI genome database under accession numbers CP129326, CP129327, CP129328, CP129329, CP129330, CP129331, and CP129322.
Conflicts of Interest
Sunita Awate and Salma Mubarka are employees of UVAXX Pte Ltd., a Singapore-based company which develops and markets vaccines for aquaculture.
Funding Statement
This research was partially supported by A*STAR grant 202D800013 to R.G.H.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.FAO . Fisheries & Aquaculture-FAO Yearbook of Fishery and Aquaculture Statistics. FAO; Rome, Italy: 2021. [Google Scholar]
- 2.FAO. IFAD. Unicef. WFP. WHO . The State of Food Security and Nutrition in the World 2017: Building Resilience for Peace and Food Security. FAO; Rome, Italy: 2017. [Google Scholar]
- 3.Rosenberg A.A., Fogarty M.J., Cooper A.B., Dickey-Collas M., Fulton E.A., Gutiérrez N.L., Hyde K.J., Kleisner K.M., Kristiansen T., Longo C., et al. Developing new approaches to global stock status assessment and fishery production potential of the seas. FAO Fish. Aquac. Circ. 2014;1:1086. E-ISBN 978-92-5-107992-8. [Google Scholar]
- 4.Mathew G. Course Manual: National Training on Cage Culture of Seabass. CMFRI & NFDB; Kochi, India: 2009. Taxonomy, identification and biology of seabass (Lates calcarifer) pp. 38–43. [Google Scholar]
- 5.Grey D.L. An overview of Lates calcarifer in Australia and Asia in Management of Wild and Cultured Sea Bass/Barramundi; Proceedings of the ACIAR Proceedings; Darwin, Australia. 24–30 September 1987; No. 20. [Google Scholar]
- 6.Chang S.F., Ng K.S., Grisez L., De Groof A., Vogels W., Van Der Hoek L., Deijs M. Novel Fish Pathogenic Virus. WO2018029301A1. U.S. Patent. 2018 February 15;
- 7.de Groof A., Guelen L., Deijs M., van der Wal Y., Miyata M., Ng K.S., van Grinsven L., Simmelink B., Biermann Y., Grisez L., et al. A Novel Virus Causes Scale Drop Disease in Lates calcarifer. PLoS Pathog. 2015;11:e1005074. doi: 10.1371/journal.ppat.1005074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong H.T., Jitrakorn S., Kayansamruaj P., Pirarat N., Rodkhum C., Rattanarojpong T., Senapin S., Saksmerprome V. Infectious spleen and kidney necrosis disease (ISKND) outbreaks in farmed barramundi (Lates calcarifer) in Vietnam. Fish Shellfish Immunol. 2017;68:65–73. doi: 10.1016/j.fsi.2017.06.054. [DOI] [PubMed] [Google Scholar]
- 9.Gibson-Kueh S., Chee D., Chen J., Wang Y.H., Tay S., Leong L.N., Ng M.L., Jones J.B., Nicholls P.K., Ferguson H.W. The pathology of ‘scale drop syndrome’ in Asian seabass, Lates calcarifer Bloch, a first description. J. Fish Dis. 2012;35:19–27. doi: 10.1111/j.1365-2761.2011.01319.x. [DOI] [PubMed] [Google Scholar]
- 10.Bromage E.S., Thomas A., Owens L. Streptococcus iniae, a bacterial infection in barramundi Lates calcarifer. Dis. Aquat. Organ. 1999;36:177–181. doi: 10.3354/dao036177. [DOI] [PubMed] [Google Scholar]
- 11.Weinstein M.R., Litt M., Kertesz D.A., Wyper P., Rose D., Coulter M., McGeer A., Facklam R., Ostach C., Willey B.M., et al. Invasive infections due to a fish pathogen, Streptococcus iniae. S. iniae Study Group. N. Engl. J. Med. 1997;337:589–594. doi: 10.1056/NEJM199708283370902. [DOI] [PubMed] [Google Scholar]
- 12.Lau S.K.P., Woo P.C.Y., Tse H., Leung K.-W., Wong S.S.Y., Yuen K.-Y. Invasive Streptococcus iniae infections outside north america. J. Clin. Microbiol. 2003;41:1004–1009. doi: 10.1128/JCM.41.3.1004-1009.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koh T.H., Kurup A., Chen J. Streptococcus iniae discitis in Singapore. Emerg. Infect. Dis. 2004;10:1694. doi: 10.3201/eid1009.040029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng S., Hu Y., Jiao X., Sun L. Identification and immunoprotective analysis of a Streptococcus iniae subunit vaccine candidate. Vaccine. 2010;28:2636–2641. doi: 10.1016/j.vaccine.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Eldar A., Horovitcz A., Bercovier H. Development and efficacy of a vaccine against Streptococcus iniae infection in farmed rainbow trout. Vet. Immunol. Immunopathol. 1997;56:175–183. doi: 10.1016/S0165-2427(96)05738-8. [DOI] [PubMed] [Google Scholar]
- 16.Deamer D., Akeson M., Branton D. Three decades of nanopore sequencing. Nat. Biotechnol. 2016;34:518–524. doi: 10.1038/nbt.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Locke J.B., Colvin K.M., Datta A.K., Patel S.K., Naidu N.N., Neely M.N., Nizet V., Buchanan J.T. Streptococcus iniae capsule impairs phagocytic clearance and contributes to virulence in fish. Am. Soc. Microbiol. 2007;189:1279–1287. doi: 10.1128/JB.01175-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Millard C.M., Baiano J.C., Chan C., Yuen B., Aviles F., Landos M., Chong R.S., Benedict S., Barnes A.C. Evolution of the capsular operon of Streptococcus iniae in response to vaccination. Appl. Environ. Microbiol. 2012;78:8219–8226. doi: 10.1128/AEM.02216-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao X., Zhang J., Zhang Q., Wang L., Tan Y., Guo Z., Yang R., Qiu J., Zhou D. Two methods for extraction of high-purity genomic DNA from mucoid Gram-negative bacteria. Afr. J. Microbiol. Res. 2011;5:4013–4018. doi: 10.5897/AJMR11.785. [DOI] [Google Scholar]
