Abstract
Infection of the central nervous system (CNS) is a significant cause of morbidity and mortality, and treatments available to combat the highly debilitating symptoms of CNS infection are limited. The mechanisms by which pathogens in the circulation overcome host immunity and breach the blood–brain barrier are active areas of investigation. In this review, we discuss recent work that has significantly advanced our understanding of the avenues of pathogen dissemination to the CNS for four eukaryotic pathogens of global health importance: Toxoplasma gondii, Plasmodium falciparum, Trypanosoma brucei, and Cryptococcus neoformans. These studies highlight the remarkable diversity of pathogen strategies for trafficking to the brain and will ultimately contribute to an improved ability to combat life-threatening CNS disease.
Introduction
The central nervous system (CNS) is protected by a formidable and unique barrier system. Remarkably, several pathogens have evolved mechanisms to breach this barrier and cause disease. Pathogens circulating in the bloodstream may access the brain parenchyma by crossing the blood–brain barrier (BBB) or through other portals of entry, such as the peripheral nerve root ganglia or the choroid plexus, which generates the cerebrospinal fluid (CSF). The blood vessels of the BBB are comprised of densely packed endothelial cells that are linked by tight junctions and surrounded by pericytes and astrocyte end-feet [1]. Collectively, these cells function to restrict the passage of molecules, pathogens, and leukocytes into the parenchyma. In contrast, endothelial cells of the choroid plexus are fenestrated, permitting molecules from the bloodstream into the choroid plexus stroma. Tight junctions interconnect plexus epithelial cells, which line the choroid plexus and form the blood–CSF barrier (BCB) [2]. These barriers physically protect the brain from microbial invasion and toxins and mediate the selective permeability of nutrients and ions. As discussed below, the pathogens that can overcome this unique barrier and enter the CNS often cause severe, life-threatening disease.
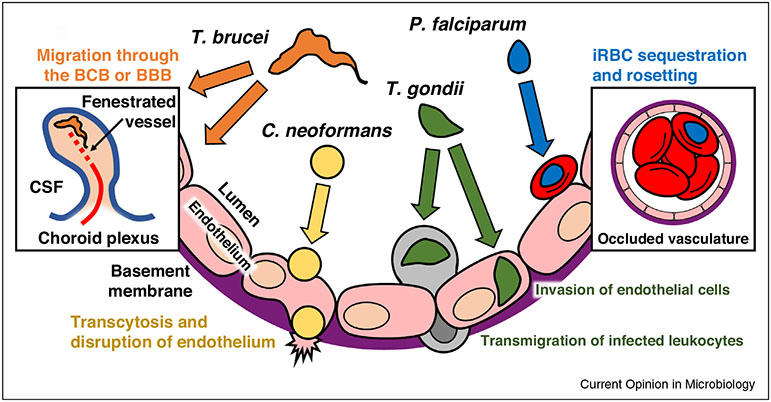
In this review, we will highlight recent findings on four eukaryotic pathogens of global health relevance. Although many researchers have made valuable contributions to the field of CNS infection, the focus for this review will be on research published in the past three years. Interestingly, the pathogens discussed here appear to use distinct routes for trafficking to the CNS (Figure 1) and cause a diverse range of disease symptoms. Toxoplasma gondii establishes a chronic infection in the parenchyma, and parasite reactivation can cause encephalitis in immune compromised individuals. Trypanosoma brucei invasion of the brain leads to the neurological disorders and sleep disturbances that characterize African sleeping sickness. In contrast, red blood cells infected with Plasmodium falciparum do not enter brain tissue, but instead become sequestered in the cerebral microvessels, resulting in vascular obstruction, a defining feature of cerebral malaria (CM). The fungal pathogen Cryptococcus neoformans causes a respiratory infection that can undergo hematogenous spread to the brain and is a major cause of CNS disease in HIV/AIDS patients. The outcomes of these CNS infections vary depending on the pathogen, the immune status of the host, and the stage of infection that occurs in the brain. Recent work in these fields and the use of intravital imaging technologies have significantly expanded our understanding of pathogen entry into the brain as well as revealed areas that require further investigation.
Figure 1.
Eukaryotic pathogens utilize a diverse range of strategies to migrate from the blood to the CNS. A schematic shows the cross section of a blood vessel of the BBB and potential pathways of CNS entry for four global pathogens. T. gondii can invade and replicate in brain endothelial cells and may undergo transendothelial migration either as a free tachyzoite or inside an infected leukocyte. Cerebral malaria is associated with sequestration of P. falciparum-infected red blood cells (iRBCs) in the brain microvasculature and binding of iRBCs to uninfected RBCs in a process known as rosetting. This leads to the obstruction of blood flow and may contribute to breakdown of the BBB and vascular leakage. T. brucei likely crosses the BCB via fenestrated vessels inside the choroid plexus, followed by trafficking to the meninges. Some evidence suggests that enhanced vascular permeability induced by host inflammatory responses may allow the parasite to directly cross the BBB. C. neoformans can cytoadhere to endothelium, often at narrow points in the blood vessels and undergo transcytosis. Endothelial cells that internalize yeast lose their structural integrity, resulting in cell stress and injury.
