Abstract
Therapies modulating the immune system offer the prospect of treating a wide range of conditions including infectious diseases, cancer and autoimmunity. Biomaterials can promote specific targeting of immune cell subsets in peripheral or lymphoid tissues and modulate the dosage, timing and location of stimulation, thereby improving safety and efficacy of vaccines and immunotherapies. Here we review recent advances in biomaterials-based strategies, focusing on targeting of lymphoid tissues, circulating leukocytes, tissue-resident immune cells and immune cells at disease sites. These approaches can improve the potency and efficacy of immunotherapies by promoting immunity or tolerance against different diseases.
Short Summary
This Review discusses biomaterials that promote therapeutic targeting of immune cells by modulating the dosage, timing and location of stimulation, thereby improving the safety and efficacy of vaccines and immunotherapies.
Introduction
Modulating the immune system is a pivotal treatment strategy of modern medicine. In 2021, seven of the top 10 drugs by sales act on the immune system, including, notably, the messenger RNA (mRNA)-based vaccines for COVID-191. Beyond its established role in vaccine development, immunomodulation holds therapeutic potential against different conditions including autoimmunity and cancer, as well as inflammatory, fibrotic and infectious diseases. However, immunomodulation is a two-edged sword, as most clearly demonstrated in the field of cancer immunotherapy, where despite inducing substantial anti-tumour immunity, systemic administration of many immunotherapy drugs has resulted in immune-related adverse events distal to tumour sites2. Therefore, delivering precise dosages of immunomodulatory drugs with spatiotemporal control to specific cells and tissues, while avoiding unwanted off-target stimulation, is essential to ensure a safe and effective immune response.
Targeting of immune cells can be achieved using traditional protein engineering strategies, employing monoclonal antibodies, engineered binding proteins or recombinant native ligands for immune cell surface receptors. These approaches are at various stages of clinical development, with mono- and multi-specific antibodies being the most advanced3–7. However, the only cell surface protein that truly defines a lymphocyte as disease relevant is its antigen receptor, making highly specific targeting challenging. In addition, simply linking an antibody domain to an immunomodulatory drug is not always sufficient to achieve effective targeting, as the therapeutic payload may dominate the biodistribution of such fusions8. 8 Engineered biomaterials can introduce additional functionality beyond simple binding to target cells by concentrating immunotherapy agents in specific tissue sites, controlling their release kinetics and their intracellular localization.
The immune system consists of well-defined regional control centres (lymphoid organs), important tissue-resident cell populations (especially at barrier tissues such as mucosal surfaces) and mobile cell populations that constantly recirculate through the blood and tissues, providing both challenges and opportunities as a therapeutic target (Fig. 1a). Here we review recent advances of biomaterials-enabled therapeutic strategies for in vivo targeted modulation of the immune system. We focus on approaches targeting immune cells and lymphoid organs, excluding direct targeting of pathogens such as viruses or bacteria, followed by a discussion on prospects and challenges for future developments in this area.
Figure 1 |. Primary and secondary lymphoid organs.
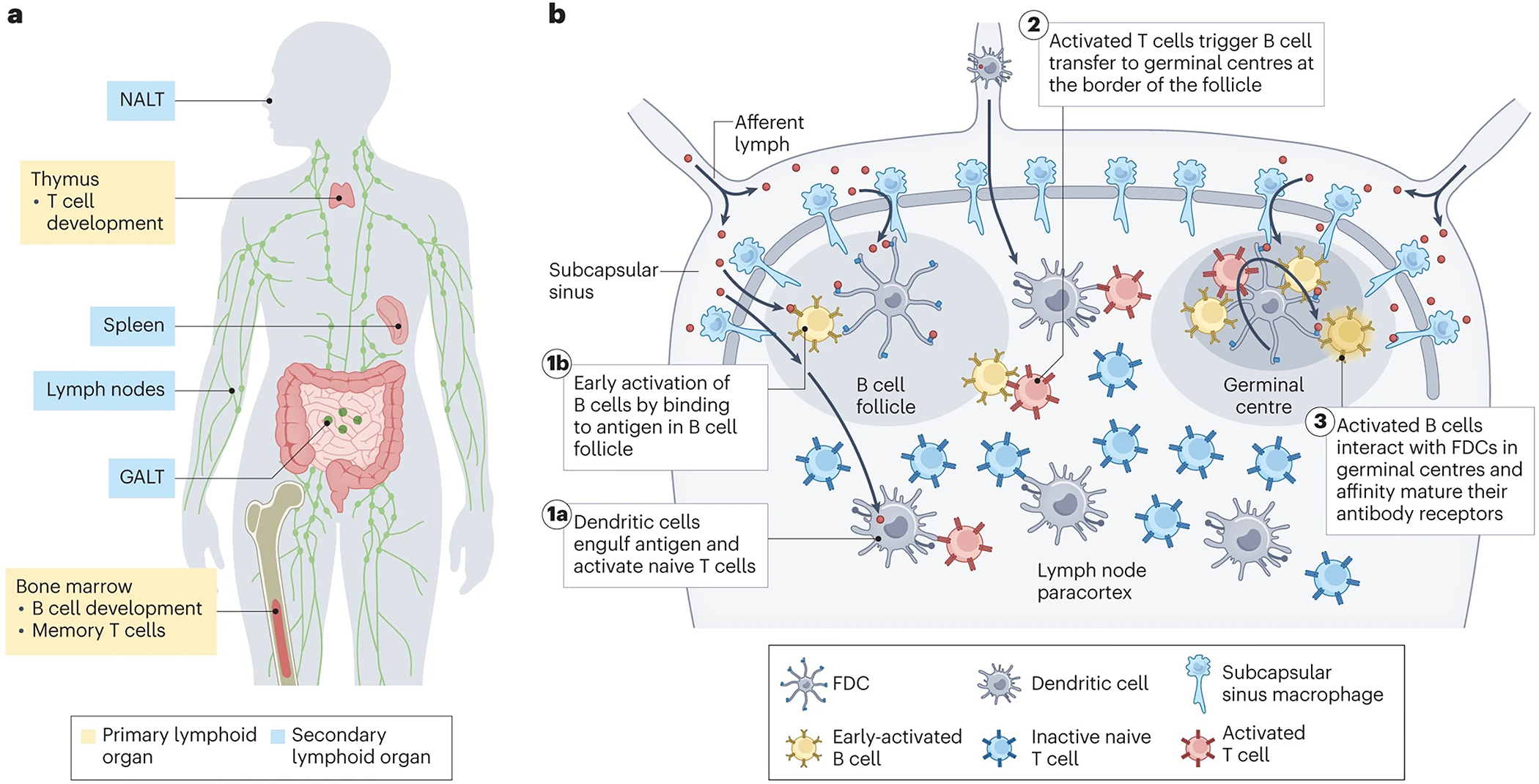
a | Anatomic distribution of primary and secondary lymphoid organs. NALT, nasal-associated lymphoid tissue; GALT, gut-associated lymphoid tissue. b | General organization of secondary lymphoid organs and sites of key interactions leading to adaptive immunity, using the lymph node (LN) as an example. Shown are orchestrated steps in the early activation of adaptive immune response in response to an antigen (orange): (1a) dendritic cells (DCs, light purple) acquire antigens trafficked into the LN or migrate to the LN from peripheral tissues carrying antigens, which they then present to naïve T cells (blue) to drive T cell activation and proliferation. (1b) B cells (orange) bind to antigens arriving in follicles, triggering initial B cell activation and proliferation. (2) Early-activated B cells receive help signals from CD4+ T cells at the T zone-follicle border, providing signals to drive entry into germinal centres. (3) Activated B cells enter germinal centres where they undergo proliferation and somatic hypermutation to affinity mature their antibody receptor through interactions with follicular helper T cells and the antigens captured on the dendrites of follicular dendritic cells (FDCs).
Lymphoid organs as target
Lymphoid organs coordinate the maturation and migration of immune cells while organizing and regulating immune responses (Fig. 1a). Primary lymphoid organs in adults include the bone marrow and thymus, which serve as niches for lymphocyte development. Secondary lymphoid organs, which include 600–800 lymph nodes (LNs) distributed across the body, the spleen and mucosa-associated lymphoid tissue, house and organize T cells, B cells and antigen presenting cells (APCs). These organs serve as command centres of adaptive immunity by activating naïve B and T lymphocytes (Fig. 1a–b), and are thus natural targets for vaccines and immunotherapies.
Principles of lymph node targeting
Passive targeting of therapeutics to lymphatic vessels.
LNs interface with peripheral tissues through lymphatic vessels, which drain lymph fluid from all tissues and provide a conduit for immune cell trafficking. Drugs and vaccines can be targeted to LNs through lymphatic uptake following parenteral injection. A major factor influencing lymphatic targeting is the physical size of the injected agents: following injection into tissue (including tumours), particles larger than 50–100 nm in diameter become trapped in the extracellular matrix (ECM), whereas particles in the size range of 5–50 nm convect with lymph into the lymphatic vessels, and flow to draining LNs (dLNs). By contrast, particles smaller than 5 nm partition preferentially into the blood rather than the lymph (Fig. 2a)9,10. These are approximate size ranges that depend on the tissue site of injection (which can vary in ECM and lymphatic composition) and factors such as injection volume and rate (which can cause mechanical expansion of flaps mediating entry into lymphatic vessels), especially in small animal models. Furthermore, particle shape, charge and surface chemistry can also play a role by promoting or hindering convection through the tissue and lymphatic entry11. Notably, capturing particles at the downstream dLN is a less-studied but equally important prerequisite for LN modulation, as evidenced by data showing that proteins12 and small hydrophilic nanoparticles (NPs)13 can pass through the entire lymphatic chain, reach the thoracic duct and enter the systemic circulation following a parenteral injection. In this regard, using adjuvants that promote compound entry into LNs14, or designing carriers that stimulate recognition by macrophages lining the subcapsular and medullary sinuses can prove useful15.
Figure 2 |. Targeting therapeutics to tissue-draining lymph nodes.
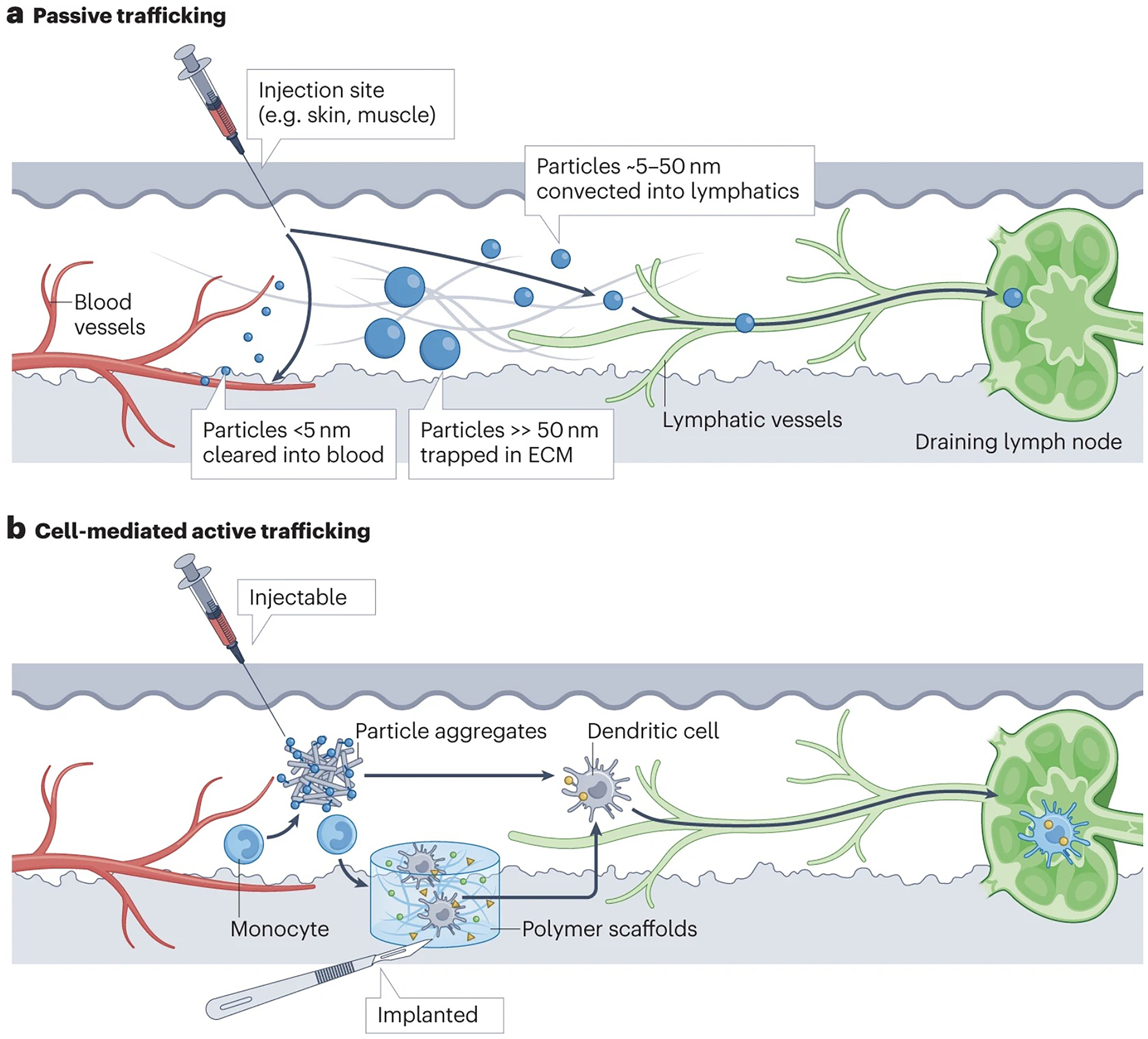
a | Passive targeting of lymphatics through particle size. Particles < 5 nm in diam. are preferentially cleared into the blood vasculature, while particles > 50 nm in diam. tend to become trapped in the tissue. Intermediate sized particles (5–50 nm diam.) exhibit preferential trafficking into lymphatic vessels. ECM, extracellular matrix. b | Targeting migratory leukocytes to traffic vaccines and therapeutics to draining lymph nodes. Shown are examples of injectable or implantable biomaterials that attract monocytes or dendritic cells from the local tissue and peripheral blood. The cells are then stimulated by the implanted material, triggering activation and differentiation into migratory antigen presenting cells that carry antigens or other compounds to the draining lymph nodes.
Protein NPs with surface-arrayed antigens and a size optimized for efficient lymphatic uptake enhance the immunogenicity of vaccines16–20. Protein NP vaccines are currently in clinical trials for human immunodeficiency virus (HIV), SARS-CoV-2 and influenza, and have been shown to be safe and elicit potent neutralizing antibody responses in humans21,22 (Table 1). Lipid nanodiscs and block copolymer micelles smaller than 50 nm also efficiently traffic and deliver compounds into lymphatics 23,24. Cage-like NPs formed by the self-assembly of saponin and lipids (termed ISCOMs) are potent vaccine adjuvants with an ideal size (~40 nm) for LN targeting, and recently received emergency use approval in the US against SARS-CoV-2 (EudraCT: 2020–004123-16)25. Another strategy to promote lymphatic uptake is to exploit albumin and lipoproteins that naturally traffic from blood to lymph. Amphiphilic conjugates consisting of peptides, proteins or small molecules linked to an albumin-binding lipid tail through a hydrophilic poly(ethylene glycol) (PEG) spacer (amph-vaccines), bind to endogenous albumin in the tissue following parenteral injection, improving LN targeting26–29. In one example, conjugation of an albumin-binding lipid tail to a CpG oligonucleotide adjuvant resulted in a 12-fold greater area-under-the-curve for total LN exposure following subcutaneous immunization in mice, compared with unmodified CpG26. This approach is currently being tested in the clinic for LN targeting of a cancer vaccine (NCT04853017; Table 1)30. LN-targeting NPs are also being used to deliver latency-reverting drugs in HIV cure strategies31 and to induce immunological tolerance32–35. NPs composed of self-assembled proteins, synthetic bioresorbable polymers or lipid assemblies, have different in vivo lifetimes and stabilities that can impact their functionality. For example, proteolysis of protein NPs vaccines could lead to particle disassembly and loss of antigen multimerization. However, such effects have received limited attention to date.