- 20.Lin Y., Yuan J., Kolmogorov M., Shen M.W., Chaisson M., Pevzner P.A. Assembly of long error-prone reads using de Bruijn graphs. Proc. Natl. Acad. Sci. USA. 2016;113:E8396–E8405. doi: 10.1073/pnas.1604560113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolmogorov M., Yuan J., Lin Y., Pevzner P.A. Assembly of long, error-prone reads using repeat graphs. Nat. Biotechnol. 2019;37:540–546. doi: 10.1038/s41587-019-0072-8. [DOI] [PubMed] [Google Scholar]
- 22.Alsheikh-Hussain A.S., Zakour NLBen Forde B.M., Silayeva O., Barnes A.C., Beatson S.A. A high-quality reference genome for the fish pathogen Streptococcus iniae. Microb. Genome. 2022;8:000777. doi: 10.1099/mgen.0.000777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tatusova T., DiCuccio M., Badretdin A., Chetvernin V., Nawrocki E.P., Zaslavsky L., Lomsadze A., Pruitt K.D., Borodovsky M., Ostell J. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016;44:6614–6624. doi: 10.1093/nar/gkw569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emms D.M., Kelly S. OrthoFinder: Solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biol. 2015;16:157. doi: 10.1186/s13059-015-0721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emms D.M., Kelly S. OrthoFinder: Phylogenetic orthology inference for comparative genomics. Genome Biol. 2019;20:1–14. doi: 10.1186/s13059-019-1832-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 27.Emms D.M., Kelly S. STAG: Species tree inference from all genes. BioRxiv. 2018:267914. doi: 10.1101/267914. [DOI] [Google Scholar]
- 28.Katoh K., Misawa K., Kuma K., Miyata T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajoo S., Jeon W., Park K., Yoo S., Yoon I., Lee H., Ahn J. Complete genome sequence of Streptococcus iniae YSFST01-82, isolated from olive flounder in Jeju, South Korea. Genome Announc. 2015;3:e00319-15. doi: 10.1128/genomeA.00319-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pridgeon J.W., Zhang D., Zhang L. Complete genome sequence of a virulent strain, Streptococcus iniae ISET0901, isolated from diseased tilapia. Genome Announc. 2014;2:e00553-14. doi: 10.1128/genomeA.00553-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pridgeon J.W., Zhang D., Zhang L. Complete genome sequence of the attenuated Novobiocin-resistant streptococcus iniae vaccine strain ISNO. Genome Announc. 2014;2:e00510-14. doi: 10.1128/genomeA.00510-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goh S.H., Driedger D., Gillett S., Low D.E., Hemmingsen S.M., Amos M., Chan D., Lovgren M., Willey B.M., Shaw C., et al. Streptococcus iniae, a Human and Animal Pathogen: Specific Identification by the Chaperonin 60 Gene Identification Method. J. Clin. Microbiol. 1998;36:2164. doi: 10.1128/JCM.36.7.2164-2166.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manzer H.S., Nobbs A.H., Doran K.S. The Multifaceted Nature of Streptococcal Antigen I/II Proteins in Colonization and Disease Pathogenesis. Front. Microbiol. 2020;11:602305. doi: 10.3389/fmicb.2020.602305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Q., Fu T., Li X., Luo Q., Huang J., Sun Y., Wang X. Cross-immunity in Nile tilapia vaccinated with Streptococcus agalactiae and Streptococcus iniae vaccines. Fish Shellfish Immunol. 2020;97:382–389. doi: 10.1016/j.fsi.2019.12.021. [DOI] [PubMed] [Google Scholar]
- 35.Liu J., Cao Y., Ma H., Du H., Liu T., Wang G., Liu M., Wang Q., Li P., Wang E. Enolase-based nanovaccine immersion immunization induces robust immunity and protection against Streptococcus infection in tilapia. Aquaculture. 2023;576:739849. doi: 10.1016/j.aquaculture.2023.739849. [DOI] [Google Scholar]
- 36.Wang J., Wang E., Liu T., He Y., Wang K. The highly conserved α-enolase stimulats cross-protective immunity against serotype I and II Streptococcus iniae infection in channel catfish (Ictalurus punctatus) Aquaculture. 2022;550:737854. doi: 10.1016/j.aquaculture.2021.737854. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The genome assemblies of all strains are deposited in the NCBI genome database under accession numbers CP129326, CP129327, CP129328, CP129329, CP129330, CP129331, and CP129322.