T. gondii entry into the brain: free parasites or ‘Trojan horse’?
Infection by T. gondii typically occurs via the oral route through the ingestion of parasite cysts. Evidence suggests that the circulation is a major avenue for T. gondii dissemination to distal organs, including the brain. The replicative tachyzoite form of the parasite multiplies in the small intestine [3] and encounters an influx of neutrophils and inflammatory monocytes [4]. Within days after oral infection of mice with tissue cysts, T. gondii can be detected both inside monocytes and as extracellular parasites in the blood [5]. Systemic inflammation is characteristic of in vivo T. gondii infection, and increased BBB permeability has been associated with the development of toxoplasmic encephalitis [6]. Ultimately, the parasites enter the brain parenchyma and establish a chronic infection as bradyzoite-containing tissue cysts.
The precise mechanism by which T. gondii breaches the BBB remains unknown; however, several possibilities have been investigated. Extracellular T. gondii tachyzoites can adhere to human vascular endothelium in conditions of shear stress by using the parasite adhesin MIC2 [7]. After adhesion, tachyzoites may either transmigrate across the endothelial barrier [8] or invade endothelial cells [9], and invasion appears to predominate [7]. Interestingly, human brain endothelial cells are more permissive to T. gondii replication than neurons or microglia [10]. The extracellular parasite can also migrate through multiple tissue layers of the human retina [11]. Infection enhances the expression of the host adhesion molecules ICAM-1, VCAM-1, and ALCAM in the CNS [6,12]. In particular, ICAM-1 interacts with MIC2 to facilitate the migration of extracellular tachyzoites across epithelium [13] and retinal endothelium [8] without disrupting monolayer integrity.
Recent studies have also expanded our understanding of the ‘Trojan horse’ mechanism of dissemination, by which the extravasation of parasitized leukocytes facilitates tachyzoite translocation across barriers. Following oral infection of mice, T. gondii are found in the brain in CD11b+/CD11c− monocytes [5], and a direct comparison of GFP+ intracellular and DsRed+ extracellular parasites injected into mice resulted in a significantly greater number of GFP+ tachyzoites in the brain [14]. More recently, infected human dendritic cells (DCs) and murine monocytes were shown to efficiently transmigrate across retinal and brain endothelium, respectively [12,15], supporting the idea that infected cells may facilitate T. gondii entry into the CNS.
T. gondii infection induces hypermotility in migratory cells, and this phenotype correlates with cytoskeletal rearrangement in infected DCs [16] and monocytes [17]. Interestingly, the DC hypermotility is linked to signaling by the neurotransmitter GABA [18••]. The degree to which hypermotility plays a role in crossing the BBB is still not well understood. Under fluidic shear stress, T. gondii delays the firm adhesion of infected human monocytes on vascular endothelium [17] and enhances their subsequent crawling [19]. This could be due in part to changes in adhesion molecules, since infection impairs integrin clustering [16,17]. T. gondii-infected monocytes appear to undergo TEM (transendothelial migration) via the paracellular route in vitro by a process involving the monocyte surface integrin Mac-1 (CD11b/CD18) and its binding partner ICAM-1 [19]. These findings are consistent with the up-regulation of ICAM-1 on brain endothelial cells and the accumulation of CD11b+ monocytes in the brain during T. gondii infection in vivo [12]. Taken together, hypermotility may facilitate the mobilization of parasitized leukocytes to the CNS while altering the dynamics of their adhesion and motility in the cerebral vasculature. Additionally, since inflammation and vascular permeability change during the course of infection, T. gondii may access the CNS using distinct mechanisms at different stages of the infection. A major focus in the coming years will be to translate many of the in vitro findings described above into in vivo studies. Experiments employing intravital imaging of the brain during acute infection will significantly improve our understanding of the parasite’s remarkable ability to colonize the CNS.
Sequestration of Plasmodium-infected red blood cells in cerebral vessels
In contrast to T. gondii, infection with Plasmodium spp. occurs through an insect vector, during a blood meal of the female Anopheles mosquito. Cerebral malaria (CM) is a severe and often fatal complication of P. falciparum infection, and most cases occur in children. Notably, CM is characterized by vascular dysregulation rather than entry into the brain parenchyma. The cytoadherence of infected red blood cells (iRBCs) in the brain microvasculature is a hallmark of this disease and is associated with vessel obstruction. Post-mortem brain tissue analysis of Malawian children with clinically and pathologically defined CM revealed that iRBC sequestration is associated with myelin and axonal damage, breakdown of the BBB, and glial cell responses [20]. iRBC sequestration is a defining feature of infection with P. falciparum. These parasites harbor the highly polymorphic var genes that encode the virulence factor erythrocyte membrane protein 1 (PfEMP1). PfEMP1 is exported to the knob structures on the surface of the iRBC through a process that relies on various parasite proteins, including the Plasmodium translocon of exported proteins (PTEX) [21] and PfEMP1 trafficking protein 1 (PfPTP1) [22].