Table 1 |.
Ongoing clinical trials of immune cell- and tissue-targeted biomaterials therapeutics
| Immune cell and target site | Technology | Phase | Condition or disease | Clinical trial identifier | Refs. |
|---|---|---|---|---|---|
| Migratory APCs | Polymer scaffolds carrying tumor antigen, GM-CSF and CpG as a cancer vaccine | 1 | Stage IV melanoma | NCT01753089 | 36,37 |
| B cell follicles in LNs | eOD-GT8 60mer protein NP vaccine | 1 | HIV and AIDS | NCT03547245 | 17,68,70 |
| B cell follicles in LNs | Influenza HA ferritin NP vaccine | 1 | Influenza |
NCT03186781
|
21 |
| LNs | GBP510 SARS-CoV-2 designed protein NP vaccine | 1/2, 3 |
COVID-19 |
NCT04750343, NCT05007951 | 22 |
| LNs | Amph-CpG7909 combined with amph-KRAS peptide antigen cancer vaccine |
1 | KRAS mutated PDAC and other solid tumor cancers | NCT04853017 | 26–28,30 |
| LNs | Matrix M adjuvant in Novavax COVID-19 vaccine | approved | COVID-19 | NCT05463068, NCT05468736, NCT05112848 | 25 |
| Systemic APCs | LNP vaccine encapsulating mRNA encoding the viral oncogenes E6 and E7 | 2 | Head and neck cancer | NCT04534205 | 79 |
| Systemic APCs | LNP vaccine encapsulating mRNA encoding the viral oncogenes E6 and E7 | 1, 2 | Advanced HPV16+ cancer (head and neck, anogenital, penile or cervical) | NCT03418480 | 79 |
| Systemic APCs | Liposomal RNA vaccine | 1 | Melanoma |
NCT02410733 | 78,80 |
| Systemic APCs | PLGA NPs vaccine carrying NY-ESO-1 antigen and IMM60 invariant NK T cell activator | 1 | NY-ESO-1+ tumours |
NCT04751786
|
82 |
| Spleen and liver APCs | Rapamycin-encapsulating NPs | 3 | Chronic Gout |
NCT04596540
|
84 |
| Splenic B cells | Liposomes carrying iNK T cell ligand | 1 | Treatment for graft versus host disease post allogeneic stem cell transplant |
NCT04014790, NCT01379209 |
89–91 |
| Adoptively transferred T cells | IL-15 nanogels backpacked on T cells | 1 | Selected solid tumours, lymphoma |
NCT03815682 | 108 |
| TLR9+ myeloid cells | TLR-9 agonist-presenting spherical nucleic acids | 1b/2 | Advanced solid tumours |
NCT03684785
|
183,184 |
| Intratumoral lymphocytes | LNPs encapsulating mRNAs encoding IL-12 | 1 | Solid tumours | NCT03946800 | 232 |
| Intratumoural lymphocytes | Saline-formulated mRNA cocktail encoding single-chain IL-12, interferon-α2b, GM-CSF and IL-15 superagonist | 1 | Metastatic neoplasm | NCT03871348 | 198 |
| Intratumoural lymphocytes | LNPs encapsulating mRNAs encoding OX40L, IL-23 and IL-36γ |
1 | Selected solid tumours, lymphoma | NCT03739931 | 197,200 |
| Intratumoural lymphocytes | LNPs encapsulating mRNA encoding OX40L |
1/2 | Relapsed and refractory solid tumours, lymphoma and ovarian cancer | NCT03323398 | 197 |
Ref., reference; APC, antigen presenting cells; GM-CSF, granulocyte-macrophage colony-stimulating factor; CpG, unmethylated cytosine–guanine dinucleotide; LN, lymph node; NP, nanoparticle; HIV, human immunodeficiency virus; AIDS, acquired immunodeficiency syndrome; HA, hemagglutinin; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; COVID-19, coronavirus disease-19; Amph, amphiphilic; KRAS, Kirsten rat sarcoma; PDAC, pancreatic ductal adenocarcinoma; mRNA, messenger ribonucleic acid; HPV, human papillomavirus virus; PLGA, poly(lactide-co-glycolide); NY-ESO-1, New York esophageal squamous cell carcinoma 1; NK T, natural killer T; IL, interleukin; TLR, toll-like receptor
Targeting lymph node-bound migratory immune cells in peripheral tissues.
Complementary to the strategy of direct lymphatic targeting, this approach leverages the natural process by which immune cells, including dendritic cells (DCs), monocytes and neutrophils, internalize antigens in peripheral tissues and transport them to LNs as part of their constitutive immune surveillance (Fig. 2b). For example, subcutaneously-implanted porous polymer scaffolds releasing the cytokine granulocyte-macrophage colony-stimulating factor (GM-CSF) attract and differentiate monocytes into the DC lineage. When such scaffolds also carry molecular adjuvants and tumour antigens, these are taken up by the recruited cells and transported to dLNs (Fig. 2b)36,37. This approach improved anti-tumour immunity, eliciting ~50% complete responses in an aggressive mouse model of melanoma, and leading to a first-in-humans clinical trial of this technology (NCT01753089; Table 1). Data from this trial are not yet published, but should provide important insights into the safety of generating a strong localized inflammatory reaction at the implant site, an approach that could be extended to applications beyond cancer. To avoid implantation, injectable polymeric hydrogels38 or suspensions of biodegradable silica particles39 have been employed, resulting in both cellular and humoral immune responses against cancer or microbial antigens40. Similarly, microparticles loaded with tumour lysates as antigen and adenosine triphosphate (ATP) as a chemotaxis-inducing ‘find-me’ signal promoted recruitment of DCs and increased levels of mature DCs migrating to tumour dLNs, resulting in reduced tumour growth.41
DC-attracting biomaterials are also being developed to program tolerance rather than immunity. Dual-sized poly(lactide-co-glycolide) (PLGA) particles consisting of small (0.5–2.5 μm diam.) phagocytosable microparticles loaded with the tolerogenic metabolite vitamin D3 and autoantigens, combined with large (10–65 μm diam.) non-phagocytosable microparticles designed to release extracellular transforming growth factor-beta (TGF-β) and GM-CSF, prevented hyperglycemia in a mouse model of type 1 diabetes42 and induced antigen-specific tolerance in mouse models of multiple sclerosis (MS) and collagen-induced arthritis43,44. This example highlights the potential of DCs-attracting approaches not only to modulate immunity, but also to program tolerance.
Targeting niches within lymph nodes
Organization of lymph nodes.
LNs are highly organized organs with defined compartments enriched in different immune cell subsets. Afferent lymph enters the subcapsular sinus (SCS) of the LN, a fluid space between the LN capsule and the LN parenchyma, lined by lymphatic endothelial cells and macrophages (Fig. 1b). Within the parenchyma, many of the key cell populations regulating adaptive immunity have specific niches; B cells and follicular DCs reside in follicles arrayed around the exterior of the node, whereas T cells are localized deeper in the LN paracortex (Fig. 1b)45,46. This physical segregation plays an important role in orchestrating sequential steps in the immune response, therefore, targeting distinct niches in the LN could offer valuable therapeutic opportunities.
Entry into lymph nodes.
Under physiological conditions, substances in the lymph can pass into the parenchyma through narrow collagen conduits, or be captured and transferred into the LN interior by subcapsular sinus (SCS) macrophages (Fig. 1b)47–49. The conduits exclude globular proteins larger than ~70 kDa49,50, although evidence suggests that this size limit can change in the presence of a live infection51. Vaccine adjuvants such as oil-in-water nanoemulsions and saponin NPs have been used to boost entry of compounds into LNs by inducing rapid death of SCS macrophages (Fig. 3a)14,52,53. Similarly, liposomal formulations of clodronate, widely used to deplete SCS macrophages, improve antigen entry into LNs54. In another strategy, a two-stage delivery system was developed, in which CpG oligonucleotides were coupled to ~30 nm poly(propylene sulfide) (PPS) NPs through cleavable oxanorbornadiene linkers and then injected intradermally in a murine model of lymphoma55. The NPs efficiently trafficked from the injection site to dLNs, but were largely trapped in the sinuses after 24 hr. By tuning the chemistry of the oxanorbornadiene linker, the CpG adjuvant payload remained bound to the NPs during trafficking to the dLN, and were later released deep into the LN parenchyma (Fig. 3b).
Figure 3 |. Targeting lymph node-resident cells and lymph node subregions.
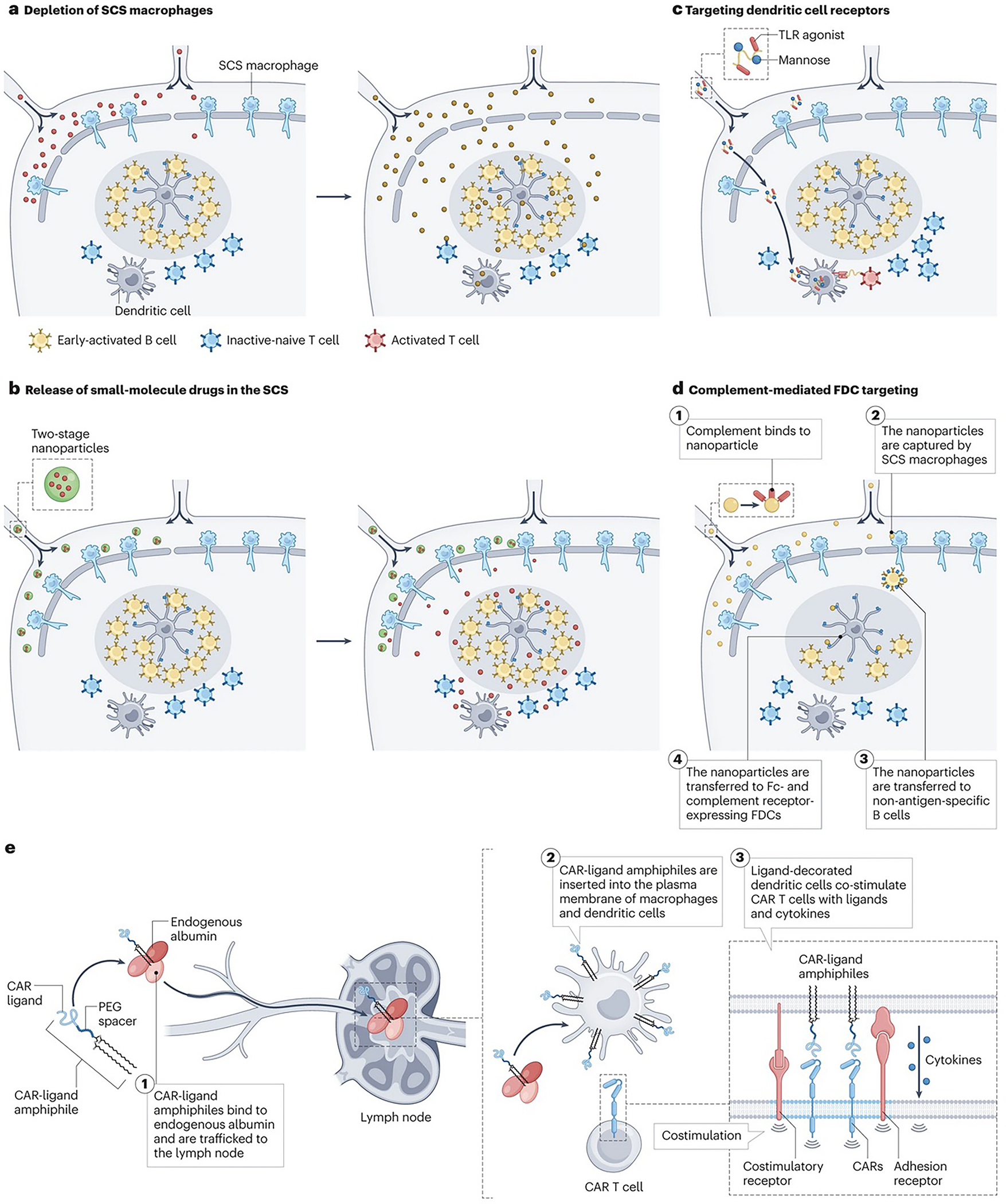
a | Promoting therapeutic entry into lymph nodes (LNs) through depletion of subcapsular sinus (SCS) macrophages. Vaccine adjuvants (red) induce rapid death of SCS macrophages, leading to enhanced entry of antigens and other compounds (orange) into LNs. b | Polymer nanoparticles (blue) efficiently traffic into lymphatic vessels (20–30 nm) but are too large to penetrate the LN paracortex. They chemically release small molecule payloads (red) that rapidly permeate throughout the node. c | Soluble polymers and polymer nanoparticles (NPs) conjugated with ligands for receptors expressed by specific target cell types (such as dendritic cells (DCs)), are taken up by antigen presenting cells (APCs) in LNs and co-deliver vaccine antigens or DC activating compounds. d | (1) NPs activate complement, (2) are captured by SCS macrophages (3) and transferred to non-antigen-specific B cells through complement receptors. (4) These NPs are then transferred to follicular dendritic cells (FDCs), which express high levels of complement and (fragment crystallisable) Fc receptors. e | (1) Ligands for chimeric antigen receptor (CAR) T cells linked to albumin-binding poly(ethylene glycol) (PEG)-lipid moieties traffic from an injection site and bind to endogenous albumin. These compounds are then transferred to LNs (2) where they are inserted into the plasma membrane of macrophages and DCs. Subsequent encounter of CAR T cells with the ligand displayed on the surface of DCs will lead to CAR T cell tandem stimulation by natural costimulatory receptors and cytokine signals provided by the ligand-decorated DCs.
Targeting specific cells and niches within the lymph node.