PfEMP1 mediates iRBC attachment to vascular endothelium, and both ICAM-1 [23] and CD36 [24,25] have been identified as receptors. P. falciparum isolates expressing the domain cassettes 8 and 13 of PfEMP1 are associated with severe childhood malaria, including severe anemia and CM [26]. Recently, domain cassettes 8 and 13 were found to bind to the endothelial protein C receptor (EPCR) and interfere with the binding of its natural ligand, protein C [27••,28•], which functions in endothelial cytoprotection and the anticoagulant pathway. By examining children in Malawi with CM, Moxon et al. found that EPCR is lost from endothelial cells at sites of iRBC sequestration [29], and in a study of children in Benin, high levels of soluble EPCR in the blood positively correlated with pediatric CM and mortality [30]. These data suggest a link between EPCR dysregulation and coagulation during acute malaria in children.
Rosetting, the binding of multiple uninfected RBCs to sequestered iRBCs, contributes to the formation of aggregates and vascular obstruction. Based on studies using human brain microvascular endothelial cells, distinct domains of PfEMP1 were found to mediate rosetting and cytoadhesion [31]. STEVOR, which is encoded by a multi-gene family, is expressed on the iRBC surface and interacts with Glycophorin C to mediate rosette formation independently of PfEMP1 [32]. The binding of pentameric IgM to iRBCs is also associated with rosetting, and recent data suggest that IgM may function by binding to PfEMP1 and strengthening the interactions between RBCs and iRBCs [33].
There has been debate about the relevance of rodent models of experimental cerebral malaria (ECM) for studying the histopathology of human CM, since the predominant model of ECM, P. berghei ANKA (PbA) infection in susceptible mouse strains, is associated more with leukocyte accumulation in postcapillary venules than iRBC sequestration [34]. Indeed, recent work in ECM models has focused on the role of CD8+ T cells in vessel obstruction. Intravital imaging of PbA-infected mice revealed extensive vascular leakage in postcapillary venules that is associated with platelet margination, leukocyte adhesion, the deposition of fibrin, and death from ECM [35]. This was prevented by treatment with anti-LFA-1 or with the sphingosine analog FTY720, which inhibits lymphocyte egress from lymph nodes [35]. The recruitment of CD8+ T cells, macrophages, and neutrophils to postcapillary venules restricts blood flow, increasing intracranial pressure, and potentially contributing to ECM [36•]. In the PbA model, cross-presentation of an immunodominant CD8+ T cell epitope from glideosome-associated protein 50 (GAP50) was found to contribute to BBB breakdown and ECM development [37]. Despite the notable differences in the pathogenesis of human malaria and rodent models of ECM, an improved understanding of the host response to PbA infection may provide avenues of inquiry for investigation in human cell systems or clinical studies to inform our understanding of human disease.
Two-step invasion by T. brucei spp.: the blood–brain barrier versus the blood–cerebrospinal fluid barrier
T.b. gambiense and T.b. rhodesiense are vector-borne diseases that are transmitted by the tsetse fly and cause African sleeping sickness in humans. Unlike Toxoplasma and Plasmodium, which are obligate intracellular parasites, the trypanosomes are extracellular, free-swimming parasites. The parasite surface is decorated with many copies of a variant surface glycoprotein (VSG) coat that under-goes antigenic variation during the blood stage to evade humoral immunity [38]. CNS infection induces human African trypanosomiasis, and the mechanism by which trypanosomes enter the CNS is a topic of active investigation and debate [39]. The long-standing model suggests a ‘two-step’ mode of invasion, by which parasites in the blood initially cross the BCB in the choroid plexus but ultimately penetrate the BBB and enter the brain parenchyma at a late stage of infection. In infected mice, T.b. brucei migration across the BBB may follow the extravasation of lymphocytes. TNF-α and IFN-α/β release upon TLR-mediated recognition of the parasite up-regulates ICAM-1 and VCAM-1 expression in the CNS and leads to the accumulation of T cells and parasites in the parenchyma [40••]. This could be due in part to MyD88-induced production of IFN-γ and CXCL10, which are upregulated in blood vessel-associated cells during infection [41]. In vitro studies have suggested that T.b. gambiense potentially migrates across human brain endothelium via both paracellular and transcellular pathways [42]. Brucipain, a cysteine protease secreted by T.b. gambiense, enhances parasite TEM, possibly by targeting GPCR-mediated calcium flux in endothelium to dysregulate barrier permeability [43].
Emerging reports build upon the above model by further dissecting the avenues of T. brucei spp. migration into the CNS. Wolburg et al. used an electron microscopy approach to visualize the sequential stages of T.b. brucei brain infection in rats [44•]. Beginning around 20 days post-injection, trypanosomes were found inside the fenestrated vessels, the stroma, and the ventricle of the choroid plexus and within the meninges, near the pial cells. Parasites were found in the blood vessels but not in the surrounding parenchyma. The authors thus concluded that the direct access to the parenchyma by the bloodstream form is unlikely and that T.b. brucei likely migrates out of the choroid plexus and to the leptomeninges, either by crossing the BCB into the CSF current to the subarachnoid space or via the Virchow–Robin space, which extends from the pia mater alongside blood vessels into the brain [44•]. Frevert et al. utilized intravital microscopy in mice to characterize T.b. brucei interactions in the brain. They visualized parasite entry into the parenchyma as early as 5 hours post-intravenous (IV) injection and captured subsequent parasite replication in the parenchyma [45•]. Notably, parasite transmigration occurs at postcapillary venules even before the onset of host inflammation, leukocyte recruitment, and vascular leakage [45•]. These contrasting studies are just beginning to broaden our appreciation for the complexity of trypanosome entry into the CNS.
The observations by Wolberg et al. and Frevert et al. could be reconciled by differences between early and late stages of encephalitic trypanosomiasis as well as by recent findings demonstrating the dynamic and cyclical nature by which T.b. brucei appears in the CSF [46]. While the majority of trypanosomes likely invade the CNS via the choroid plexus, it is possible that a subset of parasites extravasates across the BBB, triggering the production of glial cell cytokines and chemokines that promote endothelial permeability [41]. Trafficking across the BCB may not be a requirement for reaching the meninges, and this is supported by the detection of T.b. brucei in the meningeal blood vessels and in the extravascular space by intravital microscopy within days post-IV injection in mice [47]. However, it is important to note that the stromal matrix, which is easily accessed via the fenestrated vessels, is also populated by resident macrophages and DCs [48], which could amplify host innate immune responses upon parasite recognition by TLRs. Therefore, T. brucei spp. may employ a variant of the two-step invasion model: in combination with direct migration across the BBB, entry via the choroid plexus may represent the path of least resistance into the CNS. Further work will certainly lead to a better understanding of this important aspect of T. brucei spp. infection and of how CNS infection ultimately leads to chronic cerebral trypanosomiasis.
Transcellular migration: C. neoformans penetration of the brain vascular endothelium
C. neoformans is a prevalent yeast fungal pathogen and the leading cause of CNS infection in HIV/AIDS patients. The pathology of cryptococcal meningitis has been well reviewed elsewhere [49]. Unlike the pathogens discussed above, transmission of Cryptococcus occurs through the airways. Following the inhalation of fungal spores, C. neoformans establishes infection in the lungs. During reactivation due to immune compromise, C. neoformans can spread in the blood as extracellular yeast cells. The infection most frequently presents as meningitis, but parenchymal infection also occurs. Transcytosis of the yeast can occur across human brain microvascular endothelial cells, and in a mouse model of IV infection, C. neoformans associates with endothelial cells in the brain [50]. The molecular interactions that mediate CNS infection are of particular interest. C. neoformans utilizes inositol, an abundant metabolite in the CNS, to synthesize hyaluronic acid [51], which decorates the yeast capsule [52]. CD44, the hyaluronic acid receptor on brain endothelial cells, induces the lipid raft-mediated endocytosis of C. neoformans [53]. Consistent with these findings, mice deficient in CD44 have reduced C. neoformans brain invasion and fungal burden and enhanced survival [50,54•,55]. Similarly, the ablation of inositol metabolism by yeast cells decreases the formation of cystic lesions in the parenchyma and enhances survival in mice [51]. Additionally, transcytosis of C. neoformans may be accompanied by modification of the endothelium by yeast factors [56,57,58••,59].
Advances in intravital imaging have permitted visualization of yeast cells lodging in brain capillaries, frequently at branch points in the vessels, prior to crossing the BBB [56]. Although C. neoforms can undergo passive uptake by endothelial cells [55], studies indicate that entry into the CNS is actively driven by the pathogen: BBB penetration by C. neoformans is dependent on yeast urease, which can lead to breakdown of the endothelium via the production of highly toxic ammonia from urea [56]. Additionally, C. neoformans-derived serine proteases compromise barrier resistance of bovine [60] and human brain endothelium [59,61] in vitro. Mpr1, a newly identified secreted fungal metalloprotease, facilitates C. neoformans cytoadherence and transendothelial migration (TEM) across human cerebral microvascular endothelium [58••]. Yeast deficient in Mpr1 fail to induce brain pathology, which prolongs survival in mice. Strikingly, mutants lacking Mpr1 establish a fungal burden comparable to that of wild-type yeast, suggesting that Mpr1 is a virulence factor specific for CNS invasion [58••].
The host cell response to the uptake of C. neoformans is an area of active study. A proteomic survey of human brain endothelial cells following exposure to C. neoformans demonstrated marked changes in the expression of metabolic and cytoskeletal factors, and analysis by transmission electron microscopy revealed structural damage to the organelles as well as cell injury and death [62•]. Rearrangement of host actin may be a critical step in C. neoformans transcellular passage across the BBB, and evidence supports the targeting of host Rho GTPases and downstream activation of focal adhesion kinase and ezrin by yeast, possibly due to binding to CD44 [63]. Furthermore, the C. neoformans phospholipase Plb1 associates with and activates the Rho GTPase Rac1 for TEM across human brain endothelium [57]. Collectively, these studies suggest that C. neoformans traffics through brain endothelial cells, disrupting endothelial barrier integrity, and that the pathogen possesses a diverse set of highly evolved virulence factors for facilitating this process.