To facilitate intra-nodal delivery, specific cell populations can be targeted within the LN parenchyma, in particular DCs and macrophages. For example, oral or systemic administration of rapamycin promotes tolerance by acting on DCs and myeloid cells (for example, in organ transplants); however, with considerable systemic side effects 56. Subcutaneous injection of rapamycin encapsulated in PEG-b-PPS polymer vesicles (~100 nm diam.) efficiently targeted the LNs in a diabetic mouse model, where it was preferentially phagocytosed by DCs and other myeloid cells and induced a regulatory DC phenotype enabling allogeneic pancreatic islet transplants to be maintained over 100 days, without side effects57. To drive antigen-specific tolerance, LN DCs were targeted using liposomes co-delivering a tolerance-driving aryl hydrocarbon receptor agonist drug together with a multiple sclerosis (MS)-related peptide in a murine MS model58. Cancer neoantigens linked to hydrophobic peptides bearing toll-like receptor-7 (TLR7) agonists were developed to self-assemble into ~20 nm micelles, which efficiently trafficked from s.c. injection sites to dLNs, achieving uptake in 80% of LN DCs, leading to anti-tumour immunity in multiple murine tumor models59. DC targeting has also been achieved by targeting specific receptors expressed by these cells. Following intradermal vaccination, polymer chains functionalized with antigens, TLR7 agonists and mannose moieties to target mannose receptor+ DCs improved uptake of antigens in LNs (Fig. 3c), generating antigen-specific CD8+ T cell responses and increasing serum antibody concentrations (1.7- and 3.6-fold compared to potent alternative adjuvant formulations AS01EL and poly(I:C), respectively)60. These studies highlight the potential of co-delivering antigens and adjuvant compounds to the same cell through physical conjugation or encapsulation within the polymer carrier, an already widely-adopted strategy59,61,62. Moreover, the physical chemistry of antigen and adjuvant compounds, together with a selective design of NPs carriers that enable release into DCs upon arrival in the LNs, are important parameters to consider to ensure optimal T cell priming and functional polarization62–64. For example, autoimmune-related peptide antigens coupled to ~10 nm quantum dots (QDs) trafficked from s.c. injection sites to dLNs, where they accumulated in macrophage receptor with collagenous structure (MARCO)+ macrophages65. These cells have been associated with immune tolerance, and expanded regulatory T cells (Tregs) contributing to disease control in a mouse model of MS65. Therefore, targeting both of these LN APC populations appears promising for promoting both immunity and tolerance. Of note, in this work QDs were used owing to their bright fluorescent properties, but are known to release toxic metals over time66. In general, an important question to address with any polymer or NP-based DC targeting strategy is whether the material is effectively captured in the draining lymph node chain following injection, or whether they it passes through the thoracic duct and enters the systemic circulation67, raising potential toxicity concerns, in particular for potent immunostimulants.
B cell follicles are another important niche regulating humoral immune responses. NPs engineered to activate the complement system can target antigens or therapeutics to B cell follicles. For example, NPs bearing a high density of mannose-containing glycans are recognized by the innate immune protein mannose-binding lectin, which triggers complement deposition on the particles. Upon arrival to the LNs, SCS macrophages transfer complement-decorated particles to migratory B cells in the LN parenchyma, which in turn pass the particle to follicular DCs (FDCs) (Fig. 3d)53,68. For vaccines, this strategy is glycan density-dependent, amplifies germinal centre and serum antibody responses and can be engineered into synthetic particles to promote follicle localization68,69. A protein nanoparticle vaccine that triggers the FDC targeting process completed a phase I trial (NCT03547245) and demonstrated 97% efficacy in initiating broadly-neutralizing antibody B cell lineages in humans70. In principle, particles triggering complement activation through natural immunoglobulin M (IgM) or the alternative pathway should also be capable of such FDC localization. However, this trafficking is size dependent; complement-decorated particles smaller than 15 nm were internalized and cleared by FDCs, whereas particles 50 nm or larger were retained on FDC dendrites for several weeks, resulting in improved antibody responses71. An important parameter to consider when designing carriers targeting B cells, is that rigid particles carrying multivalent copies of an antigen are more effective than flexible polymers in triggering B cell activation72.
T cells are another key cell type in LNs, which are located in the paracortex and interfollicular regions (Fig. 1b). Polymer NPs coated with DC-derived plasma membrane fragments and conjugated with anti-CD3 antibodies showed efficient accumulation in dLNs, activated and expanded CD8+ T cells, and, in combination with anti-programmed cell death protein 1 (PD-1) therapy, eliciting antitumor immunity73. To provide vaccine-like stimulation of chimeric antigen receptor (CAR) T cells in LNs, albumin-binding ‘amph-vaccine’ molecules were designed carrying CAR ligands. The lipid tails of these conjugates inserted into cell membranes of DCs and macrophages in the dLN, enabling activation of CAR T cells in the LN and improving anti-tumor activity (Fig. 3e)74.
Targeting lymph nodes, spleen and liver
Unlike live infections, current vaccines fail to elicit high-magnitude CD8 T cell responses in humans, which has motivated the field to explore new ways to prime cellular immunity75. Vaccines commonly act locally on a chain of LNs draining at the injection site such as the muscle76. However, this means that reacting immune cells are confined to a few LNs, resulting in competition for a limited resources (for example cytokines or antigens), which theoretically might restrain the eventual systemic immune response. One hypothesis is that systemic administration of antigens to several LNs could alleviate such resource constraints and enable more potent immune priming. A key consideration is that adjuvant and antigen signals need to be delivered together to ensure that immunity, rather than tolerance, is elicited. Moreover, intravenously (i.v.) administered materials are rapidly opsonized and captured by macrophages in the liver, spleen and bone marrow, in particular for particles larger than 100 nm. By contrast, particles smaller than 5 nm are rapidly cleared through the kidneys77. Therefore, to systemically target lymphoid organs, opsonization-resistant (e.g., PEGylated) particles in the range of 5–100 nm would be ideal, although this size dependency could be altered if circulating immune cells take up the particles and actively transport them into systemic lymph nodes. For example, i.v. administration of negatively charged interferon-inducing lipid nanoparticles (LNPs) carrying antigen-encoding mRNA efficiently targeted DCs in the spleen and peripheral LNs as well as myeloid cells in the liver and bone marrow, resulting in a substantial antigen-specific T cells response (30–60% of the total CD8+ T cell compartment specific for the targeted antigens), and consequent tumour rejection in aggressive murine tumour models78 (Fig. 4). These findings spurred ongoing clinical trials of mRNA LNPs in multiple cancers, with promising interim results including objective response rates in 6 out of 17 patients with advanced melanoma79,80 (NCT04534205, NCT03418480 and NCT02410733; Table 1). Similarly, i.v. vaccination with self-assembled polymer NPs carrying cancer neoantigen peptides and small molecule TLR-7/8 agonists primed a larger TCF-1+ stem-like antigen-specific T cell population compared to parenteral vaccination with the same material, leading to improved anti-tumour immunity81. These examples highlight the potential beneficial effects of systemic immunization on the immune response. Following these principles, a clinical trial in the Netherlands is currently evaluating i.v.-administered PLGA-based nanoparticles delivering a tumour antigen together with a small molecule as innate immune activator designed to promote DC activation through invariant NK T cells (NCT04751786, Table 1)82.
Figure 4 |. Targeting systemic lymphoid organs.
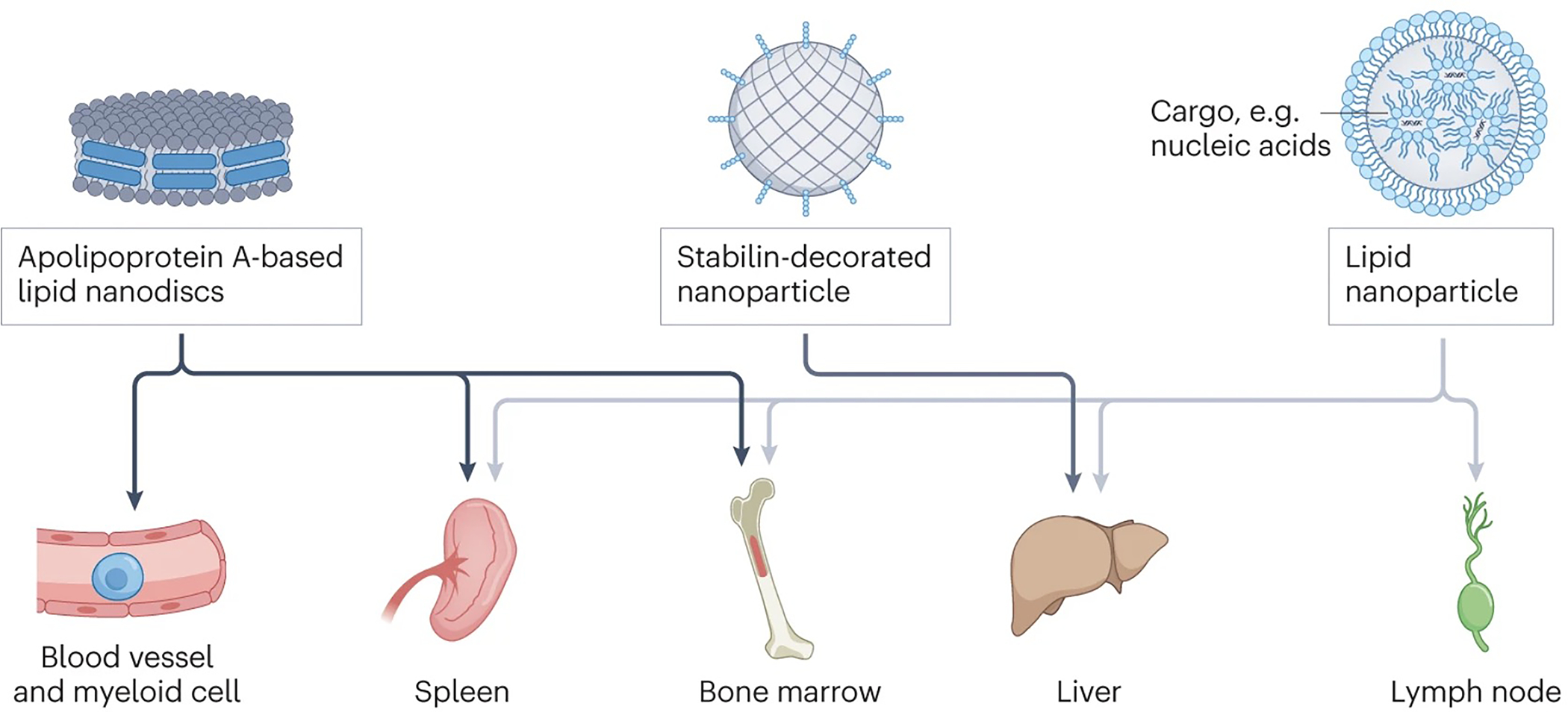
Nanoparticle (NP) carriers can target lymphoid organs systemically, including lymph nodes, spleen, bone marrow and tolerogenic antigen presenting cells in the liver. For example, Apolipoprotein-based lipid nanodiscs a preferentially taken up by myeloid cells in the liver, spleen, and bone marrow. Stabilin-funcitonalized nanoparticles are efficiently scavenged by endothelial cells in the liver. Anionic LNPs target antigen presenting cells in the liver, spleen, lymph nodes, and bone marrow.
APC populations in the liver and spleen are known to play important roles in systemic tolerance, and are therefore being targeted for tolerance and autoimmunity. For example, i.v.-administered biodegradable PLGA NPs carrying rapamycin were efficiently captured by DCs in the spleen, and elicit a Treg-generating, tolerizing phenotype in these cells that blocked generation of cellular and humoral responses against co-administered antigens83. Following this approach, a clinical trial aimed at blocking anti-drug antibody responses against recombinant uricase in gout patients demonstrated promising efficacy and safety data in humans (NCT02648269)84. Similarly, i.v. administration of biodegradable polymer NPs coupled to autoantigens for tolerance induction led to efficient uptake in tolerance-promoting MARCO+ APCs in the spleen and liver, fostering protection in mouse models of diabetes and other autoimmune conditions85 86. Subcutaneously-injected diabetes autoantigens coupled to hyaluronic acid polymers were delivered to both disseminated LNs and spleen, promoting systemic expansion of CD4+ T cells with an immunosuppressive phenotype87. I.v. injection of biodegradable PLGA NPs loaded with a model antigen and functionalized with stabilin to target scavenger receptors expressed by liver sinusoidal endothelial cells resulted in an almost exclusive accumulation in the liver, providing protection from airway-induced allergic inflammation (Fig. 4)88. As a final example, liposomes were administered intraperitoneally to deliver alpha-galactosyl ceramide to marginal zone B cells in the spleen. Presentation of the ligand to invariant NK T cells promoted the development of regulatory T cells and tolerogenic DCs in the spleen of mice89. This treatment suppressed graft versus host disease (GVHD) following bone marrow transplant90, and is now moving into early stage clinical trials (NCT04014790 and NCT01379209; Table 1)91. In these examples, different materials were used to target antigens and stimuli to relevant immune cells. An important but understudied parameter is how the duration of cargo release in recipient target cells affects their tolerizing effect. For example, loaded PLGA particles could be engineered to release antigens over weeks, whereas fast-degrading surface-conjugated particles or liposomes could provide rapid release following endocytosis. Whether these kinetics have a substantial impact remain unknown.
Targeting immune cells in bone marrow
The bone marrow is a primary lymphoid organ for hematopoiesis and myelopoiesis, and also a site housing a large pool of memory T cells (Fig. 1a)92. Myeloid precursors, in particular, have received recent attention because they drive a process known as “trained immunity”. This phenomenon occurs when innate immune cells or their progenitors receive certain microbial stimuli (including Bacillus Calmette-Guérin bacteria, Candida albicans yeast, β-glucan, or peptidoglycan93) that drive epigenetic changes rewiring their metabolic and functional state94,95. When induced in progenitor cells, these epigenetic modifications are passed on to their progeny and confer a prolonged and elevated state of responsiveness to microbial stimuli in monocytes, macrophages and natural killer cells. Therapeutic induction (or suppression) of trained immunity is now being considered as a promising approach to treat diverse diseases93. For example, i.v. injection of apolipoprotein-A1-based lipid nanodiscs carrying the minimal peptidoglycan muramyl dipeptide preferentially accumulated in myeloid cells and their precursors in the blood, spleen and tumours in a mouse model of melanoma, resulting in enhanced cytokine production and increased efficacy of checkpoint blockade therapy (Fig. 4) 96. Such innate immune modulation could be a powerful complement to therapeutic strategies focused on boosting adaptive immunity.
Targeting circulating leukocytes
Many immune cells continuously recirculate between secondary lymphoid organs, the blood and tissues, patrolling for cognate foreign antigens. The ability of immune cells to home from the blood into disease sites forms the basis of adoptive cell therapies, where engineered immune cells are infused i.v. in patients and subsequently home to targeted distal sites. These cells can be targeted while in the blood to serve as chaperones to transport drugs into disease sites.
‘Backpacking’ cells
Adoptive cell therapies based on the injection of autologous cells engineered to have disease-specific functions is a steadily expanding approach for immunotherapy97,98. To date, the most clinically successful example is chimeric antigen receptor (CAR) T cell therapy, where lymphocytes isolated from cancer patients are transduced to express a CAR that enables recognition and selective killing of tumour cells. CAR T cells are then expanded ex-vivo and re-infused into the patient99. So far, six different CAR T cell products have been approved for hematologic malignancies, with several ongoing clinical trials to extend CAR T therapy to solid tumours and infectious diseases100–102. Adoptive cell therapies are also being developed using natural or transgenic T cell receptor (TCR)-expressing T cells, natural killer cells, macrophages, Tregs (for autoimmune disease and transplant tolerance) and other immune cell populations103. Adoptively transferred cells benefit from supporting stimulation to maintain their function or promote their expansion and survival, however, as with other immunomodulators, systemic co-administration of supporting drugs can be toxic owing to non-specific effects on endogenous immune cells.