Concluding remarks
There is a tremendous interest in defining the mechanisms of entry into the brain for pathogens that cause debilitating CNS disease. Recent studies have yielded valuable insight into the diverse routes of eukaryotic pathogen entry into the CNS, and as noted for T. gondii and T. brucei, some pathogens may utilize more than one mechanism to breach the CNS barrier. Despite the growing body of work in this area, more studies are needed to precisely define the molecular interactions mediating CNS infection. Future studies using transgenic or knock-out mice, genetic manipulation of the pathogen, and high resolution intravital imaging techniques will undoubtedly provide novel insight into this important area of pathogenesis.
Acknowledgements
We would like to thank all the members of the Lodoen lab as well as Naomi Morrissette, Kent Hill, Anita Koshy, and Christopher Hunter for insightful discussions about pathogen dissemination and infection of the CNS.
References and recommended reading
Papers of particular interest, published recently, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Serlin Y, Shelef I, Knyazer B, Friedman A. Anatomy and Physiology of the Blood–Brain Barrier. Seminars in Cell & Developmental Biology. Elsevier; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbott NJ, Ronnback L, Hansson E: Astrocyte–endothelial interactions at the blood–brain barrier. Nat Rev Neurosci 2006, 7:41–53. [DOI] [PubMed] [Google Scholar]
- 3.Gregg B, Taylor BC, John B, Tait-Wojno ED, Girgis NM, Miller N, Wagage S, Roos DS, Hunter CA: Replication and distribution of Toxoplasma gondii in the small intestine after oral infection with tissue cysts. Infect Immun 2013, 81:1635–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coombes JL, Charsar BA, Han SJ, Halkias J, Chan SW, Koshy AA, Striepen B, Robey EA: Motile invaded neutrophils in the small intestine of Toxoplasma gondii-infected mice reveal a potential mechanism for parasite spread. Proc Natl Acad Sci USA 2013, 110:E1913–E1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Courret N, Darche S, Sonigo P, Milon G, Buzoni-Gatel D, Tardieux I: CD11c- and CD11b-expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood 2006, 107:309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silva NM, Manzan RM, Carneiro WP, Milanezi CM, Silva JS, Ferro EA, Mineo JR: Toxoplasma gondii: the severity of toxoplasmic encephalitis in C57BL/6 mice is associated with increased ALCAM and VCAM-1 expression in the central nervous system and higher blood–brain barrier permeability. Exp Parasitol 2010, 126:167–177. [DOI] [PubMed] [Google Scholar]
- 7.Harker KS, Jivan E, McWhorter FY, Liu WF, Lodoen MB: Shear forces enhance Toxoplasma gondii tachyzoite motility on vascular endothelium. mBio 2014, 5:e01111–e01113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furtado JM, Bharadwaj AS, Chipps TJ, Pan Y, Ashander LM, Smith JR: Toxoplasma gondii tachyzoites cross retinal endothelium assisted by intercellular adhesion molecule-1 in vitro. Immunol Cell Biol 2012, 90:912–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zamora DO, Rosenbaum JT, Smith JR: Invasion of human retinal vascular endothelial cells by Toxoplasma gondii tachyzoites. Br J Ophthalmol 2008, 92:852–855. [DOI] [PubMed] [Google Scholar]
- 10.Mammari N, Vignoles P, Halabi MA, Darde ML, Courtioux B: In vitro infection of human nervous cells by two strains of Toxoplasma gondii: a kinetic analysis of immune mediators and parasite multiplication. PLoS One 2014, 9:e98491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furtado JM, Ashander LM, Mohs K, Chipps TJ, Appukuttan B, Smith JR: Toxoplasma gondii migration within and infection of human retina. PLoS One 2013, 8:e54358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lachenmaier SM, Deli MA, Meissner M, Liesenfeld O: Intracellular transport of Toxoplasma gondii through the blood–brain barrier. J Neuroimmunol 2011, 232:119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barragan A, Brossier F, Sibley LD: Transepithelial migration of Toxoplasma gondii involves an interaction of intercellular adhesion molecule 1 (ICAM-1) with the parasite adhesin MIC2. Cell Microbiol 2005, 7:561–568. [DOI] [PubMed] [Google Scholar]
- 14.Unno A, Suzuki K, Xuan X, Nishikawa Y, Kitoh K, Takashima Y: Dissemination of extracellular and intracellular Toxoplasma gondii tachyzoites in the blood flow. Parasitol Int 2008, 57:515–518. [DOI] [PubMed] [Google Scholar]
- 15.Furtado JM, Bharadwaj AS, Ashander LM, Olivas A, Smith JR: Migration of Toxoplasma gondii-infected dendritic cells across human retinal vascular endothelium. Invest Ophthalmol Vis Sci 2012, 53:6856–6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weidner JM, Kanatani S, Hernandez-Castaneda MA, Fuks JM, Rethi B, Wallin RP, Barragan A: Rapid cytoskeleton remodelling in dendritic cells following invasion by Toxoplasma gondii coincides with the onset of a hypermigratory phenotype. Cell Microbiol 2013, 15:1735–1752. [DOI] [PubMed] [Google Scholar]
- 17.Harker KS, Ueno N, Wang T, Bonhomme C, Liu W, Lodoen MB: Toxoplasma gondii modulates the dynamics of human monocyte adhesion to vascular endothelium under fluidic shear stress. J Leukocyte Biol 2013, 93:789–800. [DOI] [PubMed] [Google Scholar]
- 18.••. Fuks JM, Arrighi RB, Weidner JM, Kumar Mendu S, Jin Z, Wallin RP, Rethi B, Birnir B, Barragan A: GABAergic signaling is linked to a hypermigratory phenotype in dendritic cells infected by Toxoplasma gondii. PLoS Pathog 2012, 8:e1003051. Demonstrates a role for the neurotransmitter GABA in T. gondii-induced DC hypermotility.
- 19. Ueno N, Harker KS, Clarke EV, McWhorter FY, Liu WF, Tenner AJ, Lodoen MB: Real-time imaging of Toxoplasma-infected human monocytes under fluidic shear stress reveals rapid translocation of intracellular parasites across endothelial barriers. Cell Microbiol 2014, 16:580–595. Uses real-time imaging to show that T. gondii-infected monocytes undergo rapid TEM in shear stress conditions by a process dependent on Mac-1.
- 20.Dorovini-Zis K, Schmidt K, Huynh H, Fu W, Whitten RO, Milner D, Kamiza S, Molyneux M, Taylor TE: The neuropathology of fatal cerebral malaria in Malawian children. Am J Pathol 2011, 178:2146–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elsworth B, Matthews K, Nie CQ, Kalanon M, Charnaud SC, Sanders pR, Chisholm SA, Counihan NA, Shaw PJ, Pino P et al. : PTEX is an essential nexus for protein export in malaria parasites. Nature 2014, 511:587–591. [DOI] [PubMed] [Google Scholar]
- 22.Rug M, Cyrklaff M, Mikkonen A, Lemgruber L, Kuelzer S, Sanchez CP, Thompson J, Hanssen E, O’Neill M, Langer C et al. : Export of virulence proteins by malaria-infected erythrocytes involves remodeling of host actin cytoskeleton. Blood 2014, 124:3459–3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berendt AR, Simmons DL, Tansey J, Newbold CI, Marsh K: Intercellular adhesion molecule-1 is an endothelial cell adhesion receptor for Plasmodium falciparum. Nature 1989, 341:57–59. [DOI] [PubMed] [Google Scholar]
- 24.Barnwell JW, Asch AS, Nachman RL, Yamaya M, Aikawa M, Ingravallo P: A human 88-kD membrane glycoprotein (CD36) functions in vitro as a receptor for a cytoadherence ligand on Plasmodium falciparum-infected erythrocytes. J Clin Invest 1989, 84:765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oquendo P, Hundt E, Lawler J, Seed B: CD36 directly mediates cytoadherence of Plasmodium falciparum parasitized erythrocytes. Cell 1989, 58:95–101. [DOI] [PubMed] [Google Scholar]
- 26.Lavstsen T, Turner L, Saguti F, Magistrado P, Rask TS, Jespersen JS, Wang CW, Berger SS, Baraka V, Marquard AM et al. : Plasmodium falciparum erythrocyte membrane protein 1 domain cassettes 8 and 13 are associated with severe malaria in children. Proc Natl Acad Sci USA 2012, 109:E1791–E1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.••. Turner L, Lavstsen T, Berger SS, Wang CW, Petersen JE, Avril M, Brazier AJ, Freeth J, Jespersen JS, Nielsen MA et al. : Severe malaria is associated with parasite binding to endothelial protein C receptor. Nature 2013, 498:502–505. Identifies the endothelial protein C receptor (EPCR) as a novel ligand for PfEMP1.
- 28.•. Lau CK, Turner L, Jespersen JS, Lowe ED, Petersen B, Wang CW, Petersen JE, Lusingu J, Theander TG, Lavstsen T et al. : Structural conservation despite huge sequence diversity allows EPCR binding by the PfEMP1 family implicated in severe childhood malaria. Cell Host Microbe 2015, 17:118–129. Demonstrates that highly diverse variants of the CIDR(alpha)1 domain of PfEMP1 can bind to EPCR and interferes with binding by activated protein C.