To provide sustained and localized stimulation, therapeutic carriers have been directly attached to the surface of donor cells prior to infusion (an approach termed ‘backpacking’) (Fig. 5a). This strategy has been used to load tumour-specific T cells and Tregs with cytokines, small molecule drugs or chemotherapeutic agents104–107. Early studies showed that LNP backbacks carrying cytokines such as IL-15 remained on the cell surface for extended periods, inducing potent autocrine stimulation and proliferation of adopted antigen-specific T cells in vivo107. 107–104, 6Backpacking protein nanogels onto T cells enabled a more controlled release of supporting cytokines in response to altered cell surface reduction activity after TCR or CAR stimulation in mice108. This strategy restricted donor cell stimulation to tumours and tumour-dLNs (where the antigen is encountered), improving therapeutic efficacy and safety108. Interim results from a phase I clinical trial of this approach in cancer patients (NCT03815682; Table 1) showed that only one tenth the amount of administered interleukin (IL)-15 was detectable in the blood despite backpacking 3 times the maximum tolerated dose of free systemic Fc-fused IL-15109 onto T cells, leading to stable disease in 10 out of 17 patients110. A limitation of this approach is that the therapeutic payload is by definition finite, and will be diluted over time as T cells proliferate in vivo. However, it could still provide immediate autocrine stimulation following adoptive transfer, enabling cells to successfully engraft and initiate tumour rejection. Owing to the cytokine carrier being directly conjugated to the cell membrane, the concentration of newly-released cytokines at the cell surface is substantial, and it was estimated that the IL-15 nanogels will continue to stimulate T cells through at least 7 cell divisions before stimulatory capacity is lost108.
Figure 5 |. Targeting circulating and tissue-resident immune cells.
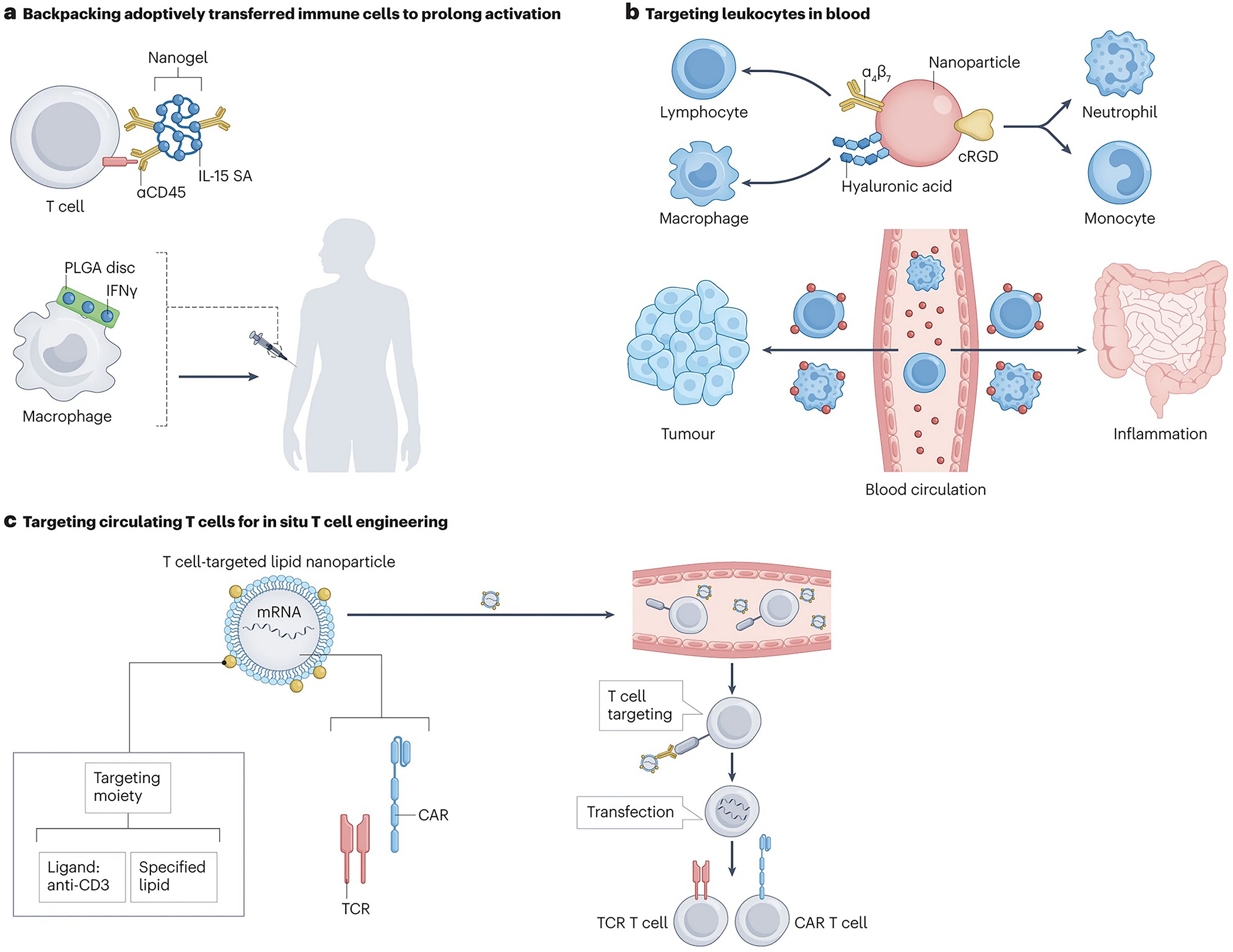
a | “Backpacking” cells by attaching drug-releasing materials to cells ex vivo prior to adoptive transfer. IL-15 SA, interleukin-15 super agonist; PLGA, poly(lactide-co-glycolide); IFN, interferon. b | Nanoparticles (NPs) functionalized with targeting moieties can bind to circulating immune cells in the blood, which then traffic into different tissues sites with the drug carrier. cRGD, cyclic arginine-glycine-aspartic acid. c | T cell-targeted NPs deliver nucleic acid payloads (DNA or RNA) to circulating lymphocytes, resulting in the expression of chimeric antigen receptors (CARs) and other immunomodulatory gene payloads in situ. d | Albumin is used as a chaperone to transport vaccines across the epithelial barrier in the nasal and respiratory pathways through the neonatal fragment crystallisable (FcRn) receptor. NPs functionalized with hyaluronic acid are phagocytosed by macrophages and other myeloid cells at sites of intestinal inflammation in the gut. e | Systemically-injected NPs passively accumulate in tumours through the enhanced permeation and retention (EPR) effect, or functionalized NPs actively target cells such as programmed death-1 (PD-1+) lymphocytes. Alternatively, biomaterials can be directly injected into tumours to locally present or release immunostimulatory agents in the tumour microenvironment, or promote gene delivery to tumour cells for localized expression of immunomodulatory factors. f | NPs can be modified with targeting moieties or opsonization-triggering components to bind to the endothelial cells of HEVs of the inflamed draining lymph nodes or target different innate immune cells that home to sites of inflammation. cRGD, cyclic arginine-glycine-aspartic acid; HDL, high-density lipoprotein; HEV, high endothelial venules; PNAd, Peripheral lymph node addressin.
Backpacking approaches have also been developed for innate immune cells; discoid polymer particles carrying interferon gamma (IFN-γ), adhered to the surface of macrophages without inducing phagocytosis, resulting in persistent polarization into an ‘M1’ phenotype that promoted tumour rejection in a murine breast cancer model111. Importantly, the use of a discoid morphology was crucial to avoiding phagocytosis of the backpack (Fig. 5a)112.
Targeting immune cells in blood
Targeting circulating cells that traffic drugs into tissues
Targeting immune cells in the blood is an attractive strategy to circumvent physical barriers in tissues. For example, lymphocytes populating Peyer’s patches and mesenteric LNs express well-defined adhesion and chemokine receptors (α4β7 integrin and CCR9, respectively113,114) that direct their homing to these sites (Fig. 5b). I.v. administration of lipid-coated polymer NPs loaded with an HIV antiretroviral drug and surface-functionalized with antibodies against α4β7 promoted uptake in gut lamina propria cells, which transported the particles to the gut of mice115. Functionalizing silencing-RNA (siRNA)-loaded LNPs with recombinant mucosal vascular addressin cell adhesion molecule 1 (MAdCAM-1), the ligand recognized by the high-affinity conformation of α4β7, increased the particle’s specificity to gut-homing lymphocytes resulting in silencing of IFN-γ in a murine model of colitis116.
NPs have also been used to target circulating immune cells that home into tumours or sites of inflammation. Intraperitoneally-injected hyaluronic acid-polyethyleneimine hybrid NPs carrying miR-125b, a microRNA known to induce an anti-tumour ‘M1’ phenotype in macrophages117, were taken up by these cells which then migrated to lung tumours and repolarized tumour-associated macrophages towards an M1 phenotype. Of note, choosing the right administration route is important to avoid unexpected transduction of macrophages in the liver or bone marrow. I.v. administration of liposomes or lipid nanoemulsions conjugated with arginylglycylaspartic acid (RGD) peptides, known to bind to αV integrins which are highly expressed by monocytes and neutrophils, led to rapid uptake by these cells in the blood, and these innate cells subsequently carried the particles to tumours in mice118 (Fig. 5b). The same approach enabled the delivery of neuroprotective drugs across the blood-brain barrier to sites of ischemia in mice119. Thus, targeting of immune cells in the blood is a promising strategy to hitchhike drugs into disease sites.
Antigen-specific targeting of circulating T cells.
Targeting of antigen-specific T cells in the blood has been pursued to enhance adoptive cell therapy and promote protective T cell responses. For example, in mouse models of autoimmunity, i.v. injection of iron oxide NPs coated with disease-relevant peptide-major histocompatibility complexes (pMHC) induced expansion of CD4+ or CD8+ T cells with a regulatory phenotype120,121. I.v.-injected class II pMHC-displaying NPs can further expand Tregs to control liver autoimmune diseases without systemically suppressing immunity in other organs122. In the setting of cancer, artificial APCs were generated by conjugating biodegradable PLGA/poly(β-amino-ester) (PBAE) microparticles with pMHC and anti-CD28 antibodies123. I.v. administration of these particles expanded antigen-specific cytotoxic CD8+ T cells in vivo, and, in combination with checkpoint blockade therapy, increased the median survival time by 31% compared to single checkpoint blockade therapy in a mouse model of melanoma.123 The same strategy was used to develop tolerogenic particles that promoted the expansion of forkhead box P3 (Foxp3+) regulatory T cells, which, after a single i.v. injection, resulted in a 20% increase of these cells in lymph nodes compared to untreated mice.124
Targeting circulating T cells for gene delivery in vivo.
CAR T cell therapy is having substantial clinical impact against hematologic malignancies; however, its patient-specific nature and complex manufacturing process of transducing T cells ex vivo, limits its widespread application125. A potential alternative is to genetically modify T cells in vivo. Engineered viral vectors are being developed for this purpose126,127; however, viral vectors raise concerns of strong immune reactions and toxicity128–130. Another option is to use synthetic NP gene delivery vectors. I.v.-injected poly(β-amino ester) NPs carrying transposon DNA encoding a CAR, which targets T cells through anti-CD3 antibody fragments, proved safe and effective in mouse models of leukaemia (Fig. 5c)131. Recently, in vivo mRNA delivery to T cells generated transient CAR T cells capable of eliminating fibrosis-promoting fibroblasts in models of heart failure132. Of note, immune cells (including T cells) are notoriously resistant to conventional transfection methods, therefore, it is important to identify more effective lipid/polymer compositions for nucleic acid delivery133–135. CAR T cells have also been produced in vivo using an implantable macroporous alginate scaffold, which provides a contact interface for T cells and retroviruses and facilitates vector-mediated CAR gene transfer136. Subcutaneous implantation of these scaffolds seeded with human peripheral blood mononuclear cells and CD19-encoding retroviral particles reduced ex vivo cell processing times to a single day, compared to 2–4 weeks for conventional CAR T cells, while keeping similar anti-tumour efficacy 136.
Targeting tissue-resident cells
Certain innate and adaptive immune cell populations permanently reside in tissues and provide functions such as immediate immune defence at barrier tissues, local production of protective antibodies and tissue-resident immune memory. In addition, these cells infiltrate sites of tissue damage, inflammation and tumours, making them important therapeutic targets for disease treatment.
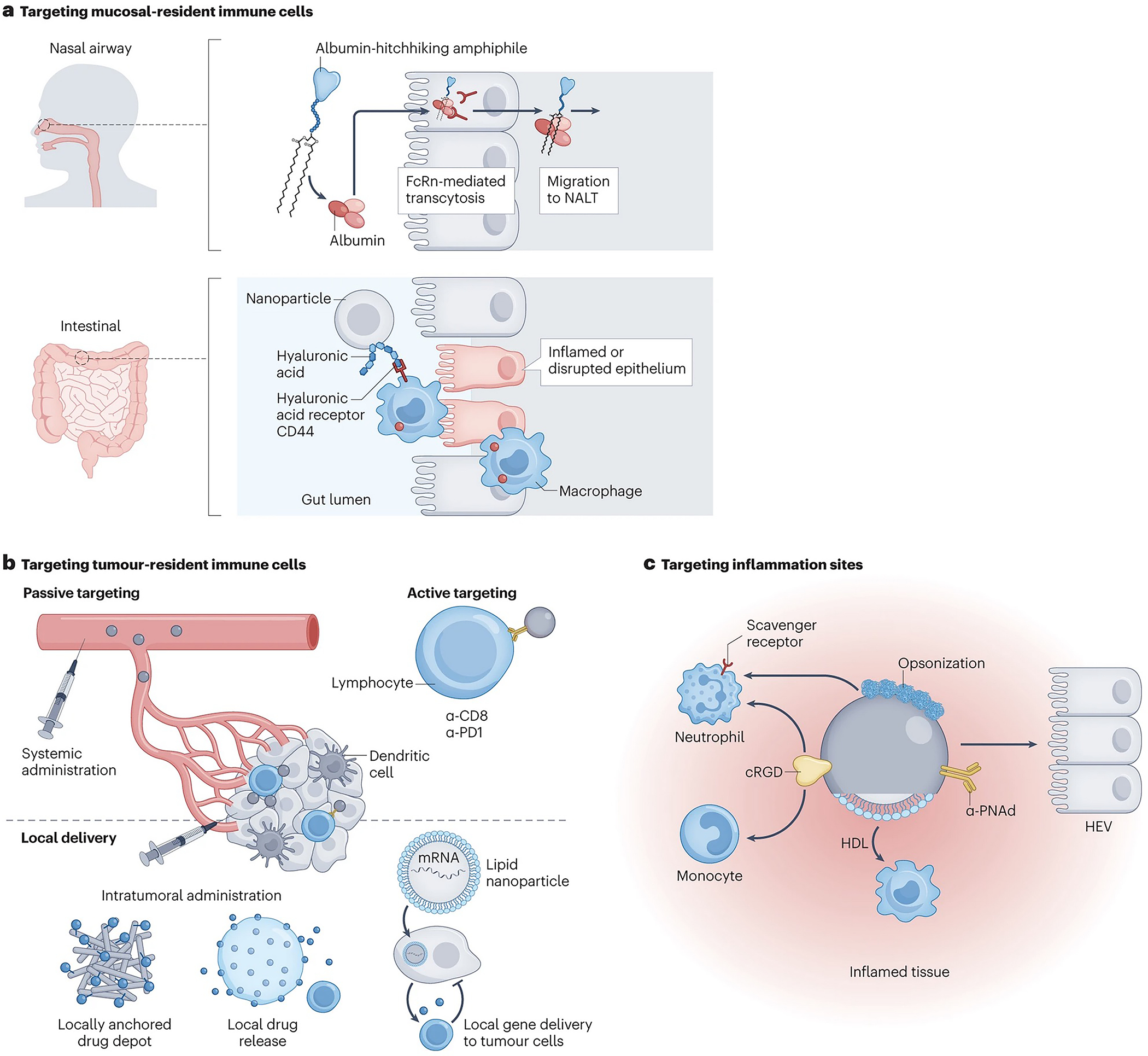
Delivery at mucosal barriers
Several immune cell populations reside at mucosal barriers, such as the skin, airways, gastrointestinal and reproductive tract. DCs serve as sentinels of pathogen entry by sampling foreign antigens; tissue-resident memory lymphocytes and plasma cells provide frontline adaptive immune protection137,138; while mucosa-associated lymphoid tissues, such as the Peyer’s patches and nasal-associated lymphoid tissue (NALT), serve as secondary lymphoid organs that rapidly respond to antigens entering through the mucosae139. These cells are important targets for vaccination, because antigens taken up across mucosal barriers prime lymphocytes in the draining lymphoid tissues that home back to these sites137. Moreover, mucosa-resident immune cells can be preferential targets of infection (for example, gut-resident memory CD4+ T cells in HIV infection), posing them as interesting therapeutic targets for viral latency-reverting drugs against HIV31,140. A key challenge in targeting immune cells at these sites is that mucosal barriers have evolved to efficiently block incoming pathogens and foreign materials141–142.
One promising strategy for effective drug delivery across mucosal surfaces is to exploit a natural bidirectional transport pathway for crossing the epithelial barrier. Following endocytosis in epithelial cells, immunoglobulin G (IgG) and albumin bind to the neonatal fragment crystallisable (Fc) receptor (FcRn) in endosomes, triggering transcytosis and release at the apical surface143. Fusing therapeutic proteins with Fc domains or albumin promotes uptake at the mucosal surface144 (Fig. 5d). Similar to the backpacking approach, protein or peptide antigens linked to PEG-lipids (amph-vaccines) associate with albumin in the airway fluid and ‘hitchhike’ through the mucus across the epithelial barrier in an FcRn-dependent manner28,145. Peptide amph-vaccines which were administered into the lungs primed robust lung tissue-resident memory T cells that enhanced vaccine protection against respiratory viral or tumour challenge28. By contrast, intranasal administration of protein amph-vaccines amplified germinal centre responses in the NALT, promoting higher mucosal antibody titers in both mice (100- to 1000-fold increase) and non-human primates (10-fold increase), compared to unmodified proteins145.
Biomaterials are also being developed to target immune cells at sites of mucosal inflammation. For example, conjugation of the hydrophilic ECM polymer hyaluronic acid with the natural gut anti-oxidant bilirubin led to the formation of NPs 80–400 nm in size. After oral administration in mouse models of colitis, these NPs were efficiently taken up at the inflamed epithelium by macrophages expressing the hyaluronic acid receptor CD44, leading to polarization of these cells toward an anti-inflammatory phenotype146 (Fig. 5d). Interestingly, this treatment caused an increase in microbial diversity in the microbiome, which promoted epithelial healing and inflammation reduction.
Targeting immune cells in tumours
Systemic targeting of immune cells in tumours
Instead of targeting circulating immune cells destined to migrate into tumours, there is also interest in delivering drugs directly to tumour-resident immune cells. For many years, biomaterials scientists have sought to target tumours by exploiting the enhanced permeation and retention (EPR) effect, a phenomenon first described in animal models in the late 1980s; here, large proteins or NPs accumulate in tumours owing to their hyperpermeable vasculature and reduced lymphatic clearance77 (Fig. 5e). However, the efficiency of EPR-based tumour targeting remain low and penetration of NPs deep into tumours is often poor147,148. Therefore, new approaches are being pursued to facilitate targeting of immune cells in tumours. For example, the STimulator of Interferon Genes (STING) pathway, a cytosolic danger sensor in host immune cells149, have gained particular interest. However, its natural ligands cyclic dinucleotides are labile and poorly taken up by cells in vivo. Conjugating PEGylated lipid nanodiscs with cyclic dinucleotide prodrugs enabled penetration into solid tumours, activating DCs to drive T cell-mediated tumour rejection150. Passive NP uptake in tumours can be further exploited to localize immune cell activation to tumors. For example, i.v.-injected PEGylated gold nanorods accumulated in tumours and then were irradiated by a near-infrared laser to induce photothermal heating of the tumour-resident nanorods151. This heating activated thermally-responsive gene cassettes in co-administered engineered T cells and induced localized CAR or cytokine expression in mice151. Nonetheless, the clearing mechanism of gold nanoparticles remains unclear, because these materials are not resorbable in vivo152. (Fig. 5e). NPs have also been targeted to T cells in tumours via conjugation with antibody fragments binding to T cell receptors. In one example, PD-1-targeted PLGA/PEG-based NPs were detected on intratumoural T cells within 1 hr of i.v. injection and enabled pronounced therapeutic modulation of tumours through delivery of a small molecule TLR-7/8 agonist153.
One factor limiting NP dissemination deep into the tumour parenchyma is their propensity to be captured by tumour-associated macrophages residing near blood vessels154–156. This affinity is now being exploited to reprogram tumour myeloid cells into a pro-immune phenotype using polymeric or lipid carriers loaded with immunomodulators such as TLR-7/8 agonists and colony stimulating factor 1 receptor inhibitors157,158. As another example, polymer NPs carrying mRNA targeted to myeloid cells through di-mannose moieties reprogramed tumour-associated macrophages toward an M1 phenotype159. Although promising, these approaches will need to assess potential side effects of non-specific uptake by macrophages in the liver, spleen and bone marrow, given the tendency of nanoparticles to be captured by macrophages in these organs160,161.
In addition to capture by macrophages, abnormal tumour vasculature limits dissemination of NPs and immune cells in the TME. Inhibiting proangiogenic factors, such as vascular endothelial growth factor (VEGF) and angiopoietin 2 (ANG2) –at low to intermediate doses– normalizes tumour vasculature and perfusion, thereby improving the efficacy of anticancer treatments, including immunotherapy162–167. For example, tumour biopsies from breast cancer patients treated with an anti-VEGF antibody in a phase 3 clinical trial (NCT00546156) showed increased infiltration of CD4+ T, CD8+ T and mature dendritic cells168. Moreover, combining antiangiogenic agents with immunotherapies, including cancer vaccines and immune-checkpoint inhibitors, enhanced tumour infiltration of effector immune cells and improved therapeutic efficacy in murine cancer models169–176.
Intratumoural delivery of biomaterials for immune cell targeting
Biomaterials can also be directly injected into the tumour. Importantly, to ensure efficacy, the delivered payload needs to remain in the tumour (or tumour- dLNs) after injection. Conjugation of immunomodulatory payloads to antibodies or engineered proteins that bind to tumour cell surface antigens177 or ECM components such as collagen178–180 improves safety and efficacy compared to intratumoral or peritumoral administration of free immunotherapy drugs. However, these approaches suffer from short-lived tumour stimulation (typically only a few days or less) because these agents are internalized by tumour cells or released out of the tumour microenvironment (TME)178. Biomaterial-based treatments can extend the window of drug exposure and enable efficacy from single injections, which is important for treating deep visceral lesions that require surgical access. For example, IL-12 bearing a phosphoserine peptide tag enables stable high-avidity binding to aluminium hydroxide (alum), a common clinical vaccine adjuvant181. Intratumoural injection of cytokine-loaded alum particles allowed drug retention in tumours for more than 2 weeks, resulting in pronounced regressions and complete responses in several tumour models in mice (Fig. 5e). Similarly, spherical nucleic acids, nanoparticles with surface-conjugated oligonucleotides as ligands for TLR-9, led to strong immunomodulatory activity182. In a phase I b/2 clinical trial, as of the data cut-off date of July 1st 2021, two and one out of 14 patients had a complete and partial response, respectively, and side effects were mostly limited to injection site reactions and flu-like symptoms183,184 (NCT03684785; Table 1). Another promising approach consists of in situ vaccination using viral NPs derived from the virus cowpea mosaic virus, which shifts the immune composition of the TME towards a pro-inflammatory profile185,186.
Biomaterials are also being considered as depots for the local release of immunotherapy payloads to the TME (Fig. 5e). For example, chitosan microparticles were developed that following intratumoural injection released IL-12 over 1–2 weeks and elicited complete tumour regression in 80–100% of animals in murine cancer models187,188. Highlighting its synergistic potential, in pancreatic cancer models in mice, intratumoral injection of IL-12-loaded polymer microspheres at 24 hr following local stereotactic body radiation therapy resulted in increased TME reprogramming, systemic T cell responses and antitumor efficacy compared with IL-12-loaded microspheres or radiation only treatments. 189. Hydrogels releasing innate immune stimulating small molecule drugs, such as TLR7/8 or STING agonists, decreased tumour recurrence when implanted at surgical resection sites in mouse models of breast and lung cancer190. Sprayable fibrin hydrogels releasing an anti-CD47 antibody at tumour resection sites stimulated macrophage phagocytic activity while neutralizing the acidic pH at the tumour site, triggering myeloid cells to eliminate residual tumour cells191.
Biomaterials have also been used to deliver and locally stimulate adoptively transferred T cells in tumours. For example, intratumoural implantation of alginate hydrogels loaded with polyclonal tumour-specific T cells or CAR T cells, cytokines and immunostimulatory antibodies, led to enhanced tumour elimination in mouse models of breast and ovarian cancer192. In the adjuvant setting, hyaluronic acid hydrogels encapsulating CAR T cells together with stimulatory factors for localized immunostimulation implanted into the tumour bed following surgical resection protected against tumour recurrence and inhibited distant tumour growth193. In another report, nitinol metal scaffolds, a material routinely used as cardiovascular stents, were functionalized with T cell-stimulatory antibodies and loaded with CAR T cells for implantation peritumorally. In a mouse model of non-resectable ovarian cancer, these scaffolds eradicated tumours in 70% of animals and extended the average tumour survival time 2.7-fold compared with untreated controls194. All of the described materials have different in vivo lifetimes and might require surgical retrieval. Clinical implications are still unclear as none of these approaches have entered clinical testing yet.
Engineered materials can also be used to increase uptake and promote cytosolic delivery of therapeutics in immune cells following localized delivery to tumour sites. For example, intratumoural administration of endosome-disrupting polymersomes delivering cyclic dinucleotides into phagocytes improved STING activation, indicated by an 11-fold decrease in melanoma growth rate and complete tumour rejection in one-third of the mice 195. Similarly, intratumoural injection of CDN-loaded lipid-calcium phosphate NPs surface-treated with phosphatidyl serine, promoted CDN uptake by myeloid cells and DCs in the pleural space in a mouse model of malignant pleural effusion 196. mRNA also requires cytosolic delivery to immune cells (Fig. 5e). Intratumoural injection of mixtures of LNPs delivering mRNA encoding cytokines and membrane-bound T cell costimulatory receptor OX40L, led to pronounced immune activation and complete tumour regression in 50% of animals bearing s.c. hepatoma tumours197. Similarly, intratumoural injection of saline formulations or polymer NPs delivering mRNAs encoding selected potent cytokine combinations (e.g. IL-12, granulocyte-macrophage colony-stimulating factor, IL-15, and IFN-α) led to robust tumour regressions and induction of systemic T cell responses that regressed even distal untreated lesions198,199. Interim data from a follow-up phase I clinical trial showed that LNPs encapsulating mRNAs encoding OX40L, IL-23 and IL-36γ, in combination with a PD-1 immune checkpoint inhibitor, resulted in increased levels of proinflammatory cytokines in both plasma and tumour biopsies (NCT03739931; Table 1)200. These LNP formulations were shown to primarily transduce cancer cells and myeloid cells. Another approach is to deliver mRNA directly into tumour-infiltrating T-cells; LNPs incorporating ionizable lipids transfected up to over 5 and 10% of tumour infiltrating CD4+ and CD8+ primary T cells respectively following intratumoral injection in a murine melanoma model, and when used to deliver mRNA encoding OX40 in combination with systemic administration of anti-OX40 agonist antibodies, resulted in 60% tumour rejection in a murine lymphoma model.133.
Targeting sites of inflammation
Immune cells are central to the pathology of inflammatory diseases, therefore, targeting them at sites of inflammation is a potentially effective strategy for disease treatment. Phagocytes are involved in generating persistent inflammation, thereby providing a first-line target choice for drug development. 201For example, polymersomes, vesicles formed by the self-assembly of amphiphilic block copolymers, were efficiently taken up by neutrophils through scavenger receptors202. Using pH-responsive polymersomes that disrupt endosomes in response to acidification of these compartments, cytosolic delivery of cyclin-dependent kinase inhibitor (R)-roscovitine induced neutrophil apoptosis and suppressed inflammation in a sterile injury zebrafish model. Immune cells can also serve as chaperones to concentrate nanocarriers at sites of inflammation and angiogenesis; for example, i.v. administration of nanoemulsions functionalized with cyclic RGD peptides that bind to αVβ3 integrins expressed on inflamed blood vessels rapidly adhered to circulating neutrophils and monoyctes in a mouse model of acute wound-derived inflammation within minutes. These NPs also directly bound to the endothelium of inflamed vessels at later times, suggesting transfer from leukocytes to endothelial cells203. Neutrophils are known to effectively capture opsonized particles in the blood, a process exploited to target these cells at sites of lung inflammation using NPs of different size, charge and composition, provided they are rapidly opsonized with complement upon injection201 (Fig. 5f). I.v.-injected synthetic high density lipoprotein NPs carrying the mammalian target of rapamycin (mTOR) inhibitor efficiently targeted myeloid cells and blocked the expression of inflammatory cytokines by macrophages infiltrating heart allografts, resulting in long-term allograft survival204. Interestingly, i.v.-injected empty PLGA NPs were reported to be taken up by monocytes and neutrophils in the blood, leading to their reprogramming into an anti-inflammatory phenotype and localization to sites of spinal cord injury, promoting a pro-regenerative milieu that enabled axon recovery205.
Biomaterials are also being explored to modulate adaptive immune responses at tissue transplant sites. For example, biodegradable polymer microspheres releasing TGF-β and naïve CD4+ T cells promoted Treg development in vitro, and promoted Treg infiltration of allogeneic pancreatic β cell transplants for treatment of an in vivo diabetes model206. In a rat model of vascularized hindlimb allogeneic tissue grafting, local administration of microspheres releasing TGF-β, rapamycin and IL-2 promoted induction and expansion of Tregs at the implant site, leading to long-term tissue engraftment207.
LNs draining sites of disease are also modulated by upstream inflammation. For example, high endothelial venules (HEVs) are specialized blood vessels of the LNs that express the lymphocyte homing protein peripheral node addressin (PNAd), which supports naïve lymphocyte entry into LNs. Notably, PNAd expression is upregulated in LNs draining sites of chronic inflammation208. Exploiting this process, i.v.-injected PLGA microparticles carrying the immunosuppressive drug tacrolimus and functionalized with a PNAd-targeting antibody enabled accumulation of the drug at LNs draining the transplanted tissues209. Because microparticles are too large to passively transport across the endothelium, this finding suggests particle accumulation on the luminal surfaces of the HEVs, followed by drug diffusion into the tissue across the endothelial barrier. Building on these findings, PNAd-targeted PLGA NPs were found not only bind to the HEVs of inflamed dLNs, but were also further transported into the LN parenchyma, leading to substantially prolonged allograft survival in a model of MHC-mismatched heart tissue transplantation210 (Fig. 5f).
Outlook
Biomaterials-mediated targeting of immune cells and tissues is starting to have clinical impact, holding great promise for the future of vaccines and immunotherapy. However, there remain important fundamental aspects of immunology to be understood, and a number of technological and translational drawbacks remain to be solved for these technologies to reach their full potential.