- 29.Moxon CA, Wassmer SC, Milner DA Jr, Chisala NV, Taylor TE, Seydel KB, Molyneux ME, Faragher B, Esmon CT, Downey C et al. : Loss of endothelial protein C receptors links coagulation and inflammation to parasite sequestration in cerebral malaria in African children. Blood 2013, 122:842–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moussiliou A, Alao MJ, Denoeud-Ndam L, Tahar R, Ezimegnon S, Sagbo G, Amoussou A, Luty AJ, Deloron P, Tuikue Ndam N: High plasma levels of soluble endothelial protein C receptor are associated with increased mortality among children with cerebral malaria in benin. J Infect Dis 2014, 211:1484–1488. [DOI] [PubMed] [Google Scholar]
- 31.Adams Y, Kuhnrae P, Higgins MK, Ghumra A, Rowe JA: Rosetting Plasmodium falciparum-infected erythrocytes bind to human brain microvascular endothelial cells in vitro, demonstrating a dual adhesion phenotype mediated by distinct P. falciparum erythrocyte membrane protein 1 domains. Infect Immun 2014, 82:949–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niang M, Bei AK, Madnani KG, Pelly S, Dankwa S, Kanjee U, Gunalan K, Amaladoss A, Yeo KP, Bob NS et al. : STEVOR is a Plasmodium falciparum erythrocyte binding protein that mediates merozoite invasion and rosetting. Cell Host Microbe 2014, 16:81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevenson L, Huda P, Jeppesen A, Laursen E, Rowe JA, Craig A, Streicher W, Barfod L, Hviid L: Investigating the function of F-specific binding of IgM to Plasmodium falciparum erythrocyte membrane protein 1 mediating erythrocyte rosetting. Cell Microbiol 2014, 17:819–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Craig AG, Grau GE, Janse C, Kazura JW, Milner D, Barnwell JW, Turner G, Langhorne J: The role of animal models for research on severe malaria. PLoS Pathog 2012, 8:e1002401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nacer A, Movila A, Baer K, Mikolajczak SA, Kappe SH, Frevert U: Neuroimmunological blood brain barrier opening in experimental cerebral malaria. PLoS Pathog 2012, 8:e1002982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.•. Nacer A, Movila A, Sohet F, Girgis NM, Gundra UM, Loke P, Daneman R, Frevert U: Experimental cerebral malaria pathogenesis – hemodynamics at the blood brain barrier. PLoS Pathog 2014, 10:e1004528. Uses intravital microscopy to compare rodent models of Plasmodium infection that develop either ECM or hyperparasitemia and shows that ECM is associated with the accumulation of activated leukocytes in the cerebral microvasculature.
- 37.Howland SW, Poh CM, Gun SY, Claser C, Malleret B, Shastri N, Ginhoux F, Grotenbreg GM, Renia L: Brain microvessel cross-presentation is a hallmark of experimental cerebral malaria. EMBO Mol Med 2013, 5:916–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horn D: Antigenic variation in African trypanosomes. Mol Biochem Parasitol 2014, 195:123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mogk S, Meiwes A, Bosselmann CM, Wolburg H, Duszenko M: The lane to the brain: how African trypanosomes invade the CNS. Trends Parasitol 2014, 30:470–477. [DOI] [PubMed] [Google Scholar]
- 40.••. Amin DN, Vodnala SK, Masocha W, Sun B, Kristensson K, Rottenberg ME: Distinct Toll-like receptor signals regulate cerebral parasite load and interferon alpha/beta and tumor necrosis factor alpha-dependent T-cell infiltration in the brains of Trypanosoma brucei-infected mice. J Infect Dis 2012, 205:320–332. Shows TLR-MyD88-mediated host inflammation in response to T.b. brucei mediates the infiltration of T cells and parasites into the brain parenchyma.
- 41.Amin DN, Rottenberg ME, Thomsen AR, Mumba D, Fenger C, Kristensson K, Buscher P, Finsen B, Masocha W: Expression and role of CXCL10 during the encephalitic stage of experimental and clinical African trypanosomiasis. J Infect Dis 2009, 200:1556–1565. [DOI] [PubMed] [Google Scholar]
- 42.Nikolskaia OV, Kim YV, Kovbasnjuk O, Kim KJ, Grab DJ: Entry of Trypanosoma brucei gambiense into microvascular endothelial cells of the human blood–brain barrier. Int J Parasitol 2006, 36:513–519. [DOI] [PubMed] [Google Scholar]
- 43.Grab DJ, Garcia-Garcia JC, Nikolskaia OV, Kim YV, Brown A, Pardo CA, Zhang Y, Becker KG, Wilson BA, de ALAP et al. : Protease activated receptor signaling is required for African trypanosome traversal of human brain microvascular endothelial cells. PLoS Negl Trop Dis 2009, 3:e479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.•. Wolburg H, Mogk S, Acker S, Frey C, Meinert M, Schonfeld C, Lazarus M, Urade Y, Kubata BK, Duszenko M: Late stage infection in sleeping sickness. PLoS One 2012, 7:e34304. Uses intraperitoneally infected rats to show that T.b. brucei migrates through the choroid plexus and resides in the meninges at late stages of brain infection.
- 45.•. Frevert U, Movila A, Nikolskaia OV, Raper J, Mackey ZB, Abdulla M, McKerrow J, Grab DJ: Early invasion of brain parenchyma by African trypanosomes. PLoS One 2012, 7:e43913. Visualizes rapid T. b. brucei migration across the BBB and subsequent replication in the parenchyma of IV-injected mice using intravital microscopy.
- 46.Mogk S, Meiwes A, Shtopel S, Schraermeyer U, Lazarus M, Kubata B, Wolburg H, Duszenko M: Cyclical appearance of African trypanosomes in the cerebrospinal fluid: new insights in how trypanosomes enter the CNS. PLoS One 2014, 9:e91372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Myburgh E, Coles JA, Ritchie R, Kennedy PG, McLatchie AP, Rodgers J, Taylor MC, Barrett mP, Brewer JM, Mottram JC: In vivo imaging of trypanosome-brain interactions and development of a rapid screening test for drugs against CNS stage trypanosomiasis. PLoS Negl Trop Dis 2013, 7:e2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meeker RB, Williams K, Killebrew DA, Hudson LC: Cell trafficking through the choroid plexus. Cell Adhes Migrat 2012, 6:390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu TB, Perlin DS, Xue C: Molecular mechanisms of cryptococcal meningitis. Virulence 2012, 3:173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang YC, Stins MF, McCaffery MJ, Miller GF, Pare DR, Dam T, Paul-Satyaseela M, Kim KS, Kwon-Chung KJ: Cryptococcal yeast cells invade the central nervous system via transcellular penetration of the blood–brain barrier. Infect Immun 2004, 72:4985–4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu TB, Kim JC, Wang Y, Toffaletti DL, Eugenin E, Perfect JR, Kim KJ, Xue C: Brain inositol is a novel stimulator for promoting Cryptococcus penetration of the blood–brain barrier. PLoS Pathog 2013, 9:e1003247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jong A, Wu CH, Chen HM, Luo F, Kwon-Chung KJ, Chang YC, Lamunyon CW, Plaas A, Huang SH: Identification and characterization of CPS1 as a hyaluronic acid synthase contributing to the pathogenesis of Cryptococcus neoformans infection. Eukaryotic Cell 2007, 6:1486–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang SH, Long M, Wu CH, Kwon-Chung KJ, Chang YC, Chi F, Lee S, Jong A: Invasion of Cryptococcus neoformans into human brain microvascular endothelial cells is mediated through the lipid rafts-endocytic pathway via the dual specificity tyrosine phosphorylation-regulated kinase 3 (DYRK3). J Biol Chem 2011, 286:34761–34769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.•. Jong A, Wu CH, Gonzales-Gomez I, Kwon-Chung KJ, Chang YC, Tseng HK, Cho WL, Huang SH: Hyaluronic acid receptor CD44 deficiency is associated with decreased Cryptococcus neoformans brain infection. J Biol Chem 2012, 287:15298–15306. Uses a knock-out model to demonstrate the role of CD44 in C. neoformans colonization in the infected mouse brain.
- 55.Sabiiti W, May RC: Capsule independent uptake of the fungal pathogen Cryptococcus neoformans into brain microvascular endothelial cells. PLoS One 2012, 7:e35455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi M, Li SS, Zheng C, Jones GJ, Kim KS, Zhou H, Kubes P, Mody CH: Real-time imaging of trapping and urease-dependent transmigration of Cryptococcus neoformans in mouse brain. J Clin Invest 2010, 120:1683–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maruvada R, Zhu L, Pearce D, Zheng Y, Perfect J, Kwon-Chung KJ, Kim KS: Cryptococcus neoformans phospholipase B1 activates host cell Rac1 for traversal across the blood–brain barrier. Cell Microbiol 2012, 14:1544–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.••. Vu K, Tham R, Uhrig JP, Thompson GR 3rd, Na Pombejra S, Jamklang M, Bautos JM, Gelli A: Invasion of the central nervous system by Cryptococcus neoformans requires a secreted fungal metalloprotease. mBio 2014, 5:e01101–e01114. Identifies novel metalloprotease Mpr1 that specifically targets brain endothelium and is required for establishing fungal disease in the CNS.
- 59.Xu CY, Zhu HM, Wu JH, Wen H, Liu CJ: Increased permeability of blood–brain barrier is mediated by serine protease during Cryptococcus meningitis. J Int Med Res 2014, 42:85–92. [DOI] [PubMed] [Google Scholar]
- 60.Stie J, Fox D: Blood–brain barrier invasion by Cryptococcus neoformans is enhanced by functional interactions with plasmin. Microbiology 2012, 158:240–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stie J, Fox D: Induction of brain microvascular endothelial cell urokinase expression by Cryptococcus neoformans facilitates blood–brain barrier invasion. PLoS One 2012, 7:e49402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.•. Vu K, Eigenheer RA, Phinney BS, Gelli A: Cryptococcus neoformans promotes its transmigration into the central nervous system by inducing molecular and cellular changes in brain endothelial cells. Infect Immun 2013, 81:3139–3147. Reveals dysregulation of several cytoskeletal and metabolic proteins in C. neoformans-associated endothelial cells and visualizes host cell stress and injury.
- 63.Kim JC, Crary B, Chang YC, Kwon-Chung KJ, Kim KJ: Cryptococcus neoformans activates RhoGTPase proteins followed by protein kinase C, focal adhesion kinase, and ezrin to promote traversal across the blood–brain barrier. J Biol Chem 2012, 287:36147–36157. [DOI] [PMC free article] [PubMed] [Google Scholar]