For example, there is still much to learn about the dynamics of immune cells during disease and treatment. The reciprocal trafficking of lymphocytes from LNs to blood and tumours, as well as between tumours, has received limited attention211,212. However, this information is essential for the design of targeted immune-oncology therapeutics that will ‘hit’ cells at a desired location or timepoint in their life cycle. The role of stem-like CD8+ T cells in diseases, such as autoimmunity213, infections214 and cancer215,216, is gaining particular attention, because these cells appear to play an important role as responders to immunotherapies such as checkpoint blockade214,217, by producing progeny that are important effector cells (either in LNs or directly at peripheral tissue sites). These cells are promising targets for immunotherapy, but how they can be generated and expanded in vivo remains poorly understood.
Biomaterial interactions with the immune system also need further assessment. The SARS-CoV-2 pandemic revealed that LNPs used for mRNA delivery have direct adjuvant activity in vaccines218–220 by triggering inflammatory cytokine production in LNs; however, the exact mechanisms remain to be elucidated. Other biomaterials, for example, biodegradable polymers, such as PLGA, are generally considered passive scaffolds or drug-release matrices; however, lactic acid produced by PLGA hydrolysis is known to be immunomodulatory,221–223 and PLGA particles promote a pro-regenerative phenotypic state when phagocytosed by innate immune cells205. These effects can promote tolerance in conditions such as autoimmune disease224, but would be undesirable in other settings (e.g., cancer immunotherapy).
One reality that the field of biomaterials-based immune therapies must face is that complex multicomponent formulations requiring the production of multiple recombinant proteins and exotic multi-step chemistries using different clinical-grade materials, are unlikely to be clinically translated owing to the prohibitive cost and complexities involved in the good manufacturing practice (GMP) of such systems. To ensure clinical translation, “less is more” should be a motivating mantra. This reality is evident by looking at the technologies undergoing clinical trials (Table 1). Furthermore, different technologies are often confined to the expertise of individual laboratories. Sharing methods in the field will enable broader experimental validation and testing in different disease models.
The success of LNP-delivered SARS-CoV-2 mRNA vaccines provides an important proof-of-concept in humans, but there is much room for improvement to realize their full potential. Despite important advances over the past 20 years, LNPs remain substantially inferior to biological vectors, such as viruses, at transfecting cells. Systemic or intratumoural injection of LNPs to deliver mRNA to T cells transfects only 2% or less of T cells131,198. In addition, what makes LNPs suitable for packaging nucleic acids (for example, their charge) also makes them prone to opsonization and non-specific binding to cells and matrices. Moreover, allergic reactions elicited in a small proportion of individuals with current PEG-stabilized LNP formulations represent a potential safety issue 225,226. Despite the clinical success of LNPs as delivery materials for mRNA and siRNA, other materials, such as endosome-responsive polymers,227–229 can outperform LNPs for nucleic acid delivery in some cell types. Furthermore, combinatorial library studies of LNP compositions revealed that LNP formulations can be tuned to target specific tissues and immune cell types without even the need for specific antibody or other binder-based targeting201,230,231. Materials with improved delivery efficiency will increase the utility of mRNA, as well as DNA and CRISPR-based gene editing systems.
Despite existing knowledge gaps and challenges, the use of biomaterials to enable cell- and organ-specific targeted immune modulation is an important and exciting area of research and clinical development, which can unlock the full potential of immunotherapies for different diseases.
Figure 6.
Targeting tissue-resident immune cells.
Box 1 |. Defining optimal immune cell targets for disease modulation.
Modulation of the immune system can only be effective if the targeted cells and their responses are well understood. For example, different phenotypic and functional states exist for antigen-specific T cells; Inactivated precursors, early activated cells (including short-lived and memory precursor effector cells), activated effector cells, memory cells and memory stem cells and ‘exhausted’ effector cells233 have been defined, all with different functionality and ability to respond to immunostimulation. ‘Stem-like’ CD8+ T cells, in particular, share characteristics between memory cells and more activated cells 215–217,234,235, and express specific transcriptional and protein signatures (for example, T cell factor-1 (TCF-1+), programmed cell death protein 1 (PD-1+) and self-ligand receptor of the signalling lymphocytic activation molecule 6 (SLAM6+)), which are important modulators of anti-tumour immune response. Fundamental studies identifying these important but possibly rare cell types will be required to develop targeted immunotherapies and vaccines.
Key points.
In immunotherapy, choosing the right target cell, tissue and treatment duration is essential to ensure effective immunomodulation while avoiding toxicity
Biomaterials-mediated targeting of immune cells in lymph nodes improves the potency and efficacy of vaccines by promoting immunity or tolerance
Circulating migratory immune cells can be targeted to perform as living chaperones to carry therapeutics into tissues
Systemic administration or intratumoural injection of nanomaterials and therapeutic depots can selectively accumulate and target immune cells in tumours
Reducing biomaterial complexity is essential to facilitate clinical translation
Acknowledgements
This work was supported in part by the Marble Center for Nanomedicine, the Ragon Institute of MGH, MIT, and Harvard, the NIH (awards CA247632, EB031082, U01-CA265706, AI147845, AI162307, AI161297, and CA235375 to DJI), and the Mark Foundation for Cancer Research. This material is based upon work supported in part by the U. S. Army Research Office through the Institute for Soldier Nanotechnologies at MIT, under Cooperative Agreement Number W911NF-1-2-0048. DJI is an investigator of the Howard Hughes Medical Institute.
Footnotes
Competing interests
DJ is an inventor on patents related to “albumin hitchhiking” discussed under lymph node targeting, nanoparticle modification of T cells discussed under “Backpacking adoptively transferred cells” and alum-binding cytokines discussed under “Intratumoural delivery of biomaterials”. These patents have been licensed to Elicio Therapeutics, Repertoire Immune Medicines and Ankyra Therapeutics, respectively, and DJI holds equity in these companies. The remaining authors declare no competing interests.
References
- 1.Urquhart L Top companies and drugs by sales in 2021. Nat Rev Drug Discov 21, 251–251 (2022). [DOI] [PubMed] [Google Scholar]
- 2.Dougan M, Luoma AM, Dougan SK & Wucherpfennig KW Understanding and treating the inflammatory adverse events of cancer immunotherapy. Cell (2021) doi: 10.1016/j.cell.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seung E et al. A trispecific antibody targeting HER2 and T cells inhibits breast cancer growth via CD4 cells. Nature 603, 328–334 (2022). [DOI] [PubMed] [Google Scholar]
- 4.Muik A et al. Preclinical Characterization and Phase I Trial Results of a Bispecific Antibody Targeting PD-L1 and 4–1BB (GEN1046) in Patients with Advanced Refractory Solid TumorsGEN1046, a Bispecific Antibody Targeting PD-L1 and 4–1BB. Cancer Discov 12, 1248–1265 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neri D Antibody-Cytokine Fusions: Versatile Products for the Modulation of Anticancer Immunity. Cancer Immunology Research 7, 348–354 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.II DSJ et al. Cell surface–tethered IL-12 repolarizes the tumor immune microenvironment to enhance the efficacy of adoptive T cell therapy. Sci Adv 8, eabi8075 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ren Z et al. Selective delivery of low-affinity IL-2 to PD-1+ T cells rejuvenates antitumor immunity with reduced toxicity. J Clin Investigation 132, e153604 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tzeng A, Kwan BH, Opel CF, Navaratna T & Wittrup KD Antigen specificity can be irrelevant to immunocytokine efficacy and biodistribution. Proc National Acad Sci 112, 3320–3325 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reddy ST et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol 25, 1159–1164 (2007). [DOI] [PubMed] [Google Scholar]
- 10.McLennan DN, Porter CJH & Charman SA Subcutaneous drug delivery and the role of the lymphatics. Drug Discov Today Technologies 2, 89–96 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Schudel A, Francis DM & Thomas SN Material design for lymph node drug delivery. Nat Rev Mater 4, 415–428 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta NK et al. Pharmacokinetic tuning of protein–antigen fusions enhances the immunogenicity of T-cell vaccines. Nat Biomed Eng 4, 636–648 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kourtis IC et al. Peripherally Administered Nanoparticles Target Monocytic Myeloid Cells, Secondary Lymphoid Organs and Tumors in Mice. PLoS ONE 8, e61646 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silva M et al. A particulate saponin/TLR agonist vaccine adjuvant alters lymph flow and modulates adaptive immunity. Sci Immunol 6, eabf1152 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moon JJ et al. Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proc National Acad Sci 109, 1080–1085 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyoglu-Barnum S et al. Quadrivalent influenza nanoparticle vaccines induce broad protection. Nature 1–6 (2021) doi: 10.1038/s41586-021-03365-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jardine JG et al. HIV-1 broadly neutralizing antibody precursor B cells revealed by germline-targeting immunogen. Science 351, 1458–1463 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walls AC et al. Elicitation of potent neutralizing antibody responses by designed protein nanoparticle vaccines for SARS-CoV-2. Cell 183, 1367–1382.e17 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcandalli J et al. Induction of potent neutralizing antibody responses by a designed protein nanoparticle vaccine for respiratory syncytial virus. Cell 176, 1420–1431.e17 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walls AC et al. Elicitation of broadly protective sarbecovirus immunity by receptor-binding domain nanoparticle vaccines. Cell 184, 5432–5447.e16 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houser KV et al. Safety and immunogenicity of a ferritin nanoparticle H2 influenza vaccine in healthy adults: a phase 1 trial. Nat Med 28, 383–391 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song JY et al. Safety and immunogenicity of a SARS-CoV-2 recombinant protein nanoparticle vaccine (GBP510) adjuvanted with AS03: a phase 1/2, randomized, placebo-controlled, observer-blinded trial. Medrxiv 2022.March.30.22273143 (2022) doi: 10.1101/2022.03.30.22273143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuai R, Ochyl LJ, Bahjat KS, Schwendeman A & Moon JJ Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat Mater 16, 489–496 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karabin NB et al. Sustained micellar delivery via inducible transitions in nanostructure morphology. Nat Commun 9, 624 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heath PT et al. Safety and Efficacy of NVX-CoV2373 Covid-19 Vaccine. New Engl J Med (2021) doi: 10.1056/nejmoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu H et al. Structure-based programming of lymph-node targeting in molecular vaccines. Nature 507, 519–522 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moynihan KD et al. Enhancement of peptide vaccine immunogenicity by increasing lymphatic drainage and boosting serum stability. Cancer Immunology Research 6, 1025–1038 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rakhra K et al. Exploiting albumin as a mucosal vaccine chaperone for robust generation of lung-resident memory T cells. Sci Immunol 6, eabd8003 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vrieze JD et al. Potent lymphatic translocation and spatial control over innate immune activation by polymer-lipid amphiphile conjugates of small-molecule TLR7/8 agonists. Angewandte Chemie International Edition 58, 15390–15395 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Pant S et al. First-in-human phase 1 trial of ELI-002 immunotherapy as treatment for subjects with Kirsten rat sarcoma (KRAS)-mutated pancreatic ductal adenocarcinoma and other solid tumors. Journal of Clinical Oncology 40, TPS2701–TPS2701 (2022). [Google Scholar]
- 31.Cao S et al. Hybrid nanocarriers incorporating mechanistically distinct drugs for lymphatic CD4+ T cell activation and HIV-1 latency reversal. Sci Adv 5, eaav6322 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Capini C et al. Antigen-specific suppression of inflammatory arthritis using liposomes. J Immunol 182, 3556–3565 (2009). [DOI] [PubMed] [Google Scholar]
- 33.Zhou K et al. Targeting peripheral immune organs with self-assembling prodrug nanoparticles ameliorates allogeneic heart transplant rejection. Am J Transplant 21, 3871–3882 (2021). [DOI] [PubMed] [Google Scholar]
- 34.Kishimoto TK & Maldonado RA Nanoparticles for the induction of antigen-specific immunological tolerance. Front Immunol 9, 230 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maldonado RA et al. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc National Acad Sci 112, E156–E165 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ali OA, Huebsch N, Cao L, Dranoff G & Mooney DJ Infection-mimicking materials to program dendritic cells in situ. Nat Mater 8, 151–158 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ali OA, Emerich D, Dranoff G & Mooney DJ In situ regulation of DC subsets and T cells mediates tumor regression in mice. Sci Transl Med 1, 8ra19 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shah NJ et al. A biomaterial-based vaccine eliciting durable tumour-specific responses against acute myeloid leukaemia. Nat Biomed Eng 4, 40–51 (2020). [DOI] [PubMed] [Google Scholar]
- 39.Kim J et al. Injectable, spontaneously assembling inorganic scaffolds modulate immune cells in vivo and increase vaccine efficacy. Nat Biotechnol 33, 64–72 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Super M et al. Biomaterial vaccines capturing pathogen-associated molecular patterns protect against bacterial infections and septic shock. Nat Biomed Eng 6, 8–18 (2022). [DOI] [PubMed] [Google Scholar]
- 41.Lee JA et al. Recruitment of dendritic cells using ‘find-me’ signaling microparticles for personalized cancer immunotherapy. Biomaterials 282, 121412 (2022). [DOI] [PubMed] [Google Scholar]
- 42.Lewis JS et al. A combination dual-sized microparticle system modulates dendritic cells and prevents type 1 diabetes in prediabetic NOD mice. Clin Immunol 160, 90–102 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allen R, Chizari S, Ma JA, Raychaudhuri S & Lewis JS Combinatorial, microparticle-based delivery of immune modulators reprograms the dendritic cell phenotype and promotes remission of collagen-induced arthritis in mice. Acs Appl Bio Mater 2, 2388–2404 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Cho JJ et al. An antigen-specific semi-therapeutic treatment with local delivery of tolerogenic factors through a dual-sized microparticle system blocks experimental autoimmune encephalomyelitis. Biomaterials 143, 79–92 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerner MY, Casey KA, Kastenmuller W & Germain RN Dendritic cell and antigen dispersal landscapes regulate T cell immunity. The Journal of Experimental Medicine jem.20170335–25 (2017) doi: 10.1084/jem.20170335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eickhoff S et al. Robust anti-viral immunity requires multiple distinct T cell-dendritic cell interactions. Cell 162, 1322–1337 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Junt T et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature 450, 110–114 (2007). [DOI] [PubMed] [Google Scholar]
- 48.Gretz JE, Norbury CC, Anderson AO, Proudfoot AEI & Shaw S Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J Exp Med 192, 1425–1440 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sixt M et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity 22, 19–29 (2005). [DOI] [PubMed] [Google Scholar]
- 50.Roozendaal R et al. Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity 30, 264–276 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reynoso GV et al. Lymph node conduits transport virions for rapid T cell activation. Nat Immunol 20, 602–612 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim EH et al. Squalene emulsion-based vaccine adjuvants stimulate CD8 T cell, but not antibody responses, through a RIPK3-dependent pathway. Elife 9, e52687 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Detienne S et al. Central role of CD169+ lymph node resident macrophages in the adjuvanticity of the QS-21 component of AS01. Sci Rep-uk 6, 1–14 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Y-N, Poon W, Sefton E & Chan WCW Suppressing subcapsular sinus macrophages enhances transport of nanovaccines to lymph node follicles for robust humoral immunity. Acs Nano (2020) doi: 10.1021/acsnano.0c02240. [DOI] [PubMed] [Google Scholar]
- 55.Schudel A et al. Programmable multistage drug delivery to lymph nodes. Nat Nanotechnol 15, 491–499 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hafiz MM et al. Immunosuppression and Procedure-Related Complications in 26 Patients with Type 1 Diabetes Mellitus Receiving Allogeneic Islet Cell Transplantation. Transplantation 80, 1718–1728 (2005). [DOI] [PubMed] [Google Scholar]
- 57.Burke JA et al. Subcutaneous nanotherapy repurposes the immunosuppressive mechanism of rapamycin to enhance allogeneic islet graft viability. Nat Nanotechnol 17, 319–330 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kenison JE et al. Tolerogenic nanoparticles suppress central nervous system inflammation. Proc National Acad Sci 202016451 (2020) doi: 10.1073/pnas.2016451117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lynn GM et al. Peptide–TLR-7/8a conjugate vaccines chemically programmed for nanoparticle self-assembly enhance CD8 T-cell immunity to tumor antigens. Nat Biotechnol 38, 1–19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilson DS et al. Antigens reversibly conjugated to a polymeric glyco-adjuvant induce protective humoral and cellular immunity. Nature Materials 18, 1–18 (2019). [DOI] [PubMed] [Google Scholar]
- 61.Shae D et al. Co-delivery of peptide neoantigens and stimulator of interferon genes (STING) agonists enhances response to cancer vaccines. Acs Nano (2020) doi: 10.1021/acsnano.0c02765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang S et al. Rational vaccinology with spherical nucleic acids. Proceedings Of The National Academy Of Sciences Of The United States Of America 33, 201902805–9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Callmann CE et al. Tumor cell lysate-loaded immunostimulatory spherical nucleic acids as therapeutics for triple-negative breast cancer. Proc National Acad Sci 202005794 (2020) doi: 10.1073/pnas.2005794117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Froimchuk E, Oakes RS, Kapnick SM, Yanes AA & Jewell CM Biophysical properties of self-assembled immune signals impact signal processing and the nature of regulatory immune function. Nano Lett 21, 3762–3771 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hess KL et al. Engineering immunological tolerance using quantum dots to tune the density of self-antigen display. Adv Funct Mater 27, 1700290 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liang Y, Zhang T & Tang M Toxicity of quantum dots on target organs and immune system. J Appl Toxicol 42, 17–40 (2022). [DOI] [PubMed] [Google Scholar]
- 67.Kourtis IC et al. Peripherally Administered Nanoparticles Target Monocytic Myeloid Cells, Secondary Lymphoid Organs and Tumors in Mice. PLoS ONE 8, e61646 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tokatlian T et al. Innate immune recognition of glycans targets HIV nanoparticle immunogens to germinal centers. Science 363, 649–654 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Read BJ et al. Mannose-binding lectin and complement mediate follicular localization and enhanced immunogenicity of diverse protein nanoparticle immunogens. Cell Reports 38, 110217 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Venkatesan P Preliminary phase 1 results from an HIV vaccine candidate trial. Lancet Microbe 2, e95 (2021). [DOI] [PubMed] [Google Scholar]
- 71.Zhang Y-N et al. Nanoparticle size influences antigen retention and presentation in lymph node follicles for humoral immunity. Nano Lett 19, 7226–7235 (2019). [DOI] [PubMed] [Google Scholar]
- 72.Veneziano R et al. Role of nanoscale antigen organization on B-cell activation probed using DNA origami. Nat Nanotechnol 15, 716–723 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xiao P et al. Engineering nanoscale artificial antigen-presenting cells by metabolic dendritic cell labeling to potentiate cancer immunotherapy. Nano Lett 21, 2094–2103 (2021). [DOI] [PubMed] [Google Scholar]
- 74.Ma L et al. Enhanced CAR–T cell activity against solid tumors by vaccine boosting through the chimeric receptor. Science 365, 162–168 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pennock ND, Kedl JD & Kedl RM T Cell Vaccinology: Beyond the Reflection of Infectious Responses. TRENDS in Immunology 37, 170–180 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ols S et al. Route of Vaccine Administration Alters Antigen Trafficking but Not Innate or Adaptive Immunity. Cell Reports 30, 3964–3971.e7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Golombek SK et al. Tumor targeting via EPR: Strategies to enhance patient responses. Advanced Drug Delivery Reviews 130, 17–38 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kranz LM et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 534, 396–401 (2016). [DOI] [PubMed] [Google Scholar]
- 79.Beyaert S, Machiels J-P & Schmitz S Vaccine-based immunotherapy for head and neck cancers. Cancers 13, 6041 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sahin U et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature 585, 107–112 (2020). [DOI] [PubMed] [Google Scholar]
- 81.Baharom F et al. Intravenous nanoparticle vaccination generates stem-like TCF1+ neoantigen-specific CD8+ T cells. Nat Immunol 1–12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Creemers JHA et al. Assessing the safety, tolerability and efficacy of PLGA-based immunomodulatory nanoparticles in patients with advanced NY-ESO-1-positive cancers: a first-in-human phase I open-label dose-escalation study protocol. Bmj Open 11, e050725 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kishimoto TK et al. Improving the efficacy and safety of biologic drugs with tolerogenic nanoparticles. Nature Nanotechnology 11, 890–899 (2016). [DOI] [PubMed] [Google Scholar]
- 84.Sands E et al. Tolerogenic nanoparticles mitigate the formation of anti-drug antibodies against pegylated uricase in patients with hyperuricemia. Nat Commun 13, 272 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Prasad S et al. Tolerogenic Ag-PLG nanoparticles induce tregs to suppress activated diabetogenic CD4 and CD8 T cells. J Autoimmun 89, 112–124 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Luo X, Miller SD & Shea LD Immune tolerance for autoimmune disease and cell transplantation. Annual review of biomedical engineering 18, 181–205 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leon MA et al. Soluble antigen arrays displaying mimotopes direct the response of diabetogenic T cells. Acs Chem Biol 14, 1436–1448 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu Q et al. Use of polymeric nanoparticle platform targeting the liver to induce Treg-mediated antigen-specific immune tolerance in a pulmonary allergen sensitization model. ACS Nano 13, 4778–4794 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ishii Y et al. Alpha-galactosylceramide-driven immunotherapy for allergy. Front Biosci Volume, 6214 (2008). [DOI] [PubMed] [Google Scholar]
- 90.Duramad O, Laysang A, Li J, Ishii Y & Namikawa R Pharmacologic Expansion of Donor-Derived, Naturally Occurring CD4+Foxp3+ Regulatory T Cells Reduces Acute Graft-versus-Host Disease Lethality Without Abrogating the Graft-versus-Leukemia Effect in Murine Models. Biol Blood Marrow Tr 17, 1154–1168 (2011). [DOI] [PubMed] [Google Scholar]
- 91.Chen Y-B et al. Pharmacokinetics and Safety of KRN7000 Following Intravenous Infusion of RGI-2001 in Allogeneic Transplant Patients. Blood 136, 15–16 (2020). [Google Scholar]
- 92.Okhrimenko A et al. Human memory T cells from the bone marrow are resting and maintain long-lasting systemic memory. Proc National Acad Sci 111, 9229–9234 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mulder WJM, Ochando J, Joosten LAB, Fayad ZA & Netea MG Therapeutic targeting of trained immunity. Nature Reviews Drug Discovery 18, 1–14 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Netea MG et al. Trained immunity: A program of innate immune memory in health and disease. Science 352, aaf1098 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Netea MG & Meer JWM van der. Trained Immunity: An Ancient Way of Remembering. Cell host & microbe 21, 297–300 (2017). [DOI] [PubMed] [Google Scholar]
- 96.Priem B et al. Trained Immunity-Promoting Nanobiologic Therapy Suppresses Tumor Growth and Potentiates Checkpoint Inhibition. Cell 183, 786–801.e19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Finck AV, Blanchard T, Roselle CP, Golinelli G & June CH Engineered cellular immunotherapies in cancer and beyond. Nat Med 28, 678–689 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ribas A T cells as the future of cancer Therapy. Cancer Discovery (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hong M, Clubb JD & Chen YY Engineering CAR-T Cells for Next-Generation Cancer Therapy. Cancer Cell (2020) doi: 10.1016/j.ccell.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 100.Barros LRC et al. Systematic review of available CAR-T cell trials around the world. Cancers 14, 2667 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.CAR T Cells: Engineering Patients’ Immune Cells to Treat Their Cancers. https://www.cancer.gov/about-cancer/treatment/research/car-t-cells (2022).
- 102.Larson RC & Maus MV Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat Rev Cancer 21, 145–161 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Finck AV, Blanchard T, Roselle CP, Golinelli G & June CH Engineered cellular immunotherapies in cancer and beyond. Nat Med 28, 678–689 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Siriwon N et al. CAR-T cells surface-engineered with drug-encapsulated nanoparticles can ameliorate intratumoral T-cell hypofunction. Cancer Immunology Research 6, 812–824 (2018). [DOI] [PubMed] [Google Scholar]
- 105.Huang B et al. Active targeting of chemotherapy to disseminated tumors using nanoparticle-carrying T cells. Science Translational Medicine 7, 291ra94–291ra94 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Eskandari SK et al. Regulatory T cells engineered with TCR signaling–responsive IL-2 nanogels suppress alloimmunity in sites of antigen encounter. Sci Transl Med 12, eaaw4744 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stephan MT, Moon JJ, Um SH, Bershteyn A & Irvine DJ Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat Med 16, 1035–1041 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tang L et al. Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery. Nature Biotechnology 36, 707–716 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Romee R et al. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood 131, 2515–2527 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hamilton E et al. 801 PRIME™ IL-15 (RPTR-147): Preliminary clinical results and biomarker analysis from a first-in-human Phase 1 study of IL-15 loaded peripherally-derived autologous T cell therapy in solid tumor patients. J Immunother Cancer 8, A850–A850 (2020). [Google Scholar]
- 111.ShieldsIV CW et al. Cellular backpacks for macrophage immunotherapy. Sci Adv 6, eaaz6579 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Champion JA & Mitragotri S Role of target geometry in phagocytosis. Proceedings Of The National Academy Of Sciences Of The United States Of America 103, 4930–4934 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Berlin C et al. α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 74, 185–195 (1993). [DOI] [PubMed] [Google Scholar]
- 114.Mora JR et al. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature 424, 88–93 (2003). [DOI] [PubMed] [Google Scholar]
- 115.Cao S, Jiang Y, Zhang H, Kondza N & Woodrow KA Core-shell nanoparticles for targeted and combination antiretroviral activity in gut-homing T cells. Nanomed Nanotechnol Biology Medicine 14, 2143–2153 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dammes N et al. Conformation-sensitive targeting of lipid nanoparticles for RNA therapeutics. Nat Nanotechnol 16, 1030–1038 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Parayath NN, Parikh A & Amiji MM Repolarization of tumor-associated macrophages in a genetically engineered nonsmall cell lung cancer model by intraperitoneal administration of hyaluronic acid-based nanoparticles encapsulating microRNA-125b. Nano Lett 18, 3571–3579 (2018). [DOI] [PubMed] [Google Scholar]
- 118.Sofias AM et al. Tumor targeting by αvβ3 integrin-specific lipid nanoparticles occurs via phagocyte hitchhiking. Acs Nano 14, 7832–7846 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hou J et al. Accessing neuroinflammation sites: Monocyte/neutrophil-mediated drug delivery for cerebral ischemia. Sci Adv 5, eaau8301 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tsai S et al. Reversal of autoimmunity by boosting memory-like autoregulatory T cells. Immunity 32, 568–580 (2010). [DOI] [PubMed] [Google Scholar]
- 121.Singha S et al. Peptide–MHC-based nanomedicines for autoimmunity function as T-cell receptor microclustering devices. Nat Nanotechnol 12, 701–710 (2017). [DOI] [PubMed] [Google Scholar]
- 122.Umeshappa CS et al. Suppression of a broad spectrum of liver autoimmune pathologies by single peptide-MHC-based nanomedicines. Nat Commun 10, 2150 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rhodes KR et al. Biodegradable Cationic Polymer Blends for Fabrication of Enhanced Artificial Antigen Presenting Cells to Treat Melanoma. Acs Appl Mater Inter 13, 7913–7923 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rhodes KR, Meyer RA, Wang J, Tzeng SY & Green JJ Biomimetic tolerogenic artificial antigen presenting cells for regulatory T cell induction. Acta Biomater 112, 136–148 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yip A & Webster RM The market for chimeric antigen receptor T cell therapies. Nat Rev Drug Discov 17, 161–162 (2018). [DOI] [PubMed] [Google Scholar]
- 126.Agarwal S, Weidner T, Thalheimer FB & Buchholz CJ In vivo generated human CAR T cells eradicate tumor cells. Oncoimmunology 8, 1–7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Parayath NN & Stephan MT In situ programming of CAR T cells. Annu Rev Biomed Eng 23, 1–21 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nayak S & Herzog RW Progress and prospects: immune responses to viral vectors. Gene Ther 17, 295–304 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Barnes C, Scheideler O & Schaffer D Engineering the AAV capsid to evade immune responses. Curr Opin Biotech 60, 99–103 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hinderer C et al. Severe toxicity in nonhuman primates and piglets following high-dose intravenous administration of an adeno-associated virus vector expressing human SMN. Hum Gene Ther 29, 285–298 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Smith TT et al. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat Nanotechnol 12, 813–820 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rurik JG et al. CAR T cells produced in vivo to treat cardiac injury. Science 375, 91–96 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li W et al. Biomimetic nanoparticles deliver mRNAs encoding costimulatory receptors and enhance T cell mediated cancer immunotherapy. Nat Commun 12, 7264 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Billingsley MM et al. Ionizable lipid nanoparticle-mediated mRNA delivery for human CAR T cell engineering. Nano Lett 20, 1578–1589 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McKinlay CJ, Benner NL, Haabeth OA, Waymouth RM & Wender PA Enhanced mRNA delivery into lymphocytes enabled by lipid-varied libraries of charge-altering releasable transporters. Proc National Acad Sci 115, 201805358 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Agarwalla P et al. Bioinstructive implantable scaffolds for rapid in vivo manufacture and release of CAR-T cells. Nat Biotechnol 1–9 (2022) doi: 10.1038/s41587-022-01245-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mora JR et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science 314, 1157–1160 (2006). [DOI] [PubMed] [Google Scholar]
- 138.Allie SR et al. The establishment of resident memory B cells in the lung requires local antigen encounter. Nat Immunol 20, 97–108 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kiyono H & Fukuyama S NALT- versus PEYER’S-patch-mediated mucosal immunity. Nat Rev Immunol 4, 699–710 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bowen A, Sweeney EE & Fernandes R Nanoparticle-based immunoengineered approaches for combating HIV. Front Immunol 11, 789 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li M et al. Mucosal vaccines: Strategies and challenges. Immunol Lett 217, 116–125 (2019). [DOI] [PubMed] [Google Scholar]
- 142.Iwasaki A Exploiting mucosal immunity for antiviral vaccines. Annual Review of Immunology 34, 575–608 (2016). [DOI] [PubMed] [Google Scholar]
- 143.Sockolosky JT & Szoka FC The neonatal Fc receptor, FcRn, as a target for drug delivery and therapy. Adv Drug Deliver Rev 91, 109–124 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fieux M et al. FcRn as a transporter for nasal delivery of biologics: A systematic review. Int J Mol Sci 22, 6475 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hartwell BL et al. Intranasal vaccination via lipid-conjugated immunogens promotes antigen persistence and transmucosal uptake to drive mucosal and systemic immunity. Science Translational Medicine 14, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lee Y et al. Hyaluronic acid–bilirubin nanomedicine for targeted modulation of dysregulated intestinal barrier, microbiome and immune responses in colitis. Nature Materials 19, 1–15 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lázaro I. de & Mooney DJ Obstacles and opportunities in a forward vision for cancer nanomedicine. Nat Mater 1–11 (2021) doi: 10.1038/s41563-021-01047-7. [DOI] [PubMed] [Google Scholar]
- 148.Wilhelm S et al. Analysis of nanoparticle delivery to tumours. Nat Rev Mater 1, 16014 (2016). [Google Scholar]
- 149.Barber GN STING: infection, inflammation and cancer. Nat Rev Immunol 15, 760–770 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dane EL et al. STING agonist delivery by tumour-penetrating PEG-lipid nanodiscs primes robust anticancer immunity. Nat Mater 21, 710–720 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Miller IC et al. Enhanced intratumoural activity of CAR T cells engineered to produce immunomodulators under photothermal control. Nat Biomed Eng 5, 1348–1359 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Longmire M, Choyke PL & Kobayashi H Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine-uk 3, 703–717 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schmid D et al. T cell-targeting nanoparticles focus delivery of immunotherapy to improve antitumor immunity. Nature communications 8, 1–11 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Turk MJ, Waters DJ & Low PS Folate-conjugated liposomes preferentially target macrophages associated with ovarian carcinoma. Cancer letters 213, 165–172 (2004). [DOI] [PubMed] [Google Scholar]
- 155.Miller MA et al. Tumour-associated macrophages act as a slow-release reservoir of nano-therapeutic Pt(IV) pro-drug. Nat Commun 6, 1–13 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dai Q et al. Quantifying the ligand-coated nanoparticle delivery to cancer cells in solid tumors. ACS Nano 12, 8423–8435 (2018). [DOI] [PubMed] [Google Scholar]
- 157.Rodell CB et al. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat Biomed Eng 2, 1–15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kulkarni A et al. A designer self-assembled supramolecule amplifies macrophage immune responses against aggressive cancer. Nature Biomedical Engineering 2, 1–15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang F et al. Genetic programming of macrophages to perform anti-tumor functions using targeted mRNA nanocarriers. Nat Commun 10, 3974 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gustafson HH, Holt-Casper D, Grainger DW & Ghandehari H Nanoparticle uptake: The phagocyte problem. Nano Today 10, 487–510 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chen D et al. NIR-II fluorescence imaging reveals bone marrow retention of small polymer nanoparticles. Nano Lett 21, 798–805 (2021). [DOI] [PubMed] [Google Scholar]
- 162.Sorensen AG et al. A “vascular normalization index” as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res 69, 5296–5300 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dickson PV et al. Bevacizumab-induced transient remodeling of the vasculature in neuroblastoma xenografts results in improved delivery and efficacy of systemically administered chemotherapy. Clin Cancer Res 13, 3942–3950 (2007). [DOI] [PubMed] [Google Scholar]
- 164.Willett CG et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med 10, 145–147 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Inai T et al. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am J Pathology 165, 35–52 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Goel S, Wong AH-K & Jain RK Vascular normalization as a therapeutic strategy for malignant and nonmalignant disease. Csh Perspect Med 2, a006486 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tong RT et al. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and Improves drug penetration in tumors. Cancer Research 11, 3731–3736 (2004). [DOI] [PubMed] [Google Scholar]
- 168.Boucher Y et al. Bevacizumab improves tumor infiltration of mature dendritic cells and effector T-cells in triple-negative breast cancer patients. Npj Precis Oncol 5, 62 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fukumura D, Kloepper J, Amoozgar Z, Duda DG & Jain RK Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol 15, 325–340 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Shigeta K et al. Regorafenib combined with PD1 blockade increases CD8 T-cell infiltration by inducing CXCL10 expression in hepatocellular carcinoma. J Immunother Cancer 8, e001435 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Manning EA et al. A Vascular endothelial growth factor receptor-2 inhibitor enhances antitumor immunity through an immune-based mechanism. Clin Cancer Res 13, 3951–3959 (2007). [DOI] [PubMed] [Google Scholar]
- 172.Yasuda S et al. Simultaneous blockade of programmed death 1 and vascular endothelial growth factor receptor 2 (VEGFR2) induces synergistic anti-tumour effect in vivo. Clin Exp Immunol 172, 500–506 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Li B et al. Vascular Endothelial Growth Factor Blockade Reduces Intratumoral Regulatory T Cells and Enhances the Efficacy of a GM-CSF–Secreting Cancer Immunotherapy. Clin Cancer Res 12, 6808–6816 (2006). [DOI] [PubMed] [Google Scholar]
- 174.Voron T et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med 212, 139–148 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Allen E et al. Combined antiangiogenic and anti–PD-L1 therapy stimulates tumor immunity through HEV formation. Sci Transl Med 9, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Huang Y et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc National Acad Sci 109, 17561–17566 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yang RK et al. Intratumoral hu14.18-IL-2 (IC) induces local and systemic antitumor effects that involve both activated T and NK cells as well as enhanced IC retention. (2012) doi: 10.4049/jimmunol.1200934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Momin N et al. Maximizing response to intratumoral immunotherapy in mice by tuning local retention. Nat Commun 13, 109 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Momin N et al. Anchoring of intratumorally administered cytokines to collagen safely potentiates systemic cancer immunotherapy. Sci Transl Med 11, eaaw2614 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ishihara J et al. Matrix-binding checkpoint immunotherapies enhance antitumor efficacy and reduce adverse events. Sci Transl Med 9, eaan0401 (2017). [DOI] [PubMed] [Google Scholar]
- 181.Agarwal Y et al. Intratumourally injected alum-tethered cytokines elicit potent and safer local and systemic anticancer immunity. Nat Biomed Eng 6, 129–143 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Huang ZN, Callmann CE, Cole LE, Wang S & Mirkin CA Synergistic Immunostimulation through the Dual Activation of Toll-like Receptor 3/9 with Spherical Nucleic Acids. Acs Nano 15, 13329–13338 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.O’Day S et al. 423 Safety and preliminary efficacy of intratumoral cavrotolimod (AST-008), a spherical nucleic acid TLR9 agonist, in combination with pembrolizumab in patients with advanced solid tumors. Regul Young Investigator Award Abstr A257.2–A258 (2020) doi: 10.1136/jitc-2020-sitc2020.0423. [DOI] [Google Scholar]
- 184.Exicure Provides Interim Results from Ongoing Phase 1b/2 Clinical Trial of Cavrotolimod (AST-008). Exhibit 99.2 https://sec.report/Document/0001698530-21-000079/exhibit992pressrelease0805.htm (2021). [Google Scholar]
- 185.Wang C, Fiering SN & Steinmetz NF Cowpea mosaic virus promotes anti-tumor activity and immune memory in a mouse ovarian tumor model. Adv Ther 2, 1900003 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lizotte PH et al. In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat Nanotechnol 11, 295–303 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Nguyen KG et al. Localized interleukin-12 for cancer immunotherapy. Front Immunol 11, 575597 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zaharoff DA, Hance KW, Rogers CJ, Schlom J & Greiner JW Intratumoral immunotherapy of established solid tumors with chitosan/IL-12. J Immunother 33, 697–705 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mills BN et al. Stereotactic body radiation and interleukin-12 combination therapy eradicates pancreatic tumors by repolarizing the immune microenvironment. Cell Reports 29, 406–421.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Park CG et al. Extended release of perioperative immunotherapy prevents tumor recurrence and eliminates metastases. Sci Transl Med 10, eaar1916 (2018). [DOI] [PubMed] [Google Scholar]
- 191.Chen Q et al. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat Nanotechnol 14, 89–97 (2018). [DOI] [PubMed] [Google Scholar]
- 192.Stephan SB et al. Biopolymer implants enhance the efficacy of adoptive T cell therapy. Nat Biotechnol 33, 97–101 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hu Q et al. Inhibition of post-surgery tumour recurrence via a hydrogel releasing CAR-T cells and anti-PDL1-conjugated platelets. Nat Biomed Eng 5, 1038–1047 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Coon ME, Stephan SB, Gupta V, Kealey CP & Stephan MT Nitinol thin films functionalized with CAR-T cells for the treatment of solid tumours. Nature Biomedical Engineering 528, 1–15 (2019). [DOI] [PubMed] [Google Scholar]
- 195.Shae D et al. Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nat Nanotechnol 14, 1–16 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Liu Y et al. Intrapleural nano-immunotherapy promotes innate and adaptive immune responses to enhance anti-PD-L1 therapy for malignant pleural effusion. Nat Nanotechnol 17, 206–216 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hewitt SL et al. Durable anticancer immunity from intratumoral administration of IL-23, IL-36γ, and OX40L mRNAs. Sci Transl Med 11, eaat9143 (2019). [DOI] [PubMed] [Google Scholar]
- 198.Hotz C et al. Local delivery of mRNA-encoding cytokines promotes antitumor immunity and tumor eradication across multiple preclinical tumor models. Sci Transl Med 13, eabc7804 (2021). [DOI] [PubMed] [Google Scholar]
- 199.Tzeng SY et al. In situ genetic engineering of tumors for long-lasting and systemic immunotherapy. P Natl Acad Sci Usa 117, 4043–4052 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Patel M et al. 539 Phase 1 study of mRNA-2752, a lipid nanoparticle encapsulating mRNAs encoding human OX40L/IL-23/IL-36γ, for intratumoral (ITu) injection+/−durvalumab in advanced solid tumors and lymphoma. (2021). [Google Scholar]
- 201.Myerson JW et al. Supramolecular arrangement of protein in nanoparticle structures predicts nanoparticle tropism for neutrophils in acute lung inflammation. Nat Nanotechnol 17, 86–97 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Robertson JD, Ward JR, Avila-Olias M, Battaglia G & Renshaw SA Targeting neutrophilic inflammation using polymersome-mediated cellular delivery. J Immunol 198, 3596–3604 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sofias AM et al. Cyclic Arginine–Glycine–Aspartate-Decorated Lipid Nanoparticle Targeting toward Inflammatory Lesions Involves Hitchhiking with Phagocytes. Adv Sci 8, 2100370 (2021). [Google Scholar]
- 204.Braza MS et al. Inhibiting inflammation with myeloid cell-specific nanobiologics promotes organ transplant acceptance. Immunity 49, 819–828.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Park J et al. Intravascular innate immune cells reprogrammed via intravenous nanoparticles to promote functional recovery after spinal cord injury. Proc National Acad Sci 116, 14947–14954 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Li Y et al. Immunosuppressive PLGA TGF-β1 microparticles induce polyclonal and antigen-specific regulatory T cells for local immunomodulation of allogeneic islet transplants. Front Immunol 12, 653088 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Fisher JD et al. Treg-inducing microparticles promote donor-specific tolerance in experimental vascularized composite allotransplantation. Proc National Acad Sci 116, 25784–25789 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hautz T et al. Lymphoid neogenesis in skin of human hand, nonhuman primate, and rat vascularized composite allografts. Transplant Int 27, 966–976 (2014). [DOI] [PubMed] [Google Scholar]
- 209.Azzi J et al. Targeted Delivery of Immunomodulators to Lymph Nodes. Cell Reports 15, 1202–1213 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bahmani B et al. Targeted delivery of immune therapeutics to lymph nodes prolongs cardiac allograft survival. The Journal of clinical investigation 128, 4770–4786 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Li Z et al. In vivo labeling reveals continuous trafficking of TCF-1+ T cells between tumor and lymphoid tissue. J Exp Med 219, e20210749 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Torcellan T et al. In vivo photolabeling of tumor-infiltrating cells reveals highly regulated egress of T-cell subsets from tumors. Proc National Acad Sci 114, 5677–5682 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Gearty SV et al. An autoimmune stem-like CD8 T cell population drives type 1 diabetes. Nature 602, 156–161 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Im SJ et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537, 417–421 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Connolly KA et al. A reservoir of stem-like CD8+ T cells in the tumor-draining lymph node preserves the ongoing anti-tumor immune response. Sci Immunol 6, eabg7836 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Schenkel JM et al. Conventional type I dendric cells maintain a reservoir of proliferative tumor-antigen specific TCF-1+ CD8+ T cells in tumor-draining lymph nodes. Immunity (2021) doi: 10.1016/j.immuni.2021.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Siddiqui I et al. Intratumoral Tcf1+PD-1+CD8+ T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity 50, 195–211.e10 (2018). [DOI] [PubMed] [Google Scholar]
- 218.Tahtinen S et al. IL-1 and IL-1ra are key regulators of the inflammatory response to RNA vaccines. Nat Immunol 1–11 (2022) doi: 10.1038/s41590-022-01160-y. [DOI] [PubMed] [Google Scholar]
- 219.Alameh M-G et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 54, 2877–2892.e7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Li C et al. Mechanisms of innate and adaptive immunity to the Pfizer-BioNTech BNT162b2 vaccine. Nat Immunol 1–13 (2022) doi: 10.1038/s41590-022-01163-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Yang X et al. Lactate-modulated immunosuppression of myeloid-derived suppressor cells contributes to the radioresistance of pancreatic cancer. Cancer Immunol Res (2020) doi: 10.1158/2326-6066.cir-20-0111. [DOI] [PubMed] [Google Scholar]
- 222.Certo M, Tsai C-H, Pucino V, Ho P-C & Mauro C Lactate modulation of immune responses in inflammatory versus tumour microenvironments. Nat Rev Immunol 21, 151–161 (2021). [DOI] [PubMed] [Google Scholar]
- 223.Sangsuwan R et al. Lactate exposure promotes immunosuppressive phenotypes in innate immune cells. Cell Mol Bioeng 13, 541–557 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Allen RP, Bolandparvaz A, Ma JA, Manickam VA & Lewis JS Latent, immunosuppressive nature of poly(lactic-co-glycolic acid) microparticles. Acs Biomater Sci Eng 4, 900–918 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Shimabukuro TT, Cole M & Su JR Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US—December 14, 2020-January 18, 2021. Jama 325, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Szebeni J et al. Applying lessons learned from nanomedicines to understand rare hypersensitivity reactions to mRNA-based SARS-CoV-2 vaccines. Nat Nanotechnol 17, 337–346 (2022). [DOI] [PubMed] [Google Scholar]
- 227.McKinlay CJ et al. Charge-altering releasable transporters (CARTs) for the delivery and release of mRNA in living animals. Proceedings Of The National Academy Of Sciences Of The United States Of America 114, E448–E456 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kowalski PS, Rudra A, Miao L & Anderson DG Delivering the messenger: advances in Technologies for therapeutic mRNA delivery. Mol Ther 27, 710–728 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kong N et al. Synthetic mRNA nanoparticle-mediated restoration of p53 tumor suppressor sensitizes p53-deficient cancers to mTOR inhibition. Sci Transl Med 11, eaaw1565 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Leuschner F et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nature Biotechnology 29, 1005–1010 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Cheng Q et al. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat Nanotechnol 15, 313–320 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hewitt SL et al. Intratumoral IL12 mRNA therapy promotes TH1 transformation of the tumor microenvironment. Clin Cancer Res 26, 6284–6298 (2020). [DOI] [PubMed] [Google Scholar]
- 233.Youngblood B, Hale JS & Ahmed R T-cell memory differentiation: insights from transcriptional signatures and epigenetics. Immunology 139, 277–284 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Gounari F & Khazaie K TCF-1: a maverick in T cell development and function. Nat Immunol 23, 671–678 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zhao X, Shan Q & Xue H-H TCF1 in T cell immunity: a broadened frontier. Nat Rev Immunol 22, 147–157 (2022). [DOI] [PubMed] [Google Scholar]


